10 Cervical and Posterior Arch Augmentation
Luigi Manfre, Nicole S. Carter, Joshua A. Hirsch, and Ronil V. Chandra
Summary
Cervical vertebroplasty is performed far less often than thoracic and lumbar vertebroplasty but is an important treatment for patients with fractures and neoplastic involvement of the cervical spine. Osseous augmentation of the cervical spine has greater technical challenges due to the surrounding neurovascular structures and the small size of the vertebral bodies and should be performed by experienced practitioners. Cervical vertebroplasty is typically performed under fluoroscopic or CT guidance with various approaches utilized such as the transoral, translateral, and transpedicular approaches. Posterior arch osseous augmentation can be performed for neoplastic and non-neoplastic pathologies located in the pedicles, laminae, and in the spinous process. Injection of cement into the pedicles or laminae must be done with care as important nerves and vessels are located directly adjacent to these osseous structures and CT guidance can be very useful when augmenting the posterior arch. Spinoplasty can be very useful prior to placement of interspinous spacers to augment the strength of the spinous process in patients at high risk of fracture or osseous erosion around the spacer.
Keywords: osseous augmentation, cervical, posterior arch, transoral, translateral, pediculoplasty, laminoplasty, spinoplasty
10.1 Cervical Augmentation
10.1.1 Introduction
Percutaneous vertebroplasty is widely considered an effective procedure in the treatment of selected patients with vertebral compression fractures (VCFs) related to osteoporotic disease, primary and secondary osteolytic tumors, and some cases of trauma.1 The majority of vertebroplasty procedures are performed in the thoracic and lumbar spine by fluoroscopic or computed tomography (CT) guidance, where established radiological landmarks and approaches to the body allow safe and rapid augmentation. VCFs also occur in the cervical spine, in around 1% of patients affected by primary or secondary spinal neoplasms.2,3 For these cases, cervical vertebroplasty presents an alternative or an adjunct treatment to radiotherapy (RT) or cervical spinal surgery in reducing pain and improving stability of the VCF. However, compared with thoracolumbar vertebroplasty, augmentation in the cervical spine presents technical challenges due to the small size of target vertebral bodies, the difficulty in visualizing bony landmarks, and the surrounding neurovascular and airway structures. Cervical vertebroplasty thus requires special considerations in regard to the procedural approach, sedation, and radiological guidance, and should be performed by experienced operators.4
Operative surgery of the cervical spine remains essential when the spinal canal is compromised and/or when there is spinal instability.5,6 Even when cervical lesions generate instability, anterior fixation of the spine may not be well tolerated, particularly in patients with short life expectancy, immune compromise, or significant debilitation. Moreover, even in patients with longer life expectancy and greater baseline functional status, surgical treatment carries risk of restricted cervical movement, which may not be desirable for younger patients.
Palliative RT has demonstrated powerful effects in the treatment of secondary cervical lesions that require pain relief and vertebral bone remineralization.7 It does not require general anesthesia, and can be performed in patients who are in extremely poor clinical condition. However, post radiation remineralization generally occurs in 3 to 6 months after treatment, with interval risk of vertebral collapse or radiation-specific complications such as radiation-induced myelitis or soft-tissue radionecrosis.8
10.1.2 Historical Perspective
The first vertebral augmentation procedure reported in the literature was performed in the cervical spine by Galibert et al, who performed vertebroplasty to treat an aggressive C2 hemangioma.9 The procedure provided rapid, almost complete pain relief for the patient, leading to an increased uptake of the procedure and application to treat thoracolumbar fractures. It is currently considered highly effective for cervical lesions and can be considered the best treatment option after stringent patient selection, even in extremely aggressive osteolytic lesions (▶Fig. 10.1a, b).
10.1.3 Image Guidance
Conventional vertebroplasty can be safely performed under either fluoroscopic or CT guidance; the choice of either method is at the discretion of the practitioner. In cervical vertebroplasty, both fluoroscopy and CT can be used if necessary along with multiplanar reconstructions. Prior to the procedure, comprehensive radiological analysis is advisable, including both CT and magnetic resonance imaging (MRI), with cervical vessel imaging with CT or MR angiography. Careful pre-procedural planning reduces the likelihood of complications.
