18 Predisposing Factors to Vertebral Fractures
Olivier Clerk-Lamalice
Summary
The risk factors for vertebral compression fractures (VCFs) include infection, trauma, and cancer but the vast majority of vertebral fractures are due to osteoporosis. There are contributing factors to osteoporosis that are modifiable including excessive alcohol and tobacco use, insufficient weight bearing activity, and low body mass. One of the predisposing fractures that is unmodifiable is a person’s genetic predisposition to osteoporosis. There have been at least fifteen genes that have been confirmed as susceptibility genes and the number could be as high as thirty. After vertebral augmentation it appears that the primary risk factor associated with an additional vertebral fracture is the presence of osteoporosis and the treatment of that underlying disorder is important to limit or prevent additional fractures. Pathologic fractures due to metastatic disease may account for up to 25% of the Medicare patients treated for VCFs and the most common metastases to the spine include breast, kidney, prostate, lung, and, thyroid carcinomas. Given the relatively high prevalence of these lesions, a bone biopsy is indicated in focal lesions or with infiltrated bone marrow. Vertebral fractures due to infection should be treated with antibiotics and the infection eradicated before any additional structural support is provided by vertebral augmentation. The majority of vertebral fractures due to trauma happen in younger and healthier patients with a high energy fall being the most common cause. Most traumatic fractures are of the compression variety and involve the thoracolumbar junction. Traumatic fractures are most often not treated with vertebral augmentation but with the development of implant augmentation these fractures may commonly be treated by percutaneous implant augmentation in the future.
Keywords: vertebral compression fractures, osteoporosis, vertebral augmentation, metastatic disease, spondylodiskitis
18.1 Introduction
Approximately 1.5 million osteoporotic fractures occur each year in the United States, of which more than 50% are VCFs.1 Osteoporosis is responsible for the vast majority of VCFs; however, trauma, infection, and neoplasm are also predisposing factors to fractures. The risk factors of osteoporotic VCFs are categorized as potentially modifiable and nonmodifiable. Nonmodifiable risk factors include being Caucasian of Northern European descent, female gender, advanced age, susceptibility to fall, presence of dementia, and history of fractures in a first-degree relative. On the other hand, potentially modifiable risk factors include estrogen deficiency, alcohol/tobacco use, frailty, impaired eyesight, insufficient physical activity, and low body weight. Pathologic vertebral fractures are surprisingly prevalent, responsible for up to 25% of the reported Medicare VCF volume. These fractures can be treated with vertebral augmentation especially when radiotherapy is considered. Traumatic vertebral fractures are the most prevalent in patients younger than 50 years. The most common causes of accidents responsible for those fractures are high-energy falls, followed by automobile accidents. Although vertebral augmentation has not been routinely adopted in treatment of traumatic VCFs, preliminary reports have concluded that such interventions could be performed in well-selected cases.
18.2 Current Information Based on Recent Literature and State-of-the-Art Practice
18.2.1 Osteoporosis
The World Health Organization has developed a definition of osteoporosis using dual-energy X-ray absorptiometry as a means of defining bone mass. The bone density is compared to the ideal peak bone mineral density (BMD) of a healthy 30-year-old adult. This comparison results in a T-score. A score of 0 means your BMD is equal to the norm for a healthy young adult. The bones are considered normal or healthy with a bone density from +1 to −1 standard deviation (SD). If the measurement reveals a bone mass between −1 and -2.5 SD, the patient is considered to have low bone mass. Individuals with measurements lower than −2.5 SD are considered osteoporotic. In addition, individuals with a T-score lower than −2.5 SD with an osteoporotic fragility fracture are considered to have severe osteoporosis (▶Table 18.1).
