Hard substrata provide habitat for sedentary and sessile organisms, and the density and diversity of these epifaunal communities are determined by the persistence of the habitat in relation to prevailing hydrodynamic regimes. In low-energy environments accumulations of dead shell on soft, muddy sea floors may support clumps of large hydroids or sea squirts, tolerant of silty, turbid waters. Offshore shell gravels, in higher-energy environments, may be habitat for diverse communities of encrusting animals, but these will have a sheet-like, or otherwise low-profile, form, able to withstand a high degree of current scour. Mussel beds and banks of coralline algae, maerl, may be the bases of high-diversity assemblages, with numerous encrusting species, an associated fauna of small, free-living species, and a variety of predators, large and small. However, although biogenic habitats may persist for decades, they are not truly permanent: mussels and coralline algae grow and die, though at dramatically different rates, and the structure of the habitats they provide, and the faunal assemblages they support, are continually changing. Boulder habitats are also less stable than they appear. They are characteristic of turbulent hydrodynamic environments, often scoured by sand and gravel, and sporadically overturned, with a frequency related to the size of each boulder. Bedrock provides the only natural, stable, hard substratum habitat, and while rock is subject to slow solution, abrasion and occasional catastrophic collapse, in comparison with other hard substrata it is effectively permanent. Offshore hard grounds, consisting of bedrock interspersed with patches of unconsolidated coarse sands and gravels, cobbles and boulders, and shell debris, extend across much of the shelf to the northwest, populated by sessile and motile epifaunas. A degree of inclination often results in a change in faunal assemblages, and steep to vertical rock faces may be carpeted by sessile animals, benefiting from a refuge from competitors, predators and physical scour.
Hard-ground benthos consists of an encrusting epifauna of bivalves, tubeworms, large, solitary sea squirts (or ascidians) and, especially, colonial, or modular, animals (Box 3), including sponges, cnidarians, bryozoans and small ascidians, and non-modular clonal species, such as sea anemones. There is also a motile fauna of small, non-colonial or unitary organisms, particularly polychaetes, small crustaceans, molluscs and echinoderms, that varies in density and diversity in relation to water flow and the incline of the rock substratum. Many of these will build and occupy fine silt tubes, weaving between the primary space occupiers, and will be suspension feeders or detritivores; others will be free-roving detritivores or micropredators. There may also be guilds of larger predators, each species often with a narrowly defined array of preferred prey.
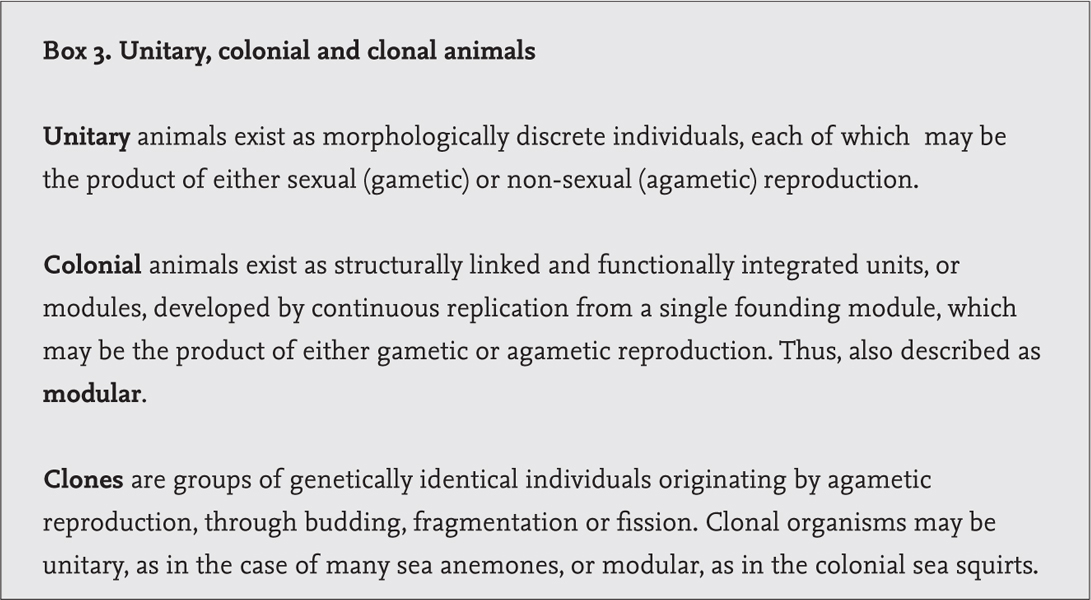
The composition, diversity and density of hard-ground benthic communities is initially defined by physical factors, and may change sharply with depth, water flow, rock type and inclination, and the abundance and diversity of the motile component depend largely upon the shelter and food provided by the attached epifauna. Gradient is important: flat areas in low-energy environments tend to accumulate silt, the finest silt collecting in the stillest water, and silt is inimical to many suspension feeders. Captorial suspension feeders, such as some of the larger hydroids, may tolerate considerable siltation, but filter-feeding bryozoans cannot, and the aquiferous systems of sponges are soon clogged. On sloping and vertical rock surfaces silt does not persist, and as water flow increases towards more exposed habitats, sand scour has another structuring effect on sessile benthos. The bushy bryozoan Flustra foliacea (Fig. 47) lives in aggregations on current-swept sea floors. Its colonies are largely chitinous, and flexible, and the orifice of each constituent module, or autozooid, through which the feeding lophophore of tentacles is exserted, is ringed by a palisade of thick, chitinous spines. Flustra foliacea seems highly resistant to sand scour; it is long-lived – individual colony ages are known to exceed 15 years – and while its populations may increase by larval settlement and recruitment, colonies are also able to regenerate though new growth from the base. On steep and vertical faces, above the turbulent bottom layers of the water column, F. foliacea may be displaced by populations of the gregarious tubeworm Sabellaria spinulosa and the clonal anemone Metridium senile (Fig. 80). This apparent zonation may reflect not only the tolerance of each species to physical stress, but perhaps also feeding functions. Anemones collect large food items from strong water currents, and sabellid worms require finer materials, while bryozoans are microphagous filter feeders.


