Virtually all our food is of biological origin and comes from the tree of life. We eat living organisms, or parts of living organisms that were once alive, from a broad range of sources: plants, animals, fungi, and algae. Even bacteria contribute to our nutritional intake, although we rarely give this a second thought when we eat a dairy product, such as yogurt, that contains live lactic acid bacteria. More amazingly, these microorganisms take up residence in our digestive system, which is host to a thriving community of approximately 100 trillion essential bacteria, or about ten times as many of these unicellular organisms as there are total cells in the human body itself. This is equivalent to about 4.4 pounds (2 kg) of bacteria and may include up to 1,000 different types.
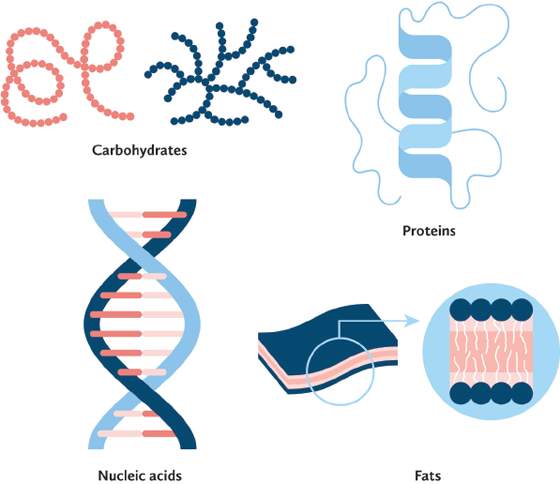
When these diverse sources are turned into food, we can think of them as raw materials that are made up of the same building blocks as living organisms—that is, proteins, carbohydrates, fats, and nucleic acids. Added to these are minerals, trace elements, vitamins, and above all, water, the largest component.
Seen through the eyes of a physicist, biological materials are what are called soft condensed matter—they are flexible, they can be bent, and their shape can be altered. Living organisms also avail themselves of rigid materials to support and protect their soft parts—for example, inner skeletons and carapaces made of calcium and chitin. Nevertheless, biological materials are first and foremost characterized as soft, a condition that is absolutely necessary for their very existence and for being able to carry out those functions that we associate with life.
In the course of human evolution, our sensory system, including mouthfeel, has been especially designed and fine-tuned to explore this characteristic of softness so as to determine whether biological materials are potentially edible.
Foodstuffs derived directly from raw ingredients that have not been altered in any substantial way and that do not contain food additives are commonly thought of as natural foods. Other foods, known as processed foods, are produced from raw materials that have usually been transformed to such an extent that it is no longer possible by simple inspection to determine their origins. This latter category includes a wide range of products, from those that contain mostly natural ingredients, such as butter, cheese, bread, and ketchup, to others that are highly processed and contain significant amounts of preservatives, additives, flavorings, and coloring. Finally, futuristic synthetic food, assembled entirely from chemical compounds, has recently become a reality. In the case of both processed and synthetic food, it is necessary to pay a great deal of attention to producing an appropriate texture that will result in a good mouthfeel. To a large extent, this has to be created as part of the manufacturing process, whereas the texture of natural foods generally mirrors the structure of the living organisms from which they are derived.
Almost every part that is not poisonous of all living organisms has been incorporated into the human diet somewhere in the world. Nevertheless, there are vast differences as to what is regarded as edible. Brains, chicken feet, pig ears, jellyfish, insects, and seaweed are featured in a number of food cultures, while in others they are nowhere to be found. Some people prefer the fleshy meat of land animals, but others are happy to eat the internal organs, referred to as offal. A connection can sometimes be made between preferences for certain raw materials and the extent to which a particular cultural group is attuned to texture and mouthfeel. An example of this is seaweed, which is a staple food in Japan and highly prized for its special texture.
The mouthfeel of edible biological materials varies enormously from one species to another and is, to a large extent, dependent on the parts of the organism from which they were derived, how old those organisms were, and under which conditions they lived or grew. Of course, how the ingredients are prepared also plays an important part. Nevertheless, a number of dominant conditions characterize the different types of raw materials and are determined by their biological origin and physiological function. For instance, food that comes from the sea, especially fish, shellfish, and seaweeds, has a texture that is distinct from that of land organisms in a number of ways. This is because aquatic organisms do not have to support their own weight, whereas terrestrial animals and plants do. Another example is the texture of plants, which reflects that, unlike animals, they are unable to move about and are bound to the place where they are growing.
Plants
Plants are our most diversified food source; we eat a much greater variety of them than of animals. Vascular plants, also called higher plants, have some combination of roots, taproots, tubers, rhizomes, stems, branches, trunks, leaves, flowers, seeds, and fruits, each of which has a distinct structure. Many fruits, such as ripe berries, are juicy and soft, whereas seeds can be hard, crisp, oily, or creamy. Taproots and tubers, which tend to be hard, crunchy, and possibly stringy, can be cooked to make them soft and mealy. Stems and leaves in vegetables might be tough and fibrous, but can be prepared so that they become soft, crisp, or firm.
(Top) Natural foods whose biological origins are easy to identify; (middle) processed foods made from ingredients that have been transformed to such an extent that the raw materials cannot be recognized; (bottom); examples of pure substances that make up food: carbohydrates, proteins, and fats.
Cell structure of a seaweed (dulse) before and after it has been cooked. The cells are around 20 micrometers in size. Cooking loosens the cell structure so that the seaweed is softer and easier to chew.
The potential of plants as a food source is mainly attributable to their lack of mobility and the necessity to survive in places where they have put down roots. For support, plants have firm cells walls reinforced with cellulose to give them form and structure. Not all parts of the plants are equally stiff, as illustrated by the differences between leaves, stems, and roots. While all plants need sunlight to photosynthesize, some are able to thrive growing right along the ground, while others have to be sufficiently rigid to keep themselves very upright to gain sufficient exposure to light. This is why their cells have stiff walls that are strengthened with cellulose, but that are not equally stiff in all parts of the plants. To avoid being eaten, plants may also be protected by having hard or poisonous tissue or a bitter taste. Conversely, they may depend on the harvesting of their fruits and seeds by humans and animals so that these are scattered and the species can be propagated. Ripe fruits, therefore, are often soft and tasty, whereas the unripe ones may be sour, bitter, or very hard. Plant cells contain oils and starch, whose function is energy storage for the plant.
For humans to be able to eat and derive sufficient nutrition from plants, or parts thereof, it is necessary to take many of them on a trip through the kitchen to alter their texture and mouthfeel. Simple mechanical treatment sometimes does the trick. Chopping, crushing, pureeing, and grinding partially break down the cell walls, releasing the contents of the cells. The most effective method, however, is heating. Heating and cooking gelatinize the starch and soften the cell walls, with the result that the plant tissue becomes softer and easier to chew. The cellulose content, which humans cannot digest, is not broken down, though, and cooked plants still contain a considerable amount of insoluble fiber. Some plants contain poisonous substances—for example, hemagglutinin, found in kidney beans; and cyanide, in cassava—and they absolutely must be cooked to render them harmless for human consumption. Other ways of processing plants include salting, drying, marinating, and fermenting, all of which alter their nutritional content and taste as well as mouthfeel.
Two polysaccharides, called pectin and hemicellulose, bind the cells together. In contrast to cellulose, both of these substances are water soluble, and they can actually bind so much water that they can be used as gelling agents. When they are heated in water, they absorb water and the cell walls are softened to such an extent that the parts of the plant are easier to separate into small pieces and are softer to eat. This takes place at moderate temperatures in the range of 176°–198°F (80°–92°C). Using heat to prepare plant-based foods is much less challenging than cooking meat, which involves finding a delicate balance between the different ways in which muscle proteins and collagen behave. And as the cells in animals do not have rigid cell walls, the juices in them are able to seep out during heating, which can result in a dry, unpalatable piece of meat. In some cases, the cell walls of plants retain some of their properties during heating, and water can seep out of the cells—for example, when cabbage and spinach are heated; in other cases, water can be absorbed—for example, when rice is cooked. Although it is easier to preserve the texture of plants than that of meat, there is a greater risk of spoiling their good taste substances and aromas.
