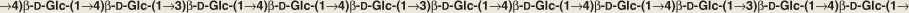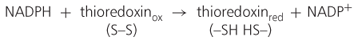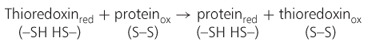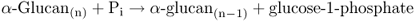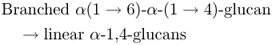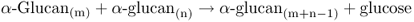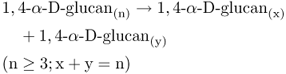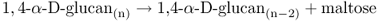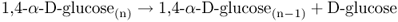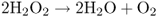Chapter 6
Seed to seedling: germination and mobilization of food reserves
6.1 Introduction to Seeds and their Germination
The life of a plant begins with a seed and is renewed when the seed germinates and the embryo grows and develops into a seedling. The seed initially develops in a hydrated state but loses water and dries during maturation. In the dry (resting or quiescent) state, the seed can survive for extended periods, often years, in some species tens or even hundreds of years, before it takes up water and germinates. Some seeds are dormant when they mature; this dormancy must be overcome before germination can occur.
The visible sign that germination is complete is the penetration of the seed coat by the embryonic root, the radicle. As the embryo grows and develops, the reserves within the storage tissues of the seed are mobilized to support seedling growth and development. Germination typically occurs below the surface of the soil and the young seedling is often dependent on the stored reserves in the seed. In this regard seedling growth can be entirely heterotrophic and independent of photosynthesis.
Seeds are an important source of food for humans and livestock and it has been estimated that as much as 50% of human caloric intake and 47% of protein comes from seeds. Seventeen plant species provide 90% of human food and, of these, cereal grains make up the bulk of edible dry matter consumed. Wheat, maize, rice and barley grains make up about 70% of the food and feed consumed worldwide. The prevalence of cereal grains in the human diet is of relatively recent origin. Agriculture had its origins in the Fertile Crescent, an area encompassing what is now Turkey, Iraq, Israel, Palestine and Jordan, less that 10 000 years ago—a relatively short time in the context of human evolution. A consequence is that humans are not particularly well adapted to a diet rich in starch and seed proteins. Many human diseases are recognized as being linked to a diet that relies on cereal grains.
The impact of plant breeding on agricultural production has been profound. Despite Malthusian claims that population growth will outstrip the food supply, conventional plant breeders have succeeded in increasing the yield of cereals on an almost annual basis. The high-yielding wheat and rice varieties that were the basis of the Green Revolution are testament to the breeders' skills. The Nobel Peace Prize was awarded to Norman Borlaug for his work in developing many of these new wheat varieties.
Molecular approaches are now becoming more widely used to develop new and improved varieties of seed plants. New maize varieties carrying resistance to various pests, and soybeans that are herbicide-resistant, have been introduced, and rice varieties with enhanced vitamin D synthesis and elevated iron levels have been successfully developed using recombinant DNA methods. There have also been dramatic improvements in the genetic modification of oil seeds; for example, the development of canola plants having very low levels (<2%) of the toxic fatty acid erucic acid (see Chapter 17). Molecular engineering has also led to improvements in the protein quality of various seeds. Perhaps the biggest hurdle faced by biotechnologists will be the acceptance of genetically modified foods by consumers.
In this chapter we will examine seed structure in more detail and describe the types of reserves that are stored in seeds. Then we will look at the processes involved in seed germination, and examine how stored reserves are mobilized during early seedling growth.
6.2 Seed Structure
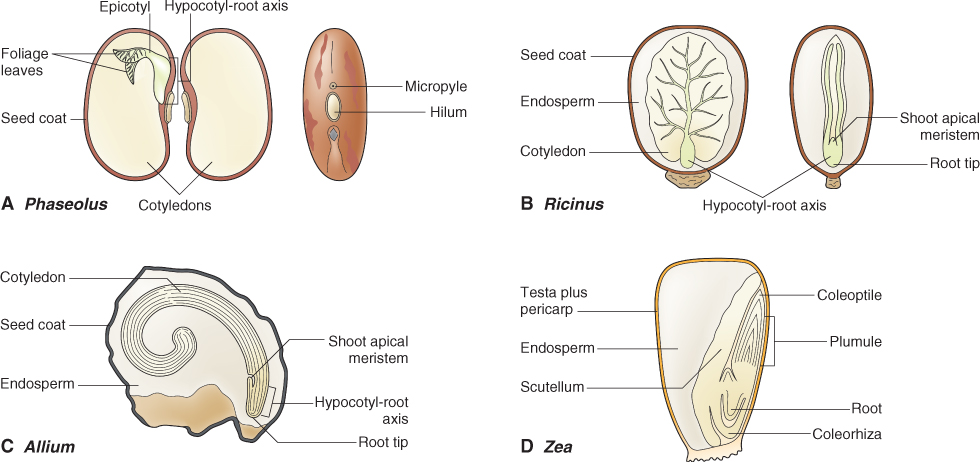
The mature seed consists of an embryo enclosed by a protective seed coat (Figure 6.1). In addition, endosperm is present in the seeds of all monocots and many eudicots; these are endospermous seeds. The eudicot endosperm can vary in size from a single layer of cells as in Arabidopsis seeds (Figure 6.2), to the large, oil-storing endosperm of castor bean (Ricinus communis; Figure 6.1B) and the starch-storing endosperm of cereals such as maize (Zea mays; Figure 6.1D). Seeds of some eudicots such as the green bean (Phaseolus vulgaris; Figure 6.1A) are non-endospermous, i.e. they lack endosperm at maturity. We will now examine the parts of a seed in more detail.
6.2.1 Seeds Contain an Embryonic Plant
With few exceptions the eudicot embryo has an axis consisting of an embryonic root, the radicle, and an embryonic shoot, the plumule, which bears a pair of seedling leaves, the cotyledons. Monocot embryos show more variation in their structure. They vary from embryos of onion that are similar in many respects to eudicot embryos except that they have only one cotyledon (Figure 6.1C), to the embryos of grasses with their relatively more complex structure (Figure 6.1D). In grass embryos the single cotyledon has been modified to form the scutellum, an absorptive organ that lies adjacent to the endosperm. In addition, a sheath called a coleoptile surrounds the plumule of the grass embryo and a second sheath, the coleorhiza, surrounds the radicle.
6.2.2 Seed Coats, made of Layers of Dead Cells, Protect the Embryo
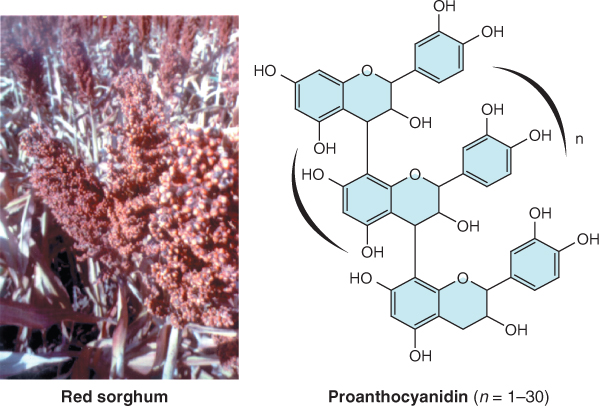
The testa, derived from the integuments of the ovule (see Figure 1.18), consists of several layers of dead and compressed cells (Figure 6.2). It is often impregnated with phenolic compounds that impart a red to brown color to many seeds and grains (Figure 6.3A). These phenolic compounds protect the seed from attack by herbivores and pathogens, filter out harmful short wavelength light, and can impose dormancy by making the seed coat rigid or impermeable to water or oxygen. Tannins and other polyphenols in seed coats contribute to the astringency of many fruits and seeds and deter feeding by herbivores. The effectiveness of polyphenolic compounds in protecting embryos from the damaging effects of ultraviolet light becomes clear from their structure (Figure 6.3B). The presence of a large number of double bonds in polyphenols such as the proanthocyanidins makes these compounds effective in absorbing short wavelength light, protecting the embryo from the mutagenic effects of these wavelengths.
The testa often has an exterior mucilage layer that hydrates quickly when seeds are exposed to water (Figure 6.4). In Arabidopsis seeds, mucilage is composed largely of esterified pectins made up of polygalacturonic acid polymers. This mucilage is deposited on the outer surface of the testa as the seed matures. Its presence is thought to be important in the uptake and retention of water by the imbibing seed, allowing for uniform water absorption over its surface.
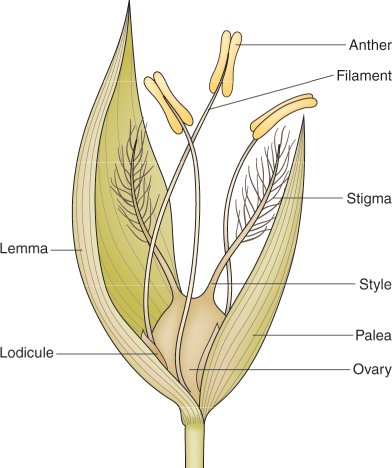
The coats of many seeds are much more complex because the testa is fused to the pericarp. The pericarp arises from the ovary wall; therefore, seeds enclosed by a testa and pericarp are technically fruits. Examples of fruits in which a fused testa and pericarp surround a single embryo are the caryopses of grasses (Figure 6.1D) and the achenes of strawberry, dandelion and lettuce (Figure 6.5). Bracts called the palea and lemma, which are modified leaves, also enclose the grass caryopsis and are often referred to as hulls (Figure 6.6). Hulls are a major component of the chaff that is produced when grain has been threshed.
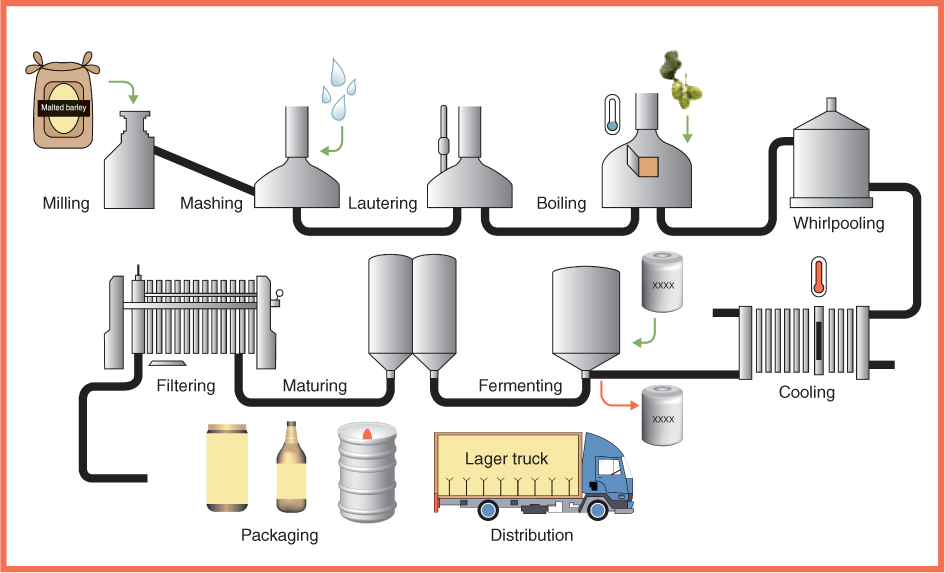
Seed coats are believed to be beneficial for human health when included as part of a normal diet. They may provide dietary fiber. Because seed coat lignins and polysaccharides are largely indigestible they may aid in the passage of food through the gut. They may also be a rich source of antioxidants. Among the compounds found in seed coats, the proanthocyanidins (Figure 6.3) are known to be strong antioxidants. The hulls of barley grains play an important role in traditional beer brewing methods, where they are used as filters in the beer-making process (Figure 6.7).
Key Points
The seed consists of an embryo and food reserves surrounded by a seed coat of varying complexity; food may be stored in the embryo or in specialized, polyploid tissue called endosperm. The eudicot embryo axis includes the seedling root, stem and leaves, in the form of the radicle, plumule and cotyledons, respectively. Monocot embryos either have one cotyledon, as in the case of the onion embryo, or lack a true cotyledon. In grasses the scutellum is a modified cotyledon that absorbs nutrients from the endosperm. The seed coat or testa surrounds the embryo. It can consist of one to many layers of dead cells in the mature seed and is often impregnated with phenolic compounds.
