
We knew the world would not be the same. Few people laughed, few people cried, most people were silent. I remembered the line from the Hindu scripture, the Bhagavad-Gita. Vishnu is trying to persuade the Prince that he should do his duty and to impress him takes on his multi-armed form and says, “Now I am become Death, the destroyer of worlds.” I suppose we all thought that, one way or another.
—Robert Oppenheimer1
NUCLEI
Since 1932, it has been known that the nuclei of atoms are composed of protons and neutrons, generically referred to as nucleons. The proton carries an electric charge of +e, where e is the unit electric charge and the electron's charge is –e. The neutron is electrically neutral and is just slightly heavier than the proton. Each nucleon is over 1,830 times more massive than an electron, and so the masses of atoms are essentially contained in their nuclei.
The diameters of nuclei range from 0.8 femtometers for hydrogen to 15 femtometers for uranium, where 1 femtometer equals 10–15 meter. The diameter of the hydrogen atom is about 0.11 nanometer, while that of the uranium atom is 0.35 nanometer, where 1 nanometer equals 10–9 meter. So, roughly speaking, the nucleus of the atom is a million times smaller than the atom itself. Matter is, indeed, mostly empty space.
In chemical and nuclear reactions, the sum of the atomic numbers of the reactants does not change even though the reactants themselves can change. This follows from the principle of charge conservation: the total charge in a reaction is unchanged in the reaction.
Likewise, we observe a principle of nucleon number conservation: the total number of nucleons in a reaction is unchanged during the reaction. (Later this will be modified when we get to particle physics.)
In this chapter, all nuclear and subnuclear particles X will be designated with the notation ZXA, where Z is the charge in units of the unit electric charge (the atomic number in the case of nuclei) and A is the nucleon number, usually (but not always) the atomic weight to the nearest integer. X is the chemical symbol for the atom found in the periodic table, or the particle symbol in the case of subatomic particles.
In chapter 6, it was mentioned that Ernest Rutherford had transmuted nitrogen into oxygen by bombarding it with alpha rays. Although he did not recognize it at the time, the specific reaction was:
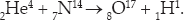
Adding up the subscripts and superscripts, we see that the sums are conserved. Note that the oxygen isotope 8O17 is produced, which has one more neutron than the most common form of oxygen, 8O16.
THE NUCLEAR FORCES
Because the protons in a nucleus are all positively charged, they will repel one another electrostatically. Something needs to hold them together to make a stable nucleus. Gravity is far too weak for such small masses, so another attractive force is needed. This force must also apply to neutrons in order to keep them in the nucleus. In fact, the neutrons aid in keeping the nucleus together; as we add more and more protons, the mutual repulsion makes it harder to keep the nucleus together and more neutrons are needed.
The force that attracts protons and neutrons is called the strong nuclear force or the strong interaction. Experiments determine that this force is extremely short range. Unlike the gravitational and electromagnetic forces that reach across the universe, the strong nuclear force goes rapidly to zero beyond about 2.0 femtometers.
A second, even shorter-distance nuclear force exists called the weak nuclear force or the weak interaction. Recall that three types of nuclear radiation are observed: alpha, which are helium nuclei (2He4); beta, which are electrons (–1e0) or positrons (+1e0); and gamma (0γ0), which are high-energy photons. Alpha radiation is a manifestation of the strong nuclear force. Beta radiation results from the weak nuclear force. Gamma radiation occurs by means of the electromagnetic force. Here are examples of each:
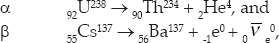
where 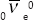 is an anti-electron neutrino, which will be discussed later, and
is an anti-electron neutrino, which will be discussed later, and
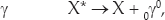
where X is any generic particle and X* is the same particle in an electromagnetically excited state. This process is analogous to the photon emission from an excited atom. Here we have photon emission from an excited nucleus, only the energy of the photon is much greater than that of an atom, MeVs rather than eVs or keVs.
