
For severe native and prosthetic valve dysfunction, valve replacement is often the treatment of choice with over 280,000 implants worldwide every year. This chapter discusses types of prosthetic valves, specific considerations for valve selection, and prosthetic valve dysfunction.
I.Prosthetic valve types and selection
A.Types of prosthetic valves. Prosthetic valves are classified as mechanical or bioprosthetic (Table 18.1). Overall, 80% of replacements are with bioprosthetic valves, and 20% are with mechanical valves. For bioprosthetic valves, replacement may occur via a surgical or transcatheter approach. Each valve differs in its durability, thrombogenicity, and hemodynamic performance. Various mechanical and bioprosthetic valves are shown in Figure 18.1.
1.Bioprosthetic valves (Table 18.2). These resemble native valves but have a less optimal hemodynamic performance, in part because of the reduction in flow profile by interposed stents and the sewing ring.
a.Heterografts
(1)Stented bioprostheses are the most frequently implanted biologic valves. The Carpentier-Edwards standard and Hancock standard are whole porcine valves, but more commonly, a composite valve is created by taking a cusp from three different pigs (e.g., St. Jude Epic, Carbomedics Synergy, or Medtronic Mosaic). Alternatively, stented bioprostheses may be made from pericardium, usually bovine. The pericardium may be sewn inside or outside the stent posts (e.g., Mitroflow, Trifecta). The durability of bioprosthetic bovine pericardial versus porcine valves is controversial, although the pericardial valves may have an advantage in younger patients.
TABLE 18.1 Types of Prosthetic Heart Valves |
Biologic |
Stented: pericardial or porcine bioprosthesis |
Stentless: pericardial or porcine bioprosthesis, aortic homograft, pulmonary autograft (Ross procedure) |
Sutureless |
Transcatheter |
Mechanical |
Bileaflet |
Single tilting disc |
Caged ball |

FIGURE 18.1 Photographic and radiographic appearance of different prosthetic heart valves. From left to right: Starr-Edwards caged ball, Kay-Suzuki caged disk, Björk-Shiley single tilting disk, St. Jude’s bileaflet tilting disk mechanical valves, and Carpentier-Edwards xenograft. (Reprinted with permission from Garcia M. Principles of imaging. In: Topol EJ, ed. Comprehensive Cardiovascular Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 1998:610.)
TABLE 18.2 Surgical Biologic Valve Replacement |
Stented pericardial replacement valve: Carpentier-Edwards Perimount, Carpentier-Edwards Magna, Labcor pericardial, Mitroflow Synergy, Sorin Pericarbon MOREa, St. Jude Biocor Pericardial, St. Jude Trifecta |
Stented porcine replacement valve: AorTech Aspire, Carbomedics Synergy, Carpentier-Edwards standard and supra-annular, Hancock standard and Hancock II, Labcor, Medtronic Mosaica, St. Jude Biocor, St. Jude Bioimplant, St. Jude Epic |
Stentless pericardial: 3F-SAVR, Freedom Solo, Sorin Pericarbon |
Stentless porcine: AorTech Aspire, Cryolife-O’Briena, Cryolife-Ross Stentless porcine pulmonary, Edwards Prima Plus, Labcor, Medtronic Freestyle, Medtronic-Venpro Contegra pulmonary valve conduit, Shelhigh Skeletorized Super-Stentless aortic porcine and pulmonic, St. Jude Medical Torontoa, St. Jude Quattro stentless mitral |
Sutureless: 3F Enable (ATS Medical), Edwards Intuity, Perceval S (Sorin), Trilogy (Arbor Surgical Technologies) |
aWithdrawn from market.
(2)Stentless bioprostheses are most often composed of porcine aorta, although some are tricomposite (e.g., Biocor, Cryolife-O’Brien), and others are made from bovine pericardium (e.g., Sorin Freedom). The porcine aorta may be long (e.g., Medtronic Freestyle) or designed for implantation under the coronary arteries (e.g., St. Jude Medical Toronto). Even though the stentless valves offer a better hemodynamic profile owing to the larger effective orifice area (EOA), convincing advantages in terms of mortality, left ventricular (LV) mass regression, and durability have not been demonstrated.
(3)Since the first-in-man TAVR done by Cribier in 2002, more than 200,000 patients have undergone TAVR worldwide. Currently, TAVR is the standard of care for inoperable patients and is comparable to surgical aortic valve replacement in high- and intermediate-risk patients. The technology has evolved rapidly with lower profile catheter and delivery systems, wider range of valve sizes, more precise valve positioning as well as retrievable and repositionable features, and reduced paravalvular aortic regurgitation. In the United States, the Edwards SAPIEN and Medtronic CoreValve are approved for commercial use, whereas more TAVR systems are available in Europe (Fig. 18.2).

FIGURE 18.2 Transcatheter aortic valve systems. A: Edwards Lifesciences SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA); B: Medtronic CoreValve Evolut R (Medtronic, Minneapolis, MN); C: Symetis Acurate neo valve (Symetis, Écublens Vaud, Switzerland); D: JenaValve (JVT Research & Development Corporation, Irvine, CA); E: St. Jude Medical Portico valve (St. Jude Medical, St. Paul, MN); F: Direct flow medical valve (Direct Flow Medical, Inc., Santa Rosa, CA); G: Medtronic Engager valve (Medtronic, Minneapolis, MN); and H: Boston Scientific Lotus valve (Boston Scientific, Marlborough, MA). Two TAVR systems are approved by the U.S. Food & Drug Administration (A and B). (Adapted from Vahl TP, Kodali SK, Leon MB. Transcatheter aortic valve replacement 2016. J Am Coll Cardiol. 2016;67:1472–1487. Copyright © 2016 American College of Cardiology Foundation. With permission.)
b.Aortic homografts are cryopreserved cadaveric human aortic valves. These are typically implanted stentless, with a short segment of the donor’s aortic root for support. The coronary arteries require reimplantation. The hemodynamic profile of the homograft is similar to that of the native valve. Availability of homografts of different sizes can be a limiting factor.
c.Autograft. An autograft is a procedure in which the patient’s own valve is moved from its normal anatomical position to another site. Typically, this is done with the pulmonary valve in patients with significant aortic valve disease. A pulmonary homograft is placed at the native pulmonary position. This operation is called the Ross procedure, after the surgeon who popularized it. This procedure has the advantage of placing a native valve at the hemodynamically most important position. It has been advocated for younger patients, and some reports suggest that the autograft may grow with the patient, which is advantageous in the adolescent age group. However, the initial enthusiasm with this procedure has been tempered by suboptimal outcomes in many adult patients, which can involve the autograft or the pulmonic homograft. Additionally, progressive root enlargement may occur in patients who have bicuspid aortic valves, which can also lead to autograft failure. The decision to proceed to autograft implantation in adults should be considered carefully and in consultation with a surgeon with extensive experience with this procedure.