6.2.3 Endosperm, a Tissue Unique to Angiosperms, Contains Stored Food
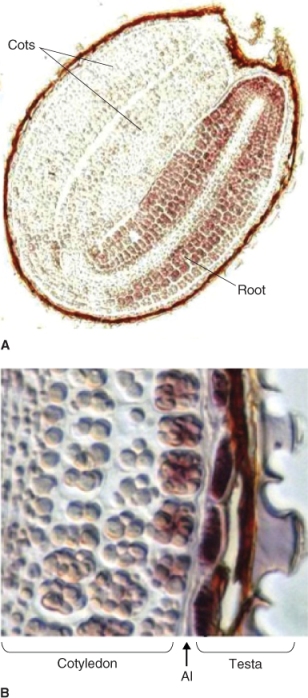
Almost all seeds contain stored food, which will be used to fuel the growth of the seedling after germination. The dust-like seeds of orchids are an exception as these lack stored food reserves and germination requires the participation of symbiotic endomycorrhizal fungi. In non-endospermous seeds, such as those of many legumes, food is stored in the fleshy cotyledons of the embryo. In most endospermous seeds, the endosperm is specialized for storage of food reserves. However, in some species, it plays only a minor role in food storage. In Arabidopsis seeds, for example, the endosperm consists of only a single layer of cells that have relatively thick cell walls (see Figure 6.2). As we shall see later, the endosperm and testa of Arabidopsis impose dormancy by restricting growth of the embryo. These more specialized endosperm cells also store protein and lipid reserves, but the bulk of food reserves in Arabidopsis are found in cotyledons.
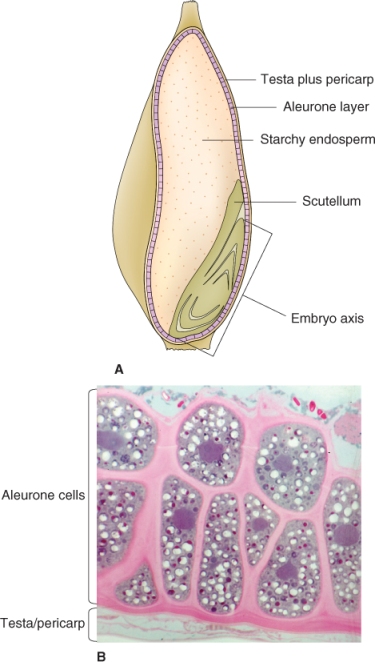
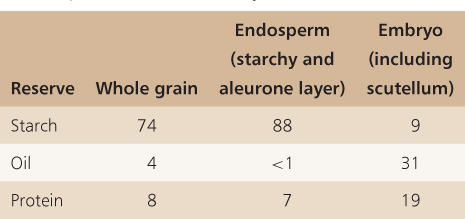
The endosperm of many seeds is often not uniform; it may be differentiated into areas of varying thickness. In grasses the endosperm differentiates into two structurally and functionally distinct tissues: the starchy endosperm and the aleurone layer (Figure 6.8). Although they contain enzymes that function during germination, starchy endosperm cells are dead at maturity; these cells store protein as well as starch. The aleurone layer is alive in the mature seed. After germination, it functions as a digestive tissue that synthesizes and secretes large quantities of enzymes such as α-amylase to mobilize food reserves that are located in the starchy endosperm.
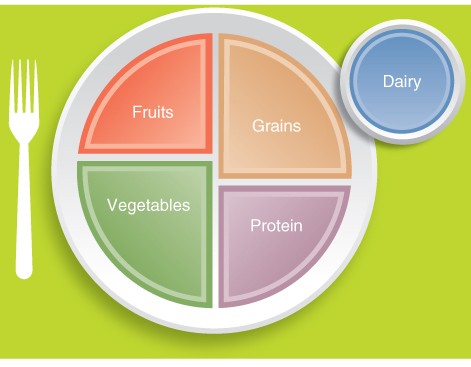
Endospermous cereals and non-endospermous legumes make up the bulk of the human diet. Recent figures show that more than 75% of dry matter consumed by humans comes from cereal grains and about 6% from legume seeds. Table 6.1 shows the tonnage of various seed crops produced worldwide and illustrates how cereal grains represent by far the largest proportion of seed crops grown. Indeed, the diet recommended by the United State Department of Agriculture (USDA) advocates 6–11 servings per day of cereals or cereal products, and USDA's food plate includes cereals as a large portion of the human diet (Figure 6.9).
Table 6.1 Annual production of seed crops worldwide.
| Cereals |
|
| Wheat (Triticum spp.) |
468 |
| Maize (Zea mays) |
429 |
| Rice (Oryza sativa) |
330 |
| Barley (Hordeum spp.) |
160 |
| Sorghum spp. |
60 |
| Oats (Avena sativa) |
43 |
| Rye (Secale cereale) |
29 |
| Millets and other small-grained cereals |
26 |
| Legumes |
|
| Soybean (Glycine max) |
88 |
| Pea (Pisum sativum) |
12 |
| Bean |
14 |
| Peanut (Arachis hypogaea) |
13 |
| Other |
|
| Rapeseed (Brassica napus) |
19 |
| Sunflower (Helianthus annuus) |
10 |
| Cottonseed (Gossypium sp.) |
5 |
The prominent role of cereals in the human diet is relatively recent in the context of human evolution and is a result of the rise of agriculture. The domestication of cereals began about 10 000 years ago and it is likely that cereals only became a staple in the human diet from around 3000 to 4000 years ago. Because human metabolism evolved for at least 100 000 years largely on a meat-based, hunter-gatherer diet (in which starchy roots and tubers may have played an important role; see Chapter 17), it is perhaps not surprising that we are relatively poorly adapted to cereal-based food. Numerous diseases, including wheat allergy and celiac disease, can be traced to the preponderance of cereals in the human diet. The latter disease is caused by intolerance to gluten, a storage protein found in some cereal grains. Other less well-recognized illnesses are also associated with a high intake of cereals, including deficiencies of certain essential amino acids (lysine, methionine, tryptophan) and minerals (calcium, iron, zinc) (see Section 6.3.5).
6.3 Use of Seed Storage Reserves by the Germinating Embryo
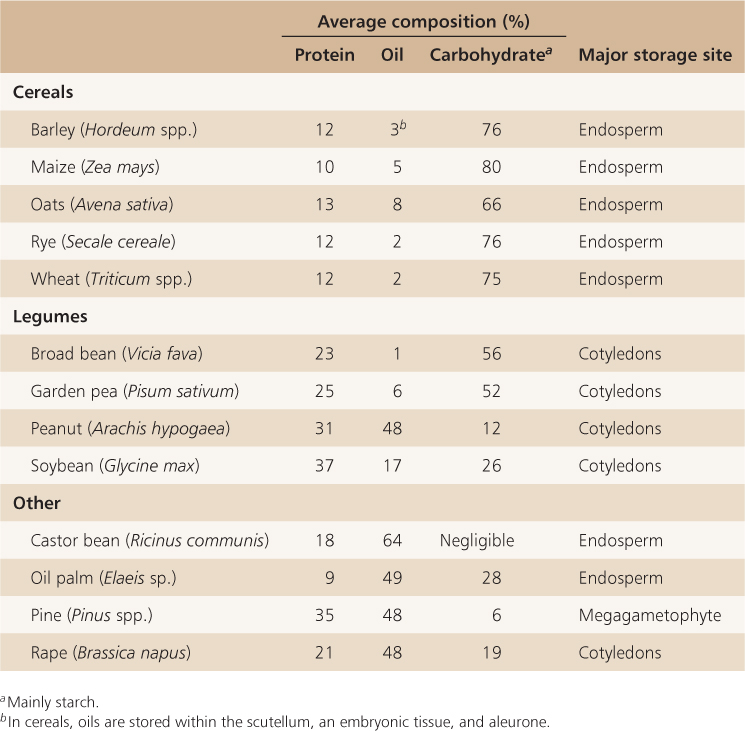
Seeds store polysaccharides, lipids, proteins and minerals as well as a wide range of other molecules that will be used by the embryo when it starts to grow (Tables 6.2 and 6.3). As mentioned earlier, these reserves may be stored in the endosperm, the cotyledons and in other tissues that make up the embryo (Table 6.4). For example, in cereals the scutellum is a major site of reserve storage and corn oil is obtained largely from this organ. While the starchy endosperm is a major site for the storage of protein and starch, the aleurone layer, scutellum and the rest of the embryo also contain stored protein and lipid. Small-grain cereals such as rice (Oryza sativa) are processed so that the oil of the aleurone layer can be harvested. In oil palm (Elaeis spp.) and castor bean (Ricinus communis), oil is stored in the endosperm. In brazil nut (Bertholletia excelsa), on the other hand, the fleshy portion of the seed that is eaten by humans is the swollen hypocotyl/radicle of the embryo. In pine seeds (Pinus spp.), reserves are stored in cells of the megagametophyte (see Figure 1.15).
Table 6.2 Vitamin and mineral content of the unprocessed grains of five cereals (100 g samples).
Table 6.3 The food reserves of some important crop species.
Table 6.4 Percent composition of stored reserves in different parts of a maize (Zea mays cv. Iowa 939) kernel.
6.3.1 Starch is the Major Carbohydrate Reserve of Plants
Starch is the major carbohydrate reserve in most plants. It forms the bulk of the storage carbohydrate in many seeds and grains, especially cereals, but also in legumes such as garden pea (Pisum sativum) and broad bean (Vicia faba) (Table 6.3).
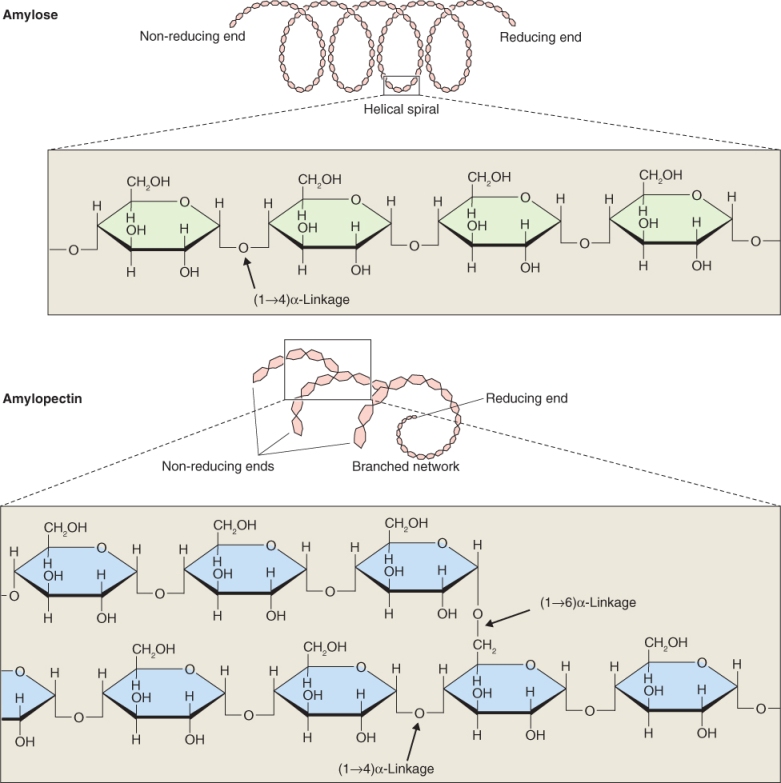
Starch is made up of unbranched amylose chains and branched amylopectin (Figure 6.10). Amylose is a polymer of up to about 300–3000 glucose residues joined by α(1 → 4) linkages. The backbone of the amylopectin polymer is much longer than that of amylose. It can be up to 105 glucose residues joined by α (1 → 4) linkages with side-chains of about 20–25 α(1 → 4)-linked glucose monomers that are attached by α(1 → 6) linkages to the backbone (Figure 6.10).
