Preparation of the patient for urological surgery
The degree of preparation is related to the complexity of the procedure. Certain aspects of examination (pulse rate, BP) and certain tests (Hb, electrolytes, creatinine) are important, not only to assess fitness for surgery, but also as a baseline against which changes in the post-operative period may be measured.
•Assess cardiac status (angina, arrhythmias, previous myocardial infarction) with BP, electrocardiogram (ECG), and CXR.
•Arrange an anaesthetic review, as needed. Cardiopulmonary exercise testing is offered to some patients with cardiac and/or lung disease prior to major surgery.
•Culture urine; treat active (symptomatic) infection with an appropriate antibiotic, starting a week before surgery, and give prophylactic antibiotics at induction of anaesthesia.
•Consider stopping anticoagulation (with or without bridging therapy) 7–10 days prior to surgery.
•Obtain consent.
•Measure Hb and serum creatinine, and investigate and correct anaemia, electrolyte disturbance, and abnormal renal function. If blood loss is anticipated, group and save a sample of serum or cross-match several units of blood, the precise number depending on the speed with which your blood bank can deliver blood, if needed. In our own unit, our policy is (other units may have a different policy) (Table 17.1):
•The patient may choose to store their own blood prior to the procedure.
Table 17.1 The Group and Save and Cross Match policy in Oxford University Hospitals NHS Foundation Trust (consult your local hospital policy)
| TURBT |
Group and save |
| TURP |
Group and save |
| Open prostatectomy |
Cross-match 2U |
| Simple nephrectomy |
Cross-match 2U |
| Radical nephrectomy |
Cross-match 4U |
| (Renal vein or IVC extension) |
Cross match 6U |
| Cystectomy |
Cross-match 4U |
| RP |
Cross-match 2U |
| PCNL |
Group and save |
Should anticoagulation be stopped prior to minor urological procedures and urological surgery?
Aspirin and TRUS biopsy
In the UK, 65% of urologists routinely stop aspirin prior to TRUS biopsy; 35% do not.1 Four of 297 urologists (1.3%) reported cerebrovascular side effects from stopping aspirin. There remains no consensus guidance on whether to stop or continue aspirin.
Aspirin and TURP
There is wide variation in the management of aspirin in men undergoing TURP. In a recent audit of UK urologists, 38% said they did not stop aspirin prior to TURP, but of those that said they did stop it, a substantial number still proceeded with TURP if the aspirin had inadvertently not been stopped.2 Overall, 75% either did not bother stopping aspirin or proceeded with TURP if patients were inadvertently still taking it, presumably because of a perceived  risk of serious cardiovascular events. Some studies suggest an
risk of serious cardiovascular events. Some studies suggest an  risk of bleeding and the need for blood transfusion in those on aspirin, while others report no
risk of bleeding and the need for blood transfusion in those on aspirin, while others report no  risk. There is only one RCT, and this showed that aspirin did increase blood loss after TURP, but not enough to increase the requirement for blood transfusion.3 The risks of short-term withdrawal of aspirin prior to TURP have not been established, although there are anecdotal reports of serious adverse cardiovascular events. So should aspirin be stopped or continued prior to TURP? The short answer is that there is no substantial body of evidence to support stopping it or continuing it, and as the majority continue to do TURP with patients on aspirin, but a substantial minority stop it, either behaviour is reasonable. Since bleeding times return to normal within 48h of stopping aspirin (the time taken for new platelets to reach sufficient numbers to compensate for impaired function of circulating platelets), it seems reasonable to stop it 2 days before surgery and to restart it within a few days of surgery when it is obvious that post-operative bleeding has stopped (usually when it is deemed safe to remove the catheter).
risk. There is only one RCT, and this showed that aspirin did increase blood loss after TURP, but not enough to increase the requirement for blood transfusion.3 The risks of short-term withdrawal of aspirin prior to TURP have not been established, although there are anecdotal reports of serious adverse cardiovascular events. So should aspirin be stopped or continued prior to TURP? The short answer is that there is no substantial body of evidence to support stopping it or continuing it, and as the majority continue to do TURP with patients on aspirin, but a substantial minority stop it, either behaviour is reasonable. Since bleeding times return to normal within 48h of stopping aspirin (the time taken for new platelets to reach sufficient numbers to compensate for impaired function of circulating platelets), it seems reasonable to stop it 2 days before surgery and to restart it within a few days of surgery when it is obvious that post-operative bleeding has stopped (usually when it is deemed safe to remove the catheter).
Drug-eluting cardiac stents and antiplatelet agents
Be careful in patients receiving the newer antiplatelet drugs such as clopidogrel or ticlopidine (with or without aspirin), since bleeding times can increase 3-fold.4 Severe intractable bleeding can occur following ‘minor’ procedures such as prostate biopsy or bladder biopsy. Patients with coronary artery stents are treated with dual anticoagulation with aspirin and clopidogrel for several months after stent insertion to reduce the risk of stent thrombosis. The precise duration of antiplatelet therapy has not been established, and while recent evidence suggests 6 months might be non-inferior to longer regimens, 12 months is a common treatment. Seek advice from a cardiologist about the safety of stopping these drugs. Consider delaying invasive procedures (e.g. prostate or bladder biopsy) if the risk of bleeding is deemed to be unacceptable in the presence of the continued need for anticoagulation.
New oral anticoagulants
Patients on one of the new oral anticoagulants (NOACs), such as dabigatran (inhibitor of free thrombin, fibrin-bound thrombin, and thrombin-induced platelet aggregation), apixaban, edoxaban, and rivaroxaban (inhibitors of factor Xa), are advised to discontinue treatment for at least 2 days preoperatively (3–5 days if renal impairment) if surgery is associated with a high risk of bleeding (PCNL, radical cancer surgery, TURP). Treatment can be restarted when post-operative bleeding has settled, usually at 1–2 days.
If emergency surgery is required for a patient taking a NOAC, idarucizumab (to reverse dabigatran) or andexanet alfa (to reverse apixaban, edoxaban, or rivaroxaban) can be used, depending on availability.
Bowel preparation
Indicated if large bowel is to be used (bowel prep is not required if small bowel alone is to be used, e.g. ileal conduit, ileal neobladder reconstruction). Use a simple mechanical prep (Citramag® or Picolax®—magnesium salts), two doses starting the morning before surgery, with a clear, fluid-only diet.
References
1Masood J, Hafeez, Calleary J, Barua JM (2007). Aspirin use and transrectal ultrasonography-guided prostate biopsy: a national survey. BJU Int 99:965–6.
2Enver MK, Hoh I, Chinegwundoh FI (2006). The management of aspirin in transurethral prostatectomy: current practice in the UK. Ann R Coll Surg Engl 88:280–3.
3Nielsen JD, Holm-Nielsen A, Jespersen J, et al. (2000). The effect of low-dose acetylsalicylic acid on bleeding after transurethral prostatectomy—a prospective, randomized, double-blind, placebo-controlled study. Scand J Urol Nephrol 34:194–8.
4Stephen Jones J (2007). Urologists: be aware of significant risks of stopping anticoagulants in patients with drug eluting coronary stents. BJU Int 99:1330–1.
Antibiotic prophylaxis in urological surgery
The precise antibiotic prophylaxis policy that you use will depend on your local microbiological flora. Your local microbiology department will provide regular advice and updates on which antibiotics should be used, both for prophylaxis and treatment. The policy shown here and in Table 17.2 is our own local policy.
We do not routinely administer prophylaxis for flexible cystoscopy, SWL, diagnostic cystoscopy and biopsy, transperineal prostate biopsy, circumcision, inguinoscrotal surgery, or upper tract surgery.
There is a move away from the use of cefuroxime (to reduce the risk of antibiotic-induced Clostridium difficile colitis) and fluoroquinolones (to reduce the risk of C. difficile-associated diarrhoea, pseudomembranous colitis, and MRSA).* Trimethoprim, gentamicin, penicillin, and co-amoxiclav are less likely to cause C. difficile-associated disease.
Culture urine before any procedure, and use specific prophylaxis (based on sensitivities) if culture positive.
We avoid ciprofloxacin in inpatients because it is secreted onto the skin and causes MRSA colonization. For most purposes, nitrofurantoin provides equivalent cover without being secreted onto the skin. We do use ciprofloxacin if there is known Proteus infection (all Proteus species are resistant to nitrofurantoin).
* C. difficile is a Gram-positive, anaerobic, spore-forming bacillus. The commonest cause of nosocomial diarrhoea and antibiotic-associated colitis. Disease arises as a consequence of faeco-oral transmission of C. difficile spores (ribotype 027 seems to be particularly pathogenic). Once colonization has occurred, progression to diarrhoea or colitis depends on coexisting conditions and host immune response. C. difficile toxins A and B are responsible for pathogenicity. They bind to intestinal epithelial receptors. Inflammatory cytokines cause fluid secretion, mucosal destruction, and tissue necrosis. Other risk factors for C. difficile-associated disease: age >65y, use of proton pump inhibitors, laxatives, nasogastric tubes, and prolonged hospital stay. Treatment for diarrhoea and colitis: stop causative antibiotics, isolate and barrier nurse (wash hands with soap and water, as alcohol hand rubs are ineffective against spores), and oral metronidazole (oral vancomycin reserved for serious or recurrent infection).
Patients with artificial heart valves
Antibiotic prophylaxis against infective endocarditis is not recommended routinely for urological procedures (NICE guidelines, 2015).1
Patients with joint replacements
The advice is conflicting.
AAOS/AUA advice
Joint advice of the American Academy of Orthopaedic Surgeons (AAOS) and the AUA—antibiotic prophylaxis is not indicated for urological patients with pins, plates, or screws or for most patients with total joint replacements. It is recommended for all patients undergoing urological procedures, including TURP within 2y of a prosthetic joint replacement, those who are immunocompromised (e.g. rheumatoid patients, those with SLE, drug-induced immunosuppression, including steroids), and those with a history of previous joint infection, haemophilia, HIV infection, diabetes, and malignancy.
Antibiotic regime
Single dose of a quinolone, such as ciprofloxacin 500mg, 1–2h preoperatively + ampicillin 2g IV + gentamicin 1.5mg/kg 30–60min preoperatively (substituting vancomycin 1g IV for penicillin-allergic patients).
UK advice
In the UK, a Working Party of the British Society for Antimicrobial Chemotherapy has stated that patients with prosthetic joint implants (including total hip replacements) do not require antibiotic prophylaxis and considers that it is unacceptable to expose patients to the adverse effects of antibiotics when there is no evidence that such prophylaxis is of any benefit. This advice is based on the rationale that joint infections are caused by skin organisms that get onto the prosthesis at the time of the operation and that the role of bacteraemia as a cause of seeding outside the immediate post-operative period has never been established.
We use the same antibiotic prophylaxis as for patients without joint prostheses.
Table 17.2 Oxford Urology procedure: specific antibiotic prophylaxis protocol for urological surgery
| Procedure |
Antibiotic prophylaxis |
| Change of long-term catheter (only if symptomatic UTI after previous change, acute UTI, after traumatic catheterization) |
Nitrofurantoin 100mg PO 20min before (gentamicin 3mg/kg IV if eGFR <45) |
| Transrectal prostatic biopsy |
Ciprofloxacin 500mg PO and metronidazole 400mg 60min pre-biopsy and for 48h post-biopsy (ciprofloxacin 500mg bd, metronidazole 400mg tds) |
| PCNL |
Co-amoxiclav 1.2g IV and gentamicin 3mg/kg IV at induction (also 24h preoperatively if spinal injury patient) |
| Ureteroscopy, cystolitholopaxy |
Gentamicin 3mg/kg IV at induction |
| Urogynaecological procedures (e.g. colposuspension) |
Co-amoxiclav 1.2g IV and metronidazole 500mg IV at induction of anaesthesia |
| TURP, HoLEP, prostate artery embolization, prostate HIFU |
Gentamicin 3mg/kg at induction |
| Radical or Millin’s prostatectomy (open, laparoscopic, or robotic) |
Gentamicin 3mg/kg IV at induction |
| BNI, urethrotomy, TURBT |
Gentamicin 3mg/kg IV at induction |
| Cystectomy or other procedures involving the use of bowel (e.g. augmentation cystoplasty) |
Co-amoxiclav 1.2g IV + metronidazole 500mg IV at induction |
| AUS insertion |
Teicoplanin 800mg IV two doses (24 and 12h preoperatively), gentamicin 3mg/kg IV at induction, and teicoplanin 800mg IV + gentamicin 3mg/kg IV 24h post-operatively |
Reference
1National Institute for Health and Care Excellence (2008, updated 2016). Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. Clinical guideline [CG64]. Paragraph 1.1.3, p. 5.
Complications of surgery in general: DVT and PE
VTE is uncommon after urological surgery, but it is considered the most important non-surgical complication of major urological procedures. Following TURP, 0.1–0.2% of patients experience a PE,1 and 1–5% of patients undergoing major urological surgery experience symptomatic VTE.2 The mortality of PE is in the order of 1%.3
Risk factors for DVT and PE
 risk: open (vs endoscopic) procedures, malignancy, increasing age, duration of procedure.
risk: open (vs endoscopic) procedures, malignancy, increasing age, duration of procedure.
Categorization of VTE risk
The American College of Chest Physicians (ACCP) guidelines on the prevention of VTE2 and the British Thromboembolic Risk Factors (THRIFT) Consensus Group4 categorize the risk of VTE as:
•Low-risk patients: those <40 undergoing minor surgery (surgery lasting <30min) and no additional risk factors. No specific measures to prevent DVT are required in such patients other than early mobilization. Increasing age and duration of surgery increase the risk of VTE.
•High-risk patients: include those undergoing major surgery (surgery lasting >30min) who are aged >60.
Additional risk factors (that indicate the requirement for additional prophylactic measures, e.g. the addition of SC heparin and/or intermittent pneumatic calf compression (IPC)
•Active heart or respiratory failure.
•Active cancer or cancer treatment.
•Acute medical illness.
•Age >40y.
•Antiphospholipid syndrome.
•Behçet’s disease.
•Central venous catheter in situ.
•Continuous travel >3h up to 4wk before surgery.
•Immobility (paralysis or limb in plaster).
•Inflammatory bowel disease (Crohn’s disease/ulcerative colitis).
•Myeloproliferative diseases.
•Nephrotic syndrome.
•Obesity (BMI >30kg/m2).
•Paraproteinaemia.
•Paroxysmal nocturnal haemoglobinuria.
•Personal or family history of VTE.
•Recent myocardial infarction or stroke.
•Severe infection.
•Use of oral contraceptive or hormone replacement therapy.
•Varicose veins with associated phlebitis.
•Inherited thrombophilia.
•Factor V Leiden.
•Prothrombin 2021A gene mutation.
•Antithrombin deficiency.
•Protein C or S deficiency.
•Hyperhomocysteinaemia.
•Elevated coagulation factors (e.g. factor VIII).
Prevention of DVT and PE
(See Table 17.3.)
Diagnosis of DVT
Signs of DVT are non-specific (i.e. cellulitis and DVT share common signs—low-grade fever, calf swelling, and tenderness). If you suspect a DVT, arrange a Doppler USS. If the ultrasound probe can compress the popliteal and femoral veins, there is no DVT; if it cannot, there is a DVT.
Diagnosis of PE
Small PEs may be asymptomatic. Symptoms: include breathlessness, pleuritic chest pain, haemoptysis. Signs: tachycardia, tachypnoea, raised jugular venous pressure (JVP), hypotension, pleural rub, pleural effusion.
Tests
•CXR: may be normal or show linear atelectasis, dilated pulmonary artery, oligaemia of affected segment, small pleural effusion.
•ECG: may be normal or show tachycardia, right bundle branch block, inverted T waves in V1–V4 (evidence of right ventricular strain). The ‘classic’ S1, Q3, T3 pattern is rare.
•ABGs: low PO2 and low PCO2.
•Imaging: CT pulmonary angiogram (CTPA)—superior specificity and sensitivity, when compared with ventilation–perfusion (V/Q) radioisotope scan.
•Spiral CT: a negative CTPA rules out a PE with similar accuracy to a normal isotope lung scan or a negative pulmonary angiogram.
Treatment of established DVT
•Below-knee DVT: AK-TEDs if no peripheral arterial disease (enquire for claudication and check pulses) + unfractionated heparin 5000U SC 12-hourly.
•Above-knee DVT: start LMWH and warfarin, and stop heparin when the INR is between 2 and 3. Continue treatment for 6wk for post-surgical patient; lifelong if underlying cause (e.g. malignancy).
•LMWH.
Treatment of established PE
Fixed dose of SC LMWH seems to be as effective as adjusted-dose IV unfractionated heparin for the treatment of PE found in conjunction with symptomatic DVT.3 Rates of haemorrhage are similar with both forms of heparin treatment. Start warfarin at the same time, and stop heparin when the INR is 2–3. Continue warfarin for 3 months.
Options for prevention of VTE
•Early mobilization.
