WHILE A YOUNG WIFE kept house, washed her baby daughter and put pans on the fire, in a wretched laboratory at the School of Physics a woman physicist was making the most important discovery of modern science.
At the end of 1897 the balance sheet of Marie’s activity showed two university degrees, a fellowship and a monograph on the magnetization of tempered steel. No sooner had she recovered from childbirth than she was back again at the laboratory.
The next stage in the logical development of her career was the doctor’s degree. Several weeks of indecision came in here. She had to choose a subject of research which would furnish fertile and original material. Like a writer who hesitates and asks himself questions before settling the subject of his next novel, Marie, reviewing the most recent work in physics with Pierre, was in search of a subject for a thesis.
At this critical moment Pierre’s advice had an importance which cannot be neglected. With respect to her husband, the young woman regarded herself as an apprentice: he was an older physicist, much more experienced than she. He was even, to put it exactly, her chief, her “boss.”
But without a doubt Marie’s character, her intimate nature, had a great part in this all-important choice. From childhood the Polish girl had carried the curiosity and daring of an explorer within her. This was the instinct that had driven her to leave Warsaw for Paris and the Sorbonne, and had made her prefer a solitary room in the Latin Quarter to the Dluskis’ downy nest. In her walks in the woods she always chose the wild trail or the unfrequented road.
At this moment she was like a traveler musing on a long voyage. Bent over the globe and pointing out, in some far country, a strange name that excites his imagination, the traveler suddenly decides to go there and nowhere else: so Marie, going through the reports of the latest experimental studies, was attracted by the publication of the French scientist Henri Becquerel of the preceding year. She and Pierre already knew this work; she read it over again and studied it with her usual care.
After Roentgen’s discovery of X rays, Henri Poincaré conceived the idea of determining whether rays like the X ray were emitted by “fluorescent” bodies under the action of light. Attracted by the same problem, Henri Becquerel examined the salts of a “rare metal,” uranium. Instead of finding the phenomenon he had expected, he observed another, altogether different and incomprehensible: he found that uranium salts spontaneously emitted, without exposure to light, some rays of unknown nature. A compound of uranium, placed on a photographic plate surrounded by black paper, made an impression on the plate through the paper. And, like the X ray these astonishing “uranic” salts discharged an electroscope by rendering the surrounding air a conductor.
Henri Becquerel made sure that these surprising properties were not caused by a preliminary exposure to the sun and that they persisted when the uranium compound had been maintained in darkness for several months. For the first time, a physicist had observed the phenomenon to which Marie Curie was later to give the name of radioactivity. But the nature of the radiation and its origin remained an enigma.
Becquerel’s discovery fascinated the Curies. They asked themselves whence came the energy—tiny, to be sure—which uranium compounds constantly disengaged in the form of radiation. And what was the nature of this radiation? Here was an engrossing subject of research, a doctor’s thesis! The subject tempted Marie most because it was a virgin field: Becquerel’s work was very recent and so far as she knew nobody in the laboratories of Europe had yet attempted to make a fundamental study of uranium rays. As a point of departure, and as the only bibliography, there existed some communications presented by Henri Becquerel at the Academy of Science during the year 1896. It was a leap into great adventure, into an unknown realm.
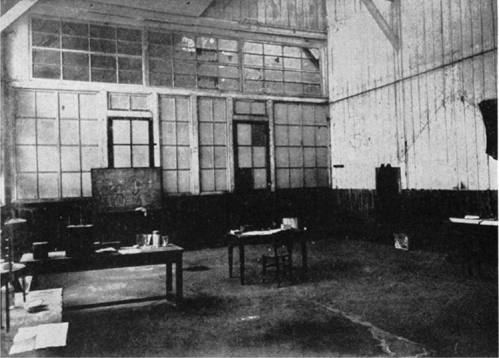
There remained the question of where she was to make her experiments—and here the difficulties began. Pierre made several approaches to the director of the School of Physics with practically no results: Marie was given the free use of a little glassed-in studio on the ground floor of the school. It was a kind of storeroom, sweating with damp, where unused machines and lumber were put away. Its technical equipment was rudimentary and its comfort nil.
Deprived of an adequate electrical installation and of everything that forms material for the beginning of scientific research, she kept her patience, sought and found a means of making her apparatus work in this hole.
