Figure 8.1 (A, B) Cucumber and (C, D) maize seedlings grown for 1 week in the light (A, C) or in the dark (B, D). H, apical hook; M, mesocotyl–coleoptile border.

Chapter 8
Light perception and transduction
Light is the ultimate source of energy for most of life on Earth. Plants and other photoautotrophs capture light energy in the process of photosynthesis (see Chapter 9). But light is more than the driver of energy-demanding metabolic activities; it also reports on the state of the environment through its quality (the balance of photons of different wavelengths), intensity (energy flux) and interactions with other environmental factors.
Plants are acutely sensitive to seasonal, daily and moment-to-moment variations in solar radiation. Light has a dramatic effect on growth and development. When seedlings germinate and begin to grow underground, their stems elongate rapidly and their cotyledons and/or leaves do not expand. They are pale in color because they lack chlorophyll. In eudicot seedlings, the apical portion of the stem is hooked so that the shoot apex faces downwards. In cereals and other grasses, the coleoptile encloses the shoot apex and young leaves. Seedlings grown in the dark are said to be etiolated. This type of growth is called skotomorphogenesis (skoto = dark). In the light, photomorphogenesis begins. Photomorphogenesis is defined as the developmental response of an organism to information in light, which may be its quantity, quality (i.e. wavelengths present) and direction or the relative length of day and night (photoperiod). On exposure to light, stem elongation slows; cotyledons and/or leaves expand and become green. In eudicots, the apical hook straightens and in grasses coleoptile growth decelerates and stops as expanding leaves pierce its tip (Figure 8.1).
Figure 8.1 (A, B) Cucumber and (C, D) maize seedlings grown for 1 week in the light (A, C) or in the dark (B, D). H, apical hook; M, mesocotyl–coleoptile border.

In order to respond to light, organisms must possess photoreceptors, molecules that absorb light and set in motion a cascade of events leading to biological responses. In this chapter we will review the general properties of light, discuss the way plants detect light, and consider how light perception leads to biological responses.
The sun is the ultimate source of all non-nuclear energy on Earth. Solar energy is the product of the sun's nuclear fusion reaction that converts protons into helium nuclei at a rate equivalent to almost 1017 kg of TNT per second. The total annual solar energy absorbed by the Earth's atmosphere, oceans and landmasses amounts to some 5.62 × 1024 joules, of which photosynthesis captures 3.16 × 1021 joules per year (Table 8.1).
Table 8.1 The fate of solar power reaching Earth.
| Global solar power balance | Amount in terawattsa |
| Solar power inputb | 178 000 |
| Reflected to space immediately | 53 000 |
| Absorbed and then reflected as heat | 82 000 |
| Used to evaporate water (weather) | 40 000 |
| Captured by photosynthesis (net primary productivity)c | 100 |
| Total power used by human society: | |
| In 2005 | 13 |
| Projected use in 2100 | 46 |
| Total used for food | 0.6 |
a The watt is the unit of power and is related to the joule, the unit of energy, by watts = joules per unit time. A terawatt is 1012 watts and is equal to 1012 joules s−1.
b Total solar energy input per year = 5.62 × 1012 terawatts (5.62 × 1024 joules).
c Total solar energy per year captured by photosynthetic organisms = 3.16 × 109 terawatts (3.16 × 1021 joules).
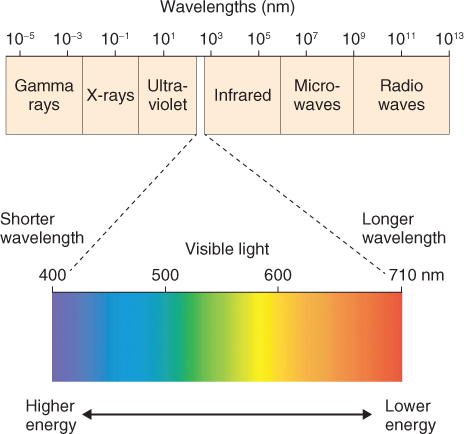
Visible light is just one part of the electromagnetic spectrum, which stretches from γ- and X-rays at one extreme through to radiowaves at the other (Figure 8.2). Light has the properties simultaneously of a wave and a particle. In simple terms it can be thought of as individual packets of energy or quanta that move in waves. A quantum of light energy is called a photon. The wavelength (λ, Greek letter lambda) of visible light is usually expressed in nanometers (nm). The visible spectrum, which we see as the colors of the rainbow, runs from a wavelength of about 380 nm (violet) through to 760 nm (far red). Equation 8.1 expresses the relationship between wavelength (in meters) and frequency (υ, Greek letter nu; units = s−1) and speed of light (c, units = m s−1).
Figure 8.2 The electromagnetic spectrum with the portion from 400 to 710 nm expanded to show the colors of the visible wavelengths. The limits of human perception can extend beyond this range, as far as 380 nm at the blue end and 760 nm at the red end.
The energy of a quantum is directly related to its frequency and inversely related to its wavelength, as given in Equation 8.2. The proportionality constant h is called Planck's constant. E, for photons, is expressed in units of electron-volts (eV): 1 eV is equal to 1.6 × 10−19 joules. The term hυ is often used to represent a photon.
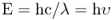
where c = speed of light (approximately 300 × 106 m s−1) and h = Planck's constant (4.14 × 10−15 eV · s). It follows that the energy of a given wavelength of light (in the commonly expressed unit of nm) is:
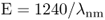
Gamma- and X-rays are at the short wavelength end of the electromagnetic spectrum and are very energetic (E > 105 eV). Radiowaves have long wavelengths and are comparatively low energy ( < 10−6 eV). The photon energy of visible light at the short wavelength (blue-violet) end of the spectrum is higher than that at long wavelengths (red)—about 3.3 eV compared with 1.6 eV. Ultraviolet (UV) light is generally subdivided into UVA (wavelength range 400–320 nm, photon energy 3.1–3.94 eV), UVB (320–280 nm, 3.94–4.43 eV) and UVC (280–100 nm, 4.43–12.4 eV).
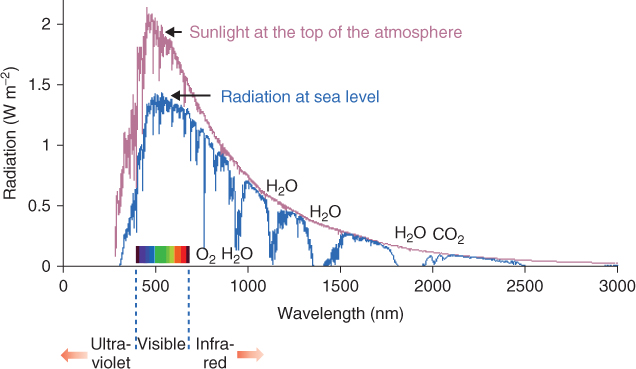
The interaction of light with living organisms can be characterized by the principles of quantum physics. A photon colliding with an atom may transfer its energy to an electron. The subsequent fate of the electron and photon will depend on the energy level of the photon and the nature of the atom. Short wavelength photons such as γ- or X-rays are energetic enough to give the electron sufficient kinetic energy to break free of the atom altogether. The short wavelength portion of the electromagnetic spectrum is therefore often referred to as the source of ionizing radiation. Life on Earth is possible because solar energy reaching the planet's surface (Figure 8.3) has been filtered by the atmosphere to remove most of the ionizing radiation (wavelengths < 295 nm) that would otherwise be hazardous to living matter. Even so, enough short wavelength photons (principally UVB) reach the biosphere to make it necessary for organisms to equip themselves with antioxidant defenses, repair mechanisms and sunblockers (discussed in detail in Chapter 15).
Figure 8.3 The spectrum of solar energy reaching the Earth. The red and blue lines are the solar spectra, respectively, before and after filtering by the Earth's atmosphere. The visible spectrum, ultraviolet and infra-red regions, and bands of radiation absorbed by atmospheric O2, H2O and CO2 are identified.
A molecule that can interact with photons within the visible part of the electromagnetic spectrum is called a pigment. Pigments are colored to the human eye because they selectively absorb certain parts of the visible wavelength range. When light of visible wavelengths impinges upon such a pigment molecule, it is not energetic enough to cause ionization but may make an electron jump to a higher energy level within the atom (Figure 8.4). When the atom of a pigment absorbs a light quantum with an energy that matches the energy difference between the molecule's non-excited (ground) state (Eg) and excited state (Ee), one of its electrons is shifted from a lower-energy to a higher-energy molecular orbital (Equation 8.3).
Figure 8.4 Energy levels within a pigment molecule interacting with light. The example shows a molecule such as chlorophyll that absorbs light in both the blue and red regions of the spectrum. During transitions between excited states, energy is lost as heat, in accordance with the second law of thermodynamics. Fluorescence is the re-emission of light from the lowest excited state. The wavelength maximum of photons emitted through fluorescence is longer (i.e. they are of lower energy) than the maxima of photons absorbed by the pigment molecule. The spectra for fluorescence and absorption are shown at the right of the figure. The short wavelength absorption band corresponds to a transition to the higher excited state, and the long wavelength absorption band corresponds to a transition to the lower excited state.
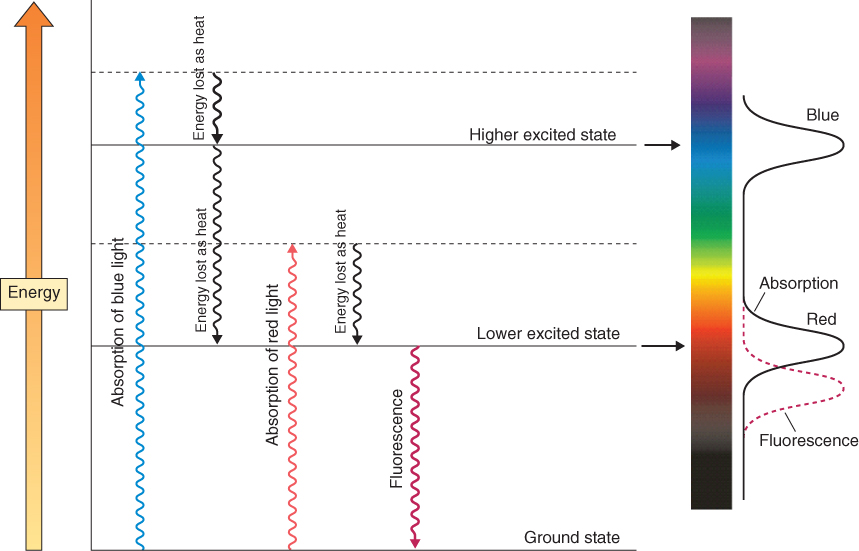
Because a pigment molecule consists of many different atoms and electrons, each with its own Ee and Eg, it will absorb light across broad wavelength ranges (for example, one wave band in the blue and one in the red spectral region in the case of chlorophyll) rather than the sharp bands characteristic of individual atoms (Figure 8.4). An energized electron (sometimes called an exciton) in this molecular environment may have one of a number of possible fates. As Figure 8.4 shows, it may immediately fall back to its original energy level, re-emitting energy as light (fluorescence) and/or heat (infra-red energy). It may remain in the high-energy state for a relatively prolonged period until it gives up its energy and reverts to the low-energy state (phosphorescence). It may be transferred to another molecule, leaving the donor pigment molecule positively charged and the acceptor negatively charged (a process called charge separation, of central importance in photosynthesis; see Chapter 9). The energy levels of infra-red and other long wavelength photons are too low to make electrons jump, but their absorption by molecules can translate into vibrational energy in bonding systems. Absorption of infra-red radiation by ‘greenhouse gases’ such as carbon dioxide and methane traps heat in the atmosphere and is of international concern as the cause of global warming and climate change. In all cases the energy transfers from photons to atoms strictly obey the laws of thermodynamics, as shown by the higher entropy levels of the system and the increased wavelength (decreased energy) of photons re-emitted when excitons revert to the low-energy state (Figure 8.4).
In vivo, if pigment molecules absorb light energy in excess of the capacity of normal energy metabolism to use it, photosensitization may occur. In this case the energized pigment brings about chemical alteration of another molecule in the system. Photosensitization is frequently harmful and makes it necessary for defenses against damage by excess light to be built into cell structure and function. This is particularly important in plants, whose primary energy source is light. Chapter 9 describes how photosynthesis is organized to deal with the hazards of photosensitivity, and defenses against toxic chemical products of excess energy are discussed in Chapter 15.
