ACCORDING TO THE ORDNANCE SURVEY’S 1:10 000 map, the length of the coastline of Britain is about 17,820 km or, if the larger off-shore islands are taken into account, 31,370 km. The length of the Irish coast has been estimated at 5631 and 6437 km. We should not worry about these discrepancies, because the ‘length’ of any irregular boundary depends on the scale at which you measure it, a well-known mathematical problem (Mandelbrot 1967). By any measure, our two islands have a lot of coast, and our coasts account for some very distinctive vegetation and a significant part of the biodiversity of Britain and Ireland. But there are different kinds of coast: ‘soft’ coastlines – saltmarshes, sand-dunes and shingle – and rocky shores and cliffs, each with its own suite of characteristic species. These are the subject of the next three chapters.
Saltmarshes, sand-dunes and shingle are all ‘accretional’ habitats, built up from material water-borne by coastal tides and currents, with a continuous range of particle size from the finest clay particles of saltmarsh mud to beach pebbles. The clay and silt particles (less than 0.05 mm diameter) that predominate in saltmarsh mud are kept in suspension by even slight turbulence and only settle out in estuaries and sheltered bays. Sand particles (0.05–2 mm) settle out quickly but are readily moved by waves and currents, and of course dry sand is picked up and carried by wind. Shingle (2–200 mm) is too large to be carried by wind but small enough to be moved by the combination of waves and longshore currents. Each kind of coast has its own suite of land-forms and distinctive vegetation and species. Rocky shores and sea-cliffs can only be eroded by the elements; they have a degree of permanence, but for plants they can be among the most stressful of habitats.
FIG 273. Saltmarsh in the estuary of the Urr Water, 5 km south of Dalbeattie, Dumfriesshire. Sediment erodes from the outer and downstream sides of the bends of the meandering river channel, and is deposited on the inner, lower sides of the loops. Various stages in the development of saltmarsh channels can be seen. (© Patricia & Angus Macdonald/SNH).
SALTMARSHES
Saltmarshes sometimes develop on open coasts, provided these are not too exposed, but more usually in estuaries (Fig. 273), at the head of sheltered bays or inlets, or in the shelter of shingle spits. They occur all round our coasts below high tide mark, with particular concentrations in the Poole Harbour–Solent– Chichester Harbour area, the north Kent, Essex and south Suffolk estuaries, northwestern Norfolk and the Wash, and the Humber estuary on the east coast, and the Bristol Channel, the northern Welsh and Lancashire estuaries and the Solway on the west. There are numerous saltmarshes, all of them small, in the Scottish sea-lochs, and many, mostly small, in Ireland.
The tides and saltmarsh zonation
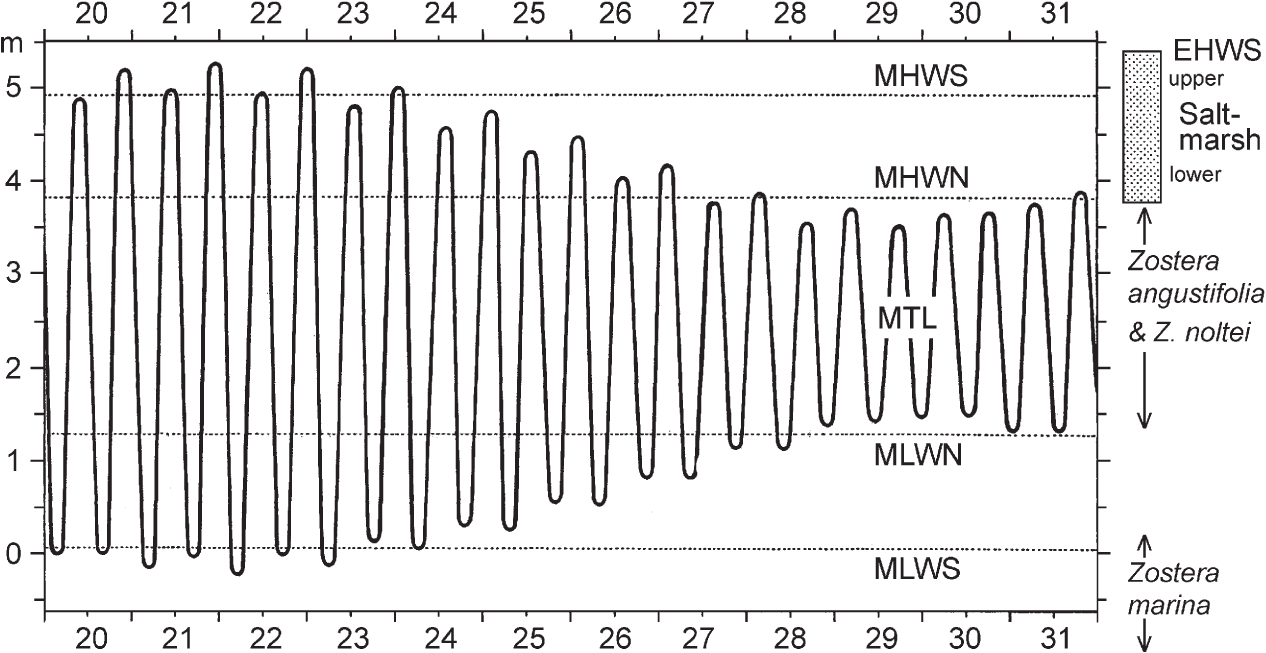
The tides are caused by the gravitational pull of the sun and moon on the water of the oceans. There are generally two high tides and two low tides each day. When the sun, earth and moon are lined up (new and full moon) the tides are at a maximum; these are spring tides. When the moon is at right angles to the sun (half moon) tides are at a minimum, giving neap tides. Tides are about 51 minutes later each day because the moon is orbiting round the earth, but spring and neap tides always occur at the same time of day at any particular place. Figure 274 shows the twice-daily rise and fall of the tides through a spring–neap cycle, with the mean high water of spring tides (MHWS), mean high water of neap tides (MHWN), and mean low water of neaps (MLWN) and springs (MLWS) – calculated on tides for the whole year, and based on ‘Chart datum’, which is lower than mean sea level. The exact height of the tide varies with the relative position of the sun and moon and with the seasons, so additional levels can be defined, such as extreme high and low water of spring tides (EHWS and ELWS) and mean tide level (MTL). These are all based on astronomical predictions. Weather also influences tidal level. The sea acts as a ‘water barometer’, so if atmospheric pressure is low, sea level is raised by about 1.3 cm per millibar, and vice versa. Onshore winds also raise tidal level, especially when they funnel water into confined spaces such as the English Channel or the southern North Sea.