FIG 80. The Plumose Anemone, Metridium senile. (J. S. Porter)
SPATIAL COMPETITION
For hard-substratum sessile communities, space, to which to attach, and on which to grow and expand, is a limiting resource, and competition for space is constant and intense. On well-lit surfaces, in clear water, algae will prevail and encrusting invertebrates will be restricted to impermanent refuges on stipes and holdfasts of some of the larger brown seaweeds. In shaded habitats, and in deeper or turbid water, algal populations decline, and hard substrata support communities of encrusting invertebrates (Fig. 81). New space on crowded surfaces becomes available following the death of an occupant, through senescence, predation or random accident, and is soon colonised by new settlers. Unitary organisms, such as serpulid tubeworms, saddle oysters and solitary ascidians, can only retain living space by resisting overgrowth or dislodgement by spatial competitors, and some species may be only transitory components of the assemblage. Competitively inferior species may persist only through a strategy of rapid growth to maturity and early reproduction, and populations are sustained entirely through continuous larval recruitment. In the case of modular and non-modular clonal animals, a single larval recruit establishes the species in the community, and the newly settled juvenile then grows by replication of identical functional units, modules, polyps or zooids, or through fission, to expand into all available space. Colony form of modular animals is often indeterminate, with clones having no regular pattern or distribution, and spatial competitors may be surrounded and enclosed, and prevented from expanding, or dislodged, or simply overgrown and smothered.
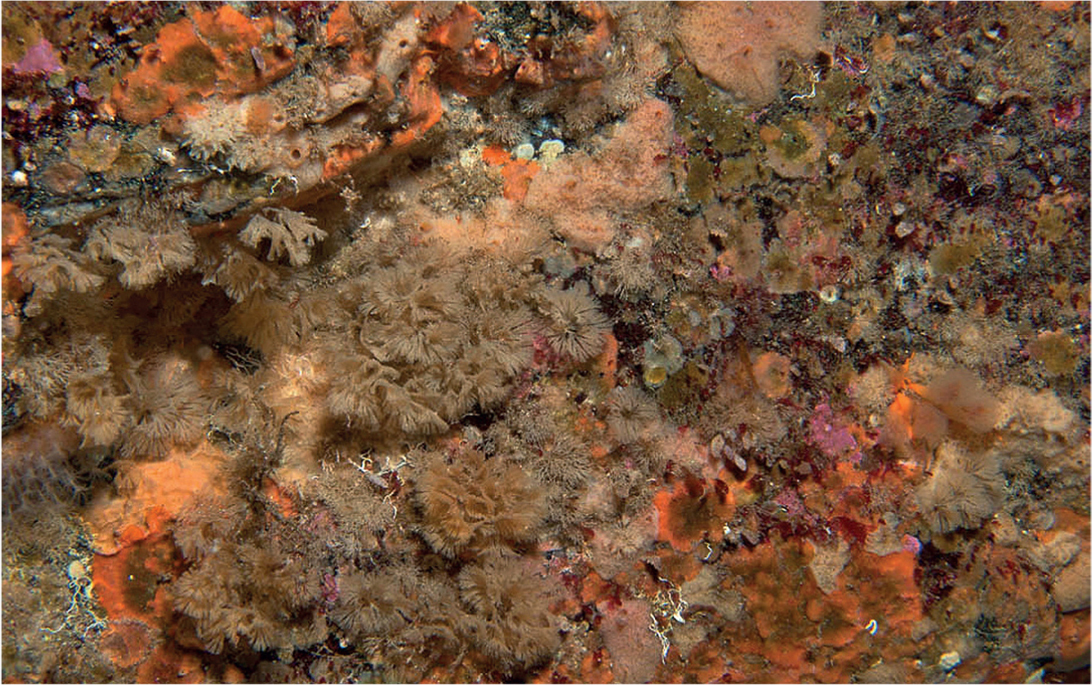
FIG 81. An epilithic community of sessile, suspension-feeding, modular animals, with bryozoans especially predominant. (J. S. Porter)
Colonies and clones are the competitive elite among hard-substratum epifaunal assemblages, and employ a range of strategies to hold and increase the territory they occupy. Sponges have the most sophisticated defences; they are profoundly sedentary animals and rely upon an arsenal of chemical repellants to discourage both spatial competitors and predators. There is a number of specialised predators, mostly small sea slugs, that are resistant to sponge toxins, and a suite of small hydroids, polychaetes and crustaceans may live as symbionts of sponges, but almost all marine sponge species, in northwest shelf environments, are invulnerable to overgrowth. Cnidarians are equipped with cnidocytes, stinging cells that serve to subdue prey and to deter territorial invaders, and tightly packed anemone clones provide a formidable defence against intruders. The soft coral Alcyonium digitatum (Fig. 82), dependent, lobed colonies of which earned it the epithet ‘dead men’s fingers’, is a long-lived animal, and individual colonies have been recorded occupying the same spot for as much as a decade, achieving heights above 10 cm, from a much broader base, and weighing more than 1 kg. Through spring and summer A. digitatum colonies feed through daylight hours, and are in active growth, but from late summer they enter a period of quiescence, when feeding polyps are permanently contracted, and gonads ripen in preparation for winter reproduction (Hartnoll, 1975). The surfaces of the colony, usually covered with an accumulation of mucus-bound sand and silt, are then likely to be settled by diatoms and algal spores, which develop into communities of erect algae and hydroids, which are colonised by small tube-building amphipods and polychaetes. The soft coral appears to have no defence against this occupation, but when growth and feeding resume in the spring it simply sheds its outer epithelial layer, together with the burden of epibionts.

