10
Genetic Modification of Plants
Advances in medicine and agriculture have saved vastly more lives than have been lost in all the wars in history.
From The Demon‐Haunted World: Science as a Candle in the Dark, Carl Sagan (1995)
The widespread belief that our food still resembles what our ancestors domesticated out of ‘nature’ is only a demonstration of how little we understand history and science.
From The Non‐GMO Food Label Is a Lie, Steven Savage (2016)
10.1 Introduction and Definitions
For those of us who are observers of the science, technology and bioethics scenes, it is difficult to remember a topic, which, in the United Kingdom and in some other European countries, has generated as much opposition as has GM crops. Even research on human embryonic stem cells, which some people find morally objectionable (see Chapter 5), has not generated anything like as much antipathy. It is for this reason that we have devoted a whole chapter to this topic.
In Section 10.3 we examine the basic methodology of plant GM and in Section 10.4, the main elements of the opposition to GM crops but first it is necessary to set out some definitions. The term GM crop is self‐defining – a crop variety that carries a genetic character as a result of genetic modification. However, a GM crop is not necessarily a GM food because products of GM crops such as oils, proteins or starch may well not have been modified by the GM process. The products from such plants are termed ‘nature identical’. Herbicide‐tolerant oilseed rape (canola) and soya bean fall into this category: the lipids and proteins remain unaltered. A GM food, strictly speaking, is a food where the genetic modification of the crop from which the food is derived was aimed at changing the composition of the food itself; for example, the plant breeders may have used GM in order to change the biochemical composition of seed storage compounds. Golden Rice, with enhanced amounts of β‐carotene (which is turned into vitamin A in the human body), is an example. The enhanced β‐carotene content is important because many people in SE Asia lack adequate supplies of vitamin A. Thousands of children go blind and some die because of this and it is hoped that Golden Rice will be one of the ways in which the deficiency is addressed. It was hoped that Golden Rice would come to the market in 2014, 15 years after the initial successful experiments.1 Many commentators put the delay down to protests by anti‐GM campaigners, although others say that the crop simply is not yet ready – it requires more development.2 However, there are only a few other examples of such foods under consideration for commercial growth but more may come on stream in the future. Finally, the term GM food may also be applied to situations in which whole plants or parts of plant are consumed but the genetic modification was aimed not at the food content but at some other trait, such as insect resistance. Here, the importance of the extensive food safety tests that are applied to GM crops becomes very obvious. We also need to say that in some of the anti‐GM campaigning, especially in the United States, which we will discuss later in this chapter; these distinctions are lost and the term ‘GMO food’ may apply to any of the three categories described here.
10.2 Back to the Beginning
Genetic modification of plants was first achieved, albeit initially with a low success rate, in 1983. The pioneer scientist was Marc van Montagu at Ghent, Belgium. These initial successes in Europe were very quickly followed up in the United States by Mary‐Dell Chilton and Robert Frayley. In 2013, van Montagu, Chilton and Fraley were awarded the prestigious World Food Prize (the Nobel Prize for food and agriculture).3 We note in passing that one of the researchers in van Montagu’s group was a young visiting Mexican scientist, Louis Herrera‐Estrella who was able to take knowledge of the techniques back to his own country at the end of his period of work in Belgium. It is therefore somewhat ironic that Mexico has banned the growth and import of a GM maize variety bred in the United States (see Section 10.4.4).
