2
Roots
Roots are often an understudied component of plants. Their largely underground nature results in an ‘out of sight, out of mind’ attitude among people, and the inherent difficulty in accessing them makes those who study them resort to increasingly creative approaches.
However, roots and the underground processes to which they contribute should not be taken for granted. Like leaves, their above-ground counterparts, roots supply required resources to the plant, often through a number of unique adaptations to their environment. Unlike leaves, roots can, in many cases, directly modify their environment through growth, chemical interactions and associations with other organisms, making the soil environment a bustling epicentre of activity invisible to the naked eye.
Deepening our understanding of roots – including where they are located (not always where you would expect), what they are doing and how they adapt to their surroundings – will hopefully increase our appreciation of these important plant structures.

The purpose of roots
Roots serve necessary functions for the plant, including resource acquisition, storage of those resources and anchorage to the substrate. During the evolution of roots from underground rhizomes over the last hundreds of millions of years, they have adopted a multitude of adaptations allowing them to deal more efficiently with their environment. Considering the wide range of habitats in which we find current true roots of terrestrial plants, we can begin to appreciate the competitive and heterogeneous environments that affect root morphology and physiology in a way that favours one or more root functions.

One rarely gets a glimpse of plant roots – in a way, they are like the unseen portion of an iceberg. In the case of many ficus trees (Ficus spp.), however, a portion of the root system can be seen growing above ground. Here, the root system spreads laterally far beyond the canopy to support this massive Ficus tree.
Root evolution
During the Devonian period, spanning the period 416–358 million years ago, plants that occupied land underwent immense changes. In particular, the evolution of roots impacted nutrient cycling and soil formation, thus affecting plant development and spread. The earliest ‘roots’ within the fossil record were discovered in present-day Scotland, on such species as Horneophyton lignieri and Nothia aphylla, and are estimated to be more than 400 million years old. These early structures were most likely not true roots but rhizoids, unicellular protuberances extending from a rhizome, or underground stem. In fact, most early root structures appear to have arisen from stems that grew under or along the ground and behaved physiologically as roots. In contrast, true roots are defined by their endogenous origin (the pericycle), the occurrence of a root cap, and the presence of an endodermis and protostele. In extant bryophytes, rhizoids are still found on the gametophyte stage, and even though they lack a true vascular system, they behave in a similar way to roots (see box). Interestingly, the genes that regulate rhizoid development in mosses are similar to the gene network regulating root-hair formation in the sporophyte stage (the multicellular generation) of vascular plants.
As time progressed and new, larger, vascular plant species arose, a variety of root types evolved to serve the new functional requirements of support in addition to transport and uptake. It is quite difficult to ascertain a clear timeline for this development as roots are less frequently preserved within the fossil record. However, as roots evolved and plants became larger, particularly with the continued evolution of trees (including the prehistoric genera Wattieza and Archaeopteris), root biomass increased, contributing immensely to the formation and weathering of soil through organic acid secretion and carbon fixation.
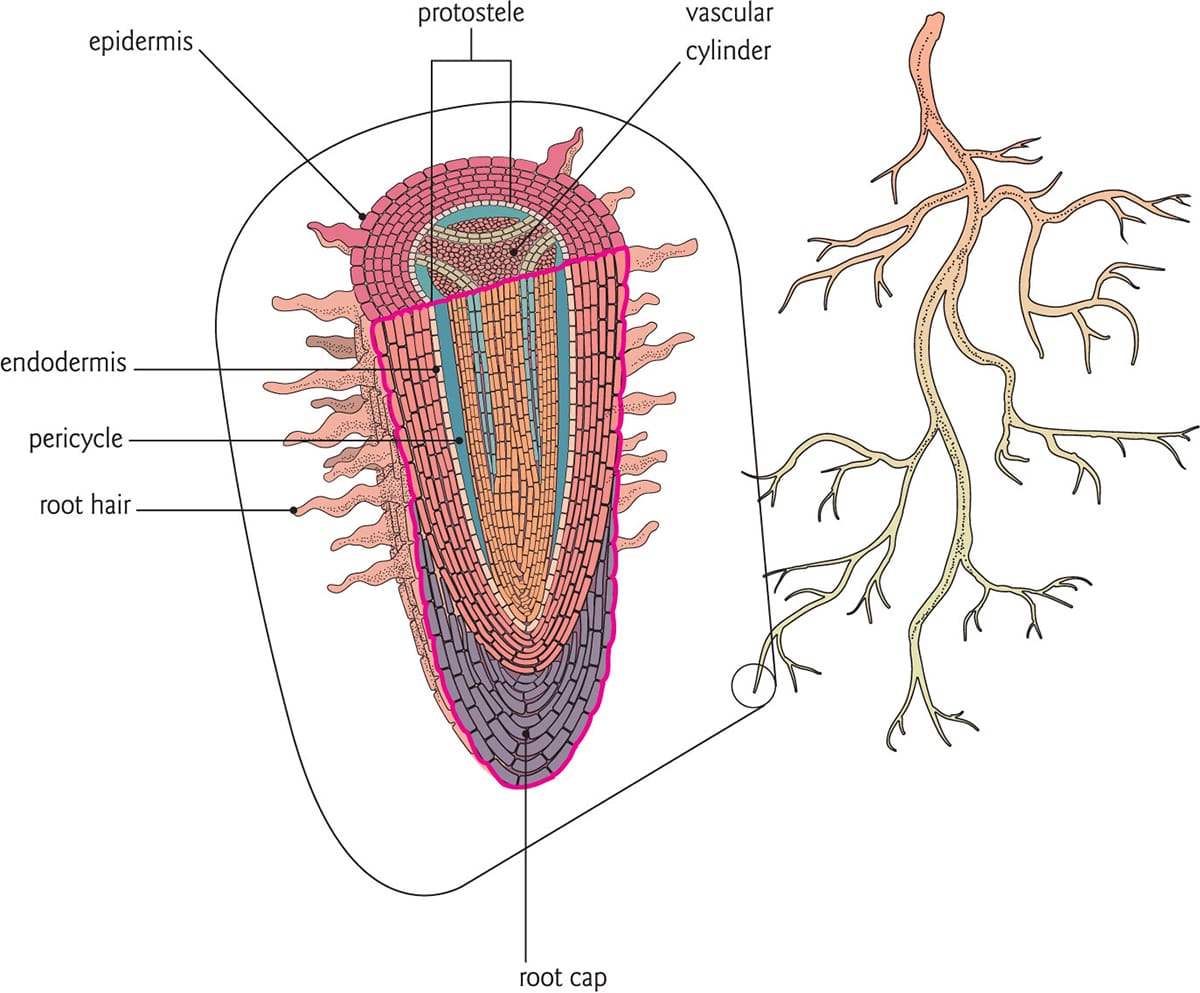
Anatomy of a root. Roots evolved to serve unique functions of support and uptake for the plant. Non-woody roots can anatomically be distinguished from stems by the presence of a root cap, endodermis, pericycle and, in some cases, root hairs.
The multiple functions of roots
To those who do not study roots, it may appear that a root is just a root. However, root systems are made up of diverse types of roots that serve multiple functions for the plant. Generally, root types are divided into the coarse roots, whose primary functions include stability, storage and transport; and the fine roots, the most distal (distant from the centre) portions of the root system, which are largely involved in water and nutrient uptake.
It may seem mundane to mention the importance of coarse roots for anchorage, but ecologically this function is attained through the compilation of several complex root characteristics, including shear strength, branching angles, root length and the amount of secondary thickening. In order to build a ‘strong’ root system that withstands uprooting, plants must invest considerable amounts of carbon into it. In other words, a strong root system comes at a price to the plant. In many cases, however, this cost is worth the sheer benefit of staying upright. For example, trees that grow on a slope must ensure proper anchorage of their root system to prevent an undesirable incline downhill. In fact, trees will change the shape of their root system in such situations to a more asymmetrical form, with enhanced root production on both the upward and downward slopes to provide extra stability in such uneven situations.

Loading forces on the stems of trees are transferred to the roots and result in modified root growth patterns. Coordination over the entire root system is required to maintain stability in uneven environments.
Coarse roots are also well equipped to transport and store water and nutrients acquired from the finer roots throughout the soil profile. Interconnected xylem and phloem cells that run the length of the plant allow the water and nutrient resources to be allocated from roots to shoots, or even from roots to other roots, as can be the case in internal water redistribution. In larger woody roots, anatomical shifts may occur, as in the production of xylem cells with smaller diameters during extended periods of drought to help protect the tree from mortality.
Of the entire root system, the finest roots are the most specialized for water and nutrient uptake. The membranes across which water moves from the soil into roots are highly permeable to allow for the largely passive process. However, not all root systems can acquire the same amounts of water. Factors such as root age, levels of suberin (a waxy substance) and connection to the soil can all drastically affect the ability of a plant to take up water. The uptake of solutes or nutrients, on the other hand, is driven by plant metabolism and to a large extent is controlled by the plant.

Root systems are made up of diverse root types and sizes, which serve multiple functions for the plant. Small changes in root diameter can indicate large differences in function within a root system.
All plants require the same nutrients for growth, reproduction and maintaining proper function. As nutrients are taken up by the plant, their concentration in the soil naturally decreases, resulting in a gradient that encourages the diffusion of nutrients from higher concentrations in the soil toward the lower concentrations just outside the root. The specialized proteins at the root surface that move nutrients into the root vary across plant species, and nutrient uptake itself can be quite a complex process that depends on protein regulation, nutrient concentration and the properties of the soil. Physiological changes in roots in patches of high nutrient concentration in the soil allow root production and uptake rates to be increased in order to take advantage of these resources.
Complex coordination between above- and below-ground organs regulates multiple plant functions, including nutrient and water uptake. The functional attributes of coarse and fine roots aid the plant’s response to its soil environment.
Root hairs
Root hairs are extensions of single epidermal cells that have a very thin cell wall and so are tightly coupled to their environment, making them ideal for gas exchange and water and nutrient uptake. Additionally, the small diameters of the hairs allow them to reach soil regions that thicker roots cannot penetrate, thus making them even more important for accessing nutrients that do not readily move through the soil.

The radish (Raphanus sativus) radicle produces dense root hairs upon germination to aid in soil contact and therefore nutrient and water uptake.
The occurrence of root hairs
Despite the specialized function of root hairs, they are not found on all higher plants. For example, while most flowering plants have root hairs, gymnosperms are less likely to form root hairs and most aquatic plants have few to none. Moreover, plants that form an association with ectomycorrhizal fungi are less likely to form root hairs, probably because the fungi can encase the entire root, thus covering any existing hairs.
In the large number of species that do produce root hairs, there can be substantial variation in their size and number. For example, a single plant can produce up to 14 billion root hairs, but that number can be drastically decreased in plants that are rooted in soils with high levels of phosphorus, high pH or low soil moisture. Root-hair formation is also influenced by the aeration of the growth medium. Submerging root hairs in water severely truncates their lifespan and usually results in disintegration. This could likely be why they are absent in many aquatic plant species.

