14 Appropriateness Criteria for Vertebral Augmentation
Alexios Kelekis and Dimitrios K. Filippiadis
Summary
Clinical practice decisions regarding how to treat certain fractures remain heterogeneous and traditionally there has been little consensus of what type of vertebral augmentation procedure should be used to treat specific fracture types. Various medical societies have produced their own recommendations for treating vertebral compression fractures but the most important document that has been developed to establish a clinical care pathway for the treatment of Vertebral compression fractures (VCFs) has been the UCLA/RAND appropriateness criteria recommendations published in 2018 by a multispecialty group of physicians. These criteria included clinical signs and symptoms of VCFs that need vertebral augmentation as well as imaging criteria for choosing vertebral augmentation over nonsurgical management (NSM). There were seven key factors identified that determined the appropriateness of proceeding with vertebral augmentation as opposed to treating with NSM. The treatment choice was strongly influenced by the clinical variables and the difference in patient characteristics. The clinical factors that determined the overall choice of treatment included the clinical exam and imaging findings, the duration of pain, the impact of the VCF on daily functioning, the degree of height reduction, and kyphotic deformity, whether there was progressive vertebral body height loss and the overall evolution of symptoms. The contraindications for performing vertebral augmentation were also narrowed and refined and treatment recommendations were put forth for dealing with the underlying condition (i.e. osteoporosis) that gave rise to the fracture.
Keywords: appropriateness criteria, Magerl classification, UCLA/RAND methodology, clinical care pathway
14.1 Introduction
VCFs can be related to osteoporosis, trauma, or malignancy. The end result is pain and mobility impairment with a high impact on life quality as well as morbidities resulting in reduced life expectancy.1 Therapeutic options include NSM (pain medication, bracing, bed rest, etc.) and vertebral augmentation by means of vertebroplasty (VP), balloon kyphoplasty (BKP), or spinal implants (stents, jacks, peek cages, etc.).1,2 The wide variety in the causes and the characteristics of a VCF necessitate a tailored-based therapeutic approach taking into account both the advantages and limitations of each treatment. At the moment, clinical practice decisions are driven by operator’s preference and/or international guidelines, which can be divergent and sometimes contradictory. There is no predictive tool available that will be able to identify the ideal therapeutic approach for a given VCF with specific characteristics. Furthermore, there is a clear lack of appropriateness criteria that will govern a therapy based on the expected benefits that should outweigh the potential complications by a sufficient margin in order to render the technique worth doing. A recent systematic review of evidence-based guidelines for the management of VCFs showed considerable inconsistencies in the treatment recommendations as well as in the recommendations for the diagnostic evaluation and the prevention of future fractures.3
14.2 Indications, Guidelines, and Recommendations
Standard indications for vertebral augmentation include symptomatic (painful) type A fractures according to the Magerl classification (most commonly type A1 fractures) with bone edema on MRI (▶Fig. 14.1) and/or positive bone scan scintigraphy. The patient should be an adult reporting at least moderate pain (Visual Analog Scale >4 units) at the respective level, with absence of neurologic impairment and no absolute contraindications to therapy (e.g., active infection at the surgical site or the presence of an untreated blood borne infection).1,2,4 Depending on the fracture’s specific characteristics, different techniques of vertebral augmentation can be utilized.
