7
Seeds and fruits
A seed is a survival capsule, enabling a plant to endure through space and time. It consists of the plant embryo and a food supply enclosed in a tough coat, and may comprise more than three internal components to enable it to sense and respond to its environment. In addition, it may have external components to aid its movement away from the parent plant, although seed dispersal is also manipulated by the fruit.
A fruit is a structure that develops from the ovary, particularly its wall. To recap, the ovary is the region at the base of the carpel, which is the female part of the flower of an angiosperm. In this large group of species, it is inevitable that there will be a correspondingly large diversity of seeds and fruits in terms of size and structure.
The emergence of seeds, approximately 365 million years ago, was one of the most important events in the history of life on Earth. And yet for nearly ten times that length of time they did not exist, and for about 100 million years even land plants did perfectly well without them.

Before there were seeds
It is widely accepted that the first plants emerged about 2.1 billion years ago. This followed the ‘endosymbiotic event’ that resulted in a photosynthetic cyanobacterium being engulfed, but not digested, by another cell (see here). The two organisms began to cooperate, and the result was a photosynthetic organism powered by the chloroplasts that evolved from the cyanobacterium. These simple plants lived in oceans and freshwater lakes for 1.5 billion years, and so it is perfectly possible to be a plant and survive without seed. However, every plant needs a survival capsule.

Before there were vascular plants, the world would have been very drab, with no colourful flowers and very little diversity of form. The world would have been dominated at first by simple plants living in the oceans and freshwater before the first land plant evolved. The world would perhaps have looked something like this contemporary view of a river clogged with cyanobacteria as the result of a hot summer.
The perils of moving a plant
Plants are immobile, and yet they are found all over the world, and from altitudes above 4,000 m (13,000 ft) to depths below 50 m (160 ft). This means that although they cannot move themselves, they can be moved. Moving a terrestrial plant that is rooted to the spot is a traumatic experience for the plant because it deprives it of its water supply. This water is required for a plethora of purposes, including cellular metabolism, photosynthesis and turgor, which keeps the plant upright. Aquatic plants have a different but equally stressful relationship with their water supply. It may be too salty, and thus able to draw water from the plant, or if it is fresh, excess water may enter the cells or nutrients may diffuse out. Clearly, isolating life from the medium in which it exists is a ubiquitous and unending activity. When a plant is moved, it may experience very inhospitable conditions.
Spores
Both aquatic plants and seedless land plants use spores as their survival capsules. (Seed plants also produce spores, but these do not leave the safety of the plant that produces them.) A spore is a unicellular stage in the life history of the plants. Generally, its production is preceded by cell division, when the number of chromosomes is halved and thus there is just one copy of the genome. Spores are capable of growing into the body that produces sperm and eggs in the plant’s life history, but before they can do this the right conditions must be present. They are, however, amazingly resilient to the problems the environment throws at them thanks to a covering of vegetable Kevlar called sporopollenin (see box).
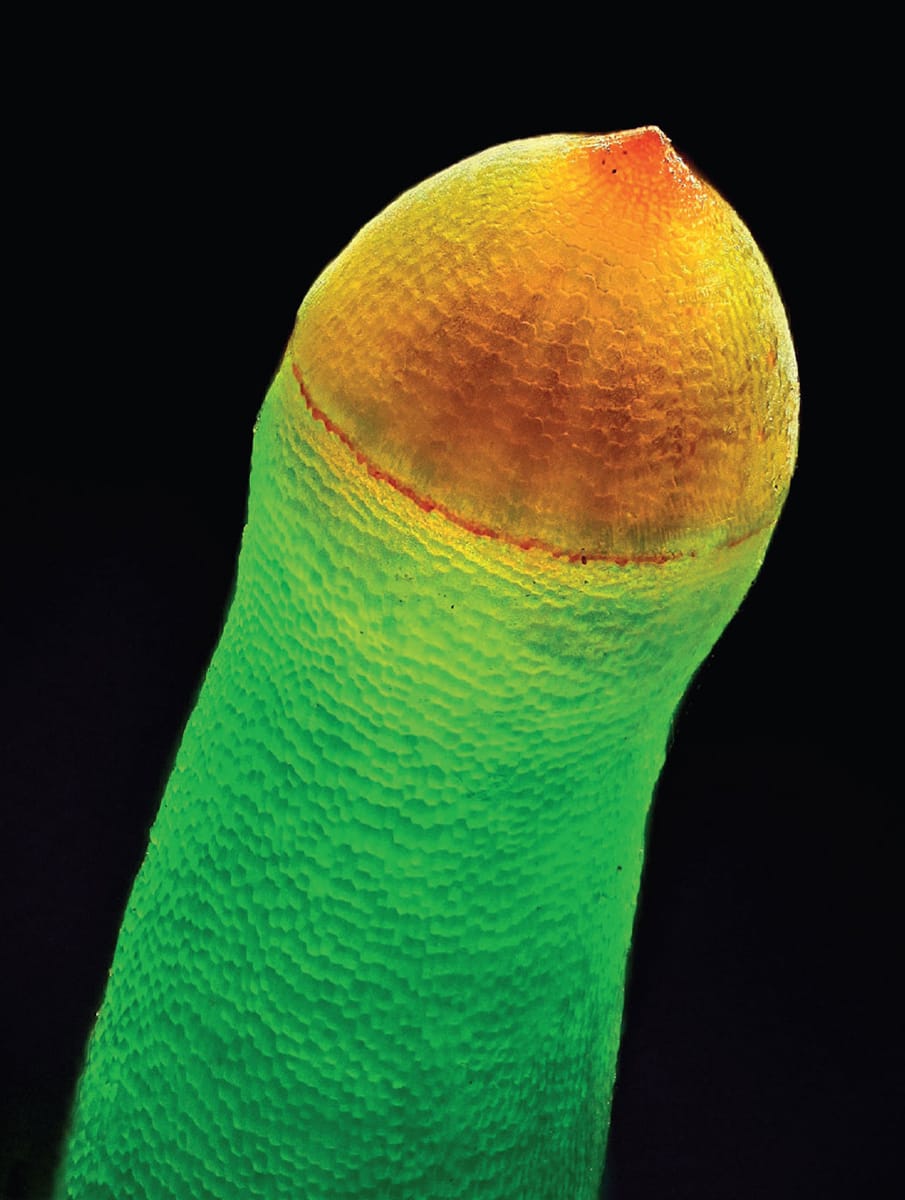
Spore capsules, such as this one of the many-fruited thyme moss (Plagiomnium affine), contain myriad tiny haploid spores – survival capsules that enable a plant to endure through space and time.
The first seeds
Charles Darwin proposed that no organism has acquired a novel feature or adaptation for the benefit of any other than itself. He did, however, acknowledge that this does not preclude the possibility that the new feature is exploited by other organisms, and this is certainly true for seeds, which now provide the primary food supply for thousands of species. The seed evolved because it was beneficial for land plants to have a survival capsule, and initially it was not associated with modern structures like flowers and cones.

A fossil specimen of Archaeopteris halliana, an extinct genus of tree-like plants with fern-like leaves dating from 383–323 million years ago.
The lack of evidence
The evolution of seeds is not clearly understood. This is for several reasons, one of which is that it happened long ago. Perhaps as far back as 386 million years ago, the evolutionary ball started rolling with the production of pre-ovules, which are found in a range of fossils. However, not one of the fossils found to date is the missing plant that links the two extant groups of seed plants – the gymnosperms and angiosperms. The best we can say at the moment is that, a very long time ago, an extinct group of plants gave rise to two lineages, one of which is the present gymnosperms and the other the angiosperms. We have no clear idea what this missing link looked like, but we do know that several other groups of early seed plants have petered out and no longer exist.
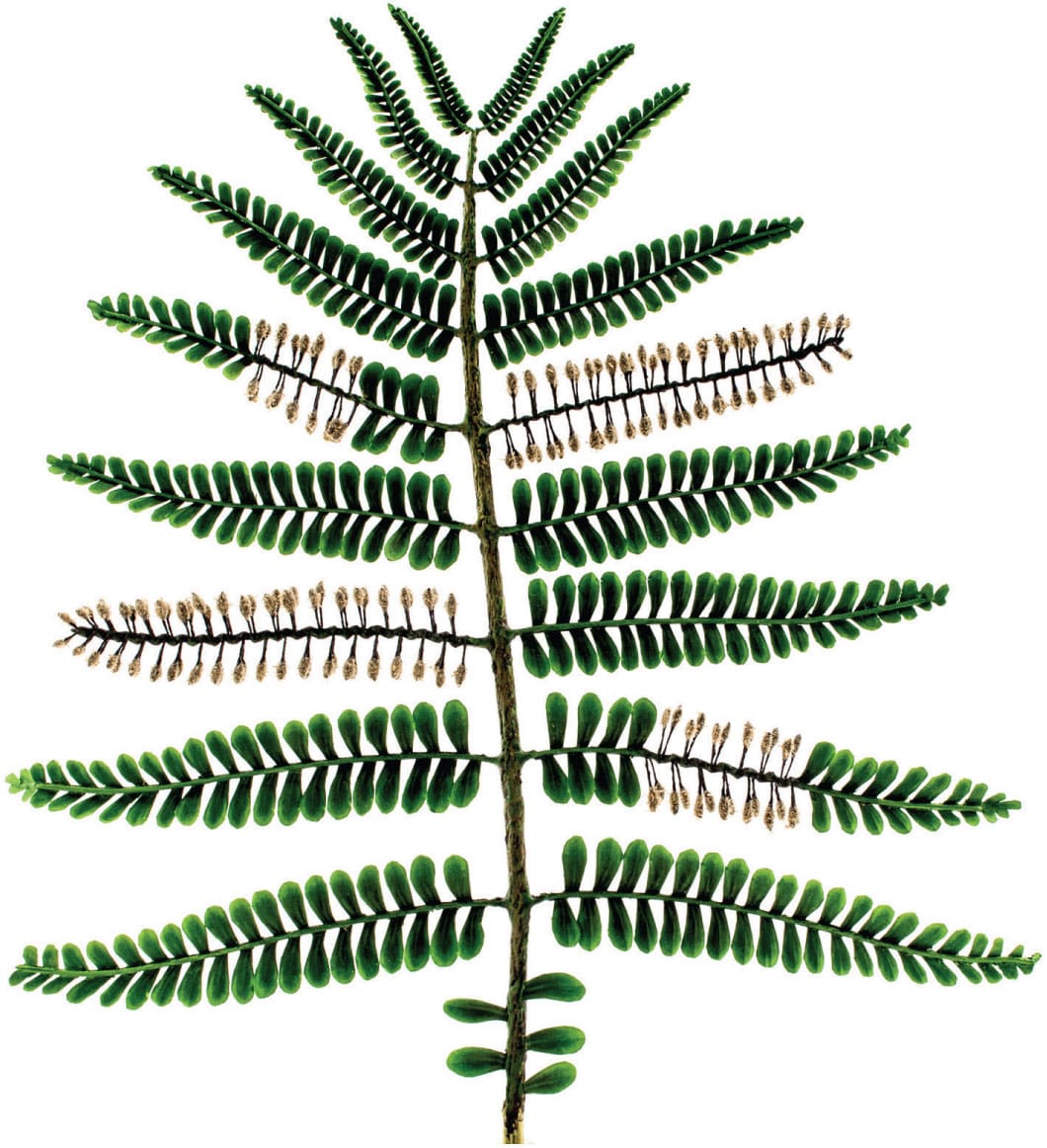
Despite the similarity between the leaves of Archaeopteris and a fern frond, Archaeopteris is thought to be one of the earliest known trees and bears some resemblance to gymnosperms.
Where on the plant did the seeds evolve?
For seeds to be produced, a number of innovations are required. For a start, two types of spores are required (heterospory), to allow for the retention of the female egg-producing gametophyte within the sporophyte and the release of a smaller sperm-producing male gametophyte (the pollen grain) to seek out the female. Another essential innovation is the development of a structure to receive the pollen. You may have noticed that no mention has yet been made of flowers and cones. That is because it is generally considered inconceivable that the evolution of seeds occurred on the aerial parts of the plant, because that would have demanded the simultaneous development of too many novel features. Among the failed theories about the evolution of seeds is the idea that they evolved from the hard-coated sporocarps of aquatic ferns such as Marsilea species, which can survive for up to 100 years in mud. While these were not the first proto-seeds, they did evolve in the right place – at soil level.

