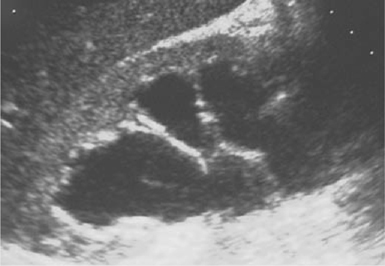
Fig. 10.1 Hydronephrosis as seen on renal ultrasonography.
Management of ureteric strictures (other than PUJO)
Pathophysiology of urinary tract obstruction
Dilatation of the renal pelvis and calyces (Fig. 10.1). When combined with dilatation of the ureters, known as hydroureteronephrosis. For causes, see Box 10.1.
Obstructive nephropathy is damage to the renal parenchyma, resulting from an obstruction to the flow of urine anywhere along the urinary tract.
Dilatation of the renal pelvis and calyces can occur without obstruction, and therefore, hydronephrosis should not be taken to necessarily imply the presence of obstructive uropathy.
•False negative (i.e. obstruction present, no hydronephrosis): acute onset of obstruction; in the presence of an intrarenal collecting system; with dehydration; misdiagnosis of dilatation of the calyces as renal cortical cysts (in acute ureteric colic, ultrasonography fails to detect hydronephrosis in up to 35% of patients with proven acute obstruction on IVU).
•False positive (i.e. hydronephrosis, no obstruction): capacious extrarenal pelvis; parapelvic cysts; VUR; high urine flow.
Patients with hydronephrosis may present either as an incidental finding of hydronephrosis on USS or CT done because of non-specific symptoms or it may be identified in a patient with raised creatinine or presenting with loin pain. Symptoms, if present, will depend on the rapidity of onset of obstruction of the kidney (if that is the cause of hydronephrosis), whether the obstruction is complete or partial, unilateral or bilateral, and whether the obstruction to the ureter is extrinsic to the ureter or is within its lumen.
•Severe flank pain suggests a more acute onset of obstruction, and if very sudden in onset, a ureteric stone may well be the cause. Pain induced by diuresis (Dietl’s crisis, e.g. following consumption of alcohol) suggests a possible PUJO.
•Anuria (the symptom of bilateral ureteric obstruction or complete obstruction of a solitary kidney).
•If renal function is impaired, symptoms of renal failure may be present (e.g. nausea, lethargy, anorexia).
•Extrinsic causes of obstruction (e.g. compression of the ureters by retroperitoneal malignancy) usually have a more insidious onset, whereas intrinsic obstruction (ureteric stone) is often present with severe pain of very sudden onset.
•An increase in urine output may be reported by the patient due to poor renal concentrating ability.
•Obstruction in the presence of bacterial UTI—signs and symptoms of pyelonephritis (flank pain and tenderness, fever) or sepsis.

Fig. 10.1 Hydronephrosis as seen on renal ultrasonography.
•Measure BP: elevated in HPCR due to BPO (caused by fluid overload).
•Bilateral oedema (due to fluid overload).
•Abdominal examination: percuss and palpate for an enlarged bladder.
•DRE (? prostate or rectal cancer) and, in women, vaginal examination (? cervical cancer).
•Check serum creatinine, potassium, and the acid–base balance to determine the functional effect of the hydronephrosis.
•Renal ultrasonography (if not already done).
•An obstructive (dense) nephrogram.
•A delay in filling of the collecting system with contrast material.
•Dilatation of the collecting system.
•Rupture of fornices (junction between the renal papilla and its calyx) with urinary extravasation.
•Ureteric dilatation and tortuosity.
•A standing column of contrast material in the ureter.
KUB X-ray (a ureteric stone may be seen); CTU (or IVU) if stone suspected.
•If no stone seen, but hydronephrosis is confirmed and the ureter is non-dilated, the obstruction must be at the PUJ (PUJO).
•If no stone seen and the ureter is dilated, as well as the kidney, ureteric TCC is likely. Arrange retrograde ureterography to identify the site of obstruction and ureteroscopy/ureteric biopsy.