The scientific background is interesting because it demonstrates how information gained in one field may be used in another. Van Montagu, with a colleague, Jeff Schell, had worked for several years on a bacterium, Agrobacterium tumefaciens that causes tumours in plants. They showed that the formation of the tumour required the transfer to the plant of DNA sequences from the bacterium. These sequences, now known as T‐DNA, are located within a circular DNA molecule known as a plasmid. This is now known as the tumour inducing or Ti plasmid. The induction of the tumour thus relies on the excision of the T‐DNA from the plasmid, its transfer to the host plant’s nuclei and its integration into the plant’s DNA (see Figure 10.1). Since 1983 we have uncovered more and more details about how all this happens; one of the authors of this book has been privileged to be part of that research.4
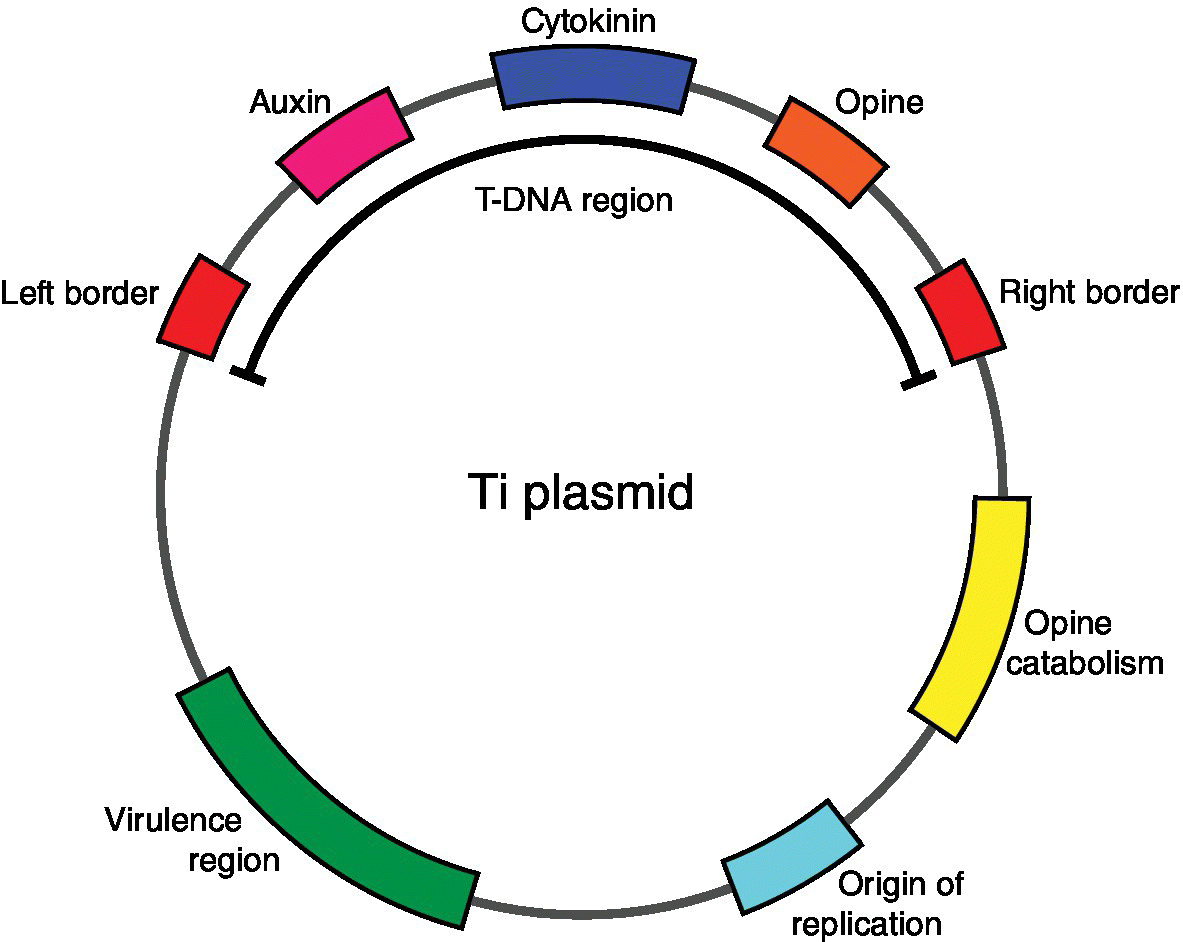
Figure 10.1 Diagram of the tumour‐inducing (Ti) plasmid of Agrobacterium tumefaciens. Note that for the sake of clarity, the various important sequences of the plasmid are not drawn to scale with each other. We thank Richard Tennant, University of Exeter, for drawing this diagram. The earliest successful plant GM experiments were carried out with this vector and many of the more modern vectors employ the key features of this gene transfer system. When Agrobacterium tumefaciens infects a plant host, a copy of the T‐region of the plasmid, the T‐DNA, is transferred to the host cells. Proteins encoded by the Virulence (vir) genes mediate this process. Inside the host cell’s nucleus, the T‐DNA is integrated into the host’s DNA by plant enzymes. The 24‐base‐pair left and right border sequences are essential for the process. In a natural infection, the host cells are transformed to a tumorous phenotype under the action of enzymes encoded by the auxin and cytokinin genes, causing formation of a ‘crown gall’. The cells of the gall synthesise amino acid derivatives called opines using enzymes encoded in the opine region of the T‐DNA. Enzymes that mediate opine catabolism are encoded by genes on the Ti plasmid, thus enabling the bacterium to use these compounds as sources of carbon and nitrogen. In forming a useful vector from the Ti plasmid, the first stage is to remove the genes that cause the tumorous phenotype and replace them with the genes that it is wished to transfer to the plant. In some early applications, the opine genes were left in as markers but other marker systems were quickly developed.