The study of the interaction between light and life is called photobiology. Light-absorbing photoreceptor molecules allow an organism to monitor environmental rhythms and fluctuations and to adjust its physiology accordingly. In humans and other animals, rhodopsin is one of the photoreceptors for vision. Plants have a number of different photoreceptors. These include the photosynthetic pigments (see Section 8.4 and Chapter 9), phytochromes (see Section 8.2), and cryptochromes and phototropins (see Section 8.3). Each photoreceptor has a characteristic absorption spectrum. The wavelengths of light that are absorbed by a photoreceptor activate specific responses. Plotting the intensity of a particular physiological response against the wavelengths that trigger it produces an action spectrum. Measuring the action spectrum for a photoresponse helps to identify the photoreceptor for the response. Examples of action spectra will be introduced in Section 8.2.3.
Most aspects of vegetative and reproductive growth and development are attuned to the light environment, as illustrated in Figure 8.5. For instance buried, light-sensitive seeds remain dormant until the soil is disturbed and they are exposed to light (see Chapter 17). Many plant species will not switch from the vegetative to reproductive state unless particular daylength conditions for flower induction are met. Seasonal behavior, like bud dormancy and the shedding of parts such as leaves, is also determined by changing light conditions over the annual cycle. Furthermore, plants respond to the direction of light. Charles Darwin and his son Francis provided one of the earliest scientific accounts of phototropism, the growth of plants and their parts towards or away from a light source (tropism = directional growth). In the following sections we will look at the major photoreceptors and present examples of how absorption of light by the photoreceptor leads to a biological response.
Figure 8.5 Light-regulated plant development mediated by red and blue light receptors. The clock indicates circadian control.
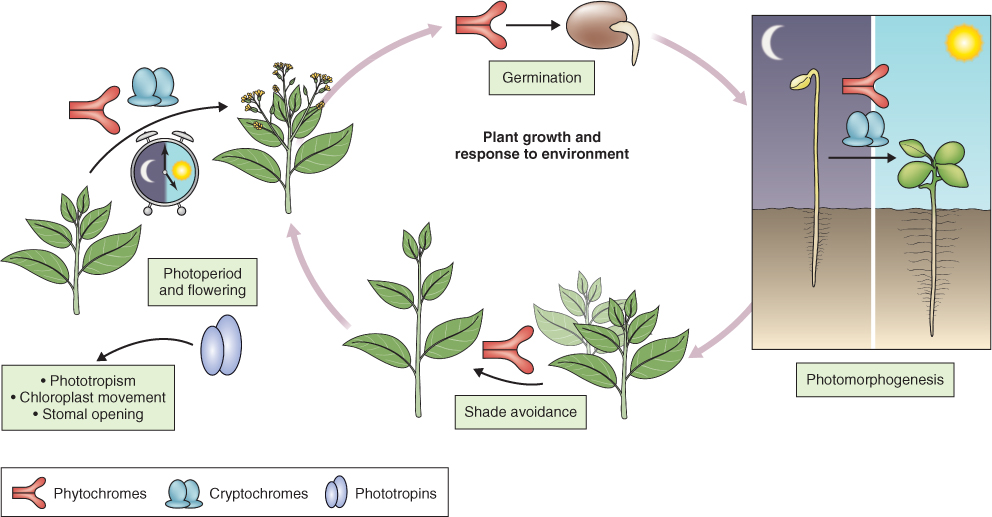
Germination of light-sensitive lettuce seeds is promoted by exposure to red light (R; λ around 660 nm). If the red light stimulus is followed by exposure to far-red light (FR; λ 730 nm), seeds do not germinate (see Figure 6.24). In turn, the effect of far-red light can be reversed by red light. The property of R/FR photoreversibility allowed scientists to use spectrophotometry to isolate and identify the photoreceptor pigment phytochrome. Phytochrome has been purified and the genes that encode it have been identified. Developmental processes regulated by phytochrome include: induction of germination in light-sensitive seeds; de-etiolation and increased chlorophyll synthesis (see Figure 8.1); decreased rate of stem elongation; promotion of leaf expansion; shade avoidance; photoperiodism; and flowering (see Figure 8.5).
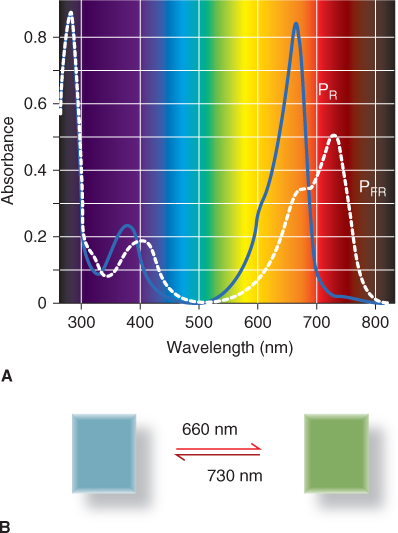
Phytochrome is a biliprotein, consisting of a 120 kDa apoprotein with a photoreactive prosthetic group (chromophore). The chromophore is a straight-chain tetrapyrrole, a bilin designated (3E)-phytochromobilin or PΦB (3E refers to the isomeric form of the photoactive molecule). Phytochromobilin is structurally related to, and shares its biosynthetic origin with, cyclic tetrapyrroles such as heme and chlorophyll (Section 8.4). The four pyrrole rings are designated A, B, C and D (Figure 8.6). Phytochromobilin adopts two molecular configurations, PΦBR and PΦBFR. PΦBR, the red-absorbing form, has a peak of absorbance at a wavelength of 660 nm. On exposure to red light, PΦBR is converted to PΦBFR and the absorbance peak is shifted to 730 nm in the far-red region of the spectrum. In far-red light, PΦBFR changes back to PΦBR. The phytochrome biliprotein with the chromophore in the PΦBR configuration is the red-absorbing form and is referred to as PR; the far-red-absorbing form is PFR, with the bilin chromophore in the PΦBFR configuration. The light absorption profiles of PR and PFR are illustrated in Figure 8.7A and the corresponding difference in color between PR and PFR in solution is shown in Figure 8.7B. PFR has considerable absorbance at red wavelengths. For this reason, under red light the population of phytochrome molecules consists of a maximum of 85% PFR. Conversely, the proportion of PR under far-red light can reach 95%. Photoreversible PΦBR–PΦBFR interconversion is the chemical basis of the phytochrome effect in photomorphogenesis.
Figure 8.6 Cis–trans isomerization of the phytochrome chromophore (PΦB) following exposure to red or far-red light. Note the change in configuration about the double bond between carbon atom 15 and the D-ring.

Figure 8.7 Light perception is mediated by the photoreversible receptor protein phytochrome. (A) Absorption of light in the visible wavelength range by the two molecular forms of phytochrome. (B) Reversible photoconversion of the phytochrome chromophore is associated with a visible change in the color of the photoreceptor in solution.
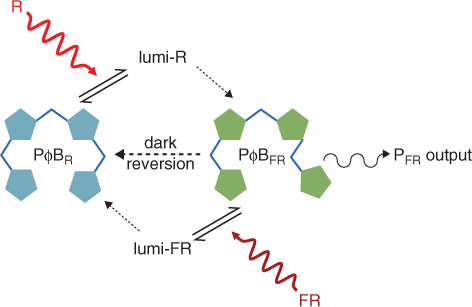
The A pyrrole ring of the chromophore is linked to a cysteine residue in the polypeptide chain of the phytochrome apoprotein. In PΦBR the C and D pyrroles are attached to each other through a methine bridge (–CH—) in the cis configuration (Figure 8.6). PΦBFR is the trans isomer. Red light flips the cis bond into the trans conformation and far-red light drives the reverse reaction. High-resolution spectroscopy has identified transient intermediate states of the chromophore between PΦBR and PΦBFR, known as lumi- forms, arising from the effects of protein conformation changes. In addition, PΦBFR slowly converts to PΦBR in the absence of light in a process referred to as dark reversion. PΦBFR is the form that triggers photomorphogenesis. The biological outputs from the phytochrome cycle are determined by the ratio of PΦBR and PΦBFR forms, which in turn reflects the relative levels of red and far red in the light environment and the rate of dark reversion. The phytochrome photocycle is summarized in Figure 8.8.
Figure 8.8 The phytochrome photocycle driven by red and far-red light. Lumi-R and lumi-FR are transient intermediate states of the chromophore during its conversion from one configuration to the other.
Rapid advances in our understanding of phytochrome structure and mode of action have come from comparative studies of photoreceptive biliproteins across a range of plants and microorganisms, from seed plants to fungi to cyanobacteria. Alignments of protein sequences and modeling of three-dimensional structures based on X-ray crystallography data have identified functional and regulatory motifs within the phytochrome molecule (Figure 8.9). The phytochrome polypeptide consists of an N-terminal photosensory region and a C-terminal regulatory domain, with a hinge between them. The protein has a modular architecture within which a number of structural motifs are recognized. The photosensory part has four domains (Figure 8.9A). P1, at the N terminus, and P4 function in the inhibition of PFR to PR reversion in the dark. P2 is a motif with the so-called PAS fold, a conserved structural feature that is found in a wide range of proteins in prokaryotes and eukaryotes and which is involved in signal sensing. P3 is the binding site of phytochromobilin. The three-dimensional model of phytochrome shows that the chromophore occupies a pocket formed by the folding of the P3 domain. The regulatory fragment of phytochrome consists of two PAS-type domains and a C-terminal kinase region (Figure 8.9A).
Figure 8.9 Domain structure and conformations of phytochrome. (A) Domain configuration, showing the P1–P4 regions of the photosensory domain, the PAS motif repeated in the regulatory region of the molecule, and the C-terminal histidine kinase module. The chromophore is shown attached to P3. (B) Dimerization of phytochrome and conformations of PR and PFR.
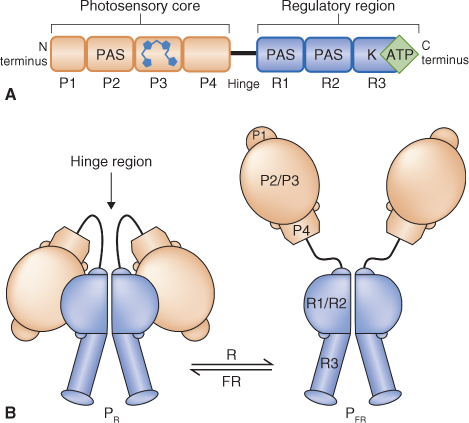
In cells phytochrome exists as a dimer (Figure 8.9B). In the red-absorbing form of the holoprotein, PR, both subunits are folded at the hinge so that the N-terminal photosensory and C-terminal regulatory regions are brought into contact with each other. Photoconversion of the chromophore by red light results in disruption of the interaction between the N- and C-terminal domains, opening up the structure and exposing buried surfaces (Figure 8.9B) in the far-red-absorbing form of phytochrome, PFR, This makes these surfaces accessible to phytochrome-interacting factors (PIFs) in the signal transduction pathway. The relationship between PFR and such PIFs is described in Section 8.2.4. The conformational change also allows for light-dependent phosphorylation of exposed serine and threonine residues which may result from autophosphorylation by the R3 domain kinase. A PFR-specific phosphatase has been identified that removes phosphate groups from amino acids in the hinge region; this increases their affinity for PIFs and susceptibility to proteolysis. Phytochrome has also been shown to phosphorylate other sensory photoreceptors, including cryptochromes (see Section 8.3) and proteins of the auxin signaling pathways (see Chapter 10), which can promote cross-talk between light and other regulators of plant development. Another consequence of the conformational difference between PR and PFR is the difference in stability of the two forms in vivo. The half-life of PR is about 1 week, whereas that of PFR is 1–2 hours. In part this reflects the difference in compactness of the protein which reveals peptide linkages in PFR that are susceptible to hydrolysis by proteolytic enzymes. Thus the combination of red/far-red light modulation, phosphorylation/dephosphorylation and differential proteolytic stability allows phytochrome action to be subject to a fine degree of control.