2.Mechanical valves (Table 18.3)
a.Bileaflet. The most frequently implanted mechanical valves, bileaflet valves differ in their composition of pyrolytic carbon, the shape and opening angles of the leaflets, and the design of the pivots and sewing ring. For the aortic site, these valves also differ by implantation position, which can be intra-annular, partially supra-annular, or entirely supra-annular. In the open position, bileaflet valves have two large lateral orifices and a smaller central space. A built-in leakage volume is designed to reduce thrombus formation on disks.
TABLE 18.3 Mechanical Valve Replacement |
Bileaflet mechanical valves: ATS, Carbomedics (standard, reduced cuff, Optifrom, Orbis, Top Hat), Edwards (Mira, Tekna), Jyros, Medtronic Advantage, On-X, St. Jude medical (standard, HP, Masters, Regent) |
Tilting disc: Björk-Shiley monostruta, Medtronic-Hall, Omnicarbon, Sorin Monoleaflet allcarbon, Ultracor |
Caged ball: Starr-Edwards, Smeloff-Cutter |
aWithdrawn from market.
b.Single-leaflet tilting disk. This valve (e.g., Björk-Shiley, Medtronic-Hall, and Omniscience) consists of a metallic sewing ring attached to a tilting disk made of pyrolytic carbon that rotates about an off-centered pivot axis, with a range of about 60° to 85° from the occluded to the open position. When open, the prosthesis has two orifices separated by the occluder. The major orifice is formed as the disk swings downstream to the open position. The disk on the other side of the pivot axis swings proximally and forms the minor orifice.
c.Caged ball. The Starr-Edwards valve consists of a silicone ball within a cage attached to a metallic alloy ring. The ball is free to travel along the cage over a distance of 1 to 2 cm. Flow across the prosthesis is directed circumferentially around the ball. The hemodynamic profile is less favorable, but this valve has the longest follow-up, over 30 years in some studies.
B.Selection of valves. Table 18.4 summarizes the clinical factors that favor selection of a bioprosthetic versus a mechanical valve. The choice is largely dependent upon the age of the patient and the risks of anticoagulation therapy for a mechanical valve versus the risks of SVD and reintervention for biologic valves. Contemporary data to inform this decision are observational because there have been no randomized trials comparing biologic and mechanical valves in the past three decades. Importantly, patient preferences are emphasized, and there has been a shift toward using bioprosthetic valves in younger patients.
TABLE 18.4 Clinical Factors Leading to Selection of a Bioprosthetic versus a Mechanical Valve |
||
Factors Favoring Bioprosthesis |
Factors Favoring Aortic Homograft |
Factors Favoring Mechanical Prosthesis |
Age > 70 y |
Endocarditis |
Age < 50 y |
Bleeding diathesis |
Small aortic annulus in older patients |
Combined multivalvular placement |
High risk of trauma |
Other indications for chronic anticoagulation |
|
Poor compliance |
Completed childbearing in younger woman |
|
Young woman considering pregnancy |
High risk for reoperation (e.g., radiation heart disease) |
|
1.Valve repair. The feasibility of native valve repair instead of replacement should always be considered prior to surgery (Table 18.5). Currently, the greatest experience is with mitral valve repair. If feasible, mitral valve repair offers several potential advantages over replacement, including preservation of LV function via conservation of the subvalvular apparatus, lower operative mortality, higher long-term survival rate, and freedom from anticoagulation. Mitral valve repair may be considered for asymptomatic patients with severe primary mitral regurgitation, if there is a high chance of repair at high-volume centers.
TABLE 18.5 Characteristics Favoring Valve Repair versus Replacement |
|
Favoring Valve Replacement |
Favoring Valve Repair |
Rheumatic valve disease |
Mitral valve prolapse |
Calcified and fibrosed valve |
Excessive mitral valve leaflet mobility |
Extensive leaflet destruction |
Noncalcified bicuspid aortic valve with prolapse |
Inexperienced surgeon |
Aortic annular dilation with normal leaflets |
An aortic valve with predominant regurgitation because of prolapse, but without severe stenosis or calcification, can also be repaired.
2.Bioprosthetic valves are indicated in patients with a contraindication to chronic anticoagulation and are preferred for older patients (≥65 years old for the aortic position and ≥70 years old in the mitral position) because of reasonable durability, favorable hemodynamic profile, and freedom from chronic anticoagulation. Approximately 30% of heterograft bioprostheses fail within 10 to 15 years of implantation, although the incidence of bioprosthesis failure is age-dependent (Table 18.6). Overall complication rates for aortic bioprosthetic and mechanical valves are similar at 12 years, with a higher rate of reintervention for bioprosthetic valves and a higher rate of hemorrhage with mechanical valves. The advent of newer low-profile bioprostheses and the apparent improved durability of later models have led to an increase in their use, especially in patients who wish to avoid anticoagulation. Currently, for patients 50 to 70 years old, the choice of a bioprosthetic or mechanical valve is individualized.
TABLE 18.6 Heterograft Valve Failure Rate 10 Years after Valve Replacement Relative to the Patient’s Age |
|
Patient’s Age (y) |
Failure Rate at 10 Y (%) |
<40 |
40 |
40–49 |
30 |
50–59 |
20 |
60–69 |
15 |
≥70 |
10 |
From Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med. 1996;335:412, by permission of the Massachusetts Medical Society. Modified with permission from Massachusetts Medical Society.
3.Transcatheter aortic valve replacement, as mentioned, is now an option for patients with severe aortic stenosis who are either inoperable or high or intermediate risk for open heart surgery. A comprehensive evaluation for procedural eligibility and candidacy is required including coronary angiography to exclude significant coronary artery disease and computed tomography (CT) angiography to size the aortic annulus and assess suitability of iliofemoral access.
4.Homografts. The homograft is the valve of choice in aortic valve endocarditis and has the lowest valvular gradient among the bioprosthetic valves. Durability was thought to be superior to that of heterografts, but recent studies have not confirmed this finding, and only 10% are still functioning after 20 years. The primary operation is more difficult with homografts, because the coronary arteries require implantation. Reoperation is also more complex, because the homograft frequently calcifies and is difficult to remove and replace. The main indication for an aortic homograft is invasive endocarditis, especially with an aortic root abscess, where the risk of reinfection is high. Another indication is in older patients with a small aortic root and left ventricular outflow tract (LVOT), in order to maximize hemodynamics and minimize the transaortic gradient.