Fossils of the Devonian plant Chaleuria cirrosa demonstrate heterospory, with both microsporangia and megasporangia being present on the fronds. Heterospory is a prerequisite for the evolution of seeds in the gymnosperms and angiosperms.
Protection
All land plants produce embryos – hence their scientific name, Embryophyta or embryophytes. It is the character that clearly distinguished them from the ancestral algae. In all embryophytes, the embryo has to be fed, otherwise it could not survive. This means that the second of the three parts of a seed – the food supply – existed already. However, the embryo was not protected, at least not in a way that would prevent anything but desiccation. This required a new structure, the integument. In gymnosperms, the integument consists of one layer of tissue enclosing the female gametophyte and in angiosperms it consists of two layers. There is a small opening in the integument through which the pollen gains access to the female gametophyte and thus to its eggs. At its most simple and primitive, the seed is an integumented female gametophyte.
Gymnosperm seeds
While the seeds of gymnosperms and those of angiosperms share the same name, that should not be taken as evidence of homology. And although they also share the same three basic parts (coat, embryo and food store), the origins of these components is not necessarily the same in both groups. Gymnosperm embryos are sustained by the female gametophyte, as in ferns, making them appear to be more primitive than the angiosperms. This led to the belief that evolution proceeded in a line from ferns to gymnosperms to angiosperms, but this is now known to be untrue.
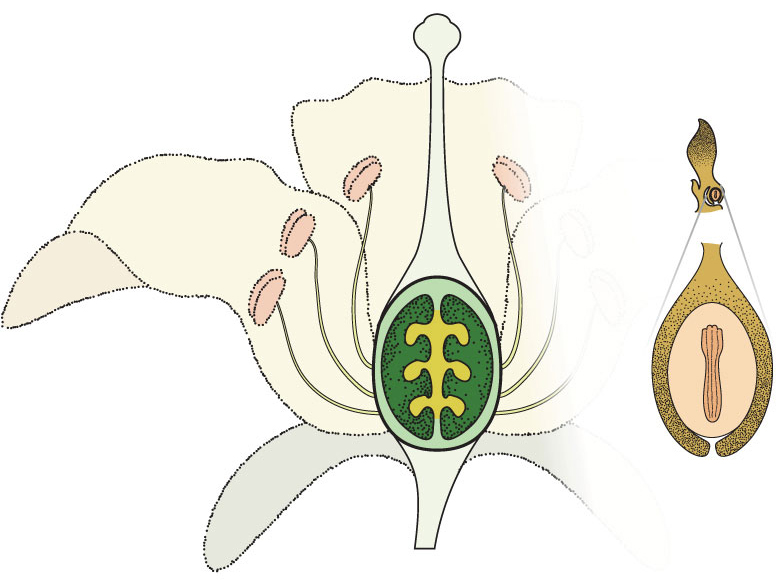
A typical angiosperm flower, if there is such a thing, showing typical sexual parts of the flower and the development of the seed.
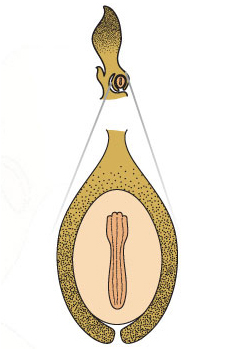
The food supply in a gymnosperm seed is the haploid female gametophyte.
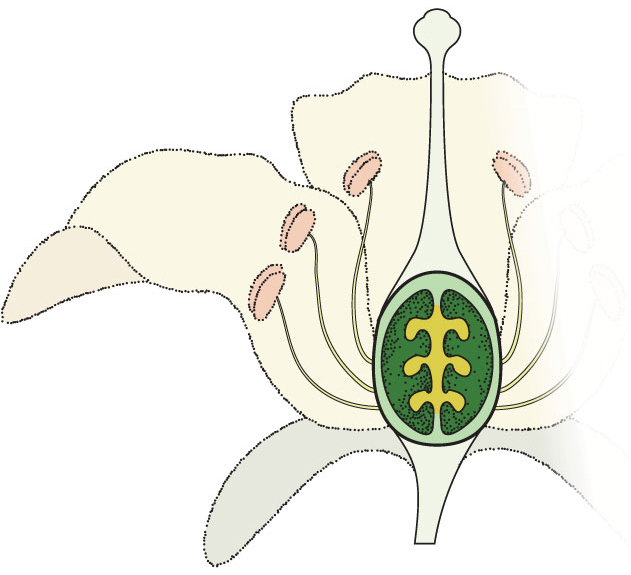
the food supply in an angiosperm seed is the triploid endosperm formed from the doubly haploid central cell of the female gametophyte and a single haploid sperm.
Bigger on average
The differences between present-day gymnosperms and present-day angiosperms reflect differences in both their current and past biology. We can map these differences onto a phylogeny (an evolutionary tree) to try to decide the extent to which they are due to the past or to the present. One clear difference between the two groups is the size of their seeds: gymnosperm seeds are 59 times bigger on average and they do not vary as much as those of angiosperms. This difference goes back a long way, with the earliest angiosperm fossils having seeds with a volume of 1 mm3, compared to a volume of 200 mm3 for fossilized gymnosperm seeds of the same age. The smallest extant gymnosperm seeds weigh 0.63 mg, 10,000 times heavier than the lightest angiosperm seeds.

Gymnosperms such as Cycas armstrongii, an endemic of Australia’s Northern Territory, have much larger seeds than most of the present-day angiosperms.
Determining seed size
It appears that there is something about the ways in which gymnosperms and angiosperms produce their seeds that imposes different upper and lower limits on their sizes. The ways in which the food supply for the embryo is produced varies greatly, and the fact that gymnosperms produce this food supply before the embryo is formed puts constraints on the size of the seeds. The size of the integument, embryo and food supply in a gymnosperm seed are linked, whereas in the angiosperms the three parts can develop independently. Surprisingly, the gymnosperms are just as diverse when it comes to methods of dormancy. The size of seeds in relation to the size of the organism and the size of that plant’s genome have also been investigated, and there is some correlation here although the significance of this is not yet clear.

The large, bright red, fleshy seeds of the cardboard cycad (Zamia furfuracia) are borne within a cone structure typical of the gymnosperms.
Fleshy conifer fruits
The term ‘gymnosperm’ is derived from the Greek words meaning ‘naked seeds’, because the seed is not enclosed in a structure. This means that the 1,000 or so species of gymnosperm do not produce fruits in the same way that the angiosperms do (even though the fleshy fruits of angiosperms are produced in a wide variety of ways). However, in many gymnosperm genera the species produce structures that, to the casual observer, look like a fruit. One of these, the Japanese plum yew (Cephalotaxus harringtonia var. drupacea), was even named by botanists after the drupe, a single-seeded fleshy fruit.

The fleshy fruit of the Japanese plum yew (Cephalotaxus harringtonia var. drupacea) is remarkably similar to an angiosperm drupe, but is formed from the naked seeds typical of the gymnosperms.
Different origin, same function
Many gymnosperms bear fleshy ‘fruits’, but these are produced in different ways. For example, the fleshy red aril on the seeds of European yews (Taxus baccata) is an outgrowth of the peduncle. The aril is the only part of the tree that is not toxic – even the seed coat contains some very nasty cyanogenic glycosides. In contrast to the yew tree, the fleshy parts of the ‘fruits’ of ginkgo (Ginkgo biloba) are derived from the outer of the two integuments that surround the seed. This is sometimes the way angiosperms make fleshy fruits, and when the genes controlling the development of ginkgo ‘fruits’ were identified, they turned out to be the same as those used by angiosperms.

