 pp. 518–519).
pp. 518–519).Initial resuscitation of the traumatized patient
Renal trauma: classification, mechanism, grading
Renal trauma: clinical and radiological assessment
Ureteric injuries: mechanisms and diagnosis
Pelvic fractures: bladder and ureteric injuries
Posterior urethral injuries in males and urethral injuries in females
Torsion of the testis and testicular appendages
The resuscitation of the traumatized patient is usually initiated in the field by the paramedic team and is continued systematically once the patient reaches the emergency department by a rapid, multidisciplinary, priority-based approach.
Goals of resuscitation are:
•Restoration of cardiac, pulmonary, and neurological function.
•Diagnosis of immediate life-threatening conditions.
•Prevention of complications from multisystem injuries.
The initial resuscitation process can be divided into three phases: the primary survey, the secondary survey, the definitive survey.
ABC: assess the patient’s Airway, Breathing, and Circulation.
•Ventilate by oxygen mask or endotracheal intubation and mechanical ventilation.
•Immobilize the cervical spine.
Assess circulatory function by pulse rate and BP.
The commonest cause of hypotension in the polytraumatized patient is hypovolaemia secondary to haemorrhage. With hypovolaemic shock, an immediate bolus of IV isotonic crystalloid solution should be given and the patient’s response (pulse rate, BP) is assessed.
Determined by local facilities. Increasingly, in the severely traumatized patient, CT of the chest, abdomen, and pelvis is used to identify significant chest, abdominal, and pelvic injuries. If not available, arrange supine chest, abdomen, and pelvic X-rays to identify the presence of rib and pelvic fractures and to identify the presence of significant quantities of blood in the chest, abdomen, and pelvis, and in patients with persistent hypotension from presumed bleeding, search for occult haemorrhage using a diagnostic peritoneal lavage or focused abdominal USS.
Hypovolaemic shock is not always associated with hypotension. In young patients, compensatory mechanisms, e.g. rapid vasoconstriction, can compensate for as much as a 35% volume loss, without significant decreases in BP.
Remember the non-hypovolaemic causes of hypotension:
Routinely performed in every trauma patient because it provides valuable information regarding the likelihood of injuries to the upper and lower urinary tract. The absence of haematuria, however, does not exclude a urinary tract injury, e.g. haematuria may be absent in acceleration/deceleration renal injuries (see  pp. 518–519).
pp. 518–519).
As life-threatening injuries are found during the primary survey, resuscitation efforts are initiated concurrently (e.g. chest drain for pneumothorax). The decision to transfer a patient from the emergency room to either the operating room or angiography suite is made during the primary survey.
Performed after completion of the primary survey. Take a complete history, and perform a physical examination from head to toe. Arrange selective skeletal X-rays according to physical findings.
During this phase, focus attention on identifying specific organ injuries using clinical and radiographic means. Genitourinary injuries are usually recognized during the definitive survey.
During all phases of the initial resuscitation, assess vital signs (BP, respiratory rate, blood gases, urinary output, and body temperature) continually. Vascular pressure monitoring, using central venous and pulmonary arterial catheters, can be performed selectively. Frequent re-evaluation should be performed to detect changes in the patient’s condition, and the appropriate actions taken.
(See Table 11.1.)
Proportion of all renal injuries that are blunt: Europe 97%; USA 90%; and South Africa 25–85%. Proportion depends on whether urban or non-urban community. This classification is useful because it predicts the likely need for surgical exploration to control bleeding. Experience from large series shows that 95% of blunt injuries can be managed conservatively, whereas 50% of stab injuries and 75% of gunshot wounds require exploration. For staging, see Box 11.1.
•Rapid acceleration or rapid deceleration.
Rapid deceleration frequently causes renal pedicle injuries (renal artery and vein tears or thrombosis, PUJ disruption), because the renal pedicle is the site of attachment of the kidney to other fixed retroperitoneal structures.
Commonest cause: motor vehicle accidents (e.g. pedestrian hit by a car, direct blow combined with rapid acceleration and then deceleration). Seemingly trivial injuries (e.g. fall from a ladder), direct falls onto the flank, or sporting injuries can lead to significant renal injuries.
Stab or gunshot injuries to the flank, lower chest, and anterior abdominal area may inflict renal damage. Fifty per cent of patients with penetrating trauma and haematuria have grade III, IV, or V renal injuries. Penetrating injuries anterior to the anterior axillary line are more likely to injure the renal vessels and renal pelvis, compared with injuries posterior to this line where less serious parenchymal injuries are more likely. Thus, renal injuries from stab wounds to the flank (i.e. posterior to the anterior axillary line) can often be managed non-operatively.
The wound profile of a low-velocity gunshot wound is similar to that of a stab wound. High-velocity gunshot wounds (>350m/s) cause greater tissue damage due to stretching of surrounding tissues (‘temporary cavity’).
The kidneys are retroperitoneal structures surrounded by perirenal fat, the vertebral column and spinal muscles, the lower ribs, and abdominal contents. They are therefore relatively protected from injury, and a considerable degree of force is usually required to injure them (only 1.5–3% of trauma patients have renal injuries). Associated injuries are therefore common (e.g. spleen, liver, mesentery of the bowel). Renal injuries may not initially be obvious, hidden as they are by other structures. To confirm or exclude a renal injury, imaging studies are required. In children, there is proportionately less perirenal fat to cushion the kidneys against injury and thus, renal injuries occur with lesser degrees of trauma.
Box 11.1 Staging of the renal injury
Using CT, renal injuries can be staged according to the American Association for the Surgery of Trauma (AAST) Organ Injury Severity Scale. Higher-injury severity scales are associated with poorer outcomes.
•Grade I: contusion (normal CT) or subcapsular haematoma with no parenchymal laceration.
•Grade II: <1cm deep parenchymal laceration of the cortex, no extravasation of urine (i.e. collecting system intact).
•Grade III: >1cm deep parenchymal laceration of the cortex, no extravasation of urine (i.e. collecting system intact).
•Grade IV: parenchymal laceration involving the cortex, medulla, and collecting system OR renal artery or renal vein injury with contained haemorrhage.
•Grade V: completely shattered kidney OR avulsion of the renal hilum.
The kidneys are said to be more prone to injury in children because of the relatively greater size of the kidneys in children, the smaller protective muscle mass and cushion of perirenal fat, and the more pliable rib cage.
Table 11.1 Summary of mechanisms, causes, grading, and treatment of renal disease
| Mechanisms and cause | Blunt: direct blow or acceleration/deceleration [road traffic accidents (RTAs), falls from a height, fall onto flank] |
| Penetrating: knives, gunshots, iatrogenic (e.g. PCNL) | |
| Imaging and grading | CT: accurate, rapid, images other intra-abdominal structures |
|
Staging—AAST Organ Injury Severity Scale: |
|
| Treatment | Conservative: 95% of blunt injuries, 50% of stab injuries, and 25% of gunshot wounds can be managed non-operatively (cross-match, bed rest, observation) |
| Exploration if:
Persistent bleeding (persistent tachycardia and/or hypotension not responding to appropriate fluid and blood replacement) Expanding perirenal haematuria Pulsatile perirenal haematoma |
See also: Santucci RA, Wessells H, Bartsch G, et al. (2004). Consensus on genitourinary trauma. Evaluation and management of renal injuries: consensus statement of the renal trauma subcommittee. BJU Int 93:937–54.
Examination: pulse rate, systolic BP, respiratory rate, location of entry and exit wounds, flank bruising, rib fractures. The lowest recorded systolic BP is used to determine the need for renal imaging.
Urinalysis: crucial for determining the likelihood of renal injury, and therefore the need for radiological tests.
Haematuria (defined as >5 erythrocytes per HPF or dipstick positive) suggests the possibility of a renal injury; however, the amount of haematuria does not correlate consistently with the degree of renal injury.
Do FBC and serum chemistry profile.
•Penetrating chest and abdominal wounds (knives, bullets).