Although many scientific organizations have published guidelines regarding the indications for vertebral augmentation, none provides a tailored approach based upon the unique characteristics of a specific vertebral fracture. Furthermore, almost all the documents mention standard VP and balloon augmentation omitting spinal implants, which constitute a paradigm shift away from cement injection performed either with or without using bone tamps. According to the 2013 NICE guidelines and position paper 5, standard VP and balloon augmentation without stenting should be offered to patients with severe ongoing pain and in whom the pain has been confirmed to be at the level of the fracture by physical examination and imaging after a recent, unhealed vertebral fracture that continues to produce significant discomfort despite optimal pain management.5 According to a position paper released by multiple scientific societies including the Society of Interventional Radiology (SIR), American Society of Neuroradiology (ASNR), American College of Radiology (ACR), American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS), vertebral augmentation remains a proven medically appropriate therapy for treatment of painful VCFs refractory to nonoperative medical therapy and for vertebrae weakened by neoplasia when performed for the medical indications outlined in the published standards.6–10 The American Academy of Orthopaedic Surgeons (AAOS) guidelines are a distinct outlier with recommendations against VP and for BKP as a treatment option for painful VCFs.11 According to ACR guidelines, both vertebral augmentation techniques are similar and should be offered as a second-line therapy after NSM with balloon augmentation resulting in better angular correction and fracture reduction.12 The Standards and Guidelines Committee of the SNIS reports that both VP and kyphoplasty are indicated in symptomatic osteoporotic or cancer-related VCFs refractory to medical therapy.13 The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) in the recently published guidelines for vertebral augmentation include in the indications for VP painful osteoporotic fractures, painful vertebrae due to benign tumors or malignant infiltration, Kummels’ disease, symptomatic vertebrae plana, acute stable A1 and A3 fractures according to the Magerl classification, and chronic traumatic fractures (▶Fig. 14.2, ▶Fig. 14.3, ▶Fig. 14.4). In the same document, indications for balloon augmentation include all the aforementioned, but it is reported that the best indication for the technique is a traumatic acute (<7–10 days) fracture (particularly Magerl A1) with a local kyphotic angle less than 15 degrees.4 The CIRSE guidelines include indications for the application of spinal implants that, according to the authors, can be used in all the indications valid for VP and balloon augmentation.4 The spine metastatic disease working group suggests that vertebral augmentation can be proposed in the cases in which there is no metastatic epidural spinal cord compression and in the cases of fracture prophylaxis after radiation therapy or after percutaneous ablation for local tumor control.14 These recommendations are especially important in patients with relatively good prognoses.14 Additionally, according to the same authors, vertebral augmentation techniques are recommended for first-line pain palliation treatment, related to stable pathologic VCFs.14 The American Society for Radiation Oncology (ASTRO) guidelines comment that there are no prospective data suggesting that either kyphoplasty or VP would obviate the need for EBRT (external beam radiotherapy) for painful bone metastases, but these two different therapies can certainly be complimentary.15
Fig. 14.1 Sagittal short tau inversion recovery (STIR) MR image showing acute or subacute vertebral compression fractures with bone marrow edema (areas of increased signal as shown by the white arrows). In L1 vertebral body there is suspected initial cavity formation with high signal intensity in the center (area within white oval). A fluid-filled cavity is seen under the upper end plate of L3 vertebral body (white arrowheads).
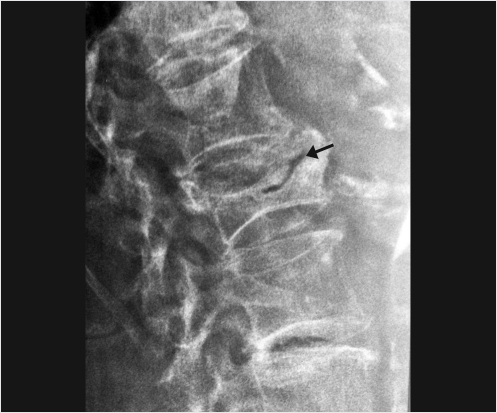
Fig. 14.2 Lateral X-ray view of the lumbar spine showing vacuum cleft phenomenon (black arrow) in an upper lumbar vertebral body indicative of a fracture nonunion and an unstable vertebral body.
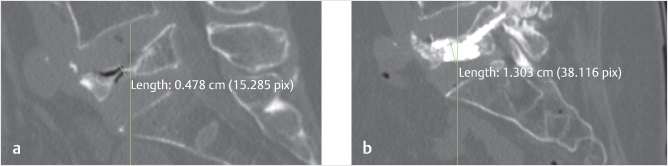
Fig. 14.3 Sagittal CT reconstructions of the lumbar spine showing a vertebra plana at L5 in a symptomatic patient (a) before and (b) after vertebral augmentation. The height of the midportion of the vertebral body was measured at 0.478 cm prior to vertebral augmentation and 1.303 cm afterward.
