29 Treatment of Neoplastic Vertebral Compression Fractures
Kyung-Hoon Kim
Summary
The spine is the most frequently involved bony structure in cancer metastasis, the order of frequency being the thoracic (70%), lumbosacral (20%), and cervical (10%) spine. The spine metastasis usually originates from a cancer of the adjacent organs: the thoracic spine from the lung and breast; the lumbosacral spine from the colon, prostate, urinary bladder, and uterine cervix; the cervical spine from the thyroid or lung.
Diagnosis is rendered by a history of weight-bearing pain, tenderness in the supraspinous area with or without adjacent facet area tenderness on physical examination, loss of a pedicle on a plain film x-ray with or without vertebral height loss on the lateral view, active bone lesions on bone scans, and posterior wall destruction of the compressed vertebral body on computed tomography (CT) or magnetic resonance imaging (MRI).
Treatment for painful osteolytic and osteoblastic metastatic fractures, such as percutaneous vertebroplasty (PVP) or kyphoplasty, may start with performing facet-joint injections to determine the exact painful level in patients with multilevel spine metastases. This is done with the patient in the prone position with care taken to minimize facet-mediated pain. The levels are localized so as to minimize obscuration of the underlying anatomy by the potentially overlying radio-opaque bone cement.
A vertebral needle should be placed at the anterior one-third to one-fourth of the vertebral body to prevent leakage of bone cement into the anterior epidural space through the damaged posterior wall of the vertebral body. Injecting bone cement equal to 20 to 25% of the volume of the vertebral body is sufficient.
The mechanism of pain relief is not only augmentation by bone cement that provides pain relief by stabilizing the vertebral body but also denervation (by both thermal and chemical means) of basivertebral nerve branches that carry pain from the end plates through the vertebral. If leakage of bone cement is avoided, the result of PVP is similar to that of percutaneous kyphoplasty.
PVP for the treatment of painful metastatic osteolytic or osteoblastic compression fractures provides immediate pain relief and allows for early ambulation.
Keywords: augmentation, bone cement, denervation, kyphoplasty, neoplasm, metastasis, spine, vertebroplasty, zygapophyseal joint
29.1 Introduction
Percutaneous vertebroplasty (PVP) was first reported for the treatment of painful benign vertebral angioma in 1987.1 The procedure has been widely used to treat painful osteoporotic compression fractures, commonly in the thoracolumbar junction.
Increased life expectancy after cancer diagnoses and a higher prevalence of bony metastases has increased the number of cases of painful metastatic vertebral compression fractures (VCFs). As with osteoporosis, metastatic vertebral fractures mainly occur in the thoracic spine, followed in frequency by the lumbosacral and cervical spines.2
Spinal metastases usually develop from cancers of the adjacent organs, and most common types of spinal metastases (60%) originate from breast, lung, or prostate, and far less frequently from the kidney, urinary bladder, and thyroid.2 The segment of the spine involved with metastatic disease is usually the portion of the spine nearest to the affected organ. For example, the thoracic spine is usually affected by cancers of the lung and breast; the lumbosacral spine by cancers from the colon, prostate, urinary bladder, and uterine cervix; the cervical spine by cancers from the thyroid or lung.
Spinal metastases from breast cancer are common in women in their forties and fifties but spinal metastases from lung cancer are more common in men in their sixties and seventies. About 10% of all cancers eventually metastasize to the spine.2
29.2 Diagnosis
29.2.1 History
Patients who have painful metastatic osteolytic or osteoblastic fractures are more frequently referred from the oncology department, rather than from a pain or spine clinic. In patients with spine metastases, it is easier to find the metastasis to the spine if there are red flags, such as recent sudden weight loss, combined motor deficits, or if the patient is less than 40 years old and has severe unremitting back pain.
On the contrary, in cases referred by way of findings on cross-sectional imaging usually come to the physician’s attention after an emergency room visit or following a scan obtained after surgery, chemotherapy, or radiotherapy. The typical complaints are of sudden and severe back pain that is exacerbated with the patient changing position or in transition. Many times the patient is debilitated to the point where they are unable to sit and stand by themselves and are relegated to lying on their back.