However, at the time of the discovery that DNA was transferred to the host, few of the details were known. Nevertheless, enough was known for van Montagu and Schell to realise that here was the potential to develop a method, based on a naturally occurring mechanism, for genetic modification of plants. By 1983, genetic modification of bacteria was ten years old. It was already being used to produce pharmaceuticals on an industrial scale (Chapter 9) and in research on genes (including plant genes), for example, by ‘growing’ genes in bacterial cells. Genetic modification of animals (Chapters 9 and 13) had followed relatively quickly but the world of plant science had been frustrated by lack of progress. A range of experiments, some of them rather bizarre, had been carried in a number of laboratories and although some positive claims were made, the fact was that no consistent results had been reported. The announcement from van Montagu’s lab, made at a conference in Florida, was therefore widely welcomed by plant scientists across the world. We were of course unaware of the storms that would follow.
10.3 Basic Methodology
The key features that we need for our discussion here are firstly that genes carried on the Ti plasmid, but not within the T‐DNA, are essential for the transfer process (Figure 10.1). These are the virulence or VIR genes. Secondly, the T‐DNA is bounded at each end by essential border sequences of 25 base pairs, which are direct repeats of each other. These are essential for transfer and integration of the T‐DNA. Any gene placed between the two borders will be transferred to the plant host’s genome. Thus, to make a ‘vector’ for plant GM, it was necessary to remove from the T‐DNA the genes involved in the formation and metabolism of the tumour and to replace them with the gene(s) of interest for transfer to the plant. The plasmid is reintroduced into Agrobacterium, which then does the rest. Plant scientists, including one of the authors of this book, have used and continue to use this technique and variants of it in laboratories all over the world. In fact, however, the technique may be simplified still further. It is the VIR genes that enable the DNA to be transferred but it is actually the border sequences that provide the essential sequence information for integration. So, if a DNA molecule containing genes of interest, spliced between two border sequences, can be delivered in some way to a plant cell without using Agrobacterium, then the gene(s) of interest will be transferred to the host DNA. Thus a range of methods and devices has been developed for injecting or shooting (gene guns) DNA into plant cells. Again, one of us has had experience with this.
GM techniques embody at least two advantages over ‘conventional’ breeding techniques. The first is precision. GM is precise because, unlike ‘traditional’ plant‐breeding techniques, one or a few specific genes, conferring desired characters, are transferred to a plant, the rest of whose genetic characteristics are not altered. Thus, single desirable genes can be moved into particular crop varieties without bringing in any unwanted traits. It is regarded as more efficient than conventional breeding because it does not bring two complete genomes together and thus avoids the need for generations of backcrossing and selection. The second advantage is that the transferred genes may come from a wide variety of organisms. Conventional breeding is confined to crossing of varieties within species or to hybridisations between related species. In respect of the latter, plant breeders have become very adept at achieving ‘unnatural’ hybridisations but still cannot incorporate, for example, a gene from a benign bacterium into a crop plant (as with the insect‐resistance trait) without using GM techniques.
However, genetic modification is also imprecise because of position effects: until recently there has no way of controlling where in the plant chromosomes the incoming genes are inserted. This causes great variation from plant to plant in the first ‘GM generation’ in the level of expression of the new gene or genes. Therefore there is a need for selection of the first generation of GM plants followed by observation of the stability of inheritance in subsequent generations. However, in practice, the overall process is still much faster than sorting, backcrossing and reselection that has to occur in conventional breeding. Further, recent advances in understanding mechanisms of genetic recombination in plants have now made it possible to target genes to specific sites (as well as removing marker genes used in the early stages of the GM process). These methods are being applied in developments of new GM crops.