5.Mechanical valves. Mechanical valves are more durable than bioprosthetic valves; some can last >20 years. Mechanical prostheses are generally recommended for patients ≤50 years because of greater durability and for patients already on permanent anticoagulation for previous stroke or arrhythmia. The stroke risk of about 1% per annum for patients with a mechanical valve receiving appropriate anticoagulation management is similar to that for a bioprosthetic valve without anticoagulation. In younger patients requiring combined aortic and mitral valve replacement, mechanical valves may be preferred, given the more rapid rate of prosthesis deterioration in the mitral position. Pregnancy should be discouraged in patients with mechanical prostheses because of the high risk to the mother and the fetus. Given their lower profile, mechanical prostheses may be preferred in patients with small ventricles. Issues of compliance with anticoagulation and risks of trauma should be integrated into the selection of a mechanical valve.
a.Bileaflet valves are the most popular mechanical prosthetic valves because of their favorable hemodynamic performance, longevity, and low rates of complications.
b.Starr-Edwards valves are older and have demonstrated durability. However, they are less popular today because of their thrombogenicity and suboptimal hemodynamic performance in comparison with tilting disk valves.
c.Manufacture of the Björk-Shiley valve was discontinued in 1986 following published reports of complications with strut fracture.
6.The decisions regarding valve type is a shared decision-making process that takes into account patient preferences, indications for and risks of anticoagulation, and risks of reintervention.
C.Follow-up after valve surgery. There is a wide spectrum of clinical practice in the follow-up of the asymptomatic patient after valve surgery. An echocardiogram should be performed between 4 and 6 weeks following surgery, after resolution of postoperative anemia, as a baseline for future reference. For mechanical valves, anticoagulation should be monitored regularly for life. Endocarditis prophylaxis is imperative for prosthetic valves, and patients should receive appropriate education. Annual or biannual echocardiography is reasonable 5 to 10 years after surgery.
D.Anticoagulation. Table 18.7 summarizes the recommended targets for anticoagulation therapy in patients with mechanical heart valves. Patients should receive a vitamin K antagonist, and oral direct thrombin inhibitors or anti-Xa agents should not be used. The embolic event rate is greater for mitral than for aortic prostheses.
TABLE 18.7 Recommended Anticoagulation Therapy for Patients with Mechanical Prosthetic Valves |
|
Prosthesis Type |
Recommended INR |
Aortic valve, mechanical bileaflet, or current generation single tilting disk without risk factorsa |
2.0–3.0 |
Mechanical aortic valve, with risk factorsb |
2.5–3.5 |
Mechanical mitral valve |
2.5–3.5 |
INR, international normalized ratio.
aIn patients with a mechanical On-X aortic valve replacement and no risk factors, a lower target INR of 1.5 to 2.0 may be reasonable.
bRisk factors include atrial fibrillation, previous thromboembolism, LV dysfunction, hypercoagulable condition, or an older generation mechanical valve.
1.Immediate postoperative period
a.Mechanical valves. The approach to postoperative anticoagulation for mechanical prostheses varies widely. Early anticoagulation increases the risk of bleeding and tamponade. One approach is warfarin, but not heparin, 3 to 4 days following surgery when the epicardial wires are removed. Other centers recommend low-dose intravenous heparin, targeted for upper normal limits of activated partial thromboplastin time within 6 to 12 hours after valve replacement, and full-dose intravenous heparin once the chest tubes are removed. Warfarin is initiated within 24 to 48 hours following valve replacement. Chronic anticoagulation for mechanical valves is associated with rates of minor hemorrhage of 2% to 4% per year, major hemorrhage of 1% to 2% per year, and death of 0.2% to 0.5% per year. The bleeding risk is 5% to 6% in patients aged ≥70 years. Patient-related risk factors for thromboembolism are older age, atrial fibrillation, and LV dysfunction. All patients should also receive aspirin (75 to 100 mg daily).
b.Bioprosthetic valves. The need for anticoagulation in bioprosthetic valves is controversial. The risk of embolism is greatest in the early postoperative period, declines after 3 months, and is greater for mitral (7%) compared with aortic valves (3%). In patients with a low risk of bleeding, anticoagulation with a goal international normalized ratio (INR) of 2.5 can be considered for 3 months following bioprosthetic aortic valve replacement and up to 6 months after bioprosthetic mitral valve replacement. All patients should receive aspirin (75 to 100 mg daily). After TAVR, clopidogrel, in addition to life-long low-dose aspirin, is reasonable for 6 months. The use of anticoagulation after TAVR is evolving, given that thrombosis has been observed with four-dimensional (4D) CT, followed by resolution after warfarin therapy.
2.Management of anticoagulation in patients with prosthetic valves undergoing noncardiac surgery. Although the risk of thromboembolism increases when anticoagulant therapy is briefly discontinued, the decision to suspend therapy should be individualized.
a.For major procedures in which substantial blood loss is expected, warfarin should be discontinued at least 3 days prior to the procedure to achieve an INR of 1.6 or less. Hospital admission for intravenous heparin administration is often recommended for patients with caged ball prosthetic valves, atrial fibrillation, severe LV dysfunction, or previous embolization. Postoperatively, intravenous heparin therapy should be resumed when it is considered safe and continued until therapeutic anticoagulation is achieved with warfarin. Low-molecular-weight heparin (LMWH) may be considered for patients with prosthetic valves as bridging therapy.
b.For minor procedures (e.g., dental extraction) where blood loss is minimal, anticoagulation can be continued.
3.Pregnancy. Pregnant women have an increased incidence of thromboembolic complications. The use of warfarin through the entire course of pregnancy is associated with warfarin embryopathy in as many as 6.4% of live births. Given its teratogenic effects, warfarin should be discontinued during the first trimester of pregnancy, especially if the dose is greater than 5 mg daily. However, with ≤ 5 mg of daily warfarin, the risk of embryopathy is low (<3%), and after careful discussion, may be continued. If warfarin is discontinued, dose-adjusted LMWH can be used during the first trimester with twice daily dosing and target anti-Xa level of 0.8 to 1.2 U/mL 4 to 6 hours after the dose. Anti-Xa monitoring is essential because the therapeutic dose can increase by 50% during pregnancy. Alternatively, a continuous infusion of unfractionated intravenous heparin can be used. Warfarin is used in the second and third trimesters, typically in conjunction with low-dose aspirin (75 to 100 mg). Prior to planned delivery, warfarin is discontinued, and continuous infusion of unfractionated heparin is used.