FIG 82. The soft coral Alcyonium digitatum. (J. S. Ryland)
As with sponges, there are specialised predators of all the larger cnidarian species, and a number of symbionts, but none is easily dislodged or overgrown by spatial competitors. Large, solitary ascidians have fewer defences than either sponges or cnidarians, but their outer tests are tough, they will tolerate a degree of overgrowth or encrustation by other sessile animals, and their extensible siphons and strong respiratory currents repel settling larvae from these critical areas. Encrusting bryozoans tend to be the least successful spatial competitors, but they may be both common and taxonomically diverse in many sessile, epilithic communities, and there is intense competition for space between them. Some species produce bioactive compounds that deter prospecting larvae from settling on colony surfaces, and possibly also prevent overgrowth in spatial interactions. Other strategies include blocking the growing edge of a competitor, overgrowth and smothering, and overarching an opponent’s bell of feeding tentacles to deplete its food resources. Among these communities of small sessile species, competitive networks have been observed, with the advantage passing from one species to another, perhaps in relation to season, reproductive period, settlement and growth rate, and perhaps mediated by interactions between sessile and motile components of the community.
SITES AND SETTLEMENT
Life cycles and reproductive strategies of hard-substratum sessile invertebrates show interesting contrasts with those of the soft-sediment macrofauna. For example, modular animals, and non-modular clones, may be very long-lived, and persistent, with some individuals occupying the same territory for decades; yet their reproductive output may be small, and some species may increase only by non-sexual replication. A majority of the spatially dominant encrusting species have perennial life cycles that begin with a lecithotrophic larva. Rocky habitats are sparsely distributed, and space on which to settle is scarce; over-dispersal risks high mortality, and the larvae of rock-encrusting species are most often characterised by short free-swimming periods. Lecithotrophic larvae are produced in much smaller numbers than the planktotrophic larvae characteristic of most soft-sediment benthos, and adult populations of encrusting species may be limited by the rate of larval supply: they are said to be recruitment-limited. In soft sediments food is a more significant factor limiting adult populations than space; larval supply is not generally limiting, and is not a major determinant of the abundance and distribution of adult populations. However, planktotrophic larvae experience higher mortality during the free-swimming phase than lecithotrophic larvae, and are often subject to higher post-settlement mortality. Consequently, soft-sediment species tend to show strong interannual variation in year-class strength, while hard-substratum sessile species show fairly constant patterns in distribution and density between successive years.
The most important distinction between larval modes and strategies of hard- versus soft-substratum benthos relates to settlement processes. On soft sediments the distribution of settling larvae is largely dependent upon hydrodynamic factors. Some species are able to delay metamorphosis until currents carry them into habitats suitable for settlement; others may secrete mucus drift anchors that carry them towards their optimum habitat. In either case it must be assumed that the final stimulus to settlement is simply a change in current flow, and it is not apparent that any soft-sediment species exerts further choice in the selection of a settlement site; once landed, metamorphosis ensues and the settlers undergo severe post-settlement mortality before the surviving proportion recruits to the population (Hunt & Scheibling, 1997). In contrast, larvae of hard-substratum sessile species exhibit complex pre-settlement behaviours that serve to narrow the choice of settlement site by the prospecting larva (Chia, 1989). Cues for settlement may be negative as well as positive: prospecting larvae encountering sponges, if not killed or disabled by the chemical defences of the sponge, will quickly move away. Other primary space occupiers, high in the competitive hierarchy, such as some modular ascidians, exude substances that do not damage exploring larvae but instead seem to promote renewed swimming, and thus discourage settlement on, or in proximity to, the spatial dominant.
The settlement behaviour of lecithotrophic larvae of sessile rocky benthos reflects a sequence of responses to successive cues, and may show a number of distinct phases. Initial cues may be provided by physical processes. An important first response might be to illumination, with larvae displaying either positive or negative phototaxis, and swimming towards or away from light. This may be followed by, or in synchrony with, a geotactic response, the larva displaying a positive, downward-swimming, geotaxis, or perhaps a negative response, leading up towards a downward-facing shaded overhang. Additional cues might arise from water flow and turbulence, with some species selecting current-swept surfaces and others attracted to shelter. As the larva begins to explore the surface its behavioural responses have directed it to – in light or shade, open surface or overhang, high- or low-energy hydrodynamic regime – it will respond to textural features, and for most species pits and rugosities provide positive cues. Rough surfaces perhaps provide protection from physical stress, such as current speed and abrasion, and from competition and predation, and ensure firm, permanent attachment by the newly metamorphosed larva. Settlement site is finally determined by larval responses to a sequence of biological cues. Larvae generally avoid clean surfaces – those only recently submerged, or abraded by large epilithic grazers, such as chitons and echinoids – but are attracted to films of microorganisms, developed by bacterial communities, fungi and diatoms. The significance of this response is unclear; it may have a physical basis, the larva attaching more easily to a sticky filmed surface; or there might be a chemical basis to the attraction, reflecting the composition of a film, and it might then be supposed that there are unattractive films that instead repel prospecting larvae. The relative attractiveness of a biological film to a settling larva, perhaps, might be related to the proximity of conspecifics, because it is certain that for most encrusting sessile animals it is the presence of adults, or newly settled juveniles, that provides the final cue to settlement and metamorphosis. Gregarious larval settlement is characteristic of many hard-substratum sessile animals: the new settler thereby finds itself in optimum habitat, and aggregated adult populations are essential to successful sexual reproduction in sessile animals.
Larval behaviours governing site selection and gregarious settlement have been investigated, through both field and laboratory experiments, among many intertidal species, especially barnacles, spirorbid tubeworms and bryozoans. There are fewer data for strictly subtidal species, but what are available suggest that larval settlement behaviour among sessile epilithic invertebrates is similar to that observed in intertidal communities. The large, purplish sea squirt Ascidia mentula (Fig. 83) is often common on sloping to vertical rock surfaces, from the shallowest subtidal habitats to at least the edge of the continental shelf, and at the perhaps optimum depth range of 25–40 m may occur in aggregated populations of 100 to almost 400 individuals per square metre. It grows to around 18 cm in length, and in some populations may achieve a life span of 5–7 years. Monitoring A. mentula populations in the field reveals that new recruits tend to be clustered around adults, and that as the population density increases the rate of recruitment also increases, suggesting that prospecting larvae are positively attracted to established adult populations (Havenhand & Svane, 1989). This was demonstrated experimentally: larvae of A. mentula will settle in response to a chemical inducer present in the tunic tissue of the adult sea squirt. This stimulus is essential in order for the larva to metamorphose successfully, and in its absence, in filtered seawater, only 20% of settling larvae were able to complete metamorphosis.
FIG 83. The sea squirt Ascidia mentula, with its usual community of encrusting bryozoans. (J. S. Ryland)
SAMPLING AND MONITORING
Ecological research on hard-ground benthos is beset with practical difficulties; baseline surveys and continuous monitoring programmes are both time-consuming and expensive, and probably always underestimate the density and diversity of particular components of the community sampled. Remote sampling techniques formerly employed heavy bucket dredges to collect cobbles and boulders, and even to break off pieces of bedrock, and more recently suction dredges deployed by divers have been used to collect material scraped from rock surfaces. Such techniques are inefficient and destructive, and the epifauna is always under-sampled as a consequence; biological material dislodged from the substratum may be swept away by water flow, or by the turbulence created by the sampling gear, while such specimens as are collected may be damaged beyond use as scientific information. Damage to the habitat caused by trawls and dredges may also be severe and long lasting; its extent has only come to be appreciated over the past couple of decades, and the resulting environmental perturbation is now seen to be unacceptable in scientific research. Less damaging sampling techniques have utilised photographic, video and digital recorders, deployed by remotely operated vehicles (ROVs) or divers, and will probably become more widely employed as equipment, image quality and techniques for analysis improve. However, image-based recording and monitoring techniques have limitations that will not be easily resolved, and it is probable that benthic ecologists will have to adopt environmentally sensitive methods for collecting samples from narrowly defined sampling areas; for example, mounting a video camera above a van Veen grab allows accurate location of the grab, and sampling with minimal damage to the wider habitat (Mortensen et al., 2000). Large, unitary organisms such as anemones, ascidians, sedentary bivalves and tube-building polychaetes are easily identified and counted in photographic quadrats. Some large, erect sponges, modular cnidarians and ascidians, and the more distinctive species of bryozoans, might also be positively identified, and numbers counted, or areas estimated, from image samples. It is thus possible to measure density or area of the most conspicuous, and spatially dominant, sessile species, and to estimate biomass, and to monitor seasonal and annual change. However, many sessile epifaunal animals are not easily identified in the field, and the morphological characteristics that determine each species may not be apparent in photographic and digital images. In particular, sheet-forming sponges, hydroids, bryozoans and modular ascidians often can only be identified to species level by microscopic examination. In addition, sessile epifaunal communities provide habitat for numerous small motile organisms, including numerous species of ostracod, amphipod and isopod, vagile polychaetes, small decapods and molluscs, that require at the least a ×10 hand lens in order to identify them. A few species of individually large, spatially dominant sessile animals may comprise the greater part of the biomass of a sessile epifaunal community, but species richness derives from a large number of smaller organisms. These represent only a minor proportion of the biomass, but the greater part of the taxonomic diversity, and usually the majority of the total number of individuals present in the community.
Ecologists have employed photographic recording, in-situ mapping or individual counting and area estimation to survey and monitor sessile communities of rocky habitats. A comparison of epilithic faunas on the coasts of Denmark, Sweden and Norway, Orkney and Northumberland, and the Netherlands, Brittany and southwest Ireland employed the latter method, identifying and counting, and estimating area covered by, macroalgae and sessile animals, in randomly placed 0.25 m2 quadrats at a number of sites at each location (de Kluijver, 1997). On North Sea coasts the taxonomic diversity of the communities sampled was remarkably similar between locations, with 52–58 species of macroalgae and 97–105 species of sessile invertebrate recorded. Vagile species, mostly large predators that could be identified accurately in the field, totalled around 30, and there was a positive correlation between the numbers of sessile and vagile species recorded at each location. On Atlantic coasts, at Morlaix Bay, Brittany, and Roaringwater Bay, southwest Ireland, algal diversity was recorded at 152 and 109 species respectively, and sessile invertebrates at 152 and 180 species. Rocky habitats in Trondheimsfjord, Norway, monitored by photographic recording over a five-year period, were dominated by two solitary ascidians, Dendrodoa grossularia and Ciona intestinalis, and a tubeworm, Pomatoceros triqueter, which also constituted the bulk of the biomass as well as the greatest coverage; the population of C. intestinalis (Fig. 84) a soft-bodied animal that may grow to 15 cm in length, exceeded 400 individuals per square metre (Gulliksen, 1980). Yet suction sampling of the habitat yielded more than 150 additional species, mostly consisting of tube-building amphipods and polychaetes, and small molluscs. Dense populations of C. intestinalis accumulate large quantities of fine silt, drawn by the combined inhalant currents of the animals, which becomes packed between them, and augmented by faecal pellets. This creates a turbid environment that results in decreasing densities and diversity of other filter-feeding species, but provides perfect conditions for the small detritivorous amphipods and polychaetes.
Rocky habitats, at around 17 m depth, in Kinsale Harbour on the south coast of Ireland were found to be populated by sparsely distributed kelps, Laminaria hyperborea, and beds of the brittlestar Ophiothrix fragilis (Ball et al., 1995). Vertical surfaces bore dense assemblages of sessile animals, with no apparent spatial dominants; large ascidians, in particular, seemed rare, but the community consisted of 116 species, of which around 100 were modular organisms. Some of these were perhaps encrusting kelp holdfasts, but none was strictly epiphytic in habit, and the sessile faunas of the holdfasts would have developed through the settlement of larvae shed by rock-encrusting species in immediate proximity to the kelps. More than half of the total number of sessile species recorded from natural rock habitats were bryozoans, 57 species, indicating how extraordinarily diverse bryozoan assemblages may be in the absence of large, superior spatial competitors. The potential diversity of the epilithic faunas of this area was suggested by an additional 44 species, including 16 bryozoans, that were recovered from artificial settlement panels.

