33 Cementoplasty Outside the Spine
Peter L. Munk, Joshua A. Hirsch, Tyler M. Coupal, and Paul I. Mallinson
Summary
Metastatic disease to the skeletal system is a common occurrence that can be very painful and very debilitating. The metastatic tumors can weaken the bone leading to fracture or directly invade the bone and surrounding soft tissues. In addition to the conventional treatments for symptomatic metastases such as chemotherapy and radiation, percutaneous techniques such as tumor ablation and cementoplasty may be used to ameliorate symptoms produced by the metastases. While surgery may be appropriate for controlling symptoms produced by local invasion of tumor, patients who are significantly debilitated or those with a very short life expectancy may benefit from less invasive options such as percutaneous treatment. There are disadvantages to some of the conventional therapy including lack of structural stability after chemotherapy and radiation. Radiation is well known to cause regional osteoporosis and increase the risk of fracture in weight bearing bones. Cementoplasty also does not preclude the use of any of the other neoplastic treatments and can help provide structural stability to the axial and appendicular skeleton. Cementoplasty and the various ablative therapies can be effectively used alone or in combination with the conventional therapies for optimal local control of symptomatic metastatic disease. When utilizing the percutaneous therapies it is important to keep in mind the goals of providing adequate stabilization, ablating the interface between the tumor and normal bone, ablating the soft tissue component of the lesion that impinges on surrounding structures, and being mindful to not injure critical surrounding structures. When performing extraspinal cementoplasty and ablative therapies it is important to have adequate information prior to performing the procedure including adequate pre-procedure imaging and knowledge of the patient’s most debilitating symptoms. Clear treatment goals should be established prior to the procedure and multiple sessions may be necessary to adequately treat the full extent of the tumor without putting the patient at undue risk. Computed tomography and fluoroscopy are the most commonly used imaging modalities to guide the treatment. The location of percutaneous treatments most often involves the pelvis and acetabulum and reinforcement of the acetabulum often produces profound pain relief by stabilizing this area of prominent weight bearing. In addition to bone cement, some interventionalists may also use percutaneous screws to supplement the strength of the construct especially when large metastases are treated. Although care should be taken to avoid cement extravasation into the normal anatomy surrounding the metastasis, small amounts of extravasation are surprisingly well tolerated even into such important structures such as weight bearing joints. Other axial and appendicular osseous areas are also responsive to cementoplasty including the inferior pubic ramus, pubis, ilium, and long bones of the upper and lower extremities. When using cement in any osseous structure it should be kept in mind that although bone cement offers excellent support and resistance to compression, it tends to break with tensile or rotational forces so its use in long bones and other areas undergoing these forces should be in combination with other supporting devices such as nails, screws, or wires. There have also been reports of cementoplasty in more unusual locations such as the sternum, scapula, and clavicle. Complications associated with cementoplasty are rare but care should be taken to avoid cement extravasation and large volume cement injections causing displacement of a large amount of bone marrow especially in patients with compromised pulmonary function. It is not known whether cement injection into metastases causes spread of the malignancy but the use of ablative treatments could serve to limit the potential tumor spread. A combination of ablative techniques and cementoplasty could also ablate the tumor all the way out to its margins and reduce the tumor volume thereby making the injection of cement into the tumor easier. Cryoablation can impede the inflow of cement and complete thawing of the ablation zone is necessary to adequately inject cement into the area previously ablated. There has been considerable experience demonstrating that cementoplasty of the pelvis is an important technique for improving patient’s pain and quality of life in those patients suffering from painful pelvic metastases. Additional cementoplasty of metastatic disease outside the pelvis is limited but appears equally promising. The use of a combination of ablative treatments and cementoplasty can be a very effective way to manage patient with symptomatic metastases and should be considered in the appropriate clinical scenarios.
Keywords: cementoplasty, metastatic disease, tumor, radiation, chemotherapy, ablation, axial skeleton
33.1 Introduction
Metastatic bone disease is common in patients with certain types of neoplasms, and patients that die with metastatic disease often have metastatic bone deposits.1,2 These can be intensely symptomatic, and significantly degrade the quality of their life. Successfully treating symptomatic bone disease can be challenging, often requiring coordinated multidisciplinary efforts. Bone pain can make even simple things such as patient transfer or rolling over in bed an excruciatingly painful ordeal.
