3
Stems
No forests can exist without trees, and no trees would be complete without stems. Woody, herbaceous, climbing or underground, stems are integral components of the plant world, yet are rarely appreciated for all they do. In a superficial sense, they are the scaffold that keeps a forest ecosystem intact, but closer inspection reveals that they are more than an assembly of inert beams. They are complex structures that conform to essential principles of engineering and fluid flow. Water transport is essential to all plant organs, and plant stems house the long-distance plumbing that supplies the plant’s need for water. Likewise, transport of sugars from leaves to roots requires specific vascular tissue, an abundance of which is found in the stem. Stems are the bridge between earth and sky.
So critical are conduction, structural support and optimal leaf exposure to the life of a plant that natural selection has acted strongly on numerous aspects of stem architecture. Plants cannot move about like animals, so stems must be resistant to drought, freezing and pathogen attack. Resilient, economical and diverse in form and physiology, plants have evolved to function under such stress, and in the case of trees, stems faithfully record these events as they continue to grow. Stems are a portal to the past, and key to our planet’s future.

General stem structure and development
Given the diversity of colours, shapes and textures of the plants around us, it may come as a surprise that plant development is rather similar across species. In fact, flowering plants share a nearly identical pattern of root and shoot development once the embryo begins to emerge from the seed. Over time, a plant’s appearance becomes largely a function of hormonal signals, environmental cues and genetic make-up.
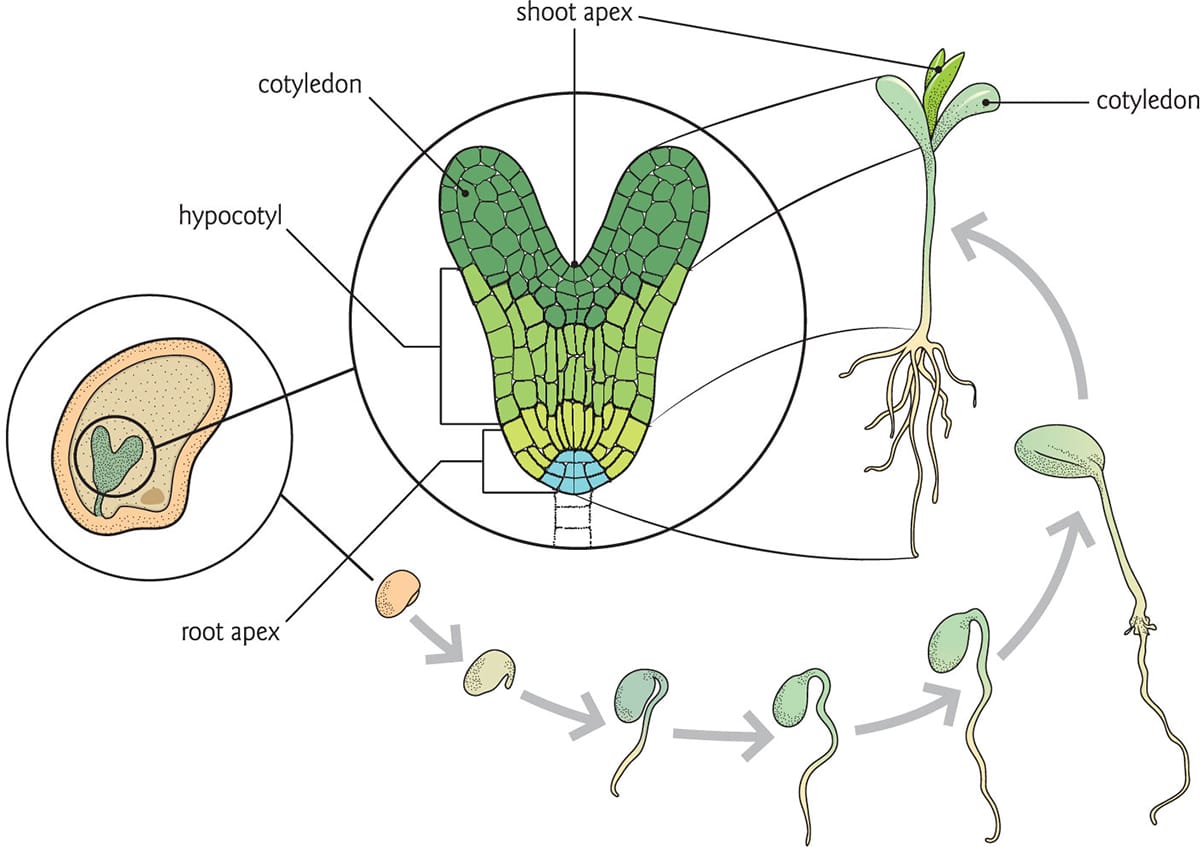
Early plant development from embryo to seedling. The cotyledons provide nourishment for the growing seedling, but it is the shoot apical meristem that generates the first true leaves and stems. The root apical meristem gives rise to the radicle, and matures into roots.
Initial stem development
The tiny embryo within a seed bears little resemblance to a mature plant, but it contains all the information it needs to develop into one. As germination begins, the base of the embryo will develop roots, while the hypocotyl will expand into a primordial stem possessing two leaf-like appendages, the cotyledons. With time, the hypocotyl will develop into a true stem with leaves and buds, while the cotyledons, depleted of their nourishment, will drop off. At this point, the juvenile plant is the first complete module of what will become a mature tree or herb. As the stem gains in length and girth, each module will develop a node, some leaves and an internode. In the node, axillary buds are sometimes nestled in the nook between the stem and leaf petiole; these buds may give rise to lateral stems. The internode is simply a zone of elongation. Over time, the iterative growth and expansion of these modules will generate a mature plant.
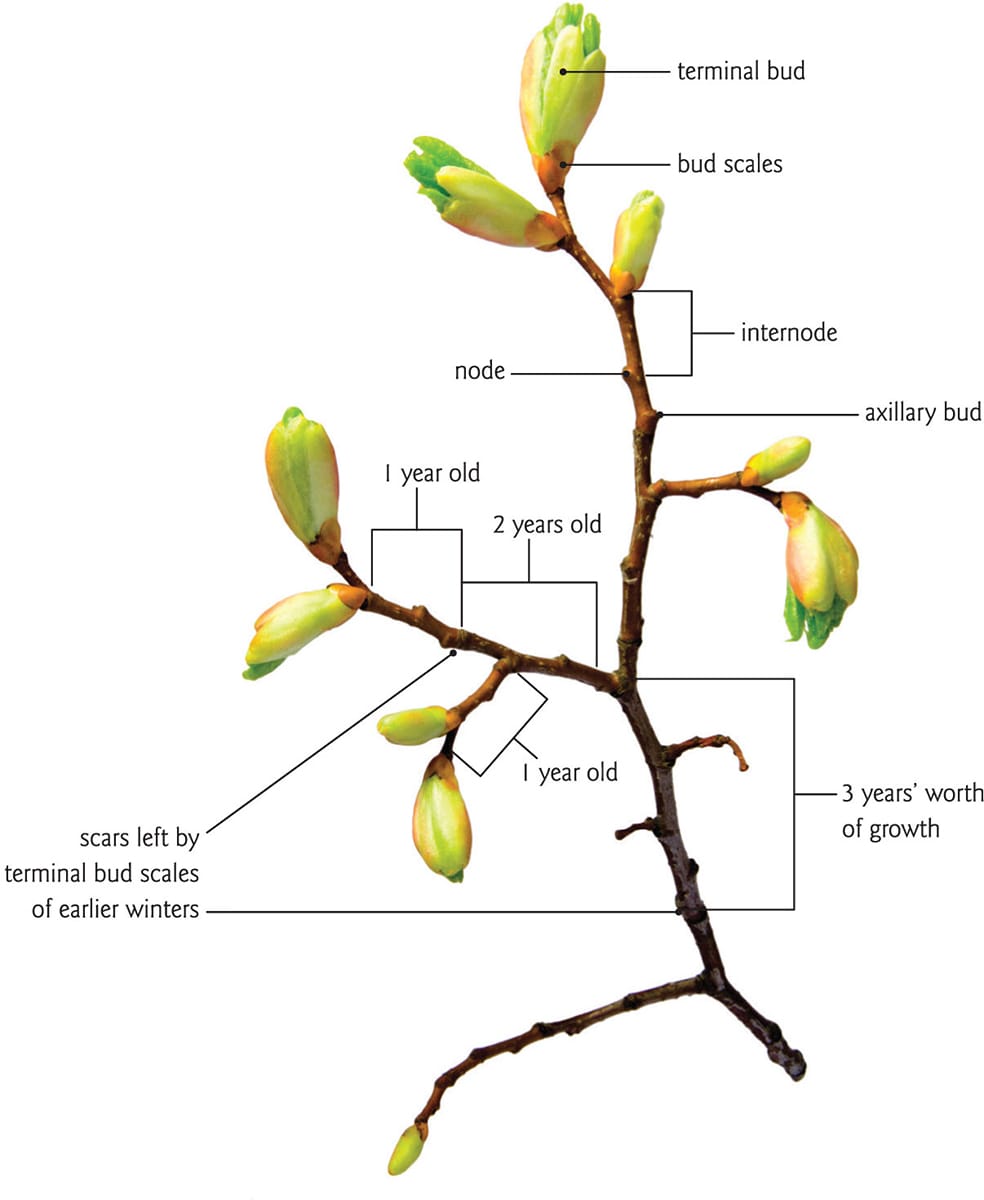
Anatomy of a young twig. Stem elongation occurs in the internodes; annual growth increments are evident as scars in the bark.
The apical meristem
The terminal buds we normally see on a mature stem or twig also produce modules with nodes, internodes and leaves. More precisely, these structures arise in the apical meristems within the buds. The term meristem refers to zones of undifferentiated tissues in which growth occurs. Three basic tissue types form the developing module in the apical meristem, and these tissues ultimately comprise all of the vegetative organs in a mature plant. Epidermal tissue covers the exterior of all plant organs, while the ground tissue comprises the bulk of the stem and surrounds the centrally located vascular tissues, which transport water and sugars. The growth of vascular and ground tissues below the apical meristem is responsible for the increase in stem length and thickness.
The height of a plant, as well as the arrangement of its leaves and branches, is determined in part by the apical meristem. Apical dominance is the extent by which a leader shoot suppresses lateral branch growth. For example, most temperate conifers, with their central bole, exhibit a high degree of apical dominance in comparison to oaks (Quercus spp.), which tend to have an expansive canopy composed of numerous stems roughly equivalent in size. Controlling apical dominance is the hormone auxin, which is produced in the apical meristem; high levels of auxin suppress lateral branching, and this is indeed the case in conifers. In contrast, dicot species such as oaks are less dependent on auxin and thus benefit from a higher degree of morphological flexibility.

With its expansive canopy and multitude of branches, this valley oak (Quercus lobata) exhibits little apical dominance.
Evolution of stems
The first plants to colonize land had no blueprint for leaves, roots and stems, and faced a nearly constant threat of desiccation. In the face of such challenges, why would selection favour terrestrial plant life? Certainly, higher light levels, increased availability of carbon dioxide and less competition from other organisms would have offset the costs. But not for long. Crowding meant that plants needed to grow taller and put down an anchor to stake their territory. It was the evolution of stems that allowed them to grow vertically, eventually forming dense forests of ferns and other fern-like plants.

Fossil of early clubmoss genus Baragwanathia. Clubmosses possess a central cylinder of vascular tissue; the tiny leaves also have a vascular strand.
Fossil clues
Early Devonian (419–393 million years ago) localities have yielded some remarkable plant fossils, giving researchers valuable insight into the evolution of plant form and structure. Northern Scotland’s Rhynie Chert, once an ancient hot spring, is especially rich in fossil plants that most closely resemble modern clubmosses. The 40 cm-tall (16 in) Aglaophyton major lacked true roots and leaves, and was composed of creeping rhizomes and bifurcating vertical stems, which terminated in spore-bearing sporangia. Like some existing mosses, A. major had no true vascular tissue, but rather elongated, tubular cells that functioned in transport (see box). By comparison, larger plants such as Asteroxylon were vascularized and bore small leaf-like scales. Interestingly, neither deep roots nor leaves would have been essential to the survival of early land plants: hydric habitats provided essential water, while an atmosphere rich in carbon dioxide ensured relatively high levels of photosynthesis.
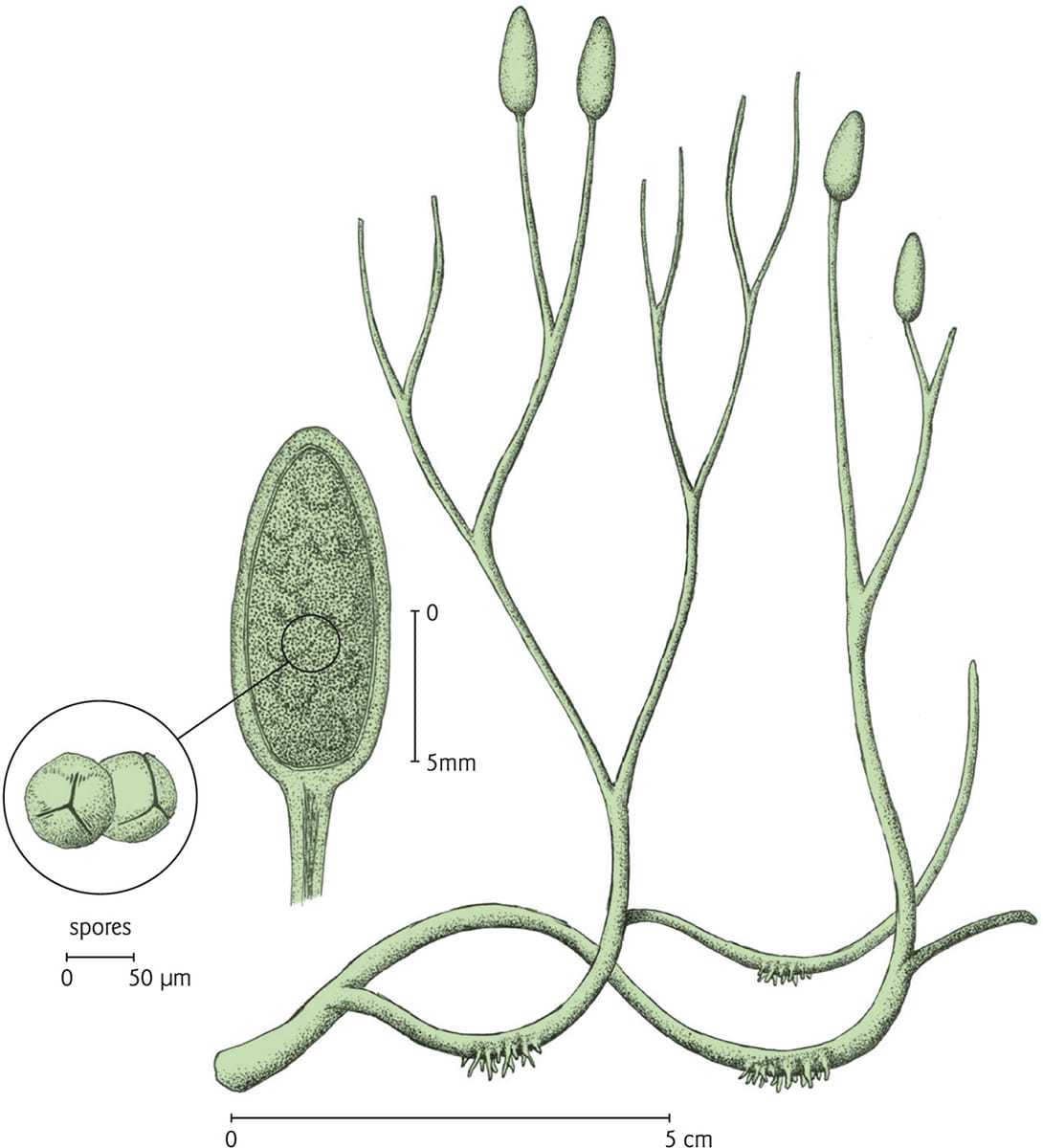
A rendering of Aglaophyton major, a Devonian-era plant that most closely resembles today’s mosses. It is composed of stems, some of which terminate in spore-bearing structures.
Support and transport
The proliferation and increasing diversity of plants during the Devonian created competition that selected for true roots, broad leaves and tall stems. Arborescent clubmoss relatives, ancestral seed plants and fern-like vegetation comprised Earth’s first forests, and these were dense and complex, with an organismal array that rivalled contemporary ecosystems. But to leap from Aglaophyton to Carboniferous forests is to forget that in order to grow tall, Devonian flora had to contend with gravity and the need for efficient long-distance conduction. This was especially important for those plants colonizing drier habitats. Without a dependable source of water, plants needed root and vascular systems to ensure consistent hydration. Trees efficiently achieved both conduction and canopy support by way of wood, and the fossil record shows that woody stems appeared in the Middle Devonian, nearly contemporaneously with the Rhynie Chert flora. New discoveries may push this date even earlier and challenge our present interpretation of the evolution of early land plants.
The middle Palaeozoic saw fascinating variation in stem morphology, much of which has no analogues in contemporary vegetation but speaks to the diversity that is possible in plant structure and function. Countless species and scores of botanical experiments have been transformed into coal beds or have disappeared altogether, leaving us to wonder about their botanical mysteries.