•If the patient is in retention or has a substantial PVR urine volume, pass a catheter. If the elevated creatinine falls (and the hydronephrosis improves), the diagnosis is BOO due to, for example, BPH, PC, urethral stricture, and DSD. If the creatinine remains elevated, the obstruction affecting both ureters is higher ‘upstream’.
•TRUS and prostatic biopsy if PC suspected on DRE; CT scan looking for malignant bilateral ureteric obstruction and AAA.
Box 10.1 Causes of hydronephrosis
•Obstructing clot in the ureter.
•(Any of the causes listed below where the pathologic process has not yet extended to involve both ureters).
•Bilateral ureteric obstruction at their level of entry into the bladder.
•Locally advanced cervical cancer.
•Locally advanced rectal cancer.
•Poor bladder compliance (often combined with DSD): neuropathic bladder (SCI, spina bifida), post-pelvic RT.
•From adjacent bowel involved with inflammatory bowel disease (e.g. Crohn’s, ulcerative colitis) or diverticular disease, endometriosis.
•Idiopathic 70% (diagnosed following exclusion of other causes; consider IgG4-related disease).
•Periarteritis—aortic aneurysm, iliac artery aneurysm.
•Drugs—methysergide, hydralazine, haloperidol, lysergic acid diethylamide (LSD), methyldopa, β-blockers, phenacetin, amphetamines.
•Malignant—retroperitoneal malignancy (lymphoma, metastatic disease from, e.g. breast cancer), post-chemotherapy.
•Infection—TB, schistosomiasis, syphilis, Actinomyces, gonorrhoea, chronic UTI.
•Hydronephrosis of pregnancy (partly due to smooth muscle relaxant effect of progesterone, partly obstruction of the ureters by the fetus).
•Hydronephrosis in association with an ileal conduit (a substantial proportion of patients with ileal conduit urinary diversion have bilateral hydronephrosis in the absence of obstruction).
A normal ureter undergoes peristalsis, and therefore, at any one moment, at least one area of the ureter will be physiologically narrowed. A ureteric stricture is a segment of the ureter that is narrowed and remains so on several images (i.e. it is a length of the ureter that is constantly narrow).
Most ureteric strictures are benign and iatrogenic. Some follow the impaction of a ureteric stone for a prolonged period; malignant strictures—within the wall of the ureter (e.g. TCC ureter), extrinsic compression from the outside wall of the ureter (e.g. lymphoma, malignant retroperitoneal lymphadenopathy); and retroperitoneal fibrosis (RPF) which may be benign (idiopathic, aortic aneurysm, post-irradiation, analgesic abuse) or malignant (retroperitoneal malignancy, post-chemotherapy).
Normally ischaemic:
•Usually injury at the time of open or endoscopic surgery (e.g. damage to ureteric blood supply or direct damage to the ureter at the time of colorectal resection, AAA graft, hysterectomy); at ureteroscopy—mucosal trauma (from ureteroscope or EHL), perforation of the ureter (urine extravasation, leading to fibrosis).
•RT in the vicinity of the ureter.
•Stricture of ureteroneocystostomy of renal transplant.
The stricture may be diagnosed following investigation for symptoms (loin pain, upper tract infection) or may be an incidental finding on an investigation done for some other reason. The stricture may be diagnosed on renal USS (hydronephrosis), IVU, or CTU. A MAG3 renogram will confirm the presence of obstruction (some minor strictures may cause no renal obstruction) and establish split renal function. Where ureteric TCC is possible, proceed with urine cytology, ureteroscopy, and biopsy.
•Nothing (symptomless stricture in an old patient with significant comorbidity or <25% function in an otherwise healthy patient with a normally functioning contralateral kidney).
•Permanent JJ stent or nephrostomy, changed at regular intervals (symptomatic stricture in an old patient with significant comorbidity or <25% function in the affected kidney with compromised overall renal function).
•Dilatation (balloon or graduated dilator) (Figs. 10.2 and 10.3).
•Incision + balloon dilatation (endoureterotomy by Acucise® balloon; ureteroscopy or nephrostomy and incision, e.g. by laser). Leave a 12Ch stent for 4wk.
