1
Presentation of an Aeronautical Unidirectional Composite
1.1. Introduction
As it stands, the two main materials in use in aircraft structures are aluminum and a carbon fiber-based material. These two materials make up approximately 70% of the mass of the structure of a typical commercial plane such as the Boeing 787 (Figure 1.1).
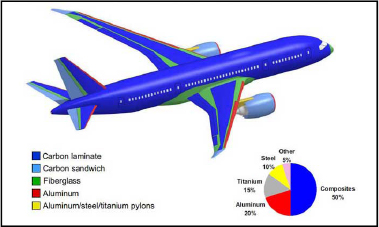
Figure 1.1. Material breakdown of the Boeing 787 (according to http://www.boeing.fr). For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
The remaining structural materials are glass fiber-based composites, sandwich structures (a honeycomb core covered with two composite sheets), titanium, steel, etc.
Keep in mind that the weight ratio of composite materials is of over 50% of the overall mass for the Boeing 787 or the Airbus A350, but more standard commercial aircrafts such as the Airbus A320 or A380 are primarily composed of aluminum alloy, which makes up over 60% of its mass.
1.2. Carbon/epoxy composite T300/914
We will now look at a carbon/epoxy composite that is widely used in aircraft structures, called T300/914. The T300 portion of the name refers to a carbon fiber produced by Toray® [TOR 16], while 914 is a reference to an epoxy resin produced by Hexcel® [HEX 16]. T300/914 is a first generation carbon/epoxy composite that is 50% (in volume) carbon fiber and 50% epoxy resin. It takes the form of a thin fabric (less than a millimeter thick) that can subsequently be cut and draped to obtain a desired thickness (Figures 1.2 and 1.3).
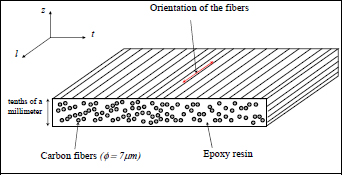
Figure 1.2. Unidirectional carbon/epoxy laminate
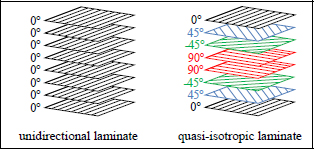
Figure 1.3. Unidirectional and quasi-isotropic laminate
Performing a test along the direction of the fibers, also called the longitudinal direction, we can observe a brittle elastic behavior comparable to the fibers. The elastic limit is obviously lower than that of the fibers, since approximately 50% of the resin has been added, which has a relatively low elastic limit (Figure 1.4). This resin is necessary in order to obtain a less brittle material and to shape it. The carbon fibers are indeed thoroughly interesting elements from the aspect of their mechanical characteristics but cannot be used to adopt a desired geometry. Furthermore, when a crack appears in the material and propagates perpendicularly to the fibers, it will cause a lot of fiber failures and fiber debonding, thus requiring an elevated dissipation of energy; the material will therefore be less brittle (Figure 1.5). In practice, a crack would travel parallel to the fibers if it could, which is why there are plies in other directions, so as to reinforce the material in different orientations of loading (in practice, we can demonstrate that four directions 0°, +45°, −45° and 90° are enough). It is then a case of a composite laminate, as opposed to a composite with fibers facing only one direction, which is called a unidirectional composite (Figure 1.3).
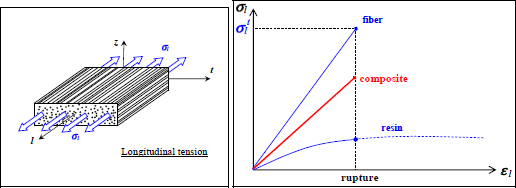
Figure 1.4. Tension along the longitudinal direction of a composite: behavior of fiber, resin and composite. For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
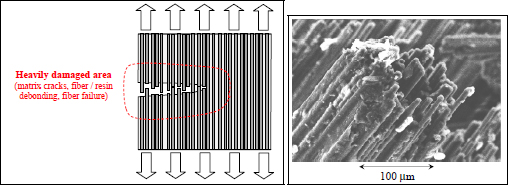
Figure 1.5. Tension along the longitudinal direction: damaged area
Comparing Young’s modulus and the strength of the main structural materials according to density, we note that composite materials are very well positioned compared to the metals (Figures 1.6 and 1.7). Ceramic materials are also very interesting but often too brittle for any structural use.