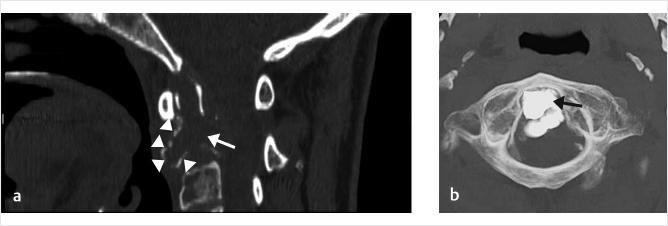
Fig. 10.1 Multiple myeloma with subtotal C2 osteolysis (white arrow). Only a faint cortical bone can be appreciated (white arrowheads), with dramatic cervical pain and need for immediate stabilization (a). After the procedure, complete C2 augmentation, reproducing the dens (black arrow), is obtained, regaining the conventional C1–C2 joint function, avoiding more severe posterior occipital bone–C3 surgical fixation (b).
10.1.4 Procedural Approaches
Transoral
The transoral approach (utilized by Galibert et al in the first cervical vertebroplasty procedures) is considered the preferred approach in the case of a lesion involving the upper cervical spine (C1–C4).10–12 In this approach, the patient is positioned supine, with mild hyperextension of the neck and an oropharyngeal retractor utilized to achieve sufficient mouth opening. The needle is introduced directly through the oral cavity along the midline, traversing the oropharynx to reach the vertebral body directly (▶Fig. 10.2a). In certain cases, even the clivus can be reached through this method, allowing the treatment of large osteolytic lesions of the skull base.13
Benefits
In contrast to alternative approaches, the transoral method avoids almost all the main neurovascular cervical structures, as thin pharyngeal muscles, fascia, and anterior ligaments are the only structures that will be perforated by a correctly placed transoral needle.14 This approach is a known neurosurgical route for treating intradural and extradural diseases and, despite not being a percutaneous approach, it remains one of the safest ways to reach the upper cervical vertebrae.
Limitations
General anesthesia and intubation are mandatory for the transoral approach. Oral intubation is possible but generally not recommended, as oral cavity should not be occupied by tools other than the vertebroplasty equipment; this allows more freedom when mediolateral or craniocaudal inclination of the needle is preferred, depending on the location of the disease. Nasotracheal intubation is thus accomplished, and may be performed with fiberoptic assistance. Infection is a key source of potential complication in the transoral approach, with wound infections occurring in less than 2% and meningitis in less than 5%.15,16 For this reason, perioperative and postoperative antibiotic prophylaxis is recommended for preventing infection, as preparation of the posterior oropharynx with iodine is not always sufficient to obtain 100% oral cavity sterility.
Translateral
Translateral approaches (TLAs; ▶Fig. 10.2c) have been described for cervical lesions at C1 to C5, and allow direct percutaneous treatment of the vertebral body. Three translateral methods are differentiated: the anterolateral approach (ALA), the TLA, and the posterolateral approach (PLA). In the ALA,17,18 with moderate hyperextension of the head, the carotid is manually compressed and pushed laterally by the operator’s fingers. The needle is carefully introduced under the mandibular angle, between the trachea and the carotid–jugular axis.17,18 The TLA for vertebral augmentation has usually been described in the literature as a combined fluoroscopy-CT guided treatment. In some cases, this approach may be somewhat difficult to perform due to the risk of damaging nearby neurovascular structures.17 The PLA19,20 may be performed in certain cases: the needle is placed posterolaterally through the posterior cervical space, bypassing the main lateral neurovascular structures.19,20 However, in the case of anatomical variations or tumoral mass displacement (▶Fig. 10.3), the vertebral artery may prevent the PLA from being utilized.21
Benefits
In some cases, translateral procedures can be performed under simple local anesthesia. Nevertheless, considering the difficult anatomical area to be accessed, sedation of the patient is strongly advised. Moreover, since these procedures utilize a fully percutaneous approach, there is no significant risk of infection, and a simple skin disinfection is considered sufficient for infection prevention. A TLA is typically the optimal choice in case of obese or short-necked patients.22
Limitations
TLAs require the needle has to be advanced slowly and carefully in the soft local neck tissues. In contrast to posterior thoracic or lumbar procedures, where the main paravertebral muscles offer moderate resistance and support to maintain the needle position, in TLAs the needle almost immediately reaches deep structures under only mild pressure. Moreover, potential damage to the main vascular and neural structures of the neck presents a life-threating potential risk. These structures may even be located in nonstandard areas in the case of anatomical variation or displacement.
