3
Lucy and her sisters
The next big event in the story of our origins was the rise of the australopithecines: a group of more human-like creatures that thrived in Africa between about 4 and 2 million years ago. Australopithecus gave us perhaps the most famous single fossil in all of palaeoanthropology: the legendary ‘Lucy’. These creatures were also a crucial step on the path to humanity.
The Taung Child
In 1924 Raymond Dart made a discovery that would change his life, and upend the established wisdom in anthropology.
Quarrymen had found the fossilized skull of an infant ape-like creature at a site called Taung in South Africa. Dart identified the skull as that of an early human ancestor. The fossil became known as the Taung Child, the first small-brained hominid to be discovered. Dart named it Australopithecus africanus, which means ‘southern ape from Africa’.
At the time, most anthropologists thought that Asia was where humanity evolved. The Taung Child was the first fossil of a human ancestor found in Africa. It provided the first concrete evidence that this continent, not Asia, was the cradle of humankind. For his insight in suggesting that this little ape-like creature played a significant role in human evolution, Dart was derided and, finally, ignored. Two decades were to pass before his ideas became accepted as a central part of anthropological thinking.
Lucy
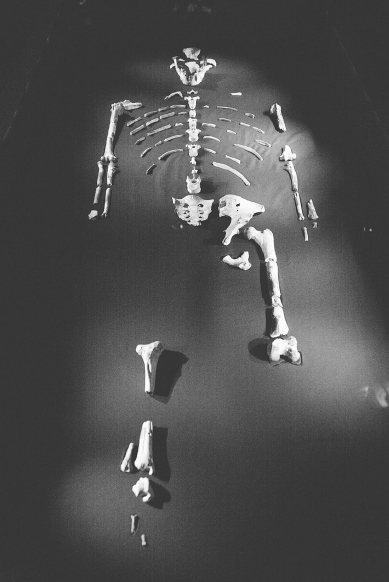
In late 1974 anthropologists were digging for fossils in the isolated Afar region of Ethiopia. One of them, Donald Johanson, spotted several bones sticking out of the ground – ground that was known to be 3.2 million years old. At the time (decades before the discoveries of Orrorin, Sahelanthropus and Ardi), this was the oldest hominin fossil ever found (see Figure 3.1).
FIGURE 3.1 When the first specimen, famously named ‘Lucy’, was discovered in 1974, Australopithecus afarensis was the oldest hominin fossil ever found. Anthropologists have since identified the remains of more than 300 individuals of the species.
That evening, Johanson played a Beatles recording he had brought with him, and the song ‘Lucy in the Sky with Diamonds’ came on. Someone in the group suggested that they could call the new fossil ‘Lucy’, and the name stuck. However, her scientific name is Australopithecus afarensis, a species distinct from A. africanus, the Taung Child.
What was Lucy like? Scans of her skeleton published in 2016 confirm that she had an exceptionally powerful upper body, thanks to spending a lot of time climbing trees. The finding suggests that moving in trees may have remained important to some early human ancestors for millions of years after they developed the ability to walk on the ground.
Lucy had long chimp-like arms and fingers – features that would seem ideal if her life involved a great deal of tree climbing. But her legs and human-like feet show that she was what researchers call a ‘terrestrial biped’ – she could walk in a human-like manner. So Lucy’s chimp-like arms might simply be features she inherited from a tree-climbing ancestor but no longer really used.
Later analysis found that Lucy’s arm bones were thick-walled, implying that her arms were unusually strong. This is a ‘use it or lose it’ trait: bone strength is a direct consequence of an animal’s behaviour, not something inherited. What this means is that some australopiths were equally at home in trees as on the ground – they did not have to defy their physical traits to exist in the trees. It also seems that Lucy’s skeleton has injuries that suggest she died in a fall from a great height – possibly from a tall tree.