“ATOMIC” ENERGY
Let us consider a generic reaction, be it chemical, nuclear, or subnuclear:
A + B → C + D.
The number of reactants need not be two; they can be any number on either side of the arrow. And you can reverse the arrow at any time because all such reactions are reversible, although not necessarily with equal reaction rates. The principle of conservation of energy then says that the total energy before the reaction equals the total energy after, where the energy of each reactant is the sum of its rest and kinetic energies.2
The rest energy of a body of mass m is, of course, mc2. If the total rest energy before the reaction exceeds the total rest energy after, an increase in kinetic energy results and the reaction produces energy, usually in the form of heat as the rise in kinetic energy corresponds to a rise in temperature. This type of reaction is called exothermic.
If the total rest energy before the reaction is less than the total rest energy after, a decrease in kinetic energy and cooling results. This type of reaction is called endothermic. If the energy difference is high enough, new particles can be produced. This does not happen in chemistry, but it is common in nuclear and particle physics. For example, since the neutron is slightly heavier than the proton, it decays producing a proton, electron, and electron antineutrino:
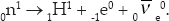
In the case of chemical reactions, the increase or decrease in kinetic energy is a tiny fraction of the rest energies of the reactants involved, and the mass differences are unmeasurable. Nevertheless, the energy produced in an exothermic chemical reaction results from the loss of rest energy in the reaction, a fact that was not appreciated until Einstein showed the equivalence of mass and rest energy, and it is still rarely explained that way in physics or chemistry classes. For example, in the chemical reaction
C + O2 → C O2,
4.1 eV of energy is released, compared to the rest energy of CO2, which is 41 GeV (1 GeV = 109 eV).
On the other hand, if we consider the reverse endothermic reaction
CO2 → C + O2,
4.1 eV must be provided to split CO2 into carbon and oxygen, since the rest energy of carbon dioxide is less than C + O2 by that amount.
Let us define the efficiency of an exothermic reaction as the fraction of the rest energy that is converted into kinetic energy. While this definition is not what chemists or engineers generally use, it provides the precise measure of how well a reaction is able to convert its available rest energy to kinetic. For example, the efficiency of burning pure carbon to produce energy is 4.1/41,000,000,000 = 10–10. That is, for every unit of energy produced, ten billion units of the energy that is fundamentally available in principle are wasted, usually exhausted into the environment. This should drive home that the world's current use of carbon burning as its primary source of energy is incredibly inefficient.
NUCLEAR FUSION
Nuclear and particle reactions are far more efficient than chemical reactions in converting rest energy to kinetic energy. In these cases, the energies produced are a much larger fraction of the rest energies of the reactants. For example, the hydrogen isotopes deuterium and tritium will interact to produce a helium nucleus and a neutron
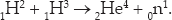
The energy released is 17.6 MeV compared to the rest energy of 2He4, which is 3,726 MeV, for an efficiency of 17.6/3,726 = 0.005. Not great, but 50 million times better than chemical reactions. This reaction is an example of nuclear fusion. Basically, you can think of it as bringing two protons and two neutrons together to form a helium nucleus. Fusion is the source of energy in stars such as our sun and in the hydrogen bomb.
The primary processes that provide the energy in the sun are:
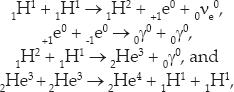
where +1e0 is an antielectron or positron. The second reaction is matter-antimatter annihilation. Note that the first reaction is actually a weak interaction (signaled by the neutrino), the second and third are electromagnetic interactions, and only the fourth is a strong interaction. Note also that each of the first three reactions must occur twice before the final reaction. If you put them all together, you have
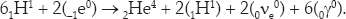
The net release of energy is 26 MeV.
The proton-proton interactions are very difficult to produce in most natural environments, since protons have the same charge and so repel each other. Only in the very high pressure that exists at the center of stars are the speeds of the protons high enough for them to penetrate the barrier between them. Even then, a process called quantum tunneling in which particles are able to tunnel through barriers, like you and I walking through a wall, is required for the reaction to take place.
