5
CHEMISTRY
Jay Labinger
The evolving relationship between literature and chemistry is intriguingly intertwined with the history of chemistry and its perception. We might even consider the origins of chemistry as primarily literary, not scientific, since the core concept of atomic theory was initially expounded by the ancients (Democritus, Epicurus, Lucretius, etc.) with little if any appeal to observational (let alone experimental) support. Experimental work was much more central to alchemy, the precursor of modern chemistry that flourished during the Middle Ages and beyond. Indeed, one commentator has proposed that many nineteenth-century representations of what would then be considered modern chemistry are actually responses to persisting images of alchemists (Schummer 2007). A bibliography of literature and science includes references to alchemy in works of authors right up to the present, including Hawthorne, E.T.A. Hoffmann, Poe, Strindberg, Yeats, Joyce, Nin, and Pynchon (Schatzberg et al. 1987). Alchemy and literature is treated at length elsewhere in this volume.
Modern chemistry is usually considered to begin with Lavoisier, towards the end of the eighteenth century, although many developments earlier in the eighteenth century – particularly those centered on the concept of affinity – are much more appropriately classified as chemistry than as alchemy (Kim 2003). And we have another reason to focus on Lavoisier: the chemical revolution that he helped initiate was not just experimental but literary.
For Lavoisier restructured chemistry from fundamental principles [and] provided it with a new language and fresh goals. … A modern chemist, on looking at a chemical treatise published before Lavoisier’s time, would find it incomprehensible; but everything written by Lavoisier himself, or composed a few years after his death, would cause a modern reader little difficulty.
(Brock 1992: 88; italics added)
The importance of this linguistic turn can be seen in the opposition it generated: phlogistonists and others attacked Lavoisier’s nomenclature, demanding one based on observable facts, not biased by theory (Golinski 1992). Some of the conservatives eventually acknowledged the impossibility of that goal, in language that rather strikingly anticipates much more recent developments.
Since the Lavoisian theory was still controversial, [William] Nicholson tried to give impartial expositions of it and the phlogistic alternative, couched in a purportedly neutral terminology. … His views about language had been shaken by the arrival of a new scheme of interpretation that exposed a purportedly factual discourse as itself theory-laden.
(Golinski 1992: 247–48)
Rhetorical analysis in all fields of scientific writing is of course commonplace today; some recent examples include chemist Roald Hoffmann’s article about the scientific article (Hoffmann 1988) and a couple of self-described “deconstructions” of early reports on the discovery of the Buckyball (Aldersey-Williams 1995: 78–90; 1996).
The following discussion of literary representations of the chemical sciences makes no attempt at comprehensive coverage;1 rather I will try to illustrate the range of themes that have interested writers.2 The division into two sections, nineteenth and twentieth century, is not so arbitrary as one might think, for the status of chemistry within science in general underwent a dramatic shift sometime around, or shortly after, 1900, resulting in a considerable change in the relationship between literature and chemistry.
The nineteenth century
Chemistry at the turn of the century, after the discoveries of Lavoisier and his introduction of the new nomenclature, was a pioneer science in which exciting progress was being made. It was natural that it should acquire a fashionable appeal.
(Sharrock 1962: 60)
During the nineteenth century, chemistry in many ways stood as an exemplar of experimental science and was a natural focus for writers who wished to portray aspects of contemporary science, even if those portrayals continued to be tinted by the residues of alchemy (Schummer 2007: 39). In England, chemist (and poet) Humphrey Davy was most responsible for connections between Lavoisier’s new chemistry and literature. A close friend of Coleridge and Wordsworth, he influenced them and others – not only through his writing and lectures, but perhaps in some cases even physically, by promulgating experiments on the mental effects of nitrous oxide (Knight 1970: 63).
