
I.Aortic Stenosis
A.Introduction. Aortic stenosis (AS) causes progressive obstruction of the left ventricular outflow tract (LVOT), resulting in pressure hypertrophy of the left ventricle and the classic symptoms of heart failure, syncope, and angina pectoris. Stenosis most commonly occurs at the level of the valve; however, subaortic stenosis and supravalvular stenosis are also well-defined entities. Untreated AS is associated with significant morbidity and mortality. As prompt recognition and treatment are associated with improved life expectancy in those patients with symptomatic severe AS, careful evaluation and management can have a significant impact on survival.
B.Etiology
1.Valvular AS has several causes, including congenital, rheumatic, bicuspid, and most commonly inflammation with resultant calcification.
a.The most common cause of AS in the United States is calcific degeneration. Although initially thought to be the result of normal “wear and tear” of the valve leaflets, there is now ample evidence that suggests that the progression of stenosis is related to an active process of inflammation involving the renin–angiotensin system, lipid accumulation, and resultant calcification. Several inflammatory pathways are implicated, including those that utilize osteopontin, bone morphogenic proteins, and receptor activator of nuclear factor-κB ligand. Recent studies suggest that those with excess lipoprotein (a) are at greater risk of aortic valve calcification and progression to stenosis.
Aortic sclerosis is caused by calcification and thickening of the aortic valve without the increased gradients seen in AS. Both aortic sclerosis and calcific AS have been associated with traditional risk factors for atherosclerosis, such as smoking, hypertension, and hyperlipidemia. Aortic sclerosis is associated with increased risk of cardiovascular death and myocardial infarction and can progress to AS. Other conditions associated with calcific AS include Paget disease and end-stage renal disease.
b.Bicuspid aortic valve (BAV) is the most common congenital heart defect with a prevalence estimated between 0.5% and 2% with a 3:1 male predominance. It is present in ~10% of first-degree relatives; thus, all first-degree relative of patients with BAV should undergo echocardiographic screening for BAV. The most common abnormality seen in bicuspid valves is fusion of the right and left coronary cusps (79.3%); whereas right and noncoronary cusps (19.4%) are less common, and least common is left and noncoronary cusp fusion (0.5%; Fig. 15.1). Concurrent dilation of the thoracic aorta occurs in as many as 50% of patients, while coarctation, aortic dissection, and coronary anomalies are seen in a minority of patients. BAV may be associated with Shone syndrome (in which multiple left-sided lesions of inflow/outflow obstruction occur), Williams syndrome with supravalvular stenosis, and Turner syndrome with coarctation. Severe AS usually develops by the fifth or sixth decade; however, earlier and later presentations are common.

FIGURE 15.1 A schematic representation of parasternal short axis of a congenitally abnormal aortic valve.
(1)Diagnosis. The mainstay of diagnosis is echocardiography (transthoracic or transesophageal if transthoracic imaging is suboptimal; 92% sensitivity and 96% specificity if adequate images are obtained). The diagnosis is made during systole in the short-axis view and classically the valve opens as an oval rather than as a triangle in normal people. In situations when echocardiography is nondiagnostic, cardiac magnetic resonance imaging (MRI) and computed tomography (CT) can be used to improve the diagnostic accuracy (ACC/AHA class I indication).
(2)Complications
(a)Infective endocarditis. The lifetime risk of infective endocarditis (IE) in the current era is 3%. On the basis of these data, prophylactic antibiotic therapy for dental procedures in isolated BAV is no longer recommended except in patients with prior IE. Unfortunately, when IE does occur in BAVs, it is associated with a higher incidence of perivalvular abscess and worse outcomes compared with tricuspid aortic valves.
(b)Aortopathy is present in ~50% of individuals with BAV and typically involves the aortic annulus, sinus, and proximal ascending aorta. In patients undergoing aortic valve replacement (AVR), 30% will require concomitant aortic root surgery. Per the 2014 ACC/AHA valve guidelines, aortic surgery is indicated when the aorta is ≥5.5 cm (class I) and there is no indication for valve surgery. However, if the patient is undergoing concurrent AVR and aorta is ≥4.5 cm concomitant aortic replacement is a class IIa indication. When the aorta is ≥5.0 cm and the rate of increase in diameter is ≥0.5 cm/y, this is considered a class IIa indication for aortic replacement. In addition, if the surgery is to be performed by an experienced aortic surgical team and the patient has a low surgical risk, it is reasonable to perform aortic surgery once the aorta is ≥5.0 cm. Increasingly, the impact of patient size on aorta size is being appreciated and therefore when the maximal ascending/aortic root area in square centimeter divided by patient’s height in meters exceeds 10, this is considered an indication for surgical intervention by some authorities. In addition, serial evaluation of the aortic sinuses and ascending aorta by echocardiography, cardiac MRI, or CT angiography is recommended in all patients with BAV and should be performed annually in patients with aortic diameter >4.0 cm (ACC/AHA class I).
c.Unicuspid aortic valve (UAV) is a rare valvular anomaly and is described as being either pinhole-shaped acommissural UAV (typically presents at birth) or slit-shaped unicommissural UAV (Fig. 15.1). Patients typically present for cardiac surgery in their 30s and UAV shares many of the features of BAV including risk of aortopathy, aortic dissection, IE, coronary artery anomalies, PDA, and coarctation.
d.Rheumatic AS often coexists with aortic regurgitation (AR) and mitral valve lesions, especially mitral stenosis. It is a rare cause of isolated severe AS in the industrialized world. Fusion of the commissures occurs, leaving a small central orifice.
2.Subvalvular AS is a congenital condition, although it may not be apparent at birth. Typically, a circumferential fibromuscular membrane involving the anterior mitral valve leaflet is present in the LVOT below the aortic valve. In more extreme cases, a tunnel-like obstruction may be present, rather than a discrete membrane. The pathogenesis of this condition is not perfectly understood but is thought to represent a maladaptive response to abnormal flow dynamics in the LVOT. It may exist with other left-sided obstruction lesions, such as coarctation or as part of Shone syndrome. The condition may recur even after successful membrane resection. Subvalvular AS may be difficult to distinguish from hypertrophic cardiomyopathy, especially when secondary left ventricular hypertrophy (LVH) is pronounced.
3.Supravalvular AS is uncommon and may occur as part of a congenital syndrome such as Williams syndrome in which a mutation in the elastin gene occurs. Characteristic features of Williams syndrome include hypercalcemia, elfin facies, developmental delay, small stature, and multiple stenoses in the aortic and peripheral arteries. Lipid deposits in severe forms of familial hypercholesterolemia may also cause obstruction above the valve in the ascending aorta.
C.Pathophysiology
1.Pressure overload. All forms of AS are characterized by progressive narrowing of the LVOT. To maintain cardiac output in the face of increased afterload, the left ventricle must generate higher systolic pressures, which increases LV wall stress. In response to the pressure overload and increased wall stress, the left ventricle undergoes compensatory concentric hypertrophy. The increase in LV wall thickness allows the wall stress to normalize according to Laplace’s law: wall stress = (pressure × radius)/(2 × thickness). Eventually, the LV is unable to adequately compensate for the pressure overload and LV dilatation and systolic dysfunction ensue (Fig. 15.2).
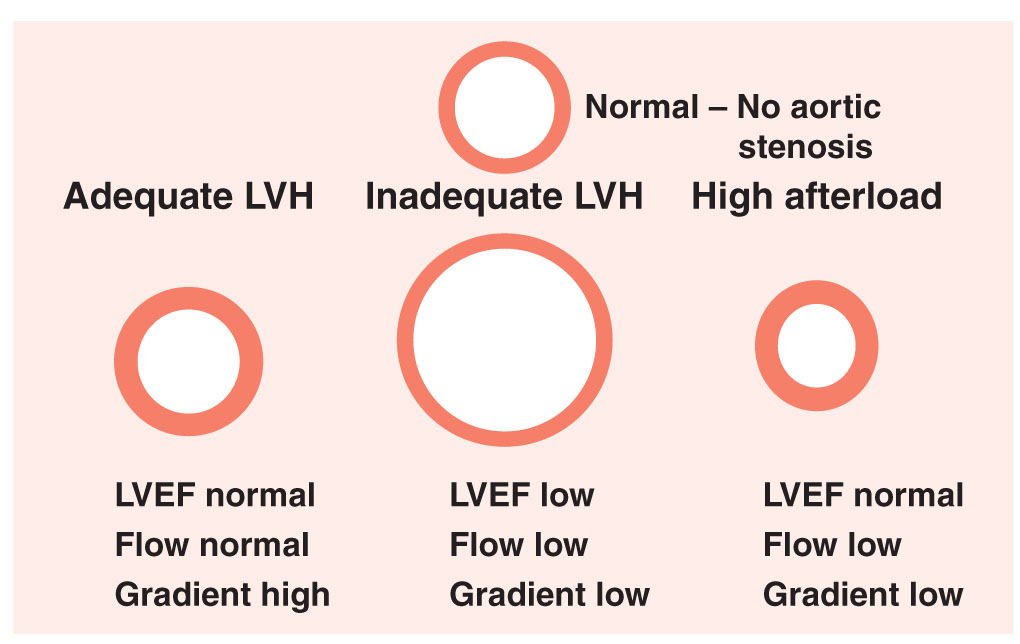
FIGURE 15.2 Aortic stenosis: left ventricular compensatory response. LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy.
2.Diastolic dysfunction. LV diastolic function is determined by LV relaxation properties and LV compliance (i.e., change in volume with change in pressure [dV/dP]). Increased afterload and LVH lead to a reduction in LV compliance. Furthermore, there are changes in strain and torsion characteristics of the left ventricle in AS. Passive early diastolic filling is reduced, and maintenance of an adequate LV preload becomes more dependent on active left atrial contraction.
3.Supply–demand mismatch. Myocardial oxygen is determined by heart rate, contractility, and myocardial wall stress imposed on the left ventricle by progressive pressure overload. As the AS becomes more severe, wall stress and myocardial oxygen demand increase in parallel. Concurrently, AS is associated with a decrease in myocardial oxygen supply. Progressive LVH and diastolic dysfunction lead to an elevation in left ventricular end-diastolic pressure (LVEDP). Elevated LVEDP leads to decreased perfusion pressure across the coronary bed and causes endocardial compression of small intramyocardial arteries, impairing coronary flow reserve. The imbalance between myocardial oxygen supply and demand can precipitate ischemia during exertion even in the absence of significant obstructive coronary disease.
D.Natural history. The classic survival curve for patients with untreated AS, as described by Ross and Braunwald, is shown in Figure 15.3.

FIGURE 15.3 Patient survival in aortic stenosis.