•AK-TEDs—provide graduated, static compression of the calves, thereby reducing venous stasis. More effective than below-knee TEDS for DVT prevention.5
•SC heparin [low-dose unfractionated heparin (LDUH) or LMWH]. In unfractionated preparations, heparin molecules are polymerized—molecular weights from 5000–30 000Da. LMWH is depolymerized—molecular weight 4000–5000Da.
•IPC boots, which are placed around the calves, are intermittently inflated and deflated, thereby increasing the flow of blood in calf veins.6
•For patients undergoing major urological surgery (RP, cystectomy, nephrectomy), AK-TEDS with IPC intraoperatively, followed by SC heparin (LDUH or LMWH) should be used. For TURP, many urologists use a combination of AK-TEDS and IPCs; relatively few use SC heparin.7
Contraindications to AK-TEDS
•Any local leg conditions with which stockings would interfere such as dermatitis, vein ligation, gangrene, and recent skin grafts.
•Peripheral artery occlusive disease (PAOD).
•Massive oedema of the legs or pulmonary oedema from congestive cardiac failure.
•Extreme deformity of the legs.
Contraindications to heparin
•Allergy to heparin.
•History of haemorrhagic stroke.
•Active bleeding.
•Significant liver impairment—check clotting first.
•Thrombocytopenia (platelet count <100 × 109/L).
Management of anticoagulation in the perioperative period
Liaise with whoever is responsible for the patient’s anticoagulation (e.g. anticoagulant clinic). Warfarin should be stopped either 4 days (if the target INR is 2.5) or 5 days (if the target INR is higher) before surgery. Determine the INR the day before surgery to reduce the risk of cancellation. Administer oral vitamin K (2.5mg) if the INR is ≥2.0. Check the INR on the day of surgery.
The main decision is whether to give bridging therapy with treatment-dose heparin (unfractionated heparin or LMWH) and, if not, whether preoperative prophylactic LMWH is advised when the INR is <2.0. For pragmatic purposes, to save monitoring the INR as an outpatient, this could be instituted 2–3 days after warfarin is stopped, i.e. on the morning after two doses have been omitted.
A controversial group of patients are those with a prosthetic (non-caged) aortic valve and no other risk factor. It is acceptable not to use bridging therapy with treatment-dose heparin in these patients, particularly if the bleeding risk is high.8,9
Table 17.3 Pre- and post-operative risks
|
Preoperative |
Post-operative* |
| High risk, e.g. VTE within 1 month. Prosthetic mitral valve, AF, and history of stroke |
Treatment-dose heparin (either IV unfractionated heparin or SC LMWH)** |
Treatment dose heparin (either IV unfractionated heparin or SC LMWH) |
| Non-high risk, e.g. AF without previous stroke |
Nil/prophylactic LMWH*** |
Prophylactic LMWH |
* Continue until INR >2.0 for 2 consecutive days.
** Stop full-dose IV unfractionated heparin 6h preoperatively and check APTT; omit full-dose SC LMWH on the day of surgery.
*** For patients with VTE within 1–3 months or cancer, we would suggest prophylactic LMWH preoperatively.
References
1Donat R, Mancey–Jones B (2002). Incidence of thromboembolism after transurethral resection of the prostate (TURP). Scand J Urol Nephrol 36:119–23.
2Geerts WH, Heit JA, Clagett PG, et al. (2001). Prevention of venous thromboembolism. (American College of Chest Physicians (ACCP) guidelines on prevention of venous thrombo-embolism). Chest 119:132S–75S.
3Quinlan DJ, McQuillan A, Eikelboom JW (2004). Low molecular weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism. Ann Intern Med 140:175–83.
4Lowe GDO, Greer IA, Cooke TG, et al. (1992). Risk of and prophylaxis for venous thromboembolism in hospital patients. Thromboembolic Risk Factors (THRIFT) Consensus Group. BMJ 305:567–74.
5Howard A, Zaccagnini D, Ellis M, Williams A, Davies AH, Greenhalgh RM (2004). Randomized clinical trial of low molecular weight heparin with thigh-length or knee-length antiembolism stockings for patients undergoing surgery. Br J Surg 91:842–7.
6Soderdahl DW, Henderson SR, Hansberry KL (1997). A comparison of intermittent pneumatic compression of the calf and whole leg in preventing deep venous thrombosis in urological surgery. J Urol 157:1774–6.
7Golash A, Collins PW, Kynaston HG, Jenkins BJ (2002). Venous thromboembolic prophylaxis for transurethral prostatectomy: practice among British urologists. J R Soc Med 95:130–1.
8Dunn AS, Turpie AG (2003). Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med 163:901–8.
9Kearon C (2003). Management of anticoagulation before and after elective surgery. In: Broudy VC, Prchal JT, Tricot GJ (eds). Hematology, American Society of Hematology Educational Programme Book. Washington DC: American Society of Haematology; pp. 528–34.
Fluid balance and the management of shock in the surgical patient
Daily fluid requirement
Can be calculated according to the patient’s weight:
•For the first 10kg: 100mL/kg per 24h (= 1000mL).
•For the next 10kg (i.e. from 10–20kg): 50mL/kg per 24h (= 500mL).
•For every kg above 20kg: 20mL/kg per 24h (= 1000mL for a patient weighing 70kg).
Thus, for every 24h, a 70kg adult will require 1000mL for their first 10kg of weight, plus 500mL for their next 10kg of weight, and 1000mL for their last 50kg of weight = total 24h fluid requirement, 2500mL.
Daily sodium (Na+) requirement is 100mmol, and for potassium (K+) 70mmol. Thus, a standard 24h fluid regimen is 2L of 5% glucose + 1L of normal saline (equivalent to about 150mmol of Na+), with 20mmol K+ for every litre of infused fluid.
Fluid losses from drains or nasogastric aspirate are similar in composition to plasma and should be replaced principally with normal saline.
Shock due to blood loss
Inadequate organ perfusion and tissue oxygenation. The causes are hypovolaemia, cardiogenic, septic, anaphylactic, and neurogenic. The commonest cause in the surgical patient is hypovolaemia due to blood and other fluid loss. Haemorrhage is an acute loss of circulating blood volume.
Haemorrhagic shock may be classified as:
•Class I: up to 750mL of blood loss (15% of blood volume); normal pulse rate (PR), respiratory rate (RR), BP, urine output, and mental status.
•Class II: 750–1500mL (15–30% of blood volume); PR >100;  pulse pressure due to
pulse pressure due to  diastolic pressure; RR 20–30; urinary output 20–30mL/h.
diastolic pressure; RR 20–30; urinary output 20–30mL/h.
•Class III: 1500–2000mL (30–40% of blood volume); PR >120;  BP and pulse pressure due to
BP and pulse pressure due to  systolic pressure; RR 30–40; urine output 5–15mL/h; confusion.
systolic pressure; RR 30–40; urine output 5–15mL/h; confusion.
•Class IV: >2000mL (>40% of blood volume); PR >140;  pulse pressure and BP; RR >35; urine output <5mL/h; cold, clammy skin.
pulse pressure and BP; RR >35; urine output <5mL/h; cold, clammy skin.
Management
•Remember ‘ABC’: 100% oxygen to improve tissue oxygenation.
•ECG, cardiac monitor, pulse oximetry.
•Insert two short and wide IV cannulae in the antecubital fossa (e.g. 16G). A central venous line may be required.
•Infuse 500mL of crystalloid (e.g. Hartmann’s) over <15min. Aim for a urinary output of 0.5mL/kg/h and maintenance of BP.
•Check FBC, coagulation screen, U&E, and cardiac enzymes.
•Consider early (<3h) tranexamic acid 1g IV (trials of use in trauma patients show significantly  survival).
survival).
•Cross-match 6U of blood.
•ABGs to assess oxygenation and pH.
•Obvious and excessive blood loss may be seen from drains, but drains can block, so assume there is covert bleeding if there is tachycardia (and low BP). If this regimen fails to stabilize pulse and BP, return the patient to the operating room for exploratory surgery.
Patient safety in the urology theatre
In 2009, the WHO developed guidelines for safe surgery, and the WHO checklist, which is now in use worldwide, has been shown to significantly reduce morbidity and mortality. It has three components, although modifications and additions to the WHO checklist are incorporated according to local practices:
•Sign in: before induction of anaesthesia, with at least the anaesthetist and a nurse. Verbal check (ideally with the patient) of identity, procedure and site, consent, operative site mark, and functioning pulse oximeter. Review of the patient’s risk of blood loss (if >500mL, ensure blood available), airway difficulty or aspiration risk, allergies, and anaesthesia safety checks.
•Time out: after anaesthesia induction, before surgical incision, all team (introductions if not previously done). Confirm correct operation for correct patient on correct site; review anticipated critical events; confirm prophylactic antibiotics (as required), VTE prophylaxis, patient warming, and glycaemic control; and display any essential imaging.
•Sign out: during or immediately after wound closure (with surgeon present in operating theatre). Confirm operation performed and recorded; check instrument, swab, and needle counts complete and correct; check surgical specimens labelled correctly; highlight any equipment issues, and verbalize concerns for post-operative recovery.
Develop an approach to operating that involves members of your team. Listen to the opinions of junior staff; they may sometimes be able to identify errors that are not obvious to you. Cultivate the respect of the recovery room staff. They may express concern about a patient under their care—listen to their concerns, take them seriously, and if all is well, reassure them. It does no harm for your patients or for your reputation to develop the habit of visiting every patient in the recovery room to check that all is well. You may be able to identify a problem before it has developed into a crisis, and at the very least, you will gain a reputation for being a caring surgeon.
Transurethral resection syndrome
Arises from the infusion of a large volume of hypotonic irrigating solution into the circulation during endoscopic procedures (e.g. TURP, TURBT, PCNL). Occurs in 0.5% of TURPs.
Pathophysiology
Biochemical, haemodynamic, and neurological disturbances occur.
•Dilutional hyponatraemia is the most important—and serious—factor leading to the symptoms and signs. Serum sodium usually has to fall to <125mmol/L before the patient becomes unwell.
•Hypertension—due to fluid overload.
•Visual disturbances may be due to the fact that glycine is a neurotransmitter in the retina.
Diagnosis: symptoms, signs, and tests
Confusion, nausea, vomiting, hypertension, bradycardia, visual disturbances, seizures. If the patient is awake (spinal anaesthesia), they may report visual disturbances (e.g. flashing lights).
Preventing the development of TUR syndrome and definitive treatment
Use a continuous irrigating cystoscope (provides low-pressure irrigation), limit the resection time (<60min), avoid aggressive resection near the capsule, and reduce the height of the irrigant solution (<70cm).1
Early identification of TUR syndrome is important, particularly by less experienced surgeons and during resection of a large prostate. For prolonged procedures, where a greater degree of fluid absorption may occur, measure serum sodium and give 20–40mg of IV furosemide to start offloading the excess fluid that has been absorbed. If serum sodium comes back as being normal, you will have done little harm by giving furosemide, but if it comes back at <125mmol/L, you will have started treatment already and thereby may have prevented the development of severe TUR syndrome.
Techniques for measuring fluid overload
•Weighing machines can be added to the ordinary operating table.2
•Adding a little alcohol to the irrigating fluid and constantly monitoring the expired air with a breathalyser3 allow an estimation of the volume of excess fluid which has been absorbed.
References
1Madsen PO, Naber KG (1973). The importance of the pressure in the prostatic fossa and absorption of irrigating fluid during transurethral resection of the prostate. J Urol 109:446–52.
2Coppinger SW, Lewis CA, Milroy EJG (1995). A method of measuring fluid balance during transurethral resection of the prostate. Br J Urol 76:66–72.
3Hahn RG (1993). Ethanol monitoring of extravascular absorption of irrigating fluid. Br J Urol 72:766–9.
Catheters and drains in urological surgery
Catheters
Made from latex or silastic (for patients with latex allergy or for long-term use—better tolerated by the urethral mucosa).
Types
•Self-retaining (also known as a Foley, balloon, or 2-way catheter) (Fig. 17.1). An inflation channel can be used to inflate and deflate a balloon at the end of the catheter, which prevents the catheter from falling out.
•A 3-way catheter (also known as an irrigating catheter). Has a third channel (in addition to the balloon inflation and drainage channels) which allows fluid to be run into the bladder at the same time as it is drained from the bladder (Fig. 17.2).
Size
The size of a catheter is denoted by its circumference in millimetre. This is known as the ‘French’ or ‘Charrière’ (hence Ch) gauge. Thus, a 12Ch catheter has a circumference of 12mm.
Uses
•Relief of obstruction (e.g. BOO due to BPE causing urinary retention—use the smallest catheter that you can pass; usually a 12Ch or 14Ch is sufficient in an adult).
•Irrigation of the bladder for clot retention (use a 20Ch or 22Ch 3-way catheter).
•Drainage of urine to allow the bladder to heal if it has been opened (trauma or deliberately, as part of a surgical operation).
•Prevention of ureteric reflux, maintenance of a low bladder pressure, where the ureter has been stented (post-pyeloplasty for PUJO).
•To empty the bladder before an operation on the abdomen or pelvis (deflating the bladder gets it out of harm’s way).
•Monitoring of urine output post-operatively or in the unwell patient.
•For delivery of bladder instillations (e.g. intravesical chemotherapy or immunotherapy).
•To allow identification of the bladder neck during surgery (e.g. RP, operations on or around the bladder neck).
Drains
Principally indicated for the prevention of accumulation of urine, blood, lymph, or other fluids. Particularly used after the urinary tract has been opened and closed by suture repair. A suture line takes some days to become completely watertight, and during this time, urine leaks from the closure site. A drain prevents accumulation of urine (a urinoma), the very presence of which can cause an ileus, and if it becomes infected, an abscess can develop.
•Tube drains (e.g. Robinson’s drain) (Figs. 17.3 and 17.4): provide passive drainage (i.e. no applied pressure). Used to drain suture lines at a site of repair or anastomosis of the urinary tract. Avoid placing the drain tip on the suture line, as this may prevent healing of the repair. Suture it to adjacent tissues to prevent it from being dislodged.
•Suction drains (e.g. Hemovac®) (Figs. 17.5 and 17.6): provide active drainage (i.e. air in the drainage bottle is evacuated, producing a negative pressure when connected to the drain tube to encourage evacuation of fluid). Used for the prevention of accumulation of blood (a haematoma) in superficial wounds. Avoid in proximity to a suture line in the urinary tract—the suctioning effect may encourage continued flow of urine out of the hole, discouraging healing.
As a general principle, drains should be brought out through a separate stab wound, rather than through the main wound, since the latter may result in bacterial contamination of the main wound, with subsequent risk of infection. Secure the drain with a thick suture to prevent it from inadvertently ‘falling out’.
Failure to deflate a catheter balloon for removal of a urethral catheter
From time to time, an inflated catheter balloon will not deflate when the time comes for removal of the catheter.
•Try inflating the balloon with air or water—this can dislodge an obstruction.
•Leave a 10mL syringe firmly inserted in the balloon channel, and come back an hour or so later.
•Try bursting the balloon by overinflation.
•Cut the end of the catheter off, proximal to the inflation valve—the valve may be ‘stuck’, and the water may drain out of the balloon.
•In the ♀ patient, introduce a needle alongside your finger into the vagina, and burst the balloon by advancing the needle through the anterior vaginal and bladder wall.
•In ♂ patients, balloon deflation with a needle can also be done under USS guidance. Fill the bladder with saline, using a bladder syringe, so that the needle can be introduced percutaneously and directed towards the balloon of the catheter under USS control.
•Pass a ureteroscope alongside the catheter, and deflate the balloon with the rigid end of a guidewire or with a laser fibre (the end of which is sharp).
Guidewires
An essential tool for endourological procedures.
Uses
As a track over which catheters or instruments can be passed into the ureter, collecting system of the kidney (retrograde or antegrade), or bladder.
Types
Many different types of guidewire are available. They are classified according to their size, tip design, rigidity, and surface coating. These specific properties determine their use. All are radio-opaque, so X-ray screening can be used to determine their position. They come prepackaged in a coiled sheath to allow ease of handling and storage (Fig. 17.7).
Size
‘Size’ refers to the diameter measured in inches (the length is usually around 150cm). The commonest sizes are 0.035 inches (2.7Ch) and 0.038 inches (2.9Ch). Also available as 0.032 inches (2.5Ch).
Tip design
Shape of the tip—straight or angle (Fig. 17.8); a straight tip is usually adequate for most uses. Occasionally, an angled tip is useful for negotiating an impacted stone or for placing the guidewire in a specific position. Similarly, a J-shaped tip can negotiate an impacted stone—the curved leading edge of this guidewire type can sometimes suddenly flick past the stone (in this situation, a straight guidewire can inadvertently perforate the ureter, thereby creating a false passage).
Surface coating
Most standard guidewires are coated with polytetrafluoroethylene (PTFE), which has a low coefficient of friction, thus allowing easy passage of the guidewire through the ureter and of instruments over them. Some guidewires are coated with a polymer which, when wet, is very slippery (hydrophilic coating). In some cases, the entire length of the guidewire is so coated (e.g. Terumo Glidewire), and in others, just the tip (e.g. Sensor guidewire). The virtually friction-free surface of Glidewires makes them liable to slip out of the ureter, and they therefore make unreliable safety wires (they can be exchanged for a wire with greater friction via a ureteric catheter). If allowed to become dry, these wires have a high coefficient of friction, which makes them difficult to manipulate.