Key Points
Seed storage reserves function to nourish the embryo; they may be found in a variety of tissues. In pine seeds, protein and oil are stored in megagametophyte tissue. In angiosperms, seed reserves are stored in the endosperm and/or in the embryo itself. In cereal grains, the endosperm consists of dead cells that contain stored starch and proteins whereas oil is found mostly in the scutellum of the embryo. In other monocots such as coconut, the endosperm in a mature seed contains living cells that store starch, carbohydrate and lipids. In many eudicot seeds, the endosperm is reduced to a single layer of cells that surrounds the embryo and the bulk of reserves are found in the embryonic cotyledons. Some endospermous eudicot seeds, for example castor bean, have a fleshy endosperm that stores reserves such as oil.
Amylose can be distinguished from amylopectin by its reaction with an iodine and potassium iodide (I2KI) mixture. The α(1 → 4) linkages in amylose give the molecule a helical structure whereas amylopectin, because it is branched, does not form helices. Iodine is able to intercalate into the helical amylose molecule and, when it does, it changes color from golden brown to blue-black. Because the amylopectin molecule is not a helix, iodine does not bind to it and its color remains unchanged.
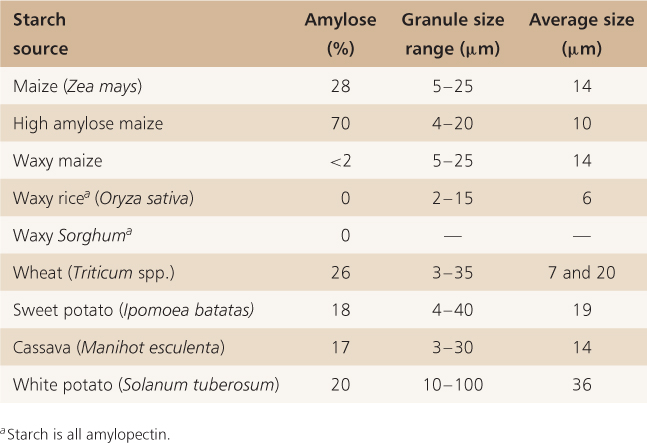
The degree of branching in amylopectin can vary considerably and this changes the property of the starch that is synthesized. The ratio of amylose to amylopectin also varies according to species; in most cereals starch is on average 30% amylose and 70% amylopectin. Table 6.5 shows the extremes in amylose content in some commercial crops. In waxy cereals, such as waxy mutants of maize, starch is composed entirely of amylopectin, whereas in sweet maize mutants deficient in enzymes catalyzing the conversion of sugar to starch, amylose content can be as high as 70%.
Table 6.5 Amylose content and granule size of starch grains from various species.
Sugars imported from the parent plant into developing seeds and grains are converted into starch within the amyloplast, a non-green plastid that is modified for starch synthesis and accumulation (see Figure 4.24). Starch accumulates in starch grains that can vary in size from 2 μm to more than 50 μm, such as those found in the tubers of white potato (Solanum tuberosum) (Table 6.5). The starch grains of cereal endosperm show considerable variation in size even within one cell, as is shown in the electron micrograph in Figure 6.11A. Starch is added to grains in layers, which remain distinct in the growing grain and appear as concentric rings when examined by electron microscopy (Figure 6.11B).
Starch has many uses beyond its predominance as a basic food source for humans and livestock. For example, it is widely used in the making of paper, in the printing industry as a bonding agent or glue, as a stiffening agent in the laundering of clothes, in the molding of candy and as a thickening agent in a wide variety of food stuffs.
6.3.2 Cell Walls are also an Important Store of Polysaccharides in many Seeds
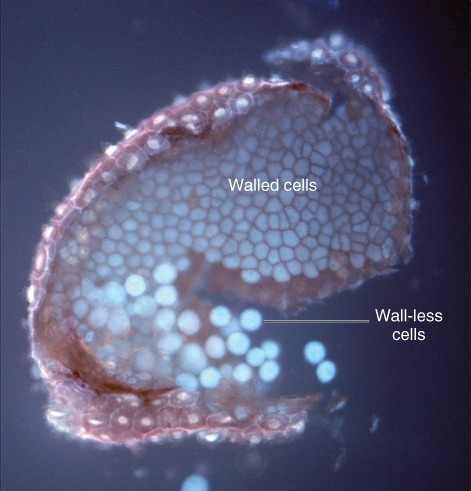
Although starch is clearly an important source of stored polysaccharide, cell walls may be the predominant site for carbohydrate storage in many seeds. The cell walls of almost all tissues of a seed can be modified for polysaccharide storage. Cell walls of the endosperm are especially important storage sites. In seeds such as date (Phoenix dactylifera) and fenugreek (Trigonella foenum-graecum), the cell walls are the principal reserve of mannan polysaccharides. Endosperm walls in these species become extensively thickened so that they almost totally eliminate the cytoplasm (Figure 6.12).
Several types of polysaccharide may function as storage polymers in cell walls. In the cell walls of many legume seeds, as well as those of date and fenugreek, polymers with a mannose backbone are the principal storage polymers. Pure mannans form a hard, almost crystalline, matrix similar to that of cellulose; in galactomannans galactose side-chains change the structural properties of the polymer (Figure 6.13). Glucomannans, in which the backbone of the polymer is made up of varying proportions of mannose and glucose, are found in the seeds of many lilies (Lilium spp.), whereas the seeds of tamarind (Tamarindus indica) have polysaccharides that are galactose-rich xyloglucans.
The cell walls of the cereal endosperm have a characteristic polysaccharide known as mixed-link glucan or β-glucan (Figure 6.14). This polymer consists of alternating runs of cellotriose (three glucose units) and cellotetraose (four glucose units) linked by β(1 → 3) and β(1 → 4) linkages. Barley and oat endosperm can contain up to 4–5% β-glucan. It forms a highly viscous polymer and as such creates problems for brewers, causing haze in beer at cold temperatures as well as interfering with the filtering of finished beer. Cereal β-glucans are thought to benefit human health by lowering serum cholesterol levels. They are frequently marketed as ‘soluble fiber’.
6.3.3 Storage Proteins in Eudicot Seeds Include Globulins and Albumins
Storage proteins make up as much as 40% of the dry weight of legume seeds but comprise only 10–12% of cereal grains (see Table 6.3). Despite this difference in protein content, cereal storage proteins represent a far higher proportion of human and livestock protein intake (about 2 × 105 kg annually) than do those of legumes (about 7 × 104 kg) (see Table 6.1). Work at the beginning of the 20th century led to the classification of plant proteins according to their solubility in water, saline solution, dilute alkali and alcohol (Table 6.6), Water-soluble proteins are called albumins, those soluble in dilute salt and alkali solutions are globulins and glutelins, respectively, and those proteins soluble in 70% ethanol are prolamins.
Table 6.6 Classification of plant proteins.
| Albumin |
Water |
| Globulin |
Dilute salt (0.1 m NaCl) |
| Glutelin |
Dilute alkali (0.1 m NaOH) |
| Prolamin |
70% aqueous ethanol |
Salt-soluble globulins and water-soluble albumins make up a large fraction of the storage proteins of eudicot seeds. In seeds of alfalfa (Medicago sativa), for example, globulins make up around 40% of seed storage proteins and albumins make up about 20%. The globulins of legumes are made up of two classes of oligomeric (multisubunit) proteins, the legumins and the vicilins. Legumins and vicilins are distinguished based on their sedimentation coefficients in Svedberg units (S). Proteins of the legumin class are between 11S and 14S in size whereas vicilins are 7S–8S. Both classes of proteins are made up of variable numbers of subunits, up to eight in vicilin and seven in legumin of Pisum sativum. Other eudicot seeds may contain legumin-like and/or vicilin-like proteins. Arabidopsis seeds lack globulins of the 7S vicilin class and about 85% of its storage proteins are made up of 12S legumin and 2S albumin.
6.3.4 Storage Proteins in Cereal Grains differ from those Found in Eudicot Seeds
The storage protein composition of cereal grains is markedly different from that of eudicot seeds. Cereal grains contain high concentrations of alcohol-soluble prolamins and alkali-soluble glutelins, proteins that are essentially absent from the seeds of eudicot plants (Table 6.7). Despite these differences in overall protein composition, the storage proteins of cereal grains and eudicot seeds share some interesting homologies. The globulins of cereals have sedimentation coefficients of about 7S and show sequence homologies with the 7S vicilins of legumes. Similarly, the globulins found in the starchy endosperm and aleurone of cereals show sequence similarities with the 11S family of legumins.
Table 6.7 The approximate protein composition (%) of some cereal grains and the common names of some storage proteins.
About one-half of wheat endosperm protein is a mixture of proteins, named gluten, which is made up largely of prolamins and glutelins. These proteins are crucial for the properties of foodstuffs such as bread and pasta. The baking properties of bread are strongly influenced by the presence of disulfide bonds (S–S) between adjacent gluten chains; the elasticity of the dough is a function of the oxidation status of gluten. During bread-making, dough is kneaded to evenly distribute the gluten in the flour and to oxidize the SH groups, enabling the formation of S-S bonds between adjacent gluten molecules. This forms a protein network that traps the carbon dioxide produced by the added fermenting yeast and allows the bread to rise. Flour that is low in the sulfur-containing amino acid cysteine produces bread that does not rise, probably because it forms fewer S-S bonds. On the other hand, excess cysteine in the flour also adversely affects bread quality (Figure 6.15). Agents such as potassium bromate that oxidize cysteine and promote the formation of the disulfide network are often used to enhance dough quality.
6.3.5 The Amino Acid Content of Seed Proteins Affects their Nutritional Value for Humans and Livestock
Globulins, abundant in legume seeds, have high levels of the amino acids arginine, asparagine and glutamine and are therefore an important source of amino-nitrogen for the developing seedling. These proteins are nutritionally poor for humans and livestock as they are relatively depleted in several essential amino acids, including the sulfur-containing amino acids cysteine and methionine, as well as lysine and tryptophan. In contrast several seed proteins of the albumin class are especially rich in methionine; for example, the 2S albumins from Brazil nut (Bertholletia excelsa) and sunflower (Helianthus annuus) seeds have 18% and 16% methionine, respectively. Because legume crops are widely used as animal feed, attempts have been made to increase their methionine content. To this end, 2S albumins from sunflower have been successfully introduced into narrow-leafed lupine (Lupinus angustifolius), a major grain legume. Transgenic lupines carrying the sunflower albumin protein contain higher methionine levels and are nutritionally superior to wild-type lupines when fed to test animals. A cautionary note, however, should be added regarding attempts to improve the nutritional quality of seeds by introducing genes that encode proteins of a desired amino acid content. Experiments to improve the nutritional qualities of soybean (Glycine max) by introducing a gene from Brazil nut that encodes a 2S albumin inadvertently introduced a strong allergen into transgenic plants. Indeed, it is well known that among the 2S albumins of seeds are inhibitors of the amylases and proteases that are necessary for the digestion of seed storage products as well as proteins that are highly allergenic. The antinutritional and allergenic properties of these albumins are usually retained when transgenes encoding them are introduced into other species.
Seed storage proteins of the legumin, vicilin and prolamin classes are localized in specialized protein storage vacuoles, also called protein bodies, and aleurone grains (Figures 6.16 and 6.17). All seed storage proteins are synthesized on rough endoplasmic reticulum (ER) but they can follow distinct paths from the ER to the vacuole. The globulins of dicot and monocot seeds are transported from the ER to vacuoles via dense vesicles produced by the Golgi apparatus (Figure 6.18). Proteins such as pumpkin seed albumin and the prolamins of maize and rice do not pass through the Golgi apparatus. In the case of pumpkin (Cucurbita pepo), albumin synthesized in the ER is transported directly to the vacuole by precursor accumulating vesicles (Figure 6.18). On the other hand, cereal prolamins accumulate in the lumen of the ER to form an ER-derived protein storage vacuole (Figure 6.17).
6.3.6 Seed Storage Proteins may Act as Antinutrients
Certain seed storage proteins possess undesirable properties and can be the cause of many diseases. Among these proteins are lectins, enzyme inhibitors and thionins. Enzyme inhibitors fall into two general classes, the α-amylase inhibitors and the protease inhibitors. Seeds contain a wide range of amylase inhibitors that have specificity for α-amylases in a wide range of animals, from insects to humans. They are especially abundant proteins in grasses that belong to the tribe Triticeae, which includes barley, rye and wheat. Amylase inhibitors make up a large proportion (up to 80%) of the water-soluble albumin fraction of seed storage proteins. They function by binding near the active site of α-amylase, preventing hydrolysis of the glucosyl linkages in the amylose chain. It is thought that these proteins evolved as part of the plant's defense mechanism against insects. Certainly many seed amylase inhibitors show high specificity toward insect α-amylases, but cereal amylase inhibitors also inhibit cereal α-amylases as well as human salivary and pancreatic α-amylases.