1.Asymptomatic patients
a.The disease process of AS is characterized by a long latent phase, during which the patient has no symptoms. This period is associated with near-normal survival. The risk of sudden cardiac death in asymptomatic patients with critical AS is <2% per year.
b.Although the underlying cause helps predict the age of symptom onset, there is marked individual variability in the length of the latent period and the subsequent rate of progression of disease. In general, among asymptomatic valvular AS patients, the mean aortic valve gradient rises by 7 mm Hg/y, peak transvalvular velocity increases by 0.1 to 0.3 m/s/y, and aortic valve area (AVA) decreases by 0.1 cm2/y.
c.Because of the variable rate of disease progression, all patients with AS should be advised to report the onset of any symptoms to their physician and should be followed clinically and with Doppler echocardiography with increasing frequency as the lesion progresses.
(1)Frequency of echocardiograms per 2014 ACC/AHA guidelines
(a)Mild—Every 3 to 5 years
(b)Moderate—Every 1 to 2 years
(c)Severe—Every 6 to 12 months
d.Once the valve becomes severely stenotic, as evidenced by a peak Doppler velocity of 4 m/s, the likelihood of development of symptoms or requiring surgical intervention over the following 2 years is very high. This likelihood is increased if the valve is heavily calcified.
2.Symptomatic patients. When symptoms of AS develop, the survival rate decreases markedly, unless AVR is performed.
a.Patients with angina have a 50%, 5-year survival rate without surgical intervention. Those with syncope have a 50%, 3-year survival rate without surgical intervention. Patients with heart failure have a mean survival time of <2 years if treated medically.
b.In patients with severe, symptomatic AS, sudden cardiac death can occur in the setting of hypotension or arrhythmia due to ischemia, LVH, or impaired LV function. Resuscitation in this situation is difficult because of the difficulty of obtaining adequate transmural LV perfusion and cardiac output.
c.Signs and symptoms of severe AS may be subtle in some patients. Since AS is a slowly progressive disease, patients may subconsciously adapt their activities and thus remain “asymptomatic.” Information concerning activity levels from family members may be useful in this situation, as may a symptom-limited stress echocardiogram to quantify the patient’s functional capacity. Exercise stress testing is absolutely contraindicated in the setting of definite symptoms (class III).
E.Clinical manifestations
1.Signs and symptoms. The onset of symptoms usually indicates progression to severe AS and heralds the need for surgical evaluation.
a.Angina. Patients with severe AS can experience ischemia from myocardial supply–demand mismatch due to high LV diastolic pressures, decreased myocardial perfusion, and increased wall stress. Angina can also result from underlying coronary artery disease (CAD). CAD is common among patients with severe AS and occurs in 40% to 80% of patients with angina and 25% of patients without angina.
b.Syncope. Because of a fixed LVOT obstruction, patients with severe AS are unable to augment their cardiac output under conditions of low systemic vascular resistance (SVR) (i.e., induced by certain medications or vasovagal reactions). The ensuing hypotension can cause presyncope, syncope, or even cardiovascular collapse and death. Syncope can also result from atrial or ventricular arrhythmias, abnormal baroreceptor function, or abnormal vasodepressor responses induced by LV pressure overload.
c.Heart failure symptoms, such as exertional dyspnea, orthopnea, or paroxysmal nocturnal dyspnea, and fatigue, may result from LV systolic or diastolic dysfunction.
2.Physical findings
a.Arterial examination. A hallmark finding in AS is a diminished and delayed carotid upstroke, pulsus parvus et tardus. However, elderly patients with noncompliant vessels or patients with concomitant AR may often maintain a normal carotid pulsation, despite severe AS. These findings are rare with obstruction above or below the valve. It is classically thought that severe AS is not associated with hypertension, as the narrowed valve limits the flow into the arterial system and thus gives rise to a narrowed pulse pressure and relative hypotension. In fact, in the elderly, hypertension and severe AS may often coexist, likely as a result of impaired elasticity of the aortic walls, and the finding of arterial hypertension does not preclude significant associated AS.
b.Palpation. With LVH and normal LV cavity dimensions, the apical impulse is usually nondisplaced, diffuse, and sustained. However, the apical impulse may later be displaced when there is LV systolic dysfunction. A double apical impulse represents a palpable a wave or S4, caused by a noncompliant left ventricle. A systolic thrill may be palpable in the second right intercostal space.
c.Auscultation. The main auscultatory findings are shown in Figure 15.4.
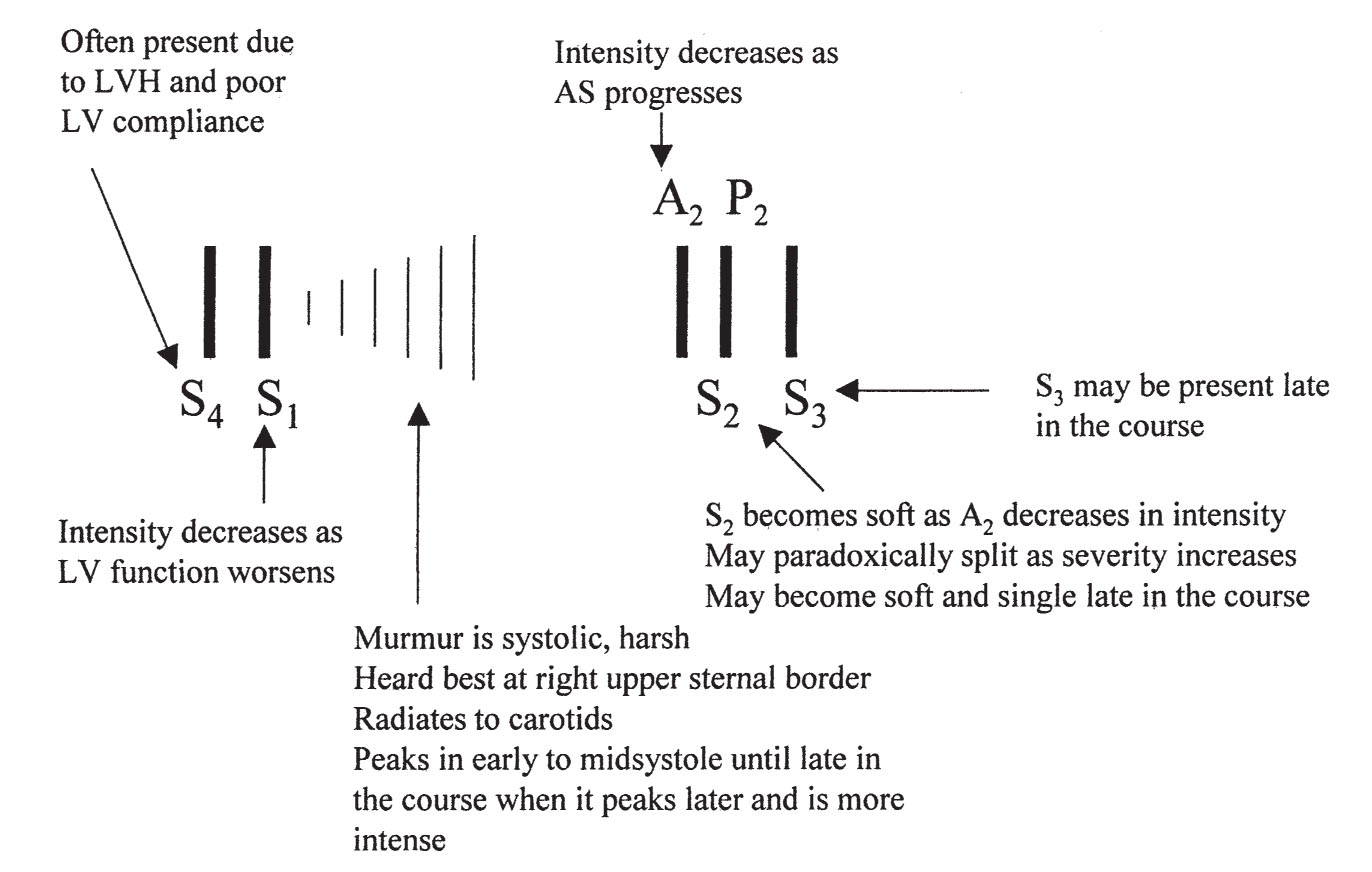
FIGURE 15.4 Auscultatory findings in aortic stenosis. LVH, left ventricular hypertrophy; LV, left ventricle.
(1)The typical murmur of AS is a systolic ejection murmur heard at the right upper sternal border that radiates to the neck. With a mobile bicuspid valve, an aortic opening sound or click may precede the murmur. As the severity of the stenosis increases, the murmur becomes longer and peaks later in systole. The intensity of the murmur does not necessarily correspond to the severity of AS. S1 is usually normal in AS. As the AS becomes more severe, the aortic component of S2 diminishes and eventually disappears, resulting in a soft, single S2. Often, with severe AS, S2 is paradoxically split because of the prolonged ejection duration through the severely narrowed valve. S3 is indicative of poor LV systolic function. An S4 is common because of reduced LV compliance.
(2)Careful examination for other murmurs should be performed. AS is often accompanied by AR. Maneuvers performed during the physical examination can help differentiate different types of LV outflow obstruction, whether this is at, below, or above the valve. These are summarized in Table 15.1.
TABLE 15.1 Physical Findings and Maneuvers Useful in Distinguishing Various Forms of Left Ventricle Outflow Tract Obstruction |
||||
Maneuver Finding |
Valvular |
Supravalvular |
Subvalvular |
Hypertrophic Cardiomyopathy |
Pulse volume after PVC |
Increases |
Increases |
Increases |
Decreases |
Valsalva effect on systolic murmur |
Decreases |
Decreases |
Decreases |
Increases |
AR |
Common |
Rare |
Common |
Rare |
S4 |
Common |
Uncommon |
Uncommon |
Common |
Carotid pulse |
Normal to anacrotic (parvus et tardus) |
Unequal |
Normal to anacrotic |
Rapid jerky upstroke |
Abbreviations: AR, aortic regurgitation; PVC, premature ventricular contraction.
3.Diagnostic testing
a.The typical electrocardiogram (ECG) of a patient with isolated severe AS usually demonstrates left atrial abnormality (80% of cases) and LVH (85% of cases) but a normal electrocardiogram does not exclude severe AS.
b.Chest radiography can be entirely normal, even in patients with critical LVH. The cardiac silhouette may become boot-shaped because of the concentric LVH. Cardiomegaly may be identified if there is LV dysfunction or coexisting AR. Aortic valve and root calcification can be seen in adults with severe calcific, degenerative AS. Poststenotic dilation of the ascending aorta may also be evident.
c.Natriuretic peptides are released in response to pressure overload of the LV. There is increasing evidence that supranormal levels of these peptides may indicate early decompensation of the LV even in the presence of normal left ventricular ejection fraction (LVEF). Anemia commonly coexists with AS in the elderly who appear to be at increased risk of dysplastic vascular lesions in the bowel, which may bleed. Increased risk of bleeding may also occur because of disruption of Von Willebrand molecules with turbulent flow at the stenotic aortic valve (Heyde disease). Rarely, AS is so severe and turbulent to lead to intravascular hemolysis in a native valve.