•Excision of stricture and repair of the ureter (open or laparoscopic approach).
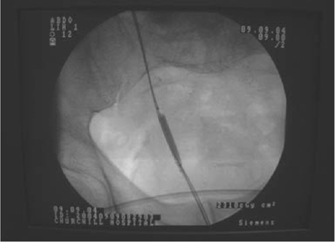
Fig. 10.2 Balloon dilatation of a lower ureteric stricture.

Fig. 10.3 The catheter used for balloon dilatation.
•Mid-ureteric stricture (compared with upper and lower)—tenuous blood supply.
These are due to ischaemia and/or periureteral urine leak in the immediate post-operative period, which leads to fibrosis in the tissues around the ureter (~5% in Bricker and 3% in Wallace anastomoses). In ileal conduits, the left ureter is affected more frequently than the right, because greater mobilization is required to bring it to the right side and it may be compressed under the sigmoid mesocolon, both of which impair blood flow to the distal end of the ureter.
Leads to a triphasic relationship between renal blood flow (RBF) and ureteric pressure.
•Phase 1 (up to 1.5h post-obstruction): ureteric pressure rises, RBF rises (afferent arteriole dilatation mediated by prostaglandins, nitric oxide, and angiotensin II).
•Phase 2 (from 1.5–5h post-obstruction): ureteric pressure continues to rise, RBF falls (efferent arteriole vasoconstriction, with shunting of blood from the outer to inner cortex).
•Phase 3 (beyond 5h): ureteric pressure falls, RBF continues to fall [afferent arteriole vasoconstriction mediated by thromboxanes, prostaglandin E2 (PGE2), endothelin-1, and platelet-activating factor].
The early haemodynamic response seen in UUO is not observed, or markedly reduced.
•Phase 1 (up to 1.5h post-obstruction): ureteric pressure rises.
•Phase 2 (from 1.5–5h post-obstruction): ureteric pressure continues to rise, RBF is significantly lower than that during UUO.
•Phase 3 (beyond 5h): ureteric pressure remains elevated (in contrast to UUO). By 24h, RBF has declined to the same level as for UUO.
Leads to a biphasic pattern, in contrast to acute UUO.
•Phase 1 (up to 1h post-obstruction): ureteric pressure rises, short-lived rise in RBF (afferent arteriole dilatation).
•Phase 2 (from 1h post-obstruction): ureteric pressure continues to rise until stabilizing around 24h, RBF rapidly falls (efferent arteriole vasoconstriction, with shunting of blood).
•No afferent arteriole vasoconstriction phase, due to release of atrial natriuretic peptide (ANP) (promotes diuresis and natriuresis via afferent arteriole vasodilatation and efferent arteriole vasoconstriction).
In UUO, the decrease in urine flow through the nephron results in a greater degree of sodium absorption, so sodium excretion falls. Water loss from the obstructed kidney increases.
Release of BUO is followed by marked natriuresis,  potassium excretion, and diuresis (solute diuresis).This is due to:
potassium excretion, and diuresis (solute diuresis).This is due to:
•An appropriate (physiological) natriuresis to excrete excessive sodium, which is a consequence of BUO.
•A solute diuresis from the accumulation of urea in the extracellular fluid (ECF).
A diminution of the corticomedullary concentration gradient, which is normally established by the countercurrent mechanism of the loop of Henle (LoH) and is dependent on maintenance of flow through the nephron—a reduction of flow, as occurs in BUO, reduces the efficiency of the countercurrent mechanism (effectively, the corticomedullary concentration gradient is ‘washed out’).
There may also be accumulation of natriuretic peptides (e.g. ANP) during BUO, which contributes to natriuresis following the release of the obstruction.
In dogs with completely obstructed kidneys, full recovery of renal function after 7 days of UUO occurs within 2wk of relief of obstruction. A total of 14 days of obstruction leads to a permanent reduction in renal function to 70% of control levels (recovery to this level taking 3–6 months after reversal of obstruction). There is some recovery of function after 4wk of obstruction, but after 6wk of complete obstruction, there is no recovery. In humans, there is no clear relationship between the duration of BUO and the degree of recovery of renal function after relief of obstruction. Two phases of recovery: (1) tubular, by 2wk (improved serum creatinine, sodium, and volume), and (2) glomerular, by 3 months (improved GFR).