FIG 84. A group of the solitary ascidian Ciona intestinalis. (J. S. Ryland)
POPULATIONS AND COMMUNITIES
Some of the larger spatial dominants in sessile benthic communities may be relatively short-lived. Ciona intestinalis, for example, has a maximum life span of two years, and in sparse populations individuals probably do not survive even that long, being outgrown by competitive superiors or succumbing to predation. A strong year class, following from high reproductive output and good recruitment, may establish a dense population by late summer that persists not through competitive superiority but rather through the blanketing effect of the silt and faecal material it generates. As well as discouraging other suspension feeders, an example of amensalism (in which the life mode of one species precludes co-occurrence with others, as opposed to commensalism), the inhalant and exhalant streams of the ascidian population prevent settlement of prospecting larvae, which are either engulfed or swept away. Community structure may change as a consequence: larvae of C. intestinalis suffer the same fate as all others, the demography of the population does not change, and it collapses and disappears at the termination of the two-year life cycle (Fig. 85).
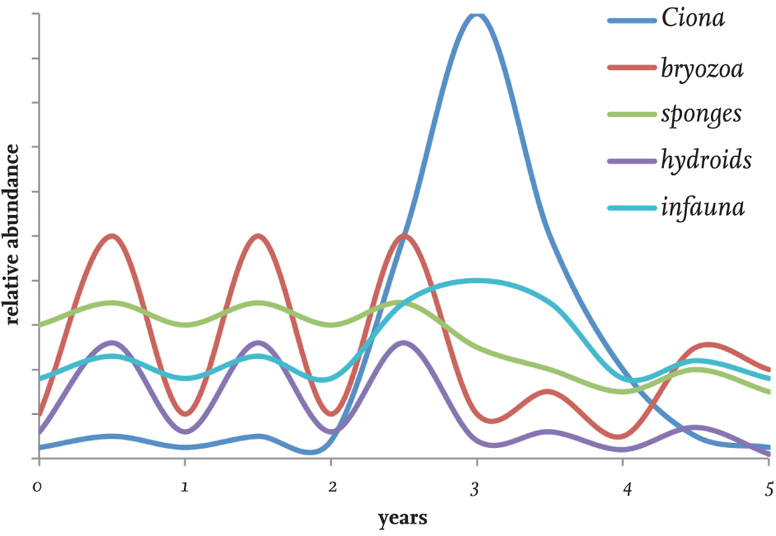
FIG 85. Hypothetical fluctuations in the population densities of components of an epilithic community colonised by Ciona intestinalis. (After Gulliksen, 1980)
Such exceptions aside, most spatially dominant sessile animals tend to be long-lived; clones of Metridium senile, for example, seem to persist for decades. The Plumose Anemone is a large animal, with presumably a high energy demand; it is reliant upon water flow for its food supply, and is common in high-energy environments, tolerating current velocities up to 75 cm per second. Turbulent water flow and the cnidocyte-laden tentacles of the anemones usually result in a high-density but low-diversity assemblage. The upright, perennial colonies of the large bryozoans Flustra foliacea (Fig. 47) and Pentapora foliacea (Fig. 86) will also be found in high-energy habitats, while the solitary ascidian Ascidia mentula favours a quieter milieu, with current velocities no greater than 18 cm per second, but all three have a characteristic associated fauna of both sessile and vagile species, and constitute high-density mesocosms within the broader hard-substratum habitat. The firm outer test of A. mentula is often encrusted with bryozoans, hydroids and small tubicolous polychaetes, and as it grows it gathers a coating of small red algae. In the field these epizootic communities effectively camouflage the ascidian, which is sometimes only recognised by its open siphons. The capacious branchial chamber of A. mentula, through which flows a constant stream of food-laden, oxygen-rich water, provides a secure environment for a number of endosymbiotic species, especially small crustaceans. These include several species of cyclopoid copepod within the genus Notodelphys, the systematic affinities of which are patent (Fig. 87a), and the extravagantly dimorphic Notopterophorus papilio (Fig. 87b), which may not be immediately recognised as a copepod at all. A large amphipod, Leucothoe spinicarpa, growing to almost 2 cm in length and with distinctively chelate gnathopods (Fig. 87c), is also likely to be found in the branchial sac of A. mentula, as well as in other large, solitary ascidians, and in the aquiferous chambers of sponges.

FIG 86. (a) The Ross Coral, Pentapora foliacea. (b) Detail showing the growing edge and feeding lophophores. (J. S. Porter)
An especially interesting symbiont of A. mentula is a small mussel, Modiolarca subpicta (Fig. 88), which can be found embedded within the outer surface of the test, its siphons communicating to the exterior through a small opening. Modiolarca subpicta is not restricted to A. mentula, but can be found in association with several species of solitary ascidian, and its biology has been investigated in a population commensal with Ascidiella aspersa (Fig. 89), a smaller species than Ascidia mentula with a maximum length of 12 cm and a life span of no more than two years (Morton & Dinesen, 2011). Ascidiella aspersa is a protandrous hermaphrodite; its lecithotrophic larvae settle in midsummer following a brief free-swimming period and limited dispersal. Modiolarca subpicta has a single short spawning period in summer; planktotrophic larvae develop during a protracted pelagic phase of up to six weeks, before settling and metamorphosing on the ascidian host. Newly settled spat are first apparent on A. aspersa individuals of 10–25 mm in length; adult mussels are most frequently found on ascidians 35–60 mm long, and are most abundant, to a recorded maximum of 11, in those in the 45 mm size class. It does not burrow into the outer tunic of the host, contrary to previous conceptions. Instead the newly settled mussel uses its elongate foot to attach byssal threads on its dorsal, hinge-bearing side, securing it to the host tunic before turning onto its dorsal side so that the ventral shell gape and the mantle siphons face upward and outward. As the host grows, folds of tunic enclose and envelop the mussel, so that only its siphons protrude to the exterior. The life cycles of host and symbiont seem synchronised: both breed during a short summer period, and the smallest mussels occur on the smaller size classes of the ascidian. The association terminates with the death of the host. Ascidiella aspersa breeds once in its second summer, and then dies in its second winter, and it is possible that the mussel also has just a two-year life span, determined by that of its host. Yet individuals of M. subpicta have been recorded from interstices of kelp holdfasts and attached beneath shells and stones by byssal threads, so it is equally possible that it survives the death of the initial host, and is perhaps able to seek and attach to a second host. An investigation into size frequencies of M. subpicta populations on longer-lived hosts, such as A. mentula, would be an interesting preliminary test of this possibility.
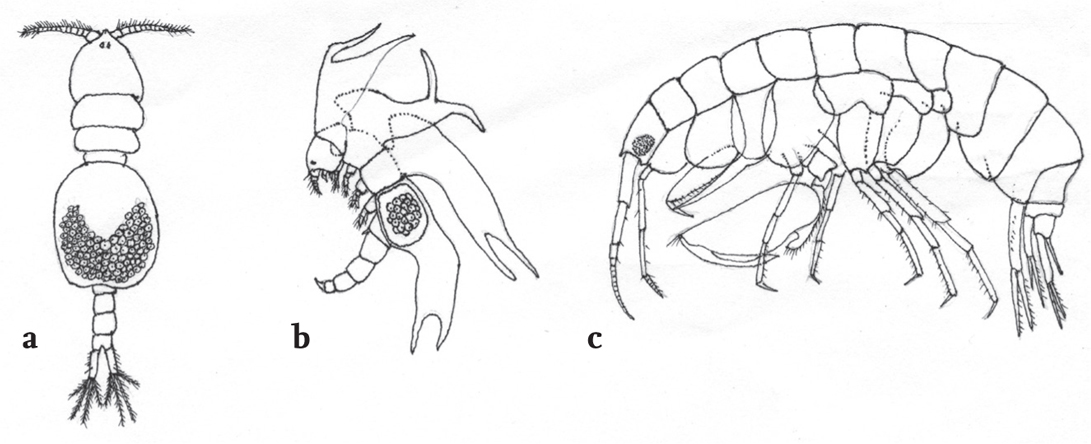
FIG 87. Some common endosymbionts of Ascidia mentula: the cyclopoid copepods (a) Notodelphys allmani and (b) Notopterophorus papilio, and (c) the amphipod Leucothoe spinicarpa, with distinctive chelate (clawed) ganathopods (first limbs). Not to scale.

FIG 88. The small mussel Modiolarca subpicta. Scale bar: 2 cm.