4.Severity of AS. The severity of AS as defined by the 2014 ACC/AHA valve guidelines is summarized in Table 15.2. A normal aortic valve opens 3 to 4 cm2.
TABLE 15.2 Aortic Stenosis Severity Parameters |
||||
Valve Severity |
AVA (cm2) |
Mean Gradient (mm Hg) |
Jet Velocity (Vmax) (m/s) |
Miscellaneous |
Mild |
>1.5 |
<20 |
2.0–2.9 |
|
Moderate |
1.0–1.5 |
20–30 |
3.0–3.9 |
|
Severe |
0.6–1.0 |
≥40 |
≥4.0 |
|
Very severe |
<0.6 |
≥60 |
≥5 |
|
LFLG severe |
<1.0 |
<40 |
<4 |
LVEF < 50%; DSE increases AVAa by <0.3 and <1.0 cm2 |
Pseudosevere |
<1.0 |
<40 |
<4 |
LVEF < 50%; DSE increases AVAa by >0.3 cm2 |
Paradoxical LFLG |
<1.0 |
<40 |
<4 |
LVEF ≥ 50%; stroke volume index < 35 mL/m2 DSE increases AVA <0.3 and <1.0 cm2 |
aDependent on the presence of contractile reserve (20% increase in stroke volume with DSE).
Abbreviations: AVA, aortic valve area; DSE, dobutamine stress echocardiography; LFLG, low-flow/low-gradient; LVEF, left ventricular ejection fraction; Vmax, aortic valve maximum velocity.
a.Transthoracic Doppler echocardiography is the test of choice to establish the diagnosis of AS, to determine the cause and location, and to assess its severity. It should be performed when the diagnosis of AS is first suspected and information such as LV wall thickness, size, and function should also be obtained. After the diagnosis is established, patients should have frequent regular clinical follow-up examinations to look for the development of symptoms. Development of new symptoms and signs should quickly prompt a repeat evaluation.
(1)The parasternal long-axis views, two-dimensional and M-mode, provide valuable information for determining the mechanism and severity of AS. In this view, the coaptation line of the aortic valve is normally centered within the LVOT in a trileaflet valve. The leaflets of a bicuspid valve often have an eccentric closure line, typically posterior to the midline. Systolic leaflet doming can be seen in congenital AS and rheumatic AS. The degree of LVH, chamber enlargement, or left atrial enlargement can be quantitated using two-dimensional and M-mode imaging. The LVOT diameter used in the continuity equation is measured in the two-dimensional, parasternal long-axis view.
Subaortic stenosis and supravalvular AS may also be detected in this view. Subaortic stenosis may be evident as a membrane below the aortic valve with normal opening of the valve. Pulsed Doppler may indicate that the obstruction is occurring below the valve, and two-dimensional echocardiogram often shows AR because of the turbulent jet hitting the aortic valve leaflets and causing leaflet scarring and impaired coaptation. In supravalvular AS, narrowing above the valve is evident on imaging.
(2)The parasternal short-axis view is the most useful view for establishing the cause of congenital AS. The number of commissures and the shape of the valve orifice should be assessed (Fig. 15.1). In diastole, the valve appears as three commissures and three cusps of equal size creating an inverted Y configuration or “Mercedes-Benz sign.” However, this view alone should not be used to determine the anatomic morphology of the valve because in “functionally” bicuspid valves from commissural fusion, a raphe is present creating the appearance of a trileaflet valve. Thus a systolic image should be used to evaluate whether there is a triangular opening (trileaflet) or ellipsoid appearance (bicuspid valve). In UAV, the opening is elliptical but occurs across a radius rather than the diameter of the valve.
(3)The apical five-chamber and three-chamber views are well aligned with flow through the aortic valve. Continuous wave Doppler recordings across the aortic valve and pulsed wave Doppler flow in the LVOT proximal to the aortic valve are recorded in these views for the continuity equation.
(4)Continuous wave Doppler should be performed at multiple sites, including the suprasternal notch and the right sternal border, to ensure that the maximal velocity across the aortic valve is recorded. Failure to obtain the maximum velocities will result in underestimation of the severity of AS. The dimensions of the ascending aorta should be sought, especially in those with BAV.
(5)Newer technologies such as strain imaging are increasingly being employed in the assessment of ventricular function especially in those with normal LVEF. There is an increasing body of evidence that global myocardial strain may be abnormal before the LVEF deteriorates and may of itself be an indicator of higher long-term risk.
b.Transesophageal echocardiography (TEE). Planimetry of the aortic valve orifice is often possible with TEE and relates well to that measured by cardiac catheterization. Planimetry is difficult when the valve is extremely calcified. In a bicuspid valve, the smallest area should be sought carefully, as the valve opening in not planar but rather forms a cone because of the doming of the valve as it opens. TEE is particularly useful for determining the morphologic features of the valve in congenital AS. TEE is often necessary to confirm the diagnosis of subaortic membrane and to differentiate it from hypertrophic cardiomyopathy or valvular AS.
c.Dobutamine stress echocardiography (DSE) and exercise stress echocardiography (ESE). When discrepancy exists in regard to the severity of valve disease and symptoms, exercise stress testing can be helpful in objectively quantifying their functional capacity and appropriate classification of their valve disease. In patients with asymptomatic severe AS, two-thirds of ESEs are abnormal including one-third being attributed to development of symptoms alone. In addition, a normal test provides reassurance with an excellent 1-year prognosis with continued medical management and monitoring for the development of symptoms.
Low-dose DSE is also helpful in appropriately classifying the low-flow, low-gradient AS patients into true severe AS, pseudosevere AS, or paradoxical severe AS.
d.Other imaging modalities. CT scanning is increasingly used in the assessment of AS patients particularly those in whom transcatheter aortic valve replacement (TAVR) is a consideration to determine appropriate valve and peripheral vessel sizing and to avoid complications such as coronary impingement, aortic size and calcification, and other comorbidities. When there is doubt about severity of AS, the degree of calcification of the aortic valve by CT imaging may provide useful information. MRI is helpful in patients with congenital forms of AS associated with other anomalies and in assessment of the aorta or concomitant AR.
6.Hemodynamic calculations
a.Doppler echocardiography is the standard modality used for the assessment of transvalvular pressure gradients and AVA.
(1)Simplified Bernoulli equation (ΔP = 4 v2), in which P is pressure and v is peak velocity of flow across the aortic valve, is used to estimate the peak instantaneous gradient. The mean gradient across the valve can be determined by measuring the area under the Doppler envelope. The peak velocity of flow across the aortic valve should be measured in three areas: the LV apex, the right sternal border, and the suprasternal notch. The highest measured velocity is used to calculate the peak transvalvular gradient. When stenosis is present at two levels (i.e., in LVOT and at the valve), the gradient across the LVOT reflects the integrated effects of the obstruction at both levels. It is usually impossible with Doppler to precisely differentiate the contribution of each level of obstruction to the total. This may be inferred by analysis of the images, by TEE, or by direct measurement by cardiac catheterization.
(2)Calculation of AVA based on the continuity principle, which states that flow of an incompressible fluid in a closed system must remain constant. Flow in a vessel is the product of the cross-sectional area (A) of the vessel and the velocity (V). Area is calculated as πR2 or πD2/4 = 0.785D2, where R is the radius of the vessel and D is the diameter. A schematic representation of the variables for calculating AVA is shown in Figure 15.5. The continuity equation for the aortic valve is as follows:
Areaaortic valve = diameterLVOT2 × 0.785 × VTILVOT/VTIaortic valve
In the above equation, VTI is the time–velocity integral. The continuity equation is valid only for valvular AS. It cannot be used to assess valve area when there are stenoses in series such as valvular and subvalvular narrowing occurring simultaneously.
(3)Care should be taken to avoid measuring post-extrasystolic beats. If the patient is in atrial fibrillation, ideally 10 consecutive beats should be measured and averaged for both velocity measurements.
(4)During evaluation of an aortic valve prosthesis, the standard continuity equation cannot be used. Instead, the velocity ratio or dimensionless index is used to estimate the severity of prosthetic stenosis. It is calculated by dividing the peak velocity in the LVOT by the peak velocity through the aortic valve. A dimensionless index of <0.25 is generally accepted to represent severe stenosis. This is also useful if the LVOT diameter is difficult to ascertain.

FIGURE 15.5 A schematic representation of parasternal long-axis view and the continuity principle. LA, left atrium; LV, left ventricle.
b.Cardiac catheterization was once considered the gold standard for the quantification of AS but is now only indicated in symptomatic patients when there is discrepancy between physical examination and noninvasive testing or when noninvasive tests are inconclusive in regard to the severity of the valve lesion (ACC/AHA class I indication).
(1)Preoperative left heart catheterization is indicated before valve intervention in patients with symptoms of angina, objective evidence of ischemia, decreased LV systolic function, history of CAD, or coronary risk factors (including men >40 years old and postmenopausal women) (ACC/AHA class I indication).
(2)Catheter-derived hemodynamics often differ from those of echocardiography and thus it is important to understand the differences in what is being measured. The mean gradients obtained during catheterization should be equivalent to the mean gradients obtained by echocardiography. These correlate well when performed expertly and simultaneously. The peak gradient measured during catheterization is the peak-to-peak gradient, which is lower than the peak instantaneous gradient obtained with echocardiography (Fig. 15.6). In the setting of reduced cardiac output of any cause, the aortic gradient may be lower and may be <20 mm Hg in severe LV dysfunction despite critical AS.
(3)The most precise measurement of transaortic valvular gradient is made with two different catheters (one in the LV cavity and the other in the ascending aorta or with a double lumen pigtail catheter). A less optimal method is measuring the peak-to-peak gradient by catheter pullback from the left ventricle to the ascending aorta. A typical pressure tracing of simultaneous LV and aortic pressures is shown in Figure 15.6.
(4)Catheterization of the patient with severe AS should be performed with low-osmolar, nonionic contrast agents. These cause less hypotension because of peripheral arterial vasodilation, less bradycardia, less transient myocardial dysfunction, and less osmotic diuresis after the procedure. Left ventriculography should be avoided.