II.Assessment of prosthetic valves
A.Clinical presentation. The clinical presentations of prosthetic valve dysfunction can vary substantially. A discussion of the various entities is detailed in Section III.
1.History. This should include a thorough cardiovascular review in addition to questions pertinent to the function of the prosthesis.
a.The indication for placement of valve prosthesis, position of implantation, type of prosthesis, and the year of implantation should be elicited. The model and size of the prosthesis can be verified by the identification card provided by the manufacturer.
b.Other important questions involve compliance with anticoagulation, previous endocarditis, thromboembolism, fever, and perceived change in the quality of the valvular click.
2.Physical findings
a.The physical examination may be remarkable for a new murmur, muffled prosthetic valve sounds, or evidence of embolic events.
b.Prosthetic valves are associated with distinct auscultatory events caused by prosthesis motion or altered flow patterns. The prosthesis sounds may mask the normal heart sounds; significant valvular dysfunction may occur without audible changes. However, familiarity with the normal auscultatory findings in the prosthetic valve examination can provide valuable clues on prosthesis dysfunction prior to the more definitive imaging examination. Figure 18.3 summarizes the acoustic characteristics of common valve prostheses.

FIGURE 18.3 Acoustic characteristics of various mechanical and bioprosthetic valves. AC, aortic bioprosthetic closing sound; CC, closing click; DM, diastolic murmur; MC, mitral bioprosthetic closing sound; MO, mitral bioprosthetic opening sound; OC, opening click; P, pulmonary component of second heart sound; SEM, systolic ejection murmur; s1, first heart sound; S2, second heart sound. (From Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med. 1996;335:410, with permission from the Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.)
B.Laboratory examination and diagnostic testing. The diagnosis of prosthetic valve dysfunction relies predominantly on echocardiographic findings, which can often identify degeneration prior to the onset of symptoms.
1.Two-dimensional echocardiography. By their design, almost all replacement valves are stenotic compared with normal native valves. The degree of stenosis varies with the type and size of the valve. Thus, it may be difficult to differentiate mild obstruction from valve design, structural valve degeneration (SVD), or patient–prosthesis mismatch (PPM). Most mechanical valves and many biologic valves are associated with trace or mild transprosthetic regurgitation. The pattern of this “physiologic” regurgitation varies with the design of the replacement valve.
The interrogation of the prosthetic valve requires a systematic approach to the prosthetic apparatus, peak and mean gradients, and regurgitant flow. Oftentimes, transesophageal echocardiography (TEE) is performed to evaluate symptomatic patients and patients with known or suspected endocarditis. The 2D assessment of prosthetic valves is similar to that of the native valve, but is limited by reverberation artifacts and acoustic shadowing. In general, echocardiographic evaluation should be done to assess the following:
a.Occluders and leaflets. Failure of the leaflet or occluder to open or coapt properly may result from pannus ingrowth (see Section III.H), thrombus formation (see Section III.E), or calcification of bioprosthetic leaflets. Imaging of leaflets and occluders may be suboptimal with transthoracic echocardiography (TTE). Multiplane TEE provides higher temporal and spatial resolution of the prosthesis than TTE and allows improved assessment of leaflet mobility and abnormalities. Although the aortic prosthesis is less well visualized relative to the mitral prosthesis, TEE still provides a better visual inspection of the posterior aspect of the prosthesis and perivalvular structures than TTE. In particular, mechanical aortic valve leaflets can be difficult to assess when a mechanical mitral valve is also present.
b.Sewing ring. The orientation of the prosthetic valve in the annulus can be variable; however, excessive motion (“rocking”) of the sewing ring is consistent with dehiscence of the prosthesis. Concomitant paravalvular regurgitation can be commonly identified with the use of color-flow mapping. Furthermore, adjacent echolucent structures identified in the evaluation of endocarditis may represent a pseudoaneurysm. In general, flow into an adjacent echolucent space is pathologic.
c.Three-dimensional echocardiography. 3D echocardiography is essential in the evaluation of prosthetic valves. Occluder motion and the sewing ring are often well evaluated, and the precise location of an abnormality relative to the sewing ring can be optimally demonstrated.
2.Doppler evaluation. Doppler evaluation complements the 2D examination and provides a reliable indirect assessment of the prosthetic valve performance. Pulsed-wave and continuous-wave Doppler are used to assess transvalvular gradients, from which EOAs can be derived.
a.Imaging planes for TTE. Prosthetic mitral and aortic regurgitation can be visualized in the parasternal long- and short-axis views. Acoustic shadowing from the aortic and mitral prosthesis can interfere with the color-flow map in the proximal portion of the aortic and mitral regurgitant jets. The apical views allow assessment of transvalvular pressure gradients but may underestimate the size of the mitral regurgitant jets because of acoustic shadowing. Pulmonary vein flows may not be available for the same reason. Prosthetic aortic regurgitation is also characterized from the apical window.
b.Imaging planes for TEE. On a short-axis view of the aortic valve (~40°), the origin of regurgitation (intravalvular or paravalvular) can be identified. The extent of the aortic regurgitant jet into the LV cavity can be visualized with a long-axis view (~120°). By systematically sweeping through the mitral valve from 0° to 120°, the origin and severity of mitral regurgitation is appreciated. Continuous-wave Doppler, usually at 0° and 120°, is used to measure the peak and mean gradients across the prosthesis.
(1)Continuous-wave Doppler evaluation of the aortic valve is performed with a deep transgastric view using anteflexion to bring the aortic valve in line for Doppler interrogation.
(2)Continuous-wave Doppler can also be used to assess mechanical prosthetic regurgitation. Advantages of continuous-wave Doppler include excellent temporal resolution to allow identification of specific periods in the cardiac cycle and the ability to indicate the severity of a regurgitant jet by its signal intensity. Using 2D images and the color-flow map as a guide, continuous-wave Doppler allows interrogation of different parts of the prosthesis and can help to detect eccentric jets.
c.Normal Doppler findings
(1)Prosthetic valve clicks. The opening and closure of mechanical valve leaflets create a brief intense Doppler signal that appears as a narrow band on the spectral display.
(2)Prosthetic valve velocities/pressure gradients. The systolic spectral Doppler contour is frequently triangular, with an earlier systolic peak velocity. The expected normal velocities and pressure gradients for commonly used prosthetic valves are presented in Table 18.8. However, there is a large variability in these numbers depending on flow and other factors. Therefore, a postoperative baseline study, usually 4 to 6 weeks after surgery, is indicated for patients with prosthetic valves.