FIG 89. Two individuals of the solitary ascidian Ascidiella aspersa with (left) cushion- shaped colonies of the modular species Polyclinum aurantium and (right) a laminar colony of Botryllus schlosseri. (J. S. Ryland)
Two perennial bryozoans, Flustra foliacea and Pentapora foliacea, contribute importantly to habitat heterogeneity. Flustra foliacea populations occur as patches on level coarse grounds and on inclined rock surfaces, often forming permanent shrubberies over several square metres. Its flexible, horn-coloured colonies, from which it derives its vernacular name, Hornwrack, have been aged at up to 12 years, and provide ideal surfaces for small, encrusting organisms, and the flabellate fronds offer protection from scour. Encrusting bryozoans are particularly frequent on F. foliacea colonies, together with small hydroids and a suite of motile predators. More than 50 species of bryozoan have been recorded encrusting F. foliacea, across a geographical range encompassing the entire shallow shelf region of northwest Europe. An ecological study of a population of Hornwrack at 15–20 m depth on the south Wales coast reported an associated fauna of 32 sessile invertebrate species, and noted, unfortunately without detail, large numbers of the small, suspension-feeding porcelain crab Pisidia longicornis, and the regular echinoid Psammechinus miliaris, and ‘thousands’ of caprellid amphipods, which were assumed to be living amongst the F. foiliacea fronds (Stebbing, 1971). Colony form of the bryozoan species reported from F. foliacea encompasses most known morphologies, with the exception of heavily calcified, branching, rigid forms. Apart from a few ubiquitous species, such as Electra pilosa (Fig. 90), the most frequently occurring bryozoan associates of Hornwrack are characterised by delicate, flexible, branching colonies. Predominant among these are stiff, white colonies of Crisia eburnea, species of Scrupocellaria (Fig. 91) with open, fan-like colonies, and flabellate fronds of Dendrobeania and Bugula species. Crisia eburnea and Scrupocellaria reptans were abundant in the south Wales population, with 260 and 210 individual colonies, respectively, on a single 10.5 cm high F. foliacea colony. Bugula flabellata (Fig. 92) was the third most abundant bryozoan species found, and had the most intimate association with its host. The branching rootlets – rhizoids – that attach each B. flabellata colony to its substratum penetrated the autozooids of the Flustra colony, and ramified throughout the frond. This phenomenon is not unique, however, as B. flabellata can be found in similarly close association with many other species of bryozoan, and none of the sessile species recorded from Hornwrack is restricted to it. There is no characteristic, recurring sessile community associated with F. foliacea, probably because its populations tend to be patchily distributed, and the colonising fauna is determined by local larval supply.
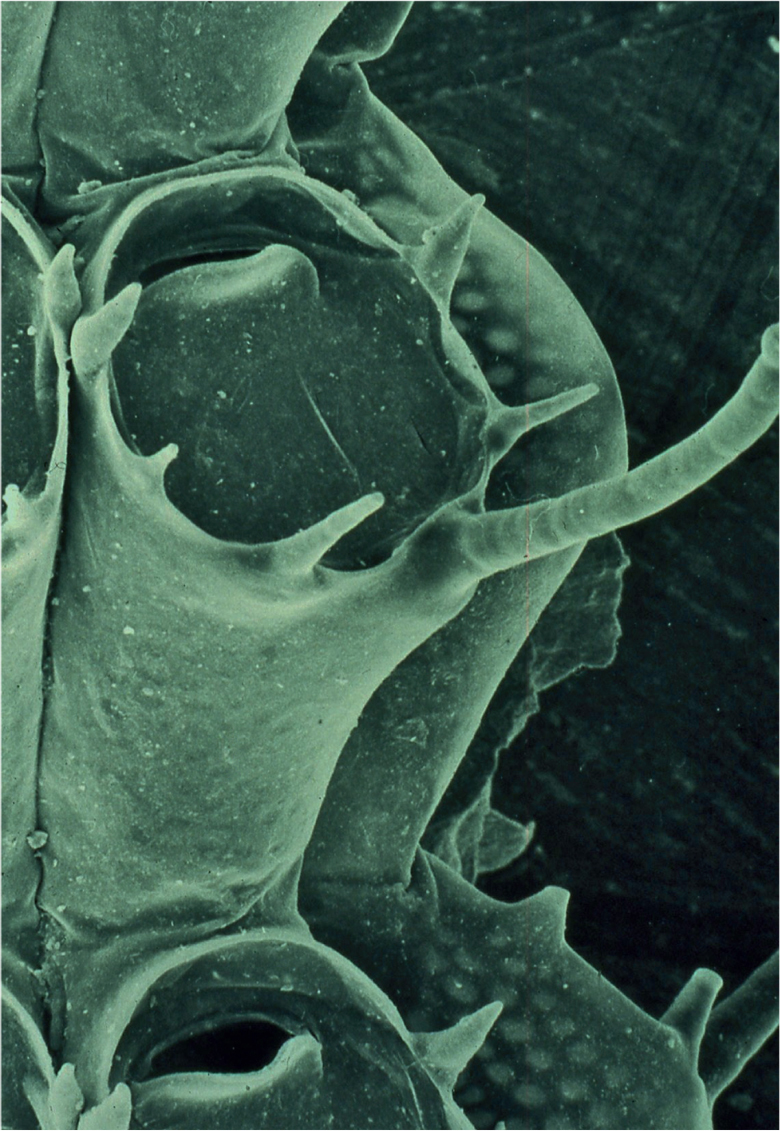
FIG 90. Scanning electron micrograph showing autozooids (modules) of the bryozoan Electra pilosa. For scale, the elongate frontal spine is approximately 0.25 mm long.

FIG 91. Colonies of the erect bryozoan Scrupocellaria. (J. S. Porter)
Colonies of Pentapora foliacea, commonly referred to as the Ross Coral (Fig. 86), are heavily calcified and rigid, built from bilaminar plates of autozooids that divide and anastomose along the growing edges. They frequently exceed diameters of 20 cm, for a height of 10 cm, and some huge specimens have been reported in the past, during times when the benthic environment was less disturbed by human activity. The frequency of division and fusion of the growing edges is perhaps determined by local environmental factors, such as hydrodynamic regime. Colonies may have an open lattice structure, with large interstices between the plates of autozooids, while in others the growing edge may be closely folded, with small spaces in a honeycomb-like structure; the latter growth form might be an adaptation to especially turbulent conditions, but the possibility has not been investigated. The biology and ecology of P. foliacea have been little studied. It is present on coarse grounds and in rocky habitats along the south coast of England, sparsely from the Solent eastwards to West Sussex, increasingly common westwards, and abundant around Cornwall; it is found off Pembrokeshire, the Isle of Man and north Wales, but is otherwise absent from most of the Irish Sea, and from the southern and western coasts of Ireland northwards to the Hebrides. It seems to be scarce around Orkney and Shetland, where it perhaps reaches its northern limit. Population densities, colony growth rates, individual maximum sizes and longevity have not been documented with any precision. Oxygen isotope analysis has suggested growth rates of around 2 cm vertical extension per year; actual age has not been established for any specimen, although a minimum age of three years has been estimated for a colony from Pembrokeshire (Pätzold et al., 1987). The colony is attached to the substratum by an encrusting sheet of zooids; erect plates may be produced during the first year’s growth, or perhaps commence in the second, and it is probable that P. foliacea has the same facility for repair and regeneration as most cheilostome bryozoans, so that accurate ageing of any individual is likely to be very difficult to achieve. Pentapora foliacea colonies contribute significantly to hard-ground habitat heterogeneity, providing secure refuges for an abundance of other organisms. As in the case of Flustra foliacea, it supports a diverse community of encrusting bryozoans, although no similar inventory of species associated with it has been made. Amphiblestrum flemingii, Callopora dumerilii, Membraniporella nitida and Smittoidea reticulata, all developing encrusting sheets, are commonly found on P. foliacea colonies, and possibly represent a perennial community, regenerating annually from overwintered fragments. Erect, bushy bryozoans seem to be uncommon, perhaps because of turbulent water flow, or because the rigid structure of the Pentapora colony supports an assemblage of small browsers, grazers and predators, as well as relatively large suspension feeders. Sedentary bivalves, especially small species of scallop and mussel, may be permanent residents within the chambers defined by the anastomosed plates of the Pentapora colony; suspension-feeding decapods, such as Pisidia longicornis, may also be abundant, but they are probably outnumbered by hosts of lesser crustaceans, especially amphipods. By analogy with coralliform bryozoan biotopes described from other regions, including the eastern Mediterranean and New Zealand, dense populations of Ross Coral are probably utilised for food and shelter by juveniles of many species of large decapod and fish, and represent foci of high biomass and high density in moderate- to high-energy habitats.