(5)The Gorlin formula is used to estimate the AVA:
AVA (cm2) = cardiac output/(44.3 × heart rate × SEP × √MVG)
In the above equation, SEP is the systolic ejection period, defined as the time from aortic valve opening to closing (in seconds), and MVG is the mean valvular gradient (mm Hg). The Gorlin formula measures the true anatomic area of the aortic valve, as it has a correction factor (the discharge coefficient) to account for the difference of flow across the true anatomic valve versus the flow at the level of the vena contracta. The continuity equation measures the physiologic area (vena contracta) and as such is smaller than that measured by Gorlin.

FIGURE 15.6 Simultaneous recording of LV and aortic pressures. AO, aorta; LV, left ventricle.
An alternative measurement can be made by the Hakki equation, which is a simplification of the Gorlin formula, where the observation of heart rate × SEP approximates 1,000. This then simplifies the estimation of AVA:
AVA (cm2) = cardiac output/√MVG
F.Therapy
1.Medical therapy. The mainstay of therapy for AS is replacement of the aortic valve. Onset of symptoms in patients with severe AS is associated with a marked reduction in lifespan when treated medically, rather than replacement. Medical therapy alone is ineffective for severe symptomatic valvular AS.
a.Antibiotic prophylaxis. The 2007 AHA guidelines for the Prevention of Infective Endocarditis do not recommend antibiotic prophylaxis before dental procedures with valvular pathology including BAVs unless the patient has a valve prosthesis or prior history of IE.
b.Medical therapy in patient at risk for developing AS and asymptomatic AS. Therapy should be directed at primary prevention of CAD, maintenance of sinus rhythm, and blood pressure control. Patient with hypertension should be treated according to guideline-directed medical therapy and started at a low dose, and slowly titrated upward as needed with appropriate clinical monitoring (ACC/AHA class I). ACE-inhibitors are no longer contraindicated and are potentially advantageous through a reduction in LV fibrosis. Alternatively, diuretics should be avoided solely for the use as an anti-hypertensive if the LV size is small as this can result in a decrease in cardiac output.
c.Medical therapy in symptomatic patients. Medical therapies may be necessary in patients with symptomatic severe AS who are awaiting surgery or who are considered inoperable and require palliation. Therapy for heart failure is directed at relief of pulmonary congestion. This is usually achieved with cautious use of diuretics. Overly aggressive diuresis may cause hypotension if hypovolemia significant impairs cardiac output by diminishing preload. Nitrates may also cause hypotension and syncope by reducing preload and should be avoided or used with extreme caution. Thus, the management of symptomatic patients with AS and CAD is difficult, and urgent surgery is the optimal treatment where feasible. Digoxin is used for symptom relief in the setting of impaired LV systolic function and volume overload, particularly if atrial fibrillation develops.
d.Vasodilator therapy has been formerly relatively contraindicated in patients with AS because of the concern about lowering SVR in the setting of a fixed cardiac output causing syncope, especially in the ambulatory setting. However, patients with severe heart failure and LV dysfunction with severe AS may benefit from the careful titration of intravenous nitroprusside in the intensive care unit with concomitant invasive arterial and pulmonary artery catheter monitoring (ACC/AHA class IIb). One study suggests an improvement in hemodynamic indices with this approach. Intra-aortic balloon counterpulsation is another strategy that can be used while patients with LV dysfunction and severe AS in cardiogenic shock are worked up toward urgent surgery. Asymptomatic patients who have been successfully treated with vasodilator therapy for hypertension over a period of years do not necessarily need to have this adjusted on diagnosis of AS unless there is evidence of significant hypotension.
e.Treatment of hyperlipidemia. The association between AS and risk factors for atherosclerosis has prompted trials with statins to retard the progression of AS. Several studies suggested that when statin therapy is indicated on the basis of current guidelines for hyperlipidemia, it is associated with a modest effect in slowing the rate of progression of AS. However, in more recent randomized controlled trials of patients with calcific AS, where statin therapy was not otherwise mandated, statins had little effect on AS progression or need for AVR. As such, statin therapy is not indicated for prevention of progression of calcific AS in patients with mild-to-moderate disease (ACC/AHA class III). However, aggressive low-density lipoprotein (LDL) lowering with statins appears warranted in patients who have an indication for statin therapy. In patients with supravalvular AS due to severe familial hyperlipidemia, improvement in the obstruction may occur after LDL apheresis or aggressive lipid lowering by other means.
2.Percutaneous aortic balloon valvuloplasty (PABV) is a procedure involving inflation of a balloon across a stenotic aortic valve resulting in an increase in the AVA and cardiac output. Because of the risk of worsening AR, this procedure is contraindicated in patient with concomitant moderate or severe AR. With the advancement in percutaneous AVR, the usage of PABV has increased significantly as a bridge to valve replacement.
a.In pediatric congenital, noncalcific AS, PABV is a safe and effective therapy comparable to surgical repair or replacement. The goal of PABV in congenital AS is to achieve a 60% to 70% reduction in measured peak-to-peak transvalvular gradient. Redilatation or AVR becomes necessary within 10 years of the initial PABV in >50% of children. AR is well recognized as a potential early or late complication of PABV, although moderate-to-severe AR only occurs in a minority of cases.
b.In adults, PABV has limited utility and long-term effectiveness while also being associated with substantial risks. On average, the effective orifice area is increased by 0.44 cm2 with a decrease in mean aortic gradient of 24 mm Hg. However, the procedures effects are short lived with an ~50% restenosis rate at 5 months and 80% at 15 months. No survival benefit with PABV has been reported even in those with the largest increment in valve area. Complication rates are diminishing with improvements in technology and techniques, but there are still considerable risks of vascular complications (6.8%), respiratory failure (6.5%), and strokes (2.9%).
The general indications for PABV are (1) as a bridge to definite replacement with either TAVR or surgical AVR (SAVR; ACC/AHA class IIb); (2) to assess degree to symptomatology due to AS when the patient has other comorbidities (i.e., severe COPD) which may mimic the symptoms of AS; and (3) as a means of palliation for patients who are not candidates for TAVR/SVR. PABV is generally not recommended before moderate-risk elective noncardiac surgery in patients with asymptomatic severe AS. Such patients are better served with appropriate intraoperative and postoperative hemodynamic monitoring to avoid significant periprocedural hypotension (ACC/AHA class IIa).
3.TAVR. An exciting and evolving strategy is the placement of a stented bioprosthetic valve over the native aortic valve, either percutaneously from an arterial site (usually transfemoral, subclavian/axillary, or rarely carotid) or transapically from an incision made at the LV apex on the chest wall (usually via mini-sternotomy or anterior thoracotomy). The first TAVR was performed in 2002 by Cribier.
a.Indications. Patients with intermediate or high surgical risk being considered for TAVR should be evaluated by a multidisciplinary group including healthcare professionals in valvular heart disease, cardiac imaging, interventional cardiology, cardiac anesthesia, and cardiac surgery (ACC/AHA class I).
(1)Symptomatic severe AS, inoperable or prohibitive risk for SAVR. TAVR is recommended in these patients if predicted post-TAVR survival is greater than 12 months (ACC/AHA class I).
(2)Symptomatic severe AS with high surgical risk for SAVR. Either TAVR or surgical AVR is recommended on the basis of patient specifics and preferences (ACC/AHA class I).
(3)Symptomatic severe AS with intermediate surgical risk. TAVR is a reasonable alternative to surgical AVR (ACC/AHA class IIa). The safety and efficacy of TAVR in this group have shown noninferiority to surgical AVR (see Section I.F.3.c).
b.Valve types. Five percutaneous transcatheter aortic valves have received US Food and Drug Administration approval in the United States: Edwards Sapien XT and S3 valves; Medtronic CoreValve, CoreValve Evolut R, and CoreValve Evolut PRO. Two additional valves have received Conformité Européene (CE) approval in Europe and are investigation use only in the United States: Boston Scientific Lotus and St. Jude Portico valves. Specifics of these valves are displayed in Table 15.3.
TABLE 15.3 Current Available Transcatheter Aortic Valve Replacements Valves |
||||
Deployment |
Mechanism to Help Prevent Paravalvular Leak |
FDA Approval in the United States for Severe AS |
Sizes (mm) |
|
|
Boston Scientific |
||||
Lotus |
Controlled mechanical expanding |
Adaptive polycarbonate seal |
No. Limited to investigational use only in the United States |
23, 25, 27 |
|
Edwards Sapien |
||||
SAPIEN |
Balloon expandable |
Yes, but no longer available |
23, 26 |
|
SAPIEN XT |
Balloon expandable |
Inoperable, intermediate and high risk; valve-in-valve for AS/AR |
20, 23, 26, 29 |
|
SAPIENT 3 (S3) |
Balloon expandable |
Polyethylene terephthalate outer skirt |
Intermediate-, high-, or extreme-risk patients |
23, 26, 29 |
|
Medtronic |
||||
CoreValve |
Self-expanding |
High- or extreme-risk patientsa; valve-in-valve for AS/AR |
23, 26, 29, 31 |
|
CoreValve Evolut R |
Self-expanding |
Extended skirt |
High- or extreme-risk patientsa |
23, 26, 29 |
CoreValve Evolut PRO |
Self-expanding |
Porcine pericardial skirt |
High- or extreme-risk patientsa |
23, 26, 29 |
|
St. Jude |
||||
Portico |
Self-expanding |
Pericardial cuff |
No. Limited to investigational use only in the United States |
23, 25, 27, 29 |
Abbreviations: AR, aortic regurgitation; AS, aortic stenosis; FDA, US Food and Drug Administration.
aFDA approval for use in intermediate-risk patients is currently under review.
(1)Edward Sapien
(a)Sapien valve was a balloon-expandable bovine pericardial valve attached to a stainless steel stent with a polyethylene terephthalate fabric cuff. This valve is no longer available.
(b)XT is a balloon-expandable bovine pericardial valve attached to a stainless steel mesh frame with a polyester wrap.
(c)S3 is a balloon-expandable bovine pericardial valve with polyethylene terephthalate outer skirt to minimize the potential for paravalvular leak.
(2)Medtronic
(a)CoreValve is a self-expanding porcine pericardial valve attached to a flexible nitinol frame.
(b)CoreValve Evolut R is a self-expanding porcine pericardial valve attached to a nitinol frame with an extended skirt to minimize the potential for paravalvular leak.
(c)CoreValve Evolut PRO is a self-expanding, repositionable, supra-annular porcine pericardial valve with a porcine pericardial tissue wrap to minimize potential for paravalvular leak.
(3)Boston Scientific
(a)Lotus valve is a controlled mechanical expanding bovine pericardial valve on a nitinol frame with polycarbonate seal to minimize potential for paravalvular leak.