Fungi
Fungi make up a large class of organisms. Their cell walls, unlike those of plants, are reinforced with chitin. Many of the small unicellular fungi, including yeast, are not in themselves important contributors to mouthfeel as they are not usually eaten directly. But they play a central role in fermentation, which alters the texture of other raw materials, sourced from both plants and animals. The much larger multicellular fungi, such as button mushrooms, oyster mushrooms, and shiitake, have an edible fruiting body, called the sporocarp, which is the part of the organism that grows aboveground. Truffles are distinctly unusual in that the sporocarp grows beneath the soil near the roots of trees. Approximately 80–90 percent of the fruiting body, which can be fragile, juicy, or tough, is composed of water. When they are heated, the fungi lose most of their liquid content and they shrink, but as their cell walls are not water soluble, they retain their shape, become softer, and do not turn to mush. Soaking dried fungi in water can more or less restore them to their original shape. Some fungi—for example, wood ear mushrooms (Auricularia auricula-judae)—contain water-soluble carbohydrates, with the result that they take on a sticky texture when they are heated.

Button mushrooms growing on spawn.
Button mushrooms that have been cultivated on a bed of dry soil have a special taste and firm texture. The gastronomic value of these extra-dry champignons is often overlooked.
Algae
Algae make up a large, diverse heterogeneous group of organisms, ranging in size from tiny unicellular specimens to large multicellular ones. The smallest algae are unicellular cyanobacteria or phytoplankton that is more closely related to plants. These are consumed mostly in the form of freeze-dried powders, with spirulina and chlorella being the best-known examples. They are used primarily as protein supplements or to add intense green color and have no impact on mouthfeel. The largest algae are the multicellular aquatic organisms that we know as seaweeds, of which there are about 10,000 different species, most of which are edible.
THE DAY BEFORE
• Divide the oil evenly among four small ramekins.
• Separate the eggs and place one yolk in each prepared ramekin. Reserve the whites for another use.
• Turn on a small smoking oven. Or place smoking chips in a pot and set it over heat.
• Place the ramekins in the smoking oven or the pot, turn off the heat, and let stand, covered, for 5 minutes. Taste the oil to find out whether it has taken on a smoky taste; if not, repeat the process. Freeze the yolks, still in the oil, in their ramekins.
• Cut the individual leaves from the endives, until only a single long stalk remains. Reserve the stalk for some other use.
• Season the apple cider vinegar with a little sugar and salt and place in a resealable plastic bag together with the endive leaves. Refrigerate until ready to serve.
• Brush the mushrooms carefully to remove any dirt. Shave the mushrooms very finely and refrigerate.
• Crumble the blue cheese and refrigerate or freeze it whole so that it can be grated over the dish just before serving.
• Whisk together the mayonnaise, yogurt, the vinegar mixture from the endives, and the mustard and Worcestershire sauce. Stir in the parsley and chives.
TO SERVE
• Spread a little of the crème on the bottom of a serving dish.
• Distribute the marinated endive leaves on the crème and heap the mushroom slices on top.
• Sprinkle or grate the blue cheese over the dish.
• Remove the egg yolks from the freezer, take them out of the oil, and grate them on top of the other ingredients.
• To finish, sprinkle a little Maldon salt on top and serve immediately. As an extra touch, add a few croutons and decorate with chive blossoms, if available.
Serves 4
1⅔ cups (400 ml) neutral-tasting oil
4 organic eggs
2 Belgian endives
1 tablespoon (15 ml) apple cider vinegar
Sugar
Salt
7 ounces (200 g) extra-dry champignons
1¾ ounces (50 g) Roquefort or Danablu cheese
Maldon sea salt
CRÈME
3½ tablespoons (50 ml) mayonnaise
3½ ounces (100 ml) low-fat, thick yogurt
1 teaspoon (5 ml) Dijon mustard
1 tablespoon (15 ml) Worcestershire sauce
A little fresh parsley and chives, chopped finely
Extra-Dry Champignons, Endive, and Umami Crème with Grated, Smoked, and Frozen Egg Yolks and Roquefort.

Seaweeds: sugar kelp and winged kelp.
Like those of plants, the cell walls of marine algae are reinforced with cellulose or water-insoluble carbohydrates that resemble it. But their makeup also includes water-soluble carbohydrates that have an important relationship to mouthfeel, especially carrageenan, alginate, and agar. These carbohydrates are a source of soluble dietary fiber and have the ability to bind large volumes of water, making them suitable as gelling agents. Seaweed extracts can be used in this way to add texture to other foodstuffs and are often incorporated into yogurt and desserts. The carbohydrates in some other marine algae, such as those in sugar kelp, produce a slimy mouthfeel. Seaweeds in their natural state may be tough, crunchy, crisp, soft, or hard, depending on the species. In many parts of Asia, seaweeds are prized precisely because of their special contribution to mouthfeel.
In contrast to terrestrial plants, the large marine algae have no need to develop roots and circulatory systems for the transport of water and nutrients. As everything they require is in their immediate surroundings, each individual cell is able to look after itself. An example of how this is reflected in their role as food is the giant kelp (Macrocystis pyrifera), which can grow up to 180 feet (60 m) in length, forming enormous kelp forests, yet in spite of its size is still edible and has a delicate mouthfeel. We can hardly make a similar statement about the largest plants in the world, the giant sequoia trees (Sequoiadendron giganteum), which can grow to a height of over 240 feet (80 m) and are totally inedible.
Terrestrial Animals: Meat from Muscles and Organs
The structure of animals reflects their ability to move around and their consequent need for muscles, a heart, and internal energy depots that are readily accessible. Muscles are composed of bundles of muscle fibers, connective tissue, and fat. The muscle fibers enable the muscles to contract, while the connective tissue holds them all together. Although there are three different types of muscles, it is primarily the striated muscles, which connect bones and tendons, and to a lesser extent cardiac muscles that are eaten. The striated muscles consist of parallel fibers, whereas the cardiac muscles are branchlike rather than linear. This difference has a major impact on their respective mouthfeel.
An essential feature of meat is its fat and protein content. Unlike plants, which store their energy in the form of starch, animals do so in the form of fat. The muscle fibers consist of proteins—myosin and actin—and the connective tissue of collagen, which is also a protein. What is special about the muscles from land animals is that their fibers are very long and run the length of the entire muscle. Collagen is relatively stiff and melts only at temperatures of 140°–158°F (60°–70°C) or higher, at which point it turns to gelatin.
Raw beef heart.
Heart meat, along with that of other organ meats, has fallen out of favor in many Western cuisines, which is a pity because it is exceptionally nutritious. We might bear in mind the old Chinese saying: “Eat the organ you want to heal.”
• Rinse the beef heart and trim off any excess fat and hard bits of tissue and veins around the top.
• Cut it into four or five large pieces and freeze them only as long as it takes for the surface to become a little hard.
• Slice the heart into thin slices, a little under ½ inch (1.3 cm) thick. Use a meat slicer if that is more convenient.
• Dry the meat with paper toweling, brush the slices with olive oil on one side only, and grill, oiled side down, just before serving. Important: remove the slices from the pan while they are still rare.
• Season thoroughly with salt and pepper and serve grilled side up.
Serves 6
1 beef heart (about 3 pounds [1.5 kg])
Olive oil
Salt
Pepper
Grilled Beef Heart.
The muscle fibers are firmly attached to the bones with connective tissue. As a result, raw meat from land animals is usually tough and elastic. Heating it denatures the proteins in the meat and the connective tissue is softened, completely changing the texture of the meat, making it tender and easier to chew.
The fat content in muscles has only a minor effect on the tenderness of the meat. On the contrary, it is the collagen content and the actual structure of the muscles that determine how tender the meat is or can become when it is heated.