29.2.2 Physical Examination
It is important to check combined motor deficits in a supine position and it is especially important for both doctors and patients to recognize existing motor deficits that may be present prior to the procedure. After discovery of any motor deficits, the doctor should inform the patient as to their presence. The motor deficits may eventually progress, whether or not the PVP is performed and it is optimal to document this prior to performing any intervention. The initial examination must include palpation of the paraspinal region and overlying the posterior superior iliac spine keeping in mind that it may not be easy to put the patient in the prone in order to be able to examine them.3
If the metastatic vertebral fractures are combined with diskogenic back pain or facet-joint pain, it may be very difficult to determine the exact region that is producing the pain. In this case it may be better to perform intradiskal or facet-joint injections prior to the PVP to be able to further localize the pain generator. The merits of performing injections prior to the PVP are (1) after relieving pain from the painful disk or facet joint, the residual presence of supraspinous tenderness has a value in choosing the correct level for the PVP among the multiple metastatic levels, (2) after relieving the patients’ degenerative back pain, they can lie in a prone position and better cooperate with the augmentation procedure, and (3) if the PVP is performed prior to the injections, the cement may obscure the injection targets, making it difficult to perform the appropriate injections.3
29.2.3 Imaging Diagnosis
• Plain film X-rays: It is difficult to diagnose neoplastic VCFs until they are prominent or even severe. However, if there is a loss of the pedicle on the anteroposterior view, this provides strong evidence of spinal metastasis. This is in contradistinction to a vertebra with intact pedicles but a compressed vertebral body. This type of compression fracture can be difficult to determine whether the fracture is a result of osteoporosis or tumor (▶Fig. 29.1).
• Bone scans: Nuclear medicine bone scanning tends to be an optimal diagnostic tool if the lesions detected are in the posterior wall of the vertebral body or pedicle. In the sacrum and pelvis, a signature sign of a benign sacral insufficient fracture is classically seen as a ‘Honda sign’ on bone scan, composed of vertically oriented fractures primarily through S1 and S2 vertebral bodies and a horizontally oriented fracture through S2 (▶Fig. 29.2a).
• Computed tomography (CT): It is helpful to reveal whether the posterior wall of the vertebral body is intact or not and where the exact fracture lines in the vertebrae are located.
• Positron emission computed tomography (PET/CT): Is effective in evaluating the status of both the primary focus of the cancer and spinal metastasis and when combined with CT or MRI scanning can be highly accurate at localizing the exact anatomic location of the neoplastic involvement.
• Magnetic resonance imaging (MRI): The soft tissues such as the intervertebral disks, spinal cord, cauda equina, and dorsal nerve root ganglion are seen clearly, as well as other areas of interest such as the anterior epidural space and the cerebral spinal fluid. The extent of the tumor or metastasis involvement is typically well characterized by MRI.
29.2.4 Valuable Scoring Systems for Evaluation of the Patients with Metastatic Bone Lesions
• Karnofsky performance status (KPS) scale (▶Table 29.1): It is very helpful to evaluate patients’ physical performance status before and after PVP. If the score is 50% or higher, patients can be discharged from the hospital.4
• Tokuhashi revised scoring system for preoperative evaluation of metastatic spine tumor prognosis (▶Table 29.2): It is important to predict the prognosis of a metastatic spine tumor before the PVP.5
29.3 Treatment
29.3.1 Thoracic and Lumbar Vertebrae
In a typical vertebra of the thoracic or lumbar spine, a transpedicular approach is a common method for accessing the painful vertebral bodies. However, if there is metastatic destruction on one side of the pedicle, it may be difficult to choose between accessing the involved or uninvolved pedicle and deciding between a transpedicular, peripedicular, or extrapedicular approach.
In most of the cases, if the needle can be placed in the anterior one-third or one-fourth of the vertebral body there is confidence that leakage into the anterior epidural space can be avoided. It is okay to access the vertebral body through an involved pedicle and not necessary to have bone cement completely filling the entire anteroposterior extent of the vertebral body. Pain relief by PVP is obtained not only by augmentation of the structure of the vertebral body, but also by thermal and chemical ablation of the ingrown nerves and in-dwelling tumor.