(4)St. Jude
(a)Portico valve is a self-expanding bovine pericardial valve on a nitinol frame with a porcine pericardial cuff within the stent to minimize the potential for paravalvular leak.
c.Outcomes. TAVR was developed to offer an alternative for patients with severe symptomatic AS who were not a candidate for open surgical replacement because of either an unacceptably high estimated surgical risk or where this is prohibited because of technical challenges (i.e., porcelain aorta, radiation heart disease). Outcomes data from recent studies are summarized below. However, it is always important to keep in mind that morbidity and mortality figures from clinical trials, although useful, should not replace knowledge of these risks for individual procedures at one’s own institution. When choosing between surgical AVR and TAVR consideration of surgical risk, comorbidities including severe CAD who may be best served with surgical AVR and coronary artery bypass grafting (CABG), and patient preference should be taken into account. In addition, there is a relative lack of data on the long-term durability of TAVRs compared with surgical AVR.
(1)TAVR versus medical therapy in inoperable patients. The Placement of Aortic Transcatheter Valves (PARTNER) cohort B trial was performed with the Edwards SAPIEN valve compared to medical therapy including PABV. The results of this study showed that the SAPIEN valve met noninferiority criteria with a significant reduction in mortality (20% absolute survival advantage) that persisted out to 5 years and a higher percentage of patients with NYHA class I or II symptoms at 1-, 2-, 3-, and 5-year follow-up. The Medtronic CoreValve underwent a prospective, nonrandomized trial, which showed favorable outcomes at both 1- and 2-year follow-up and with outcomes being driven by the patients underlying comorbid conditions rather than valve performance.
(2)TAVR in high-risk patients. PARTNER cohort A trial compared the SAPIEN valve (transapical or transfemoral approach) to surgical AVR in high-risk patients (mean STS-PROM 11.7%). The results showed similar mortality rates at 1 and 5 years with a stroke risk that was significantly higher at 1 year in TAVR but did not remain significant at 3 and 5 years. The CoreValve high-risk trial compared the CoreValve to surgical AVR showing noninferiority as well as superiority for 1- and 2-year mortality data based on prespecified criteria. The CoreValve had similar stroke risk at 1 year with a trend toward a lower stroke risk at 2 years compared with surgical AVR.
(3)TAVR in intermediate-risk patients. Three major trials have evaluated the outcomes of TAVR in an intermediate-risk population. The PARTNER IIA trial was a large randomized trial of over 2,000 patients comparing the SAPIEN XT valve to surgical AVR and showed similar rates for both 2-year mortality and risk of disabling stroke. The SAPIENT 3 valve was evaluated as an observational study via propensity score analysis with the surgical AVR population in the PARTNER IIA trial. These results showed that the SAPIENT 3 valve was noninferior and superior to surgical AVR for the primary composite endpoint at 1 year for all-cause mortality, disabling strokes, and moderate or severe AR. Last, the SURTAVI trial evaluated the combined results of the CoreValve (84%) and CoreValve Evolut R (16%) valves compared with surgical AVR and found it to be noninferior for the primary composite endpoint of all-cause mortality and disabling stroke at 2 years.
(4)BAVs. TAVR in BAVs is challenging because of association with a dilated aortic annulus, elliptical and eccentric opening complicating frame apposition, and increased risk of damage to the aorta. On the basis of registry data, BAV has been associated with a higher rate of moderate-to-severe paravalvular leak, which may be improved with the use of CT-guided valve sizing as compared with echocardiography.
(5)Prosthetic dysfunction treated with valve-in-valve. To date, no trial has evaluated valve-in-valve TAVR but a multinational registry has reported outcomes matched of 757 cases of valve-in-valve compared with native valve TAVR placed between November 2011 and September 2015. These results showed that valve-in-valve TAVR had superior safety outcomes including lower all-cause mortality (2.3% vs. 4.1%, p = 0.03), all-cause 1-year mortality (13.3% vs. 23.1%, p < 0.001), in-hospital stroke (0.4% vs. 2.1%, p = 0.002), stroke at 1 year (2.0% vs. 4.3%, p = 0.002), and lower rate of complication including major bleeding, vascular complication, and new-onset atrial fibrillation. Overall, the results show that valve-in-valve TAVR is a safe procedure and should be considered in inoperable patients and possibly in those with high surgical risk.
(6)Future TAVR trials include comparing the use of TAVR versus surgical AVR in patients with low surgical risk, asymptomatic severe AS (EARLY-TAVR), and moderate AS in patients with symptomatic heart failure (NYHA ≥ II) and reduced ejection fraction (LVEF < 50%; TAVR UNLOAD).
4.Surgical therapy. AVR is the surgical treatment of choice. It is preferred over repair because debridement of the aortic valve calcification often results in early postoperative AR from leaflet fibrosis and retraction, a process that progresses over time.
a.Recommendations for the use of surgical AVR in patients with AS according to the 2014 ACC/AHA valvular heart disease guidelines and 2017 Focused Update are given in Table 15.4. The major indications are severe AS with symptoms, when other cardiac surgery is needed, or LV systolic dysfunction develops as a result of severe AS.
TABLE 15.4 Recommendations for Aortic Valve Replacement in Patients with Aortic Stenosis |
Class I |
1.Symptomatic patients with severe AS 2.Asymptomatic patients with severe AS and LVEF < 50% 3.Either surgical AVR or TAVR is recommended for symptomatic severe AS if high surgical risk. 4.TAVR is recommended for symptomatic severe AS and deemed inoperable for surgical AVR if life expectancy >12 mo. 5.Severe AS and having heart surgery for another indication |
Class IIa |
1.Asymptomatic patients with very severe AS (aortic velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg) if low surgical risk 2.“Asymptomatic” severe AS patients with decreased exercise tolerance or drop in systolic blood pressure during exercise testing 3.Symptomatic patients with low-flow/low-gradient AS with LVEF < 50% and low-dose dobutamine stress echo showing aortic velocity >4.0 m/s or mean pressure gradients ≥40 mm Hg and AVA <1.0 cm2 4.Symptomatic patients with low-flow/low-gradient severe AS with LVEF ≥ 50%, systolic blood pressure < 140 mm Hg, and low stroke volume index (<35 mL/m2) if the valve disease is believed to be the most likely cause of symptoms 5.If intermediate surgical risk, TAVR is a reasonable alternative to surgical AVR for symptomatic severe AS 6.Moderate AS and having heart surgery for another indication |
Class IIb 1.Asymptomatic patients with severe AS, low surgical risk, and evidence of rapid disease progression (increase in aortic velocity ≥0.3 m/s/y) |
These recommendations apply to both surgical AVR and TAVR.
Abbreviations: AS, aortic stenosis; AVA, aortic valve area; AVR, aortic valve replacement; LVEF, left ventricular ejection fraction; TAVR, transcatheter aortic valve replacement.
Adapted with permission from Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57–e185; Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252–289. Copyright © 2017 by the American Heart Association, Inc., and the American College of Cardiology Foundation.
b.Surgical mortality rate varies among patients with AS, depending on age and other comorbidities including concomitant CAD. In an otherwise healthy individual, mortality rate for isolated AVR in experienced centers should be <1%. Successful AVR is feasible and improves life expectancy, even in very elderly patients without multiple comorbidities. Surgical options include pulmonary valve autograft (i.e., Ross procedure), aortic valve homograft conduit, a pericardial or porcine bioprosthesis, mechanical valve, or rarely surgical repair. The relative advantages, disadvantages, and indications for use of different prostheses are outlined in Chapter 18.
(1)In the Ross procedure, the pulmonary valve and main pulmonary artery are removed as a unit and placed in the aortic position with reimplantation of the coronary arteries. A pulmonary homograft is placed in the pulmonic position. This procedure is best suited for pediatric and adolescent patients with growth potential because the autograft is capable of growth, does not require anticoagulants, and has an excellent hemodynamic profile. The procedure, however, is long and technically difficult and subsequently turns a single-valve problem into a double-valve problem. Problems with pulmonary homograft are common in adults who underwent this operation as are subsequent dilatation of the aorta in those with aortopathy such as with bicuspid valves.
Aortic valve homografts have been used to treat younger patients, especially those who wish to avoid anticoagulation, in the hope that greater durability of this valve might result than with a bioprosthesis. Unfortunately, more recent data suggest that any durability advantage of a homograft over a bioprosthesis in a middle-aged patient is slight. Moreover, the homograft tends to calcify and is often difficult to remove at subsequent reoperations. Therefore, enthusiasm for homografts has waned, except in the setting of endocarditis of native or prosthetic valves with pyogenic complications such as abscess or fistula or when the LVOT is small, in which case homografts maximize the flow area and minimize the pressure gradient.
c.Bioprostheses include porcine heterografts and bovine pericardial prostheses. These valves are most often used to treat patient older than 60 years because structural deterioration is much slower in this age group compared with younger patients. These valves have a low risk for thromboembolism and do not necessitate long-term anticoagulation. Because of the sewing ring and struts, all prostheses, both mechanical and biologic, have a pressure gradient across them, even with normal function. The largest possible valve should be inserted to minimize this pressure gradient. The threshold to insert a bioprosthesis at a younger age continues to decline given the excellent quality of life these afford and with the developing innovation of valve-in-valve TAVR.
d.Mechanical valves. The most commonly used mechanical prostheses include the St. Jude, Onyx, and CarboMedics prostheses. These all require anticoagulation to minimize the risk of valve thrombosis and thromboembolism. These valves are durable if anticoagulation is maintained and careful antibiotic prophylaxis is used over the years. Mechanical valves are used with caution in older patients (>65 years) given the substantial increase in anticoagulation-related hemorrhage and resultant mortality in this population.
G.Special considerations
1.Management of asymptomatic patients with severe AS
a.High-risk patients. Most asymptomatic AS patients have low mortality and morbidity rates. A minority of asymptomatic patients, however, may die suddenly or have rapid progression of disease. These patients may benefit from AVR in the absence of symptoms. Accurate identification of such patients has been difficult. A transaortic flow velocity of >4 m/s predicts a 70% likelihood of needing an AVR within the subsequent 2 years, whereas a velocity of <3 m/s corresponds to a low likelihood (<15%) of needing an AVR in the subsequent 5 years. Patients with highly calcified valves and a rapid progression of disease (aortic velocity ≥ 0.3 m/s/y) or critical AS (aortic velocity ≥ 5.0 m/s or mean pressure gradient ≥ 60 mm Hg) may be considered for elective AVR if the transaortic flow velocity is >4 m/s. Other reasonable indications for AVR in patients with severe asymptomatic AS including LV dysfunction attributed to AS, exercise-induced hypotension, pulmonary hypertension (PASP > 60 mm Hg), a high likelihood of rapid progression, and before pregnancy. Additional high-risk features on exercise testing includes an increase in mean gradient ≥ 18 to 20 mm Hg with exercise, increase in PASP ≥ 60 mm Hg with exercise, lack of contractile reserve, and impaired functional capacity. Operating on an asymptomatic patient with severe AS is only reasonable when the surgical center performing the operation has a low mortality and morbidity for this procedure.
b.CABG and moderate AS. Studies suggest a benefit of concomitant AVR in patients undergoing CABG who have an AVA of <1.5 cm2. Although concomitant AVR increases the risk of the initial surgery, the need for reoperation is significantly lower in these patients, and this may provide a survival benefit.
