Xanthasia and the Magic Numbers
Science is about refining things from the simple to the complex until everything makes sense. It’s easy to think of atomic structure as something we fixed a long time ago, thanks to the likes of Ernest Rutherford and Niels Bohr. A nucleus at the centre, and electrons orbiting in shells: the solar system in miniature (albeit obeying different rules of physics). What could be simpler? It’s an idea still taught in schools.
We’ve already added a layer of complexity. When Lise Meitner discovered fission, she was using the idea that the nucleus behaved like a drop of magnetic water. This is the ‘liquid-drop model’ and, for most things, it works rather well.
When it comes to element hunting, the liquid drop isn’t enough to explain everything. Why, for example, are some isotopes more stable than others? Why do some combinations of beam and target have higher cross sections than others, making them more likely to occur? The answers lie in a final layer of complexity, first published in 1949 in two separate papers in back-to-back issues of Physical Review. Entirely independently of each other, the US theoretical physicist Maria Goeppert Mayer and a German group led by Hans Jensen had come up with similar, radical ideas about how the nucleus worked. Rather than compete, Goeppert Mayer and Jensen had decided to work together and publish a book on the subject. It was called Elementary Theory of Nuclear Shell Structure. Today, it’s known as the nuclear shell model.1
The idea was relatively straightforward. The electrons orbiting the nucleus were already known to form shells. Rather than just being a big blob of magnetic liquid, the protons and neutrons both had these shells too, independent of each other, related to different energy levels. This was linked to an idea called spin-orbit coupling, which Goeppert Mayer explained like a ballroom full of waltzers, all going around a room in circles (the orbit), deciding which way to step and when they’ll twirl (the spin). On the dance floor, it’s much easier for the waltzers if they are all circling and twirling in the same direction as it requires less energy. When she hit certain numbers – when the nuclear shells were filled – everything was at its most tightly bound. The dancers took up the ballroom but had enough space to look elegant and ordered as they glided across the floor. Add too many dancers, though, and they interrupt the flow. By spotting when there was a big difference in energy when another proton or neutron was added – where the dance was being disrupted – Goeppert Mayer could identify where the shells started and finished.
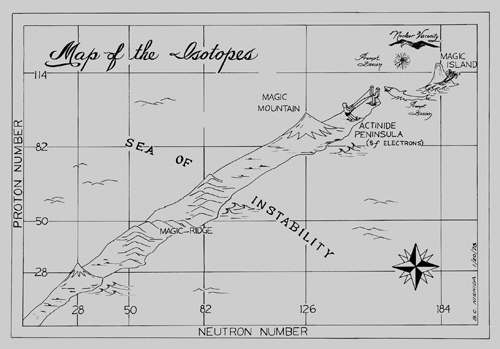
The new model of the nucleus was the biggest shift in fundamental particle science since the 1930s. The chemical world opened as if a map had been unrolled, with the number of protons and neutrons as the axes. The stable elements existed on a long, thin peninsula that arched toward the top of the chart before dipping away. On either side was a ‘sea of instability’ – where the whole nucleus would break apart. All the discovered isotopes existed on this peninsula; adding or taking away neutrons pushed the element toward the peninsula’s edge, making the structure less stable and giving it a shorter and shorter half-life. Although in theory the elements could extend to number 173, the peninsula of stability had already run out at uranium.2
This explained what was being seen with the superheavy elements: the Berkeley and Dubna teams were creating the elements at an imaginary cliff edge that marked the end of stability, where the peninsula fell away into the sea. The half-life of the most stable form of californium was 898 years; by fermium it was 100 days; for element 102 it was 58 minutes. Sooner or later even the most stable isotopes would be gone in less than a second. If that were the case, the superheavy elements would always be little more than laboratory curiosities. And what’s the use in that?
But there was another twist. If the nuclear shell idea was true, that meant there were some combinations of protons and neutrons that would be inherently more stable; just like you sometimes need to fill the ballroom to make the dance floor fun for everyone, sometimes higher numbers of protons and neutrons resulted in a sort of nuclear balancing act.