Tip rigidity
The tip of all guidewires, over at least 3cm, is soft, and therefore flexible. This reduces—although certainly does not completely remove—the risk of ureteric perforation.
Shaft rigidity
Stiff guidewires are easier to manipulate than floppy ones and help to straighten a tortuous ureter (e.g. Amplatz Ultrastiff is particularly useful for this). Very malleable wires, such as the Terumo Glidewire, can be very useful for passing an impacted stone (for the same reason as J tip wires).
Some guidewires provide a combination of properties—a soft, floppy, hydrophilic-coated tip, with the remainder of the guidewire being stiff (e.g. Sensor guidewire).
Irrigating fluids and techniques of bladder washout
Glycine (usually 1.5%) is used for endoscopic surgery requiring application of diathermy
Normal saline is used for:
•Irrigation of the bladder following TURP and TURBT.
•Irrigation during ureteroscopy and PCNL.
Blocked catheter post-TURP and clot retention
Avoiding catheter blockage following TURP—keep the catheter bag empty; ensure a sufficient supply of the irrigant solution.
The bladder will be painfully distended. Irrigant flow will have stopped. A small clot may have blocked the catheter, or a chip of the prostate may have stuck in the eye of the catheter. Attach a bladder syringe to the end of the catheter, and pull back (Fig. 17.9). This may suck out the clot or chip of the prostate, and flow may restart. If it does not, draw some irrigant up into the syringe until it is about half full, and forcefully inject this fluid into the bladder. This may dislodge (and fragment) a clot that has stuck in the eye of the catheter. If the problem persists, change the catheter. You may see the obstructing chip of the prostate on the end of the catheter, as it is withdrawn.
Blocked catheter post-TURBT
Use the same technique as for post-TURP catheter blockage, but avoid vigorous pressure on the syringe—the wall of the bladder will have been weakened at the site of tumour resection, and it is possible to perforate the bladder, particularly in elderly women who have thin bladder walls.
Blocked catheters following bladder augmentation or neo-bladder
The suture line of the augmented bladder is weak, and over-vigorous bladder washouts can rupture the bladder.
JJ stents
These are hollow tubes with a coil at each end, which are inserted through the bladder (usually) into the ureter, and thence into the renal pelvis. They are designed to bypass a ureteric obstruction (e.g. due to a stone) or drain the kidney (e.g. post-renal surgery). They have a coil at each end (hence, the alternative name of ‘double pigtail’ stent—the coils have the configuration of a pig’s tail—or the less accurate name of J stent). These prevent migration downwards (out of the ureter) or upwards (into the ureter). They are therefore ‘self-retaining’. Made of polymers of variable strength and biodurability. Some stents have a hydrophilic coating which absorbs water and thereby makes them more slippery and easier to insert. Stents are impregnated with barium- or bismuth-containing metallic salts to make them radio-opaque, so that they can be visualized radiographically to ensure correct positioning.
Types
Classified by size and length. Common sizes are 6 or 7Ch (range 4–8Ch) (Fig. 17.10). Common lengths for adults are 22–28cm (range 18–30cm). Multilength stents are of variable length, allowing them to accommodate to ureters of different length.
Stent materials
Polyurethane; silicone; C-flex; Silitek; Percuflex; biodegradable (experimental—obviates the need for stent removal and eliminates the possibility of the ‘forgotten stent’). Some are coated (by chemical bonding) with a hydrogel (e.g. HydroPlus™) which provides a low friction surface, so making insertion easier and encrustation less likely, and, in theory, makes the stent more comfortable (whether this is the case in practice has not been established). Metallic stents are sometimes used in benign strictures or malignant obstruction (e.g. Allium self-expanding stent, Resonance®), and the Detour® stent is an extra-anatomical ureteric bypass stent.
Indications and uses
•Relief of obstruction: from ureteric stones; benign (i.e. ischaemic) ureteric strictures; malignant ureteric strictures. The stent will relieve the pain caused by obstruction and reverse renal impairment, if present.
•Prevention of obstruction: post-ureteroscopy (routine stenting after ‘uncomplicated’* ureteroscopy is not necessary).
•Indications for J stenting post-ureteroscopy:
•Ureteric injury.
•Solitary kidney.
•Large residual stone burden.
•Raised creatinine (implying overall impaired renal function).
•Ureteric stricture.
•Prevention of obstruction post-ESWL.
•Indications for J stenting post-ESWL:1
•Stents reduce the incidence of steinstrasse with large renal calculi (1.5–3.5cm, 6% with a stent, and 13% without developing steinstrasse post-ESWL).
•Solitary kidney.
•Raised creatinine (implying overall impaired renal function).
•(The analysis by the Joint AUA/EAU Nephrolithiasis Guideline Panel 20072 found no improvement in stone fragmentation with stenting, i.e. stents do not enhance ESWL efficacy.)
•‘Passive’ dilatation of the ureter prior to ureteroscopy.
•To ensure antegrade flow of urine following surgery (e.g. pyeloplasty) or injury to the ureter.
•Following endopyelotomy (endopyelotomy stents have a tapered end, from 14 to 7Ch, to keep the incised ureter ‘open’).
•Post-renal transplantation (stenting of the reimplanted ureter).
* The definition of ‘uncomplicated’ ureteroscopy is not precise. ‘Complicated’ ureteroscopy has been variously defined as: (1) ureteral perforation (i.e. mucosal injury); (2) severe ureteric oedema at the site of the stone; (3) impaction (which means difficulty getting a guidewire past the stone (‘cork in a bottle’ stone); (4) prolonged operation (no precise definition of what ‘long’ is); and (5) one where ureteral dilatation was carried out (to define such ureteroscopies as ‘complicated’ is contentious, because some urologists routinely ‘dilate’ with a dual-lumen catheter to allow double guidewire placement. Does this automatically make all their ureteroscopies ‘complicated’?).
An alternative to the J stent
A short-term 4 or 6Ch ureteric catheter, attached to a 12Ch urethral catheter (to stop the ureteric catheter from falling out), is an alternative form of post-ureteroscopy drainage.
In an RCT of 24h of ureteric catheter drainage post-ureteroscopy, compared with no drainage, the non-catheterized group were more likely to report renal colic (45% vs 2%) and have loin pain (76% vs 20%) than the ureteric catheterized group.3 Analgesic use was greater in the non-catheterized group (67% vs 20%). Twenty per cent of non-catheterized patients and 5% of catheterized patients returned to hospital for analgesia (but no patient required readmission). The only disadvantage of this technique was a higher reported rate of urethral irritation (37% vs 4%) in the catheterized patients. It has the obvious advantage of ease of removal of the catheter without the need for a second procedure and avoids the potential risk of the forgotten stent.
Symptoms and complications of stents
•Stent symptoms: common (78%)—suprapubic pain, LUTS (frequency, urgency—the stent irritates the trigone), haematuria, inability to work.4 More than 80% of patients have stent-related pain that affects daily activities; 32% report sexual dysfunction, and 58% report reduced work capacity and loss of income. α-blockers may help reduce pain with voiding and overall analgesic use.
•UTI: development of bacteriuria after stenting is common. In a small proportion, sepsis can develop. In such cases, consider placement of a urethral catheter to lower the pressure in the collecting system and prevent reflux of infected urine. Stents coated with the antibacterial triclosan are no better than non-coated stents in preventing stent-associated UTI.
•Incorrect placement: too high (distal end of the stent in the ureter; subsequent stent removal requires ureteroscopy; can be technically difficult; percutaneous removal may be required). Too low (proximal end not in the renal pelvis; stent may not therefore relieve obstruction).
•Stent migration (up the ureter or down the ureter and into the bladder).
•Stent blockage: catheters and stents become coated with a biofilm when in contact with urine (a protein matrix secreted by bacteria-colonizing stent). Calcium, magnesium, and phosphate salts become deposited. Biofilm build-up can lead to stent blockage or stone formation on the stent (Fig. 17.11). Stents coated with heparin are no better than non-coated stents in preventing stent biofilm formation or encrustation.
•The ‘forgotten stent’: rare, but potentially very serious, as the biofilm may become encrusted with the stone, making removal technically very difficult. If the proximal end only is encrusted, PCNL may be required to remove the stone and then the stent. If the entire stent is encrusted, open removal via several incisions in the ureter may be necessary.
Commonly asked questions about stents
Does urine pass though the centre of the stent?
No, it passes around the outside of the stent. Reflux of urine occurs through the centre.
Should I place a JJ stent after ureteroscopy?
(See  pp. 758–762.)
pp. 758–762.)
A stent should be placed if:
•There has been ureteric injury (e.g. perforation—indicated by extravasation of contrast).
•There are residual stones that might obstruct the ureter.
•The patient has had a ureteric stricture that required dilatation.
•Solitary kidney.
•Raised creatinine (implying overall impaired renal function).
Routine stenting after ureteroscopy for distal ureteric calculi is unnecessary.5,6 Many urologists will place a stent after ureteroscopy for proximal ureteric stones.
Do stents cause obstruction?
In normal kidneys, stents cause a significant and substantial increase in intrarenal pressure, which persists for up to 3wk.7 (This can be prevented by placing a urethral catheter.)
Do stents aid stone passage?
Ureteric peristalsis requires coaptation of the wall of the ureter proximal to the bolus of urine to be transmitted down the length of the ureter. JJ stents paralyse ureteric peristalsis. In dogs, the amplitude of each peristaltic wave (measured by an intraluminal ureteric balloon) falls (from 50 to 15mmHg) and the frequency of ureteric peristalsis falls (from 11 to 3 waves/min). Peristalsis takes several weeks to recover. Ball bearings of 3mm placed within a non-stented dog ureter take 7 days to pass, compared with 24 days in a stented ureter.
Are stents able to relieve obstruction due to extrinsic compression of a ureter?
Stents are less effective at relieving obstruction due to extrinsic obstruction by, for example, a tumour or retroperitoneal obstruction.8 They are much more effective for relieving obstruction by an intrinsic problem (e.g. a stone). Placement of two stents may provide more effective drainage (figure-of-eight configuration may produce more space around the stents for drainage).
For acute ureteric stone obstruction with fever, should I place a JJ stent or a nephrostomy?
In theory, one might imagine that a nephrostomy is better than a JJ stent—it can be done under LA (JJ stent insertion may require GA); it lowers the pressure in the renal pelvis to zero or a negative value, whereas a JJ stent results in a persistently positive pressure; it is less likely to be blocked by thick pus, and it allows easier subsequent imaging (contrast can be injected down the ureter—a nephrostogram—to determine if the stone has passed). A randomized trial of 42 patients with obstructing, infected stones (temperature >38°C and/or WBC >17 000/mm3) showed J stenting (6 or 7Ch J stent with a Foley bladder catheter) and nephrostomy drainage (8Ch) to be equally effective in terms of time to normalization of temperature and WBC (~2–3 days) and in-hospital stay. As a consequence, the EAU/AUA Nephrolithiasis Guideline Panel10 recommends that the system of drainage of the obstructed, infected kidney is left to the discretion of the urologist. Whichever method is chosen, decompression should be performed as soon as possible.
References
1Al-Awadi KA, Abdul Halim H, Kehinde EO, Al-Tawheed A (1999). Steinstrasse: a comparison of incidence with and without J stenting and the effect of J stenting on subsequent management. BJU Int 84:618–21.
2Preminger GM, Tiselius HG, Assimos DG, et al. (2007). 2007 Guideline for the management of ureteral calculi, joint EAU/AUA Nephrolithiasis Guideline Panel. J Urol 178:2418–34.
3Djaladat H, Tajik P, Payandemehr P, Alehashemi S (2007). Ureteral catheterization in uncomplicated ureterolithotripsy: a randomized, controlled trial. Eur Urol 52:836–41.
4Joshi HB, Stainthorpe A, MacDonagh RP, et al. (2003). Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol 169:1065–9.
5Srivastava A, Gupta R, Kumar A, Kapoor R, Mandhani A (2003). Routine stenting after ureteroscopy for distal ureteral calculi is unnecessary: results of a randomized controlled trial. J Endourol 17:871–4.
6Netto Jr NR, Ikonomidis J, Zillo C (2001). Routine ureteral stenting after ureteroscopy for ureteral lithiasis: is it really necessary? J Urol 166:1252–4.
7Ramsay JW, Payne SR, Gosling PT, Whitfield HN, Wickham JE, Levison DA (1985). The effects of double J stenting on obstructed ureters. An experimental and clinical study. Br J Urol 57:630–4.
8Docimo SG (1989). High failure rate of indwelling ureteral stents in patients with extrinsic obstruction: experience at two institutions. J Urol 142:277–9.
9Pearle MS, Pierce HL, Miller GL, et al. (1998). Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol 160:1260–4.
10Preminger GM, Tiselius HG, Assimos DG, et al. (2007). 2007 Guideline for the management of ureteral calculi Joint EAU/AUA Nephrolithiasis Guideline Panel. J Urol 178:2418–34.
Lasers in urological surgery
Light amplification by stimulated emission of radiation.
Photons are emitted when an atom is stimulated by an external energy source and its electrons, having been so excited, revert to their steady state. In a laser, the light is coherent (all the photons are in phase with one another), collimated (the photons travel parallel to each other), and of the same wavelength (monochromatic). The light energy is thus ‘concentrated’, allowing delivery of high energy at a desired target.
The holmium:YAG laser is currently the principal urological laser. It has a wavelength of 2140nm and is highly absorbed by water, and therefore by tissues which are composed mainly of water. The majority of the holmium laser energy is absorbed superficially, resulting in a superficial cutting or ablation effect. The depth of the thermal effect is no greater than 1mm. The holmium:YAG laser produces a cavitation bubble that generates only a weak shock wave, as it expands and collapses. Holmium laser lithotripsy occurs primarily through a photothermal mechanism that causes stone vaporization.
Uses of the holmium:YAG laser
•Laser lithotripsy (ureteric stones, small intrarenal stones, bladder stones).
•Resection of the prostate (holmium laser prostatectomy).
•Division of urethral strictures.
•Division of ureteric strictures, including PUJO.
•Ablation of small bladder, ureteric, and intrarenal TCCs.
Advantages
•The holmium laser energy is delivered via a laser fibre (Fig. 17.12), which is thin enough to allow its use down a flexible instrument, without affecting the deflection of that instrument, and can therefore gain access to otherwise inaccessible parts of the kidney.
•The zone of thermal injury adjacent to the tip of the laser fibre is limited to no more than 1mm; the laser can safely be fired at a distance of 1mm from the wall of the ureter.
•Can be used for all stone types.
•Minimal stone migration effect because of minimal shock wave generation.
Disadvantages
•High cost.
•Produces a dust cloud during stone fragmentation, which temporarily obscures the view.
•Can irreparably damage endoscopes if inadvertently fired near or within the scope.
•Relatively slow stone fragmentation—the laser fibre must be ‘painted’ over the surface of the stone to vaporize it.
Greenlight PVP for TURP
The 80 or 120W KTP laser is used for photoselective vaporization of the prostate. The laser is green (hence, the name ‘greenlight’ laser) and is absorbed by Hb, generating a heating effect which causes vaporization of the targeted tissue.
The procedure is done under general or spinal anaesthetic.
Advantages
Saline is used for irrigation (therefore, no risk of TUR syndrome).
Disadvantages
No tissue for histological examination.
Diathermy
Diathermy is the coagulation or cutting of tissues through heat.
Monopolar diathermy
When an electric current passes between two contacts on the body, there is an increase in temperature in the tissues through which the current flows. This increase in temperature depends on the volume of tissue through which the current passes, the resistance of the tissues, and the strength of the current. The stronger the current, the greater the rise in temperature. If one contact is made large, the heat is dissipated over a wide area and the rise of temperature is insignificant. This is the earth or neutral electrode, and under this, the rise in temperature is only 1 or 2°C. The working electrode or diathermy loop is thin, so that the current density is maximal, and therefore, so is the heating effect.
When a direct current is switched on or off, nerves are stimulated and muscles will twitch. If the switching on and off is rapid enough, there is the sustained contraction familiar to the physiology class as the ‘tetanic contraction’. If a high-frequency alternating current is used (300kHz to 5MHz), there is no time for the cell membranes of nerve or muscle to become depolarized and nerves and muscles are not stimulated (they are stimulated at lower frequencies).
The effect of the diathermy current on the tissues depends on the heat that is generated under the diathermy loop. At relatively low temperatures, coagulation and distortion of small blood vessels occur. If the current is  to raise the temperature further, water within cells vaporizes and the cells explode. This explosive vaporization literally cuts the tissues apart.
to raise the temperature further, water within cells vaporizes and the cells explode. This explosive vaporization literally cuts the tissues apart.