Although superficially similar to angiosperm berries, the red, fleshy parts of yew tree (Taxus spp.) drupes are in fact formed from the peduncle and are the only edible parts of the plant.
An ancient strategy
Having fleshy fruits is thus an ancient strategy within land plants for the dispersal of seeds, and one that has evolved many times. Furthermore, the fruit is not a new structure belonging solely to the angiosperms, despite the fact that angiosperms are defined by having enclosed seeds. This is important from an evolutionary point of view, because it was once proposed that one of the reasons for the vast diversity of angiosperms is their novel use of animals to disperse their fleshy fruits. This is clearly not the case.
Fleshy fruits are today linked with seed dispersal by birds and mammals, particularly bats and primates, but we now know that the structures significantly pre-date these animals. Could any other animals have been co-opted into seed dispersal? The answer is yes, confirmed thanks to the discovery of a beetle with gymnosperm ‘fruits’ in its mouth preserved in amber, dated back to the Cretaceous period, 145–66 million years ago. Based on the fact that modern-day reptiles are often frugivores, it is also believed that pterosaurs and dinosaurs could have dispersed fleshy-fruited seed plants.

Gymnosperms with fleshy fruit such as ginkgo (Ginkgo biloba) adopt the same kinds of seed-dispersal strategies as angiosperms with similar fruit. The gymnosperm seed inside the ginkgo fruit is particularly prized in Asian cooking.

Strange gymnosperms
The gymnosperms include 985 species in 79 genera and 12 families. By way of comparison, the beech family (Fagaceae), just one of the 416 angiosperm families, comprise 970 species in seven genera. Care should be taken not to read too much into these figures, but the gymnosperms are a very varied group that could be described as a bunch of oddballs. There is certainly more diversity in the gymnosperms than in the beeches, even though both contain almost the same number of species. A group like the gymnosperms is therefore beguiling, and consequently it has been studied in depth. Differences between the species may prove helpful when trying to explain the mystery of the origin of the angiosperms.

Welwitschia (Welwitschia mirabilis), a member of the gnetophyte group of gymnosperms, is a remarkably resilient plant that can live up to 1,500 years in the Namib Desert.
The gnetophytes
An unpronounceable name is a good start for a group of plants that seem to defy classification. The gnetophytes comprise three genera, each in its own family, and they are clearly variations on a theme. The genus Welwitschia includes one species, found in the Namib Desert. These plants can live for 1,500 years, and so can get away with a low fecundity. The ovule in the female cone has two integuments, the outer layer later developing into a wing that aids the dispersal of the seeds. There are about 40 species of Ephedra, and despite some obvious differences between them, their ovules all have two integuments and several bracts, the outer layer of which may be fleshy or dry and winged. The third genus in the group, Gnetum, comprises 30 species. Their ovules have three integuments, the innermost extending into a tube to ‘catch’ the pollen, the middle layer becoming hard and the outer remaining fleshy. Both Gnetum and Ephedra exhibit double fertilization, a characteristic of the angiosperms, but the second fertilization results in either a second embryo or it aborts.

Ephedra species are unusual gymnosperms, not only because of their fleshy fruit, but also because they exhibit double fertilization.
Ginkgo and the cycads
Ginkgo is a species out on a branch of its own, and appears elsewhere in this book. Like ginkgo, the cycads are often portrayed as living fossils, and yet recent work has shown that the 100 or so extant species in the order are actually less than 10 million years old. While the lineage itself is ancient, the species are clearly still evolving and speciating. This is a clear example of the ubiquitous fact that no matter how old the lineage, the species themselves are much younger. Of the five oddballs mentioned here, the cycads are the easiest to recognize as gymnosperms because their cones are very superficially coniferous. However, look closer and you will see that their seeds can often have a spongy, fleshy outer layer.

Gnetum species, such as Gnetum scandens, are one of the two gymnosperm genera that have double fertilisation, a feature more characteristic of angiosperms.
The origin of fruits
It is not known how fruits evolved in the angiosperms, because according to the fossil record the carpel – from which the fruit develops – turned up out of the blue about 140 million years ago. There appears to be an advantage attached to possessing a carpel, however, because the majority of plants on land (in terms of species numbers and biomass) have one. The definition of a fruit is a ripened ovary and any other structures that are attached to it and ripen with it; the inclusion here of ‘any other structures’ means that fruits can be very diverse and adaptable.

Papaya (Carica papaya) is cultivated in most tropical countries for its sweet, fleshy fruits, which are rich in vitamin C.
Evolution and development
A rich seam of research that is capable of answering questions such as the origin of fruits is known as evo-devo, the snappy abbreviation of evolution and development. The basis of this research is that evolutionary relationships might be hidden in the genes controlling the development of organs that now look very different but share a common ancestor. This is being used to analyze and compare the development of the reproductive parts of the major groups of land plants, but while it has provided some answers as to the evolution of fruits, sadly it has not come up with the answer. A current front runner is the mostly male theory, which proposes that the female reproductive parts were fused with the male cones of the ancestral seed plants to give the prototype for the bisexual flower with its carpel in the centre.

Although they look like any other fruit, the bananas we buy in shops are parthenocarpic, or seedless.

Vegetable or fruit? Although sold in shops as a salad vegetable, tomatoes are in fact fleshy fruit containing numerous seeds.
What is a fruit?
We may be in the dark with regards the evolutionary origins of fruits, but as we saw above, they can be defined. Fruits are ripened ovaries, but they are just the final stage and function of the carpel. Prior to ripening, the ovary protects the ovules, where the seeds develop. When the seeds have matured and are ready to be released into the wild, the ovary can do one of several things. The minimum it can do is to dry and open, so that the seeds simply fall out. Alternatively, the ripened ovary can develop into something that will benefit the seeds, and thus its genes, including offering protection and transport facilitation. As many as eight out of ten species choose the first option, releasing their seeds and allocating no resources to their dispersal. Examples of this are acorns falling from an oak tree (Quercus sp.), or seeds tumbling from the dried fruit of a foxglove (Digitalis sp.).

The tamarind tree (Tamarindus indica) produces seeds without fleshy outer coverings and therefore invest little energy in the promotion of seed dispersal by animal agents, relying instead on gravity.

Oak trees (Quercus spp.) are another group that primarily rely on gravity for dispersal of their acorns, but squirrels still find them irresistible and contribute to their dispersal far away from the parent plant.
Seed size
Seeds vary in weight from 0.3 mg to 18 kg, or 0.0000003 g to 18,000 g, or to put it another way, the biggest seed is 60 billion times heavier than the smallest. Seeds also vary in size, being anything from 0.18 mm to 500 mm long, nearly a 3,000-fold difference. It is a safe bet that these extremes are produced by very different plants living in very different places, with different selective pressures and conditions, and yet the function of all seeds is the same: to grow into another seed-producing plant.

A single Cynoches ventricosum plant, a member of the orchid family, can produce a staggering 4 million tiny seeds in a single year.
Compromise, trade-offs and resource allocation
Seeds are energetically expensive to produce. To start with, the plant has to invest a lot of energy in the production of flowers, and it might even have to give energy away in the form of nectar to keep pollinators sweet. Once the egg(s) in the flowers have been fertilized, the embryo can develop and differentiate into the plumule and the radicle. Food for the embryo is stored in either its cotyledons (seed leaves) or in the endosperm that has resulted from the second fertilization event. Clearly there will be a link between the size of each seed and the number that are produced, which is generally an inverse relationship: the greater the number of seeds produced, the smaller they will be. The most seeds produced by one plant in a year is around 4 million, by the orchid Cycnoches ventricosum, while the coco de mer (Lodoicea maldivica) produces at most a few seeds per annum. This is not dissimilar to the way birds vary in terms of the number of eggs they lay and how much parental care they offer to each chick. In plants, larger seeds represent greater parental investment.
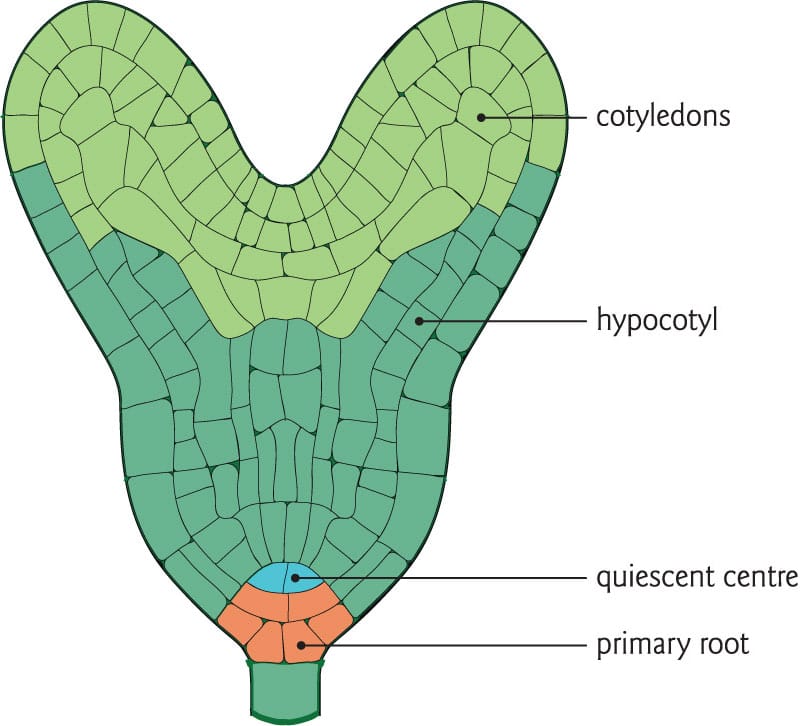
A simplified seed embryo, showing the four main regions including the cotyledons (seed leaves) that nourish the developing root and shoot.

The coco de mer (Lodoicea maldivica) invests so much energy into producing its enormous seeds (the largest in the plant kingdom), that one plant will produce only a few a year and each can take up to seven years to develop to maturity.