Urine production by the kidneys is a continuous process. Its transport from the kidneys down the ureter and into the bladder occurs intermittently by waves of peristaltic contraction of the renal pelvis and ureter (peristalsis = wave-like contractions and relaxations). The renal pelvis delivers urine to the proximal ureter. As the proximal ureter receives a bolus of urine, it is stretched and this stimulates it to contract while the segment of the ureter just distal to the bolus of urine relaxes. Thus, the bolus of urine is projected distally.
The origin of the peristaltic wave is from collections of pacemaker cells in the proximal-most regions of the renal calyces, with electrical activity dependent on the movement of potassium and calcium (not sodium) ions. In species with multiple calyces such as humans, there are multiple pacemaker sites in the proximal calyces. The frequency of contraction of the calyces is independent of urine flow rate (it is the same at high and low flow rates), and it occurs at a higher rate than that of the renal pelvis. Precisely how the frequency of contraction of each calyx is integrated into a single contraction of the renal pelvis is not known. All areas of the ureter are capable of acting as a pacemaker. Stimulation of the ureter at any site produces a contraction wave that propagates proximally and distally from the site of stimulation, but under normal conditions, electrical activity arises proximally and is conducted distally from one muscle cell to another (the proximal-most pacemakers are dominant over these latent pacemakers).
Peristalsis persists after renal transplantation and denervation and does not therefore appear to require innervation. The ureter does, however, receive both parasympathetic and sympathetic innervation, and stimulation of these systems can influence the frequency of peristalsis and the volume of urine bolus transmitted.
At normal urine flow, the frequency of calyceal and renal pelvic contractions is greater than that in the upper ureter, and there is a relative block of electrical activity at the PUJ. The renal pelvis fills; the ureter below it is collapsed and empty. As renal pelvic pressure rises, urine is extruded into the upper ureter. The ureteric contractile pressures that move the bolus of urine are higher than renal pelvic pressures. A closed PUJ may prevent back-pressure on the kidney. At higher urine flow rates, every pacemaker-induced renal pelvic contraction is transmitted to the ureter.
To propel a bolus of urine, the walls of the ureter must coapt (touch). Resting ureteric pressure is 0–5cmH2O, and ureteric contraction pressures range from 20 to 80cmH2O. Ureteric peristaltic waves occur 2–6 times per minute. The VUJ acts as a one-way valve under normal conditions, allowing urine transport into the bladder and preventing reflux back into the ureter, although decompensation occurs when the bladder pressure is >40cmH2O.
The ureter has a rich autonomic innervation.
•Sympathetic: preganglionic fibres from spinal segments T10–L2; post-ganglionic fibres arise from the coeliac, aorticorenal, mesenteric, superior, and inferior hypogastric (pelvic) autonomic plexuses.
•Parasympathetic: vagal fibres via coeliac to the upper ureter; fibres from S2–4 to the lower ureter.
The role of ureteric autonomic innervation is unclear. It is not required for ureteric peristalsis (though it may modulate this). Peristaltic waves originate from intrinsic smooth muscle pacemakers located in minor calyces of the renal collecting system.
Upper ureter—afferents pass (alongside sympathetic nerves) to T10–L2; lower ureter—afferents pass (alongside sympathetic nerves and by way of the pelvic plexus) to S2–4. Afferents subserve stretch sensation from the renal capsule, collecting system of the kidney (renal pelvis and calyces), and ureter. Stimulation of the mucosa of the renal pelvis, calyces, and ureter also stimulates nociceptors, the pain so felt being referred in a somatic distribution to T8–L2 (kidney T8–L1, ureter T10–L2), in the distribution of the subcostal, iliohypogastric, ilioinguinal, or genitofemoral nerves. Thus, ureteric pain can be felt in the flank, groin, scrotum or labia, and upper thigh, depending on the precise site in the ureter from which the pain arises.