The lifetime risk of all types of skeletal fractures for Caucasian women older than 50 years of age approaches 75%. In fact, the lifetime risk of a symptomatic vertebral fracture is 15.6% in white women and 5.0% in white men.2 This fracture risk in postmenopausal woman increases sixfold3 with 25% of additional risk increase in women with a history of prior vertebral fracture in the last 2 years.4,5 Osteoporosis decreases the BMD, disrupts the bone microarchitecture, and alters the contents of noncollagenous proteins in the bone matrix.6 This structural deterioration leads to fragile bones prone to fractures. It is estimated that approximately 44 million Americans have osteoporosis and an additional 34 million Americans have low bone mass.7
Table 18.1 World Health Organization definitions based on bone density levels
Level |
Definition |
Normal |
Bone density is within 1 SD (+1 or −1) of the young adult mean |
Low bone mass |
Bone density is between 1 and 2.5 SD below the young adult mean (–1 to −2.5 SD) |
Osteoporosis |
Bone density is 2.5 SD or more below the young adult mean (–2.5 SD or lower) |
Severe osteoporosis |
Bone density is more than 2.5 SD below the young adult mean, and there have been one or more osteoporotic fractures |
Genetic predisposition also has an important impact on VCF incidence within a studied population. There is indeed a threefold variation in VCF occurrence in Europe with higher rates seen in Scandinavian countries.8 Also, at least 15 genes have been confirmed as susceptibility genes (i.e., RANKL, OPG, RANK, SOST, LRP5) and multiple others (at least 30) have been highlighted as promising genes. Those genes are regrouped in three biological pathways: the OPG/RANK/RANKL pathway, the Wnt/β-catenin pathway, and the estrogen, endocrine pathway.9
The typical risk factors associated with osteoporotic VCFs can be categorized as potentially modifiable and nonmodifiable. Nonmodifiable risk factors include being Caucasian of Northern European descent, female gender, advanced age, susceptibility to fall, presence of dementia, and history of fractures in a first-degree relative. Potentially modifiable risk factors include estrogen deficiency, alcohol/tobacco use, frailty, impaired eyesight, insufficient physical activity, and low body weight. Interestingly, age itself has been found to be a risk factor independent of bone density.6
The fracture risk is multifactorial but highly dependent on the peak bone mass achieved at 30 years of age. However, the peak bone mass is mainly determined by genetic factors, physical activity, endocrine status, nutrition, and health during growth. Health professionals recommend being active prior to this age to maximize the bone peak mass. Indeed, animal model and human studies suggest stress loading that is of high magnitude and is rapidly applied is effective in increasing bone density prior to 30 years of age along with continued regular exercise afterward.10 Also, brief high-impact-jump training increases the BMD in premenopausal women.
The trunk muscles provide static equilibrium and appropriate response to changes in loading and displacement perturbations while ensuring stability of the vertebral column.11 Unfortunately, the current understanding of the muscle/bone interaction in older patients remains limited. Studies report that prevalent VCFs are associated with lower trunk muscle density and/or increased fat accumulation in muscle.12 It is also known that coactivation of antagonistic muscles can increase muscle stiffness and stability; however, this activation also increases spinal loads13,14 and can contribute to VCFs.
After vertebral augmentation, the only risk factor significantly associated with subsequent compression fracture is the presence of osteoporosis (low T-score).15 It has been thought and debated for many years that vertebral augmentation might predispose to adjacent-level fractures. However, meta-analyses demonstrated that there is no increase of adjacent-level fracture in patient treated with vertebral augmentation16 and even a decrease of the incidence of subsequent fractures when comparing the vertebral augmentation arm to the conservative management arm.17 This decrease in adjacent-level fractures is most likely related to corrected segmental kyphosis and decrease of the flexion moment introduced at the level of the functional spinal unit by the VCF.
18.2.2 Pathologic Fractures
The most common malignancies that metastasize to the bone and spine are breast, lung, kidney, prostate, and thyroid carcinomas. Spine metastases are 40 times more prevalent than all primary tumors combined and the vertebral body is 20 times more likely to be involved by a lesion in comparison to the posterior elements (▶Fig. 18.1). As a rule of thumb, most lesions involving the anterior column will be malignant (metastases, myeloma, lymphoma, chordoma) with the exception of hemangiomas and eosinophilic granuloma. On the other hand, lesions involving the posterior arc are typically benign such as osteoid osteoma, osteoblastoma, and aneurysmal bone cyst (benign but locally aggressive).