Seed size, habitat and life history
Seeds vary greatly in their size, and understanding and explaining this has been one of the most vexatious questions in plant biology. We are still unable to detect universal rules governing seed size, although there are some tantalizingly robust relationships in specific circumstances. For example, in the United Kingdom, increasing seed size is correlated with shade tolerance. Interestingly, the average weight of seeds increases by an order of magnitude (×10) for every 23 degrees in latitude you move closer to the Equator. Against this is the fact that within one Australian population of silver banksia trees (Banksia marginata) a fivefold variation in seed weight was discovered, and five very different floras from around the world have been found to have seed weights within very similar ranges. The prevailing conditions during seed development seem to affect seed size, with higher temperatures often leading to smaller seeds, and the position of the flower on the plant can also have an effect. Seed size therefore is difficult to explain or predict, and perhaps this is the point. It may be that the ability of a plant to produce different-sized seeds in the same year and seeds of different sizes in different conditions is the best strategy, because small and large seeds have different advantages and constraints. A variety of seed sizes is best in an unpredictable world.
Orchid seeds
The seeds of orchids are odd, which is something of a generalization bearing in mind that the orchid family (Orchidaceae) is the largest in the plant kingdom, with 25,000 species found in every habitat except deserts and submerged aquatic environments. This group of plants has been around for at least 75 million years, and their oddness is multifaceted. First, the seeds are very small, lack a carbohydrate store and have a coat that is just one cell thick. Second, the embryo grows into a protocorm rather than a seedling. And third, the orchids have formed an intimate and at times uneasy alliance with fungi.

Walker’s cattleya (Cattleya walkeriana) fruits contain thousands of tiny seeds, which are well suited to wind dispersal across long distances by virtue of their weight.
Why small is good for orchids
The seeds of orchids are 0.18–3.85 mm in size, but most are towards the lower end of this range and the largest is a hybrid and therefore the result of hybrid vigour (see here). Orchids began as terrestrial plants but many extant species are epiphytes growing on the branches of tropical trees. These plants take nothing from their host plant, so they are not parasitic, just benign lodgers. However, they share a problem with parasites like the mistletoes, namely how to get up into the crown of a tree. The majority of mistletoes use birds for transportation (see box), but orchids use wind. Dust-like orchid seeds, which also contain a lot of air, can easily be blown up onto tree branches. Among the very first plants to recolonize the Indonesian island of Krakatoa after the volcanic eruption in 1883 were four species of orchids, whose seeds must have been carried 400 km (250 miles) to get there. Wind can be a very efficient dispersal vector, and the smaller a seed is, the further it will go.
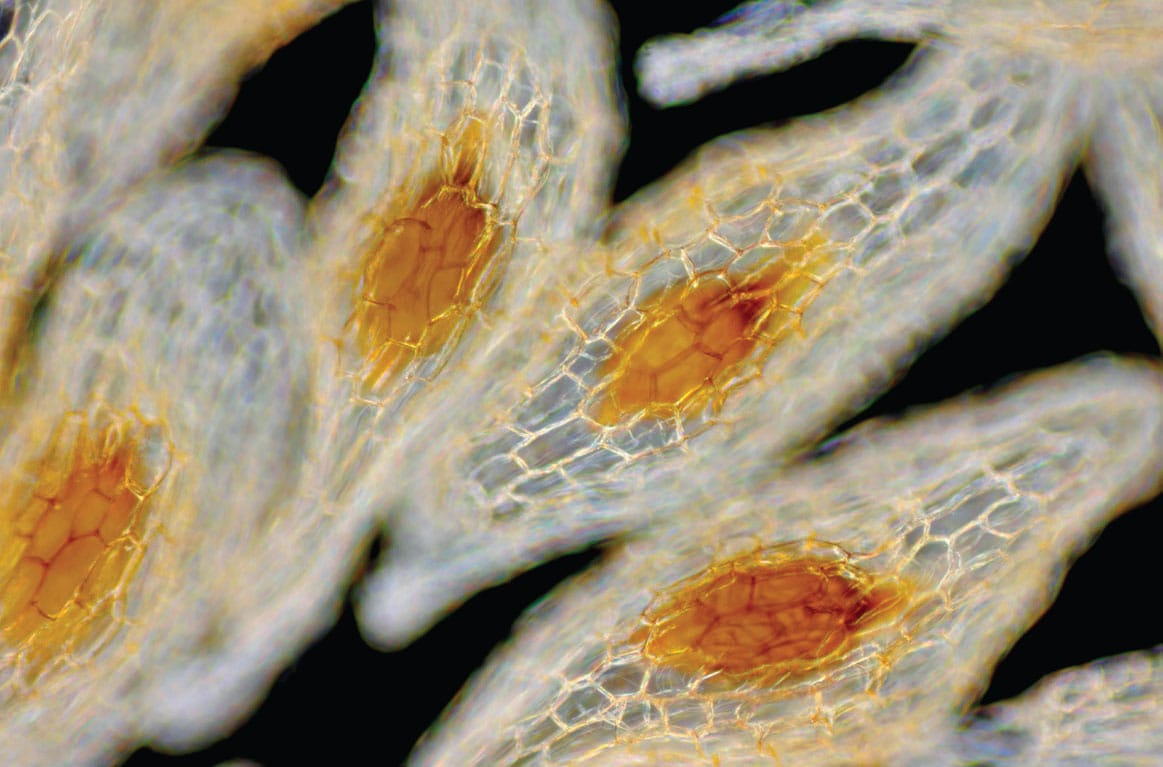
Viewed under the microscope, the tiny seed of this European terrestrial orchid is clearly visible through the one-cell-thick papery layer that surrounds it.
Why small is bad for orchids
The seeds of orchids have made several sacrifices to become so small. First, their embryos are tiny and undifferentiated, so they have to do some catching up before they can put out their first photosynthetic leaf. Second, there are few supplies in the seeds to power the growth and development of the embryo. And third, they are not protected by a thick coat but by a papery layer, which is just one cell thick and about as waterproof as a piece of paper. On the plus side, the seeds are not an attractive meal for an animal, but these significant drawbacks still have to be addressed if the embryo is to grow into a mature flowering plant that produces more seeds.
The wrong way round – again
Orchid seeds look like dust and yet their pollen in normally stuck together in clumps the size of breadcrumbs. This is the reverse of the situation in most plants, where the pollen is like dust and the seeds are much bigger. Another example of orchids getting things the wrong way round is in the formation of the protocorm. A corm is a short, solid, vertical underground stem surrounded by thin, papery leaves, while a protocorm is the first stage in the development of a storage organ in orchids. Corms are generally produced months or years after the seed has germinated, when the plant has been actively photosynthesizing for some time – they act as storage organs. The protocorm of orchids, however, is the first structure produced by the embryo. It is typically 1 mm (1/32 in) in diameter and has no top or bottom to start with, although it will usually have some rhizoids to help with water uptake. Having made the protocorm, the new plant generally stops growing, because the pitifully small amount of energy stored in the cells of the embryo have been used up. It is possible that the protocorm becomes dormant at this point, waiting for a signal such as a period of cold to tell it that it is time to grow.
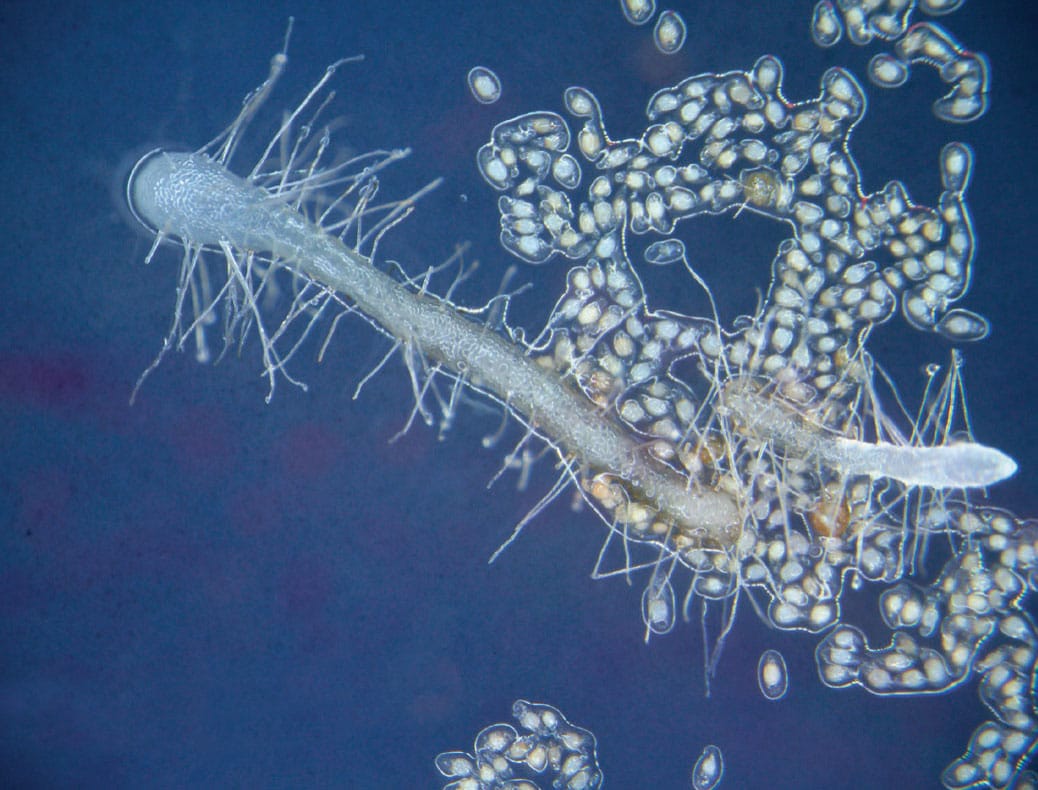
The tiny protocorms (c. 15 mm long) that develop when orchid seeds germinate rely on close mycorrhizal relationships with fungi. The growing protocorm very quickly exhausts the tiny reserves from the seed and must rely on its fungal partner for nutrients, a relationship called mycoheterotrophy.
Storage in seeds
As mentioned earlier, seeds have three basic components that allow them to fulfil their role as a plant survival capsule: the embryo, which will grow into the seedling; the seed coat, which protects the embryo on its journey; and food for the journey and resources for the first few days after germination, before the roots and leaves of the seedling are fully functioning. The storage tissue is not the same in every seed in terms of location and composition.