Coloured scanning electron micrograph of a germinating radish, showing the developing root (radicle) and root hairs.
Root hairs as a gateway
Root hairs serve as the main site of entry for microorganisms into the root system. Most likely, infection occurs here both due to the lack of a cuticle or protective outer tissue, and the higher levels of oxygen produced through respiration compared to other areas of the root system. Nitrogen-fixing microorganisms in particular infect the root through the root hairs. So-called ‘shepherds’ crooks’, a sharp bend or curl that occurs when a root hair is infected with bacteria, are a response of the hair to hormone signalling; they vary depending on the stage of root-hair development at the time of infection.
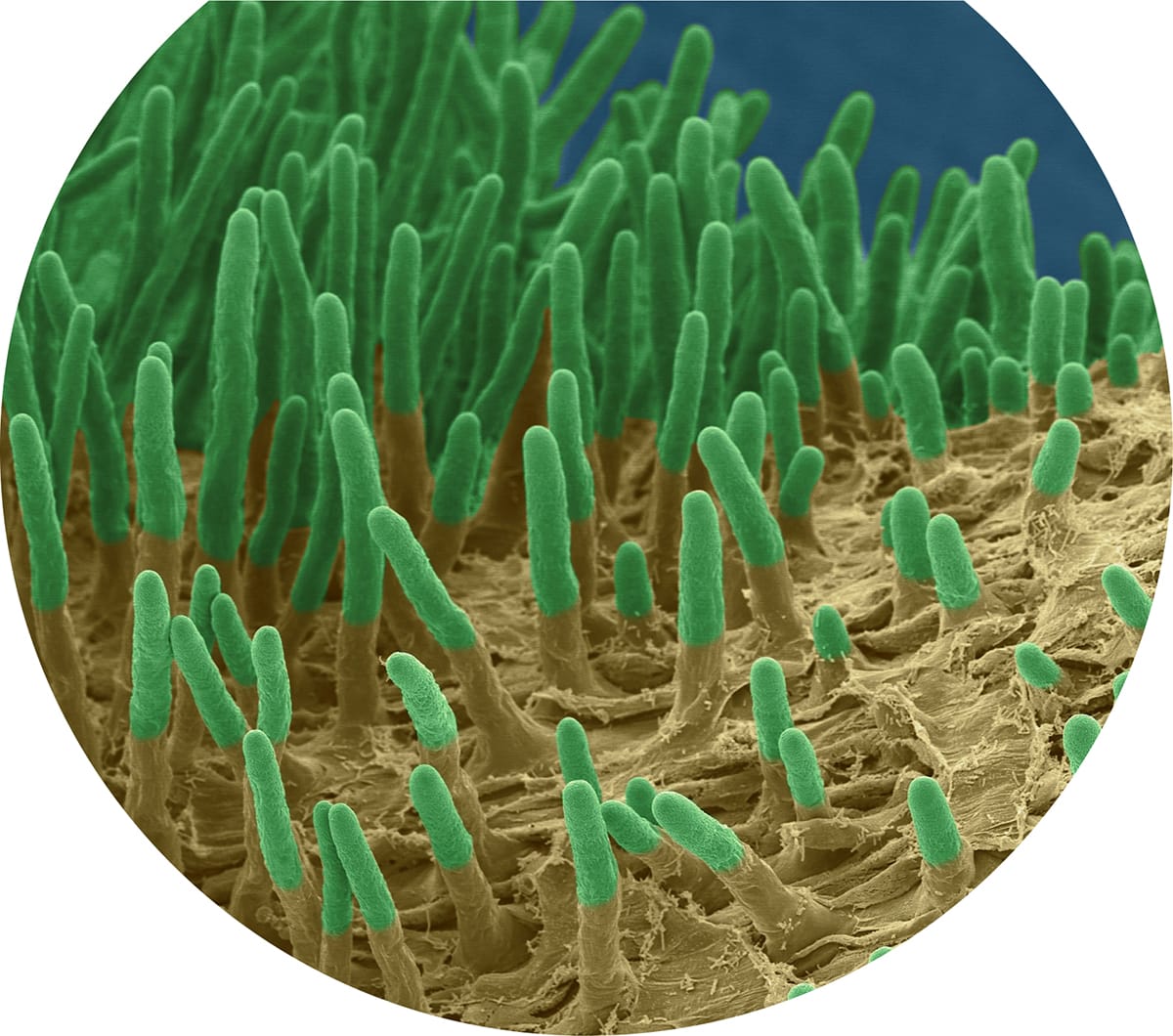
Coloured scanning electron micrograph of elongated epidermal cell root hairs of a radish (Raphanus sativus). Root hair cells can grow at a rate of more than 1 μm/minute, greatly increasing the surface area of roots over a short space of time.
Evolutionary remnant or specialized function?
Some researchers have reported the occurrence of stomata, the pore-like structure normally found on leaves (see here), within the root-hair zone. Because these structures are regulated by light, humidity and carbon dioxide, the lack of light underground prevents stomata found on roots from opening and closing, and instead they remain permanently open. The function of these root stomata is puzzling. In an evolutionary context, root hairs are closely related to their rhizoid counterparts, suggesting they are a vestigial structure. Often the stomata are lost as the root ages, making it possible that they function to aid increased gas exchange within the root-hair zone during root maturation.
Roots are not always where you expect
Determining where roots are located can sometimes be trickier than expected. Generally, we find roots in areas where resources (water and nutrients) are ample. But because resources can vary across environments, it is important to consider root distribution as a three-dimensional structure that changes with time as resources are utilized or become available. For example, in arid environments where water is located at depth, roots must grow deep to access water. Overall, roots must explore their environment to optimize resource uptake through their root placement.
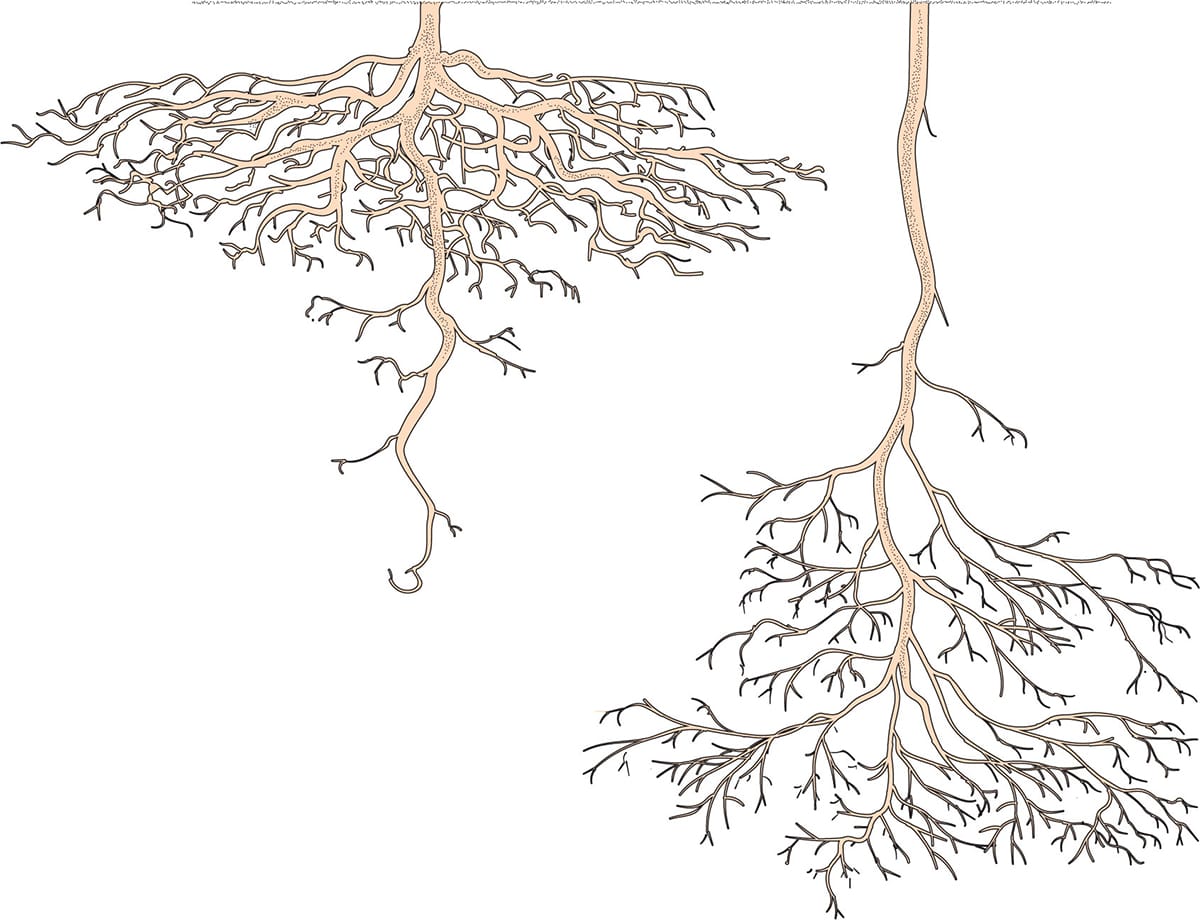
Root system architecture can vary greatly in habitats with differing soil resource availability. No single root architecture is optimal for all resources, but in general shallow roots have better access to nutrients, while deeper roots can access more stable water resources deeper in the soil profile.
Root branching
Root architecture refers to the spatial distribution and branching patterns (topology) of a root system. There are two main forces at play in determining the architecture of any given root system: those that are a direct result of genetic makeup (endogenous), and those that are a response to the biotic and abiotic environment (exogenous). Unlike animals, plants continuously grow new organs throughout their life and adjust their growth in response to their environment. Thus, root architecture can vary substantially both within and between species.
As a means to understanding the architecture of roots quantitatively, biologists borrow from fractal geometry to describe the arrangement of repetitive root branches. Standardizing how root branches are arranged allows studies on root responses to varying resource levels and provides a way of comparing branching structure with plant performance. Included in the parameters that are used to quantify a root system’s architecture are, commonly, the growth angle of new lateral roots, the rate of root growth and the maximum depth to which the root system develops. These three traits alone can help predict root system resource acquisition strategies. By linking the root system’s ability to occupy a three-dimensional space and the known resource limitations of an environment, it is possible to determine the efficiency by which roots will access water and nutrients.
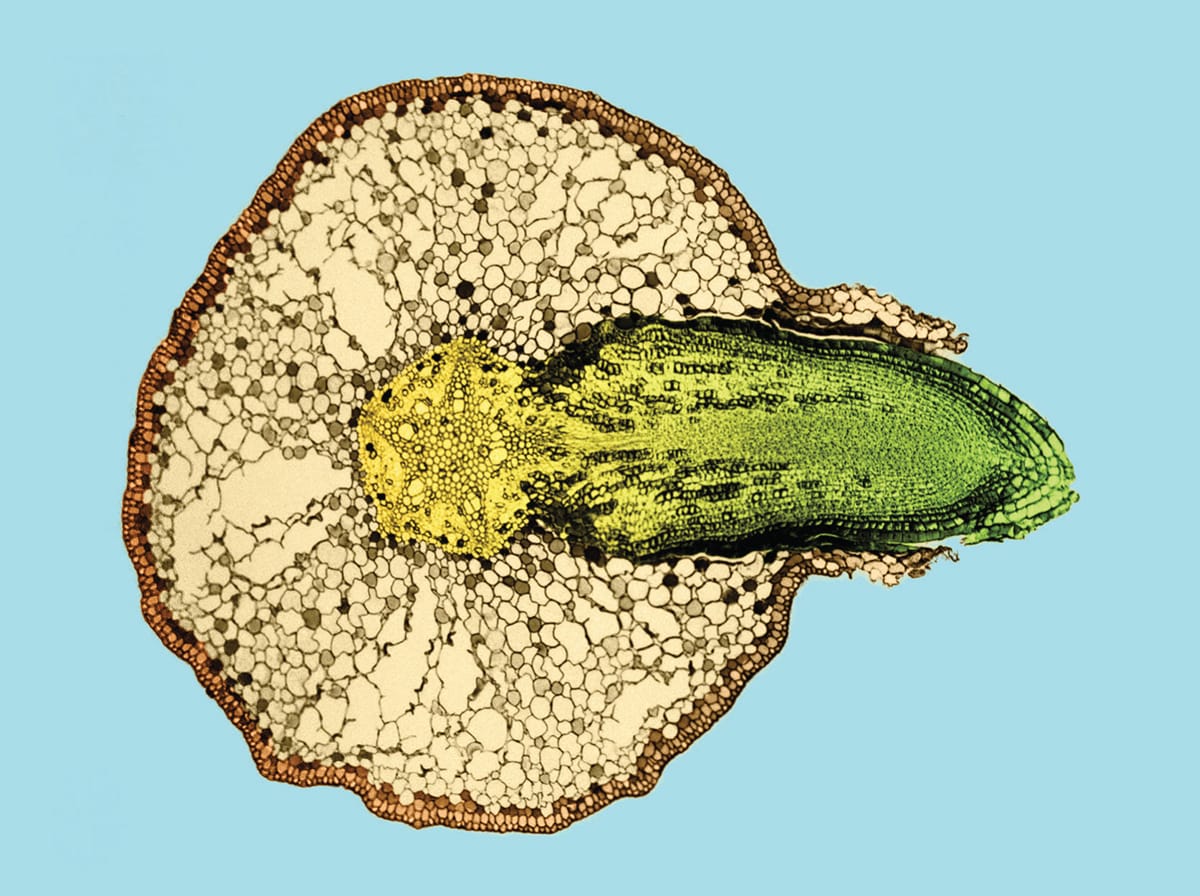
Colour enhanced light micrograph of a willow (Salix sp.) root cross section with an emerging lateral root. The arrangement of lateral root initiation is tightly regulated by the plant’s vascular tissues. Mechanical and chemical factors can alter the root’s development and ultimately change its elongation rate and/or branching angle.
Links to global food supply
Given the ever-increasing requirement for global food supplies, scientists are continually working to improve crops by selecting for traits such as root branching that will optimize a plant’s ability to access resources efficiently. Natural variation within a species’ root growth and branching angle can allow crops to be grown in many areas of the world where poor soils and limited fertilizer availability constrain people’s access to food. However, much more work needs to be done in order to optimize the growth of plants facing multiple stresses in a field setting that requires sometimes diverse root architectures.

Plants can behave quite opportunistically and establish roots in areas of high resource availability, such as shallow soil layers. Soil erosion has exposed the shallow roots of this tree, located in an urban environment.
Root depth and spread
It is difficult to find a commonality across all species that describes the development and spread of roots throughout a plant’s lifetime. Perhaps the initial germination of the seed and production of the first root is a fairly common attribute, but after that all bets are off. What we do know about root location has mostly been acquired through controlled environment studies on plants grown in pots. Large, destructive trenches have also provided clues in field-based settings, but frequently it remains difficult to locate roots precisely in their soil environment. The lack of soil uniformity is a major driver in determining where roots grow. Generally, a large fraction of a plant’s root system will be concentrated in the shallower soil layers, since this is where the majority of nutrients are located in most ecosystems.
No single root architecture is optimal for all resources within the soil. Interestingly, the shallow, long root architecture found in the common bean (Phaseolus vulgaris) and other species, which may be desirable for reaching resources that do not move readily in the soil (such as phosphorus), is in contrast to the deeper root branching that is beneficial for accessing water. It is not uncommon to find portions of the root system of a woody species at depths greater than 5 m (16 ft). These deep roots contribute substantially to meeting the plant’s daily water needs and can even add to the ecosystem’s water balance. In extreme cases, roots have been found growing into deep (20 m, or 65 ft) underground caves that foster a continual water supply through a network of subterranean streams.
Horizontal root spread can also reach surprising distances. Roots of mature trees, for example, can often span two to three times the radius of their crown, allowing them to reach potential favourable environments for root growth far from their own habitat. In urban environments, this lateral root spread can become problematic for underground utilities and sewer pipes in particular. As with the natural caves mentioned above, roots can grow into breaks in water-filled pipes and soon proliferate, causing a blockage in flow and a headache for the municipality.

Major advances in the breeding of crop species such as the common bean (Phaseolus vulgaris) have improved root architecture to deal with low soil fertility, including in tropical areas.
The idea of the root niche
Competition between plants is a ubiquitous feature across both cultivated and natural landscapes. In the context of below-ground root interactions, the effect of competition for, or sharing of, resources among plants is poorly understood. Yet it has been argued that the placement of roots and their overlap with neighbouring plants is a dominant factor in determining how plant communities are structured. This competition for resources can play a role in where roots grow, either to avoid the competition (i.e. in a location other than where the roots of other plants are growing) or to access ample resources (i.e. proliferate despite the presence of other roots). In some plant communities, plants shift where they place their roots, moving them deeper in the soil to reduce overlap with neighbouring species. The North American burro-bush (Ambrosia dumosa) is known for inhibiting its root growth when an individual root contacts another root of the same species. Other species such as the creosote bush (Larrea tridenta), a desert shrub also native to North America, are even more extreme, completely halting root growth by inhibiting elongation when the roots of other creosote bushes are nearby.