Lepidodendron, an arborescent clubmoss. Reaching heights in excess of 30 m (100 ft), these plants were abundant in Carboniferous swamps.
The structure of woody stems
Wood is a marvel of economy. It is, for the most part, a dead tissue composed primarily of cellulose, pectins and phenolic constituents such as lignin, with a small fraction of living tissue. Its maintenance costs are negligible and yet it manages to achieve two tasks at once: it provides mechanical support of the canopy, and it enables the transport of water over long distances. Trees are nature’s skyscrapers and their elegance lies in the adaptive structure of wood.
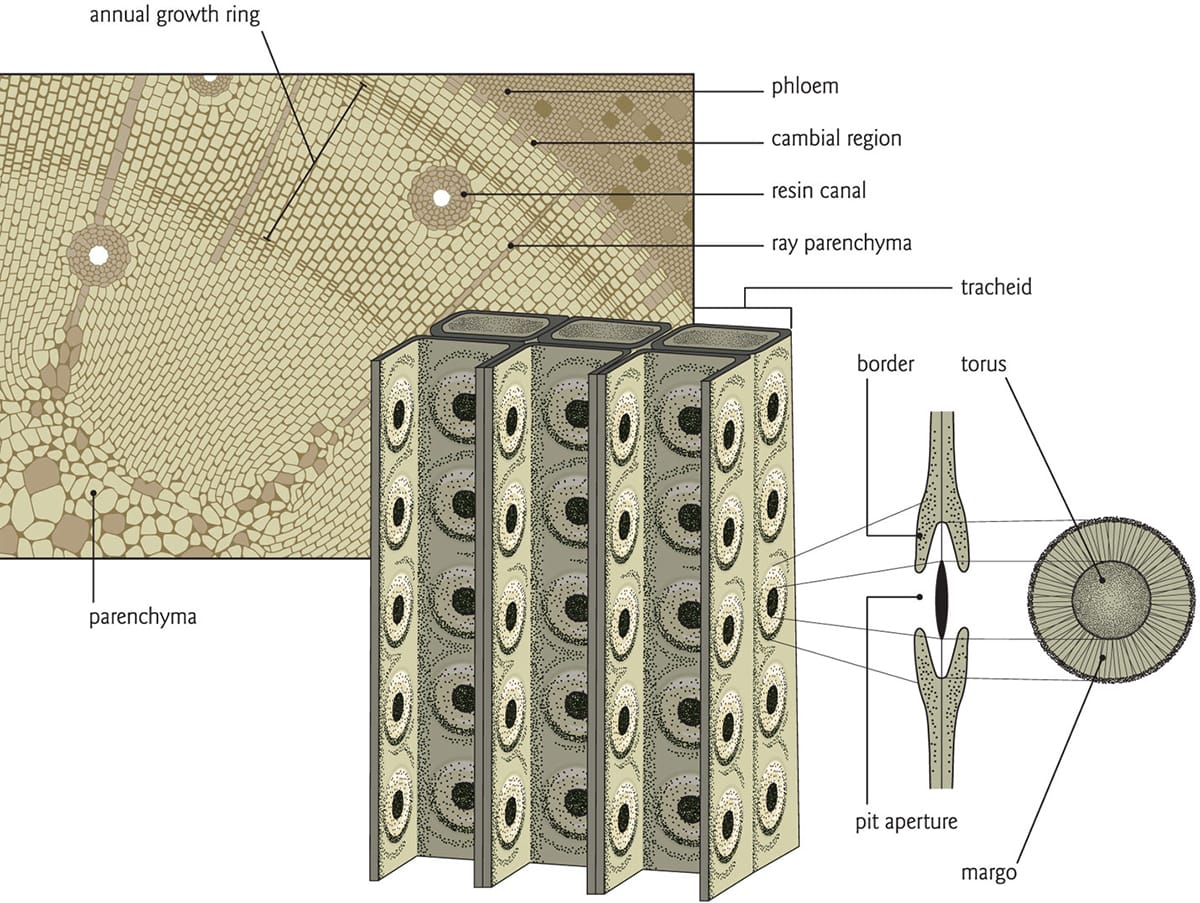
Water transport in conifers occurs in tracheids, single cells connected to one another with torus-margo pit membranes. Water moves from one tracheid to another through the pits.
Wood structure and function
Conifers and angiosperms are loosely categorized as softwoods and hardwoods, although the wood of some conifers can be just as hard as that of some angiosperms, while many angiosperm species have wood that is relatively pliant. Wood is also known as secondary xylem and the seasonal production of growth rings adds to the girth of a tree. In conifers, more than 90 per cent of wood is constructed of overlapping, dead single cells known as tracheids, which conduct water to the canopy as well as support the tree (see box). The remainder consists of parenchyma tissue, which functions in the transport and storage of sugars and other constituents within the xylem. Angiosperm wood, by contrast, is more specialized. Consisting of wide vessels for water transport, narrow fibres for mechanical reinforcement (and storage), a small fraction of tracheids and more than 15 per cent parenchyma tissue, it exhibits a high degree of developmental flexibility that supports, in large part, the great diversity of canopy structures we see in flowering trees.
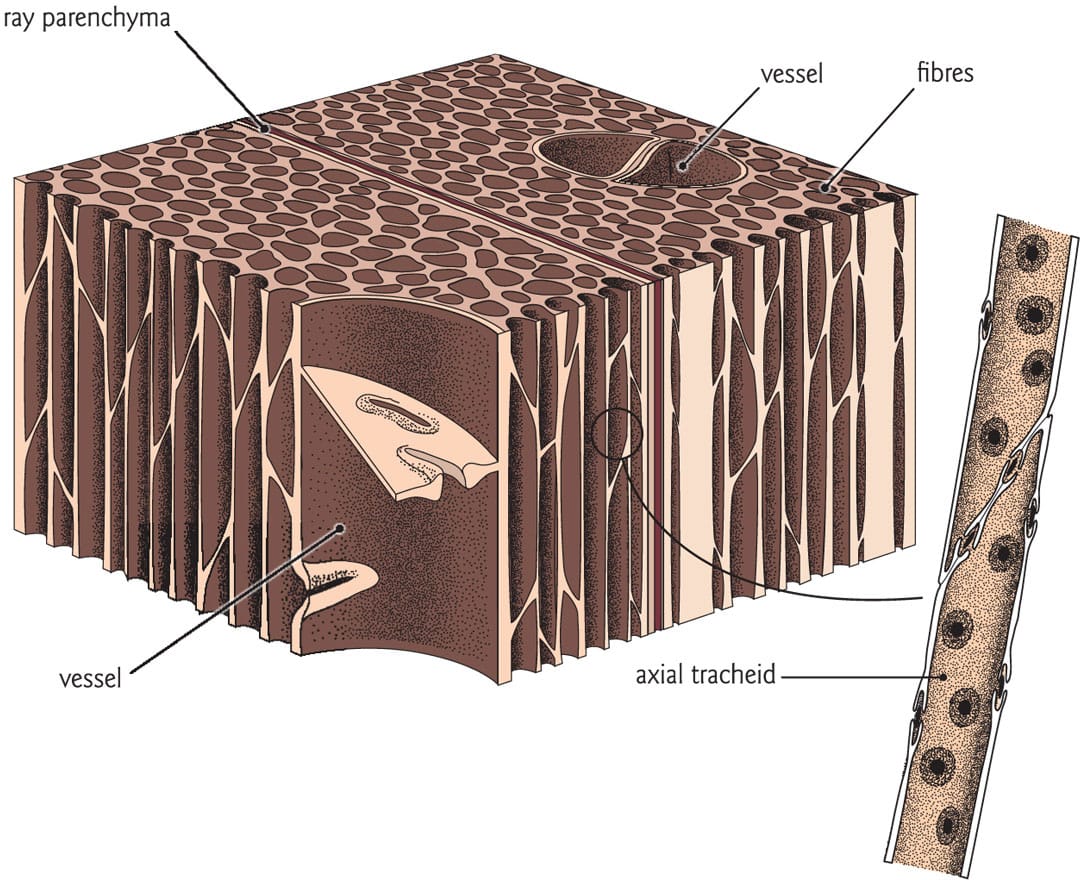
In angiosperms, water moves through vessels, which are composed of single hollow cells known as vessel elements. The vessels may be more than 2 m (6.5 ft) in length, and are provided with structural support by the surrounding fibre cells.
Growth rings
Further attributes and distinctions can be observed in a cross section of wood. Within a growth ring of either a conifer or angiosperm, earlywood is produced at the start of the growing season, and consists of relatively large xylem conduits with thin walls. Large conduits ensure efficient water delivery to the expanding new leaves. By contrast, latewood consists of smaller cells with thicker walls and is thought to function in support. The degree to which earlywood and latewood are discernible depends in large part on the habitat of the tree. In temperate zones where the winter season enforces dormancy, these patterns are reliably observed, but not so in aseasonal or tropical habitats where trees may grow continuously. It can therefore be difficult to determine the age of tropical trees using growth rings.
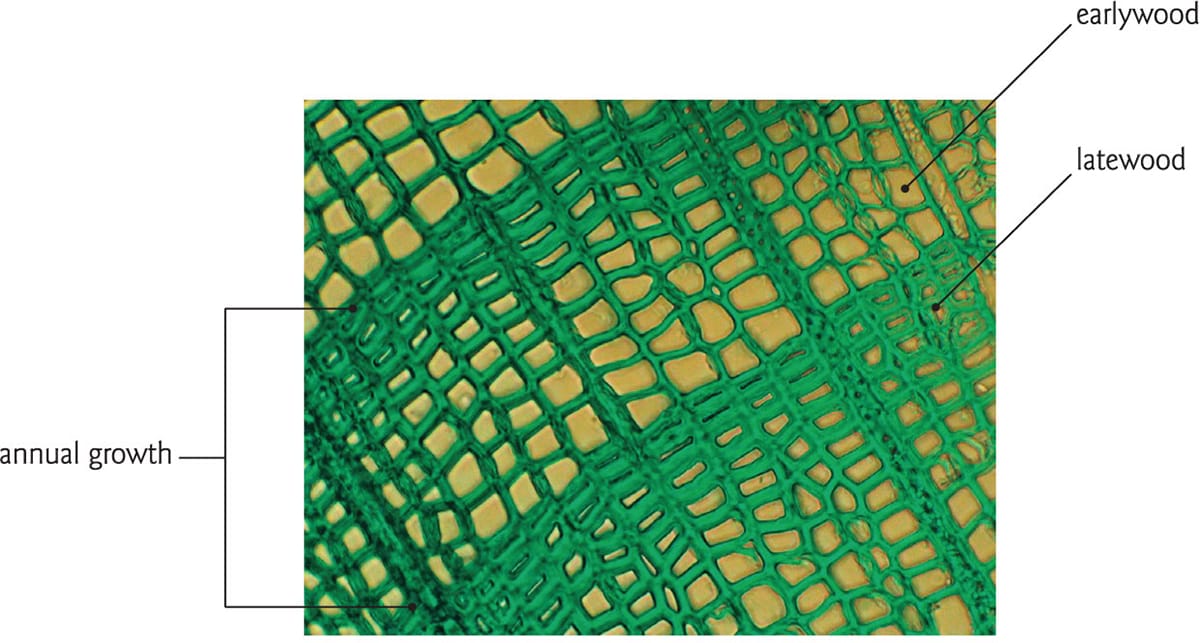
Earlywood xylem conduits are larger than those produced later in the growing season. Bigger conduits, whether conifer tracheids or angiosperm vessels, move water more efficiently than small ones, so earlywood is essential for new leaf growth in the spring.
Sapwood and heartwood
Tree trunks and older stems typically have regions of sapwood and heartwood. Sapwood forms the outer part of a woody stem, and as the name implies, conducts water to the canopy. Its pale colour is in contrast to the typically darker heartwood, which no longer has functional xylem or living parenchyma, and is thought to play a structural role. Permeated with phenolic constituents and resins, heartwood is prized for its colour and resilience, and lends its unique properties to musical instruments, fine furniture and artisanal wood products.