Bipolar diathermy
Bipolar diathermy involves the passage of electrical current between two electrodes on the same hand piece. It is inherently safer than monopolar diathermy, since the current does not pass through the patient and diathermy burns cannot therefore occur.
Potential problems with diathermy
The diathermy is not working
•Do not increase the current.
•Check that the irrigating fluid is glycine (sodium chloride conducts electricity, causing the diathermy to short-circuit).
•Check that the diathermy plate is making good contact with the skin of the patient.
•Check that the lead is undamaged.
•Check that the resectoscope loop is securely fixed to the contact.
Modern diathermy machines have warning circuits which sound an alarm when there is imperfect contact between the earth plate and the patient.
Diathermy burns
If current returns to earth through a small contact, rather than the broad area of the earth pad, then the tissues through which the current passes will be heated, just like those under the cutting loop. If the pad is making good contact, the current will find it easier to run to earth through the pad and no harm will arise, even when there is accidental contact with some metal object. The real danger arises when the diathermy pad is not making good contact with the patient. It may not be plugged in or its wire may be broken. Under these circumstances, the current must find its way to earth somehow, and any contact may then become the site of a dangerous rise in temperature.
Pacemakers and implantable cardioverter–defibrillators (ICDs) and use of diathermy
See Box 17.1 for diathermy problems and their prevention.
Box 17.1 Pacemakers, ICDs, and diathermy: problems and their prevention
Diathermy can cause electrical interference of a pacemaker or ICD, leading to inhibition, triggering of electrical output from the device, reprogramming, asynchronous pacing, damage to the circuitry of the device, or triggering of defibrillator discharge. An electrical current can also be induced in the pacemaker or ICD leads, which can, in turn, cause tissue heating, leading to myocardial damage.
•Pacemaker inhibition: the high frequency of the diathermy current may simulate the electrical activity of myocardial contraction, so the pacemaker can be inhibited. If the patient is pacemaker-dependent, the heart may stop.
•Phantom reprogramming: the diathermy current may also simulate the radiofrequency impulse by which the pacemaker can be reprogrammed to different settings. The pacemaker may then start to function in an entirely different mode.
•The internal mechanism of the pacemaker: may be damaged by the diathermy current if this is applied close to the pacemaker.
•Ventricular fibrillation: if the diathermy current is channelled along the pacemaker lead, ventricular fibrillation may be induced.
•Myocardial damage: another potential effect of channelling of the diathermy current along the pacemaker lead is burning of the myocardium at the tip of the pacemaker lead. This can subsequently result in ineffective pacing.
It was formerly recommended that a magnet was placed over the pacemaker to overcome pacemaker inhibition and to make the pacemaker function at a fixed rate. This can, however, result in phantom reprogramming. For demand pacemakers, it is better to programme the pacemaker to a fixed rate (as opposed to demand pacing) for the duration of the operation. Consult the patient’s cardiologist for advice.
Other precautions
•The patient plate should be sited, so that the current path does not go right through the pacemaker. Ensure that the indifferent plate is correctly applied, as an improper connection can cause grounding of the diathermy current through the ECG monitoring leads and this can affect pacemaker function. The indifferent plate should be placed as close as possible to the pacemaker (e.g. over the thigh or buttock).
•The diathermy machine should be placed well away from the pacemaker and should certainly not be used within 15cm of it.
•The heartbeat should be continually monitored, and a defibrillator and external pacemaker should be at hand.
•Try to use short bursts of diathermy at the lowest effective output.
•Use bipolar diathermy in preference to monopolar (not practical for many urological procedures where the only form of diathermy that can be used is monopolar).
•Give antibiotic prophylaxis (as for patients with artificial heart valves).
•Because the pacemaker-driven heart will not respond to fluid overload in the normal way, resection should be as quick as possible and fluid overload should be avoided.
Further reading
Allen M (2006). Pacemakers and implantable cardioverter defibrillators. Anaesthesia 62:852–3.
Medicines and Healthcare Products Regulatory Agency (2006). Guidelines for the perioperative management of patients with implantable cardioverter defibrillators, where the use of diathermy is anticipated. Available from:  http://www.mhra.gov.uk.
http://www.mhra.gov.uk.
Salukhe TV, Dob D, Sutton R (2004). Pacemakers and defibrillators: anaesthetic implications. Br J Anaesth 93:95–104.
Sterilization of urological equipment
Techniques for sterilization
•Autoclaving: modern cystoscopes and resectoscopes, including components such as light leads, are autoclavable. Standard autoclave regimens heat the instruments to 121°C for 15min or 134°C for 3min.
•Chemical sterilization: this involves soaking instruments in an aqueous solution of chlorine dioxide (Tristel), an aldehyde-free chemical (there has been a move away from formaldehyde because of health and environmental concerns). Chlorine dioxide solutions kill bacteria, viruses (including HIV and hepatitis B and C), spores, and mycobacteria.
Cameras cannot be autoclaved. Use a camera sleeve, or sterilize the camera between cases in solutions such as Tristel.
Sterilization and prion diseases
Variant Creutzfeldt–Jakob disease CJD (vCJD) is a neurodegenerative disease caused by a prion protein (PrP). Other examples of neurodegenerative prion diseases include classic CJD, kuru, sheep scrapie, and bovine spongiform encephalopathy (BSE). Variant CJD and BSE are caused by the same prion strain and represent a classic example of cross-species transmission of a prion disease.
There has been much recent concern about the potential for transmission of vCJD between patients via contaminated surgical instruments. Classic CJD may be transmitted by neurosurgical and other types of surgical instruments, because normal hospital sterilization procedures do not completely inactivate prions.1 It is not possible at present to quantify the risks of transmission of prion diseases by surgical instruments. To date, iatrogenic CJD remains rare, with 267 cases having been reported worldwide up to 2000.2
The risk of transmission of CJD may be higher with procedures performed on organs containing lymphoreticular tissue, such as tonsillectomy and adenoidectomy, because vCJD targets these tissues and is found in high concentrations there. For this reason, there was a move towards the use of disposable, once-only-use instruments for procedures such as tonsillectomy. However, these instruments have been associated with a higher post-operative haemorrhage rate,3 and as a consequence, ear, nose, and throat (ENT) departments in the UK are no longer obliged to use disposable instruments.
In the UK, the Advisory Committee on Dangerous Pathogens and Spongiform Encephalopathy4 provides advice on appropriate methods of cleaning and sterilization of surgical instruments. Prions are particularly resistant to conventional chemical (ethylene oxide, formaldehyde, and chlorine dioxide) and standard autoclave regimens, and dried blood or tissue remaining on an instrument could harbour prions that will not then be killed by the sterilization process. Once proteinaceous material, such as blood or tissue, has dried on an instrument, it is very difficult to subsequently be sure that the instrument has been sterilized. Sterilization should include:
•Pre-sterilization cleaning: initial low-temperature washing (<35°C) with detergents and an ultrasonic cleaning system remove and prevent coagulation of PRPs—sonic cleaners essentially ‘shake’ attached material from the instrument.
•Hot wash.
•Air drying.
•Thermal sterilization: longer autoclave cycles at 134–137°C for at least 18min (or six successive cycles with holding times of 3min) or 1h at conventional autoclave temperatures may result in a substantial reduction in the level of contamination with prions.
The latest models of pre-sterilization cleaning devices—automated thermal washer disinfectors—perform all of these cleaning tasks within one unit.
Enzymatic proteolytic inactivation methods are under development.
References
1Collinge J (1999). Variant Creutzfeldt–Jakob disease. Lancet 354:317–23.
2Collins SJ, Lawson VA, Masters CL (2004). Transmissible spongiform encephalopathies. Lancet 363:51–61.
3Nix P (2003). Prions and disposable surgical instruments. Int J Clin Pract 57:678–80.
4Advisory Committee on Dangerous Pathogens and Spongiform Encephalopathy (1998). Transmissible spongiform encephalopathy agents: safe working and the prevention of infection. London: HM Stationery Office.
Telescopes and light sources in urological endoscopy
There are three types of modern urological telescopes: rigid, semi-rigid, and flexible. These endoscopes may be used for inspection of the urethra and bladder (cystourethroscopes—usually simply called cystoscopes), the ureter and collecting system of the kidney (ureteroscopes and ureterorenoscopes), and, via a percutaneous access track, the kidney (nephroscopes). The light sources and image transmission systems are based on the innovative work of Professor Harold Hopkins from the University of Reading.
The Hopkins rod–lens system
Introduced by Professor Harold Hopkins in 1959. The great advance in telescope design was the development of the rod–lens telescope, which replaced the conventional system of glass lens with rods of glass, separated by thin air spaces which essentially were air lenses (Fig. 17.13). By changing the majority of the light transmission medium from air to glass, the quantity of light that could be transmitted was doubled. The rods of glass were also easier to handle during manufacture, and therefore, their optical quality was greater.
The angle of view of the telescope can be varied by placing a prism behind the objective lens. 0°, 12°, 30°,* and 70° scopes are available.
* In days gone by, when a tiny lamp at the end of the telescope was used for illumination, it was necessary to have a slightly angled line of vision; otherwise, the light bulb got in the way of the view. The 30° scope is a throw back to this historical requirement.
Lighting
Modern endoscopes (urological and those used to image the GI tract) use fibreoptic light bundles to transmit light to the organ being inspected (developed by Karl Storz). Each glass fibre is coated with glass of a different refractive index, so that light entering at one end is totally internally reflected and emerges at the other (Fig. 17.14). These fibreoptic bundles can also be used for image (as well as light) transmission, as long as the arrangement of the fibres at either end of the instrument is the same (coordinated fibre bundles are not required for simple light transmission). The fibre bundles are tightly bound together only at their end (for coordinated image transmission). In the middle, the bundles are not bound—this makes the instrument flexible (e.g. flexible cystoscope and flexible ureteroscope).
Digital image capture systems
Conventional analogue camera systems have a 3-chip camera with separate sensors for red, green, and blue colours. They convert analogue data into digital data for image storage and enhancement. Image distortion can reduce image quality (a ‘spectrum’ effect can occur—bands of red, green, and blue across the image). A recent innovation in scope design is chip miniaturization which allows these sensors to be placed at the tip of the flexible cystoscope or flexible ureteroscope, so allowing a totally digital imaging system (as in a digital camera). The resolution and image quality are superior to analogue systems.
Consent: general principles
Consent is required before you examine, treat, or care for a competent adult (a person aged 16 or more).
Think of obtaining consent as a process, rather than an event. In order to give consent, a patient must understand the nature, purpose, and likely effects (outcomes, risks) of the treatment. From the information they receive, the patient must be able to weigh up the risks against benefits and so arrive at an informed choice. They must not be coerced into making a decision (e.g. by the doctor in a hurry). Giving the patient time to reach a decision is a good way of avoiding any accusation that they were pressured into a decision. To reiterate—think of consent as a process, rather than an event.
Giving information and level of disclosure
How much information should you give? What options and risks should you mention? While the previous standard of adequate consent was judged against the Bolam test (which asks whether a doctor’s conduct would be supported by a responsible body of medical opinion), the Montgomery Supreme Court ruling in 2015 has resulted in a move away from the ‘reasonable doctor’ to the ‘reasonable patient’. This materiality test relates to whether a reasonable person in the patient’s position would be likely to attach significance to the risk or the doctor is or should reasonably be aware that the particular patient would be likely to attach significance to it.
You have a duty to discuss the range of treatment options available (the alternatives), regardless of their cost, in a form the patient can understand and the side effects and risks that are relevant to the individual patient’s circumstances.
Remember, it can be argued that the consent was not valid because the amount of information you gave was not enough or was in a form the patient could not understand.
Recording
Remember, record the consent discussion in the notes. If you do not record what you said, you might as well not bother saying it. If a patient later claims that they were not told of a particular risk or outcome, it will be difficult to refute this if your notes do not record what you said. Writing ‘risks explained’ is inadequate. When cases do come to court, this is usually several years after the events in question. You will have forgotten precisely what you said to the patient, and it will not take much effort on the part of a barrister to suggest that you might not have said everything that you thought you said! If you give a written information sheet, record that you have done so and put a copy of the version you gave in the notes.
The consent form
The consent form is designed to record the patient’s decision and, to some extent, the discussions that took place during the consent process (although the space available for recording the discussion, even on the new NHS consent form, is limited). It is not proof that the patient was properly informed—that valid consent was obtained. Avoid, if possible, technical abbreviations such as TURBT. A patient could reasonably claim not to have understood what this was. Try to avoid standing over the patient, waiting for them to sign the form. It is good practice to leave the form with them and to return after a few minutes—they will feel less pressured and can ask further questions if they wish.
Children
Children aged <16 may give consent, as long as they fully understand what is involved in the proposed examination or treatment (a parent cannot override the competent child’s consent to treatment). However, a child cannot refuse consent to treatment (i.e. a parent can override a child’s refusal to consent—the parent can consent on the child’s behalf if the child refuses consent, although such situations are rare).
Cystoscopy
A basic skill of the urologist. Allows direct visual inspection of the urethra and bladder.
Indications
•Haematuria.
•Irritative LUTS (marked frequency and urgency) where intravesical pathology is suspected (e.g. CIS, bladder stone).
•For bladder biopsy.
•Follow-up surveillance of patients with previously diagnosed and treated bladder cancer.
•Retrograde insertion of ureteric stents and removal.
•Cystoscopic removal of stones.
Technique
•Flexible cystoscopy: a flexible cystoscope is easily passed down the urethra and into the bladder following instillation of lubricant gel (with or without LA—a meta-analysis of nine RCTs showed no difference in pain control between lidocaine gel and plain gel lubrication).1 Principally diagnostic, but small biopsies can be taken with flexible biopsy forceps, small tumours can be fulgurated (with a diathermy probe) or vaporized (with a laser fibre), and JJ stents can be inserted and removed using this type of cystoscope.
•Rigid cystoscopy: rigid metal instrument which can be passed under LA in women (short urethra) but usually requires GA. Preferred over flexible cystoscopy where deeper biopsies will be required or as an antecedent to TURBT or cystolitholapaxy where it is anticipated that other pathology will be found (tumour, stone).
The flexible cystoscope uses fibreoptics for illumination and image transmission. It can be deflected through 270°.
Common post-operative complications and their management
Mild burning discomfort and haematuria are common after both flexible and rigid cystoscopy. It usually resolves within hours. Bacteriuria after flexible cystoscopy occurs in about 8–9% of patients (4–5% have bacteriuria before cystoscopy), and this rate is reduced by prophylactic antibiotics (Table 17.2).
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of flexible cystoscopy
Warn the patient that if cystoscopy is being done because of haematuria, it is possible that bladder cancer may be found, which may require further treatment. You should specifically seek consent for biopsy (removal of tissue if an abnormality is found).
Common
•Mild burning or bleeding on passing urine for a short period after the operation.
•Biopsy of an abnormal area in the bladder may be required.
Occasional
•Infection of the bladder requiring antibiotics.
Rare
•Temporary insertion of a catheter.
•Delayed bleeding requiring removal of clots or further surgery.
•Injury to the urethra causing delayed scar formation (a stricture).
Serious or frequently occurring complications of rigid cystoscopy
•As for flexible cystoscopy.
•The use of heat (diathermy) may be required to cauterize biopsy sites.
•Very rarely, perforation of the bladder can occur, requiring temporary insertion of a catheter or open surgical repair.
Reference
1Patel AR, Jones JS, Babineau D (2008). Lidocaine 2% gel versus plain lubricating gel for pain reduction during flexible cystoscopy: a meta-analysis of prospective, randomized controlled trials. J Urol 179:986–90.
Transurethral resection of the prostate
Indications
•Bothersome LUTS which fail to respond to changes in lifestyle or medical therapy.
•Recurrent acute urinary retention.
•Renal impairment due to BOO (high-pressure chronic urinary retention).
•Recurrent haematuria due to BPE.
•Bladder stones due to prostatic obstruction.
Post-operative care
A 3-way catheter is left in situ after the operation, through which irrigation fluid (normal saline) is run to dilute the blood so that a clot will not form to block the catheter. The rate of inflow of the saline is adjusted to keep the outflow a pale pink rosé colour, and as a rule, the rate of inflow can be cut down after about 20min. The irrigation is continued for 12–24h. The catheter is removed the day after (second post-operative day) if the urine has cleared to a normal colour [TWOC or trial of void (TOV)].
Common post-operative complications and their management
Blocked catheter post-TURP
Common
The catheter may become blocked with a clot or a prostatic ‘chip’ which was inadvertently left in the bladder at the end of the operation.
•Apply a bladder syringe to the end of the catheter to try to dislodge the obstruction.
•If this fails, withdraw some irrigant into the syringe and flush the catheter.
•If this fails, change the catheter. The obstructing chip of the prostate may be found stuck in one of the eye-holes of the catheter.
•Pass a new catheter on an introducer.
If the bladder has been allowed to become so full of clot that a simple bladder washout is unable to evacuate it all, return the patient to the theatre for clot evacuation.