•Microscopic (>5 RBCs per HPF) or dipstick haematuria in a hypotensive patient (systolic BP <90mmHg recorded at any time since the injury).*
•A history of a rapid acceleration or deceleration (e.g. fall from a height, high-speed motor vehicle accident). Falls from even a low height can cause serious renal injury in the absence of shock (systolic BP <90mmHg) and of haematuria (PUJ disruption prevents blood from reaching the bladder).
•Any child with microscopic or dipstick haematuria who has sustained trauma.
Adult patients with a history of blunt trauma and microscopic or dipstick haematuria need not have their kidneys imaged, as long as there is no history of acceleration/deceleration and no shock, since the chances of a significant injury being found are <0.2%.
* Remember, in young adults and children, hypotension is a late manifestation of hypovolaemia; BP is maintained until there has been substantial blood loss.
While significant renal injury is more likely with macroscopic haematuria, in some cases of severe renal injury, haematuria may be absent. Thus, the relationship between the presence, absence, and degree of haematuria and the severity of trauma is not absolute. Broadly speaking, in blunt trauma, macroscopic haematuria predicts the likelihood of significant renal injury (Table 11.2). Conversely, in penetrating trauma, haematuria may be absent in severe renal injury (renal vascular injury, PUJ, or ureter avulsion).
Table 11.2 Blunt trauma in adults: chance of significant renal injury vs degree of haematuria and systolic BP (SBP)
| Degree of haematuria; SBP (mmHg) | Significant renal injury (%) |
| Microhaematuria*; >90 | 0.2 |
| Macroscopic haematuria; >90 | 10 |
| Macroscopic haematuria; <90 | 10 |
* Dipstick or microscopic haematuria.
Haemodynamic instability may preclude standard imaging, such as CT, the patient having to be taken to the operating theatre immediately to control the bleeding. In this situation, an on-table IVU (Box 11.2) is indicated if:
•A retroperitoneal haematoma is found and/or
•A renal injury is found which is likely to require nephrectomy.
Box 11.2 What imaging study?
The IVU has been replaced by contrast-enhanced CT scan as the imaging study of choice in patients with suspected renal trauma. Compared with IVU, it provides clearer definition of the injury, allowing injuries to the parenchyma and collecting system to be more accurately graded, and therefore determines subsequent management. An arterial–venous phase scan is done within minutes of contrast injection, followed by a repeat scan 10–20min after contrast administration, to allow time for contrast to reach collecting system.
While ultrasound can establish the presence of two kidneys and identify blood flow in the renal vessels (power Doppler), it cannot accurately identify parenchymal tears, collecting system injuries, or extravasation of urine until a later stage when a urine collection has had time to accumulate.
Imaging is designed to:
•Document the presence and function of the contralateral kidney.
•Detect pre-existing renal pathology in the affected kidney.
On contrast-enhanced CT, look for:
•Depth of parenchymal laceration.
•Parenchymal enhancement (absence of enhancement suggests renal artery injury).
•Presence of urine extravasation (medial extravasation of contrast suggests disruption of the PUJ or renal pelvis).
•Presence, size, and position of retroperitoneal haematoma (haematoma medial to the kidney suggests a vascular injury).
•Presence of injuries to adjacent organs (bowel, spleen, liver, pancreas, etc.).
•Presence of a normal contralateral kidney.
When, because of shock and need for immediate laparotomy, a patient is transferred immediately to the operating theatre without having had a CT scan and a retroperitoneal haematoma is found, a single-shot abdominal X-ray, taken 10min after contrast administration (2mL/kg of contrast), can establish the presence/absence of a renal injury and the presence of a normally functioning contralateral kidney where the ipsilateral kidney injury is likely to necessitate a nephrectomy.
Most blunt (95%) and many penetrating renal injuries (50% of stab injuries and 25% of gunshot wounds) can be managed non-operatively.
•Dipstick or microscopic haematuria: if systolic BP since injury has always been >90mmHg and no history of acceleration or deceleration, imaging and admission are not required.
•Macroscopic haematuria: in a cardiovascularly stable patient, having staged the injury with CT, admit for bed rest (no hard and fast rules as to duration) and observation until the macroscopic haematuria, if present, resolves (cross-match in case the BP drops); give antibiotics if urinary extravasation.
•High-grade (IV and V) injuries: can be managed non-operatively if they are cardiovascularly stable. However, grade IV, and especially grade V, injuries often require nephrectomy to control bleeding (grade V injuries function poorly if repaired).
Embolization is increasingly being used for renal trauma of all grades.
Surgical exploration is indicated (whether blunt or penetrating injury) (Table 11.3) if:
•The patient develops shock which does not respond to resuscitation with fluids and/or blood transfusion.
•Hb decreases (there are no strict definitions of what represents a ‘significant’ fall in Hb).
•There is urinary extravasation and associated bowel or pancreatic injury.
•Expanding perirenal haematoma (again the patient will show signs of continued bleeding).
•Pulsatile perirenal haematoma (though embolization may be used in this situation).
An expanding and/or pulsatile perirenal haematoma suggests a renal pedicle avulsion. Haematuria is absent in 20%.
Table 11.3 Embolization and surgical exploration
Technique of renal exploration Midline incision allows: •Exposure of the renal pedicle, so allowing early control of the renal artery and vein. •Inspection for injury to other organs. Lift the small bowel upwards to allow access to the retroperitoneum. Incise the peritoneum over the aorta, above the inferior mesenteric artery. A large perirenal haematoma may obscure the correct site for this incision. If this is the case, look for the inferior mesenteric vein, and make your incision medial to this. Once on the aorta, the IVC may be exposed, then the renal veins and the renal arteries. Pass slings around all of these vessels. Expose the kidney by lifting the colon off of the retroperitoneum. Bleeding may be reduced by applying pressure to the vessels via the slings. Control bleeding vessels within the kidney with 4/0 Vicryl or Monocryl sutures. Close any defects in the collecting system with 4/0 Vicryl. If your sutures cut out, place a strip of Surgicel over the site of bleeding; place your sutures through the capsule on either side of this, and tie them over the Surgicel. This will stop them from cutting through the friable renal parenchyma. Finding a non-expanding, non-pulsatile retroperitoneal haematoma at laparotomy The finding of an expanding and/or pulsatile retroperitoneal haematoma at laparotomy will often indicate a renal pedicle injury (avulsion or laceration), and nephrectomy may be required to stop further haemorrhage. Controversy surrounds the correct management of the finding at laparotomy of a non-expanding, non-pulsatile retroperitoneal haematoma. Most can be left alone. Remember, exploration increases the chances of loss of the kidney (because of bleeding which can be controlled only by nephrectomy). The decision to explore is based on whether preoperative or on-table imaging has been done and is normal or abnormal. |
|
| Preoperative or intraoperative imaging | Action |
| Normal | Leave the haematoma alone |
| Abnormal; contralateral kidney normal | Explore and repair the renal injury |
| Abnormal; abnormal or absent contralateral kidney | Leave the haematoma alone* |
| None | Explore and repair the renal injury |
* Exploration increases the chances of loss of the kidney (because of bleeding that can be controlled only by nephrectomy), which is a disaster if the contralateral kidney is absent or damaged.
Not in itself necessarily an indication for exploration. Almost 80–90% of these injuries will heal spontaneously. The threshold for operative repair is lower with associated bowel or pancreatic injury—bowel contents mixing with urine is a recipe for overwhelming sepsis. In these situations, the renal repair should be well drained and the omentum interposed between the kidney and bowel or pancreas.
If there is substantial contrast extravasation, consider placing a JJ stent. Repeat renal imaging if the patient develops a prolonged ileus or fever, since these signs may indicate the development of a urinoma which can be drained percutaneously. Renal exploration is required for a persistent leak.