Symptoms from metastatic bone deposits arise from a variety of different mechanisms. Weakening of the bone may result in a pathologic fracture. In addition, tumor can invade the periosteum, which is richly innervated, producing severe pain or cause bleeding and subsequent elevation of the periosteum. Even without a pathologic fracture, weakened bone placed under stress, as with weight bearing, can be painful, therefore inhibiting ambulation or use of an extremity. Tumors can also produce nociceptive factors that cause inflammation or that can be irritating to nerve fibers and thus result in pain. Associated soft tissue masses arising from the metastasis can cause compression of adjacent structures, such as neurovascular bundles and other organs (e.g., bowel or bladder).
Conventional methods for treating symptomatic metastases in the pelvis and extremities include surgery, radiation therapy, and chemotherapy. More recently, percutaneous techniques such as cementoplasty and ablative methods (e.g., radiofrequency ablation, microwave ablation, and cryoablation) have been introduced. Surgery can be highly efficacious, particularly in the treatment of pathologic fractures in long bones of the extremities. At times, excision of a metastasis can also be performed and, if surgery is successful, this can provide excellent and durable pain relief. Evaluation and management of acetabular metastases comparing patients treated with surgery versus those managed with percutaneous cementoplasty has been studied.3–5 Surgical treatment may have some advantages in patients that are candidates for this type of surgery, in that symptom control and mobility may be better than with cementoplasty alone. There are several disadvantages inherent to surgery, including the fact that patients are often in such debilitated condition that they are poor surgical candidates for anesthesia and major resection. Prolonged period of convalescence may be required after surgery, which may be undesirable in an individual with a restricted lifespan. These debilitated patients are often difficult to rehabilitate. It has been argued that percutaneous imaging-guided cement injections may provide a less invasive, less expensive, but still highly effective treatment that decreases pain, and improves mobility and quality of life and stabilizes the skeletal area of interest particularly in those with limited projected survival.3,4,6
Radiation is an extremely useful tool and extensive experience with this modality exists. This technique is noninvasive and often provides excellent pain control but, unfortunately, up to 30% of patients do not receive satisfactory improvement in their symptoms.6 In addition, although radiation is useful in treatment of tumor, it does not provide mechanical reinforcement of bone so patients with symptoms on weight bearing may find that their symptom improvement after radiation is limited.7 Partial reconstitution of bone with healing can sometimes occur but may take months and the previously applied radiation may promote regional osteoporosis, thereby further increasing the risk of fracture.6,8 Chemotherapy can be a useful adjunct but a significant portion of patients may show incomplete or no response. Chemotherapy toxicity can be significant. Often the appropriate treatment approach uses multiple modalities, making multidisciplinary treatment decisions crucial. It is important to remember that cementoplasty does not preclude use of other treatment modalities. Subsequent radiotherapy can be performed in the presence of cement, and indeed may allow radiation to be held in reserve for future use if needed.
33.2 Anatomic Sites
This chapter will focus principally on cementoplasty within the pelvis, exclusive of the sacrum as this is covered elsewhere in this textbook. Pelvic cementoplasty procedures were first performed in the mid-1990s but have gained considerable popularity in the last 10 years.9–11 This technique is still not uniformly available, even in major medical centers, but is gaining in acceptance. More recently, some experience with cementoplasty of long bones and other sites have also been reported.1
33.2.1 Pelvic Cementoplasty
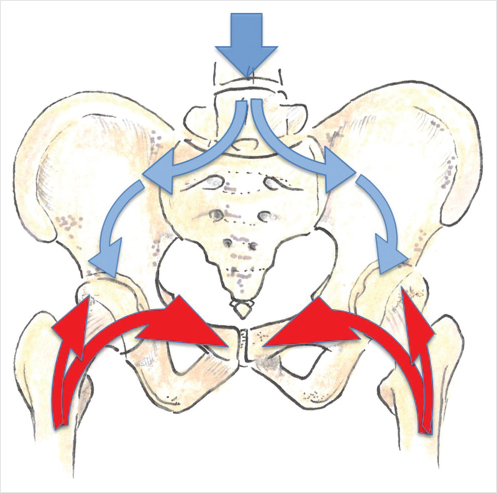
The pelvis is a large osseous structure that contains a substantial amount of cancellous bone and is an extremely common site of metastatic bone disease. Considerable force is transmitted through the pelvic ring, particularly in the upright position and on ambulation. Force is transmitted through the spine through the sacroiliac joints toward the acetabular roof and superior pubic ramus or from the lower extremity in the upright position (▶Fig. 33.1). Destruction of osseous integrity along this pathway can produce symptoms, particularly on ambulation and/or weight bearing.6
Fig. 33.1 Schematic of the transmission of forces through the lumbar spine via the pelvis into the lower limbs. Force is transmitted down from the spine into the pelvic ring (blue arrows), while force from the lower extremity is transmitted cranially (red arrows).