Underground caves can serve as a major contributor of water resources to woody plants. Roots of vines are seen here descending into a deep water source in Mexico.
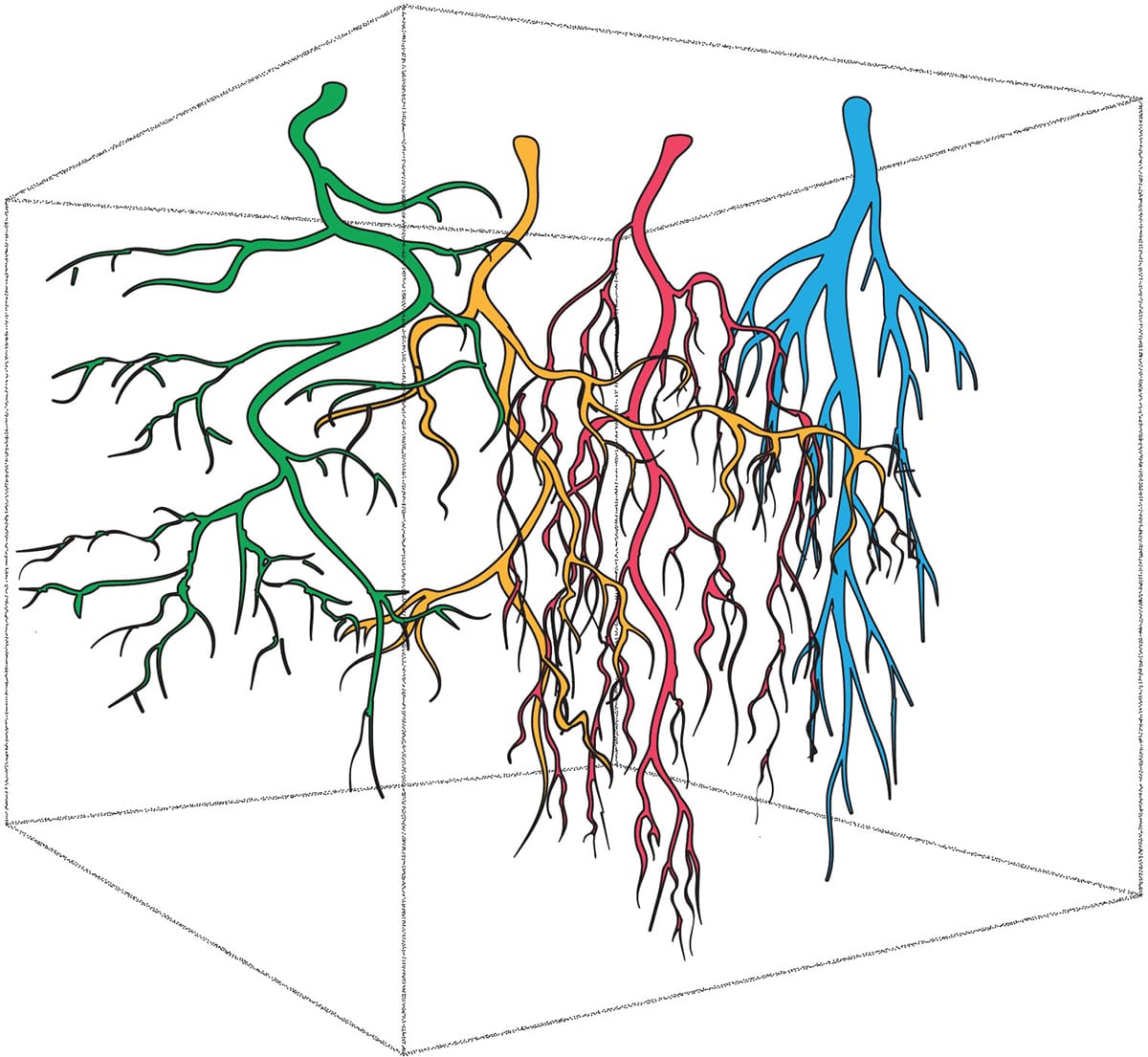
In areas with multiple plants, as represented in this diagram by the multiple coloured root systems, differences in root system architecture inherently lead to differences in root space occupation below ground. How root systems interact depending on the proximity and identity of their neighbours is often difficult to ascertain.
Root growth
The rate at which roots grow is determined by the rate at which new root cells are produced and elongate. The root cap plays a major role in determining a root’s movement through the soil and can even respond to non-biological stimuli. However, in order for roots to grow into a new soil area, they must exert pressure on the soil. If the mechanical impedance of the soil is too great, then roots may increase in diameter rather than length, inhibiting their capacity for exploration.
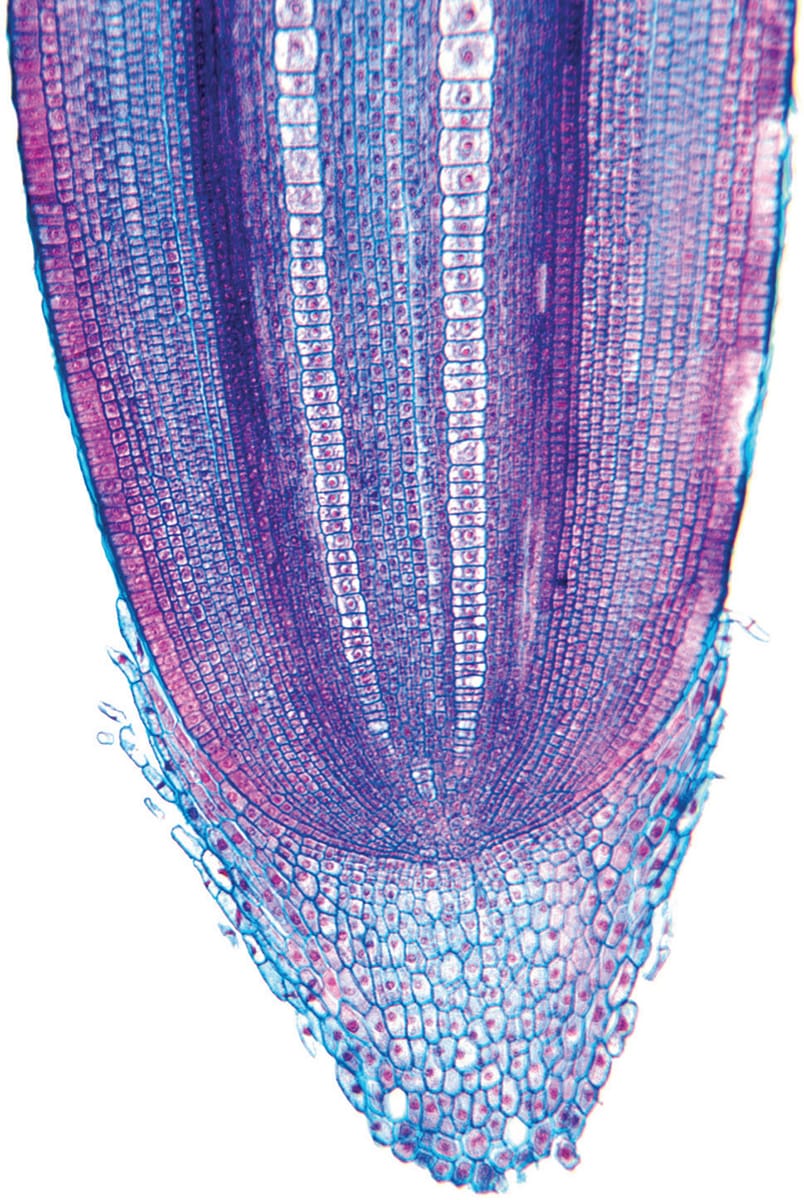
Primary root tip and root cap of maize (Zea mays). The root cap protects the growing root tip and serves in gravity perception; its removal can result in random directionality in root growth.
Mechanical impedance in the soil
Roots are built to navigate their way through the resistance of the soil matrix. This soil resistance is important, however, as without it plants would not have the required stability to remain upright, particularly during windy weather. But roots can be lazy in a manner of speaking, generally taking the path of least resistance and following cracks or large pores in the soil. For roots to push through denser soil, they require a pressure that exceeds that of the soil resistance. If the soil impedes root growth, then thick, stunted and deformed roots are produced. Root functionality will also likely change if roots do not grow and develop normally, most likely resulting in stunted growth of the plant above ground as well.

This cauliflower plant (Brassica oleracea) has minimal thickened roots as a result of high mechanical impedance in the soil. Club root symptoms, a result of fungal infestation, are also evident on the bulbous root in the foreground.
The role of the root cap in root growth
Unlike stems, roots have a group of cells produced from the actively dividing cells of the meristem at their tip. These cells, collectively called the root cap, primarily function to protect the root tip as it moves through the soil matrix. The root cap helps to protect the root by secreting a viscous substance (mucilage) as a means to aid its ability to move through the soil. But the root cap also has additional functions, including signal perception of its environment. While there is still much to be learned about how this signalling works, we know that roots are capable of growing toward water and away from toxic metals, indicating that they can change their growth patterns in response to interactions between the root cap and external stimuli.
As roots grow through the soil, border cells of the root cap are shed, leaving behind a sheath of cells and mucilage that aid the retention of root-to-soil contact and create what many refer to as the rhizosphere (see here). The meristematic region mentioned above continually replaces these lost cells with new ones throughout the root’s growth through the soil, until the root reaches the end of its elongation phase.
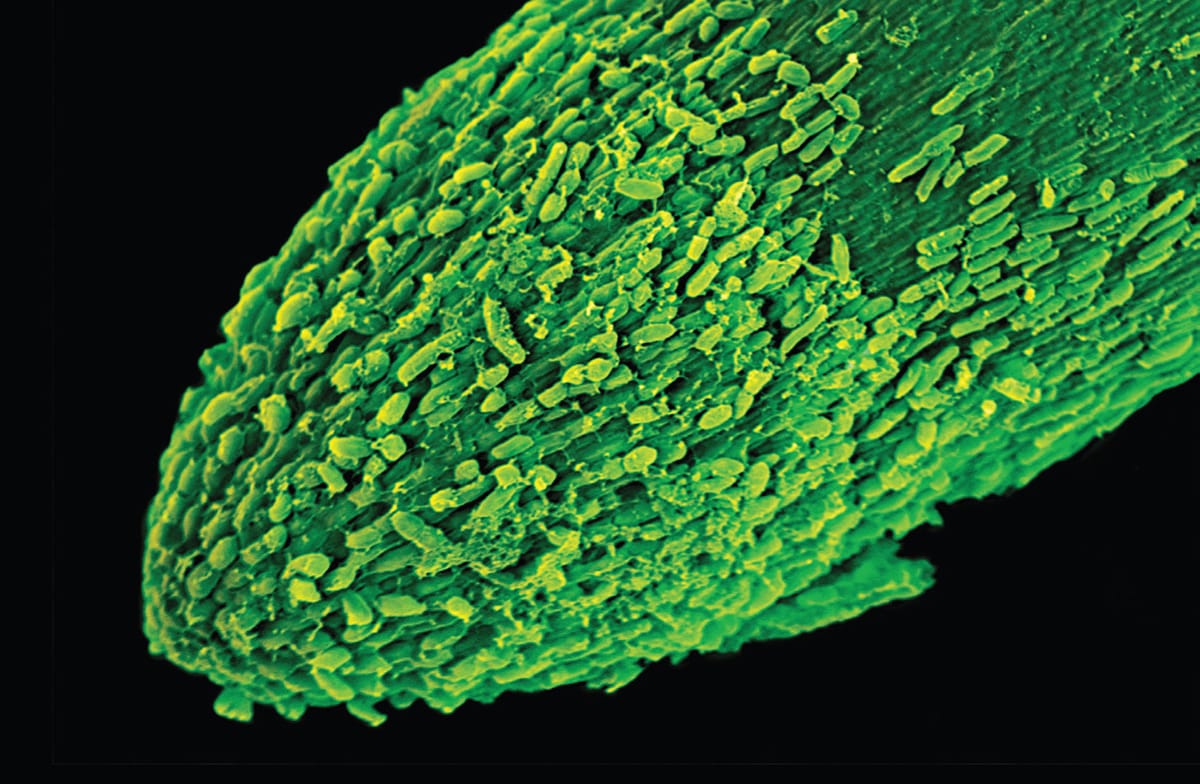
Scanning electron micrograph of the root cap of a maize plant (Zea mays), showing border cells being shed (magnification ×270). Border cells are metabolically active cells that aid the root in moving through the soil. This root tip has been growing for only a few days.
Root dynamics
Fine roots – those that are most distal from the plant – are the most ephemeral root type. When roots are produced, how long they live and when they die can have large implications for root system function and overall plant health. Due to the simple primary development of fine roots and the low levels of secondary compounds they contain, they tend to deteriorate with age and turn over at a higher rate than their woody counterparts, and are subsequently more prone to attack by pathogens and herbivores.

Cacti are well known for producing largely ephemeral root systems. During periods of rainfall, the plants produce roots to take up water, but then shed them during dry periods.
Every root has a lifespan
Production and lifespan of these finest roots are controlled by both endogenous factors (e.g. diameter and mycorrhizal associations) and environmental factors (e.g. temperature, soil moisture, nutrient availability and rooting depth), although the importance and role of these factors vary considerably over species and environments. In general, the roots of evergreen species have the longest lifespans, living for several years, whereas those of annual species and desert cacti are extremely ephemeral, perhaps living only during the season of ample precipitation to aid water uptake. In general, roots that have a mycorrhizal association (see here) benefit from a longer lifespan, likely due to the buffered nutrition and water status of the root.

Tree root systems are quite different from those of herbaceous plants. Woody roots clearly have very long lifespans, and the plant has invested substantial amounts of energy (carbon) into their production and growth, in the form of secondary compounds such as lignin and suberin.
Phenology of roots
It is easy to assess the growth and mortality of leaves, whether during major senescence events, such as the autumn months for temperate forests, or continual leaf regeneration in more tropical ecosystems. Moreover, quantifying shifts in these phenological, or life-cycle, stages in response to environmental variables can provide clues to fluctuations in leaf carbon gain – the ability of leaves to produce energy through photosynthesis – and therefore resource availability. Timing of root production and mortality, on the other hand, can be more difficult to quantify. Some level of root birth and death occurs continually, but these events can easily go unnoticed in the absence of direct root observation. While some studies on temperate tree species have found that root production and mortality are highly synchronized with foliar production, whereby root systems grow and expand prior to leaf growth in order to support necessary water and nutrient uptake, others have been unable to render clear connections in production or mortality between the above- and below-ground portions of a plant.
Roots as underground storage organs
All plant organs can serve in a storage capacity to some extent, essentially reserving carbohydrates and water for growth and maintenance of the plant body. Of all plant organs, however, roots contain the highest concentrations of carbohydrates and are therefore an extremely important energy substrate. Large fluctuations in the accumulation and utilization of storage reserves occur naturally, but stressful environmental conditions can deplete them rapidly as they are used to repair or regrow tissues or organs. If stress is prolonged and reserves become critically low, the consequences will likely be detrimental to plant survival.
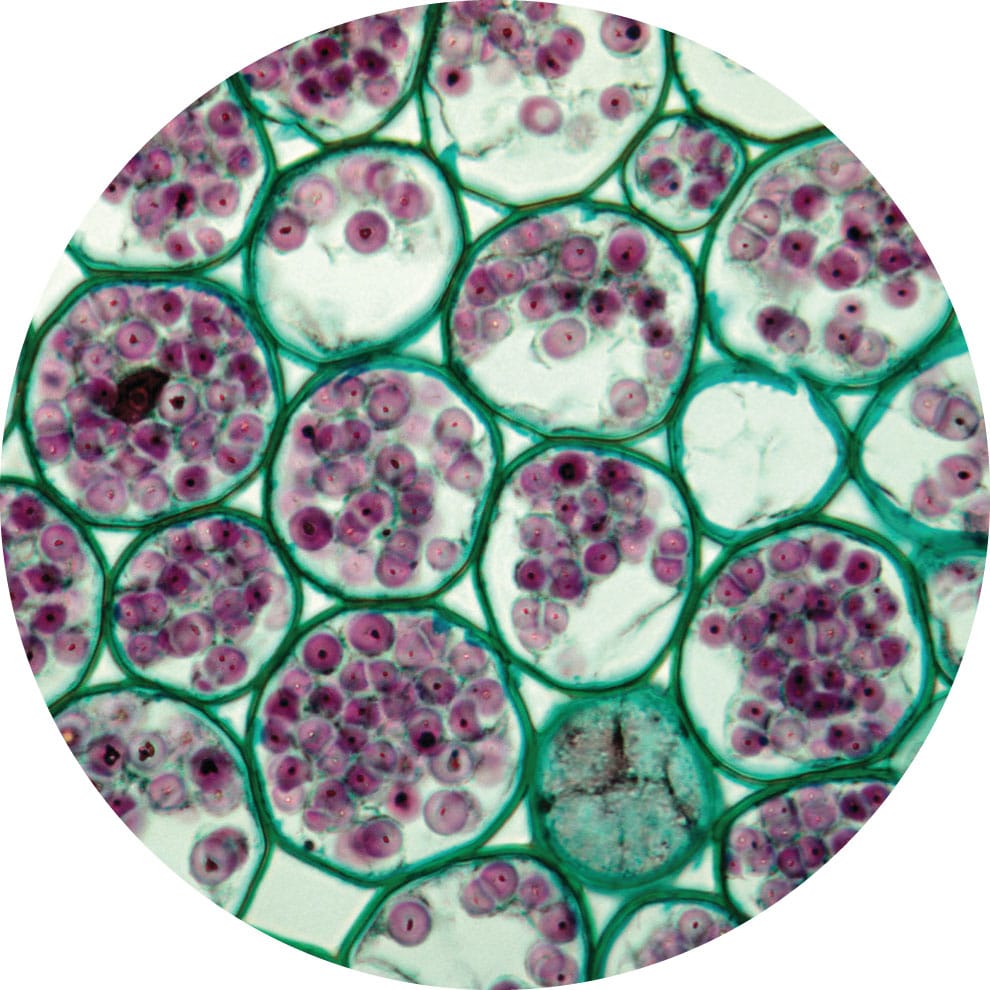
Parenchyma cells of the cortex of a buttercup (Ranunculus sp.) root, containing starch grains stained purple for visualization (magnification ×100).
Water and carbohydrate reserves
Roots that store precious energy and resources in the form of both soluble and insoluble compounds and/or water can vary from morphologically indistinct (e.g. the roots of the apple tree, Malus domestica) to morphologically distinct (e.g. the large, swollen fleshy organs of some succulents, including the soapweed yucca, Yucca glauca). Starch is typically the main form of stored carbohydrate in woody perennials, but many other forms of sugar can also be utilized as a storage reserve. Regardless of the form of carbohydrate used for storage, the parenchyma cells retain it in high concentrations for the plant.
Weedy species are often persistent due to the fact that they can store large amounts of carbohydrates in their root systems. Therefore, when only the above-ground portion of a weed is removed, the plant can easily regenerate from its root reserves. A successful agricultural practice to remove weeds is repeated tillage of a field, which forces the plants to resprout several times, thereby depleting their reserves and resulting in carbohydrate starvation.
In the case of water storage, roots contain increased amounts of the wax-like substance suberin, which retards the loss of water once absorbed. These specialized roots are also capable of dilating to prevent the breakage of cells or tissues during the swelling that naturally occurs during larger water uptake events.