These differences between GM‐based breeding and conventional breeding are well illustrated by a recent example. For a number of agronomic reasons, salt tolerance is becoming a very useful trait in crop plants. In 2012, Australian scientists reported a successful transfer into ‘pasta wheat’ (Triticum durum) of the salt‐tolerance trait from einkorn wheat (Triticum monococcum), one of the founder crops of agriculture in the Near East. The two species will not hybridise directly and it took some very sophisticated plant breeding techniques to achieve the transfer. Overall, the whole process took 15 years. However, the gene that confers salt tolerance in einkorn has been identified and could readily be transferred by GM techniques, achieving the desired result more easily and more quickly than by ‘conventional’ breeding. It is also worth noting that in the United Kingdom, in the European Union (EU) in general and in several other countries, the ‘conventionally bred’ version would not be subject to the range of evaluation procedures to which the GM version would be subject, despite the fact that they carry the same genetic trait derived from the same source.5 The majority of plant scientists consider this to be at the least, anomalous. We leave our readers to make up their own minds on this situation (see also Section 10.4.4).
10.4 The Debate
10.4.1 Introduction
During the first ten years or so after the invention of plant GM techniques, their development and application was largely unnoticed among the wider public. For the plant science community, and especially those with a professional interest in genes and gene expression, however, these were exciting times. In less than three years after the announcement from van Montagu’s lab, it was possible to regulate the expression of the inserted ‘foreign’ gene so the protein encoded by it was only produced in certain parts of the plant. Thus it would be possible, for example, to modify seed storage proteins without upsetting the range of proteins found in leaves. Small‐scale field trials were carried out (observing the appropriate national and local regulations). The first commercial product, which appeared in the United Kingdom in 1996 (1994 in the United States), was in some ways slightly odd, namely, a tomato (and products made from the tomato, such as tomato paste)6 that did not soften. In terms of post‐harvest transport and post‐harvest processing, it offered a number of advantages. It was not a typical GM crop in that the genetic modification involved not a gene from another organism but one of the tomato’s own genes turned back to front. This effectively turned off the softening process.
The non‐softening tomato was regarded as a test case for the introduction of GM crops to the market and initially it was a success. In the United Kingdom, the only product available was tomato paste, the tins of which were clearly labelled, indicating that the paste had been made from the fruit of GM plants. There were some small‐scale protests about ‘genetic foods’ but in general, the introduction of this product attracted little attention. The GM label did not seem to deter consumers and in at least one of the two supermarket chains that sold this product; it outsold its conventional rival (although admittedly the pricing policy may have had something to do with this).7
We have dealt with the non‐softening tomato in some detail because, in the United Kingdom and the EU in general, it gave a false sense of security to those with an interest in the commercialisation of crops that had been bred by GM techniques. Around the same time as the tomato/tomato products came onto the market, soybean, bred by GM techniques to be resistant to the herbicide glyphosate (Roundup™), started to be grown commercially in the United States. The products extracted from the soybean are lipids and proteins. They are completely unaffected by the GM process and are thus ‘nature identical’. Under US labelling regulations, there is no requirement to label these products as coming from GM plants.8 Soybean products are very widely used in the food industry and as the GM plants gained more and more market penetration (they were very popular with most large‐scale farmers in the United States), it was inevitable that more and more of the protein and lipid used in food processing would have come from GM plants. And thus, in about 1999 in the United Kingdom, when this was realised, the storm of protest began. Eighteen years later, opposition in the United Kingdom still has not completely disappeared; indeed, among some groups it is as strong as ever (but see Sections 10.4.4 and 10.6). As one of us has written elsewhere,9 in some sections of British society, opposition to GM crops has almost become part of ‘politically correct’ liberal thinking.
10.4.2 Conducting the Debate
In 2009, ten years on from the first widespread protests, Robert May, former UK government chief scientist, suggested ‘GM warriors have killed the debate’ and that ‘a confused public is caught in cross‐fire of the biotech battle’. This may be an overstatement but it is clear that neither ‘side’ in this polarised debate emerges with honour. Both have presented misleading and in some cases specifically untruthful propaganda and have misrepresented the other side, making genuine debate difficult. So who are these warriors? On the one hand, there were the biotechnology companies that by the mid‐1990s were already benefitting from the commercialisation of GM crops. These tended to make highly exaggerated claims about the roles of such crops in, for example, combating global hunger. Some of these companies used quite aggressive and, in some people’s eyes, unethical commercial practices to protect their intellectual property and their market share. Further, the US‐based companies opposed labelling of products from GM crops (see Section 10.6) on the grounds that labelling was not necessary for ‘nature‐identical’ products. Even in 2017, much of the ongoing debate is tinged with the perception that crop GM technology is largely owned by aggressive American companies, with one particular company often being named as especially deserving of criticism.