In the discussion of phytochrome structure to this point, we have assumed that there is just one type of phytochrome molecule. In fact, molecular analysis has shown that there is a family of phytochrome genes. For example, Arabidopsis has five genes, designated PHYA to PHYE, that encode phytochrome. Rice has three PHY genes (PHYA to PHYC). Most conifers and mosses have four PHY genes, cycads three, ferns two and lycopods one. Analysis of phylogenetic relationships, based on comparison of gene sequences, groups PHYA with PHYC and puts PHYB in a divergent group with PHYD and PHYE (Figure 8.10A). The various phytochromes encoded by these genes have distinctive molecular properties. For example, PHYA is unstable in the light and consequently is most abundant in etiolated and newly-emerged tissues, whereas all the other phytochromes are light-stable and predominate in light-grown plants (Figure 8.10B). Arabidopsis PHYA forms only homodimers, whereas the other Arabidopsis PHYs can form heterodimers with each other, though the physiological significance of such contrasting dimerization behavior is not clear.
Figure 8.10 (A) Relationships between members of the Arabidopsis PHY family based on protein sequence alignment. PHYA and PHYC have about 50% homology to each other. PHYB and PHYD are closely related with about 80% homology. PHYE has less than 50% homology with the PHYA/C group and more than 50% with PHYB/D. (B) Expression of each PHY gene in different tissues during the Arabidopsis life cycle.

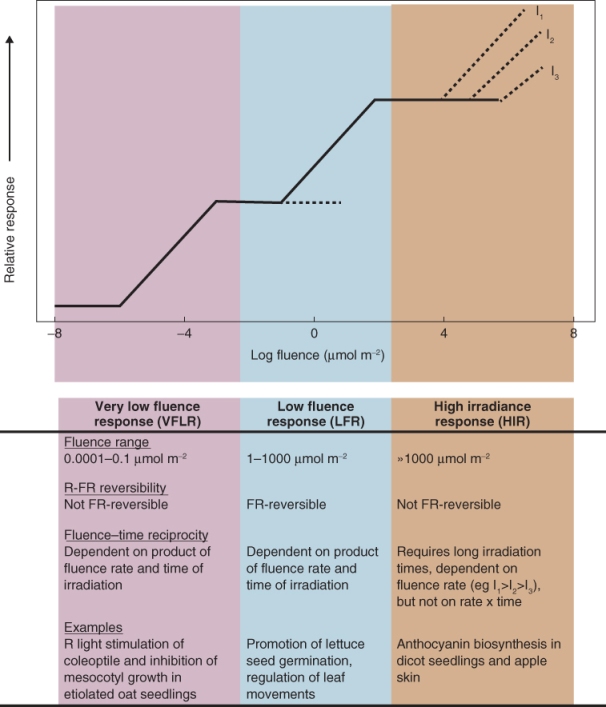
In addition to variation in expression between different tissues during the life cycle (Figure 8.10B), the diverse forms of phytochrome differ in their sensitivities to light and in the responses they control. Phytochrome-mediated responses can be classified into the high irradiance reaction (HIR) and low fluence responses. Fluence is defined as the number of photons impinging on a unit surface area and has units of mol m−2. Irradiance is fluence rate, that is, fluence per unit time, with units of mol m−2 s−1. A characteristic of low fluence responses (but not HIR) is that they obey the principle of reciprocity: they may be invoked by long exposure to dim light or short exposure to higher irradiance. Low fluence responses are further subdivided into the low fluence red/far-red reversible reaction (LFR) and the very low fluence response (VLFR). Figure 8.11 summarizes the fluence-response relationships of VLFR, LFR and HIR and gives examples of photomorphogenic events associated with each class.
Figure 8.11 Characteristics of phytochrome responses at different fluences. FR, far red; R, red.
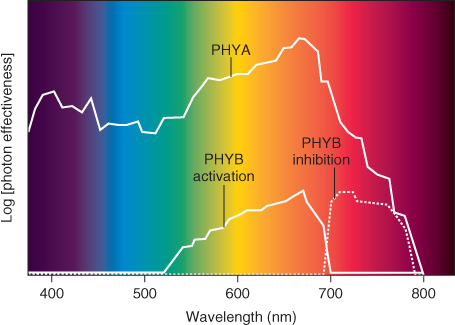
Mutations in one or other of the phytochrome genes have been identified in a number of plant species and these have allowed individual members of the phytochrome gene family to be associated with particular fluence responses. For example, the lh mutant of cucumber (Figure 8.12) and the hy3 mutant of Arabidopsis, which are deficient in PHYB, grow taller than wild-type plants in white light. This phenotype mimics the effect of shading and is consistent with the ecological role of phytochrome in proximity-sensing and competition for light in plant communities. When the PHYA-deficient aurea mutant of tomato is grown in white light, it has yellow-green leaves, elongated hypocotyls and reduced anthocyanin content. The evidence from such studies identifies PHYB and PHYA as the prime regulators of LFR and VLFR, respectively. The different functions of PHYA and PHYB are reflected in their action spectra. Figure 8.13 shows the action spectrum of PHYA, determined for the VLFR of Arabidopsis seed imbibed in water for 2 days in darkness, and the PHYB action spectrum determined in seed imbibed for very short periods. The PHYA-related reaction is not far-red reversible. By contrast, the PHYB response is a typical LFR (see Figure 8.11) in being reversed by exposure to far-red light.
Figure 8.12 Growth of wild-type and lh mutants of cucumber. Plants were grown for 20 days on a 14/10-hour light/dark cycle and exposed to 20 minutes of far-red light (FR) or darkness (control treatment, D) at the end of each day period.

Figure 8.13 Phytochrome action spectra measured as the capacity of various wavelengths of light to induce seed germination or to reverse red light-dependent promotion of germination in Arabidopsis. The PHYA action spectrum is determined on seed of a PHYB-less mutant, imbibed in water for 2 days in darkness. The action spectrum for PHYB is determined on seed of a PHYA mutant imbibed in water for 3 hours in darkness. PHYB activation of seed germination by red light is reversible by exposure to far-red light while the PHYA effect is not. Germination response is determined as photon effectiveness (fluence required for the induction or inhibition) and is expressed on a log scale.
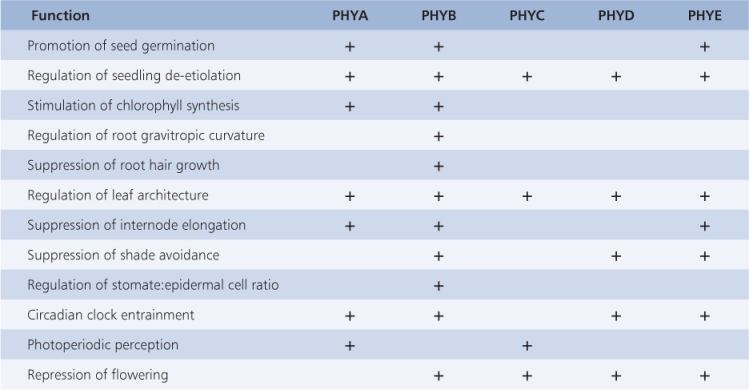
The three other phytochromes of Arabidopsis have a diversity of functions, some of which overlap (Table 8.2). Although phytochromes clearly are involved in HIR, evidence indicates that other photoreceptors that absorb UV or blue light (see Section 8.3) contribute to this control. In relation to the different fluence responses, it seems that the various phytochromes within or between species share similar functions but have diverged to adopt particular roles, reflecting evolutionary separation and particular ecophysiological adaptations.
Table 8.2 Examples of functions influenced by phytochromes A–E, determined by analysis of Arabidopsis mutants.
Induction of photomorphogenesis by phytochrome involves large changes in the patterns of gene expression. If phytochrome is to affect gene expression, a number of events must take place. First PR, which is found in the cytosol, must be converted to PFR, which then moves into the nucleus. Next PFR interacts with one of a number of phytochrome-interacting factors (PIFs). The first PIF was discovered by using yeast two-hybrid screening, a technique that allows the identification of molecules that bind to a protein of interest (Figure 8.14). When PHYB was used as bait in this technique, a PIF called PIF3 was discovered. PIF3 is a nuclear-localized basic helix-loop-helix (bHLH) transcription factor (see Chapter 3) that binds to PHYB in preference to PHYA, at the C-terminal domain. Subsequently more members of the PIF3 bHLH protein family have been identified; these include PIF1, -4, -5 and -6. PIF3 and related factors promote the transcription of skotomorphogenic genes and suppress photomorphogenesis. After PFR complexes with PIF3, the interaction leads to the phosphorylation of PIF3, which is followed by its ubiquitination and degradation via the proteasome (Figure 8.15; see Section 5.9). Thus PFR removes the transcription factors that promote skotomorphogenesis and rebalances transcription in favor of photomorphogenesis.
Figure 8.14 Yeast two-hybrid screening. (1) The transcription factor that regulates a yeast gene encoding a selectable marker is engineered so that it is separated into a DNA-binding domain (DBD) and a transcription activation domain (AD). The DBD or AD alone is unable to activate transcription. (2) If one fragment binds a protein of interest, X, usually referred to as the ‘bait’ (phytochrome in this case), and (3) the other fragment binds an interacting protein, Y, the ‘prey’ (a PIF, for example), the transcription factor is reconstituted (4) and the yeast reporter gene is transcribed.
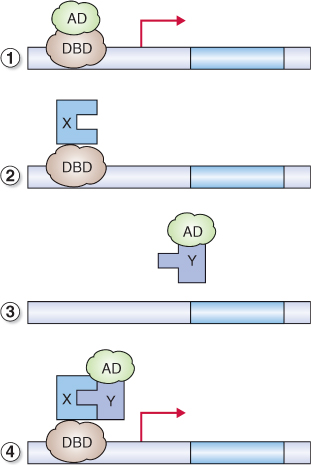
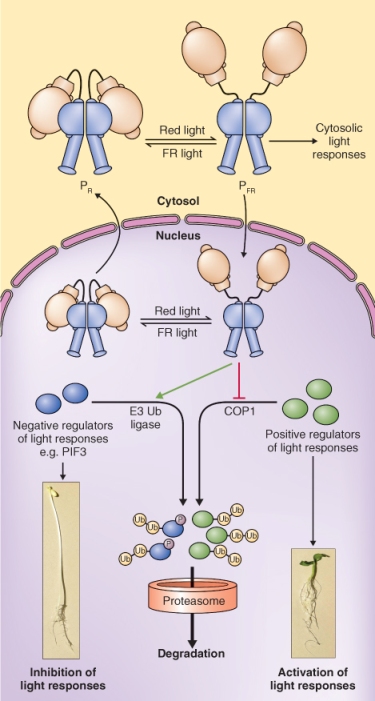
Figure 8.15 The effect of phytochrome on photomorphogenesis. PFR promotes photomorphogenesis by stimulating the degradation of negative regulators of photoresponses via the ubiquitin–proteasome system and by preventing the degradation of positive regulators. COP1 is an E3 ubiquitin ligase that specifically targets positive regulators of light responses.
There are many other types of molecule that interact with phytochrome. FHY1 (Far-red elongated Hypocotyl 1) and FHL (FHY1-like) are two PIFs that interact with the PFR form of PHYA but not PHYB and facilitate the transport of PHYA to the nucleus. Some PIFs influence phytochrome activity by stabilizing PFR. ARR4 (Arabidopsis response regulator 4) is an example of this type of PIF; it stabilizes PHYB but not PHYA. ARR4 is a member of the type A family of Arabidopsis response regulators, which act as negative regulators of cytokinin signaling (see Section 10.4.3). ARR4 protein accumulates in red light, in a PHYB-dependent manner. It binds equally to the PR and PFR forms of PHYB and inhibits dark reversion of PFR to PR. By stabilizing the PFR form of PHYB, ARR4 binding enhances phytochrome activity.
Another PIF that regulates the output activity of phytochrome is COP1 (Constitutive Photomorphogenic 1). COP1 is the master repressor of photomorphogenesis; cop1 mutants undergo constitutive photomorphogenesis, even in the dark. COP1 is an E3 ubiquitin ligase (see Section 5.9). It promotes skotomorphogenesis by targeting a number of the transcription factors that enhance expression of photoresponsive genes to the 26S proteasome for degradation. The cellular localization of COP1—cytosolic in the light, nuclear in the dark—is regulated by PHYA and PHYB (and also cryptochrome). COP1 bound to PFR is excluded from the nucleus, thus stabilizing positive regulators of light-regulated genes and permitting photomorphogenesis (Figure 8.15). COP1 also appears to play a role in the light-triggered degradation of PHYA. Binding to COP1 increases ubiquitination and degradation of PHYA; conversely, PHYA is stabilized in the cop1 mutant.