According to the Medicare data, up to approximately 25% of VCFs are neoplastic in origin.18 Because of this high prevalence, a bone biopsy is indicated in patients with either a focal lesion or evidence of infiltrated bone marrow (characterized by a lower T1-weighted bone marrow signal when using the intervertebral disks or paraspinal muscles as a reference guide; ▶Fig. 18.2).
For cases of pathologic fracture with extraosseous soft-tissue component and/or multiple metastases, multidisciplinary meetings are useful to optimize therapy approaches and patient outcomes. Indeed, discussions between the department of radiation oncology, neurosurgery, and interventional pain management help select candidates that might benefit from vertebral augmentation prior (or after) to radiation therapy. The goal of instilling polymethyl methacrylate (PMMA) in these patients is to provide anterior column support and reducing the risk of additional pathologic fractures related to the weakened trabecular bone due to radiation and tumor infiltration.
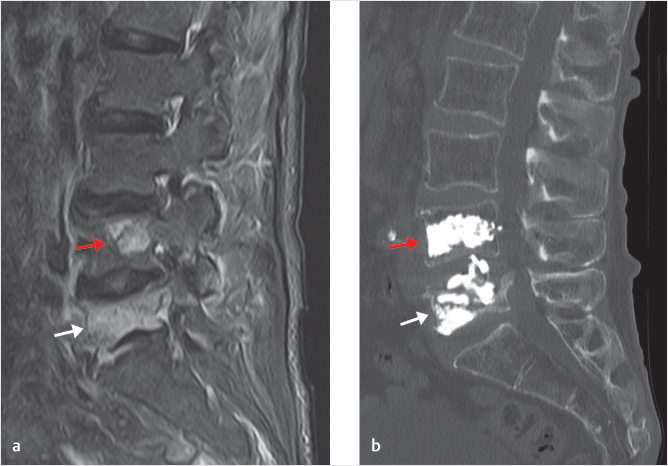
Fig. 18.1 (a) Sagittal STIR (short tau inversion recovery) MR image shows pathologic fractures secondary to prostate adenocarcinoma metastasis at L4 (red arrow) and L5 (white arrow). (b) Sagittal CT scan image after kyphoplasty of L4 and L5 demonstrates adequate PMMA (polymethyl methacrylate) filling of the vertebral bodies.
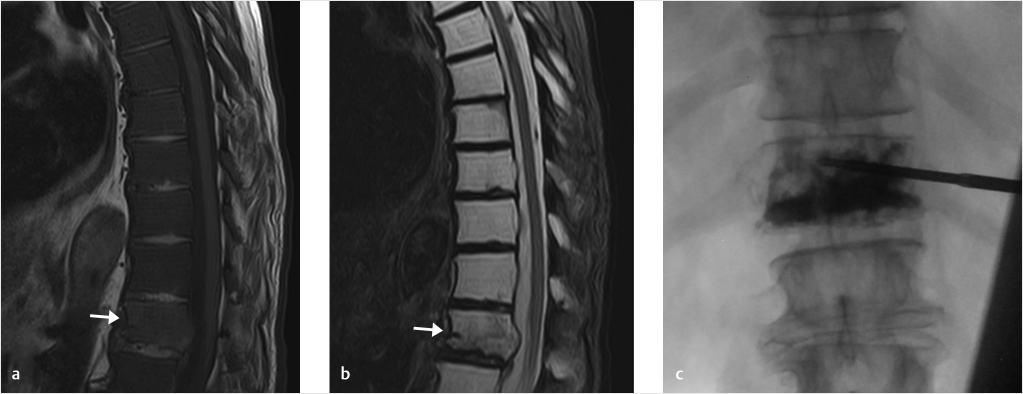
Fig. 18.2 (a) Sagittal T1-weighted sequence of the thoracic spine demonstrates diffuse metastatic infiltration of the bone marrow by a rectal mucinous adenocarcinoma (T1-weighted signal of the marrow significantly lower than the disk). (b) Sagittal T2-weighted sequence. Arrows in (a) and (b) show a T12 pathologic vertebral compression fracture. (c) Fluoroscopic image demonstrates adequate PMMA (polymethyl methacrylate) fill of the vertebral body from pedicle to pedicle and from end plate to end plate.