Due to the small surface area-to-volume ratio, the large seeds of the coconut (Cocos nucifera) would run the risk of not being able to hydrate themselves during germination if it were not for the water supply stored in each fruit.
The origin of the food store
In the thousand or so species of gymnosperm, the nutrient store in the seed consists of the tissue that was responsible for supporting the archegonium, or egg-producing organ. This means that the cells in the store have just one set of chromosomes. In the angiosperms, of which there are around 361,000 species, the food store is either in the cotyledons of the embryo or in the endosperm, which unusually has three sets of chromosomes. This is an innovation of the angiosperms and may be responsible in part for the fact that they are currently the dominant group of plants on Earth. Apart from the very different genetics of the two storage tissues, the gymnosperm seeds make their food before there is an embryo to feed, whereas the angiosperms wait until the egg has been successfully fertilized. The generally larger seeds of the gymnosperms, coupled with the presumptuous production of the nutrient store, makes this group of plants less efficient and more wasteful than the angiosperms.

Many seeds, such as those of peas (Pisum sativum), are rich in substances such as proteins, fats or carbohydrates. This is exploited by humans in agriculture where they are grown as food crops.
Baby food for plants
The nutrients in seed food stores come in many molecular forms and combinations. The major components are either carbohydrates (e.g. starch), proteins (in which case the seed, and thus the young plant, has a larger supply of nitrogen and sulfur) or fats (the most energy rich of the three). Another property of fats is that they are lighter for a given supply of calories, which makes them suitable for wind-dispersed seeds or seeds of species that need fast-growing seedlings. The legume family is known for its production of seeds rich in protein, which is often legumin. Unexpectedly, and inexplicably, the same molecule is used by ginkgo, an isolated, ancient lineage in the gymnosperms. Analysis of the mineral contents of seeds reveals that the proportions of these elements bear little or no relationship to the needs of the seedlings, which must become self-supporting as soon as they can. Nitrogen is most likely to run out first, followed by calcium.
Seeds and animals
Seeds did not evolve with the purpose of feeding animals, and yet many animals – including humans – depend on them for much of their calorific intake. The majority of seeds contain food for the embryo, the major exception to this rule being the orchids. This food, in the form of carbohydrates or fats, can provide energy for any organism – many gardeners will have seen birds such as finches feeding in the autumn on the seeds of plants in their gardens. Being eaten by an animal is, generally speaking, bad news, and yet plants have overcome this problem, and in some cases even turned it to their advantage.

A male Brambling (Fringilla montifringilla) takes advantage of the copious quantities of seeds (right) produced in a beech mast year.

The first seed-eaters
It is a reasonable proposition that the first seeds were produced at ground level. The fact that the majority of plants produce seeds shows that this is a very stable strategy from an evolutionary point of view. However, at some point since the emergence of the first seed plant, a passing animal must have taken an exploratory nibble at the seed and found that it was nutritious. Furthermore, the seed did not run away or fight back; it just accepted its fate. The plant clearly had to respond and adapt to this new threat. This response could, and did, take several forms.
A numbers game
The relationship between the density of a plant species and the density of the herbivores that feed off that plant is clearly a dynamic one. If the number of herbivores rises too high, for example, the number of plants may fall until it is no longer sufficient to support the animals and their population will crash. By producing large numbers of seeds, plant numbers can increase to acceptable levels as the likelihood is high that some seeds will avoid being eaten. One way to increase the odds further is to vary dramatically the number of seeds produced each year. This is known as masting, and is seen in tree species such as the European beech (Fagus sylvatica). In most years, this species will accept a high level of seed mortality. In a mast year, however, the production of seeds is so excessive that there is no way the herbivores that feed on them can consume all of them. The following year, there may be a crash in the herbivore population, when the reduced number of beech seeds produced cannot support the increased numbers of animals.
Seed sowing
Seed dispersal by animals can take many forms, but one of the best from the plant’s point of view is where the seed is not only taken a safe distance from its parents, but it is also sown. Scatter hoarding is a well-documented phenomenon, and generally involves vertebrates as opposed to small creatures like ants that have permanent nests. In South America, agoutis gnaw out the seeds of the Brazil nut tree (Bertholletia excelsa) and then bury them to keep them safe for later. Fortunately for the trees, the agoutis do not recover every seed.

The Brazil nut tree (Bertholletia excelsa) relies on the sharp teeth and forgetful nature of the scatter-hoarding rodents called agoutis for its seed dispersal and survival.
Protecting seeds
Seeds can fulfil their function as survival capsules only if they themselves are robust. The threats facing the embryo fall into two broad categories: there are those that are derived from the environment, such as fire, which are termed abiotic hazards; and there are those that come from other organisms, the major one being predation, which are termed biotic hazards.

Slow-growing agaves play the long game, producing flowers and seeds after many years.
Abiotic hazards
Weather can be a problem for any plant at any stage in its life. Drought, flood, frost, heat and fire can all retard growth and even kill a plant. Difficult conditions during the formation of fruits containing seeds can threaten the fecundity of a plant, and this is a particular problem for plants that fruit just once – monocarpic species. These species build up resources that can then be used for a once-in-a-lifetime final reproductive effort, and the time it takes them to do this varies with the habitat and size of the plant. For small herbs like hairy bittercress (Cardamine hirsuta), it can take just a few weeks, but for some larger, slower-growing plants such as agaves (Agave spp.), it can take many years. Generally speaking, monocarpic species put a far higher proportion of their biomass into nurturing their embryos. For example, annual grasses may put 50 per cent of their biomass into raising their seeds, whereas in perennials the figure may be as low as 10 per cent.

Jequirity (Abrus precatorius), a member of the legume family, contains extremely toxic alkaloids, but potential herbivores associate the red-and-black colour of the seeds with toxicity and thus leave them alone.
Deterring herbivores
In common with parents across the Earth, plants try to give their offspring the best start in life and prepare them for the threats that lie ahead, some of which come from other organisms. Seeds are a wonderful resource for animals, being full of carbohydrates, fats, proteins and minerals, so persuading animals not to eat them is an important survival strategy. One way plants do this is by making their seeds highly visible, advertising the fact that they are toxic in the hope that animals remember to associate the colour with toxicity. That said, if the animal does die, the seed will be inside a rotting carcass that may provide nutrients for the seedlings. Some of the world’s most notorious toxins, including ricin and hemlock, are found in seeds. These molecules are expensive for the plants to synthesize, so it is a reasonable assumption that they have a survival benefit.

Some plants, like hairy bittercress (Cardamine hirsuta), can squeeze many generations into the space of a year by completing their life cycle in a matter of weeks.
The benefit of a good coat
Chemical protection of seeds is one strategy for survival, but prevention is better than revenge. This is where the seed coat comes into the spotlight. The integument has several functions, one of which is undoubtedly to protect the embryo from enzymes in the intestines of birds and other herbivores. It is worth noting here that this is not an onerous task in the case of bird herbivory, because the gut environment of many birds is not as hostile as that of mammals. Another function of the seed coat is to endow physical dormancy, which is broken by specific conditions and not by random damage or microbial attack, as is frequently stated. In addition, the seed coat physically protects the embryo from the environment, filtering out certain wavelengths of the light and keeping water in or out – parent plants grown in drought conditions will produce seeds with thicker seed coats. There is also clear evidence that in some seeds the integument includes chemicals with antifungal properties, and occasionally with antibiotics. One example is the wild parsnip (Pastinaca sativa), in which it has been shown that an increased levels of antibiotics significantly reduces the allocation of resources to seed production.

The seeds of wild parsnip (Pastinaca sativa) pack an antibiotic punch in their coats, but at the expense of the food resources produced in the seed.

Castor-oil plants (Ricinus communis) are the source of the deadly toxin ricin, which was used to assassinate the Bulgarian dissident Georgi Markov in 1978. A minute quantity in the tip of a sharpened umbrella ferrule was sufficient to kill him within a few days.

Preventing germination
When a seed is released by a plant, it will hopefully land in soil that is appropriate for its species, and that is moist and at a suitable temperature. The seed should germinate and be growing into a healthy seedling within a month. If it does not germinate under these conditions, there are two possible explanations. First, the embryo may be dead, or it never developed in the first place (the latter is surprisingly common in garden conifers). Or second, the seed may be dormant and will not germinate until that dormancy is broken.

The seeds of the Douglas fir (Pseudotsuga menziesii) have a wing that slows their descent to the ground, enabling the wind to blow them further from their parent plant.
Why dormancy is a good idea
Dormancy is found in many, although not all, plant species. It has evolved several times and so it is assumed that in some circumstances it has a survival value. It does, however, bring with it a level of risk, because the longer the seed lies around, the greater the chance an animal will eat it. There are many potential reasons for the evolution of dormancy, which is a form of dispersal, but dispersal in time rather than space. Surviving in both unpredictable and predictable environments can be improved by optimizing the timing of seed germination, to coincide with the best conditions for seedling survival. This may involve avoiding cold, hot, wet or dry conditions, or avoiding predation. Dormancy can also be a way of preventing intergenerational breeding, a strategy that encourages genetic diversity. Habitat and life history both have an important influence on dormancy.