These examples of phytochrome interactions show that light influences morphogenesis in a very precise way through photoconversion of the phytochrome chromophore, changes in protein conformation, phosphorylation/dephosphorylation, complex formation with PIFs, subcellular compartmentation and differential protein turnover via the ubiquitin–proteasome system (UbPS). The phytochrome regulatory network is extensive. At least 15 different proteins with roles in phytochrome signal transduction have been identified as interacting with PFR, and over a dozen more are known from genomic analyses and protein-binding screens. We are only just beginning to explore the mechanisms and interactions of the pathways in which these components operate, but it is already clear that phytochrome-mediated light perception is of central importance in the control of plant growth and development.
Phytochrome provides the plant with morphogenetic information primarily from the red end of the light spectrum. Plants are also sensitive to wavelengths in the range 320–500 nm. Among the reactions to blue light are changes in plant cell plasma membrane potential (Figure 8.16), inhibition of hypocotyl elongation, phototropism, stomatal opening, circadian clock setting and anthocyanin production. Biochemical and molecular responses include changes in redox reactions, electron transport and expression of blue light-regulated genes (Table 8.3). Plants are equipped with at least three classes of photoreceptors sensitive to UVA and blue wavelengths of light. Cryptochromes and phototropins are well-established receptors that mediate the effects of UVA/blue light. More recently a third class of blue light receptor, known as zeitlupe from the German for ‘slow motion’, has been identified. Zeitlupe proteins will be discussed in more detail in Section 8.3.3. All three groups of blue light photoreceptors contain flavins as chromophores. There is incomplete but growing knowledge of the processes regulated by these photoreceptors (Table 8.3) and the underlying mechanisms of photoreceptor activation and signal transduction.
Figure 8.16 Blue light effect on plasma membrane potential and growth of etiolated hypocotyls. Individual cells of etiolated hypocotyls undergo a pronounced depolarization of the membrane potential when irradiated with blue light (white arrow). This is followed by a marked decline in their rate of growth.
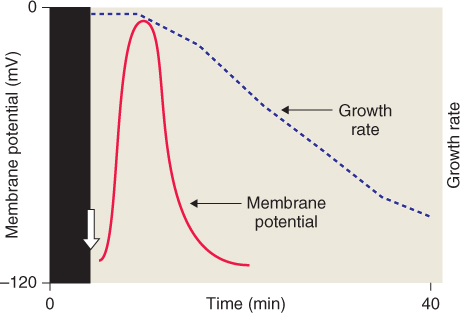
Table 8.3 Physiological responses to blue light and the corresponding photoreceptors.
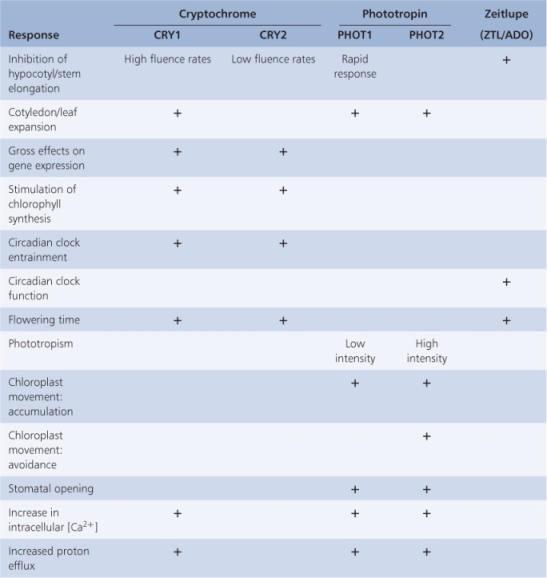
The Arabidopsis genome contains three genes, designated CRY1–3, that encode cryptochromes. CRY1 was isolated by screening mutagenized populations for individuals that have abnormally long hypocotyls when grown under blue or UVA light but have normal hypocotyl length under R or FR light. A second Arabidopsis cryptochrome gene, CRY2, was identified by sequence homology with CRY1. CRY1 and CRY2 are responsible for regulating various blue light responses, including hypocotyl and cotyledon growth, operation of the circadian clock and flowering time (see Table 8.3). The Arabidopsis genome contains a third member of the cryptochrome gene family, CRY3, whose function is unknown at present. While the CRY1 protein is stable in blue light, CRY2 is unstable in blue light and subject to rapid proteolysis. CRY2 and CRY1 are 58% identical in amino acid sequence at the amino-terminal region, but show only 14% sequence identity at the C terminus.
CRY genes encode proteins of 70–80 kDa that form dimers in the active state. Figure 8.17 compares the domain structures of the three CRY proteins of Arabidopsis. Cryptochromes contain two cofactor chromophores, flavin adenine dinucleotide (FAD) and pterin (methenyltetrahydrofolate, MTHF). FAD is non-covalently bound and transfers electrons to the protein moiety via a semiquinone intermediate when CRY1 and CRY2 are in their signaling state. Equation 8.4 shows the forms of FAD during the cryptochrome photocycle, in which the interconversions are driven not only by blue light and darkness but also by green light (see Section 8.3.5).
Figure 8.17 Domain structures of the three cryptochromes of Arabidopsis. FAD, flavin adenine dinucleotide cofactor; MTHF, methenyltetrahydrofolate (pterin) cofactor; PHR, photolyase-related domain (binds cofactors). DAS is a highly conserved motif found in cryptochromes from a wide range of organisms. Nuc shows the nuclear localization motif near the C terminus of CRY2. Imp indicates the organelle import signal at the N terminus of CRY3.
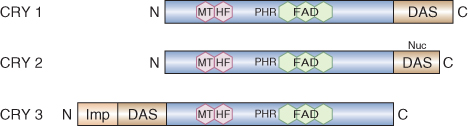
The cofactor-binding region of the protein is called the PHR (photolyase-related) domain because of its structural similarity to the bacterial repair enzyme DNA-photolyase (although plant CRYs have no photolyase enzyme activity). The PHR domain also mediates dimerization, which is essential for photoreceptor function. A conserved motif, referred to as DAS, occurs in the C-terminal domains of CRY1 and CRY2. The C-terminal region of CRY2 has also been shown to contain a nuclear localization signal. Although CRY1 lacks this sequence, it also has been shown to move between cytosol and nucleus. In CRY3 the DAS motif found between the PHR domain and an N-terminal extension (absent from CRY1 and CRY2) is required for the import of the protein into chloroplasts and mitochondria.
Several aspects of cryptochrome function in signal transduction resemble those of phytochrome. We have already seen that CRY2, like PHYA, is unstable in light, and both cryptochrome and phytochrome are active in the nucleus. Blue light stimulation of CRY1 and CRY2 results in a rapid phosphorylation that is at least partially self-mediated. There is evidence that blue light leads to a change in CRY protein conformation, exposing the C terminus to physical interaction with signaling factors. One of these is COP1, the repressor of photomorphogenesis in darkness that also interacts with phytochrome. Experiments have shown that CRY1 and CRY2 bind to phytochrome (see Section 8.2.4), and there are also reports that phytochrome can phosphorylate CRYs. The physiological significance of such CRY–PHY interactions is not clear, but it is likely that they mediate cross-talk between photoreceptor signaling pathways. CRY1 is known to be required for PHYA signaling to the circadian clock (see Section 8.5).
As the name suggests, the phototropin class of UV/blue light receptors was discovered through studies of phototropism. The search for the blue light photoreceptor for phototropism led to identification of a plasma membrane-associated protein from pea epicotyls that becomes autophosphorylated on exposure to blue light. Subsequently a homologous protein was identified in Arabidopsis through analysis of a non-phototropic mutant, nph1. Two phototropin genes (PHOT1 and PHOT2), which have partially overlapping functions, are currently known to occur in Arabidopsis (see Table 8.3). PHOT1 alone modulates cotyledon and hypocotyl growth, whereas both PHOT1 and PHOT2 are implicated in phototropism, chloroplast movement, stomatal opening and ion transport. In some cases, however, the two phototropins operate over different fluence ranges.
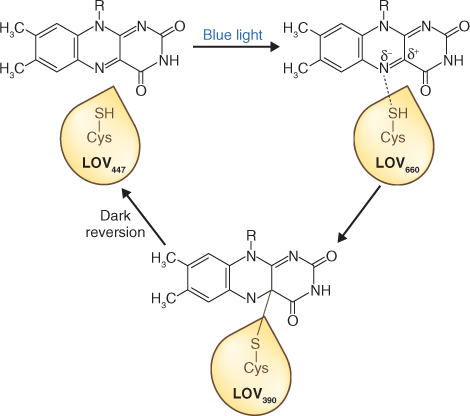
The phototropin protein (Figure 8.18A) consists of two regions: an N-terminal photosensory domain and a C-terminal serine/threonine kinase domain that are connected by a hinge. Within the photosensory region are two similar motifs of approximately 110 amino acids, called LOV1 and LOV2, that bind the light-sensing cofactor FMN (flavin mononucleotide). The acronym LOV refers to light, oxygen and voltage—the external signals that regulate proteins of the diverse superfamily to which phototropins belong. In the dark, the two domains of the molecule are appressed and the kinase is inactive. The FMN photocycle is shown in Figure 8.19. When FMN absorbs blue light it binds covalently to the LOV domain via a conserved cysteine residue. Intermediates in the photoconversion process are identifiable by their absorbance maxima. In the dark the LOV2 region interacts with a conserved helical region on the C-terminal side of the photosensory domain, the Jα-helix (Figure 8.18B). Autophosphorylation, a consequence of the disruption of this association by photostimulation, leads to activation of the C-terminal kinase. There is evidence that phosphorylation may be necessary for relocating the proteins from the plasma membrane to the Golgi apparatus, a rapid subcellular response to irradiation.
Figure 8.18 Phototropin and neochrome. (A) Domain structures of phototropin and neochrome. Neochrome, found in certain ferns and algae, is a chimeric protein consisting of a phytochrome photosensory domain fused to the N terminus of an entire phototropin receptor. (B) Mechanism of phototropin receptor activation by light. The unphosphorylated receptor is in the inactive ground state. Absorption of light by LOV2 causes the Jα-helix region to unfold. This activates the C-terminal kinase domain, which leads to autophosphorylation of the photoreceptor and (possibly) phosphorylation of other proteins.
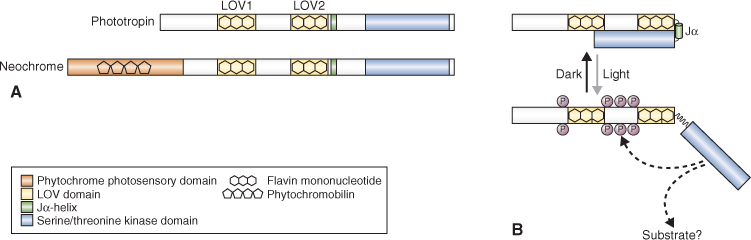
Figure 8.19 The FMN photocycle in the phototropin LOV domain. In darkness the FMN chromophore absorbs light maximally at a wavelength of 447 nm (LOV447). Blue light drives covalent binding of FMN to a cysteine residue in the LOV domain forming LOV390, via LOV660, an intermediate absorbing maximally at 660 nm. The photoreaction process is fully reversible in darkness.
How the signal is transmitted from phototropins to downstream targets is not clear. For example, blue light, absorbed by both PHOT1 and PHOT2, stimulates the opening of stomata. This response requires activation of the guard cell plasma membrane H+-ATPase and extrusion of protons (see Figure 14.32). Although activation of the H+-ATPase involves its phosphorylation, there is no evidence that phototropin is directly involved. Other factors are thought to mediate the interaction between phosphorylated PHOT and plasma membrane H+-ATPase, allowing transduction of the blue light signal into stomatal opening.
Stems and grass seedling coleoptiles are positively phototropic, growing towards a unilateral light source. This response is mediated by PHOT1. Unilateral irradiation induces a gradient of PHOT1 autophosphorylation, which in turn leads to a gradient of auxin concentration. Higher concentrations of auxin on the darker side of the organ result in more rapid growth on that side and the stem or coleoptile curves toward the light. Analysis of mutants with impaired phototropic responses has identified proteins that interact with PHOT1 and are thought to function in auxin redistribution. One of these is phytochrome kinase substrate 1 (PKS1) protein, a PIF. PKS1 is a member of the PKS gene family, which has been shown by analysis of mutants in Arabidopsis to be required for normal phototropism under weak intensities of blue light. PKS1 is thus a link between the phototropin and phytochrome signaling pathways.