These properties of amylase inhibitors have been exploited in several ways. Seeds have been engineered to synthesize amylase inhibitors as a means of reducing losses to insects, which can be very large in some parts of the world. These inhibitors have also been used as dietary supplements in humans for weight reduction. They have been shown to effectively reduce starch digestion and thus lead to weight loss. Amylase inhibitors are therefore commonly used to reduce obesity. They can also be administered in order to decrease sugar production in people with diabetes.
In addition, protease inhibitors are found in seed storage proteins; they act to inhibit protein breakdown by binding to proteases. Seeds contain a spectrum of these inhibitors with a range of specificities and, like amylase inhibitors, they are thought to have evolved as part of the plant's defense mechanism. Various protease inhibitors have been expressed in new plant hosts as a means of elevating defense against pests, especially insects.
Lectins include a large group of carbohydrate-binding proteins. Like enzyme inhibitors, lectins can affect the properties of carbohydrates and glycosylated proteins. They may bind to enzymes, to structural proteins and to molecules that are taken up as food. Wheat germ agglutinin (WGA) is a widely studied lectin because it has diverse effects on human cells and tissues. WGA binds to surface glycans on the cells that border the digestive system and it interferes with uptake from the gut and alters the gut microflora. WGA is also very stable, surviving heat during cooking as well as passage through the digestive tract. WGA is a strong immunogen, and because it survives in the gut and can be taken up into the blood stream, it can elicit a wide range of deleterious effects.
Thionins are small basic proteins common in seeds but also found in vegetative tissues. Most thionins contain 45–50 amino acids and possess several conserved cysteine residues. The structure of γ-thionins shows that they resemble toxins produced by arthropods, e.g. insect defensins and scorpion toxin. Thionins are known to be toxic to animals as well as plant pathogens and their mode of action is varied; they can function as inhibitors of enzyme synthesis and activity and affect transport systems in cells.
6.3.7 Unlike Most Plant Tissues, Seeds often Contain Storage Lipids
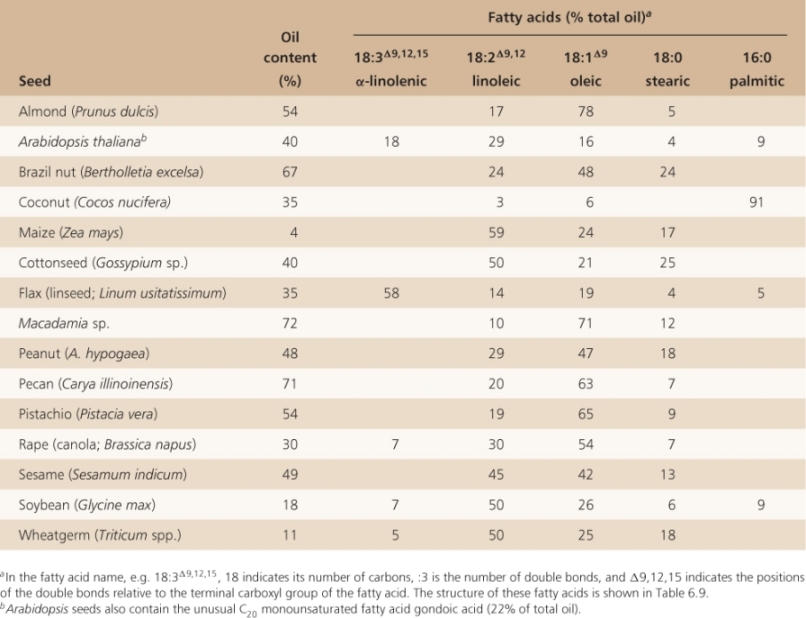
While most plant tissues store only carbohydrates, many seeds store lipids, in the form of triacylglycerols (see Figures 2.10A and 6.16, and Tables 6.3, 6.4 and 6.8). In Macadamia and pecan (Carya illinoinensis), oils can make up more than 70% of seed dry weight; seeds of many other species have oil contents in excess of 50%. Oils that are of commercial importance are derived from a relatively small number of crops including coconut (Cocos nucifera), maize (Zea mays), cotton (Gossypium sp.), flax (Linum usitatissimum), olive (Olea europaea), palm (Elaeis sp.), rapeseed/canola (Brassica napus), soybean (Glycine max) and sunflower (Helianthus annuus) (Table 6.8). Although oil makes up only 4.5% of maize kernel dry weight, it can comprise up to 50% of the weight of the embryo including the scutellum where most corn oil is stored. The embryo, called the germ, is removed from mature maize kernels during processing for animal feed and breakfast cereals. The germ, including the large scutellum, is pressed for oil production whereas the endosperm is used for feed, ethanol production and related uses. Because maize is such a widely grown crop in the USA, it is one of the major sources of vegetable oil.
Key Points
Seeds contain a broad spectrum of compounds that act as antinutrients for humans and other animals. These include phytate and storage proteins that are thought to have evolved a role in defense against bacterial and fungal pathogens, and herbivores such as insects. Phytate is a strong cation chelator that reduces the amount of calcium, iron and other cations available to livestock and humans. Diets rich in seeds can cause bone loss and anemia. Among the proteins in seeds that play a protective role are the many amylase and protease inhibitors, small molecular weight thionins, and lectins that bind and inactivate glycoproteins. Although they evolved to protect plants against predation, these antinutrients affect humans when foods containing them are consumed. Some antinutrients have useful medicinal properties. For example, amylase inhibitors reduce starch digestion and when taken orally they can be effective in promoting weight loss.
Table 6.8 Oil content and fatty acid composition of seed oils. (Note that content and composition can vary widely with variety and growing conditions. Where sources vary, average values are given).
Grains of rice, the dominant cereal grown in Asia, are also used as a source of oil. Almost all rice for human consumption is white rice, produced by pearling whole grains. Pearling is a process that removes the embryo, pericarp, testa and aleurone; you may be familiar with pearled barley, barley that is almost like white rice. The layers removed by pearling are often called bran. Bran can be pressed to produce rice bran oil that is thought to be nutritionally beneficial because it is rich in vitamin E. Rice bran oil also has a high smoking point making it excellent for frying food.
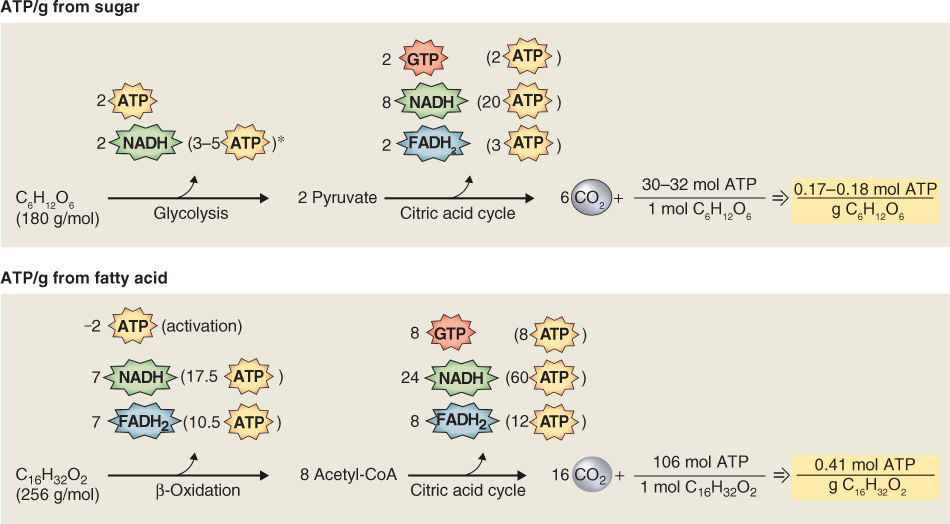
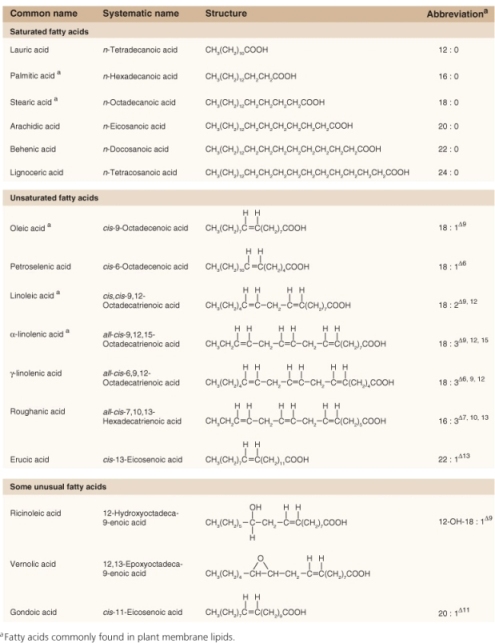
Because fatty acid molecules are more reduced than carbohydrates, lipid reserves yield more energy mole for mole than polysaccharides. Hydration of starch grains and fructans reduces their energy density, whereas triacylglycerols are largely hydrophobic and represent a more concentrated form of potential energy. As a consequence of their physicochemical properties, the ATP yield from catabolism to carbon dioxide and water is approximately twice as high for triacylglycerols as for an equivalent mass of carbohydrates (Figure 6.19). Storing carbon and energy reserves in the form of lipid rather than carbohydrate may be advantageous where a compact seed mass is a requirement for ecological fitness. The triacylglycerol reserves of seeds and fruits typically contain C16 and C18 saturated and unsaturated fatty acids, though some oils, such as that of palm kernels, are rich in fatty acids with shorter chain lengths, and Arabidopsis seeds have high levels of monounsaturated C20 gondoic acid (see Table 17.1). The polyunsaturated fatty acids linoleic acid (18:2Δ9, 12) and α-linolenic acid (18:3Δ9, 12, 15) are the major types found in plant membrane lipids (see Chapter 2).
6.3.8 The Fatty Acid Content of Seed Oil is Important for Human Uses
The fatty acid composition of seed oil is key to its end use. Table 6.9 shows the fatty acids commonly found in plants and Table 6.8 shows the distribution of these fatty acids in seeds. Saturated fatty acids, i.e. those without double bonds, are abundant in palm and coconut, where palmitic acid makes up 85–90% of the seed oil. Saturated fatty acids are also plentiful in Brazil nut, cotton seed and peanut, in which stearic acid comprises 17–24% of the fatty acid content of the seed oil. The most abundant unsaturated fatty acids in seeds are linoleic (18:2), linolenic (18:3) and oleic (18:1).
Nutritionists have identified a category of essential fatty acids that are required components of the human diet. Essential fatty acids, like essential amino acids, are those that cannot be synthesized by humans. They are called omega-3 and omega-6 fatty acids; among those made by plants are linoleic and α- and γ-linolenic acids (Table 6.10). The omega-3 and -6 terminology is based on the position of the double bond in the fatty acyl chain relative to the terminal carbon atom that is farthest away from the carboxyl end of the molecule. Omega-3 signifies that the third carbon atom from the terminal CH3 group has a double bond; in omega-6 fatty acids a double bond is found on the sixth carbon atom. Plants are a good source of essential fatty acids in the human diet and α-linolenic and linoleic acids are examples of omega-3 and omega-6 fatty acids, respectively.