Denaturing and unfolding of a protein.
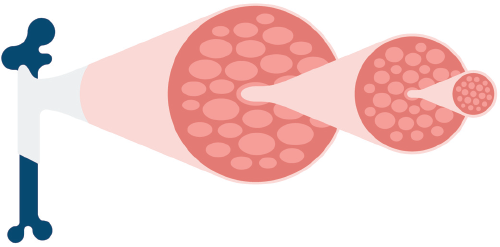
Muscles are built up hierarchically from bundles called fascicles, consisting of from ten to one hundred muscle fibers encased in connective tissue. Multiple bundles of these fascicles are then grouped together and sheathed by a tougher layer of connective tissue to form the actual muscles, which are anchored to the bones by tendons. The tenderness of the muscle meat is determined by three factors: whether the muscle fibers in the fascicles are fine or coarse, the amount of connective tissue, and whether the muscle is weak or strong. In broad terms, we can say that tender meat comes from weak muscles and tough meat from the strong ones.
Connective tissue is a network of collagen fibers and, like the fascicles, is also hierarchically constructed. Each collagen fiber consists of many fibrils, each made up of three long protein molecules, called tropocollagen, that are helically wound around one another. The individual protein molecules are, to a greater or lesser extent, chemically cross-linked. The strength of the fibrils, and consequently, that of the connective tissue as a whole, increases with the number of cross-links. Apart from how it is prepared, this is the key factor that determines how tender the meat will be. Strong muscles and muscles from older animals have more cross-links in the connective tissue.
When meat is heated quickly for a short period of time, the fibrils contract and it becomes firmer. Conversely, if it is slow-cooked at lower heat, the cross-links can be broken down, the water-insoluble collagen breaks up into small pieces and converts to water-soluble gelatin, and the meat becomes more tender. This is why strong muscles and those from older animals need to be cooked for a longer period of time to be tender.
Structure of a collagen fiber from connective tissue. The fiber consists of a collection of fibrils, each of which is made up of three long protein molecules (tropocollagen) that are wound around one another like a helix. In the intact fibril, the individual protein molecules are bound together chemically by cross links. As shown at the bottom, when the fiber is heated, these linkages are broken and the fiber eventually breaks up into smaller pieces. These are actually gelatin, which is water soluble.
Technically speaking, gelatin is hydrolyzed collagen. As we will see later, gelatin can be used to prepare jellied foods. Connective tissue also contains a certain amount of fat, which has only a minor effect on the texture of the meat, but makes a particularly important contribution to the taste.
Only about half of the total meat derived from terrestrial animals is in the form of skeletal muscles. The rest comes from organs—including tongue, heart, liver, kidney, sweetbreads, and tripe—which generally contain much more connective tissue than skeletal muscles. Some of these are quite tough and may require longer cooking times to be palatable and have a pleasant mouthfeel. Others—for example, heart or lamb kidneys—need to be sautéed for only a short time. Unlike the other organ meats, liver consists of a collection of loosely bound cells, which results in a rather delicate texture. In the case of duck and goose liver, it also has a high fat content, causing it to melt partially and to curl up when it is heated.
Eggs
Chicken eggs consist primarily of whites and yolks. Proteins account for 11–13 percent of the whites and the remainder is water. The yolks are made up of 50 percent water, 16 percent protein, and 33 percent lipids (lecithin, triglyceride, and cholesterol) that contribute to the emulsifying properties of eggs.
TWO TYPES OF STRIATED MUSCLES
In principle, there are two types of striated muscles, with their differences reflected in how they work. Slow-twitch muscles need to have endurance in order to work continuously over long periods of time—for example, those in animals that are in constant motion or in the thighs of animals that stand upright most of the time. These muscles obtain energy by oxidizing glucose. Oxygen is constantly transported around in the muscles with the help of myoglobin, a protein molecule related to hemoglobin in the blood. Because myoglobin is red or brown, the slow-twitch muscles are dark and red.
Their counterparts are the fast-twitch muscles, which are able to work in short bursts only, but exert much more force. These muscles cannot wait for the myoglobin to transport oxygen to them, so instead they anaerobically oxidize a special carbohydrate, glycogen, which is produced in the liver and is already stored up in the muscles, ready for use. It is a colorless polysaccharide, with the result that fast-twitch muscles are pale.
On poultry we can clearly see which muscles are slow-twitch and which are fast-twitch by using the examples of meat from domesticated chickens and wild pheasants. The thigh muscles, which are slow-twitch, yield dark meat in both instances. The breast meat of domesticated chickens that are barely able to fly, and for only very short distances, is made up of fast-twitch muscles and is white, whereas that from the pheasants consists of slow-twitch muscles and is dark.
The hierarchical construction of a striated muscle.
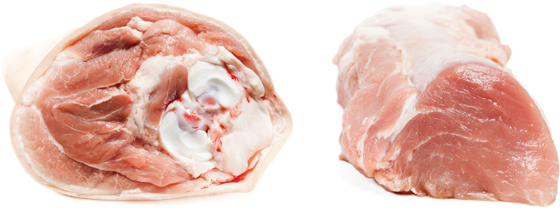
Cross-section of raw pork: (left) shank and (right) tenderloin.
TENDERIZED AND CHOPPED MEAT
Meat from tough muscles with a great deal of strong connective tissue can be tenderized by pounding it or chopping it. This reduces the cooking time so that it can be prepared without losing very much of its taste. In certain instances, it is even possible to eat chopped meat raw, for example, in beef or veal steak tartare. Popular myth has it that the name of this dish is based on the belief that Tatar horsemen used to put a piece of meat under their saddle so that it would be tenderized while they rode. The truth is more prosaic. This dish as we know it today has evolved from one that was originally served with tartar sauce—à la tartare—the sauce is gone, but the name stuck.
The yolk is actually a suspension of lipid and protein particles, collectively called lipoproteins, encased in a complex matrix of water and proteins that has many different levels of structure. The level of most importance in terms of mouthfeel is the highest one, in which the whole yolk is composed of a large quantity of small spheres, about 0.1 micrometer in diameter, which are each protected by a membrane. When the yolk is cooked, the proteins in these spheres coagulate, becoming firm and giving the yolk in a hard-boiled egg its characteristic slightly crumbly structure. The yolk is surrounded by a wall that consists of a biological membrane and a layer of glycoproteins, special proteins that are quite strong.
In contrast to the yolk, the egg white becomes uniformly firm when heated, as the proteins in it coagulate into a solid, homogeneous white mass.
Milk
Milk has a surprisingly complicated inner structure, a characteristic that is responsible for its mouthfeel as a drink and for the properties of the dairy products made from it, including cream, cheese, yogurt, and other soured or fermented products. We will return later to the texture and mouthfeel of all these products.
Whole milk consists of 87.8 percent water, 3.5 percent fat, 3.4 percent protein, and 4.8 percent carbohydrates in the form of lactose, as well as minerals and vitamins. Two types of proteins, casein and whey protein, are particularly important for milk’s role as a nutrient.
There are four different types of casein proteins. These cluster together into small, complex structures, called micelles, which each contain from 10,000 to 100,000 proteins and have a diameter of 0.01–0.3 micrometer. The micelles are made up of smaller groupings of casein molecules that are bound together by hydrophobic interactions and calcium ions, which contribute to the calcium content of milk. Seen from the outside, the casein molecules look a bit like hairs on the micelles. These have a negative electrical charge, which causes the micelles to repel one another and prevents them from clumping together. If the milk is soured, these electrical repulsions attenuate and the milk coagulates into cheese curds when the micelles form a network that captures the fat particles in the milk. Micelles can also be made to bind together into a network with the help of a particular enzyme, rennet, which clips off the hairlike casein molecules.
Whey proteins are dissolved in the milk in the form of individual molecules that contain sulfur, which contributes to the particular smell and taste of cooked milk. When warmed, whey proteins do not coagulate, but instead bind to the casein micelles. Nevertheless, if only a little casein is present, the whey proteins can clump together in the presence of an acid, producing the type of cheese curd that is used to make ricotta.