Regrettably patients may present with large metastatic deposits. Large lesions can be challenging to treat, as it may not be practical or reasonable to treat the entirety of the tumor particularly if a considerable soft tissue component is present. In this situation it is helpful to devise a plan of treatment whereby the areas most likely to provide symptom relief are targeted. Several principles should be considered in choosing the regions of the tumor to treat:
• Reinforcement of weight- or stress-bearing bone with cement or other stabilizing material.
• Cementation +/− ablation or ablation alone of the interface between tumor and normal bone.
• Ablation of soft tissue components of the lesion which impinge on adjacent structures.
• It is important to be aware of adjacent important structures, such as neurovascular bundles, bowel, bladder, and joints to minimize the possibility of leakage into the surrounding structures.
At present these procedures are relatively uncommon with only modest experience having been accrued. Both patients and referring doctors may know very little about them. Before embarking on a cementoplasty procedure in the pelvis, it is important to have clear goals as to what is hoped to be achieved. Moreover, it is crucial that the patient be aware of the potential risks and benefits and be involved in the decision making regarding the treatment goals and potential outcomes. Recent imaging (computed tomography [CT] and/or magnetic resonance imaging [MRI]) should be available and a thorough understanding of the patient’s symptoms is mandatory if a procedure is to be undertaken. It is important to know as precisely as possible where the patient’s most debilitating pain is, and what worsens or elicits the patient’s pain (e.g., weight bearing, sitting, etc.). As patients may have extensive metastatic disease, those most symptomatic areas should be treated first, with other regions being addressed later if required. Unnecessarily treating more extensive areas can put the patient at risk for more complications and potentially turn an effective palliative procedure into one that may worsen the patient’s condition.
33.2.2 Technique of Cementoplasty
The exact technique utilized varies considerably from one operator to another, as does the equipment used. A wide variety of different needles, cements, and ablation equipment have been reported in the literature with successful outcomes. Most operators performing cementoplasty already have considerable experience with vertebroplasty and can readily translate these skills to sites outside the spine.
For planning purposes reasonably contemporaneous cross-sectional imaging is required. Ideally, patients should not be coagulopathic and should not have platelet counts below 50,000 in order to minimize bleeding complications. Treatment while the patient has an active infection should also be, whenever possible, avoided. The use of prophylactic antibiotics in many procedures is common and should be employed in cementoplasty in order to minimize the possibility of infection. These procedures can at times be lengthy and uncomfortable for patients. Moreover, comorbidities and narcotic-tolerance can make analgesia difficult. Many operators utilize anesthesiology in order to safely ensure that patients are comfortable, and optimally monitored during the procedure.
Prior to undertaking the procedure, clear goals and reasonable expectations should be established. Areas of greatest stress or weight bearing should be selected to be buttressed and reinforced. Satisfactory outcomes can be achieved without complete filling of the entirety of large lesions, provided the key areas are treated. If cement can also be placed near the interface between tumor and bone, or the interface can be treated with ablation techniques, this can contribute to a good symptomatic outcome. Cementing of undisplaced or minimally displaced fracture sites can provide dramatic improvement in pain.
Although fluoroscopic guidance alone can be utilized with good success, some operators feel more comfortable with CT or cone beam CT guidance. The complex three-dimensional anatomy of the pelvis can make purely fluoroscopic guidance challenging. Combined fluoroscopy with cone beam CT facilitates confirmation of needle placement and also permits real-time evaluation of cement distribution.3,4,9,10,12
33.2.3 Assessment of Outcome
Outcomes assessment is patient dependent. Some patients are affected more by pain, others by diminished mobility or side effects of medication. The majority of patients have pain as their primary complaint. Numerous studies have shown that in the majority of treated patients pain significantly diminishes.10,11,13,14 Ideally pain should be assessed using a visual analog or ordinal scale, providing a quantitative measure of change in symptoms. The center of the lead author of this chapter typically asks the patient what their worst pain is in the week prior to treatment, on the day of treatment, and then will follow up the patient the following day, and one week later with additional assessment and pain queries as required. It is important to manage expectations and patients must realize that it is unlikely that all of their pain will disappear. Most patients are quite satisfied with a significant improvement in their symptoms. Studies have also demonstrated improved mobility and functionality following cementoplasty.1,15–17 Several authors of this chapter have treated patients who were unable to walk prior to treatment, due to pain on weight bearing, who subsequently became able to ambulate. This can significantly improve the patient’s quality of life and ability to participate in their own care.7,18, −28 Some authors have also examined the reduction in consumption of analgesics.4,29 Although patients often experience reduction in analgesic consumption it should be remembered that these patients often have multifocal disease, and may continue to require considerable analgesic use for management of symptoms remote from the site of cementoplasty.