Scientists and romantic poets might appear to be odd bedfellows, given the apparent divergence of their worldviews (mechanistic, reductionist, systematic vs. mystical, holistic, idealistic). Indeed, anti-science rhetoric from the Romantics was far from uncommon, most prominently in Blake but not absent among Davy’s friends, and there may be less than meets the eye in these connections: Wordsworth and Coleridge considered chemistry to be somewhat superficial, a relaxing diversion from the serious work of poetry (Sharrock 1962: 60). It has been observed that although Coleridge was certainly interested in chemistry (as were Keats and Shelley), little of it actually appears in his poetry (Ward 1976). Nonetheless, the English Romantics found the new science mostly congenial – “to Coleridge chemistry, ‘the striving after unity of principle, through all the diversity of forms,’ was ‘poetry, as it were, substantiated and realized’” (Knight 1970: 62) – and allowed it into their work in significant ways. Davy’s lectures, demonstrations, and treatises played a crucial role in the genesis of Frankenstein (Thoman 1998); Keats’s use of “poetic” words such as “ethereal” was colored by the more scientific meanings they acquired from Davy’s work (Sperry 1970).
Outside of England, the most notable appearance of chemistry in early nineteenth-century literature is in Goethe’s Elective Affinities, which draws its scientific inspiration not from Lavoisier but from his predecessors, particularly Swedish chemist Torbern Bergman’s work (of the same title) from the 1770s. Goethe offers chemical affinity as a metaphor for human relationships – a comfortable couple is torn apart when one (or both) is more strongly attracted to another party – with a lengthy disquisition on the process chemists used to call “double displacement” (now usually termed “metathesis”), which he describes in both abstract terms and a specific example, as represented in Figure 5.1 (Goethe 1809: 37–44). Goethe does not appear to foreshadow any causal connection between physiological chemistry and psychological behavior, a common enough theme in later work, such as The Brothers Karamazov (Schummer 2007: 58–59). Nonetheless, this early incorporation of detailed technical chemical knowledge into a fictional narrative is of considerable historical interest.3
Literary interest in chemistry was sustained through the century. Notable writers in whose work chemical themes have been identified include Balzac, Flaubert, Hawthorne, Poe, Dickens, Turgenev, and Dostoevsky (Schummer 2007; Varvogli and Varvoglis 1995; Schatzberg et al. 1987). For instance,
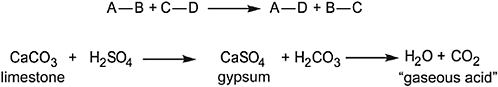
Figure 5.1 A generalized metathesis reaction, and Goethe’s example thereof.
Hawthorne’s oft-cited story “The Birthmark” alludes to the transition from alchemy to chemistry:
In the latter part of the last century there lived a man of science. … In those days … the comparatively recent discovery of electricity and other kindred mysteries of Nature seemed to open paths into the region of miracle.
(Hawthorne 1843: 264)
Here, the contents are clearly based on the former tradition, not the new science. Balzac depicted chemists and chemistry in several works, and was well informed about, if not particularly impressed by, its development as a modern science (Schummer 2007: 52, 57–58). At one point he seems to echo Goethe by analogizing chemical and psychological forces, to the point of metaphorically ascribing some “elective” powers to the former.
Are not fearful poisons set up in the soul by a swift concentration of all her energies, her enjoyments, or ideas; as modern chemistry, in its caprice, repeats the action of creation by some gas or other? Do not many men perish under the shock of the sudden expansion of some moral acid within them?
(Balzac 1831: 20)
Dickens gives us what at first seems a trivial case: the butler-made-chemist-by-simile –“Meanwhile the retainer goes round, like a gloomy Analytical Chemist: always seeming to say after ‘Chablis, sir?’–‘You wouldn’t if you knew what it’s made of’” (Dickens 1864: 52) – who is subsequently referred to simply as “the Analytical Chemist” or just “the Analytical.” Is this just a typical Dickensian fillip? Probably not: one commentator connects the character’s role in the novel to the public visibility of his titular colleagues.
So few are the scenes in which this Analytical Chemist appears and so seldom does Dickens give him a speaking part that it is easy to pass over him as just another whimsical flourish in a novel rich with imaginative embellishments of all kinds. But the Analytical’s morose presence is felt even in scenes over which he does not officially preside; indeed his grim taciturnity becomes an implicit commentary on the verbal excesses and artificial rhetoric of his employers.
(Metz 1979: 66)
This example illustrates, perhaps as well as any, the place of chemistry in nineteenth-century literature. While we find few really extensive considerations of up-to-date chemical theories and findings, authors showed increasing awareness of the prominence of modern chemistry in nineteenth-century science and society, and alluded to it almost as a matter of course. At the beginning of the twentieth century, and certainly by the end of World War I, the situation began to change.