Exploration is usually not required for patients with devitalized segments of the kidney and with urinary extravasation.1
See references for more information.2,3,4
•Delayed bleeding: 1.5% of surgically treated patients, 4% of surgically treated penetrating injuries, 1–6% of paediatric blunt injuries managed non-operatively, 20% of conservatively managed stab injuries; 75% require surgery, and of these, 60% require nephrectomy.
•Urinary extravasation and urinoma formation: blunt injury 2–20%; penetrating injury 10–25%. If low volume and non-infected, often heal spontaneously; large volume—consider a trial of JJ stenting with renal repair if extravasation persists.
•Abscess formation: flank pain, fever, ileus. CT or USS is diagnostic. Treat by percutaneous drainage.
•Renal arteriovenous fistulae: commonest cause is percutaneous renal biopsy, i.e. iatrogenic. Often small and heal spontaneously, but may manifest with retroperitoneal bleeding; collecting system bleeding (heavy haematuria); microscopic haematuria; abdominal bruit; hypertension; tachycardia; high output heart failure. Diagnosis is confirmed by selective renal arteriography. Treat by arterial embolization (treatment of choice); partial nephrectomy; complete nephrectomy.
Excess renin excretion occurs following renal ischaemia from renal artery injury or thrombosis or renal compression by haematoma or fibrosis (so-called ‘Page’ kidney). This can lead to hypertension months or years after renal injury. The exact incidence of post-traumatic hypertension is uncertain. It may occur in <1% of individuals.
Significant renal injuries can occur during PCNL for kidney stones. This is the surgical equivalent of a stab wound, and serious haemorrhage results in 1% of cases.5
Bleeding during or after a PCNL can occur from vessels in the nephrostomy track itself, from an arteriovenous fistula, or from a pseudoaneurysm which has ruptured. Track bleeding will usually tamponade around a large-bore nephrostomy tube. Traditionally, persistent bleeding through the nephrostomy tube is managed by clamping the nephrostomy tube and waiting for the clot to tamponade the bleeding. While this may control bleeding in some cases, in others, a rising or persistently elevated pulse rate (with later hypotension) indicates the possibility of persistent bleeding and is an indication for renal arteriography and embolization of the arteriovenous fistula or pseudoaneurysm (Figs 11.1 and 11.2). Failure to stop bleeding by this technique is an indication for renal exploration.
Arteriovenous fistulae can sometimes occur following open renal surgery for stones or tumours, and arteriography with embolization again can be used to stop the bleeding in these cases. However, bleeding usually occurs over a longer time course (days or even weeks), rather than as an acute haemorrhage causing shock.
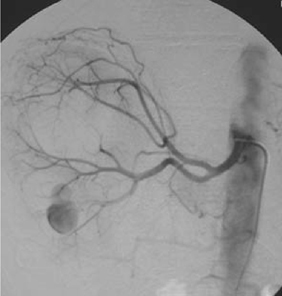
Fig. 11.1 Renal arteriography after PCNL where severe bleeding was encountered. An arteriovenous fistula was found and embolized.
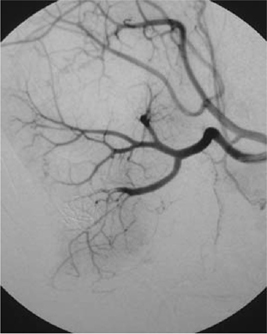
Fig. 11.2 Post-embolization of an arteriovenous fistula. Note the embolization coils in the lower pole.
References
1Toutouzas KG (2002). Non-operative management of blunt renal trauma: a prospective study. Am Surg 68:1097–103.
2McAninch JW, Carroll PR, Klosterman PW, et al. (1991). Renal reconstruction after injury. J Urol 145:932.
3Carroll PR, McAninch JW (1985). Operative indications in penetrating renal trauma. J Trauma 25:587.
4Bernath AS, Shutte H, Fernandez RRD, et al. (1983). Stab wounds of the kidney: conservative management in flank penetration. J Urol 129:468.
5Martin X (2000). Severe bleeding after nephrolithotomy: results of hyperselective embolization. Eur Urol 37:136–9.
•External: rare—blunt (e.g. high-speed RTAs, fall from a height); penetrating (knife or gunshot wounds).
•Internal trauma (= iatrogenic): during pelvic or abdominal surgery, e.g. hysterectomy, colectomy, AAA repair; ureteroscopy. The ureter may be divided, ligated, or angulated by a suture; a segment excised or damaged by diathermy.
Based on a high index of suspicion for the possibility of ureteric injury in the types of scenarios (see  p. 527). Imaging studies: IVU or CT can be used to determine the presence of a ureteric injury. If doubt remains regarding the integrity of the ureters, retrograde ureterography should be done.
p. 527). Imaging studies: IVU or CT can be used to determine the presence of a ureteric injury. If doubt remains regarding the integrity of the ureters, retrograde ureterography should be done.
The injury may be suspected at the time of surgery, but injury may not become apparent until some days or weeks post-operatively.
For ureteric contusions and perforations seen at the time of ureteroscopy, insert a JJ stent. During abdominal or pelvic surgery, first optimize exposure of the suspected injury site by packing the bowel out of the way, controlling bleeding, and ensuring the theatre lights are appropriately positioned. Examine both ureters (bilateral injuries can occur).
A good way of inspecting the ureter for injury but requires exposure of a considerable length of the ureter to establish that it has not been injured. Lower ureteric exposure is more difficult than upper ureteric.
Look for leakage of the dye from a more distant section of the ureter.
Technically difficult; does not always demonstrate the presence or site of injury.
Via an incision made in the bladder or via a cystoscope. A very accurate method of establishing the presence or absence of a ureteric injury (Fig. 11.3). Both ureters can be easily examined.
The diagnosis is usually apparent in the first few days following surgery (Box 11.3), but it may be delayed by weeks, months, or years (presentation: flank pain, post-hysterectomy incontinence—a continuous leak of urine suggests a ureterovaginal fistula).
Box 11.3 Symptoms and signs of ureteric injury
•An ileus (due to urine within the peritoneal cavity).
•Prolonged post-operative fever or overt urinary sepsis.
•Persistent drainage of fluid from drains, the abdominal wound, or the vagina. Send this for creatinine estimation. Creatinine level higher than that of serum = urine (the creatinine level will be at least 300µmol/L).
•Flank pain if the ureter has been ligated.
•Abdominal mass, representing a urinoma (a collection of urine).
•The pathology report on the organ that has been removed may note the presence of a segment of the ureter!
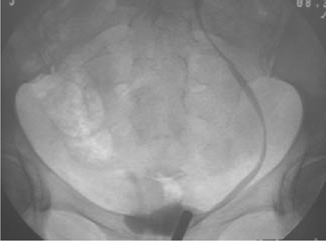
Fig. 11.3 A normal retrograde ureterogram.
IVU or retrograde ureterogram. Ultrasonography may demonstrate hydronephrosis, but hydronephrosis may be absent when urine is leaking from a transected ureter into the retroperitoneum or peritoneal cavity. The IVU usually shows an obstructed ureter or, occasionally, a contrast leak from the site of injury.
Generally, the best time to repair the ureter is as soon as the injury has been diagnosed.
Delay definitive ureteric repair when:
•The patient is unable to tolerate a prolonged procedure under GA.
•There is evidence of active infection at the site of proposed ureteric repair (infected urinoma).
A percutaneous nephrostomy should be placed, the infection drained radiologically (percutaneous drain), IV antibiotics given, and ureteric repair delayed until the patient is apyrexial.
Traditional teaching held that surgical repair should be delayed when the injury was diagnosed between roughly days 7 and 14 after ureteric injury, the time when maximal oedema and inflammation at the site of repair was believed to occur. However, favourable outcomes have been demonstrated after early repair, and the time of the original injury is nowadays seen as a less important determinant of the time of definitive repair.1
•Whether the injury is recognized immediately.
The options are:
•JJ stenting for 3–6wk (e.g. ligature injury recognized immediately).
•Primary closure of a partial transection of the ureter.
•Direct ureter-to-ureter anastomosis (primary uretero-ureterostomy)—if the defect between the ends of the ureter is of a length where a tension-free anastomosis is possible.
