Figure 15.1 Strawberries move around, and exploit, their environment by sending out runners that form roots and new plantlets at intervals.

Chapter 15
Environmental interactions
Plants have evolved a wide range of characteristics to cope with a variable and sometimes hostile environment. Even though a plant is anchored in place, its open-ended, indeterminate mode of development (see Chapter 12) gives it the option of invading its environment as a means of overcoming limitations. For example, growing roots penetrate the soil and, by following gradients of water and nutrients, are able to maintain these inputs in response to demands from the rest of the plant. Similarly, creeping plants that produce rhizomes, stolons or runners—for instance, strawberries (Fragaria spp.; Figure 15.1)—effectively travel around in their environment, foraging for resources. Where light is limiting, such as beneath the forest canopy, shoots will extend and leaves will expand to escape from shading (see Chapter 12). Dispersal of seeds and other propagules is the means by which a plant, despite being rooted at a particular location, is able to send its genes elsewhere. So plants may be sessile, but they are far from immobile.
Figure 15.1 Strawberries move around, and exploit, their environment by sending out runners that form roots and new plantlets at intervals.

Nevertheless, for an individual that stays in one place, the environment is a constant test of fitness. The inescapable cycle of day and night is a case in point: as photoautotrophic organisms, plants must make relatively rapid physiological adjustments to an ever-changing light environment. In temperate regions, day length varies with the season and invokes longer-term changes in development (see Chapters 8 and 12). It is characteristic of plants that not only do they react to deviations from optimal environmental conditions; they also use such fluctuations as a source of information to trigger adaptive changes in structure and function. This chapter starts by considering general features of plant–environment interactions, then goes on to look at the most distinctive of these responses, namely the capacity to synthesize a vast range of phytochemical constituents with known or suspected roles in resisting or avoiding environmental pressures. We then proceed to describe specific examples of responses to non-biological external influences, followed by a survey of the mechanisms by which plants react to pathogenic organisms and other biological challenges.
One approach to understanding the behavior of living organisms in response to the environment is to borrow the concepts of stress and strain from engineering. Stress is defined as any factor that invokes a corresponding strain. In this context we should be careful not to think narrowly of stress in terms of distress, as a kind of analogy with human psychological and physical reactions to pressure. As we have seen, optimal conditions are not stress-free: for a plant, which cannot run away, stress and strain are a way of life. If the environment changes, the plant has no option but to change too. We have already met an example of the application of the stress–strain concept to a plant process in the discussion of the rheological properties of the growing cell wall (see Chapter 12), where the idea of plastic and elastic deformation was introduced. We can broaden this picture of stress–strain behavior to include the whole range of responses to environmental challenge. When a physiological reaction to the experience of an environmental perturbation is reversible on removal of the stress, the system is considered to have behaved elastically. Beyond the elasticity threshold, the physiological adjustment will be irreversible and the response is plastic. A third condition is reached if the stress exceeds the capacity of the system to react elastically or plastically in a physiologically coherent way. The result is system failure, a catastrophic outcome that leads to pathological changes and death.
Factors in the environment that influence plant growth, development and survival may be divided into biotic (those that arise directly or indirectly from interactions with other living organisms) and abiotic (originating in experiences of physical, chemical and energetic conditions). The major abiotic factors are light, temperature, water and nutrients (discussed in Section 15.6). Biotic influences include other plants, pathogens, predators, pollinators and dispersers, as well as human interventions such as in selection and pollution.
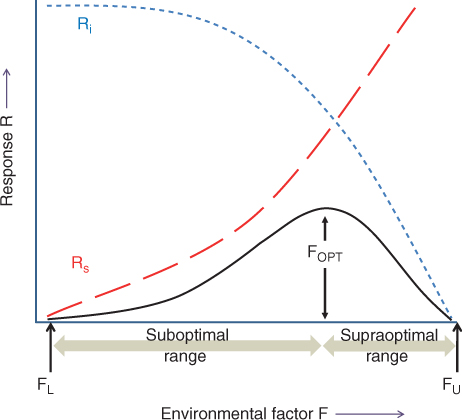
A simple model describes the interaction between environmental influences and the physiology of a plant. Imagine the case of an environmental factor F eliciting a biological response R. If F and R are measurable (for example F may be temperature, whereas R may be rate of growth), we can plot a graph to represent the relationship between stimulus and response (Figure 15.2). The curve typically rises from a minimum at a low value of F to a peak (the optimum), above which R either increases no further or (in the case of many environmental factors such as temperature) declines, ultimately to zero. The F–R curve is a combination of two opposing responses to increasing levels of the environmental factor. One, represented in Figure 15.2 as the Rs line, is the unconstrained stimulatory effect of exposure to progressively higher levels of F. For example, chemical reactions (the basis of most biological processes) will increase in a broadly exponential manner with increasing temperature. The Rs curve is counteracted by the tendency of biological processes to become progressively more disordered when overstimulated (Figure 15.2, Ri line). The combined curve is thus characterized by a suboptimal range, bounded by FL (the value of F at which R is minimal) and FOPT, and a supraoptimal range between FOPT and FU (Figure 15.2).
Figure 15.2 Generalized curves for the response, R, of a given biological process on exposure to different intensities of an environmental factor, F. The Rs and Ri lines are the positive (stimulatory) and negative (inhibitory) components, respectively, of the response to F. The combination of Rs and Ri is a curve (black line) that rises from a minimum at F = FL to a maximum (FOPT) and declines again to an upper limit beyond which there is no further response (FU).
A typical example of response curves for different plants is shown in Figure 15.3. At low soil temperatures, dry matter is accumulated slowly. As the soil gets progressively warmer, dry matter productivity increases until it reaches a maximum at the optimal growth temperature. Increasing the temperature further results in decreasing production until a lethal temperature is reached and growth ceases completely. The production versus temperature plots in Figure 15.3 have similar features to the idealized curve in Figure 15.2. Temperatures below and above the optimum for growth are sub- and supraoptimal, respectively. Different species, especially from different habitats or geographical regions, have different temperature optima and growth–temperature ranges. Figure 15.3 shows that the optimum for wheat (Triticum), a temperate crop plant, is 18 °C, about 10 °C lower than that for soybean (Glycine), which is adapted to hotter, drier environments.
Figure 15.3 Plant growth responses to soil temperature in some agricultural crop systems. Growth is expressed as rate of dry matter accumulation, normalized with respect to maximal production = 1.
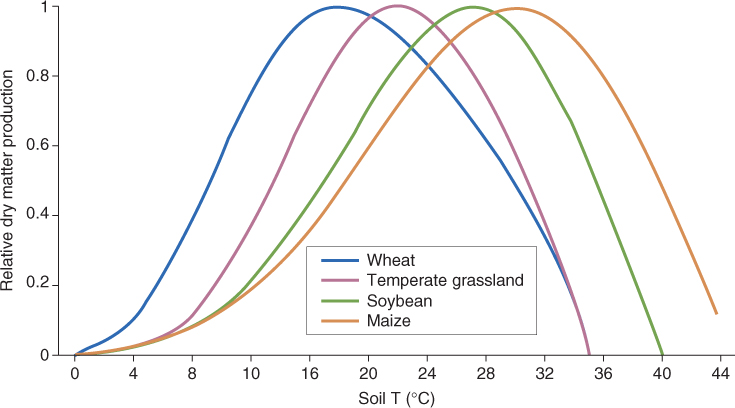
The lesson to take from this discussion of response curves is that, even at the optimum, plants experience environmental constraint. Living organisms are immensely complex combinations of individual components and processes, each of which has its own response curve for any given environmental variable. So although a Zea mays plant, for example, grows optimally at a soil temperature of 32 °C (Figure 15.3), for many constituent elements of its physiological machinery this temperature will be sub- or supraoptimal. Temperature is given here as an example of an environmental influence, but the principle is the same for any and every external factor. The optimum for a composite trait such as growth is the result of a great diversity of input–output actions and interactions among underlying processes.
Organisms deal with non-optimal environmental conditions in different ways. They may adopt a strategy of tolerance, building resilient structures and physiologies able to survive by withstanding stress. Or they may take the avoidance route, confining growth to relatively favorable periods in a fluctuating environment, through life cycle strategies that minimize exposure of vulnerable stages to extreme conditions. The capacity to survive different degrees of stress varies greatly between species, and among genotypes of a single species. For example, many desert plants are xerophytes, that is, they have morphological and physiological characteristics that enable them to tolerate extreme drought. These include water-storing succulent tissues, CAM (crassulacean acid metabolism) photosynthesis and structural features that minimize dehydration (Figure 15.4A). Another drought-tolerance mechanism, adopted by species such as honey mesquite (Prosopis glandulosa var. glandulosa), is to develop deep taproots that improve access to groundwater and increase survival during long periods without rain (Figure 15.4B). In contrast, the strategy of desert ephemerals (short-lived weedy plants of hot dry habitats) is to avoid drought by germinating and completing their life cycles while adequate water is available (Figure 15.4C).
Figure 15.4 Examples of species that exhibit stress tolerance and stress avoidance. (A) The saguaro cactus (Carnegiea giganteus) is a highly drought-tolerant xerophyte. (B) The honey mesquite (Prosopis glandulosa var. glandulosa) is a drought-avoiding species with deep roots. (C) Mohave desert star (Monoptilon bellioides) is a desert ephemeral that only flowers after adequate winter/spring rain. (D) Spinach (Spinacia oleracea) is normally sensitive to osmotic stress, but has the capacity for physiological acclimation. (E) Black spruce (Picea mariana) is a cold-hardy tree that develops freezing tolerance by acclimation.

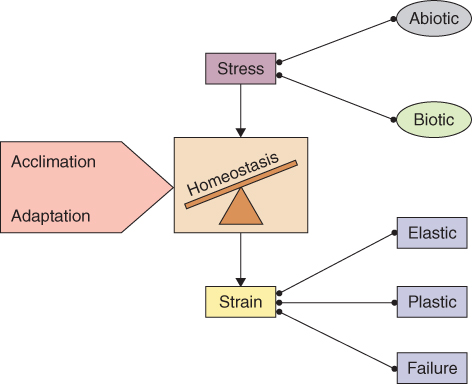
Living systems are homeostatic. They tend to adjust to stress by minimizing strain and maintaining equilibrium. A homeostatic system has three modes of response to disturbance, the first two being: elastic, in which the system bounces back and resumes its former state; and plastic, in which it deforms and settles on a new stable configuration. When the limits of elastic and plastic resilience are exceeded, a catastrophic response results and the system becomes incoherent, entropy increases, and in the case of biology, death follows.
Homeostatic adjustment of an individual organism in response to changing environmental factors is termed acclimation. An example of acclimation is the reaction of spinach plants to the presence of salt in the supply of water for growth (Figure 15.4D). These plants adjust their physiology by producing solutes that accumulate in the cytoplasm and, by sequestering salts in the vacuole, thereby maintain osmotic balance with the soil. Another example is found in trees of temperate regions. During the summer such trees generally cannot withstand freezing, but many species can acclimate in response to gradually decreasing temperatures in the fall. Eventually they may even be able to survive winter temperatures below − 50 °C (Figure 15.4E). Acclimation is familiar to gardeners in the process known as hardening off, in which an otherwise vulnerable plant can be ‘toughened up’ by exposure to non-lethal levels of stress.
Constant or recurrent environmental challenge not only invokes acclimation; it may also exert selective pressure, driving the evolution of traits that increase fitness under stress. Such adjustments, which occur over many generations and across entire populations, are termed adaptations. In the case of cacti and similar xerophytes, for example, the adaptive traits, which include sunken stomata, light-reflective spines and CAM metabolism, are genetically determined, constitutive characters. These attributes have evolved for stress resistance but are expressed whether the plants are stressed or not. Table 15.1 summarizes the contrasting features of acclimation and adaptation. By acting over the short term in individuals, acclimation represents a tactical response to stresses that are often unscheduled. In contrast, adaptation is strategic in nature and is the basis of population-scale responses. These responses, such as winter dormancy and the loss of leaves in temperate forests, are often associated with predictable seasonal challenges. Figure 15.5 summarizes the relationships between stress, strain, acclimation and adaptation.
Table 15.1 Adaptation and acclimation compared and contrasted.
| Adaptation | Acclimation | |
| Individual or population level | Population | Individual |
| Caused by | Natural selection acting on allelic variation within populations. | Local environmental conditions acting on genetically-determined physiological responsiveness |
| Heritability | Genotypic | Generally non-heritable only; some instances of epigenetic transmission |
| Reversibility | Irreversible (except by further genotypic change and selection) | Reversible |
| Response of homeostasis to perturbation | Mostly plastic | Mostly elastic |
| Timescale | From generation time of the organism up to evolutionary | Short term (minutes/hours): metabolic and physiological adjustments of existing components without significant change in gene expression |
| Long-term (up to weeks or months): altered patterns of gene expression, reallocation of resources, morphological change | ||
| Deployment in the life cycle | Strategic | Tactical |
Figure 15.5 Biotic or abiotic stress invokes strain in the form of elastic, plastic or system failure responses. The extent to which homeostasis can be maintained is conditioned by the acclimatory or adaptive capacities of the organ, individual or population.
A critical difference between acclimation and adaptation is the role of the genome in each process (Table 15.1). Acclimation responses generally involve metabolic adjustments, which may or may not require the transcription of genes and translation of their products. Over the longer-term these metabolic changes lead to significant phenotypic changes in morphology and physiology. The traits acquired during acclimation are, in the vast majority of cases, not heritable. Nevertheless, there is evidence that epigenetic changes occur under some circumstances (see Chapter 3). A classic example is seen in flax (Linum usitatissimum), where plants grown with nitrogen-potassium fertilizer lacking phosphorus are only one-third to a quarter the size of those grown on NPK. This phenotypic difference is heritable, has been maintained over 50 generations, and remains even when plants are subsequently grown on NPK (Figure 15.6). The molecular basis of this so-called genotrophic effect is not known. Epigenetic effects apart, changes in gene expression, if they are significant at all, play a reversible role in acclimation and are not associated with permanent changes to the genome. Adaptation, on the other hand, acts on allelic variation within a population, favoring those genotypes best fitted to survive the selective stress, and results in irreversible changes to the genome.
Figure 15.6 Heritable phenotypes of flax (Linum usitatissimum) derived from a genetically uniform parental line that was grown on fertilizer with (NPK) or without (NK) phosphate. The photograph shows representative individuals of the 49th generation progeny grown on NPK. Such epigenetic variants resulting from responses to nutrient availability are termed genotrophs.

Plants employ distinctive biochemical measures to deal with environmental stresses. The chemical intermediates and end-products that are directly involved in growth and development are referred to as primary metabolites. Primary metabolites are found in all plants, and include sugars, phytosterols, acyl lipids, nucleotides, amino acids and organic acids—the biochemical functions of which have been well established. But there is also an extraordinary variety of organic compounds that do not participate in primary metabolism and are differentially distributed within and between taxonomic groups within the plant kingdom. The specific functions of many of these so-called secondary compounds are unknown. It is becoming clear, however, that the principal roles of secondary metabolites are in acclimation and adaptation to environmental stresses, particularly those of biotic origin. It makes perfect sense for a sedentary, autotrophic organism to have evolved chemical defenses that react to, or prepare for, environmental challenges. In this chapter we will focus on three major groups of natural compounds with such functions in the molecular life of plants: phenolics, alkaloids and terpenoids (isoprenoids).
Phenolics (mostly phenylpropanoids), estimated to comprise at least 8000 different chemical structures, include low molecular weight phenolics, and condensation products such as tannins, lignins and flavonoids. The approximately 10 000 known alkaloids are protective, nitrogenous, secondary metabolites, derived principally from amino acids. Alkaloids are often pharmacologically active. The 25 000-plus terpenoid constituents of plants are built from five-carbon isoprenoid units. These include the carotenoids and related compounds, as well as a variety of toxins, antifeedants and attractants.
Secondary metabolites have evolved as a defense against pathogens, herbivores and other environmental hazards, thereby improving competitive ability and fitness. Secondary compounds are often toxic, antinutritional, allergenic, malodorous or bad-tasting and therefore detrimental for human consumption. Domestication of wild species and selection for desirable crop traits usually results in the reduction or elimination of such natural products. Consequently, cultivated species frequently display weakened defenses against environmental (particularly biotic) challenges. The function of pesticides and other crop protection measures is to manage this vulnerability, but this incurs environmental and economic costs that significantly impact on agricultural efficiency. Understanding plant secondary metabolism is thus of importance for food production. In addition since many existing and potential drugs are derived from plant natural products, secondary metabolism is of fundamental pharmacological interest.
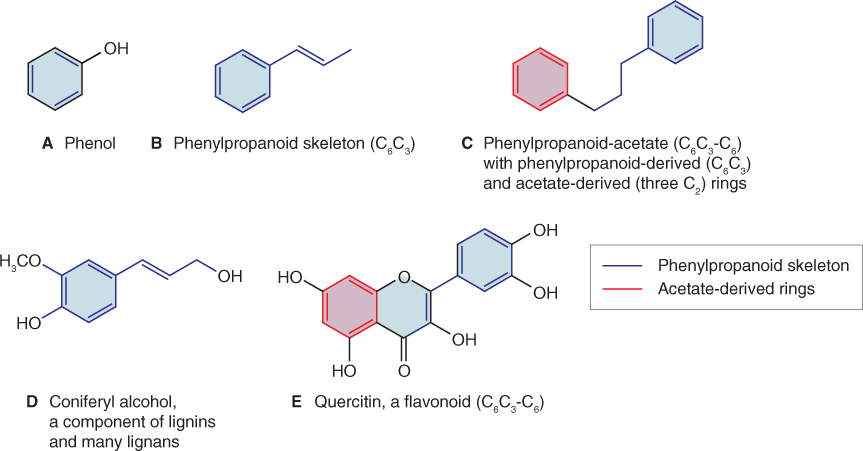
Plant phenolics include a wide diversity of secondary metabolites. The basic chemical unit of phenolic compounds is a six-carbon aromatic (phenyl) ring to which one or more hydroxyl groups are attached (Figure 15.7A). The capacity to make a huge range of phenolics with a variety of structural and physiological roles is one of the characteristics that allowed the evolution of terrestrial plants from their aquatic ancestors. Phenolic cell wall components such as lignin stiffen cell walls and are essential for holding plants upright on land in the absence of the buoyant support provided by water. Terrestrial plants have also developed a wide variety of non-structural phenolics. These contribute to hardiness, colors, tastes, odors, defense against stresses and other attributes essential for survival. It is estimated that phenolics represent about 40% of the organic carbon circulating in the biosphere. The relative chemical inertness of macromolecular phenolic derivatives such as lignins and tannins makes the biodegradation of these products a rate-limiting step in the recycling of organic carbon into CO2.