On the other side, the main organisation for organic agriculture in the United Kingdom, the Soil Association, declared that it would not validate as ‘organic’ any crop that had been bred by GM techniques, no matter whether its growth and husbandry had been conducted according to organic standards.10 The two most vociferous ‘green’ organisations, Friends of the Earth and Greenpeace, were also opposed to GM crops as were a number of smaller anti‐technology and anti‐capitalist groups. These opponents of GM crops conducted a well‐organised and well‐orchestrated campaign via the media, using such terms as Frankenfoods and Frankenstein Crops, which immediately gave the impression of mad scientists losing control of the products of their ill‐conceived work. That impression was further reinforced when protestors ripping up GM crops in field trials wore chemical protection suits in order to do so: these crops were clearly so dangerous that one had to avoid skin contact! (Of course they were not dangerous.) The public were thus persuaded that GM crops posed threats both to human health and the environment. Barry Barnes, a distinguished sociologist of science (and certainly not a supporter of the biotechnology companies), makes some very telling comments in this respect: What it was that moved…Greenpeace to attack them (i.e. GM crops) can only be conjectured… However, the attack itself was conducted along standard environmentalist lines. There was, allegedly, the unacceptable risk of gene transfer into the environment. But the consequence of the attack was that people who had no previous engagement with the topic became anxious about their diet. Hearing of yet more risks and dangers…they reacted with aversion to the indicated products, often products that they had previously been happily consuming. And as the supermarkets removed the offending boxes from their shelves, so a devastating ‘environmentalist’ victory was secured and proudly trumpeted….11 The success of this campaign meant that the GM label was not merely informative but a sign that these products should be avoided at all costs, hence their removal from supermarket shelves.
Opponents of the technology were also very critical of the commercial practices of American biotechnology companies (see above), added to which there was a perception, almost certainly unfounded, that the companies were too secretive in respect of data on topics such as food safety. Overall, in the United Kingdom and in most other countries, public opposition to GM crops became very strong. Notwithstanding Robert May’s assertion about confusion (above), the majority of the public in the United Kingdom and in much of the EU were convinced by the arguments of the anti‐GM campaigners. Public opinion thus ensured that GM‐bred crops were not quickly or widely adopted in Europe. Later in this chapter we present an update but in the meantime it needs to be said that opposition to GM crops and the perceived reasons for that opposition led on at least one occasion to tragedy. In 2002 Zambia suffered severe drought, leaving about three million people very short of food. The World Food Programme provided 3000 tonnes of American maize in order to alleviate the shortage but the Zambian government refused to accept it because much of the seed was from GM plants. Zambia’s President Levy Mwanawasa said, ‘Simply because my people are hungry, that is no justification to give them poison, to give them food that is intrinsically dangerous to their health’, showing the depth to which negative views had penetrated. Actually, there has never been any suspicion that GM crops are dangerous for human health but the idea had been subtly (although not explicitly) planted by the opponents of the technology, as clearly described by the sociologist Barry Barnes.12 Further, in the same Zambian episode, the Minister of Agriculture, Mundia Sikatana, stated that ‘The country should…refrain from actions that might adversely affect human and animal health as well as harm the environment’. It is not clear how many starving people died as a result of this refusal but we can be sure that the President and members of the Zambian government were not among them. The anti‐genetically modified organism (GMO) campaigners were strongly criticised for their role in engendering such negative attitudes and one of us (JB) was moved to ask one of the campaigning organisations if they were proud of what they had achieved. However, they remained adamant in their opposition.
Having considered the large, mainly American commercial interests pushing crop GM technology during the 1990s and the network of organisations opposing it, we now need to consider a third group, namely, the plant science research community. Many plant scientists work in universities and research institutes, in addition to those working for commercial organisations (not all of which have a financial interest in GM technology). Marc van Montagu, for example, in whose lab the first GM plants were created, worked at the University of Ghent in Belgium; Ingo Potrykus who first developed Golden Rice (see Sections 10.1 and 10.4.4) was a university professor in Zurich, Switzerland. This vitamin A‐enhanced rice is not owned in any way by commercial organisations and, encouragingly, where such organisations held patents that may have blocked progress, the non‐profit organisations developing Golden Rice were given freedom to operate.