Many economically important food crops are enlarged root systems with ample carbohydrate storage. Here, cassava (Manihot esculenta) roots, which are high in starch and other carbohydrates, are harvested in Ecuador.
Adventitious roots
Inevitably, roots can arise from two origins: the embryonic root that forms during normal plant development, or tissues such as stems or leaves that were not predetermined root tissue. It is these latter roots, which form from an organ other than the embryonic root, that are classified as adventitious. Fossil records indicate that in evolutionary terms, underground adventitious roots were likely some of the first roots that plants formed. Despite differences in origin, roots and adventitious roots are functionally similar, both structures serving to supply the plant with water and nutrients.

Tulip (Tulipa spp.) bulbs produce adventitious roots at the base of their modified stem.
Adventitious roots of terrestrial plants
In extant species, unlike early plants, adventitious roots are frequently formed only as a response to wounding and generally above ground, therefore requiring lager anatomical changes within the plant. Above-ground root formation can clearly be advantageous to plants as these roots can exploit new environments that might not otherwise be accessible. However, the ability of plants to form adventitious roots varies quite widely from species to species, with added complexity depending on the plant age and tissue type. As a general rule of thumb, groupings can be made between plants that can or cannot form adventitious roots, and those that can whether or not they require wounding to initiate production.
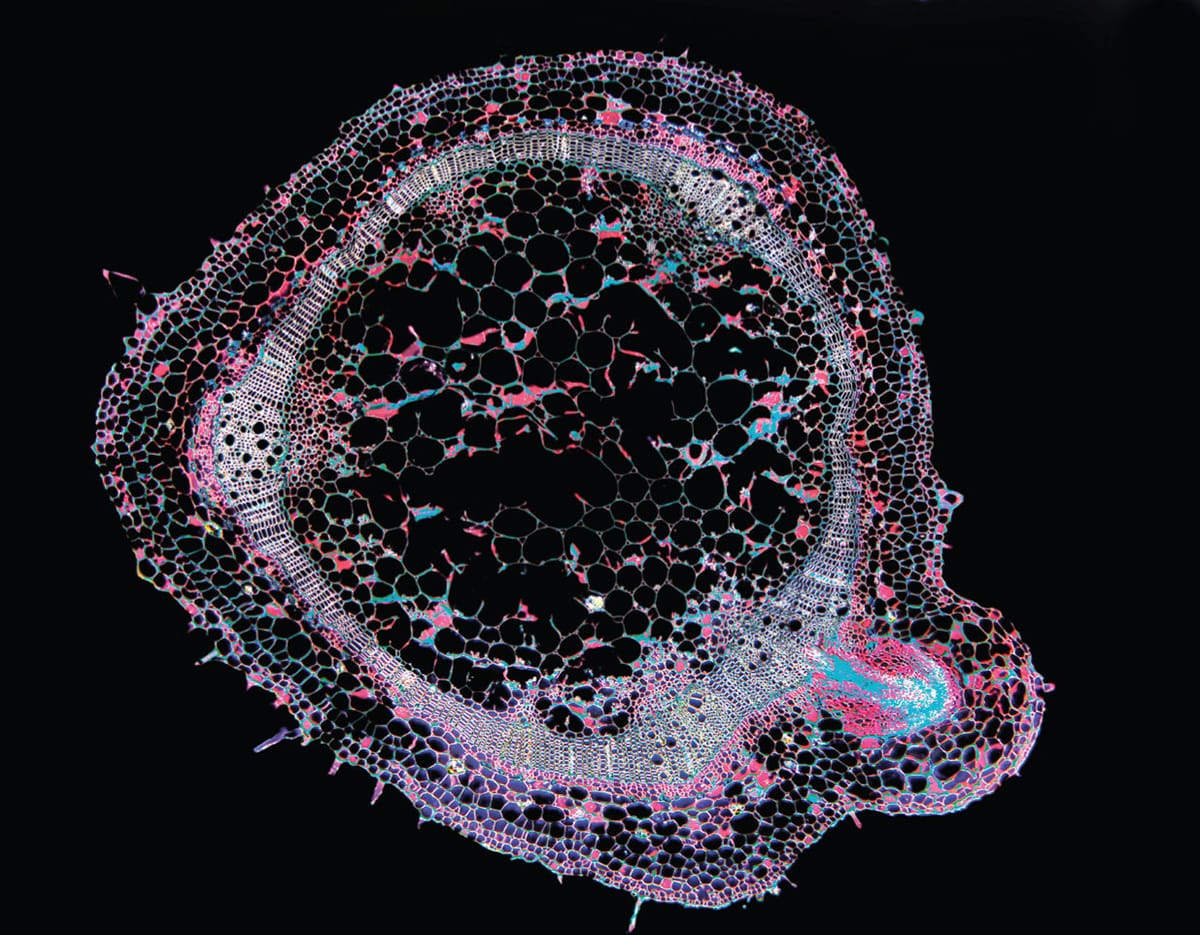
A cross section of the stem of a tomato plant (Lycopersicum esculentum), showing the development of an adventitious root. In the tomato, adventitious roots usually develop only if the stem is buried or subjected to flooding.
Rising from the stem
Most commonly and naturally, adventitious roots that arise from the shoot are formed at nodes and allow for increased plant spread. Common agricultural species such as the strawberry (Fragaria × ananassa) and the undesirable field bindweed (Convolvulus arvensis) are examples of plants that root abundantly from the nodes found on their stolons (horizontal above-ground stems) or rhizomes.
Horticultural advantages to adventitious rooting
Horticulturalists have taken advantage of the ability of plant cells to regenerate and form new adventitious root tissues through wounding. Plant propagation techniques can induce adventitious roots through the application of careful ratios of plant hormones (namely, auxins and cytokinins). These new roots can form on plant stems, petioles and even leaves, and play a key role in producing large numbers of new plants in a fast, efficient manner. Many gardening, forestry and food crops are produced using such rooted cuttings.
Root adaptations
While the soil habitat in which roots reside is in some regard buffered from environmental extremes, roots nonetheless encounter a multitude of limitations and stresses during their growth and development, including shifts in nutrient and water availability, high concentrations of heavy metals, extreme temperatures, increased salinity and mechanical resistance. Root adaptations to these stressors have resulted in a number of unique morphologies, which aid the roots in their ability either to tolerate or escape the unfavourable environment.
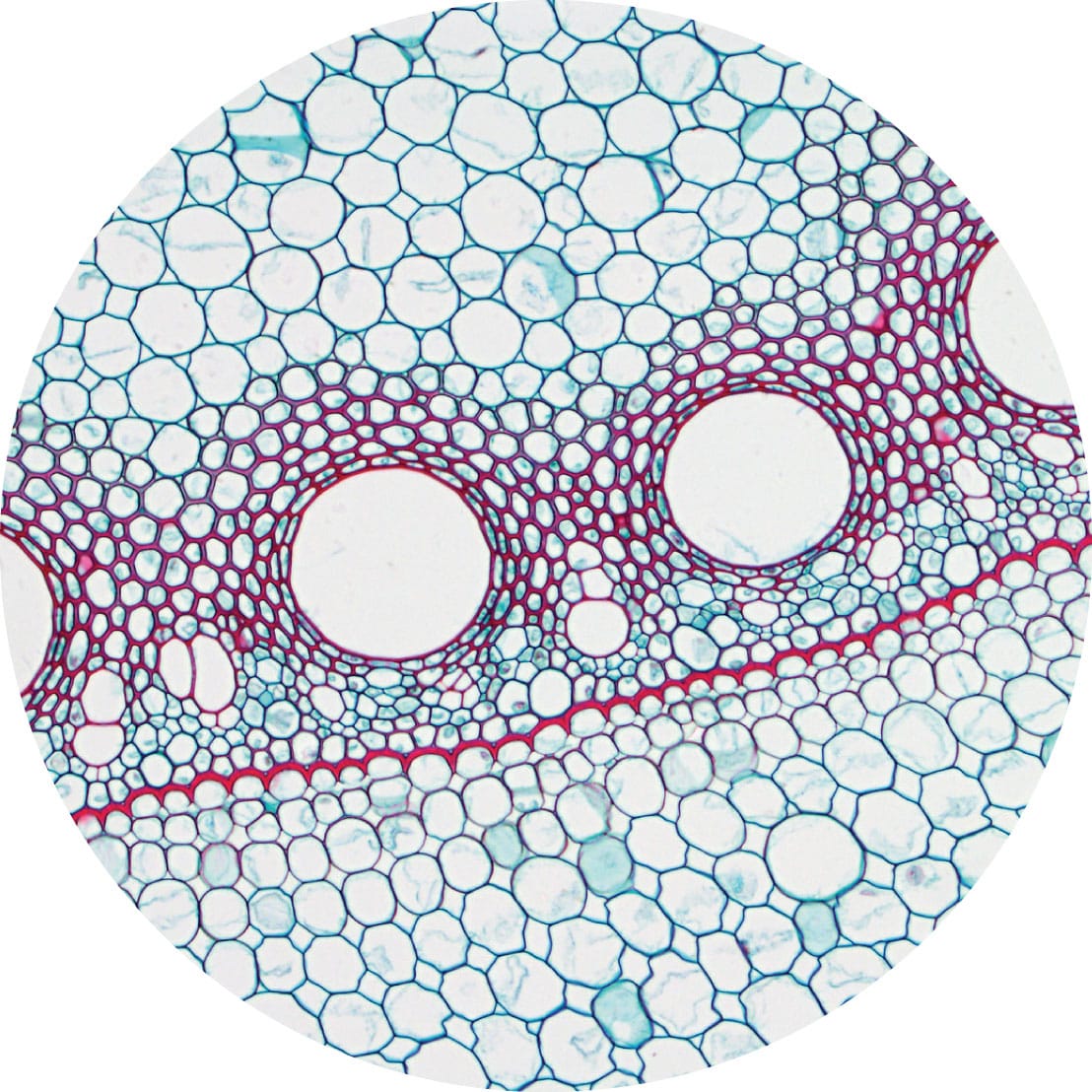
Light microscopy cross section of a maize (Zea mays) root showing the endodermis. The casparian strip (red line) is a hydrophobic cell layer that aids in water regulation, with the vascular tissue (red circular cells) lying just inside it.
Water – too much or not enough
We can compare water availability in the soil to the story of Goldilocks and the three bears (see also here). For the majority of plants, either too much or too little is not good – soil water availability must be just right. However, plants that continuously experience drought or flooding have evolved mechanisms to cope. The issue of too little water commonly produces physiological adaptations rather than morphological ones. For many species, as long as the plant can access water with a portion of its root system, the passive internal movement of water driven by gradients in water potential can redistribute it to the roots that are in dry soil, thus aiding in their continued survival and functionality. For many desert plants, portions of the root system are more ephemeral in nature and regrow only during the rainy season, when water is more readily available.
Too much water, on the other hand, can cause just as serious consequences for a plant. Roots require sufficient oxygen for cellular respiration to take place, but waterlogged soils contain insufficient levels of the gas. Plants that live in areas that are prone to flooding therefore commonly produce adventitious roots from their stem as a means to extend their root system above the waterline.

Tall-stilt mangroves (Rhizophora apiculata) produce multiple stilt roots from their stems and branches that resemble a skirt in order to provide support for the trees in their watery environment.
Temperature
The influences of light and temperature are sometimes difficult to separate when discussing plant responses. Regardless, variation in both of these abiotic (physical) factors can affect root growth, which is important considering that soil temperature is predicted to increase in the coming decades as a result of climate change. In colder environments, this could lead to longer growing seasons and hence increased above- and below-ground growth. However, if temperatures surpass the optimum for a particular species, root damage and reduced growth could occur. While roots typically grow toward warmer soils (positive thermotropism), under extreme conditions negative thermotropism could occur, whereby they grow away from soil that is too hot. Suboptimum temperatures are also undesirable and can result in stunted root systems with fewer lateral branches.
Metals
Heavy metals in the soil can cause myriad morphological changes to a root system. Of these, reduced root elongation and consequent stunted root growth is a primary response to high levels of such metals. Subsequently, roots often become thicker in diameter and produce greater levels of suberin, the protective waxy layer in the outer epidermal layers of the root. Lateral root branches and root hair production are also reduced. Collectively, these morphological responses reduce uptake by the roots and hence prevent accumulation of the toxic metals within the plant.
Salinity
While it might seem unusual for roots to grow in saline environments, select species have evolved ways to deal with high salt levels. In some cases, however, roots are unable to exclude salt from their tissues, and once it has entered the plant it ends up in the leaves, where it is compartmentalized in cells. While some salt is tolerable, an overabundance can prove toxic for the plant. In a response similar to that undertaken when heavy metals are present in the soil, plants that inhabit saline environments produce shorter, thicker roots.

Mangrove (Rhizophora sp.) pneumatophore roots stick up from the sand like snorkels, allowing part of the root to access much-needed oxygen for at least a portion of the day.
Plants in the genus Avicennia, commonly known as mangrove trees, inhabit coastlines where fresh and salt water mix, and have adopted multiple adaptations to allow them to survive in such harsh environments. Mangrove trees can translocate salt that enters through the root system to old leaves, which are then shed, thus removing the salt from the plant. Additionally, they can hold on to fresh water by minimizing evaporation in younger leaves. The most impressive way these trees deal with their brackish environment, however, is through their modified roots. Specialized aerial roots allow the mangrove trees to ‘breathe’, despite the excess salt and water they encounter on a regular basis (see box).