Heartwood is typically darker in colour due to higher concentrations of tannins, phenolics, oils or gums. The moisture content of heartwood is lower than in the sapwood.
How trees grow
For trees to grow tall, they must also expand in girth. Tree trunks become wider each year because annual xylem is added to supply water to the new season’s leaves. Thicker trunks also improve stability, so altogether, the appropriate scaling of stem height and girth is the most basic requirement of functional tree architecture.
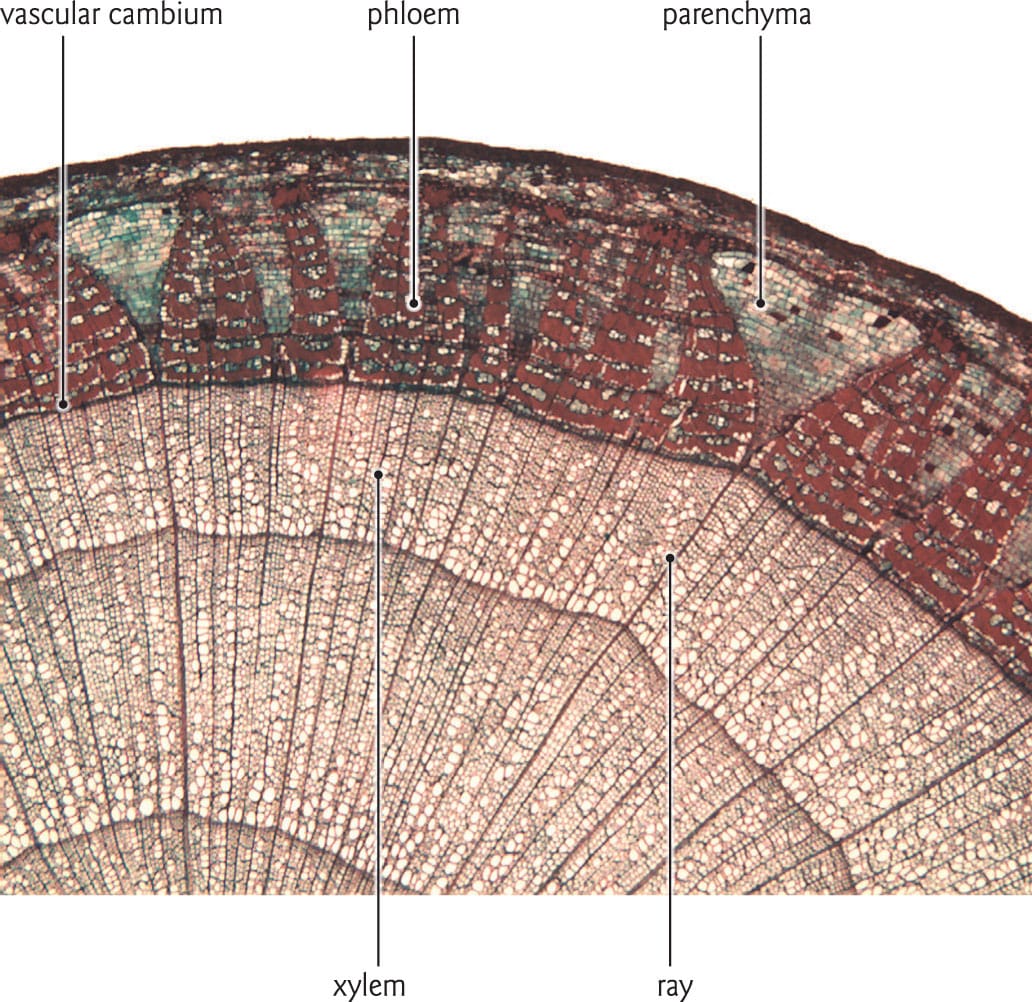
The vascular cambium generates xylem toward the centre of the stem, and phloem toward the outside. Rays connect the phloem and parenchyma tissues with the xylem.
The cambial layer
Non-woody vegetation exhibits primary growth, whereas woody plants such as trees and shrubs develop both vertical extension and radial expansion, a process known as secondary growth. Tissues such as secondary xylem (wood) and secondary phloem increase a stem’s girth, and it is the cambial zone, a thin meristematic layer of one or two cells, that facilitates this growth. The cambium is located on the periphery of the woody tissue not far below the bark, and is composed of fusiform and ray cells. The division of fusiform cells toward the centre of the stem produces annual growth rings of secondary xylem, while division toward the outside of the stem generates secondary phloem. Ray cells generate parenchyma tissue that penetrates both the xylem and the phloem. Because phloem is a soft tissue that compresses with the annual expansion of the xylem, it does not produce discernible growth rings.
In temperate climates, the cambium is dormant during the winter, but the expansion of stem buds in the spring initiates cambial cell division by the downward transport of the hormone auxin through active phloem tissue. Active cambial tissue then divides both radially and axially to increase the circumference and the height of the trunk. Lateral branches also originate from the cambium, and develop like the trunk.
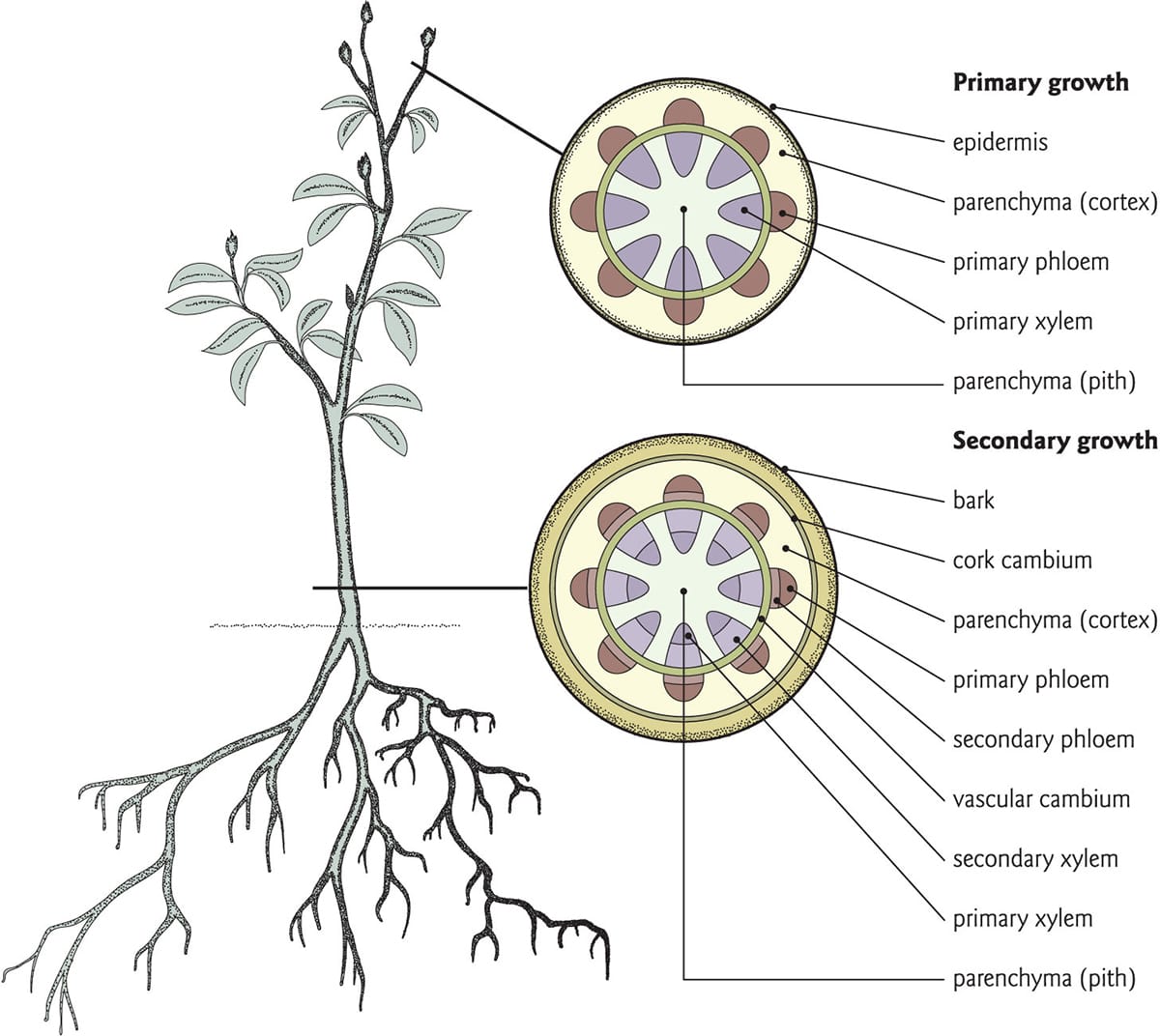
Herbaceous plants, such as sunflowers, can exhibit secondary growth. In young stems, the vascular cambium develops below the region of primary growth, and generates secondary growth by dividing laterally as well as radially. Lateral divisions add cells to the cambial cylinder, increasing its circumference and, ultimately, stem girth. Radial divisions of the cambium produce xylem towards the pith, while phloem develops in the direction of the bark. The cambium produces much more secondary xylem than phloem, so wood constitutes the highest volume fraction of a tree’s secondary growth.
Variations in cell production
The cambium gives rise to cells of various dimensions and wall thicknesses depending on the tree species and time of year the cambium is active. In conifer and temperate angiosperm trees, the cambium produces some earlywood and latewood, although the proportion of each cell type depends on climate and water availability. For example, a relatively wide growth ring with several stacks of large earlywood cells would have been produced during moist conditions early in the growing season, whereas a narrow growth ring with only a few earlywood cells indicates a short or dry summer.
In angiosperm trees, we see further diversity of cell types. Here, the cambium produces fibres, which function in storage and canopy support, as well as vessels that conduct water. In ring-porous species such as some oaks or chestnuts (Castanea spp.), the earlywood vessels form a visibly distinct band in a growth ring, whereas in diffuse-porous species such as birches (Betula spp.) and maples (Acer spp.), the vessels are similar in size throughout the growth ring.
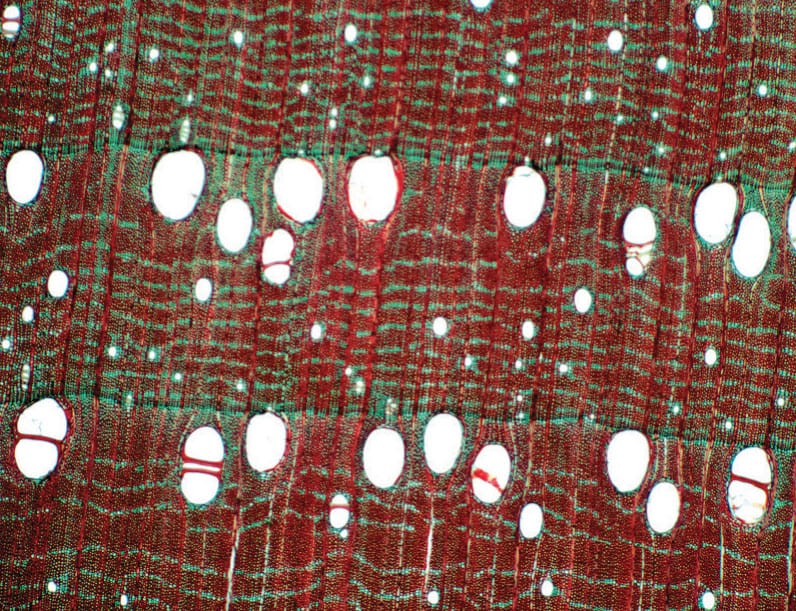
Ring-porous wood (above) is characterized by the presence of large earlywood vessels, which form a discrete band in a growth ring. By contrast, vessels in diffuse-porous wood (below) are similar in size and relatively evenly distributed throughout the growth ring. Ring-porous species tend to have fewer vessels than diffuse-porous species, but these are typically wider and longer to support efficient water transport.

The structure of herbaceous stems
Investing in wood is an unnecessary cost for herbaceous plants with short life cycles, which instead rely on their primary tissues to provide sufficient support for floral displays and the development of seeds. It is generally accepted that herbaceous dicots evolved from woody ancestors sometime during the Cretaceous (145–66 million years ago). In fact, the capacity to produce wood has re-evolved in some herbaceous dicots found in dry, stressful habitats. Monocots, on the other hand, split from dicots earlier, possibly at the end of the Jurassic 145 million years ago.
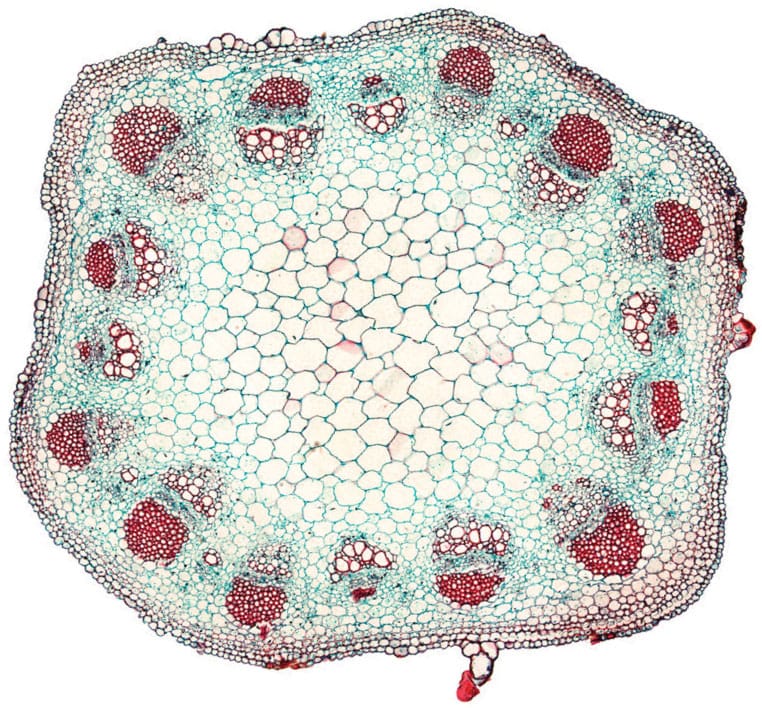
Primary growth in a transverse section of a sunflower (Helianthus) stem. Numerous vascular bundles surround the pith; each bundle consists of xylem, phloem and a dense fibre cap.
Dermal tissue
The dermal tissue is typically only one cell layer thick, and covers the exterior of the stem tissue, as well as all other plant organs. In green photosynthetic stems, it may be modified to include stomatal pores to permit the entry of carbon dioxide. Extruded by the epidermal cells is a waxy cuticle, which protects against water loss and the entry of pathogens. Epidermal cells modified into glands or trichomes are not uncommon on stems, but are usually found in greater abundance on leaves. The epidermal tissue is often under tension due to the positive pressure exerted by the hydrated ground tissue.
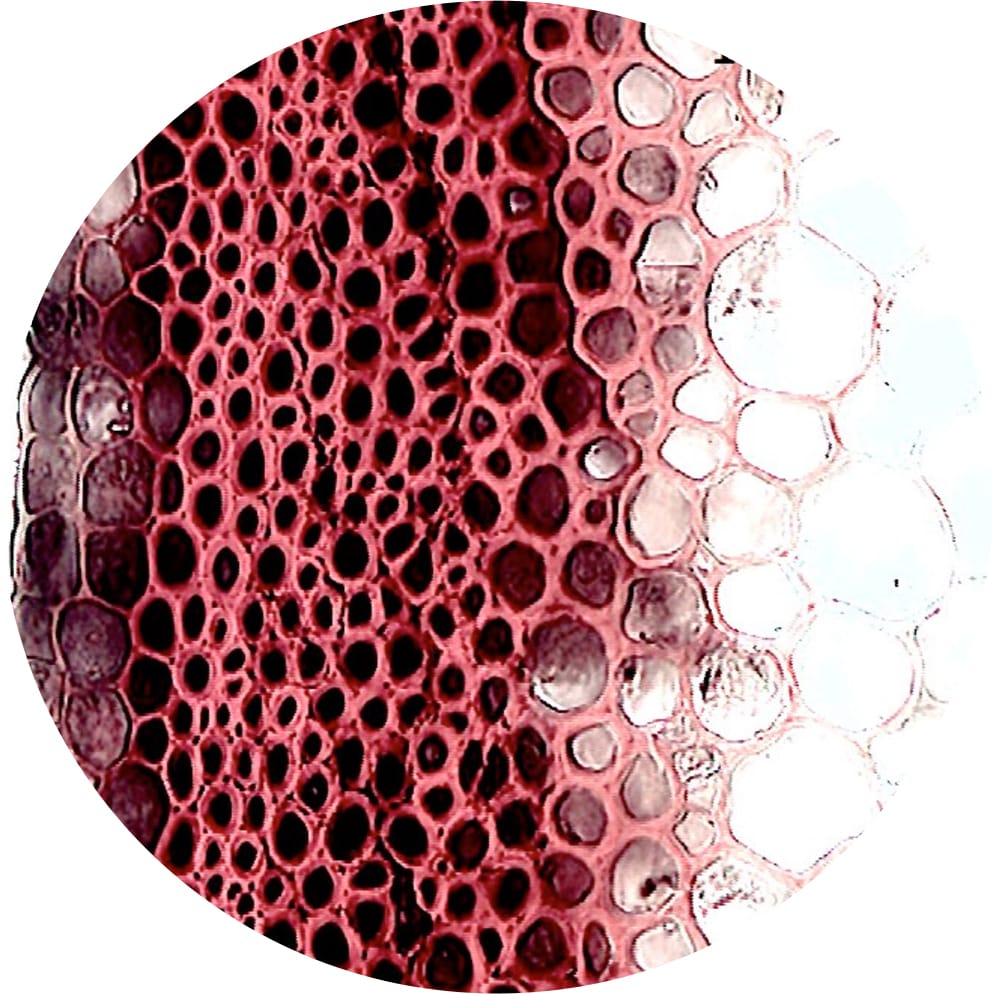
The sclerenchyma fibres have thick walls to protect the phloem and impart strength to the stem. They are typically much narrower and denser than adjacent cortex parenchyma cells.
Ground tissue
Stem ground tissue serves in storage, support, and protection from insects and pathogens. There are three types of ground tissue. Simple parenchyma comprises the bulk of a dicot stem. It is typically white and fleshy, is made up of large cells and is clearly visible in the centre of a stem cross section. Such cortex parenchyma functions in the maintenance of turgor and plant structure, as well as the storage of water, nutrients and carbohydrates. Collenchyma and sclerenchyma tissues are derived from parenchyma tissue, and function in support. Flexible support is provided by collenchyma tissue, which is composed of parenchyma cells that are much reduced in size and exhibit selective thickening of the primary cell wall. It is common in stems with ridges and corners, such as those found in mints (Mentha spp.). Sclerenchyma tissue is substantially more rigid than collenchyma, and is found in older stem sections that have stopped elongating. Much like wood, sclerenchyma cells have strong, lignified secondary cells walls, except that they are much shorter and narrower, forming fibres. Sclerenchyma may occur near the periphery of a stem, but more commonly it is found in bundles just outside the phloem tissue, protecting these delicate cells from injury or phloem-feeding insects.
Vascular tissue
Xylem and phloem constitute the plant vascular system, delivering water, minerals, sugars and signalling compounds throughout the plant body. Xylem functions in the transport of water, and in herbaceous dicots it is composed of vessels and fibres with lignified secondary walls. In vessels, the walls often form rings or helices, which provide the xylem with some flexibility as the stem develops. One can think of vessels as a series of short, wide, single-cell derivatives known as vessel elements, stacked to create a long, hollow tube. Fibres are narrow and short, and reinforce the vessels as well as support the stem. Xylem tissue becomes functional once cells reach maturity and die, leaving behind their cell walls. By contrast, phloem is a living tissue and serves to deliver sugars and signalling compounds throughout the plant. It is always associated with xylem.
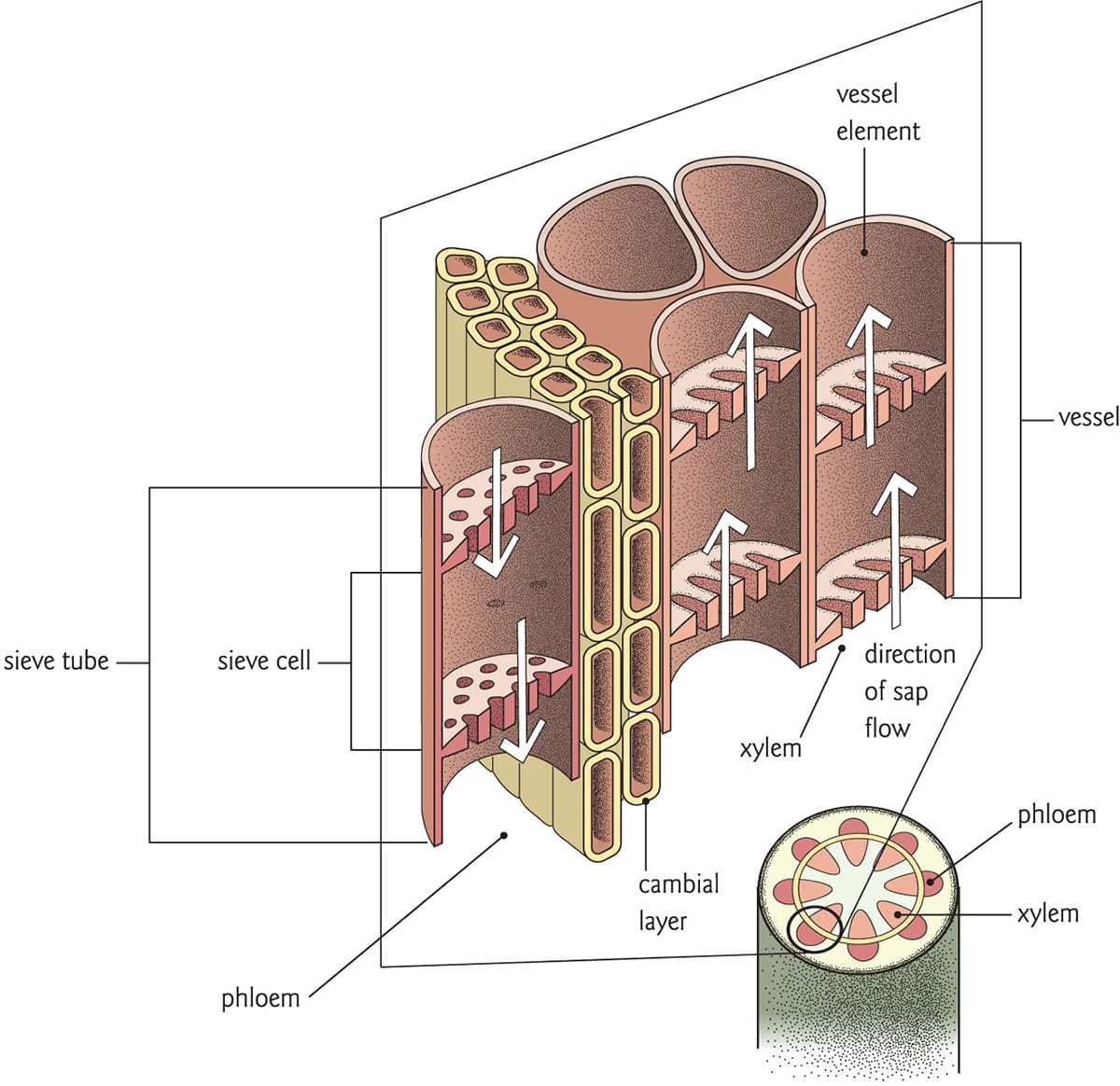
Xylem transports water, and comprises long tubes of cells called vessels. Sugars are translocated in the phloem to wherever they are needed for growth. In contrast to the xylem, phloem is composed of living cells.
Bamboos, palms and tree ferns
Similar to monocots and living ferns, bamboos, palms and tree ferns lack a true vascular cambium and are unable to produce secondary growth. This means that their trunks are generally narrower than those of woody trees and devoid of lateral branches. Despite these constraints, palms and bamboos can develop huge amounts of biomass and exceptionally strong stems very quickly, while tree ferns are recognized as resilient and are often principal entities in tropical forests.