Structures in milk span many different length scales.
Milk fats are present in the shape of large globules, typically 0.1 micrometer to 10 micrometers in diameter. Each globule is protected by a lipid membrane, which ensures that they do not merge into one another. In fresh whole milk, the fats float to the top after the liquid has been cooled for a few hours and form a layer of cream. When the milk is homogenized, the globules are broken up into fairly uniform pieces, about 1 micrometer in diameter. These are so small that they remain suspended in the liquid, turning the milk into a colloid. In addition, the casein micelles attach themselves to the surface of the fat globules, acting as a sort of emulsifier. If the cream is skimmed off instead, what remains is skim milk, which contains less fat but is rich in proteins. Removing some of the casein micelles from skim milk—for example, in the form of cheese curds—turns it into whey, which also has a low fat content but contains only whey proteins.
Although the fat globules are very stable when heated, during cooling they form crystals that destroy the membranes and the globules cluster together. When cream is churned, the membranes are broken down mechanically and the fat aggregates to form butter. The remaining liquid is buttermilk, which has little fat but retains the proteins.
Fish
In contrast to the plants and animals that live on land, the organisms that have adapted to living in aquatic conditions have developed much less capacity to support their own weight. On account of the buoyancy of water, the effect of gravity is very small because marine plants, animals, and algae have almost the same specific gravity as the surrounding water. Compared with terrestrial animals, those living in water expend no energy to stay upright, and consequently, are able to devote more of their muscle power to movement and to maintaining the shape of their body. Broadly speaking, this means that these organisms have less need for strong support mechanisms. Nevertheless, outer structures, such as shells and scales, are sometimes a practical necessity. In the case of fish, their skeleton and bone structure also depends on the type of water in which they live and how deep it is. Because salt water is more buoyant than fresh and brackish water, ocean fish are generally able to support a frame with heavier, thicker bones, which is also essential for withstanding the tremendous pressure exerted by deep water. Conversely those that inhabit fresh or brackish water tend to have many small, thin bones.
The fibers in the striated musculature of bony fish are much shorter than those in terrestrial animals, being typically on the order of 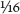 − ⅜ inch (2 mm–1 cm) in length. These short fibers are arranged in layers that are held together by some fragile layers of connective tissue, which stretch from the bones to the inner layer of skin in such a way that the fish is easily able to make swimming movements. This creates the well-known zigzag pattern, easily seen in salmon flesh, or observed less directly when a piece of cooked cod flakes apart. Because this structure is not very sturdy, fish muscles are much softer than those of land-based animals. But this does not mean that their muscles are weak. On the contrary, they can be very strong, because fish need to be able to move quickly through water, which offers much more resistance than the air in which land-based animals move about.
− ⅜ inch (2 mm–1 cm) in length. These short fibers are arranged in layers that are held together by some fragile layers of connective tissue, which stretch from the bones to the inner layer of skin in such a way that the fish is easily able to make swimming movements. This creates the well-known zigzag pattern, easily seen in salmon flesh, or observed less directly when a piece of cooked cod flakes apart. Because this structure is not very sturdy, fish muscles are much softer than those of land-based animals. But this does not mean that their muscles are weak. On the contrary, they can be very strong, because fish need to be able to move quickly through water, which offers much more resistance than the air in which land-based animals move about.
Zigzag structure of a striated fish muscle (salmon).
There are a number of differences in the collagen and proteins found in the muscles of terrestrial and aquatic animals that have implications for mouthfeel. First of all, there is considerably more connective tissue in the meat from terrestrial animals; and, second, the collagen in fish is weaker because there are comparatively fewer cross-links between the individual protein molecules. Fish muscles fall apart more quickly after the fish is killed than do meat muscles, which typically need to be aged before they are ready to eat. In addition, fish collagen melts easily and at lower temperatures; and because it is weaker, fish flesh is softer, more tender, and can, in many cases, be eaten raw. Proteins in fish muscle also denature at a lower temperature, but this can be problematic as it is more difficult to prevent fish from becoming dry and mealy when heated.
The mouthfeel of fish eggs, known as roe, varies, depending on the structure and, especially, the size of the individual eggs. In contrast to eggs from terrestrial animals, fish eggs have no outer calcified shell but only a thin outer membrane. The membrane around small eggs, from such fish as flying fish and smelt, feels hard, crisp, and crunchy, whereas it is soft and supple on the considerably larger salmon and sturgeon eggs. Changing the osmotic pressure—for example, by adding salt—can alter the crispness of the roe. The individual eggs are held together in the egg sac by a protein solution that has to be removed mechanically to isolate an individual egg. If the entire egg sac is cooked, these proteins coagulate and the roe turns into a solid mass.
Fish roe: (left to right) flying fish, lumpfish, and salmon.
Mollusks are invertebrate animals, most of which have an outer shell or two half shells—for example, snails and bivalves, respectively. Others, such as octopus, cuttlefish, and squid, have a minimal outer or inner shell or none at all.
Bivalves have one or two adductor muscles. One type can hold the shells closed tightly over a long period of time, is incredibly strong, and contains a great deal of connective tissue. It is too hard and tough to be edible. The other type, which enables the shells to open or close quickly, contains less connective tissue, and consequently, it is soft. A prime example of an edible adductor muscle is what we know as a scallop. This muscle, which is a fast-twitch muscle, and therefore white, has a very particular tender texture, because the scallop is the only bivalve that can swim over short distances by clapping its shells together.
Some aquatic mollusks—abalone, large sea snails, and geoducks (Panopea generosa), among others—have a muscular foot or siphon that is used for locomotion or food intake. These muscles are very tough, and when heated, they become even tougher. To make them suitable for eating, the uncooked muscles can be pounded or else sliced very thinly to serve raw as sushi or sashimi, in which case they will have a crisp or crunchy mouthfeel.
In some bivalves, such as blue mussels, clams, and oysters, the adductor muscle is small, hard, and basically inedible. Instead, we eat all the rest, made up mostly of the stomach and the gills. The texture of a mouthful of oyster is, therefore, very different from that of a scallop. As the inside of an oyster contains only a little muscle mass, a raw oyster is soft and viscous. It becomes firmer when heated because the proteins are denatured and at the same time bind the free amino acids, which are responsible for its characteristic sweet and umami tastes when it is raw.
Cuttlefish, razor clams, and anchovies.
Mollusks, such as octopus and cuttlefish, have longer muscle fibers and more connective tissue than do bony fish, such as anchovies. The individual muscle fibers are considerably thinner than those in fish muscles, resulting in a firm, smooth flesh. What gives these organisms their particular texture is their large collagen content. The collagen forms cross-linkages, making the muscles exceptionally strong, tough, and elastic. This special muscle structure also gives octopus and cuttlefish a great deal of flexibility, allowing them to change shape in all directions.
Octopuses are notoriously difficult to prepare. They have to be cooked either very little or for a long time. When heated for a short period of time, they become soft and juicy because the proteins are not fully denatured and the collagen is partially softened. But if we misjudge and cook them a little too long, they become tough, because the collagen contracts in all directions and the muscles lose liquid. The muscles then become soft and tender only after they have been simmered slowly so that the connective tissue breaks down to gelatin. Making crosshatches in the surface of the flesh helps somewhat to speed up this process.
Crustaceans, such as shrimps, lobsters, scampi, and crabs, have external shells, and their head often forms an integral part of the body. Shrimps, scampi, and lobsters have a very distinct striated, segmented musculature in their tail, which contains more connective tissue than the flesh of bony fish. For this reason, these muscles are tougher and dry out more readily than those from fish. A raw shrimp muscle is soft and a little soapy, but becomes firm when cooked. Because they contain large quantities of aggressive enzymes that quickly render the flesh mealy, lobsters and some other crustaceans must either be eaten immediately after they are killed or be cooked right away if they are to be kept a little longer.