It is common practice in horticulture to dry seeds in paper bags to promote dormancy, or quiescence.
Types of dormancy
Dormancy types are commonly classed as either endogenous or exogenous. In endogenous dormancy, something in the embryo imposes the state, whereas in exogenous dormancy any other part of the seed and/or fruit can prevent germination. Exogenous dormancy may be physical, chemical or mechanical, whereas endogenous dormancy can be physiological, morphological or morphophysiological. Some researchers have also grouped together chemical and mechanical dormancies with the other physiological categories to create a new type of dormancy, which is a combination of physical and physiological components. Whichever system is used, the fact is that there is more than one way to achieve dormancy and no single system fits every situation. For example, combined physical and physiological dormancy may be the best strategy where fire is a regular component of the ecology of a habitat, as seen in the North American redstem ceanothus (Ceanothus sanguineus).
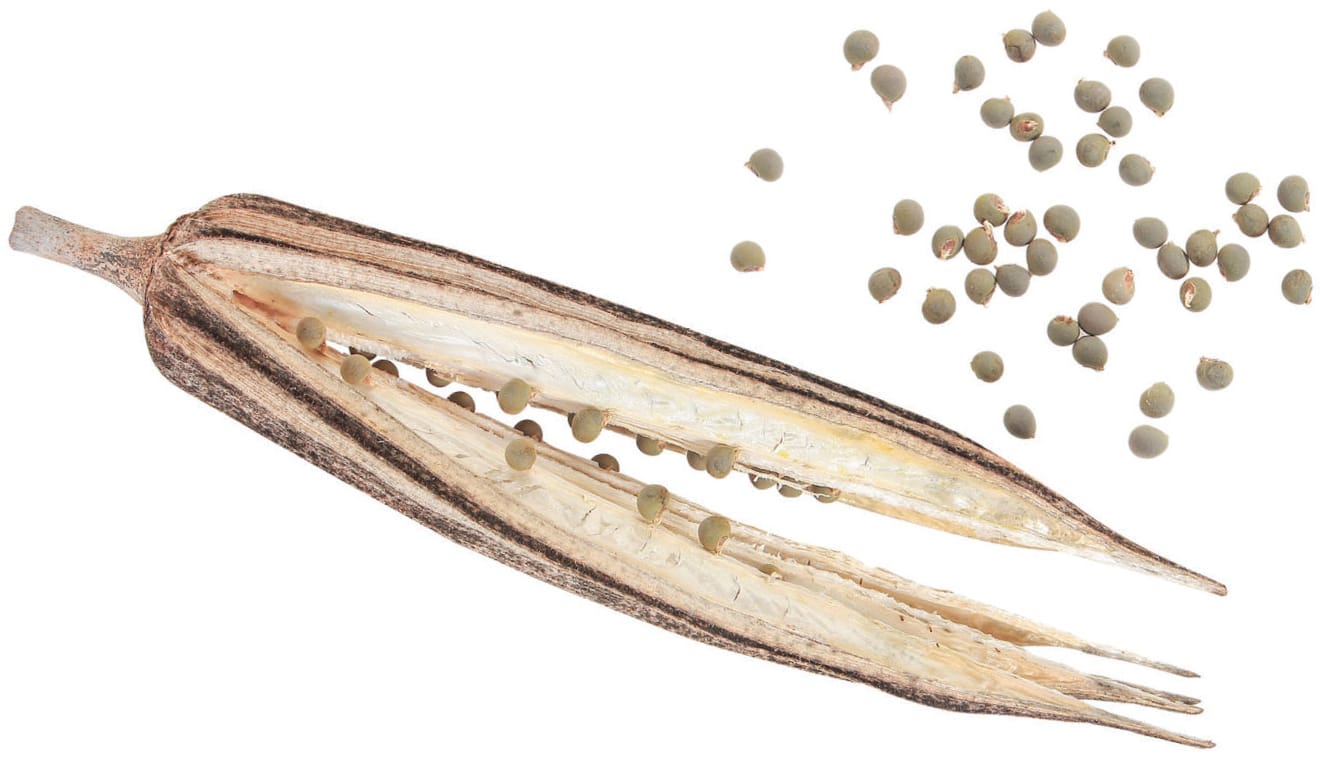
The dried seeds of okra (Hibiscus esculentus) will survive much longer than those left inside a moist fruit.

Redstem ceanothus (Ceanothus sanguinea) optimises its chances of survival in a habitat characterised by regular fire by adopting both physical and physiological seed dormancy.
Stimulating germination
Farmers, gardeners and conservation biologists all need to know how to make seeds grow into vigorous plants. This may involve breaking the seed’s dormancy, and in order to do that, the plant needs to be identified, at least to family level and preferably to genus or species. Important factors indicating the type of dormancy in a species new to you include how well developed or differentiated the embryo is, whether the embryo grows prior to germination, how impermeable the fruit and/or seed coat are to water, and finally whether the shoot and root appear at the same time or the root clearly precedes the shoot.

Many members of the legume family, such as beans (Phaseolus spp.), have thick, hard seed coats that must be ruptured before germination can begin.
Breaking physical dormancy
This is perhaps the easiest type of dormancy to crack, because it may simply involve breaking the hard, woody layer of integument cells that are impregnated with substances such as lignin and cutin, or waxes and other lipids. A typical family that uses this type of dormancy is the legume family, and wise vegetable growers know that soaking their bean or pea seeds in warm water for 24 hours before sowing can help to soften the seed coat. Some tropical woody members of the family have large seeds that can be carefully cracked with a hammer.

Seeds of the common ash (Fraxinus excelsior) contain inhibitors that must be washed or leached out before germination can begin.
Breaking physiological dormancy
In some seeds there is no physical barrier to germination, but a physiological inhibitor instead. This might be a chemical that prevents the cells of the embryo from dividing and thus stops the root from emerging, an example being the plant hormone abscisic acid (ABA). A common way in which physiological dormancy is broken is through a minimum period of warm or cold weather, or periods of both, known as stratification. Common ash (Fraxinus excelsior) and sycamore (Acer pseudoplantanus) seeds contain inhibitors that simply have to be washed, or leached, out. Sometimes, a molecular change in a layer of the fruit can be brought about by a period of cold, and this facilitates the emergence of the root. This is found particularly in members of the rose family (Rosaceae).
Breaking morphological dormancy
Morphological dormancy is when the embryo is either undifferentiated or differentiated but undeveloped at the point when the plant releases its seeds. For such dormancy to be broken, the correct conditions must be present for the embryo to continue its differentiation and development. This means that there must be a moist substrate at the right temperature and the right light conditions, which might be dark (buried) or light (not underneath other plants).
Breaking combinational dormancy
To overcome combinational dormancy, an impenetrable barrier must be broken for water to enter the seed and then a physiological barrier such as an inhibitor must be removed by cold temperatures, or there must be after-ripening of the embryo at high temperature. This being biology, and therefore nothing being simple, the order in which the two types of dormancy are broken differs between species.
Seeds and fire
When plants emerged from their watery environment about 470 million years ago, they remained wet enough to resist burning for millions of years. However, once they became lignified and grew into trees, they contained sufficient combustible material to fuel a decent conflagration. It is reasonable to suppose that any innovation that enables a plant to get through a fire has been naturally selected. Such innovations include protecting seeds.

Forest fires are not uncommon in certain habitats, for example in Mediterranean-type climates, but many plants can survive and reproduce even in these extreme conditions by adopting various survival mechanisms.
Where do you keep yours?
There are two places where seeds survive fire. First, there is the obvious place – in the soil. How deep is deep enough for seed survival depends on the temperature of the fire, but a good rule of thumb is a depth of 50 mm (2 in). Second, plants can ‘hide’ their seeds in cones that are held in the canopy. Such woody cones have evolved several times in both gymnosperms and angiosperms. Pine trees (Pinus spp.) are the best-known examples of serotiny (the release of seeds from a cone prompted by an environmental stimulus, e.g. fire) in the gymnosperms, but Australians will be just as familiar with the cones of banksias (Banksia spp.) in the angiosperms.

Giant redwoods (Sequoiadendron giganteum) are just one type of gymnosperm that are able to survive fire by protecting their seeds in woody cones. Once the fire has passed, the cones open to release their seeds into the freshly prepared and nutrient-rich soil.
No germination without smoke
Seeds are commonly buried by simply falling into a fissure in the soil, but many are also buried by ants in their nests. Such seeds often have a fat body known as an elaiosome attached to them, which is thought to contain a chemical that is irresistible to ants. Irrespective of the method by which seeds come to be buried, researchers are exploring why they germinate following a fire. While heat was the first and obvious suspect, it has been shown that in many cases it is smoke that triggers germination, and specifically the chemical butenolide in that smoke. Following a bush fire, it is common for the ground to be covered with seedlings.

In Australia, the heat of a bush fire causes Banksia cones to open and release their seeds after the fire itself has passed.
The right sort of fire
The features of wildfires are numerous and vary with every event. The time of year and the size of the area burnt are two, but the major variable is intensity, which itself is determined by factors such as the type of plants (i.e. the calorific value of the fuel) and the length of time since the last fire (i.e. the amount of fuel lying on the soil surface). The intensity, which is a combination of temperature and the length of time the fire stays in one place before burning out, is critically important to plants. If the fire is too intense, the seeds in the ground will get too hot to survive. Seeds that are held in cones in the canopy are released when heat from the fire melts the wax that is sealing the scales of the cone, but if the fire is too intense, the flames will reach into the canopy and the cones and their seeds will be incinerated.

After a bush fire, seeds stored in the seed bank in the soil germinate en masse in response to the chemicals in the smoke.
Fruits and animals
Along with flowers, fruits are a defining feature of the angiosperms. The ovary at the base of the carpel encloses and isolates the ovules, protecting them from the environment through a delicate phase. In common with any part of a plant, a fruit can be eaten, but it appears that some plants – particularly tropical woody species – go out of their way to attract herbivores, while others do the exact opposite by being toxic. There must be some advantage to the plant in developing fruits in these ways, because they often contain energy-rich carbohydrates, demanding an investment of resources.