Despite the less healthy appearance of this Arabidopsis halleri plant (left), the species is naturally found on polluted soils and is better able to hyperaccumulate cadmium and zinc than thale cress (Arabidopsis thaliana; right).
Digging deeper
There are clear advantages for a plant to have its roots and storage organs buried under adequate soil cover. Sufficient root anchorage to ensure plant stability is one, but others include protection from temperature extremes and fluctuations in water availability. In order to dig deeper into the soil, contractile roots shrink in length by 50–70 per cent, in such a manner as to exert a pulling force that allows the above-ground plant or storage structures such as a bulb or corm to descend deeper into the soil. Because the portion of the root that does the pulling is located close to the base of the plant or bulb, the root tips and root hairs mainly stay fixed in place and only the upper plant parts are drawn down. Moreover, because the main roots have already navigated through the space into which the plant is descending, it faces less resistance and so a greater movement is attainable.
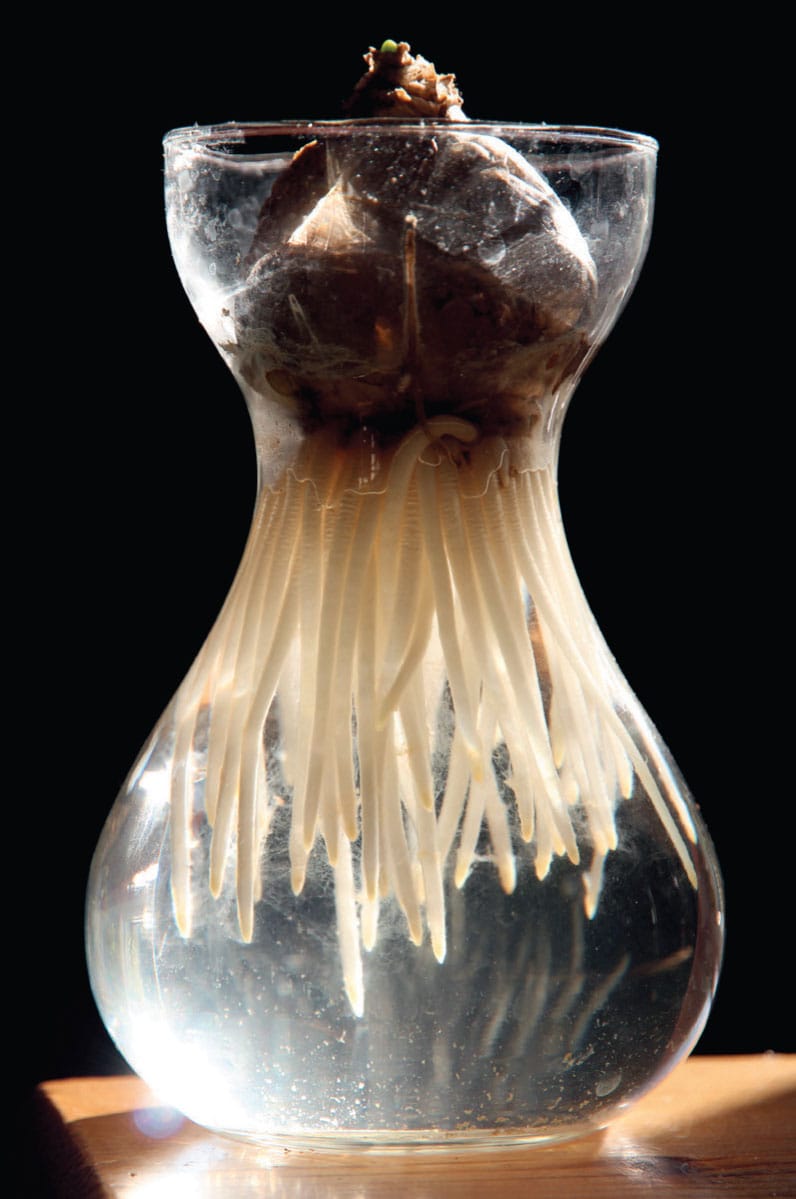
Hyacinths, including Scilla (shown here), along with other bulbs, produce contractile roots that aid in pulling the bulb deeper into the soil.
Aerial roots
Aerial roots do not differ functionally from their below-ground counterparts, but they have acquired a more diverse morphology to deal with their drier and more suspended environment. Unlike the roots of plants anchored in soil, those of plants that live on or climb up others must garner resources from the air or from decomposing materials that are caught in the canopies of taller plants, or, in a few cases, make the long journey down to the ground to access the resources that are available in the soil.

Aerial roots of the silk-cotton tree (Ceiba pentandra) can attach to numerous substrates, including man-made structures – as seen here at the twelfth-century Ta Prohm temple in Angkor, Cambodia.
Climbing vines
Vines and lianas commonly use adventitious roots along their stem to cling to the surface of trees or other vertical substrates. These roots aid the plant in climbing toward less shady environments. Unlike roots below ground, these climbing roots can be negatively gravitropic (growing away from the earth) and in most cases garner water and nutrients from the air, leaving the plant on which they are climbing relatively unharmed. Perhaps the most extreme case of climbing vines is that of the so-called strangler fig (Ficus aurea), a native of southern North America, Central America and the Caribbean. As the roots of this initially epiphytic plant descend a tree toward the ground, they wrap around the host, the two fusing with each other as they grow. However, it is not until the strangler fig’s roots have reached the ground and the tree is self-sustaining that its roots expand in girth, slowly outcompeting the host and causing its early demise.

Strangler figs (Ficus aurea) start their life as an epiphyte in a tree. But, as their name implies, they grow quickly and develop roots that can take hold of other nearby plants. These roots can completely encircle their host and constrict its growth, essentially strangling it to death.
The specialized roots of epiphytes
Epiphytes – plants that grow on the surface of another plant – have developed a myriad of root types that are specialized for holding onto their host as well as capturing resources in somewhat unusual substrates. Roots of these plants can capture resources from the atmosphere, including water from precipitation, dust particles suspended in vapour (termed atmospheric deposition), decomposing plant material that has gathered in the crux of the canopy or even ant nests that house ant excrement as well as the decomposing bodies of the ants themselves. The majority of epiphytes are found in tropical or humid environments, which provide the required moisture within the air to ensure the plant’s survival.
Roots of some epiphytes are capable of changing their function, moving from a holdfast (a root that grows minimally in length and functions, as its name suggests, to anchor the epiphyte to its substrate) to a more absorptive capacity with the introduction of valuable resources. Adaptations such as these are what allow epiphytes to thrive in such harsh environments.
Parasitic roots
Approximately 1 per cent of flowering plants are parasitic, meaning that they ‘steal’ some or all of their carbohydrates from their host. Of these, approximately two-thirds are parasitic on the roots of their host and the remaining third are parasitic on the stem. All, however, use a modified root structure called a haustorium to attach to and penetrate the host in order to fuse directly with its vascular tissue. Because parasites usurp the energy produced through photosynthesis from their host, they frequently lack chlorophyll and thus come in an array of colours.
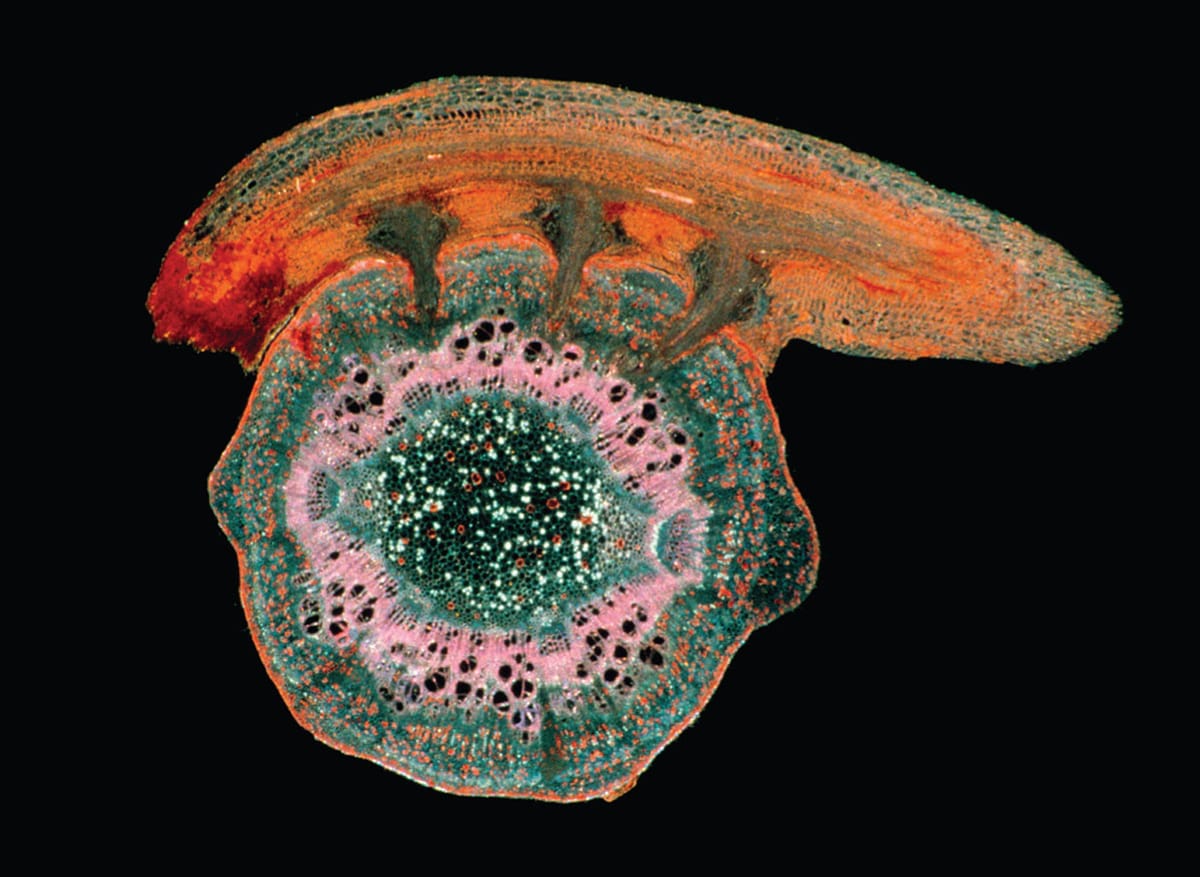
A photomicrograph cross section of three haustoria of American dodder (the upper orange structure), a parasitic climbing vine seen here infecting a host stem.
Epicortical roots
Parasites that penetrate the stems of plants climb the host using adventitious epicortical roots, or runners. Anatomically, epicortical roots resemble stems, but they remain leafless and, more importantly, produce a root cap, a critical anatomical structure that differentiates stems from roots. Usually, the parasite plant begins its life with a primary root that acquires its resources through the more traditional route – the soil. As the parasite grows, the numerous epicortical roots it produces in turn produce numerous haustoria, which are able to penetrate the host as the parasite grows along its surface. Only a few haustoria are needed before the parasitic plant loses its primary root and thus its connection to the ground. Interestingly, the haustoria of the epicortical roots develop only on the side of the root that has contact with the host, and the frequency at which they are produced is a function of the thickness and density of the host plant’s bark.

Colourful orange American dodder (Cuscuta americana), a leafless parasite, steals resources from its host without ever touching the soil by producing root-like haustoria that fuse with the host’s vascular system.
Root parasites
The seeds of parasitic plants can remain dormant in the soil for many years. Germination of root parasite seeds is initiated by a chemical signal induced when a host plant is near. The detected signal is a hormone called strigloactone, which plants utilize to form connections with fungi underground. Root parasites are also sensitive to strigloactones, however, and use this cue to produce an initial root that requires host contact in order to survive. If a host is found, the parasitic root will produce a substance that allows it to stick to the host’s roots long enough to penetrate the endodermis. As with other plant parasites, contact with the host’s vascular tissue ensures nutrient transfer. Purple witchweed (Striga hermonthica), a notable root parasite of crops such as sorghum (Sorghum bicolor), rice (Oryza sativa) and maize (Zea mays), utilizes water and photoassimilates at rates higher than its host, resulting in severe growth reduction in the host.