The movement of organelles within cells is also influenced by blue light acting through phototropin receptors. Under dim illumination the chloroplasts of mesophyll cells tend to orientate parallel to the leaf surface, thereby maximizing light capture, but in bright light they avoid possible photodamage by lining up along the cell walls perpendicular to the surface of the leaf (see Figure 4.39). Identification of Arabidopsis mutants lacking the chloroplast avoidance response has led to the identification of CHUP1 (Chloroplast Unusual Positioning 1) and a family of PMI (Plastid Movement-Impaired) genes, the expression of which is necessary for linking phototropin excitation with organelle positioning within the cell.
Proteins in the recently described zeitlupe class of blue light photoreceptors (also known as ZTL/ADO) are related to, but different from, phototropins. Like the phototropins, the zeitlupe proteins have a photoactive FMN-binding LOV region. However, they also have an F-box motif. This is a structural motif of approximately 50 amino acids that facilitates protein–protein interactions. F-box proteins are components of certain E3 ubiquitin ligases. E3 ligases with F-box proteins are associated with a range of cellular functions including signal transduction, hormone action and regulation of the cell cycle (see Chapter 10). Three zeitlupe-type genes have been identified in the Arabidopsis and rice genomes and putative homologs have been described in several other species, including poplar, maize and pine. The physiological functions of zeitlupe photoreceptors include the targeted proteolysis of components associated with the control of flowering time and the operation of the circadian clock (see Section 8.5).
Another type of photoreceptor with LOV domains is the neochromes. This family of receptors appears to have evolved independently in ferns and algae. Neochrome proteins have a phototropin-like protein sequence fused with a phytochrome chromophore-binding domain (see Figure 8.18A), making them dual red/blue light photoreceptors. The filamentous green alga Mougeotia has two neochromes and two phototropins. The single, large, ribbon-like chloroplast in each Mougeotia cell displays a striking light-avoidance response (Figure 8.20) under the control of these photoreceptors.
Figure 8.20 Neochrome- and phototropin-mediated movement of chloroplasts in cells of the filamentous green alga Mougeotia. (A) In dim light the flat side of the single ribbon-like chloroplast faces the light, while in bright light the edge faces the light. (B) Light micrographs of a rotating chloroplast taken at defined intervals. Scale bar = 10 μm.

Rhodopsin-like and flavoprotein photoreceptors occur in many algae and also in animals. Rhodopsin is well known as a visual pigment in humans and other vertebrates; its chromophore is retinal, a vitamin A derivative. The flagellated, unicellular green alga Chlamydomonas reinhardtii has a pigmented eye-spot that detects light for phototaxis (movement in response to directional light). The photoreceptors in this alga are two rhodopsin-like molecules called channel rhodopsins (ChR1 and ChR2). The channel rhodopsins are light-gated proton channels that regulate phototaxis in response to blue-green light (absorption peak at 500 nm). In addition to rhodopsin-like photoreceptors, C. reinhardtii has a single cryptochrome and a single phototropin (but no phytochrome). In the photosynthetic protist Euglena gracilis, the photoreceptor for phototaxis is a photoactivated adenylyl cyclase. This flavoprotein complex responds to directional blue light information by increasing the levels of cytosolic cyclic AMP, a regulator of flagellar motility.
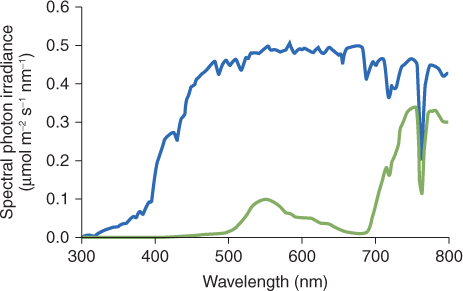
Light absorbed by the major photoreceptors is predominantly at either extreme of the visible spectrum. Likewise, light capture by photosynthetic pigments takes place largely at red and blue wavelengths. Reflection and transmittance of non-absorbed light peaks at about 560 nm, which gives vegetation its characteristic green color (Figure 8.21). The reflectance spectrum of foliage has been an important factor in the coevolution of plants and the visual systems of animals that interact with them. For example old-world primates, such as gorillas, chimpanzees and humans, have three-color (RGB) vision, whereas the photoreceptors in the eyes of new-world primates are sensitive to only two wavelength ranges, centered on green and blue. Comparative physiological and genetic studies indicate that the third photoreceptor of old-world primates (absorbance maximum at about 564 nm) evolved because it is spectrally tuned to the wavelengths reflected by leaves. This would have been an adaptive advantage, allowing the animal to pick out food and predators more easily against a background of foliage.
Figure 8.21 The spectral photon distributions of daylight (blue line) and light reflected from leaves of Japanese knotweed, Fallopia japonica (green line). Note that the light reflected from leaves is highly depleted in red quanta and relatively enriched in far-red quanta.
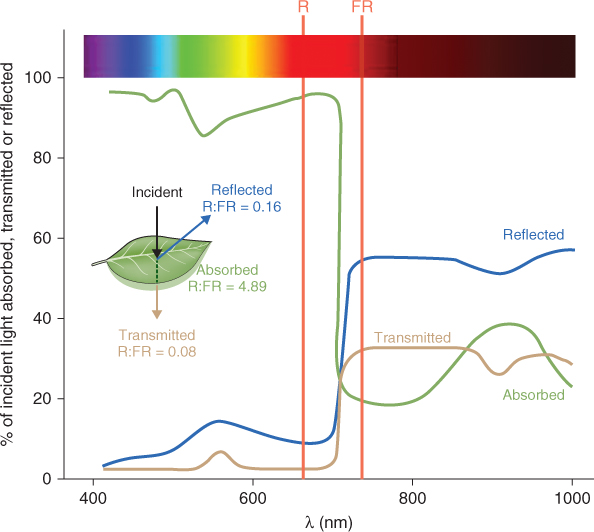
Light reflected from vegetation also has an important role in plant–plant interactions. A crop or a natural community is a population of individuals competing for resources, with foliage that forms canopies. Neighboring plants can sense each other by responding to shifts in the spectral quality of light resulting from canopy reflectance. For example, the light that is transmitted or reflected by green leaves has an increase in the ratio of far-red to red quanta (Figure 8.22). This change in R:FR ratio can be detected by phytochrome, leading to an increase in the proportion of phytochrome that is in the PR (inactive) form. Shade-intolerant plants, like Arabidopsis, respond by increasing their rate of stem and petiole elongation. This response has been called shade avoidance, since rapid stem elongation may enable plants to escape shading by other plants. Mutations that abolish this mechanism for detecting changes in R:FR ratio cause plants to grow as though shaded even when they are placed in white light, as shown for the PHYB-deficient lh mutant of cucumber in Figure 8.12. Shading also decreases blue light fluence rates that can be detected by blue/UV photoreceptors, and in some species phototropin may serve as a photoreceptor for the shade-avoidance response. This response is an essential strategy for survival and, because it directly influences light capture and photosynthetic productivity, is agriculturally important as a factor in crop yield.
Figure 8.22 Spectral characteristics of typical plant leaves over the wavelength range 400–1000 nm. Leaves absorb more than 80% of incident light between 400 and 700 nm. There is a sharp fall in absorbance in the far-red and infra-red regions. Consequently the red:far-red (R:FR) ratio of absorbed light (4.89) is much higher than that of reflected (0.16) or transmitted (0.08) light. There are consequences for the state of the phytochrome, which will be converted to the PR form in reflected and transmitted light.
In laboratory studies of photophysiology, green is widely used as a ‘safe’ light, because it has minimal effects on the plants under investigation. There is increasing evidence, however, that plant morphogenesis is not completely insensitive to green light. A number of studies suggest that the green waveband has negative effects on aspects of extension and tropic growth, stomatal opening and the expression of genes in plastids. Many of these responses may be explained in terms of the absorbance spectra of known photoreceptors. For example, Figure 8.7 shows that phytochromes, particularly PR, have appreciable absorbance in the 500–600 nm range, and the PHYA action spectrum for breaking seed dormancy extends well into the green waveband (see Figure 8.13).
Recent studies of cryptochrome show that green light can reverse the effect of blue and have led to a proposed model of the flavin photocycle in which oxidized and reduced forms interconvert in response to irradiation with blue or green light and are subject to dark reversion (see Equation 8.4). Nevertheless, some responses to green light are difficult to account for in terms of the known photoreceptors and the search for a fourth, green-sensitive, class has turned up some interesting candidates. Blue light stimulates stomatal opening. A short green light pulse eliminates the blue light response. The action spectrum for the green light effect, which shows a maximum at 540 nm and minor peaks at 490 and 580 nm, resembles the absorption spectrum of the carotenoid zeaxanthin red-shifted by about 50 nm. Stomata from a zeaxanthin-less Arabidopsis mutant lack a specific response to blue light. Zeaxanthin is also a central component in the xanthophyll cycle, a mechanism for dealing with light stress (see Chapters 9 and 15). Other pigments that could function as potential green-sensitive photoreceptors in seed plants have been described, including novel classes of flavoprotein and retinal-binding proteins.
As mentioned in Sections 8.1.1 and 8.1.2, UV light can be harmful to living organisms. In particular, nucleic acids and proteins absorb UVB and UVC strongly and may be damaged in the process. Ultraviolet damage is itself a photoperception event and triggers a range of signaling and biochemical responses. There is evidence, however, for the existence of specific ultraviolet-sensitive photoreceptors distinct from the known UV/blue receptor classes, one active in the range 280–300 nm and another at 300–320 nm. At present their chemistry and mode of action are unknown.
Seedlings germinated and grown in the dark are pale yellow, while those grown in the light are green. This change in color is due to the production of chlorophyll and a functional photosynthetic apparatus that only takes place in the light. In this section we will examine the process of chlorophyll synthesis and the basis for its dependence on light. Chlorophyll (Chl) is one of a group of molecules known as tetrapyrroles, so named because they take the form of four linked pyrrole groups. Phytochromobilin is also a tetrapyrrole. Another important tetrapyrrole is heme, best known as a component of the oxygen-carrying protein hemoglobin in blood. Leghemoglobin is a hemoprotein that is essential for the nitrogen-fixation process in plants because it sequesters oxygen to prevent inhibition of the nitrogen-fixing enzyme nitrogenase (see Section 12.4.4). Heme is also the cofactor of cytochromes, which are involved in electron transport in both respiration (see Chapter 7) and photosynthesis (see Chapter 9), and of certain enzymes such as peroxidases. The initial steps in the biosynthesis of all tetrapyrroles are identical; the pathway then branches according to the final product. In the case of Chl, all the biosynthetic enzymes are found in the plastid, although they are encoded by nuclear genes (Figure 8.23). Most of the genes needed for Chl biosynthesis are regulated in a light-dependent manner, and some of them are also expressed according to circadian rhythms. Environmental factors other than light also play a part in regulating Chl biosynthesis.
Figure 8.23 The tetrapyrrole biosynthesis pathway. Chlorophylls and hemes are biosynthesized by a branched pathway which begins with the synthesis of δ-aminolevulinic acid (ALA) from glutamic acid. In the next three steps eight molecules of ALA are combined, via the intermediate porphobilinogen (PBG), to form the ring structure of uroporphyrinogen III which undergoes further modifications to become protoporphyrin IX. After this point the heme and chlorophyll pathways diverge. Chlorophyll synthesis requires insertion of Mg into the protoporphyrin IX ring by magnesium chelatase, whereas heme contains Fe, which is inserted into the ring by a ferrochelatase. Chlorophyll synthesis involves further steps, the penultimate of which, in angiosperms, requires the light-dependent enzyme protochlorophyllide oxidoreductase. This produces chlorophyllide a, to which the phytol chain is attached to form chlorophyll a. Chlorophyll b is synthesized from chlorophyll a.

Tetrapyrrole biosynthesis begins with the formation of aminolevulinic acid (ALA). The first step in ALA biosynthesis (Equation 8.5), catalyzed by glutamyl tRNA reductase (GluTR) in plants, is rate-limiting for the whole pathway. GluTR is encoded by the gene HEMA1. The photoreceptors that have been shown to act in light stimulation of Chl biosynthesis are PHYA, PHYB, CRY1 and CRY2. They achieve this effect mainly by regulating transcription of HEMA1.