FIG 92. A luxuriant colony of the bushy bryozoan Bugula flabellata. (J. S. Porter)
The upper portions, and the plate edges, of live and actively growing P. foliacea colonies have a deep orange-red coloration, and are generally free of epibionts; the colour fades towards the older, basal regions of the colony, which are usually encrusted with sessile organisms. The clean growing edges might indicate a mismatch between the period of larval settlement and recruitment of epibiotic species, and the growth period of the host, but might also indicate that the vulnerable growing edge is a source of bioactive compounds that deter settlement of larvae and spores. However, microbial communities show an opposite pattern of distribution, with thick layers of bacteria present on the growing edges of the colony, and thin, diffuse bacterial growth on the older regions. Flustra foliacea (Fig. 47) and Alcyonidium diaphanum (Fig. 46) are known to generate metabolic by-products that may have toxic or irritant properties. Trawler fishermen constantly exposed to A. diaphanum through cleaning nets may become sensitised to an alkaloid metabolite released by the bryozoan, and develop a painful allergic dermatitis, termed ‘Dogger Bank itch’ (Pathmanaban et al., 2005); notably, A. diaphanum colonies are usually free of epibionts. Fresh, live colonies of F. foliacea have a lemon odour, and also contain alkaloid secondary metabolites, some of which have been found to disrupt functions of bacterial colonies. Bacteria were found to be differentially distributed on the surface of F. foliacea zooids, suggesting suppression of bacterial growth by secondary metabolites (Peters et al., 2003), yet colonies typically support rich communities of encrusting animals, with the larvae of some species settling preferentially along the tips of the growing fronds. The roles of secondary metabolites in the biology of bryozoans are thus still quite unclear, but the possibility that in some species their interaction with bacterial communities, and bacterial products, may have consequences for macrofouling communities is intriguing.
Spiny-armed brittlestars are abundant in hard-ground habitats, from coarse gravels to rock, and brittlestar beds, dominated by Ophiothrix fragilis (Fig. 61) but often with a small proportion of Ophiocomina nigra (Fig. 74), constitute a biotope characteristic of all moderately exposed northwest European coasts. Ophiothrix fragilis was the most common species recorded by the CEFAS surveys in the eastern English Channel, the Bristol Channel and the southern Irish Sea (here), and it ranges, at equivalent densities, along the western coastlines of the British Isles and Ireland. Ophiothrix fragilis can be found in the low intertidal on rocky shores, and offshore to the shelf edge; in coastal habitats it lives in small groups among kelp holdfasts, and on inclined, even vertical, rock faces, but beyond the limit of wave surge, below 15 m, its populations form dense aggregations, in discrete, sharply demarcated patches (Warner, 1971). The brittlestars actively associate: removed from a patch and placed alone, regardless of the type of substratum a solitary individual will begin to walk in a straight line, changing direction at intervals, and continue to walk until it encounters a group of conspecifics. A patch, or bed, of O. fragilis has a minimum population of around 100 individuals. Smaller groups tend to continue moving and eventually coalesce with other groups, and the most extensive may cover thousands of square metres of sea floor. The largest patches consist mostly of the largest adults, with disc diameter 8–12 mm, together with the smallest recruits, disc diameter < 1 mm, which cling to the arms and arm spines of the adults. At disc diameters greater than 2 mm juveniles appear to migrate, congregating as small groups in adjacent habitats, such as clumps of large hydroids, and returning to the main concentration as they grow. This behaviour perhaps serves to mitigate competition for food between adults and juveniles. Patch stability is partly related to hydrodynamic factors: patches form at current speeds of around 10 cm per second and achieve stability at an optimum speed of 25 cm per second as the closely packed individuals interlink their arms, but are scattered at speeds above 50 cm per second. Within the largest beds densities of O. fragilis are often in the range 1,500–2,000 individuals per square metre; biomass estimated by wet weight suggested a value of 340 g/m2 for a population density of 309/m2, and mean biomass in terms of ash-free dry weight (AFDW) has been measured at 210 g/m2 for a population density of 1500/m2.
TROPHIC INTERACTIONS
Ophiothrix fragilis is principally a particulate suspension feeder, holding two or more arms vertically into the water column, trapping and binding fine food items with mucus-bearing tube feet, and passing packages of material along a tube-foot conveyor to the mouth. One advantage of aggregated populations may be that they result in increased feeding success, as the dense thickets of upraised arms probably slow boundary-layer current flow and enhance particle capture. Phytoplankton provides the most significant source of nutrition through much of the year, but this is probably supplemented by detrital material of various origins. Optimal current speed for suspension feeding was found to be 20 cm per second, and duration of feeding was determined by tidal currents: in the Dover Strait O. fragilis populations were able to feed through 86% of a neap tidal cycle, but only for 37% of a spring cycle (Davoult & Gounin, 1995). Brittlestar aggregations must be a significant element of the benthic ecosystem, contributing substantially to organic carbon input and the cycling of calcium carbonate. In the Dover Strait and the eastern English Channel O. fragilis beds extend over 6,000 km2, and the Dover Strait populations have been estimated to produce 682 g CaCO3 per square metre per year, significantly boosting levels of dissolved CO2. However, their further ecological roles are still only incompletely documented, and the importance of brittlestars in marine food webs are generally poorly known. High-density O. fragilis populations affect the composition of hard-ground benthic communities, partly through resource competition with other suspension feeders, and almost certainly through the exclusion of potential spatial competitors. Ophiothrix fragilis is preyed upon by bottom-feeding fish, especially gadoids and flatfish, and by several species of decapod, but it is a principal prey item only for the large starfish Luidia ciliaris and L. sarsi.
Hard-ground sessile benthos, with its epibionts and symbionts and its associated complement of small tube builders, and the blankets of brittlestars comprise the primary consumers and detritivores that support a dependent community of secondary consumers. The most important of these are bottom-feeding fish, large decapods, numerous species of gastropod, and a number of echinoderm species. Some are scavengers, including most whelk and many decapod species, while others are carnivorous predators, such as the cottid fish Myoxocephalus scorpius and Taurulus bubalis (Fig. 93) and most starfish, or broadly omnivorous, like the epifaunal sea urchins. Echinoderms are as important to the ecology of hard-ground habitats as they are to that of soft sediments. Asterias rubens swarms can have the same devastating effect in both environments, and on rocky grounds are probably only limited by greater water turbulence, which will eventually disperse them. The Edible Sea Urchin Echinus esculentus (Fig. 94) browses kelp fronds, with a predilection for those encrusted with the bryozoan Membranipora membranacea (Fig. 95), and below the kelp forest feeds on smaller red and brown seaweeds, as well as on barnacles, bryozoans and other encrusting invertebrates. At high densities E. esculentus has a considerable impact on sessile macroalgal and invertebrate assemblages; the animals are very motile, seem to move offshore during the winter months, and migrate in response to changing food resources.

FIG 93. The bottom-feeding Sea Scorpion, Taurulus bubalis. (J. S. Porter)

FIG 94. The Edible Sea Urchin, Echinus esculentus, browsing the stipe of Laminaria hyperborea. (J. S. Porter)
Submarine cliffs and rock outcrops, offshore installations, and of course wrecks, all increase habitat complexity in hard-ground environments, and support a correspondingly broad diversity of scavengers and predators. Blennies and gobies, the small flatfish Zeugopterus punctatus, known as the Topknot, the Tadpole Fish, Raniceps raninus, and the Bull Rout, Myoxocephalus scorpius, are often permanent residents, venturing out from holes and crevices to capture prey. Individuals appear to show a degree of home-range fidelity, defending a foraging area against conspecific intruders, though not always against other species, and display rhythmic activity cycles (Nickell & Sayer, 1998). Other fish species may be only temporarily resident, seeking refuge as juveniles, visiting to feed as adults, or as seasonal visitors establishing breeding territories during part of the year. Diets of these benthic-feeding fish are not known in detail, but most seem to take small prey items, and to show little selectivity. Crabs, squat lobsters, amphipods and other minor crustaceans, molluscs and polychaetes all form part of the diet of most species. In Lough Hyne, southern Ireland, five species of small wrasse were shown to establish breeding territories on a vertical rock face, constructing and guarding nests in which their eggs develop (Bell et al., 2003). Some were observed foraging among the sea squirts Ascidia mentula (Fig. 83), Ascidiella scabra and Ascidiella aspersa (Fig. 89). The latter was the most abundant of the three species and attracted the greatest attention from the wrasse, and in one year 95% of its population was consumed. The detached tests of the sea squirts, collected from the foot of the cliff, had been eviscerated, and holes in the outer tunic showed where the fish had also extracted the bivalve symbiont Modiolarca subpicta (Fig. 88). The bulk of the biological material raining down from the cliff consisted of sponges; 22 species were recognised, but these had probably been dislodged by wrasse in the course of nest construction, or by other cumbersome foragers, particularly echinoids and spider crabs, rather than through predation.

FIG 95. A young colony of the bryozoan Membranipora membranacea, on the Serrated Wrack, Fucus serratus. (J. S. Porter)
FIG 96. The large predatory sea slug Tritonia hombergii. (J. S. Ryland)
Most of the benthic secondary consumers are generalists, consuming a wide variety of food items and displaying little selectivity. However, hard-ground benthos also provides habitat for a very diverse suite of specialist predators, some with diets so narrow that their life cycles are spent in association with just one or very few prey species. Many small arthropods fall into this category, but most familiar, and conspicuous, among them are species of nudibranch sea slug (Picton & Morrow, 1994). Tritonia hombergii is especially striking (Fig. 96): it is the largest species of sea slug found in northwest European seas, a bulky animal growing to 20 cm in length, recognised by a lobed and fringed oral veil that overhangs the head, and thick-stemmed arborescent gills in pairs along its dorsal surface. This huge sea slug feeds exclusively on Alcyonium digitatum, reportedly biting off and ingesting large pieces of its prey. Dendronotus frondosus (Fig. 97) is a rather similar species, but with a maximum length of only 10 cm, with a more slender body and proportionately longer, delicately branching gills. Its diet is less restricted than that of T. hombergii. Juveniles feed on several species of small hydroid, while adults browse clumps of the larger Tubularia indivisa and Ectopleura (formerly Tubularia) larynx (Fig. 98).
Goniodoris and Onchidoris are two genera of medium-sized sea slugs, species of which are mostly less than 4 cm long. All have firm, oval and domed bodies, the dorsal surface typically covered with rounded tubercles, with an anterior pair of sensory, club-shaped rhinophores, and a posterior ring of contractile gills. Goniodoris castanea and G. nodosa feed on encrusting bryozoans as juveniles, and on colonial ascidians such as Dendrodoa grossularia when mature. There are numerous species of Onchidoris in European shelf habitats, all of which appear to feed on encrusting, calcified bryozoans, although whether each species has a particular dietary preference is not known. Specialist sponge predators are found mostly in the superfamily Doridacea, and many dorid species seem to have quite narrowly limited diets.