Figure 15.7 Chemical structures of phenolics. (A) Phenol, the parent structure of phenylpropanoids. (B) The phenylpropanoid skeleton. (C) Structure of phenylpropanoid acetate, showing the origins of different parts of the molecule. (D) Coniferyl alcohol, a phenylpropanoid component of lignans. (E) Quercitin, a flavonol with a structure based on phenylpropanoid acetate.
When an apple is damaged and exposed to air, the familiar browning reaction occurs rapidly. This is an example of a widespread response that occurs in plant tissues; it is the result of oxidation of phenolics. It frequently yields products that form inactivating complexes with proteins and nucleic acids and can make biochemical or molecular analysis difficult. For this reason, protocols for isolating plant proteins and nucleic acids generally include special precautions designed to minimize interference by phenolic compounds.
The chemical structures of most plant phenolics are related to their biosynthetic origins in phenylpropanoid (Figure 15.7B, D) and phenylpropanoid-acetate (Figure 15.7C, E) metabolism. Polymeric lignins, which reinforce the secondary cell walls of structural and transport tissues in vascular plants (see Figure 12.15), are products of the phenylpropanoid pathway. The lignans (Figure 15.7D) are di- and oligomeric phenylpropanoids structurally and biosynthetically related to lignin. They are widely distributed throughout the plant kingdom and function as defenses against biotic and abiotic stress in flowers, seeds, stems, bark, leaves and roots. Suberin, which is found in cork, bark and periderm tissues such as potato skin, contains alternating hydrophilic and hydrophobic layers of phenolic and aliphatic structural substances. Suberization establishes a barrier that protects against water loss and pathogen attack. The most diverse group of plant phenolics is the flavonoids (Figure 15.7E). Included among the estimated 4500 flavonoids present in plants are: anthocyanins (responsible for the pink/blue/red pigmentation in many vegetative tissues, flowers and fruits); proanthocyanidins or condensed tannins (antifeedants and wood protectants); and isoflavonoids (defensive products and signaling molecules). Plants are also protected against herbivores, microbial pathogens and competing neighbors by a range of miscellaneous phenolic-related products, including coumarins, furanocoumarins and stilbenes.
Most phenolic and alkaloid secondary compounds in plants are derived from the aromatic amino acids phenylalanine, tyrosine and tryptophan. In addition to their role as monomers for protein synthesis, these primary metabolites are major points of contact between primary and secondary metabolism. Among the secondary metabolites that are derived from the aromatic amino acids are the indole hormones (e.g. indole-3-acetic acid (IAA); see Chapter 10), anthocyanin pigments, defensive phytoalexins, bioactive alkaloids and structural lignins. It is estimated that about 20% of the carbon fixed by plants flows through the common aromatic amino acid pathway, the largest proportion ending up in lignin.
Figure 15.8 is an outline of the reaction sequence leading from intermediates in primary carbon metabolism (phosphoenolpyruvate (PEP) and erythrose-4-phosphate) to phenylalanine, tyrosine and tryptophan, and onward to phenolics and alkaloids. The intermediate shikimic acid, a carboxylic acid with a hydroxylated six-carbon ring, gives its name to the shikimate pathway. The shikimate pathway is a seven-step sequence localized in the chloroplast; its product chorismic acid is the final common intermediate in the synthesis of the three aromatic amino acids. The first reaction of this pathway is the formation of 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) by DAHP synthase (Equation 15.1), an enzyme that is regulated by tryptophan and Mn2+ activation.
Figure 15.8 Biosynthesis of aromatic amino acids phenylalanine, tyrosine and tryptophan, the sources of phenolic and alkaloid natural products.
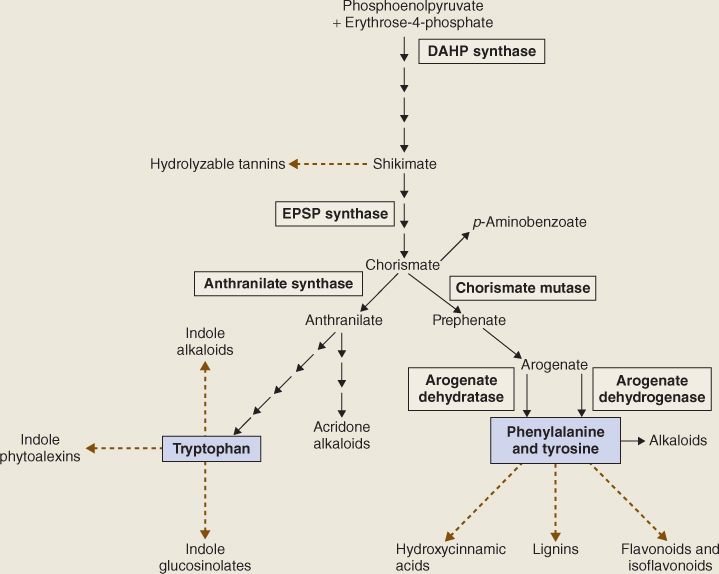
The next to last step in the shikimate pathway (Equation 15.2) is the target of the widely used herbicide glyphosate (Roundup; Figure 15.9) which is a competitive inhibitor of EPSP synthase. The lethal consequences of blocking this strategic reaction clearly demonstrate the central importance of the shikimate pathway in plant metabolism.
Figure 15.9 The herbicide glyphosate is a competitive inhibitor of EPSP (5-enolpyruvylshikimate-3-phosphate) synthase, an enzyme in the phenylalanine and tyrosine synthesis pathway.
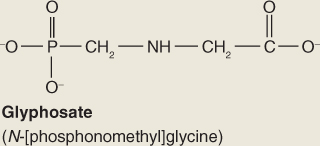
Chorismate, which is made from EPSP by the enzyme chorismate synthase, is the end-product of the shikimate pathway and the starting point for the reaction sequence that leads to the synthesis of phenylalanine and tyrosine (Figure 15.8). The enzyme chorismate mutase (CM) catalyzes the formation of prephenate from chorismate by an intramolecular rearrangement. CM1, an isoform located in the chloroplast, is a critical point for controlling entry into the phenylalanine/tyrosine branch of the aromatic amino acid synthesis pathway (Figure 15.8). Phenylalanine and tyrosine, the end-products of this pathway, modulate CM1 activity by feedback inhibition of the enzyme. Prephenate is transaminated to yield arogenate, the immediate precursor of phenylalanine and tyrosine. Arogenate dehydratase catalyzes the synthesis of phenylalanine from arogenate in a decarboxylation reaction (Equation 15.3A) and arogenate dehydrogenase produces tyrosine from arogenate in an NADP+-dependent reaction (Equation 15.3B). These two enzymes are regulated by end-product inhibition and act as sensitive control points in phenylpropanoid production.
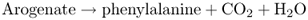
15.3B Arogenate dehydrogenase

Some phenolic compounds are generated through pathways other than the phenylalanine/tyrosine route. An example is hydrolyzable tannins (defense compounds found in leaves, fruits, pods and galls of a range of dicot species) which are derivatives of shikimate and carbohydrates. Hydrolyzable tannins are chemically distinct from condensed tannins, which are products of flavonoid metabolism, as described in Section 15.3.3.
In discussing the products of phenylalanine metabolism, it is useful to set out the basic structural features that underlie the chemical diversity of phenolic intermediates. The common molecular structure is the six-carbon aromatic ring. An unsaturated three-carbon (propene) side-chain is attached to the ring at the carbon atom designated 1 (Figure 15.10A). The other atoms of the aromatic ring are numbered clockwise with respect to C-1. Intermediate phenolic metabolites, derived from phenylalanine, vary in the atoms or groups attached to carbons 3, 4 and 5 of the aromatic ring (Figure 15.10A, groups R1, R, R2 respectively) and the terminal C atom of the propene side-chain (Figure 15.10A, R3). The enzyme phenylalanine (tyrosine) ammonia-lyase (PAL/TAL) is the point of entry of aromatic amino acids into phenylpropanoid metabolism. PAL converts its preferred substrate, phenylalanine, to cinnamic acid, although the monocot enzyme is also able to utilize tyrosine to form 4-coumaric acid (Figure 15.10B). The ammonium ion, produced in this reaction, is reassimilated by the GS-GOGAT pathway (see Chapter 13). In some species, PAL is encoded by a single gene, but in other species it is the product of a multigene family. The cinnamate product of PAL is converted to 4-coumarate by the enzyme cinnamate-4-hydroxylase (C4H) (Figure 15.11). This oxygen-requiring, NADPH-dependent enzyme has a cytochrome P450 prosthetic group that specifically hydroxylates the aromatic ring in the 4- (that is, para-) position (Equation 15.4). Cytochrome P450-type enzymes carry out oxidations of organic compounds and are encoded by a large gene family (CYP); the Arabidopsis genome has more than 270 CYP or CYP-like sequences. C4H, like other CYP enzymes, is anchored in membranes of the endoplasmic reticulum (ER). There is evidence that PAL physically associates with C4H to form a loose complex that allows the product of the former enzyme to feed directly into the second, a process known as metabolic channeling.
Figure 15.10 Chemical structures of phenolic intermediates derived from cinnamic acid. (A) Carbon atoms of the phenylpropanoid aromatic ring are numbered clockwise, beginning at the position of the propene side-chain. The convention in describing substitution patterns in aromatic rings is to refer to two substituents on adjacent carbon atoms as ortho (o-) with respect to each other; if there is one carbon atom between substituents the configuration is meta (m-), and para (p-) with two intervening carbon atoms. (B) Reactions catalyzed by phenylalanine (tyrosine) ammonia lyase. According to the convention described in (A), the hydroxylated derivative of cinnamic acid is referred to as either 4- or p-coumaric acid.
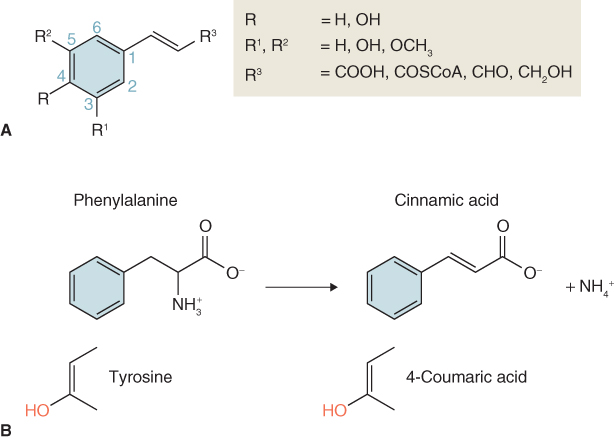
Figure 15.11 Phenylpropanoids derived from cinnamate, 4-coumarate and 4-coumaroyl-CoA.
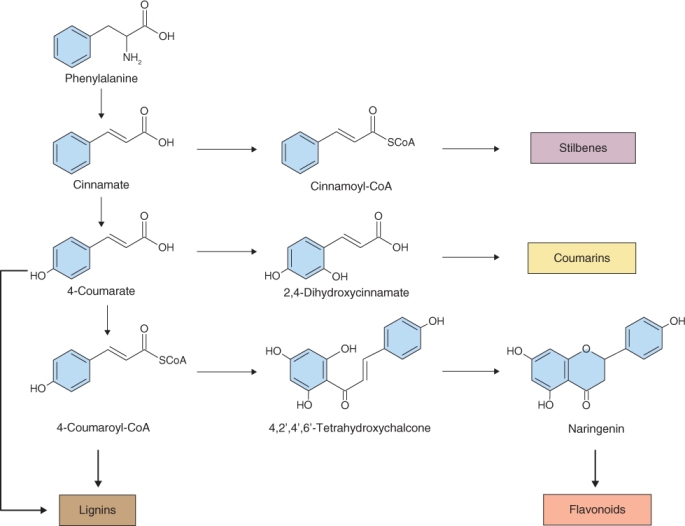
Cinnamoyl-CoA, the coenzyme A thioester synthesized from cinnamate, is the origin of the stilbene group of natural products (Figure 15.11) that are synthesized by plants in response to damage. Grapes, pines and legumes are rich sources of the resveratrol family of stilbenes, which function as phytoalexins (pathogen defense agents; see Section 15.7.1) and are of pharmaceutical interest as anticarcinogens and cardioprotectives. Hydroxylation of 4-coumarate at the 2-position yields 2,4-dihydroxycinnamate, which in turn gives rise to the coumarins (Figure 15.11), a group of fragrant but bitter-tasting products with appetite-suppressing properties. The anticoagulant warfarin (Coumadin), which is used as a rat poison and blood thinner, is a chemically-modified coumarin derivative.
4-Coumaroyl-CoA is the metabolic origin of the flavonoids (Figure 15.11). Flavonoids can occur as monomers, dimers and higher oligomers, and are distributed throughout most plant tissues. In contrast to pigments such as carotenoids and chlorophylls, which are lipophilic and usually found in plastids, most flavonoids are water-soluble and are often located in vacuoles. The flux into the flavonoid synthesis pathway through 4-coumaroyl-CoA begins with two distinctive enzymes, chalcone synthase (CHS) and chalcone isomerase (CHI). The reaction catalyzed by CHS (Equation 15.5) consists of the condensation of three molecules of malonyl-CoA, derived from acetate (see Figure 15.7E), with 4-coumaroyl-CoA to form 4,2′,4′,6′-tetrahydroxychalcone (Figure 15.11). Because of its importance in the pathway leading to floral pigmentation, the gene for CHS was one of the earliest targeted for biotechnological modification. Such interventions showed that flower color can be changed by altering CHS expression. CHS may act coordinately with chalcone reductase, an NADPH-dependent enzyme that generates isoliquiritigenin and leads to the isoflavonoid branch of phenylpropanoid metabolism (Figure 15.12).
Figure 15.12 The pathway leading from 4-coumaroyl-CoA to isoflavonoids and phytoalexins. Enzymes in common with flavonoid biosynthesis are chalcone synthase (CHS) and chalcone isomerase (CHI). Committed enzymes of isoflavonoid synthesis are chalcone reductase (CHR) and isoflavone synthase (IFS). Note that the phenyl group in isoflavonoids is attached at position 3 on the oxane ring, whereas the bond in flavonoids derived from naringenin is at position 2 (compare Figure 15.11).
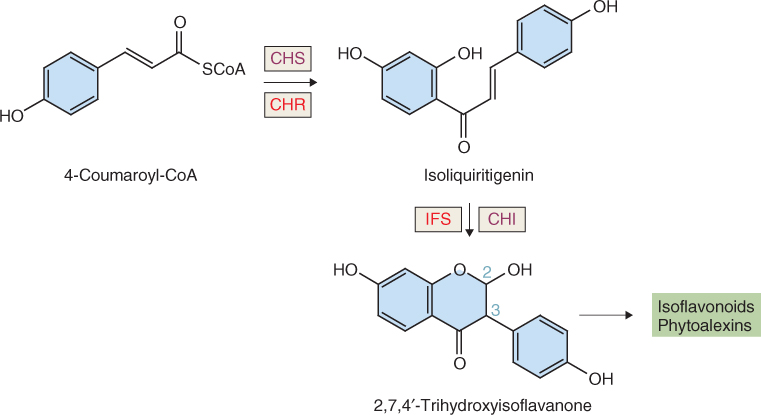
Chalcone isomerase catalyzes a stereospecific ring closure isomerization step, usually to form naringenin (Figure 15.11). In isoflavone synthesis, CHI works with isoflavone synthase (an NADPH-dependent cytochrome P450 enzyme) to yield a product with the aromatic ring attached at position 3 (rather than 2 as in flavonoids) of the oxane ring (Figure 15.12). Further metabolism of isoflavonoids yields antipathogenic phytoalexins and insecticidal rotenoids.
Naringenin is the source of flavones, flavonols, anthocyanins and condensed tannins (Figure 15.13). The abundant flavone apigenin is reported to have a range of beneficial therapeutic properties; it is the reaction product of flavone synthase. Enzymatic hydroxylation of naringenin results in dihydrokaempferol, which in turn is the source of flavonols (through the flavonol synthase-catalyzed formation of kaempferol). A highly visible fate of dihydrokaempferol is to feed a metabolic grid leading to the synthesis of the vivid red, pink, mauve, violet, blue and purple anthocyanin pigments found in many petals, leaves, stems and fruits (Figure 15.13). Dihydroquercitin and dihydromyricetin are the products of hydroxylating dihydrokaempferol at the 3′ and 5′ positions. All three flavonols may be used as substrates by dihydroflavonol-4-reductase (DFR), an NADPH-dependent enzyme. The products of the DFR reaction are leucoanthocyanidins (e.g. leucopelargonidin, derived from dihydrokaempferol), which in turn can be converted to the colored anthocyanidins (e.g. pelargonidin) through the action of anthocyanidin synthase, an α-ketoglutarate-dependent dioxygenase (Figure 15.13). The final step in anthocyanin biosynthesis is glycosylation of anthocyanidin, which stabilizes the molecule. Further modifications then include additional methylations, glycosylations and acylations of hydroxyl groups to produce the great range of anthocyanin colors present in the plant kingdom. The control of flower color is discussed further in Chapter 16, and the significance of foliar anthocyanins, responsible for many of the reds and purples of autumn leaves, is considered in Chapter 18.
Figure 15.13 Conversion of naringenin to flavones, flavonols and intermediates in the metabolic grid leading to synthesis of anthocyanins and condensed tannins. Key to enzymes: ANR, anthocyanidin reductase; ANS, anthocyanidin synthase; DFR, dihydroflavonol-4-reductase; F3H, flavanone-3-hydroxylase; F3′H, flavonoid-3′-hydroxylase; F3′5′H, flavonoid-3′,5′-hydroxylase; FLS, flavonol synthase; FNS, flavone synthase; LAR, leucoanthocyanidin reductase.