It isn't magic. You and I can, in principle, walk through a wall by quantum tunneling. It's just that the probability for us to bounce back after hitting the wall is much higher than for us to tunnel through. At the subatomic level, on the other hand, the tunneling probability is much higher. It all depends on the thickness of the barrier.
A number of other reaction cycles are also known to take place at the center of the sun. How do we know all this about the center of the sun? We can't see it with photons. But we can with neutrinos. In 1998, I participated in an underground experiment in Japan that took a picture of the center of the sun at night using neutrinos that had not only passed through the immense solar matter lying over the sun's center, but had also passed straight through Earth as well.3 A number of other experiments have detected neutrinos from the sun and provided detailed information about the reactions taking place.
Matter-antimatter annihilation provides 100 percent conversion efficiency from rest to kinetic energy. The problem, however, is that we have no sources of antimatter to mine. Even in the sun, the antimatter must first be generated by the initial proton-proton collision. The antimatter in the universe is a billion times less abundant than matter. We will later discuss why this is so. You would think that there should be equal amounts.
For over fifty years, attempts have been made to develop controlled nuclear-fusion energy sources, which would, in principle, provide an endless supply of energy out of water with negligible environmental impact. Unfortunately, the temperatures needed to overcome the repulsive barrier between the positively charged hydrogen nuclei are so high that they cannot be contained by any known materials. The required temperatures, on the order of 100 million degrees, are provided in a star by the tremendous gravitational pressure at its center, or, in the case of the hydrogen bomb, by a nuclear fission bomb used as a trigger. Nuclear fusion research continues with no practical application yet in sight.
NUCLEAR FISSION
When the United States annihilated the Japanese cities of Hiroshima and Nagasaki in August 1945, the world was introduced to a terrible new weapon called the “atomic bomb.” This was a misnomer, since the energy of conventional explosives such as TNT results from the rapid oxidation of chemical atoms and molecules and thus is “atomic” in nature. The new source of energy was nuclear fission, and what were exploded were nuclear bombs (not “nucular”).
A typical fission reaction is
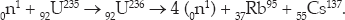
The uranium nucleus is basically split into two nuclei, each having roughly half the mass of the uranium. The net release of energy in this reaction is 191 MeV, for an efficiency of 191/(236 × 931) = 0.0009. While again this is not a large number, it is 9 million times more efficient than carbon burning.
Note that three more neutrons are produced than went in, making possible a sustained chain reaction without which nuclear energy production on a large scale would not be possible.
The bomb makers in the World War II Manhattan Project faced a formidable problem. Fissionable 92U235 constitutes only 0.72 percent of naturally occurring uranium, most of which is radioactive but nonfissionable 92U238. Separating out sufficient fissionable material for a bomb required the building of a huge facility at Oak Ridge, Tennessee. After an excruciatingly long time of centrifuging enough pure 92U235, a uranium bomb could be built. Nicknamed “Little Boy,” it was dropped over Hiroshima on August 6, 1945.
Also part of the Manhattan Project, another huge facility was built in Hanford, Washington. There, so-called breeder reactors transformed 92U238 into fissionable isotopes of plutonium, of which only trace amounts are found in nature. While several plutonium isotopes are fissionable, the primary isotope used in plutonium bombs is 94Pu239 that is produced by the reactions
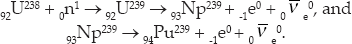
When enough plutonium was finally accumulated, it was used to build a plutonium bomb named “Fat Man.” It was dropped over Nagasaki on August 9, 1945.
Following World War II, there existed much enthusiasm that nuclear energy would solve the world's energy problems. There also existed much dread that humans would destroy the world with uncontrolled nuclear war. Neither have yet to come to pass.