Scampi (Nephros norvegicus).
In many places all around the world, humans have eaten insects for thousands of years and in many countries—for example, Mexico, Thailand, and the Democratic Republic of Congo—some species are considered a true delicacy. Even though it is estimated that 70 percent of the global population eats insects in one form or another, they are still thought of as strange and exotic in most Western countries. In terms of nutrition, insects are very important because they contain considerable quantities of proteins, fats, and vitamins. They are a sustainable food source and utilize the earth’s resources much more efficiently than domesticated animals.
With their crisp, crunchy exoskeleton and softer innards, insects could quite easily be transformed into foods with a perfect mouthfeel. The only stumbling block is figuring out how to overcome the cultural aversion that some people have to eating bee larvae, grasshoppers, ants, and a range of other insects.
ROASTED BEE LARVAE: PEAS ’N’ BEES
Researchers and cooks at the Nordic Food Lab in Copenhagen are engaged in an ongoing quest to seek out the gastronomic potential of raw materials found in the Nordic countries and to tease out their distinctive tastes. A scientist, Josh Evans, and a chef, Roberto Flore, have turned their attention to insects. They have discovered an untapped resource—the bee larvae that beekeepers routinely discard in the springtime from the frames in beehives to control the problem of mite infestation. These larvae are very rich in proteins and unsaturated fats, so why not eat them?
Peas ’n’ Bees: toasted bee larvae sprinkled on a creamy, chilled soup made from fresh peas, lovage, and toasted bee larvae sprinkled on top.
This is where the problem of texture arises. Most people are unwilling to eat insects, period. And if what is on offer are fat, creamy larvae, there are even fewer takers. The trick is to make them crunchy and mix them with other ingredients. One solution is a type of toasted muesli that combines bee larvae, honey, and different types of cereals and seeds.
Another possibility is to deep-fry the larvae. Prepared this way, they reminded the cooks of popcorn. So, Evans and Flore came up with the idea of sprinkling toasted bee larvae on top of a creamy, chilled green pea soup. This dish, which was named Peas ’n’ Bees, had its debut at an international symposium on the science of taste held in Copenhagen in 2014.
“Like everything on earth, foods are mixtures of different chemicals.” This statement by noted American food writer and scholar Harold McGee serves to remind us that what we eat is made up of molecules, in particular, proteins, carbohydrates, fats, and nucleic acids, or the products formed by breaking them down. The first three types of molecules are of importance for nutrition, taste, and mouthfeel, whereas nucleic acids in the form of their degradation products, known as free nucleotides, have an effect on taste, especially umami.
Proteins and carbohydrates are normally fairly large molecules that are composed of many smaller parts and form long chains or cross-linked networks. Such molecules are called polymers. The polymeric properties of proteins and carbohydrates are totally responsible for the way in which these molecules can provide structure and, with it, mouthfeel in food. Water plays an important role because the degree of water solubility of polymers completely determines how a foodstuff is structured. Carbohydrates are generally water soluble, whereas some proteins are and others are not. The behavior of fats in the food is to a great extent linked to their relative insolubility in water. When they do dissolve, however, they form some very special structures that have a great effect on texture.
Dried grasshoppers.
The molecules we eat: carbohydrates, proteins, nucleic acids, and fats.
Two particular properties of the proteins, carbohydrates, and fats that are found in living substances are the primary reasons that they form soft matter. One of the properties is that the molecules have very different reactions to water, being hydrophilic, hydrophobic, or amphiphilic, concepts that will be explained later. The other is that they are normally very large—that is, macromolecules—and may have complex structures and form intricate links with one another and with water.
The combination of these factors and how they interact is the secret underlying mouthfeel. Keeping this in mind, we can view the preparation of food as a collection of processes that helps transform the general characteristics of the raw ingredients in such a way that the food is edible, nutritious, palatable, and preferably safe to eat. These processes change their structure and, with it, how we experience their texture. To be able to understand how this works and how we can change it, we first have to know more about the actual molecules that make up the raw materials derived from living organisms.
Proteins
Proteins are made up of long chains of amino acids held together by strong chemical bonds. These bonds can be broken down by the action of a variety of enzymes that cut the proteins into smaller pieces, among them free amino acids and small peptides, which are important for both nutrition and taste. The enzymes can, therefore, have an effect on raw ingredients and the structure of food. An example of this process is a pineapple dessert that becomes runny because certain enzymes in the fruit degrade its pectin. Similarly, enzymes in calf rennet break down the proteins in the small micelles in milk and the milk coagulates, and amylase in the saliva breaks down starch to sugars.
A protein can incorporate both hydrophilic and hydrophobic amino acids; this causes it to fold up into a complex structure when immersed in water. This structure is important for the biological function of the protein. As proteins can also carry an electrical charge, the way in which they fold up depends on which salts and acids are present in the water. In addition, the folding process is temperature-dependent; at either low or high temperatures, the majority of proteins will undergo a change of structure; that is, they denature. We can see these effects in a cooked egg, where albumin, the protein in the egg white, becomes stiff, or in ceviche, where the proteins in the raw fish muscle denature and unfold when it is marinated in lemon juice, leaving the flesh a little firmer.
Some types of proteins that are water soluble, such as gelatin from connective tissue, can bind large quantities of water. Hence, they can be used to alter the flow behavior of liquids to make them more viscous. Under some circumstances, they then form a particular type of firm substances called gels. Gels containing a great deal of water are also known as hydrogels.
Carbohydrates
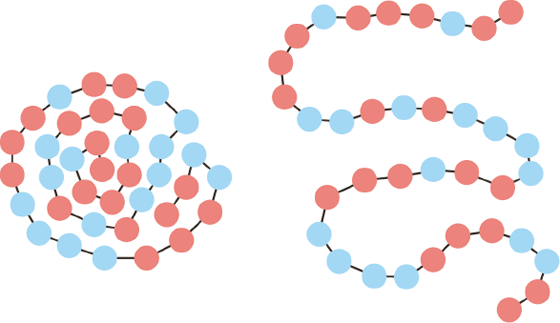
Carbohydrates are made up of a variety of sugars—for example, the monosaccharides glucose (grape sugar), fructose (fruit sugar), and galactose. When paired, they form other sugars; among the common ones are the disaccharides sucrose (fructose + glucose, ordinary white sugar), maltose (glucose + glucose, malt sugar), and lactose (glucose + galactose, milk sugar). Most sugars are not stable when heated and give off water, which affects their melting properties so that they caramelize. A larger number of sugar molecules can also bind together as polymers, called polysaccharides, in the form either of long chains as in amylose, a component of starch, or of networks, such as cellulose. Networks are stiffer than chains, which is why the support structures of plant cells and tissues are composed primarily of cellulose. As we will see later, starch is an interesting example of the interplay between chains and networks.
Starch is made up from a mixture of a linear polysaccharide, amylose, and a branched-chain carbohydrate called amylopectin. To understand the relationship between how these two types of starch work in practice, we can look at how they affect the amount of water and the cooking time needed to prepare different types of rice. More water and a longer cooking time are required to dissolve the starch crystals in long-grain rice. Short-grain rice, of the kind used for rice pudding and sushi, has a greater proportion of amylopectin, resulting in a softer mouthfeel than the harder long-grain varieties. A number of starches in powder form, commonly sourced from corn, wheat, rice, and potatoes, are often used in cooking to thicken liquids such as gravies.
In addition to starches, there is a long list of other polysaccharides that, under the right conditions, are soluble in water, can bind with it, and form hydrogels that have the properties of solid substances. Examples include fruit pectin and certain polysaccharides extracted from seaweeds. All these substances can be used to alter the flow behavior of fluids, to make them more viscous, and in certain cases to form gels.
Some enzymes can facilitate the cleavage of the bonds in carbohydrates. For example, amylase breaks down starch in the mouth and the stomach, and pectinase degrades pectin, as happens when an apple becomes overripe.