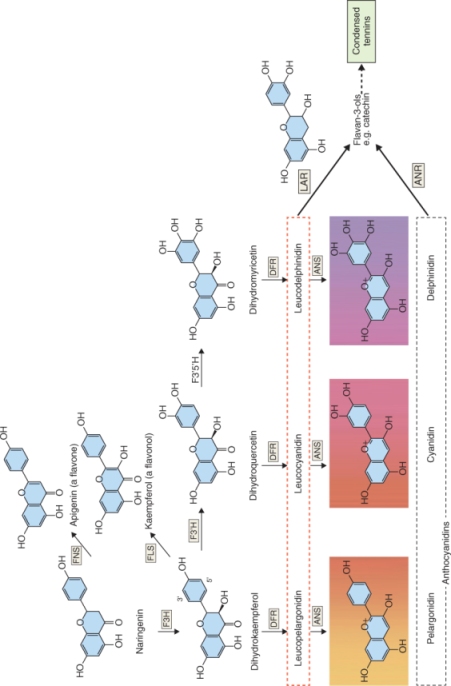
Leucoanthocyanidins and anthocyanidins can also serve as precursors of catechins and condensed tannins (Figure 15.13), widely-distributed astringent defense compounds. Tea, cocoa, chocolate, wine and many fruits and vegetables are rich sources of catechins, which contribute to taste and nutritional effects. Tannins deter pathogens and herbivores by complexing with macromolecules and inactivating enzymes.
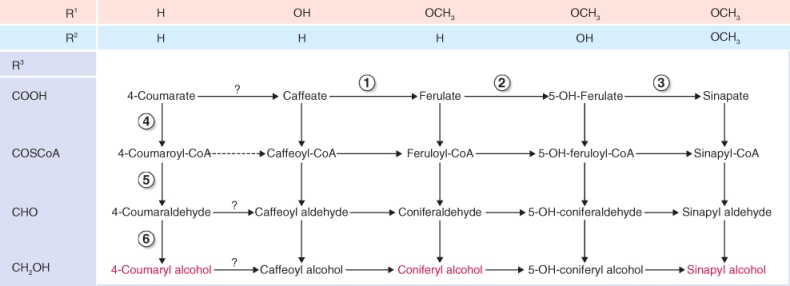
Cinnamic and 4-coumaric acids are converted to a range of phenolic products through a series of enzymatic reactions including aromatic hydroxylations, O-methylations, CoA ligations and NADPH-dependent reductions (Figure 15.14). The result is a metabolic grid that supplies the precursors of lignin biosynthesis (monolignols), 4-coumaryl, coniferyl, 5-hydroxyconiferyl and sinapyl alcohols. Most of the enzymes of the grid are multifunctional with broad substrate specificities and each catalyzes more than one reaction. Ferulate-5-hydroxylase (Figure 15.14, reaction 2) is an endomembrane-associated CYP-type enzyme. All the other enzymes of the grid are soluble, though some may be involved in metabolic channeling as described above for PAL and C4H.
Figure 15.14 The grid of phenolic lignin precursors derived from 4-coumaric acid. R1, R2 and R3 refer to the substitution pattern shown in Figure 15.10A. Monolignols are shown in red. Enzymes catalyzing horizontal reactions are: (1) O-methyl transferase, (2) ferulate-5-hydroxylase and (3) O-methyl transferase. Vertical reactions are catalyzed by: (4) 4-coumarate:coenzyme A ligase, (5) cinnamoyl-CoA reductase and (6) cinnamyl/sinapyl alcohol dehydrogenase. ? = identity of enzymes not certain. 4-Coumaroyl-CoA is converted to caffeoyl-CoA by three enzymatic steps.
Lignin synthesis takes place in the cell wall. Before their export to the wall, monolignols synthesized in the cytosol are glycosylated by a reaction with uridine diphosphate (UDP) glucose, catalyzed by UDP-glucose coniferyl alcohol glucosyltransferase (Equation 15.6A). After the monolignol glucosides are transferred to the cell wall, they are hydrolyzed by coniferin β-glucosidase (Equation 15.6B). The monolignols released undergo polymerization, catalyzed by peroxidases and laccases, and are cross-linked to cell wall polysaccharides as described in Chapter 12.
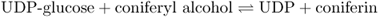
15.6B Coniferin β-glucosidase
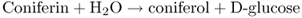
From the earliest days of human history, alkaloids have been among the most potent and socially significant plant products. They include: opium, from the latex of the opium poppy (Papaver somniferum); coniine, the poison of hemlock (Conium maculatum; Figure 15.15A), famously used for the execution of the classical Greek philosopher Socrates; and hyoscyamine, which is the active principle in extracts of a number of solanaceous plants, and deadly in high concentrations but at trace levels is an effective drug treatment for a range of medical conditions. Alkaloid-containing plants were humankind's original medicines and even today the list of effective prescription drugs for the commonest ailments is dominated by pharmaceuticals of plant origin. Plant alkaloids are also chemical models on which the design of modern synthetic drugs is based. For example, the antimalarial drug chloroquine is an indole-derived alkaloid related to the natural product quinine. Table 15.2 lists a number of the major plant-derived alkaloids used in modern medicine.
Figure 15.15 Examples of alkaloids and the plants that produce them, indicating the variety of chemical structures. (A) Coniine from poison hemlock (Conium maculatum). (B) Senecionine from ragwort (Senecio jacobaea). (C) Nicotine from tobacco (Nicotiana tabacum). (D) Caffeine from coffee (Coffea arabica).
Table 15.2 Physiologically active alkaloids used in modern medicine.
| Alkaloid | Plant source | Use |
| Ajmaline | Rauwolfia serpentina | Antiarrhythmic that functions by inhibiting glucose uptake by heart tissue mitochondria |
| Atropine | Atropa belladonna | Anticholinergic antidote to nerve gas poisoning |
| Caffeine | Coffea arabica | Widely used central nervous system stimulant |
| Camptothecin | Camptotheca acuminata | Potent anticancer agent |
| Cocaine | Erythroxylon coca | Topical anesthetic, potent central nervous system stimulant, and adrenergic blocking agent; drug of abuse |
| Codeine | Papaver somniferum | Relatively non-addictive analgesic and antitussive |
| Coniine | Conium maculatum | First alkaloid to be synthesized; extremely toxic, causes paralysis of motor nerve endings, and used in homeopathy in small doses |
| Emetine | Uragoga ipecacuanha | Orally active emetic and amoebicide |
| Morphine | Papaver somniferum | Powerful narcotic analgesic and addictive drug of abuse |
| Nicotine | Nicotiana tabacum | Highly toxic, causes respiratory paralysis; horticultural insecticide; drug of abuse |
| Pilocarpine | Pilocarpus jaborandi | Peripheral stimulant of the parasympathetic system, used to treat glaucoma |
| Quinine | Cinchona officinalis | Traditional antimalarial, important in treating Plasmodium falciparum strains that are resistant to other antimalarials |
| Sanguinarine | Eschscholzia californica | Antibacterial showing antiplaque activity, used in toothpastes and oral rinses |
| Strychnine | Strychnos nux-vomica | Violent tetanic poison, used as rat poison; used in homeopathy |
| ( + )-Tubocurarine | Chondrodendron tomentosum | Non-depolarizing muscle relaxant producing paralysis; used as an adjuvant to anesthesia |
| Vinblastine | Catharanthus roseus | Antineoplastic used to treat Hodgkin's disease and other lymphomas |
Alkaloids were originally characterized as pharmacologically active, basic compounds that contain nitrogen and are derived from plants. The term has since been extended to apply to many phytochemicals that do not strictly conform to this definition. It has also become clear that alkaloids are not confined to plants. Species of bacteria, fungi, sponges, arthropods, amphibians and mammals have been shown to accumulate alkaloids, which have significant ecological functions and in some cases are of plant origin. For example, larvae of the cinnabar moth, Tyria jacobaea, gather alkaloid precursors from Senecio jacobaea, the host plant on which they feed (Figure 15.15B), and convert them into pheromones and defense compounds. Generally, however, plants deploy alkaloids as antibiotics, herbivore deterrents or other measures against biotic stresses. Alkaloids may be either constitutive (present all the time) or synthesized in response to physical or threatened trauma. Nicotine, for example (Figure 15.15C), is a potent insecticide and has long been used by humans to protect cultivated plants. Wild plants of tobacco (Nicotiana spp.) respond to herbivory by boosting the rate of endogenous nicotine biosynthesis. Caffeine (Figure 15.15D), found in seeds and leaves of cocoa (Theobroma cacao), coffee (Coffea spp.) and tea (Camellia sinensis), is another defense chemical, effective against insects at a fraction of the concentration found in fresh coffee beans or tea leaves. The pharmaceutical, nutritional or psychotropic properties of plant alkaloids can be directly attributed to their ecological function as chemical influences (often harmful) on the physiology of interacting animals and other organisms.
Most classes of alkaloid are derived directly or indirectly from amino acids (Table 15.3), either alone or in combination with a terpenoid. Detailed biosynthetic pathways are known for relatively few alkaloids. Where information is available it often reveals exotic and complex chemical structures and mechanisms that testify to the great metabolic versatility of plants. By way of illustration, here we briefly consider the reaction sequence leading to one of the indole group of alkaloids, and in Section 15.4.2 we discuss a second example in which the product is an isoquinoline (Table 15.3).
Table 15.3 Precursors, structural groupings and examples of plant alkaloids.

The indole-type neurotoxin strychnine is a derivative of tryptophan. The first committed step in tryptophan formation is the synthesis of anthranilate from chorismate, catalyzed by anthranilate synthase (see Figure 15.8). Anthranilate synthase transfers an amine group from glutamine to chorismate and releases pyruvate (Figure 15.16). Two enzymatic steps convert anthranilate to indole-3-glycerol phosphate, the substrate for tryptophan synthase (TS). Tryptophan is the product of two reactions that are catalyzed by the α- and β-subunits of TS respectively (Equation 15.7).
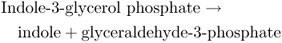
15.7B Reaction catalyzed by β-subunit
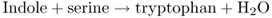
Figure 15.16 Conversion of chorismate to anthranilate by anthranilate synthase, first step in tryptophan biosynthesis.
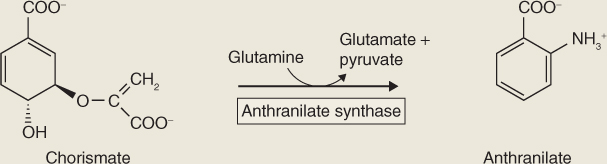
Strychnine biosynthesis begins with the decarboxylation of tryptophan by tryptophan decarboxylase to form tryptamine (Equation 15.8). Strictosidine synthase then catalyzes the stereospecific condensation of tryptamine with the terpenoid secologanin. The product is strictosidine (Figure 15.17), the starting point for species-specific enzymatic permutations that result in a multitude of diverse structures. Strychnine, one of the classic tools of poisoners in history and fiction, is just one of the many metabolic derivatives of strictosidine. Others include the antimalarial quinine, and vinblastine and vincristine, tubulin-binding alkaloids from Catharanthus spp. that are widely used in cancer therapy.
Figure 15.17 The indole alkaloid strychnine is biosynthesized from tryptophan via tryptamine (which is itself a biologically active alkaloid) and strictosidine.
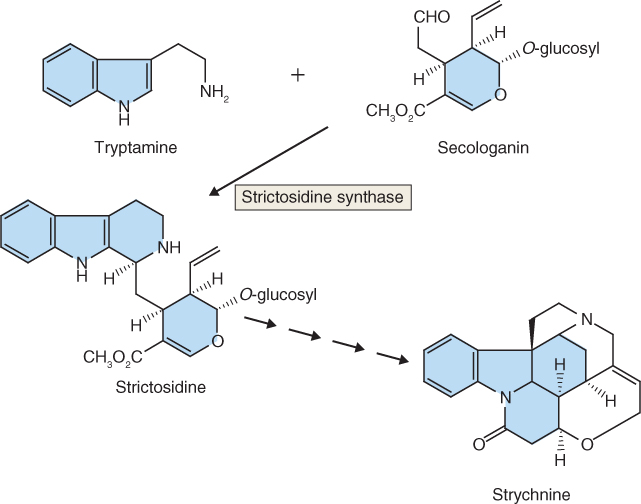
Tyrosine is the precursor of the isoquinoline alkaloid, morphine (Figure 15.18). One molecule of tyrosine is decarboxylated to form tyramine, which, in turn, is converted to 3,4-dihydroxyphenylamine (dopamine) by the action of a phenol oxidase. A second tyrosine is transaminated and decarboxylated to yield p-hydroxyphenylacetaldehyde. Dopamine and p-hydroxyphenylacetaldehyde are then stereoselectively condensed to form the first isoquinoline intermediate, (S)-norcoclaurine. A series of methylation and oxidation reactions follow that produce (S)-reticuline, a branch point metabolite. (S)-reticuline, like strictosidine, is the precursor of an enormous array of structurally diverse products, that includes not only morphine and related compounds such as codeine, but also berberine (an active component of traditional medicines as well as a dyestuff) and sanguinarine (a product, found in a number of plant species, that is harmful to animal cells).
Figure 15.18 Outline of the morphine biosynthetic pathway.
Terpenoids take their name from terpenes, the volatile constituents of turpentine (a solvent produced by the distillation of pine tree resin). They are the most structurally varied group of plant natural products and are built from isoprene units, which consist of the branched five-carbon isopentane skeleton. Terpenoids are often called isoprenoids; however, isoprene itself is not an intermediate in terpenoid synthesis. The repetitive C5 structural motif from which terpenoids are built is referred to as the prenyl group (Figure 15.19). The different classes of terpenoids are identified by the number of C5 units (Table 15.4). Thus we have hemiterpenoids (C5), monoterpenoids (C10), sesquiterpenoids (C15), diterpenoids (C20), sesterpenoids (C25), triterpenoids (C30) and tetraterpenoids (C40). Terpenoids consisting of more than eight isoprene units are referred to as polyterpenoids. Natural rubber, a polymer of isoprene consisting of 11 000 to 20 000 monomeric units, is an example (Table 15.4). Ubiquinone and plastoquinone, the electron carriers of respiration (see Figure 7.11) and photosynthesis (see Figure 9.15A), are also polyterpenoids. Norterpenoids are terpenoid derivatives that have lost one or more carbons and so are no longer C5 multiples. For example, some gibberellins (see Chapter 10) are C19 structures, the result of the removal of one C from a diterpene precursor. Natural products of mixed biosynthetic origins that are partially derived from terpenoids are sometimes called meroterpenoids. Such compounds include cytokinins (see Chapter 10) and some proteins that are prenylated by the addition of lipophilic, membrane-anchoring 15- or 20-carbon isoprenoid side-chains.
Figure 15.19 Terpenoids are built from prenyl units, based on the branched C5 hydrocarbon isoprene. A great array of terpenoid structures can be generated by joining C5 units in combinations of head–tail, head–head and head–middle configurations.
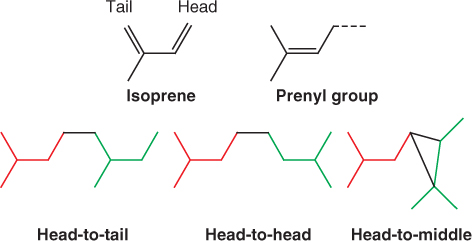
Table 15.4 Terpenoids grouped by number of carbon atoms, with examples and structures of members of each class.


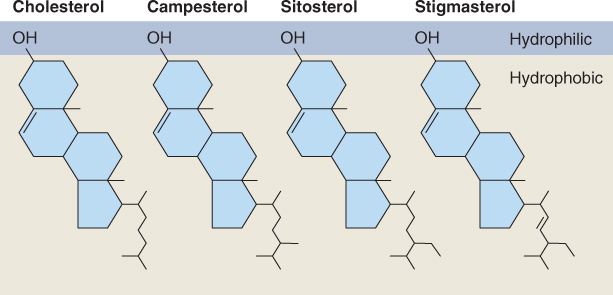
Diterpenoids and triterpenoids include both primary and secondary metabolites. For example, the diterpene kaurenoic acid is an essential intermediate in the synthesis of gibberellins in all plants (see Chapter 10) and is therefore a participant in primary metabolism. On the other hand abietic acid, similar to kaurenoic acid in structure and biosynthetic origin (Figure 15.20), is a component of resin and largely restricted to members of the Fabaceae and Pinaceae families. Because it is not involved in primary metabolism, it is by definition a secondary compound. An important chemical group derived from di- and triterpenoid precursors is the steroids. Steroids are widely distributed in plants, fungi and animals; they have essential roles in membrane structure and hormone signaling. Among the steroids of plants are phytosterols, natural constituents of vegetable oils with possible beneficial effects for human nutrition, and brassinosteroids, a class of plant hormone (see Chapter 10). Figure 15.21 shows some plant membrane sterols of triterpenoid origin. The distinctive structural feature is the sterane core, which comprises one cyclopentane (C5) and three cyclohexane (C6) rings.
Figure 15.20 Examples of a primary and a secondary diterpenoid metabolite. Kaurenoic acid is a precursor of the gibberellin group of essential hormones, and is therefore classified as a primary metabolite. The resin component abietic acid is a secondary metabolite.
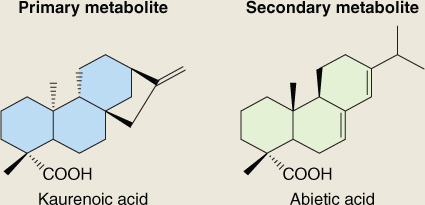
Figure 15.21 Steroids of plant membranes, showing amphipathic character. Cholesterol is a C27 molecule, campesterol is C28 and sitosterol and stigmasterol are C29. These sterols are derived from the straight-chain C30 triterpenoid precursor by cyclization to create the 4-ring C17 sterane core, followed by removal of one or more carbons.
The majority of tetraterpenoids are carotenoids, many of which (for instance, violaxanthin and lutein; see Figure 9.12) have essential roles in photobiology and oxygen metabolism across the taxonomic range of autotrophs and are therefore primary metabolites. Table 15.4 gives an example of a carotenoid secondary metabolite, capsorubin, which is responsible for the red color of the spice paprika. Apocarotenoids, the products of oxidative cleavage of tetraterpene carotenoids, are widely distributed in living organisms, and frequently perform important regulatory, receptor and signaling functions. The hormone abscisic acid (see Chapter 10) is the apocarotenoid product of asymmetrical cleavage of a C40 carotenoid.
The variety of terpenoids produced by plants is much wider than that produced by either microbes or animals. The accumulation, emission or secretion of large quantities of terpenoids by plants is almost always associated with the presence of specialized structures such as glandular trichomes, secretory cavities, resin ducts and blisters. These structures are usually non-photosynthetic and so are dependent on neighboring or remote tissues for the carbon and energy necessary to drive terpenoid synthesis. They are sources of defensive products, such as the diterpenoids of rosin from conifer species. The terpenoid essential oils emitted by the glandular epidermis of petals encourage insect pollination. Specialization of the epidermis is also associated with the formation and excretion of triterpenoid surface waxes, while lactiferous ducts produce certain triterpenoids and rubber. It is thought that, by separating bioactive and often harmful terpenoid products from the sites of metabolism in the major tissue types, a plant is able to avoid potential autotoxicity problems.