Heme, one of the major products of the pathway, acts as an allosteric inhibitor of GluTR in plants, so that if, for example, heme accumulates because its degradation is blocked, the biosynthesis of Chl precursors is also reduced. The synthesis of ALA is downregulated in darkness by the product of the FLU gene, which interacts physically with GluTR. Mutations in FLU result in plants that accumulate fluorescent Chl biosynthesis intermediates in the dark.
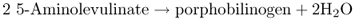
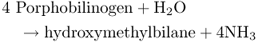
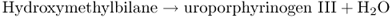
The first cyclic tetrapyrrole in the biosynthesis pathway, uroporphyrinogen III, is formed by the condensation of eight molecules of ALA (Equation 8.6). It then undergoes a three-stage oxidative conversion to protoporphyrin, during which the molecules become increasingly photoreactive and potentially damaging to the plant. When Chl and other tetrapyrroles absorb light they can transfer the energy to other molecules, leading to the formation of reactive oxygen species, which in turn can damage proteins and lipid membranes. For this reason tetrapyrrole synthesis is normally tightly regulated and mutations or chemical treatments that cause the buildup of photoreactive intermediates cause plants to become very sensitive to light damage. Mutation of the FLU gene, described in Section 8.4.1, is only one of the possible causes for accumulation of these phototoxic compounds. Their properties make intermediates in tetrapyrrole biosynthesis useful in the treatment of diseases, especially cancerous tumors by photodynamic therapy. In this approach a photosensitizing molecule is targeted to the diseased tissue. Then high-intensity light is used to induce localized production of reactive oxygen species which kill cancerous cells. ALA, the precursor for all tetrapyrrole biosynthesis, has been widely used in photodynamic therapy as have derivatives of bacterial chlorophyll. The early intermediates of chlorophyll catabolism (see Chapter 18) are also photoactive tetrapyrroles and one of them, pheophorbide, is another photosensitizer used therapeutically to cause lipid peroxidation and arrest of tumor growth.
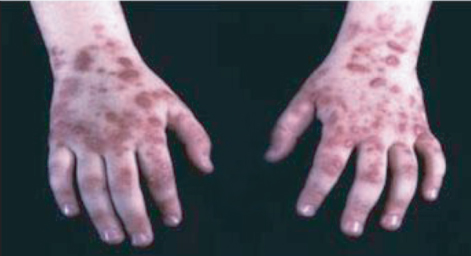
Animals, like plants, are susceptible to damage if intermediates in tetrapyrrole biosynthesis accumulate abnormally. Several of the human diseases known as porphyrias are due to deficiencies of specific enzymes in the heme biosynthetic pathway. For example, uroporphyrinogen III synthase, coproporphyrinogen III oxidase, protoporphyrinogen oxidase and ferrochelatase deficiencies can all lead to illnesses with symptoms that include photosensitivity. Sufferers need to avoid bright light as otherwise they may develop painful skin lesions (Figure 8.24).
Figure 8.24 Skin lesions in a sufferer from porphyria, a disorder affecting the heme biosynthesis pathway, induced by accumulation of tetrapyrrole biosynthesis intermediates.
All the enzymes of the pathway illustrated in Figure 8.23 up to and including coproporphyrinogen III oxidase are found in the plastid, either in the stroma or attached loosely to the plastid membranes. However, the next enzyme in the pathway, protoporphyrinogen oxidase (PPX), is located in both mitochondrial and plastid membranes. At this stage in the pathway, if the tetrapyrrole is destined for conversion into a mitochondrial cytochrome it must be transported from the plastid to the mitochondrion, though the transporters responsible have not yet been identified.
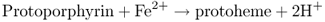
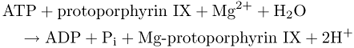
Protoporphyrin (Proto) can undergo two different fates (Equations 8.7 and 8.8). If Fe2+ is inserted into the center of the tetrapyrrole ring the molecule is destined to become heme; insertion of Mg2+ leads to the synthesis of chlorophyllide (Chlide) and thence Chl. In plants these processes are carried out by the metal chelatase enzymes ferrochelatase (FeCh) and magnesium chelatase (MgCh), respectively; when both enzymes are present in the same subcellular compartment they compete for their common substrate, Proto. FeCh is found both in plastids and in mitochondria, but there is evidence that in plants most heme biosynthesis takes place in the plastids. Plant MgCh consists of three subunits, CHLI, CHLD and CHLH. In angiosperms, the distribution of CHLH and CHLD between the plastid envelope membrane and the stroma is dependent on internal Mg2+ concentration. It is probable that the CHLI–CHLD complex provides magnesium ions, while CHLH carries the Proto substrate.
As discussed in Section 8.2.2, phytochromes are dimeric proteins. Each of the two monomers in a phytochrome molecule is covalently linked to the linear tetrapyrrole phytochromobilin (see Section 8.2.1 and Figure 8.6). This compound is synthesized from heme (Figure 8.25); the committed step in its synthesis is the cleavage of heme by heme oxygenase, producing biliverdin IXa, in a reaction that requires oxygen and the electron donor ferredoxin (Fd), and produces carbon monoxide and Fe2+ (Equation 8.9).
Figure 8.25 The biosynthesis of phytochromobilin and other plant bilins from the linear tetrapyrrole biliverdin IXα (BV). 3Z-phytochromobilin, the precursor of the 3E-phytochromobilin chromophore that is found in phytochrome, is produced by the action of phytochromobilin synthase (HY2). Phycocyanobilin:ferredoxin oxidoreductase (PcyA) is responsible for the synthesis of phycocyanobilin (PCB). Phycoerythrobilin (PEB) formation requires the sequential action of two enzymes: 15,16-dihydrobiliverdin:ferredoxin oxidoreductase (PebA), which reduces the C-15 methine bridge of BV; and phycoerythrobilin:ferredoxin oxidoreductase (PebB), which reduces the A-ring diene structure of 15,16-dihydrobiliverdin (DHBV).

The ferredoxin-dependent enzyme (3E)-phytochromobilin synthase then reduces biliverdin IXa to the so-called 3Z-isomer of phytochromobilin, which is finally isomerized by flipping a methyl group, resulting in (3E)-phytochromobilin, the active configuration of the phytochrome chromophore (see Section 8.2.1). It is not known whether this isomerization step occurs spontaneously or requires enzymatic catalysis. The biosynthetic pathway shown in Figure 8.25 also produces other bilins, including phycocyanobilin and phycoerythrobilin. These compounds are chromophores of phycobiliprotein photosynthetic pigments in cyanobacteria and some algae (see Section 9.2.1).
The last but one stage in the biosynthesis of chlorophyll a is the conversion of protochlorophyllide (Pchlide) to Chlide by reduction of the double bond between carbons C17 and C18 in ring D (see Figure 8.23). The enzyme catalyzing this reaction is NADPH-Pchlide oxidoreductase (POR; Equation 8.10).
All algae, aerobic photosynthetic bacteria, liverworts and gymnosperms contain a three-subunit light-independent form of POR. However, at some point in evolution, angiosperms lost this enzyme, and they have only a light-dependent form, which is also present in all the other photosynthetic organisms except for some anaerobic bacteriochlorophyll-containing bacteria. This means that the final steps in Chl biosynthesis in angiosperms have an absolute requirement for light; in its absence, leaf plastids become etioplasts instead of chloroplasts (Figure 8.26).
Figure 8.26 Etioplast from Arabidopsis seedling grown in darkness.

Light-dependent POR, a key enzyme in Chl synthesis, is a monomeric enzyme of 35–38 kDa; in angiosperms it is usually encoded by small families of genes which are differentially regulated by light and at different developmental stages. In barley, for example, there are two POR genes, while Arabidopsis has three. Arabidopsis PORA is specifically expressed in the dark; phytochrome downregulates its transcription. PORB is constitutively expressed and PORC is induced by light. The lattice-like structure that occupies much of the etioplast, as shown in Figure 8.26, is largely composed of a complex of Pchlide, NADPH and POR. Pchlide acts as a photoreceptive prosthetic group in the complex. When tissue containing etioplasts is illuminated, POR rapidly converts Pchlide to Chlide and the internal structure of the etioplast is reorganized into the typical chloroplast configuration (see Section 4.6.2). The polypeptide prePORA, the precursor of the major ‘dark’ form of Arabidopsis POR, is imported into plastids from the site of synthesis in the cytosol in a substrate-dependent manner, so that sufficient Pchlide must be present in the plastid for the PORA precursor to enter.
PORA is one of a small number of plant proteins, also including phytochrome, which act as photoreceptors. In seedlings germinated in the dark, the etioplast complex of POR, Pchlide and NADPH described above is stable. However, upon illumination and the conversion of PChlide to Chlide, POR is released and the free enzyme is then subject to rapid proteolysis, so that most of the POR protein disappears in the light. Thus, the photoreceptor properties of POR are comparable with those of phytochrome: POR possesses a tetrapyrrole chromophore that triggers photomorphogenic changes in cells and tissues; and the light being received by the plant tissue regulates its form and abundance. The behavior of POR proteins is best understood from studies of the etioplast-to-chloroplast conversion. Other forms of POR, such as Arabidopsis PORC, which is responsible for chlorophyll synthesis in light-grown plants, still require light for activity, but the regulation of their turnover is much less well understood.
The product of the POR-catalyzed reaction is Chlide a. In the final step of chlorophyll a biosynthesis the 18-carbon phytol side-chain is added (Equation 8.11), a process that makes Chl, in contrast to Chlide, highly hydrophobic. The enzyme that carries out this step is chlorophyll synthase, which adds phytyl pyrophosphate to the C18 propionyl group of Chl in an esterification reaction.
Formation of chlorophyll b requires the oxidation of the C-7 methyl group of Chlide a to formyl as well as the addition of the phytol side-chain. This oxidation (Equation 8.12) is carried out by the enzyme Chlide a oxygenase, a monooxygenase that contains iron and sulfur, and the substrate is Chlide a rather than Chl a, producing Chlide b as a substrate for Chl synthase.
Chlorophylls are at the core of the photosynthetic apparatus, and as this section shows, their biosynthesis as well as their function is light-dependent, reinforcing the central role of light in autotrophic plant growth and morphogenesis. The workings of the photosynthetic machinery will be described in Chapter 9.
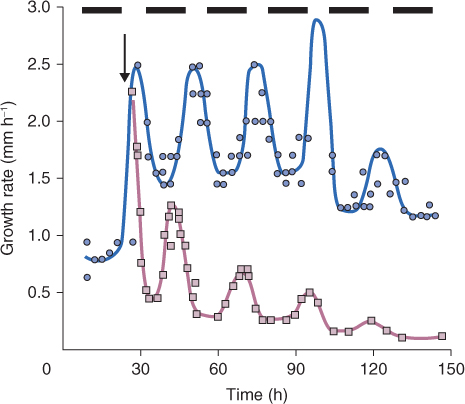
Early in evolution, living organisms evolved mechanisms to coordinate biological processes with the day–night cycle of light and temperature caused by the planet's rotation. It has been suggested that the original reason for this may have been to ensure that DNA was only replicated at night, to avoid the damaging effects of ultraviolet radiation. Present-day plants, animals, fungi and many other living organisms all show such cyclic regulation of biological activities. In some cases, as described earlier in this chapter, light itself has a direct regulatory role, but organisms also possess biological clocks which schedule events to occur on a daily basis—the so-called circadian rhythms. Under normal light–dark cycles, circadian rhythms are entrained to these cycles; that is, the duration and phase of the circadian rhythm is matched to that of the light–dark sequence. But even when an organism is removed from the normal environment of day and night and placed, for example, in continuous light, circadian rhythms continue to operate, with a time period of about 24 hours, for many days or even longer. Figure 8.27 shows an example in which the timing of leaf growth in a grass species follows a circadian rhythm even when the plant is placed in darkness, though the amplitude of the rhythm gradually declines and the cycle lengthens relative to the original 24-hour period.
Figure 8.27 Circadian rhythm in leaf growth. Growth rates of the fourth leaf of Lolium temulentum seedlings at 20°C were measured in an 8/16-hour light/dark photoperiod () and after transfer to continuous darkness () at 24 hours (arrow).