Bohemian waxwings (Bombycilla garrulus) rely on the fleshy fruit of many plants to survive the winter, and in doing so disperse the seeds away from the parent plant.
Dispersal syndromes
The most common explanation for the evolution of fleshy fruit is that fruit characteristics are associated with the dispersal of seeds, in much the same way that flower characteristics are associated with pollination. In pollination, the correlation between pollinator and flower is often so predictable that there are well-defined pollination syndromes (see here). Despite various efforts over that past 100 years, however, no one has come up with a comparable set of seed-dispersal syndromes. For example, tubular red flowers with lots of sweet nectar are often pollinated by birds, but sweet-tasting, bright red fruits are not the preserve of birds. It seems that many animals eat many different fruits, and that many fruits are eaten by many different animals. Pollination in particular, and herbivory in general, are very well studied, but frugivory and seed dispersal are lagging behind.
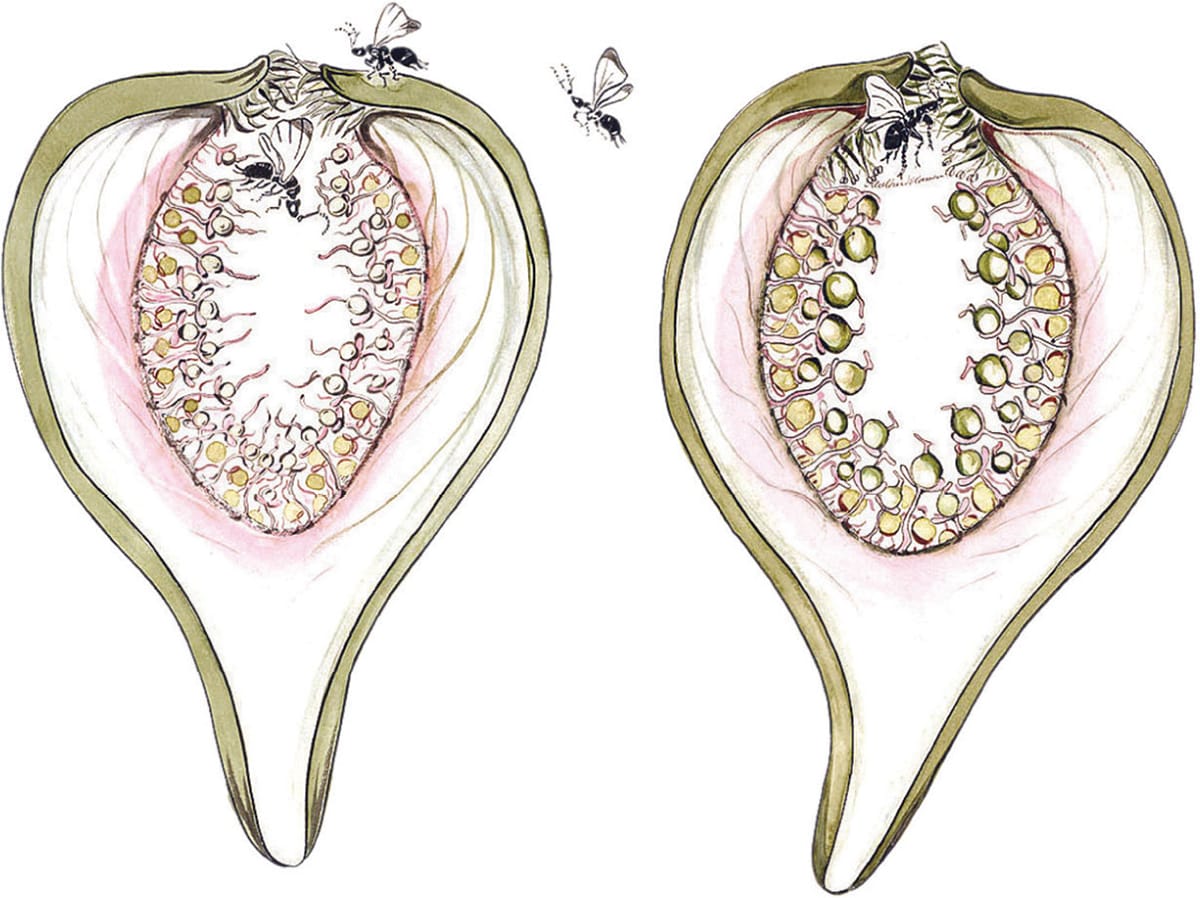
The variation in length of the ovaries in the female flowers of the fig (Ficus sp.), mean that some are parasitized and some are pollinated by visiting fig wasps.
It is not all about dispersal
Some pollinator–plant relationships involve the fruits as well as the other components of the flowers. Fig wasps are able to lay their eggs in some of the ovaries of female fig (Ficus spp.) flowers because the styles attached to these ovaries are short enough for the ovipositor to reach them (see here). Flowers with long styles are not parasitized but are pollinated. Yucca flowers are similarly parasitized by the egg-laying females of yucca moths (see here), although there appears to be some restraint on the part of the moths because they lay eggs in only a few of the ovules. This means that some viable seeds will be produced to continue the host plant lineage.
Dry fruits
The majority of fruits produced by plants are dry fruits, including all the grains harvested from cereal crops. These are a particular type of dry fruit called a caryopsis, in which the seed and ovary wall are fused together. Dry fruits are often associated with abiotic methods of dispersal, because they lack a nutritious fleshy tissue to act as an inducement to an animal. There are, however, many well-documented examples of animals scatter hoarding dry fruits. This is clearly similar to the dispersal of seeds inside fleshy, edible fruits, but it differs in one very important way: the reward is the seed itself, and the parent plant ‘sacrifices’ some of its offspring so that others may survive.

The helicopter wings of sycamore (Acer pseudoplatanus) seeds keep them aloft for short distances.

The seeds of goat’s-beard (Tragopogon pratensis) take advantage of their parachutes, which let them float through the air away from the parent plant.

Ash (Fraxinus spp.) seeds, or keys, have dry wing-like appendages that aid wind dispersal.
On a wing and a prayer
The wind can transport seeds long distances – 400 km (250 miles) in the case of orchid seeds to Krakatoa after its eruption (see here), and 100 km (60 miles) for a conifer seed in North America. These are probably exceptions, and most wind dispersal is over far shorter distances because adaptations to flight are more to do with slowing the rate of vertical fall than movement in a horizontal direction – as seen in the hairy, parachute-like structures on the fruits of many members of the daisy family (Asteraceae). The samaras (winged fruits) of maples like the sycamore look like the rotary blades of a helicopter but they do not keep the seeds in the air permanently. Pine (Pinus spp.) and ash (Fraxinus spp.) trees have fruits that are a variation on the theme of helicopter blades, while birch (Betula spp.) fruits have two wings and look like a small butterfly. An important consideration here is the lack of control over the direction of travel. Prevailing winds will result in a seed shadow, with distribution being asymmetrical. This may sound trivial, but the colonization of the oceanic Canary Islands off Africa has been significantly influenced by the fact that the prevailing winds come from the northeast, blowing seeds from southwest Europe and northwest Africa.
Hanging on
The fruits of many families of flowering plants have small hooks so that they can attach themselves to passing mammals and birds. So successful is this strategy that the ability of the tiny hooks on the fruits of burdock (Arctium spp.) to hang onto clothing was the inspiration for the invention of Velcro by Swiss engineer George de Mestral. The fruits of plantains (Plantago spp.) do not have hooks but become adhesive when wet, sticking to mammal fur and bird feathers. Plants in this genus are pollinated by wind but dispersed by animals. There appears to be very little relationship between choice of pollinator and dispersal vector in plants.

Seeds of burdock (Arctium lappa) adopt a ‘Velcro’ method of dispersal, using hooks to attach themselves to passing animals. The burs are fruit heads covered in involucral bracts that become woody and hooked after fertilization.
Fleshy fruits
Fleshy fruits are familiar to us because we eat them, as do many other animals (see also here). In fact, this may be the main function of fleshy fruits – to be eaten. However, it is not out of an altruistic desire to feed animals that the plant puts resources into fruit, but to have its seeds dispersed. Many parts of the plant associated with flowers can become fleshy, and to an animal it matters not one jot where its food comes from. To a botanist, however, a fleshy fruit is one in which the fleshy tissue is derived from the wall of the ovary.

Left to right: stinking iris (Iris foetidissima), asparagus (Asparagus officinalis) and plum (Prunus domestica). Although these are members of different families, they all produce drupes, fleshy fruits containing a single seed.


Drupes
Fleshy fruits with just one seed, called drupes, are found in many families of plants across the evolutionary tree. Within the monocots, drupes are common in the palm family (Arecaceae) and asparagus family (Asparagaceae). The bright orange fruits of cuckoo-pint (Arum maculatum), in the monocotyledonous arum family (Araceae), are a familiar sight in European woodlands, and look remarkably similar to the seeds of another shade-dwelling plant, the stinking iris (Iris foetidissima). The arum fruits are seriously toxic and it appears that the iris may be mimicking them to avoid predation. Other familiar drupes are cherries, plums and peaches. When lots of little drupes, or drupelets, are arranged on a receptacle, it is known as an aggregate fruit – blackberries and raspberries are good examples.

Confusingly, the blackberry (Rubus fruticosus) is not a berry but is an aggregate of drupes, individual fleshy fruits each containing a single seed. On the other hand, the melon (Cucumis sp.) is a berry, because it is a fleshy fruit containing many seeds.

Berries
This being botany, raspberries are not berries, while tomatoes, bananas, grapes and aubergines are. The definition of a berry is a many-seeded fleshy fruit, and they include countless variations that look very different from one another. Citrus fruits like oranges and lemons are a type of berry, in which the fleshy tissue is made up of fluid-filled hairs. Melons and cucumbers are berries with thick rinds and are known as pepos.

Purple aubergines, red tomatoes, yellow lemons and green melons. Fleshy fruit comes in a wide range of different colours that may or may not have a bearing on their function in biology.
The colour of fruit
Fleshy fruits tend to be colourful, whereas dry fruits tend to be brown. While some biologists like everything that has been adapted by natural selection to have a function, naturalist Alfred Russel Wallace cautioned in 1879 that, when it comes to fruit characters such as colour, looking for a function could be a waste of time. However, that has not stopped people speculating and researching the area. Colour in fruits may be a signal, encouraging animals to eat them, or discouraging animals from doing so because they are toxic. The signal could change with time from the latter to the former, to ensure that the seeds are removed only when ripe. This might be a change to red from green to make the fruit more obvious when ripe, because green and red are contrasting colours that make each other more saturated. As Wallace warned, however, colour transformation might just be a consequence of chemical changes during ripening or varying temperatures at night. How plants work is still a mystery in many ways.