Footprints in rock
Laetoli in northern Tanzania is the site of iconic ancient footprints, capturing the moment – 3.66 million years ago – when three members of Lucy’s species (Australopithecus afarensis) strode out across the landscape.
The Laetoli footprints were discovered in 1976. Nothing quite like them had ever been found before. They remain by far the oldest hominin footprints we know, fortuitously preserved because a group of australopiths walked across damp volcanic ash during the brief window of time before it turned from soft powder into hard rock.
In 2016 something quite unexpected came to light: the footprints of two other individuals. Researchers described 13 prints belonging to a large individual – dubbed S1 – and a single print belonging to a smaller S2 australopith. S1 seems to have been walking in the same direction, at the same speed – and in all probability at the same time – as the australopiths whose footprints were uncovered in the 1970s.
It has been all too tempting to interpret the original trackways – often reconstructed as belonging to two adults and one juvenile – as evidence of a prehistoric ‘nuclear family’. The new footprints show that more adults were present, including one who was much larger, the S1 individual. That has spawned a new hypothesis about australopith social groups: that they lived in societies similar to those of gorillas, with a single dominant large male accompanied by several females and offspring.
Toolmakers
A growing body of evidence suggests that the ape-like Australopithecus may have figured out how to make stone tools, long before the rise of modern humans.
In 2010 German researchers working in Ethiopia discovered markings on two animal bones that were about 3.4 million years old. The cut marks had clearly been made using a sharp stone, and they were at a site that was used by Lucy’s species, Australopithecus afarensis.
The study, led by Shannon McPherron of the Max Planck Institute for Evolutionary Anthropology in Leipzig, Germany, was controversial. The bones were 800,000 years older than the oldest uncontested stone tools, and at the time few seriously thought that australopithecines had been tool users. In addition to this, McPherron had not found the tool itself.
In a 2015 study, Matthew Skinner at the University of Kent in the UK and his colleagues looked at the hands of species that would have held tools. Specifically, they looked at metacarpal bones – the five bones in the palm of the hand that connect to the digits. Because the bone ends are made of soft, spongy bone tissue, they are shaped over a lifetime of use and moulded by what that hand has done.
A chimp, for instance, spends a lot of time swinging from branches and knuckle-walking. That exerts a great deal of force on the joints in its hands, in a specific way. Skinner and his colleagues predicted how this should shape the soft bone in ape hands, then looked at modern ape bones, finding that their predictions were right.
Modern human metacarpals look different because we use our hands differently. Most of our activities involve some kind of pinching – think of how you hold a pencil or pick up a cup. This precision squeeze between thumb and fingers is uniquely human and a legacy from our flint-wielding ancestors.
When Skinner and his colleagues looked at the metacarpals of early human species and Neanderthals – who also used stone flakes for tasks like scraping and butchering – they found bone ends that were shaped like those of modern human, and unlike ape bones. Finally, they looked at metacarpals from four A. africanus individuals, up to 3 million years old. This revealed that their owners had been tree swingers but had also spent a lot of energy tightly pinching small objects, suggesting that they were indeed early tool users.
This is not proof that A. africanus used stone tools. They might have been using their strong precision grips to get at food in new ways, such as peeling tough skins off fruit. But the study does suggest that 3 million years ago – 400,000 years before the oldest known hand axes – A. africanus was already starting to use its hands differently from its ancestors.
You are what you eat
Australopithecines tackled a significantly different range of foods compared to their more ape-like ancestors. In fact, they might have been crackers – specialized crackers of tough nuts and seeds, that is. Australopithecines boasted mouths ideal for accessing such well-protected food, according to a 2009 study.
Australopithecus possessed jaws and teeth larger and more powerful than those of its ape ancestors. Some argued that this was for munching small, hard objects such as seeds. Others have proposed that their bigger mouths merely allowed them to eat more food with each bite. However, the 2009 results cast doubt on both explanations.