It was not easy. Instruments of precision have sneaking enemies: humidity, changes of temperature. Incidentally the climate of this little workroom, fatal to the sensitive electrometer, was not much better for Marie’s health. But this had no importance. When she was cold, the young woman took her revenge by noting the degrees of temperature in centigrade in her notebook. On February 6, 1898, we find, among the formulas and figures: “Temperature here 6°25.” Six degrees …!* Marie, to show her disapproval, added ten little exclamation points.
The candidate for the doctor’s degree set her first task to be the measurement of the “power of ionization” of uranium rays—that is to say, their power to render the air a conductor of electricity and so to discharge an electroscope. The excellent method she used, which was to be the key to the success of her experiments, had been invented for the study of other phenomena by two physicists well known to her: Pierre and Jacques Curie. Her technical installation consisted of an “ionization chamber,” a Curie electrometer and a piezoelectric quartz.
At the end of several weeks the first result appeared: Marie acquired the certainty that the intensity of this surprising radiation was proportional to the quantity of uranium contained in the samples under examination, and that this radiation, which could be measured with precision, was not affected either by the chemical state of combination of the uranium or by external factors such as lighting or temperature.
These observations were perhaps not very sensational to the uninitiated, but they were of passionate interest to the scientist. It often happens in physics that an inexplicable phenomenon can be subjected, after some investigation, to laws already known, and by this very fact loses its interest for the research worker. Thus, in a badly constructed detective story, if we are told in the third chapter that the woman of sinister appearance who might have committed the crime is in reality only an honest little housewife who leads a life without secrets, we feel discouraged and cease to read.
Nothing of the kind happened here. The more Marie penetrated into intimacy with uranium rays, the more they seemed without precedent, essentially unknown. They were like nothing else. Nothing affected them. In spite of their very feeble power, they had an extraordinary individuality.
Turning this mystery over and over in her head, and pointing toward the truth, Marie felt and could soon affirm that the incomprehensible radiation was an atomic property. She questioned: Even though the phenomenon had only been observed with uranium, nothing proved that uranium was the only chemical element capable of emitting such radiation. Why should not other bodies possess the same power? Perhaps it was only by chance that this radiation had been observed in uranium first, and had remained attached to uranium in the minds of physicists. Now it must be sought for elsewhere.…
No sooner said than done. Abandoning the study of uranium, Marie undertook to examine all known chemical bodies, either in the pure state or in compounds. And the result was not long in appearing: compounds of another element, thorium, also emitted spontaneous rays like those of uranium and of similar intensity. The physicist had been right: the surprising phenomenon was by no means the property of uranium alone, and it became necessary to give it a distinct name. Mme Curie suggested the name of radioactivity. Chemical substances like uranium and thorium, endowed with this particular “radiance,” were called radio elements.
Radioactivity so fascinated the young scientist that she never tired of examining the most diverse forms of matter, always by the same method. Curiosity, a marvelous feminine curiosity, the first virtue of a scientist, was developed in Marie to the highest degree. Instead of limiting her observation to simple compounds, salts and oxides, she had the desire to assemble samples of minerals from the collection at the School of Physics, and of making them undergo almost at hazard, for her own amusement, a kind of customs inspection which is an electrometer test. Pierre approved, and chose with her the veined fragments, hard or crumbly, oddly shaped, which she wanted to examine.
Marie’s idea was simple—simple as the stroke of genius. At the crossroads where Marie now stood, hundreds of research workers might have remained, nonplussed, for months or even years. After examining all known chemical substances, and discovering—as Marie had done—the radiation of thorium, they would have continued to ask themselves in vain whence came this mysterious radioactivity. Marie, too, questioned and wondered. But her surprise was translated into fruitful acts. She had used up all evident possibilities. Now she turned toward the un-plumbed and the unknown.
She knew in advance what she would learn from an examination of the minerals, or rather she thought she knew. The specimens which contained neither uranium nor thorium would be revealed as totally “inactive.” The others, containing uranium or thorium, would be radioactive.
Experiment confirmed this prevision. Rejecting the inactive minerals, Marie applied herself to the others and measured their radioactivity. Then came a dramatic revelation: the radioactivity was a great deal stronger than could have been normally foreseen by the quantity of uranium or thorium contained in the products examined!