33.2.4 Acetabulum/Pelvis
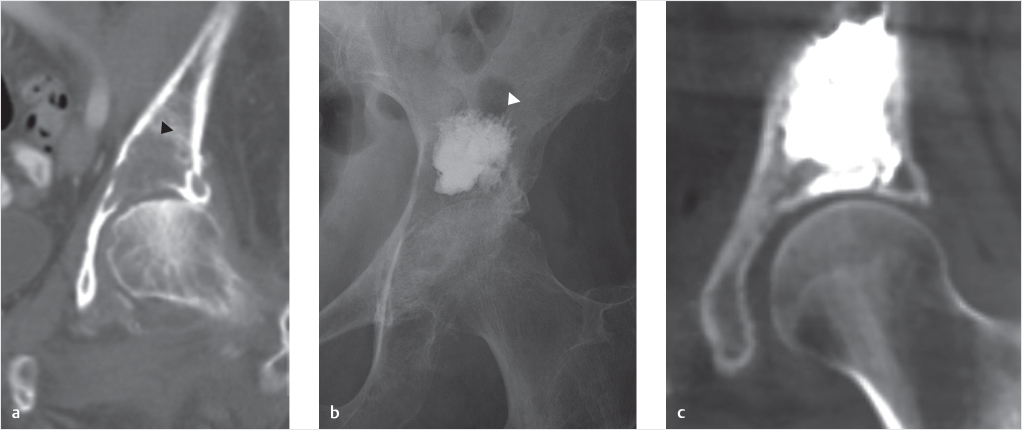
The first site outside the spine where cementoplasty was extensively utilized was the acetabulum. Metastases to the acetabular roof can be highly symptomatic even when quite small due to the weight-bearing stress placed on this site.4,17 It was quickly noted that these lesions could be treated with small volumes of cement, resulting in dramatic improvement in patient pain and mobility (▶Fig. 33.2).
Reinforcement of the acetabular roof by placement of a needle from an anterolateral approach allows cement to be injected safely using a route well clear of the femoral neurovascular structures. Continuous fluoroscopic observation during injection of cement can be performed when these procedures are performed under fluoroscopy. If imaging is done under CT guidance or fluoro-CT, frequent CT imaging following injection of small aliquots of cement is done to monitor the cement injection.14 Any extravasation of cement into the joint or blood vessels can therefore be immediately visualized and the injection terminated.
Some operators have also advocated the use of cannulated screws to supplement the cement injection for lesions of the acetabulum, ilium, and pubic ramus. Screws can be placed quite precisely under imaging guidance and may provide additional structural support, particularly in the presence of large metastatic lesions.30–32 Although, intuitively, this would seem to be advantageous, as of the writing of this chapter no firm data comparing screw placement with cementoplasty alone is available.
Extrusion of small amounts of cement into surrounding soft tissue extension of the metastases is not usually symptomatic. Although every effort should be made to avoid injection of cement into the joint space, this is often surprisingly well-tolerated by patients. Complications from intra-articular cement injection includes grinding, clicking, and pain from intra-articular loose bodies and rarely chondrolysis.33
Fig. 33.2 (a) Coronal computed tomography (CT) of the left hip of a patient with breast cancer demonstrated a destructive metastasis eroding into the acetabular roof (black arrowhead). (b) A post-procedural frontal pelvic radiograph demonstrates successful reinforcement by acetabuloplasty (white arrowhead). (c) A coronal CT of the left hip demonstrates a similar result in a patient with prostate cancer.
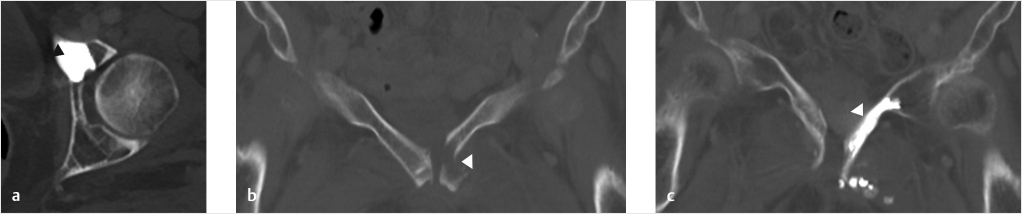
Fig. 33.3 (a) Axial post-procedural computed tomography (CT) demonstrates successful deployment of cement into the anterior acetabulum/lateral aspect of the left superior pubic ramus in a patient with a prostate cancer metastasis (black arrowhead). (b) A pretreatment coronal CT image demonstrates a patient with a painful pathological fracture secondary to osteonecrosis (white arrowhead). (c) The post-procedure image shows successful cement deployment along the length of the superior pubic ramus, which bridged the fracture (white arrowhead).