Table 6.10 Examples of omega-3 and omega-6 fatty acids.
| Omega-3 fatty acids |
|
|
| Roughanic acid |
16:3Δ7, 10, 13 |
all-cis-7,10,13-hexadecatrienoic acid |
| α-Linolenic acid (ALA) |
18:3Δ9, 12, 15 |
all-cis-9,12,15-octadecatrienoic acid |
| Stearidonic acid (STD) |
18:4Δ6, 9, 12, 15 |
all-cis-6,9,12,15-octadecatetraenoic acid |
| Eicosatrienoic acid (ETE) |
20:3Δ11, 14, 17 |
all-cis-11,14,17-eicosatrienoic acid |
| Eicosatetraenoic acid (ETA) |
20:4Δ8, 11, 14, 17 |
all-cis-8,11,14,17-eicosatetraenoic acid |
| Eicosapentaenoic acid (EPA) |
20:5Δ5, 8, 11, 14, 17 |
all-cis-5,8,11,14,17-eicosapentaenoic acid |
| Docosapentaenoic acid (DPA), clupanodonic acid |
22:5Δ7, 10, 13, 16, 19 |
all-cis-7,10,13,16,19-docosapentaenoic acid |
| Docosahexaenoic acid (DHA) |
22:6Δ4, 7, 10, 13, 16, 19 |
all-cis-4,7,10,13,16,19-docosahexaenoic acid |
| Tetracosapentaenoic acid |
24:5Δ9, 12, 15, 18, 21 |
all-cis-9,12,15,18,21-docosahexaenoic acid |
| Tetracosahexaenoic acid (nisinic acid) |
24:6Δ6, 9, 12, 15, 18, 21 |
all-cis-6,9,12,15,18,21-tetracosenoic acid |
| Omega-6 fatty acids |
|
|
| Linoleic acid |
18:2Δ9, 12 |
all-cis-9,12-octadecadienoic acid |
| γ-Linolenic acid |
18:3Δ6, 9, 12 |
all-cis-6,9,12-octadecatrienoic acid |
| Eicosadienoic acid |
20:2Δ11, 14 |
all-cis-11,14-eicosadienoic acid |
| Dihomo-γ-linolenic acid |
20:3Δ8, 11, 14 |
all-cis-8,11,14-eicosatrienoic acid |
| Arachidonic acid |
20:4Δ5, 8, 11, 14 |
all-cis-5,8,11,14-eicosatetraenoic acid |
| Docosadienoic acid |
22:2Δ13, 16 |
all-cis-13,16-docosadienoic acid |
| Adrenic acid |
22:4Δ7, 10, 13, 16 |
all-cis-7,10,13,16-docosatetraenoic acid |
| Docosapentaenoic acid |
22:5Δ4, 7, 10, 13, 16 |
all-cis-4,7,10,13,16-docosapentaenoic acid |
The fatty acid composition and degree of saturation in vegetable oils vary (see Table 6.8), and have been subject to chemical and biotechnological interventions on a large scale to tailor them to particular culinary and industrial uses. Catalytic hydrogenation reduces double bonds, increases the ratio of saturated to unsaturated fatty acids and raises the oil's melting point, but is implicated in the formation of so-called trans-fats which are under suspicion as unhealthy components of the human diet. In many species there is substantial natural variation in oil composition which can be exploited by plant breeders. For example, the oleic acid (18:1Δ9) composition of canola oil may be anything from 43% to 78% of total fatty acids. Canola is a cultivar of Brassica napus (oilseed rape) and takes its name from Canadian oil low acid. Rapeseed oil naturally contains high levels of erucic acid (22:1Δ13). Accumulation of this long-chain fatty acid has been reduced in canola by naturally-occurring knockout mutation of a gene encoding an elongase that specifically extends the chains of C18 fatty acids. There are concerns that dietary erucic acid is harmful to heart tissue. Mutagenesis to increase the range of genetic variation in oil composition has been successfully applied to a number of species, including flax and sunflower. In recent years the modification of oil properties by recombinant DNA technology has been directed, with varying degrees of success, towards reducing relative levels of saturates, decreasing the tendency of plant fatty acids to become oxidized to potentially hazardous products during cooking or storage in air, increasing dietary antioxidants, reducing trans-acids and the production of essential long-chain fatty acids. The contribution of such approaches to human nutrition will depend on the safety and acceptability of foodstuffs from genetically modified crop sources. In addition to their importance as sources of feed and food, there are many industrial uses of seed oils and these include their use in the manufacture of cosmetics, detergents, lubricants and paints (Table 6.11).
Table 6.11 Some non-food uses of plant fatty acids.
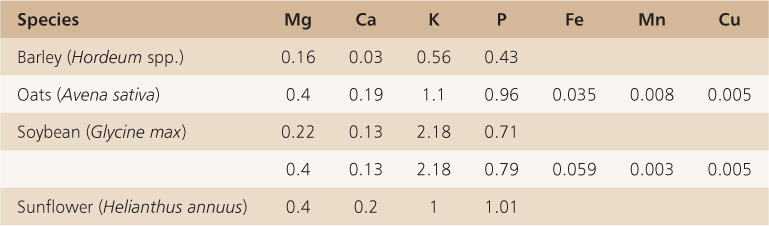
6.3.9 Seeds Store the Bulk of Mineral Elements in a Complexed Form
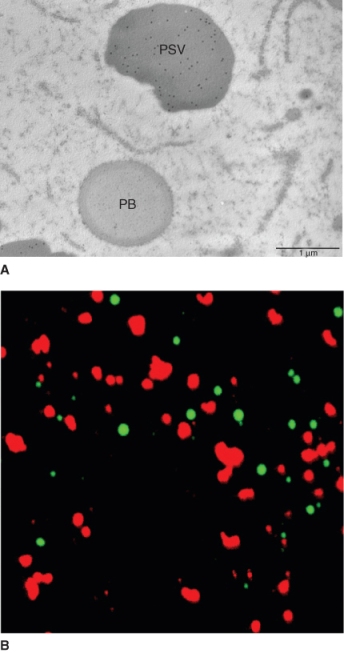
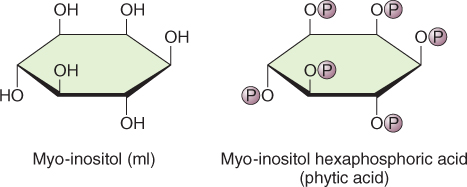
Seeds store the bulk of mineral elements in a complex with phytic acid, myo-inositol hexaphosphoric acid (IP6) (Figure 6.20). More than 90% of the phosphorus and major cations including K+, Mg2+, Ca2+, Zn2+ and Fe3+ in seeds are found as salts of phytic acid collectively called phytate (Table 6.12). Phytic acid is synthesized from glucose-6-phosphate; myo-inositol monophosphate synthase converts glucose-6-phosphate (G6P) to inositol-1-P and by successive phosphorylation reactions, with ATP as the phosphate (Pi) donor, to IP3. Two inositol phosphate kinases convert IP3 to IP6. The subcellular location of IP6 synthesis is not known although it is likely that it occurs in the cytosol. Phytate accumulates in protein storage vacuoles as a crystalline deposit known as a globoid surrounded by an envelope. Globoid crystals can vary greatly in size even in tissues of the same species. Figure 6.16 shows electron micrographs of the cotyledon and endosperm cells of Arabidopsis seeds. Both cell types contain protein storage vacuoles containing phytate but the globoid crystals in cotyledonary cells are much larger than those found in the endosperm.
Key Points
Oils, polysaccharides and proteins make up the bulk of storage reserves in seeds. Mineral elements are generally stored as a chelated complex with inositol hexaphosphate in a compound called phytate. Starch grains stored in amyloplasts and cell wall carbohydrates make up the bulk of storage polysaccharides. In some seeds, such as date and fenugreek, cell wall polymers make up the bulk of storage polysaccharide. Seed storage proteins are complex and are classified according to their solubility in water, dilute salt or dilute alcohol solutions. They are found in protein storage vacuoles and protein bodies. Oils, as triglycerides, are stored in specialized oil bodies (oleosomes). They are also chemically diverse, varying both in the length of the fatty acid chain and in their degree of saturation.
Table 6.12 The content of the main inorganic elements of phytate in various species of seeds, expressed on a percent dry weight basis.
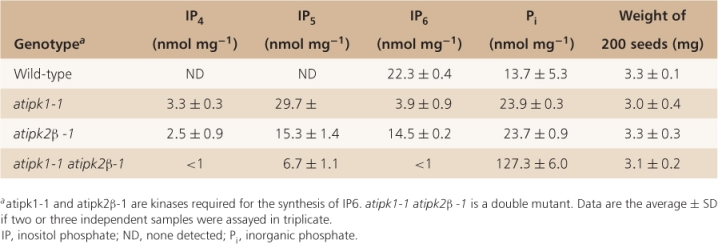
Recent molecular genetic experiments show that phytate is not required for the production of viable seed. Arabidopsis double mutants that lack both of the inositol phosphate kinases that catalyze the last two steps in IP6 synthesis, produce seeds that lack phytate and have soluble phosphate concentrations that are almost ten times higher than wild-type (Table 6.13). Seed yield is not affected by the absence of phytate and germination of phytate-free seeds is normal.
Table 6.13 Inositol phosphate, free phosphate and seed yield analysis in Arabidopsis.
6.3.10 Phytate is another Antinutrient in Seeds
Phytate is a potent antinutrient because of its ability to chelate cations such as Ca2+, Fe3+ and Zn2+. Diets rich in seeds, such as those in countries where a mixture of beans and corn form the staple diet, may lead to defects in bone mineralization because Ca2+ is sequestered by phytate and is therefore not taken up by the digestive system. Other diseases that result from diets high in phytate are anemia from reduced iron availability, and hypogonadal dwarfism from zinc deficiency. Seeds also act as antinutrients in animal feed, especially for monogastric animals with single stomachs (as opposed to bovines and other ruminants with multiple stomachs) that lack the intestinal flora to hydrolyze phytate and mobilize nutrients. Birds, especially turkeys, that are fed on high cereal diets suffer from weakened limb bones. In pigs, much of the phytate that is ingested is lost via feces, leading to eutrophication of rivers and streams because of increased phosphorus levels. Treatment of seed-based livestock feed with the enzyme phytase can alleviate some of the problems associated with high phytate content, as can cooking methods that hydrolyze phytate. Another strategy being tested to reduce the adverse effects of phytate in human and livestock diets is to develop crop plants that are deficient in phytate; this approach has been successful in the model plant Arabidopsis (Table 6.13).
6.3.11 Seed Maturation Produces Seeds that can Survive for Long Periods
Mature seeds have a water content below 10%, a condition that is doubtless required for seeds to remain viable during extended periods of dormancy or quiescence. Seeds with higher water content are known to have a higher metabolic rate and will oxidize their food reserves more rapidly than do quiescent or dormant seeds. In addition to providing the reserves required to support growth of a new seedling, the polymerization of stored reserves is required to ensure water loss as the seed matures. Glucose is polymerized to starch where, as noted above, the degree of polymerization can easily exceed 105 glucose residues. The organization of starch into the complex starch grain ensures its insolubility. Similarly, incorporation of sugars into cell wall polymers ensures that the osmotic properties of the cytoplasm are unaffected by the accumulation of polysaccharide.
Storage proteins with a large molecular mass are packaged into protein storage vacuoles where they form deposits that are often soluble only in saline solution or aqueous alcohol. Triacylglycerols are neutral, water-insoluble oils that are partitioned from the cytoplasm by the half-unit membrane of the oleosome (oil body; see Chapter 4). The ultimate sequestration of mineral elements as crystalline phytate within vacuoles further ensures that the water potential of the cytosol will allow water to be easily lost from the seed by evaporation. In the next sections we will describe how the process of reserve deposition is reversed when a seed germinates and the seedling starts to grow. These processes will support survival of the seedling as a heterotrophic organism until its photosynthetic machinery is developed and becomes active.
6.4 Germination and Early Seedling Growth
Germination is defined as the penetration of the seed coat by the elongating embryonic root (Figure 6.21). In addition to the pericarp, testa and endosperm as in the example of Lepidium shown in this figure, the radicle of a cereal embryo must also penetrate the coleorhiza and often the hulls (palea and lemma) (see Figure 6.6). After germination, the embryo starts to grow and the seedling emerges from the seed coats. The elongation of the embryonic stem may take place in the epicotyl (above the cotyledons) or hypocotyl (below the cotyledons), depending on whether the cotyledon(s) of the germinating seedling remain below ground or above ground (Figure 6.22). In hypogeal seedlings such as garden pea (Pisum sativum) the cotyledon(s) remain below ground after germination and the first true leaves of the seedling are borne on an epicotyl. In peas, the epicotyl forms a hook that pulls the shoot apical bud upward, while in cereal seedlings, the coleoptile protects the plumule of the embryo as it is pushed upward through the soil. In epigeal seedlings such as garden bean (Phaseolus vulgaris), castor bean (Ricinus communis) and onion (Allium cepa) the cotyledons are propelled upwards into the air by growth of the hypocotyl (Figure 6.22). In onion and castor bean seedlings, the cotyledons serve not only as a source of stored food, but also as the first photosynthetic organs of the seedling.