“It must be an error in experiment,” the young woman thought; for doubt is the scientist’s first response to an unexpected phenomenon.
She started her measurements over again, unmoved, using the same products. She started over again ten times, twenty times. And she was forced to yield to the evidence: the quantities of uranium and of thorium found in these minerals were by no means sufficient to justify the exceptional intensity of the radiation she observed.
Where did this excessive and abnormal radiation come from? Only one explanation was possible: the minerals must contain, in small quantity, a much more powerfully radioactive substance than uranium and thorium.
But what substance? In her preceding experiments, Marie had already examined all known chemical elements.
The scientist replied to the question with the sure logic and the magnificent audaciousness of a great mind: The minerals certainly contained a radioactive substance, which was at the same time a chemical element unknown until this day: a new element.
A new element! It was a fascinating and alluring hypothesis—but still a hypothesis. For the moment this powerfully radioactive substance existed only in the imagination of Marie and of Pierre. But it did exist there. It existed strongly enough to make the young woman go to see Bronya one day and tell her in a restrained, ardent voice:
“You know, Bronya, the radiation that I couldn’t explain comes from a new chemical element. The element is there and I’ve got to find it. We are sure! The physicists we have spoken to believe we have made an error in experiment and advise us to be careful. But I am convinced that I am not mistaken.”
These were unique moments in her unique life. The layman forms a theatrical—and wholly false—idea of the research worker and of his discoveries. “The moment of discovery” does not always exist: the scientist’s work is too tenuous, too divided, for the certainty of success to crackle out suddenly in the midst of his laborious toil like a stroke of lightning, dazzling him by its fire. Marie, standing in front of her apparatus, perhaps never experienced the sudden intoxication of triumph. This intoxication was spread over several days of decisive labor, made feverish by a magnificent hope. But it must have been an exultant moment when, convinced by the rigorous reasoning of her brain that she was on the trail of new matter, she confided the secret to her elder sister, her ally always.… Without exchanging one affectionate word, the two sisters must have lived again, in a dizzying breath of memory, their years of waiting, their mutual sacrifices, their bleak lives as students, full of hope and faith.
It was barely four years before that Marie had written:
Life is not easy for any of us. But what of that? we must have perseverance and above all confidence in ourselves. We must believe that we are gifted for something, and that this thing, at whatever cost, must be attained.
That “something” was to throw science upon a path hitherto unsuspected.
In a first communication to the Academy, presented by Prof. Lippmann and published in the Proceedings on April 12, 1898, “Marie Sklodovska Curie” announced the probable presence in pitchblende ores of a new element endowed with powerful radioactivity. This was the first stage of the discovery of radium.
By the force of her own intuition the physicist had shown to herself that the wonderful substance must exist. She decreed its existence. But its incognito still had to be broken. Now she would have to verify hypothesis by experiment, isolate this material and see it. She must be able to announce with certainty: “It is there.”
Pierre Curie had followed the rapid progress of his wife’s experiments with passionate interest. Without directly taking part in Marie’s work, he had frequently helped her by his remarks and advice. In view of the stupefying character of her results, he did not hesitate to abandon his study of crystals for the time being in order to join his efforts to hers in the search for the new substance.
Thus, when the immensity of a pressing task suggested and exacted collaboration, a great physicist was at Marie’s side—a physicist who was the companion of her life. Three years earlier, love had joined this exceptional man and woman together—love, and perhaps some mysterious foreknowledge, some sublime instinct for the work in common.
The available force was now doubled. Two brains, four hands, now sought the unknown element in the damp little workroom in the Rue Lhomond. From this moment onward it is impossible to distinguish each one’s part in the work of the Curies. We know that Marie, having chosen to study the radiation of uranium as the subject of her thesis, discovered that other substances were also radioactive. We know that after the examination of minerals she was able to announce the existence of a new chemical element, powerfully radioactive, and that it was the capital importance of this result which decided Pierre Curie to interrupt his very different research in order to try to isolate this element with his wife. At that time—May or June 1898—a collaboration began which was to last for eight years, until it was destroyed by a fatal accident.