•Reimplantation of the ureter into the bladder (uretero-neocystostomy), using either a psoas hitch or a Boari flap (Figs. 11.4 and 11.5).
•Transuretero-ureterostomy (Fig. 11.6).
•Autotransplantation of the kidney into the pelvis—where the segment of the damaged ureter is very long.
•Replacement of the ureter with the ileum—where the segment of the damaged ureter is very long.
•Permanent cutaneous ureterostomy—where the patient’s life expectancy is very limited.
•Nephrectomy—traditionally advocated for ureteric injury during vascular graft procedures (e.g. aortobifemoral graft for AAA), but the trend is towards ureteric repair and renal preservation, reserving nephrectomy only where a urine leak develops post-operatively (continuing drainage of urine from the drain placed at the site of the ureteric anastomosis).2
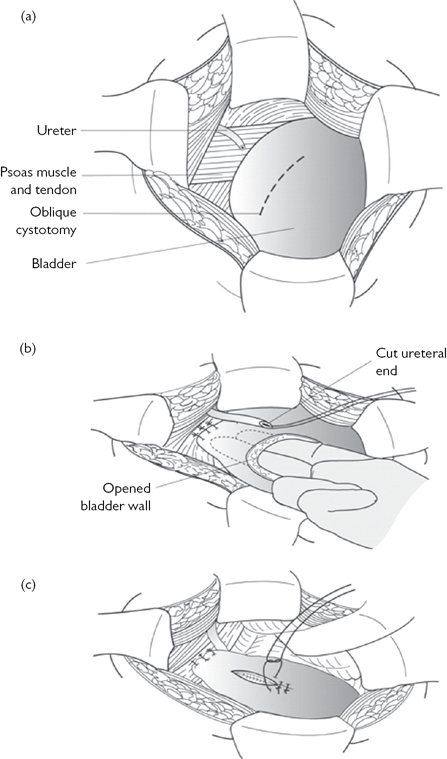
Fig. 11.4 A psoas hitch.
Reproduced from Reynard, J, Mark, S. et al, (2008) Urological Surgery. Oxford University Press, with permission from Oxford University Press.
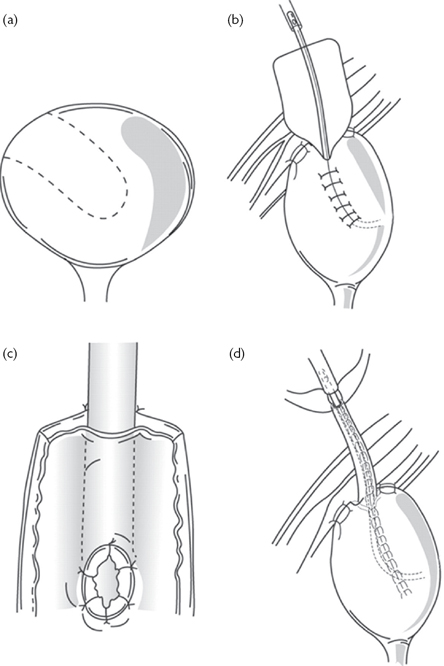
Fig. 11.5 A Boari flap.
Reproduced from Reynard, J, Mark, S. et al, (2008) Urological Surgery. Oxford University Press, with permission from Oxford University Press.
For some injuries, JJ stenting may be adequate for definitive treatment, particularly where the injury does not involve the entire circumference of the ureter, and continuity is therefore maintained across the region of the ureteric injury. In situations where a ligature has been applied around the ureter and this has been immediately recognized such that viability of the ureter has probably not been compromised, remove the ligature and place a JJ stent (cystoscopically if this is feasible or, if not, by opening the bladder). If there has been a delay in recognition of a ligature injury to the ureter, it is probably safer to remove the affected segment of the ureter and perform a uretero-ureterostomy. Generally speaking, the stent is maintained in position for somewhere between 3 and 6wk (no hard and fast rules). At the time of stent removal, perform a retrograde ureterogram to confirm that there is no persistent leakage of contrast from the original site of injury and to see if there is evidence of ureteric stricturing.
Factors other than the level of injury are important in determining the type of repair (Box 11.4). Blast injuries characteristically cause considerable ‘collateral’ damage to the ureter and surrounding tissues, and this may not be apparent at the time of surgery. Delayed necrosis can occur in such apparently normal-looking ureters.
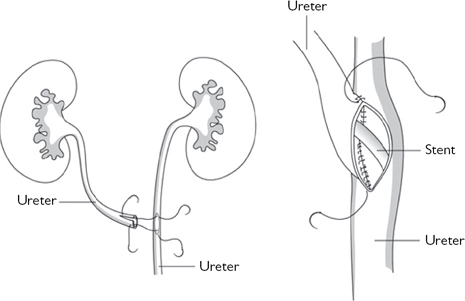
Fig. 11.6 Transuretero-ureterostomy.
Reproduced from Reynard, J, Mark, S. et al, (2008) Urological Surgery. Oxford University Press, with permission from Oxford University Press.
Box 11.4 General principles of ureteric repair
•The ends of the ureter should be debrided, so that the edges to be anastomosed are bleeding freely.
•The anastomosis should be tension-free.
•For complete transection, the ends of the ureter should be spatulated to allow a wide anastomosis to be done.
•A stent should be placed across the repair.
•Mucosa-to-mucosal anastomosis should be done to achieve a watertight closure.
References
1Blandy JP, Badenoch DF, Fowler CG, Jenkins BJ, Thomas NW (1991). Early repair of iatrogenic injury to the ureter and bladder after gynecological surgery. J Urol 146:761–5.
2McAninch JW (2002). In: Walsh PC, Retik AB, Vaughan ED, Wein AJ (eds). Campbell’s Urology, 8th edn. Philadelphia, PA: WB Saunders; pp. 3703–14.
Pelvic fractures are usually due to run-over or crush injuries where massive force is applied to the pelvis. Associated head, chest, intra-abdominal (spleen, liver, mesentery of the bowel), pelvic (bladder, urethra, vagina, rectum), and genital injuries are common, and these injuries and massive blood loss from torn pelvic veins and arteries account for the substantial (20%) mortality after pelvic fracture.
Pelvic fractures are often occult. Screen run-over or crush victims with a pelvic X-ray. Assess:
•Vital signs (pulse rate, systolic BP).
•Neurovascular integrity of the lower limb (lumbosacral plexus, peripheral nerves, and major vessels may be damaged).
•Examine for head, chest, abdominal, and perineal injuries.
•Determine stability/instability of the fracture from pelvic X-rays.
(See Box 11.5 and Table 11.4.)
•Abdominal/pelvic CT: establishes the presence/absence of associated pelvic (rectum, bladder) and abdominal organ injury (liver, bowel, spleen).
•Retrograde urethrogram: to detect urethral injury. Some hospitals perform retrograde urethrography only when blood is present at the meatus; others do this in all pelvic fracture patients where the pubic rami have been disrupted.
•If the urethra is intact, a retrograde cystogram is done to assess the integrity of the bladder.
Box 11.5 Is the fracture stable or unstable?
Stable = the fracture can withstand normal physiologic forces.
Unstable = the fracture cannot withstand normal physiologic forces.
Instability suggests a greater degree of trauma to the pelvis and increases the likelihood of serious associated injuries. In addition, fixation of an unstable fracture reduces blood loss, mortality, hospital stay, leg length discrepancy, and long-term disability; makes nursing care easier; and reduces analgesic consumption. Stability can be defined according to the Tile classification system of pelvic ring fractures (Table 11.4).
Of unstable pelvic fractures, 70% are B2 and B3, 10–20% are open-book type (B1), and 10–20% are type C.
•Open-book pelvic fracture (B1): caused by anteroposterior compression. A dramatic rise in pelvic volume stretches vessels, nerves, and organs (e.g. bladder) (Figs. 11.7 and 11.8).