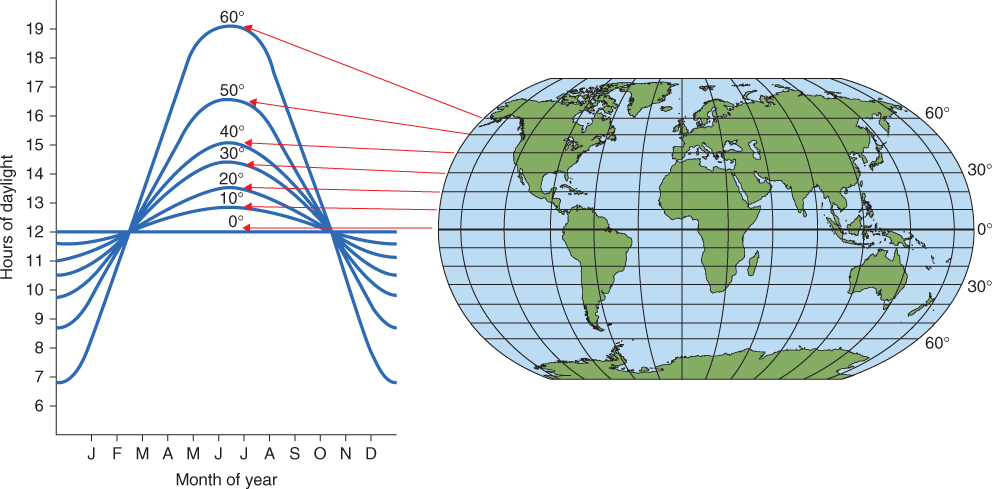
In regions near the equator, the cycle of light and darkness varies little throughout the year. In more northerly and southerly parts of the Earth, however, the planet's tilt on its axis means that the ratio of dark to light varies continuously (Figure 8.28). Photoperiodic control is the term given to the regulation of biological processes according to the relative lengths of day and night. As we shall see later, for photoperiodic control to operate properly a plant needs to ‘compare’ the external signal provided by light or its absence with its own endogenous circadian rhythm.
Figure 8.28 The relationship between day length throughout the year (measured on the 20th of each month) and latitude.
Proper functioning of circadian rhythms is important for all organisms. In humans, for example, the phenomenon of jet-lag is caused when travel through a number of time zones means that the body's clocks are out of phase with the new day–night cycle under which the person is now living. Over a period of days, the clocks are reset thanks to exposure to the altered dark/light pattern, but different bodily processes are reset at different rates, leading to unpleasant symptoms that can include sleep disturbance, digestive problems, headache and disorientation. For plants too, research has shown that those whose circadian clock is correctly aligned with the current day–night cycle fix more carbon, grow faster and have a competitive advantage over plants of the same species with incorrect circadian clocks due to mutation. Selective pressure on all species must have favored the existence of accurate clocks.
Circadian rhythms are cell autonomous—that is, they are set largely independently in each cell of an organism. It has been shown that the clocks of individual cells in a leaf are weakly synchronized with one another, even in the prolonged absence of light, so that rhythmic behaviors such as stomatal opening occur together across the whole leaf. However, roots use only a subset of clock genes for circadian regulation. Because roots are not normally exposed to light it is believed that they depend on a signal from the leaves to maintain their clocks in synchrony with the day–light cycle.
Circadian clocks regulate a high proportion of plant genes—it has been estimated that 25–30% of the protein-coding genes in Arabidopsis are subject to circadian control. These genes participate in many essential processes, as illustrated in Figure 8.29. Plants also use the circadian clock to measure the duration of day and night, thus sensing the season and influencing processes such as the onset of flowering or the start of leaf senescence, which must occur at the right time of year if the plant is to flourish. We will first discuss features of circadian clocks in plants and then examine photoperiodism, the response to seasonal changes in the lengths of day and night.
Figure 8.29 The circadian clock regulates many aspects of the plant life cycle: (A) germination; (B) elongation growth and shade avoidance; (C, D) leaf and flower movements; (E) flowering time and flower opening; (F) scent production; (G) tuberization; (H) winter dormancy; (I) stomatal opening; (J) photosynthesis; (K, L) protection from extremes of temperature.
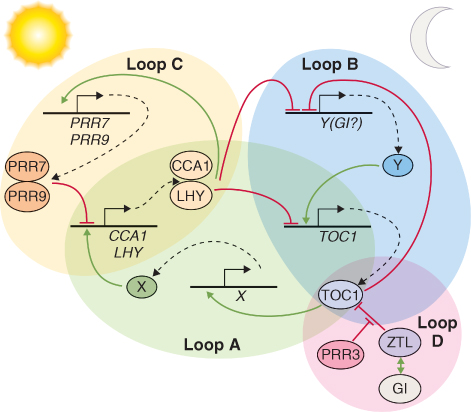
Although the way circadian clocks function appears similar in all organisms, the genes that control the clock mechanisms in plants are very different from those in animals or fungi. Because possessing a clock confers several selective advantages, there were probably many independent evolutionary events that led to their presence in these different organisms. Studies at the molecular and genetic levels have shown that the synchronizing of clocks to the Earth's natural 24-hour cycle occurs via a series of feedback loops that control the expression of a fairly small number of so-called clock genes. Typically, in a simple feedback loop (Figure 8.30), a stimulus causes transcription of a gene to increase, leading to synthesis of the corresponding protein and other downstream effects. Increasing abundance of the protein causes transcription of the gene to be downregulated, usually after a delay, and the net effect is an oscillation in the abundance of the protein and in any processes controlled by it. More sophisticated regulation can be achieved when interlocking loops participate, as illustrated in Figure 8.31. All circadian clocks are regulated by such systems of interlocking feedback loops, and research on plant clocks is uncovering increasing degrees of subtlety. Figure 8.31 shows the current explanation for clock regulation in the leaves of Arabidopsis in which the proteins CCA1 (Circadian Clock Associated 1), LHY (Late Elongated Hypocotyl) and TOC1 (Timing of CAB Expression 1), along with other, as yet unidentified, factors, control a morning and an evening loop. The third, more recently discovered, morning loop also involves CCA1 and LHY together with PRR7 and PRR9 (Pseudo-Response Regulators 7 and 9). At dawn, LHY and CCA1 proteins are synthesized, and this upregulates the genes encoding their inhibitors, PRR7 and PPR9, which in turn downregulate expression of the LHY and CCA1 genes in an example of negative feedback. LHY and CCA1 also inhibit the expression of evening genes in the morning. The most important of these evening genes, TOC1, is expressed at dusk and it regulates expression of its own activator, a hypothetical protein encoded by gene Y, which has not yet been isolated but whose existence has been inferred from the properties of the clock. Another hypothetical gene, X, believed to be upregulated by TOC1, feeds back from the evening to the morning loop, activating expression of LHY/CCA1 late in the night. Expression of the genes encoding LHY/CCA1, PRR9 and Y is stimulated by light, providing a means by which the clock can be maintained in synchrony with the length of the day.
Figure 8.30 A feedback loop. Transcription of gene A is upregulated by an inducer which leads to the synthesis of a protein D, and to a pathway leading to a response C. Increasing concentration of D leads to downregulation of A. The net result is an oscillation in the abundance of D and of the response C.
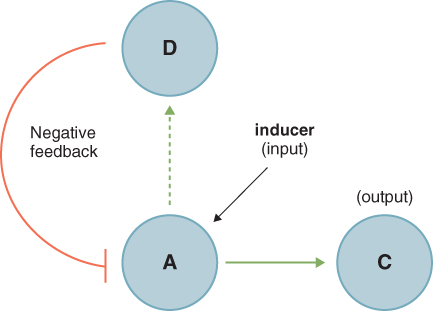
Figure 8.31 Model of the plant clock. Loop A consists of the dawn-phased factors CCA1 and LHY, negative regulators of TOC1 expression. TOC1 directly or indirectly activates the postulated component X, which induces CCA1 and LHY expression. Loop B comprises two or more evening-phased genes, an unknown factor Y (the activity of which may be partially provided by GI, the product of the gene Gigantea) and TOC1. Loop C consists of morning-phased PRR7, PRR9, CCA1 and LHY. Loop D shows post-transcriptional regulatory interactions between ZTL, TOC1, GI and PRR3.
While the best-understood components of the clock mechanism use transcriptional-level regulation, post-transcriptional modifications also play an important role, as in the case of the zeitlupe protein ZTL. ZTL negatively regulates abundance of the protein product of the key TOC1 gene by binding to TOC1 and targeting it for degradation by the proteasome. Although it is clear that the relative abundances of the clock gene products at different times of day exert a regulatory effect over other plant genes, the mechanisms by which they do so—the outputs of the plant circadian clock—are still poorly understood.
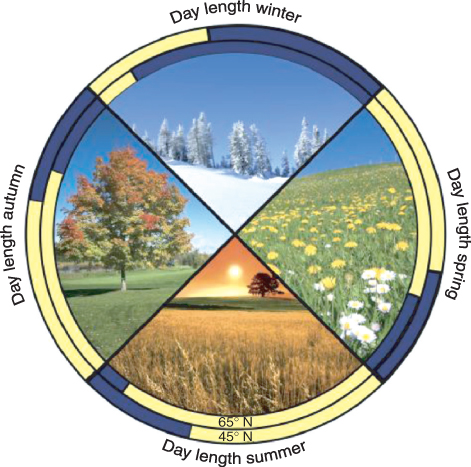
Many stages in plant reproductive development are under photoperiodic control. They include the initiation of flowering, onset of tuber development in species like potato, bulb initiation in onions and garlic, bud set in some trees, and entry into dormancy for many perennial species. Such regulation allows plants to anticipate and prepare for an oncoming season that may be unfavorable for growth. For example, shortening day lengths in mid to late summer are the most reliable predictor of the cold days of late fall and winter. Photoperiodic sensitivity also allows the plant to time its key developmental stages so that it can make the best possible use of light throughout the growing season to set seed, or produce vegetative storage structures, before the arrival of adverse growth conditions. Light availability can vary dramatically from season to season, particularly in far northern and southern latitudes (Figure 8.32). The best-studied photoperiodic system is undoubtedly the control of flowering by day length.
Figure 8.32 Variation of day length over latitude and season.; 65° and 45° north are compared (65°N is approximately the latitude of Reykjavík, Iceland and Anchorage, Alaska, USA while 45°N is the approximate latitude of Milan, Italy and Montreal, Canada). The start date of winter is taken to be January 1, spring May 1, summer June 20 and fall August 10.
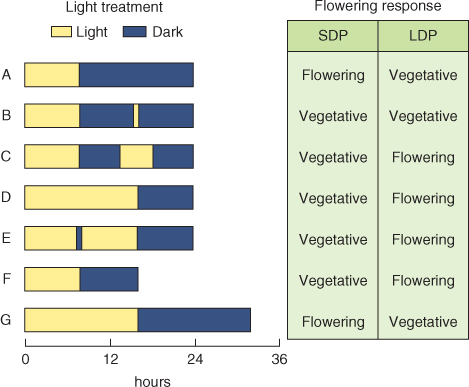
The earliest experiments on environmental control of the switch from vegetative to reproductive development established that exposure to short days (long nights) promotes flowering in Humulus (hop) and Cannabis (hemp), whereas Sempervivum funckii (Funck's houseleek) flowers under long days (short nights). Subsequently, day/night duration (photoperiod) was found to regulate flowering and many other aspects of development in a wide variety of plants. Species in which flowering is promoted by long-night/short-day conditions are classified as short-day plants (SDPs); S. funckii is an example of a long-day plant (LDP) species (Figure 8.33). In general, LDPs bloom in spring or early summer as the days are lengthening, whereas SDPs blossom in late summer and early fall at a time of shortening days and lengthening nights. Flowering in many plant species is insensitive to photoperiod: such species are classified as day-neutral.
Figure 8.33 Typical responses of short-day plants (SDPs) and long-day plants (LDPs) to combinations of light and dark periods of different durations: (A–E), normal 24-hour day–night cycle; (F), artificially shortened day–night cycle; (G), artificially lengthened day–night cycle. SDPs flower in response to long nights (A), whether the light period is short (A) or long (G). Interrupting the long dark period with a short or long light break suppresses the flowering response (B, C). Most LDPs flower in response to short nights (D, E) even when the light period is also short (F) and remain vegetative under long nights with (B) or without (A) a short light break, even when the day is also long (G); but a longer light break in a long dark period will induce flowering (C). A dark break in a long light period does not modify the response of SDPs or LDPs (compare D and E).