A portion of a witchweed (Striga sp.) haustorium digests the surface of, and then penetrates, a maize (Zea mays) root. The entire process, from the recognition of the host to access of the host’s cortex cells, takes less than 72 hours.
Root associations
Roots form a number of symbiotic associations with other organisms in the soil. Mycorrhizae and rhizobia – fungi and bacteria, respectively – are the most ubiquitous and well studied of these organisms. These symbiotic associations facilitate the uptake of nutrients and water from the soil, and in the case of mycorrhizae form an underground fungal network that connects plant roots to their neighbours. The benefits of these associations outweigh the carbon costs to the plant, and allow for its establishment – and thus a competitive advantage – in habitats that might not otherwise be favourable.
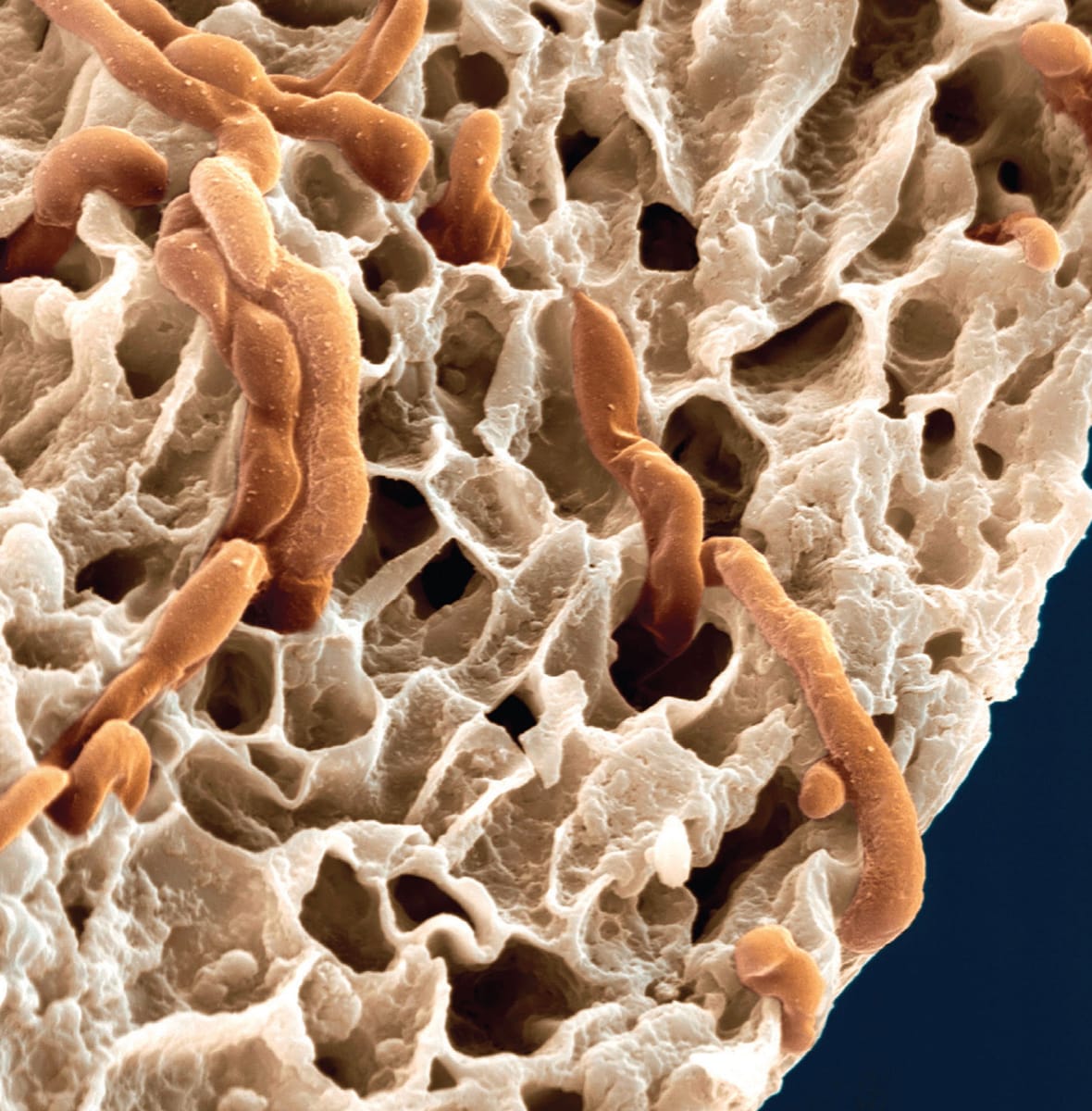
Mycorrhiza hyphae (orange) can be seen within the cortex of a plant root in this coloured scanning electron micrograph (magnification ×3,400). The fungus is able to access nutrient forms unavailable to the plant, process them and pass them on to the roots.
Mycorrhizae
The majority of seed-bearing plants form a mycorrhiza–root association. In the simplest sense, there are two types of host inoculation with a fungus, defined by where the fungus resides within the root. In ectomycorrhizae, the long strands of the vegetative fungal body (the hyphae) are located outside the root and do not penetrate the root cells, whereas in endomycorrhizae the majority of the hyphae are housed within the root and the fungus enters individual root cells to form a close association for nutrient exchange. Depending on the plant species, a single plant can range from having one tight-knit association with a solitary fungus, to multiple mycorrhizal associations with both ectomycorrhizae and endomycorrhizae. Furthermore, those associations can shift with plant development and environmental constraints.
Ectomycorrhizae are recognizable by the visible sheath of hyphae (appropriately called the mantle) that encases the tip of the plant root. A portion of the hyphae do penetrate the epidermis and enter the root, but then simply surround the individual root cells to form a highly branched fungal network, called the Hartig net. The root’s association with the fungus results in a lack of root-hair production, a visual swelling in the root tip due to the mantle that covers the outer root, and an overall stunted root length, most likely due to the fungus preferentially colonizing slow-growing roots.
There are several classifications of endomycorrhizae that, in some cases, are quite specific to their plant host. Arbuscular mycorrhizae are the most common and associate with approximately 80 per cent of plant species. More specific endomycorrhizae include ericoid mycorrhizae, specific to the heather family (Ericaceae), and orchidaceous mycorrhizae, specific to the germination of orchid seeds. A third type of mycorrhizae, termed ectendomycorrhizae, are primarily found in pine, spruce and larch trees. They possess characteristics of both ecto- and endomycorrhizae, in that the fungal body is located both externally and internally within the plant tissue.
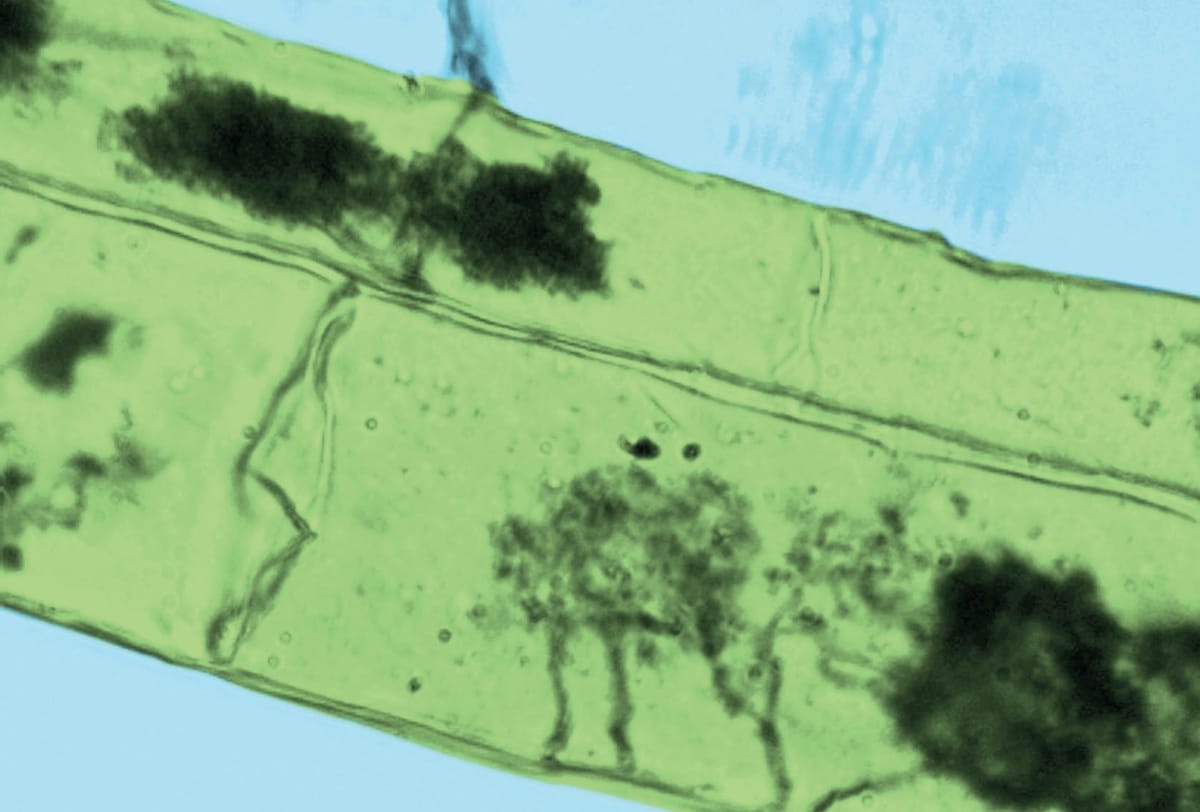
Colour-enhanced light micrograph of mycorrhizal root cells, showing arbuscules (dark lines) inside cortical root cells of a maize (Zea mays) plant. Arbuscular mycorrhizal fungi help plants capture nutrients such as phosphorus from the soil, exchanging them for carbon.
The development of the connection between the plant and fungus is an intricate process, and one that is still not fully understood. Chemical compounds produced by the roots and the fungus alike suggest that primary infection involves specific signals that either initiate the germination of the fungal spore or induce branching of the fungal hyphae. Likewise, root elongation and branching may occur during infection.
The benefits to plants that occur as a result of their association with mycorrhizae have been the focus of numerous studies. Regardless of the type of infection, the general outcome is the same, namely increased access to nutrients via the extensive below-ground hyphal network. The ability of the fungal hyphae to reach distances that the plant roots alone could not – up to several metres for ectomycorrhizae – provides the plant with access to nutrients that have slow diffusion rates in the soil, most notably phosphorus. For some plant species, the mycorrhizal association is imperative to their ability to complete their life cycle. In most cases, however, the association forms only in habitats where the plant confers a benefit, and may not be formed at all if adequate and accessible nutrients are available to the root system. More recently, researchers are discovering that hyphal networks also connect several plants underground, thereby creating a network of carbon and nutrient transfer. This idea should make us take pause to consider the extensive and interconnected environment below ground and its role in ecosystem function.

The mycelium (white) of an ecotmycorrhizal fungus has completely enveloped the roots of this pine tree (Pinus sp.) (magnification ×41). Ectomycorrhizae remain on the outside of the root, and do not penetrate its cells.
Root nodules and nitrogen fixation
One of the more well-studied interactions with roots is that of nitrogen-fixing bacteria. This symbiotic relationship is of great importance to plants, in that nitrogen is the most limiting factor for the majority of species despite being required for numerous cellular processes. Rhizobia, the soil bacteria responsible for enabling plants to fix atmospheric nitrogen, are enveloped by the roots and enter via the root or root hairs after specific chemical signalling is recognized. Once inside the root, the bacteria differentiate to become millions of bacteroids and initiate cell division in the plant, causing it to produce characteristic nodules on the root that house the bacteroids.

Bacteria nodules on this pea (Pisum sativum) root fix nitrogen from the atmosphere for the plant. Most members of the legume family (Fabaceae), including peas and beans, harbour symbiotic Rhizobium bacteria, which aid in their assimilation of nitrogen.
One small problem remains with the process that converts nitrogen into a form that can be used by plants: the bacterial enzymes that fix nitrogen are extremely sensitive to oxygen. The answer is haemoglobin, the familiar protein that plays an important role in oxygen transfer between cells in animals, including humans. The root nodules, therefore, take on a red colour due to the presence of high levels of haemoglobin. In plants, haemoglobins – more precisely leghaemoglobins because they are primarily found in the legume family (Fabaceae), function to reduce the oxygen within the root nodule to protect nitrogen production and to provide oxygen to the residing respiring bacteroids.
For plants that are unable to fix their own nitrogen through this valuable symbiotic association, a small amount of nitrogen is fixed in soil through lightning, and man has developed nitrogen-containing fertilizers through the artificial Haber–Bosch process.
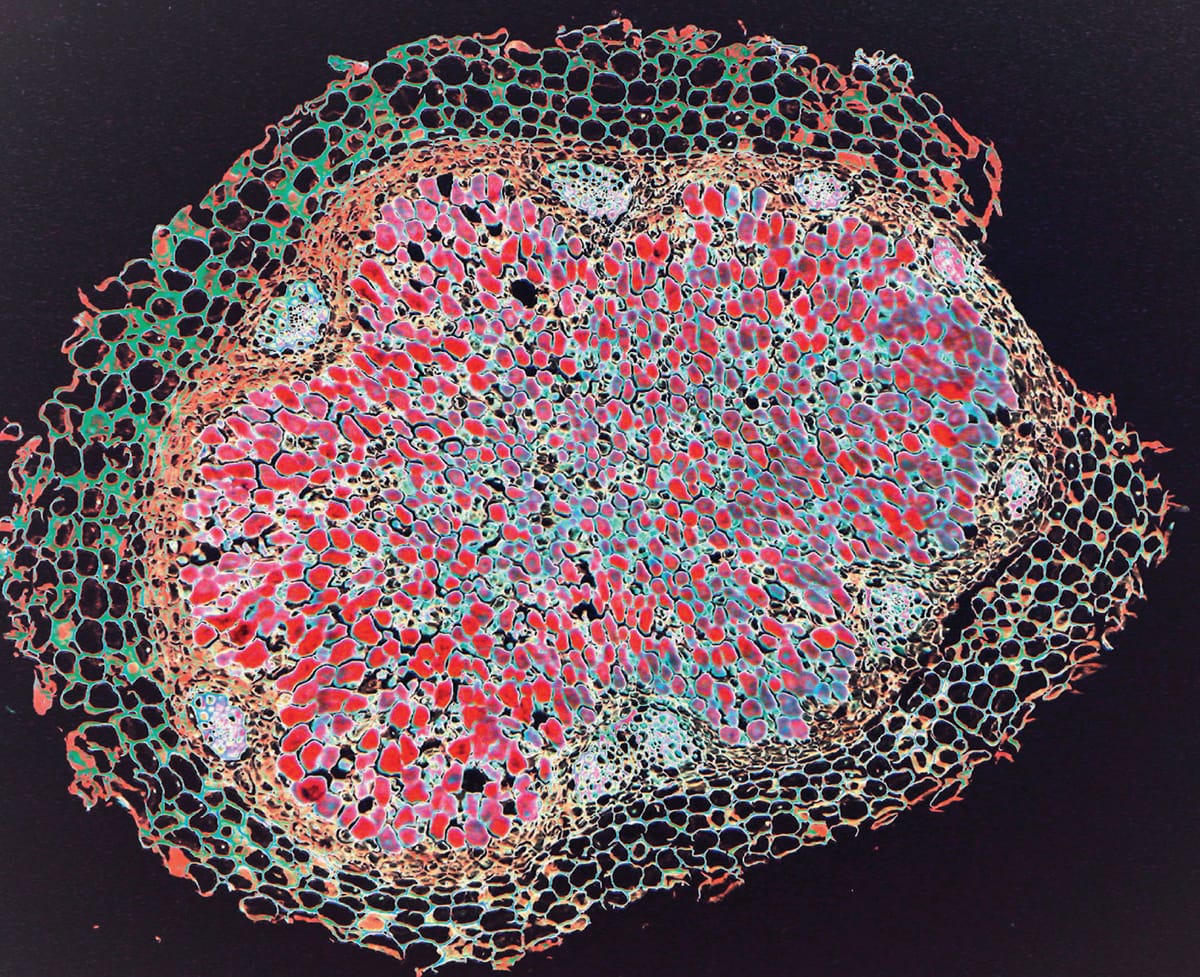
A coloured cross section of a broad bean (Vicia faba) nitrogen-fixing root nodule, showing cells filled with red-stained Rhizobium bacteria. The bacteria modify the plant’s metabolic and molecular cellular processes to allow root cell colonization, and live in symbiosis with the plant.
Endophytes
As discussed earlier (see box), an endophyte is an organism that lives within another organism. In plants, fungal and bacterial endophytes reside within plant cells, forming relationships that range from beneficial to pathogenic. These so-called endosymbionts impart a suite of costs and benefits to the varied and globally distributed hosts they infect. For example, endophyte colonization changes host response to herbivory, competition, biomass allocation, reproductive success and ability to invade new communities. When plants are infected by endosymbionts, the resulting symbiotum has a unique phenotype that is distinct from an uninfected counterpart. For roots in particular, endophytes can impart increased nutrient uptake and resistance to soil-borne pathogens.
Root fungal endophytes are largely composed of dark septate endophytes. While this terminology is somewhat vague and simply refers to the dark pigmentation and partitions that can be found within the fungal hyphae, the characterization allows for the differentiation of fungal endophytes from other mycorrhizal associations. It is as yet unclear whether these fungi can grow through the soil to reach their host, or remain sedentary in the soil until a suitable host root is in the vicinity. What is known is that endophytes have been found in the roots of all plant species.
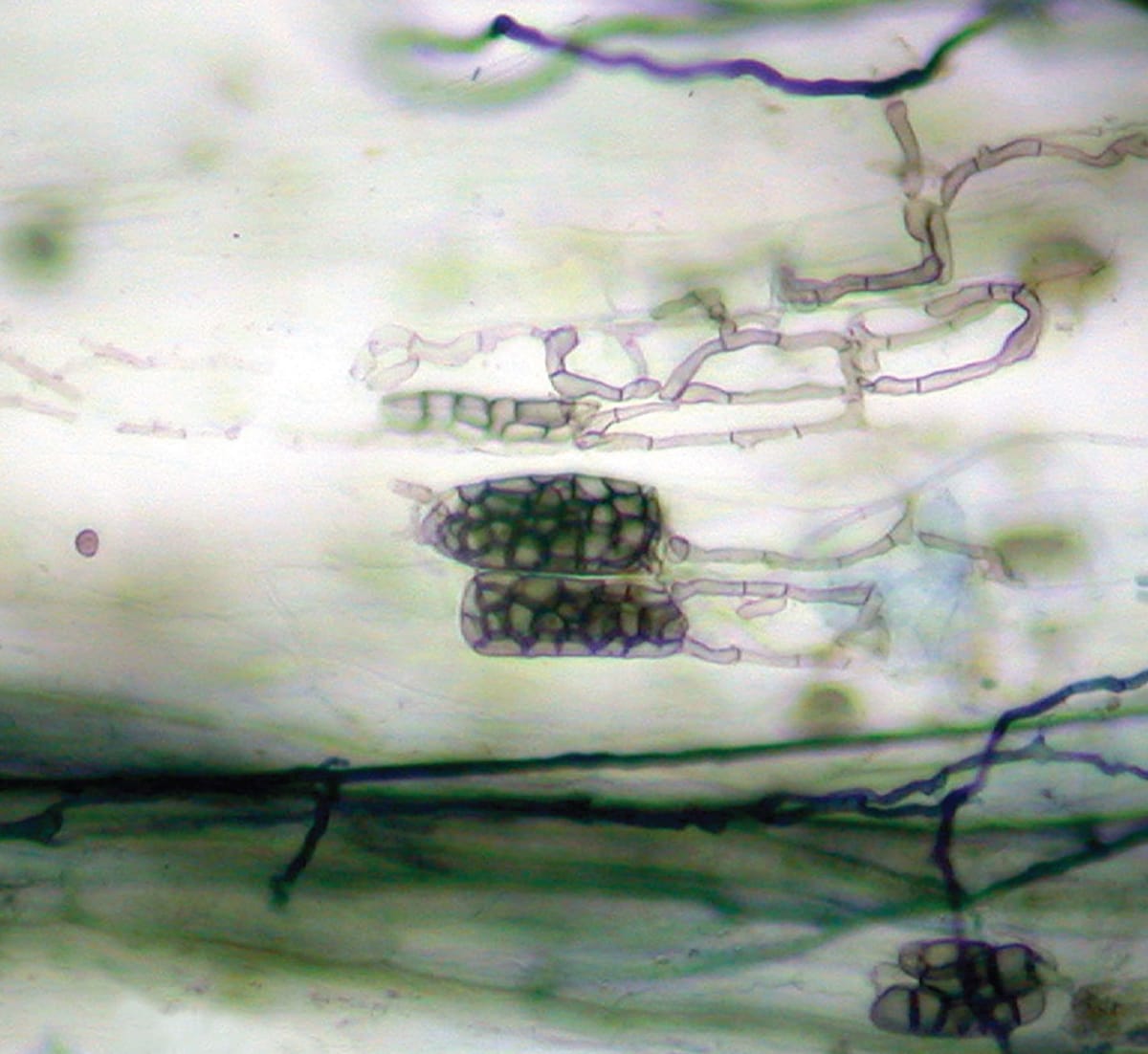
This image depicts several fungal hyphae ending in microsclerotia, which appear as grape-like clusters. These multicellular resting structures are common among plant endophytes that originate from swollen hyphae. Dark septate endophytes characteristically produce microsclerotia within the roots of their host.
Endophytic bacteria, like their fungal counterparts, most likely rely on chance followed by chemical signals to gain entry into a plant root. Cracks formed at the junctions of lateral roots and/or lesions produced by insect probing are likely locations for the bacteria to gain entry into the root and begin colonization. Once inside the plant, bacterial endophytes can also confer numerous physiological and developmental benefits to the host.
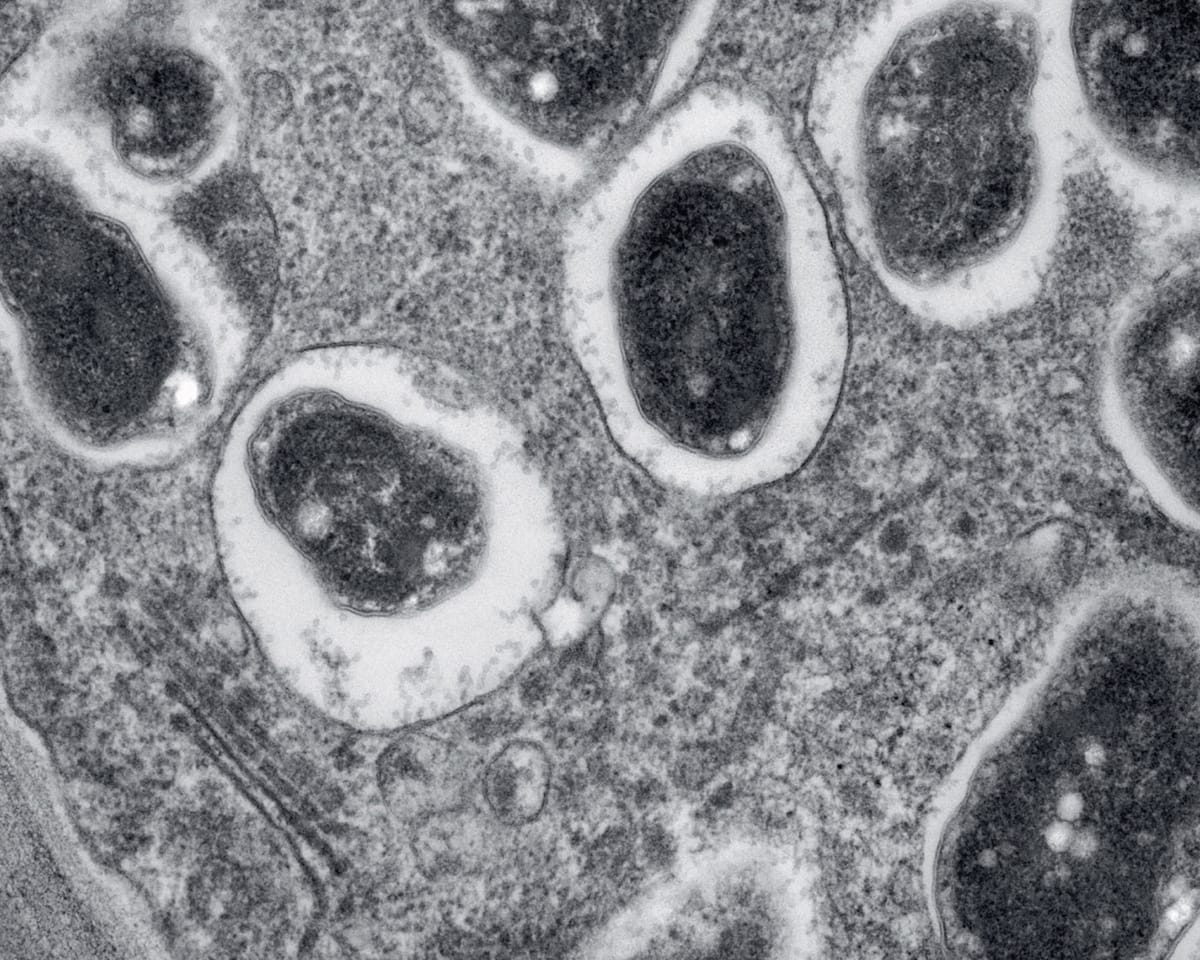
Transmission electron microscope image of a cross section through a soya bean (Glycine max) root nodule. The bacterium Bradyrhizobium japonicum infects the roots and establishes a nitrogen-fixing symbiosis with the plant.
Root pests
Roots encounter a plethora of biological activity below ground. Among the organisms with which they cohabit the soil, nematodes (roundworms), arthropods, insects and earthworms may affect their growth and function, either directly through feeding or indirectly through the production of abnormal cell growth. These organisms feed on both live and dead roots, affecting plant health, the cycling of nutrients in the soil, plant hormone production and plant distribution. Not surprisingly, the difficulties of observing and measuring root pests and their interactions with plants has deprived this field of adequate study.