FIG 274. The relation of saltmarsh (shaded band at top right) to tidal levels. Tidal data for Holyhead, July 1959, from Lewis 1964. See text for further explanation.
Saltmarshes as we usually think of them occupy only the shore between MHWN and EHWS – roughly the top quarter of the whole tidal range. This means that they spend a large proportion of time exposed to the air. At mean tide level a mud-flat (or beach) is submerged (and exposed) twice every (tidal) day – about 630 submergences a year. At about mean high water level the marsh is submerged 360 times a year, for an average of 1.2 hours a day (c. 440 hours a year), and never exposed for more than nine days at a stretch. In his work on the saltmarsh at Scolt Head Island on the Norfolk coast, V. J. Chapman took this as the boundary between lower marsh, dominated by time of submergence, and upper marsh, in which factors related to emergence are more important. Clearly this boundary cannot be sharp, but it enshrines a useful concept in thinking about saltmarsh ecology. Above mean high water level the saltmarsh can be exposed continuously to the air for many days on end, until at EHWS it is inundated only on rare occasions.
Below mean high water, the mud- or sand-flats may be colonised by eelgrasses (Zostera spp.), of which we have three species around our coasts. Zostera marina, the largest, is not generally part of the saltmarsh zonation at all, but is usually to be found on sandy substrata at or below low water of spring tides, to a depth of 3 or 4 m. The other two species, narrow-leaved eelgrass (Z. angustifolia, Fig. 275) and dwarf eelgrass (Z. noltei) are smaller, and Z. noltei is distinctive in being unbranched. These two species can form quite dense stands in suitable sites (Fig. 276), and are an important food resource for migrant brent geese and wigeon. Where the two grow together, as they commonly do, Z. noltei tends to grow on the slightly raised areas of the undulating mud, while Z. angustifolia prefers the slight depressions. These two have similar geographical distributions with us, strikingly different from the big Z. marina.
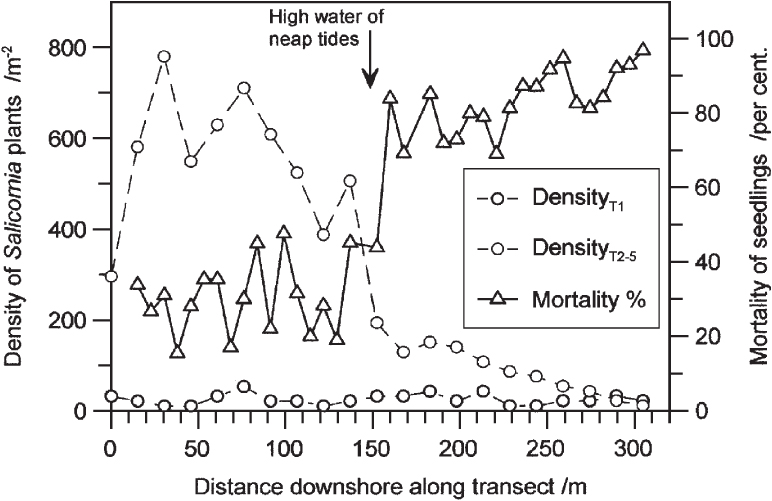
Above MHWN there is a steep rise in the average number of days the surface is undisturbed by tidal ebb and flow. This is crucial for establishment of seedlings. On the Dovey marshes in Wales, Wiehe (1935) found a dramatic drop in the density of mature glasswort (Salicornia) plants in midsummer at about high water of neap tides, and that the mortality of seedlings between April and July was 70–90% within reach of neap tides, but abruptly fell to around 30% in the zone between high water of neaps and high water of springs (Fig. 277). The two plants that generally form the lower fringe of the saltmarsh proper, the annual Salicornia species (Fig. 278a) and the perennial grass Spartina anglica (Fig. 278d), share a need for two or three days free of tidal disturbance for seedlings to establish themselves. Two other short-lived species that often become established with the Salicornia are annual sea-blite (Suaeda maritima) and sea aster (Aster tripolium), often in its rayless form. Typically there follows a broad zone dominated by common saltmarsh-grass (Fig. 279), often with sea-purslane (Fig. 280) and sea-lavenders (Limonium vulgare and L. humile; only the latter in Ireland), greater sea-spurrey (Fig. 278c) and English scurvygrass (Cochlearia anglica). This gives way upwards to a zone in which the saltmarsh-grass (with a prominent ligule) is replaced by red fescue (Festuca rubra, without a ligule) with a very characteristic list of associates including saltmarsh rush (Fig. 281c), sea-milkwort (Fig. 281a), sea plantain (Plantago maritima), sea arrowgrass (Triglochin maritimum) and thrift (Armeria maritima).