A cross section (above) of a bamboo stem. The vascular bundles can easily be seen in products such as chopsticks or flooring. They appear as dark spots (below) due to the sclerenchyma fibres that surround the vascular tissue.

Bamboo stems
Bamboo stems have long been prized for their utility in building and construction, and their rapid growth rates make them a reliable and sustainable material resource. The exceptional material properties of bamboo can be attributed to several factors. Unlike wood, bamboo is a composite structure made of about 50 per cent parenchyma and 40 per cent lignified fibres, with vascular tissues comprising the balance. Fibres and vascular tissues are packed in primary vascular bundles that are scattered throughout the ground parenchyma in a standard monocot arrangement (see box), with a higher density of bundles located near the periphery of the stem, where bending stresses are highest. In addition, the cylindrical shape of the stem, in combination with solid nodes, makes bamboo especially resistant to deformation.

Mature green bamboo stem (Phyllostachis sp.) behind a young brownish shoot. The nodes are evident as white rings on the mature stem.
Palm trunks
The trunks of palm trees also subscribe to the standard vascular bundle arrangement of monocots, but unlike most of their relatives, they have exaggerated both the girth and the height of the trunk to achieve tree-like stature. Palm trees are able to withstand the extraordinary bending stresses imposed by hurricanes and floods by capitalizing on trunk architecture that derives its strength from a combination of cross-sectional thickness and the heterogeneous distribution of fibrous vascular bundles, similar to bamboos. Palms develop from large-diameter juvenile stems that allow the mature plant to meet expected canopy support requirements; some small degree of cell division and expansion may allow for additional increases in trunk girth. However, the capacity of palms to stiffen with age is the key means by which they accommodate increased loading with height. Reduced water content and increased peripheral tissue density and stiffness together serve to strengthen the ageing trunk (see box).
Tree fern trunks
The structure of tree ferns is markedly different from bamboos and palms. The trunks of these plants derive their strength primarily from the overlapping remains of senesced petioles, which surround the ground tissues like a basket. Furthermore, the leaf bases are encased in a thick layer of sclerenchyma fibres, adding enormous rigidity and strength to the trunk. Indeed, such massive amounts of sclerenchyma have given tree ferns a reputation for ruining saws. As in bamboos and palms, transport in tree ferns occurs via primary xylem and phloem, but unlike in monocots, these tissues form a cylinder toward the outside of the trunk.
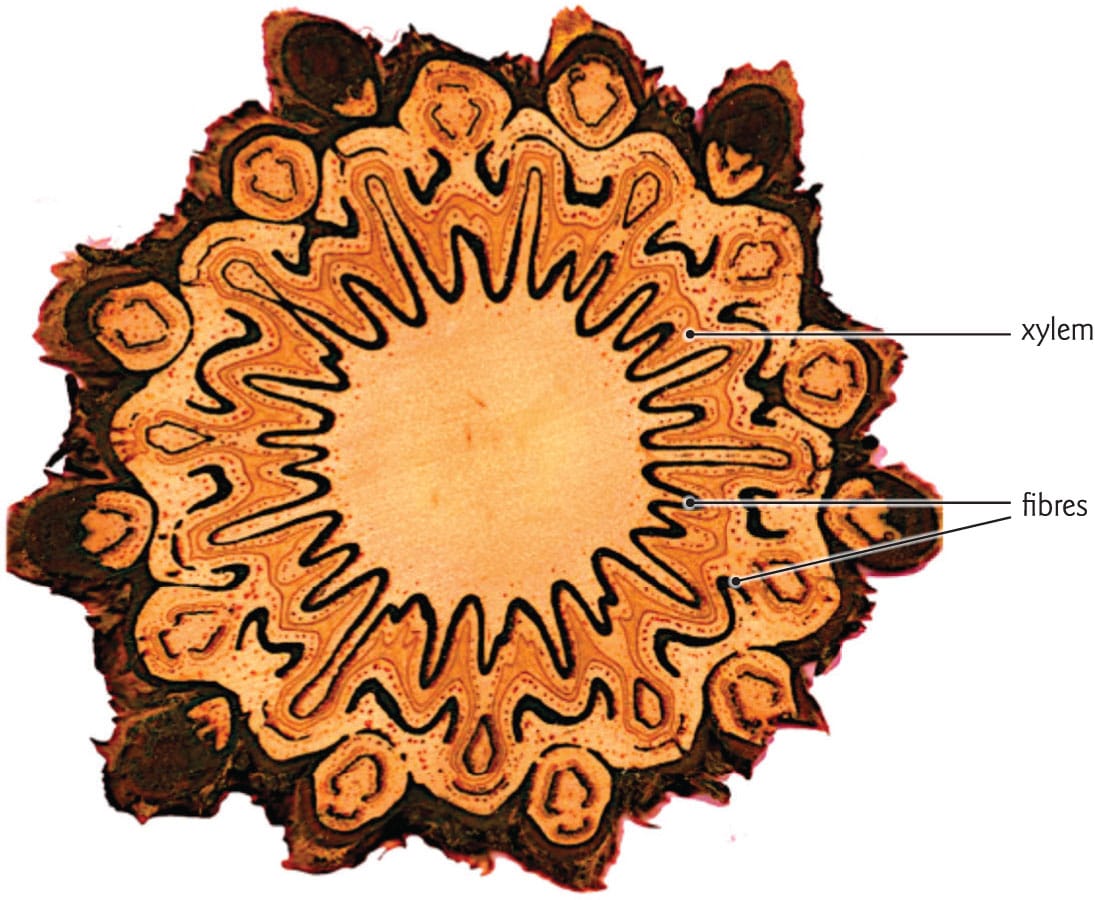
A cross section of the trunk of a soft tree fern (Dicksonia antarctica). Overlapping frond bases comprise the exterior of the trunk. An undulating ribbon of vascular tissue is sandwiched between dark layers of sclerenchyma.
Plant water transport
Most plants consume a tremendous amount of water to sustain themselves. While the average human requires 2–3 litres (4–6 pints) of water per day for good health, a maple tree will move 200–400 litres (50–100 gallons) of water from the soil to the leaves. Why so much? It is because transpiration is an inescapable side effect of photosynthesis. Leaves are sufficiently porous to allow the entry of carbon dioxide, but at the cost of water loss through these same pores. It is the efficient delivery of water that keeps transpiring leaves from wilting.

The xylem vessels in the stem’s sapwood are sufficiently conductive to deliver water from the roots to the sprawling, thirsty canopy of this leafy tree.
Tug of war
The upward movement of water in a plant begins soon after the first leaves appear. Water moves passively from regions of high water availability, such as the soil, into the roots and up to the canopy, which is significantly drier. This long-distance transport takes place at practically no cost to the plant. Moisture and sunshine are all that is needed to open stomatal pores, initiating transpiration and with it the conduction of water through the xylem. To achieve this, plants take advantage of some useful properties of water. For a start, the hydrogen bonds between water molecules make water cohesive and allow it to sustain tension similar to a string pulled from both ends. So in a transpiring plant, water is effectively pulled from the ground to the leaves in a tug of war between soil particles and the relatively parched atmosphere.
Through the roots, to the stem…
Water first enters the xylem after filtration through the root’s endodermis. Once there, it moves both upward and radially through the stem via vessels or tracheids. Friction from conduit walls slows the flow of sap, as can the movement of water from one conduit to another through pit membranes. This resistance adds to the tension of the water column. When water finally reaches the canopy, it enters numerous small veins, which irrigate the leaf mesophyll tissue. It is here, in the heart of the leaf, where the crux of plant water transport lies.

Water loss is an inescapable consequence of leaf function. This is because carbon dioxide must diffuse into the leaf for photosynthesis to take place. Many more molecules of water are lost from the leaf than carbon dioxide molecules are fixed.
… and the leaves
Mesophyll tissue is moist, but as the leaf warms in the sun, water trapped in the cell walls turns to vapour, which exits through the stomata. Plant cell walls are constructed of cellulose fibres (see box), so evaporation causes the slight retraction of menisci in the cell wall pores. This is an energetically unfavourable state, so hydrogen bonding continually rectifies the curved menisci. However, levelling the air–water interface requires additional water molecules, and these are supplied by the xylem stream, which remains cohesive as long as sufficient water exists to replenish that lost from the leaf. Taken together, this cohesion–tension mechanism explains plant water transport.
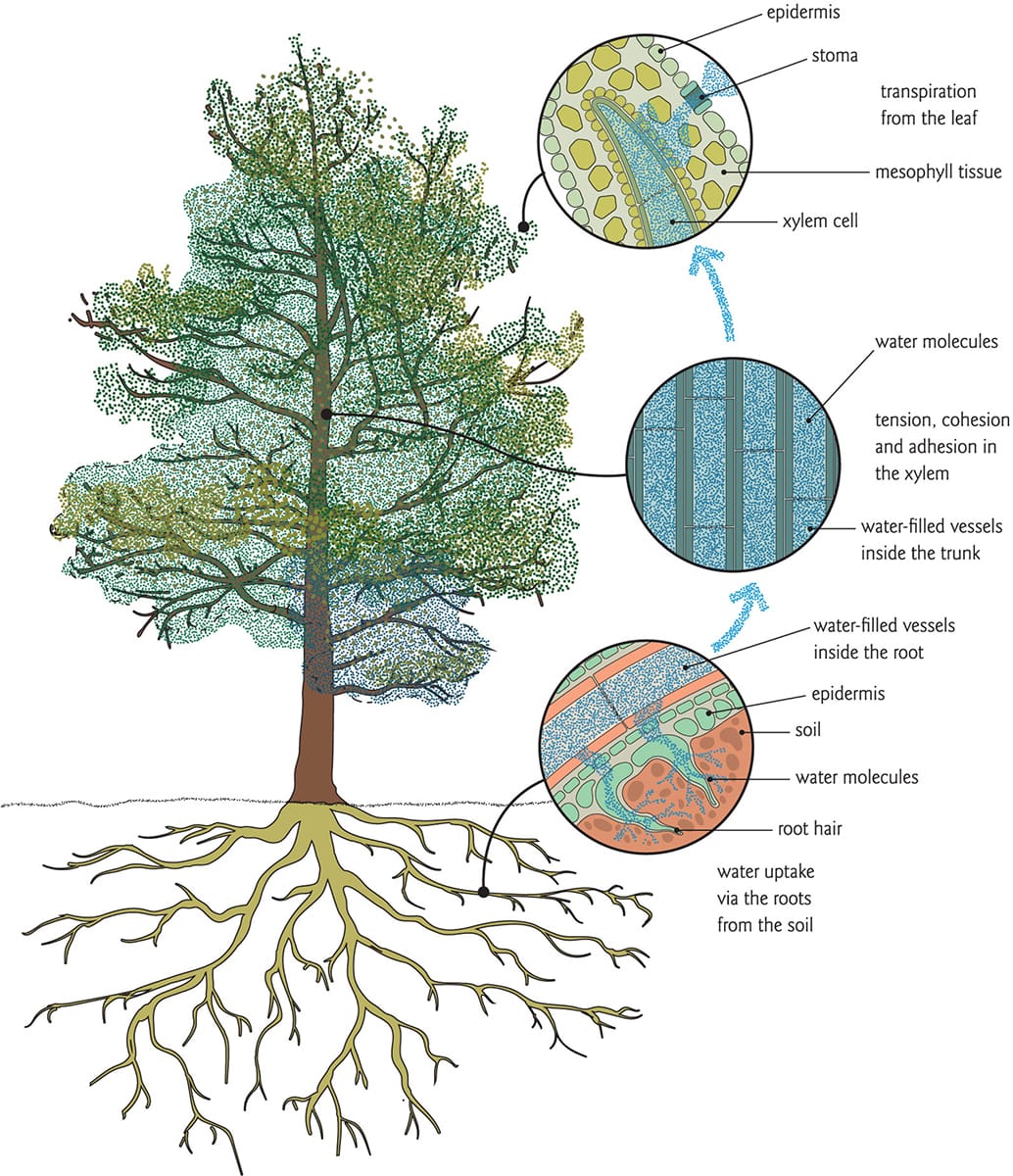
Plant water transport is a passive process in which water is ‘pulled’ up from the soil into the roots and vascular system, and into the canopy, where much of it is transpired to the atmosphere through stomata. The water thus moves from regions of high to low water availability under some degree of tension.
Water transport during drought
During episodes of water deficit, it becomes more difficult to pull water molecules from soil particles to replenish what is lost from leaves during transpiration, so significant degrees of tension can arise in the xylem sap. Plants are prepared for this. First, they fortify their xylem conduits with appropriately thick walls or supportive fibres to prevent buckling of conduits under tension. Second, they have evolved ways of coping with the tension-induced transport failure that frequently occurs under drought.

Insufficient water transport through the stem may lead to wilting of leaves and flowers as seen here in a poppy.
Cavitation and embolism
Maintaining functional water transport is one of the biggest challenges a plant faces during dry conditions. This is because xylem sap under tension is susceptible to cavitation, or the rapid conversion of liquid water into vapour – effectively a break in the water column. Cavitation produces embolized conduits, in which the water in the cell is replaced by a mixture of liquid and vapour, such that the cell can no longer transport water. Many embolized conduits impede water flow through the xylem; this can contribute to the dehydration and subsequent death of the plant.
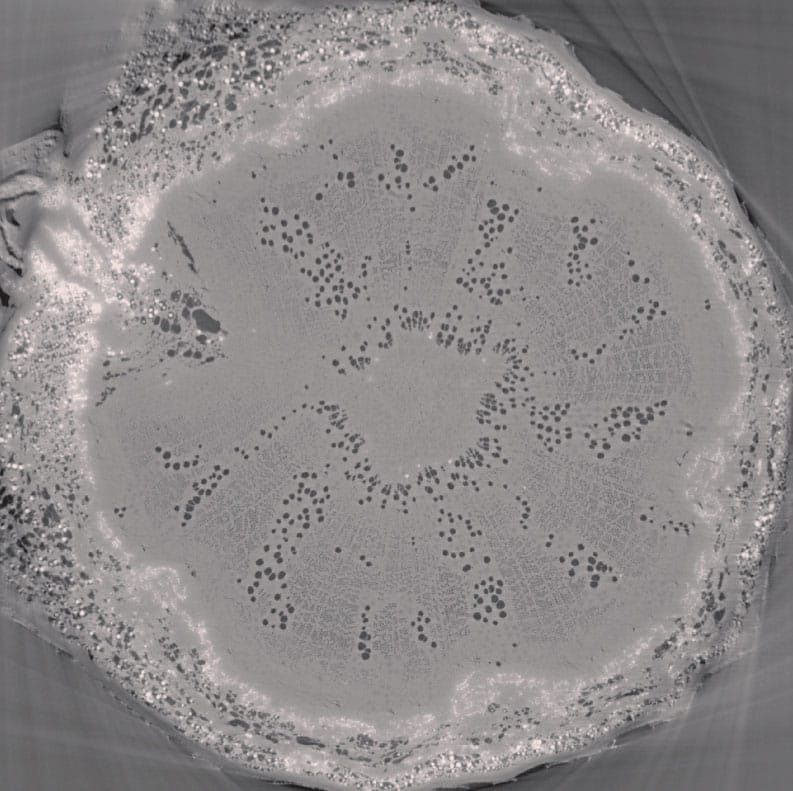
Air-filled vessels appear as dark holes in this image of oak (Quercus sp.) xylem, generated using high-resolution computed tomography.
Cavitation is largely caused by the presence of air bubbles in the xylem stream. Bubbles expand when the tension in the xylem exceeds the inward force exerted by the surface tension of the bubble’s air–water interface. The bubble then begins to expand until it fills the conduit, with the displaced water moving into a functional neighbouring cell. We know that the endodermis in the roots keeps air bubbles and impurities from entering the xylem stream, so where does the air come from? Oxygen is needed to support living stem tissues. Therefore, a plant is not hermetically sealed; air can enter the xylem either from spaces that surround the cells or from an injury to the stem. Nanobubble phenomena are also currently under investigation.
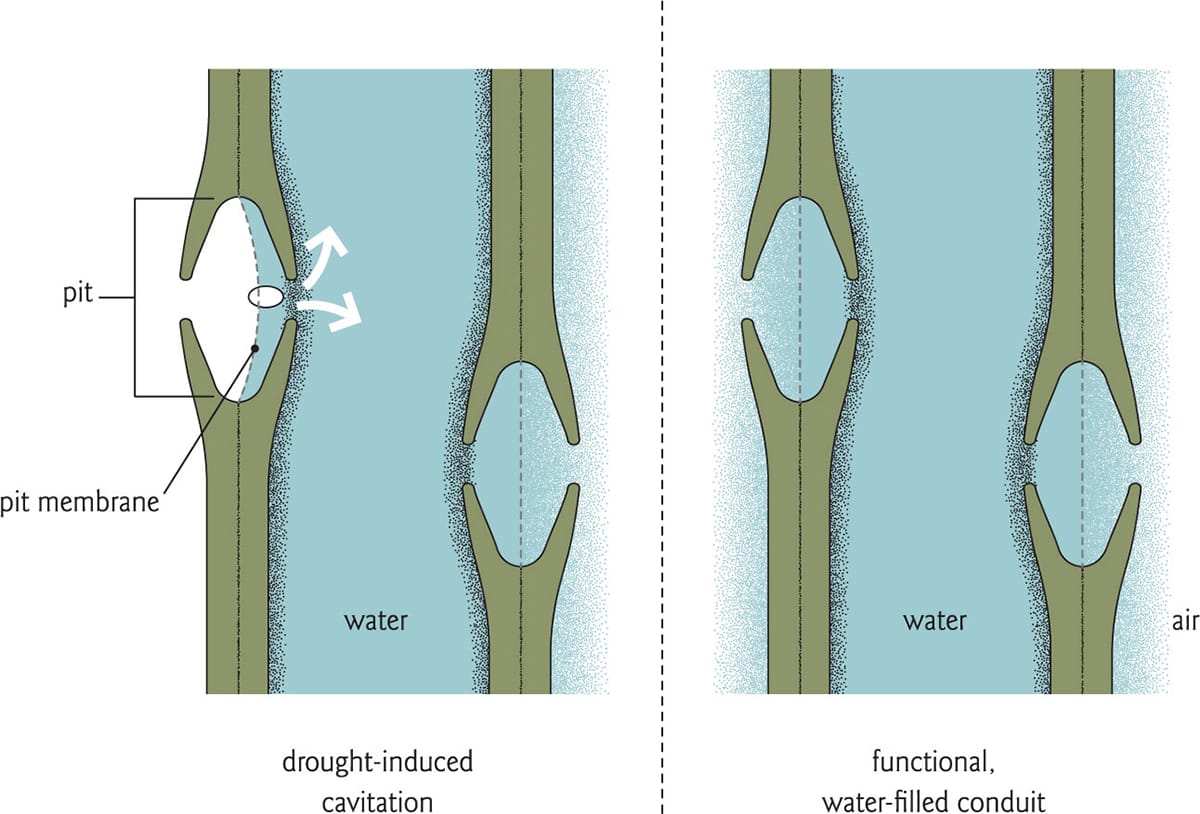
Drought-induced cavitation occurs when air is pulled from a dysfunctional (embolized) vessel to one that is water-filled through the largest pore in the shared pit membrane. This air bubble can expand, creating another embolized vessel.
Adaptations to prevent cavitation
Most air bubbles enter the xylem stream through pit membranes in the conduit wall. Composed primarily of cellulose and pectins, these structures allow water to flow from one conduit to another, but because they are porous, they also permit the spread of air. In flowering plants, pit membranes have a homogenous, fabric-like structure, and they are often clustered along the vessel walls. Air bubbles spread through the largest pore in the pit membrane, a developmental lesion that is the so-called weakest link. There is evidence to suggest that species with thicker pit membranes are less likely to develop large pores, and are thus more resistant to drought-induced cavitation than those with more porous membranes. Thicker pit membranes are not always advantageous, however: reduced vulnerability to cavitation may come at the price of increased resistance to water transport.
Ecological xylem anatomy
Stems are core elements of plant structure and must maintain their functional integrity despite episodes of drought or freezing. The transport of water and phloem sap is key to growth and carbon acquisition, so the structure and function of plant vascular tissues, especially the xylem, has evolved to respond to climate cues. This can happen within individuals or populations, as well as across a broad range of temporal and spatial scales. Ecological xylem anatomy is as much a reflection of species’ natural history, as it is an indication of physiological constraints.