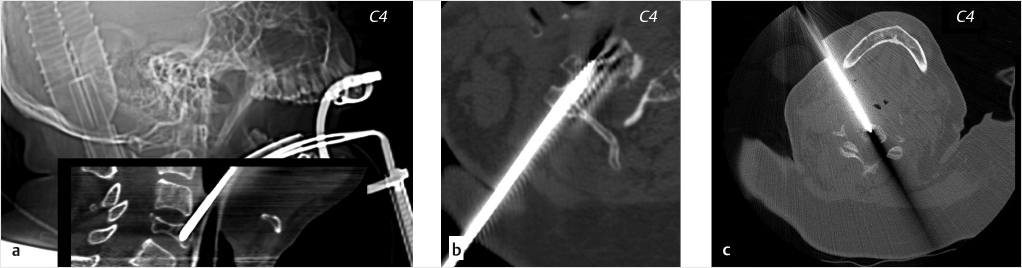
Fig. 10.2 Cervical vertebroplasty of C4: different technical approach. The same vertebra (C4 in this case) can be reached on different routes: transoral (a), transpedicular (b), or anterolateral (c).
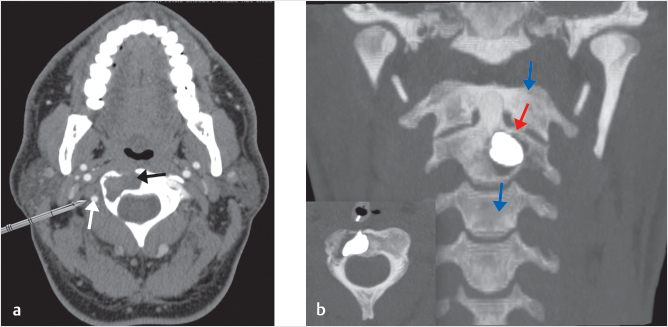
Fig. 10.3 Right-sided C2 osteolysis related to multiple myeloma. (a) A posterolateral approach, despite ostensibly easier than the transoral approach, can be extremely dangerous in the case of displacement of the vertebral artery (white arrow) by a tumor (black arrow). (b) In this patient, a transoral approach was selected and complete augmentation was obtained (red arrow) to sustain the axial load (blue arrows).
Transpedicular
A transpedicular approach can be utilized in some cases when the target lesion is located from C4 to C7. The fundamental requirement for this approach is a sufficiently large posterior vertebral pedicle, allowing transpedicular needle access (▶Fig. 10.2b). This approach is almost identical to the more familiar approach adopted for conventional thoracic and lumbar vertebroplasty.
Benefits
As in the TLA, there is minimal risk of infection for transpedicular procedures. The procedure may be performed under local anesthesia.
Limitations
Transpedicular approaches are not suitable when the cervical pedicles are very small or require a needle smaller than 15 gauge. As precision is required in needle placement, CT guidance may be utilized to protect against accidental damage to the vertebral artery or the spinal cord.
10.1.5 Prevention of Infection
In transoral approaches, a key potential risk is infection, generally related to disruption of the protective mucosal barrier, allowing pathogens to enter the deep neck tissues. The most common pathogens are Staphylococcus aureus, Streptococcus, Candida, as well as gram-negative bacteria including Escherichia coli, Proteus, and Klebsiella. Furthermore, even resident oral flora such as Corynebacterium and Bacteroides may cause infection, considering that many patients that undergo treatment are immunocompromised patients due to underlying neoplastic disease and/or chemoradiotherapy treatments.23 It is important to recognize that the oral cavity cannot be sterilized completely and that systemic antibiotic treatment is important for infection prevention.