2.Patients with AS and severely reduced ejection fraction. LV systolic dysfunction in patients with AS can result from afterload stress imposed on the left ventricle by the stenotic valve or from primary contractile dysfunction (e.g., results from other causes of cardiomyopathy; Fig. 15.2). When LV systolic dysfunction results primarily from afterload mismatch, AVR often results in improvement or normalization or LV function. In contrast, patients with primary contractile dysfunction have an overall poor prognosis and are unlikely to benefit from AVR. It is important to determine the cause of LV dysfunction in patients with severe AS for prognostic and therapeutic purposes. These patients should be considered in two groups: high transvalvular gradients (mean gradient > 40 mm Hg) and low transvalvular gradients (mean gradient < 30 mm Hg). The patients with low transvalvular gradients consist of three entities: true severe AS with systolic dysfunction, pseudosevere AS, and paradoxical AS with preserved systolic function with reduced stroke volume index due to marked LV hypertrophy and small LV size.
a.High transvalvular gradient. A high transvalvular gradient is a surrogate measure of high afterload mismatch. When the transvalvular gradient is substantial (e.g., mean gradient > 40 mm Hg), AVR can result in normalization of LV function and a relatively low operative mortality.
b.Low transvalvular gradient
(1)Low-flow, low-gradient severe AS. Patients with true anatomically severe AS (AVA < 1.0 cm2) and low transvalvular gradients (mean gradient < 30 mm Hg) have a very poor prognosis without surgery. Despite a substantial operative mortality, survival appears improved in those treated surgically compared with medical management, especially if they demonstrate contractile reserve when challenged with dobutamine. Contractile reserve is defined as the ability to increase in stroke volume by >20% from baseline.
DSE helps appropriately differentiate this subset of patients from pseudosevere AS. DSE evaluates the compliance of the valve to determine if the small AVA is secondary to a fixed AS with afterload mismatch (true severe AS) or secondary to myocardial dysfunction with inadequate stroke volume to appropriately open a mild-to-moderately stenotic valve (pseudosevere AS). When administered dobutamine, patients with true severe AS experience an increase in both cardiac output and transvalvular pressure gradient and the calculated valve area increases by <0.3 cm2 with the valve area remaining <1.0 cm2. These patients are typically older, have CAD and may have significant mitral regurgitation (MR) and pulmonary hypertension. Because of their comorbidities, TAVR is increasingly the procedure of choice when this is feasible.
(2)Pseudosevere AS is identified as patients with an inappropriately low AVA <1.0 cm2 arising secondary to impaired left ventricular function and submaximal opening of a mild-to-moderately stenotic valve. Dobutamine infusion will generate an increase in cardiac output without a significant increase in the transvalvular pressure gradient. As a result, the calculated AVA increases significantly (≥0.3 cm2) and the valve area increases to more than 1 cm2.
(3)Paradoxical AS results in low transvalvular gradients in the setting of preserved systolic function secondary to reduced stroke volume index (<35 mL/m2) that occurs in the setting of marked LV hypertrophy and resultant small LV size or increased afterload due to other reasons such as hypertension or reduced aorta compliance. These patients are typically elderly women with a history of hypertension. It is important to recognize this group of individuals also benefits from AVR once they become symptomatic. Low transvalvular gradients can also be seen in patients in which the peak aortic valve gradients are not accurately detected or there are errors in measurement. Careful evaluation of valve hemodynamics and valve anatomy is important to ensure that the valve is truly severely narrowed. Heavy calcification of the valve on echo or CT is a useful pointer to this. Dobutamine echocardiography may also be used in a manner similar to those patients with reduced LVEF to determine whether valve area remains low and valve gradients increase with higher cardiac output.
3.Subaortic stenosis. Surgical removal of the membrane leading to subaortic obstruction is indicated for symptomatic patients or for asymptomatic patients with a peak pressure gradient >50 mm Hg. In patients with peak pressure gradient <50 mm Hg, surgical intervention can also be considered if there is evidence of LV systolic dysfunction, concomitant moderate/severe AR, or a VSD. Surgery can also be considered in asymptomatic patients with peak gradient >30 mm Hg if they are planning to become pregnant or wishing to participate in competitive sports.
A.Introduction. AR can develop from primary disease of the valve leaflets or from abnormalities of the aortic root or ascending aorta. The chronic and acute forms of AR are distinct disease entities, with different causes, clinical presentations, natural histories, and treatment strategies.
B.Etiology
1.Chronic AR. Disease of the valve leaflets can cause AR by inadequate leaflet coaptation, leaflet perforation, or leaflet prolapse. The most common causes of leaflet abnormalities and aortic root abnormalities that lead to the gradual development of AR are given in Table 15.5. Subaortic stenosis can also cause AR because of a high-velocity jet of blood that is a result of the outflow obstruction hitting the aortic valve, causing damage to the leaflets. Perimembranous ventricular septal defects are associated with AR as well. In addition to disease of native valve leaflets, structural deterioration of bioprosthetic or homograft valve leaflets are an important cause of chronic AR.
TABLE 15.5 Major Causes of Chronic Aortic Regurgitation |
|
Leaflet Abnormalities |
Aortic Root or Ascending Aorta Abnormalities |
Rheumatic fever |
Age-related aortic dilation |
Infective endocarditis |
Annuloaortic ectasia |
Trauma |
Cystic medial necrosis of the aorta (isolated biscuspid aortic valve or Marfan syndrome) |
Myxomatous degeneration |
Systemic hypertension |
Congenital aortic regurgitation |
Aortitis (syphilis and giant cell arteritis) |
Systemic lupus erythematosus |
Reiter syndrome |
Rheumatoid arthritis |
Ankylosing spondylitis |
Ankylosing spondylitis |
Behçet syndrome |
Takayasu arteritis |
Psoriatic arthritis |
Whipple disease |
Osteogenesis imperfecta |
Crohn disease |
Relapsing polychondritis |
Drug-induced valvulopathy |
Ehlers–Danlos syndrome |
2.Acute AR. Acute AR can also result from abnormalities in the valve leaflets or in the aortic root. The causes of acute AR are limited (Table 15.6).
TABLE 15.6 Major Causes of Acute Aortic Regurgitation |
|
Leaflet Abnormalities |
Aortic Root or Ascending Aorta Abnormalities |
Acute infective endocarditis |
Acute aortic dissection |
Traumatic rupture of the valve |
Traumatic injury to aortic root |
Acute prosthetic valve dysfunction |
|
Post aortic balloon valvuloplasty |
|
Perivalvular leak or dehiscence of prosthetic valves |
|
C.Pathophysiology
1.Chronic AR. AR results in diastolic regurgitation of LV stroke volume. This produces an increase in LV end-diastolic volume, thereby raising wall tension (i.e., Laplace’s law). The ventricle responds to added wall tension by compensatory eccentric hypertrophy of myocytes. As a result, during the chronic compensated phase of AR, the left ventricle is able to adapt to an increase in diastolic volume without a significant increase in end-diastolic pressure. The left ventricle produces a larger total stroke volume with each contraction, preserving normal effective forward stroke volume. Over time, however, progressive interstitial fibrosis reduces LV compliance, leading to the chronic decompensated phase. Chronic volume overload results in impaired LV emptying, an increase in LV end-systolic volume and end-diastolic pressure, further cardiac dilation, and a fall in the ejection fraction and forward cardiac output.
2.Acute AR. Acute AR is usually a hemodynamic emergency because the left ventricle does not have sufficient time to adapt to the rapid increase in LV volume. The effective forward stroke volume and cardiac output fall acutely, potentially resulting in hypotension and cardiogenic shock. The sudden increase in LV diastolic pressure initially causes preclosure of the mitral valve in early diastole, protecting the pulmonary vasculature from elevated diastolic pressure. However, further LV decompensation leads to diastolic MR, which allows transmission of elevated diastolic pressure to the pulmonary vascular bed, resulting in pulmonary edema. The tachycardia that accompanies cardiac deterioration helps shorten the diastolic-filling period during which the mitral valve is open.
D.History and clinical presentation
1.Chronic AR is usually asymptomatic for a long time. The natural history of disease progression in asymptomatic severe AR is much slower compared with AS with an estimated annual progression (requiring AVR or death) rate of ~6%. After the development of LV dysfunction, patients gradually experience symptoms related to pulmonary congestion, including increased dyspnea with exertion, orthopnea, and paroxysmal nocturnal dyspnea. LV enlargement frequently produces an uncomfortable sensation in the chest that is exaggerated after premature ventricular contractions and in the supine position. Although angina is uncommon, it can be produced by latent CAD, decreased diastolic coronary perfusion pressure, nocturnal bradycardia and fall in arterial diastolic pressure, marked LVH, and subendocardial ischemia.
2.Acute AR. Patients with acute, severe AR usually present with signs of sudden hemodynamic deterioration such as weakness, altered mental status, severe shortness of breath, or syncope. If left untreated, these patients quickly progress to total cardiovascular collapse. When severe chest pain is part of the initial clinical presentation, aortic dissection must be strongly suspected.
E.Physical findings
1.Chronic AR. Patients with chronic AR can have a wide array of physical findings, especially during examination of the peripheral pulses and cardiac auscultation. The physical examination may yield clues about the cause of AR. Patients with AR should be examined for the peripheral manifestations of IE, signs of Marfan syndrome, evidence of chronic aortic dissection, and signs of collagen vascular disorders.
a.Peripheral pulse examination. The increased total stroke volume in chronic AR leads to an abrupt increase in arterial pressure during systole, followed by a rapid fall in arterial pressure during diastole. The widened pulse pressure accounts for a number of physical findings associated with chronic AR (Table 15.7). Patients with chronic AR may exhibit a bisferiens pulse, characterized by double systolic peaks with increased amplitude. The signs of hyperdynamic circulation are not specific to AR and can be seen in conditions that cause high-output heart failure, including sepsis, anemia, thyrotoxicosis, beriberi, and arteriovenous fistula.