•Closed-book pelvic fracture (B2 or B3): caused by a lateral compression force to the pelvis. The pubic rami fracture and overlap, and the ilium and sacral wings may be fractured. Nerves and vessels are not stretched, but the urethra is more likely to be damaged by scissor-like action of overlapping pubic rami.
•Vertically unstable pelvic fracture (C): vessels and nerves can be damaged by stretching.
Based on inlet (for anteroposterior displacement) and outlet views (for vertical displacement) of the pelvis, with the X-ray beam being angled accordingly. CT provides better definition of sacral, sacroiliac, and acetabular fractures and dislocations.
If there is no blood present at the meatus, a gentle attempt at urethral catheterization may be made. It has been suggested that this could convert a partial urethral rupture into a complete rupture. However, leading trauma centres in the USA state, ‘We and others have not seen any evidence that this can convert an incomplete into a complete transection … and we usually make one gentle attempt to place a urethral catheter in suspected urethral disruption’.1 If any resistance is encountered, stop and obtain a retrograde urethrogram. If the retrograde urethrogram demonstrates a normal urethra, proceed with another attempt at catheterization, using plenty of lubricant. If there is a urethral rupture, insert an SPC via a formal open approach to allow inspection of the bladder (and repair of injuries, if present).
Ten per cent of ♂ and 5% of ♀ pelvic fractures are associated with a bladder injury (the fracture type leading to bladder injury is usually an anteroposterior pelvic compression fracture, i.e. open-book pelvic fracture; Tile classification B1) (Figs 11.7 and 11.8). Sixty per cent of pelvic fracture bladder ruptures are extraperitoneal, 30% intraperitoneal, and 10% combined extraperitoneal and intraperitoneal.
Table 11.4 The Tile classification system of pelvic ring fractures
| Type A—stable | |
| Type B—rotationally (horizontally) unstable |
Lateral compression: ipsilateral fracture B3, closed book Lateral compression: contralateral fracture (bucket handle fracture) |
| Type C—rotationally (horizontally) and vertically unstable |
The posterior urethra (essentially the membranous urethra) is injured, with roughly the same frequency as the bladder, in subjects who sustain a pelvic fracture, occurring in 5–15% of such cases. Most posterior urethral injuries occur in association with pelvic fractures.2 Cass found bladder ruptures in 6% of pelvic fractures, urethral rupture in 2%, and combined bladder and urethral rupture in 0.5%.3
One-third of patients with a traumatic bladder rupture have injuries to other urinary structures, most commonly the urethra. Ten to 20% of patients with a pelvic fracture and bladder rupture also have a posterior urethral rupture (Box 11.6).
•Blood at the meatus—in 40–50% of patients (no blood at the meatus in 50–60%).
•Perineal or scrotal bruising.
•Inability to pass a urethral catheter.
The prostate and bladder become detached from the membranous urethra and are pushed upwards by the expanding pelvic haematoma. The high-riding prostate is said to be a classic sign of posterior urethral rupture. Traditional teaching states that a DRE should be done in cases of pelvic trauma to determine the prostatic position. However, the presence of a high-riding prostate is an unreliable sign.4 The pelvic haematoma may make it impossible to feel the prostate, so the patient may be thought to have a high-riding prostate when, in fact, it is in a normal position. Conversely, what may be thought to be a normal prostate in a normal position may actually be a palpable pelvic haematoma. In pelvic fracture, a DRE is done not to identify a high-riding prostate, but rather to establish the presence of an associated rectal injury (blood seen on the examining finger). However, rectal injury can still occur in the absence of rectal blood.
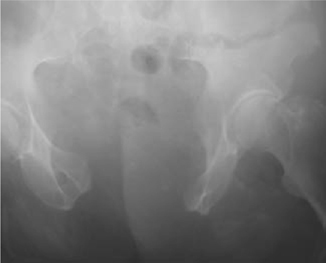
Fig. 11.7 An open-book pelvic fracture before fixation.
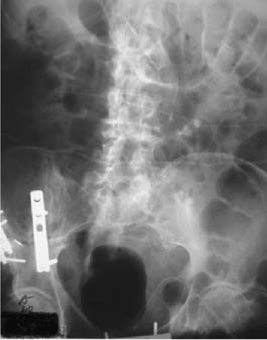
Fig. 11.8 An open book pelvic fracture after fixation.
Box 11.6 Management of bladder injuries associated with pelvic fractures
Extraperitoneal: urethral catheter until the bladder has healed (usually 2–3wk).
Intraperitoneal: open surgical repair.
SPC: placement via an open approach is generally better than a percutaneous approach, partly because it allows inspection of the bladder for associated injuries which may require repair, but also because the catheter may inadvertently be placed into the large pelvic haematoma which always accompanies such fractures. Not only does this mean that the bladder is not being drained (so urine will leak into the pelvic haematoma and fracture site), but the SPC can also act as a potential source of infection of the pelvic haematoma, which can lead to life-threatening sepsis.
If a urethral catheter can be passed and a cystogram shows an extraperitoneal bladder rupture, leave a urethral catheter in place until the bladder has healed (usually 2–3wk).
If a urethral catheter cannot be passed (because of a complete urethral rupture), an SPC should be placed via an open approach (rather than percutaneously) to allow inspection of the bladder (and repair if the bladder has been torn) at the same time that the SPC is placed. The urethral rupture will prevent a cystogram from being done, so direct inspection of the bladder is required to establish the presence/absence of a bladder injury.
References
1McAninch JW (2002). In: Walsh PC, Retik AB, Vaughan ED, Wein AJ (eds). Campbell’s Urology, 8th edn. Philadelphia, PA: WB Saunders; pp. 3703–14.
2Cass AS (1984). Simultaneous bladder and prostato membranous urethral rupture from external trauma. J Urol 132:907–8.
3Cass AS (1988). Genitourinary Trauma. Boston, MA: Blackwell Scientific Publications.
4Elliott DS, Barrett DM (1997). Long-term follow-up and evaluation of primary realignment of posterior urethral disruptions. J Urol 157:814–16.
TURBT (Figs. 11.9 and 11.10), cystoscopic bladder biopsy, TURP, cystolitholapaxy, penetrating trauma to the lower abdomen or back, Caesarean section (especially as an emergency), blunt pelvic trauma—in association with pelvic fracture or ‘minor’ trauma in the inebriated patient, rapid deceleration injury (e.g. seat belt injury with a full bladder in the absence of a pelvic fracture), spontaneous rupture after bladder augmentation, total hip replacement (very rare).
•Intraperitoneal perforation: the peritoneum overlying the bladder is breached, allowing urine to escape into the peritoneal cavity.
•Extraperitoneal perforation: the peritoneum is intact and urine escapes into the space around the bladder, but not into the peritoneal cavity.
During endoscopic urological operations (e.g. TURBT, cystolitholapaxy), the diagnosis is usually obvious on visual inspection alone—a dark hole is seen in the bladder, and loops of bowel may be seen on the other side. No further diagnostic tests are required.
In cases of trauma, the classic triad of symptoms and signs suggesting a bladder rupture is:
•Suprapubic pain and tenderness.
•Difficulty or inability in passing urine.
Additional signs:
•Absent bowel sounds (indicating an ileus from urine in the peritoneal cavity).
These symptoms and signs are an indication for a retrograde cystogram. The diagnosis may be made only at operation for fixation of a pelvic fracture.
•Ensure the bladder is adequately distended with contrast. With inadequate distension, a clot, omentum, or small bowel may ‘plug’ the perforation, which may not therefore be diagnosed. Use at least 400mL of contrast in an adult, and 60mL plus 30mL per year of age in children up to a maximum of 400mL in children.
•Obtain images after the contrast agent has been completely drained from the bladder (a post-drainage film). A whisper of contrast from a posterior perforation may be obscured by a bladder distended with contrast.
•In extraperitoneal perforations, extravasation of contrast is limited to the immediate area surrounding the bladder. In intraperitoneal perforations, loops of bowel may be outlined by the contrast.