•Contraction: α-adrenoceptor stimulation, tachykinins, histamine, angiotensin, PGF2-α, possibly opiates.
•Relaxation: β-adrenoceptor stimulation, calcitonin GRP, PGE1/2, progesterone, calcium channel antagonists.
RPF was first clearly described by the French urologist Albarran in 1905. Further cases were described by Ormond in 1948.
•Autoimmune: idiopathic RPF comprises two-thirds of cases. Considered to be a response to an insoluble lipid called ceroid that has leaked through a thinned arterial wall from atheromatous plaques, a fibrous plaque extends laterally and downwards from the renal arteries encasing the aorta, IVC, and ureters but rarely extends into the pelvis. The central portion of the plaque consists of woody scar tissue, while the growing margins have the histological appearance of chronic inflammation. It may be associated with AAA, intra-arterial stents, and angioplasty; mediastinal, mesenteric, or bile duct fibrosis.
•IgG4-related: approximately half of previously documented ‘idiopathic’ RPF. Histological features include lymphoplasmacytic infiltrate of IgG4-positive plasma cells, storiform fibrosis, tissue eosinophilia, and obliterative phlebitis.
•Drugs, including methysergide, β-blockers, hydralazine, haloperidol, amphetamines, and LSD; methyl methacrylate cement used for joint replacement.
•Chronic urinary infection, including TB.
•Inflammatory conditions such as Crohn’s disease, Reidel’s thyroiditis, or sarcoidosis.
•Amyloidosis and periaortic haematoma may mimic RPF.
•Lymphoma is the commonest cause, also sarcoma.
•Metastatic or locally infiltrative carcinoma of the breast, stomach, pancreas, colon, bladder, prostate, and carcinoid tumours.
•RT may cause RPF, although rare in recent years with precise field localization.
•Chemotherapy, especially following treatment of metastatic testicular tumours, may leave fibrous masses encasing the ureters. These may or may not contain residual tumour.
•Idiopathic RPF classically occurs in the fifth or sixth decade of life.
•Men are affected twice as commonly as women.
•In the early stage, symptoms are relatively non-specific, including loss of appetite and weight, low-grade fever, sweating, and malaise. Lower limb swelling may develop. Dull, non-colicky abdominal or back pain is described in up to 90% of patients.
•Later, the major complication of the disease develops—bilateral ureteric obstruction, causing anuria and renal failure.
•Examination may reveal hypertension in up to 60% of patients and an underlying cause such as an AAA.
•Inflammatory serum markers and IgG4 levels are elevated in idiopathic RPF [60–90% elevated erythrocyte sedimentation rate (ESR)].
•Pyuria or bacteriuria are common.
•Ultrasound will demonstrate uni- or bilateral hydronephrosis.
•CT, IVU, or ureterography reveal tapering medial displacement of the mid ureters, with proximal dilatation, and will exclude calculus disease. Up to one-third of patients will have a non-functioning kidney at the time of presentation due to long-standing obstruction.
•CT-guided fine-needle biopsy of the mass may confirm the presence of malignant disease, infection, or IgG4-positive cells. A negative result does not exclude malignancy.
•FDG-PET shows avidity associated with lymphoma or sarcoma.
•Emergency management of a patient presenting with established renal failure requires relief of the obstruction by percutaneous nephrostomy or ureteric stenting.
•Replacement of fluid and electrolyte losses following relief of BUO is vital due to frequent post-obstructive diuresis.
•Assess with daily weighing and measurement of BP (lying and standing).
•Steroids may decrease oedema often associated with RPF and, in this way, help reduce the obstruction (typical regimen of prednisolone 60mg PO on alternate days for 2 months, reassess; if improvement, taper the dose over 6–8wk to 5mg maintenance). If used, steroids are usually discontinued when inflammatory markers return to normal. Azathioprine, tamoxifen, and cyclophosphamide have been used successfully in some patients.
•Surgical ureterolysis with omental wrap may be necessary to free and insulate the ureters from the encasing fibrous tissue.
•Monitor for recurrent disease with serum creatinine and ultrasound 3- to 6-monthly or annual DMSA renography for 5y.