After the retracting forceps have been applied, the oral cavity is sterilized, generally using chlorhexidine or Betadine. For antibiotic prophylaxis, we generally use cefazolin (1–2 g, IV in adults, or 3 g in patients weighing in excess of 120 kg) immediately administered 30 to 60 minutes before the beginning of the surgical procedure. Alternatively, other protocols include a combination of 600-mg clindamycin and 500-mg levofloxacin 30 minutes preprocedure or a combination of 1,200-mg clindamycin and gentamycin (2 mg/kg). Regardless, antibiotics should be continued for at least 48 hours postprocedure after transoral needle access. For translateral and transpedicular approaches, the initial preprocedure antibiotic prophylaxis is usually sufficient.
10.1.6 Complications
There is little existing literature on the complications of cervical vertebroplasty. Chiras described a 16.7% complication rate (2 out of 12 patients in his series): 1 patient had a C1–C2 perivertebral leakage and occipital neuralgia, and the other patient had cerebellar symptoms related to polymethyl methacrylate (PMMA) embolization seen after treatment of their hypervascular pheochromocytoma metastasis. In both cases, symptoms regressed after a few weeks.24 One fatal complication has been described in a 4-year-old patient affected by a C2 aneurysmal bone cyst, with leakage into the venous compartment and embolization of the vertebrobasilar system.25 For this reason, an injection of contrast media directly into the vertebra body is useful prior to the injection of PMMA, to detect possible retrograde filling of the vertebrobasilar system.26
10.1.7 Conclusion
Cervical vertebroplasty plays an important role in the management of VCFs due to disease of the cervical spine. Successful implementation of the procedure requires a specific approach to preoperative planning, procedural techniques, and postoperative care. When patients are carefully selected, and meticulous care is taken in procedural technique, vertebroplasty can be considered a safe procedure for the treatment of osteoporotic, traumatic, and neoplastic fractures in the cervical spine. It may be utilized not only for simple pain relief but also for mechanical stabilization and prevention of life-threatening vertebral collapse.27
10.2 Posterior Arch Augmentation
10.2.1 Introduction
Lesions located in the posterior arch have previously been considered a contraindication to vertebral augmentation. However, posterior arch procedures have been described by several authors as a safe and effective option for pain relief in both neoplastic and non-neoplastic lesions.28–30
10.2.2 Pediculoplasty
The pedicle can be involved both in traumatic (stress fracture with or without osteoporosis) and metastatic or neoplastic disease. The risk of vertebral body collapse is related not only to the volume of disease within the vertebral body but also to the direct involvement of the pedicle. This is particularly significant in the case of disease in the thoracic spine where, according to several biomechanical studies, fracture risk increases with pedicle or costovertebral involvement. Posterior arch augmentation of the pedicle thus should be considered not only an adjunct procedure to vertebral body augmentation but as a key treatment option to stabilize the vertebral body in thoracic-level fractures.31
Pediculoplasty in Vertebral Tumors