All terpenoids are biosynthesized from simple primary metabolites by a four-stage process. Stage 1 is the formation of isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP), the C5 structural units from which all isoprenoids are built. During stage 2 a series of prenyl diphosphate homologs are made by repetitive additions of IPP. Stage 3 is the synthesis of terpenoid skeletons from prenyl diphosphates, catalyzed by specific terpenoid synthases. Secondary enzymatic modifications of terpenoid skeletons during stage 4 result in the diversity of functional properties and chemical structures that characterize this family of natural products.
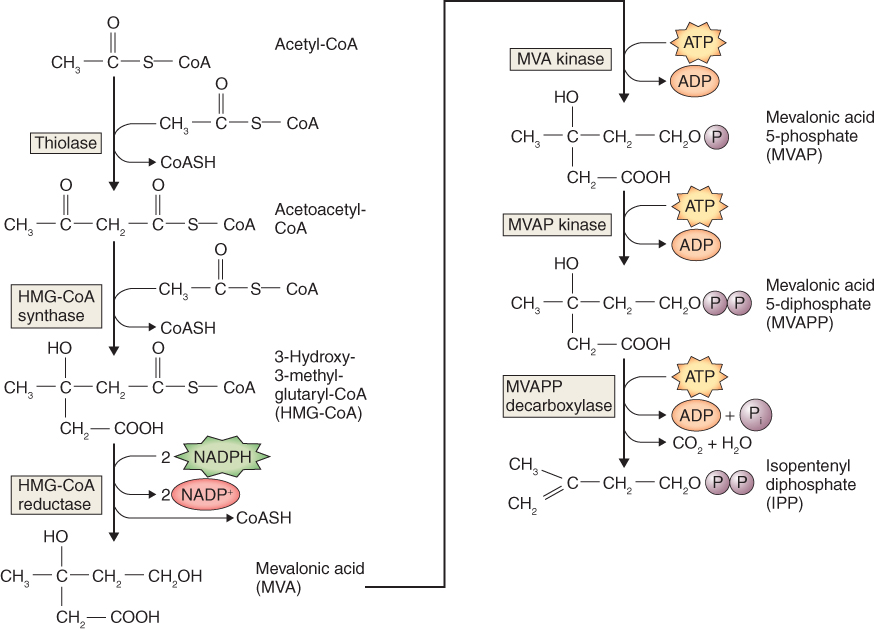
There are two pathways of IPP synthesis, one that occurs in the cytosol and a second that takes place in plastids. The synthesis of IPP in the cytosol (Figure 15.22) begins with a two-step condensation of three molecules of acetyl-CoA and a subsequent reduction to yield mevalonic acid (MVA), which gives this pathway its name. MVA is converted to IPP via two sequential, ATP-dependent phosphorylations and a decarboxylation. A critical regulatory reaction in the MVA pathway is that catalyzed by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR). HMGR is an ER-targeted enzyme and is encoded by a family of two to four genes, depending on species. Differential expression of HMG genes is thought to lead to tissue- and stress-specific biosynthesis of different types of isoprenoids.
Figure 15.22 The mevalonic acid (cytosolic) pathway of isopentenyl diphosphate biosynthesis.
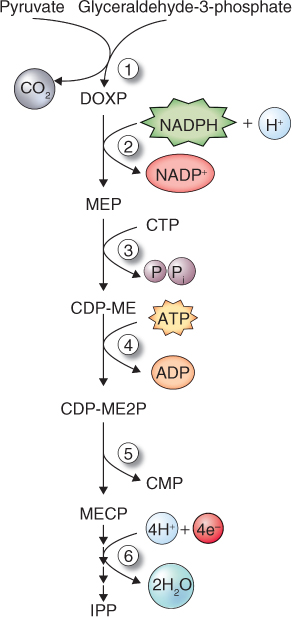
Recently, an alternative pathway of IPP formation has been shown to operate in the plastids of land plants and algae, as well as in cyanobacteria and other eubacteria, and certain eukaryotic parasites. It begins with the formation of 1-deoxy-D-xylulose-5-phosphate (DOXP; Figure 15.23). In general, the MVA pathway of IPP synthesis is the origin of phytosterols, sesquiterpenes and triterpenoids, whereas the DOXP pathway supports synthesis of plastid isoprenoids, notably carotenoids, phytol, plastoquinones and tocopherols as well as the hormones gibberellin and abscisic acid.
Figure 15.23 The DOXP pathway of IPP synthesis. Enzymes in the pathway are: (1) DOXP synthase, (2) DOXP reductoisomerase, (3) MEP cytidylyltransferase, (4) CDP-ME kinase, (5) MECP synthase and (6) synthase and reductase. CDP-ME, 4-diphosphocytidyl-2C-methyl-D-erythritol; CDP-ME2P, 4-diphosphocytidyl-2C-methyl-D-erythritol-2-phosphate; CMP, cytidine monophosphate; CTP, cytidine triphosphate; DOXP, 1-deoxy-D-xylulose-5-phosphate; IPP, isopentenyl diphosphate; MECP, 2C-methyl-D-erythritol-2,4-cyclodiphosphate; MEP, 4-diphosphocytidyl-2C-methyl-D-erythritol.
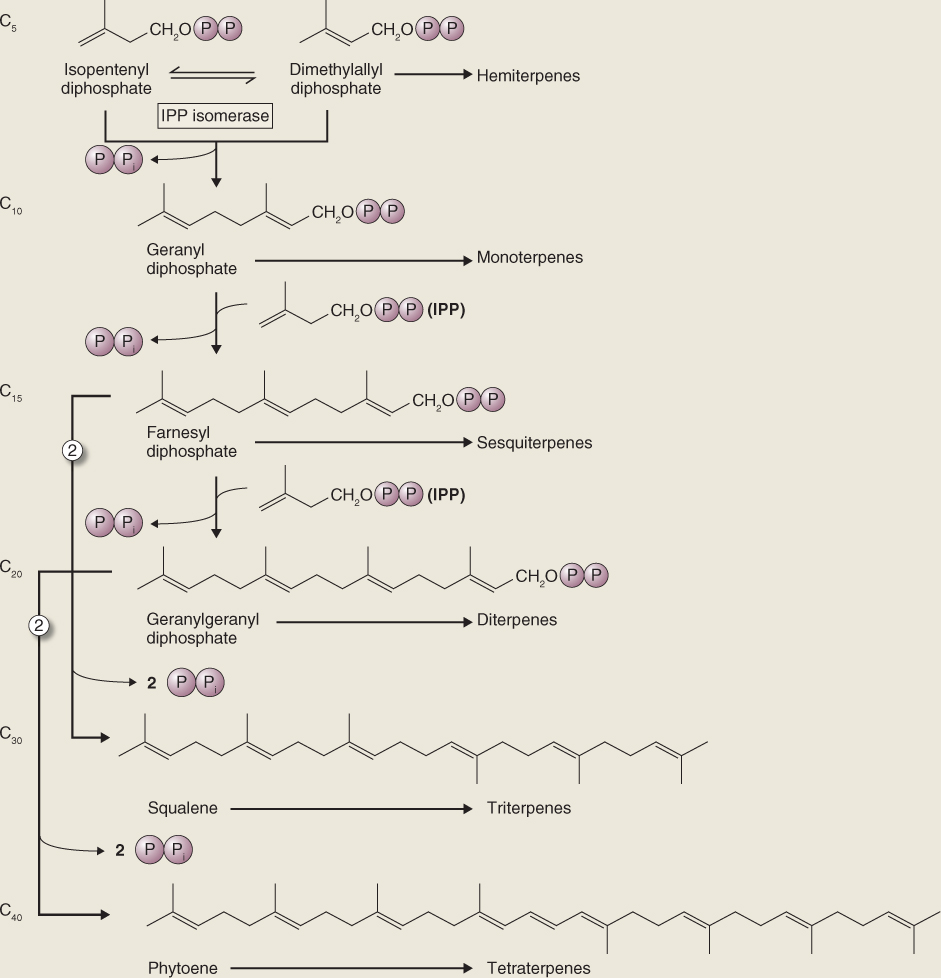
Isoprenoid biosynthesis requires both IPP and its isomer DMAPP. IPP and DMAPP are used by prenyltransferases to form the C10, C15, C20 and larger prenyl diphosphates that serve as precursors in terpenoid biosynthetic pathways (Figure 15.24). Successive units of IPP are added to an initial DMAPP primer, usually via head-to-tail condensations (see Figure 15.19). The IPP-forming step of the plastid DOXP pathway (Figure 15.23) also yields DMAPP, though the precise mechanism is not known. On the other hand, the cytosolic MVA pathway produces only IPP (Figure 15.22) and requires the action of the enzyme IPP isomerase to sustain equilibrium between IPP and DMAPP (Figure 15.24). IPP isomerase is of regulatory importance for the synthesis of the major terpenoid classes because the molar ratio of IPP to DMAPP needed to synthesize different terpenoid classes varies from 1:1 for monoterpenoids to 2:1 for sesquiterpenes and sterols, 3:1 for diterpenoids, carotenoids and phytol, and much higher for long-chain polyprenols and polyterpenoids.
Figure 15.24 Biosynthesis of the major subclasses of terpenoids from isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate. Prenyl transferases catalyze the formation of monoterpenes (C10), sesquiterpenes (C15) and diterpenes (C20) from the corresponding intermediates by sequential head-to-tail addition of C5 units. Triterpenes (C30) are formed from two C15 (farnesyl) units joined head to head, and tetraterpenes (C40) are formed from two C20 (geranylgeranyl) units joined head to head.
Hemiterpenoids are derived from the IPP–DMAPP equilibrium. For example, DMAPP is the direct precursor of isoprene, a volatile hemiterpenoid released from photosynthetically active tissues. Isoprene synthase (Equation 15.9) is a plastid-localized enzyme that catalyzes the light-dependent formation of isoprene from DMAPP. The ecophysiological function, if any, of isoprene production is unknown, although it is proposed that isoprene is involved in the process of acclimation to elevated temperatures and the synthetase enzyme has an unusually high temperature optimum. At about 500 million metric tons of carbon, annual foliar emissions of isoprene are of the same order of magnitude as those of the greenhouse gas methane. Isoprene is also a principal reactant in the formation of ozone in the troposphere.
Geranyl diphosphate (GPP), farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP) are the diphosphate ester precursors of mono-, sesqui- and diterpenoids, respectively, and are generated by the activities of prenyltransferase enzymes (Figure 15.24). The addition of one IPP moiety to the DMAPP primer yields the C10 intermediate GPP. Further reaction cycles result in FPP (C15) and GGPP (C20). Although prenyl units are mostly added head to tail, there are many instances of exceptions to this pattern, involving head-to-head and head-to-middle patterns (see Figure 15.19). An example is the pyrethrins, monoterpenoids with head-to-middle configurations. These compounds were originally identified in members of the family Asteraceae and have been widely used as insecticides both in their native form and as synthetic derivatives. Most of the new allylic double bonds introduced by prenyl transferases are in the trans configuration, though occasionally cis double bonds are created. This is the case in rubber, for example, where cis bonds account for the polymer's elasticity. Prenyl transferase reactions can attach groups other than IPP and are responsible for adding prenyl side-chains to compounds such as proteins and other non-terpenoids.
Terpene synthases are families of enzymes that are responsible for the formation of the enormous diversity of carbon skeletons characteristic of terpenoids; prenyl diphosphate esters are the substrates for these enzymes. Terpenoid synthases that produce cyclic products, the commonest type of isoprenoid structure, are also referred to as cyclases. Abietic acid (see Figure 15.20), a common diterpenoid acid of conifer resin, is an example of a product of synthase/cyclase activities. Resin is important for wound-sealing and is familiar as its fossilized form, amber. In some cases a particular terpene synthase can generate more than one reaction product. For example, pinene synthase yields both α- and β-pinene, widely distributed monoterpene toxins that are active against bark beetles and their pathogenic fungal symbionts. There are sesquiterpene synthases, also involved in the production of conifer resin, that are known to be capable of individually making more than 25 different products.
The C30 triterpenoids are generated by the head-to-head joining of two C15 FPP chains to produce squalene. Similarly, the C40 tetraterpenoids are derived from phytoene, the product of combining two molecules of C20 GGPP in head-to-head fashion (Figure 15.24). Squalene synthase and phytoene synthase are prenyl transferases with similar reaction mechanisms that involve a complex series of rearrangements necessary to bring the head end (C-1) carbons of the two prenyl diphosphate precursors together.
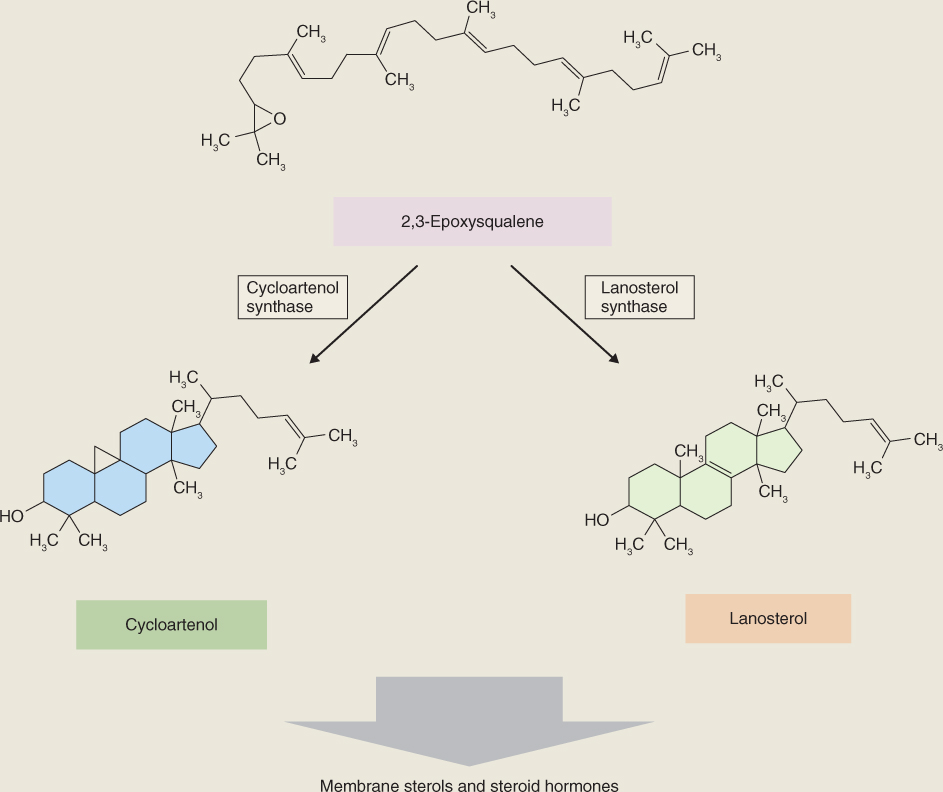
Steroids are cyclization products of squalene. An NADPH-dependent epoxidase (Equation 15.10) oxidizes squalene to 2,3-epoxysqualene, which is then cyclized to form a range of intermediates in phytosterol biosynthesis. Figure 15.25 shows cycloartenol and lanosterol, major cyclization products which, through a series of methylation and demethylation reactions, give rise to membrane sterols such as cholesterol, campesterol, sitosterol and stigmasterol (see Figure 15.21) and the brassinosteroid hormones (see Chapter 10).
Carotenoids are derived from phytoene via a series of desaturation and cyclization steps. Metabolic interconversions of carotenoids are important in protection against photoinhibition and oxidative damage, and are described further in Section 15.6. Carotenoid synthesis is also a central feature of fruit development in many species, contributing to color changes and other aspects of ripening, as discussed in Chapter 18.
Figure 15.25 Precursors of phytosteroids, formed by cyclization of epoxysqualene.
The final step in the overall scheme of terpenoid biosynthesis involves modifications of the parent skeletons produced by terpenoid synthases and results in the rich array of different structures and functional properties found among the isoprenoids of the plant kingdom. Among these secondary transformations are oxidations, reductions, isomerizations and conjugations. Some terpenoid skeletons are highly decorated. For instance, the anticancer drug taxol, obtained from yew (Taxus) species, is a diterpenoid nucleus (taxadiene; see Table 15.4) that is extensively modified by a complex pattern of hydroxylations and acylations. Saponins and cardenolides are powerful bioactive compounds that are toxic to herbivores. In these molecules, the basic terpenoid structure is modified by glycosylation. The widely used cardioactive drug digitoxigenin is the aglycone (sugarless) moiety derived from foxglove (Digitalis) cardenolides.
The experience of abiotic stress is not only normal for plants, it is the essential route by which information about the state of the environment is sensed and the physiological adjustments needed to insure survival are cued. Responses depend on the severity and duration of the stress, stage of development, tissue type and interactions between multiple stresses. Experiencing stress typically promotes changes in gene expression and metabolism. Reactions are frequently centered on altered patterns of secondary compounds as described in Section 15.2.4, and ultimately lead to localized or systemic modifications of physiology, development and life cycle. In the present section we will look at plant reactions to physico-chemical variables in the environment, particularly water (lack and excess), oxygen and its reactive derivatives, radiant energy (both heat and light) and mechanical influences. Although this discussion considers each type of factor individually, it is important to be aware of the extent to which different agents may have common modes of action on plant tissues. For example, salinity damage, drought stress and freezing injury can all be aspects of cytoplasmic trauma caused by dehydration.
The principles governing the uptake of water by plant cells, including the role of water potential (Ψw), were discussed in Chapter 14. Plant structure and growth are dependent on turgor pressure (see Chapter 12). The water content of a plant can be measured by subtracting its dry weight from its wet weight. The relative water content (RWC) with respect to a fully turgid plant is a useful parameter for measuring a plant's water status (Equation 15.11).
Leaf RWC for a plant in which root uptake of water and shoot transpiration are in balance is typically in the 85–95% range. For a given organ there is a critical RWC, below which there is tissue death. This value varies from species to species but is often less than 50%. In general, when the water potential of the soil drops, the plant's RWC also declines. This may occur as a result of drought or osmotic limitation of water availability due to salinity. Most plants, however, have the capacity to adjust water potential by internally accumulating osmotically active solutes. This has the effect of decreasing Ψw and sustaining the influx of water along the steeper gradient in water potential between soil and cell (see Chapter 14), thereby preserving a high RWC and maintaining viability. Here we discuss the cell biology of osmotic adjustment, mechanisms of water flow into cells, the products and regulation of water stress-related gene expression, and the mechanisms by which water status is sensed and signaled.