TABLE 18.8 Normal Doppler Values of Prosthetic Valves |
||
Prosthetic Valve |
Peak Velocity (m/s) |
Mean Gradient (mm Hg) |
|
Aortic Position |
||
Starr-Edwards |
3.1 ± 0.5 |
24 ± 4 |
Björk-Shiley |
2.5 ± 0.6 |
14 ± 5 |
St. Jude |
3.0 ± 0.8 |
11 ± 6 |
Medtronic-Hall |
2.6 ± 0.3 |
12 ± 3 |
Aortic homograft |
0.8 ± 0.4 |
7 ± 3 |
Hancock |
2.4 ± 0.4 |
11 ± 2 |
Carpentier-Edwards |
2.4 ± 0.5 |
14 ± 6 |
|
Mitral Position |
||
Starr-Edwards |
1.8 ± 0.4 |
5 ± 2 |
Björk-Shiley |
1.6 ± 0.3 |
5 ± 2 |
St. Jude |
1.6 ± 0.3 |
5 ± 2 |
Medtronic-Hall |
1.7 ± 0.3 |
3 ± 1 |
Hancock |
1.5 ± 0.3 |
4 ± 2 |
Carpentier-Edwards |
1.8 ± 0.2 |
7 ± 2 |
Modified from Nottestad SY, Zabalgoitia M. Echocardiographic recognition and quantitation of prosthetic valve dysfunction. In: Otto CM, ed. The Practice of Clinical Echocardiography. Philadelphia, PA: WB Saunders; 1997:803. Copyright © 1997 Elsevier. With permission.
(3)Physiologic prosthetic valve regurgitation. Many prosthetic valves have regurgitant flow characterized by uniform color without aliasing. For a mechanical prosthesis, the physiologic prosthetic regurgitant flow typically has a regurgitant jet area of <2 cm2 and jet length of <2.5 cm in the mitral position and a jet area of <1 cm2 and jet length of <1.5 cm in the aortic position.
d.Assessment of prosthetic valve dysfunction
(1)Prosthetic valve stenosis
(a)Transvalvular gradients. Assessment of transvalvular gradients is the mainstay of the Doppler evaluation. Each prosthetic valve is inherently stenotic and thus has a higher than normal peak velocity across it. The continuous-wave Doppler gradient across the prosthesis obtained 4 to 6 weeks following implantation serves as a baseline for subsequent evaluations. High gradients may also be obtained in nonobstructive situations, such as high-output states, tachycardia, anemia, severe prosthetic leaks, or from the pressure recovery phenomenon. Pressure recovery occurs secondary to flow acceleration through a narrowed orifice, especially in the central orifice of a bileaflet valve in the aortic position. In this setting, the highest pressure measured through the prosthesis by Doppler overestimates the true pressure gradient by approximately one-third, and manufacturers take into account pressure recovery when determining normal gradients.
(b)Valve area calculations. Calculation of orifice area in prosthetic valves is difficult given the complexity of the orifice (struts/disks), especially in mechanical prostheses. The following methods have been used to approximate orifice area:
a.Continuity equation. The continuity equation can be used to estimate the EOA of prosthetic aortic and mitral valves. For calculation of the prosthetic valve area in the aortic position,
Areaaortic prosthesis = (LVOTd)2 × 0.785 TVILVOT/TVIaortic prosthesis
where TVI is time–velocity integral.
The aortic prosthesis TVI is determined from continuous-wave Doppler velocity through the prosthesis. LVOT TVI is determined by pulsed-wave Doppler. Mitral valve prosthesis TVI is determined from continuous-wave Doppler. For the mitral position,
Area = (LVOT diameter)2 × 0.785 × TVILVOT/TVImitral prosthesis
(c)Pressure half-time (PHT). For a mitral valve prosthesis, the PHT method is useful for assessing prosthetic valvular stenosis, especially in comparison to prior values obtained at similar heart rates, although it does not accurately estimate EOA. The PHT can help determine whether increased velocity is secondary to increased flow or to obstruction. If the peak velocity is increased but the PHT is not prolonged, then the increased velocity is most likely due to increased forward flow.
(d)Dimensionless index. The LVOT and aortic valve prosthesis velocity ratio is the most helpful index for the evaluation of prosthetic valve stenosis, especially in the absence of a reliable LVOT diameter. The higher the index, the larger the EOA, and a normal aortic prosthesis has a ratio ≥0.30 to 0.35. A value ≤0.25 suggests prosthesis stenosis:
Dimensionless index = velocityLVOT/velocityaortic prosthesis OR TVILVOT/TVIaortic prosthesis
where TVI is time–velocity integral.
(2)Pathologic prosthetic valve regurgitation. The pathologic flow disturbance is larger and wider than that seen with physiologic regurgitation. Pathologic regurgitation may be related to calcified and fibrosed leaflets, disruption of the sutures securing the valve, or a perivalvular abscess with adjacent tissue destruction. Single or multiple jets may be present.
(a)Severe mitral prosthetic regurgitation is suggested by increased peak early diastolic velocity (>1.2 m/s) and normal mitral inflow PHT (≤150 m/s) (see Chapter 16).
(b)Severe aortic regurgitation is usually present when there is diastolic flow reversal in the descending thoracic or abdominal aorta (see Chapter 15).
(3)Cinefluoroscopy. Cinefluoroscopy is useful for assessing mechanical prosthetic valves. The image intensifier is moved to a position with x-rays parallel to the valve ring plane to determine the occluder’s excursions in a caged valve. Despite the radiolucency of pyrolytic carbon disk valves, the opening angle can be measured from positioning the image intensifier parallel to the plane of the open leaflets. The mitral prosthesis is best visualized from the right anterior oblique (RAO) cranial projections. The aortic prosthesis can be viewed from RAO caudal or left anterior oblique cranial projection.
(a)Diminished motion of the disks suggests valve obstruction, whereas excessive rocking of the base ring suggests partial dehiscence of the valve.
(4)Cardiac catheterization
(a)Invasive assessment of the left ventricle can be performed safely in patients with bioprosthetic aortic valves. However, catheter-based evaluation of the mechanical aortic valves should be performed with a transseptal technique. Transseptal access may also be necessary for accurate measurement of prosthetic mitral valve gradients, because catheter-based assessment overestimates the mitral valve gradient because of a dampening of the pressure contour and intrinsic delay in the pulmonary capillary wedge tracing.