The pediculoplasty procedure requires additional technical considerations. When the pedicle is involved by an osteolytic lesion, conventional radiological landmarks can no longer be reliably detected on fluoroscopy. Moreover, injecting PMMA cement inside the pedicle by fluoroscopic guidance could potentially be considered unsafe, as it is not possible to detect potential leakage inside the spinal canal or neural foramina. Thus, the CT-guided technique can be used to optimize patient safety in performing augmentation of the pedicle. The needle (generally 13–15 gauge in size) is introduced into the pedicle deep to the vertebral body, followed by the slow injection of a small volume of PMMA (approximately 1 mL) while the needle is gently retrieved from the vertebral body (▶Fig. 10.4). As the delivery of the PMMA can be directed from the tip of the needle, the use of a beveled needle is advised.32
Pediculoplasty in Spondylolysis
Pedicle discontinuity is found with lysis of a portion of the pedicle. This is sometimes associated with listhesis. Unilateral involvement is possible, which reduces the stability of the local spinal unit and acts as an important trigger point for pain. Stress fractures of the pedicle related to intense sport activity have also been described.33 Fractures of the pedicles have also been reported to result from abnormal stresses resulting from posterior screw and rod instrumentation.34,35 In these patients, pediculoplasty is a potential treatment option to provide pain relief and improved stability. The combination of percutaneous posterior intrapedicular screw fixation and PMMA injection, a treatment originally named “Buck’s technique,”36 has been described as a highly successful solution for restoring vertebral stability.37
Pediculoplasty in Osteoporotic Disease
Pedicular involvement is not typically reported on routine radiological studies for osteoporotic disease, as attention is generally focused on the vertebral body. However, the association between more typical osteoporotic VCFs and unsuspected pedicular fractures has been reported in the literature.38 Jung et al reported involvement of the pedicle in 51% of patients affected by vertebral body fracture (▶Fig. 10.5), with typical bone discontinuity on CT scan as well as signal abnormalities of the bone marrow on MR imaging.39 Osteoporotic fractures of the pedicle may also be detected as double symmetrical uptake spots on bone scanning.40 In patients with this type of fracture, pedicle strength can be significantly improved by cement injection in which the PMMA-filled needle is pulled out while the stylet remains unreplaced, thereby creating a long continuous tube of cement within the pedicle.38
10.2.3 Laminoplasty and Spinoplasty
New-generation interspinous spacers generally cause minimal stress to the spinous process and laminae. However, particularly in the case of full PEEK systems, larger interspinous implants or in elderly patients with advanced osteoporosis, bone remodeling or fracture of the spinous process can occur. Younger patients treated with interspinous spacers for spinal canal stenosis or spinal foramina stenosis may suffer a posterior element fracture due to the effects of stress upon the bone surrounding the device. Bone remodeling of the posterior laminae has been described in approximately 13% of patients undergoing interspinous spacer insertion.41
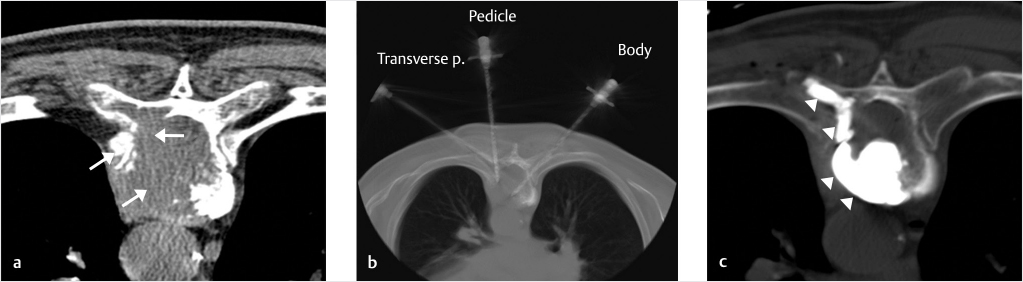
Fig. 10.4 Metastasis at the level of T9, with extensive osteolysis of the left vertebral body, pedicle, and costovertebral process (white arrows; a): three needles were introduced into the vertebral body, the left pedicle, and the left costovertebral process (b). (c) PMMA injection was performed, with total bone remodeling of the osteolytic area (white arrowheads).
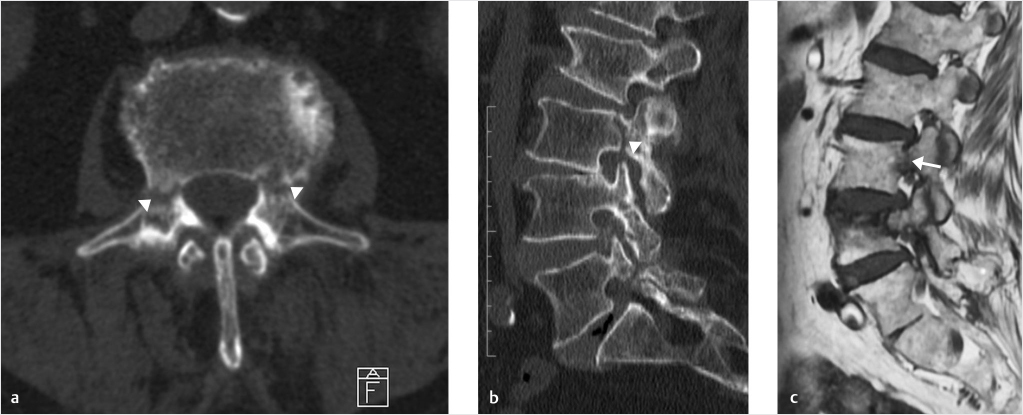
Fig. 10.5 Bilateral L3 pedicle fractures in a patient with severe osteoporotic disease (white arrowheads). On axial (a) and 2D recon sagittal (b) CT scans, interruption of the cortical bone can be appreciated on both the pedicles of the third lumbar vertebra (white arrowheads). Signal abnormality is easily depicted on sagittal T1-weighted scan (c; white arrow).