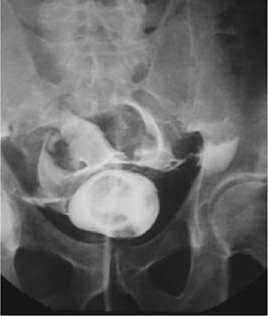
Fig. 11.9 An intraperitoneal bladder perforation following TURBT, as demonstrated on a cystogram (anteroposterior view).
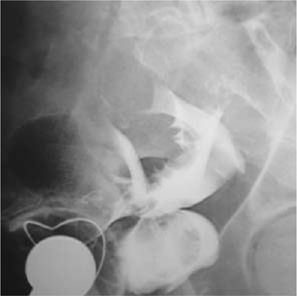
Fig. 11.10 A bladder perforation following TURBT, as demonstrated on a cystogram (lateral view).
(See Box 11.7.)
Box 11.7 Treatment of bladder rupture
Bladder drainage with a urethral catheter for 2wk, followed by a cystogram to confirm the perforation has healed.
Indications for surgical repair of extraperitoneal bladder perforation:
•If you have opened the bladder to place an SPC for a urethral injury.
•A bone spike protruding into the bladder on CT.
•Associated rectal or vaginal perforation.
•Where the patient is undergoing open fixation of a pelvic fracture, the bladder can be simultaneously repaired.
Usually repaired surgically to prevent complications from leakage of urine into the peritoneal cavity.
Spontaneous bladder rupture occasionally occurs months or years after bladder augmentation and usually with no history of trauma. If the patient has spina bifida or an SCI, they usually have limited awareness of bladder fullness and pelvic pain. Their abdominal pain may therefore be mild and vague in onset and nature. Fever or other signs of sepsis may be present. Have a high index of suspicion in patients with augmentation who present with non-specific signs of illness. A cystogram usually, although not always, confirms the diagnosis. If doubt exists, consider exploratory laparotomy.
•External blunt: pelvic fracture—RTAs, falls from a height, crush injuries—commonest cause.
•External penetrating: gunshot—rare; stab—rare.
•Internal, iatrogenic: endoscopic surgery; RP; TURP (more likely with vascular prostate, PC, inexperienced surgeon).
•Internal, self-inflicted: foreign bodies inserted into the urethra—rare.
The great majority of posterior urethral injuries are an associated injury following pelvic fracture, and their diagnosis and initial management are discussed on  p. 535. Immediate (within 48h) open repair of posterior urethral injuries is associated with a high incidence of urethral strictures (70%) and subsequent restenosis after stricture repair, incontinence (20%), and impotence (40%). The surrounding haematoma and tissue swelling make it difficult to identify structures and to mobilize the two ends of the urethra to allow tension-free anastomosis.
p. 535. Immediate (within 48h) open repair of posterior urethral injuries is associated with a high incidence of urethral strictures (70%) and subsequent restenosis after stricture repair, incontinence (20%), and impotence (40%). The surrounding haematoma and tissue swelling make it difficult to identify structures and to mobilize the two ends of the urethra to allow tension-free anastomosis.
In the majority of ♂ posterior urethral injuries, treatment should be deferred for 3 months to allow the oedema and haematoma to completely resolve. As this occurs, the two distracted ends of the urethra come closer together, thereby reducing the amount of mobilization that the surgeon has to do. Most such injuries can be repaired by an anastomotic urethroplasty. Optical urethrotomy (division of the stricture using an endoscopic knife or laser via a cystoscope inserted into the urethra) is generally not recommended.
Immediate repair is indicated where there is an open wound, as long as the urethral ends are close (i.e. not distracted by a large haematoma).
Rare because the ♀ urethra is short and its attachments to the pubic bone are weak, such that it is less prone to tearing during pubic bone fracture. When they do occur, such injuries are usually associated with rectal or vaginal injuries. In developing countries, prolonged labour can cause ischaemic injury to the urethra and bladder neck, leading to urethrovaginal or vesicovaginal fistula formation.
•External blunt: straddle injury (e.g. forceful contact of the perineum with a bicycle cross-bar*)—commonest cause of injury; kick to the perineum; penile fracture.
•External penetrating: gunshot; stab.
•Internal, iatrogenic: catheter balloon inflated in the urethra; endoscopic surgery; penile surgery.
•Internal, self-inflicted: foreign bodies inserted into urethra.
* The bulbar urethra being crushed against the pubic bone.
The patient usually presents with difficulty in passing urine and frank haematuria in the context of a straddle injury. Blood may be present at the end of the penis and a haematoma around the site of the rupture. If Buck’s fascia has been ruptured (the deep layer of the superficial fascia of the penis), urine and blood track into the scrotum, causing swelling and a ‘butterfly wing’ pattern of bruising, reflecting the anatomical attachments of Colles’ fascia—the membranous layer of the superficial fascia of the groin and perineum (Figs. 11.11 and 11.12; Box 11.8).
Retrograde urethrography delineates the extent of urethral injury.
Extravasation of urine can create a collection of urine around the urethra (a urinoma) and generates an inflammatory reaction, with subsequent stricture formation. Superadded infection can lead to abscess formation which may burst onto the surface of the skin, leading to a urethrocutaneous fistula. More rarely, Fournier’s gangrene supervenes. Urinary diversion (urethral or suprapubic catheter) prevents further extravasation of urine, and antibiotics may reduce the likelihood of superadded infection.
Typical history: blood at the meatus, no extravasation of contrast on retrograde urethrogram. Pass a small-gauge urethral catheter (12Ch in an adult), and remove a week or so later.
Leak of contrast from the urethra, with retrograde flow into the bladder. Most can be managed by a period of suprapubic urinary diversion. Seventy per cent heal without stricture formation (primary closure can be difficult because of oedema and haematoma at the site of injury and can convert a short area of urethral injury into a longer one). Give a broad-spectrum antibiotic to prevent infection of extravasated urine and blood. If a voiding cystogram 2wk later confirms urethral healing, remove the SPC. If contrast still extravasates, leave it in place a little longer.
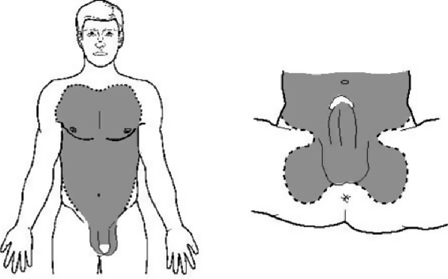
Fig. 11.11 Butterfly bruising following rupture of Buck’s fascia.
Box 11.8 Anatomical explanation for the ‘butterfly wing’ pattern of bruising in anterior urethral rupture
Fascial layers of the penis, from superficial to deep (Fig. 11.12):
•Superficial fascia of the penis (= dartos fascia)—continuous with the membranous layer of the superficial fascia of the groin and perineum (= Colles’ fascia).
•Buck’s fascia (= the deep layer of the superficial fascia).
•Deep fascia of the penis (the tunica albuginea) which covers the two dorsal rods of erectile tissue, the corpora cavernosa, and the ventrally located corpus spongiosum that surrounds the urethra.
If Buck’s fascia is intact, bruising from a urethral rupture is confined in a sleeve-like configuration along the length of the penis. If Buck’s fascia has ruptured, extravasation of blood, and thus subsequent bruising, is limited by the attachments of Colles’ fascia which forms a ‘butterfly’-like pattern in the perineum and is continuous in the upper abdomen and chest with Scarpa’s fascia.
•Urografin 150® (sodium amidotrizoate and meglumine amidotrizoate), but other contrast agents can be used.
•Position the patient at an oblique angle (bottom leg flexed at the hip and knee).
•A 12Ch catheter is placed in the fossa navicularis of the penis, 1–2cm from the external meatus, with the catheter balloon with 2mL of water or with a penile clamp applied to prevent contrast from spilling out of the urethra and to hold the catheter in place.