FIG 97. (a) The sea slug Dendronotus frondosus browsing on a hydroid colony. (b) A larger individual with especially arborescent gills. (J. S. Porter)
FIG 98. A colony of the hydroid Ectopleura larynx. (J. S. Ryland)

FIG 99. The sea slug Doto fragilis – recognised by its knobbly, brown cerata – on its prey, the hydroid Nemertesia antennina, with its spiralled, white spawn ribbon. (J. S. Porter)
The most attractive of the nudibranch predators are the many species of Doto (Fig. 99), a genus usually classified with Tritonia and Dendronotus in the Dendronotacea. They are all tiny animals, usually less than 2 cm in length and many not exceeding 1 cm, with often prettily patterned bodies bearing pairs of clubbed, tuberculate processes – cerata – dorsally. They are an interesting contrast to larger carnivorous sea slugs in that their prey is also their habitat. Doto species live among clumps of hydroids, on which they deposit their spawn, and to which their larvae must be attracted at settlement. At least 18 species of Doto have been described from northwest European seas, and more perhaps remain to be recognised. They are not easily distinguished from each other by size, colour or morphology, and it is probable that apparently widely occurring species, reported from several different species of hydroid, may each represent a number of superficially similar species, each with only a limited diet, and perhaps restricted to one, or very few, hydroid host species. Cryptic species groups have already been revealed among some Doto populations through molecular genetic studies (Morrow et al., 1992)
SETTLEMENT AND SUCCESSION
Newly available substratum soon becomes encrusted with assemblages of sessile organisms. Fresh surfaces provided by rock falls, boulder overturn, coastal constructions and offshore installations become coated with bacterial films, diatoms and microalgae, and then colonised by macroalgal propagules and invertebrate larvae. Settlement may seem to show succession, with hydroids, bryozoans and serpulid tubeworms often among the early colonists, and ascidians and anemones arriving later. The composition of the community thus changes with time, but there is no predictable climax community. Development of the community is determined by the season in which the new habitat is created, its depth, larval supply, and the growth rates and longevity of each colonising species. The species founding the new community might be barnacles and other winter breeders, in the case of habitat newly available in spring, while hydroids, tubeworms and bryozoans might be the pioneers in summer colonisations, and these early successional species might be displaced by late successional invaders, or may persist as the spatial dominants, partly depending on the season in which the community begins to develop. Spatial competition between the filter-feeding sessile component will further mediate development of the assemblage, and biomass and diversity will fluctuate accordingly. Complexity increases as vagile suspension feeders, scavengers and predators begin to colonise.
Where hard-substratum benthic assemblages form on anthropogenic surfaces they may become an expensive nuisance. They may develop an especial luxuriance, as the conveniently provided habitat may benefit from enhanced water flow, increased volumes of particulate food, more light and higher water temperatures, and even decreased competitive pressure and a much reduced risk of predation. Such assemblages, on docks and pontoons, on and within structures associated with coastal power stations and cooling systems, on the hulls of inshore pleasure craft and oceanic shipping, and on oil and gas rigs, are referred to as ‘fouling communities’ (Fig. 100). Marine biofouling may have severe economic impacts, obstructing cooling-water intakes, modifying water flow regimes and creating additional frictional drag; on ships’ hulls fouling may affect speeds, and result in increased fuel consumption, while on stationary structures, such as offshore rigs, it may result in increased stress, and in both cases encrusting communities may conceal potentially damaging phenomena, such as cracks and corrosion. Marine biologists have researched the ecology of fouling communities for decades, through field observation and manipulation, and through the use of artificial settlement panels to record the settlement and succession of epifaunal assemblages on different substrata and under differing environmental conditions. These approaches still have importance for understanding the structure and development of fouling assemblages, and in order to devise methods for control and eradication, but now have an added significance in relation to environmental management and conservation. Information on the settlement, succession and development of sessile benthic communities may be useful in evaluating the sensitivity of habitats to disturbance, and for estimating rates of recovery following disturbance. Further, such data might be important when considering the creation of artificial reefs, which may prove to be useful means of increasing habitat heterogeneity, and hence biodiversity.

FIG 100. (a) The invasive tubeworm Ficopotamus enigmaticus in a dense band on a pontoon float, Swansea marina. (b) Detail to show regenerated tubes intergrown with small barnacles.
The composition of epilithic communities varies according to the type of rock they encrust. Carboniferous limestone, with frequent bedding planes and numerous fissures, and pocked by solution, biogenic boring and physical erosion, offers a greater range of niches than hard schists and soft shales. Communities established on artificial substrata are often structurally different from those on naturally occurring hard substrata, and it has been suggested that they will only approximate to a ‘natural’ state if the artificial substratum encompasses the same degree of physical heterogeneity as natural substrata. Submerged wooden pilings, concrete, smooth steel and corroded iron surfaces each comprise distinct, new microhabitats, and comparisons between benthic communities on different natural and artificial hard substrata must be carefully drawn. Nevertheless, research into fouling communities on artificial substrata supplies some interesting insights into the ecology of natural sessile benthos. Hydroids are among the earliest invertebrate colonisers of newly available substrata. In March 2004 an artificial reef was created in Whitesand Bay, south Cornwall, when the decommissioned frigate HMS Scylla was scuttled, and over a number of years successive settlements of algae and invertebrates were recorded by diving biologists (Hiscock et al., 2010). Barnacles (Balanus crenatus), tubeworms (Pomatoceros triqueter) and the hydroid Obelia dichotoma settled within a few weeks of the establishment of the reef, but all three species seemed to have disappeared by the end of the first summer. The first two remained as inconspicuous, and probably insignificant, components of the developing assemblage, but by summer the most abundant species of hydroid were Ectopleura larynx (Fig. 98) and Tubularia indivisa (Fig. 101), both of which had also first appeared soon after the ship had been sunk, and continued to show brief annual peaks in abundance during the five-year duration of the monitoring programme.
These early successional species have sometimes been termed ‘pioneer’ species, expanding rapidly into often extensive populations that are eventually displaced, though not entirely obliterated, by competitively superior, late-successional species that may come to constitute the spatial dominants of the community. Fifty-nine taxa of algae and invertebrates had colonised Scylla by the end of 2004, and the number increased annually to a total of 148 by the end of the five-year survey. These consisted mostly of ‘conspicuous’ species that could be identified with confidence in the field, under water, together with species identified from a number of samples scraped from upper surfaces of the ship, on a single occasion halfway through the survey. It is probable that the actual diversity of the new reef’s epifauna was underestimated, and that regular (but, alas, expensive) sampling would have greatly increased the number of species recorded. The ascidian Ciona intestinalis was an early colonist, increased in abundance in the second year of the survey, and thereafter maintained a more or less prominent presence in the communities. Metridium senile and Ascidiella aspersa were not recorded until the year following the creation of the reef, but increased in abundance in the third year, and as potential spatial dominants would probably have established a permanent presence by year five. The authors of this study noted that an artificial reef of concrete blocks installed in Poole Bay attracted a settlement of 90 species within a year, with the total number of taxa recorded rising to 220 during the second year and to 240 in the third year following the submersion of the blocks. They also noted that natural rock habitats in the vicinity of Scylla supported a community of 122 conspicuous species, 39 of which had not been recorded on the artificial reef during the five years it was monitored. Three different types of hard substrata, one natural and two artificial, appeared to support three different benthic assemblages.