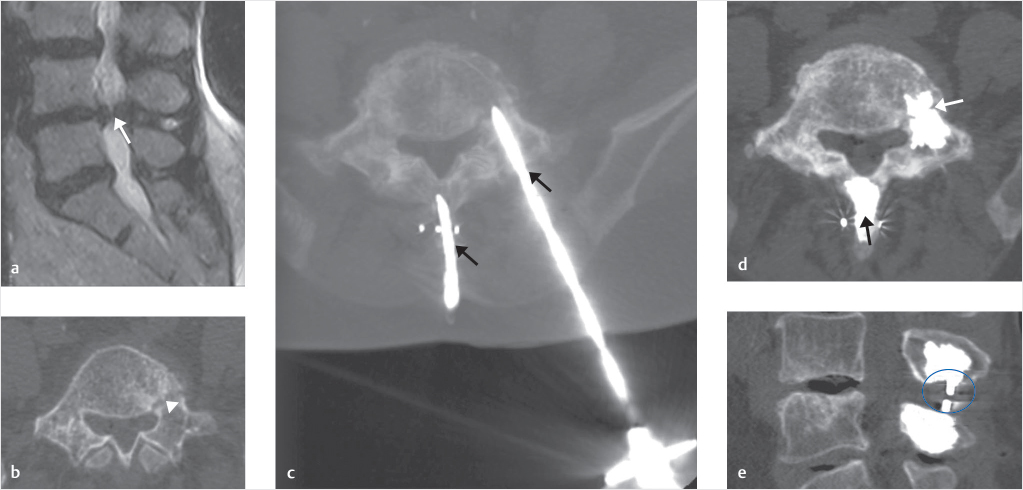
Fig. 10.6 Prophylactic spinoplasty and pediculoplasty in a patient affected by severe L4–L5 spinal canal stenosis (SCS) associated with left pedicle stress fracture. On sagittal T2-weighted scan (a), grade D spinal canal stenosis (according to Schizas’ classification) was detected (white arrow). Pre-op axial CT scan of L4 revealed left pedicle stress fracture (white arrowhead) as an incidental finding (b). Two needles were introduced on a sagittal route inside the spinous processes of L4 and L5 (black arrow), and a third needle was introduced into the left L4 pedicle (black arrow; c). After injection of PMMA, spinoplasty of L4 and L5 (black arrow) as well as left-side pediculoplasty of L4 (white arrow) was obtained (d), making possible the subsequent safe introduction of the interspinous spacer (blue circle; e).
To perform spinoplasty, PMMA can be prophylactically introduced directly into the spinous processes via CT or fluoroscopic guidance with a small needle (13–15 gauge). In the case of a very thin spinous processes, an oblique posterolateral route may be utilized to reach the laminae crus for retrograde filling of the spinous process (▶Fig. 10.6). As this area is far from the main neural structures, and the bone cortex of the laminae crus is particularly compact (which protects the spinal canal and foramina), no significant contraindication or complication generally occurs.42 By performing prophylactic spinoplasty, bone remodeling and fracture related to interspinous spacers can be decreased or avoided, increasing the efficacy of the spacer itself.42
10.2.4 Key Points
• Vertebroplasty (cervical).
• Vertebroplasty (pedicle).
• Spinoplasty.
• Prophylaxis.
• Transoral.
• Spondylolisthesis.