•Continuous screening (fluoroscopy) is done as contrast is instilled until the entire length of the urethra is demonstrated. Remember, as the urethra passes through the pelvic floor (the membranous urethra), there is a normal narrowing and similarly, the prostatic urethra is narrower than the bulbar urethra.
Suprapubic catheterization (percutaneously) is preferred over urethral catheterization, because a partial rupture can be converted to a complete rupture. If the bladder cannot be palpated, such that an SPC cannot be safely inserted, then perform an open suprapubic cystostomy (under GA).
Leak of contrast from the urethra on retrograde urethrogram, no filling of the posterior urethra or bladder. Either the urethra may be immediately repaired (if a surgeon with sufficient experience is available) or an SPC can be placed with delayed repair.
Knife or gunshot wound: primary (i.e. immediate) repair may be carried out if a surgeon experienced in these techniques is available; if not, suprapubic diversion and subsequent repair by an appropriate surgeon.
Immediate surgical repair of anterior urethral injuries is only done in the context of penile fracture or where there is an open wound.
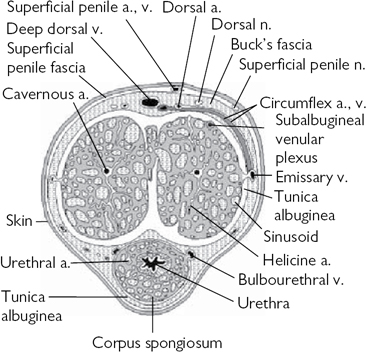
Fig. 11.12 The fascial layers of the penis.
Testicular injuries are uncommon.
Blunt or penetrating. Most in civilian practice are blunt, a blow forcing the testicle against the pubis or the thigh. Bleeding occurs into the parenchyma of the testis, and if sufficient force is applied, the tunica albuginea of the testis (the tough fibrous coat surrounding the parenchyma) ruptures, allowing extrusion of the seminiferous tubules.
Penetrating injuries occur as a consequence of gunshot and knife wounds and from bomb blasts; associated limb (e.g. femoral vessel), perineal (penis, urethra, rectum), pelvic, abdominal, and chest wounds may occur.
Where bleeding is confined by the tunica vaginalis, a haematocele is said to exist. Intraparenchymal (intratesticular) haemorrhage and bleeding beneath the parietal layer of the tunica vaginalis will cause the testis to enlarge slightly. The testis may be under great pressure as a consequence of the intratesticular haemorrhage confined by the tunica vaginalis. This can lead to ischaemia, necrosis, and atrophy of the testis.
The force is usually sufficient to rupture the tunica albuginea and tunica vaginalis, and the seminiferous tubules and blood extrude into the layers of the scrotum. This is a haematoma.
Severe pain is common, as are nausea and vomiting. If the testis is surrounded by haematoma, it will not be palpable. If it is possible to palpate the testis, it is usually very tender. The resulting scrotal haematoma can be very large, and the bruising and swelling so caused may spread into the inguinal region and lower abdomen.
A normal parenchymal echo pattern suggests there is no significant testicular injury (i.e. no testicular rupture). Hypoechoic areas within the testis (indicating intraparenchymal haemorrhage) suggests testicular rupture.
•Testicular rupture: exploration allows evacuation of the haematoma, excision of extruded seminiferous tubules, and repair of the tear in the tunica albuginea.
•Penetrating trauma: exploration allows repair to damaged structures (e.g. the vas deferens may have been severed and can be repaired).
Blood loss can be severe; resuscitate the shocked patient, and cross-match blood. Place the penis, if found, in a wet swab inside a plastic bag, which is then placed inside another bag containing ice (‘bag in a bag’). It can survive for 24h.
Associated injuries are common (e.g. scrotum, major vessels of the lower limb). Most injuries, other than minor ones, should undergo primary repair. Remove debris from the wound (e.g. particles of clothing) and debride necrotic tissue, and repair as for penile fractures (Box 11.9).
Rupture of the tunica albuginea of the erect penis (i.e. rupture of one or both corpora cavernosa, rupture of the corpus spongiosum with rupture of the urethra). The tunica albuginea is 2mm thick in the flaccid penis. It thins to 0.25mm during erection and is therefore vulnerable to rupture if the penis is forcibly bent (e.g. during vigorous sexual intercourse). The patient usually reports a sudden ‘snapping’ or ‘popping’ sound and/or sensation with sudden penile pain and detumescence of the erection.
The penis is swollen and bruised, sometimes resembling an aubergine. If Buck’s fascia has ruptured, bruising extends onto the lower abdominal wall and into the perineum and scrotum. A tender, palpable defect may be felt over the site of the tear in the tunica albuginea. If the urethra is damaged, there may be blood at the meatus or haematuria (dipstick/microscopic or macroscopic) and pain on voiding or urinary retention. Arrange a retrograde urethrogram in such cases.
There has been a trend away from conservative management towards surgical repair (lower complication rate, e.g. reduced penile deformity, less chance of penile scar tissue, and prolonged penile pain).
•Conservative: application of cold compresses to the penis; analgesics and anti-inflammatory drugs; abstinence from sexual activity for 6–8wk to allow healing.
•Surgery: expose the fracture site in the tunica albuginea; evacuate the haematoma, and close the defect in the tunica.
Box 11.9 Surgical reimplantation of amputated penis
Repair the urethra first over a catheter to provide a stable base for subsequent neurovascular repair. Close the tunica albuginea of the corpora (4/0 absorbable suture). Cavernosal artery repair is technically very difficult and does not improve penile viability. Anastomose the dorsal artery of the penis (11/0 nylon), then the dorsal vein (9/0 nylon) to provide venous drainage, and finally the dorsal penile nerve (10/0 nylon).
Expose the fracture site by degloving the penis via a circumcising incision around the subcoronal sulcus or by an incision directly over the defect, if palpable. A degloving incision allows better exposure of the urethra for associated urethral injuries. Alternatively, use a midline incision extending distally from the midline raphe of the scrotum along the shaft of the penis. This latter incision, along with a degloving incision, allows excellent exposure of both corpora cavernosa so that an unexpected bilateral injury can be repaired easily, as can a urethral injury, should this have occurred.
Close the defect in the tunica with absorbable sutures or by non-absorbable sutures (bury the knots so that the patient is unable to palpate them). Non-absorbable sutures may possibly be associated with prolonged post-operative pain. Leave a urethral catheter (voiding can be difficult immediately post-operatively). Repair a urethral rupture, if present, with a spatulated single- or two-layer urethral anastomosis and splint repair with a urethral catheter for 3wk.
Clean the wound. Give broad-spectrum antibiotics (e.g. cephalosporin and amoxicillin).
If the penis is still caught in the zipper, use lubricant jelly and gently attempt to open it. The zipper may have to be cut with orthopaedic cutters or prised apart with a pair of surgical clips on either side of the zipper.
A testicular torsion is a twist of the spermatic cord, resulting in strangulation of the blood supply to the testis and epididymis. Testicular torsion occurs most frequently between the ages of 10 and 30 (peak incidence 13–15y of age), but any age group may be affected.
Sudden onset of severe pain in the hemiscrotum, sometimes waking the patient from sleep. It may radiate to the groin, loin, or epigastrium (reflecting its origin from the dorsal abdominal wall of the embryo and its nerve supply from T10/11). There is sometimes a history of minor trauma to the testis. Some patients report previous episodes, with spontaneous resolution of the pain (suggesting previous torsion with spontaneous detorsion). The patient may have a slight fever. The testis is usually slightly swollen and very tender to touch. It may be high-riding (lying at a higher-than-normal position in the testis) and may be in a horizontal position due to twisting of the cord. The cremasteric reflex is usually, but not always, absent (positive Rabinowitz’s sign). The cremasteric reflex may normally be elicited by stroking the finger along the inside of the thigh, which results in an upward movement of the ipsilateral testis. Elevation of the involved testicle does not ameliorate the symptoms (negative Prehn’s sign).