(5)Magnetic resonance imaging can be performed safely in patients with most prosthetic valves, because they are not ferromagnetic, but imaging susceptibility artifacts often preclude assessment of prosthetic valve leaflets. However, dedicated sequences can provide information about blood flow velocities and regurgitant fractions.
(6)Multislice gated cardiac CT with retrospective image acquisition (4D CT) allows adequate evaluation of prosthetic leaflet motion and abnormalities of both bioprosthetic and mechanical valves. Like cinefluoroscopy, 4D CT can be used to measure opening and closing angles of mechanical valves. In addition, 4D CT can assess bioprosthetic leaflet thickening, thrombosis, and calcification. Unlike echocardiography, 4D CT displays only a single heart cycle.
III.Valve dysfunction and complications related to prosthetic valves
A.Atrial fibrillation. Up to 50% of patients undergoing valve surgery experience postoperative atrial fibrillation. Management of atrial fibrillation is discussed elsewhere.
1.In patients without a previous history of atrial fibrillation, the arrhythmia is often self-limited.
2.For patients with persistent atrial fibrillation beyond 24 hours, anticoagulation, direct current cardioversion, and a short course of antiarrhythmic therapy can be considered.
B.Conduction disturbances. High-grade heart block requiring permanent pacemaker implantation has been described in 2% to 3% of patients after valve replacement and 8% following repeat valve surgery. It is caused by trauma to the bundle of His or from postoperative edema of the periannular tissue. Aortic or mitral annular calcification, preoperative conduction disturbance, advanced age, and infectious endocarditis are associated with higher rates of postoperative conduction abnormalities, leading to permanent pacemaker implantation.
C.Endocarditis. Approximately 3% to 6% of patients with prosthetic heart valves will experience prosthetic valve endocarditis.
1.Early prosthetic valve endocarditis (<60 days following implantation) is typically caused by Staphylococcus epidermidis.
2.Late prosthetic valve endocarditis has a microbiology similar to community-acquired native valve endocarditis.
3.TEE is the imaging modality of choice, with sensitivity of 95% and specificity of 90% in diagnosis. TEE is also useful in detecting invasive complications such as abscess, valve dehiscence, and fistula formation.
4.Medical therapy. Medical cure for prosthetic valve endocarditis caused by staphylococci, gram-negative organisms, or fungi is rare. Streptococcal prosthetic valve endocarditis responds to medical therapy alone in 50% of cases. A high index of suspicion should be maintained for the presence of residual infection, and surgical reevaluation should be considered if medical treatment fails.
5.Surgical therapy. Valve replacement surgery is indicated in the setting of
a.Persistent bacteremia despite intravenous antibiotics
b.Tissue invasion or fistula formation
c.Recurrent embolization
d.Fungal infection
e.Prosthesis dehiscence or obstruction
f.New or worsening heart block
g.New-onset or worsening congestive heart failure
D.Hemolysis. Subclinical hemolysis is present in many patients with mechanical valves but rarely results in significant anemia.
1.Pathophysiology and etiology. Clinical hemolysis occurs in 6% to 15% of patients with caged ball valves but is uncommon with normal bioprosthetic or tilting disk valves. Clinical hemolysis is also associated with multiple prosthetic valves, small prostheses, periprosthetic leaks, and prosthetic valve endocarditis. Mechanisms involved in the generation of hemolysis include high shear stress or turbulence across the prosthesis, interaction with foreign surfaces such as cloth, and rapid deceleration of erythrocytes following collision with adjoining structures (e.g., struts or cardiac walls).
2.Laboratory examination and diagnostic testing
a.Diagnosis is made by elevated lactate dehydrogenase, reticulocyte count, unconjugated bilirubin, urinary haptoglobin, and presence of schistocytes on blood smear.
b.Echocardiographic findings consistent with mechanical hemolysis include abnormal rocking of the prosthesis or regurgitant jets of high shear stress (e.g., eccentric or periprosthetic regurgitant jets or those impacting a solid surface such as the left atrial appendage or sewing ring).
3.Therapy
a.Medical therapy. Mild hemolytic anemia can be managed with iron, folic acid supplement, and if needed, blood transfusion. β-Blockade and blood pressure control may reduce the severity of hemolysis. Paradoxically, treatment of the anemia may reduce the degree of hemolysis by limiting the need for high flow through the defective valve.
b.Surgical therapy. Repair of perivalvular leaks or valve replacement is indicated in patients with severe hemolysis requiring repeated transfusions or in those with congestive heart failure. Percutaneous approaches can also be considered, but are not feasible with extensive dehiscence or when there is active infection.
E.Thrombosis. Mechanisms of prosthetic valve dysfunction are highlighted in Figure 18.4. For thrombosis, the annual incidence of mechanical prosthetic valves is 0.2% to 1.8%. The incidence is highest in the tricuspid position, followed by the mitral and then the aortic position. Thrombus is suspected in patients with an acute onset of symptoms, an embolic event, or inadequate anticoagulation. Thrombosis at bioprostheses is uncommon, but may also occur.