In order to take up water, a plant must maintain a water potential gradient between root and soil. Wilting is an early symptom that the rate of transpiration by leaves and stems exceeds the rate of water uptake from the soil. Many plants that tolerate drought or saline conditions avoid the harmful effects of declining RWC by regulating their solute potentials. Osmotic adjustment is an acclimatory process that involves the synthesis of compounds which increase the osmotic concentration of the cell and protect it from dehydration damage. Comparative physiological studies show that some of these compounds function in acclimation not only in plants but also in bacteria and animals. In view of the fundamental importance of water for life, it is not surprising that cellular mechanisms of regulating water potential will show a degree of commonality in all living organisms.
In plants, the compounds used for osmotic adjustments in the central vacuole are different from those used in the remainder of the cell. In the vacuole, which occupies most of the volume of the cell, inorganic salts are usually the major osmoticum. Vacuolar enzymes tolerate high salt concentrations. In the cytosol and the rest of the organelles, high salt concentrations are lethal and small organic molecules are used instead. Compounds that function in osmotic adjustment in the cytosol and non-vacuole organelles are referred to as compatible solutes or osmolytes. They are members of a relatively small group of chemically diverse, water-soluble, organic compounds (Figure 15.26) that can be accumulated to high concentrations without significantly interfering with cellular metabolism (hence the name ‘compatible’). For example, the cytosol and chloroplasts of salt-stressed spinach leaves (see Figure 15.4D) accumulate glycine betaine to more than 250 mm, whereas the concentration of this osmolyte in vacuolar sap is vanishingly small. The movement and differential distribution of osmolytes within and between cells are regulated by membrane-associated transporters. Accumulation of compatible osmolytes may have the additional benefit of protecting against other stresses. For example, mannitol and proline (Figure 15.26) are scavengers of hydroxyl radicals and could have a role in moderating the effects of reactive oxygen species (see Section 15.6.3).
Figure 15.26 Structures of some common cell osmolytes.
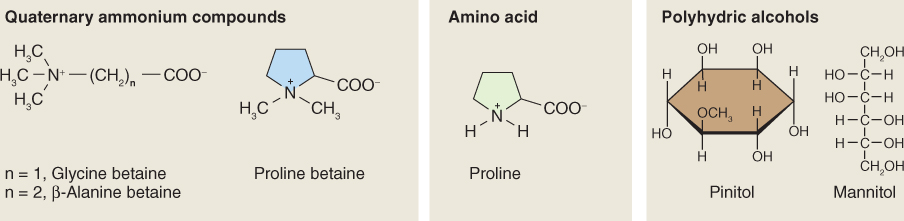
Some compatible solutes, e.g. proline, occur widely throughout the plant kingdom. Others are more limited in their distribution; for example β-alanine betaine (Figure 15.26) is only found in a few species of the family Plumbaginaceae. Monomeric sugars derived from polymers, notably glucose from starch and fructose from fructans, can act as effective compatible solutes under stress conditions. Different mechanisms for solute accumulation are recognized. In some cases there is irreversible synthesis en masse. In others, buildup is achieved by shifting normal turnover toward synthesis and away from breakdown. Glucose derived from starch is an example of a solute released from an osmotically inactive polymer that can be readily repolymerized when the stress is relieved.
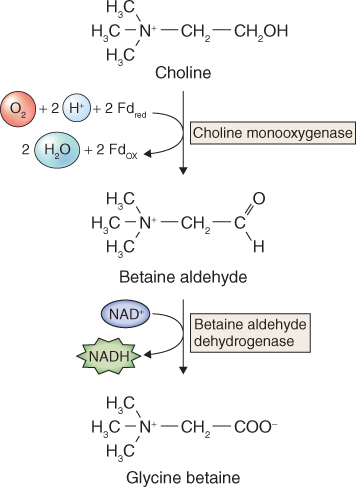
Mass synthesis of a compatible osmolyte in response to water limitation is illustrated by glycine betaine (GB) (Figure 15.26). Glycine betaine is a quaternary ammonium compound that acts as a compatible solute in a wide range of animals, bacteria and some halophytic (salt-tolerant) and drought-tolerant angiosperms. In angiosperms, it is abundant mainly in chloroplasts, where it maintains photosynthetic efficiency under osmotic stress by protecting the integrity of thylakoid membranes. Species such as maize (Zea mays), sugar beet (Beta vulgaris), barley (Hordeum vulgare) and spinach (Spinacia oleracea) are natural accumulators of GB in response to salt, drought or low-temperature stresses. GB is synthesized in chloroplasts from choline, which in turn is derived from the amino acid serine and the turnover of membrane phospholipid. Choline is dehydrogenated to betaine aldehyde by a monooxygenase that uses photosynthetically-reduced ferredoxin as a co-substrate. Betaine aldehyde is then converted to GB by betaine aldehyde dehydrogenase (Figure 15.27). The supply of choline is often the rate-determining step in GB biosynthesis. The activities of both enzymes, and the abundance of the mRNA transcripts of the genes that encode them, increase markedly under conditions of osmotic stress. Transcript levels decline when the stress is removed, but in the absence of a breakdown pathway, accumulation of GB itself is irreversible. Other routes of GB biosynthesis are known, but the one that originates with choline is common to all GB-accumulating plant species. Genetically engineering enzymes of GB biosynthesis into plants that normally fail to accumulate GB has been shown to improve tolerance of osmotic stress.
Figure 15.27 Biosynthesis of glycine betaine from choline.
The amino acid proline (see Figure 15.26) is an example of a compatible osmolyte that accumulates in the cytosol in response to a range of environmental stresses. Unlike GB, proline is rapidly catabolized when the stress is relieved; this provides breakdown products that are thought to contribute to the energy requirements of recovery and repair. In addition to its role as an osmolyte, proline supports the stabilization of proteins, membranes and other subcellular structures, acts as a free radical scavenger, and buffers cellular metabolism against fluctuations in redox and pH conditions. Moreover, expression of a number of salt stress-responsive genes is known to be under the control of promoters that possess proline responsive elements. There are two different biosynthetic pathways for proline in plants. The one that originates in the amino acid ornithine is less well understood than the major route from glutamate (Equation 15.12), which is also the pathway that operates in bacteria.

15.12B Spontaneous cyclization of glutamate γ-semialdehyde
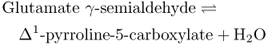
15.12C Δ1-pyrroline-5-carboxylate reductase
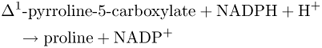
Formation of glutamate γ-semialdehyde (GSA) by the bifunctional enzyme Δ1-pyrroline-5-carboxylate synthetase is the rate-limiting step and is subject to allosteric feedback regulation by proline. In coordination with tissue proline levels, transcripts of the gene encoding Δ1-pyrroline-5-carboxylate synthetase accumulate rapidly when osmotic stress is imposed and decrease when the plant is rehydrated. The enzyme proline dehydrogenase catalyzes conversion of proline back to pyrroline-5-carboxylate. The abundance of proline dehydrogenase mRNA under dehydration/rehydration conditions is the reciprocal of proline content and pyrroline-5-carboxylate synthetase transcription. By balancing stress-sensitive synthesis and catabolism in this way, efficient control of proline content can be exerted through metabolic turnover.
A final group of compatible solutes considered here is the polyhydric alcohols, which include mannitol and pinitol (see Figure 15.26). Mannitol, a sugar alcohol, represents a substantial proportion of carbohydrates in some plant species, such as celery (Apium graveolens). It accumulates in response to osmotic stress as a result of decreased rates of consumption and reduced competition from sucrose synthesis for precursors. High concentrations of the cyclic sugar alcohol pinitol, found in members of the Pinaceae, Fabaceae and Caryophyllaceae families, are characteristic of halophytic and drought-adapted species. Pinitol is located in chloroplasts and the cytosol but is absent from vacuoles. Pinitol synthesis is transcriptionally regulated. Plants that have been genetically engineered to accumulate pinitol, mannitol or proline have been shown to have enhanced resistance to osmotic stresses.
The accumulation of compatible solutes in response to drought and salt stress enables a plant to acclimate to low water potentials. For osmotic adjustment to maintain integrity of cell structure and function, it must also control ion concentrations and transport. This is particularly significant for plants growing under saline conditions, in the presence of potentially toxic amounts of specific ions, notably Na+. In addition to its direct osmotic effect, Na+ can be toxic as a consequence of interference with the uptake of K+, an essential mineral nutrient (see Chapter 13). No Na+-selective ion channels have been found in plants. The major route of Na+ influx across the plasma membrane is via voltage-independent non-specific cation channels (VI-NSCCs). Intracellular Ca2+ status has a direct influence on Na+ flux since Ca2+ blocks VI-NSCC activity. Active export of Na+ across the plasma membrane counteracts accumulation of Na+ in the cytosol. Additionally, Na+/H+ antiporters in the tonoplast become active in response to salt stress and can move cytosolic Na+ into the vacuole.
Acclimatory responses to drought and salinity, such as the accumulation of compatible solutes and redistribution of ions, are associated with alterations in gene expression under the control of regulatory networks sensitive to abiotic stresses. A comparison of global transcription patterns in water-deficient and -sufficient tissues reveals a range of stress-inducible genes, many of which have identifiable roles in acclimation. The protein products of such genes can be broadly classified into two groups (Figure 15.28). The functional group includes chaperones, antifreeze proteins, enzymes of osmolyte biosynthesis, aquaporins, sugar and proline transporters, detoxification enzymes, and various proteases. The regulatory group, comprising protein factors involved in further regulation of signal transduction and stress-responsive gene expression, includes transcription factors, protein kinases and phosphatases, enzymes of phospholipid metabolism, and components of the calmodulin system (see Chapter 13).
Figure 15.28 Drought stress-inducible genes classified into those whose products are responsible for executing stress responses (functional proteins) and those concerned with signal transduction and control of gene expression (regulatory proteins).

Among the functional gene products, late embryogenesis-abundant (LEA) proteins are of particular interest in the context of stress responses during the plant life cycle. Desiccation is a normal phase of embryo maturation, as the seed dries out in preparation for quiescence and dispersal. Desiccation-related induction of LEA genes was originally identified in studies of seed maturation, but subsequently LEA proteins have also been observed to increase in abundance in vegetative tissues experiencing water deficit. The 400 or so LEA proteins are classified into up to seven families which differ in species of origin and structural motifs. LEAs are in turn members of a widespread group of proteins referred to as hydrophilins, characterized by high hydrophilicity and a glycine content of more than 6%. Hydrophilins occur throughout the archeal, eubacterial and eukaryotic domains and are believed to be components of a ubiquitous stress-response mechanism. Overexpression, in one species, of LEA genes from another species has been observed to improve drought and salinity resistance in plants, bacteria and yeast. There are, however, reports of instances of no or detrimental effects. It is not clear how LEAs work. A number of LEA proteins have been shown to protect against dehydration-induced enzyme inactivation in vitro. The common molecular motifs among the members of different LEA families indicate that most LEAs are intrinsically unstructured proteins, existing principally as random coils in solution. This may allow LEAs to prevent enzyme inactivation by closing in on their target enzymes and maintaining a water-rich environment that preserves protein integrity. LEAs may also form a tight hydrogen-bonding network in dehydrating cytoplasm that retains residual water and stabilizes cell structures.
As Figure 15.28 indicates, the hormone abscisic acid (ABA) has a role in plant responses to water limitation. Drought and high salinity trigger strong increases in endogenous ABA content, accompanied by major changes in gene expression and acclimatory and adaptive physiological responses. Mutants deficient in ABA biosynthesis are impaired in their responses to drought. Stomatal closure, mediated by ABA-triggered changes of ion fluxes in guard cells, is a fast response to water limitation (see Chapter 14). ABA-deficient mutants are abnormally wilty due to unrestrained transpiration. Drought sends an immediate hydraulic signal (possibly a rapid change in xylem tension) that is able to stimulate ABA biosynthesis over long distances. ABA is formed primarily in the vascular system and is rapidly distributed to neighboring tissues. ABA synthesized in response to water deficiency is swiftly broken down on rehydration. Abscisic acid 8′-hydroxylase, a key enzyme in ABA catabolism, is activated in vascular tissues and stomatal guard cells within minutes of exposing shoots to high humidity, resulting in the formation of inactive phaseic acid.
Several drought-sensitive genes are induced by exogenous ABA treatment, including those that encode enzymes of compatible solute metabolism. For example, P5CS1, the gene encoding the proline-synthesizing enzyme Δ1-pyrroline-5-carboxylate synthetase (see Equation 15.12A), is regulated by an ABA-responsive subfamily of bZIP (basic leucine Zipper domain) transcription factors. A bZIP-binding ABA responsive element has also been identified in the gene for Em, a wheat LEA. Transcription factors of the MYC, MYB and NAC families have also been implicated in ABA regulation of responses to, and tolerance of, drought and salinity stress. ABA does not regulate all stress-induced genes, however. Accumulation of many carbohydrate-related compatible solutes, notably oligosaccharides of the raffinose family, occurs via an ABA-independent pathway. Genes in this pathway have a drought-responsive element in their promoter regions and are probably regulated by distinct or interacting signal transduction networks.
From water deficiency we turn now to the problems of too much water. Plants, like most eukaryotes, are obligate aerobes. Because the diffusion coefficient of oxygen in air is about 10 000 times greater than that in water, flooding has the effect of blocking the entry of O2 into the soil so that roots and underground stems cannot carry out respiration. Normal aerobic respiration yields up to 32 mol of ATP per mole of hexose, whereas respiration in the absence of oxygen produces only 2 mol ATP (see Chapter 7). To survive short-term flooding, plants must be able to make sufficient ATP, regenerate NADP+ and NAD+, and avoid accumulation of toxic metabolites. Flooding stimulates fermentative respiration, ethanol formation and the Pasteur effect (see Chapter 7). Many aquatic, wetland and shoreline species have evolved adaptations that allow them to survive or thrive under conditions of periodic or continuous immersion. However, tolerance of flooding is limited in vascular plants. In addition to its ecological significance in determining vegetation patterns, response to flooding is agriculturally important since waterlogged soil severely depresses crop yields.
Plant or cellular oxygen status can be defined as normoxic (normal O2 levels), hypoxic (reduced O2 levels) or anoxic (lacking O2) (Table 15.5). Periods of oxygen deficit cause a shift from aerobic to anaerobic metabolism. Initially this is a direct metabolic response to O2 deprivation, which is then followed by changes in gene expression. Over the longer term, acclimation to hypoxic or anoxic conditions can take the form of developmental responses involving modifications in growth behavior, morphology and anatomy. Injury caused by anoxia or hypoxia is a consequence of acidification of cytoplasm, which in turn results in greatly diminished protein synthesis, mitochondrial degradation, inhibition of cell division and elongation, disrupted ion transport, and root meristem cell death. Flooding-tolerant plants are able to avoid cytoplasmic acidosis and continue to make ATP during short-term inundation by stimulating ethanolic fermentation. Seedlings of maize (Zea mays), for example, can survive anoxia for up to 5 days before experiencing injury. Exposure to an episode of hypoxia can greatly enhance survival in a subsequent period of anoxia, a classic acclimatory or hardening response. Hypoxic pretreatment results in greater specific activity of hexokinase, fructokinase, pyruvate kinase and other enzymes of glycolysis and ethanolic fermentation (see Chapter 7). This, in turn stimulates glycolytic flux, ATP production and the production of cytoplasm-acidifying lactate. The shift from aerobic metabolism to glycolytic fermentation is accompanied by rapid and dramatic changes in gene expression patterns and activities, which are regulated at both the transcriptional and the post-transcriptional level.
Table 15.5 Responses of respiratory metabolism to oxygen deprivation.
| Oxygen status | Effect on metabolism |
| Normoxic (aerobic) | Aerobic respiration proceeds normally. Almost all ATP production results from oxidative phosphorylation |
| Hypoxic | The partial pressure of O2 limits ATP production by oxidative phosphorylation. Glycolysis accounts for a larger proportion of ATP yield than under normoxic conditions. Metabolic and developmental changes are stimulated that result in adaptation to a low-oxygen environment |
| Anoxic (anaerobic) | ATP is produced only by way of glycolysis. Cells exhibit low ATP contents, diminished protein synthesis and impaired division and elongation. If anoxic conditions persist, many plant cells die |
The mechanism by which plants sense oxygen deprivation is not known in detail, but there is clear evidence of interaction with metabolic and signaling networks associated with reactive oxygen species (ROS; see Section 15.6.3). Oxygen deprivation represses the expression of most genes, but upregulates an important subset, including genes encoding enzymes of sucrose and starch degradation, glycolysis and ethanol fermentation. Isoforms of alcohol dehydrogenase are differentially expressed in aerobic and anoxic cells because of sequence differences in their promoters. The promoters of many hypoxia-stimulated genes include the so-called anaerobic response element that interacts with hypoxia-responsive transcription regulators of the ERF (ethylene responsive factor) family.
Wetland plants, which are adapted to long-term flooding, possess anatomical, morphological and physiological features that permit survival in waterlogged soils. A particularly striking structural response to flooding and long-term anoxia is the differentiation of aerenchyma, continuous columnar intracellular spaces developed in root cortical tissues which facilitate the transport of O2 from aerial structures to submerged roots. The production of aerenchyma, which is under the control of ethylene, is an example of programmed cell death; this is discussed in further detail in Chapter 18. Other adaptive features are adventitious roots, a thickened root hypodermis that reduces O2 loss to the anaerobic soil, and lenticels (openings in the periderm of the stem that allow gas exchange). In addition some plants produce pneumatophores, shallow, negatively gravitropic roots that grow out of the water.
Rice (Oryza sativa) is the most important staple food for much of the world's population. Yield of rice per hectare has more than doubled since the 1960s, but further doubling will be necessary to meet projected nutritional requirements by the middle of the present century. More than 30% by area of Asian and 40% of African rice is grown in paddy fields with water depths from 15 cm to more than 50 cm. High-yielding rice varieties cannot survive deep water and prolonged submergence. Rice varieties that are adapted to deep water and those that are submergence-tolerant endure flooding but tend to be less productive.