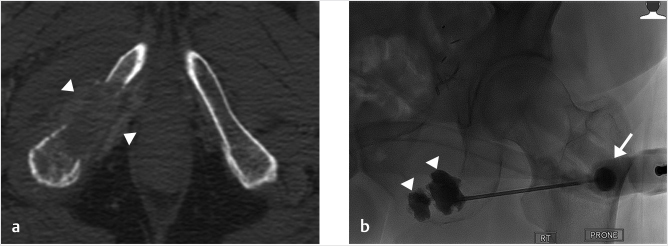
Fig. 33.4 (a) An axial computed tomography (CT) demonstrates destruction of the right inferior pubic ramus and ischial tuberosity by a metastatic soft tissue deposit (white arrowheads). This was determined clinically to be the source of significant pain for the patient during sitting. (b) A posteroanterior fluoroscopic image shows the right ramus and tuberosity successfully reconstructed with cement (white arrowheads) via a 13G vertebroplasty needle (white arrow).
33.3 Other Sites
Force and stress through the pelvic ring is also transmitted through the pubic body, superior pubic ramus, as well as the portion of the iliac bone adjacent to the sacroiliac joint.14,34 Fractures, usually undisplaced or minimally displaced, are common at these sites and can respond to injections of small volumes of cement (▶Fig. 33.3). Although below and outside the kinetic chain of force transmission between lumbar spine and the femora, the inferior pubic rami, including the ischial tuberosity, are important transmitters of compressive force during sitting. They are, therefore, potentially responsive sites to cementoplasty (▶Fig. 33.4). In the case of pubic lesions, it can be helpful to decompress the bladder with a urinary catheter prior to the procedure. With large lytic lesions of the ilium, it should be remembered that the lumbar plexus is located nearby anterior to the sacral ala so care must be exercised not to extravasate cement into this area.
At the time of this writing, only modest experience exists with cementoplasty of the long bones. Although cement is extremely good in dealing with compressive forces, cement tends to fracture or shatter with rotational or torque forces, which are typically experienced by long bones, such as the femur.30 Regardless, reports have appeared which have shown that some patients appear to have significant pain reduction following injection of cement with good clinical outcomes.1,8,12,15,17,35,36 It would appear that patients with large cortical breaches are poor candidates for treatment. In the proximal femur, an intact calcar in particular may be an important treatment selection criterion.30 Purely intramedullary metastatic deposits may have a more favorable outcome. The presence of cement within long bones may function as a stress riser in spite of intact overlying cortex, with resultant fractures on additional weight bearing.17,35,37
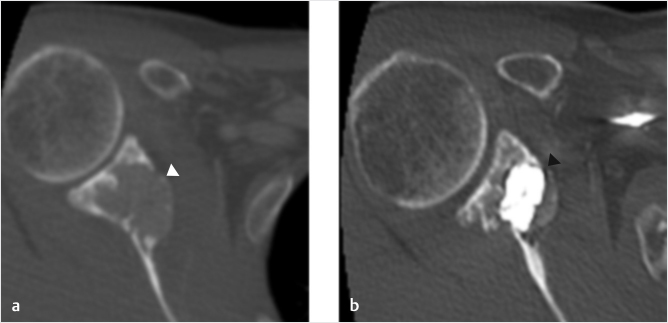
Fig. 33.5 (a) Axial CT image demonstrates a lytic metastasis in the neck of the scapula (white arrowhead). (b) This area of the scapula was treated by cementoplasty (black arrowhead).
In order to further reinforce the bone and minimize the chance of post-procedural fracturing, other investigators have percutaneously placed thin rods, wires, or guide wires across the tumor site undergoing cementoplasty. Reports of percutaneous placement of multiside large-bore catheters into medullary space of bones, with subsequent injection of bone cement through the catheter, have claimed good clinical results.37,38 These techniques should be viewed as developmental but may play a valuable role, particularly in patients who are poor candidates for traditional surgical fixation.
Scattered reports of successful cementoplasty of other osseous structures have also appeared in the literature.18,39,−41 Sternal metastases and fractures can be highly symptomatic but can show significant improvement with injection of small volumes of cement. Similar results have been reported in the clavicle and scapula (▶Fig. 33.5). Particular attention to the nearby neurovascular structures is clearly necessary, emphasizing the importance of using cross-sectional imaging guidance when necessary.