References
[1] Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 1997;18(10):1897–1904
[2] Sherk HH. Lesions of the atlas and axis. Clin Orthop Relat Res 1975(109):33–41
[3] Moulding HD, Bilsky MH. Metastases to the craniovertebral junction. Neurosurgery 2010;66(3, Suppl):113–118
[4] Cotten A, Boutry N, Cortet B, et al. Percutaneous vertebroplasty: state of the art. Radiographics 1998;18(2):311–320, discussion 320–323
[5] Delank KS, Wendtner C, Eich HT, Eysel P. The treatment of spinal metastases. Dtsch Arztebl Int 2011;108(5):71–79, quiz 80
[6] Boschi V, Pogorelić Z, Gulan G, Perko Z, Grandić L, Radonić V. Management of cement vertebroplasty in the treatment of vertebral hemangioma. Scand J Surg 2011;100(2):120–124
[7] Rades D, Schild SE, Abrahm JL. Treatment of painful bone metastases. Nat Rev Clin Oncol 2010;7(4):220–229
[8] Guedea F, Majó J, Guardia E, Canals E, Craven-Bartle J. The role of radiation therapy in vertebral hemangiomas without neurological signs. Int Orthop 1994;18(2):77–79
[9] Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty Neurochirurgie 1987;33(2):166–168
[10] Gailloud P, Martin JB, Olivi A, Rüfenacht DA, Murphy KJ. Transoral vertebroplasty for a fractured C2 aneurysmal bone cyst. J Vasc Interv Radiol 2002;13(3):340–341
[11] Tong FC, Cloft HJ, Joseph GJ, Rodts GR, Dion JE. Transoral approach to cervical vertebroplasty for multiple myeloma. AJR Am J Roentgenol 2000;175(5): 1322–1324
[12] Reddy AS, Hochman M, Loh S, Rachlin J, Li J, Hirsch JA. CT guided direct transoral approach to C2 for percutaneous vertebroplasty. Pain Physician 2005;8(2):235–238
[13] Wright CH, Kusyk D, Rosenberg WS, Sweet JA. Percutaneous transoral clivoplasty and upper cervical vertebroplasties for multifocal skeletal lymphangiomatosis resulting in complete resolution of pain: case report. J Neurosurg Spine 2017;26(2):171–176
[14] Menezes AH, VanGilder JC. Transoral-transpharyngeal approach to the anterior craniocervical junction. Ten-year experience with 72 patients. J Neurosurg 1988;69(6):895–903
[15] Kingdom TT, Nockels RP, Kaplan MJ. Transoral-transpharyngeal approach to the craniocervical junction. Otolaryngol Head Neck Surg 1995;113(4):393–400
[16] Hadley MN, Spetzler RF, Sonntag VK. The transoral approach to the superior cervical spine. A review of 53 cases of extradural cervicomedullary compression. J Neurosurg 1989;71(1):16–23
[17] Anselmetti GC, Manca A, Chiara G, Regge D. Painful osteolytic metastasis involving the anterior and posterior arches of C1: percutaneous vertebroplasty with local anesthesia. J Vasc Interv Radiol 2009;20(12):1645–1647
[18] Wetzel SG, Martin JB, Somon T, Wilhelm K, Rufenacht DA. Painful osteolytic metastasis of the atlas: treatment with percutaneous vertebroplasty. Spine 2002;27(22):E493–E495
[19] Huegli RW, Schaeren S, Jacob AL, Martin JB, Wetzel SG. Percutaneous cervical vertebroplasty in a multifunctional image-guided therapy suite: hybrid lateral approach to C1 and C4 under CT and fluoroscopic guidance. Cardiovasc Intervent Radiol 2005;28(5):649–652
[20] Sun G, Jin P, Li M, et al. Percutaneous vertebroplasty for treatment of osteolytic metastases of the C2 vertebral body using anterolateral and posterolateral approach. Technol Cancer Res Treat 2010;9(4):417–422
[21] Sun HY, Lee JW, Kim KJ, Yeom JS, Kang HS. Percutaneous intervention of the C2 vertebral body using a CT-guided posterolateral approach. AJR Am J Roentgenol 2009;193(6):1703–1705
[22] Guo WH, Meng MB, You X, et al. CT-guided percutaneous vertebroplasty of the upper cervical spine via a translateral approach. Pain Physician 2012;15(5):E733–E741