Deep-water rice survives by outgrowing slowly rising floodwaters, which can reach 4 m in depth. The contrasting strategy of submergence-tolerant rice is to suppress elongation and adopt a quiescent state (Figure 15.29). The control of stature by gibberellin and ‘Green Revolution’ genes of the Rht family is described in detail in Chapter 12. Elongation growth in deep-water rice is under the control of SNORKEL and SUBMERGENCE ethylene response factor genes. Transcription factors encoded by SNORKEL1 and SNORKEL2 (SK1 and SK2) are activated by ethylene and promote gibberellic acid (GA)-stimulated extension growth. Ethylene accumulated in submerged tissues also inhibits the synthesis, and promotes the breakdown, of ABA, a negative regulator of GA action. In the quiescence strategy the ethylene-induced action of the SUBMERGENCE (SUB1A-1) transcription factor on SLENDER RICE-1 (SLR1) and SLR LIKE-1 (SLRL1) expression suppresses GA-mediated growth. Transfer of SK or SUB genes into high-yielding varieties that are normally flooding-intolerant has the prospect of contributing significantly to improving the quality and quantity of rice produced on marginal land.
Figure 15.29 Strategies adopted by rice in response to flooding. The escape strategy of deep-water rice (shown on the left) is mediated by two SNORKEL genes, SK1 and SK2. In submergence-tolerant varieties (shown on the right), quiescence is the result of interaction between the ethylene-sensitive SUBMERGENCE gene SUB1A-1 and the stature-determining genes SLR1 and SLRL1. Abscisic acid (ABA) inhibits gibberellic acid (GA)-mediated growth. Ethylene accumulated under submergence conditions promotes elongation by downregulating ABA synthesis and stimulating ABA degradation.
Molecular oxygen (O2) accounts for about 20% by volume of the Earth's atmosphere. Living organisms have been exposed to atmospheric oxygen since the first oxygenic photoautotrophs evolved 2.7 billion years ago. Because many partially reduced oxygen derivatives are highly reactive and potentially toxic to cells, evolution has equipped all aerobic organisms with efficient mechanisms for sensing, scavenging and exploiting reactive oxygen species (ROS). ROS have multiple physiological roles in plants. Reactive oxygen is a substrate in a number of important biochemical reactions, for example the formation of lignin. ROS also participate in signaling networks, redox control of metabolism and development, and responses to biotic stress. Most significantly for the present discussion, many environmental factors promote the formation of active oxygen species that are highly destructive to lipids, nucleic acids and proteins, resulting in cell damage or death. Oxidative stress mediated by ROS is a common factor in adverse reactions to air pollution, oxidant-forming herbicides, heavy metals, drought, heat and cold, wounding, and ultraviolet and excess visible light (Figure 15.30).
Figure 15.30 Oxidative stress mediated by reactive oxygen species underlies the entire range of plant responses to unfavorable abiotic and biotic factors in the environment.
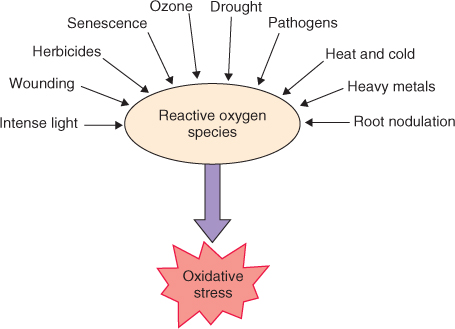
The various forms of reactive oxygen are given in Table 15.6. Singlet oxygen is the product of photosensitization and is discussed in the context of responses to light in Section 15.6.6. Here we consider the biological effects and metabolism of the superoxide anion (O2·−), hydrogen peroxide (H2O2) and ozone (O3). Interconversion, scavenging and elimination of these reactive molecules are the task of defense systems, comprising antioxidant enzymes and non-enzymatic antioxidant compounds that are present in various subcellular compartments. Figure 15.31 summarizes the activities and components of the major antioxidant systems of plants. The central importance of these systems is emphasized by the improvement in oxidative stress tolerance observed in plants selected or genetically engineered for increased synthesis of antioxidants and antioxidant enzymes.
Figure 15.31 Reactive oxygen species interconversions and the functions of the major enzyme and non-enzymatic antioxidants. The ascorbate–glutathione cycle is highlighted. Superoxide radicals are eliminated by conversion to hydrogen peroxide (H2O2), a reaction catalyzed by superoxide dismutase. Catalase converts H2O2 to O2 and H2O. H2O2 may also be reduced to water by reaction with ascorbate. Ascorbate is regenerated by enzymatic reduction of monodehydroascorbate in the plastids. Alternatively, monodehydroascorbate may spontaneously dismutate to dehydroascorbate. Dehydroascorbate reductase then catalyzes the reaction of dehydroascorbate with reduced glutathione (GSH) to produce ascorbate and oxidized glutathione (GSSG). GSSG is reduced to GSH in an NADPH-dependent reaction catalyzed by glutathione reductase. Hydroxyl ions and singlet oxygen are eliminated in the glutathione pathway; damage by singlet oxygen is also diminished by non-enzymatic antioxidants.
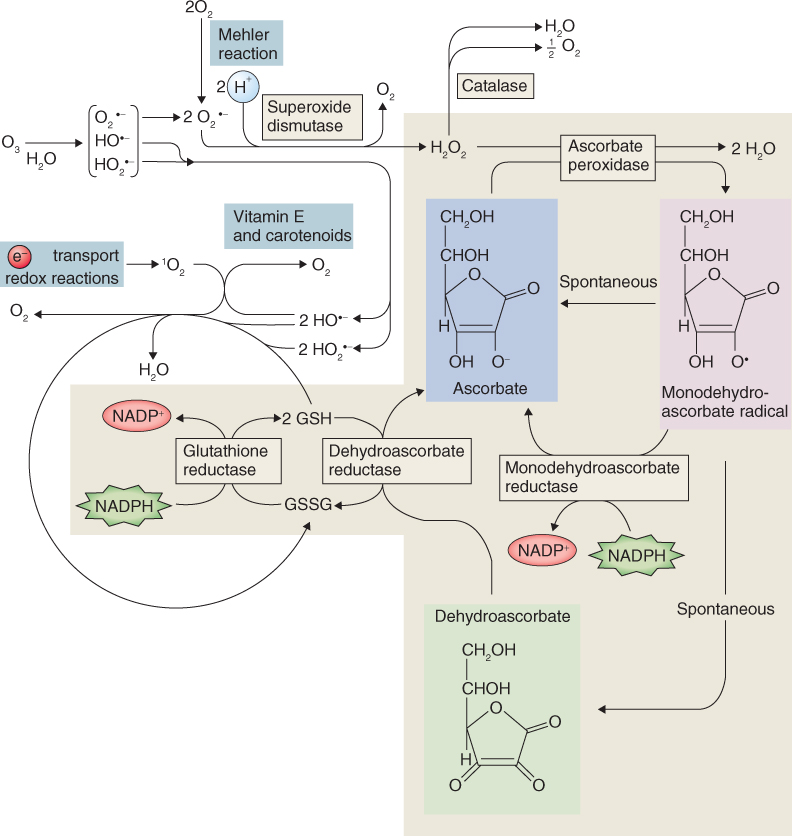
Table 15.6 Forms of reactive oxygen.
| Compound | Shorthand notation(s) | Sources |
| Molecular oxygen (triplet ground state) | O2; 3Σ | Most common form of dioxygen gas |
| Singlet oxygen (first excited singlet state) | 1O2; 1Δ | UV irradiation, photoinhibition, photosystem II e− transfer reactions (chloroplasts) |
| Superoxide anion | O2·− | Mitochondrial e− transfer reactions, Mehler reaction in chloroplasts (reduction of O2 by iron–sulfur center Fx of photosystem I), glyoxysomal photorespiration, peroxisome activity, plasma membrane, oxidation of paraquat, nitrogen fixation, defense against pathogens, reaction of O3 and OH− in apoplastic space |
| Hydrogen peroxide | H2O2 | Photorespiration, β-oxidation, proton-induced decomposition of O2·−, defense against pathogens |
| Hydroxyl radical | HO·− | Decomposition of O3 in presence of protons in apoplastic space, defense against pathogens |
| Perhydroxyl radical | HO2·− | Reaction of O3 and OH− in apoplastic space |
| Ozone | O3 | Electrical discharge or UV radiation in stratosphere, reactions involving combustion products of fossil fuels and UV radiation in troposphere |
Superoxide is the product of electron transport chains in the chloroplast and mitochondrion as well as a range of enzymes, including NAD(P)H oxidases, xanthine oxidase, lipoxygenase and cytochrome P450 monooxygenases. Superoxide anions are also produced in the chloroplast by the so-called Mehler reaction, when electrons are transferred directly from photosystem I to oxygen.
Hydrogen peroxide is formed from superoxide in a type of reaction known as dismutation, in which a chemical species is simultaneously reduced and oxidized so as to form two different products. The biochemical reaction between O2·− and H+ to produce H2O2 and O2 is catalyzed by the enzyme superoxide dismutase (SOD; Figure 15.31). Different SODs are distinguished by their metal cofactors and subcellular location. Cu/Zn SOD occurs in cytosol, peroxisomes and plastids. It is also found in root nodules. Mn SOD is mitochondrial, and Fe SOD is located in the plastid. H2O2 is also a normal product of photosynthesis and photorespiration (see Chapter 9) as well as of a number of enzymes including glucose oxidases, amino acid oxidases, cell wall-bound peroxidases and plasma membrane NADPH oxidases. Enzymatic detoxification of H2O2 occurs via catalase or through a redox cycle catalyzed by ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase and glutathione reductase (Figure 15.31). Ascorbate (vitamin C), one of the reactants in this cycle, is a soluble, sugar acid antioxidant distributed throughout cytosol, plastids, vacuoles and apoplast. Another reactant, glutathione (see Chapter 13), is a tripeptide thiol antioxidant found in cytosol, mitochondria and plastids. H2O2, unlike other reactive forms of oxygen, is not a labile, short-lived radical and is a normal, measurable constituent of unstressed plant tissues. Under some circumstances, however, such as the presence of transition metal ions (Fenton reaction; see Chapter 13), H2O2 can be a source of the extremely harmful hydroxyl (HO·−) and perhydroxyl (HO2·−) radicals.
Ozone is a pollutant that causes injury to living organisms by oxidative stress mediated by ROS. Ozone is a normal component of the stratosphere, where it carries out the essential job of shielding the Earth from harmful ultraviolet radiation. However, living organisms are increasingly exposed to damaging levels of ozone in the lower atmosphere (troposphere) that are generated from reactions of O2 with anthropogenic hydrocarbons and oxides of nitrogen (NOx) and sulfur (SOx) under the influence of solar ultraviolet radiation. Plant responses to ozone include impaired photosynthesis, leaf injury, reduced growth of shoots and roots and accelerated senescence, all of which contribute to significant reductions in yields from crop species.
Because of the potential for injury that results from the uncontrolled propagation of oxygen-containing radicals, the steady-state level of ROS in cells needs to be tightly managed. More than 150 genes have been identified in the ROS regulatory network, encoding a range of ROS-scavenging and ROS-producing proteins with a high degree of functional redundancy (a protein is functionally redundant if its activity can be substituted by that of one or more of the other proteins in the network). Plant cells sense ROS through receptor proteins and redox-sensitive transcription factors and by direct inhibition of signal-transducing phosphorylation cascades. Most of the proposed mechanisms for sensing cell redox status are based on the reversible formation and breakage of disulfide bridges between cysteine residues in polypeptide chains. Equation 15.13 shows the making and breaking of an S-S bridge between polypeptides P1 and P2 under the influence of the redox mediator A. The resulting change in protein conformation and/or enzyme activity triggers the signal transduction network, which in turn leads to changes in gene expression and the corresponding acclimatory or injury response.
Prominent among the genes whose expression is enhanced by oxidative stresses are those that encode enzymes of antioxidant synthesis and metabolism. Table 15.7 lists some example enzymes and illustrates the degree of cross-talk between oxidative and other stresses. This interaction between stress pathways is mediated in part by plant hormones such as abscisic acid, salicylic acid, jasmonic acid and ethylene (see Chapter 10). The regulation of catalase during aging and senescence, as described in Chapter 18, is an example of such an interaction.
Table 15.7 Antioxidants and antioxidant enzymes and the stress conditions that stimulate an increase in their levels or activities.
| Antioxidant or antioxidant enzyme | Stress conditions |
| Ascorbate peroxidase | Drought, high CO2, high light intensity, ozone, paraquat |
| Catalase | Chilling, aging |
| Glutathione | Chilling, drought, γ-irradiation, heat stress, high CO2, ozone, SO2 |
| Glutathione reductase | Chilling, drought, high CO2, ozone, paraquat |
| Superoxide dismutase | Chilling, high CO2, high light, increased O2, ozone, paraquat, SO2 |
The nature of the stress experienced by plants exposed to suboptimal temperatures is dependent on the physical states of water and cell membranes. Cooling to below the optimal temperature range causes metabolism, growth and development to slow. Initially this is a direct thermodynamic effect on rates of chemical reactions. Longer periods of exposure to low temperatures have more complex physiological consequences that depend on the temperature range, the adaptive features of the species and the extent to which it is able to acclimate. In discussing plant responses to low temperature, it is important to distinguish between chilling (temperatures in the range 0–15 °C, where water is in the liquid state) and freezing (below the temperature of ice formation). Plants of temperate regions are generally able to survive chilling temperatures. Species adapted to warmer climates are often chilling-sensitive and will show signs of damage after quite short exposures to temperatures below a defined lower limit which, for some tropical plants, could be well above 10 °C. Response to freezing is quantitative and statistical; that is, death in a genetically uniform population occurs over a temperature range rather than at a single threshold value. A widely used index of freezing sensitivity is LT50, the temperature that is lethal for 50% of the population. LT50 varies with species and genotype, and can be altered by acclimation at low, non-lethal temperatures. Thus non-acclimated plants of winter varieties of rye (Secale cereale)—among the most freezing-tolerant crop species—may have an LT50 of − 5 °C, but exposure to a period of chilling temperatures can lower this to − 30 °C. Some trees of northern latitudes may experience winter temperatures of − 70 °C and acclimated tissues of such species have been shown to have correspondingly low LT50 values.
Many of the mechanisms underlying responses to low-temperature stresses are shared with those related to water limitation (see Section 15.6.1) and the influence of ROS (Section 15.6.3). Freezing injury, for example, is largely a result of the formation of ice crystals, which in turn lead to dehydration, ionic imbalance and damage to membrane structure and function (Figure 15.32). Conditions that support acclimation to water stress frequently improve freezing tolerance and vice versa. Compatible solutes (see Section 15.6.1) are effective cryoprotectants (that is, chemicals that prevent low-temperature damage) and acclimation to freezing is commonly associated with the accumulation of carbohydrates, amino acids and amines. Freezing sensitivity and susceptibility to pathogens is a further example of cross-acclimation between stresses. A number of pathogenesis-related (PR) proteins (see Section 15.7.1) have been shown to have antifreeze properties.
Figure 15.32 Freezing damage to plant cells. Ice formation in the wall withdraws water from the cell and results in dehydration injury, which shares a number of physiological features with drought stress responses.
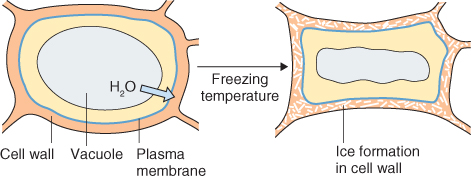
The primary cellular basis of chilling injury differs from that of freezing damage. Much research has been aimed at linking the threshold below which chilling-sensitive species display symptoms of damage to a so-called phase transition in the state of membrane lipids, from liquid crystalline to gel (Figure 15.33). Gel phase lipids disrupt membrane fluidity, integrity and function and lead to disturbed metabolism, injury and death. The critical phase transition temperature for a given lipid is related to the degree of saturation of its constituent fatty acids (see Chapter 2). For example, pure phosphatidylcholine in which the glycerol moiety is esterified with two stearic acid residues (saturated C18 fatty acid) has a gel–liquid crystalline transition temperature in water of 58 °C. Replacing one of the stearates with the 18:1 unsaturated fatty acid oleic acid reduces the phase transition temperature to 3 °C. The relationship between chilling sensitivity and degree of saturation of membrane lipids is complex, however, and only broad correlations have been established. The precise cellular mechanism of injury in chilling-sensitive species remains to be established.
Figure 15.33 Membrane phospholipid undergoes a reversible state transition from gel (Lβ) to liquid crystalline (Lα) at a critical temperature. The change of state, which is strongly endothermic, is detected by calorimetric measurements as a peak of heat absorption.
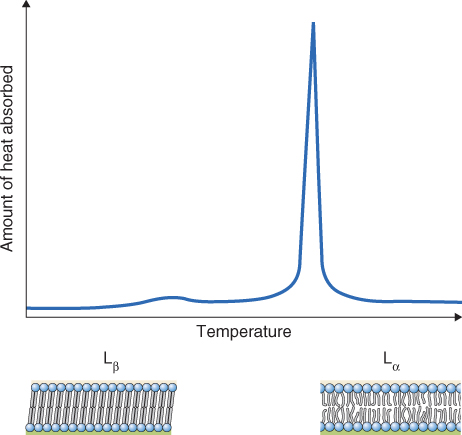
Membrane fluidity is one possible way in which plant cells can sense cold stress. Metabolite concentrations, protein conformation and redox status are other temperature-sensitive factors through which chilling may be perceived and transduced into changes in COR (COLD RESPONSIVE) gene expression. Recent evidence also implicates chromosomal DNA–histone interactions (nucleosomes; see Chapter 3) in the coordinated activation and deactivation of genes in response to shifts in temperature. Among the CORs are genes encoding transcription factors of the ERF family, genes of the ABA-responsive and ABA-independent drought response pathways (see Section 15.6.1) and genes for antioxidant metabolism (see Table 15.7). A distinctive and visible reaction to chilling (and other stresses) in many plant species is the accumulation of red anthocyanins, indicative of activation of phenylpropanoid and other secondary metabolic pathways. The function of such pigments in stressed tissue is not certain, but there is evidence for a role in defense against light and/or oxidative damage.
In contrast to the diversity of responses to suboptimal temperatures, most plants react uniformly to heat stress by invoking a physiological syndrome that shares basic features with that of organisms across the taxonomic range, from bacteria to vertebrates. Gene expression is altered, leading to the accumulation of heat shock proteins (HSPs; Figure 15.34), a family of proteins whose structure is conserved among different organisms. HSPs generally function as molecular chaperones; that is, they interact with mature or newly synthesized proteins to prevent aggregation or promote disaggregation, to assist folding or refolding of polypeptide chains, to facilitate protein import and translocation, and to participate in signal transduction and transcriptional activation (see Chapter 5).