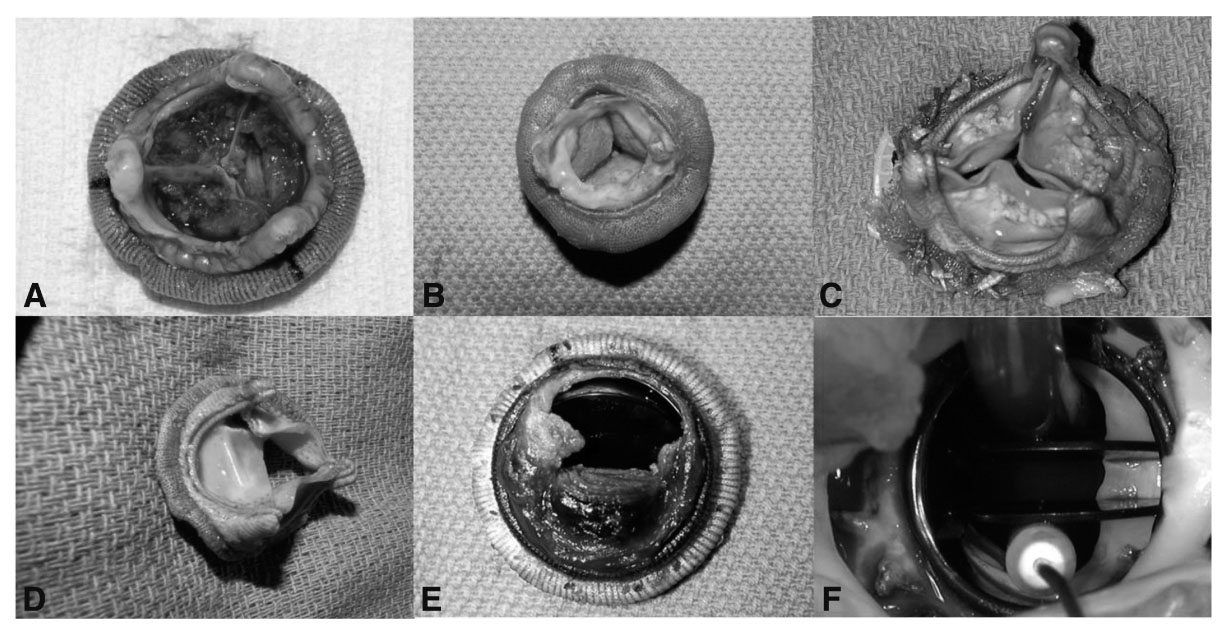
FIGURE 18.4 Modes of prosthetic valve dysfunction. A: Layering thrombi on the nonflow side of stented bioprosthesis; B: A ring of pannus on the flow side (subvalvular) of a stented bioprosthesis; C: Nodular cuspal calcifications of a stented bioprosthesis; D: Leaflet teat of a stented bioprosthesis; E: Thrombosed bileaflet mechanical valve; F: Subvalvular pannus ingrowth of a bileaflet mechanical valve. (Photos courtesy of Gosta B. Pettersson.)
1.Laboratory examination and diagnostic testing. TEE is the most widely employed diagnostic technique, although 4D CT is also useful. 4D CT or cinefluoroscopy can also be used to document restriction in occluder mobility. Echocardiographic features suggestive of thrombus include an irregular and mobile mass.
2.Therapy
a.Priority of therapy
(1)Heparin is typically initiated early in the course of evaluation.
(2)Warfarin is continued unless surgery is planned.
(3)TTE, TEE, 4D CT, or cinefluoroscopy can be performed as needed.
b.Medical therapy
(1)Fibrinolytic therapy is considered the treatment of choice for right-sided prosthetic valve thrombosis because the consequences of distal embolization are less severe than in left-sided prosthesis. Fibrinolytic therapy has an initial success rate of 82%, overall thromboembolism rate of 12%, and a 5% incidence of major bleeding episodes. For left-sided valves, there is a similarly high success rate (82%) with fibrinolytic therapy; however, the associated risks of death (10%) or systemic embolism (12.5%) are high. Thrombolysis should be considered for left-sided valves in patients with contraindications to surgery. Thrombolysis may be a reasonable alternative to surgery for mitral or aortic prosthetic valve thrombosis in patients with a small thrombus burden.
(a)The classical regimen for streptokinase is a 500,000 IU bolus given over 20 minutes, followed by an infusion of 1.5 million IU infused over 10 hours.
(b)The regimen for rtPA (recombinant tissue plasminogen activator) is 10 mg bolus followed by 90 mg an hour for 9 hours
(c)The use of slow infusion low dose fibrinolytic regimens have been reported in mechanical valve prosthetic thrombosis in pregnancy where 25 mg TPA without bolus was given over 6 hours and repeated after 24 hours as needed. This has been associated with low embolic and bleeding rates and successful thrombolysis in >90%and is endorsed for consideration in the 2017 ACC/AHA Valve Guidelines update.
(d)Thrombolysis should be stopped if there is no hemodynamic improvement after 24 to 72 hours. TEE is useful in the assessment of progress.
(e)Following successful thrombolysis, close follow-up of anticoagulation along with serial Doppler echocardiography is recommended.
(2)Anticoagulation with heparin and warfarin is generally recommended for a small thrombus (≤5 mm). The regimen consists of intravenous heparin followed by warfarin.
c.Surgical approach. The lowest surgical mortality reported has been approximately 5%. The risk profile of the individual patient must be balanced against the expertise and experience at each center.
(1)Valve replacement and debridement are generally performed for left-sided prosthetic valve thrombosis, unless the thrombus is small or the patient has a prohibitive surgical risk.
(2)Surgery is also indicated in the case of unsuccessful thrombolysis 24 hours following discontinuation of the infusion.
F.Dehiscence. Detachment of the sewing ring from the annulus may occur in the early postoperative period because of poor surgical techniques, excessive annular calcification, chronic steroid use, fragility of the annular tissue (particularly following prior valve operations), or infection. Late dehiscence occurs mainly from infectious endocarditis. Abnormal rocking of the prosthesis on echocardiography is an indication for urgent surgery.
G.Patient Prosthesis Mismatch (PPM). All prosthetic valves, with the exception of stentless aortic homografts, have effective orifices that are smaller than those of native valves. There is an inherent pressure gradient and relative stenosis with each prosthesis. Occasionally, when an inappropriately small prosthesis is placed, the low EOA may cause symptoms. PPM should be considered moderate if indexed valve EOA is >0.65 cm2/m2 but ≤0.85 cm2/m2, or severe if <0.65 cm2/m2. The impact and prevalence of PPM are controversial. Depending on the definition and surgical series used, this mismatch may occur between 20% and 70% of cases after aortic valve replacement. It has been shown in some series to be associated with worse hemodynamic function, less regression of LV hypertrophy, more cardiac events, and lower survival. Unlike most of the other risk factors, PPM can be avoided or its severity lessened by putting in place a prevention strategy at the time of the operation. It is rare that PPM occurs to a degree that surgical explantation is necessary.
Some important points to consider:
1.The projected indexed EOA should be systematically calculated at the time of the operation to estimate the risk of PPM.
2.In a patient with a small annulus, a hemodynamically favorable prosthesis like a stentless bioprosthesis, aortic homograft, or a tilting disk valve is preferred. Alternatively, the aortic annulus may be enlarged surgically in order to accommodate a prosthesis of acceptable size.
3.Aortic prostheses <21 mm in diameter are not recommended for a large or physically active patient.
4.Young patients in particular, as well as those with poor LV function and/or severe LV hypertrophy, are more vulnerable to PPM.
H.Pannus formation. Valve obstruction occurs in up to 5% of mechanical valves per year. Valve thrombosis and pannus formation are responsible for the majority of mechanical prosthesis obstructions. Frequently, thrombus coexists with pannus. Little is known about the causes of fibroblastic proliferation in pannus formation. Foreign body reactions to the prosthesis, inadequate anticoagulation, and endocarditis have been implicated as potential causes. Pannus formation begins around the annulus of the valve and is more common in aortic than at mitral valve prostheses. A subacute presentation of fatigue or dyspnea in a patient who is well anticoagulated can suggest pannus formation. TEE and/or 4D CT are/is generally required to identify the cause of prosthetic valve obstruction, although pannus is difficult to image.