Mutually assured destruction (MAD) seems to have kept another nuclear bomb from being used against an enemy since the tragedies of Hiroshima and Nagasaki. The end of the Cold War makes large-scale nuclear war increasingly unlikely, while at the same time, nuclear proliferation makes small-scale nuclear conflicts and terrorist attacks a serious threat.
POISONING THE ATMOSPHERE
Currently more than 80 percent of the world's energy is obtained from fossil fuels: petroleum, coal, and natural gas. Another 10 percent comes from biomass and waste, leaving only 10 percent from sources other than carbon burning. The CO2 and other compounds produced by these fuels are filling the atmosphere with pollutants. The World Health Organization estimates that urban air pollution results in 1.3 million premature deaths worldwide each year. Indoor air pollution is estimated to cause approximately 2 million premature deaths, mostly in developing countries. Almost half of these deaths are due to pneumonia in children under five years of age.4
Observations unequivocally show that carbon dioxide levels in the atmosphere have risen to levels higher and faster than any on record and that Earth is definitely warming by way of the greenhouse effect. The overwhelming majority of climate scientists agree that this warming will result in severe climate change in a few years, if it hasn't already begun, and that the burning of fossil fuels by human industry is the primary cause.
In my previous book, God and the Folly of Faith, I described how powerful corporations with economic interests in fossil fuels have used their vast funds to foment groundless doubt about these solid scientific results. As part of their strategy to preserve their freedom to exploit resources for profit, they have allied themselves with the antiscience Religious Right, which believes God created Earth's resources for us, the pinnacle of Creation, to consume. And so, the majority of evangelical Christians, including prominent Republican politicians, believe that God will assure that Earth remains in perfect environmental balance—at least until Jesus returns.5
The sad part of the story is that with nuclear technology that was developed over sixty years ago, we could have preserved a clean atmosphere with safe levels of pollutants. The happy part of the story is, we can still do it.
NUCLEAR POWER
As of December 2011, thirty countries worldwide were operating 435 nuclear reactors for generating electricity, and 63 new nuclear plants were under construction in fourteen countries. Nuclear power now provides 13.5 percent of the world's electricity. France is the largest percentage user, with 74.1 percent of its electricity coming from nuclear reactors. In 2010, the United States obtained 19.6 percent of its electricity from nuclear reactors.6
However, nuclear energy has not proved to be “too cheap to meter,” as the chair of the Atomic Energy Commission, Lewis Strauss, had enthused in 1954.7 The technologies involved turned out to be much tougher, and nuclear power plants are far more costly than originally imagined. Radioactive waste disposal is a major issue. Still, the environmental hazards of this waste dwarf in comparison to those from carbon burning, which as we have seen results in over a million deaths each year. Despite this fact, nuclear power today is feared by much of the populace.
On March 28, 1979, a series of equipment malfunctions and operator errors led to a partial meltdown of the nuclear reactor at Three Mile Island in Pennsylvania. While negligible radiation was released, and no one was injured, the event caused widespread panic and triggered a moratorium on nuclear power plant construction in the United States that has continued to this day. Not a single new plant was approved until 2012, with two recently being approved. The last plant to go into operation in the United States was one ordered in 1976.
A far more serious nuclear disaster occurred at Chernobyl in Ukraine on April 26, 1986. Once again, operator errors were a major contributing cause and explosions blew the top off a reactor and set off a fire. Radioactive clouds spread over a large portion of the continent. A 2005 report by the International Atomic Energy Agency, the World Health Organization, and the United Nations Development Agency estimated that up to four thousand people could ultimately die of radiation exposure from the accident—bad, but still orders of magnitude less than the deaths from carbon burning.8
Chernobyl resulted in an even more intense opposition to nuclear energy, especially in Europe. Only France continued its program at full speed, while Germany began phasing its program out. Beyond Europe, Japan lacked any significant energy sources and, despite being the only nation ever to have suffered a nuclear attack, continued building nuclear power plants. Dozens more came online after Chernobyl. By the beginning of 2011, Japan had fifty-four operating plants supplying 30 percent of its energy.9 But then, on March 11 of that year, nature triggered a disaster of immense proportions that once again affected the whole nuclear picture.