29.3.2 Sacrum
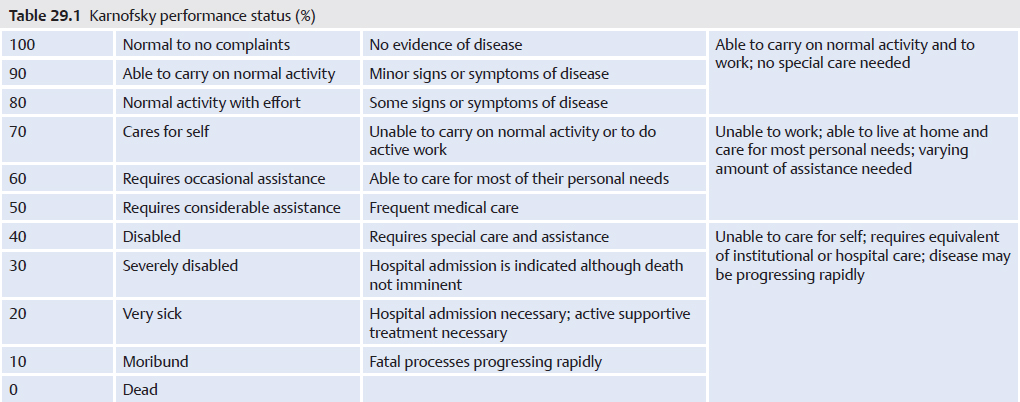
The sacral body and sacral ala are connected but are separate anatomic structures (▶Fig. 29.2b). It is not difficult, however, to place the needle(s) into both anatomic locations during one sacroplasty session and to fill two to three or more adjacent levels of the sacral ala with bone cement at one time, due to the fused structure of the sacral segments (▶Fig. 29.2c).
Fig. 29.1 (a) Osteolytic metastatic fracture at T11. The right pedicle cannot be seen, but the height of the vertebral body is preserved. (b) Osteoporotic compression fracture at T11. Both pedicles can be seen clearly, but the height of the body is reduced.

Fig. 29.2 Sacral insufficiency fractures. (a) A “Honda sign,” which is composed of the fractured sacral body with the two vertically oriented fracture lines through the sacral ala, is apparent in the posterior view on the nuclear medicine bone scan. (b) A needle (black arrowheads) is positioned through the patient’s left sacroiliac joint into the left sacral ala after having been placed through the sacral body and into the right sacral ala where bone cement was injected (white arrowheads) to treat the patients’ sacral fractures. (c) Anteroposterior (left image) and lateral (right image) views show needles placed into the sacral ala (white arrows). A single needle is placed into the center of the sacral body (black arrow) from an inferior position. The lateral view shows the needles entering the sacrum at the mid-portion of S3 (white arrowhead) and extending to the superior portion of S1 (black arrowhead). (d) Anteroposterior (left) and lateral (right) views show bone cement injected into the sacral ala and sacral body (black arrows). (e) Anteroposterior (left) and lateral (right) fluoroscopic images show a 22-gauge needle (black arrows) entering the sacroiliac joint. The contrast within the joint is best seen on the anteroposterior view (within the circle on the AP view).
When performing sacral augmentation, caution should be taken not to pierce or penetrate the sacral foramina when the needle is advanced into the sacral body or ala. A safe insertion of a needle into the sacral ala involves placing it halfway between the lateral border of the sacral foramina and the medial border of the sacroiliac joint. If the sacral foramina are difficult to visualize, contrast can be injected into the epidural space via the sacral hiatus to highlight the foramina. A sacroiliac joint injection may also be performed to outline the anatomy of the sacroiliac joint (▶Fig. 29.2e).
Table 29.2 Tokuhashi revised scoring system for preoperative evaluation of metastatic spine tumor prognosis
Characteristic |
Score |
1. General condition (performance scale) |
Poor (10–40%) |
0 |
Moderate (50–70%) |
1 |
Good (80–100%) |
2 |
2. Number of extraspinal bone metastases foci |
≥3 |
0 |
1–2 |
1 |
0 |
2 |
3. Number of metastases in the vertebral body |
≥3 |
0 |
1–2 |
1 |
0 |
2 |
4. Metastases to the major internal organs |
Lung, osteosarcoma, stomach, bladder, esophagus, pancreas |
0 |
Liver, gall bladder, unidentified |
1 |
Others |
2 |
Kidney, uterus |
3 |
Rectum |
4 |
Thyroid, breast, prostate, carcinoid tumor |
5 |
5. Palsy |
Complete (Frankel A, B) |
0 |
Incomplete (Frankel C, D) |
1 |
None (Frankel E) |
2 |
Criteria of predicted prognosis |
Total score 0–8 < 6 months |
|
Total score 9–11 ≥ 6 months |
|
Total score 12–15 ≥ 1 year |
|
29.3.3 Cervical vertebrae
C1 Vertebra (The Atlas)
The atlas or C1 vertebra is composed of the anterior arch, posterior arch, lateral masses with superior and inferior articular facets, vertebral foramen, and transverse processes with the transverse foramina. The superior facets of the lateral masses become weight-bearing units articulating at the occiput of the head. When the structural integrity of the lateral masses is compromised, cement augmentation may be performed to re-establish the weight-bearing capability of the lateral masses of the atlas.