The twentieth century
What strikes one first about chemistry in twentieth-century literature is that, in comparison to physics, biology and mathematics, there is so little of it.
(Ball 2007: 97)
Chemistry certainly does not disappear from twentieth-century literature: authors continued to make use of chemical themes, metaphors, and allusions, much as in the previous century. Some examples: a character in Zola’s turn-of-the-century Paris can be identified with noted French chemist Marcellin Berthelot (Gratzer 1989: 423–24); substantial passages in Proust’s À la recherche du temps perdu are expressed metaphorically in terms of chemical research (Large 1998); chemists and chemistry are found in a number of Updike novels (Varvogli and Varvoglis 1995: 45). But chemistry does appear to take a back seat to other sciences, a trend that has been ascribed to the absence of “grand themes”: chemistry is perceived to have become more of a technology, concerned with synthesizing things, than a science asking deep questions about the world (Ball 2007: 98).4 Contemporary chemistry has been described as falling “in-between” physics and biology in the public eye (Laszlo 2007: 335), without the fundamental underpinnings that would attract philosophers of science (Schummer et al. 2007: 1).5 The new developments that really caught the eye of the public (including authors) came mainly from physics (relativity, quantum mechanics) in the first half of the century, and biology (DNA and the genetic code) in the second half.
Thinking of chemistry as more an applied art than a profound science harks back, in some ways, to the image of alchemy. To be sure, appreciation of chemistry primarily for its potential practical use was not a new development: it is found even in the Romantics’ reaction to the new chemistry. Wordsworth proclaimed that “the discoveries of the chemist will become as proper subjects of the poet’s art as any others … familiar not in the laboratory but in their application throughout the general framework of social life” (Sharrock 1962: 72). However, in contrast to that earlier period, many manifestations of chemical discoveries in social life in the twentieth century came to be known for undesirable consequences – claims about “better things for better living through chemistry” notwithstanding. At the same time, “chemophobic” themes begin to appear in twentieth-century literature.
Perhaps the first such theme one might think of – at least in terms of popular visibility – comes from detective and crime fiction, whose authors always look to chemistry for ever-more-subtle and ingenious ways to poison victims. This topic, usually taken to date back to Sherlock Holmes,6 has attracted a great deal of attention; two entire collections (mostly concerned with non-fictional forensic science) devoted to “Chemistry and Crime” (Gerber 1983; Gerber and Saferstein 1997) feature several articles on fiction. But in fact we do not often see any real anti-chemistry feelings here. The majority of cases involve familiar poisons – cyanide, arsenic, strychnine, etc. – and even when more exotic agents do appear, the net effect seems oddly pro-science. Agatha Christie’s work provides some notable examples, as when a series of apparently supernaturally induced deaths, or the observation of a mysterious aura about an imminent victim, are explained respectively by thallium and phosphorus poisoning (Christie 1962, 1937). Relatively few unfavorable images of chemistry are found in this genre; one such is in the Bulldog Drummond series of the 1920s, which features “a poison which is absorbed through the skin … with [a] chemist fashioning it into a weapon so horrible as to make war unthinkable” (Rae 1983: 565).7
The most elaborate treatment of chemistry in detective literature is found in The Documents in the Case (Sayers and Eustace 1930), in which a man who appears to have died from eating the wrong kind of mushrooms (Amanita muscaria instead of A. rubescens) was actually poisoned by the addition of synthetic muscarine to an innocuous dish; the murder is exposed by the discovery that the remnants show no trace of optical activity (the rotation of polarized light), which the natural (but not the synthetic) toxin would. The exposition includes a synthetic route to muscarine and its chemical formula (both were incorrect, unfortunately8) and, of greater interest, some remarks about the key role of chirality in life, which the authors did get pretty much right.
[U]p to the present, it is only living substance that has found the trick of transforming a symmetric, optically inactive compound into a single, asymmetric, optically active compound. …“At the moment when Life first arose, a directive force came into play … to select one crystallised enantiomorph and reject its asymmetric opposite.”