18.2.3 Fractures Secondary to Infection
VCF can be caused by underlying infection (spondylitis or spondylodiskitis). If an underlying infection is suspected, meticulous evaluation of the patient should be performed to make an adequate diagnosis and avoid catastrophic consequences related to instillation of PMMA within an infected nidus. It is important to recognize pyogenic spondylitis and to treat it accordingly with antibiotics. An infectious process is an absolute contraindication for an augmentation procedure, just like a suspected systemic infection.
The MR signal characteristics can sometimes be similar for both a metastasis and a spondylitis/spondylodiskitis. Both can have hypointense T1-wighted signal and hyperintense T2/short tau inversion recovery (STIR) weighted signal on MRI. However, in 95% of the infectious processes, the disk will be involved (▶Fig. 18.3) and, due to its avascular characteristics, this anatomical location will be affected in only 1% of the neoplasm/metastasis.19 Also, classic radiological findings can be useful to differentiate both entities (▶Table 18.2). The patient history and clinical symptoms are also keys to differentiate both entities. The following points can provide arguments in favor of spondylitis/spondylodiskitis: history of intravenous (IV) drug usage, chronic preexisting disease, prior spinal surgery, and penetrating trauma.
With a suspected spondylitis, laboratory and microbiological tests should also be performed. An elevated C-reactive protein (CRP) will be highly suggestive of infection and will be seen in 90 to 98% of cases.19 Blood sedimentation rate can also be helpful but is less specific than CRP. Elevated white cell count may or may not be present.
Aerobic and anaerobic cultures should also be obtained via blood culture (at least two blood culture pairs should be obtained). However, the pathogen is identified only in 25 to 60% of cases. A biopsy of the disk, end plate, or paravertebral soft tissue has a specificity close to 90% and should be considered whenever possible. Despite the high specificity, needle biopsies of the intervertebral disk may have a sensitivity of only 33%, so a negative culture result after a biopsy should not preclude empiric treatment for a suspected diskitis.20
Most commonly, spondylodiskitis occurs via hematogenous route. Typically, in adults, the bacteria will first affect the end plates and then will extend to the intervertebral disk. Another possible seeding mechanism is via direct extension, for instance in penetrating trauma or infection from instrumentation.
Spondylodiskitis is overwhelmingly caused by Staphylococcus aureus (50–70% of cases) in developed countries.21,22 However, tuberculous spondylodiskitis, most commonly resulting from hematogenous spread, is the most common type worldwide.
Other gram-positive and gram-negative bacteria are responsible for spondylodiskitis such as the following: Enterobacteriaceae species such as E. coli (e.g., patients with active urinary tract infections); P. aeruginosa (e.g., patients with a history of IV drug abuse); Brucellosis (e.g., patients living in Mediterranean countries and in the Middle East); less commonly Streptococcus pneumoniae (e.g., patients with diabetes); and more rarely Salmonella species (e.g., patients with sickle cell disease or asplenia). Brucella, fungi, and parasites such as hydatid disease are other possible infectious pathogens, but are rarely seen.