6.4.1 Imbibition of Water is Necessary for Seed Germination
As noted above, mature seeds have a low water content, often below 10% of seed dry weight. The water content of seeds will vary with the water content of the atmosphere in which they are stored as well as with the type of reserves stored within the seed. In general, oil seeds have a lower water content (about 5%) than starchy seeds (about 10%) stored at the same relative humidity. When seeds are placed in water, they rapidly rehydrate, a process known as imbibition. Imbibition takes place in both living and dead seeds; it is a physical process resulting from hydration of seed storage polymers. A micropyle is present in the testa of most seeds (see Figure 6.1A); this may be a thinner area of tissue or a microscopic hole in the testa. It is a remnant of the gap between the integuments of the ovule where the pollen tube entered the ovule (see Figure 1.18). Hydration of the seed often begins by water uptake through the micropyle. Uniform water uptake by seeds, especially under conditions of limited water availability, is aided by the presence of mucilage layers on the surface of seed coats (see Figure 6.4). The mucilage hydrates very quickly, often within seconds of exposure to water. Very large forces, up to 10–20 MPa (equal to 100–200 atmospheres of pressure, sufficient to break rocks!) can be generated by imbibitional water uptake. Splitting of the testa and pericarp often occurs during imbibition, but this may not result in germination.
6.4.2 Dormant Seeds do not Germinate after Imbibition
Viable seeds that do not germinate under normally favorable conditions of water and oxygen availability are said to be in a state of dormancy. Like other dormant structures—resting buds, for example—dormant seeds require additional stimuli to commence and sustain growth. Different types of dormancy in different organs have distinctive features but also share some common physiological and regulatory mechanisms; these aspects are described in Chapter 17. Here we focus on the major ways in which dormancy inhibits seed germination.
Dormancy is a complex characteristic. Seeds that have primary dormancy are dormant when they are shed from the mature plant, but seeds can acquire secondary dormancy in response to environmental conditions that they experience after shedding. Secondary dormancy is most commonly imposed on non-dormant seeds by extremes of temperature but the presence or absence of light may also induce secondary dormancy. Primary dormancy ensures that seeds do not germinate prematurely while they are still attached to the parent plant. Secondary dormancy delays germination until environmental conditions favor successful growth of the emerging seedling.
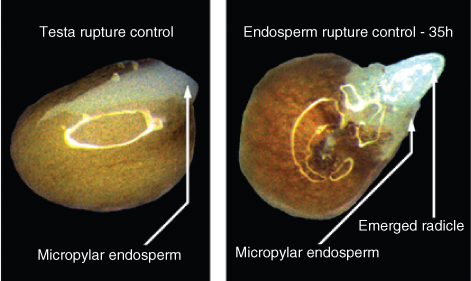
Dormancy may be a property of the seed coats or of the embryo. Seed coat-enhanced dormancy is found in a wide variety of species including dormant varieties of Arabidopsis, barley, lettuce, rice and wild oats. A basic characteristic of seed coat dormancy is that when the embryo from a dormant seed is separated from the enveloping seed coats and is incubated in water, it is able to grow and develop into a seedling (Figure 6.23). The growth of embryos removed from dormant seeds is indistinguishable from that of embryos in non-dormant seeds. As discussed further in Chapter 17 (see Sections 17.2.1 and 17.4.1), mechanisms proposed to explain seed coat dormancy include: mechanical restraint of the embryo; impermeability of seed coats to the uptake of water or gases; the coat acting as a barrier preventing inhibitory compounds such as the plant hormone abscisic acid (ABA) from being removed by leaching; or a combination of these factors.
Embryos that do not grow when seed coats have been removed are said to show embryo dormancy. An example of embryo dormancy is found in freshly harvested onion (Allium) seeds that must be stored for several months before they will germinate. Less is known about why such embryos do not grow but investigators suspect that metabolic or developmental deficiencies in the embryo play a role (see Section 17.4.2).
6.4.3 Environmental Signals may Trigger the Breaking of Dormancy
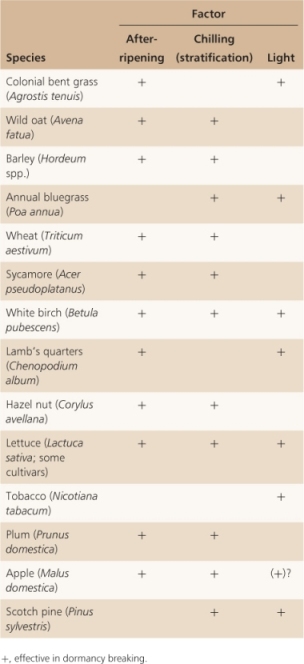
Seeds that are dormant eventually germinate, and some of the signals that bring about germination are now well understood. Dormant seeds normally germinate in response to environmental signals—usually temperature or light—or as a result of prolonged storage. As Table 6.14 shows, seeds often respond to more than one germination-triggering signal. Grains of many cereals are among the seeds that are dormant when freshly harvested but germinate after storage under dry conditions, a process called after-ripening. A requirement for after-ripening in cereals means that freshly harvested grain cannot be sown immediately but must be stored under suitable conditions, often for several months, before dormancy is completely broken.
Table 6.14 Termination of dormancy by various factors.
Many seeds lose dormancy after storage under moist, cool conditions, a treatment called stratification (Table 6.14). Most seeds that respond to chilling are from species that grow in temperate regions; this suggests that stratification may have adaptive advantages. Because seeds shed in late summer or early fall must receive an over-wintering period so that dormancy can be lost, these seeds germinate only in the following spring or summer when conditions are more favorable for growth. Dormancy of vegetative resting buds and vascular cambium is also attuned to winter conditions, as discussed further in Chapter 17 (see Sections 17.4.2 and 17.4.5).
6.4.4 Light can be an Important Trigger for Germination
Light is clearly an important trigger for germination; light of specific wavelengths, intensity or duration may be the effective stimulus (Table 6.15). For example, brief pulses of red or blue light can trigger germination, as can long periods of light or light given as a series of either short days or long days, the equivalent of winter and summer day lengths, respectively.
Table 6.15 Illumination conditions required for the breaking of dormancy.
| Seconds or minutes |
Colonial bentgrass (Agrostis tenuis)
Lamb's quarters (Chenopodium album)
Lettuce (Lactuca sativa cv. Grand Rapids)
Tobacco (Nicotiana tabacum) |
| Several hours |
Pig nut (Hyptis suaveolens)
Purple loosestrife (Lythrum salicaria) |
| Days |
Epilobium cephalostigma
Kalanchoe blossfeldiana |
| Long days |
Begonia evansiana
White birch (Betula pubescens) (at 15 °C)
Jerusalem oak (Chenopodium botrys) (at 30 °C) |
| Short days |
Jerusalem oak (>30 °C)
Eastern hemlock (Tsuga canadensis)
White birch (>15 °C) |
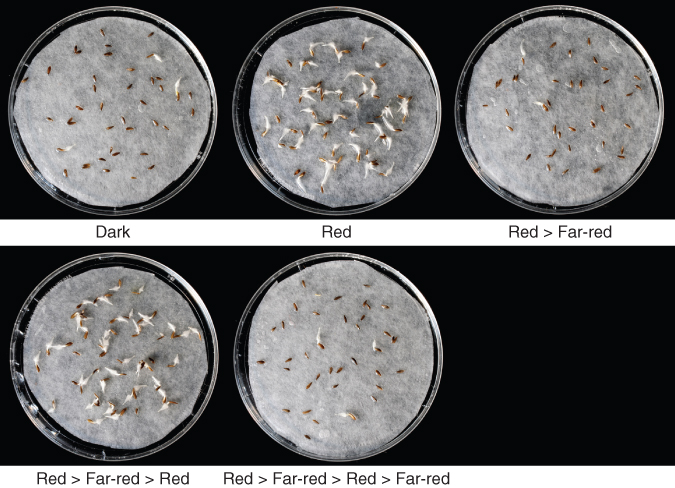
The discovery that red light at around 660 nm could break dormancy in lettuce (Lactuca sativa) seeds led to a landmark discovery in the 1950s. Photodormant lettuce seeds were shown to germinate after exposure to a brief pulse of red light, an experiment that eventually led to the discovery of the photoreversible pigment, phytochrome (see Chapter 8). A key experiment showed that a brief pulse of far-red (FR) light (approximately 710 nm) could reverse the promotive effects of a brief pulse of red (R) light (about 660 nm) on germination (Figure 6.24). Indeed, it was shown that when alternating pulses of R and FR light were given to dormant lettuce, seeds remained dormant or germinated depending on the wavelength of light that was given last (Table 6.16). These experiments were interpreted as showing that a photoreversible pigment was present in lettuce seeds and that the form of the pigment present in seeds after R light treatment favored germination. It was further hypothesized that exposure of this form of the pigment to FR light caused its photoconversion to a form that would block germination. We now know this pigment to be phytochrome (P). The relationship between the PR and PFR forms of phytochrome that exist after FR and R light exposure is shown in the simplified diagram in Figure 6.25. You will learn more about phytochrome and its role in plant growth and development in Chapter 8 and about the adaptive and ecological significance of dormancy photoregulation in Chapter 17 (see Section 17.4.3 and 17.4.5).
Table 6.16 Phytochrome photoreversibility and the breaking of dormancy.a
| None (darkness) |
4 |
| R |
98 |
| FR |
3 |
| R, FR |
2 |
| R, FR, R |
97 |
| R, FR, R, FR |
0 |
| R, FR, R, FR, R |
95 |
6.4.5 Plant Hormones Play Important Roles in the Maintenance and Breaking of Seed Dormancy
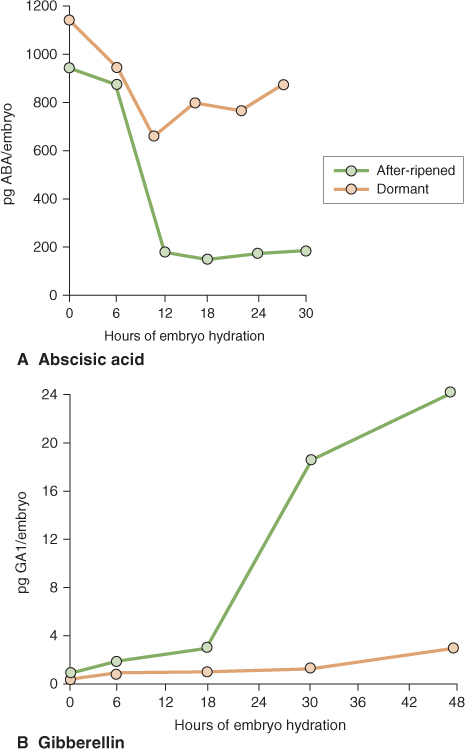
Although a range of environmental signals can trigger germination of dormant seeds, it is likely that the underlying molecular mechanisms for breaking dormancy are similar. Plant growth hormones have been implicated in the regulation of dormancy. Genetic and physiological experiments point to important roles for ABA and gibberellins (GAs). Mutants that overproduce GAs, or that have a constitutive GA response in the absence of elevated GA levels, lack dormancy. Similar behavior is shown by mutants that lack ABA, have reduced ABA levels or have a compromised response to ABA. A useful rule-of-thumb is that in most seeds ABA imposes dormancy and a reduction in its concentration and an increase in GA concentration lead to breaking of dormancy.
In barley, the ABA concentration is high in dormant and non-dormant dry grain but following imbibition its level declines much more dramatically in embryos of non-dormant grain than in dormant grain (Figure 6.26A). GA concentration in barley grains also changes during the breaking of dormancy, increasing rapidly in non-dormant embryos while remaining low in dormant embryos (Figure 6.26B). Taken together, the reciprocal changes in ABA and GA concentrations in dormant and non-dormant embryos result in the breaking of dormancy. In Arabidopsis seeds, red light acting via phytochrome can stimulate the breaking of dormancy. In this case, light triggers the expression of GA biosynthetic genes and this in turn leads to an increase in bioactive GAs in the seed.