On that fateful day, an earthquake just off the northeastern coast of Honshu generated an immense tsunami that swept ashore, destroying everything in its path. Unfortunately, that path included the Fukushima Daiichi nuclear station. While the radiation released was not as bad as Chernobyl, it was bad enough, and another blow was struck against the use of nuclear energy.
Many lessons were learned from the mistakes that resulted in the three nuclear plant accidents. Designs were poor and certainly could have been improved, especially with regard to safety. It surely was a major error in judgment to locate the Fukushima station so close to a fault line. While both Three Mile Island and Chernobyl exhibited design flaws, the actual equipment failures were minor. However, plant operators made wrong decisions that reflected inadequate training that ended up doing more harm than good. Put simply, the standards were simply too low and the designs were inadequate.10
But, as Pulitzer Prize–winning author Daniel Yergin says in his in-depth study of the economics and politics of world energy, nuclear power remains “the only large-scale, well-established, broadly deployable source of electric generation currently available that is carbon-free.”11 Despite the fact that the United States has not put a new nuclear power plant into operation for decades, during which time power consumption in the country has more than doubled, nuclear energy still provides 20 percent of America's electricity needs, showing that nuclear technology continues to improve.
LIQUID FLUORIDE THORIUM REACTORS
Unfortunately, all existing nuclear power plants utilize the fission of 92U235 and 94Pu239. This was a terrible choice that was based on making bombs and powering submarines and other ships rather than achieving optimal energy production. An alternative that has been available from the beginning is the fission of 92U233. While 92U233 does not occur naturally, it can be bred from 90Th232 (thorium), which is four times as abundant in nature as 92U238, by the reactions
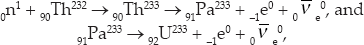
where Pa is protactinium. The same initial reaction produces other reaction products
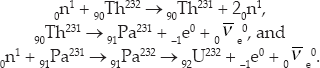
In particular, 92U232 is nasty because gamma rays with energies of 2.6 MeV are radiated in its decay chain. This makes 92U233 undesirable for weapons use because a weapon should not radiate much until it is detonated so that it needs only minimal shielding. Gamma rays are dangerous to the personnel who have to handle the weapon and to the weapon's instrumentation. However, these gamma rays are not a factor for use in reactors, which are already sufficiently shielded.
Fission bombs were based on 92U235 or 94Pu239, where the gamma radiation was minimal and of lower energy. So when the time came to build nuclear reactors for power production, including propulsion for navy ships and submarines, this was where the knowledge and expertise was developed. While seven different designs of nuclear power plants are currently in commercial operation, all are based on uranium or internally bred plutonium, with Japan and Russia each having one plant that utilizes plutonium alone. The most common design is the pressurized-water reactor, developed by Alvin Weinberg at Oak Ridge in 1946, based on the earlier designs of Enrico Fermi in Chicago. These comprise 265 of the 439 reactors in use.
Unfortunately, reactors based on 92U235 and 94Pu239 have designs and other inherent flaws that contributed to the nuclear power plant disasters that turned the public, and many scientists, against nuclear power:
- They require costly separation of uranium isotopes or breeding of plutonium.
- Most use solid fuels that must be removed before they have given off 1 percent of their potential energy availability, because of depletion of the fissionable material and radiation damage.
- Water or other coolants under high pressure must be pumped through from the outside to extract heat. Meltdown can occur when coolant flow is disrupted, as with power failures.
- High pressure requires high-strength piping and pressure vessels that are degraded by radiation and corrosion. If weakened, the reactor vessel or parts of the pressure boundary can rupture, causing a loss of cooling and the potential release of radioactivity to the environment.
- Even after shutdown, fission products continue to generate heat and must be cooled with water for one to three years. If this flow is disrupted, meltdown can occur.
- The radioactive waste takes 10 million years before it decays to naturally occurring levels.