Fig. 14.4 (a) Coronal and (b) sagittal CT reconstructions and (c) an axial CT image of the lumbar spine showing a Magerl A3.3 vertebral fracture treated with a vertebral implant (Kiva device) and PMMA (polymethyl methacrylate; white arrows).
14.3 Appropriateness Criteria
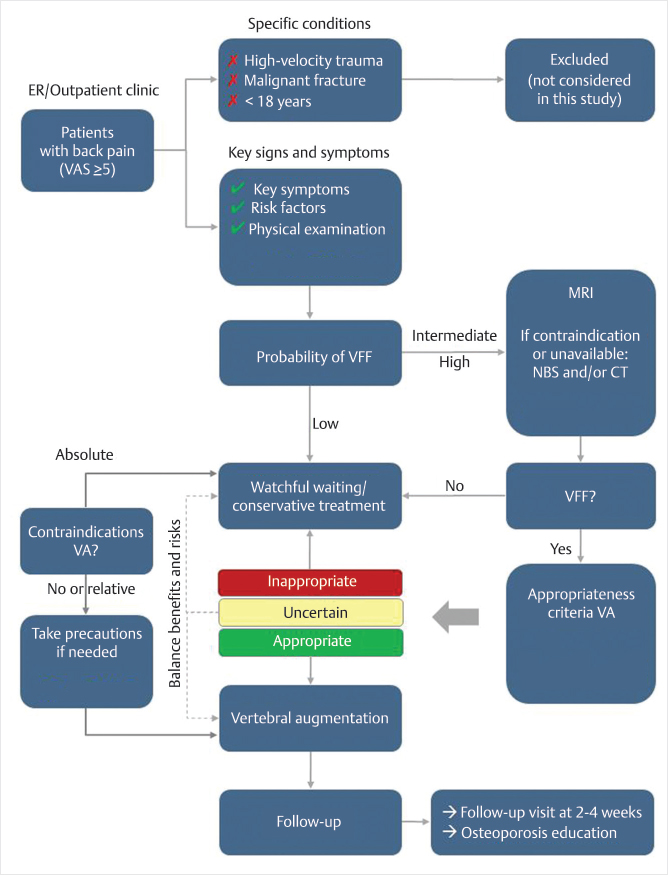
In order to establish a clinical care pathway for VCFs, criteria to be considered include clinical signs and symptoms, imaging criteria for choosing vertebral augmentation techniques over NSM, contraindications for vertebral augmentation, and posttherapy follow-up.16 Decisive variables for performing or not performing vertebral augmentation as well as for selecting one technique over the other include time passed since the fracture occurrence, MR imaging findings including number and type of fractured vertebrae, computed tomography (CT) findings of bone deformity, impact of the fracture on daily functioning, evolution of symptoms, spinal deformity (including kyphotic angle), proof of ongoing fracture process, and pulmonary dysfunction. An international, multispecialty utilization review showed excellent applicability of and good adherence to RAND/UCLA-based recommendations on treatment choice in osteoporotic VCFs. The treatment choice was strongly associated with the clinical variables used in the panel study. Differences in patient characteristics largely determined the different treatment decisions made by the clinicians and time since fracture was the most dominant clinical factor.17 The UCLA/RAND recommendations published in 2018 by a multispecialty group of physicians from the United States found several key factors in determining the clinical appropriateness of vertebral augmentation, but in these recommendations (▶Fig. 14.5) duration of time since fracture occurrence was considered far less relevant for determining the appropriate treatment choice (▶Table. 14.1).16 Overall some of the important clinical factors that determine the treatment choice for the patient are as follows:
Fig. 14.5 Clinical care pathway for the treatment of osteoporotic vertebral compression fractures.16
• The clinical exam should include pain, tenderness on palpation or closed fist percussion, vertebral height reduction and progression of height loss, as well as evolution of symptoms.16
• MR imaging should be the preferred imaging modality for the evaluation of VCFs, although scintigraphy can help indicate the appropriate level. CT can be used for bone fragment identification.