Fats
In contrast to proteins and carbohydrates, fats are not polymers, even though the molecules themselves can be large. Fats in raw ingredients are found in a variety of forms, both as building blocks in cells or in designated fat deposits. In animals they take the form of fatty tissue and in plants they are stored as oil in seeds, nuts, and fruits.
Some of the fat in a raw ingredient can often be rendered by boiling, steaming, simmering, or frying it, thereby breaking down the fatty tissues. The gentler methods, cooking in liquid or steaming, allow the fat to retain some of its character, whereas the other ways of heating the fat often degrade it and change its taste. Fats that are released from the meat can be used for the preparation of other foodstuffs, to add both taste and texture.
“Fat” and “oil” are merely different names for the same class of substances. Those that are liquid at room temperature are usually referred to as oils, whereas those that are solid, including butter, are called fats. These phases are entirely a function of their melting point, which is very dependent on the degree of saturation of the fat—the more unsaturated, the lower the melting point. Fats from plants, and especially, those from fish, are often unsaturated and typically have a low melting point, whereas fats from terrestrial animals are more saturated and generally have a higher melting point. Because they have a lower melting point, the loss of unsaturated fats during the cooking process is often greater than that of saturated fats, which can result in a drier mouthfeel, such as that of fish.
In relation to taste, there is a risk that foods containing fats may turn rancid due to the oxidation and breakdown of unsaturated fats if stored for too long a period of time or in an environment that is too warm.
Fats in food are typically made up of fatty acids that are bound in pairs or in threes to other molecules, such as glycerol, and that can also be attached to carbohydrates. Oils and fats are often composed of what are called triglycerides; frequently many different types mixed together, which means that they do not have a unique melting point, but melt over a temperature interval. Triglycerides are hydrophobic and are immiscible in water. But fats, such as the lipid lecithin, can also be amphiphilic and their solubility in water can vary significantly, a property that is important for the formation of emulsions, as discussed later.
The melting properties of fats are crucial for the mouthfeel of a food. Think of chocolate—if it does not melt on the tongue, it feels completely different, resulting in another type of flavor impression. This is a primary reason for the use of cocoa butter in chocolate making. Its melting point is around 95°F (35°C), which is considerably higher than the average room temperature, but a little under body temperature, and therefore, it melts in the mouth.
| Fats and oils |
Melting point (°F/°C) |
| Clarified butter (from cow’s milk) |
206–210/96–99 |
| Beef fat |
129/54 |
| Duck and chicken fat |
129/54 |
| Solid margarine |
113/45 |
| Palm oil |
99/37 |
| Cocoa butter |
93–100/34–38 |
| Soft margarine |
91–109/33–43 |
| Pork fat |
91/33 |
| Butter |
82–100/28–38 |
| Coconut oil |
77/25 |
| Palm olein oil |
50/10 |
| Sesame oil |
23/−5 |
| Olive oil |
21/−6 |
| Grape seed oil |
14/−10 |
| Rapeseed oil |
14/−10 |
| Corn oil |
12/−11 |
| Soybean oil |
3/−16 |
| Thistle oil |
1/−17 |
| Sunflower oil |
1/−17 |
Source: N. Myhrvold, Modernist Cuisine: The Art and Science of Cooking (Bellevue, Wash.: Cooking Lab, 2010), 2:126.
Fats with high melting points form small crystals in the food. These small crystals can organize themselves into a sort of network that imparts properties resembling those of a gel or a solid. Some examples are pork fat, butter, and margarine at room temperature, all of which melt over a fairly broad range of temperatures. Unless they are very cold, these substances are quite pliable, allowing them to be spread easily on the surface of another food.
The fat content of foods is normally a mixture of many different types, which means that it exhibits complex behavior, with solid and crystalline phases that melt over a broad range of temperatures. In many cases, the structure of the fat is not in equilibrium and can change over time. Also, its stability depends on how the foodstuff has been handled. As a result, an otherwise fluid fat can on occasion become solid and crystalline when heated, the reverse of what we might expect. Chocolate again serves as an example: when it is melted and then cooled, the cocoa butter does not return to its original crystalline structure but becomes another that has a lumpy mouthfeel.
A common characteristic of all the materials from which living organisms are composed is that their structures come in many sizes, from the tiniest at the atomic and molecular level right up to that of an entire organism. Another characteristic is that the design principles behind biological soft materials are based on self-organization and self-assembly, what is known as a bottom-up approach. As we have already seen in the case of muscles and collagen, this means that the different molecular building blocks—proteins, fats, carbohydrates, and nucleic acids—initially organize themselves into larger structures, which in turn organize themselves into still larger ones, and so on. The result is a sort of hierarchical construction, which endows biological materials with their very special properties and which is a precondition for their ability to support life and life functions. This construction also determines the texture of a substance when it is used for food, especially how it is transformed in the kitchen and changes when we put it in our mouth.
An example is the complex inner structure of a cell, which is a combination of small and large molecules that together make up higher-level units, including the cell nucleus, organelles, fibers, and communication systems. All of these are enclosed in a container, the cell, surrounded by a wall, the cell membrane, which is a very thin, highly structured layer of lipids, proteins, and carbohydrates. Several cells can join together to form much bigger entities, such as muscles, organs, nervous systems, and circulatory systems, eventually ending up as a complete organism at a higher level of complexity.
Biological soft materials exhibit the properties of both fluids and solids, being something in between the two. They are often called structured or complex fluids or macromolecular materials. They resemble fluids in that they are flexible, their shape can be altered easily, and they can adapt to their surroundings. They resemble solids in that they are elastic, pliable, very strong, and can usually retain their shape. All these characteristics, which are vital for their function in living organisms, are due to their being composed of polymers. These are either long-chained or branched macromolecules that are self-assembled from other, often large, molecules, typically proteins, fats, and carbohydrates.
Biological soft materials—both natural and processed foods.
Soft matter is endowed with a very special property that is due to its being formed by self-assembly with bottom-up design principles. Within certain limits, it can repair or heal itself, an ability unique to living organisms and unknown in all other materials. Just think of how fantastic it would be if a computer could correct a hardware malfunction after it had been dropped on the floor or if the outside of a building could clean itself or mend storm damage on its own. A self-organized soft material, whether it is dead or alive, can often autonomously rebuild a structure after it has broken down, possibly due to external forces. We will encounter this principle again and again in the kitchen, for example, when we make mixtures, when we add sugar, salt, and acids, and when we whip, heat, or cool ingredients, all processes that can dramatically alter mouthfeel.
To truly understand what is responsible for the special properties of biological soft materials and, consequently, their particular effect on the texture of food, we need to explore the role of their most overlooked component—water.
All living organisms contain massive quantities of water in liquid form and depend on it for their very existence. In fact, it is by far the most prevalent component of the raw ingredients that we eat and can account for up to 90 percent of the total weight of fresh fruits and vegetables.
Some Properties of Water
Water is unique in that no other substance can match its amazing stability, which is due to the extraordinary ability of its molecules to form hydrogen bonds. This implies that water has both a high melting point and a high boiling point. In liquid form, water simply sticks together incredibly well, in some ways even better than ice. Actually, the individual molecules are so content to bind to each other that they are reluctant to mix with molecules that lack hydrogen bonds, such as oils and fats. As a consequence, mixtures of oil and water separate out, unless extra effort is made to bind them together.
HOW MUCH WATER?
Water is life and life is water. Every form of life as we know it is dependent on water—water in liquid form. We humans are actually mostly made up of water: 95 percent of our weight when we are still in the womb, 75 percent while we are babies, 60 percent in adulthood, and even after we have expired of old age, the corpse is still 50 percent water.
Everything that we eat contains varying amounts of water, reflecting the biological origins of our food from organisms that were once alive. Raw ingredients are mostly water. Fresh meat has a water content of about 70 percent; while in fruits, vegetables, and fungi, it is in the range of 70 to 95 percent. Prepared foods also contain reasonable quantities of water. For example, cooked rice and cooked eggs have a water content of 73 percent; bread, about 35 percent; and butter, 16 percent, but a dried cracker or biscuit has only about 5 percent.