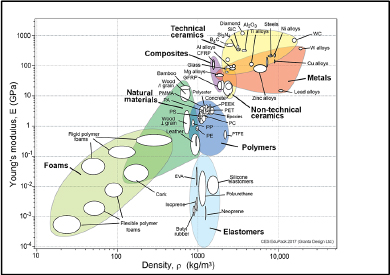
Figure 1.6. Young’s modulus according to density [ASH 00a] CFRP: carbon fiber reinforced plastic; GFRP: glass fiber reinforced plastic. For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
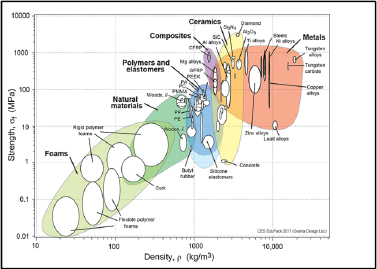
Figure 1.7. Strength according to density [ASH 00a] CFRP: carbon fiber reinforced plastic; GFRP: glass fiber reinforced plastic. For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
The way to better understand a composite material is to take a close look at its composition and microstructure, in particular for epoxy resin.
1.3. Polymers
The epoxy matrix is part of the polymer family commonly referred to as plastics. The word plastic comes from the mechanical behavior of polymers that present plastic strains, i.e. the deformation does not return to its original point when loading is released.
Polymers are composed, as indicated by the name, of chains of monomers linked together with covalent bonds. In this particular work, we will limit ourselves to organic polymers. Keep in mind that organic matter is created by living creatures (plants, mushrooms, animals, microorganisms), particularly by their decomposition. In contrast, inorganic or mineral matter is composed of metals, glass, ceramics, rocks, etc.
Organic polymers are therefore based on chains of monomers linked together by carbon atoms. The carbon–carbon covalent bond is strong and will provide elevated mechanical properties. These carbon–carbon bonds serve as a basis for macromolecules that make up the skeleton of the polymer material. On top of these strong bonds, these macromolecules are linked together via the intermediary of weak bonds (hydrogen bonds, Van der Waals bonds, etc.). It is the deformation of these weak bonds which will induce important plastic strain on behalf of the polymers.
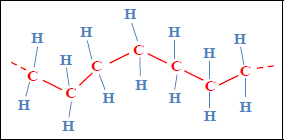
Take for instance, the case of polyethylene, one of the simplest and cheapest polymers. It is made up of the polymerization of ethylene monomers (CH2=CH2) which leads to the creation of long chains. These chains are simply linked together via weak bonds. The mechanical characteristics obtained are therefore relatively weak and depend on temperature.

If we trace, for example, the Young’s modulus according to temperature, we then obtain a characteristic three-part curve that demonstrates the existence of two distinct temperatures points within the studied material; its glass transition temperature Tg (g for glass; this glass appellation will be refined further on) and its melting temperature Tm. Below Tg, the behavior of a material is typical of a solid material; beyond Tm, it takes on a practically fluid (more or less viscous) behavior and in between the two, we observe a rubbery behavior characterized by very low rigidity and a high capacity for deformation. Typically, polymers cannot be used as structure materials, in particular as a resin for a composite material, for a temperature beyond Tg (there are, nonetheless, exceptions which we will see further along).
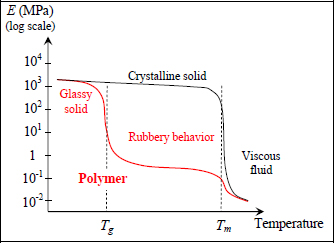
Figure 1.10. Rigidity of a polymer depending on temperature. For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
This rubber-like behavior is made possible because the molecular chains present only weak bonds (hydrogen bonds, Van der Waals bonds, etc.) linking them. They can therefore reorient and align when loaded. This type of polymer is called thermoplastic, because its plastic behavior depends on temperature.