Cement augmentation on atypical vertebrae, including C1 and C2, should be performed while paying appropriate attention to avoid injury to the internal carotid artery, internal jugular vein, vertebral artery, and nerve roots (▶Fig. 29.3). An ultrasound guided or a manual compression anterolateral approach technique with or without using a blocking needle provides safe guidance for a larger gauge vertebral needle.
C2 Vertebra (The Axis)
The other atypical cervical vertebra, the C2, or atlas, also has specific anatomic features. The anterior structures include the vertebral body and dens (odontoid process) and lateral masses with superior and inferior articular facets. Laterally there are transverse processes with foramina called the foramina transversarium. Posteriorly the structures include the laminae and spinous process.
The odontoid process of the axis is one of the more commonly injured vertebral structures. There are three types of the C2 fractures: type I (an oblique fracture through the upper part of the dens) which is least common type of dens injury and has the potential to be unstable; type II (a fracture across the base of the dens at the dens-body junction) which is unstable and has a high risk of non-union; type III (a fracture through the dens that extends into the vertebral body and lateral masses). The type III dens fracture has the best prognosis for healing.6 Metastatic osteolytic fractures at C2 show similar patterns to the three types of fractures from trauma.
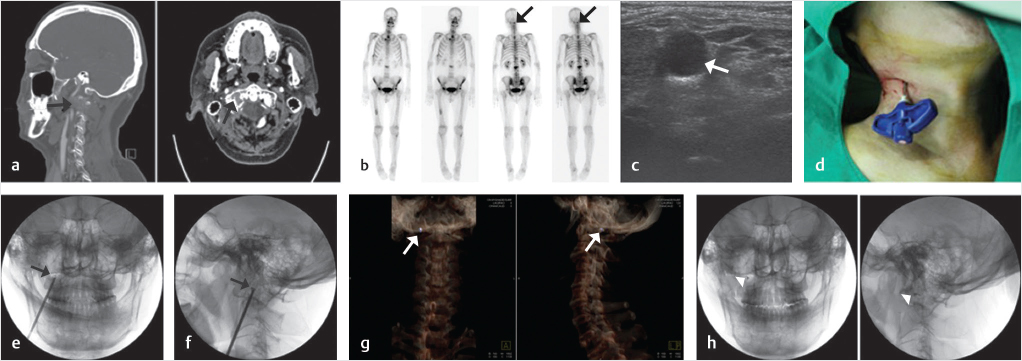
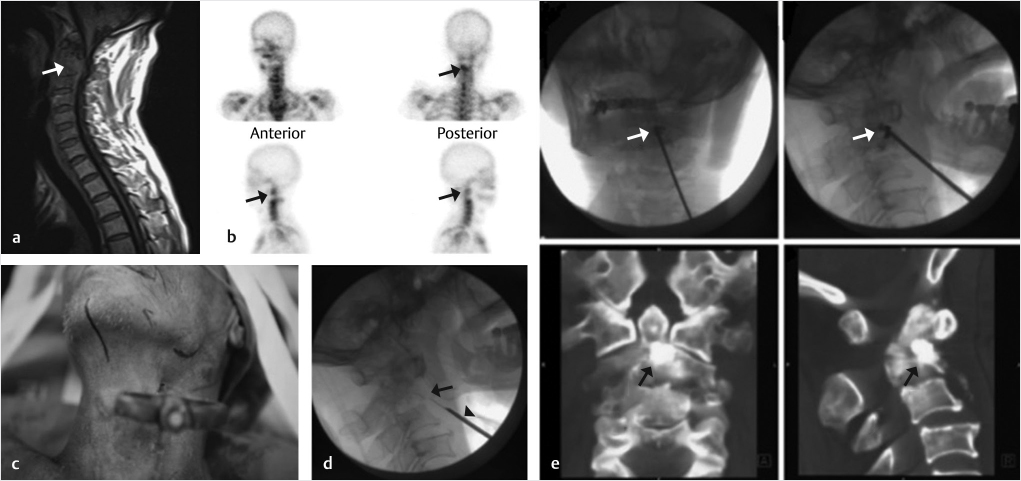
Fig. 29.3 Percutaneous osteoplasty (POP) of the right lateral mass of the atlas (C1). (a) An osteolytic lesion is present in the right lateral mass as seen on sagittal reconstruction and axial computed tomography (CT) images (black arrows). (b) Nuclear medicine bone scan images show an active lesion in the location of the right lateral mass. (c) An ultrasound-guided approach can reduce the risk of piercing the major vessels and nerves in the neck. An axial ultrasound image shows the