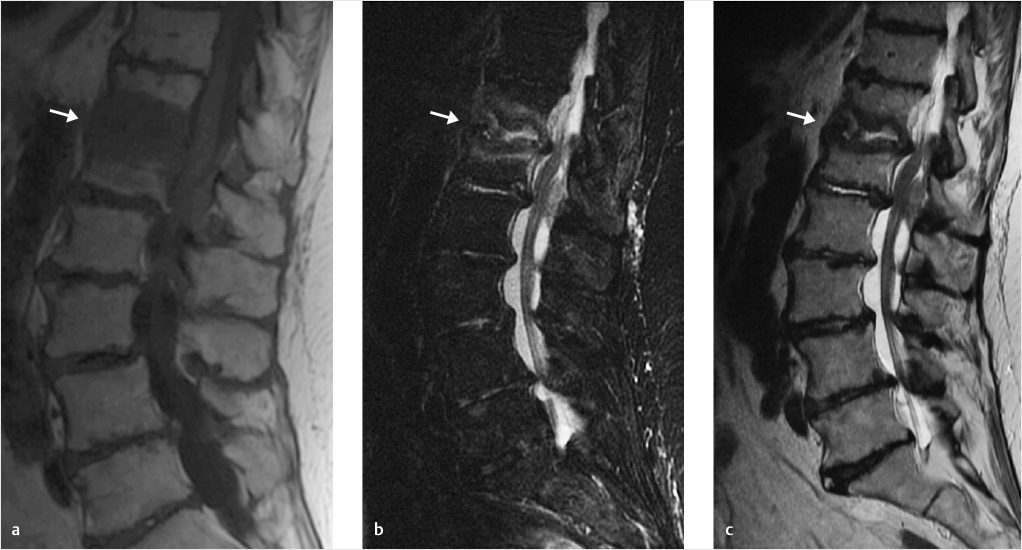
Fig. 18.3 Classic case of T12–L1 pyogenic spondylodiskitis. (a) T1-weighted, (b) STIR (short tau inversion recovery), and (c) T2-weighted sequences demonstrate a T1 hypointense focus with central T2 fluidlike signal centered on the disk space (white arrows).
Table 18.2 Radiological findings differentiating spondylitis/spondylodiskitis of metastases
MRI findings |
In favor of spondylitis/spondylodiskitis |
In favor of metastases |
Hyperintense T2/STIR signal |
X |
X |
Hypointense T1 signal |
X |
X |
Disk involvement |
X |
|
End plate erosion |
X |
|
Paravertebral/psoas/epidural inflammation or abscess |
X |
|
|
18.2.4 Traumatic Fractures
Traumatic spine fractures are more prevalent in the males, with a male-to-female ratio of 1.6:1.23 In comparison to osteoporotic fractures that virtually happen all after 60 years of age, the majority of patients with traumatic vertebral fracture are between the age of 20 and 50 years. The most common cause of fracture is a high-energy fall (40%), followed by traffic accidents (25%). The fractures related to falls can happen anywhere along the spine, while fractures related to motor vehicle accident preferentially affect the thoracic and cervical spine. The majority of fractures, however, happen in the thoracolumbar region (L1: 29%; T12: 14%; L2: 12%).23
This high prevalence of fractures along the thoracolumbar region can be explained with underlying biomechanical reasons. The mid to upper thoracic region (T1–T8) is stabilized by the ribs. At T9–L2, there is a transition between the rigid/kyphotic midthoracic region and the flexible lordotic lumbar spine. Also, the positions of the center of gravity of the thoracic and lumbar spine are located completely opposite from one to the other; in the upper and midthoracic spine, the center of gravity is located anteriorly to the spine, while in the lumbar spine the center of gravity is located posteriorly. For these reasons, the thoracolumbar transitional region is susceptible to traumatic fractures.
The majority of traumatic spine fractures will be of compressive origin (55%); however, distraction (17%), rotation (18%), and fracture-dislocation (20%) are also prevalent mechanisms. Indeed, most of the fractures of the thoracic and lumbar region that have failure under axial compression without injury to the posterior ligamentous complex (PLC) are classified as Magerl or AO type A fractures. To date, only few studies have evaluated the use of vertebroplasty to treat traumatic vertebral fractures. This is mainly due to the younger age of the patients with traumatic fractures, uncertainties regarding the biomechanics alteration after PMMA cement instillation, and possible risk of cement leakage.24 However, as discussed in prior chapters, these concerns have been addressed in multiple publications.
Our expectation is that more cases of vertebral augmentation will be performed in patients with vertebral traumatic fracture. However, it is worth noting that some of those cases require advanced expertise, and consultation between colleagues would be considered good practice, especially when younger patients are being treated. In the upcoming years, percutaneous implants will have an important role to play in traumatic vertebral fractures, both by ensuring an optimal correction of the kyphotic angle and by providing for decreased cement leakage.
18.3 Key Points
• VCFs are extremely prevalent, affecting close to 25% of the population of our society.
• Osteoporosis is responsible for the vast majority of VCFs; however, trauma, infection, and neoplasm are other predisposing factors to consider.