(Sayers and Eustace 1930: 244–46)
Later in the century, as the hard-boiled detective and the police procedural took over the field, chemistry was relegated to supporting forensic work, as in the series of novels by Patricia Cornwell (Gerber 1997), or vanished altogether. A notable exception to the latter trend is Joseph Wambaugh’s The Delta Star (Wambaugh 1983), which (though undeniably in the hard-boiled school) includes a murder by scientific instrument, as well as some serious scientific content (photochemistry).9
The role of chemistry in science fiction, another genre in which it might be expected to feature predominantly, appears to be rather limited (again, in comparison to physics and biology). Much of the material in a collection of essays on the topic (Stocker 1998) addresses science fiction in general rather than focusing specifically on chemistry, although a number of interesting illustrations are presented, including two works by chemist-author Isaac Asimov – a mock-scientific article concerning thiotimiline, a substance so soluble that it dissolves before water is added, and a short story about a goose that really does lay golden eggs – both reprinted in full.
A much more significant factor in the increasingly negative image of chemistry was the introduction of gas warfare in World War I; much of the onus thereof centers on the figure of Fritz Haber, who appears (explicitly or thinly disguised) in several literary works (Hoffmann and Laszlo 2001). The most notable of these is the verse play Square Rounds (Harrison 1992), which includes several other historical figures (Justus von Liebig, William Crookes) along with Haber, and includes a great deal of exposition of chemical detail, using a variety of theatrical devices (including stage magic) to leaven the potential didacticism. The play does not take a purely anti-chemistry moral stance: characters question whether gas warfare is inherently any more inhumane than explosives (or, for that matter, the eponymous square bullets invented by an eighteenth-century Englishman to increase the pain inflicted on non-Christian opponents); chemistry’s positive contributions are also highlighted, although by no means as an unmixed blessing.
Nitrogen fixation giving ammonia NH3
Makes fertilizers, yes, but also TNT.
Nitrogen as nitrates could make all Europe green
But it blasts in even blacker as tri-ni-tro-to-lu-ene.
The nitrogen you brought from way up high
Now blows the men you saved into the sky.
Those nitrates you produced for fertilizer
Now serve the warlike purpose of the Kaiser.
(Harrison 1992: 27)
An earlier (but far less entertaining) dramatic portrayal of technological advancement as a double-edged sword is Kaiser’s two-part play Gas, in which an unspecified gas powers the world economy, but occasionally explodes and kills people; a popular uprising against its use is quelled with the aid of a newly invented poison gas (Kaiser 1918, 1920). This theme is common in twentieth-century literature, with numerous cautionary tales about environmental degradation and adverse health effects, all the way up to global-scale catastrophic events.
Truly apocalyptic literary disasters relate to chemistry less frequently than to physics or biology (think of On the Beach or Oryx and Crake); perhaps the most familiar end-of-the-world story involving chemistry is Kurt Vonnegut’s Cat’s Cradle, in which a high-melting crystalline modification of water (“ice-nine”) nucleates the solidification of the entire terrestrial water supply (Vonnegut 1963).10 In Don DeLillo’s White Noise there is a literal “airborne toxic event”–a release of a mythical waste product: “Nyodene D. is a whole bunch of things thrown together that are byproducts of the manufacture of insecticide. The original stuff kills roaches, the byproducts kill everything left over” (DeLillo 1986: 131). But it affects only a limited area and does not actually kill anybody. More important, though, is the metaphoric significance of the event: it stands for death in general, the referent of the book’s title: “What if death is nothing but sound?”“Electrical noise. … Uniform, white” (DeLillo 1986: 198). DeLillo’s choice of a chemical metaphor emphasizes the synthetic character of contemporary society and its inability to deal with death as a natural part of life.11
Richard Powers’s Gain, which intertwines the history of a chemical company with the story of a woman dying of ovarian cancer – possibly caused by exposure to the company’s discharges – looks at a larger, more complex picture. Chemical synthesis is responsible not only for the toxic wastes that may have caused the cancer, but also for the therapeutic chemicals that might cure it; the point is reinforced by the fact that one of the agents used is taxol, originally a “natural” product but now made synthetically, with the company in question providing some of the precursors for the process (Powers 1998: 75, 151). There is also a modest dose of hard chemistry; Powers’s comparison of metathesis reactions involved in making soda to dancers exchanging partners (130–31) recalls Goethe’s much earlier analogy.
There has been some counterbalance to chemophobic themes in the twentieth century, much of it provided by practicing chemists who turned to literature. Primo Levi, a chemist and survivor of Auschwitz who wrote extensively on both of those experiences, gives us in The Periodic Table a group of autobiographical vignettes and short stories, each taking its inspiration from one of the chemical elements.12 The last one, on carbon, eloquently rebuts the chemistry–death connection by reconnecting it to life.