Rather than analyse microscopic cracks in tooth enamel or the chemical composition of bone, as others had done, David Strait of the University of Albany, New York and his team took an approach more common to mechanical engineering. Using a CT scanner, they measured jawbones and teeth from A. africanus, the species to which the Taung Child belonged. Then, with estimates of muscle strength, they calculated the maximum force that each tooth could exert before shattering.
These calculations suggest that A. africanus’s premolars – teeth just behind the sharper canines – were strong enough to crush the shells of nuts that would have been too large to fit between the even more powerful molars further back in the mouth. Nuts and large seeds were probably not Australopithecus’s favourite snack, but munching on these foods might have helped them survive lean periods.
There is also evidence that australopithecines began eating grass soon after they started leaving the trees. Australopithecines living 3 to 3.5 million years ago got more than half their nutrition from grasses, unlike their predecessors, who preferred fruit and insects. This is the earliest evidence of hominins eating savannah plants.
A 2012 study found high levels of carbon-13 in the bones of A. bahrelghazali, which lived on savannahs near Lake Chad in Africa. This is typical of animals that eat a lot of grasses and sedges. Previously, the oldest evidence of grass eating was from 2.8 million years ago. The 4.4-million-year-old Ardipithecus ramidus did not eat grass. A. bahrelghazali may have eaten roots and tubers rather than tough grass blades. Adding these to their diet may have helped them leave their ancestral home in East Africa for Lake Chad. The question is whether hominins moved on to savannahs permanently, or went between woodland and savannah when it suited them.
Australopithecus sediba
In 2010 the world of anthropology was rocked when another long-lost human cousin was unearthed in South Africa. Of all the australopithecine primates yet found, its anatomy is the closest to the true humans that evolved into us.
The find came in the form of two partial skeletons of Australopithecus sediba that were dug up in the Cradle of Humankind World Heritage Site near Johannesburg, South Africa, by Lee Berger of the University of the Witwatersrand in Johannesburg and colleagues. The skeletons are between 1.95 and 1.78 million years old.
A. sediba’s physical features are closer to human than other australopithecines, but the skeletons are hundreds of thousands of years younger than the oldest fossils assigned to the genus Homo. To many, this implied that it was unlikely to be our direct ancestor.
Berger found the bones of a male child, aged 9 to 13 years, and an adult female in a previously unknown cave in the Malapa cave system, an area with 13 previous hominid fossil finds. Notably, the juvenile is the most complete australopithecine skeleton yet found from the period. It includes much of the skull and large parts of an arm, leg and pelvis. Both skeletons were about 1.2 metres tall and lightly built, with ape-sized brains and bodies resembling A. africanus, which is thought to have been a direct ancestor of humans. With long, muscular arms and strong hands, they would have been well adapted for both tree climbing and walking.
The modern human lineage is widely believed to have evolved from a line of ‘gracile’, or lightly built, australopithecines that goes back some 4 million years. However, the full family tree remains unclear. With the notable exception of the famed 3.2-million-year-old Lucy, an A. afarensis, most australopithecine and early Homo fossils have been very scrappy, making it hard to determine their features and relationships.
The fossils’ traits do not neatly fit A. sediba into the hominin family tree, which includes only humans and our ancestors and extinct cousins. In 2010 Berger wrote that ‘it is most likely descended from A. africanus’, but that it was ‘not possible to establish the precise phylogenetic position of A. sediba in relation to the various species assigned to early Homo’.
Timing is a key problem in determining ancestry, because the A. sediba fossils lived after the earliest humans. They may have been a relict population of a group that earlier gave rise to Homo, or a surviving sister group to the ancestral lineage.