[23] Todar K. The normal bacterial flora of humans. In: Todar K, ed. Todar’s Online Textbook of Bacteriology (online book). Madison, WI: Keith Todar; 2008–2012
[24] Mont’Alverne F, Vallée JN, Cormier E, et al. Percutaneous vertebroplasty for metastatic involvement of the axis. AJNR Am J Neuroradiol 2005;26(7): 1641–1645
[25] Peraud A, Drake JM, Armstrong D, Hedden D, Babyn P, Wilson G. Fatal ethibloc embolization of vertebrobasilar system following percutaneous injection into aneurysmal bone cyst of the second cervical vertebra. AJNR Am J Neuroradiol 2004;25(6):1116–1120
[26] Turowski B, Schellhammer F, Herdmann J, Rommel F. Fatal Ethibloc embolization of vertebrobasilar system following percutaneous injection into aneurysmal bone cyst of the second cervical vertebra. AJNR Am J Neuroradiol 2005;26(7):1883–1884
[27] De la Garza-Ramos R, Benvenutti-Regato M, Caro-Osorio E. Vertebroplasty and kyphoplasty for cervical spine metastases: a systematic review and meta-analysis. Int J Spine Surg 2016;10:7
[28] Anselmetti GC, Bonaldi G, Carpeggiani P, Manfrè L, Masala S, Muto M. Vertebral augmentation: 7 years’ experience. In: Alexandre A, Masini M, Menchetti P, eds. Advances in Minimal Invasive Therapy of the Spine and Nerves. Vienna: Springer; 2011:147–161
[29] Reyes M, Georgy M, Brook L, et al. Multicenter clinical and imaging evaluation of targeted radiofrequency ablation (t-RFA) and cement augmentation of neoplastic vertebral lesions. J Neurointerv Surg 2018;10(2):176–182
[30] Manfrè L. Posterior arch and extravertebral augmentation. Neuroradiology 2014;56(I):109–110
[31] Taneichi H, Kaneda K, Takeda N, Abumi K, Satoh S. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine 1997;22(3):239–245
[32] Martin JB, Wetzel SG, Seium Y, et al. Percutaneous vertebroplasty in metastatic disease: transpedicular access and treatment of lysed pedicles—initial experience. Radiology 2003;229(2):593–597
[33] Guillodo Y, Botton E, Saraux A, et al. Contralateral spondylolysis and fracture of the lumbar pedicle in an elite female gymnast. Spine 2000;25(19): 2541–2543
[34] Maurer SG, Wright KE, Bendo JA. Iatrogenic spondylolysis leading to contralateral pedicular stress fracture and unstable spondylolisthesis. Spine 2000;25(7):895–898
[35] Macdessi SJ, Leong AK, Bentivoglio JEC. Pedicle fracture after instrumented posterolateral lumbar fusion: a case report. Spine 2001;26(5):580–582
[36] Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br 1970;52(3):432–437
[37] Rajasekaran S, Subbiah M, Shetty AP. Direct repair of lumbar spondylolysis by Buck’s technique. Indian J Orthop 2011;45(2):136–140
[38] Eyheremendy EP, De Luca SE, Sanabria E. Percutaneous pediculoplasty in osteoporotic compression fractures. J Vasc Interv Radiol 2004;15(8):869–874
[39] Jung HS, Jee WH, McCauley TR, Ha KY, Choi KH. Discrimination of metastatic from acute osteoporotic compression spinal fractures with MR imaging. Radiographics 2003;23(1):179–187
[40] Traughber PD, Havlina JM Jr. Bilateral pedicle stress fractures: SPECT and CT features. J Comput Assist Tomogr 1991;15(2):338–340
[41] Miller JD, Miller MC, Lucas MG. Erosion of the spinous process: a potential cause of interspinous process spacer failure. J Neurosurg Spine 2010;12(2):210–213
[42] Manfré L. Posterior arch augmentation (spinoplasty) before and after single and double interspinous spacer introduction at the same level: preventing and treating the failure? Interv Neuroradiol 2014;20(5):626–631