internal carotid neurovascular bundle with the internal jugular vein as the most prominent hypoechoic structure (white arrow). (d) A vertebral needle is placed into the right lateral mass from a right paravertebral and submental approach. (e) AP and (f) lateral fluoroscopic images show the trajectory of the needle and the needle tip within the right lateral mass of C1 (black arrows). (g) Three-dimensional CT reconstruction images show a bright purple-colored bone cement located in the destroyed right lateral mass of C1 (white arrows). (h) The cement is seen as a black dot on AP and lateral fluoroscopic images (white arrowheads).
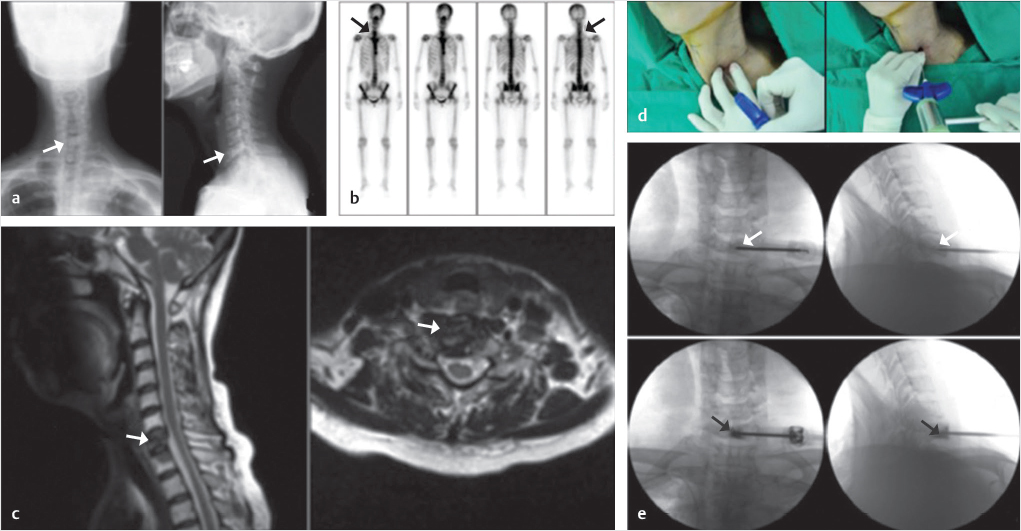
Fig. 29.4 Percutaneous osteoplasty (POP) in the dens and body of the axis. (a) Sagittal T1-weighted image shows an osteolytic lesion in the left dens and body (white arrow). (b) Anteroposterior, posteroanterior, and oblique views of a nuclear medicine bone scan shows an active lesion in the body and dens of C2 (black arrows). (c) A vertebral needle with an anteromedial approach is inserted into the body and dens of the C2 vertebrae (black arrow in d) with the guidance of a blocking needle (black arrowhead in d). (e) Anteroposterior and lateral fluoroscopic views show a needle injecting bone cement into the junction between the dens and body of C2 (white arrows on the upper images). Anteroposterior and lateral CT images demonstrate the cement at the dens/body junction (black arrows on the lower images).
As with C1, percutaneous osteoplasty (POP) of C2 can be performed with fluoroscopic or ultrasound guidance approach and the body of C2 may be accessed via anterolateral, posterolateral, or transoral approaches (▶Fig. 29.4).7
Typical Vertebrae (C3–C7)
The anatomic components of the typical cervical vertebrae from C3 to C7 consist of the vertebral body with the uncinate processes, the transverse processes with the foramen transversarium (for the vertebral artery), superior and inferior articular facets, the vertebral arch composed of the pedicles and lamina, and the spinous process.