The idea seemed almost beyond belief. One of Goeppert Mayer’s colleagues, Eugene Wigner (the man who had transformed Oak Ridge into a national laboratory), admitted that the evidence for her theory was compelling, but felt the supposed moments of increased stability seemed to occur at ‘magic numbers’. The name stuck. In 1963 Goeppert Mayer, Jensen and Wigner won the Nobel Prize for their work. In doing so, Goeppert Mayer became the first woman since Marie Curie to win the highest prize in physics.
The idea of magic numbers changed the face of element discovery. In both the US and USSR, the teams realised that if you could create a nucleus with a ‘magic number’ of protons or neutrons, the newly created element would be far more stable than anything they had made before. Continuing the theme of peninsulas and seas, they began to refer to these nuclides as being on ‘the island of stability’. If, somehow, the element makers could leapfrog over the unstable elements they were currently discovering, it would change everything. ‘You would have a very long half-life,’ Oganessian explains. ‘I can show you calculations of that time. The half-lives would not be seconds. They would be a million years. Maybe a billion years.’
The idea captured the element-hunting community. ‘A remarkable shift from pessimism to optimism occurred,’ Seaborg wrote. ‘These new superheavy elements may be much more stable than elements 99–105 [… and] it may be possible to study their chemical properties and determine how they fit into the periodic system.’
Both Seaborg and Flerov latched onto the idea immediately. They drew up fanciful cartoons, imagining the element pioneers sailing off the end of the peninsula in small boats, exploring new territories and trying to reach the ‘islands’ of elements with a magic number of protons. Ghiorso, ever the contrarian, made a $100 bet with Seaborg: the elements were going to keep becoming more and more unstable and his magic island didn’t exist.
No one was really sure where the ‘island of stability’ would begin and end, but the best theory suggested that its centre would be around the undiscovered element 114. This wasn’t just a magic number: if you could create an isotope with 184 neutrons, it would be doubly magic, with exactly the right number of protons and neutrons to keep its stability. Suddenly, interest in the newly created elements blossomed again. If the element hunters could make a stable version of element 114, atom by atom, it would potentially be the greatest discovery in modern history. An amount the size of a pea could power a city.

Figure 6 Map of the isotopes, showing the ‘magic island’ of stability, drawn for Glenn Seaborg by B.C. Nishida in 1978. The ‘magic mountain’ shows the increased stability of lead compared with the rest of the elements.
Previously, nobody believed superheavy elements could exist on our planet any more. All of that changed with the island of stability. The solar system formed 4.6 billion years ago; if the island of stability meant half of a sample broke down every 460 million years, there would still be large enough amounts of superheavy elements created in those stellar collisions to find them on Earth.
And if that were the case, where were they?
* * *
It’s possible to find trace amounts of elements beyond uranium on Earth. As early as 1943, natural plutonium-239 had been found from neutron capture in uranium pitchblende ore. We know that the Earth used to have ‘natural nuclear reactors’ some 1.7 billion years ago in what is now Gabon, on the west coast of Africa. Sadly, these are long gone, and the latest estimates figure you’d need 2.1x1032kg of uranium ore to produce a single atom of fermium on Earth. That’s 100 times greater than the mass of the Sun.
Another route heavy elements can take to end up on Earth is from the debris of supernovae and neutron stars. Through these, elements as heavy as curium have arrived on Earth through cosmic rays – basically high energy particle showers from outer space.
But neither of these finds show an element heavier than uranium is still present since the Earth actually formed.
The island of stability caused the first superheavy element gold rush. Almost overnight, the element hunt moved away from high-end ion cannons and turned to techniques almost anyone could do. ‘Everyone was encouraged to participate,’ German physicist Günter Herrmann recorded. ‘Almost nothing was needed to perform these experiments … an intelligent choice of a natural sample and a corner in the kitchen at home could be sufficient to make an outstanding discovery.’ People began to look for superheavy elements everywhere.
The different factions of the superheavy community split up, each looking in different areas for an answer. Flerov’s technique was, unsurprisingly, to look for evidence of spontaneous fission having occurred in nature. If an atom of a superheavy element broke up, it would produce about 10 neutrons. If a superheavy element had a half-life of, say, a billion years, that would still mean that 1mg would produce around 400 disintegrations a second – the kind of burst that was easily detected. The only challenge was that cosmic rays (high-energy particle showers from space) could give false positives. ‘We used salt mines, deep underground,’ Andrey Popeko says. ‘They shielded [our detectors] from cosmic rays. We set up neutron multiplicity counters.’