In late 2011 Berger reported that A. sediba appeared to mark a halfway stage between primitive ‘ape-men’ and our direct ancestors. A year of detailed study had revealed that the skeletons were a hodgepodge of anatomical features: some bones looked almost human while others were chimpanzee-like. One area of particular interest was the brain size, which was small even for an australopith, with a volume of just 420 cubic centimetres. A. afarensis, by contrast, averaged 459 cubic centimetres, despite being an earlier species. This suggests that there was no overall increase in brain size over the course of australopith evolution. But there was evidence that the brain had been subtly reorganized. The orbitofrontal region, which sits just behind the eyes, is a different shape from those of other australopiths and apes, and may have been rewired into a more human-like design.
A second group looked at A. sediba’s hands. The fingers are long, thin and slightly curved, just like those of apes, which would have allowed A. sediba to grip branches firmly. But the thumb is proportionately longer than in apes, a distinctly human trait that would have allowed A. sediba to grip small objects precisely. These hands may have made and used stone tools. So far, no tools have been found but, given the evidence that other australopiths used stone tools, it would not be a huge shock if A. sediba also did.
Other groups have focused on the pelvis, feet and ankles. They all come to the same conclusion: A. sediba is halfway between Australopithecus and Homo. Berger says it is not surprising that the fossil is a confusing mixture, pointing out that that is exactly what we would expect in a transitional fossil.
Eats bark, fruit and leaves
In 2012 Berger returned to the fray. His collaborators announced that they had discovered what A. sediba ate. On the menu: bark. It turned out that A. sediba had poor dental hygiene. From plaque on the fossils’ teeth, the team extracted ‘phytoliths’ – mineral traces of A. sediba’s food. They found signs of fruit, bark and woody tissues.
Berger was surprised, but primatologists were not. Bark represents a considerable fraction of orang-utan diets, and other primates also chew on the hard stuff: species from the golden snub-nosed monkey to chimpanzees eat bark when times are tough.
However, greater dietary surprises were in store. The team looked at sediment samples and fossilized animal faeces – coprolites – to get an idea of what the environment in which A. sediba lived was like. They found remnants of savannah grasses in the sediment, while pollen and woody fragments in the coprolites suggested that there might have been some woodland in the vicinity.
The team then looked at the carbon isotopes in A. sediba teeth to see what types of plants they ate. A ‘C4’ signature is typical of savannah plants like grasses and the grains they carry. These plants fix carbon in a four-carbon molecule. ‘C3’ indicates fruits and leaves foraged from a more forested environment.
The team expected a C4 signature – it is what most hominins have and it fits the evidence that A. sediba lived in an open savannah. They found the exact opposite. Clearly, the diet of A. sediba was different from the diet of other early hominins, but why A. sediba had such an unusual diet is still a mystery.
What is A. sediba?
The two Australopithecus sediba skeletons keep yielding new secrets. A set of studies published in 2013 offered further evidence that A. sediba may bridge the gap between the ape-like australopiths and our own genus, Homo.
The skeletal analyses confirmed that A. sediba has a mosaic of ancient australopith and modern Homo features. For instance, its teeth are remarkably human-like. Whereas most australopiths have large, prominent canines, A. sediba’s are small, like ours, according to Darryl de Ruiter at Texas A&M University in College Station and his colleagues.
The skeletons also suggest that A. sediba had the early makings of a tapering human waist. Peter Schmid of the University of Witwatersrand, South Africa, led a team which found that its lower ribs sweep inwards, as ours do. This allowed abdominal muscles to be arranged in a way that makes walking more efficient. Other australopiths are thought to have lacked a waist, says Schmid.
In other ways, A. sediba was very unlike early humans. Jeremy DeSilva at Boston University in Massachusetts and his colleagues found that its legs and feet were those you would expect of a tree climber. Humans – like most other australopiths – have a rigid foot. A. sediba’s foot was much more flexible, making it perfect for gripping tree trunks and branches.
This poses a puzzle. If australopiths spent more time walking on the savannah and less time in the trees than their ancestors, why was A. sediba, the most human-like of all australopiths, so well adapted to tree living? Conceivably, some australopiths returned to life in the trees – or it could be evidence for a deeper tree-dwelling lineage in South Africa.