How are changes in the concentration of ABA and GA translated into radicle emergence in dormant seeds? There is widespread agreement that seed coat dormancy is lost when the coat can no longer restrain growth of the embryo axis. In dormant seeds of Arabidopsis, lettuce (Lactuca), peppergrass (Lepidium) and tobacco (Nicotiana), the seed coat and especially the endosperm do not yield in response to pressure from the growing embryo and they thereby prevent radicle emergence. When the seeds of these species lose dormancy, the radicle is able to penetrate the weakened walls of the endosperm. In Arabidopsis and Lactuca, hormonal treatments affect the strength of endosperm cell walls. In GA-treated seeds, endosperm cells have reduced cell wall thickness while added ABA prevents the weakening of these cell walls. Furthermore, Arabidopsis mutants that have a constitutive GA response show extreme cell wall weakening in the endosperm adjacent to the radicle (Figure 6.27).
Most general models of the molecular regulation of dormancy include ABA–GA interaction as a central feature. This is discussed further in Section 17.4.5.
Key Points
Not all seeds germinate under favorable conditions of moisture and temperature. Seeds that fail to germinate are dormant. Dormancy is an important property because it prevents precocious germination of seeds under conditions that may not support seedling growth. Many environmental signals and endogenously produced compounds break dormancy. Among the environmental triggers are alterations in temperature, especially cold periods, the presence or absence of light, and concentration of soil anions, in particular nitrate and nitrite. The hormones abscisic acid (ABA), gibberellins (GAs) and nitric oxide (NO) all play a role in regulating dormancy. Genetic and biochemical evidence point to a key role of ABA in establishing dormancy, whereas GA and NO are known to break dormancy.
6.5 Mobilization of Stored Reserves to Support Seedling Growth
Germination usually takes place underground. The growing seedling must depend on food stored in the seed to support its growth until it reaches light and can become photosynthetic. As we have seen, seeds contain reserves of protein, carbohydrate, lipid and minerals. The growing seedling will use the minerals as a source of elements, such as P, K, Mg and Fe, and the organic molecules as sources of energy and carbon skeletons, all of which are needed to make new macromolecules. Before the growing seedling can use its stored reserves, they must be broken down into smaller subunits that can be transported throughout the developing seedling. We will look at how each of the major storage molecules is mobilized and used by the growing seedling.
6.5.1 Mobilization of Protein Involves the Enzymatic Breakdown of Proteins to Amino Acids
All seeds store some protein. During early seedling development, these proteins are broken down to yield amino acids that are required for growth. The amino acids formed during proteolysis may be reused directly for new protein synthesis or, following loss of the amino group, they may be oxidized to release energy. Hydrolysis of stored proteins to their constituent amino acids requires the action of a variety of proteases. Some of these catalyze the total hydrolysis of protein to amino acids whereas others break down proteins to small polypeptides that must be degraded further by peptidases. A large number of proteolytic enzymes have been identified and characterized in seeds. All of these appear to be members of the metallo-, cysteine and serine protease families.
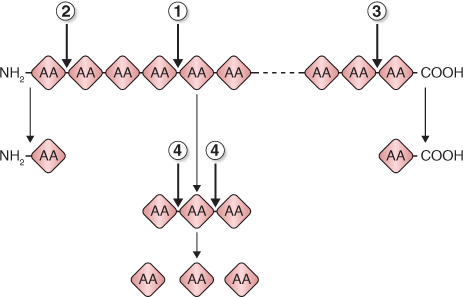
The proteases that are active during seed germination can be grouped by the way in which they hydrolyze (break) peptide bonds of their substrates (Figure 6.28):
- Endopeptidases: cleave internal peptide bonds to yield smaller peptides.
- Aminopeptidases: sequentially cleave the terminal amino acids from the free amino acid end of the polypeptide chain.
- Carboxypeptidases: resemble aminopeptidases except amino acids are cleaved from the carboxyl end of the chain.
- Peptidases: degrade small peptides to amino acids.
Recently, it has been shown that thioredoxin, a regulatory disulfide protein, facilitates the mobilization of both protein and carbohydrate reserves in developing seedlings. In the cereal endosperm, thioredoxin is reduced by NADPH and the flavin enzyme NADP-thioredoxin reductase (NTR) (Equation 6.1).
6.1A
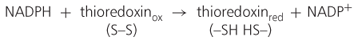
6.1B
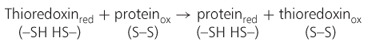
Reduced thioredoxin then reduces disulfide (S–S) groups of a number of target seed proteins. For example, when storage proteins of the seed are reduced by thioredoxin, their solubility increases and they are more susceptible to proteolysis. In addition, thioredoxin increases the activity of selected enzymes, either directly by reducing them or indirectly by inactivating disulfide inhibitor proteins. One enzyme that is inhibited by a disulfide protein is starch debranching enzyme, which is involved in starch breakdown (see Equation 6.3). Thioredoxin has been shown to affect enzyme activity in cereal grains and legume seeds.
Transgenic experiments in which expression of thioredoxin was altered have confirmed the central role it plays in germination. Cereal grains that overexpress thioredoxin were found to germinate more rapidly than control seeds, and grains in which the expression of thioredoxin was downregulated germinated more slowly. Development of transgenic wheat cultivars with reduced thioredoxin levels may lead to the solution of a long-standing agricultural problem. In very wet seasons, wheat grains may begin to germinate while still attached to the parent plant, a condition known as preharvest sprouting. Wheat cultivars with lowered thioredoxin levels show reduced preharvest sprouting (Figure 6.29).
Some of the proteases that mobilize storage proteins are synthesized de novo but there is evidence that others may already be present in the seed in an inactive form. In cereals, for example, the living aleurone layer that surrounds the starchy endosperm synthesizes and secretes a wide range of acid hydrolases, i.e. hydrolytic enzymes that have pH optima between 4 and 5. Among these acid hydrolases are a number of proteases that break down storage proteins in the endosperm. These secreted enzymes complement those present in the starchy endosperm of dry grain.
Key Points
The mobilization of stored reserves in seeds is brought about by enzymes. In the dead starchy endosperm of cereals, starch and proteins are accessible to enzymes synthesized and secreted from the aleurone layer. In storage cotyledons of eudicots, reserves are located in living cells, proteins in protein storage vacuoles (PSVs) and starch in amyloplasts. The synthesis of enzymes that break down storage protein in eudicot cotyledons occurs at the ER. These enzymes are then delivered to vacuoles by Golgi-derived vesicles. A similar process occurs for protein degradation in the PSVs of cereal aleurone. Synthesis of starch-degrading enzymes occurs on free ribosomes; these enzymes are taken up into the amyloplast via specific transporters in the outer and inner plastid membranes.
6.5.2 Stored Protein Mobilization in Eudicots takes Place in Living Cells
The digestion of proteins present in the cotyledons and endosperm of eudicot seeds is different from that in the cereal endosperm. Cells in these tissues are alive and their stored protein reserves are localized in protein storage vacuoles. There is evidence that both newly synthesized and pre-existing proteases break down protein reserves in vacuoles. One hypothesis suggests that a newly synthesized protease is transported to the vacuole where it initiates a cascade of proteolytic activities that bring about the breakdown of stored proteins. According to this hypothesis, the role of the newly synthesized protease is to activate preformed but inactive proteases in the lumen of the protein storage vacuole. The pH of the protein storage vacuole may also play an important role in regulating the degradation of storage proteins. In Arabidopsis a vacuolar processing enzyme (VPE) has been identified that participates in the mobilization of storage proteins by initiating a proteolytic cascade. VPE is synthesized in the ER as an inactive precursor; it is self-activated at an acidic pH, presumably in the vacuole. VPE then activates other proteases. Because proteases that degrade storage protein have acidic pH optima, it is easy to see how acidification of the vacuole lumen can govern the rate at which these proteases break down their substrates. The protein mobilization process in seed germination has many points of similarity with the proteolysis that occurs in senescence and cell death, as discussed further in Chapter 18.
Key Points
Proteases that hydrolyze storage proteins to peptides and amino acids fall into four broad categories, depending on how the enzyme attacks the polypeptide chain. Aminopeptidases digest from the amino terminal end of the polypeptide, carboxypeptidases from the carboxyl terminus, and endopeptidases at random points between the amino and carboxyl ends. Peptidases cleave the small peptides produced by the action of endopeptidases to amino acids. Amino acids formed as a result of proteolysis of stored protein can be used in the synthesis of new enzymes (e.g. the α-amylase produced by the cereal aleurone) or transported to the embryo to support new protein synthesis in the developing seedling.
6.5.3 Mobilization of Stored Starch may be Catalyzed by Phosphorolytic Enzymes
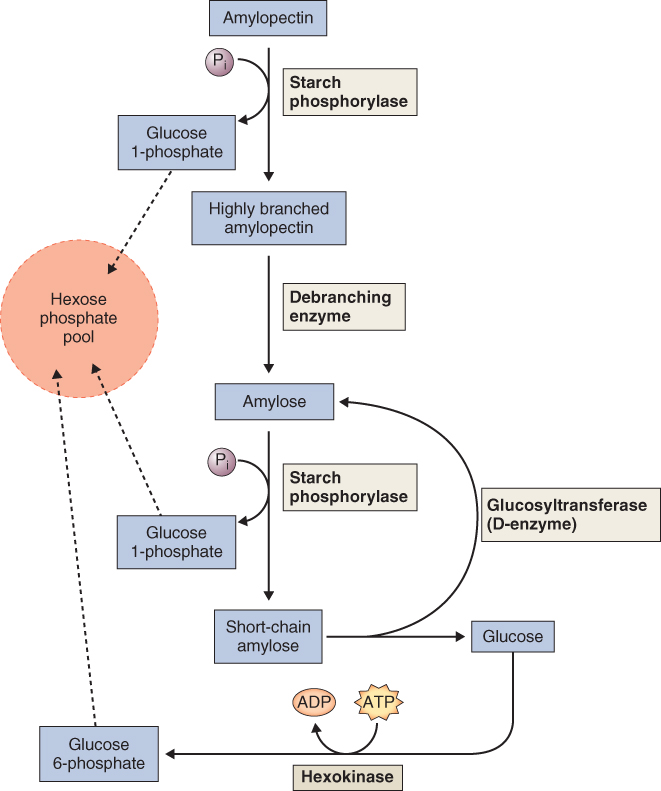
Starch is the major carbohydrate reserve in most plants. It consists of two molecules, amylose and amylopectin (Section 6.3.1). Starch may be cleaved into smaller polymers or monomers by either of two classes of enzymes: phosphorolytic enzymes that use Pi to break glycosidic (glucose to glucose) bonds and hydrolytic enzymes that use water to break these bonds (Figure 6.30).
At least three enzymes contribute to phosphorolytic starch degradation: starch phosphorylase, debranching enzyme and glucosyltransferase (Equations 6.2–6.4).
Equation 6.2 Starch phosphorylase
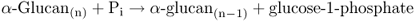
Equation 6.4 Glucosyltransferase (D-enzyme)
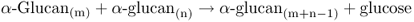
Starch phosphorylase cleaves individual glucose residues from the non-reducing end of the starch molecule, generating glucose-1-phosphate. Starch phosphorylase can attack only bonds located at least four glucose residues from a branch point. Continued phosphorolytic attack on the branched starch molecule is made possible by a debranching enzyme (also called pullulanase or R-enzyme) that cleaves the α(1 → 6) bonds, releasing linear chains on which the starch phosphorylase can act. In addition, the D-enzyme, or glucosyltransferase, can condense short glucan polymers to produce new substrate for the phosphorylase (Figure 6.30).
Although starch phosphorylase plays a central role in carbohydrate metabolism, how the activity of this enzyme is regulated in plants has not been discovered. One hypothesis is that it may be regulated by the availability of inorganic phosphate, which fluctuates in response to changes in growth conditions including different stresses. This pattern of regulation of plant starch phosphorylase is different from that of its glycogen phosphorylase counterpart in animals. The latter enzyme is regulated by a sophisticated protein phosphorylation/dephosphorylation network.