The Americans had the same idea. Rather than salt mines, the Berkeley team asked a favour of their local subway system, the Bay Area Rapid Transit (BART), which had just dug a 250m (820ft)-deep tunnel between Berkeley and Orinda to expand the line. In May 1970 Glenn and Helen Seaborg, accompanied by Stanley Thompson, hiked more than 2km (1.2 miles) into the BART tunnels and set up their own neutron multiplicity counters.
Some of the Russian experiments showed signs of neutron counts above background, but they weren’t enough to convince the community.
Next, the two teams began to look for traces left behind by the superheavy element fission. The Russians started looking at rock samples, investigating olivine crystals – green, transparent rocks commonly found in the Earth’s subsurface. Olivine is easily damaged by its surrounding environment, and if a fission fragment hits the crystal, it leaves a little track that’s easily identified under a microscope. By looking at the depth of the track, you can tell how heavy an impact was – and therefore how large the atom causing it was. In America, one group started the analysis of 60-million-year-old shark’s teeth, hoping for similar superheavy element traces.
Still nothing conclusive.
Flerov and Seaborg weren’t willing to give up quite so easily. The elements heavier than uranium had to be there somewhere. Instead, they turned to the periodic table. As mentioned before, elements form groups with similar properties (called homologues). The undiscovered superheavy elements would, if the periodic table was right, fill up the seventh row. Element 114, if it existed, was likely to behave in a similar way to lead; perhaps it would even be concealed in lead. Following this piece of chemical sleuthing, Popeko went to church: specifically, to look at stained-glass windows. ‘The idea was that in churches, the glass was mounted in lead frames. If there was a superheavy element spontaneously fissioning [from something hidden in the lead], then the glass would react chemically.’ The Russian team also began gathering flue dust at an industrial lead processing plant for the same reason.
Using similar chemical nous, Thompson’s Berkeley group started looking for element 110, which would behave in a similar way to platinum. Taking platinum samples, they managed to analyse them to an accuracy of one part per billion. Thompson also collected more than 40 large samples of natural minerals, including gold nuggets, hoping to spot element 111. Searches also began to look for stable isotopes of gases such as krypton and xenon, which were possible products of superheavy element decay.
Once again, the Russians reported signs, but the community remained unmoved.
The search turned to the bottom of the oceans and the outer reaches of the heavens. ‘We looked in manganese nodules, forming in the deep sea,’ Heinz Gäggeler, then working at JINR, says. ‘They grow slowly, and they are made of condensed heavy metals, so Flerov started acquiring tonnes of these nodules. I got a nodule, heated it up and evaporated the lead from it.’ Gäggeler didn’t find anything. Down the hall, Zvara found evidence of spontaneous fission after processing 100m3 (3,530ft3) of brine water from the Caspian Sea, but the rate – one atom a day – was far lower than expected. Thinking along similar lines, the US team sent an expedition to California’s Salton Sea, a shallow saltwater lake located directly on the San Andreas Fault, to investigate the metals bubbling up from the Earth’s mantle. Again, the results were inconclusive.
Meanwhile, the Americans persuaded NASA to give them 3kg (6.5lb) of lunar rocks brought back by the Apollo astronauts. Later, they would collect data from the orbiting Skylab space station. High-altitude balloons were used to reap the skies, all just hoping for a trace – a glimmer – of a superheavy element. The Russians, not having access to the Moon, contented themselves with investigating sacks of meteorites, hoping to find some evidence of a superheavy element that had fallen to Earth. In an act of lab cooperation, Berkeley even sent Dubna 20kg (45lb) of space debris to sift through. Finally, results seemed promising: signs that could indicate past superheavies were found in the Allende meteorite, which had fallen to Earth in Mexico in 1969 and was the largest meteorite of its kind ever found on the planet.
But there simply no way to be sure.