In an anterior approach to the vertebral body it is important to avoid damage to the tracheoesophageal complex, internal carotid artery, internal jugular vein, spinal cord, dorsal nerve root ganglion, and spinal nerves. A vertebral needle may be inserted into the cervical vertebral body using an anteromedial approach by pushing aside the tracheoesophageal complex and major vessels with one’s second and third fingers. The needle should be placed in the center or anterior one-third of the vertebral body (▶Fig. 29.5).8
Fig. 29.5 Percutaneous vertebroplasty (PVP) at C7. (a) An osteoblastic lesion is observed in a minimally compressed C7 vertebral body on the anteroposterior and lateral X-ray views of the cervical spine (white arrows). (b) The nuclear medicine bone scan shows an active lesion in the C7 vertebral body (black arrows). (c) The metastatic lesion is seen on sagittal and axial T2-weighted MR images (white arrows). (d) A vertebral needle, using an anteromedial approach, is inserted into the vertebral body while pushing aside the tracheoesophageal complex and major vessels with the second and third fingers (left image). (e) The vertebral needle is placed into the center and anterior portion of the C7 vertebrae (white arrows on the upper AP and lateral fluoroscopic images) followed by injection of bone cement into the vertebral body (black arrows on the lower AP and lateral fluoroscopic images).
Extraspinal Percutaneous Osteoplasty (POP)
PVP is performed on the vertebral body whereas POP is a general term that describes osteoplasty with the injection of bone cement into various types of osseous structures including flat bones, long bones, and extravertebral axial skeletal bones.9 Although the most frequent site of bony metastasis is known to be the spine and vertebroplasty is commonly performed in painful osteolytic or osteoblastic metastases or VCFs associated with osseous metastases, the treatment for painful extraspinal bony metastasis is also a challenging field of palliative care that can involve osteoplasty of the affected area.
There are precautions to be taken when inserting a needle into the targeted bone to avoid predictable complications. The most important thing is to choose an appropriately sized needle with a suitable bevel, in order to be able to direct the needle to the correct position while avoiding over-penetrating or falling short of the target area. It is also important to avoid major vessels and nerves. Additionally, it is better to choose the shortest available route to the targeted bone, which can reduce patient pain and procedure time.
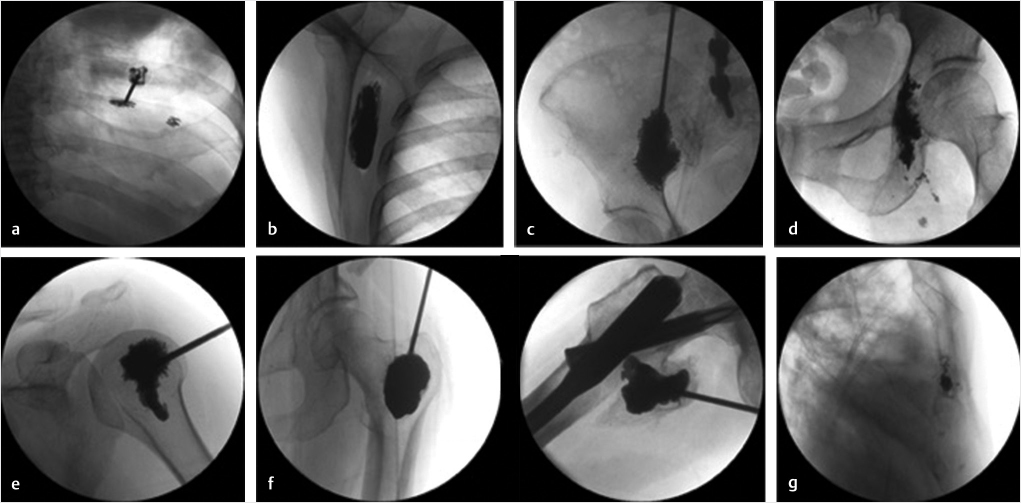
The most common sites for the origin of the metastatic disease, in order of decreasing frequency, are the lung, liver, breast, colon, and kidney.10 The sites where extraspinal POP is most frequently performed include the rib, scapula, ilium, humeral head, ischium, femur, and sternum, in that order (▶Fig. 29.6). Injections into the joints adjacent to the extraspinal POP may also be necessary.11,12
29.4 Controversies
29.4.1 Vertebroplasty versus Kyphoplasty
• Even though the merits of the reduced risk of cement leakage using balloon kyphoplasty for the treatment of osteolytic metastatic compression fracture has been much emphasized,13 augmentation using only a vertebral needle may be sufficient because (1) the target point of cement insertion is the anterior one-third or one-fourth of the vertebral body, (2) the insertion of slices of gelatin sponge after detection of leakage using an injection of a contrast medium prior to cement injection prevents cement leakage into the disrupted posterior wall of the vertebral body, and (3) vertebral body cement fill equal to 15 to 25% of the thoracolumbar vertebrae is a sufficient amount to treat the pain from the neoplasm as well as to re-establish the strength and stiffness of the vertebral body.14–16