Snow-covered Norway spruce (Picea abies) in the Austrian Alps. This species’ narrow tracheids are adaptive in cold, high elevation habitats.

The chaparral ecosystem of southern California is dominated by shrubs. Challenged by drought, freezing and fire, these plants have adopted numerous life history strategies, which are reflected in their xylem anatomy.
Dealing with drought
A number of traits are typically associated with drought-resistant xylem, but species-specific variation, plasticity and varied life history can easily complicate even the simplest generalizations. For example, there is good evidence that tracheids are small and narrow in drought-adapted conifers like junipers (Juniperus spp.), whereas they are long and wide in water-loving species such as bald cypress (Taxodium distichum). Many small conduits increase redundancy, whereas fewer larger conduits favour efficient water transport. So why is it that not all conifers are as drought resistant as junipers? It turns out that xylem composed of many small tracheids is dense, making it more expensive to build. Faced with a certain carbon budget, conifers, and indeed all plants, have evolved to balance the economy of stem structure with respect to climate and competition.
The angiosperm shrubs that occupy Mediterranean and high desert ecosystems employ varied strategies of xylem structure and function. On the whole, species that are drought resistant exhibit narrow vessels embedded in a matrix of thick-walled fibres, which fortify the vessels from collapse under tension; these plants exhibit high wood density. Alternatively, deep-rooted species are less resistant to embolism due to a more constant water supply, and therefore allocate less carbon to xylem reinforcement.
Examples of xylem in the cypress family of conifers (Cupressaceae). From left to right: Clanwilliam cypress (Widdringtonia wallichii),

Coping with cold
In temperate climates, freezing temperatures selected for three general categories of wood, each associated with a unique life history strategy. This is because freeze–thaw cycles may also cause cavitation. Frozen xylem sap contains air bubbles that coalesce as the liquid sap turns into ice. As the ice thaws and tension resumes in the xylem, the largest of these bubbles may expand, cavitate the water column and embolize the conduit. Because the likelihood of cavitation increases with bubble size, which is directly proportional to conduit diameter, species with large-diameter conduits are more vulnerable to freezing-induced embolism. Ring-porous species such as some oaks and walnuts (Juglans spp.) have large vessels, and they abbreviate their growing season to avoid freezing. Diffuse-porous trees like aspens (Populus spp.) have narrower vessels, so they leaf out in early spring and lose their leaves in mid-autumn. Active year-round, conifers may photosynthesize in the winter providing that temperatures are mild, and so suffer frequent exposure to freeze–thaw cycles. It is their narrow tracheids that protect them from extensive embolism (see box).

The xylem of the drought-tolerant sugar sumac (Rhus ovata). Fewer and narrower vessels in combination with thick fibres help make this species resistant to transport failure by cavitation.
Phloem function
The movement of sugars and other substances throughout the plant occurs in the phloem. This living tissue is always adjacent to the xylem, but unlike xylem, it can deliver contents in any direction within the plant, from a source such as a mature, photosynthesizing leaf, to where a product is needed such as new foliage or developing fruit. Because phloem is dependent on xylem for hydration, prolonged drought can significantly compromise the delivery of sugars and other substances throughout the plant.
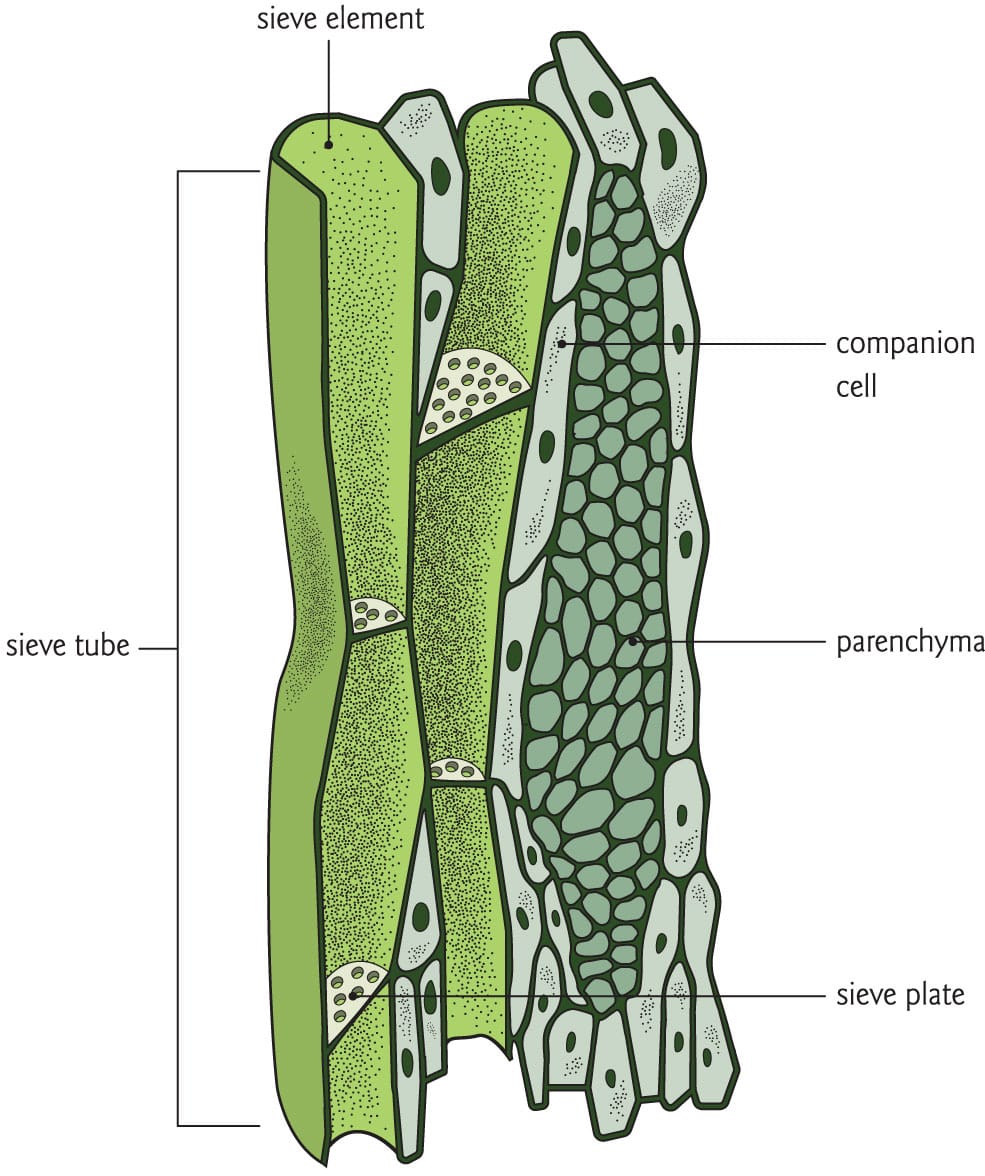
The structure of phloem. Phloem sap moves through sieve tubes, for which companion cells provide metabolic support.
Structure of phloem
In angiosperms, phloem is composed of two general cell types: sieve tubes and companion cells. Similar cells are found in the phloem of gymnosperms, but these are less well studied. Phloem sap moves through the sieve tubes, which are made of stacked individuals cells (sieve elements) connected to one another through porous end-wall regions known as sieve plates. Sieve elements lack nuclei as well as other essential organelles, and are thus dependent on adjacent companion cells for metabolic support. Companion cells have the full complement of plant organelles, so in addition to assuming the sieve elements’ metabolic workload, they also aid in the transport of sugars and solutes from leaf cells into the sieve elements. Copious plasmodesmatal pores connect companion cells and sieve elements, making phloem one of the most biophysically and metabolically dynamic tissues in the plant.
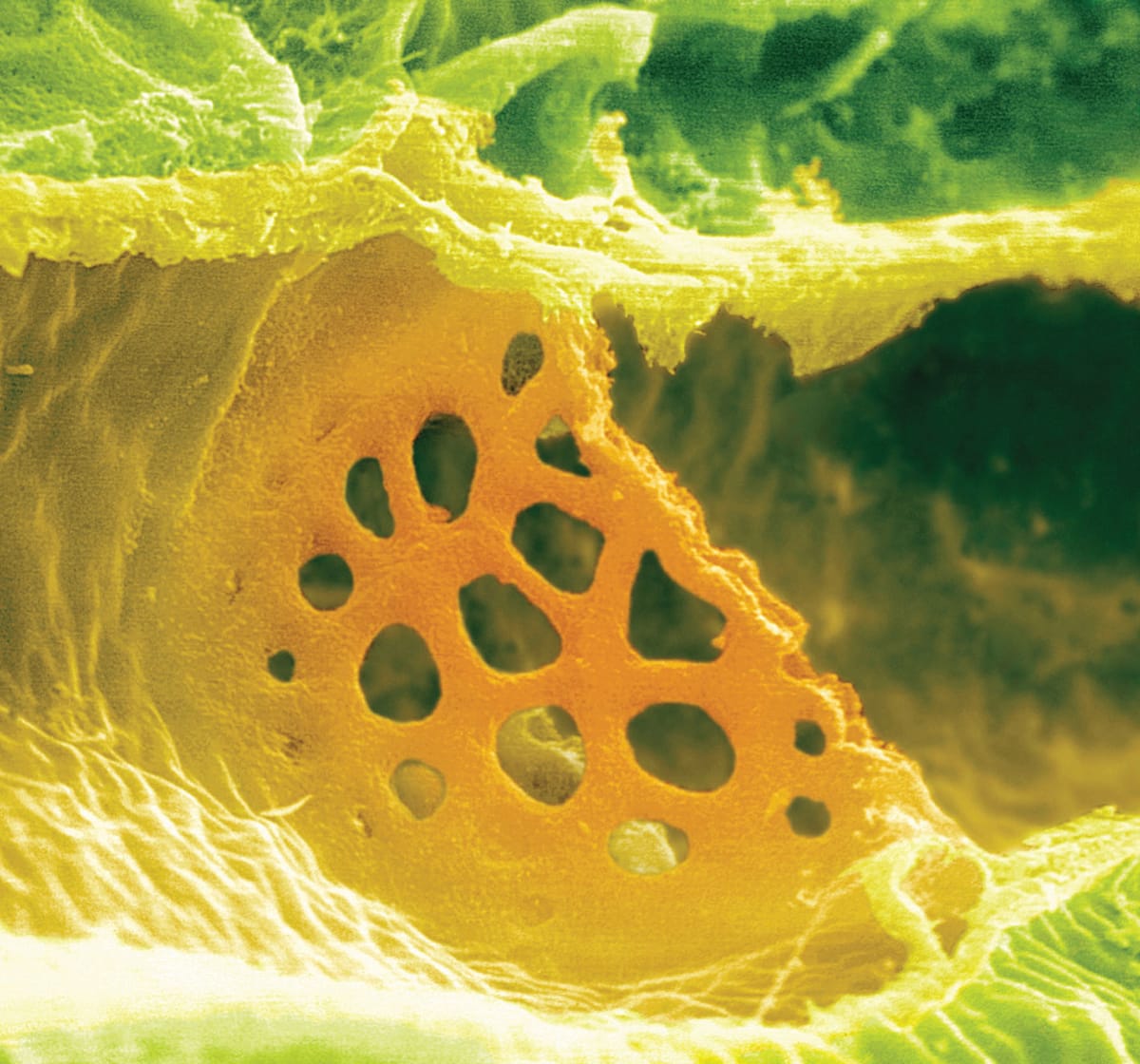
A sieve plate connecting two sieve elements. Wounding of the phloem tissue can trigger the occlusion of the sieve plate, and thus stop the flow of sap.
Transport through phloem
There is good evidence to suggest that long-distance transport of phloem sap is driven by osmotically generated turgor pressure in what is known as the pressure-flow model. Sugars produced in photosynthesizing leaves (or other sources) are loaded into the sieve tubes via companion cells, which are in contact with leaf mesophyll tissue. Elevated sucrose concentrations in the sieve tube decrease the osmotic potential of the sap, attracting water from adjacent xylem tissue into the sieve tube. This has the effect of increasing the turgor pressure in the sieve tube. With positive pressure driving flow, the phloem sap then passively moves from source to sink tissues. Sugars are unloaded in the sinks, which causes the turgor pressure of these sieve cells to drop. As a last step, water in the phloem is recycled back into the xylem stream. This cyclical process of loading, delivery and unloading of sugars is continuous, and much like water transport, an invisible yet critical activity inherent to all vascular plants.
The loading and unloading of sugars into and out of sieve tubes can either be a passive or metabolically assisted process. In simple passive phloem loading, sugars move down a concentration gradient from the leaf mesophyll through copious plasmodesmata. In the polymer trap model of phloem loading, sucrose in the companion cells is modified into progressively larger sugars, such that they become too large to diffuse back into the mesophyll and must move into the sieve cells. In some plants, metabolically assisted, or ‘active’, loading requires the coenzyme adenosine triphosphate to deliver sugars into the sieve cells against a concentration gradient. The means by which sugars are unloaded from the phloem varies depending on the sink. For example, unloading sugars into fruits or seeds requires energetic input because transport must occur against a sucrose concentration gradient.
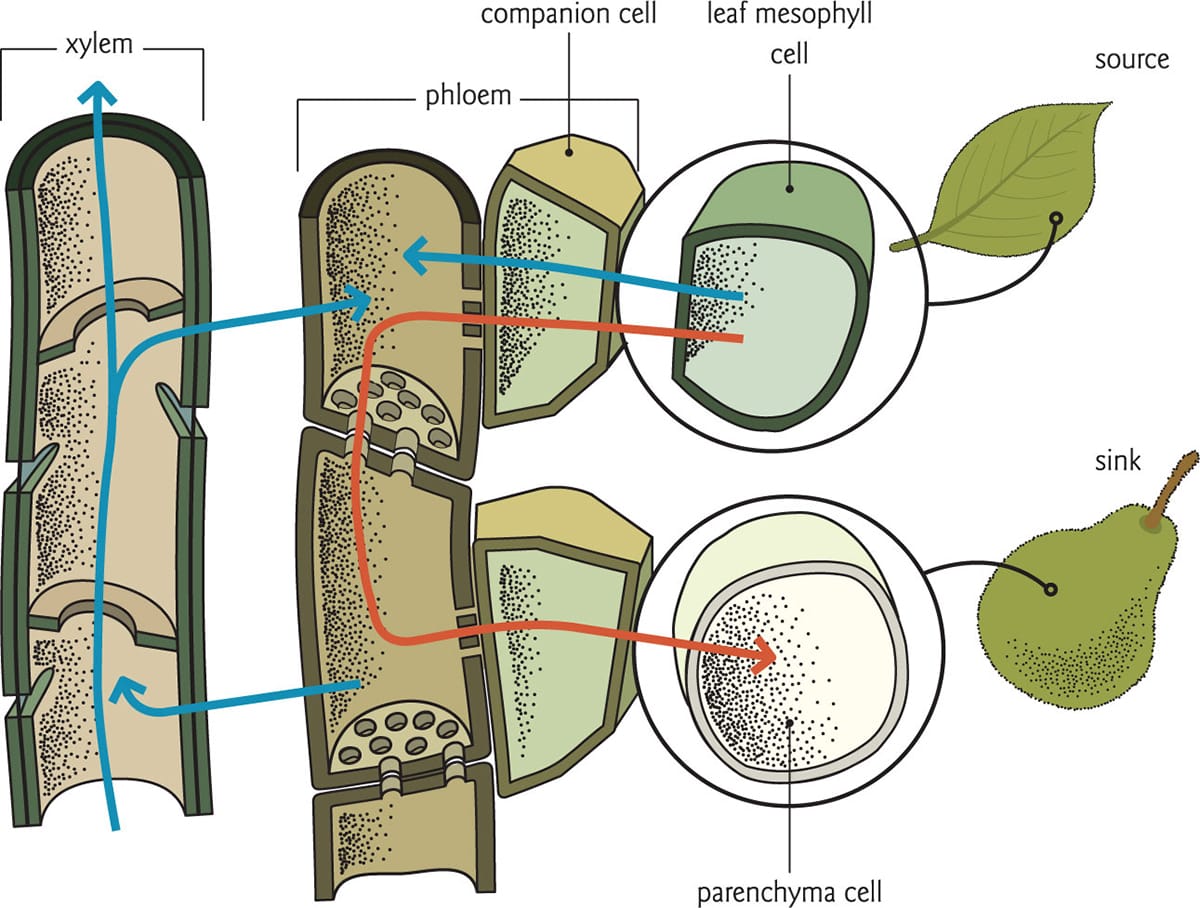
Translocation in phloem. Phloem sap (red line) moves from source to sink, relying on water (blue lines) from xylem tissue to generate positive pressure in the sieve tubes.
Structure and function of bark
A simple epidermal layer suffices to defend the internal tissues of a herbaceous plant, but trees require bark to protect their long-term investment. While only one or two layers of cells comprise the epidermis, bark tissue is made of many different cell types in various quantities, resulting in a diversity of patterns and a range of thicknesses. In addition to protection, bark can also serve other functions, including photosynthesis, the storage of water and carbohydrates, and mechanical support.