If we want to increase the mechanical characteristics of a polymer, we need to lock the relative movement of molecules by creating covalent bonds between molecule chains; this phenomenon is called cross-linking. We can then describe the polymer as thermoset if the level of cross-linking is significant, while it is referred to as thermoplastic in the absence of these cross-links. We note that the term thermoset stems from the fact that the chemical reaction that allows this cross-linking is activated by temperature; in other words, the material hardens with temperature. This is true during manufacturing, but is no longer the case once the polymer is already cross-linked.
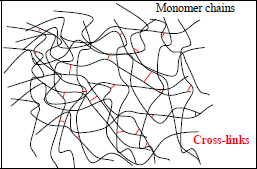
Figure 1.11. Secondary links between monomer chains: cross-linking. For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
There is a third class of polymers, elastomers, that present an intermediary behavior somewhere between thermoset and thermoplastic. Elastomers generally present a low level of cross-linking and these cross-links are realized from sulfur-based covalent bonds (a process referred to as vulcanization). These sulfur-based secondary bonds grant the material a high level of elasticity (nonlinear in general).
Going back to Young’s modulus depending on temperature, we distinguish a thermosetting polymer from a thermoplastic polymer via a less pronounced – if not non-existent – glass transition, and the absence of a melting temperature. The glass transition is a result of the dissociation of weak bonds by thermal agitation. Nonetheless, because thermosetting materials present high levels of cross-linking, this network of cross-links will last beyond Tg and grant the thermosetting material a decent mechanical behavior after Tg. Increasing the temperature further, we reach the material’s decomposition pyrolysis, i.e. decomposition into different forms of gas and residue (CO, CO2, H2, etc.).
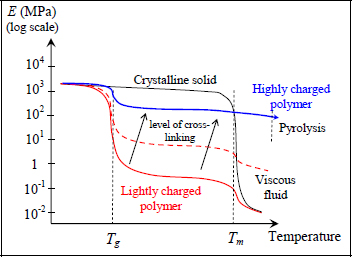
Figure 1.12. Rigidity of a polymer depending on temperature and cross-linking. For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
From a chemical standpoint, this thermoset/thermoplastic difference is due (upon first approximation) to the type of chemical reaction used to synthesize the polymer. Thermoplastics are obtained using a simple reaction of addition or polyaddition (making it difficult to create cross-links), while thermosetting polymers are obtained using a condensation or polycondensation reaction (that induces many cross-links). We can also talk about polymerization reactions, which cover both of these types of reactions.
The presence (or lack thereof) of cross-links in the network of a polymer modifies its mechanical behavior entirely, and also changes the processes for manufacturing and recycling it. Thermosetting materials are obtained once and only once during the polycondensation reaction. It therefore needs to be shaped before this reaction and cannot be reshaped afterwards. Going back to the carbon/epoxy T300/914 composite discussed previously (the epoxy is a thermosetting polymer), it is currently sold in the form of a thin film, 0.125 or 0.25 mm thick, made up of approximately 50% epoxy matrix and 50% carbon fiber (with diameter measuring approximately 7 μm), called pre-impregnated or pre-preg. This designation comes from the fact that the fibers are impregnated with resin beforehand. This resin is composed of monomers and a hardener, which improves the polymerization reaction. In order to avoid polymerizing the pre-preg, it is conserved at a low temperature (typically in the freezer at −20 °C) and it has a short shelf life (generally no more than 2 years). It is then a matter of assembling the pre-pregs into the desired shape for the part required, and curing the whole thing. This curing will serve, first of all, to melt the resin (or more exactly reduce its viscosity) and shape it. This phase is particularly critical as it evacuates any air bubbles trapped in the pre-preg, in particular between the layers of pre-preg which avoids porosity (which can cause cracks, making the material more brittle) within the final material. In order to improve this evacuation of the air bubbles, the thermosetting compound is generally place in a vacuum sealed bag, which is sealed tight to begin with, and then placed under pressure (between 5 and 12 bars) and heated. To achieve this, an autoclave is used (essentially a big pressure cooker), which must obviously be larger than the part that is being treated; something that may be an issue when constructing a wing for a plane.