Larvae of the European chafer beetle (Rhizotrogus majalis) are common invasive pests of plant roots and a significant nuisance on residential lawns. The chafer beetle feeds on grass roots, compromising the plant’s ability to deal with other environmental stresses, and usually results in dead or brown turf.
Root herbivory – modes of root damage
A number of soil animals consume roots at some point during their life cycle. While some plants can compensate for their loss of below-ground biomass with little to no effect on performance, root pests and root herbivory can cause substantial damage. Unfortunately, this loss of biomass is difficult to quantify and in many cases is not perceived until the plant’s health declines – a particularly detrimental factor in crop production.
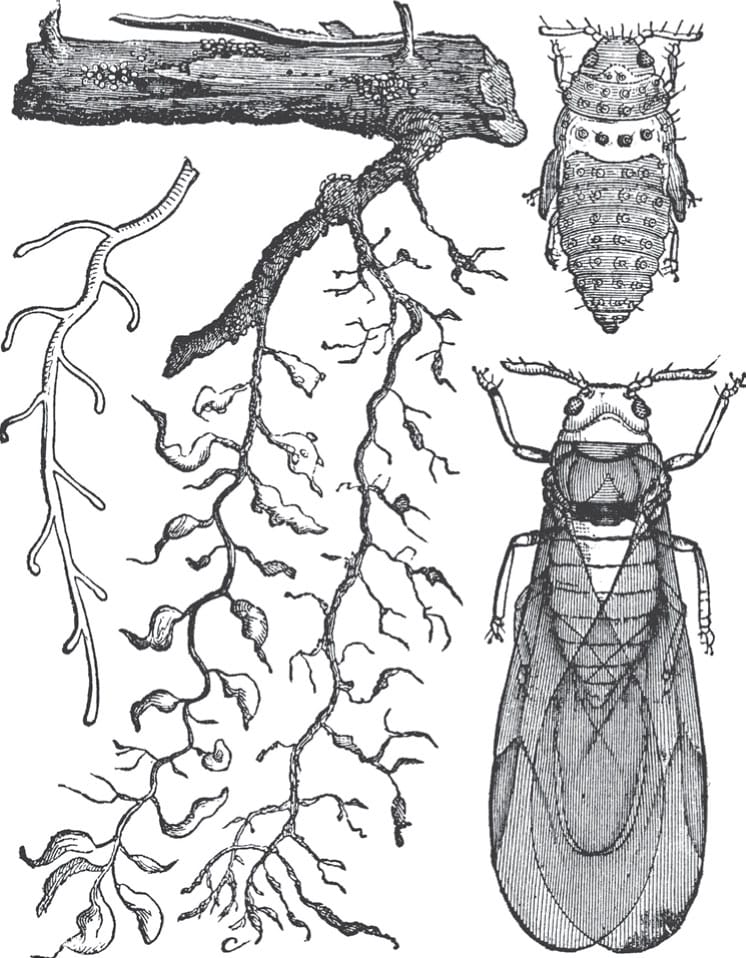
The root aphid Phylloxera radicicola can cause root tip swelling and deformity, as seen in the centre of this image. When roots start growing in the spring, these asexual insects lay eggs and produce several generations through the year. Fungal infection in roots is more severe in the presence of the aphid.
Chewing herbivores cause direct damage to roots that are consumed, or by disrupting the flow of resources through root grazing. The outcome of root chewing can disrupt water uptake and carbohydrate storage reserves. Common culprits of root damage include weevils, beetles and cutworms, moth larvae that are so called for their ability to cut off roots as they feed. The relationship between the root herbivore and the specific plant species can vary, with some species being highly host specific while others are generalists, burrowing from plant to plant through the soil in search of food.
Indirect damage to roots can be just as damaging to plant functionality. Many below-ground herbivores exploit roots as a food supply by living within them or by tapping into the phloem sugar supply as a means for more sustained survival. Aphids, scales and nematodes are common root feeders that select a feeding site on the root and then penetrate it, usually inducing visible enlarged or prolific cell production and expansion called galls. Over time, as the number of galls increases, root functionality declines. Additionally, the introduction of secondary infection is increased due to the open lesions and reduced health of the root caused by the probing of the herbivore on the root itself and its ultimate entrance into the root.
The rhizosphere
The rhizosphere comprises the volume of soil that is affected by the root, which amounts to just a few millimetres beyond the root surface. Although small, it is a virtual hotspot of activity, where the interplay between the growth of roots, root chemistry, bacteria, microbial fungi and insects results in fluctuations that affect plant nutrient availability, soil water accessibility, hormone signalling and, ultimately, plant growth. In practical terms, uncovering plant–soil interactions requires the consideration of the plant and its soil environment as a single system and not separate entities.
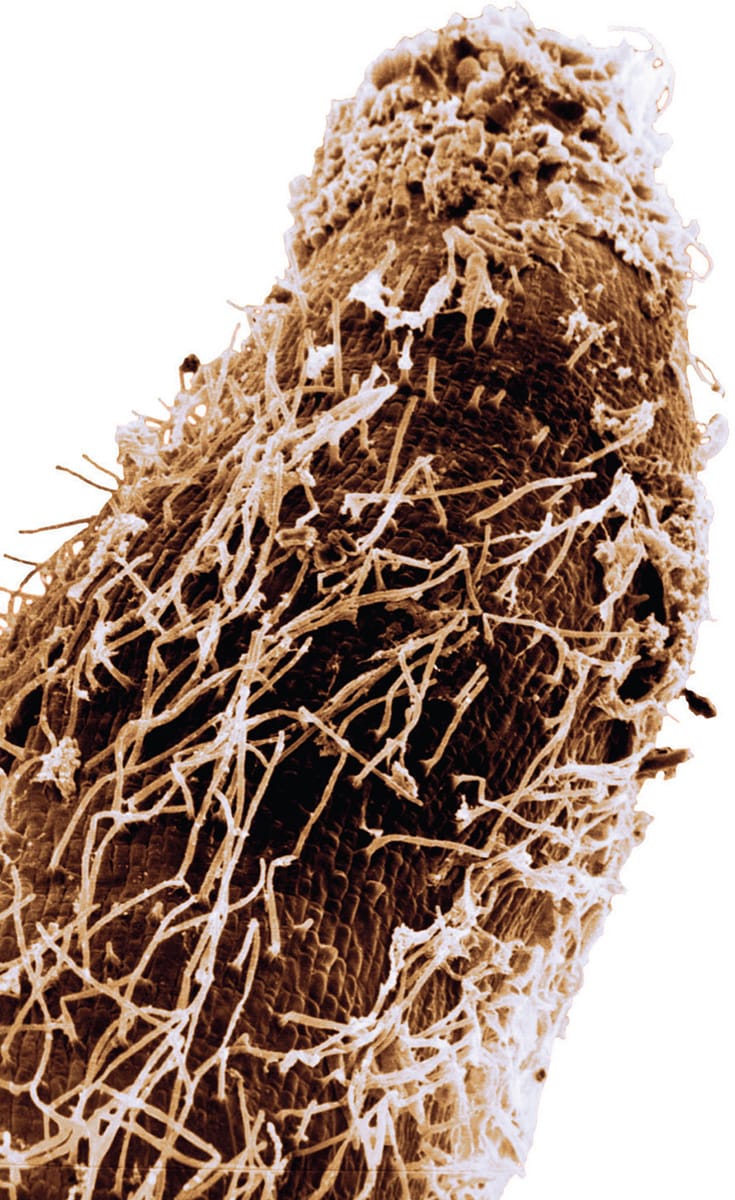
A scanning electron micrograph of the root tip of a sorghum (Sorghum bicolor) plant, with kidney bean-shaped Azospirillum brasilense bacteria on its surface.
Root exudation
Roots secrete diverse chemicals into the rhizosphere that are yet to be fully identified. Root exudates – defined as all organic and inorganic compounds released into the soil by healthy roots – are mostly composed of carbon and can influence plants, microbes and nutrient availability. Root exudates are made up of primary and secondary plant metabolites. While primary ones are essential for plant growth, secondary ones are not required to complete the life cycle, but instead are chemicals that aid in protecting plants from stress and are used in communication with other organisms.

An artistic rendition of a root minus its soil environment, showing the diversity of organisms that live in the rhizosphere, the zone around the root.
Root exudates provide a readily available carbon resource for soil microbes. In the context of their associations with microbes, general classifications group the exudates according to their mode of utilization – for example, exudates that are easily assimilated by microbes, versus more complex compounds that must be broken down into simpler forms before they can be utilized. Depending on their chemical structure and concentration, root exudates can function as either a food source or toxin for microbes.
On a strictly chemical basis, root exudates can interact with nutrients in the soil, changing the availability of nutrients. The effects include increased nutrient availability through changes in soil pH, nutrient ion solubility or metal detoxification. The size of a root system can have an effect on the ability of roots to influence the nutrient status of the rhizosphere, and there is a direct relationship between the number of roots and the size of the zone of influence.