Left to right: the external bark tissue of milkwood (Alstonia actinophylla, Australia), Eucalyptus sp. (Australia), and Ficus sp. (lowland Costa Rica). Thick bark protects against fire, whereas peeling bark deters the establishment of epiphytes.
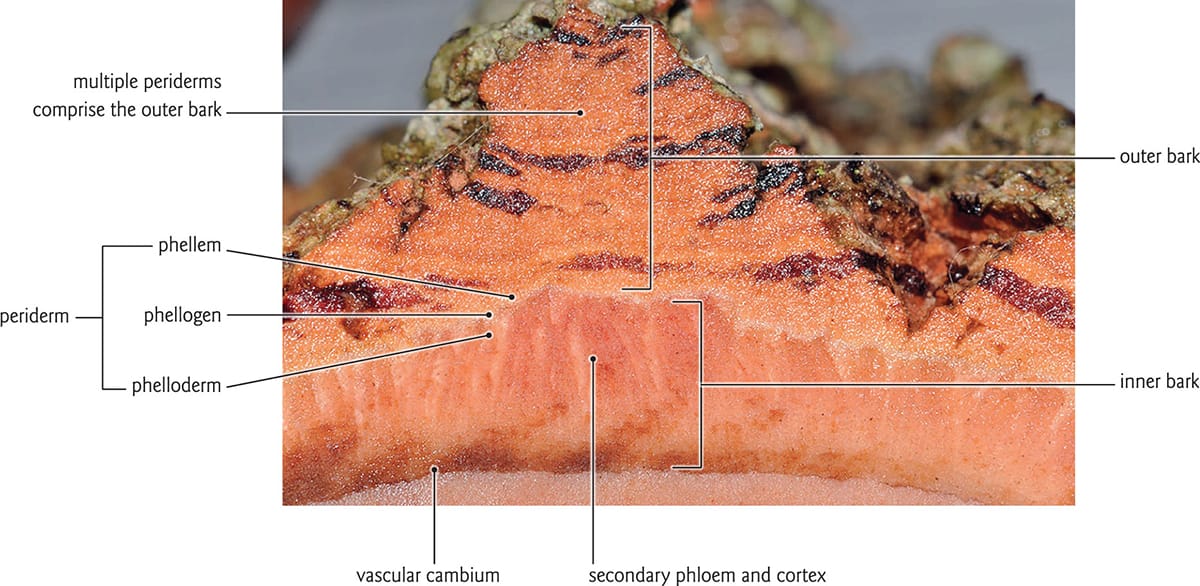
The internal structure of bark tissue. Multiple layers of dead tissue comprise the outer bark. The inner bark and the adjacent periderm layer are living tissues.
Development of bark
What is collectively known as bark is really an aggregation of several secondary tissues outside of the vascular cambium. The visible outer bark is composed of layers of dead tissues known as periderms that overlay the living inner bark, which includes secondary phloem and phloem parenchyma (cortex). The periderm is composed of three tissues: the cork cambium (also known as phellogen) is a meristematic layer that gives rise to cork (phellem) to the outside, and the phelloderm toward the inside. Additional periderms develop as the stem increases in girth. These periderms originate from phloem parenchyma cells, which become meristematic and form new cork cambia. Expansion of the tree trunk causes the most distal periderms to crack or peel, giving bark its characteristic appearance. In a young shoot, the periderm is first derived from epidermal tissue.

The papery, peeling bark of narrow-leaved geebung (Persoonia linearis, Australia), a fire-resistant species. Right: quaking aspen (Populus tremuloides, Canada). Lenticels, branch scars and wounds are evident on the white bark.
Properties of bark
The form and function of bark is surprisingly varied, and researchers are beginning to uncover the protective properties of its many layers. Trees found in fire-prone systems, such as savannahs and temperate and dry tropical forests, tend to have thicker outer bark layers than those inhabiting moister habitats where fire is rare. Generally, the thicker the bark, the better its insulating properties, and the greater the likelihood that the vascular cambium will escape fire damage. Because the thickness of the bark scales closely with tree height, larger trees show higher rates of post-fire survival. Increased water content in the bark tissues is also protective against fire, as is higher bark density.
The relationships between bark attributes and biotic agents such as insects and epiphytes are more difficult to study. Some barks, such as those of eucalypts (Eucalyptus spp.) and plane trees (Platanus spp.) constantly peel or slough, making the establishment of epiphytes, lichens or parasites on the tree trunk a difficult task. Studies in the tropics demonstrate that epiphytes prefer to establish on barks that are stable, with a rougher surface and some capacity for water retention. Here, bark chemistry also plays a role. The presence of bryophytes and lichens is often related to the nutrient content of the bark, while tannins are known to inhibit the fungal and bacterial degradation of bark and wood tissues. Along similar lines, thicker barks tend to have higher quantities of liquid latex, which protects against insect attack by gumming up mandibles.
Limits to tree height
The appearance of true secondary xylem in the evolutionary history of plants allowed trees to compete for light by growing tall while remaining well hydrated. But what sets the ceiling for tree height? Models indicate that trees are well short of what could be sustained by the material properties of wood, and the idea that photosynthetic carbon uptake cannot sufficiently compensate for rising respiratory costs with height has also been ruled out. It appears that a combination of environmental and intrinsic plant traits conspires to set the upper bounds for vertical growth.

Tree ferns and woody trees compete for light in a New Zealand forest. These ferns are often taller than angiosperm trees thanks to abundant rain and high humidity.
Trade-offs associated with growing tall
Numerous physiological and life history traits govern the size of trees. For example, trees growing in shallow or severely nutrient-depleted soils will not overcome stunting unless they are planted in better soil. Similarly, severe waterlogging will inhibit the diffusion of oxygen into roots and consequently limit respiration. Adequate carbon resources are needed to meet the basic demands of metabolism, pathogen defence, wound repair and reproduction, but because surplus carbon is required for growth, photosynthetic activity must be sufficient to meet the costs of stem construction. Consequently, tree height is greatest in bright, fertile habitats in which a mild climate and abundant precipitation can support healthy leaf function.
Currently, the most convincing explanations for limits on tree height centre around the connection between plant water transport and photosynthesis. The biggest obstacle for tall trees is the decline in leaf hydration with increasing height. This is because water transport is challenged by the effect of gravity in combination with greater friction along a longer transport pathway. Stomata in leaves at the top of the canopy may respond to drought stress by closing and thus reducing photosynthetic rates, while turgor pressure may be insufficient to support cell expansion for stem and leaf development. Simply put, the branches at the top of a tree typically experience drought stress to a greater degree than those at mid-canopy. Drought stress also raises the likelihood of xylem embolism, which could further impede the delivery of water.
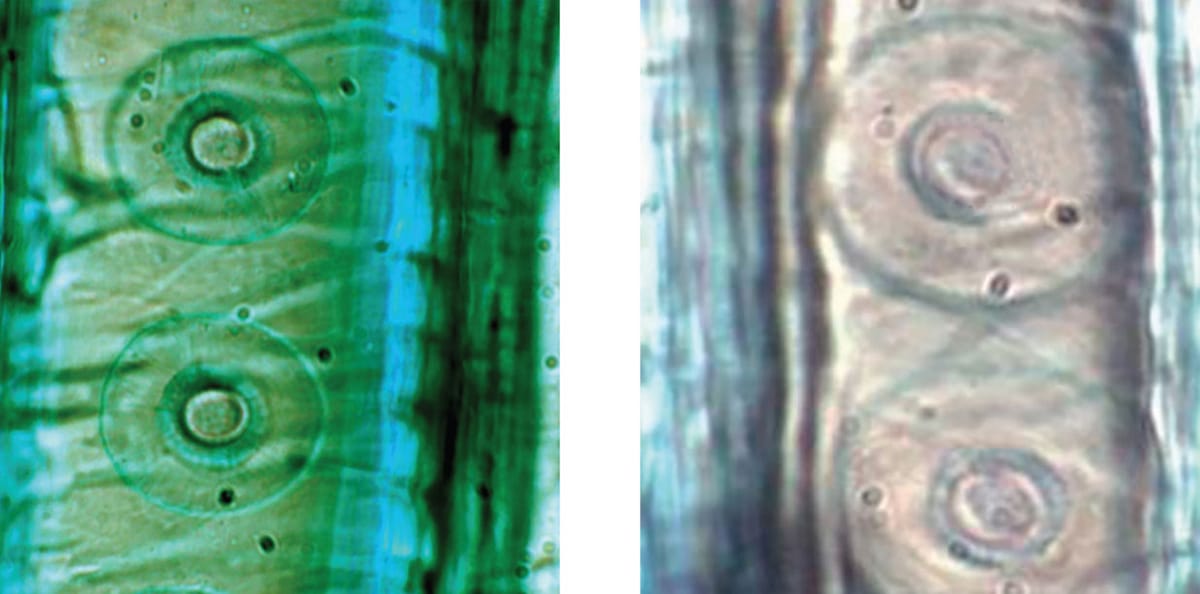
In the Douglas fir (Pseudotsuga menziesii), the aperture of the torus-margo pit membrane narrows with increasing height (left, 81 m [266 ft] and right, 14 m [46 ft] above ground). Narrow apertures improve cavitation resistance but at the cost of reducing the water flow.
Researchers surveying some the world’s tallest redwood (Sequoia sempervirens) trees have discovered that the physiology of a tree at the top of its canopy is markedly different from its branches near the base. On a sunny day, leaves at the top of a 110 m-tall (360 ft) redwood exhibit a nearly twofold drop in leaf hydration relative to leaves at 30 m (100 ft), a response coupled with substantially reduced rates of photosynthesis. Others have shown that as leaf water stress increases with height, so do the costs associated with preventing embolism. In the Douglas fir (Pseudotsuga menziesii), the aperture of the torus-margo pit becomes narrower with height, presumably to generate a stronger torus seal. This eventually creates a problem, because such narrow apertures generate additional friction for water flow. The propensity for greater water stress at the top of the canopy may help explain why the tallest trees in the world inhabit wet, often foggy climates.

Despite being more than 80 years old, pygmy trees in coastal California barely reach 2 m (6.5 ft) in height due to the extremely nutrient-deficient soil on which they grow.
Climbing plants
The movements and habits of climbing plants have fascinated many naturalists, including Charles Darwin, who first published an extensive essay on the subject in 1865. Having devised a way to reach sunlight at a fraction of the cost faced by trees, climbers seem ingenious. They are also structurally efficient: the leaf area of climbing plants occupies roughly one-third of the total leaf area of a forest, yet represents less than one-tenth of the total forest biomass. This economy of form explains both the success and the limits of these structural parasites.

A scanning electron micrograph of hooks on a herbaceous stem. These hooks catch on the surface of the host, and also increase friction to prevent slipping.
Holding on by loop, hook and cranny
Vines, lianas and climbing monocots have evolved clever mechanisms that allow them to find vertical support, then quickly ascend. Many such plants bear tendrils, which upon reaching a certain length begin to oscillate in all directions in what is known as circumnutation. Common to all plant stems but amplified in tendrils, circumnutation is the result of coordinated directional changes in cell volume. After establishing a contact coil, the tendril squeezes the host structure to avoid slipping off. A free coil formed earlier may then help draw the stem closer. Leaf climbers such as Clematis species use modified leaf petioles to wrap around support structures, while others employ irritable organs – modified branches and peduncles that swell in response to touch. The herbaceous stems of hops (Humulus lupulus) and twining palms use sharp hooks for support, while clinging climbers, such as vines in the genus Parthenocissus, secrete a polysaccharide-based cement for support, or force their holdfasts into crevices, where they thicken and remain tenaciously wedged.

An aggressive climber, the trumpet creeper (Distictis buccinatoria) produces trifurcating tendrils that coil in response to contact with a host. Once established, the young and flexible apical stem will become thick and woody with time.
Internal structure
The internal stem structure of a climbing plant assumes very different shapes and mechanical properties from self-supporting upright plants. Woody climbers such as lianas are slender, and often have anomalous cambial architecture, giving rise to lobed secondary xylem and flattened, ribbon-like main stems. Lianas may fall off their host, so these complex structures are thought to prevent compressive damage to vascular tissues. In the absence of a cambial layer, monocot climbers have fewer options with which to modify their mechanical attributes, but it has been suggested that the scattered vascular bundles found in the pith may act as cables within a composite structure. Leaf sheaths and aerial roots may confer additional mechanical benefits to the climbing monocot. In both monocots and dicots, the younger, distal regions of a climbing stem are more pliant and less reinforced with fibres or secondary growth than older portions of the stem. This strategic allocation of resources ensures that younger stems respond adaptively to their support structure, while the basal sections establish long-lasting structural and resource connections to the host and the soil.

A woody climber winds its way up to the canopy. The smooth vines are actually roots of canopy-dwelling monocots. Woody vines such as this are common in the tropics, where abundant precipitation supports high rates of water transport. Vines may have xylem vessels over 2 m (6.5ft) long.
Transition trade-offs
The transition from a self-supporting to a climbing growth form (and back) has occurred numerous times across several plant lineages. Invariably, these transitions invoke structural and functional trade-offs. For example, erect forms of Pacific poison oak (Toxicodendron diversilobum) build stems that are, on average, three times as wide as those of climbers; wider stems are more structurally stable. Other plants invest more in fibres and secondary tissues prior to their transition to climbing, with higher fractions of parenchyma and wider vessels comprising the ‘cheaper’ climbing stems they subsequently develop.
The importance of stem shape
Because reproductive structures are ultimately more valuable to plant success than the shoots and leaves per se, selection builds plants that are robust yet economical. Despite comprising more than 95 per cent of a plant’s biomass, stems and leaves are simply a means to an end, so from the plant’s point of view there is every reason to be spendthrift. Hollow stems in horsetails (Equisetum spp.), bamboo and even old trees are fine examples of structural economy, because the plant loses little mechanical resilience by divesting carbon from the centre of the stem.

The hollow stem-like peduncles of dandelions (Taraxacum spp.). Cylindrical stems are economical to build and are resistant to bending stress, but preclude storage in the absence of a pith; they may also crimp irreparably.