The temperature will then secondly activate the polymerization reaction (polycondensation in this instance) and consolidate the material. If the composite is heated a second time, nothing else will happen, unless pyrolysis temperature is achieved. It is therefore impossible to shape the material a second time or recycle it. This inability to recycle it and the obligation to keep it at a low temperature over a short period of time are the major drawbacks of thermosetting composites.

Unlike thermosetting materials, thermoplastics present no cross-linking and can therefore be easily reshaped by heating past the melting point. They can therefore be recycled with ease and maintained at ambient temperature with no sell-by date. Their major disadvantage is that they present inferior mechanic characteristics to thermosetting materials. Despite this, they are currently being tested to hopefully replace thermosetting materials in aeronautics. They are also being studied for the automotive industry. They can be used for thermoforming or SMC (sheet molding compound), allowing high volume production at a fast rate. For comparison, the typical curing cycle for a thermosetting compound such as T300/914 is approximately 2 h as opposed to a few minutes for thermoformed thermoplastic. Only the use of thermoplastics is possible for the automotive industry (other than a few high-end vehicles which use thermosetting materials).
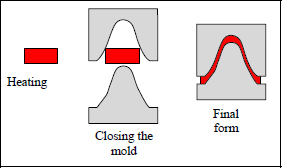
Figure 1.15. Thermoforming (SMC) for curing and shaping a thermoplastic
It is nonetheless possible to increase the mechanical characteristics of a thermoplastic by playing with the morphology of the molecular chains. Simply aligning the molecular chains increases rigidity in the direction of the elongation. Consider, for instance, a tensile test for a thermoplastic polymer (Figure 1.16), we then observe a typical three-phase behavior:
- – Phase 1 corresponds to the linear elasticity of the material.
- – If we continue to stretch, we observe a necking (localized narrowing of the cross-section) and a plastic behavior (if we release the stress, the strain does not return to its original position). We then observe a stress plateau corresponding to the material stretching and the development of the necking zone to the whole specimen. We typically observe maximum strain in the order of 100–300%!
- – Finally, once the whole specimen has been stretched, we observe a strong stiffening of mechanical behavior. If we continue to stretch, we see that the molecular chains begin to break. This stiffening phase is very interesting, because it allows us to obtain a much more rigid material than the initial one.
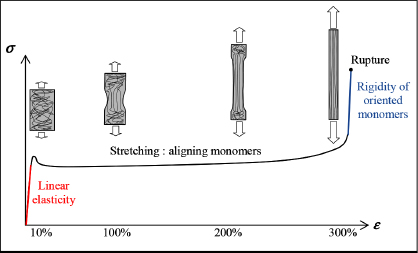
Figure 1.16. Tensile test of a polymer
In practice, this stretching can provide fibers (nylon, for example, is obtained by stretching under liquid form) or thin films that can be stretched in one or two directions. Obviously, this technique does not result in a composite material matrix for which we need rigidity in all directions.
This alignment of molecular chains can also be naturally obtained during cooldown; this is called crystallization. We can demonstrate that to minimize their energy, the molecules need to align in certain preferred directions. This alignment is only possible in the absence of cross-linking, and this is only possible for thermoplastics.
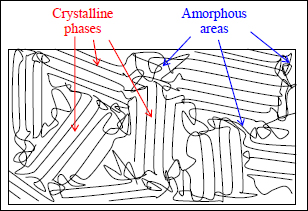
Figure 1.17. Crystalline phases and amorphous areas of a thermoplastic polymer
In practice, the degree of crystallization (or degree of crystallinity), i.e. the proportion of crystalline phase in the material, can reach 80–90% in thermoplastics and depends on the cooldown rate. This level of crystallization is even more important when there is a low cooldown rate; in other words, the macromolecules need time to reorganize if we wish to obtain crystals. If cooldown is too fast, the material will not present any particular order, and the result is an amorphous material: this state is also referred to as glassy. This is obviously where the term “glass transition” comes from, since, as it passes through the glass transition temperature Tg, these crystals disappear and the material return to an amorphous state. It is also in part due to this disappearance of the crystalline phase that the mechanical characteristics of thermoplastics reduce when passing through Tg.