FIG 275. Narrow-leaved eel-grass (Zostera angustifolia), Cockwood, Exe estuary, Devon, Aug. 1981.
FIG 276. Eelgrass (Zostera angustifolia) beds: (a) Lympstone, Exe estuary, Devon, May 1980; (b) Oranmore, Co. Galway, with Zostera rooted in the mud, seaweeds (Fucus spp.) attached to the stones and an eroded edge of saltmarsh in the middle distance on the right. September 1959.
FIG 277. Establishment of glasswort (Salicornia) seedlings along transects in the Dyfi estuary, North Wales. Transect 1 (T1) was entirely covered at every tide; transects 2–5 (T2–5) were half above, half below high water of neap tides. Triangles and the full line show mortality of seedlings between April and July on transect 2. (Data from Wiehe 1935).
At its upper edge the saltmarsh passes, gradually or abruptly, into whatever vegetation lies behind it, and as this neighbouring vegetation can be diverse, upper saltmarsh vegetation is very variable. Common transitions are to grassland, freshwater marsh, sand-dune and shingle.
Saltmarsh accretion and erosion
Saltmarshes grow by accretion of silt, but this is by no means inevitable. Rivers bring down silt in suspension, and if they debouch into a wide sheltered estuary they deposit a large part of this. The consequence is that the estuary silts up, creating mud-flats, which sooner or later reach a level at which the pioneer plants of the lower saltmarsh can become established. Further accretion of silt raises the surface, so that plants of successively higher levels take over, and this process continues until the marsh is inundated by only the highest spring tides. Upper saltmarsh turf affords good grazing, so by this stage the marsh may well have been enclosed from the sea and added, behind an embankment, to the farmland to landward. This process (‘inning’) has obviously continued for millennia in the past, and the lower courses of many of our rivers flow through tracts of flat farmland that were formerly saltmarsh. The evidence for this is often apparent in maps and aerial photographs. The river becomes confined to a channel a fraction of the width of the former estuary. It may still support saltmarshes, but deposition and erosion of sediment are likely to be much more nearly in balance. The other situation in which saltmarsh can develop de novo is in sheltered inlets and behind growing shingle spits, of which Scolt Head Island in Norfolk provides classic examples (Figs 310, 311). Wave action on exposed shores stirs up sand and silt, which then settles in less turbulent places.
FIG 278. Saltmarsh plants: (a) an annual glasswort, Salicornia dolichostachya, Budleigh Salterton, Devon; (b) common sea-lavender (Limonium vulgare), Thornham, Norfolk, July 1971; (c) greater sea-spurrey (Spergularia media), Budleigh Salterton, Devon, June 1988; (d) common cord-grass (Spartina anglica), Cockwood, Devon, July 1980.
FIG 279. Salt-marsh grass (Puccinellia maritima) colonising a dense stand of Salicornia with scattered sea aster (Aster tripolium), Blakeney, Norfolk, August 1970.
FIG 280. Sea-lavender (Limonium vulgare) marsh; with sea-purslane (Atriplex portulacoides) lining the creeks, Thornham, Norfolk, July 1971.
Accretion and erosion of silt, and their interplay, are complex. Maximum accretion of silt in a growing marsh can reach 3 cm a year in the Salicornia zone, and 10 cm a year or more in a vigorous closed turf of Puccinellia or Spartina, but these are maxima and 10–15% of these figures would be more usual. Accretion on emergence marshes is commonly 0.2–1 cm a year. In relatively young and uniform marsh, most accretion takes place near the landward margin, but the zone of maximum accretion tends to move seaward as the marsh builds up and matures, so the marsh becomes flatter (approaching high water of spring tides) as it ages. Accretion is not uniform round the year. Fine clayey mud tends to be deposited more around the seaward edges of the marsh during spring and summer. In autumn, with the vegetation full grown and at maximum trapping capacity, silt mobilised by autumn storms is deposited generally over the marsh; there may be a small net loss of sediment during the winter.