• On the contrary, if not performed correctly with incorporation of cement into the surrounding interstices of the bone and/or neoplasm, balloon kyphoplasty sometimes produces a separated cement ball within the void. It may also be difficult to expand the balloon in case of an osteoblastic lesion from prostate, liver, or breast cancers. Displacement of metastatic foci may also theoretically contribute to tumor spread into the adjacent tissue during expansion of the inflatable bone tamp.17
Fig. 29.6 Extraspinal percutaneous osteoplasties (POPs). (a) Cosoplasty, (b) scapuloplasty, (c) ilioplasty, (d) ischioplasty, (e) humeroplasty, (f) femoroplasty (greater trochanter and lesser trochanter), and (g) sternoplasty.
29.4.2 Increased Risk of VCFs after Radiotherapy
• Even though an increased risk of VCFs, ranging from 11 to 39%, after radiotherapy has been observed,18,19 radiotherapy along with PVP is a standard regimen for painful metastatic vertebrae. It is supposed that radiation damages the (organic) collagen rather than mineral (hydroxyapatite) components, and produces osteoradionecrosis. In cases using ≥20 Gy/fraction in patients with a lytic tumor or spinal misalignment and a baseline VCF, caution should be used.19
• The spinal instability neoplastic score (SINS) (▶Table 29.3)18–20 was developed for assessing patients with spinal neoplasia and it identifies patients who may be at risk for spinal instability and would therefore benefit from surgical intervention to stabilize the spine. This scoring system is an objective measurement tool to predict the degree of stability of the metastatic spine.
29.5 Conclusion
The spine is the most common osseous structure for bony metastases. The thoracic vertebrae are the most common site of metastatic disease, followed by the lumbosacral and cervical vertebrae.
A common feature of painful metastatic compression fractures is the presence of intractable pain when the patient is in a weight-bearing position or transitional pain when changing positions from standing to sitting or sitting to laying down. Access to diagnostic imaging modalities such as conventional X-rays, bone scans, CT, PET-CT, and MRI is essential.
Vertebral body cement fill equal to 15 to 25% of the thoracolumbar vertebrae (approximately 3.5–6.0 mL) is a sufficient amount to treat the pain from the neoplasm as well as to reestablish the strength and stiffness of the vertebral body.14,15 Pain relief from PVP originates from not only the stabilization provided by the augmentation but also the thermal and chemical ablation from the polymethyl methacrylate (PMMA). Due to its strength, resistance to compression, and exothermic reaction, PMMA is preferred to calcium phosphate for painful metastatic compression fractures.
It is important when treating the cervical and sacral vertebrae to understand their anatomy and adjacent major neurovascular structures. Technical accuracy and knowledge of important surrounding structures are essential when performing cervical PVP, especially at C1 and C2, to avoid unnecessary complications or injury to the cervical neurovascular structures. Cervical procedures may require multiple imaging modalities for guidance, including ultrasound, fluoroscopy, and CT.
In conclusion, PVP provides immediate and durable pain relief in patients with painful metastatic VCFs. The pain relief from stabilizing the vertebral body is substantial and cannot be provided by analgesics, including opioids and nonsteroidal anti-inflammatory drugs. Medications rendered unnecessary by PVP, especially sensorium altering medications, may be reduced or eliminated following a successful vertebroplasty.