6.5.4 Amylases also Play a Role in Starch Breakdown
Starch can also be cleaved by a group of hydrolytic enzymes known as amylases (Equations 6.5–6.7) that break down linear α(1 → 4)-glucans. α-Amylase catalyzes the cleavage of internal glucosyl bonds, giving rise to short glucans called dextrins. The smallest unit formed by α-amylase is the disaccharide maltose. β-Amylase hydrolyzes starch by cleaving maltose residues from the non-reducing end of the starch molecule. The maltose and short glucan molecules that are the products of amylase digestion are further degraded to glucose molecules by the action of α-glucosidase. Complete digestion of amylopectin requires the action of debranching enzyme (Equation 6.3) as well as the amylases and glucosidase (Figure 6.31).
Equation 6.5 α-Amylase
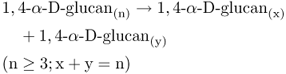
Equation 6.6 β-Amylase
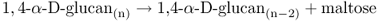
Equation 6.7 α-Glucosidase
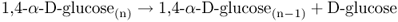
The enzymes involved in hydrolytic starch cleavage are particularly active in seed germination. During germination of cereals, there is rapid breakdown of starch in the endosperm. The most studied of the starch-hydrolyzing enzymes is α-amylase, which is synthesized in the scutellum and the aleurone layer and secreted into the starchy endosperm (Figure 6.32). The de novo synthesis of α-amylase by the aleurone is promoted by GA and repressed by ABA. All secretory proteins are synthesized on the ER and transported to the cell exterior by vesicles produced by the Golgi apparatus. Like the proteases secreted by the aleurone layer and scutellum, α-amylases have an acidic pH optimum and show maximum activity at around pH 4.
β-Amylase of cereal grains is not synthesized de novo by the aleurone layer. In this case, a precursor of the enzyme is present in bound form in the starchy endosperm before germination. It is released during germination and is activated either by removal of a small peptide from the carboxyl terminus of the enzyme or by the action of sulfhydryl agents such as thioredoxin (see Section 6.5.1). Some α-glucosidase is present in the dry seed, but during germination large amounts of this enzyme are synthesized by the aleurone in response to increased GA levels. Seed-localized debranching enzyme and α-amylase activities are further regulated by small, specific disulfide proteins that act as inhibitors. The hydrolytic enzymes associated with starch breakdown in storage tissues such as cotyledons also appear to play a role in the mobilization of starch stored temporarily in chloroplasts in foliage leaves. In this case hydrolytic enzymes are synthesized on free ribosomes and targeted to the chloroplast.
6.5.5 Cell Walls are another Source of Carbohydrates
Cell walls are a major source of stored polysaccharide in many seeds, and enzymes secreted by living cells also mobilize these. In the case of eudicot seeds that have endosperm, cell wall-digesting enzymes—such as mannanases that hydrolyze mannans and galactomannans—are synthesized by endosperm cells and secreted into the cell wall. These enzymes break down the endosperm cell walls. In the case of seeds such as those of Arabidopsis, Lactuca and Lepidium, the weakening of the cell walls by digestive enzymes breaks dormancy and allows the radicle to emerge from the seed. The aleurone and scutellum of cereals also secrete glucanases that digest the walls of starchy endosperm cells. In eudicots, the cell walls of storage cotyledons are also extensively degraded. It is thought that the cell wall-degrading enzymes in cotyledons are synthesized de novo in the cytosol and secreted into the cell wall.
6.5.6 Mobilization of Stored Lipids Involves Breakdown of Triacylglycerols
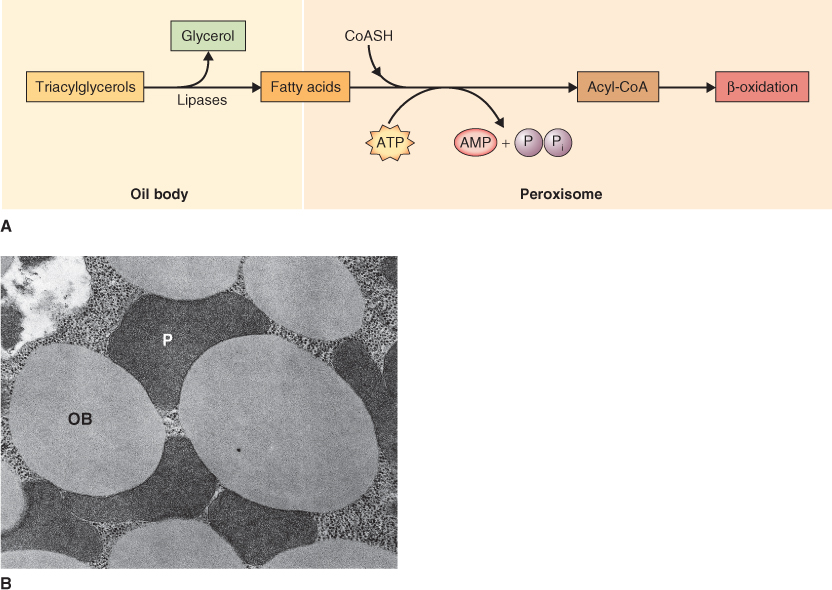
During germination and early seedling growth, triacylglycerols, stored in seeds, are degraded and converted into glucose and a wide variety of other essential metabolites. Within the cells of storage tissue, triacylglycerols may be converted to sucrose for transport to other tissues in the growing seedling. In seeds, the mobilization of triacylglycerols involves three organelles: (i) the oil body (oleosome), where stored triacylglycerols are converted to glycerol and fatty acids; (ii) the specialized peroxisome, often called a glyoxysome, where fatty acids are converted by β-oxidation to acetyl-coenzyme A (CoA) and then to succinate by the glyoxylate cycle; and (iii) the mitochondrion, where succinate is either respired or converted to malate which is transported to the cytosol for final conversion to hexoses by the reactions of gluconeogenesis. Here we will discuss the breakdown of triacylglycerols to glycerol and fatty acids and of fatty acids to acetyl-CoA. Subsequent conversion of acetyl-CoA to hexoses will be addressed in Chapter 7.
Key Points
Storage polysaccharides are broken down by a wide range of carbohydrases, enzymes that cleave glycosyl bonds in carbohydrates. Starch is broken down by a family of enzymes that include debranching enzyme, α- and β-amylases, and α-glucosidase. Debranching enzyme attacks the β(1 → 6) linkages in amylopectin producing linear chains of β(1 → 4)-linked amylose. Amylose is attacked by α-amylase at random points producing short oligomeric dextrins. β-Amylase attacks amylose chains and dextrins from the non-reducing end to form maltose, a disaccharide. α-Glucosidase breaks down maltose to glucose. Cell wall polysaccharides are broken down by specific carbohydrases, for example xylans are hydrolyzed by xylanases, β-glucans by β-glucanases, etc.
Triacylglycerols are stored in unusual organelles called oil bodies (see Chapter 4). Because of their insolubility in water, triacylglycerols must be hydrolyzed to fatty acids and glycerol by lipases before they are available for metabolism (Figure 6.33). The glycerol released in the lipase reaction is converted in the cytosol to triose phosphates, which, in turn, are used to synthesize sucrose via gluconeogenesis (see Chapter 7). In contrast, the fatty acids are transferred to peroxisomes for the next stage in the mobilization process. The mechanism by which this transfer is accomplished is not known although oil bodies are usually found adjacent to peroxisomes (see Figure 6.33B).
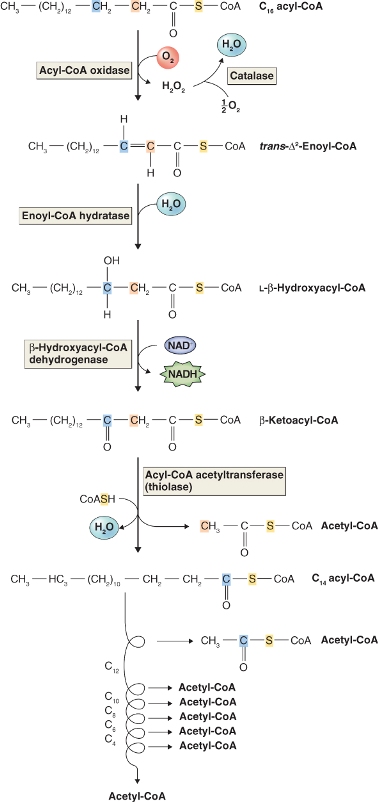
In plants, the oxidation of fatty acids takes place in peroxisomes of germinating seeds and leaves (Figure 6.33). This is in contrast to animal cells in which the major site of fatty oxidation is the mitochondrial matrix. Fatty acids are broken down to two carbon units (C2) during a cyclic process called β-oxidation because the number 2 (or β) carbon of the fatty acid is the carbon that undergoes oxidation (Figure 6.34). Fatty acids are first activated by the addition of coenzyme A using an enzyme called fatty acid-CoA synthase. They are then oxidized in a four-step process shown in Figure 6.34. The energy released in the first oxidative step of fatty acid breakdown is dissipated as heat, because acyl-CoA oxidase transfers electrons directly to oxygen, producing H2O2. The hydrogen peroxide produced is a strong and potentially damaging oxidant; it is immediately cleaved by catalase to water and oxygen (Equation 6.8).
In contrast, animal cell β-oxidation occurs in mitochondria and the electrons removed in the first oxidation step pass through the respiratory chain to oxygen, a process that is accompanied by the capture of energy by means of ATP synthesis (see Chapter 7). As we will see in Chapter 7, reactions similar to those catalyzed by enoyl-CoA hydratase and β-hydroxyacyl-CoA dehydrogenase in β-oxidation are found in the citric acid cycle phase of aerobic respiration (e.g. fumarase and malate dehydrogenase) in mitochondria.
6.5.7 Stored Minerals are Mobilized by Breaking Down Phytic Acid
As discussed in Section 6.3.9, mineral elements are stored in seeds in a complex with phytic acid, myo-inositol hexaphosphoric acid (IP6). During germination IP6 is hydrolyzed by the enzyme phytase, a phosphatase that releases phosphate and chelated cations. Phytase levels increase following seed germination making phosphate and other minerals available to support embryo growth and development. The hydrolysis of IP6 leads to the formation of a series of inositol phosphates ranging from inositol-1-phosphate to IP6. It is well known that certain inositol phosphates, especially inositol-1,4,5-trisphosphate, are important regulatory molecules in eukaryotic cells. There is as yet no evidence that IP6 hydrolysis products play a regulatory role in seeds; indeed Arabidopsis mutants lacking phytate germinate normally. The presence of phytate makes the seed and young seedling independent of an external supply of essential minerals for at least the first several days after germination.
We have seen how protein, starch and lipid reserves are mobilized and converted to, respectively, amino acids, simple sugars and acetyl-CoA. These compounds (or their derivatives) are transported from storage tissues to growing tissues. In the cereal grain the scutellum plays a prominent role in the transfer of solutes from the endosperm to the growing shoot and root systems. These solutes may be used by the embryo either as a source of carbon skeletons or as a source of energy to power metabolism. Mobilization of lipid reserves is more complex since acetyl-CoA and glycerol must be converted to sucrose or succinate for transport and further utilization. The processes involved in this conversion, including the tricarboxylic acid and glyoxylate cycles and gluconeogenesis, are discussed in detail in Chapter 7.
Key Points
Triglycerides are broken down to glycerol and fatty acyl chains in the oil body by the action of lipase. Glycerol can be further metabolized by glycolysis. Fatty acids are converted to acetyl-CoA by the reactions of β-oxidation and then oxidized in a four-step series of reactions in the peroxisome to form the two carbon molecule acetyl-CoA. The first step in this process is catalyzed by acyl-CoA oxidase, an enzyme that yields H2O2 and heat but no usable energy. The H2O2 is broken down to H2O and O2 by the enzyme catalase that is abundant in all peroxisomes. The acetyl-CoA produced in the peroxisome is further metabolized in mitochondria and cytoplasm to eventually yield sugars, reactions called gluconeogenesis. Minerals such as K, Mg and Fe are stored in the vacuoles of seed tissues as insoluble salts of phytic acid. During germination the enzyme phytase hydrolyzes phytate and the resulting phosphate salts ionize. releasing minerals for transport to growing seedling tissues.