Haemorrhage
Minor bleeding after TURP is common and will stop spontaneously. A simple system to allow communication between staff is to describe the colour of the urine draining through the catheter as the same as rosé wine (minor haematuria), dark red wine (moderate haematuria), or frank blood (bright red bleeding, suggesting serious haemorrhage). Rosé urine requires no action. Dark red urine should be managed by increasing the flow of irrigant and by applying gentle traction to the catheter (with the balloon inflated to 40–50mL), thereby pulling it onto the bladder neck or into the prostatic fossa to tamponade bleeding for 20min or so. This will usually result in the urine clearing. An attempt at controlling heavier bleeding by these techniques may be tried, but at the same time, you should make preparations to return the patient to theatre because it is unlikely that bleeding of this degree will stop. The bleeding vessel(s), if seen, is controlled with diathermy. If bleeding persists, open surgical control is required—the prostatic capsule is opened, the bleeding vessels sutured, and the prostatic bed packed. Post-operative bleeding requiring a return to theatre occurs in 0.5% of cases.1
BAUS procedure-specific consent form—recommended discussion of adverse events
Serious or frequently occurring complications of TURP
•Temporary mild burning on passing urine, urinary frequency, haematuria.
•Retrograde ejaculation in 75% of patients.
•Failure of symptom resolution.
•Permanent inability to achieve an erection adequate for sexual activity.
•UTI requiring antibiotic therapy.
•10% of patients require redo surgery for recurrent prostatic obstruction.
•Failure to pass urine after the post-operative catheter has been removed.
•In 10% of patients, PC is found on subsequent pathological examination of the resected tissue.
•Urethral stricture formation requiring subsequent treatment.
•Incontinence (loss of urinary control)—may be temporary or permanent.
•Absorption of irrigating fluid, causing confusion and heart failure (TUR syndrome).
•Very rarely, perforation of the bladder requiring a temporary urinary catheter or open surgical repair.
Alternative therapy
Observation, drugs, catheter, stent, laser prostatectomy, open operation.
Reference
1Emberton M, Neal DE, Black N, et al. (1995). The National Prostatectomy Audit: the clinical management of patients during hospital admission. Br J Urol 75:301–16.
Transurethral resection of bladder tumour
Indications
•Local control of NMI bladder cancer (i.e. stops bleeding tumours).
•Staging of bladder cancer: to determine whether the cancer is NMI or MI, so that subsequent treatment and appropriate follow-up can be arranged.
Post-operative care
A 2- or 3-way catheter is left in situ after the operation, depending on the size of the tumour, and therefore on the likelihood that bleeding requiring irrigation will be required. As for TURP, normal saline is run through the catheter to dilute the blood, so that a clot will not form to block the catheter. It is particularly important to avoid catheter blockage post-TURBT, since this could lead to distension of the bladder already weakened by resection of a tumour. The period of irrigation is usually shorter than that required after TURP and for small tumours; the catheter may be removed the day after TURBT. For larger tumours, remove it 2 days later.
Common operative and post-operative complications and their management
Bladder perforation during TURBT
Small perforations into the perivesical tissues (extraperitoneal) are not uncommon when resecting small tumours of the bladder, and so long as you have secured good haemostasis and all the irrigating fluid is being recovered, no additional steps are required, except that perhaps one should leave the catheter in for 4, rather than 2, days.
Intraperitoneal perforations (through the wall of the bladder, through the peritoneum, and into the peritoneal cavity) are uncommon but far more serious.
Is it an extraperitoneal or intraperitoneal perforation? Establishing this can be difficult. Both can cause marked distension of the lower abdomen—an intraperitoneal perforation by allowing escape of irrigating solution directly into the abdominal cavity and an extraperitoneal perforation by expanding the retroperitoneal space, with fluid then diffusing directly into the peritoneal cavity. The fact that a suspected intraperitoneal perforation was actually extraperitoneal becomes apparent only at laparotomy when no hole can be found in the peritoneum overlying the bladder (the peritoneum over the bladder is not breached in an extraperitoneal perforation).
When there is no abdominal distension, the volume of extravasated fluid is likely to be low, and if the perforation is small, it is reasonable to manage the case conservatively. Achieve haemostasis, and pass a catheter. Make frequent post-operative assessments of the patient’s vital signs and abdomen (worsening abdominal pain, distension, and tenderness suggest the need for laparotomy).
Where there is marked abdominal distension, whether the perforation is extraperitoneal or intraperitoneal, explore the abdomen, principally to drain the large amount of fluid (which can compromise respiration in an elderly patient) by splinting the diaphragm, but also to check that loops of bowel adjacent to the site of perforation have not been injured at the same time. Failing to make the diagnosis of an intraperitoneal perforation, particularly if the bowel has been injured, is a worse situation to be in than performing a laparotomy for a suspected intraperitoneal perforation, but then finding that the perforation was ‘only’ extraperitoneal.
Open bladder repair
A Pfannenstiel incision or a lower midline abdominal incision; open the bladder, evacuate the clot, control bleeding, and repair the hole. Open the peritoneum, and inspect the small and large bowel for perforations. Leave a urethral catheter and a drain in place.
Blocked catheter post-TURBT
The catheter may become blocked with clot. Use the same technique for unblocking it as for TURP, but avoid vigorous washouts of the bladder because of the risk of bladder perforation.
Haemorrhage
Minor bleeding after TURBT is common and will stop spontaneously. The only ‘technique’ for controlling it is to ensure that an adequate flow of irrigant is maintained (to dilute the blood, thereby preventing clots from forming). If bleeding persists, return the patient to theatre for endoscopic control.
TUR syndrome
Uncommon after TURBT, unless the tumour is large and the resection therefore long.
BAUS procedure-specific consent form—recommended discussion of adverse events
Serious or frequently occurring complications of TURBT
Common complications
•Mild burning on passing urine.
•Additional treatment (intravesical chemotherapy or immunotherapy) may be required to reduce the risk of future tumour recurrence.
•UTI.
•No guarantee of bladder cancer cure.
•Tumour recurrence is common.
Rare complications
•Delayed bleeding requiring removal of clots or further surgery.
•Damage to drainage tubes from the kidney (ureters) requiring additional therapy.
•Development of a urethral stricture.
•Bladder perforation requiring a temporary urinary catheter or open surgical repair.
Alternative treatment
Open removal of the bladder; chemotherapy, radiation.
Optical urethrotomy
Indications
•Bulbar urethral stricture.
•Also used for penile urethral strictures.
Anaesthesia
Regional or general.
Post-operative care
•Leave a catheter for 3–5 days (longer catheterization does not reduce long-term re-stricturing).
•Consider ISC for 3–6 months, starting several times daily, reducing to once or twice a week towards the end of this period.
Common post-operative complications and their management
•Septicaemia.
•Re-stricturing: is the commonest long-term problem occurring after optical urethrotomy.
BAUS procedure-specific consent form—recommended discussion of adverse events
Common
•Mild burning on passing urine for short periods of time after operation.
•Temporary insertion of a catheter.
•Need for self-catheterization to keep the narrowing from closing down again.
Occasional
•Infection of the bladder requiring antibiotics.
•Permission for telescopic removal/biopsy of bladder abnormality/stone, if found.
•Recurrence of the stricture necessitating further procedures or repeat incision.
Rare
Decrease in quality of erections, requiring treatment.
Alternative therapy
Observation, urethral dilatation, open (non-telescopic) repair of the stricture.
Circumcision
Indications
•Phimosis.
•Recurrent paraphimosis.
•Penile cancer confined to the foreskin.
•Lesions on the foreskin of uncertain histological nature.
Contraindications
•In neonates—hypospadias, chordee with hypospadias, microphallus.
•In all patients—bleeding diatheses.
Circumcision in HIV prevention
♂ circumcision has a significant protective effect against HIV infection.1,2 This is thought to be due to the presence of large numbers of HIV-binding target cells being present on the inner layer of the prepuce, compared to the glans and outer prepuce (which is lined by squamous epithelium).
Clearly, mass circumcision programmes in Africa and other high-risk areas would be a huge task. In addition, concerns have been expressed that this beneficial effect will be negated by  behaviour that increases HIV risk (e.g. a drop in condom use, a rise in sexual partners).
behaviour that increases HIV risk (e.g. a drop in condom use, a rise in sexual partners).
Anaesthesia
Local or general.
Post-operative care
A non-adhesive dressing may be applied to the end of the penis, but this is difficult to keep on for more than an hour or two and is unnecessary. Warn the patient that the penis may be bruised and swollen after the operation but that this resolves spontaneously over a week or two. Sexual intercourse or masturbation should be avoided until the absorbable skin sutures have dissolved.
Common post-operative complications and their management
You might think that circumcision is about as simple an operation as you can get, but it can cause both the patient (or, in the case of little boys, their parents) and you considerable concern if the cosmetic result is not what was expected or if ‘complications’ occur about which the patient was not warned. As with any procedure, it should be performed with care and with the potential complications always in mind, so that steps can be taken to avoid these. If complications do occur, manage them appropriately.
Haemorrhage
Most frequently occurs from the frenular artery on the ventral surface of the penis. If local pressure does not stop the bleeding (and if it is from the frenular artery, it usually will not), return the patient to theatre and, either under ring-block LA or GA, suture ligature the bleeding vessel. Be careful not to place the suture through the urethra!
Necrosis of the skin of the shaft of the penis
In most cases of suspected skin necrosis, there is none. Not infrequently, a crust of coagulated blood develops around the circumference of the penis after circumcision. As blood oxidizes, it turns black and this appearance can be mistaken for necrosis of the end of the penis. Reassurance of the patient (and the referring doctor!) is all that is needed. If necrosis has occurred because, for example, adrenaline was used in the LA, wait for the necrotic tissue to demarcate before assessing the extent of the problem. The penis has a superb blood supply and has remarkable healing characteristics.
•Separation of the skin of the coronal sulcus from the shaft skin: if limited to a small area, this will heal spontaneously. If a larger circumference of the wound has ‘dehisced’, resuture in theatre.
•Wound infection: rare.
•Urethrocutaneous fistula: due to haemostatic sutures (placed to control bleeding from the frenulum) passing through the urethra, the wound later breaking down.
•Urethral damage: due to a stitch placed through the urethra as the frenular artery is suture ligatured.
•Excessive removal of skin: re-epithelialization can occur if the defect between the glans and the shaft skin is not too great. If the defect is too great, the end-result will be a buried penis—the glans retracts towards the skin at the base of the penis.
BAUS procedure-specific consent form—recommended discussion of adverse events
Serious or frequently occurring complications of circumcision
•Bleeding of the wound, occasionally needing a further procedure.
•Infection of incision requiring further treatment.
•Permanent altered sensation of the penis.
•Persistence of absorbable stitches after 3–4wk, requiring removal.
•Scar tenderness, rarely long term.
•You may not be completely cosmetically satisfied.
•Occasional need for removal of excessive skin at a later date.
•Permission for biopsy of an abnormal area of the glans if malignancy is a concern.
Alternative therapy
Drugs to relieve inflammation, leave uncircumcised.
References
1Bailey RC, Moses S, Parker CB, et al. (2007). Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomized controlled trial. Lancet 369:643–56.
2Gray RH, Kigozi G, Serwadda D, et al. (2007). Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369:657–66.
Hydrocele and epididymal cyst removal
Hydrocele repair (removal)
Indications
Primary (idiopathic) hydrocele repair; not indicated for secondary hydrocele repair.
Anaesthesia
Local or general.
Techniques
•Lord’s plication technique: for small- to medium-sized hydroceles (minimal interference with surrounding scrotal tissues, which minimizes the risk of post-operative haematoma).
•Jaboulay procedure: for large hydroceles; excision of hydrocele sac.
Hydrocele aspiration
Strict attention to asepsis is vital, since the introduction of infection into a closed space could lead to abscess formation. Avoid superficial blood vessels (if you hit them, a large haematoma can result).
Post-operative care
Nothing specific.
Post-operative complications and their management
•Scrotal swelling: resolves spontaneously.
•Haematoma formation: if it is large, surgical drainage is best performed, as spontaneous resolution may take many weeks. It can be difficult to identify the bleeding vessel. Leave a small drain to prevent reaccumulation of the haematoma.
•Hydrocele recurrence.
Epididymal cyst removal (spermatocelectomy)
•Avoid in young men who wish to maintain fertility, since epididymal obstruction can occur.
•An alternative to surgical removal is aspiration, though recurrence is usual.
BAUS procedure-specific consent form: recommended discussion of adverse events
Hydrocele removal
Occasional
•Recurrence of fluid collection can occur.
•Collection of blood around the testes that resolves slowly or requires surgical removal.
•Possible infection of the incision or testis, requiring further treatment.
Alternative therapy
•Observation.
•Removal of fluid with a needle.
Epididymal cyst removal
Occasional
•Recurrence of fluid collection can occur.
•Collection of blood around the testes that resolves slowly or requires surgical removal.
•Possible infection of the incision or testis, requiring further treatment.
Rare
•Scarring can damage the epididymis, causing subfertility.
Alternative therapy
Observation, removal of fluid with a needle.
Nesbit’s procedure
Penile straightening procedure for correcting penile curvature. Wait for at least 6 months after the patient has experienced no more pain, and wait for the penile curvature to stabilize (there is no point in repairing the curvature if it is still progressing).
Indications
Peyronie’s disease.
Anaesthesia
Local or general.
Post-operative care
Avoid intercourse for 2 months. Oedema can be managed with cold compresses.
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications
Common
•Some shortening of the penis.
•Possible dissatisfaction with the cosmetic or functional result.
•Temporary swelling and bruising of the penis and scrotum.
Occasional
•Circumcision is sometimes required as part of the procedure.
•There is no guarantee of total correction of the bend.
•Bleeding or infection: may require further treatment.
Rare
•Impotence or difficulty maintaining an erection.
•Nerve injury, with temporary or permanent numbness of the penis.
Alternative therapy
Observation, drugs, other surgical procedures.
Vasectomy and vasovasostomy
Vasectomy
This is the removal of a section of the vas deferens from each side, with the aim of achieving infertility.
Indications
A method of birth control.
Anaesthesia
Local or general.
Post-operative care and common post-operative complications and their management
Post-operative haematoma can occur. If large, evacuation may be required. Infection can occur but is usually superficial. The 2016 guidelines from the Association of Biomedical Andrologists, the British Andrology Society, and the BAUS recommend a single post-vasectomy semen analysis be performed at 12wk (minimum 20 ejaculations) within 4h of production (1h if non-motile sperm are observed), and if no sperm are observed, then clearance can be given. Occasionally, a persistently positive semen analysis is an indication that the vas was not correctly identified at the time of surgery and has not been ligated (or, very rarely, that there were two vas deferens on one side). If <100 000/mL of non-motile sperm are observed in two samples, special clearance can be given (overall around 2% of vasectomy patients, not associated with pregnancies in large studies).
The potential for fertility remains in those with positive semen analysis, and re-exploration is indicated. Even if clearance is given, warn the patient that the vas deferens can later recanalize, thereby restoring fertility.
Sperm granuloma
A hard, pea-sized lump in the region of the cut ends of the vas forming as a result of an inflammatory response to sperm leaking out of the proximal cut end of the vas. It can be a cause of persistent pain, in which case, it may have to be excised or evacuated and the vas cauterized or re-ligated.
Vasovasostomy
Vasectomy reversal.
Anaesthesia
This tends to be done under general or spinal anaesthesia, as it takes far longer than a vasectomy.
Post-operative care and common post-operative complications and their management
Much the same as for vasectomy. The patient should avoid sexual intercourse for 2wk or so.
Vasectomy: BAUS procedure-specific consent form—recommended discussion of adverse events
Serious or frequently occurring complications
Common
•Irreversible.
•Small amount of scrotal bruising.
Occasional
•Bleeding requiring further surgery or bruising.
•Early failure (1:200).
Rare
•Inflammation or infection of the testis or epididymis, requiring antibiotics.
•Rejoining of the vas ends, resulting in fertility and pregnancy (1 in 2000).
•Chronic testicular pain (5%) or sperm granuloma.
Alternative treatment
Other forms of contraception (♂ or ♀).
Vasovasostomy: BAUS procedure-specific consent form—recommended discussion of adverse events
Serious or frequently occurring complications
Common
•Small amount of scrotal bruising.
•No guarantee that sperm will return to semen.
•Sperm may return, but pregnancy not always achieved.
•If storing sperm, check that appropriate forms have been filled out.
Occasional
•Bleeding requiring further surgery.
Rare
•Inflammation or infection of the testes or epididymis, requiring antibiotics.
•Chronic testicular pain (5%) or sperm granuloma.
Alternative therapy
IVF, sperm aspiration, ICSI.
Orchidectomy
Indications
Two types: radical orchidectomy and simple orchidectomy.
Radical (inguinal) orchidectomy
For excision of TC. This approach is used for three reasons:
•To allow ligation of the testicular lymphatics as high as possible as they pass in the spermatic cord and through the internal inguinal ring, thereby removing any cancer cells which might have started to metastasize along the cord.
•To allow cross-clamping of the cord prior to manipulation of the testis which, theoretically at least, could promote dissemination of cancer cells along the lymphatics. (In reality, this probably does not occur.)