FIG 281. Upper saltmarsh plants. (a) Sea-milkwort (Glaux maritima), Cuckmere Haven, Sussex, June 1986; (b) lesser sea-spurrey (Spergularia marina), Turf Lock, Devon, May 1977; (c) saltmarsh rush (Juncus gerardii), Holme-next-the-Sea, Norfolk, July 1981; (d) parsley water-dropwort (Oenanthe lachenalii), Cuckmere Haven, July 1989.
Changing current patterns may lead to a switch from accretion to erosion – which typically takes place at the seaward edge of the marsh, creating a small cliff. If the sediment regime switches back to accretion, saltmarsh development begins anew on the eroded surface. Erosion features are very common on long-established saltmarshes, often manifested as a series of ‘steps’ as one walks down the marsh. But the possibilities are almost endless. A common pattern in estuaries is for the river channel to follow a meandering course through the deposited silt, eroding the outer and downstream sides of the meanders, and depositing silt on the more sheltered inner and lower side. The result is a series of lobes of saltmarsh in successive loops of the river, each one youngest on the downstream side, and progressively truncated on the upstream side. Thus the whole pattern slowly moves downstream. This process is well illustrated in Figure 273, which will repay close scrutiny.
Pans and creeks, and aeration
Even if the original surface is level, small irregularities inevitably arise as the plant cover develops. More silt tends to be deposited where plants slow the flow of the water, and minor depressions can become accentuated as primary pans. As accretion goes on, the water covering the marsh at flood tide becomes concentrated in regular creeks (or channels) as the water drains off at the ebb. These often become intricately branched and meandering, especially if the marsh is extensive and flat. The creeks are erosion features subject to tidal scour on the ebb, even on an accreting marsh, but they are not necessarily permanent. The vegetation on either side of the creek continues to trap silt, so the surface of the marsh remains flat. Narrow creeks can be roofed over by encroachment of the turf on either side, or blocked by collapse of their walls. The former creek becomes stagnant and detached from the drainage, and it can break up into a string of channel pans, whose origin is often obvious from their shape and orientation. Most pans in a mature saltmarsh have originated in this way. Some pans may be formed where persistent masses of tidal debris on the marsh have killed the underlying vegetation, initiating a trash pan. Whatever their origin, pans are subject to wide variations in temperature and salinity, many are permanently waterlogged, and they are generally an unfavourable environment for plant growth (Fig. 282).
FIG 282. Saltmarsh pans, Malltraeth, Anglesey. The pans are dry in a spell of fine weather; a more normal water level is marked by the line of bleached algae.
The better-aerated strip a few metres wide along the margin of a channel is often marked by the abundance of the grey-leaved sea-purslane. Even below the Salicornia zone, the surface is constantly burrowed by lugworms, cockles and the small crustacean Corophium volutator, but the saltmarsh contains a great deal of organic matter needing oxygen for its breakdown. Chapman found that when he poked a hole through the mud of a flooded saltmarsh, bubbles of air were released. At low levels on the marsh (Aster zone) the oxygen concentration in this air was only around 1%, but in sea-lavender marsh it was 10.5–17.5%. Measurement of oxidation–reduction (redox) potential in a Humber saltmarsh showed that low on the marsh under Spartina, even at 5 cm depth the soil was permanently waterlogged and highly reducing for all but a brief period at neap tides in midsummer (Armstrong et al. 1985). In Puccinellia marsh, conditions were only permanently reducing at 30 cm depth; at 10 cm or less high spring tides caused episodes of waterlogging and lowered redox potential. Rather higher, in the ‘general saltmarsh’, the soil at all levels was oxidising most of the time, broken only by periods of waterlogging following unusually high spring tides. Pans remain waterlogged and reducing most of the time. Highly reducing conditions favour anaerobic bacteria, which reduce iron from the ferric to the ferrous state, and sulphate to sulphide. Anyone incautious enough to go up to their thighs in a saltmarsh pan is likely to emerge covered in stinking black mud!
Geographical (and other) variation in British and Irish saltmarshes
The details of saltmarsh zonation and of individual saltmarshes vary greatly from place to place, depending on the geographic setting of the marsh, its exposure to wave action and currents, the nature of the substratum (muddy, sandy or peaty), whether the marsh is grazed or not, the age of the marsh and whether it is accreting or eroding. These paragraphs give only the barest outline.