In the main, the plant science community had welcomed the advent of GM techniques and the majority were supportive of their adoption (with appropriate safeguards) in agriculture, although most were not actually involved directly in this work. However, in the eyes of the anti‐GM organisations and of the concerned public, there was no perceived difference; the anger and aggression directed at scientists who expressed any support for GM crops at times reached an intensity that bordered on frightening (as one of us can testify).
Before examining the main points within the debate, one more thing needs to be said. Looking back to the start of the protests, we can see that it was an opportune time to question the progress of science within agriculture and the food industry. Over a period of about seven years leading up to 1996, the epidemic among cattle of the brain‐affecting prion disease, bovine spongiform encephalopathy (BSE), had received increasing exposure in the media. The epidemic occurred because of the practice of including in cattle feed, homogenised offal, including parts of the central nervous system, from sheep (which may carry another prion disease, scrapie). Further, it became apparent that BSE could be transmitted to humans via their diet, leading to the development of variant Creutzfeldt–Jakob disease (vCJD). At least 450,000 infected cattle entered the human food chain in Britain and subsequent to the discovery of the problem, over four million infected cattle were slaughtered. In 1996, the EU and Russia imposed bans on the import of British beef (lifted in the EU in 2006 and in Russia in 2012). An epidemic of vCJD was predicted but has not happened, although, up to the end of 2013, 177 deaths had been reported in the United Kingdom. The incubation period is long and variable but the bulk of the diagnoses were made between 1996 and 2003. There were no new cases in 2012 and only one in 2013.
It is thus clear that in 1996 there was a lot of public anxiety about the safety of food and about ‘unnatural practices’ within the food chain. GM seemed to present another form of unnatural ‘tampering’ with our food with possible but unknown risks (see above comments by Barry Barnes). When this was coupled with a suspicion of science and technology that has its roots in postmodernism (see Chapter 1), the climate was clearly right for stimulating public opposition to GM crops.
10.4.3 The Key Issues
It needs to be said that many scientists were very surprised at the strength of the opposition to GM crops. Some suggested, perhaps arrogantly, that opposition was based on ignorance but others recognised a strong ethical element in the arguments, even if they did not agree with them. And of course, those who support the adoption of GM crops in EU agriculture have their corresponding counter‐arguments (although they will recognise that there is no counter to intrinsic or deontological objections – see below).
The key points made by those opposed actively to GM crops fall into four groups:
- Intrinsic objections
- Risk
- Lack of consumer choice
- Wider social issues
At this point we discuss intrinsic objections and risk. Consumer choice is dealt with in Section 10.6; wider social issues are addressed in Chapter 11.
10.4.3.1 Intrinsic Objections
As we saw in Chapter 9, there are some opponents of GM technology who believe that moving genes between organisms is intrinsically wrong. This view was first expressed when genetic modification of bacteria was developed in the 1970s but little more was heard of it until the widespread use of GM crops became a real possibility. In the United Kingdom, some of the most ardent and vocal of the anti‐GM campaigners hold this view, which some regard as being almost religious in character; for them it is important to resist as far as possible the use in the United Kingdom of these crops. Even though this is likely to be a very minority view in wider society, we still need to consider whether it is possible to make provision for people who hold this position (see Section 10.6).
10.4.3.2 Risk
Risk and the precautionary principle were discussed in the previous chapter13. In this chapter we show how these relate to the debate on GM crops. The main risk factors raised by those who oppose GM crops are as follows:
- Containment. Crop plants, by their very nature, are not contained. Some have described growth of GM crops outdoors as letting the genie out of the bottle on the grounds that these crops present threats that conventionally bred crops do not.
- Marker genes. Marker genes are those added, along with the gene of interest, in order to identify the cells that have been successfully genetically modified. The first commercial GM crop varieties contain antibiotic resistance marker genes and concerns have been expressed that there is a chance, albeit very remote indeed, that such genes may find their way from the crop plant to bacteria that infect humans or farm animals.
- Gene flow and superweeds. Will the GM crop be able to cross with a related species that grows in the wild, thus allowing movement into the wild species of genes that may change its character with the possibility that it may be transformed, for example, into an aggressive weed? Another possibility suggested is that the GM crop itself may be able to establish itself as a ‘superweed’.