• Timing of the fracture (acute or chronic) should not be confused with the presence of bone edema on the short tau inversion recovery (STIR) sequence of the MR imaging examination. Both acute and chronic fractures can show bone edema (illustrated as increased signal intensity in STIR sequence), which is a sign of bone marrow activity.
• According to the literature, vertebral body height restoration and kyphotic angle correction seem to be better achieved by BKP.18–20 However, the number of vertebrae to be treated is significant for kyphotic angle correction with a proportionally greater improvement in sagittal alignment seen with the treatment of a greater number of vertebral bodies.21
• Standard techniques of vertebral augmentation are effective in treating low-velocity burst fractures but, at the same time, there is an increased risk of secondary in situ fracture.22 Spinal implants along with cement could work as an additional intrinsic support structure providing the extra stability necessary in these cases.
• The impact of the fracture on daily functioning, evolution of symptoms, progression of vertebral body height loss, pulmonary dysfunction, and the degree of spinal and kyphotic deformity are important decision-making factors for performance of vertebral augmentation (▶Fig. 14.6).16 The clinical consequences of kyphosis and vertebral fractures include decreased pulmonary function, decreased appetite with resultant nutritional impact, frailty, and increased future VCF risk, as well as central nervous system symptoms such as increased sensitivity and intolerance to pain and balance problems.23,24
• A cavity containing either vacuum phenomenon or fluid is an indication for vertebral augmentation.
Table 14.1 Key factors in determining the appropriateness of vertebral augmentation*16
Variable |
Value |
p-value |
Duration of pain |
<1 wk†
1–3 wk
3–6 wk
>6 wk |
<0.001 |
Advanced image findings |
Negative†
Positive |
<0.001 |
Impact of vertebral fragility fracture on daily functioning |
Moderate† Severe |
<0.001 |
Degree of height reduction |
Mild (<25%)†
Moderate (25–40%) |
<0.001 |
Kyphotic deformity |
Severe (>40%) |
<0.01 |
Progression of height loss |
No†
Yes |
<0.001 |
Evolution of symptoms |
No†
Yes |
<0.001 |
* Outcomes of logistic regression analysis for the panel outcome that VA = appropriate (vs. NSM = appropriate + equivocal/uncertain).
† Reference category. |
Fig. 14.6 Vertebral augmentation is appropriate in patients with positive imaging findings, with worsening symptoms and in patients with two to four unfavorable factors.
Table 14.2 Absolute and relative contraindications for vertebral augmentation (VA)16
Condition |
Panel recommendation |
Active infection at surgical site |
Absolute contraindication for current VA. |
Untreated blood-borne infection |
Absolute contraindication. Preoperative antibiotic (parenteral) therapy is required. Once cultures are negative, following an appropriate period of antibiotic therapy, one can proceed with caution. |
Osteomyelitis |
Usually a strong contraindication for VA. In rare situations, VA may be considered, for example, if the patient is not stable for an open procedure and the infection is chronic and caused by a less virulent organism. The infection may then be controlled locally with antibiotic-loaded cement and long-term antibiotic suppression. |
Pregnancy |
Although VA is usually contraindicated in pregnant patients, there may be exceptional situations in which benefits could prevail over risks. Radiation exposure to the fetus should be minimized. |
Allergy to fill material |
Relative contraindication, depending on the severity of the allergy. If prior reactions were not associated with severe anaphylaxis, the allergy can be pretreated with steroids, Tylenol, and Benadryl. Alternatively, another fill material can be chosen. |
Coagulopathy |