There was one near miss. In 1976 a combined team from Oak Ridge, Florida State University and the University of California, Davis announced they had found a superheavy element in nature. In certain materials, radioactivity produces a strange halo effect – a spherical zone of discolouration caused by radiation damage. Usually, slicing through these halos and looking at them under a microscope gives a neat ring structure, much like cutting through a tree, with each ring showing just how far some alpha particles managed to scatter. The new team had been looking at the halos in biotite, dark black mica, found on the beaches of Madagascar. It had halos corresponding to an alpha particle energy predicted for element 126.
If the results were correct, it would mean that tonnes of element 126 were just lying on the beaches of the Indian Ocean, waiting to be found. If that were the case, presumably the undiscovered elements in its alpha decay chain (124, 122, 120, 118, 116, etc.) were within easy reach. Better still, crustaceans in the ocean were known to regularly gobble up polonium – which meant they likely ate its homologue, element 116 too. Discovery of a superheavy element was just waiting for the first scientist to head to the Maldives, build a sand castle and enjoy a prawn cocktail. Sadly, within a year, the team’s results were explained away: the ‘126’ trace was the result of a reaction with cerium, a radioactive element found naturally in the crystal. The discovery was yet another bust.
Despite the faint glimmers and glimpses of something, the hunt for superheavy elements in nature had failed. ‘Manganese samples, deep-sea brine samples, geological samples, meteorites …’ Gäggeler sighs. ‘We did not find anything.’ The teams’ equipment was so good the detection limit was down to a precision of 10-23g/g:3 a limit beyond any other measure on Earth. Nothing was there; if there was, it would have been found.
However, there was one gem of surprising news. For over 200 years, scientists had assumed that the heaviest element left from when the Earth formed was uranium. But in 1971, while the superheavy community looked for undiscovered elements, a group from Los Alamos proved that assumption was wrong.
The team’s leader was Darleane Hoffman. And she had just made one of the most remarkable – but forgotten – scientific discoveries of the twentieth century.
* * *
In the spring of 1945, 18-year-old Darleane Christian sat in front of her counsellor at Iowa State College, her arms folded and eyes narrowed. Christian hailed from West Union, a small town in the flat sea of sleepy, idyllic corn farms that make up America’s heartland, where her father was the school superintendent. Petite at only 1.52m (5ft) tall, she had honey-golden hair that cascaded down to her shoulders and a rosy smile that had won the heart of several boys – men – who had gone off to war. She was the all-American girl next door. And she was not going to take sexist crap from anyone.
Christian was in the office because she had dared to switch majors. She had initially signed up for applied art, but one of the required courses had been home economics chemistry, a subject she had never encountered before. The professor, Nellie Naylor, was an inspiration. ‘I found myself more interested in chemistry than anything I had ever studied,’ she later recalled in The Transuranium People. ‘[The lecturer] made it all seem so beautifully logical as well as relevant to a host of everyday problems.’ Screw home economics: Darleane Christian was going to be a chemist.
Her counsellor disagreed and had summoned Christian to set her straight. ‘Do you really think chemistry is a suitable profession for a woman?’
Christian smiled sweetly. ‘I’m quite sure it is,’ she replied.
Christian had graduated from her high school with the highest grades the institution had ever recorded. In her spare time, she had breezed through advanced mathematics and trigonometry correspondence courses, taken up the saxophone and played basketball for her school (which, given her height disadvantage, was a feat unto itself). Her heroine was Marie Curie, a scientist who had discovered elements, explored radioactivity and won two Nobel Prizes all while raising two children. Darleane Christian was a real-life Lisa Simpson, with the cartoon character’s same stubborn streak and tenacity. If Curie could do it, why couldn’t she?
The counsellor was prepared for Christian’s attitude and played a trump card: even if Christian made it as a chemist, there was no way she’d find a job in the chemical industry. She’d end up a chemistry teacher – and female teachers were expected to resign if they married. Christian was far too interested in boys to end up a spinster. The counsellor expected her to give up and stick to art.
Christian didn’t flinch. ‘So,’ she decided with a defiant grin, ‘I’ll just never teach. I’m going to follow Marie Curie’s model. I’ll marry if I want and I’ll have children if I choose.’
The counsellor had run out of objections. That was fine for Christian: she wasn’t asking permission anyway. For the rest of her undergraduate course she was usually the only woman in the chemistry lectures, even though the Second World War had plucked a generation of fighting men from their classes. She excelled, of course.