33.4 Complications
As with any percutaneous procedure, both infection and bleeding can occur but are rare.
Pathologic fracture following cementation can occasionally occur in the pelvis, but is more common in the long bones.2,11,12,39,42–44 Nontarget embolization of cement into the venous system, lungs, joints, and soft tissues can occur, mandating close fluoroscopic monitoring while injecting where ever possible. If injection must be done under CT guidance, injection of small aliquots with frequent imaging must be performed.
Injection of cement into any osseous structure causes embolization of bone marrow elements, and probably tumor into the venous system. This may be a significant consideration in patients with chronic obstructive lung disease who have limited physiologic pulmonary reserve. Promotion of spread of metastatic disease by embolization of tumor is a theoretical consideration. Clear data on whether cementoplasty promotes spread of malignancy in the clinical setting is not readily available at the present time, but is unlikely to be a significant consideration in those patients who have short life expectancy. The use of accompanying ablative techniques prior to cement injection may diminish the significance of this potential problem.
33.5 Concomitant Use of Ablative Techniques
An expanding body of literature describes the use of combination ablative techniques and cementoplasty in treatment of metastases.2,29,32,34,42,45–49 The greatest experience at present is with radiofrequency ablation and cryoablation. Ablation provides a theoretical advantage in assisting in the reduction of the volume of viable tumor, compared with what could be achieved with cement injection alone. Ablation of the tumor bone interface prevents further invasion of normal bone. Concomitant ablation of soft tissue extension of the bone metastases can be performed which may be helpful if tumor is causing symptomatic compression or displacement of adjacent nerves, blood vessels, and organs (e.g., bladder, rectum). A theoretical advantage of destroying potentially embolizable tumor on injection of cement has previously been noted above.
Radiofrequency ablation may potentially increase the ease of injection of cement by disrupting the tissue integrity of metastasis. Cryoablation, on the other hand, may impede distribution of cement if the ice ball is incompletely thawed, therefore ensuring complete thawing prior to cement injection is recommended. Impeding the spread of cement in the presence of an ice ball has also been used as a useful tool to guide cement distribution.50
33.6 Conclusion
Considerable experience has shown that cementoplasty in the pelvis is a valuable tool in improving patients’ symptoms and quality of life in the setting of metastatic disease. Experience outside of the pelvis is limited, but it appears promising that specific clinical situations exist to which cementoplasty is likely helpful. The use of concomitant ablative techniques in selective patients is strongly suggested by the literature.
33.7 Key Points
• Reinforcement of weight- and stress-bearing bone is likely to produce significant patient symptom improvement.
• Significant patient improvement can be achieved with only partial selective treatment in the setting of large metastatic disease and/or extensive deposits.
• Detailed cross-sectional imaging CT +/− MRI is vital in planning the procedure, and the imaging should be recent.
• A detailed understanding of the patient’s symptoms is required for tailoring the procedure to be performed, particularly in patients with extensive disease.
References
[1] Feng H, Feng J, Li Z, et al. Percutaneous femoroplasty for the treatment of proximal femoral metastases. Eur J Surg Oncol 2014;40(4):402–405
[2] Kurup AN, Callstrom MR. Ablation of musculoskeletal metastases: pain palliation, fracture risk reduction, and oligometastatic disease. Tech Vasc Interv Radiol 2013;16(4):253–261
[3] Colman MW, Karim SM, Hirsch JA, et al. Percutaneous acetabuloplasty compared with open reconstruction for extensive periacetabular carcinoma metastases. J Arthroplasty 2015;30(9):1586–1591
[4] Gupta AC, Hirsch JA, Chaudhry ZA, et al. Evaluating the safety and effectiveness of percutaneous acetabuloplasty. J Neurointerv Surg 2012;4(2): 134–138