The classical studies on British saltmarsh vegetation were concentrated on Blakeney Point and Scolt Head Island in north Norfolk, and the Dyfi estuary in Cardigan Bay, in the years before the Second World War. A much broader and more representative picture was provided in the 1970s by Paul Adam’s detailed survey of 133 saltmarshes all round the coasts of Britain (Adam 1978, 1981). He suggested a division of saltmarsh sites round the British coast into three rough categories. Type A marshes, in which communities of the lower and middle marsh, dominated by such plants as Spartina anglicaSM6, Salicornia spp.SM8, the rayless form of sea asterSM11, common saltmarsh-grassSM13 and sea-purslaneSM14 are particularly prominent, are concentrated along the south and east coasts of England. They are seldom grazed, are typically backed by low coasts (sometimes dunes), and are the habitat of some southern species that are scarce or absent elsewhere on our coasts, notably matted sea-lavender (Limonium bellidifolium, confined with us to north Norfolk), shrubby sea-blite (Suaeda vera) at the upper fringe of the marshSM21 and sea-heath (Frankenia laevis) often growing with sea-purslaneSM22. Golden-samphire (Inula crithmoides) is more widespread than the last two (often on sea-cliffs) but grows in saltmarshes only from Essex to HampshireSM26. Sea wormwood (Seriphidium maritimum) occurs more widely again but is commonest in Type A saltmarshes in southeast England.
In type B marshes the emphasis shifts towards the upper marsh and to communities in which such species as red fescue, sea-milkwort, thrift, saltmarsh rushSM16, sea rush (Juncus maritimus)SM15,18, autumn hawkbit (Leontodon autumnalis) and parsley water-dropwort (Fig. 281d) are prominent. Most of the lower-marsh species are still present, but zonation tends to be less clearly marked than in type A marshes. Type B marshes are often grazed, usually by sheep, less often by cattle, and are particularly concentrated on the Irish Sea coastline of Wales, northern England and Galloway. They typically combine an abundance of the common saltmarsh species – thrift can make spectacular displays of pink in early summer (Fig. 283) – with rich upper-marsh transitions to non-saline wet or dry grasslands. These are marked by some characteristic species, such as autumn hawkbit, parsley water-dropwort and silverweed (Potentilla anserina), but nothing particularly notable or rare.
FIG 283. Thrift (Armeria maritima) in flower on upper saltmarsh, Holy Island, Northumberland, June 1999.
The saltmarshes in the Scottish sea-lochs are a category of their own (type C), characterised as much as anything by absences. Common saltmarsh-grass is still there, but the turf is largely dominated by the ubiquitous red fescue. Sea-milkwort, thrift, sea arrowgrass, sea plantain and buck’s-horn plantain (Plantago coronopus) are abundant. Salicornia and annual sea-blite are sparse, and Spartina is absent altogether. Features very characteristic of these Scottish saltmarshes are the ‘turf fucoids’, depauperate brown seaweeds growing mixed with Puccinellia, the detached floating masses of seaweeds (usually Ascophyllum nodosum) in the most sheltered sea-lochs (Fig. 284), and the yellow iris (Iris pseudacorus) and meadowsweet (Filipendula ulmaria) in the fringe at the head of the saltmarshM28 (Fig. 285). Western Scottish saltmarshes are the headquarters in Britain and Ireland for saltmarsh flat-sedge (Blysmus rufus)SM19 and slender spike-rush (Eleocharis uniglumis)SM20, both regularly with sea-milkwort, saltmarsh rush, creeping bent (Agrostis stolonifera), sea arrowgrass and red fescue, and often accompanied by long-bracted sedge (Carex extensa).
Tom Curtis and Micheline Sheehy Skeffington (1998) made an inventory of the saltmarshes of Ireland, noting their substrata (sand, mud or peat), and whether they were ungrazed or grazed, and if grazed, by what kind of livestock. Generally, marshes in estuaries or sheltered bays are on mud, and these predominate, especially on the south and east coasts. The saltmarshes on the east coast of Ireland would pass with little remark in southeast England, and most are ungrazed. Sea-purslane and Spartina are generally present, as they are in saltmarshes of most of the south-coast estuaries; they too are mostly ungrazed. On the west coast the marshes are generally grazed, mostly by cattle. A particular feature of the west, especially in west Galway and Mayo, is the number of saltmarshes overlying freshwater peat – a testimony to Post-glacial changes of land and sea level. These west-coast Irish saltmarshes are mostly small, and have much in common with similar marshes in western Britain, at low levels dominated by common saltmarsh-grass, with sea plantain, sea aster, sea-milkwort, thrift, greater sea-spurrey and, locally, lax-flowered sea-lavender (Limonium humile). ‘Turf fucoids’ are often prominent, as in the western Scottish sea-lochs. Salicornia and annual sea-blite are scattered among the other saltmarsh plants but, as in British west-coast marshes, seldom form extensive pioneer stands. At higher levels red fescue and saltmarsh rush become dominant, accompanied by all the common mid- and upper-saltmarsh plants, and such species as long-bracted sedge, sea rush and brookweed (Samolus valerandi).
FIG 284. Free-floating masses of a finely-divided form of knotted wrack (Ascophyllum nodosum) in the very sheltered water of Loch Sunart, Strontian, September 1973. The inset shows a close-up of the seaweed with a clump of red fescue and sea-plantain for comparison. ‘Turf fucoids’ are similarly depauperate fucoid seaweeds amongst the saltmarsh plants.
FIG 285. Upper saltmarsh with yellow iris (Iris pseudacorus), Drynoch, Skye, June 1981.