We cannot and must not attempt to find out what should be credited to Marie and what to Pierre during these eight years. It would be exactly what the husband and wife did not want. The personal genius of Pierre Curie is known to us by the original work he had accomplished before this collaboration. His wife’s genius appears to us in the first intuition of discovery, the brilliant start; and it was to reappear to us again, solitary, when Marie Curie the widow unflinchingly carried the weight of a new science and conducted it, through research, step by step, to its harmonious expansion. We therefore have formal proof that in the fusion of their two efforts, in this superior alliance of man and woman, the exchange was equal.
Let this certainty suffice for our curiosity and admiration. Let us not attempt to separate these creatures full of love, whose handwriting alternates and combines in the working notebooks covered with formulae, these creatures who were to sign nearly all their scientific publications together. They were to write “We found” and “We observed”; and when they were constrained by fact to distinguish between their parts, they were to employ this moving locution:
Certain minerals containing uranium and thorium (pitchblende, chalcolite, uranite) are very active from the point of view of the emission of Becquerel rays. In a preceding communication, one of us showed that their activity was even greater than that of uranium and thorium, and stated the opinion that this effect was due to some other very active substance contained in small quantity in these minerals.
(Pierre and Marie Curie: Proceedings of the Academy of Science, July 18, 1898.)
Marie and Pierre looked for this “very active” substance in an ore of uranium called pitchblende, which in the crude state had shown itself to be four times more radioactive than the pure oxide of uranium that could be extracted from it. But the composition of this ore had been known for a long time with considerable precision. The new element must therefore be present in very small quantity or it would not have escaped the notice of scientists and their chemical analysis.
According to their calculations—“pessimistic” calculations, like those of true physicists, who always take the less attractive of two probabilities—the collaborators thought the ore should contain the new element to a maximum quantity of one per cent. They decided that this was very little. They would have been in consternation if they had known that the radioactive element they were hunting down did not count for more than a millionth part of pitchblende ore.
They began their prospecting patiently, using a method of chemical research invented by themselves, based on radioactivity: they separated all the elements in pitchblende by ordinary chemical analysis and then measured the radioactivity of each of the bodies thus obtained. By successive eliminations they saw the “abnormal” radioactivity take refuge in certain parts of the ore. As they went on, the field of investigation was narrowed. It was exactly the technique used by the police when they search the houses of a neighborhood, one by one, to isolate and arrest a malefactor.
But there was more than one malefactor here: the radioactivity was concentrated principally in two different chemical fractions of the pitchblende. For M. and Mme Curie it indicated the existence of two new elements instead of one. By July 1898 they were able to announce the discovery of one of these substances with certainty.
“You will have to name it,” Pierre said to his young wife, in the same tone as if it were a question of choosing a name for little Irène.
The one-time Mlle Sklodovska reflected in silence for a moment. Then, her heart turning toward her own country which had been erased from the map of the world, she wondered vaguely if the scientific event would be published in Russia, Germany and Austria—the oppressor countries—and answered timidly:
“Could we call it ‘polonium’?”
In the Proceedings of the Academy for July 1898 we read:
We believe the substance we have extracted from pitchblende contains a metal not yet observed, related to bismuth by its analytical properties. If the existence of this new metal is confirmed we propose to call it polonium, from the name of the original country of one of us.
The choice of this name proves that in becoming a Frenchwoman and a physicist Marie had not disowned her former enthusiasms. Another thing proves it for us: even before the note “On a New Radioactive Substance Contained in Pitchblende” had appeared in the Proceedings of the Academy, Marie had sent the manuscript to her native country, to that Joseph Boguski who directed the little laboratory at the Museum of Industry and Agriculture where she had made her first experiments. The communication was published in Warsaw in a monthly photographic review called Swiatlo almost as soon as in Paris.
Life was unchanged in the little flat in the Rue de la Glacière. Marie and Pierre worked even more than usual; that was all. When the heat of summer came, the young wife found time to buy some baskets of fruit in the markets and, as usual, she cooked and put away preserves for the winter, according to the recipes used in the Curie family. Then she locked the shutters on her windows, which gave on burned leaves; she registered their two bicycles at the Orleans station, and, like thousands of other young women in Paris, went off on holiday with her husband and her child.