TABLE 15.7 Physical Signs Associated with Hyperdynamic Pulse in Chronic Aortic Regurgitation |
|
Physical Sign |
Description |
Water hammer pulse |
Rapid upstroke followed by quick collapse |
de Musset sign |
Head bob with each heartbeat |
Traube sign |
Pistol shot sounds heard over the femoral arteries in both systole and diastole |
Müller sign |
Systolic pulsation of the uvula |
Duroziez sign |
Systolic murmur over the femoral artery when compressed proximally and diastolic murmur when compressed distally or systolic–diastolic murmur with increasing compression over femoral artery |
Quincke sign |
Capillary pulsations visible in the lunula of the nail bed |
Hill sign |
Popliteal cuff systolic pressure exceeding brachial cuff systolic pressure by >60 mm Hg |
Becker sign |
Arterial pulsations visible in the retinal arteries and pupils |
b.Palpation. With severe AR, the apical impulse is typically enlarged and displaced lateral to the midclavicular line in the fifth intercostal space because of LV enlargement. The impulse may be sustained and hyperdynamic. A diastolic thrill may be palpable in the second left intercostal space, as may a systolic thrill caused by increased aortic flow.
c.Auscultation. The main auscultatory findings are outlined in Figure 15.7.

FIGURE 15.7 Physical findings in aortic regurgitation. LV, left ventricle; LVH, left ventricular hypertrophy.
(1)Heart sounds. S1 may be diminished in the presence of PR-interval prolongation, LV dysfunction, or preclosure of the mitral valve. S2 may be soft, singly split (P2 obscured by the diastolic murmur) or paradoxically split. An S3 may be heard with severe LV dysfunction. An S4 is often present and represents left atrial contraction into a poorly compliant left ventricle.
(2)Diastolic murmur. The hallmark murmur of AR is a blowing, diastolic, decrescendo murmur that starts immediately after A2 and is best heard in the left upper sternal border with the patient sitting up and leaning forward slightly in full expiration. In general, the severity of AR correlates with the duration of the murmur more than with its intensity. Early in the course of disease, the murmur is typically short. As the disease progresses, the murmur may become pandiastolic. In the end stages of AR, the murmur may shorten again because of rapid equilibration of pressures in the aorta and left ventricle from an elevated LVEDP. In this situation, other signs of severe AR are usually present.
(3)A second diastolic murmur may be audible at the apex in severe AR. The Austin Flint murmur is a middle-to-late diastolic rumble that is believed to be caused by vibration of the anterior mitral leaflet as it is struck by the regurgitant jet or by turbulence in the mitral inflow from partial closure of the mitral valve by the regurgitant jet. Unlike the murmur of true valvular mitral stenosis, the Austin Flint murmur is not associated with a loud S1 or with an opening snap.
(4)A short midsystolic ejection murmur may be audible at the base of the heart, radiating to the neck. It reflects the increased ejection rate and large stroke volume traversing the aortic valve.
2.Acute AR. The physical examination of patients with acute AR differs considerably from that of patients with chronic AR. The physical examination may be most notable for signs of hemodynamic compromise, such as hypotension, tachycardia, pallor, cyanosis, diaphoresis, cool extremities, pulmonary congestion, and altered mental status.
a.Peripheral examination. The signs of hyperdynamic circulation that characterize chronic AR are often absent in acute AR. The pulse pressure may be normal or only slightly widened. The heart size is often normal, and the point of maximal intensity is not displaced laterally. When aortic dissection is suspected, blood pressures should be taken in all extremities to detect the differences.
b.Heart sounds. S1 may be diminished because of preclosure of the mitral valve. An S3 often accompanies cardiac decompensation.
c.Murmurs. The early diastolic murmur of acute AR is shorter and lower in pitch than the murmur of chronic AR. In severe, acute AR, the murmur may not be audible when the diastolic pressure in the left ventricle and aorta equilibrates. The systolic murmur reflecting increased flow across the aortic valve may be heard but is usually not loud. The Austin Flint murmur, if present, is short.
F.Laboratory evaluation
1.ECG. The typical ECG in chronic AR shows LVH, left-axis deviation, and left atrial abnormality. Conduction abnormalities are unusual but can occur after the development of LV dysfunction. Premature atrial and ventricular beats are common. Sustained supraventricular or ventricular tachyarrhythmias are uncommon in the absence of LV dysfunction or concomitant mitral valve disease. In acute AR, the ECG is usually notable only for nonspecific ST-T–wave abnormalities.
2.Chest radiograph. In chronic AR, the chest radiograph may reveal marked cardiomegaly, with the heart being displaced inferiorly and leftward. Dilation of the aortic knob and root may be seen. In acute AR, the LV and left atrial dimensions are usually normal. Aortic dissection can lead to a widened mediastinum and/or a widened cardiac silhouette due to pericardial effusion. The chest radiograph is notable for signs of pulmonary congestion.
3.Laboratory testing. Chronic AR may lead to increased natriuretic peptides and evidence of their elevation is helpful when a decision about surgical timing is equivocal. Other blood tests may help in elucidating suspected underlying conditions such as connective tissue disorders or if endocarditis is possible.
4.Echocardiography. Two-dimensional and M-mode echocardiography are useful in determining the cause of AR, evaluating the aortic root, and assessing the overall LV size and function. Doppler echocardiography is useful for detecting AR and estimating severity. There are several different methods of estimating the severity of AR with color Doppler, pulsed wave Doppler, and continuous wave Doppler ultrasonography.
a.Two-dimensional and M-mode echocardiography. The cause of AR can be assessed using two-dimensional echocardiography. Rheumatic AR typically causes thickening and retraction of the leaflet tips, leading to failure of cusp apposition. Bacterial endocarditis, which can cause leaflet fibrosis and retraction, leaflet perforation, or flail of the valve cusp, should be suspected if a vegetation is detected. Prolapse of the aortic valve cusps can occur in many conditions, including IE, BAV, myxomatous degeneration, and Marfan syndrome. Aortic root abnormalities are also well visualized in the parasternal long-axis view. Aortic root dilation is most often idiopathic, although Marfan syndrome, Ehlers–Danlos syndrome, ankylosing spondylitis, Reiter syndrome, rheumatoid arthritis, syphilis, and giant cell arteritis are other potential causes. Symmetric dilation of the aortic root produces a central jet of AR, and focal dilation causes an eccentric jet. In the parasternal long axis, the transducer should be moved up one interspace to assess the ascending aorta. Infective destruction of the aortic wall and proximal aortic dissection flaps may occasionally be visualized on transthoracic images. M-mode echocardiography may reveal premature closure of the mitral valve in severe, acute AR. In acute AR and chronic AR, the regurgitant jet can strike the anterior mitral valve leaflet, causing it to reverberate or “flutter” in diastole. Reversed doming of the anterior mitral leaflet may be seen on two-dimensional imaging and generally indicates grade 3 to 4+ AR.
b.Doppler and color flow imaging. Doppler and color flow echocardiography is used to detect AR and to assess its severity. AR is identified by Doppler imaging as high-velocity pandiastolic flow originating immediately under the aortic valve. Color flow imaging allows the assessment of jet origin, size, and direction. Continuous wave Doppler provides measurement of jet velocity and timing of flow. The maximum length of the AR jet correlates poorly with severity of regurgitation when assessed angiographically. Several other Doppler measures are used to estimate the severity of AR (Table 15.8). The ratio of the jet width to LVOT diameter is measured in the parasternal long-axis view and correlates well with the angiographic severity of AR. The pressure half-time of the aortic regurgitant velocity is defined as the time required for the pressure gradient across the aortic valve to fall to half of its initial value. The pressure half-times of patients with mild, moderate, and severe AR have demonstrated considerable overlap. In general, shorter pressure half-times are associated with increased severity of AR, and a pressure half-time of <200 ms is nearly always associated with severe AR. Quantitation of regurgitant volume and regurgitant fraction provides the most direct correlation with quantitative angiographic estimates of AR severity. Regurgitant volume is the difference between the stroke volume across the LVOT (representing the sum of forward flow and regurgitant flow) and that across the mitral valve inflow (representing forward flow), provided there is no significant MR. The regurgitant fraction is the ratio of the regurgitant volume divided by the LVOT stroke volume. The proximal isovelocity surface area (PISA) method is also used for estimating AR severity. The PISA method is used to calculate the effective regurgitant orifice (ERO) area. An ERO area ≥0.30 cm2 is indicative of severe AR. The presence of a proximal convergence area on transthoracic echocardiogram at the aortic valve is indicative of at least moderate AR. Pulsed wave Doppler echocardiography should be performed in the proximal descending aorta to establish the presence of diastolic flow reversal. Some degree of flow reversal is normally seen early in diastole because of reflux of blood into the coronary vasculature, but if this is >40 cm/s and continues throughout diastole, then severe AR is likely, especially if this persists in the abdominal aorta. Flow reversal may also be seen with other conditions that cause blood to leak out of the arterial system such as patent ductus arteriosus or sizeable arteriovenous fistula.
c.TEE is used to rule out vegetation or aortic valve ring abscess in patients who may have bacterial endocarditis. In pure AR, vegetation typically occurs on the LV side of the aortic valve. TEE is also used to visualize congenital valvular abnormalities (e.g., bicuspid valve, subaortic membrane) and to exclude aortic dissection.
d.Stress echocardiography is useful for assessing functional capacity and unmasking symptoms in patient previously classified as being asymptomatic or with equivocal symptoms. It can also assess for contractile reserve, which if absent is predictive or the development of systolic dysfunction both at follow-up (medical therapy) and postoperatively. Recent research also suggests that exercise tricuspid annular plane systolic excursion (TAPSE) <21 mm Hg is associated with RV dysfunction and independently associated with need for earlier AVR. Although contractile reserve and exercise TAPSE are not included as indication for AVR, they can be considered for use to help anticipate surgical timing in higher risk patients nearing recommendations for AVR including LVEF 50% to 55% or left ventricular end-systolic dimension (LVESD) approaching 50 mm or 25 mm/m2 (Table 15.7). It is also important to acknowledge that afterload increases substantially with exercise, which can precipitate a fall in ejection fraction. This exercise-induced fall in LVEF is nonspecific and it should not of itself be used to indicate need for surgical intervention.
e.Newer techniques. Strain imaging is increasingly used to define LV dysfunction before this is apparent using LVEF. Abnormalities in global longitudinal strain may indicate early decompensation in AR patients. 3D echocardiography has the potential to provide more accurate LV volumes and dimensions in AR but is not yet widely used for this purpose.
5.Cardiac catheterization. Cardiac catheterization is not necessary for all patients with chronic AR unless there are concerns about AR severity, hemodynamic abnormalities, or LV function, despite noninvasive testing and the physical examination. All patients older than 50 years with severe AR should undergo coronary cineangiography before any definitive surgical procedure on the valve to detect CAD. The decision to perform cardiac catheterization in younger patients should be made on an individual basis after assessment of the patient’s cardiac risk profile. Catheter manipulation in patients with AR may be difficult because of dilation of the ascending aorta. Caution should be exercised when manipulating catheters in patients with Marfan syndrome or cystic medial necrosis of the aortic wall to minimize the risk of vascular trauma. In addition to conventional coronary cineangiography, aortography may be performed to evaluate the degree of AR when doubt about true severity remains after noninvasive testing. The grading of AR by angiography is given in Table 15.9. Right heart catheterization may be helpful in certain circumstances, such as new-onset heart failure or combined AR and AS.