The south- and east-coast marshes stand in a similar relationship to the west-coast marshes as the southeastern (type A) marshes do to the west-coast (type B and C) marshes in Britain. The distributions of thrift and sea-milkwort are noticeably biased toward the west side of Ireland, while sea-purslane is heavily concentrated along the south and east coasts – its distribution is nicely symmetrical around the Irish Sea. Spartina anglica is mainly a south- and east-coast plant in Ireland, with the conspicuous exception of the Shannon estuary. Lax-flowered sea-lavender, greater sea-spurrey, annual sea-blite and Salicornia, all lower-marsh species, all show a bias towards the estuarine marshes of the south and east.
SPARTINA IN BRITAIN AND IRELAND
The long-standing native small cord-grass (Spartina maritima), once recorded all along the south and east coasts of England from Devon to Lincolnshire, is now a very local plant with its headquarters in the Essex and Suffolk estuaries, and outlying localities on the Lincolnshire shore of the Wash and in the Chichester Harbour–Solent area. The American smooth cord-grass (S. alterniflora) was first recorded in the River Itchen near Southampton in 1829, having presumably arrived accidentally with shipping. By the 1870s it had spread widely and become abundant in the Itchen and as far down Southampton Water as Hamble. About 1870 a sterile hybrid between S. maritima and S. alterniflora was noticed, and in 1880 it was described by H. & J. Groves as a new hybrid species under the name Spartina × townsendii. The sterile hybrid has a chromosome number of 2n = 62; S. maritima has 2n = 60 and S. alterniflora 2n = 62. The sterile hybrid spread by rhizome growth and fragmentation, and by the early 1900s was established throughout the Solent area and had reached Poole Harbour. Probably about 1890 a fertile amphidiploid plant appeared, with a chromosome number of 2n = 122. This was vegetatively as vigorous as the sterile hybrid, but it could spread by seed. In the early decades of the twentieth century both continued to spread, and ‘Spartina townsendii sensu lato’ (including S. anglica) was widely planted for reclamation work from 1907 onwards. Goodman et al. (1959) estimated that by then the plant covered at least 12,000 ha of tidal flats in Britain and Ireland. Patches of die-back in formerly vigorous Spartina, with death and decay of the rhizomes (Fig. 286), are perhaps due to the growth of the plant itself along with accumulation of fine sediment leading to waterlogging and locally toxic anaerobic conditions, because no possibly causal disease organism has been found.
Spartina anglica is notable for the wide range of conditions it can tolerate. Its lower limit is normally about (or just below) MHWN, but in Poole Harbour, in sheltered conditions and with an unusually narrow tidal range, it grows almost down to MLWN. It can grow as high as MHWS, but is a poor competitor in dry soils, so its upper limit is usually 20–30 cm lower. It can thus cover a large part of the normal saltmarsh range. It is now generally distributed round the shores of Britain and Ireland north to Galloway and the Firth of Tay.
FIG 286. Spartina dieback. Exe estuary, behind Dawlish Warren, Devon, July 1980.
SALINITY
Saltmarsh plants are halophytes, that is, they can tolerate relatively high concentrations of salt (sodium chloride, NaCl). Halophytes face twin problems. To take up water, the cells of a plant must be at a lower water potential than the soil water; that means in practice that the cell sap has to be more concentrated than the soil water. Many of the enzymes essential for normal metabolism are inhibited by raised concentrations of NaCl, and in a saline habitat the soil water is at a lower water potential (more concentrated) than in normal soils, and contains a large excess of salt. This means that the plant’s cell-sap must be more concentrated too, but at the same time the plant must keep the sodium and chloride concentrations in its cells within bounds. Selective uptake of potassium (K) and exclusion of sodium (Na) is important to all halophytes, as it is in a lesser degree to non-halophytes. Grasses and other monocotyledonous halophytes tend to rely mainly on excluding Na, but many, including Spartina, have glands excreting salt to the exterior; their (molar) ratio of Na to K is commonly around 1:1. Many dicotyledonous halophytes take up salt, and have higher Na:K ratios, often around 10:1. They rely on various combinations of secreting salt through specialised glands into hairs or bladders on the leaf surface, or segregating it into the cell vacuoles, and balancing the osmotic potential of the vacuoles and cytoplasm by synthesising compatible solutes in the cytoplasm – substances like proline, glycine betaine and sugars – which can adjust osmotic potential but are benign to enzyme activity. In plants with this mechanism the vacuoles often make up a large proportion of their bulk, which is largely why succulence is so common in saline habitats. These two mechanisms are not mutually exclusive, and any simple explanation sidesteps a great deal of variation and physiological detail.