I.Embolic stroke. Following an embolic stroke, the risk of recurrent stroke is approximately 1% per day for the first 2 weeks.
1.If no evidence of hemorrhage is detected on CT scan at 24 to 48 hours, intravenous heparin may be administered after a small to moderate embolic stroke. Maintaining anticoagulation reduces the risk of recurrent stroke to one-third but carries an increased risk of hemorrhagic transformation of 8% to 24%, particularly during the first 48 hours.
2.In patients with larger infarcts, anticoagulation is generally withheld for 5 to 7 days.
3.Anticoagulation is withheld for 1 to 2 weeks in the setting of hemorrhagic transformation based on recommendations from neurosurgical and neurology consultants.
4.Reoperation with placement of a tissue valve may be needed for recurrent embolization.
J.Mechanical failure
1.SVD is defined as deterioration in bioprosthetic leaflets or supporting structures, not related to thrombus or endocarditis, which eventually results in hemodynamic dysfunction. As valves age, bioprosthetic SVD is expected and may manifest as stenosis, regurgitation, or both. SVD is usually gradual and due to the deposition of calcium on the leaflets. However, leaflet tears may produce a sudden clinical deterioration with the onset of severe regurgitation. Indications for reintervention are similar to those for native valve lesions, although repeat intervention is reasonable in asymptomatic patients with severe regurgitation given that further dysfunction could result in rapid clinical deterioration.
2.Failure of the current generation of mechanical prostheses is rare but may precipitate sudden hemodynamic compromise. Catastrophic failure occurs when a strut holding the occluder breaks, allowing the occluder to embolize, resulting in overwhelming regurgitation. Strut failure has been reported most commonly with the Björk-Shiley valve and results from fatigue of a metal weld.
3.In older ball-in-cage prostheses, ball variance, a structural deterioration of the occluder, can occur, giving rise to impaired occluder motion, sticking, and thromboembolism. This is rarely seen nowadays with improved prosthetic materials.
ACKNOWLEDGMENTS: The author thanks Drs. Ron Jacob, Richard Troughton, and João L. Cavalcante for their previous contributions to this chapter.
Important Articles
Acar J, Iung B, Boissel JP, et al. Multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation. 1996;94:2107–2112.
Adams DH, Popma KK, Reardon ML, et al. Transcatheter aortic valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798.
Akins CW. Results with mechanical cardiac valvular prosthesis. Ann Thorac Surg. 1995;60:1836–1844.
Birkmeyer JD, Marrin CA, O’Connor GT. Should patients with Björk-Shiley valves undergo prophylactic replacement? Lancet. 1992;340:520–523.
Cannegieter SC, Rosendaal FR, Wintzen AR, et al. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11–17.
David TE, Armstrong S, Maganti M. Hancock II bioprosthesis for aortic valve replacement: the gold standard of bioprosthetic valves durability? Ann Thorac Surg. 2010;90(3):775–781.
Davis EA, Greene PS, Cameron DE, et al. Bioprosthetic versus mechanical prosthesis for aortic valve replacement in the elderly. Circulation. 1996;94:II-121–II-125.
Dumesnil JG, Pibarot P. Prosthesis-patient mismatch: an update. Curr Cardiol Rep. 2011;13(3):250–257.
Green CE, Glass-Royal M, Bream PR, et al. Cinefluoroscopic evaluation of periprosthetic cardiac valve regurgitation. Am J Radiol. 1988;151:455–459.
Israel DH, Sharma SK, Fuster V. Antithrombotic therapy in prosthetic heart valve replacement. Am Heart J. 1994;127:400–411.
Jaeger FJ, Trohman RG, Brener S, et al. Permanent pacing following repeat cardiac valve surgery. Am J Cardiol. 1994;74:505–507.
LaBounty TM, Agarwal PP, Chugtai A, et al. Evaluation of mechanical valve size and function with ECG-gated 64-MDCT. Am J Roentgenol. 2009;193(5):W389–W396.
Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic valves: a reports from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:589–590.
le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging. 2009;2(8):931–939.
Leipsic J, Gurvitch R, Labounty TM, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging. 2011;4(4):416–429.
Lengyel M, Fuster V, Keltai M, et al. Guidelines for management of left-sided prosthetic valve thrombosis: a role for thrombolytic therapy. J Am Coll Cardiol. 1997;30:1521–1526.
Leon MB, Smith CR, Mack MJ, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607.
Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620.
Özkan M, Çakal B, Karakoyun S, et al.Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation. 2013; 128: 532-540
Rahimtoola SH. Choice of prosthetic heart valve in adults: an update. J Am Coll Cardiol. 2010;55(22):2413–2426.
Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331.
Roudaut R, Lafitte S, Roudaut MF, et al. Fibrinolysis of mechanical prosthetic valve thrombosis: a single-center study of 127 cases. J Am Coll Cardiol. 2003;41:653–658.
Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart. 2007;93:137–142.
Smith CR, Leon MB, Mach MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198.
Van der Meulen JH, Steyerberg EW, Van der Graaf Y, et al. Age thresholds for prophylactic replacement of Björk Shiley convexo-concave heart valves. A clinical and economic evaluation. Circulation. 1993;88:156–164.
Vogel W, Stoll HP, Bay W, et al. Cineradiography for determination of normal and abnormal function in mechanical heart valves. Am J Cardiol. 1993;71:225–232.
Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med. 1996;335:407–416.
Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975–1014.
Key Reviews
Grunkemeier GL, Starr A, Rahimtoola SH. Prosthetic heart valve performance: long-term follow-up. Curr Probl Cardiol. 1992;6:334–406.
Nishimura R, Otto CM,. Bonow RO, et al. AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57-185
Nishimura R, Otto CM, Bonow R et al. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017 ;135:e1159-e1195.
Zabalgoitia M. Echocardiographic assessment of prosthetic heart valves. Curr Probl Cardiol. 1992;5:270–325.
Relevant Book Chapters
Garcia MJ. Prosthetic valve disease. In: Topol EJ, ed. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:389–398.
Otto CM, Bonow RO. Valvular heart disease. In: Bonow RO, ed. Braunwald’s Heart Disease. 8th ed. Philadelphia, PA: Saunders, Elsevier; 2008:1625–1712.