•To prevent the potential for dissemination of tumour cells into the lymphatics that drain the scrotal skin that could occur if a scrotal approach is used. These lymphatics drain to the inguinal nodes. Thus, direct spread of the tumour to the scrotal skin and ‘violation’ of another lymphatic field (the groin nodes) are avoided. Historically, this was important because the only adjuvant therapy for metastatic disease was RT. The morbidity of groin and scrotal irradiation was not inconsiderable (severe skin reactions to RT, irradiation of the femoral artery and nerve).
Obtain serum markers before surgery (AFP, β-hCG, and LDH), and get a CXR. For full-staging CT scan, wait till after surgery. If the contralateral testis has been removed or is small, offer sperm storage—there is usually time to do this. Warn the patient that, very occasionally, what appears clinically and on ultrasound to be a malignant testis tumour turns out to be a benign tumour on subsequent histological examination.
Simple orchidectomy
For hormonal control of advanced PC. Done via a scrotal incision, with ligation and division of the cord and complete removal of the testis and epididymis. Alternatively, a subcapsular orchidectomy may be done where the tunica of the testis is incised and the seminiferous tubules contained within are excised. There is the potential with this approach to leave a small number of Leydig cells which can continue to produce testosterone.
Anaesthesia
Local, regional, general. Few men will require or opt for local.
Post-operative care and common post-operative complications and their management
For both simple and radical orchidectomy
Scrotal haematoma. Drain it if large or enlarging or if there are signs of infection (fever, discharge of pus from the wound).
For radical orchidectomy
Damage to the ilioinguinal nerve, leading to an area of loss of sensation overlying the scrotum.
Orchidectomy ± testicular implant: BAUS procedure-specific consent form—recommended discussion of adverse events
Serious or frequently occurring complications
Occasional
•Cancer, if found, may not be cured by orchidectomy alone.
•There may be a need for additional surgery, RT, or chemotherapy.
•Loss of future fertility.
•Biopsy of the contralateral testis may be required if an abnormality is found (small testis or history of maldescent).
Rare
•On pathological examination, cancer may not be found or the pathologic diagnosis may be uncertain.
•Infection of incision may occur, requiring further treatment and possibly removal of the implant if this has been inserted.
•Pain requiring removal of the implant.
•Cosmetic expectation not always met.
•The implant may lie higher in the scrotum than the normal testis did.
•A palpable stitch may be felt at one end of the implant.
•Long-term risks of silicone implants are not known.
Urological incisions
Midline, transperitoneal
Indications
Access to the peritoneal cavity and pelvis for radical nephrectomy, cystectomy, reconstructive procedures, etc.
Technique
Divide the skin and subcutaneous fat. Divide the fascia in the midline. Find the midline between the rectus muscles. Dissect the muscles free from the underlying peritoneum. Place two clips on either side of the midline; pinch between the two to ensure no bowel has been trapped; elevate the clips, and divide between them with a knife. Extend the incision in the peritoneum up and down, ensuring no bowel is in the way.
Closure
Use a non-absorbable (e.g. nylon) or very slowly absorbable (e.g. PDS) suture, using Jenkins rule to reduce the risk of dehiscence (suture length four times the wound length).
Specific complications
Dehiscence (classically around day 10 post-operatively and preceded by pink serous discharge, then sudden herniation of the bowel through the incision).
Lower midline, extraperitoneal
Indications
Access to the pelvis (e.g. RP, colposuspension).
Technique
Divide the skin and subcutaneous fat. Divide the fascia in the midline. Find the midline between the rectus muscles, and dissect the muscles free from the underlying peritoneum. If you make a hole in it, repair the defect with Vicryl. Divide the fascia posterior to the rectus muscles in the midline, so exposing the extravesical space.
Closure
As for midline, transperitoneal.
Pfannenstiel
Indications
Access to pelvis (e.g. colposuspension, open prostatectomy, open cystolithotomy).
Technique
Divide the skin 2cm above the pubis and the tissues down to the rectus sheath which is cut in an arc, avoiding the inguinal canal. Apply clips to the top flap (and afterwards the bottom flap), and use a combination of scissors and your fingers to separate the rectus muscle from the sheath. For maximum exposure, you must elevate the anterior rectus sheath from the recti cranially to just below the umbilicus and caudally to the pubis. Take care to diathermy a perforating branch of the inferior epigastric artery on each side. Apply two Babcock’s forceps to the inferior belly of the rectus on either side of the midline. Elevate and cut in the midline, the lower part of the fascia (transversalis fascia) between the recti. Separate the recti in the midline (do not divide them).
Closure
Tack the divided transversalis fascia together, and then close the transversely divided rectus sheath with Vicryl.
Supra-twelfth rib incision
Indications
Access to the kidneys, renal pelvis, and upper ureter.
Technique
Make the incision over the tip of the twelfth rib through the skin and subcutaneous fascia. Palpate the tip of the twelfth rib. Make a 3cm cut with diathermy through the muscle (latissimus dorsi) overlying the tip of the twelfth rib, so you come down onto the tip of the twelfth rib, and then cut anterior to the tip of the twelfth rib, down through the external and internal obliques and the transversus abdominis, to Gerota’s fascia and the perirenal fat. Sweep anteriorly with a finger to push the peritoneum and intraperitoneal organs out of harm’s way. Cut the muscles overlying the rib, cutting centrally along the length of the rib, in so doing avoiding the pleura. Cut with scissors along the top edge of the rib to free the intercostal muscle from the rib—beware the pleura! Insert Gillie’s forceps between the pleura and the overlying intercostal muscle, and divide the muscle fibres, so protecting the pleura. Dissect the fibres of the diaphragm away from the inner surface of the twelfth rib—as you do so, the pleura will rise upwards, with the detached diaphragmatic fibres out of harm’s way. At the posterior end of the incision, feel for the sharp edge of the costovertebral ligament. Insert heavy scissors, with the blades just open on the top of the rib (to avoid the eleventh intercostal nerve), and divide the costovertebral ligament. You should now be on top of Gerota’s fascia.
Specific complications
•Damage to the pleura: if you make a hole in the pleura, repair it at the end of the operation. Pass a small-bore catheter (e.g. Jacques) through the hole; close all the muscle layers; inflate the lung, and then before closing the skin, remove the catheter.
Complications common to all incisions
•Hernia, wound infection, chronic wound pain.
JJ stent insertion
Preparation
Can be done under sedation or GA, using either a rigid or flexible cystoscope. The latter is particularly useful for patients who are not fit enough for GA. The technique described is that used with the flexible cystoscope, but this is essentially the same if using a rigid scope.
With sedation
Oral ciprofloxacin 250mg; lidocaine gel for urethral anaesthesia and lubrication; sedoanalgesia (Diazemuls® 2.5–10mg IV, pethidine 50–100mg IV). Monitor pulse and oxygen saturation with a pulse oximeter.
Technique
A flexible cystoscope is passed into the bladder and rotated through 180°. This allows greater deviation of the end of the cystoscope and makes identification of the ureteric orifice easier. A 0.9mm hydrophilic guidewire (Terumo Corporation, Tokyo, Japan) is passed into the ureter under direct vision. The guidewire is manipulated into the renal pelvis using C-arm digital fluoroscopy. The cystoscope is placed close to the ureteric orifice, and its position, relative to bony landmarks in the pelvis, is recorded by frame-grabbing a fluoroscopic image. The flexible cystoscope is then removed, and a 4Ch ureteric catheter is passed over the guidewire into the renal pelvis. A small quantity of non-ionic contrast medium is injected into the renal collecting system to outline its position and to dilate it. The Terumo guidewire is replaced with an ultra-stiff guidewire (Cook UK Ltd, Letchworth, UK), and the 4Ch ureteric catheter is removed. We use a variety of stent sizes, depending on the patient’s size (6–8Ch, 20–26cm; Boston Scientific Ltd, St Albans, UK). The stent is advanced to the renal pelvis under fluoroscopic control using a ‘pusher’ (a hollow tube inserted over the guidewire), checking that the lower end of the stent is not inadvertently pushed up the ureter by checking the position of the ureteric orifice on the previously frame-grabbed image. The guidewire is then removed, while the pusher holds the stent in position (so that the stent is not pulled out along with the wire).
Further reading
Hellawell GO, Cowan NC, Holt SJ, Mutch SJ (2002). A radiation perspective for treating loin pain in pregnancy by double-pigtail stents. BJU Int 90:801–8.
McFarlane J, Cowan N, Holt S, Cowan M (2001). Outpatient ureteric procedures: a new method for retrograde ureteropyelography and ureteric stent placement. BJU Int 87:172–6.
Nephrectomy and nephro-ureterectomy
Indications for nephrectomy
•Renal cell cancer.
•Non-functioning kidney containing a staghorn calculus.
•Persistent haemorrhage following renal trauma.
Indications for nephro-ureterectomy
Transitional carcinoma of the renal pelvis and/or ureter.
Anaesthesia
General.
Post-operative care
Nephrectomy
Cardiovascular status and urine output should be carefully monitored in the immediate post-operative period. Haemorrhage from the renal pedicle or, for left-sided nephrectomy, the spleen is rare but will present with increasing tachycardia, cool peripheries, falling urine output, and eventually a drop in BP. A drain is usually not left in place, but if it is, there may be excessive drainage of blood from the drain. However, do not be lulled into a false sense of security by the absence of drainage—this does not mean that haemorrhage is not occurring, as the drain may be blocked, but haemorrhage may be ongoing.
For nephrectomy via a posterolateral (rib-based) incision, watch for pneumothorax. Arrange a CXR on return from the recovery room. Arrange routine chest physiotherapy to reduce the risk of chest infection. Regular chest examination is important, looking specifically for pneumothorax and pleural effusion.
Mobilize the patient as quickly as possible to reduce the risk of DVT and PE.
Nephro-ureterectomy
Where the ureter has been excised from the bladder, a urethral catheter is left in place at the end of the procedure to allow the hole in the bladder to heal. This is usually removed 10–14 days after surgery.
Common post-operative complications and their management
•Haemorrhage.
•Wound infection: rare. If superficial, treat with antibiotics. If an underlying collection of pus is suspected, open the wound to allow free drainage and pack the wound daily.
•Pancreatic injury: rare, but would be indicated by excessive drainage of fluid from the drain, if present, which will have a high amylase level. If no drain is present, an abdominal collection will develop, which may be manifested by a prolonged ileus.
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of nephrectomy/nephro-ureterectomy
Simple nephrectomy
•Common.
•Temporary insertion of a bladder catheter.
•Occasional insertion of a wound drain.
•Occasional.
•Bleeding requiring further surgery or transfusion.
•Entry into the lung, requiring temporary insertion of a drainage tube.
•Rare.
•Involvement or injury to nearby structures—blood vessels, spleen, lung, liver, pancreas, bowel, requiring further extensive surgery.
•Infection, pain, or hernia of incision, requiring further treatment.
•Anaesthetic or cardiovascular problems, possibly requiring intensive care admission (including chest infection, PE, stroke, DVT, heart attack).
•Alternative therapy: observation, laparoscopic approach.
Radical nephrectomy
As above plus:
•Occasional.
•Need for further therapy for cancer.
•Rare.
•May be an abnormality other than cancer on microscopic analysis.
•Alternative therapy: observation, embolization, immunotherapy, laparoscopic approach.
Nephro-ureterectomy
As above.
Radical prostatectomy
Indications
Localized PC.
Anaesthesia
General or regional.
Post-operative care
Mobilize as quickly as possible, and continue SC heparin and AK-TEDS until discharge, to reduce the risk of DVT and PE. Remove the drains when drainage is minimal. If there is persistent leak of fluid from the drains, send a sample for urea and creatinine, and if it is urine, get a cystogram to determine the size of the leak at the vesicourethral junction. Urethral catheters are left in situ post-RP for a variable time, depending on the surgeon who performs the operation. Some surgeons leave a catheter for 3 wk, and others for just 1wk.
Common post-operative complications and their management
Haemorrhage
Managed in the usual way (transfusion; return to theatre where bleeding persists or where there is cardiovascular compromise).
Ureteric obstruction
Usually results from oedema of the bladder, obstructing the ureteric orifices. Retrograde ureteric catheterization is rarely possible (this would require urethral catheter removal, and it is difficult to see the ureteric orifices because of oedema). Arrange placement of percutaneous nephrostomies.
Lymphocele
Drain by radiologically assisted drain placement. If lymphocele recurs after drain removal, create a window from the lymph collection into the peritoneal cavity, so the lymph drains into the peritoneum from which it is absorbed.
Displaced catheter post-radical prostatectomy
If the catheter falls out a week after surgery, the patient may well void successfully, and in this situation, no further action needs be taken. If, however, the catheter inadvertently falls out the day after surgery, gently attempt to replace it with a 12Ch catheter which has been well lubricated. If this fails, pass a flexible cystoscope under LA into the bulbar urethra, and attempt to pass a guidewire into the bladder, over which a catheter can then safely be passed. If this is not possible, another option is to hope that the patient voids spontaneously and does not leak urine at the site of the anastomosis. An ascending urethrogram may provide reassurance that there is no leak of contrast and that the anastomosis is watertight. If there is a leak or the patient is unable to void, an SPC can be placed (percutaneously or under GA via an open cystostomy).
Faecal fistula
Due to rectal injury, either recognized and repaired at the time of surgery and later breaking down or not immediately recognized. Formal closure is often required.
Contracture at the vesicourethral anastomosis
Gentle dilatation may be tried. If the stricture recurs, instruct the patient on ISC in an attempt to keep the stricture open. If this fails, BNI may be tried.
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of radical prostatectomy
Common
•Temporary insertion of a bladder catheter and wound drain.
•High chance of impotence due to unavoidable nerve damage.
•No semen is produced during orgasm, causing subfertility.
Occasional
•Blood loss, requiring transfusion or repeat surgery.
•Urinary incontinence—temporary or permanent, requiring pads or further surgery.
•Discovery that cancer cells are already outside the prostate, needing observation or further treatment at a later date, if required, including RT or hormonal therapy.
Rare
•Anaesthetic or cardiovascular problems, possibly requiring intensive care admission (including chest infection, PE, stroke, DVT, heart attack).
•Pain, infection, or hernia in area of incision.
•Rectal injury, very rarely needing temporary colostomy.
Alternative therapy
WW, RT, BT, hormonal therapy, and perineal or laparoscopic removal.
Radical cystectomy
Indications
•MI bladder cancer.
•Adenocarcinoma of the bladder (radioresistant).
•Squamous carcinoma of the bladder (relatively radioresistant).
•NMI TCC of the bladder, which has failed to respond to intravesical chemotherapy or immunotherapy.
•Recurrent TCC of the bladder post-RT.
Combined with urethrectomy if:
•Multiple bladder tumours.
•Involvement of the bladder neck or prostatic urethra.
Anaesthesia
General.
Post-operative care and common post-operative complications and their management
Monitor cardiovascular status, urine output, and respiratory status carefully in the first 48h. Routine chest physiotherapy is started early in the post-operative period to reduce the chance of chest infection. Mobilize the patient as early as possible to minimize the risk of DVT and PE. Drains are removed when they stop draining. Some surgeons prefer to leave them for a week or so, so that late leaks (urine, intestinal contents) will drain via the drain track and not cause peritonitis. Try to remove the nasogastric tube, if used, as soon as possible to assist respiration and reduce the risks of chest infection. The patient usually starts to resume their diet within a week or so. If the ileus is prolonged, start parenteral nutrition.
Haemorrhage
Persistent bleeding that fails to respond to transfusion should be managed by re-exploration.
Wound dehiscence
Requires resuturing under GA.
Ileus
Common. Usually resolves spontaneously within a few days.
Small bowel obstruction
From herniation of the small bowel through the mesenteric defect created at the junction between the two bowel ends. Continue nasogastric aspiration. The obstruction will usually resolve spontaneously. Reoperation is occasionally required where the obstruction persists or where there are signs of bowel ischaemia.
Leakage from the intestinal anastomosis
Leading to:
•Peritonitis: requiring reoperation and repair or refashioning of the anastomosis.
•Enterocutaneous fistula: bowel contents leak from the intestine and through a fistulous track onto the skin. If low-volume leak (<500mL/24h), will usually heal spontaneously. Normal (enteral) nutrition may be maintained until the fistula closes (which usually occurs within a matter of days or a few weeks). If high-volume leak, spontaneous closure is less likely and reoperation to close the fistula may be required.
Pelvic abscess
Formal surgical (open) exploration of the pelvis is indicated with drainage of the abscess and careful inspection to see if the underlying cause is a rectal injury, in which case a defunctioning colostomy should be performed.
Partial cystectomy
Indications
Primary, solitary bladder tumours at a site that allows 2cm of normal tissue around it to be removed in a bladder that will have adequate capacity and compliance after operation. There should be:
•No prior history of bladder cancer.
•No CIS.
•A solitary MI tumour located well away from the ureteral orifices, which includes 2cm of normal surrounding bladder.