- Biodiversity and sustainability. It has been suggested that several of the traits introduced into crops by GM techniques threaten biodiversity. For example, the herbicide treatment regime applied to herbicide‐tolerant crops may reduce weed populations to such an extent that there is a negative effect on organisms ranging from insects to birds that depend directly or indirectly on the weed species for food. Another example raised by opponents of GM crops is the possibility that genes conferring resistance to insect pests may, by virtue of their presence in pollen, have an adverse effect on beneficial insects. Use of GM crops, therefore, it is said, is incompatible with farming sustainably.
- Food safety. It has been suggested that the presence of foreign genes may cause the synthesis of unknown by‐products or of new allergens.
Consideration of all these factors leads to the strongly held view that the precautionary principle in its ‘strongest’ form should be applied. This is essentially a consequentialist argument: there are risks that the consequences of growing GM crops may turn out to be disastrous. These risks, however theoretical they may seem to be, mean that we should not go ahead with the adoption of GM crops into agriculture (meaning specifically, the agriculture of most of the EU since it is a little late to prevent its use in the United States and many other countries). Indeed, a recent ‘battle cry’ from campaigners in the United Kingdom is that they must seize the chance to prevent commercial growth of GM crops while there is still time. However, supporters of GM technology have their counter‐arguments, as follows:
- Containment. Supporters of GM technology maintain that there is no evidence that GM crops are any more dangerous than other crops and there is therefore no need for special containment. They cite data from long‐term experiments on gene flow and on the behaviour of GM and other crops in the field, from the UK’s farm‐scale evaluations and from the large‐scale commercial growth in other countries. In respect of the latter, in 2016, about 185 million hectares of arable land across the world were devoted to growth of crops bred by GM methods.14 If there was a genie, it has well and truly escaped!
- Marker genes. It is accepted that there is a very remote chance of antibiotic resistance genes being transferred to bacteria but it is contended that the risk is so small that it cannot be quantified. Nevertheless, those involved in the development of GM crops acknowledge the importance of ensuring that, where these antibiotic resistance marker genes are used, the antibiotic in question should not be one that has applications in human or veterinary medicine. Furthermore, methods have been developed for removal of marker genes, and in any case, more recently developed GM crops contain different types of markers.
- Superweeds and gene flow. Supporters of GM crops point out that all crop varieties are selected for growth and yield in agricultural systems and in general they perform poorly in the natural environment. Although it is true that some species can grow as ‘volunteers’ in the wild, they do not become established as ongoing populations. Data to support this view have already been alluded to above. Nevertheless, it remains possible that a new genetic trait may make a crop species a better competitor so that it threatens native species or becomes a nuisance. However, there is one effect of the use of a particular genetic trait that has led to the development of what we might call superweeds. Herbicide tolerance is one of the two most widespread traits used in GM crops (the other is insect resistance), enabling the farmer to apply herbicides that kill the weeds but not the crops. In any population of plants, there are likely to be very rare mutant individuals that are resistant to the herbicide, in this instance, glyphosate. Extensive use of glyphosate on GM herbicide‐tolerant crops in parts of the United States has selected for these very rare mutant weeds, which are now spreading through the weed population. It is emphasised that this cannot be laid at the door of the GM technology itself; it would have happened if glyphosate had been overused with a conventionally bred glyphosate‐tolerant crop. A similar phenomenon is seen when overuse of a pesticide selects for pesticide‐resistant pests or when a virus mutates to overcome the resistance mechanism bred into a particular crop.
Gene flow is the movement of genes from one crop variety to another or even from the crop to a related wild species. Supporters of the technology point out that in the United Kingdom there are very few crops that are capable of outcrossing readily with wild species. However, two of them, beet and oil‐seed rape, are among the crops for which GM techniques have been developed. There have been some very extensive studies of the formation of hybrids between oilseed rape and wild mustard and wild radish; these hybrids occur at very low frequencies and there is no evidence that they may become established in the wild (but readers are reminded of the discussion about risks and proving negatives in Chapter 9). However, scientists point out that GM crops are no more or no less likely to outcross than conventionally bred crops and thus the consequences of any gene flow depend on the genetic trait itself and not on the breeding method. Genes from GM crops are no more likely to ‘flow’ than genes from conventionally bred crops.