FIG 101. A clump of the hydroid Tubularia indivisa. (J. S. Ryland)
As a pioneer species, Tubularia indivisa seems to have a role in facilitating the settlement of newcomers, and thereby promoting the diversity of the developing community. This may be in part a passive process: T. indivisa is a non-modular hydroid, but its solitary polyps grow in clumps, as a result of larval settlement behaviour. Its intertwined hydrocauli (‘stalks’), each often in excess of 15 cm high, provide stable frameworks for tube-building amphipods, and habitat for caprellid amphipods. Species of Jassa, in particular, may be abundant within Tubularia clumps, and caprellids such as Pseudoprotella phasma find ideal vantages from which to seize small crustacean prey items or, as effective kleptoparasites, to steal food items from suspension-feeding amphipods, or even from the tentacles of the host hydroid. There is also a possibility that the larvae of some ascidians, Ciona intestinalis and Ascidiella aspersa in particular, may respond positively to a cue originating from the chemical constituents of the outer chitinous covering of the hydroid, the perisarc, when exploring settlement sites. Tubularia indivisa has a life span of around one year (Hughes, 1983). It breeds more or less continuously, with reproductive rate perhaps regulated by ambient temperature, but recruitment of juveniles shows a large peak in the summer months, May and June, with a lesser peak in late September. It does not have a pelagic larval stage. Instead it releases a creeping, tentaculate larva resembling a tiny polyp, termed an actinula, which settles on and attaches to the perisarc of the adult. However, the larva must have sufficient dispersal potential to enable the species to colonise new substrata so swiftly. It develops attached to the hydranth (the tentacle-bearing distal end of the polyp) of the adult, at the top of the hydrocaulus (polyp stem), and is released into the water column up to 20 cm above the substratum, With an estimated sinking rate of 1 mm per second, the actinula might achieve significant dispersal in an average tidal current flow of 10 mm per second. There are no data available on the duration of the free larva phase of T. indivisa, but it must be reckoned in days, or even weeks, as it colonises offshore structures as readily as coastal buoys and pontoons. Prospecting actinulae appear to settle preferentially on adult perisarc, but as a general rule marine invertebrate larvae show decreased discrimination in choosing a settlement site with time, as competence to settle also decreases, and this explains their colonisation of new habitat following perhaps extended dispersal.
The biomass of T. indivisa populations fluctuates annually and seasonally, and usually shows an early peak, reflecting high adult growth rates, and a high proportion of juveniles. Belgian scientists studied T. indivisa populations on a shipwreck 17 km offshore and at a depth of 30 m (Zintzen et al., 2008). Over a two-year sampling programme the total biomass of the epifauna on the wreck ranged from an October minimum of 9 g AFDW/m2 to a summer maximum of 1,106 g AFDW/m2. This range largely reflected seasonal and annual variation in the biomass of T. indivisa, which comprised from 59% to 82% of the total biomass. Mean biomass of T. indivisa ranged from 60 to 324 g AFDW/m2 in late winter, rising to 362–912 g AFDW/m2 in early summer, but dropping to only 5–14 g AFDW/m2 in October. The sharp decline in biomass in late summer and autumn, probably characteristic of all populations of the species, may have had several causes. Predation is one important factor: pycnogonids, polychaetes and several species of small nudibranch are known to feed on T. indivisa, mostly as partial predators, but the large sea slug Dendronotus frondosus will ingest entire hydranths as well as a proportion of the supporting hydrocaulus. A further cause may be a sharp decline in the number of recruits in late summer as the adult clumps become crowded with a dense associated fauna, and perisarc becomes encrusted with sessile taxa, especially bryozoans, preventing settlement of actinulae. Tube-dwelling suspension feeders may disperse or disable prospecting larvae, and other symbionts may even eat them.
Much of the southern North Sea is floored with mixed sands, and wrecks are islands of otherwise scarce hard substrata. On these T. indivisa forms a constant component of an epifaunal assemblage, in which it is dominant for part of the year, providing habitat for a diverse associated fauna. Numbers of individuals inhabiting T. indivisa clumps fluctuated seasonally, in concert with varying biomass of the host, from 6,500 ± 56 individuals per square metre in October to 445,800 ± 189,800 in July; the most abundant species was the amphipod Jassa herdmani, with a minimum 1,000 ± 385 individuals per square metre in October and a July maximum of 398,000 ± 189,000. The number of species recorded was 102, with a sample total of 15 in October and 42 in August, and for 24 species their abundance was positively correlated with the biomass of T. indivisa. The caprellid amphipods Phtisica marina and Caprella tuberculata and the porcelain crab Pisidia longicornis were the next most abundant crustaceans in the community, while cnidarians, annelids and molluscs showed maximum abundances of 6,100, 1,500 and 5,000 individuals per square metre respectively. These communities were considered unusual in that pioneer T. indivisa was the main structural element every year, while elsewhere it seems to be eventually displaced by competitively superior late-successional species. It is not clear how these Belgian wreck communities are maintained, but it is possible that environmental pressures, such as current flow, turbidity and abrasion by suspended material, modify community succession, preventing settlement of late colonisers, and larval supply must also be an important factor where the wrecks are remote from natural hard-substratum habitats.
Offshore drilling rigs provide good opportunities for observing colonisation and succession of sessile biota, in essentially simplified systems isolated from natural, coastal benthos. Two oil-drilling rigs in the northern sector of the North Sea, in waters 158 m and 167 m deep, and two in the central sector in 80 m and 85 m, were monitored over an 11-year period (Whomersley & Picken, 2003). High-resolution video cameras were employed to record fouling communities on clamps securing the oil riser pipe, carrying oil from the well head to the surface, to the drilling rig. Composition of the communities varied with depth and over time. Riser clamps on all rigs were settled by mussels, Mytilus edulis, and green algae, forming a narrow zone, close to the surface, in the upper few metres of the water column, that persisted through the entire survey period. Below the mussel zone, hydroids and tubeworms occupied all areas monitored, at all depths, but were displaced within 3–6 years by the Plumose Anemone, Metridium senile (Fig. 80). On the two northerly rigs M. senile initially settled on the lower riser clamps, but gradually extended its range upwards, outcompeting and displacing the hydroid assemblages, and subsequently-settled colonies of the soft coral Alcyonium digitatum (Fig. 82). On the rigs in the central sector hydroids persisted longest, but after six years they were eventually crowded out by overlapping zones of M. senile, which was dominant at 20–59.9 m, and occurred as deep as 79.9 m, and A. digitatum, which was the spatial dominant between 60 m and 89.9 m. The early-successional, essentially r-selected species were outcompeted by K-selected species, and the developing communities were defined by competition for space and food. The mussel community, in the upper reaches of the water column, might also be considered to be K-selected, but the most important pressure determining their community structure was predation. On the Scylla reef mussels settled in the first summer of its creation but were obliterated by the end of the year, and absent thereafter. The wave-beaten environment of the offshore oil rigs was inimical to the most significant predators of Mytilus edulis, namely Asterias rubens and Carcinus maenas, and the mussels flourished in a predation refuge.
The Stone Coral, Lophelia pertusa (here), was an unexpected colonist of several oil-drilling rigs in the northern North Sea, sited in deep water beyond the 50 m isobath (Gass & Roberts, 2006). Lophelia pertusa is adapted to cold oceanic waters, requiring an optimal temperature within the range 4–12 °C and a minimum salinity of 33 psu. In the northeast Atlantic it is found below the thermocline, but was not known to occur in the deep shelf areas of the North Sea. It is present off Shetland, and in deep, cold Norwegian fjords, but had not been recorded from the North Sea until colonies were found on the decommissioned Brent Spar oil storage buoy in the 1990s. Subsequently it was discovered encrusting 13 out of 14 drilling platforms installed in the northern North Sea between 1975 and 1988, in waters deeper than 100 m. Two platforms studied in detail were found to bear a total of 947 colonies of L. pertusa, within depth ranges of 59–122 m and 62–118 m, and at densities of 0.02–0.39 and 1.23–2.8 colonies per square metre. The period of time that each platform had been established was known, and on the assumption that the largest colonies represented the earliest colonists, growth rates, in terms of radial expansion, were estimated at 6–26 mm per year and 24–33 mm per year for populations on each of the two platforms. This extension of the coral’s range southwards into the deep North Sea was facilitated by the provision of new hard substratum that the founding planula larvae require. Recruitment into a particular rig community would depend upon its distance from the nearest source of larvae, and its proximity to south-flowing cold currents, and would probably have been infrequent and sparse. However, size frequency distributions of the two populations sampled were bimodal, suggesting that the initial colonists had begun to reproduce, and that the new populations had begun to increase through self-recruitment.