Epididymo-orchitis, torsion of a testicular appendage, and causes of flank pain with radiation into the groin and testis (e.g. a ureteric stone). Colour Doppler USS (reduced arterial blood flow in the testicular artery) and radionuclide scanning ( radioisotope uptake) can be used to diagnose testicular torsion, but in many hospitals, these tests are not readily available and the diagnosis is based on symptoms and signs.
radioisotope uptake) can be used to diagnose testicular torsion, but in many hospitals, these tests are not readily available and the diagnosis is based on symptoms and signs.
Scrotal exploration should be undertaken as a matter of urgency. Delay in relieving the twisted testis results in permanent ischaemic damage to the testis, causing atrophy, loss of hormone and sperm production, and, as the testis undergoes necrosis and the blood–testis barrier breaks down, an autoimmune reaction against the contralateral testis (sympathetic orchidopathia). Fix BOTH testes, since the bell-clapper abnormality, which predisposes to torsion, can occur bilaterally.
The appendix testis (hydatid of Morgagni—a remnant of the Müllerian duct) and the appendix epididymis (a remnant of a cranial mesonephric tubule of the Wolffian duct) can undergo torsion, causing pain that mimics a testicular torsion. At scrotal exploration, they are easily removed with scissors or a diathermy probe.
This is where the foreskin is retracted from over the glans of the penis, becomes oedematous, and cannot then be pulled back over the glans into its normal anatomical position. It occurs most commonly in teenagers or young men and also in elderly men (who have had the foreskin retracted during catheterization, but where it has not been returned to its normal position). Paraphimosis is usually painful. The foreskin is oedematous, and a small area of ulceration of the foreskin may have developed.
•The ‘iced glove’ method: apply topical lidocaine gel to the glans and foreskin for 5min. Place ice and water in a rubber glove, and tie a knot in the cuff of the glove to prevent the contents from pouring out. Invaginate the penis into the thumb of the glove. This may reduce the swelling and allow reduction of the foreskin.
•Granulated sugar: placed in a condom or glove and applied over the end of the penis, has been used to reduce oedema by osmosis.
•The Dundee technique: 1 give the patient a broad-spectrum antibiotic, such as 500mg of ciprofloxacin, by mouth. Apply a ring block to the base of the penis using a 26G needle and 10–20mL of 0.5% plain bupivacaine (children usually require GA). Clean the skin of the foreskin and the glans with cleaning solution. Using a 25G needle; make ~20 punctures into the oedematous foreskin. Squeeze the oedema fluid out of the foreskin, and return to its normal position. Approximately one-third of patients subsequently require elective circumcision for an underlying phimosis.
If this fails, the traditional surgical treatment is a dorsal slit under GA or ring block. A longitudinal incision is made in the tight band of constricting tissue, and the foreskin is pulled back over the glans. Close the incision transversely to lengthen the circumference of the foreskin and prevent recurrences.
Reference
1Reynard JM, Barua JM (1999). Reduction of paraphimosis the simple way—the Dundee technique. BJU Int 83:859–60.
Locally advanced PC, and bladder or ureteric cancer may cause unilateral or bilateral ureteric obstruction. Locally advanced non-urological malignancies can also obstruct the ureters (e.g. cervical cancer, rectal cancer, lymphoma).
Often asymptomatic; an incidental USS finding that requires no specific treatment in the presence of a normal contralateral kidney. Occasionally, loin pain and systemic symptoms may develop due to infection of the obstructed upper urinary tract. In this circumstance, drainage by nephrostomy or stenting is required.
A urological emergency. The patient presents either with symptoms and signs of renal failure or anuric without a palpable bladder. A mass will probably be palpable on rectal examination.
•Investigations: renal USS will demonstrate bilateral hydronephrosis and an empty bladder; CT-KUB will confirm the presence of dilated ureters down to a mass at the bladder base.
After treating any life-threatening hyperkalaemia, options include bilateral percutaneous nephrostomy or ureteric stenting. A clotting screen is required prior to nephrostomy insertion. Insertion of retrograde ureteric stents in this setting is usually unsuccessful, because a tumour involving the trigone obscures the location of the ureteric orifices. More successful is antegrade ureteric stenting following nephrostomy insertion, both of which are performed under sedo-analgesia. The full-length double-J silicone or polyurethane ureteric stents require periodic (4- to 6-monthly) changes to prevent calcification or blockage. In the case of PC, hormone therapy should be commenced, if not previously used; even in patients with androgen-independent disease, high-dose parenteral oestrogens may relieve ureteric obstruction.
Longer-term treatment options include urinary diversion by formation of an ileal conduit, ureteric reimplantation, insertion of short ‘permanent’ metallic ureteric stents, or ureteric replacement with isolated ileal segments or prosthetic graft material. Such procedures are often complicated and inappropriate in those patients with poor prognosis.
This is an oncological emergency; failure to diagnose and treat promptly can lead to permanent paraplegia and autonomic dysfunction. It is defined as spinal cord or cauda equina compression by direct pressure and/or induction of vertebral collapse or instability by metastatic spread or direct extension of malignancy that threatens or causes neurological disability.1
Ninety-five per cent of patients will complain of back or nerve root pain and have a positive bone scan. Five per cent of patients do not exhibit these features because their disease is paravertebral. The majority of urological cases of metastatic spinal cord compression (MSCC) are due to PC. Patients with back or nerve root pain should be examined neurologically and evaluated radiologically. Pain, sometimes worsened by straining or coughing, usually precedes clinical cord compression by ~4 months. Clinical features include sensory changes and muscle weakness in the lower limbs; 50% of patients present unable to stand or walk. Only two-thirds of patients presenting as such will recover any function within 1 month.
If cord compression is suspected, the patient should be nursed flat with neutral spine alignment (including ‘log rolling’ or turning beds, with use of a slipper pan for toilet), until bony and neurological stability are ensured and cautious remobilization may begin. Every acute UK NHS Cancer Centre should have access to an MSCC coordinator who should be informed. The investigation of choice is emergency spinal MRI. Short TI inversion recovery (STIR) and sagittal T2-weighted sequences will reveal the bone deposits and level (multiple in 20% of cases) of the soft tissue cord compression. If MRI is not possible, CT scan or myelography should be considered.
Initial treatment is with high-dose IV corticosteroids, e.g. dexamethasone 16mg, followed by 4mg 6-hourly for 2–3wk. Analgesics and bisphosphonates are administered for pain relief. Within 24h, definitive treatment is with fractionated RT or neurosurgical decompression. Surgery to achieve decompression and spinal stability is considered preferable if there is pathological fracture, an unknown tissue diagnosis, or a history of previous RT. Subsequently, care should include pressure sore and VTE prophylaxis measures. Bladder and bowel dysfunction may also occur, requiring catheterization and stool softeners. Rehabilitation and community support will also be required.
The adult spinal cord tapers below L2 vertebral level into the conus medullaris. The cauda equina consists of the nerve roots of all spinal cord segments below L2, as they run in the subarachnoid space to their exit levels in the lower lumbar and sacral spines.
•Pathophysiology: the cauda equina may be compressed by central intervertebral disc prolapse (1–15% of cases), spinal stenosis, or a benign or malignant tumour within the lower lumbar or sacral vertebral canal.
•Symptoms: the diagnosis should be considered in any ♀ or young ♂ presenting with difficulty voiding or in urinary retention. There may be back pain.
•Signs: palpable bladder, loss of perianal (S2–4) and lateral foot sensation (S1–2), reduced anal tone; priapism.
•Investigations: MRI lumbosacral spine; urodynamic studies reveal a normally compliant, but areflexic, bladder.
•Treatment: ISC, neurosurgery.
Reference
1National Institute for Health and Care Excellence (2008). Metastatic spinal cord compression in adults: risk assessment, diagnosis and management. Clinical guideline [CG75]. Available from:  https://www.nice.org.uk/guidance/cg75.
https://www.nice.org.uk/guidance/cg75.