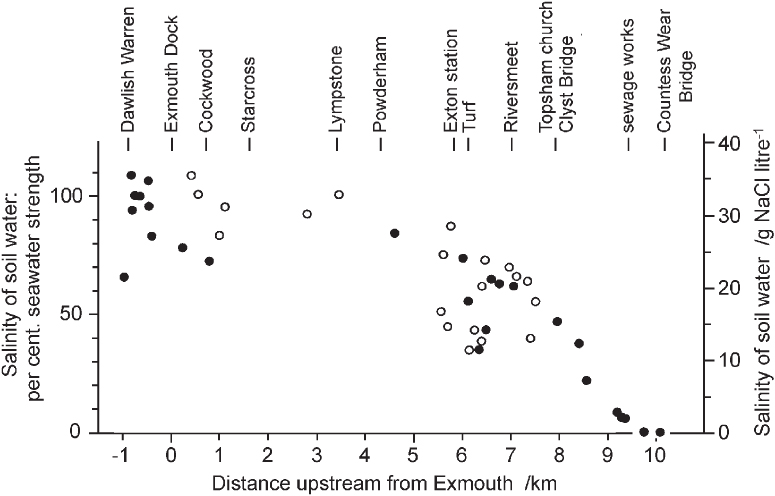
The salinity at the seaward edge of a saltmarsh or at the mouth of an estuary is roughly that of the open sea, about 35 g per litre. This is around 500 times the concentration of sodium and chloride in an average fresh water. Measurements of the salinity of the soil-water at 10 cm depth in the saltmarshes of the Exe estuary are plotted in relation to distance from the mouth in Figure 287. The effect of the river in diluting the seawater entering the estuary at every tide is immediately apparent, and minor inflows into the sheltered bay behind the sand-spit of Dawlish Warren result in a good deal of variation even close to the mouth. However, for the first 5 or 6 km, where the estuary remains a broad expanse of water at high tide, salinity does not fall greatly below the open-sea concentration. It is only as the estuary begins to narrow above Turf Lock and the confluence with the River Clyst at Riversmeet that the influence of fresh water is felt strongly. From here, there is a steep gradient of salinity to fully fresh water at Countess Wear on the Exe, and about 1 km below Clyst St Mary on the Clyst. Of the dominant species in the Exe saltmarshes, the eelgrasses (Zostera angustifolia and Z. noltei) occur only where salinity is above about 25 g per litre – about 70% of seawater. Sea-purslane occurs down to about half seawater, and Spartina anglica and Puccinellia maritima down to about a third of seawater strength. Sea club-rush (Bolboschoenus maritimus) and common reed (Phragmites australis) by contrast are restricted to the less saline parts of the estuary; sea club-rush was found at salinities from 7% to 65% of seawater and common reed up to just over 35% seawater.
FIG 287. Salinity at 10 cm depth in muds in the Exe estuary from its mouth to near the tidal limit, projected onto a straight line through Exmouth dock and Topsham church. Solid dots, west shore; open circles, east shore. From Proctor (1980).
The picture is essentially similar in the saltmarsh in the neighbouring estuary of the River Otter at Budleigh Salterton – a small estuary now, but larger and longer in the past. When the first edition of the Ordnance Survey was surveyed early in the nineteenth century, unreclaimed saltmarsh occupied much of the width of the valley for almost 2 km, and the river followed the parish boundary west of the embankment constraining the river to its present course. The present saltmarsh has grown up in the last 150 years. The first edition of the 25-inch map, surveyed in 1888, shows 6.26 ha of ‘saltings’ above high water mark of ordinary tides. By 1933 this had increased to 8.90 ha, and by 1955 to 10.45 ha. The map surveyed in 1970 gives the area of saltings as 12.13 ha, largely filling the estuary, and a recent satellite image shows little further change.
The lower-marsh species are frequently covered by the tide and are seldom exposed for more than a week at a stretch. The salinity around their roots (Fig. 288) thus mostly stays close to that of the flooding water – the central trend of the salinity curve of Figure 287. Salinity is much more variable in the upper marsh, subject both to seepage of fresh water and leaching by rain, and to concentration of the water of the high spring tides during spells of hot fine weather in summer. Hence the highest salinities are encountered between high water of neaps and high water of springs, and are seasonal and erratic. Five samples of the mud under Zostera angustifolia in the Exe estuary spanned a range of salinities of only 26–34 g per litre, with a median of 28. The common lower and middle-marsh species generally give median salinities of 25–30 g per litre, with maxima around or a little above the salinity of seawater. The few upper-saltmarsh species listed in Figure 288 all have lower median salinities, but red fescue (Festuca rubra) and sea plantain (Plantago maritima) stand out for the wide range of salinities they can tolerate. This can lead to the apparent paradox, in upper reaches of estuaries, of the frequently flooded lower levels being occupied by brackish-water species, while the more salt-tolerant species are confined to the higher parts of the zonation (Fig. 289).
FIG 288. Box plot of salinity at 10 cm depth under saltmarsh plant species in 39 1 m2 quadrats from the Otter estuary, Devon, with some comparative data from the Exe estuary. The line dividing each box is the median value. The dotted line shows the composition of average seawater. Data from Proctor (1980) and Brooks et al. (1988).