Relative contraindication. Try to normalize/correct clotting function if possible (INR [international normalized ratio] < 1.7). The risk of bleeding should be balanced against the complications from bed rest. Caution in patients with thrombocytopenia (platelets < 30,000/μL). |
Spinal instability |
Relative contraindication, depending on the degree of instability and level of fracture. If needed, plan an additional intervention to address instability, possibly but not necessarily in the same session. |
Myelopathy from the fracture |
Relative contraindication. Decompression and stabilization is the preferred option, but VA may be considered if the patient is unable to undergo surgery. Coordination with spine surgeon and neurologist is mandatory. |
Neurologic deficit |
Relative contraindication. Additional decompression with or without stabilization may be required. Patients should be informed about the risk of cement in the spinal canal. Coordination with spine surgeon and neurologist is mandatory. |
Neural impingement |
Relative contraindication, depending on the degree. Take extra care to avoid delivery of cement into canal or neural foramen. An additional open procedure may be needed. |
Fracture retropulsion/canal compromise |
Generally not a contraindication. Avoid hyperextension or aggravating stenosis. A CT scan may be used to determine integrity of the posterior wall. |
Table 14.3 Patient follow-up after vertebral augmentation (VA) for Vertebral compression fracture (VCF)
Follow-up after treatment for VCF |
1. |
After either vertebral augmentation (VA) or conservative treatment, a follow-up visit should be planned at 2–4 wk. |
2. |
In patients with a satisfactory result of VA at first follow-up (2–4 wk after the procedure), there is generally no need for further postoperative monitoring. Follow-up for management of the underlying pathology does not need to be managed by the proceduralist. |
3. |
All patients presenting with VCF should be referred for evaluation of bone mineral density and osteoporosis education for subsequent treatment as indicated. |
4. |
All patients with VCF should be instructed to take part in an osteoporosis prevention/treatment program. |
5. |
If symptoms are not resolved at follow-up, repeat imaging (preferably MRI) is mandatory. |
6. |
If the pain is not resolved after VA, repeat augmentation (at the same level) may be considered, but it does require a careful diagnostic evaluation to identify any other sources of pain (additional fractures, facet arthropathy, etc.). |
• Absolute contraindications for vertebral augmentation include infection at the surgical site such as diskitis/osteomyelitis or an untreated blood-borne infection and relative contraindications are listed in ▶Table 14.2.16
• Follow-up evaluation should be performed at 2 to 4 weeks after treatment. Usually no further follow-up for fracture treatment is needed upon a satisfactory result, but the patient will almost always need further treatment for the underlying disorder (i.e., osteoporosis) that produced the fracture (▶Table 14.3).16
A literature meta-analysis comparing VP and kyphoplasty for single-level VCF treatment concludes that both techniques are similar in terms of long-term pain relief, function outcome, and new adjacent fracture rate, but there is superiority with kyphoplasty in terms of injected cement volume, short-term pain relief, improvement in short- and long-term kyphotic angle, and a lower cement leakage rate. This superiority comes at the cost of longer procedural times and higher material expenses.18 Similar results have been reported by Evans et al and Liu et al in two prospective randomized trials.19,20 At the moment, according to the literature, there is no clearly proven superiority of one technique over the other. An alternative approach may be that of modeling detailed treatment algorithms for patients with specific fracture types and clinical characteristics including the variables mentioned in this chapter.