The water content of liquid foods varies enormously, from almost entirely water to liquids such as olive oil that contain absolutely none.
The structure of a foodstuff can sometimes make it difficult for us to judge how much water it contains. Who would have thought that firm, fresh carrots contain just as much water as whole milk, about 88 percent, or that 25 percent of the volume of a crisp, juicy apple is air?
| Foodstuff |
Water (%, by weight) |
| Tomatoes, lettuce |
95 |
| Strawberries, green beans, cabbage |
90–95 |
| Carrots |
88 |
| Eggs: whites (yolks) |
88 (51) |
| Apples, oranges, grapefruit |
85–90 |
| Beets, broccoli, potatoes |
80–90 |
| Fresh poultry |
72 |
| Fish |
65–81 |
| Lean beef |
60 |
| Fresh pork |
55–60 |
| Cheese |
37 |
| White bread |
35 |
| Jam |
28 |
| Honey |
20 |
| Dried fruit |
18 |
| Butter, margarine |
16 |
| Starch |
13 |
| All-purpose flour |
12 |
| Dried pasta |
12 |
| Milk powder |
4 |
| Beer |
90 |
| Whole milk |
88 |
| Fruit juice |
87 |
| Whiskey |
60 |
| Olive oil |
~0 |
Sources: T. P. Coultate, Food: The Chemistry of Its Components (Cambridge: Royal Society of Chemistry, 2002); J. W. Brady, Introductory Food Chemistry (Ithaca, N.Y.: Cornell University Press, 2013).
Pure water freezes at 32°F (0°C), forming hard ice crystals. This is of major importance for all foodstuffs that contain water. The entire water content in a particular substance does not turn to ice at the same temperature because much of the hydrogen-bond network has been disrupted and a small portion of the water, up to about 0.5 percent, is so tightly bound that it can neither freeze nor melt. In addition, biological material incorporates a host of substances that function as a sort of anti-freeze. This effect is based on the fundamental physico-chemical law called freezing point depression. Small quantities of other substances—for example, salt, sugar, carbohydrates, and proteins—that are dissolved in liquid water have the effect of lowering the temperature at which it freezes to below 32°F (0°C). For example, water in a saturated solution of table salt (NaCl) freezes only at −6°F (−21°C), a phenomenon that was utilized before the advent of the freezer to make ice cream by chilling the ingredients in pots immersed in these ultracold liquid solutions. A variety of substances are dissolved in the cells of biological materials, contributing to their tolerance for cold so that ice crystals in cold weather do not form and destroy them.
In the kitchen, we can use freezing point depression to prepare such dishes as sorbets and ice cream that contain liquid water at low temperatures and have few and relatively small ice crystals, resulting in a better mouthfeel. Two well-known gastronomic “antifreezes” are sugar and alcohol. The size of the ice crystals can also be reduced by freezing the ice cream mixture rapidly. Unfortunately, after sitting in the freezer for a period of time, these small ice crystals will recrystallize into other forms, and in the process, will join together into larger crystals, affecting the mouthfeel of the dessert.
The boiling point for pure water at normal atmospheric pressure is 212°F (100°C). This boiling point can be elevated, however, by adding another substance—for instance, sugar or salt—to the water. An example of where this comes into effect is when fruit juice and sugar are boiled together to make jelly; the boiling point is elevated as the water gradually evaporates.
How Do Other Substances Feel About Water?
Substances that are able to form some hydrogen bonds or that have polar moieties can be dissolved in water. These are characterized as hydrophilic, meaning they love water. Sugar or certain proteins and carbohydrates that can bind large amounts of water, such as fruit pectin and starch from flour, are good examples. At the other end of the spectrum are hydrophobic, or water-hating, substances that are unable to form hydrogen bonds, are not polarized and, consequently, are minimally soluble in water. Examples include olive oil and milk protein (casein). Finally, there are substances made up of molecules with two different parts, one of which is hydrophilic and the other hydrophobic. As they have mixed feelings toward water, they are called amphiphilic.
Amphiphilic substances can bind both oil and water to form what is known as an emulsion and they are, therefore, called emulsifiers. This effect is seen when lecithin from egg yolks binds together edible oils and vinegar in mayonnaise. The structure of mayonnaise, which can be regarded as a self-assembly of oil, vinegar, and lecithin (an oil-in-water emulsion), determines its mouthfeel. The effect is also at work when the amphiphilic glycoproteins found in the shells of mustard seeds are used as the binding agent in a vinaigrette.
Micrograph of mayonnaise, an oil-in-water emulsion. The spheres are droplets of oil, typically 2–5 micrometers in size, and the blue areas in between them are water.
Biological materials contain an abundance of amphiphilic molecules, including lipids and certain proteins and carbohydrates. Together with ions and salts, these molecules help water, which is normally present in great quantities, join up with hydrophobic substances such as fats and some proteins. As a consequence, the structure and stability of the materials is determined by a balance between the propensity of water for forming hydrogen bonds on the one side and the strength of the bonds between water and the other substances on the other side. In this way, the unique properties of water have an indirect, but extremely vital, role in shaping the mouthfeel of food.
How Does Water Interact with Other Substances?
Even though water is essential for life, the water content of food can be problematic and cause spoilage due to microbes that can grow only in the presence of water. It is not the total amount of water that counts as much as what is described as its activity.
The activity of water depends on the extent to which its molecules are accessible; if they have already formed tight bonds, their activity is lower. A food product—for example, dried fish—can easily have a water content of 20 percent but, as this water is tightly bound the fish is fully preserved, and the bacteria will not be able to thrive on it. Similarly, a number of enzymes that help decompose food components, such as fats, are dependent on water activity.
Other methods of binding water in a foodstuff involve the use of special substances that can bind a large amount of water, and in that way, compete for the water in the food. The best known of these are carbohydrates, such as sugar and polysaccharides, and salt.
In a polysaccharide, an individual sugar such as glucose can react with more than five hundred water molecules. This is what permits the formation of a hydrogel consisting of more than 99 percent water. We can think of this type of hydrogel as a sort of sponge, where the polysaccharides build walls that keep the water bound in a network of tubes and empty spaces. Water-soluble proteins behave more or less like polysaccharides. Their surfaces consist overwhelmingly of amino acids with chemical groups that can bind water. The extent to which they can do so is strongly dependent on the ion content and the acidity of the water. As the pH of the water decreases, the amount of bound water increases. Also, when they are unfolded and denatured, the proteins are usually able to bind more water, unless they clump together. This happens, for example, when eggs coagulate into scrambled eggs or when meat is fried, releasing some of its juices. Small ions from salts increase the ability of proteins to bind water but only up to a certain point. If the salt concentration is too great, the proteins will precipitate because the salt ions will bind the water so that they themselves remain dissolved. Normally we do not add so much salt to foods that this happens. But when moderate amounts of salt are injected into foods, such as ham, the muscle proteins are able to bind more water and the meat is moister. By way of contrast, salt that is sprinkled or rubbed on the surface of a food, such as Bayonne ham, helps draw out the water, which has a preservative effect.
Dehydration: Removing Water
There is a whole range of very different processes that can be used to alter water content and water activity; often they affect the texture of the food. Dehydration is the most common method. Conversely, wetting, soaking, hydration, or rehydration can be used before a dried food is added to a dish so as to incorporate liquid. Rehydration can occur quite automatically—for example, when we put such foods as potato chips or dried fruit into our mouth.
Drying a raw ingredient is a way of drawing out the water. The exact amount depends on the length of time involved, the humidity of the surrounding air, and the specific properties of the raw ingredient itself. There is a long list of methods that can be used to remove water; these include air drying, vacuum packing, filtering, microwave dehydration, oven heating, spray drying, freeze drying, and extrusion.