[E]very element says something to everyone (something different to each). … One must perhaps make an exception for carbon, because it says everything to everyone. … To carbon, the element of life, my first literary dream was turned, insistently framed in an hour and a place when my life was not worth much: yes, I wanted to tell the story of an atom of carbon.
(Levi 1975: 225)
Roald Hoffmann and Carl Djerassi are two more recent chemists-turned-authors. The former is best known for his poetry and essays; in one book on the relation of science to Jewish traditions he examines the aforementioned topic of chirality and its origins (Hoffmann and Schmidt 1997), while another expounds on the positive aspects of synthesis in chemistry, emphasizing creativity rather than artificiality (Hoffmann 1995: 85–100). Djerassi has written a series of novels and plays that he describes as “science-in-fiction” (Djerassi 1998: ix); they are as much (or more) about the scientific profession in the contemporary world as the science itself.
Hoffmann and Djerassi have collaborated on a play, Oxygen, which is a good place to conclude, as the subject of the play – to whom should an (imaginary) “retro-Nobel Prize” for the discovery of oxygen be given? – takes us full circle to the starting point for this essay: Lavoisier is one of the three candidates (along with Priestley and Scheele). Oxygen attempts to communicate both the findings and the politics of science without sacrificing comprehensibility or entertainment value – an ambitious goal, as the authors suggest in their dialogue: “Who’d like to come up with some simple phrases to explain to [the] public that without the discovery of oxygen there would’ve been no Chemical Revolution … no chemistry as we now know it?” (Djerassi and Hoffmann 2001: 28). Works of this sort inspire hope that chemistry can reclaim its stature as a subject for serious exploration in literature, by chemist and non-chemist authors alike, as we move forward in the twenty-first century.
Acknowledgments
Thanks to Steve Weininger, Jan Golinski, and Rolf Selbmann for helpful discussions and/or providing copies of hard-to-access articles.
Notes
1 To the best of my knowledge there is no such treatment of the field. There are several essays on the general topic of literature and chemistry (Rae 1983;Varvogli and Varvoglis 1995; Weininger 2002); others focus on the nineteenth (Schummer 2007; Selbmann 1996) or twentieth (Krätz 1991; Ball 2007) century. An annotated bibliography on literature and science (Schatzberg et al. 1987) provides many useful references; annual updates can be found in various issues of the journal Configurations. A compendium of excerpts (Gratzer 1989) contains a large number of chemistry-related examples, along with useful commentaries thereon; an entire volume of another collection is devoted to chemistry (Dolan 2004) but contains primarily non-fiction and runs only through 1834.
2 One that I will not address, although it arguably falls into the realm of chemistry, is thermodynamics, which has its own separate chapter in this volume. Entropy in particular constitutes an important subject in works such as The Education of Henry Adams, Pynchon’s The Crying of Lot 49, Stoppard’s Arcadia, among many others. Medical aspects of chemistry are largely left to the chapter on Literature and Medicine.
3 Not enough, though, to redeem what I would otherwise characterize as a rather tedious tragedy of manners.
4 In a similar vein, Horgan suggests that whereas all of science is running out of great questions by the end of the twentieth century, chemistry did so around 1930 (Horgan 1996). Few chemists would share this perception (Ball has a PhD in physics, although he did study chemistry as well).
5 Again, chemists (including Laszlo) dispute these characterizations, replacing “in-between” with “central” (Breslow 1997); there has also been a recent strong resurgence of interest in philosophy of chemistry (Scerri and McIntyre 1997).
6 Actually, this is a misconception: although Holmes is a formidable amateur chemist, often found in the middle of recreational experiments, one finds surprisingly little chemistry in his actual detective work, as others have also remarked (O’Brien 1993).
7 The idea of a poison so lethal and penetrating as to kill virtually instantly on contact continued to fascinate later authors (Stout 1937; Francis 1978); the former’s method, which drastically overestimates the toxicity of the proposed agent (nitrobenzene), would certainly not work.