Pacific madrone trees (Arbutus menziesii), said to have allelopathic properties, line a path in Santa Clara county, California.
Root exudates have been shown to have an effect on neighbouring plant species. In extreme cases, their presence can prevent the growth of some plants. Black walnut trees (Juglans nigra), native to eastern North America, is the most famous species for this natural toxic herbicidal activity, termed allelopathy (the inhibition of growth by one plant on another plant). The chemical juglone, produced by black walnut roots, can persist in the soil for long periods of time, inhibiting plant growth within the tree’s root zone. The Mediterranean species Johnson grass (Sorghum halepense) has also been known to suppress weeds, through sorgoleone root exudates produced in the root hairs. These golden-yellow allelochemical droplets are taken up by neighbouring crop species such as maize and soya bean (Glycine max), and are translocated into their cells, where the chemical disrupts proper cell function, leading to plant yellowing and potential death. The tree of heaven (Ailanthus altissima), native to China and once regarded as a choice specimen for ornamental gardens in Europe in the late eighteenth century, has fallen from favour due in part to its production of ailanthone, a toxic chemical that leaches from the root bark into the soil and inhibits the growth of other plants. The species can also sprout easily from stumps and produces large quantities of seeds, further contributing to its invasive tendencies.

Phytophthora infestans, the microorganism that causes potato blight, can spread through, and devastate, a crop in just a few days.
Microbial community
As a root passes through the soil, chemical signalling drives a response from microbes, which may result in positive (growth-promoting) or deleterious (pathogenic) effects. While uncertainty remains regarding the mechanisms involved, the root-associated microbiome has been shown to facilitate rhizosphere responses to changing environments in ways that are potentially beneficial for the plant. For example, rhizosphere microorganisms increase plant tolerance to water stress and even improve overall performance (growth) in many systems. Some hypotheses suggest that plants with a similar root morphology might also have similar root-associated microbial communities. For example, root systems that have little branching tend to rely more heavily on myccorhizal associations. However, this is a gross oversimplification and the field warrants research to determine other driving factors.
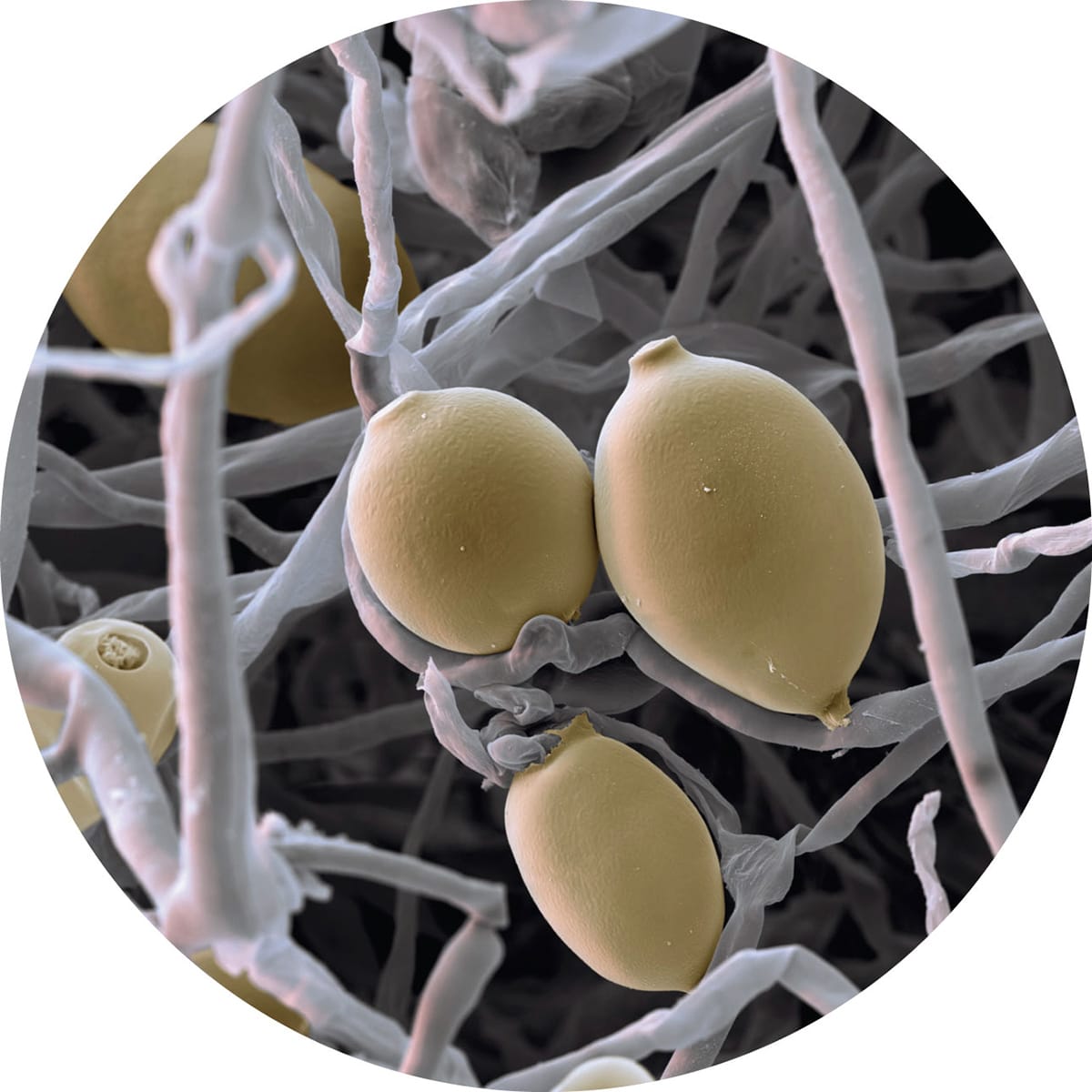
Scanning electron micrograph of Phytophthora infestans. The lemon-shaped structures are microscopic asexual spores called sporangia, which open to release spores in cool, wet conditions.
As mentioned above, not all microbes are beneficial to plant roots, and a plethora of pathogenic microbes also inhabit the rhizosphere. Root infection by species of pathogenic genera such as the fungus-like eukaryotes Phytophthora and Pythium is greater in root tips compared to higher-order roots. This may result from localization of infection events at the root tip, plant deployment of defences or constraints on tissue functionality. Ageing root tissues are a preferred route of entry for many generalist pathogens in the rhizosphere, so regions where this is occurring or particular patches of sloughing cells may provide potential infection sites.
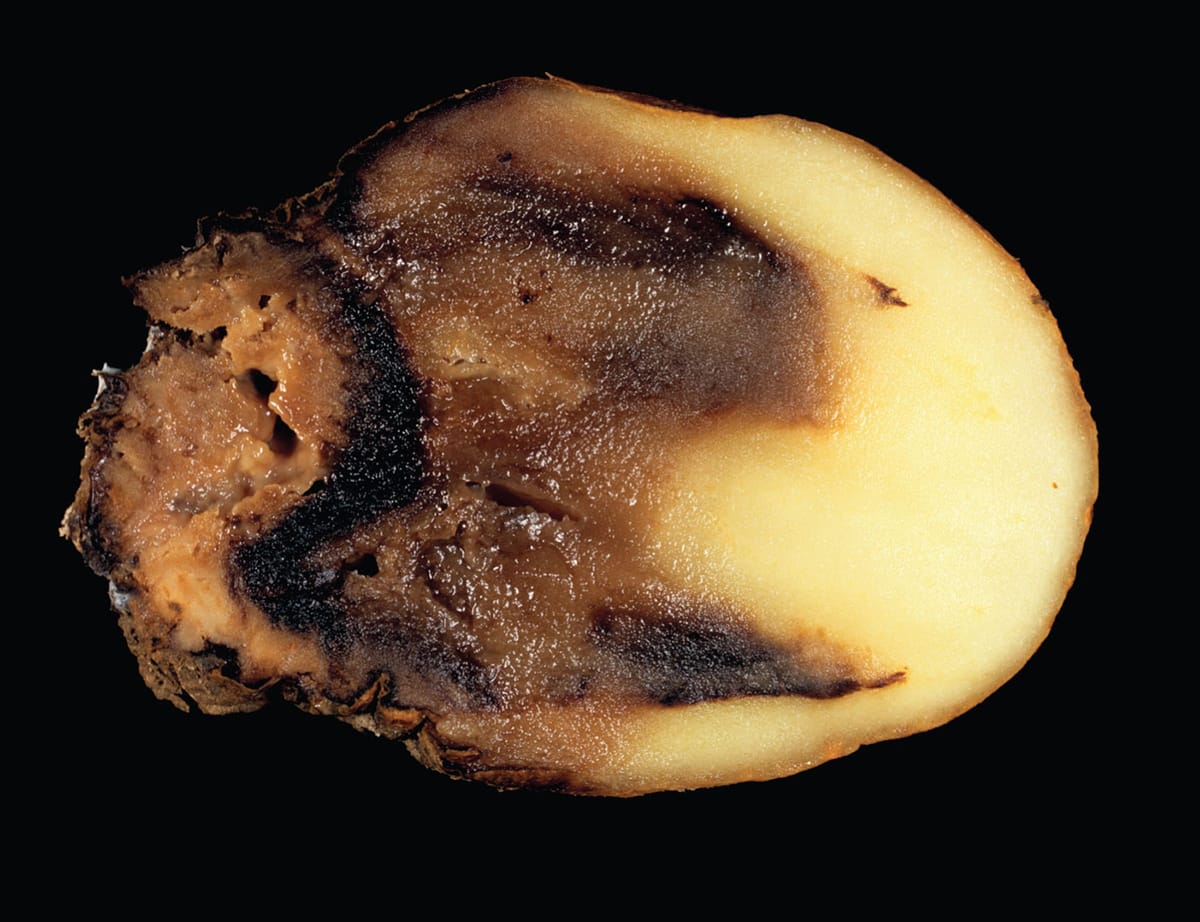
The plant pathogen Pythium ultimum is a downy mildew that infects many agricultural crops, including this potato (Solanum tuberosum) tuber. Plant infection leads to reduced plant growth and death. The pathogen can lie dormant in the soil for several years, making management difficult.
The rhizosphere and a changing climate
Climate change is altering biotic interactions in a multitude of ways. There are clear advantages to increasing our understanding of the intertwined living and non-living rhizosphere matrix and how the root–rhizosphere environment is influenced by water, temperature, toxic metals and so on. This complex environment can incur substantial changes as a result of environmental shifts induced by climate change, which can ultimately have impacts on agriculture and plant community assemblages. While this may seem hard to conceptualize as the rhizosphere of an individual root itself is very small, the rhizosphere of an entire plant or a community of plants constitutes a substantial below-ground area. As shifts in environmental variables occur with a changing climate, direct effects on the rhizosphere – including microbe interactions with other microbes and/or the plant roots – will impact on levels of carbon sequestration, decomposition and, ultimately, how plants function.
Previous studies suggest that plant controls over rhizosphere microbial communities may be more pronounced in disturbed environments and that stress may enhance plant investment in root exudation, thus potentially shifting rhizosphere functionality. Moreover, the microbial component of the rhizosphere may shift its metabolism or composition in response to altered root substrates. As if studying roots themselves were not difficult enough, research targeting the rhizosphere provides its own unique challenges. Yet, there remains ample work to be done within this focus area and in the unique feedback loops that drastically affect roots and therefore plant stability.
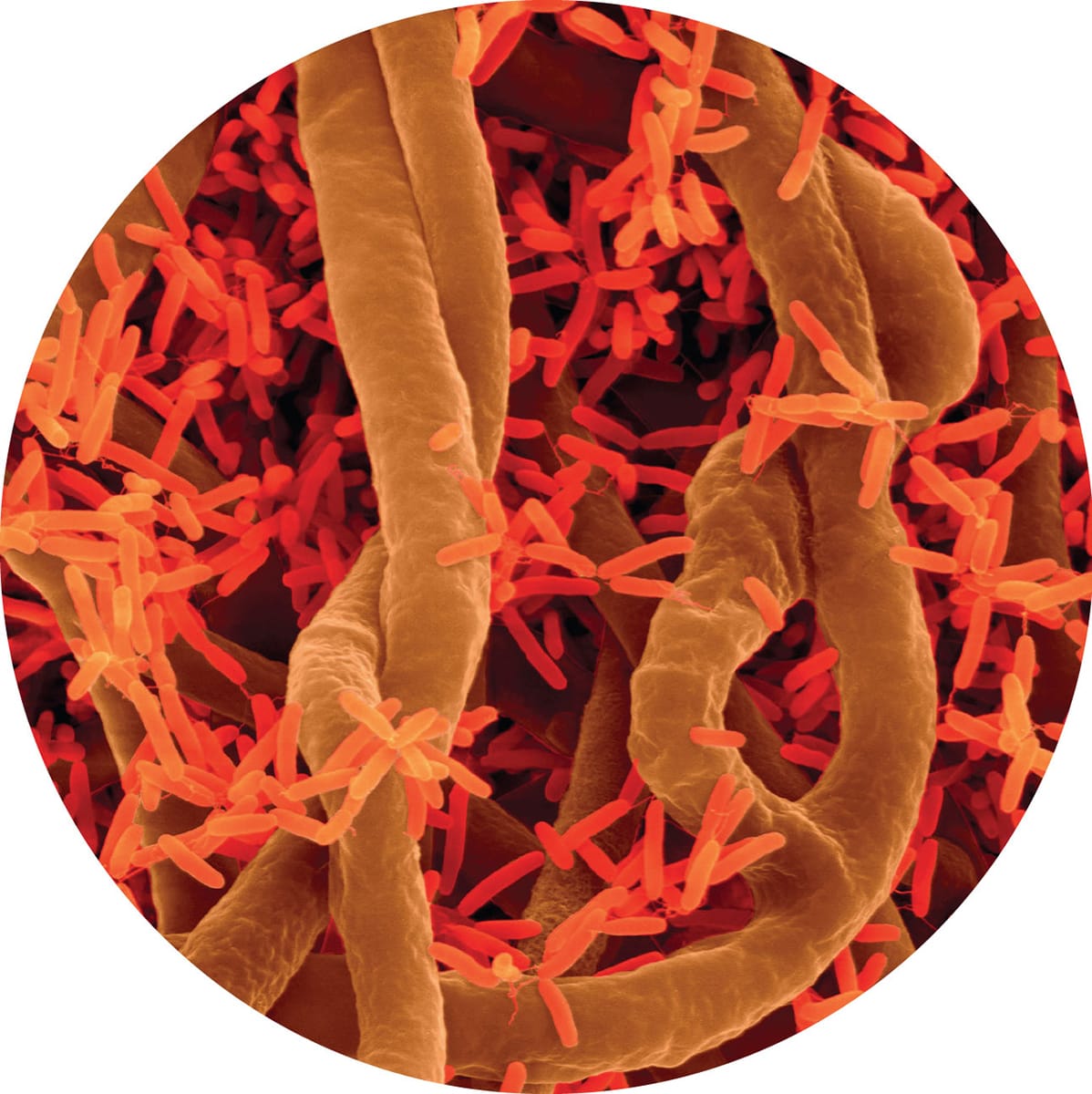
Soil fungi (ochre strands) and bacteria (red structures), imaged with scanning electron microscopy, are components of the microorganism community in the soil that potentially interact with roots.