Stem shape and resistance to bending
The vast majority of herbaceous stems are round, but hollow, square and triangular stems are also common. To appreciate the significance of these shapes, it is important to understand how geometry alters bending resistance away from the geometric centre, a property known as the second moment of area. Consider a dandelion (Taraxacum sp.) stem with a flowerhead perched on top. Built hollow like a straw, the stem is resistant to deformation, but if one were to arrange the stem tissue into a solid cylinder of equal length, then the stem would become narrower, and thus much more bendy – and certainly more expensive to build. Perhaps a very short, solid stem could do the job, but this might compromise seed dispersal. The tall, hollow stem benefits from having a higher second moment of area than its hypothetical solid equivalent, and is a much more economical structure. Given these trade-offs, one might rightly wonder why not all stems are hollow. One idea is that hollow stems are prone to buckling (crimping) under lateral and top loads, such as those imposed by canopies. This, along with developmental constraints, may explain why large branches are notably absent in bamboos, horsetails and grasses.
Stem symmetry and resistance to bending
Symmetry also has a profound effect on bending. Round stems, whether solid or hollow, can flex in any direction, but the bilaterally symmetrical square stems of mints (Mentha spp.) and triangular stems of sedges (Carex spp.) have fewer bending planes. In the case of mints, square stems may not present a biomechanical advantage so much as improved light acquisition by virtue of the alternating leaf arrangement, which may reduce self-shading. In contrast, triangular sedge stems may be useful for stabilizing the sizeable leaf and floral displays at their tip. At the far end of the spectrum are climbing plants, whose specialized stems exhibit extreme variation in their second moment of area. For example, some members of the genus Clematis have oval stems that twine around a trellis much like a ribbon, because they are more pliant in one bending plane than the other. The cost is that in the absence of external support, Clematis might never get off the ground.
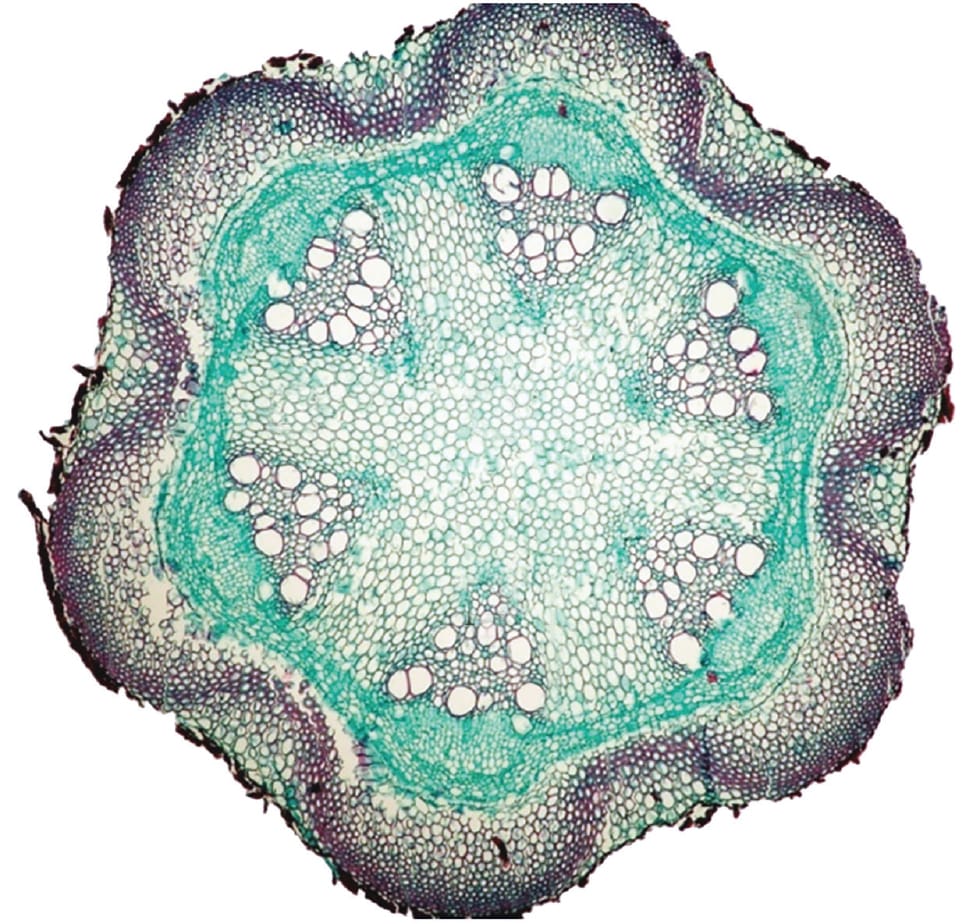
The multifaceted stem of Clematis is pliant yet strong; fortified ridges of fibres protect the vascular tissue.

The outer corners of square stems, such as in mints and nettles (Lamiaceae), are reinforced with collenchyma tissue, which is supportive yet flexible.

Many species of sedge (Cyperaceae) have stiff triangular stems. This shape may improve light capture and impart rigidity by virtue of only three bending planes.
Underwater stems
Aquatic plants have evolved physiological traits that allow them to occupy surprisingly stressful habitats. In contrast to terrestrial species, they may deal with low light levels, turbulence, drag, low carbon dioxide and oxygen diffusion, and the completely anaerobic conditions in which their roots and rhizomes are submerged. Taken together, water lilies, ferns and rushes have arrived at several convergent solutions to these problems. In contrast, species such as water hyacinth (Eichhornia crassipes) and duckweed (such as Lemna spp.) have avoided them altogether by becoming buoyant rafts.

Water lily (Nuphar sp.). The submerged rhizomes of aquatic plants can grow in habitats depleted of oxygen. Their leaves and stems have aerenchyma tissue to support gas exchange and buoyancy.
Gas exchange in water lilies
Water lilies (Nymphaea spp.) are perennial, and rely on extensive underwater rhizomes for anchorage and carbohydrate storage. In temperate climates where lakes freeze, the plants shed their leaves, leaving the rhizomes to overwinter at the bottom until the spring, when they produce new growth. Critical for the lilies’ survival, these modified stems may be many metres in length and up to 10 cm (4 in) in diameter. In some cases, the rhizomes are buried in soil 2 m (6.5 ft) below the surface of the water, but even in shallower waters they almost always occupy a stagnant, anaerobic environment in which the absence of oxygen in combination with microbial toxins creates a noxious habitat. The survival of the rhizome thus depends on an efficient means of acquiring oxygen and eliminating respired carbon dioxide.
It is thanks to the evolution of a passive, thermally driven mechanism of ventilation delivering atmospheric oxygen to the rhizome that water lilies can thrive in anoxic substrates. Gases move within the plant through lacunae, which are large, empty spaces in the plant organs. The lacunae are connected, and comprise more than 60 per cent of the volume of petioles and 40 per cent of rhizomes (see here). Gas flow originates in the lacunae of the young, recently emerged lily pads, which are pressurized in response to heating by the sun. The pressure-driven downward movement of gases through the petiole creates a vacuum within the leaf, which again draws air into its air spaces, and thus keeps the flow of gases continuous. It has been suggested that more than 22 litres (46 pints) of air may perfuse the rhizome over the course of a warm day. The efflux flow of gases occurs through older leaves, which over time become quite porous and unable to sustain pressure. Old lily pads thus act as a vent, and are the point source of much of the air bubbling up in lily ponds.
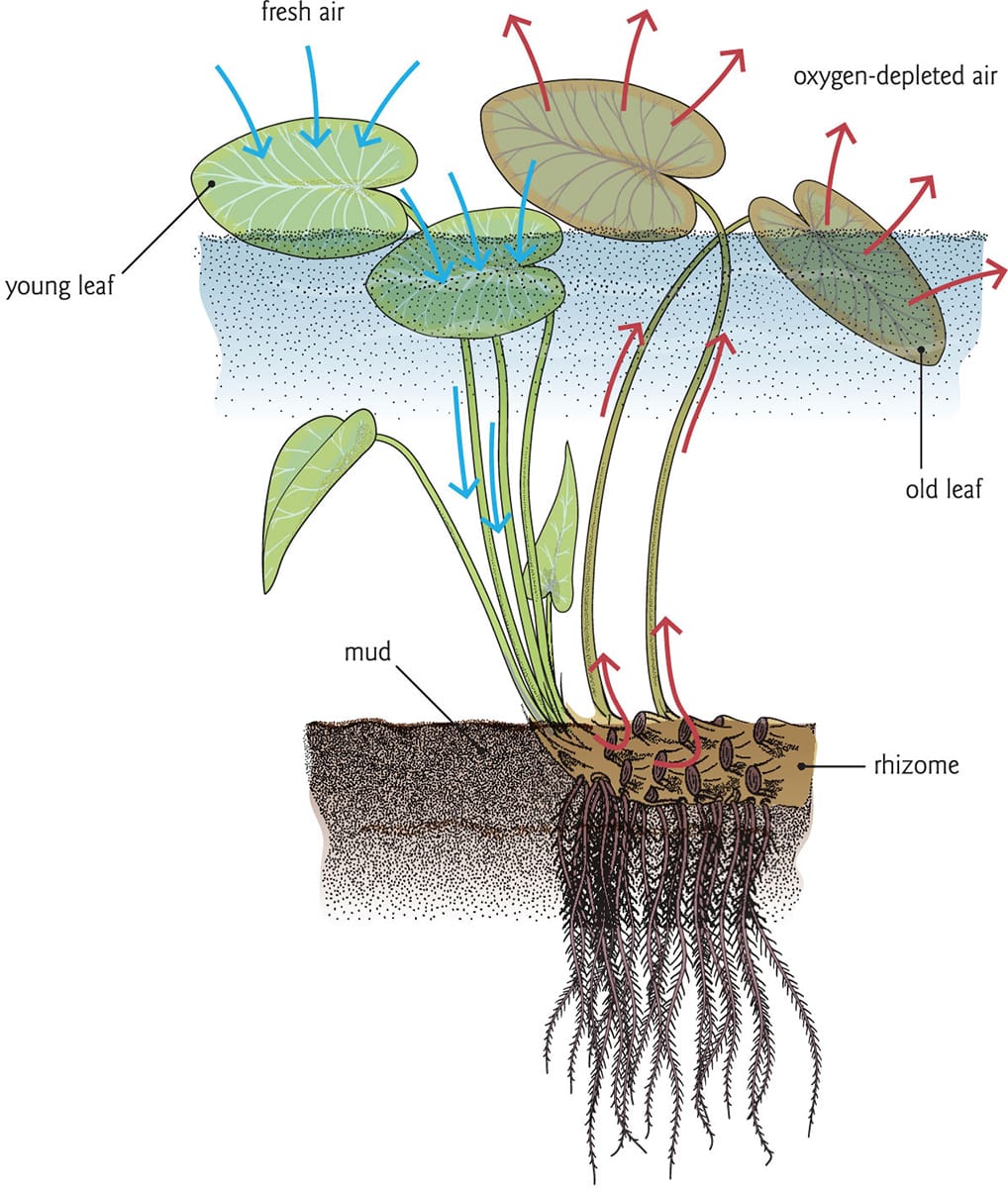
The flow of air through a water lily (Nuphar sp.). Fresh air (blue arrows) enters the young leaves, moving into the rhizome via pressure-driven flow. Oxygen-depleted air (red arrows) exits through older leaves.
This type of convective throughflow ventilation has been found in other aquatic rhizomatous plants, including lotus (Nelumbo spp.), the perennial Phragmites grasses, bulrushes or cattails (Typha spp.) and spikerushes (Eleocharis spp.). Research has shown that sun-induced stomatal opening facilitates the uptake of air, and that the low internal resistance to gas flow gives these plants a competitive advantage over those relying on diffusive gas flux. Convective flow is also ecologically important. The rates of methane and carbon dioxide flux are up to 15 times greater in wetlands that harbour species with convective ventilation than in systems where the plants rely on gas diffusion. Global warming may be consequential for wetlands.
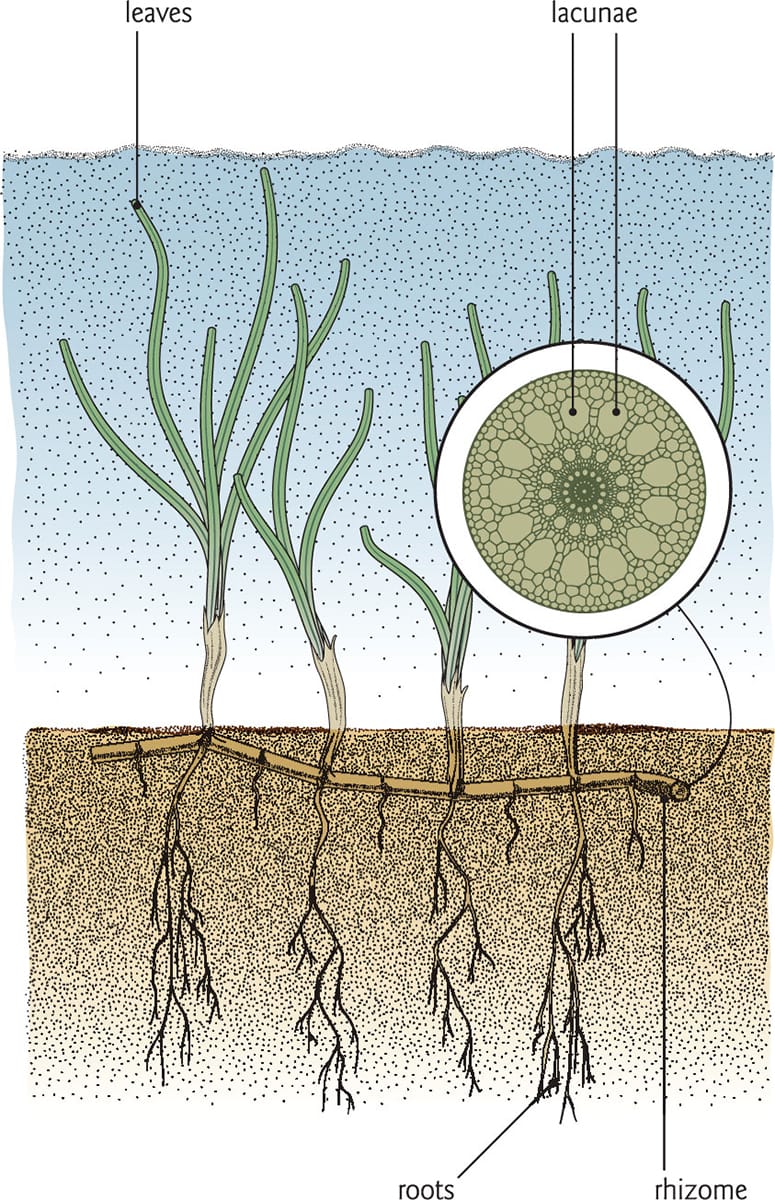
Seagrasses are monocots that are fully submerged in salt water. Forming extensive beds in bright, shallow habitats, seagrasses are anchored in the sand by a creeping rhizome, often creating large colonies. Water transport is not critical for these plants, so the xylem tissue in the rhizome is highly reduced in comparison with the phloem. Most of the rhizome is occupied by either parenchyma or aerenchyma tissues.
Stem modifications
Plants have managed to modify nearly any organ to serve a need beyond its original purpose. Some stem modifications occur above ground, some are subterranean, and still others are found in epiphytic or aquatic plants. In many cases, it can be difficult to differentiate between a modified stem and a storage root without knowledge of a species’ life history, development and even anatomy. Some generalities can help, however. Modified stems give rise to leaves, flowers, roots or other stems, they may have lateral nodes, and importantly, they are always found between roots and leaves.
Form and function
If the overall gestalt of a plant lies in stem architecture, then modified stems greatly extend the possibilities of diversity in plant form and function. In some climbers such as the common grape vine (Vitis vinifera), distal stems are modified into clasping tendrils that can produce leaves and flowers upon maturity. Cladophylls, which are stems that evolved to resemble foliage, form the edible paddle in Opuntia cacti; the small, insidious spines are actually modified leaves. In addition to spines, physical defences include thorns and prickles, but of these, only thorns are proper stems because they arise in the axils of leaves. Prickles, such as those found on roses (Rosa spp.), are outgrowths of the epidermis and cortex. Stems modified for the storage of water and food may be familiar to most. Columnar cacti such as the saguaro (Carnegiea gigantea) have swollen stems, in which parenchyma cells with large vacuoles store copious amounts of water. Shallow-rooted and subject to infrequent precipitation, saguaro relies on its stem for hydration, support and photosynthesis. Water-filled stems can also be seen in the spurge family (Euphorbiaceae), and in the trunks of baobab trees (Adansonia spp.).

Fishhook cactus (Sclerocactus parviflorus). Composed primarily of parenchyma tissue, the stem of this cactus is a reservoir of water that allows it to survive prolonged episodes of water deficit and heat.
Below-ground modifications
Stems vary considerably below ground as well, forming tubers, bulbs, corms and rhizomes, all functioning in the storage of starch. They are highly reduced in the case of bulbs (for example, garlic), which are aggregates of succulent leaves adhered to a flat stem disc. Globally, the most widely consumed tubers are potatoes. These develop from the tips of slender stems known as stolons, or runners, which may grow in both the air and the soil. The ‘eyes’ of a potato (or a yam) are the nodes that produce shoots, and these must be included in potato cuttings for successful propagation. Rhizomes may or may not be subterranean. In Iris species, the rhizomes extend underground, whereas in ferns, they can occupy terrestrial or epiphytic habitats.

The trunks of the baobab (Adansonia sp.), a Madagascan endemic tree, store copious amounts of water, which supports the flush of new leaves at the end of the dry season.
The rewards of runners
The survival value of most stem modifications is self-evident, but the functional role of stolons remains open to scrutiny. Studies have shown that they are advantageous in beachy habitats where the instability of shifting sand dunes threatens to dislodge or bury slow-growing plants. Stolons quickly extend a plant’s reach, allowing it to establish and tolerate frequent disturbance. Furthermore, stolons facilitate resource sharing in clonal species whereby nutrients, water and sugars are translocated from thriving individuals to other parts of the clone. The adaptive flexibility of stems has allowed plants to thrive in the most challenging conditions.