Naturalist Alfred Russel Wallace (1823–1913) warned against relying on function as a cause of fruit colour.
False fruits
Flthough a fruit is defined as a ripened ovary and any other structures attached to it that ripen with it, there are times when a line has to be drawn and structures that appear to be attached to the ovary must be declared ineligible. These ‘fruits’ were previously referred to as false fruits, which would not have been a problem except that many of them are found in any greengrocer’s shop, including strawberries, figs, apples and pears. The term false fruit has now largely been replaced with accessory fruit, which is more appropriate because it implies correctly that the structure is a fruit with some additional structures.
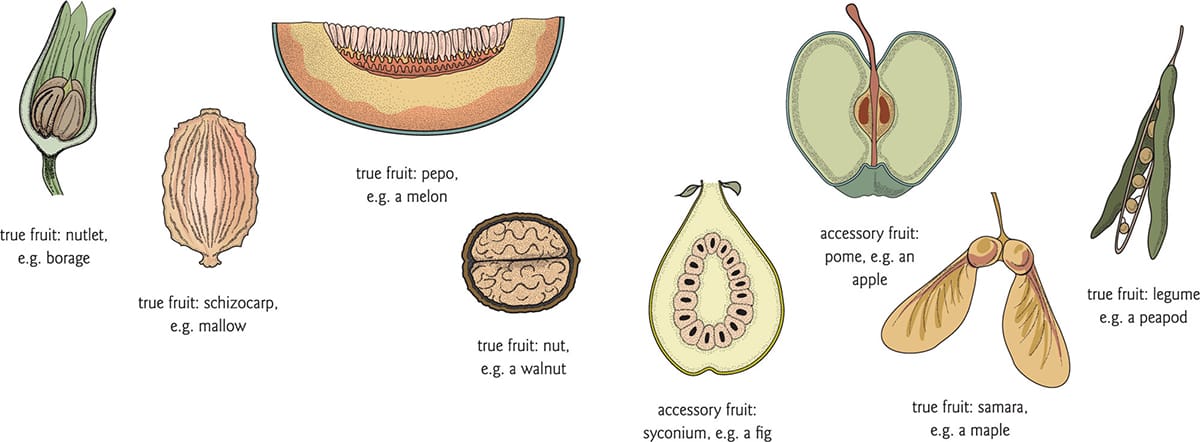
Some examples of true and accessory fruits, demonstrating that accessory fruits are very often the ones we search for in the fruit section of the supermarket.
Accessorizing fruits
Being imaginative with fruits is a trait that members of the rose family have taken to the highest level. Some species bear fruits that are unambiguously, botanically, fruits – for example, cherries, nectarines, and peaches are drupes. Another example of a conformist is the genus Dryas (see box), whose members produce achenes. However, within the rose family we also find perhaps the easiest accessory fruit to understand, the strawberry. Rosaceae species have many things in common, including the hypanthium and receptacle. The hypanthium is a cup in which the carpels sit, and around the edge of which are attached the perianth and the stamens. The receptacle is a cone in the centre of the hypanthium on which the carpels sit. In the strawberry (Fragaria spp.) flower, the carpels develop into achenes – true fruit. These achenes are embedded in the receptacle, which turns bright red when the achenes are ripe.

Strawberries may look a ‘normal’ fruit, but the sweet, juicy flesh is derived from the receptacle. To be a true fruit requires that the flesh is derived from any part of the ovary.
Apples and pears
The fruits of apples (Malus spp.) and pears (Pyrus spp.) are examples of a type of accessory fruit found only in the rose family – the pome. It appears in several other genera and consists of a core, which is the fruit, and an expanded receptacle, which makes up the flesh of the fruit. In cotoneasters (Cotoneaster spp.), hawthorns (Crataegus spp.) and medlars (Mespilus spp.), the flesh of the fruit is derived from the hypanthium rather than the receptacle.
Pineapples and mulberries
Another group of fruits that are not quite what they seem are those formed from many flowers, which then merge together or are contained in one fruit-like structure. One subset of these are two multiple fruits that are in very distantly related groups: pineapples in the bromeliad family (Bromeliaceae) and mulberries in the mulberry family (Moraceae). In both of these, many small flowers on the receptacle each contribute a carpel and thus fruit to the ‘fruit’.

Medlars (Mespilus germanica; top) and hawthorns (Crataegus monogyna; bottom) are accessory fruits, with the flesh derived from the hypanthium, a structure characteristic of Rosaceae species.

Pineapples (left) and mulberries (right) are examples of aggregations of many smaller fruits.


Seed dispersal
Seed dispersal occurs through both time and space. Dispersal in time enables plants to ride out unfavourable conditions – the most extreme example being 30,000 years (see opposite). In contrast, dispersal in space enables plants to get their offspring away from their roots. The record here is 18,000 km (11,000 miles), verified for a species of Acacia that appears to have made the journey from Hawai’i in the Pacific Ocean to Réunion in the Indian Ocean. It is assumed that the seeds were in mud on the body of a bird, which travelled between the islands around 1.4 million years ago.

Dispersal of seeds through time relies on the longevity of the seed. 2,000-year-old seeds of the date palm (Phoenix dactylifera) have been shown to be still viable.
Why leave home?
Dispersal is something into which plants invest a good deal of resources, and so it is a reasonable assumption that it is an evolutionarily stable strategy. But why is this? A plant living in a habitat that has everything it needs to survive and reproduce is a successful, fit plant. Would it not want the same for its offspring? The answer here is yes and no. If it is a monocarpic plant that dies after fruiting, there is no need for it to disperse its seeds because it will leave a gap on its death. However, if it is a long-lived perennial, it needs to send its offspring away because the most severe competition any organism faces is from members of the same species. Dispersal also has the advantage of enabling the species to try out new habitats that might suit it better. In addition, it helps to reduce breeding with close relatives, which is never a good idea. Finally, it can enable plants to get away from predators and pathogens.

Seeds of goosegrass (Galium aparine) are covered in tiny hooks and are dispersed in space by sticking to the coats of passing animals.
The dispersal options
The options for seed dispersal fall into two categories: abiotic dispersal, which uses wind or water, sometimes aided by modifications to the seed such as wings or buoyancy tanks, respectively; and biotic dispersal, which relies on an animal as the vector. When animals are used, there are three subcategories: endozoochory, where the seed is transported on the inside of the animal following ingestion but (hopefully) not digestion; epizoochory, where the seed is attached to the outside of the animal, such as the adhesive fruits of goosegrass (Galium aparine); and dyszoochory, or scatter hoarding of seeds, where the hoarder fails to recover all the seeds. All of these options may be aided by ballistic dispersal, in which the seeds are ejected explosively, as in the caper spurge (Euphorbia lathyris).
Fruit dispersal
Fruit dispersal is influenced by fruit structures in the same way that flower pollination is influenced by flower structures. Of those fruits and seeds that are modified to enhance dispersal, dry fruits and seeds have become smaller and lighter to be moved passively by wind and water, or have adopted hooked or sticky surfaces to cling to the outsides of animals. Fleshy fruits and seeds, irrespective of the origin of the flesh, are modified to be eaten by animals to promote dispersal. Pollination and fruit dispersal by animals are considered to be examples of mutualism relationships, whereby both parties benefit. Darwin proposed that the flower–pollinator relationship is very close, and there has been a great deal of research carried out both to support and, recently, refute this idea. Frugivory and seed dispersal are lagging behind in this respect, but research in these areas is catching up.

Fruit dispersal is not the exclusive domain of mammals and birds – reptiles such as tortoises are not averse to eating fleshy fruits.
The start of the affair
As soon as fleshy fruits (here meaning any seed(s) with a squidgy coat) appeared, animals tried to eat them. Initially, those animals might have been invertebrates, choosing rotting ovary tissue as an appealing place to lay their eggs. The plants with the tissue most likely to rot may have been preferentially selected, and from this fleshy fruits may have evolved. Once there were fleshy fruits, more animals would eat them, but these animals were very different from the herbivores we see today. Reptiles and dinosaurs did and, in the case of the former, still do eat fruits. The role of reptiles in fruit dispersal today is often dismissed, yet it exists and it has a name – saurochory. This group seems to be particularly attracted to oily fruits and those that are smelly and colourful. Furthermore, the fruits eaten by reptiles are often attached to the plant near the ground. The significance of this is that the first seeds and fruits were attached close to the ground. This might be serendipity, but it might also account for the importance of ancestral reptiles as fruit dispersers before mammals and birds diversified.

Reptiles such as iguanas are particularly attracted to colourful, smelly, oily fruits that are easily accessible at ground level.
Mammals and birds
Saurochory predicts which fruits will appeal to reptiles. Birds, which tend to have a poorly developed sense of smell, generally disperse fruits that are odourless but are brightly coloured red, black or blue. Frugivorous mammals tend to be nocturnal, and the fruits they disperse are therefore generally dull in colour but highly fragrant. Studying dispersal by animals is difficult, because proving a connection between a specific animal and a specific seedling requires meticulous observation. The reliability of vertebrates as seed dispersers has also been questioned, because where an animal deposits the seed is determined by the needs of the animal and not the needs of the plant – roosts, nests and breeding or display territories may all be unsuited to the plant. However, one research project has clearly and unambiguously revealed that macaws (three species in the genus Ara) are the perfect dispersers of seeds of the motacú palm (Attalea princeps).

This Galloway cow has become the unwitting dispersal agent for the Velcro-like fruit of a burdock (Arctium sp.) plant.

Frugivorous animals such as this crowned lemur (Eulemur coronatus) have yet to be proved as reliable seed dispersers, and plants are very often at the mercy of their behaviour.