Short on money, Christian paid her way through college working summers as a waitress and then as a bank teller. By 1947 she was sick of doing boring summer jobs and applied for a research position at ‘Little Ankeny’, the Ames Laboratory at Iowa State. This was a clutch of small, single-storey buildings on the edge of campus, and college legends spoke about secret experiments and strange, late-night flashes of light sparking from inside. The stories were true: Ames had been another of the Manhattan Project’s satellites and was focused on improving uranium production. Christian applied and, like Al Ghiorso before her, got a job building Geiger counters. Unlike Ghiorso, Christian loved it – she was being paid $170 a month for something she’d have happily done for nothing.

Figure 7 Darleane Christian at the Ames Laboratory, 1950.
At Ames, barriers were thrown in her career path – barriers that seemed conveniently forgotten for men. Security passes, for example, required a person to have three initials. Christian had no middle name. But if the lab workers thought that was going to deter her, they didn’t know who they were dealing with. With a shrug, she put ‘DXC’ on the form and told them she now wanted to be called Darleane Xanthasia. Nobody was dumb enough to argue.
Christian was soon heading to be a nuclear chemist. But the roadblocks only became harder. Two years into her course, her father died. Despite her grief, she plucked up the composure to head to the university and ask if she could be excused from the next day’s quantum chemistry exam to arrange the funeral. Her professor – in an act of cruelty masked as compassion – forced her to take the test on the spot. Tears in her eyes and mind elsewhere, Christian still got a B.
Without her father’s income, Christian’s family were destitute. Their home was lost and possessions auctioned. Christian was the only breadwinner in the family and arranged for her mother and younger brother to live with her on campus. After graduation she started to complete her doctorate but found that her cramped quarters weren’t ideal for study. Instead, she spent time each evening with Marvin Hoffman, a man who had exactly what a woman like Darleane was looking for: late-night access to a synchrotron for photonuclear-induced Szilard–Chalmers reactions. In December 1951 she finished her PhD and married him.
The roadblocks continued. Now Darleane Hoffman, the young chemist worked for a time at Oak Ridge, before moving to Los Alamos to lead a nuclear chemistry group. On arrival, the staff at the personnel office looked at her with barely hidden contempt. ‘There must be some misunderstanding,’ she was told. ‘We don’t hire women in that division.’ It took a month of waiting around trying to sort out the paperwork before she ran into her supposed supervisor at a cocktail party. The supervisor immediately contacted the personnel office and got things straightened out. Yet still the roadblocks continued. Now at least acknowledged as being supposed to be at Los Alamos, Hoffman’s clearance was mysteriously lost. After another three months on the sidelines, Hoffman grew tired of waiting and called in the FBI. The ‘lost’ credentials turned up almost immediately.
While Hoffman had been trapped in bureaucratic hell, she missed out on the discovery of two elements. Los Alamos had been the first stop in the US for the filters collected from the Ivy Mike hydrogen bomb test (see Chapter 6). By the time Hoffman was cleared, the filters had been analysed and passed on to other labs – which had resulted in the discovery of elements 99 and 100. It was, for Hoffman, an unforgivable slight that had cost her a place in history. ‘I missed being a discoverer of einsteinium and fermium … while I was sitting in a small apartment in Los Alamos raging against the system,’ she later wrote in in The Transuranium People. ‘I will never again trust personnel offices, not just for saying “we don’t hire women in that division”, which was untrue, but for their general insensitivity, incompetence and bias.’
It would be a lie to say it got easier. Jacklyn Gates remembers Hoffman telling her of times she walked into machine shops where Playboy pin-ups had been left on walls deliberately to intimidate her; of casual sexism from people who assumed she was a secretary; of times she was underestimated because she was just a ‘sweet little lady’. Anyone who thought Darleane Hoffman would be a pushover or would back down soon thought twice. Thanks to her, any notions the Los Alamos personnel office had about women in science were quickly dispelled.