Figure 15.34 Heat shock proteins (HSPs) accumulated by soybean seedlings in response to high temperature (40 °C compared with 28 °C control), separated by two-dimensional gel electrophoresis. (A, C) Silver-stained total proteins. (B, D) Newly synthesized proteins in gels (A) and (C), visualized by labeling with radioactive amino acid and fluorography. The vertical number scale alongside each gel represents polypeptide molecular weight (kDa) based on mobilities of standard proteins.

The heat shock response can often be detected in tissues experiencing temperatures as little as 5 °C above the growth optimum. It defends against damage to organelles, cytoskeleton and membrane function. Plants can acclimate to heat stress if exposed to a period of non-lethal supraoptimal temperatures. It is normal for plants under field conditions to experience such circumstances regularly—for example in sunny, dry conditions when the irradiance load is high and stomata are partially closed—and to go through cycles of acclimation and de-acclimation as environmental conditions fluctuate.
HSPs are classified into five groups according to molecular size: HSP100, HSP90, HSP70, HSP60 and small (sm) HSPs. The HSP100 group consists of 100–140 kDa proteins. Included in this group are members of the Clp family of proteases, most of which are located in plastids or mitochondria, where they are thought to function in ATP-dependent protein disaggregation and turnover. Representatives of the HSP90 (80–94 kDa) and HSP70 (69–71 kDa) groups are molecular chaperones that are, in many cases, ATP-dependent and are essential for normal cell function. They include constitutive members and those induced by cold stress as well as heat. They are present in several subcellular compartments—cytosol, ER, mitochondria and plastids. Members of the HSP60 group (57–60 kDa), which are characteristically found in the mitochondrial matrix and chloroplast stroma, are abundant even at normal temperatures. This protein family includes chaperonin 60, a nuclear-encoded chloroplast protein required for assembling the subunits of rubisco into the functioning holoenzyme (see Chapter 9). The diversity of low molecular size HSPs (smHSPs, 15–30 kDa) is a unique feature of the plant heat shock response (Figure 15.34). They are thought not to be required for normal cell function, but to participate in the development of thermotolerance by promoting ATP-independent protein stabilization and preventing aggregation.
The promoter regions of genes encoding HSPs have the heat shock element (HSE), a conserved DNA sequence that is recognized by members of the heat shock transcription factor (HSF) family. The promoters of several of the Hsf genes also contain the HSE motif. Heat stress is required to convert monomeric HSF proteins to the active HSE-binding oligomeric form. Three HSF classes are recognized, A, B and C. Based on expression patterns of HsfA, HsfB and HsfC gene family members in Arabidopsis (Figure 15.35), tomato and rice, together with functional and comparative analyses, a picture is emerging of the roles and interactions of heat shock factors in development and stress responses. HsfA2, HsfA7 and HsfB1 are strongly upregulated in heat-stressed roots and shoots (Figure 15.35). Transcription of HsfB1 is also stimulated in roots by the full range of abiotic stresses. The transcription factor HsfA1a (encoded by a member of the HsfA gene family) has been shown to act as a master regulator of induced thermotolerance in tomato, by triggering the expression of heat shock genes including HsfA2 and HsfB1. The HsfA2 gene encodes the dominant HSF in thermotolerant cells, and HsfA3 functions in cross-talk with drought stress signaling. HsfA4c is expressed more or less uniformly in all tissues and all stress treatments (Figure 15.35). This kind of genomics study reveals the richness and interconnectedness of regulatory processes governing stress responses and plant development.
Figure 15.35 Relative abundances of mRNA transcripts of Hsf (heat shock transcription factor) genes in response to a range of abiotic and biotic stress treatments. Transcript levels (signal intensities) are presented in the form of a ‘heat map’, on a color scale between low (blue) and high (red). Abiotic stresses were applied to hydroponically grown Arabidopsis seedlings. Biotic stresses consist of infiltration with Pseudomonas syringae pv. tomato DC3000 (DC3000), or Phytophthora infestans (Pinf), or elicitation with flagellin (Flg22), lipopolysaccharide (LPS) or harpinZ (HrpZ).

Plants are dependent on light for photomorphogenesis (see Chapter 8) and photosynthesis (see Chapter 9). However, light can be harmful if the absorbed photon energy is greater than the capacity of the plant to use it. Figure 8.4 showed how absorption of a photon raises the energy level of a pigment molecule from the ground state to an excited state. Excessive excitation of chlorophylls can result in the formation of singlet oxygen (1O2; see Table 15.6). Chlorophyll acts as a photosensitizer (S), providing the energy required to convert the stable triplet state O2 into 1O2. As shown in Equation 15.14A, a photosensitizer is a pigment that, like chlorophyll, absorbs light and flips from the excited singlet state (*1S) into the excited triplet state (*3S). Singlet oxygen is the product of reaction between *3S and O2 (Equation 15.4B). Decreased efficiency of photosynthesis as a result of oxidative injury caused by 1O2 and its reactive products is termed photoinhibition. The location and function of the D1 protein of the photosystem II (PSII) reaction center (see Chapter 9) make it particularly vulnerable to photooxidative damage. The requirement for constant synthesis to replace it accounts for the unusually high rate of turnover of this protein. Under extreme conditions unrestrained production of singlet oxygen can be lethal. Plants are equipped with a range of mechanisms that protect against (i.e. quench) photosensitization and oxyradical damage.
15.14B Reaction between excited triplet photosensitizer and triplet state oxygen
Triplet state chlorophyll can also cause free radical damage by directly reacting with the fatty acids of lipids. An important function of chloroplast carotenoids is to act as quenchers. Carotenoids are able to accept excitation energy from triplet chlorophyll (and excited singlet state chlorophyll in some cases), thereby blocking the generation of singlet oxygen and free radical cascades. Carotenoid biosynthesis (Figure 15.36) also provides a route for dissipating excess excitation energy as heat by pH-dependent quenching of chlorophyll fluorescence via the xanthophyll cycle. The biosynthetic pathway from the C40 tetraterpenoid precursor phytoene (see Figure 15.24) branches at the intermediate lycopene. One branch leads to the most abundant chloroplast xanthophyll lutein via α-carotene; the other leads from β-carotene to the xanthophyll cycle and neoxanthin (Figure 15.36). Under conditions of low irradiance the carotenoid violaxanthin functions as a photosynthetic antenna pigment by transferring energy to chlorophyll a. In high light the thylakoid lumen becomes more acidic, driven by the water splitting reaction of PSII. Acidification of the lumen activates the enzyme violaxanthin de-epoxidase, which rapidly converts violaxanthin to antheraxanthin and thence to zeaxanthin, a quencher of chlorophyll excited singlet states. In this way the combination of zeaxanthin, antheraxanthin and low pH in the thylakoid lumen facilitates non-photochemical quenching—the harmless dissipation as heat of excess excitation energy directly within the light-harvesting antennae. Zeaxanthin epoxidase recycles zeaxanthin to violaxanthin under reduced light flux (Figure 15.36). The essential protective functions of carotenoids in photosynthetic tissues explain why mutations or chemical treatments that block carotenoid biosynthesis usually result in the formation of lethal concentrations of singlet oxygen under high light intensity.
Figure 15.36 Carotenoid biosynthesis and the xanthophyll cycle. Phytoene is converted to lycopene in four steps by two desaturases. The double bonds introduced by these desaturases are in blue circles. Lycopene is cyclized to form α- and β-carotenes, precursors of the major plastid xanthophylls lutein, zeaxanthin, violaxanthin and neoxanthin. The xanthophyll cycle, an important mechanism for dissipating excess light energy, is highlighted.
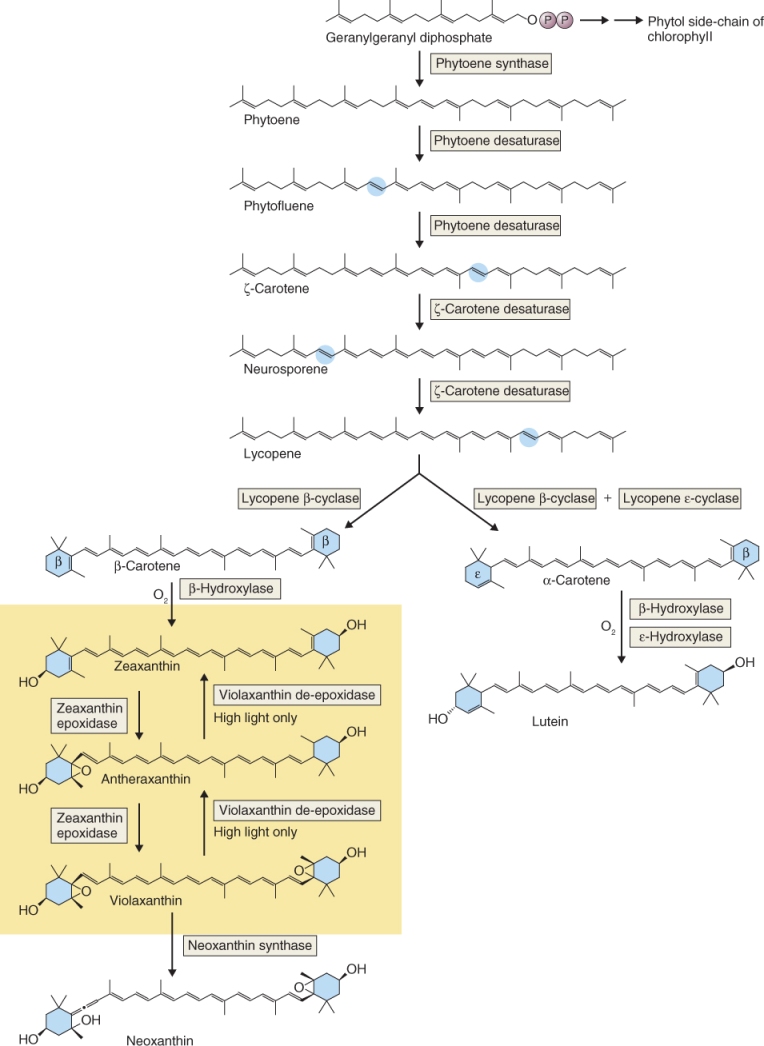
Fluctuating light quantity and quality is a normal feature of irradiance in many environments: for example shading and sunflecks are commonly experienced by forest floor plants. When sunlight passes through a leaf canopy, the ratio of red to far-red wavelengths is greatly reduced (see Figure 8.22). Photosystem I (PSI) is able to use far-red light (>660 nm) more efficiently than PSII, whereas at red wavelengths (<660 nm) PSII drives photosynthesis more actively than does PSI. In red-depleted shade light there may be imbalanced excitation of the two photosystems and inefficiencies in electron transport. So-called shade plants have a number of adaptations to deal with such imbalances. These include an altered ratio of PSII to PSI centers in the thylakoid membranes. Some species accustomed to full-sun environments are able to acclimate by adjusting this ratio in response to shading. For example, the PSII:PSI ratio for chloroplasts of pea (Pisum sativum) plants grown in PSII light (550–660 nm) is 1.2; in sunlight it is 1.8; and in PSI light (>660 nm) it is 2.3. The distribution of light energy between PSI and PSII is also regulated minute-to-minute by a mechanism called state transition. When excess light is captured by PSII (which is located primarily in grana stacks; see Table 9.1), a subpopulation of PSII light-harvesting units, LHCII trimers, is phosphorylated by a redox-activated kinase. These then dissociate from PSII and move to the intergranal region of the thylakoid membrane and associate with PSI. The process is reversible and represents a sensitive way of balancing delivery of light energy captured by LHCII to PSII and PSI.
Overexcitation of the photosynthetic apparatus can result from production of NADPH and ATP in excess of that required by CO2 fixation. This can be balanced by a system that links the redox state of ferredoxin, which is reduced by the light energy capture reactions, to that of thioredoxin (Figure 15.37) which in turn regulates the activity of several Calvin–Benson cycle enzymes (see Figure 9.29). Ferredoxin–thioredoxin reductase catalyzes the reaction between reduced ferredoxin and thioredoxin. Reduced thioredoxin activates target enzymes by reducing regulatory intramolecular disulfide bonds. Calvin–Benson cycle enzymes regulated in this way include fructose-1,6-bisphosphatase, sedoheptulose-1,7-bisphosphatase, phosphoribulokinase, NADP+-glyceraldehyde-3-phosphate dehydrogenase and rubisco activase. The Calvin–Benson cycle is also directly regulated by light via changes in pH and Mg2+ concentration. Furthermore, reduced thioredoxin formed in the light stimulates the ATP synthase associated with the light energy capture reactions and the C4 enzyme NADP+-malate dehydrogenase, and inhibits the oxidative pentose phosphate pathway.
Figure 15.37 Light regulation of enzyme structure through the ferredoxin–thioredoxin system. Red arrows show reduction reactions, black arrows oxidations.
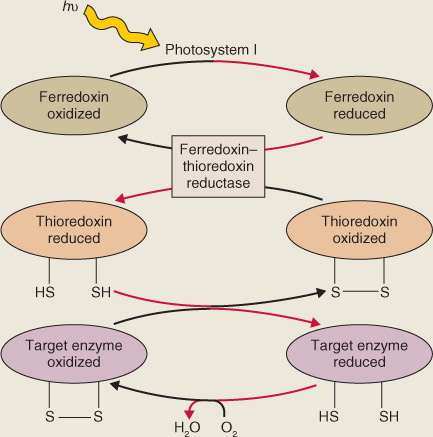
Environmental influences on plant growth and development may not be exerted uniformly on all sides. Tropisms, directional growth responses to directional stimuli, are adaptations that contribute to plant survival by aiming growth towards resources or away from potentially harmful environments. Tropic responses occur under the influence of gradients of light, temperature, water, oxygen and mineral nutrient availability (Figure 15.38). The mechanisms of light perception and signaling in phototropism are discussed in Chapter 8. Here we focus on mechanical factors, in particular the influences of gravity (gravitropism) and touch (thigmotropism).
Figure 15.38 Plant tropic responses to directional stimuli.
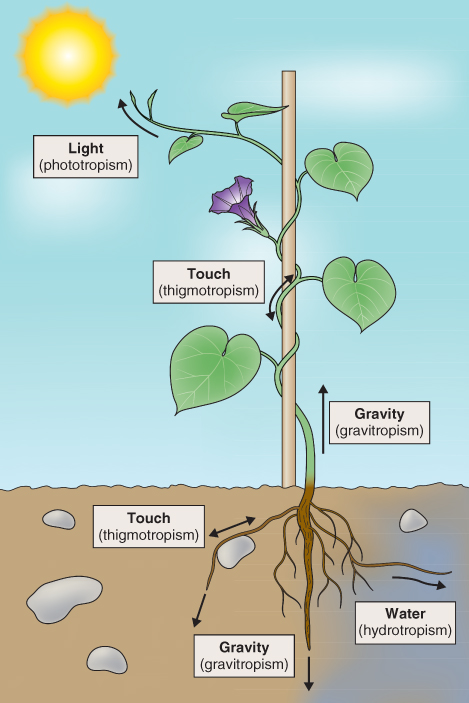
Roots are positively gravitropic, growing in the direction of gravitational pull, whereas shoots are negatively gravitropic. In general, redirection of growth during a tropic response is the consequence of asymmetrical elongation of cells on either side of an organ, in response to a lateral gradient in auxin concentration. Auxin accumulates in the downward-facing sides of roots and stems. Auxin promotes cell elongation in shoot cells leading to negative gravitropism, while it inhibits root cell elongation resulting in positive gravitropism in this organ. Polar auxin transport is mediated by localized influx (e.g. AUX1) and efflux (PINs, ABC transporters) transport carriers (see Chapters 10 and 12). In the root, gravity-sensing cells are located in the root cap while in the stem they are found toward the outside of the vascular tissue. In these sensory cells, gravity causes starch-filled amyloplasts in the cytoplasm to sediment downwards. Within the root cap, this initiates, by an as yet unknown mechanism, a biochemical signaling cascade leading to auxin redistribution (Figure 15.39). The promotion of asymmetrical growth by a lateral gradient of auxin is also the mechanism by which light perception is transduced into the phototropic response.
Figure 15.39 Auxin redistribution in the root under the influence of gravity. (1) Normal distribution of auxin in root before exposure to unilateral gravitational stimulus. (2) Root tip moved from vertical to horizontal position. (3) Active redistribution of auxin to the lower side by gravity-sensing cells. (4) Asymmetrical distribution of auxin leads to bending as a consequence of growth inhibition on the lower side and stimulation on the upper.
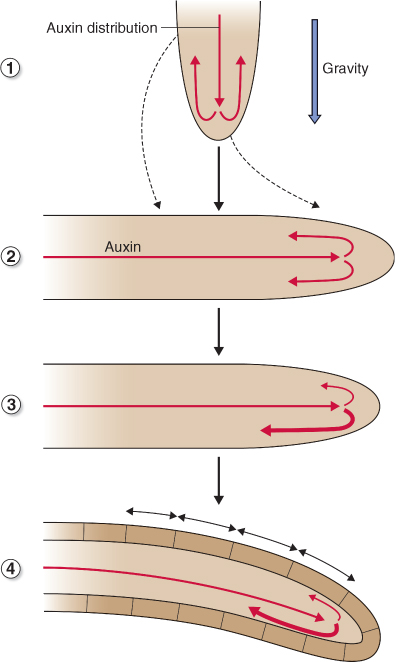
In some plants, stems, roots and/or tendrils grow around objects that they encounter (Figures 15.38 and 15.40). This response is known as thigmotropism. The identity of the touch sensor has not been established; however it is believed to activate a Ca2+-related signaling network leading ultimately to the thigmotropic response. Tropisms are integrated with other acclimatory and adaptive responses and are attuned to signaling and metabolic pathways that equip plants to deal with the diversity of abiotic stresses.
Figure 15.40 Thigmotropic growth in the stem of Phaseolus vulgaris. Note how the stem twines itself around the support.