High-grade tumours should not be excluded if these criteria are met. The lesions most commonly amenable to partial cystectomy are G2 or G3 TCCs or adenocarcinomas located on the posterior wall or dome.
Contraindications
Associated CIS, deeply invasive tumours, tumours at the bladder base (i.e. near the ureteric orifices).
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of radical cystectomy
See also consent for an ileal conduit if this is the planned form of urinary diversion.
Common
•Temporary insertion of a nasal tube, drain, and stent.
•High chance of impotence (lack of erections) due to unavoidable nerve damage.
•No semen is produced during orgasm (dry orgasm), causing subfertility.
•Blood loss, requiring transfusion or repeat surgery.
•In women, pain or difficulty with sexual intercourse due to narrowing or shortening of the vagina and need for removal of the uterus and ovaries (causing premature menopause in those who have not reached the menopause).
Occasional
•Cancer may not be cured with surgery alone.
•Need to remove penile urinary pipe as part of the procedure.
Rare
•Infection or hernia of incision, requiring further treatment.
•Anaesthetic or cardiovascular problems, possibly requiring intensive care admission (including chest infection, PE, stroke, DVT, heart attack).
• renal function with time.
renal function with time.
Very rare
•Rectal injury, very rarely needing temporary colostomy.
•Diarrhoea due to shortened bowel, vitamin deficiency requiring treatment.
•Bowel and urine leak, requiring reoperation.
•Scarring of the bowel or ureters, requiring operation in the future.
•Scarring, narrowing, or hernia formation around the stomal opening, requiring revision.
Alternative therapy
RT, neobladder formation, rather than ileal conduit urinary diversion.
Formation of neobladder with bowel
•Common: need to perform ISC if the bladder fails to empty.
Ileal conduit
Indications
•For urinary diversion following radical cystectomy.
•Intractable incontinence for which anti-incontinence surgery has failed or is not appropriate.
Post-operative care and common post-operative complications and their management
Oliguria or anuria
Try a fluid challenge.
Wound infection
Treat with antibiotics and wound care. Open the superficial layers of the wound to release pus.
Wound dehiscence
Rare. Requires resuturing in theatre under GA.
Ileus
Common. Usually resolves spontaneously within a few days.
Small bowel obstruction
From herniation of the small bowel through the mesenteric defect created at the junction between the two bowel ends. Continue nasogastric aspiration. The obstruction will usually resolve spontaneously. Reoperation is occasionally required where the obstruction persists or where there are signs of bowel ischaemia.
Leakage from the intestinal anastomosis
Leading to:
•Peritonitis: requiring reoperation and repair or refashioning of the anastomosis.
•Enterocutaneous fistula: bowel contents leak from the intestine and through a fistulous track onto the skin. If low-volume leak (<500mL/24h), will usually heal spontaneously. Normal (enteral) nutrition may be maintained until the fistula closes (which usually occurs within a matter of days or a few weeks). If high-volume leak, spontaneous closure is less likely and reoperation to close the fistula may be required.
Leakage from the uretero-ileal junction
May be suspected because of a persistently high output of fluid from the drain. Test this for urea. Urine will have a higher urea and creatinine concentration than serum. If the fluid is lymph, the urea and creatinine concentration will be the same as that of serum. Arrange a loopgram (conduitogram). This will confirm the leak. Place a soft, small catheter (12Ch) into the conduit to encourage antegrade flow of urine and assist healing of the uretero-ileal anastomosis. If the leakage continues, arrange bilateral nephrostomies to divert the flow of urine away from the area and encourage wound healing.
Occasionally, a uretero-ileal leak will present as a urinoma (this causes persistent ileus). Radiologically assisted drain insertion can result in a dramatic resolution of the ileus, with subsequent healing of the uretero-ileal leak.
Hyperchloraemic acidosis
May be associated with obstruction of the stoma at its distal end or from infrequent emptying of the stoma back (leading to back pressure on the conduit). Catheterize the stoma—this relieves the obstruction. In the long term, the conduit may have to be surgically shortened.
Acute pyelonephritis
Due to the presence of reflux combined with bacteriuria.
Stomal stenosis
The distal (cutaneous) end of the stoma may become narrowed, usually as a result of ischaemia to the distal part of the conduit. Revision surgery is required if this stenosis causes obstruction, leading to recurrent UTIs or back pressure on the kidneys.
Parastomal hernia formation
Around the site through which the conduit passes, through the fascia of the anterior abdominal wall. Many hernias can be left alone. The indications for repairing a hernia are:
•Bowel obstruction.
•Pain.
•Difficulty with applying the stoma bag (distortion of the skin around the stoma by the hernia can lead to frequent bag detachment).
Repair the hernia defect by placing mesh over the hernia site, via an incision sited as far as possible from the stoma itself, so as to reduce the risk of wound infection.
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of ileal conduit formation
Common
•Temporary drain, stents, or nasal tube.
•Urinary infections, occasionally requiring antibiotics.
Occasional
•Diarrhoea due to shortened bowel.
•Blood loss, requiring transfusion or repeat surgery.
•Infection or hernia of incision, requiring further treatment.
Rare
•Bowel and urine leakage from anastomosis, requiring reoperation.
•Scarring to the bowel or ureters, requiring operation in future.
•Scarring, narrowing, or hernia formation around the urine opening, requiring revision.
• renal function with time.
renal function with time.
Alternative therapy
Catheters, continent diversion of urine.
Percutaneous nephrolithotomy
Indications
•Stones >2cm in diameter (although with recent miniaturization, stones >1cm should also be considered).
•Stones that have failed ESWL and/or an attempt at flexible ureteroscopy and laser treatment.
•Staghorn calculi.
Preoperative preparation
•CT scan to assist planning the track position and to identify a retrorenal colon.1
•Stop aspirin 10 days prior to surgery.
•Culture urine (so appropriate antibiotic prophylaxis can be given).
•Cross-match 2U of blood.
•Start IV antibiotics the afternoon before surgery to reduce the chance of septicaemia (many of the stones treated by PCNL are infection stones). If urine is culture-negative, use 1.5g IV cefuroxime tds and IV gentamicin (3mg/kg) once daily (od). Routine antibiotic prophylaxis also reduces the incidence of post-operative UTI.2
Access
•Positioning can be either prone or supine.
•Ultrasound or fluoroscopy or both to guide puncture.
•Dilatation of the tract using balloon, metal, or Amplatz dilators.
•Access sheath size: traditional ‘maxi’ 24–30Fr, mini 14–20Fr, ultra-mini 11–13Fr, micro 4.85Fr.
Post-operative management
Once the stone has been removed, a nephrostomy tube is left in situ for several days (Fig. 17.15). This drains urine in the post-operative period and tamponades bleeding from the track. So-called ‘tubeless’ PCNL (no nephrostomy tube, although a J stent is often inserted, which has a certain morbidity) can be used in select patients (no infection—therefore, not suitable for infection staghorn stones). Less requirement for post-operative analgesia and earlier discharge have been reported.
Complications of PCNL and their management
Bleeding
Some bleeding is inevitable, but that severe enough to threaten life is uncommon. In most cases, it is venous in origin and stops following placement of a nephrostomy tube (which compresses bleeding veins in the track). If bleeding persists, clamp the tube for 10min. If bleeding continues despite this, arrange urgent angiography, looking for an arteriovenous fistula or pseudoaneurysm, both of which will require selective renal artery embolization (required in 1% of PCNLs)3 or open exposure of the kidney to control bleeding by suture ligation, partial nephrectomy, or nephrectomy.
Septicaemia
Occurs in 1–2% of cases. Incidence is reduced by prophylactic antibiotics. Track damage. Essentially minimal. Cortical loss from the track is estimated to be <0.2% of the total renal cortex in animal studies.4
Colonic perforation
The colon is usually lateral or anterolateral to the kidney and is therefore not usually at risk of injury, unless a very lateral approach is made. The colon is retrorenal in 2% of individuals (more commonly in thin ♀ with little retroperitoneal fat).1 The perforation usually occurs in an extraperitoneal part of the colon and is managed by JJ stent placement and withdrawal of the nephrostomy tube into the lumen of the colon to encourage drainage of bowel contents away from that of the urine, thereby encouraging healing without development of a fistula between the bowel and the kidney. A radiological contrast study a week or so later confirms that the colon has healed and that there is no leak of contrast from the bowel into the renal collecting system.
Damage to the liver or spleen
Very rare in the absence of splenomegaly or hepatomegaly.
Damage to the lung and pleura, leading to pneumothorax or pleural effusion
Can occur with supra-twelfth rib puncture.
Nephrocutaneous fistula
When the nephrostomy tube is removed from the kidney, a few days after surgery, the 1cm incision usually closes within a few hours to a day or so. Occasionally, urine continues to drain percutaneously for a few days and a small ‘stoma’ bag must be worn. In the majority of such cases, the urine leak will stop spontaneously, but if it fails to do so after a week or so, place a JJ stent to encourage antegrade drainage of urine.
Outcomes
For small stones, the stone-free rate after PCNL is in the order of 90–95%. For staghorn stones, the stone-free rate of PCNL, when combined with post-operative ESWL for residual stone fragments, is in the order of 80–85%.
BAUS procedure-specific consent form—recommended discussion of adverse events
Serious or frequently occurring complications of PCNL
Common
•Temporary insertion of a bladder catheter and ureteric stent/kidney tube, needing later removal.
•Transient haematuria.
•Transient temperature.
Occasional
•More than one puncture site may be required.
•No guarantee of removal of all stones and need for further operations.
•Recurrence of stones.
Rare
•Severe kidney bleeding, requiring transfusion, embolization, or, as last resort, surgical removal of the kidney.
•Damage to the lung, bowel, spleen, and liver, requiring surgical intervention.
•Kidney damage or infection, needing further treatment.
•Overabsorption of irrigating fluids into the blood system, causing strain on heart function.
Alternative therapy
External shock wave treatments, open surgical removal of stones, observation.
References
1Hopper KD, Sherman JL, Williams MD, et al. (1987). The variable anteroposterior position of the retroperitoneal colon to the kidneys. Invest Radiol 22:298–302.
2Inglis JA, Tolly DA (1988). Antibiotic prophylaxis at the time of percutaneous stone surgery. J Endourol 2:59–62.
3Martin X (2000). Severe bleeding after nephrolithotomy: results of hyperselective embolisation. Eur Urol 37:136–9.
4Clayman J (1987). Percutaneous nephrostomy: assessment of renal damage associated with semi-rigid (24F) and balloon (36F) dilation. J Urol 138:203–6.
Ureteroscopes and ureteroscopy
The instruments
Two types of ureteroscope in common use: the semi-rigid ureteroscope and the flexible ureteroscope.
Semi-rigid ureteroscopes
Have high-density fibreoptic bundles for light (‘non-coherently’ arranged) and image transmission (‘coherently’ arranged to maintain image quality). For equivalent light and image transmission using glass rod lenses, thicker lenses are required than with fibreoptic bundles. As a consequence, semi-rigid ureteroscopes can be made smaller, while maintaining the size of the instrument channel. In addition, the instrument can be bent by several degrees without the image being distorted.
The working tip of most current models is in the order of 7–8Ch, with the proximal end of the scope being in the order of 11–12Ch. There is usually at least one working channel of at least 3.4Ch.
Flexible ureteroscopes
The fibreoptic bundles in flexible ureteroscopes are the same as those in semi-rigid scopes, only of smaller diameter. Thus, image quality and light transmission are not as good as with semi-rigid scopes but are usually adequate.
The working tip of most current models is in the order of 7–8Ch, with the proximal end of the scope being in the order of 9–10Ch. There is usually at least one working channel of at least 3.6Ch.
The great advantage of the flexible ureteroscope over the semi-rigid variety is the ability to perform controlled deflection of the end of the scope (active deflection). Behind the actively deflecting tip of the scope is a segment of the scope which is more flexible than the rest of the shaft. This section is able to undergo passive deflection—when the tip is fully actively deflected by advancing the scope further, this flexible segment allows even more deflection. Flexible ureteroscopes have recently been developed which have two actively deflecting segments.
Flexible ureteroscopes are intrinsically more intricate and are therefore less durable than semi-rigid scopes.
Ureteroscopic irrigation systems
Normal saline is used (high-pressure irrigation with glycine or water would lead to fluid absorption from pyelolymphatic or venous backflow). Irrigation by gravity pressurization alone (the fluid bag suspended above the patient without any applied pressure) will produce flow that is inadequate for visualization because the long, fine-bore irrigation channels of modern ureteroscopes are inherently high resistance. Several methods are available: hand-inflated pressure bags, foot pumps, and hand-operated syringe pumps. Whatever system is chosen, use the minimal flow required to allow a safe view, so as to avoid flushing the stone out of the ureter and into the kidney, from where you may not be able to retrieve it.
Ureteric dilatation
Some surgeons do; others do not. Those who do not argue that dilatation is unnecessary in the era of modern, small-calibre ureteroscopes. Those who do cite a higher chance of being able to pass the ureteroscope all the way up to the kidney. Ureteric dilatation may be helpful where multiple passes of the ureteroscope up and down the ureter are going to be required for stone removal (alternatively, use a ureteric access sheath). Some surgeons prefer to place two guidewires into the ureter, one to pass the ureteroscope over (‘railroading’) and the other to act as a safety wire so that access to the kidney is always possible if difficulties are encountered. The second guidewire is most easily placed via a dual-lumen catheter which has a second channel through which the second guidewire can be easily passed into the ureter without requiring repeat cystoscopy. This dual-lumen catheter has the added function of gently dilating the ureteric orifice to about 10Ch. There is probably no long-term harm done to the ureter as a consequence of dilatation.1
Ureteric access sheaths, which have outer diameters from 10 to 14Ch, may facilitate access to the ureter and are particularly useful if it is anticipated that the ureteroscope will have to be passed up and down the ureter on multiple occasions (to retrieve fragments of stone). In addition, they facilitate the outflow of irrigant fluid from the pelvis or the kidney, thereby maintaining the field of view and decreasing intrarenal pressures.
Patient position
The patient is positioned as flat as possible on the operating table to ‘iron out’ the natural curves of the ureter. A cystoscopy is performed with either a flexible or rigid instrument. A retrograde ureterogram can be done to outline pelvicalyceal anatomy. A guidewire is then passed into the renal pelvis. We use a Sensor guidewire (Microvasive, Boston Scientific) which has a 3cm-long floppy, hydrophilic tip, which can usually easily be negotiated up the ureter. The remaining length of the wire is rigid and covered in smooth PTFE. Both properties aid passage of the ureteroscope.
Technique of flexible ureteroscopy and laser treatment for intrarenal stones
Flexible ureteroscopy and laser treatment can be performed with topical urethral LA and sedation. However, trying to fragment a moving stone with the laser can be difficult, and ideally, therefore, ureteroscopy is most easily done under GA, with endotracheal intubation (rather than a laryngeal mask) to allow short periods of suspension of respiration and so stopping movement of the kidney and its contained stone.
Empty the bladder to prevent ‘coiling’ of the scope in the bladder. Pass the scope over a guidewire. This requires two people—the surgeon holds the shaft of the scope, and the assistant applies tension to the guidewire to fix the latter in position without pulling it down. This allows the scope to progress easily up the ureter. The assistant also ensures that acute angulation of the scope where the handle meets the shaft does not occur. The flexible ureteroscope should slide easily up the ureter and into the renal pelvis.
With modern active secondary deflection ureteroscopes, access to most, if not all, parts of the renal collecting system is possible.
Laser lithotripsy
The main drawback of laser lithotripsy is the dust-cloud effect that occurs as the stone is fragmented. This temporarily obscures the view and must be washed away before the laser can be safely re-applied.
Use of stone baskets to retrieve stones after ureteroscopy
The aim of ureteroscopy (or flexible ureterorenoscopy) is to remove the ureteric (or renal) stone. It therefore seems intuitive to remove any large fragments—leaving them in situ runs the risk of ureteric colic post-ureteroscopy.
To stent or not to stent after ureteroscopy
JJ stent insertion does not increase stone-free rates and is therefore not required in ‘routine’ cases. A stent should be placed if:
•There has been ureteric injury (e.g. perforation—indicated by extravasation of contrast).
•There are residual stones that might obstruct the ureter.
•The patient has had a ureteric stricture that required dilatation.
•Solitary kidneys.
Routine stenting after ureteroscopy for distal ureteric calculi is unnecessary.2 Many urologists will place a stent after ureteroscopy for proximal ureteric stones.
Complications of ureteroscopy
Septicaemia; ureteric perforation, requiring either a JJ stent or, very occasionally, a nephrostomy tube where JJ stent placement is not possible; ureteric stricture (<1%).
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of ureteroscopy for treatment of ureteric stones
Common
•Mild burning or bleeding on passing urine for a short period after the operation.
•Temporary insertion of a bladder catheter may be required.
•Insertion of a stent may be required, with a further procedure to remove it.
•Urinary infections, occasionally requiring antibiotics.
Occasional
•Inability to get the stone or movement of the stone back into the kidney where it is not retrievable.
•Kidney damage or infection, requiring further treatment.