8 As has been pointed out elsewhere (Foster 1983), the correct structure of muscarine was not established until after the book was published; but the formula given was known to be incorrect, and the synthetic method could not possibly have produced a structure capable of exhibiting optical activity. Still later it was determined that muscarine is not the main toxic principle of A. muscaria (which is not all that lethal anyway, compared to other members of the Amanita family).
9 Wambaugh gleaned the latter through his friendship with my Caltech colleague, chemist Harry Gray.
10 According to H.G. Wells, the concept was originally offered to him by the famous colloid chemist Irving Langmuir as a promising basis for a science-fiction story (Gratzer 1989: 309).
11 Chemistry in both White Noise and Gain is discussed at considerably greater length in Ball 2007.
12 For an allegorical reading of The Periodic Table’s chemical elements, see Clarke 1993. Another notable element-inspired autobiography (by a non-chemist) is Oliver Sacks’s Uncle Tungsten (Sacks 2001).
Bibliography
Aldersey-Williams, H. (1995) The Most Beautiful Molecule: the discovery of the Buckyball, New York: John Wiley and Sons.
——(1996) “Reading between the lines,” The Chemical Intelligencer, 2(4): 37–41.
Ball, P. (2007) “Chemistry and power in recent American fiction,” in Schummer et al. 2007, pp. 97–135.
Balzac, H. de (1831) Le Peau de Chagrin; trans. anon., The Magic Skin,inThe Novels and Dramas of Honoré de Balzac, vol. 1, New York: George D. Sproul, 1903.
Breslow, R. (1997) Chemistry Today and Tomorrow: the central, useful, and creative science, Washington, DC: American Chemical Society.
Brock, W.H. (1992) The Norton History of Chemistry, New York: W.W. Norton.
Christie, A. (1937) Poirot Loses a Client, New York: Dodd Mead and Co.
——(1962) The Pale Horse, New York: Dodd Mead and Co.
Clarke, B. (1993) “Aspects of the daemonic in Primo Levi’s The Periodic Table,” in M.W. McRae (ed.) The Literature of Science: perspectives on popular scientific writing, Athens: University of Georgia Press, pp. 169–85.
DeLillo, D. (1986) White Noise, New York: Penguin Books.
Dickens, C. (1864) Our Mutual Friend, New York: Penguin Books, 1971.
Djerassi, C. (1998) NO, Athens: University of Georgia Press.
——and Hoffmann, R. (2001) Oxygen, Weinheim: Wiley-VCH Verlag.
Dolan, B. (ed.) (2004) Chemistry, vol. 8 of J. Hawley (ed.) Literature and Science, 1660–1834, London: Pickering & Chatto.
Foster, N. (1983) “Strong poison: chemistry in the works of Dorothy L. Sayers,” in Gerber 1983, pp. 17–29.
Francis, D. (1978) Trial Run, New York: Pocket Books.
Gerber, S.M. (ed.) (1983) Chemistry and Crime: from Sherlock Holmes to today’s courtroom, Washington, DC: American Chemical Society.
——(1997) “Forensic science in detective fiction,” in Gerber and Saferstein 1997, pp. 191–98.
——and Saferstein, R. (eds) (1997) More Chemistry and Crime: from Marsh arsenic test to DNA profile, Washington, DC: American Chemical Society.
Goethe, J.W. v. (1809) Die Wahlverwandtschaften, trans. E. Mayer and L. Bogan Elective Affinities, Chicago: Henry Regnery, 1963.
Golinski, J. (1992) “The chemical revolution and the politics of language,” The Eighteenth Century, 33: 238–51.
Gratzer, W. (ed.) (1989) A Literary Companion to Science, New York: W.W. Norton.
Harrison, T. (1992) Square Rounds, London: Faber and Faber.
Hawthorne, N. (1843) “The Birthmark,” in H.H. Waggoner (ed.) Nathaniel Hawthorne: selected tales and sketches, 3rd edn, New York: Holt, Rinehart and Winston, 1970, pp. 264–81.
Hoffmann, R. (1988) “Under the surface of the chemical article,” Angewandte Chemie, International Edition in English, 27: 1593–1602.
——(1995) The Same and Not the Same, New York: Columbia University Press.
——and Laszlo, P. (2001) “Coping with Fritz Haber’s somber literary shadow,” Angewandte Chemie, International Edition in English, 40: 4599–604.