Hoffman’s abilities matched her iron will, silencing critics with some of the most brilliant chemistry of the twentieth century. She became an expert in fission, in new isotopes and in how bacteria interact with metals. She would also become a tireless activist for women in science (although her greatest pleasure was winning awards as a chemist, not just because she was a woman). Ask any of the latest generation of US nuclear chemists working today, male or female, who helped them the most and Darleane Hoffman’s name will come up time and time again. For a decade, despite being 13 years his junior, Hoffman’s picture was hung on Glenn Seaborg’s office wall as a source of inspiration (eventually he replaced it, no doubt to Helen Seaborg’s annoyance, with a picture of him meeting the movie star Ann-Margret).
By 1971 Hoffman had 20 years of chemistry experience behind her, and was a brilliant researcher who wore distinctive cat-eye glasses as she oversaw some of the most painstaking research in nuclear chemistry. By this time, the superheavy element searches were raging across the world. Hoffman set her sights far lower: she just wanted to prove that there was something from when the Earth formed that was heavier than uranium. For this, the obvious target was plutonium-244 – the isotope first detected by Los Alamos after the Ivy Mike tests, with a half-life of 80 million years.
Hoffman’s plan of attack was simple. She would obtain a sample of an ore that had an unusually high concentration of heavy elements and test it. Searching across America, her team found a pre-Cambrian bastnäsite, a 4.6 million-year-old lump of rock, being processed by the Molybdenum Corporation of America to make magnets, lasers and parts for self-cleaning ovens. The rock was being mined for cerium oxide, as it had 500,000 times the normal quantities of CeO2 than normally found on Earth.
A diligent chemist, Hoffman knew that if plutonium was going to be anywhere, the bastnäsite was it. Better still, the way the CeO2 was extracted wouldn’t remove any of the precious plutonium. She called up the Molybdenum Corporation and asked if she could have all their waste material. The company were happy to oblige. Hoffman partnered with another researcher, Francine Lawrence, and began the delicate task of processing the sample. This was painstaking, careful chemistry – as tricky as anything Stanley Thompson had pulled off during the Manhattan Project.
Once finished, Hoffman shipped the sample off to General Electric in Schenectady, New York, where a friend had one of the most sensitive mass spectrometers (a piece of kit that weighs the different components of a sample) in the world. If plutonium was there, they would find it.
That night, Hoffman went to the open-air opera in Santa Fe. She cast her eye to the cosmos, up to the glittering sparkle of alien suns, each furiously creating elements and ready to scatter them across the worlds. ‘As I looked out at the bright stars in the clear New Mexico sky behind the stage,’ she later wrote in The Transuranium People, ‘I somehow had the feeling that this time we would find remnants of the elusive Pu-244 remaining from the last nucleosynethesis of heavy elements in our galaxy, some five billion years ago.’
She was right. When she returned to the lab the next day, the results sent back from New York were unmistakable. The sample contained 8 femtograms of plutonium – a sample that would be invisible under even the best microscopes of the time. It was a dot of pure metal that dated from the very origins of our planet, arriving from a supernova explosion somewhere near our solar system and caught in the moment a cloud of gases coalesced and cooled to create our home.
The small-town Iowa girl, through skill and smarts, had achieved something her idol Marie Curie would have been proud to claim as her own. She had found the heaviest rock on Earth – and with it, she had touched the origins of us all.4
Perhaps unsurprisingly, after her success Hoffman found herself surrounded by scientists asking why she didn’t follow up her success of detecting natural plutonium for the first time by looking for superheavy elements in nature. Her reply was easy: finding plutonium was difficult enough. Trying to find an element whose atomic number, weight and chemistry could only be guessed at? That was nearly impossible.
Notes
1 This is only the tip of nuclear physics: like a series of Russian dolls, the models become increasingly intricate as you get smaller and smaller. The good news is that this is as complicated as we need to go.
2 The peninsula of stable nuclei doesn’t directly correspond with filled shells, and there are even nuclei with filled proton and neutron shells way off the peninsula (such as tin-100, which has a half-life of about one second). Science is complicated.
3 Grams per gram (so you could find 10-23g of something hidden per 1g sample).
4 It wouldn’t be heavy element science if this weren’t disputed. Later research has been unable to reproduce Hoffman’s achievement, and there are questions over whether her experiment worked at all. There’s also some debate (it’s impossible to be sure) as to whether the plutonium she found was the result of cosmic rays, or really dates to the formation of Earth. It may not be very scientific, but after all she went through, I’m going to give Hoffman the benefit of the doubt.