- Proliferation. Material that can be used in bombs can be secretly extracted from these reactors.
- Large size and high cost of facilities.
- Like oil, coal, and natural gas, uranium probably will run out in less than a hundred years unless large numbers of fast-neutron-breeder reactors are built. These types of nuclear reactors have significant safety issues, and prototypes generally have not been successful. It seems doubtful that uranium can provide a long-term solution to the world's energy needs.
During the same postwar years, Weinberg was also responsible for another reactor design based on ideas from Fermi and Eugene Wigner. His design was successfully developed at Oak Ridge but never put into commercial use. This was the liquid-fuel reactor in which the fuel is composed of molten salt.
I will just discuss one version of the molten-salt reactor in which thorium is used as the input fuel, the thorium fluoride reactor (LFTR, referred to as “Lifter”).12 This utilizes the reaction described above in which 90Th232 is transmuted to fissionable 92U233. After successful tests of the molten-salt concept at Oak Ridge, the LFTR project was canceled in 1969 by the Nixon administration. The reason: having two fewer neutrons than 92U235, 92U233 is not as efficient in breeding plutonium for bombs. Instead, a liquid-metal fast-breeder 92U235 reactor that could efficiently breed plutonium was funded.
Today there is renewed worldwide interest in LFTR, although the United States continues to drag its feet, spending very little on research. While many scientific and engineering problems still have to be solved, LFTR seems sufficiently promising that it might, in fact, be able to provide for the increasing needs for energy, especially in developing countries, while finally reducing our reliance on fossil fuels. I will just list the advantages. Some—but not all—apply to other types of liquid-fuel reactors.13
- Thorium is plentiful and inexpensive. One ton, costing $300,000, can power a 1,000 megawatt plant for a year.
- LFTR operates at atmospheric pressure, obviating the need for a large, expensive containment dome and having no danger of explosion.
- It cannot melt down because the normal operating state is already molten.
- Stable to rising temperatures because molten salt expands, slowing the reaction. A salt plug kept solid by cooling coils will automatically melt if external power is lost; the fluid drains out to a safe dump tank.
- Salts used are solid below 300 F, so any spilled fuel solidifies instead of escaping into the environment.
- Liquid fuels use almost all the available energy, unlike solid fuels that must be removed before they have generated 1–3 percent of the available energy.
- The radiative waste is much less than from conventional plants and far more manageable.
- Air-cooling is possible where water is scarce.
- Thorium can be cheaper than coal.
- Proliferation resistant. It can't be used to build bombs.
- Are far easier to scale to meet a wide range of energy needs.
- Could provide for the world's energy needs, carbon-free, for a thousand years.
If the Three Mile Island, Chernobyl, and Fukushima reactors had been LFTRs, no radiation would have been released to the environment. Indeed, no meltdown or explosion would have occurred.
While three hundred years for the decay of radiative waste to natural levels still seems undesirable, it must be put in perspective. Even the waste from conventional nuclear plants is tiny compared to the waste from burning fossil fuels, which, although not (as) radioactive, still kills millions of people each year. A great effort is going on now to develop carbon sequestration, in which the CO2 from coal power plants is pumped back into the ground. This will be expensive, raising the price of electricity from coal an estimated 80–100 percent.14 Furthermore, the amount of underground space needed to store a year's worth of CO2 output from a single coal power plant is equivalent to six hundred football fields filled to a height of ten yards. By comparison, one football field filled to the same height is required for all the waste from the entire civilian nuclear program.15
In short, while unforeseen problems could still arise, it would seem that a major effort in the United States and worldwide should be undertaken to develop nuclear power systems based on liquid fluoride thorium. For a good summary of the world energy problem and the role LFTR can play, see the online lecture by physicist Robert Hargraves.16 After this section was written, an excellent new book appeared titled Superfuel: Thorium, the Green Energy Source for the Future, by science writer Richard Martin, that gives the full story of thorium.17