——and Schmidt, S.L. (1997) “You must not deviate to the left or the right,” in Old Wine New Flasks: reflections on science and Jewish Tradition, New York: W.H. Freeman and Co., pp. 79–121.
Horgan, J. (1996) The End of Science: facing the limits of knowledge in the twilight of the scientific age, Reading, Mass.: Helix Books.
Kaiser, G. (1918) Gas, trans. B.J. Kenworthy, in Five Plays, London: Calder and Boyars, 1971.
——(1920) Gas Zweiter Teil, trans. B.J. Kenworthy, in Five Plays, London: Calder and Boyars, 1971.
Kim, M.G. (2003) Affinity, That Elusive Dream: a genealogy of the chemical revolution, Cambridge, Mass.: MIT Press.
Knight, D.M. (1970) “The physical sciences and the romantic movement,” History of Science, 9: 54–75.
Krätz, O. (1991) “Die Chemie im Spiegel der Literatur des 20. Jahrhunderts,” Chemie in Unserer Zeit, 25: 44–50.
Large, D. (1998) “Chemical solutions: scientific paradigms in Nietzsche and Proust,” in E.S. Shaffer (ed.) The Third Culture: literature and science, New York: Walter de Gruyzer, pp. 217–36.
Laszlo, P. (2007) “On the self-image of chemists, 1950–2000,” in Schummer et al. 2007, pp. 329–67.
Levi, P. (1975) Il Sistema Periodico, trans. R. Rosenthal, The Periodic Table, New York: Schocken Books, 1984.
Metz, N.A. (1979) “The artistic reclamation of waste in Our Mutual Friend,” Nineteenth Century Fiction, 34: 59–72.
O’Brien, J.F. (1993) “What kind of a chemist was Sherlock Holmes?” Chemistry & Industry (London), 394–98.
Powers, Richard (1998) Gain, New York: Farrar, Straus and Giroux.
Rae, I.D. (1983) “Dustcoats in dustjackets,” Chemistry in Britain, 565–69.
Sacks, O. (2001) Uncle Tungsten, London: Picador.
Sayers, D.L. and Eustace, R. (1930) The Documents in the Case, New York: Harper Paperbacks, 1995.
Scerri, E.R. and McIntyre, L. (1997) “The case for the philosophy of chemistry,” Synthese, 111: 213–32.
Schatzberg, W., Waite, R.A. and Johnson, J.K. (1987) The Relations of Literature and Science: an annotated bibliography of scholarship, 1880–1980, New York: Modern Language Association of America.
Schummer, J. (2007) “Historical roots of the ‘mad scientist’: chemists in nineteenth-century literature,” in Schummer et al. 2007, pp. 37–79.
——, Bensaude-Vincent, B. and Van Tiggelen, B. (eds) (2007) The Public Image of Chemistry, Singapore: World Scientific Publishing Co.
Selbmann, R. (1996) “Auf den Menschen reimt sich die ganze Natur: über das Verhältnis von Chemie und Literatur im 19. Jahrhundert,” Euphorion – Zeitschrift für Literaturgeschichte, 90: 153–65.
Sharrock, R. (1962) “The chemist and the poet: Sir Humphrey Davy and the preface to Lyrical Ballads,” Notes and Records of the Royal Society of London, 17: 57–76.
Sperry, S.M. Jr. (1970) “Keats and the chemistry of poetic creation,” PMLA, 85: 268–77.
Stocker, J.H. (1998) Chemistry and Science Fiction, Washington, DC: American Chemical Society.
Stout, R. (1937) The Red Box, New York: Farrar & Rinehart.
Thoman, C.J. (1998) “Sir Humphrey Davy and Frankenstein,” Journal of Chemical Education, 75: 495–96.
Varvogli, A. and Varvoglis, A. (1995) “Chemists as characters and as authors in literature,” The Chemical Intelligencer, 1(2): 43–46, 55.
Vonnegut, K. (1963) Cat’s Cradle, New York: Holt, Rinehart & Winston.
Wambaugh, J. (1983) The Delta Star, New York: William Morrow and Co.
Ward, R. (1976) “What forced by fire: concerning some influences of chemical thought and practice upon English Poetry,” Ambix, 23: 80–95.
Weininger, S.J. (2002) “Chemistry,” in P. Gossin (ed.) Encyclopedia of Literature and Science, Westport, Conn.: Greenwood Press, pp. 77–79.