References
[1] Filippiadis DK, Marcia S, Masala S, Deschamps F, Kelekis A. Percutaneous vertebroplasty and kyphoplasty: current status, new developments and old controversies. Cardiovasc Intervent Radiol 2017;40(12):1815–1823
[2] Filippiadis DK, Marcia S, Ryan A, et al. New implant-based technologies in the spine. Cardiovasc Intervent Radiol 2018;41(10):1463–1473
[3] Parreira PCS, Maher CG, Megale RZ, March L, Ferreira ML. An overview of clinical guidelines for the management of vertebral compression fracture: a systematic review. Spine J 2017;17(12):1932–1938
[4] Tsoumakidou G, Too CW, Koch G, et al. CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Intervent Radiol 2017;40(3):331–342
[5] NICE guidance. Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for treating osteoporotic vertebral compression fractures. Technology appraisal guidance. 2013. Available at: nice.org.uk/guidance/ta279
[6] Barr JD, Jensen ME, Hirsch JA, et al; Society of Interventional Radiology. American Association of Neurological Surgeons. Congress of Neurological Surgeons. American College of Radiology. American Society of Neuroradiology. American Society of Spine Radiology. Canadian Interventional Radiology Association. Society of Neurointerventional Surgery. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS). J Vasc Interv Radiol 2014;25(2):171–181
[7] Barr JD, Mathis JM, Barr MS, et al. Standard for the performance of percutaneous vertebroplasty. In: American College of Radiology Standards 2000–2001. Reston, VA: American College of Radiology; 2000:441–448
[8] McGraw JK, Cardella J, Barr JD, et al; SIR Standards of Practice Committee. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol 2003;14(7):827–831
[9] Lewis CA, Barr JD, Cardella JF, et al. Practice Guidelines for the Performance of Percutaneous Vertebroplasty. Reston, VA: American College of Radiology; 2005
[10] McGraw JK, Barr JD, Cardella JF, et al. Practice Guidelines for the Performance of Percutaneous Vertebroplasty. Reston, VA: American College of Radiology; 2009
[11] Esses SI, McGuire R, Jenkins J, et al. The treatment of symptomatic osteoporotic spinal compression fractures. J Am Acad Orthop Surg 2011;19(3):176–182
[12] ACR Appropriateness Criteria. Management of vertebral compression fractures. 2013. Available at: https://acsearch.acr.org/docs/70545/Narrative/
[13] Chandra RV, Meyers PM, Hirsch JA, et al. Society of NeuroInterventional Surgery. Vertebral augmentation: report of the Standards and Guidelines Committee of the Society of NeuroInterventional Surgery. J Neurointerv Surg 2014;6(1):7–15
[14] Wallace AN, Robinson CG, Meyer J, et al. The metastatic spine disease multidisciplinary working group algorithms. Oncologist 2015;20(10):1205–1215
[15] Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol 2017;7(1):4–12
[16] Hirsch JA, Beall DP, Chambers MR, et al. Management of vertebral fragility fractures: a clinical care pathway developed by a multispecialty panel using the RAND/UCLA appropriateness method. Spine J 2018;18(11):2152–2161
[17] Schupfner R, Stoevelaar HJ, Blattert T, et al. Treatment of Osteoporotic Vertebral Compression Fractures: Applicability of Appropriateness Criteria in Clinical Practice. Pain Physician 2016;19(1):E113–E120
[18] Wang H, Sribastav SS, Ye F, et al. Comparison of percutaneous vertebroplasty and balloon kyphoplasty for the treatment of single level vertebral compression fractures: a meta-analysis of the literature. Pain Physician 2015;18(3):209–222
[19] Evans AJ, Kip KE, Brinjikji W, et al. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. J Neurointerv Surg 2016;8(7):756–763
[20] Liu JT, Liao WJ, Tan WC, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int 2010;21(2):359–364
[21] Pradhan BB, Bae HW, Kropf MA, Patel VV, Delamarter RB. Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine 2006;31(4):435–441
[22] Nieuwenhuijse MJ, Putter H, van Erkel AR, Dijkstra PD. New vertebral fractures after percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures: a clustered analysis and the relevance of intradiskal cement leakage. Radiology 2013;266(3):862–870
[23] Silverman SL. The clinical consequences of vertebral compression fracture. Bone 1992;13(Suppl 2):S27–S31
[24] Kado DM, Lui LY, Ensrud KE, Fink HA, Karlamangla AS, Cummings SR; Study of Osteoporotic Fractures. Hyperkyphosis predicts mortality independent of vertebral osteoporosis in older women. Ann Intern Med 2009;150(10):681–687