Plants form relationships with other living organisms from across the taxonomic range. These biotic interactions may be: parasite/host (one partner thrives at the expense of the other); mutualistic (mutually beneficial associations that improve access to resources or environmental resilience); predator/prey (relating to position in the food chain); allelopathic (deploying products of secondary metabolism to enhance competitiveness); or coevolutionary (exploiting other organisms through complementary adaptations). The different types of association are not mutually exclusive and, in many cases, the mechanisms underlying the interaction and the part the plant plays in it are generic. Thus wounding and the formation of symbiotic root nodules converge on the metabolic and regulatory networks of ROS-mediated oxidative stress (see Figure 15.31), and defenses against pathogens include aspects of the heat shock syndrome (see Figure 15.35) and programmed cell death (see Chapter 18). In this section we examine how the basic principles of secondary metabolism and the adaptive and acclimatory features of abiotic stress response apply to the interplay between plants and other organisms.
Plants, like all living organisms, are constantly exposed to potential pathogens. A plant pathogen is defined as an organism that must grow on or inside the plant to complete a part or all of its life cycle and in so doing has a negative effect on its host. Each type of plant pathogen invades its host in a characteristic way. Some get in via stomata or lenticels, or through previously wounded tissue, whereas others directly penetrate the epidermis by mechanical or enzymatic attack. Pathogens are classified according to the strategy they adopt to use the infected plant as a substrate (Table 15.8). Necrotrophs kill the cells of the host plant; biotrophs cause minimal cellular damage and require living host tissue; and hemibiotrophs initially keep cells alive but kill them at later stages of the infection. A pathogen strain that invokes the symptoms of disease is referred to as virulent.
Table 15.8 Strategies utilized by plant pathogens.
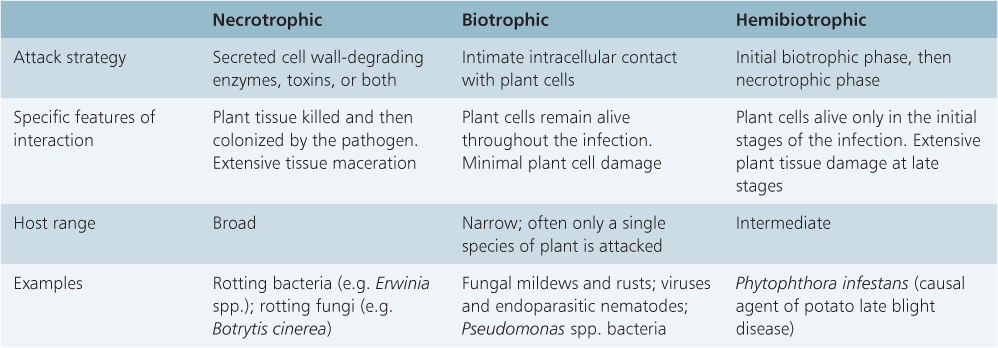
Necrotrophic fungi and bacteria injure a host by secreting cell wall-degrading enzymes, or toxins, or both. The fungi Pythium and Botrytis and bacteria of the genus Erwinia destroy cell walls and extract nutrients from the contents thus released. The fungus Fusicoccum amygdali is an example of a necrotrophic pathogen that secretes a toxin, in this case fusicoccin, which induces stomatal opening by stimulating the activity of the plasma membrane-localized H+-ATPase. This leads to wilting and cell death in many plant species.
Biotrophic fungi, such as downy and powdery mildews, are highly specialized pathogens that interact with viable host cells, often through a feeding structure or haustorium. Many biotrophs secrete cytokinins into the host tissue. This prevents host cell senescence at the infection site and results in the formation of so-called green islands. Among the biotrophic bacterial pathogens are Pseudomonas spp., which have a group of genes, collectively referred to as the hypersensitive resistant protein (hrp) cluster, that are absolutely required for bacterial pathogenesis. Rhizobium radiobacter (formerly, and widely still, known as Agrobacterium tumefaciens), the cause of crown-gall disease in thousands of dicotyledonous plant species, is a biotrophic bacterium of major importance to biotechnology. R. radiobacter genetically transforms plant cells with a DNA fragment called T-DNA (transferred DNA), which is stably integrated into the plant genome and promotes tumor formation by altering host hormonal metabolism. R. radiobacter T-DNA is the most widely used vector for stably integrating foreign DNA into the plant genome and for genetically transforming cells of higher plants with recombinant DNA molecules.
Viral pathogens are biotrophic, causing symptoms of tissue yellowing (chlorosis) or browning (necrosis), mosaic patterns (chlorotic mottling) and stunting in the host. Most plant viruses are single-stranded RNA viruses that replicate in the host cytoplasm and move between cells through plasmodesmata (see Chapter 14). Transmission from host to host is often via an invertebrate vector organism.
Among the hemibiotrophic pathogens are some of the world's most devastating plant disease organisms. For example, the Irish famine of 1846 and 1847, which resulted in the emigration of more than 1 million people from Ireland to the United States and other countries, was triggered by a catastrophic infection of Phytophthora infestans, an oomycete or water mold that causes late blight disease of potato.
Measures adopted by plants against disease-causing microorganisms include constitutive defenses in the form of antimicrobial secondary metabolites; these are located in specific cellular compartments from which they are released following cell damage. Preformed antimicrobial secondary products are sometimes referred to as phytoanticipins, to distinguish them from the phytoalexins, secondary metabolites synthesized de novo following infection. Interestingly, a given phytoalexin in one plant species may behave as a phytoanticipin in another. An example of a phytoanticipin is avenacin, a triterpenoid saponin from oat (Avena) that prevents infection by Gaeumannomyces graminis, a major pathogen of cereal roots.
In contrast to constitutive defenses, induced defense responses require detection of the pathogen by the plant and a rapid activation of defense-related genes. The resistance/avirulence gene-for-gene system (see Chapter 18) is one mechanism by which pathogens are recognized. Another is the interaction between elicitors (molecules of pathogen origin) and host receptors. Examples of bacterial elicitors are flagellins (flagellar proteins), harpins (secreted proteins) and lipopolysaccharides (see Figure 15.35). Fungal elicitors include cell wall polysaccharides, polypeptides, glycoproteins and lipid molecules. A plant–pathogen interaction that triggers the full defense syndrome is termed incompatible. It stimulates a diverse set of cellular mechanisms (Figure 15.41). The outcome is often localized cell death (hypersensitive response, HR), which impairs pathogen spread. HR is discussed in further detail, as an example of programmed cell death, in Chapter 18. It should be noted that even in compatible interactions, where the host fails to recognize the invader and there is no induced defense response, the pathogen must still overcome constitutive defenses before successful infection can occur.
Figure 15.41 Immediate, local and systemic induced responses to pathogen invasion.
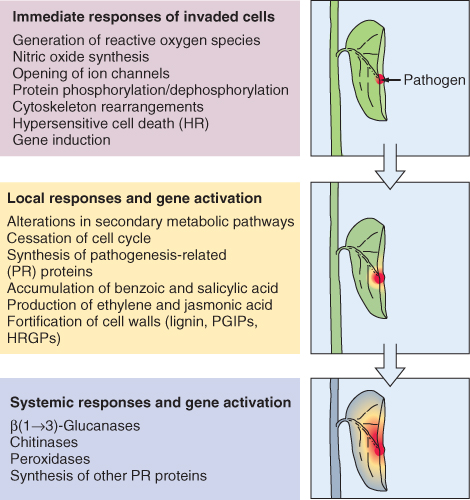
An early induced defense response is superoxide production by a plasma membrane-associated NADPH oxidase. Metabolic fates and signaling roles of O2·− and H2O2 are described in Figure 15.31 and Section 15.6.3. An early consequence of pathogen recognition is the rapid synthesis of the signaling molecule nitric oxide, which acts synergistically with ROS to activate host defenses. ROS also participate in lignin synthesis that, together with the synthesis and cross-linking of hydroxyproline-rich glycoproteins and the production of polygalacturonase inhibiting proteins, renders the plant cell wall more resistant to microbial penetration and enzymatic degradation. Initiation of resistance involves the diversion of primary precursors into secondary metabolism. For example, the synthesis of phenylpropanoid phytoalexins is the result of coordinated activation, by transcription factors of the bHLH, MYB and WRKY families, of genes that encode the pathway leading from phenylalanine to isoflavonoids (see Figures 15.11 and 15.13).
Prominent among local and systemic defense responses is the expression of pathogenesis-related (PR) proteins, a diverse group of enzymes, antifungal agents and secondary signaling components. They include glucanases and chitinases that attack fungal cell walls, endoproteinases and ribonucleases that destroy pathogen macromolecules, and lipoxygenases that generate antimicrobial volatiles and jasmonate-related signaling molecules. Transcriptional activation of many PR genes is regulated by salicylic acid- and ethylene-mediated signal transduction cascades.
Plants form non-pathogenic (endophytic) relationships with a variety of fungi. In most cases the benefits to the participants in the interaction are unclear, but some endophytic associations have known ecological and agricultural significance. For example, Neotyphodium spp., fungal endophytes of forage and turfgrasses of the genera Lolium and Festuca, have been shown to improve (by mechanisms yet to be established) host tolerance of abiotic stresses and resistance to insect and mammalian herbivores.
We have seen that roots are the source of compounds exuded into the rhizosphere that facilitate nutrient acquisition (see Chapter 13) and serve as signals to potential microbial symbionts (see Chapters 10 and 12). These functions of root exudates are only part of the story, however. Among the multiplicity of compounds found in root exudates are amino acids, organic acids, sugars, phenolics, high molecular mass polysaccharide mucilages and proteins. It has been estimated that from 5% to 20% of all carbon fixed in photosynthesis is secreted into the rhizosphere. To devote such a high level of resource to root exudation implies that secretion is important for plant survival.
An ecologically significant function of compounds secreted into the rhizosphere is to influence, in most cases negatively, the growth of neighboring plants—a phenomenon known as allelopathy. Allelopathically-active compounds (allelochemicals) are usually injurious and thus deter competitors from stealing soil resources. Many destructive or highly invasive species are very allelopathic. An example is knapweed (Centaurea maculosa), a competitive weed that can dominate large areas of landscape. Knapweed roots exude catechins, phytotoxic phenylpropanoids derived from leucoanthocyanidins and anthocyanidins (see Section 15.3.3). While allelochemicals can be an effective defense against competitors, they can also be a signal to parasites. Parasitic plants often find their hosts by using secondary metabolites secreted by host roots as signals to initiate the development of invasive roots (haustoria) (Figure 15.42). Flavonoids, p-hydroxy acids, quinones and cytokinins secreted by host roots have been shown to induce haustorium formation. Striga (Figure 15.42), a parasitic genus that causes major losses of maize, sorghum, millet and rice, is estimated to infest two-thirds of the area devoted to growing cereal crops in Africa. Striga gives its name to the strigolactones, important signaling molecules involved in many aspects of plant growth and development (see, for example, Chapters 10 and 12). These molecules also function as rhizosphere allelochemicals that are implicated in host–parasite recognition, as well as the formation of mycorrhizal associations (see Chapter 12).
Figure 15.42 The life cycle of Striga. (A) Each Striga plant can produce 100 000 seeds. (B) Seeds germinate in response to germination stimulants present in host root exudates. (C) The elongating Striga radical perceives haustorial initiation factors (HIFs) secreted by the host root and forms a functional attachment organ. (D) Sticky hairs attach the haustorium to the host root. (E) Haustorium cells form a wedge and intrude into host xylem vessels (but not phloem). (F) Following establishment of xylem continuity with the host, there is further differentiation of the haustorium, followed by the formation of cotyledons and leaf initiation. (G) The Striga shoot emerges above ground and flowers and sets seed approximately 6 weeks later. Scale bar = 250 μm (A–C).
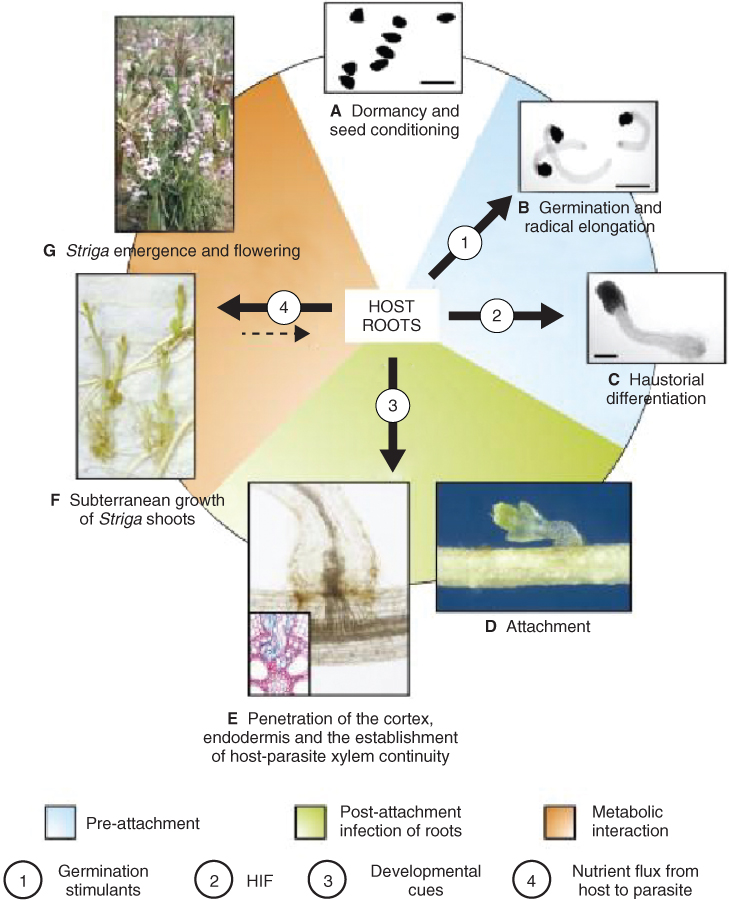
Plants are under constant attack from invertebrates, which produce physical and physiological symptoms in a plant as a result of their feeding, reproductive or sheltering behaviors. Chewing insects can be responsible for spectacular plant tissue damage. Plagues of locusts, for example, owe their fearsome reputation to their capacity to defoliate an entire crop covering several hectares within a day or so. Tissue damage caused by chewing insects frequently permits secondary infection by necrotrophic fungi and bacteria. On the other hand, sap-sucking insects, such as aphids or thrips, drain the contents of phloem sieve elements using a specialized mouth part, the stylet (see Figure 14.16). These insects rarely cause extensive physical injury, although heavy infestations can cause chronic shortages of photosynthate which lead to severe reductions in growth potential. While feeding, many sap-sucking insect pests also transmit viruses, including some agriculturally significant biotrophic pathogens such as potato virus X.
Parasitic nematodes are among the most damaging invertebrate herbivores. In most cases they infect root systems, often causing large changes to the metabolism of the entire plant and to root architecture. Cyst nematodes are endoparasites that release glandular secretions, which trigger fusion of host cells to form syncytial feeding structures. Endoparasitic root-knot nematodes stimulate DNA endoreduplication and cortical cell growth, leading to the production of giant cells. Syncytial and giant cells form connections with the phloem through transfer cells and reduce productivity of the plant by depleting it of photosynthate.
Adaptations designed to deter tissue damage by grazing or browsing animals include prickles, spines and hairs. Pasture grasses exhibit particularly striking adaptations to defoliation stress, such as intercalary shoot meristems located near the soil surface, silicified leaf surfaces and efficient mechanisms for recycling nutrients to support recovery growth. The development of the morphological characteristics of plants adapted to defoliation and trampling by grazing animals is complemented by the evolution of features that fit the grazers for a diet of vegetation—for example, teeth designed for biting and chewing foliage, digestive systems capable of processing high-bulk, low-nutrient content feed, and so forth. The relationship between herbage plants and ruminants is a coevolutionary one.
Chewing by herbivores and feeding by endoparasites trigger local and systemic wound responses in plant tissues. Wounding releases oligosaccharides from damaged cell walls, which activate a number of stress response genes, including those encoding pathogenesis-related proteins, heat shock factors (see Figure 15.35) and proteinase inhibitors (PIs). PIs are small defense proteins that interfere with the invertebrate digestive system by inhibiting serine, cysteine and aspartyl proteinases and thereby reduce the uptake of essential amino acids and retard the herbivore's growth and development. Many of the immediate biochemical changes in injured cells are shared with those of other abiotic and biotic stresses (see Figures 15.30 and 15.41): production of ROS, induction of secondary metabolism and accumulation of phenolics, tannins and phytoalexins, and increased synthesis of ethylene, jasmonates and salicylic acid.
As a consequence of the export of signal molecules from a wound site via the vascular system, wounding often stimulates systemic responses in the whole plant. Wounding promotes proteolytic processing of the 200-amino acid precursor prosystemin to form the 18-amino acid peptide hormone systemin. Systemin engages with a receptor in the plasma membrane and activates the oxylipin pathway of jasmonic acid (JA) synthesis (see Chapter 10), as well as the transcription of genes encoding a PI (PIN2). JA is believed to move through the phloem to remote undamaged tissues where it induces synthesis of PIN2 and other systemic wound response proteins (Figure 15.43).
Figure 15.43 Local and systemic responses to wounding mediated by systemin and jasmonic acid (JA). Prosystemin is proteolytically processed to the peptide hormone systemin, which activates synthesis of JA from linolenic acid (C18:3). JA stimulates the transcription of genes encoding proteinase inhibitor (PIN2) and other wound response proteins locally and in remote tissue to which JA is transported.
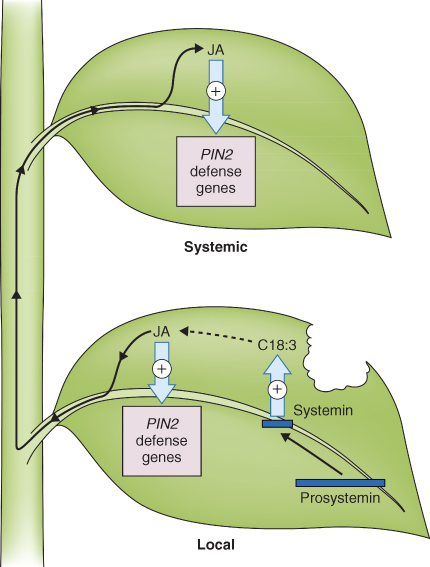
In addition to inducing physiological reactions within the plant, wounding also stimulates the production of volatile compounds that can act as an alarm signal to other parts of the same plant or even to other individuals in the community. Among the volatiles emitted by plants are methanol, acetone, formaldehyde and other short-chain carbonyl compounds, plus a host of terpenoids (including isoprene—see Section 15.5.2), phenylpropanoids and fatty acid derivatives. Plant-to-plant signaling by volatiles is a relatively new field of research and details of mechanisms and ecological significance remain to be discovered.