REMARK.– The glass that is commonly used in our windows is a glassy material (but is in no way a polymer, it is primarily composed of silica which is evidently inorganic). It is thanks to that amorphous state that it is transparent since light rays can pass through it without interacting with the crystalline network (which is absent).
To produce a thermoplastic matrix with interesting mechanical properties, an interesting molecule must be selected, and the production process to favor its crystallization established. If we now go back to the evolution curve of Young’s modulus depending on temperature, we note that a highly crystalline thermoplastic presents similar behaviors to a thermosetting material while having a lower Tg. Obviously, this thermoplastic cannot be used too close to its melting temperature so as to avoid reducing its degree of crystallinity and cause it to return to its amorphous state.
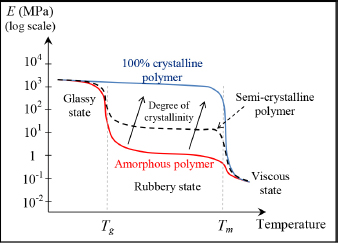
Figure 1.18. Rigidity of a thermoplastic polymer depending on temperature and degree of crystallinity. For a color version of this figure, see www.iste.co.uk/bouvet/aeronautical2.zip
Currently, composite materials in use for structural applications in the aeronautical field are for the most part epoxy matrix-based composites, i.e. thermosets. Two thermoplastic matrices are currently under consideration to replace the PPS matrix (polyphenylene sulfide) and the PEEK (polyether ether ketone) matrix. The advantage of thermoplastics is, as mentioned previously, their ease of recyclability and preservability at ambient temperature without expiring. Another advantage is their improved ductility. Thermoplastics do not have cross-linking and can therefore deform more easily before breaking and will therefore present better ductility. We restate that one of the major inconveniences of composite materials is their brittleness, in particular to impact, and that better ductility of the matrix can reduce that brittleness. Something else to keep in mind is that the ductility of a material can be characterized by fracture toughness, i.e. the energy necessary to create a crack (thus, the use of J/m2 units). Obviously, the higher the fracture toughness, the more energy the cracks need to propagate and thus the less brittle the material.
In order to compare these three materials, here is a comparison table. The values should be used cautiously, in particular the cost that depends on the supplier and the amounts produced, and the level of the mechanical properties that depend both on crystallinity and temperature. The values given here correspond to measures at ambient temperature for high levels of crystallinity and under control during the production process (essentially applying to a material created in good conditions).
Table 1.1. Comparison of the three primary resins used in aircraft structures
| Epoxy matrix (thermoset) | PPS matrix (thermoplastic) | PEEK matrix (thermoplastic) | |
|---|---|---|---|
| Density (kg/dm3) |
1.29 | 1.35 | 1.32 |
| Tg (°C) | 190 | 90 | 143 |
| Maximum useable temperature (°C) | 110 | 100 | 260 |
| Tm (°C) | – | 285 | 380 |
| Young’s Modulus (GPa) | 4 | 3.3 | 3.3 |
| Tensile stress at rupture (MPa) |
100 | 50 | 100 |
| Fracture toughness (J/m2) | 100–500 | 700 | 4,000 |
| Price (€/kg) | 10 | 10 | > 100 |
PPS has the advantage, over epoxy, of having a comparable cost, but presents inferior mechanical characteristics, except for fracture toughness that is much better. Its temperature for use is also much lower than epoxy.
PEEK is also an interesting candidate for replacing epoxy, even though its price remains prohibitive. It can be used up to 260 °C, presents similar mechanical characteristics to epoxy, except its fracture toughness which is much better. It is, however, much harder to use, as it must be heated past its Tm in order to be shaped; in practice, at around 500 °C, while epoxy resin requires a heat of around 190 °C. It should, therefore, in all logic see an increase in use in the field of aeronautics over the next few years, once its fabrication process and costs are better controlled.
The presentation of polymers in this section has been largely simplified and the reader is invited to refer to [ASH 00a, ASH 00b, BER 99, DEQ 12, DOR 86, GAY 97, SES 04, etc.].