This year the couple had rented a peasant’s house at Auroux, in Auvergne. Happy to breathe good air after the noxious atmosphere of the Rue Lhomond, the Curies made excursions to Mende, Puy, Clermont, Mont-Dore. They climbed hills, visited grottoes, bathed in rivers. Every day, alone in the country, they spoke of what they called their “new metals,” polonium and “the other”—the one that remained to be found. In September they would go back to the damp workroom and the dull minerals; with freshened ardor they would take up their search again.…
One grief interfered with Marie’s intoxication for work: the Dluskis were on the point of leaving Paris. They had decided to settle in Austrian Poland and to build a sanatorium for tubercular sufferers at Zakopane in the Carpathian Mountains. The day of separation arrived: Marie and Bronya exchanged brokenhearted farewells; Marie was losing her friend and protector, and for the first time she had the feeling of exile.
Marie to Bronya, December 2, 1898:
You can’t imagine what a hole you have made in my life. With you two, I have lost everything I clung to in Paris except my husband and child. It seems to me that Paris no longer exists, aside from our lodging and the school where we work.
Ask Mme Dluska if the green plant you left behind should be watered, and how many times a day. Does it need a great deal of heat and sun?
We are well, in spite of the bad weather, the rain and the mud. Irène is getting to be a big girl. She is very difficult about her food, and aside from milk tapioca she will eat hardly anything regularly, not even eggs. Write me what would be a suitable menu for persons of her age.…
In spite of their prosaic character—or perhaps because of it—some notes written by Mme Curie in that memorable year 1898 seem to us worth quoting. Some are to be found in the margins of a book called Family Cooking, with respect to a recipe for gooseberry jelly:
I took eight pounds of fruit and the same weight in crystallized sugar. After an ebullition of ten minutes, I passed the mixture through a rather fine sieve. I obtained fourteen pots of very good jelly, not transparent, which “took” perfectly.
In a school notebook covered with gray linen, in which the young mother had written little Irène’s weight day by day, her diet and the appearance of her first teeth, we read under the date of July 20, 1898, some days after the publication of the discovery of polonium:
Irène says “thanks” with her hand. She can walk very well now on all fours. She says “Gogli, gogli, go.” She stays in the garden all day at Sceaux on a carpet. She can roll, pick herself up, and sit down.
On August 15, at Auroux:
Irène has cut her seventh tooth, on the lower left. She can stand for half a minute alone. For the past three days we have bathed her in the river. She cries, but today (fourth bath) she stopped crying and played with her hands in the water.
She plays with the cat and chases him with war cries. She is not afraid of strangers any more. She sings a great deal. She gets up on the table when she is in her chair.
Three months later, on October 17, Marie noted with pride:
Irène can walk very well, and no longer goes on all fours.
On January 5, 1899:
Irène has fifteen teeth!
Between these two notes—that of October 17, 1898, in which Irène no longer goes on all fours, and that of January 5 in which Irène has fifteen teeth—and a few months after the note on the gooseberry preserve, we find another note worthy of remark.
It was drawn up by Marie and Pierre Curie and a collaborator called G. Bémont. Intended for the Academy of Science, and published in the Proceedings of the session of December 26, 1898, it announced the existence of a second new chemical element in pitchblende.
Some lines of this communication read as follows:
The various reasons we have just enumerated lead us to believe that the new radioactive substance contains a new element to which we propose to give the name of RADIUM.
The new radioactive substance certainly contains a very strong proportion of barium; in spite of that its radioactivity is considerable. The radioactivity of radium therefore must be enormous.

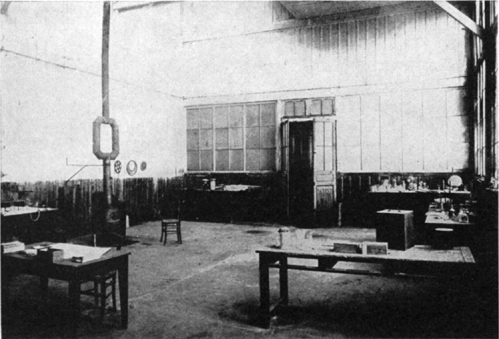
TWO VIEWS OF THE SHED AT THE SCHOOL OF PHYSICS ON THE RUE LHOMOND WHERE RADIUM WAS DISCOVERED
On the Blackboard in the Upper Picture Can Be Seen Pierre Curie’s Writing. (Illustration Credit 12.1)

PAGES FROM MARIE CURIE’S WORK BOOK, 1897-1898 (Illustration Credit 12.2)
* About 44° Fahrenheit.