Table 29.3 Spinal instability neoplastic score (SINS)
1. Patient specific |
Pain |
Mechanical pain |
3 |
Occasional pain but not mechanical |
1 |
Pain free |
0 |
2. Spine specific |
(1) Location |
Junctional spine: occiput-C2, C7–T2, T11–L1, L5–S1 |
3 |
Mobile spine: C3–C6 |
2 |
Semi-rigid spine: T3–T10 |
1 |
Rigid spine: S2–S5 |
0 |
(2) Spinal alignment |
Subluxation/translation |
4 |
Kyphosis/scoliosis |
2 |
Normal |
0 |
(3) Presence of vertebral compression fracture |
≥ 50% collapse |
3 |
< 50% collapse |
2 |
No collapse with ≥ 50% body involved |
1 |
None of the above |
0 |
3. Tumor specific |
(1) Type of lesion |
Osteolytic |
2 |
Mixed |
1 |
Osteosclerotic |
0 |
(2) Posterolateral involvement of spinal elements |
Bilateral |
3 |
Unilateral |
1 |
None |
0 |
Total SINS |
0–6 |
Stable |
7–12 |
Potentially unstable |
13–18 |
Unstable |
References
[1] Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty Neurochirurgie 1987;33(2):166–168
[2] Aebi M. Spinal metastasis in the elderly. Eur Spine J 2003;12(Suppl 2): S202–S213
[3] Kim TK, Kim KH, Kim CH, et al. Percutaneous vertebroplasty and facet joint block. J Korean Med Sci 2005;20(6):1023–1028
[4] Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 1994;73(8):2087–2098
[5] Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005;30(19):2186–2191
[6] Anderson LD, D’Alonzo RT. Fractures of the odontoid process of the axis. 1974. J Bone Joint Surg Am 2004;86(9):2081
[7] Yoon JY, Kim TK, Kim KH. Anterolateral percutaneous vertebroplasty at C2 for lung cancer metastasis and upper cervical facet joint block. Clin J Pain 2008;24(7):641–646
[8] Seo SS, Lee DH, Kim HJ, Yoon JW, Kwon OS, Kim KH. Percutaneous vertebroplasty at C7 for the treatment of painful metastases: a case report. Korean J Anesthesiol 2013;64(3):276–279
[9] Kim KH. Preoperative motion-related pain in cancer patients with extraspinal metastases treated by percutaneous osteoplasty. J Anesthe Clinic Rec 2011;S1:004
[10] Smith HS, Mohsin I. Painful boney metastases. Korean J Pain 2013;26(3): 223–241
[11] Yi YR, Lee NR, Kwon YS, Jang JS, Lim SY. Pulsed radiofrequency application for the treatment of pain secondary to sacroiliac joint metastases. Korean J Pain 2016;29(1):53–56
[12] Lee JH, Kim SY, Ok HG, Kim TK, Kim KH. Extraspinal percutaneous osteoplasty for the treatment of painful bony metastasis. J Korean Med Sci 2018;33(8):e61
[13] Berenson J, Pflugmacher R, Jarzem P, et al; Cancer Patient Fracture Evaluation (CAFE) Investigators. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12(3):225–235
[14] Martinčič D, Brojan M, Kosel F, et al. Minimum cement volume for vertebroplasty. Int Orthop 2015;39(4):727–733
[15] Luo J, Daines L, Charalambous A, Adams MA, Annesley-Williams DJ, Dolan P. Vertebroplasty: only small cement volumes are required to normalize stress distributions on the vertebral bodies. Spine 2009;34(26):2865–2873
[16] Ma XL, Xing D, Ma JX, Xu WG, Wang J, Chen Y. Balloon kyphoplasty versus percutaneous vertebroplasty in treating osteoporotic vertebral compression fracture: grading the evidence through a systematic review and meta-analysis. Eur Spine J 2012;21(9):1844–1859
[17] Cruz JP, Sahgal A, Whyne C, Fehlings MG, Smith R. Tumor extravasation following a cement augmentation procedure for vertebral compression fracture in metastatic spinal disease. J Neurosurg Spine 2014;21(3):372–377
[18] Sahgal A, Whyne CM, Ma L, Larson DA, Fehlings MG. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol 2013;14(8):e310–e320
[19] Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol 2013;31(27):3426–3431
[20] Fisher CG, Schouten R, Versteeg AL, et al. Reliability of the spinal instability neoplastic score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol 2014;9:69