The variation in salinity of upper saltmarshes is one factor in their great diversity of species composition. The widespread Festuca rubra–Juncus gerardiiSM16 and Juncus maritimusSM18 communities are never very saline in their upper parts and intergrade without a break into non-saline pastures and meadowsMG6,11,13. In sites on clayey or compacted soils, subject to tidal flooding and often to a degree of disturbance, conditions can sometimes become very saline; this is the preferred habitat of reflexed saltmarsh-grass (Puccinellia distans) and lesser sea-spurrey (Fig. 281b) – both species that (with Danish scurvygrass, Cochlearia danica) have spread inland along the verges of salt-treated roads. Many of the common middle-and upper-saltmarsh species still occur in this communitySM23, including common saltmarsh-grass, sea-milkwort, sea-purslane, sea arrowgrass, sea plantain, as well as the very characteristic little hard-grass (Parapholis strigosa).
Where a saltmarsh abuts on a shingle ridge or dune, or an embankment or sea-wall, the back of the marsh is often marked by a strand-line community. Usually these involve one or other of two species of Elytrigia, sea couch (E. atherica) or common couch (E. repens). Much the commonest is dominated by sea couch, with frequent red fescue, sea-milkwort, spear-leaved orache (Atriplex prostrata) and a scatter of other common saltmarsh plantsSM24. In the north-Norfolk saltmarshes the rare shrubby sea-blite (Suaeda vera) is constant in a community of this kind, with sea couch and sea-purslaneSM25. This is related to a Suaeda vera–Limonium binervosum (rock sea-lavender) saltmarsh community from the same marshesSM21. Common couch dominates a comparable community, mainly on the west coastSM28. It is less tied to freely drained soils, and often subject to some disturbance and deposition of drift-line debris. Sea couch, red fescue, creeping bent and spear-leaved orache are constant or nearly so, often accompanied by silverweed, parsley water-dropwort, curled dock (Rumex crispus), perennial sow-thistle (Sonchus arvensis) and creeping thistle (Cirsium arvense). Similar vegetation occurs in north Germany and Scandinavia.
FIG 289. Inverted zonation: saltmarsh grass, sea aster, sea plantain, and English scurvygrass above, sea club-rush nearest the river channel. River Exe, Topsham, Devon, May 1980.
The saline–freshwater transition: brackish habitats
Halophytes tend to be confined to the top of the tidal range near their upstream limits (e.g. sea aster, English scurvygrass), while fresh- and brackish-water species are confined to a narrow zone at the top of the tidal range near the estuary mouth, but extend down to lower levels farther upstream (e.g. sea club-rush, parsley water-dropwort). As already noted, this can lead to inversions of zonation from one part of an estuary to another. Thus sea club-rush is confined to freshwater seepage areas at the back of the more saline marshes (as in the Cefni marsh in Anglesey) but is the lowermost member of the zonation in brackish marshes.
Many species can grow in brackish habitats, but most of them have an alternative niche, either in saltmarsh or in non-saline habitats. Thus sea aster and sea plantain can grow at salinities a quarter of seawater or less, and common reed is primarily a freshwater plant, but has been found growing at a soil salinity more than half seawater strength in Poole Harbour. Parsley water-dropwort, distant sedge (Carex distans), false fox-sedge (Carex otrubae), grey club-rush (Schoenoplectus tabernaemontani) and brookweed (Fig. 300b) are other species often associated with salt-influenced habitats, but with inland freshwater habitats as well. And of course many common grassland species can grow in the less saline upper saltmarshes, including bird’s-foot trefoil (Lotus corniculatus), white clover (Trifolium repens), autumn hawkbit (Leontodon autumnalis), creeping thistle and silverweed.
Saltmarshes are fertile habitats, and estuaries tend to be hubs of human activity. Upper saltmarshes are embanked and enclosed as farmland, river channels are straightened and dredged for navigation, or for control of flooding upstream. All of these processes tend to sharpen the boundary between saline or brackish estuarine water and the freshwater drainage of the surrounding country. The species and communities of the transition are the most vulnerable to this process, and they come increasingly under pressure with improvements of the sea-walls and more sophisticated engineering of the drainage. Of the specialist brackish-water plants, the widely tolerant and versatile sea club-rush remains abundant. Other brackish-water specialists with narrower ecological niches have fared less well. Divided sedge (Carex divisa) and marsh-mallow (Althaea officinalis), both plants of brackish places, have declined over the past century and are now very local. The tasselweeds (Ruppia) are usually found in brackish ditches and pools, often behind sea-walls (see also Chapter 12). Their main habitat in the past may have been in shallow pools on mud-flats in the upper reaches of estuariesSM2, and R. maritima grows abundantly in this situation in the Cromarty Firth. It occurs in similar situations elsewhere, but not commonly. Triangular club-rush (Schoenoplectus triqueter) is a plant of mud-banks in brackish reaches of strongly flowing rivers, whose decline has certainly been due to development of the riverside. It now survives only in a single locality on the Tamar in southwest England, and by the Shannon and its tributaries in Ireland. A very different brackish-mud specialist is dwarf spike-rush (Eleocharis parvula). It occurs close to the upper tidal limit on firm estuarine mud, and in tidal pans on brackish grazing marshes. It has very few recorded localities widely scattered over England, Wales, Scotland and Northern Ireland, but it is so inconspicuous, and seems confined to so narrow an ecological niche, that it may well have been overlooked elsewhereSM3.