In spray drying, a liquid is blown into a warm air mass, possibly under reduced pressure, causing the water to evaporate and the dried particles to fall like powder snow. The individual particles in the powder are typically 100–300 micrometers in size. This process is often used to produce commercial emulsifiers.
Freeze drying is effected by reducing the pressure around an already frozen substance to such a low point that its water content sublimates; that is, it changes directly from the solid phase, ice, to water vapor. The dried substance can then be turned into powder by crushing or grinding it. A typical product made using this process is instant coffee.
Spray drying of emulsifiers.
Extrusion involves forcing a mixture, the extrudate, which usually contains water and starch, through a perforated plate or similar die, while at the same time heating it to evaporate water and turn it into a pliable mass. It is then dried so that it hardens and can take on a state called a glass, which greatly improves its shelf life. Carrying out this form of dehydration successfully depends on how well the transition to the glass state is controlled. This is a topic that we will return to later, as this state is a perfectly normal one for a whole range of goods. These include bread crusts, hard candies, and frozen foods, which are all, technically speaking, glasses.
There are a number of other ways to draw water out of raw ingredients. We can place them in a solution with salt or another substance that is good at binding water—for example, certain polymers and carbohydrates. Osmosis can be used to do this in a controlled way: the food is separated from the substances that will bind the water by a semipermeable membrane that permits the passage only of water.
All these methods decrease the water activity in the raw ingredients. But, given that water is an essential component of all biological materials, this decrease can lead to structural changes, ultimately affecting their mouthfeel.
The different methods for achieving a low level of water activity do not necessarily result in identical internal structures. In the case of some complex processes that involve drying followed by rehydration, adding water does not return the ingredients to their original state, because something irreversible has taken place. Similarly, when a sequence of dehydrating processes is involved, changing their order can lead to different outcomes. This factor is very important for achieving an appropriately crunchy mouthfeel from dried vegetables that are placed in a salt, sugar, or alcohol marinade.
Both dehydration and the way it is carried out have a major effect on the structure of a raw ingredient. This is because removing the water has the effect of condensing the carbohydrate and fat content, resulting in more solid structures in the form of crystals or glass phases. Their detailed structure depends, in turn, on the process used to remove the water.
When we prepare food from raw ingredients, such as meat, vegetables, fish, fruits, and fungi, we can usually recognize the structure of their characteristic biological tissues. But our diet includes many other types of products that are categorized as processed foods. These are put together either directly from natural ingredients or from substances that have been extracted from biological materials. The goal is to transform them in such a way that they taste good, have a pleasant mouthfeel, or introduce new elements in a dish, and as a bonus, might be less perishable.
The majority of our drinks and liquid foods, such as fruit juice, syrups, broths, and many others, have been processed at least minimally. Others—for example, beer, wine, fish sauce, and soy sauce—are so highly processed that it is no longer possible to find any traces of the structure of the original ingredients from which they are produced. This is also true of many viscous or solid foods, such as cheese, bread, ketchup, and so on, that are produced from a variety of natural ingredients.
Other products are even farther removed from their origins. Think of mayonnaise, an emulsion of water and oil, or ice cream, as well as jellies made from pectin and fruit juice. Is it possible to stray any further from the tree of life that was our first source of food? As we will see, the answer is yes.
We subconsciously think of food as something that has a biological origin—in the form of either raw ingredients that may or may not be processed in some way or extracts derived from them. But why not construct or synthesize a dish or an entire meal totally artificially using pure chemicals that are exact copies of the molecules found in natural ingredients?
A French physical chemist, Hervé This, deemed to be one of the fathers of molecular gastronomy, has become the spokesman for what he identifies as the next culinary trend: note-by-note cuisine. He uses the expression to define a way of preparing food that uses no meat, fish, plants, vegetables, fruits, fungi, or algae, and not even any complex extracts from these natural ingredients. Instead, only pure chemicals on their own or in the form of compounds are utilized. These substances can be synthesized in a laboratory or factory by chemical processes or be extracted from biological materials. This idea may seem strange, but when it comes right down to it, there is no point in discussing the origin of molecules in their purest form—they are always identical regardless of origin. Food can be constructed exclusively from chemicals, paying careful attention to their nutritional contents and health-promoting properties in such a way that it is quite possible to live on a diet consisting entirely of note-by-note cuisine.
Hervé This makes an analogy between food prepared in this way and electronic music, which is not performed by instruments but composed of individual synthesized sounds that, when put together, create a piece of music. Similarly, a chef is able to design dishes using chemical components that produce shapes, tastes, smells, colors, textural elements, and substances that stimulate the trigeminal nerve, so that they blend together, like the notes of a symphony, into a meal incorporating all the facets of flavor.
According to This, it is probably not particularly difficult to mix substances together to elicit the desired color, taste, odor, and trigeminal stimulation. It is also relatively easy to create simple textures by using coagulation, gelation, and emulsification techniques. These are already known from a range of such products as tofu, imitation crabmeat, and seitan. Reproducing the more complicated structural elements that are crucial for texture and mouthfeel presents a much greater challenge. But This thinks that perhaps the problem can be solved through advances in technology and he stresses that the goal should not be simply to make artificial copies of ingredients already available from biological sources. Discovering what he calls “a whole new continent of flavor” would be far more interesting. So, just as making culinary foam with a siphon flask and using liquid nitrogen for flash-freezing became hallmarks of molecular gastronomy, finding methods to create remarkable dishes with completely novel tastes and textures will be the distinguishing characteristic of note-by-note cuisine.
SCIENCE FICTION FOOD: LOTS OF NUTRITION, NO MOUTHFEEL
In Harry Harrison’s science fiction novel Make Room! Make Room! (1966), there is reference to a product called Soylent, a name cobbled together from the words “soy” and “lentils.” It is supposed to be the answer in some future time to the problem of food shortages due to overpopulation, lack of raw ingredients, and the breakdown of societal infrastructure. Even though Soylent is introduced more or less as a spoof in the book, where it appears in the form of a hamburger patty made of soybeans and lentils, the central idea of an artificial food that fulfills all human nutritional requirements has now become a reality. It was first marketed in 2014, inspired by its fictional forerunner and named after it. Soylent is sold as a powder, which is then emulsified in water to make a liquid food. It contains all essential proteins, amino acids, carbohydrates, fats, minerals, vitamins, trace elements, and dietary fiber in the quantities and formats necessary to meet human nutritional needs. But how boring it would be to live on nothing but Soylent—its texture is uninteresting and it never varies.
The proponents of note-by-note cuisine acknowledge that mouthfeel is an essential dimension of the gastronomic experience and the overall pleasure derived from eating. It is, therefore, of paramount importance not to confuse this way of preparing food with the mixtures of nutrients, vitamins, minerals, and so on, that have been developed with the sole purpose of satisfying the nutritional requirements of the human body without paying special attention to flavor impression and mouthfeel.











 − ⅜ inch (2 mm–1 cm) in length. These short fibers are arranged in layers that are held together by some fragile layers of connective tissue, which stretch from the bones to the inner layer of skin in such a way that the fish is easily able to make swimming movements. This creates the well-known zigzag pattern, easily seen in salmon flesh, or observed less directly when a piece of cooked cod flakes apart. Because this structure is not very sturdy, fish muscles are much softer than those of land-based animals. But this does not mean that their muscles are weak. On the contrary, they can be very strong, because fish need to be able to move quickly through water, which offers much more resistance than the air in which land-based animals move about.
− ⅜ inch (2 mm–1 cm) in length. These short fibers are arranged in layers that are held together by some fragile layers of connective tissue, which stretch from the bones to the inner layer of skin in such a way that the fish is easily able to make swimming movements. This creates the well-known zigzag pattern, easily seen in salmon flesh, or observed less directly when a piece of cooked cod flakes apart. Because this structure is not very sturdy, fish muscles are much softer than those of land-based animals. But this does not mean that their muscles are weak. On the contrary, they can be very strong, because fish need to be able to move quickly through water, which offers much more resistance than the air in which land-based animals move about.