•Failure to pass the scope if the ureter is narrow.
•Recurrence of stones.
Rare
•Damage to the ureter with need for an open operation or placement of a nephrostomy tube into the kidney.
Alternative therapy
Open surgery, shock wave therapy, or observation to allow spontaneous passage.
References
1Garvin TJ, Clayman RV (1991). Balloon dilation of the ureter for ureteroscopy. J Urol 146:742–5.
2Srivastava A, Gupta R, Kumar A, Kapoor R, Mandhani A (2003). Routine stenting after ureteroscopy for distal ureteral calculi is unnecessary: results of a randomized controlled trial. J Endourol 17:871.
Pyeloplasty
Indications
PUJO.
Anaesthesia
General.
Post-operative care
A JJ stent, bladder catheter, and drain are left in situ. The bladder catheter serves to prevent reflux of urine up the ureter, which can lead to  leakage of urine from the anastomosis site (reflux occurs because of the presence of the JJ stent). The drain is removed when the drain output is minimal. The stent is left in position for ~6wk.
leakage of urine from the anastomosis site (reflux occurs because of the presence of the JJ stent). The drain is removed when the drain output is minimal. The stent is left in position for ~6wk.
Common post-operative complications and their management
Haemorrhage
Usually arising from the nephrostomy track (if a nephrostomy tube has been left in place—some surgeons leave a JJ stent and a perinephric drain, with no nephrostomy). Clamp the nephrostomy tube in an attempt to tamponade the bleeding. If bleeding continues, consider angiography and embolization of the bleeding vessel if seen, or exploration.
Urinary leak
This can occur within the first day or so. If a urethral catheter has not been left in place, catheterize the patient to minimize bladder pressure, and therefore the chance of reflux which might be responsible for the leak. If drainage persists for more than a few days, shorten the drain—if it is in contact with the suture line of the anastomosis, it can keep the anastomosis open, rather than letting it heal. If the leak continues, identify the site of the leak by either a nephrostogram (if a nephrostomy has been left in situ) or a cystogram (if a JJ stent is in place—contrast may reflux up the ureter and identify the site of leakage) or an IVU. Some form of additional drainage may help ‘dry up’ the leak (a JJ stent if only a nephrostomy has been left in situ or a nephrostomy if one is not already in place).
Obstruction at the PUJ
This is uncommon, and if it occurs, it is usually detected once all the tubes have been removed and a follow-up renogram has been done. If the patient had symptomatic PUJO but remains asymptomatic, then no further treatment may be necessary. If they develop recurrent flank pain, reoperation may be necessary.
Acute pyelonephritis
Manage with antibiotics.
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of pyeloplasty
Common
•Temporary insertion of a bladder catheter and wound drain.
•Further procedure to remove the ureteric stent, usually under LA.
Occasional
•Bleeding, requiring further surgery or transfusion.
Rare
•Recurrent kidney or bladder infections.
•Recurrence can occur, needing further surgery.
Very rare
•Entry into the lung cavity, requiring insertion of a temporary drainage tube.
•Anaesthetic or cardiovascular problems, possibly requiring intensive care admission (including chest infection, PE, stroke, DVT, heart attack).
•Need to remove the kidney at a later time because of damage caused by recurrent obstruction.
•Infection, pain, or hernia of incision, requiring further treatment.
Alternative therapy
Observation, telescopic incision, dilatation of the area of narrowing, temporary placement of a plastic tube through the narrowing, laparoscopic repair.
Laparoscopic surgery
Virtually every urological procedure can be done laparoscopically. It is particularly suited to surgery in the retroperitoneum (nephrectomy for benign and malignant disease and for kidney donation at transplantation, pyeloplasty for PUJO), but it is also suited to pelvic surgery (lymph node biopsy, RP). Reconstructive surgery requiring laparoscopic suturing and using the bowel is technically very challenging, but possible. Laparoscopic surgery offers the advantages over open surgery of:
•Reduced post-operative pain.
•Smaller scars.
•Less disturbance of bowel function (less post-operative ileus).
•Reduced recovery time and reduced hospital stay.
Contraindications to laparoscopic surgery
•Severe chronic obstructive pulmonary disease (COPD) (avoid use of carbon dioxide for insufflation).
•Uncorrectable coagulopathy.
•Intestinal obstruction.
•Abdominal wall infection.
•Massive haemoperitoneum.
•Generalized peritonitis.
•Suspected malignant ascites.
Laparoscopic surgery is difficult or potentially hazardous in the morbidly obese (inadequate instrument length,  range of movement of instruments, higher pneumoperitoneum pressure required to lift the heavier anterior abdominal wall, excess intra-abdominal fat limiting the view); in those with extensive previous abdominal or pelvic surgery (adhesions); in previous peritonitis, leading to adhesion formation; in those with organomegaly; in the presence of ascites; in pregnancy; in patients with a diaphragmatic hernia; and in those with aneurysms.
range of movement of instruments, higher pneumoperitoneum pressure required to lift the heavier anterior abdominal wall, excess intra-abdominal fat limiting the view); in those with extensive previous abdominal or pelvic surgery (adhesions); in previous peritonitis, leading to adhesion formation; in those with organomegaly; in the presence of ascites; in pregnancy; in patients with a diaphragmatic hernia; and in those with aneurysms.
Potential complications unique to laparoscopic surgery
Gas embolism (potentially fatal), hypercarbia (acidosis affecting cardiac function, e.g. arrhythmias), post-operative abdominal crepitus (subcutaneous emphysema), pneumothorax, pneumomediastinum, pneumopericardium, barotraumas.
Bowel, vessel (aorta, common iliac vessels, IVC, anterior abdominal wall injury), and other viscus injury are not unique to laparoscopic surgery but are a particular concern during port access. Perforation of the small or large bowel is the commonest trocar injury. Rarely, the bladder is perforated. Failure to progress with a laparoscopic approach or a vessel injury with uncontrollable haemorrhage requires conversion to an open approach. Post-operatively, the bowel may become entrapped in the trocar sites or there may be bleeding from the sheath site. An acute hydrocele can develop due to irrigation fluid accumulating in the scrotum. It resorbs spontaneously. Scrotal and abdominal wall bruising not uncommonly occurs.
BAUS procedure-specific consent forms: recommended discussion of adverse events
For all laparoscopic procedures
Common
•Temporary shoulder tip pain.
•Temporary abdominal bloating.
•Temporary insertion of a bladder catheter and wound drain.
Occasional
•Infection, pain, or hernia of the incision, requiring further treatment.
Rare
•Bleeding, requiring conversion to open surgery or transfusion.
•Entry into the lung cavity, requiring insertion of a temporary drainage tube.
Very rare
•Recognized (and unrecognized) injury to organs or blood vessels, requiring conversion to open surgery or deferred open surgery.
•Anaesthetic or cardiovascular problems, possibly requiring intensive care admission (including chest infection, PE, stroke, DVT, heart attack).
Laparoscopic pyeloplasty
Common
•Further procedure to remove the ureteric stent, usually under LA.
Occasional
•Recurrence can occur, needing further surgery.
•Short-term success rates are similar to open surgery, but long-term results unknown.
Very rare
•Need to remove the kidney at a later time because of damage caused by recurrent obstruction.
Alternative therapy
Observation, telescopic incision, dilatation of the area of narrowing, temporary placement of a plastic tube through the narrowing, conventional open surgical approach.
Laparoscopic simple nephrectomy
Occasional
•Short-term success rates are similar to open surgery, but long-term results unknown.
Alternative therapy
Observation and conventional open surgical approach.
Laparoscopic radical nephrectomy
Occasional
•Short-term success rates are similar to open surgery, but long-term results unknown.
Rare
•A histological abnormality other than cancer may be found.
Alternative therapy
Observation, embolization, chemotherapy, immunotherapy, conventional open surgical approach.
Endoscopic cystolitholapaxy and (open) cystolithotomy
Indications
•Endoscopic cystolitholapaxy: generally indicated for small stones. The definition of ‘small’ is debatable. Many stones of <4cm in diameter can be removed endoscopically, but the greater the number and size of the stones, the more inclined will the surgeon be to adopt an open approach. Having said this, if you anticipate that the patient is likely to develop recurrent stones, and therefore will require multiple future procedures to remove them, then try to avoid open surgery because each redo open cystolithotomy will be more difficult (due to the presence of scar tissue).
•Open cystolithotomy: for stones of >4cm in diameter and/or multiple stones (though some surgeons will be happy to ‘take on’ larger stones endoscopically); patients with urethral obstruction which precludes endoscopic access to the bladder.
Anaesthesia
Regional or general.
Post-operative care
A catheter is left in the bladder for a day or so, since haematuria is common, particularly after fragmentation of large stones. Irrigation may be required if haematuria is heavy.
Common post-operative complications and their management
Haematuria
Requiring bladder washout or return to theatre is rare.
Septicaemia
Uncommon.
Bladder perforation
Uncommon, but can occur with the use of stone ‘punches’ which grab the stone between powerful cutting jaws. Grasping the bladder wall in the jaws of the stone forceps or punch is easily done and can cause perforation.
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of endoscopic cystolitholapaxy
Common
•Mild burning or bleeding on passing urine for short periods after the operation.
•Temporary insertion of a catheter.
Occasional
•Infection of the bladder, requiring antibiotics.
•Permission for removal/biopsy of a bladder abnormality, if found.
•Recurrence of stones or residual stone fragments.
Rare
•Delayed bleeding, requiring removal of clots or further surgery.
•Injury to the urethra, causing delayed scar formation.
Very rare
•Perforation of the bladder, requiring a temporary urinary catheter or return to theatre for open surgical repair.
Alternative therapy
Open surgery, observation.
Scrotal exploration for torsion and orchidopexy
Indications
Suspected testicular torsion.
Technique
A midline incision, since this allows access to both sides so that they may both be ‘fixed’ within the scrotum. Untwist the testis, and place in a warm, saline-soaked swab for 10min. If it remains black, remove it, having ligated the spermatic cord with a transfixion stitch of absorbable material. If it ‘pinks up’, fix it. If uncertain about its viability, make a small cut with the tip of a scalpel. If the testis bleeds actively, it should be salvaged (close the small wound with an absorbable suture). If not, it is dead and should be removed. Whatever you do, fix the other side.
Fixation technique
Some surgeons fix the testis within the scrotum with suture material, inserted at three points (3-point fixation). Some use absorbable sutures, and others non-absorbable. Those who use the latter argue that absorbable sutures may disappear, exposing the patient to the risk of retorsion.1 Those who use absorbable sutures argue that the fibrous reaction around the absorbable sutures prevents retorsion and argue that the patient may be able to feel non-absorbable sutures, which can be uncomfortable. The sutures should pass through the tunica albuginea of the testis and then through the parietal layer of the tunica vaginalis lining the inner surface of the scrotum.
Others say the testis should be fixed within a dartos pouch,2 arguing that suture fixation breaches the blood–testis barrier, exposing both testes to the risk of sympathetic orchidopathia (an autoimmune reaction caused by the development of antibodies against the testis). For dartos pouch fixation, open the tunica vaginalis; bring the testis out, and untwist it. Develop a dartos pouch in the scrotum by holding the skin with forceps and dissecting with scissors between the skin and the underlying dartos muscle. Enlarge this space by inserting your two index fingers and pulling them apart. Place the testis in this pouch. Use a few absorbable sutures to attach the cord near the testis to the inside of the dartos pouch to prevent retorsion of the testes. The dartos may then be closed over the testis, and the skin can be closed in a separate layer.
Post-operative care and potential complications and their management
As for all procedures involving scrotal exploration, a scrotal haematoma may result, which may have to be surgically drained.
BAUS procedure-specific consent form: recommended discussion of adverse events
Serious or frequently occurring complications of scrotal exploration
Common
•The testis may have to be removed if non-viable.
Occasional
•You may be able to feel the stitch used to fix the testis.
•Blood collection around the testes which slowly resolves or requires surgical removal.
•Possible infection of the incision or testis, requiring further treatment.
Rare
•Loss of testicular size or atrophy in future if the testis is saved.
•No guarantee of fertility.
Alternative therapy
Observation—risks loss of testis and autoimmune reaction, leading to subfertility and loss of hormone production in the remaining testis.
References
1Kuntze JR (1985). Testicular torsion after orchidopexy. J Urol 134:1209–10.
2Frank JD (2002). Fixation of the testis. BJU Int 89:331–3.
Electromotive drug administration
Electromotive drug administration (EMDA) is a non-invasive method of enhancing drug penetration across the bladder urothelium (and prostatic urethra), resulting in greater quantities of local drug being delivered to a greater tissue depth than is achievable by passive diffusion alone. It avoids many of the side effects seen with systemic administration.
Mechanism of action
EMDA uses an electric current to accelerate and actively transport ionized molecules into tissues. Drug administration can therefore be controlled by altering the electric current intensity. The two main electrokinetic principles are: iontophoresis (transport of ionized molecules into tissue by applying a current across a solution containing the ions, e.g. lidocaine) and electro-osmosis (transport of non-ionized solutes associated with the bulk transport of water, e.g. MMC).
Applications in urology
•LA of the bladder (and prostatic urethra) prior to other procedures: flexible and rigid cystoscopy with biopsy and cystodiathermy, TURBT, BNI, TUIP, intravesical capsaicin therapy, and BTX-A injections.
•Intravesical MMC therapy for TCC of the bladder.
•Intravesical oxybutynin therapy for OAB.
•Antibiotic administration (i.e. gentamicin) for infective recalcitrant cystitis.
•LA with anti-inflammatory drugs for cystodistension.
Method of EMDA LA
It can be performed as a day case or an outpatient procedure. A CE-DAS® UROGENICS® catheter electrode* (Fig. 17.16) is inserted urethrally, and the bladder emptied and irrigated with sterile water to remove any residual urine. A total of 150mL of 0.5% bupivacaine and 1.5mL (1.5mg) of 1/1000 adrenaline are instilled into the bladder. Two dispersive electrode pads are placed on the lower abdomen, and both the electrode pads and the catheter are connected to the PHYSIONISER® generator* set to positive polarity, 25mA current strength, and a pulsed current with a rise rate of 50μA/s for 23min. The catheter is then removed, and endoscopy can proceed. EMDA LA is effective for 60min.
* Physion Srl, Medolla, Italy.
Contraindications to EMDA LA
Allergy to LA, significant haematuria, patients on MAOIs.
Relative contraindications
Active infection of the lower genitourinary tract, protrusion of the enlarged median lobe of the prostate into the bladder, urethral stricture, bladder neck stenosis.
 risk of serious cardiovascular events. Some studies suggest an
risk of serious cardiovascular events. Some studies suggest an  risk of bleeding and the need for blood transfusion in those on aspirin, while others report no
risk of bleeding and the need for blood transfusion in those on aspirin, while others report no  risk. There is only one RCT, and this showed that aspirin did increase blood loss after TURP, but not enough to increase the requirement for blood transfusion.3 The risks of short-term withdrawal of aspirin prior to TURP have not been established, although there are anecdotal reports of serious adverse cardiovascular events. So should aspirin be stopped or continued prior to TURP? The short answer is that there is no substantial body of evidence to support stopping it or continuing it, and as the majority continue to do TURP with patients on aspirin, but a substantial minority stop it, either behaviour is reasonable. Since bleeding times return to normal within 48h of stopping aspirin (the time taken for new platelets to reach sufficient numbers to compensate for impaired function of circulating platelets), it seems reasonable to stop it 2 days before surgery and to restart it within a few days of surgery when it is obvious that post-operative bleeding has stopped (usually when it is deemed safe to remove the catheter).
risk. There is only one RCT, and this showed that aspirin did increase blood loss after TURP, but not enough to increase the requirement for blood transfusion.3 The risks of short-term withdrawal of aspirin prior to TURP have not been established, although there are anecdotal reports of serious adverse cardiovascular events. So should aspirin be stopped or continued prior to TURP? The short answer is that there is no substantial body of evidence to support stopping it or continuing it, and as the majority continue to do TURP with patients on aspirin, but a substantial minority stop it, either behaviour is reasonable. Since bleeding times return to normal within 48h of stopping aspirin (the time taken for new platelets to reach sufficient numbers to compensate for impaired function of circulating platelets), it seems reasonable to stop it 2 days before surgery and to restart it within a few days of surgery when it is obvious that post-operative bleeding has stopped (usually when it is deemed safe to remove the catheter). pulse pressure due to
pulse pressure due to 

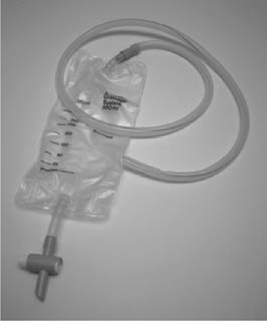



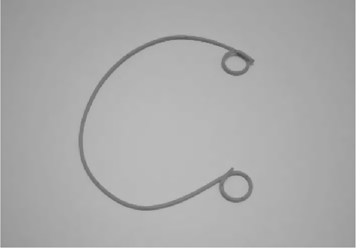
 pp.
pp.