TABLE 15.9 Angiographic Grading of Aortic Regurgitation |
||
Degree of Aortic Regurgitation |
Left Ventricular Opacification |
Rate of Clearing |
Mild (1+) |
Faint, incomplete |
Rapid |
Moderate (2+) |
Faint, complete |
Rapid |
Moderate to severe (3+) |
Equal to aortic opacification |
Intermediate |
Severe (4+) |
Greater than aortic opacification |
Slow |
6.Advanced cardiac imaging. If echocardiographic imaging is inadequate, such as poor acoustic windows, cardiac CT or cardiac magnetic resonance (CMR) imaging can be used to better evaluate both the cause and severity of AR as well as the aorta. Either modality can be used to better define the anatomy including but not limited to mechanism of AR, anatomic morphology of the valve (i.e., BAV), providing a more complete evaluation of the thoracic aorta including the degree of aortic dilatation or presence of a vascular connective tissue disorder, and using CMR to show evidence of early myocardial fibrosis. In addition to an anatomic evaluation, CMR can also provide a functional assessment including an accurate calculation of LVEF, left ventricular volumes, and quantification of regurgitation with flow mapping through calculation of regurgitant fraction. The assessment of left ventricular volumes is especially helpful in chronic severe AR when the left ventricle becomes spherical and linear dimensions may not be as representative of the degree of dilatation. CMR is highly reproducible and thus may be used in serial assessment of AR, which has a long asymptomatic period.
G.Natural history. Moderate-to-severe AR may have a good prognosis for many years, provided the patient is asymptomatic and does not exhibit signs of LV dysfunction or severe dilation. Asymptomatic patients with normal LV function require AVR at a rate of only 4% per year. Ninety percent of such patients remain asymptomatic at 3 years, 81% at 5 years, and 75% at 7 years after the diagnosis is made. Patients with mild-to-moderate AR have had a 10-year survival rate of 85% to 95%. Patients with moderate-to-severe AR treated with medical therapy have a 5-year survival rate of 75% and a 10-year survival rate of 50%. After the development of LV dysfunction, progression to symptoms is greatly accelerated, with rates approaching 25% per year. In patients who develop NYHA class III to IV symptoms, without surgical intervention survival rates are 28% at 4 years. The natural history of AR has been defined on relatively few patients and at a time when routine imaging was not widely available. There is increasing evidence that the natural history may be less favorable especially in those who do not undergo surgical intervention.
H.Therapy
1.Medical therapy
a.Chronic AR. Vasodilators, such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), calcium channel blockers, and hydralazine have historically been used in the treatment of chronic AR to reduce the severity of regurgitation. However, patients with mild-to-moderate AR and normal systolic function do not appear to benefit from vasodilator therapy. Conflicting evidence exists for vasodilator treatment in asymptomatic severe AR. Therefore, the 2014 ACC/AHA valve guidelines limit their recommendation for vasodilator use to (1) concurrent treatment of hypertension with dihydropyridine calcium channel blockers or ACEI/ARBs for systolic blood pressure >140 mm Hg (class I indication) or (2) treatment in patients with severe AR with either symptoms or LV dysfunction and not a candidate for surgery because of their comorbidities (class IIa indication). β-Blockers are also reasonable to use in these nonsurgical candidates (class IIa indication), although it is important to avoid bradycardia, which increases diastolic filling time and increases the regurgitant volume in severe AR.
b.Acute AR. The goal of medical therapy in acute AR is hemodynamic stabilization before proceeding with surgical correction. For patients presenting with cardiogenic shock, intravenous vasodilators are used to reduce the afterload stress on the left ventricle, to lower LVEDP, and to augment forward cardiac output. In severe cases, temporary transvenous atrial/ventricular pacing and/or intravenous inotropic agents may be required for temporary hemodynamic support. β-Blockers may be used with caution when acute AR is caused by an aortic dissection. β-Blockers help reduce arterial dP/dt, which reflects the transmission of force from LV ejection to the arterial wall. Although this is an essential component of the treatment of acute aortic dissection, β-blockers increase the length of diastole by slowing the heart rate, which can exacerbate acute AR and contribute to cardiovascular collapse. If acute AR is associated with endocarditis, antibiotic therapy should be instituted as soon as all culture specimens are obtained. Patients with severe acute AR who are being triaged to surgery may be treated with rapid pacing to reduce the duration of diastole if therapeutic modalities such as intravenous vasodilators fail to produce a significant tachycardia. A surgical evaluation should be performed emergently for a patient with AR caused by aortic dissection or chest trauma. The goal of medical therapy in this setting is to maximize forward cardiac output and minimize propagation of aortic dissection if present.
2.Percutaneous therapy
a.Insertion of an intra-aortic balloon counterpulsation device in patients with more than moderate AR or in the presence of aortic dissection is contraindicated.
b.Patients with combined severe AS and at least moderate AR are poor candidates for PABV because the degree of AR is likely to increase after the procedure.
c.Native AR. The use of TAVR in native severe AR is generally included as an exclusion criteria for treatment of native valve disease. However, an observational study of 31 patients in Germany was performed using the JenaValve with a 30-day mortality of 12.9% and a 6-month mortality rate of 19.3%. The JenaValve is a porcine root valve sewn to a Nitinol self-expanding stent fitted with an outer porcine pericardial patch or skirt. The device also features a fixation clip to firmly anchor the valve despite the absence of calcification. This device is only deployable by a transapical approach. SAVR remains the treatment of choice in operable patients.
3.Surgical therapy
a.Chronic AR. The 2014 ACC/AHA guidelines on the indications for AVR in patients with chronic AR are given in Table 15.10.
TABLE 15.10 Recommendations for Aortic Valve Replacement in Patients with Chronic Aortic Regurgitation |
Class I |
1.Symptomatic patients with severe AR |
2.Asymptomatic patients with severe AR and LVEF <50% (not attributed to another cause) |
3.Severe AR and having heart surgery for another indication |
Class IIa |
1.Asymptomatic patients with severe AR, normal LV function, and severe LV dilatation (LVESD > 50 mm or indexed LVESD > 25 mm/m2) |
2.Moderate AR and having heart surgery (mitral valve or CABG) or surgery on the ascending aorta |
Class IIb |
1.Asymptomatic patients with severe AR, normal LV function, and progression LV dilatation (LVEDD > 65 mm) if low surgical risk |
Abbreviations: AR, aortic regurgitation; CABG, coronary artery bypass grafting; LV, left ventricle; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension.
Adapted from Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57–e185. Copyright © 2014 American Heart Association, Inc., and the American College of Cardiology Foundation. With permission.
(1)Symptomatic patients. The 2014 ACC/AHA guidelines recommend AVR symptomatic patients with severe AR regardless of the ejection fraction.
(2)Asymptomatic patients
(a)LV systolic dysfunction. Asymptomatic patients with chronic, severe AR and LV systolic dysfunction (ejection fraction < 50%) secondary to aortic valve disease are at high risk for the development of symptomatic heart failure within 2 to 3 years and, therefore, should be considered for prompt surgical intervention. The likelihood of LV dysfunction normalizing after surgery decreases with increased duration of dysfunction.
(b)LV dilation. Asymptomatic patients with normal LV systolic function at rest and evidence of severe LV dilation have an increased risk of sudden cardiac death and perioperative mortality increases significantly once they develop symptoms or LV systolic dysfunction. However, their prognosis after AVR is excellent and they should be referred for valve replacement if surgical risk is low. It is reasonable to send patients for AVR if their LVESD is >50 mm or indexed >25 mm/m2. Surgery can also be considered in patients with left ventricular end-diastolic dimension (LVEDD) >65 mm if their surgical risk is low <1%.
(c)Other cardiac surgery. AVR is recommended for patients with chronic severe AR undergoing another cardiac surgery. AVR is also reasonable for patients with moderate AR if undergoing surgery on the mitral valve, ascending aorta, or CABG.
(d)Aortic valve repair. Many patients with prolapse of bicuspid valve as the cause of AR may be candidates for surgical repair of the aortic valve. Some patients with leaflet perforation caused by infectious endocarditis may also be candidates for repair in which a pericardial patch is sewn over the defect. Aortic valve repair has good durability when the initial result is excellent (minimal residual AR) but further surgery will be needed usually at 12 to 20 years. Leaflet sparing surgery on the aorta is increasingly feasible, and if the cause of the AR is impaired coaptation due to annular dilatation then experienced surgeons may be able to replace the aorta and reduce the severity of AR concomitantly without need for an AVR.
(e)Postoperative. Patients with severe AR may show initial worsening in LV function despite relative normalization of LV size. This is likely due to the hemodynamic changes produced by eradicating the regurgitation. Slow improvement with normalization or at least stabilization of function at about 6 months is common in these patients. Afterload reduction is indicated postoperatively in patients with impaired LV systolic function at least until there is normalization of LV systolic function.
I.Key suggestions
1.Acute, severe AR is usually a surgical emergency. Signs of congestive heart failure and mitral valve preclosure are ominous in acute AR.
2.Valve replacement can be performed without infection of the prosthesis in active endocarditis, even when antibiotics have only recently been started. An aortic valve homograft is the preferred prosthesis in the setting of endocarditis.
3.Aortic dissection should be suspected in any patient with chest pain and AR.
4.If LV systolic dysfunction is present for <12 months, LV function is likely to improve postoperatively.
5.Heart rate is usually normal until late in the course of disease, when a low effective stroke volume is compensated with tachycardia to maintain cardiac output.
6.Rapid atrial or ventricular pacing may be used as a temporary measure to manage acute AR caused by endocarditis or trauma to improve cardiac output. The diastolic-filling phase is shorter at higher heart rates; therefore, there is less time for valvular regurgitation.
ACKNOWLEDGMENTS: The authors would like to thank Dr. Anne Kanderian, Dr. Anjli Maroo, and Dr. Olcay Aksoy for their work in the previous editions.
Key Reviews
Agarwal S, Tuzcu EM, Krishnaswamy A. Transcatheter aortic valve replacement: current perspectives and future implications. Heart. 2015;101(3):169–177.
Glower DD. Management of chronic aortic regurgitation. Curr Treat Options Cardiovasc Med. 2003;5(6):511–520.
Otto CM, Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med. 2014;371(8):744–756.
Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60(19):1845–1853.
Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55(25):2789–2800.