[5] Sapkota BH, Hirsch AE, Yoo AJ, et al. Treatment of metastatic carcinoma to the hip with CT-guided percutaneous acetabuloplasty: report of four cases. J Vasc Interv Radiol 2009;20(4):548–552
[6] Zhang J, Yang Z, Wang J, et al. Study of treatment using percutaneous acetabuloplasty and interstitial implantation of (125)I seeds for patients with metastatic periacetabular tumors. World J Surg Oncol 2012;10:250
[7] Hirsch AE, Jha RM, Yoo AJ, et al. The use of vertebral augmentation and external beam radiation therapy in the multimodal management of malignant vertebral compression fractures. Pain Physician 2011;14(5):447–458
[8] Cazzato RL, Buy X, Eker O, Fabre T, Palussiere J. Percutaneous long bone cementoplasty of the limbs: experience with fifty-one non-surgical patients. Eur Radiol 2014;24(12):3059–3068
[9] Cotten A, Demondion X, Boutry N, et al. Therapeutic percutaneous injections in the treatment of malignant acetabular osteolyses. Radiographics 1999;19(3):647–653
[10] Cotten A, Deprez X, Migaud H, Chabanne B, Duquesnoy B, Chastanet P. Malignant acetabular osteolyses: percutaneous injection of acrylic bone cement. Radiology 1995;197(1):307–310
[11] Weill A, Kobaiter H, Chiras J. Acetabulum malignancies: technique and impact on pain of percutaneous injection of acrylic surgical cement. Eur Radiol 1998;8(1):123–129
[12] Wang Z, Zhen Y, Wu C, et al. CT fluoroscopy-guided percutaneous osteoplasty for the treatment of osteolytic lung cancer bone metastases to the spine and pelvis. J Vasc Interv Radiol 2012;23(9):1135–1142
[13] Deschamps F, de Baere T. Cementoplasty of bone metastases. Diagn Interv Imaging 2012;93(9):685–689
[14] Georgy BA. Percutaneous cement augmentations of malignant lesions of the sacrum and pelvis. AJNR Am J Neuroradiol 2009;30(7):1357–1359
[15] Cazzato RL, Palussière J, Buy X, et al. Percutaneous long bone cementoplasty for palliation of malignant lesions of the limbs: a systematic review. Cardiovasc Intervent Radiol 2015;38(6):1563–1572
[16] Guzik G. Treatment of metastatic lesions localized in the acetabulum. J Orthop Surg Res 2016;11(1):54
[17] Iannessi A, Amoretti N, Marcy PY, Sedat J. Percutaneous cementoplasty for the treatment of extraspinal painful bone lesion: a prospective study. Diagn Interv Imaging 2012;93(11):859–870
[18] Kamalian S, Hirsch AE, Growney ML, et al. CT guided percutaneous calcaneoplasty: a case of metastatic intra-articular calcaneus fracture. J Neurointerv Surg 2009;1(2):186–188
[19] Botsa E, Mylona S, Koutsogiannis I, Koundouraki A, Thanos L. CT image guided thermal ablation techniques for palliation of painful bone metastases. Ann Palliat Med 2014;3(2):47–53
[20] Cascella M, Muzio MR, Viscardi D, Cuomo A. Features and role of minimally invasive palliative procedures for pain management in malignant pelvic diseases: a review. Am J Hosp Palliat Care 2017;34(6):524–531
[21] Choi J, Raghavan M. Diagnostic imaging and image-guided therapy of skeletal metastases. Cancer Contr 2012;19(2):102–112
[22] Kurup AN, Morris JM, Schmit GD, et al. Balloon-assisted osteoplasty of periacetabular tumors following percutaneous cryoablation. J Vasc Interv Radiol 2015;26(4):588–594
[23] Ma Y, et al. Percutaneous image-guided ablation in the treatment of osseous metastases from non-small cell lung cancer. Cardiovasc Intervent Radiol 2017
[24] Marcy PY, Palussière J, Descamps B, et al. Percutaneous cementoplasty for pelvic bone metastasis. Support Care Cancer 2000;8(6):500–503
[25] Prologo JD, Passalacqua M, Patel I, Bohnert N, Corn DJ. Image-guided cryoablation for the treatment of painful musculoskeletal metastatic disease: a single-center experience. Skeletal Radiol 2014;43(11):1551–1559
[26] Sun G, Jin P, Liu XW, Li M, Li L. Cementoplasty for managing painful bone metastases outside the spine. Eur Radiol 2014;24(3):731–737
[27] Wallace AN, McWilliams SR, Connolly SE, et al. Percutaneous image-guided cryoablation of musculoskeletal metastases: pain palliation and local tumor control. J Vasc Interv Radiol 2016;27(12):1788–1796
[28] Buy X, Cazzato RL, Catena V, Roubaud G, Kind M, Palussiere J. Techniques de consolidation osseuse guidée par imagerie en oncologie : cimentoplastie et vissage. Bull Cancer 2017;104(5):423–432
[29] Callstrom MR, Dupuy DE, Solomon SB, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer 2013;119(5):1033–1041