• Genetic predisposition has a significant impact on osteoporosis and VCF incidence. At least 15 genes have been confirmed as susceptibility genes.
• After vertebral augmentation, the only risk factor significantly associated with subsequent compression fracture is the presence of osteoporosis (low T-score).
• Spine metastases are 40 times more prevalent than all primary tumors.
• Spondylodiskitis is overwhelmingly caused by S. aureus (50–70% of cases) in developed countries
• Traumatic vertebral fractures happen in younger patients. The most common location of these fractures is at T9–L2 because of the transition between the rigid/kyphotic midthoracic region and the flexible lordotic lumbar spine.
References
[1] Melton LJ III, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res 1997;12(1):16–23
[2] Melton LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL. How many women have osteoporosis? JBMR Anniversary Classic. JBMR, Volume 7, Number 9, 1992. J Bone Miner Res 2005;20(5):886–892
[3] Lane JM, Nydick M. Osteoporosis: current modes of prevention and treatment. J Am Acad Orthop Surg 1999;7(1):19–31
[4] Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285(3):320–323
[5] Roux C, Fechtenbaum J, Kolta S, Briot K, Girard M. Mild prevalent and incident vertebral fractures are risk factors for new fractures. Osteoporos Int 2007;18(12):1617–1624
[6] Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 2006;6(5):479–487
[7] Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166(7):514–530
[8] O’Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res 1996;11(7):1010–1018
[9] Ralston SH. Genetics of osteoporosis. Proc Nutr Soc 2007;66(2):158–165
[10] Bailey CA, Brooke-Wavell K. Exercise for optimising peak bone mass in women. Proc Nutr Soc 2008;67(1):9–18
[11] Stokes IA, Gardner-Morse M, Henry SM, Badger GJ. Decrease in trunk muscular response to perturbation with preactivation of lumbar spinal musculature. Spine 2000;25(15):1957–1964
[12] Mokhtarzadeh H, Anderson DE. The role of trunk musculature in osteoporotic vertebral fractures: implications for prediction, prevention, and management. Curr Osteoporos Rep 2016;14(3):67–76
[13] Granata KP, Marras WS. The influence of trunk muscle coactivity on dynamic spinal loads. Spine 1995;20(8):913–919
[14] Gardner-Morse MG, Stokes IA. The effects of abdominal muscle coactivation on lumbar spine stability. Spine 1998;23(1):86–91, discussion 91–92
[15] Lu K, Liang C-L, Hsieh C-H, Tsai Y-D, Chen H-J, Liliang P-C. Risk factors of subsequent vertebral compression fractures after vertebroplasty. Pain Med 2012;13(3):376–382
[16] Fan B, Wei Z, Zhou X, et al. Does vertebral augmentation lead to an increasing incidence of adjacent vertebral failure? A systematic review and meta-analysis. Int J Surg 2016;36(Pt A):369–376
[17] Papanastassiou ID, Phillips FM, Van Meirhaeghe J, et al. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J 2012;21(9):1826–1843
[18] Curtis JR, Taylor AJ, Matthews RS, et al. “Pathologic” fractures: should these be included in epidemiologic studies of osteoporotic fractures? Osteoporos Int 2009;20(11):1969–1972
[19] Herren C, Jung N, Pishnamaz M, Breuninger M, Siewe J, Sobottke R. Spondylodiscitis: diagnosis and treatment options. Dtsch Arztebl Int 2017;114(51–52):875–882
[20] Nam KH, Song GS, Han IH, Choi BK, Cha SH. Diagnostic value of biopsy techniques in lumbar spondylodiscitis: percutaneous needle biopsy and open biopsy. Korean J Spine 2011;8(4):267–271
[21] Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med 1970;282(4):198–206
[22] Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J 2014;14(2):326–330
[23] Leucht P, Fischer K, Muhr G, Mueller EJ. Epidemiology of traumatic spine fractures. Injury 2009;40(2):166–172
[24] Knavel EM, Thielen KR, Kallmes DF. Vertebroplasty for the treatment of traumatic nonosteoporotic compression fractures. AJNR Am J Neuroradiol 2009;30(2):323–327