Not all photoperiodically sensitive plants can be neatly categorized in this way; some plants require a sequence of different photoperiods, while others require sequential temperature and photoperiod treatments. Flowering is frequently a developmental reaction to non-optimal environments, and many of the regulatory networks underlying stress responses (see Chapter 15) intersect with the pathways that control flowering.
Long-day and short-day plants are further divided into qualitative daylength-sensitive, which are absolutely dependent on the correct photocycle for floral induction, and quantitative or facultative daylength-sensitive, where flowering will eventually occur autonomously under non-inductive conditions but is hastened by exposure to inductive photoperiods. Qualitative types requiring a single long day or short day are experimentally useful because photoperiodic treatments can be applied with precision, allowing the light requirements for floral induction to be analyzed in detail. Morning glory (Pharbitis nil, also referred to as Ipomoea nil) and cocklebur (Xanthium strumarium) are examples of model qualitative SDPs in which flowering is promoted by exposure to a single inductive photocycle. Flowering of the so-called Ceres form of the annual grass Lolium temulentum (Figure 8.34) is induced by a single long day. Building on the physiological foundations established by work on these qualitative long day and short day models, molecular dissection of floral induction and development has focused on Arabidopsis, a facultative LDP species.
Figure 8.34 Lolium temulentum forma Ceres is a qualitative long-day plant, requiring one inductive photocycle for floral induction. Flowering occurs when the night is shorter than 10 hours, its critical night length. Double ridge formation describes the condition of the shoot apex in grasses at the stage when the apical meristem ceases to produce leaf primordia and switches instead to initiating flowers.
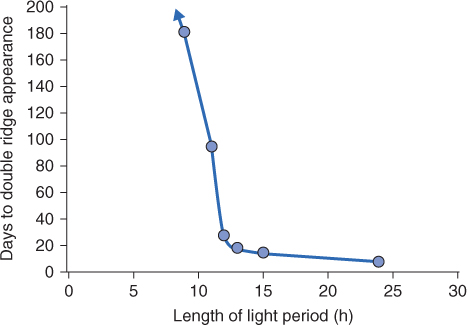
The terms LDP and SDP are a little misleading, because it is the length of the dark period rather than the length of the day that is critical in photoperiodic responses. In SDPs, short days only induce flowering if they are combined with long nights. When the plants are grown under artificial day–night cycles of less than 24 hours, in which the nights as well as the days are short, SDPs will not flower (Figure 8.33F). Moreover if a long night is interrupted with a short pulse of light, flowering is again prevented. For SDPs, therefore, it is a long night which initiates the transition from vegetative to reproductive growth. In LDPs, again the night length is critical. In this case a short night is required for induction, as shown for L. temulentum in Figure 8.34. If the night is sufficiently short, LDPs will flower even if the day is also short (Figure 8.33F), whereas a long day does not induce flowering in LDPs if the night is also long (Figure 8.33G). Thus SDPs are really long-night plants and LDPs are really short-night plants. Interrupting a non-inductive long night can induce flowering in many (but not all) LDPs, but only if the light break is long enough—a very short light break will not bring about flowering. This is in contrast to SDPs, where even a brief flash of light in the dark period is sufficient to prevent flowering.
How does a plant measure the length of its days and nights? The most widely-accepted explanation is that photoreceptors responding to the amount and quality of light in the environment control the stability of key proteins which are expressed according to circadian rhythms. These proteins in turn regulate the expression of other genes important in the process, e.g. bud set, growth cessation, tuberization and so on, which is under photoperiodic control. As for many other aspects of development, the best-understood experimental subject for analyzing the control of flowering time is Arabidopsis, a quantitative LDP in which the sensitivity to inductive floral signals increases with age.
Experiments in which different parts of the same plant are exposed to different photoperiods established that it is the leaf and not the shoot apex that perceives the inductive stimulus. Grafting a photoinduced leaf onto a vegetative shoot induces flowering. In studies of the SDP species Perilla frutescens (beefsteak plant) it has been shown that a single induced leaf can be grafted onto seven successive vegetative plants and will still sustain its inductive effect (Figure 8.35). These observations suggest that photoinduction causes a permanent change in the receptive leaf so that it becomes a continuous source of a mobile flowering stimulus. This stimulus was given the name florigen and its identification became the central objective of research on floral induction.
Figure 8.35 Permanent photoinduction of Perilla leaves. (A) A single leaf exposed to short days (SDs) induces flowering of an attached plant that has been exposed only to non-inductive long days (LDs). (B) Grafting the same SD leaf onto a second vegetative plant grown in LDs induces flowering. The same leaf can induce flowering on a third host plant (C) and so on. Neither LD leaves from plants in flower (D) nor a floral apex grafted onto a non-induced plant (E) can induce flowering in the host.
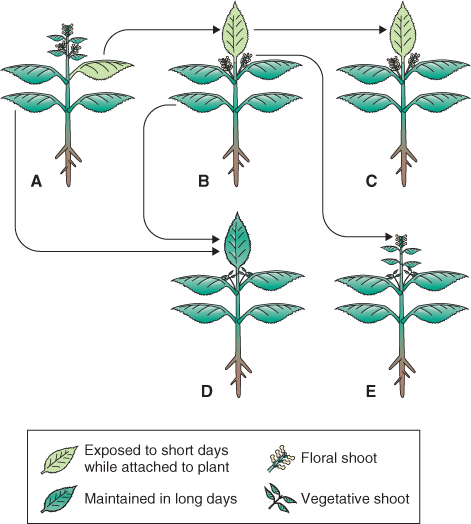
Mutants, particularly in Arabidopsis, have been essential tools for analyzing the genes and interactions controlling vegetative development (see Chapter 12). Similarly, studies of mutants that flower earlier or later than the wild-type have revealed the existence of networks that regulate the perception of a floral stimulus, transmission of the flowering signal, and transition from the vegetative to the reproductive condition in the shoot apex. In general, late-flowering genetic variants are defective in genes that promote flowering, whereas lines that flower early are indicative of genes that ordinarily repress flowering being inactivated or downregulated. Analysis of Arabidopsis late-flowering mutants led to identification of the regulatory gene CONSTANS (CO), which encodes a nuclear zinc-finger transcription factor. Under long days, CO induces transcription of the gene FLOWERING LOCUS T (FT).
FT is normally expressed in leaves and not in apices, but transgenic plants that overexpress FT in the shoot apex, under the control of a meristem-specific promoter, will flower under non-inductive conditions. When targeted to phloem, the CO protein stimulates both the synthesis of FT and the formation of a translocatable flowering signal. Such transgenic manipulations, together with grafting experiments, provide strong evidence that the FT protein is the phloem-mobile florigenic factor that transmits the inductive signal from the leaf to shoot apex. In the shoot apex FT associates with the bZIP transcription regulator FD which is encoded by the gene FLOWERING LOCUS D (FD). The FT–FD complex induces expression of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1). FT–FD and SOC1 are the transcriptional integrators that activate floral identity genes (Figure 8.36; see Chapter 16).
Figure 8.36 Gene networks connecting photoregulation and circadian control of flowering. Environmental signals upregulate the synthesis of the transcription factor CONSTANS (CO) which interacts with the floral integrator gene FLOWERING LOCUS T (FT), and, indirectly via FT, with FLOWERING LOCUS D (FD) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1). In turn, products of these genes interact with floral meristem identity genes leading to flowering.
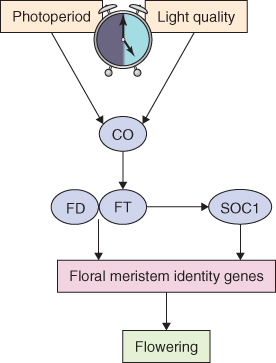
As we have seen, evidence suggests that FT, whose synthesis is promoted by CO, moves from the leaves to the shoot apical meristem where it initiates the changes in gene expression that result in a switch from vegetative growth to flowering (see Chapter 16). Figure 8.37 illustrates our current understanding of the regulation of CO expression in relation to day length in the LDP Arabidopsis. The circadian clock controls expression of the CONSTANS gene and the ability of phytochrome and cryptochrome to regulate the expression of CO or the stability of its protein product CO. When the day length is short the CO gene is expressed maximally during the night; however, the CO protein is degraded in darkness. In addition, during the early part of the day, PHYB promotes the degradation of CO. In short days CO never accumulates because it cannot be synthesized during the day and is degraded when it is made at night. On the other hand, when the day is longer a zeitlupe photoreceptor active near the end of the long day promotes the degradation of an inhibitor of CO expression. This leads to a peak of CO gene expression in the light when the CO protein that is synthesized is not subject to degradation. Additionally, phytochrome A and cryptochromes act to repress CO degradation at the end of the day, thus promoting CO stability in the light. Therefore CO accumulates and it can then activate FT expression leading to the induction of flowering. The mutants phyA and cry2 are late flowering; in contrast, phyB mutant plants are early flowering.
Figure 8.37 Clock control of CO gene expression at the transcription and protein levels. Red spheres represent intact CO protein, red split spheres are degraded CO protein, and the black line shows the clock-controlled variation in levels of CO mRNA. The clock symbol indicates genes controlled by the circadian clock. (A) In short day conditions CO mRNA is mainly synthesized in the dark, and the resulting CO protein is degraded by proteins of the SPA family. Some CO protein is also produced in the morning and this too is degraded, in a process that depends on the presence of active PHYB. Inhibition of CO gene expression by CDF1 prevents synthesis of CO in the afternoon. (B) In long days CO produced in the morning is still degraded. However, in the light FKF1 and G1 prevent repression of CO mRNA synthesis by CDF1, so the CO gene is expressed in the afternoon. The resulting CO protein is stabilized in the light by the presence of PHYA and Cry2. It is believed that this stable CO protein can form a complex with HAP (heme activator protein) and the complex binds to the promoter of the FT gene, stimulating production of FT protein. FT is transported through the phloem to the shoot apical meristem, where it induces flowering.
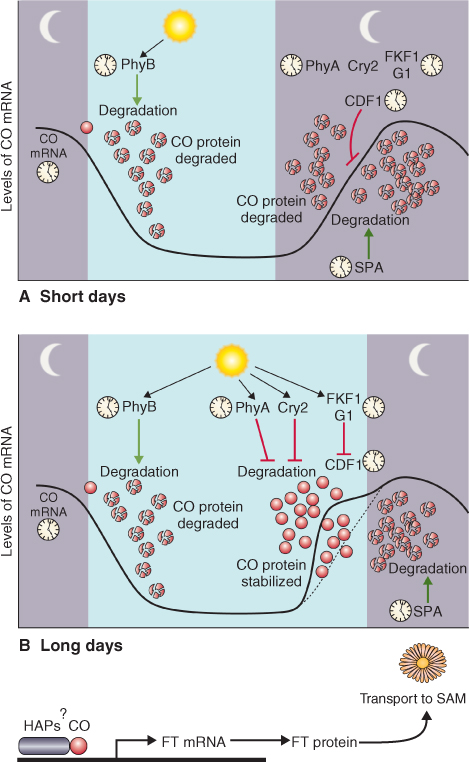
Genes homologous to CO and FT that also have diurnally-regulated patterns of expression have been identified in other cases where photoperiod controls developmental processes. Overexpression of FT in rice and winter wheat, and in a number of dicots (including Populus, Nicotiana and Pharbitis), causes extreme early flowering. Downregulating FT by mutation or RNA interference methods greatly delays flowering in Arabidopsis and rice. The evidence from such experiments strongly supports the case for FT as a universal flowering signal. The CO–FT module is highly conserved, not only in the network of genes regulating floral induction, but also in such photoperiodically determined processes as tuberization, dormancy and bud set (see Chapter 17).
An important practical implication of the conserved nature of the flowering process is that mechanistic knowledge derived from model systems can be directly applied to agricultural species, in which manipulation of reproductive development is a central objective for improving crop yields. The regulatory pathways centered on FT homologs have been studied in cereals. Flowering in Oryza (rice), an SDP, is regulated by members of the HEADING DATE (Hd) gene family. Hd3a encodes a phloem-mobile FT ortholog. Hd1 is the rice equivalent of CO and mediates the photoperiodic control of Hd3a expression, though its functions are more complex than those of its Arabidopsis homolog, since not only does Hd1 stimulate Hd3a transcription under inductive conditions but it also represses it under long days. Genetic and environmental regulation of reproductive development is considered further in Chapter 16.