Pygmy iris (Iris pumila) showing the root-like rhizome. Rhizomes are elongate, perennial non-woody stems modified for storage or support. The meristems in the nodes give rise to shoots.
Response to mechanical stress
Plants respond to an onslaught of mechanical stress in addition to the challenges presented by herbivores, too little or too much water, and changing light levels. Common examples of physical disturbance include heavy winds, rain, snow, deformation from falling canopy debris, and animal mischief such as climbing and swinging from branches. The physiological and structural responses of plants to such interference is called thigmomorphogenesis, and differs between herbaceous and woody plants. However, all plants have one thing in common: exposure to some stress makes them more resilient.

Exposed trees, such as this pine (Pinus sp.), are subject to persistent mechanical loads from wind, rain or snow. Lower leaf area reduces drag on the canopy, while reaction wood strengthens the stem.
What a (wind) drag
The general response of plants coping with wind and mechanical agitation is to shorten their overall stature and increase the flexibility of their canopy. At the cellular level, herbaceous plants develop higher collenchyma content in their stems, as well as greater amounts of lignified sclerenchyma fibres. Smaller, hardier leaves are produced from shorter, thicker stems. Whether the stems become more pliant or stiffer appears to vary depending on the context and species: plants exposed to multi-directional wind may develop more flexible stems, while point loading may result in greater stem stiffness. Building resilience comes at a cost, however – usually a decrease in flowers, fruits and seeds.
Responses in woody plants
Thigmomorphogenesis in woody plants depends on the response of the cambial layer. Xylem is a dead tissue and thus unreactive to mechanical or chemical stimuli, so only the most recently generated wood can exhibit a response to stress. Taken together, both conifers and angiosperms display shorter and smaller xylem conduits, and an increase in wood density in habitats where winds are unidirectional and cause the stem to bend. Wider, asymmetrical stems and reconfigured canopies that reduce wind drag are a common sight in windswept environments. Some species also shed or curl their leaves to increase air flow through the canopy and thus reduce stress on the trunk. In very stressful habitats such as high-elevation treelines, krummholz trees adopt permanent deformation, stunting and canopy asymmetry. The twisting of xylem in many krummholz trees is believed to increase axial flexibility under heavy loads.
Mechanosensing
The exact means by which plants sense and react to pressure is not yet fully understood. Generally, the response to stimuli is dose dependent, systemic and saturable. Studies with thale cress (Arabidopsis thaliana) have shown that more than 3 per cent of the plant’s genome – corresponding to several hundreds of genes – becomes active within 30 minutes of mechanical agitation. Mechanosensing may have its origins in cell plasma membranes, in which specialized proteins such as stretch-activated channels respond to stimulation or changes in turgor pressure, and therefore induce a cascade of signal-transduction pathways involving calcium and a suite of plant hormones.
Dendrochronology
Dendrochronology is the study of tree rings over time, and is an expanding, multifaceted discipline that seeks to date and provide chronologies for particular events, as well as understand and contextualize natural and human phenomena. It is centred around the fundamental premise that tree growth and xylem development are a reflection of aggregate signals, including climate, ageing, stand dynamics and disturbance, as well as some degree of unexplained variation.

The growth rings of a tree. Annual variation in growth ring thickness and cell attributes can be linked to climatic signals. Records from several individuals are needed for a statistically robust interpretation.
Dating events
As trees mature and develop, their xylem records numerous events and conditions, some of which will be of interest, some not. The underlying aim of dendrochronological methods is to eliminate spurious or irrelevant information through statistical analysis in order to amplify a desired signal, such as climate. To this end, researchers take great care to select appropriate sites, to replicate their sampling sufficiently within the sites, to core the trees properly, and to cross-date the rings in order to assign the correct chronology. Proper interpretation of tree rings then hinges on cross-dating. Here, the position and thickness of growth rings of an undated tree core are visually reconciled against a vetted specimen, first to determine if the sample contains all of the expected tree rings (some rings may be missing, other rings may be false), and second, to ascertain if the rings share some relevant attributes. Repeated iterations of cross-dating on progressively older trees thus yields a chronologically accurate record, the interpretation of which depends on the known relationships between the growth rings, and variations in climatic and biological factors that could affect tree-ring development.
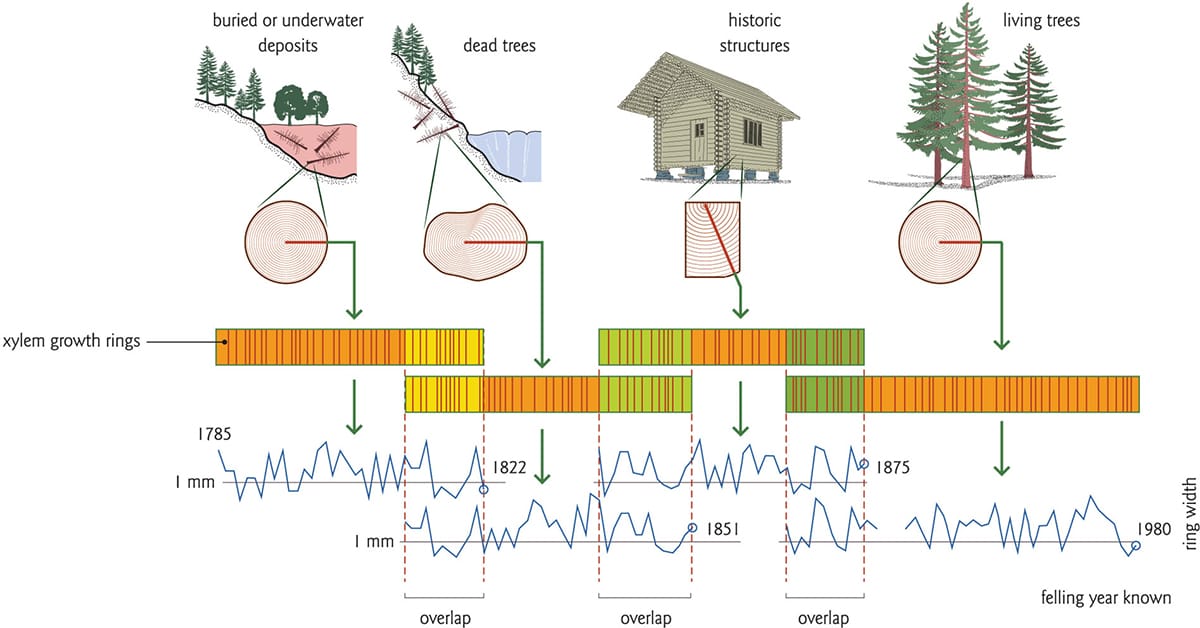
Long-reference chronologies can be generated by cross-dating live trees with ones that have died at different points in time. In this example, repeated growth ring patterns among disparate trees align several samples; this produces a continuous chronological record for the area.
Understanding events
The powerful approach of cross-dating and tree-ring analysis has yielded many important insights about human activity and asteroid impacts, among other phenomena. One of the most notable contributions to archaeology was made by American astronomer A. E. Douglass, who in the early twentieth century assembled a 700-year tree ring chronology to determine the occupation dates of numerous Native American settlements in the arid American Southwest. He achieved this by sampling in situ construction beams as well as wood artefacts from museum collections, cross-dating the rings, and subsequently anchoring this record with samples from living trees of determined age. A great deal was known about the culture of these ancient peoples, but assigning time frames to their site residency allowed archaeologists to unlock core facets of their history.
On the opposite side of the globe, Russian and American scientists have used tree rings to learn about the Tunguska event, which occurred in Siberia on 30 June 1908. Evidence suggests that a celestial body either impacted or exploded mid-air, causing an extraordinary release of energy, so much so that trees were felled over 2,000 sq km (770 sq miles) of forest. Close examination of the annual rings from trees that survived revealed that the impact most likely defoliated these individuals, as judged by their narrow and poorly developed annual rings from 1909 onwards. The researchers were then able to speculate about the magnitude of the heat impulse created by the explosion.
Stem exudates
Plant secondary metabolic processes give rise to stem exudates such as resins, latex and gums. These viscous substances are important for plant defence, but they are not critical for survival like sugars and carbohydrates, which support respiration and growth. In nature, the complex chemistry of stem exudates, in combination with their sticky, viscous quality, provides formidable protection against herbivores and fungal pathogens. Humans, on the other hand, are attracted by their useful properties and appealing aromas, and often go to great lengths to find resin-producing trees and shrubs.
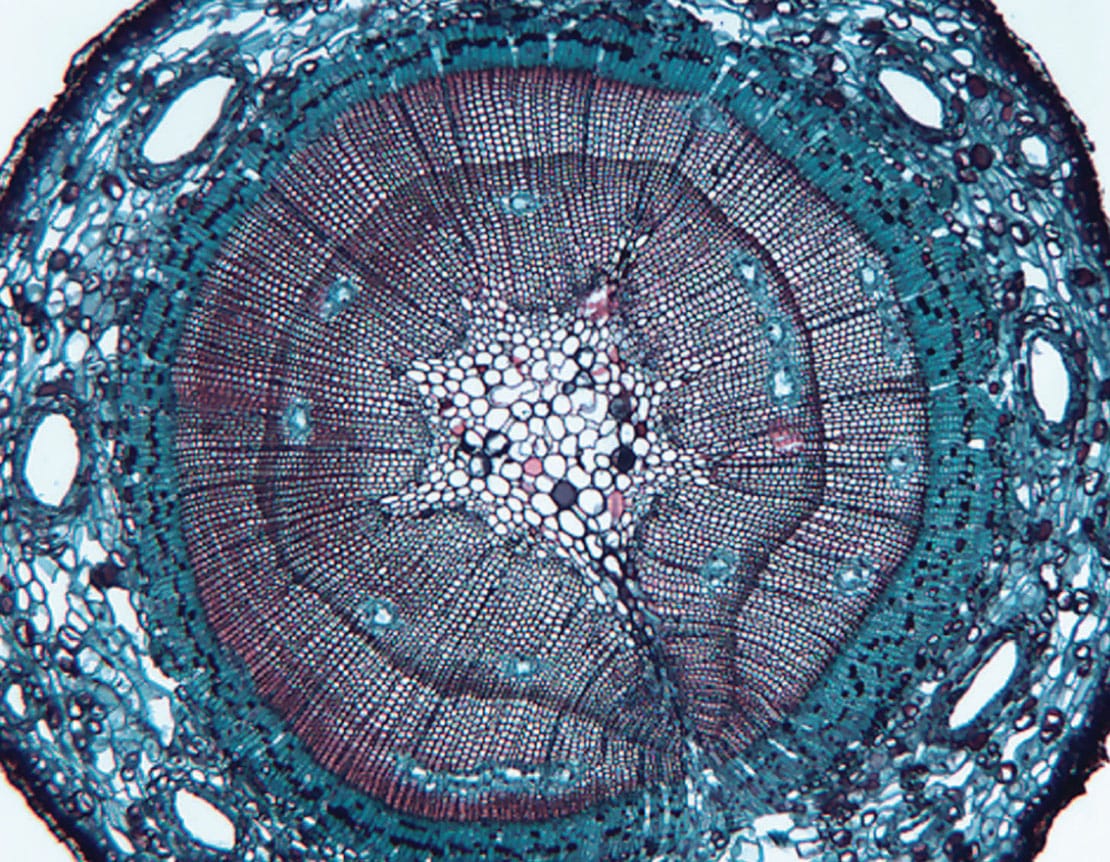
Resin canals in the bark (large voids), and in the xylem of a pine (Pinus sp.) stem (lacunae lined with blue-stained epithelial cells). The canals are under positive pressure, so damage by feeding insects will release the resin and trap the insect, or irritate its mandibles.
The biology of stem exudates
The chemical composition of exudates varies greatly among species, as does the structure of the tissues that produce them. Resins are common in conifers and several angiosperm groups, and are broadly defined as variably viscous, lipid-soluble mixtures of terpenoid and phenolic secondary compounds that have differing degrees of volatility. For example, the terpenoid alpha-pinene is volatile, and responsible for the characteristic scent of pine pitch, whereas abietic acid has low volatility, serving as the resin base. The degree of fluidity is determined by the ratio of volatiles to non-volatiles; volatile constituents tend to thin the resin. In stems, resins are produced in canals, ducts or pockets, which can be found in both the xylem and bark. These structures have an epithelial lining, which secretes the resin into the lumen.
In angiosperms, the most common stem exudate is latex, with some groups such as members of the spurge family generating amounts in such abundance as to be economically important. The colour of latex can range from milky white to red, with terpenoids, proteins, carbohydrates, tannins and other constituents comprising the bulk of the fluid. Latex occurs in specialized cells known as laticifers.
Lastly, gums are primarily found in angiosperms. Gum exudates are produced by parenchyma cells that form a cavity, which contains the polysaccharide-rich fluid. Members of the Rosaceae), such as plum (Prunus spp.) and apple (Malus spp.) trees, often secrete gums.

Pine resin. Resins serve as insect deterrents, but also help seal wounds that might otherwise allow bacteria and fungi to enter the tree. Economically important, pine resins are used to treat and finish wood products.

The sticky, milky latex that is characteristic of the Euphorbiaceae family is seen here exuding from the cut stem of petty spurge (Euphorbia peplus). The latex serves primarily to defend the plant from herbivores, kill pathogens and seal damaged tissues.

Milky latex extracted from a rubber tree (Hevea brasiliensis) as a source of natural rubber. The latex is collected by first slashing the tree’s bark in a cross-hatch pattern, and letting the liquid pour down the side of the tree into a bucket over a period of several hours.
The role of stem exudates
Stem secretions play a critical role in protection against pathogens and herbivores. Damaged stem tissue does not heal, so the sealing of cuts by resins and exudates presents a physical block against fungi and bacteria. External tissues are often a major source of resins, so it is critical to leave a collar of bark when pruning branches; this allows the tree to seal the cut.
In conifers, the release of ‘pitch’ is often related to attack from insects such as bark beetles, whose larvae bore into the cambial region to feed on phloem. In fact, so great is the selective pressure exerted by pine beetles (Dendroctonus spp.) that many members of the pine family (Pinaceae) produce resins constitutively in extensive canal ducts, and upregulate resin flow in response to an attack. Contact with the resins is usually sufficient to immobilize the insect and interfere with its mandibles. Numerous studies with ponderosa pines (Pinus ponderosa) reveal that the chemistry of the resins becomes more complex during the growing season, and yields increase in time with insect life cycles.
Stress resilience
Sealthy forests are critical to the ecological and climatic balance of our planet. Forests comprise about 30 per cent of the land surface, sequester at least as much carbon dioxide as mankind’s emissions, and influence climate via energy exchange, water transport, nutrient cycling and the release of volatiles. Up to 50 per cent of the rain in the tropics is the result of transpiration, so even modest forest removal can create shifts in humidity and air temperature. Following decades of deforestation, questions are now being raised about how we tend to our planet’s greenery.

Forests stressed by drought and beetle infestations are more vulnerable to intense fires. Increased fuel loads drive greater fire frequency and severity.
Warming and drought stress
Increases in climate-driven forest mortality have been observed around the globe, with warming and drought being the biggest culprits. Precisely how trees die varies from region to region, but there is growing consensus that two non-mutually exclusive processes may be at play: carbon stress and xylem embolism.
Carbon stress can present itself when warming and/or prolonged water deficit causes stomata to close, thereby limiting photosynthesis. Continued respiration in the absence of carbon dioxide uptake reduces starch and sugar stores, leaving the plant with few resources to fight off pathogens and recover from the drought. Evidence for ‘carbon starvation’ is limited; it is unlikely to kill trees outright.
On the other hand, the evidence for embolism-induced hydraulic limitation is gaining strength. Here, the combination of warming and soil-water deficit amplifies plant drought stress, thereby increasing sap tensions in the xylem, rendering the network extremely vulnerable to embolism. Inability to transport water to the canopy spells catastrophe for the tree. Research on aspens shows that trees now frequently exceed their hydraulic stress limits in regions struck by drought and warming, making this the most compelling explanation for the large swathes of aspen forest mortality observed in the American West. The story is far from resolved, however, especially since drought-stricken trees are more susceptible to pests.

Quaking aspen stands affected by drought in southern Colorado. Because aspen is adapted to temperate climates and higher-elevation habitats, it is vulnerable to drought stress during prolonged episodes of heat and water deficit.

Healthy aspen forest. Quaking aspen (Populus tremuloides) is a key deciduous species in high-latitude/high-elevation environments. Its characteristic white bark and trembling leaves are emblems of western North American forests.
Insect infestations and fire
Recent bark beetle outbreaks have devastated millions of conifers in forests of western Canada and the United States, causing huge economic losses and increasing atmospheric carbon dioxide levels in the process. Alongside the loss of forests often comes the loss of wildlife, and once again, the combination of warming and drought contributes to this calamity. Summer drought and higher temperatures weaken trees, all the while speeding up the insects’ life cycles. Furthermore, winter temperatures may not be sufficiently lethal to kill the larvae that persist near the cambial layer.
The degree to which pine beetle outbreaks interact with forest fires adds to the complexity. Infested, dying trees may contribute to fuel loads and alter the characteristics of fires, or alternatively, bark damage from earlier burns may make trees more susceptible to infestation. Corresponding with increased fire activity is the greater secretion and terpenoid content of resin, which finds its way to the forest floor and increases the flammability of the understorey litter. The interconnectedness of these events is perhaps inevitable, but forests are resilient and do recover, if given enough time.