[30] Deschamps F, de Baere T, Hakime A, et al. Percutaneous osteosynthesis in the pelvis in cancer patients. Eur Radiol 2016;26(6):1631–1639
[31] Pusceddu C, Fancellu A, Ballicu N, Fele RM, Sotgia B, Melis L. CT-guided percutaneous screw fixation plus cementoplasty in the treatment of painful bone metastases with fractures or a high risk of pathological fracture. Skeletal Radiol 2017;46(4):539–545
[32] Hartung MP, Tutton SM, Hohenwalter EJ, King DM, Neilson JC. Safety and efficacy of minimally invasive acetabular stabilization for periacetabular metastatic disease with thermal ablation and augmented screw fixation. J Vasc Interv Radiol 2016;27(5):682–688.e1
[33] Leclair A, Gangi A, Lacaze F, et al. Rapid chondrolysis after an intra-articular leak of bone cement in treatment of a benign acetabular subchondral cyst: an unusual complication of percutaneous injection of acrylic cement. Skeletal Radiol 2000;29(5):275–278
[34] Pusceddu C, Sotgia B, Fele RM, Ballicu N, Melis L. Combined microwave ablation and cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Intervent Radiol 2016;39(1):74–80
[35] Deschamps F, Farouil G, Hakime A, et al. Cementoplasty of metastases of the proximal femur: is it a safe palliative option? J Vasc Interv Radiol 2012;23(10):1311–1316
[36] Plancarte-Sanchez R, Guajardo-Rosas J, Cerezo-Camacho O, et al. Femoroplasty: a new option for femur metastasis. Pain Pract 2013;13(5):409–415
[37] Liu XW, Jin P, Liu K, et al. Comparison of percutaneous long bone cementoplasty with or without embedding a cement-filled catheter for painful long bone metastases with impending fracture. Eur Radiol 2017;27(1):120–127
[38] He C, Tian Q, Wu CG, Gu Y, Wang T, Li M. Feasibility of percutaneous cementoplasty combined with interventional internal fixation for impending pathologic fracture of the proximal femur. J Vasc Interv Radiol 2014;25(7): 1112–1117
[39] Basile A, Giuliano G, Scuderi V, et al. Cementoplasty in the management of painful extraspinal bone metastases: our experience. Radiol Med (Torino) 2008;113(7):1018–1028
[40] Leung OC, Poon WL, Nyaw SF, Luk SH. Percutaneous cementoplasty of osteolytic metastases induces immediate and long-lasting pain relief in oncological patients. Hong Kong Med J 2013;19(4):317–322
[41] Uri IF, Garnon J, Tsoumakidou G, Gangi A. An ice block: a novel technique of successful prevention of cement leakage using an ice ball. Cardiovascular Intervent Radiol 2015;38(2):470–4
[42] Wallace AN, Huang AJ, Vaswani D, Chang RO, Jennings JW. Combination acetabular radiofrequency ablation and cementoplasty using a navigational radiofrequency ablation device and ultrahigh viscosity cement: technical note. Skeletal Radiol 2016;45(3):401–405
[43] Coupal TM, Pennycooke K, Mallinson PI, et al. The hopeless case? Palliative cryoablation and cementoplasty procedures for palliation of large pelvic bone metastases. Pain Physician 2017;20(7):E1053–E1061
[44] Lane MD, Le HB, Lee S, et al. Combination radiofrequency ablation and cementoplasty for palliative treatment of painful neoplastic bone metastasis: experience with 53 treated lesions in 36 patients. Skeletal Radiol 2011;40(1): 25–32
[45] Callstrom MR, Atwell TD, Charboneau JW, et al. Painful metastases involving bone: percutaneous image-guided cryoablation—prospective trial interim analysis. Radiology 2006;241(2):572–580
[46] Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007;10(2): 120–131
[47] Castañeda Rodriguez WR, Callstrom MR. Effective pain palliation and prevention of fracture for axial-loading skeletal metastases using combined cryoablation and cementoplasty. Tech Vasc Interv Radiol 2011;14(3): 160–169
[48] Thacker PG, Callstrom MR, Curry TB, et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients’ immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol 2011;197(2):510–515
[49] Tian QH, Wu CG, Gu YF, He CJ, Li MH, Cheng YD. Combination radiofrequency ablation and percutaneous osteoplasty for palliative treatment of painful extraspinal bone metastasis: a single-center experience. J Vasc Interv Radiol 2014;25(7):1094–1100
[50] Swan JA, Liu DM, Clarkson PW, Munk PL. Cryoablation and cementoplasty of a pathologic fracture in the sternum. Singapore Med J 2013;54(10): e215–e217