- Biodiversity and sustainability. A moment’s thought will tell us that the aim of agriculture is to produce food and that application of that aim will lead a farmer to limit as far as possible the growth or activity of other living organisms that may compete with or damage the farmed crops (and animals). A farm, whether conventional or ‘organic’, is not a natural ecosystem. It is acknowledged that, in developed countries, much of agriculture is practised as an intensive, almost industrial system. Indeed intensive agriculture, combined with focussed breeding programmes, has contributed significantly to food security in developed countries. However, even the most ardent proponents of intensive agriculture recognise that it has had very marked effects on the landscape, land itself and wildlife. Indeed, one very clear outcome of the UK’s farm‐scale evaluations of GM crops (the results of which were published in 2003) is that we are much more aware of the effects of agriculture itself. This is a classic utilitarian trade‐off: loss of biodiversity versus production of food for people. A ‘back‐to‐nature’ approach is not workable. Nevertheless, within the farming and agricultural science communities, there is now an acceptance we should try to farm more sustainably – lessening, as far as is compatible with production of affordable food, the effects of farming on the environment. Supporters of GM technology in agriculture suggest that GM may actually help in the move to greater sustainability by the production of new crop varieties better suited to a more sustainable approach.
- Food safety. Are the fears about food safety raised by some of the opponents of GM crops actually well founded? Certainly, based on surveys and opinion polls and, more locally, in the experience of one of the authors of this book, it is apparent that some members of the public were and possibly still are concerned about food safety. However, regulatory authorities and national science academies (in the United Kingdom, the Royal Society) in several countries and in the EU itself have found no evidence at all that the technique of GM itself raises any food safety issues. In the United States, the country in which GM crops/crop products have been widely consumed for over 20 years, there has not been a single case of harm arising from their ingestion. (The same cannot be said about ‘organic’ crops.) Even so, these authorities and supporters of GM crops can envisage situations where particular genetic traits, introduced by GM, may cause problems (so, e.g. genes encoding potentially allergenic nut proteins should not be transferred to plants in which their presence would not be expected). Supporters of the technology also concede that plants containing a foreign gene which modifies a metabolic pathway, must be subject to rigorous analysis as if they were completely novel crops (because of the possibility of unusual by‐products). Overall however, as proponents of GM are keen to point out, after 20 years of large‐scale growth in the United States (and more recently in other countries), there has not been a single instance of a food safety problem with the GM crops in current production, a fact even acknowledged now by many of the opponents of the technology.
10.4.4 The Debate Continues
Since the first commercial use of GM crops in 1996, their use across the world has increased dramatically and by 2016, the number of countries in which they are grown had risen from 3 to 26 (actually a slight drop from 28 in 2014), although the ‘big five’ (United States, Brazil, Canada, Argentina and India; Table 10.1)15 account for nearly 90% of the total in terms of the area dedicated to these crops. The rapid increase in the use of GM crops in Brazil and India since 2003 is especially noteworthy. Overall, the area devoted to GM crops in 2016 amounted to about 12.3% of the world’s arable land (about 185 million hectares out of a total of 1.5 billion16). Of this total area, 54% was in 19 less‐developed countries and 46% in seven fully industrialised countries. The main crops bred by GM methods are soybean, maize (corn), cotton and oilseed rape (canola). This list hides some of the different situations in different countries. For example, Bt‐cotton has been grown in China for several years but only recently has approval been given to commercialise GM‐bred food crops.
Table 10.1 The ‘top ten’ countries growing GM‐bred crops.
Source: Data from http://www.isaaa.org/resources/publications/pocketk/16/default.asp.
| Rank | Country | 2015 | 2016 |
| 1 | United States | 70.9 million hectares | 72.9 million hectares |
| 2 | Brazil | 44.2 | 49.1 |
| 3 | Argentina | 24.5 | 23.8 |
| 4 | Canada | 11.0 | 11.6 |
| 5 | India | 11.6 | 10.8 |
| 6 | Paraguay | 3.6 | 3.6 |
| 7 | Pakistan | 2.9 | 2.9 |
| 8 | China | 3.7 | 2.8 |
| 9 | South Africa | 2.3 | 2.7 |
| 10 | Uruguay | 1.4 | 1.3 |
Of the countries growing GM crops commercially, 19 are classified by ISAAA as less developed,17 including Sudan in Africa and Bangladesh in Asia. The GM crop grown in the former is insect‐resistant cotton,18 also grown extensively in South Africa. Across the continent of Africa, GM crops are also under trial in Cameroon, Ethiopia, Ghana, Kenya, Malawi, Mozambique, Nigeria, Tanzania and Uganda19 but that does not mean that these crops are viewed favourably in all African countries, as discussed below.
In Asia, the example of Bangladesh is particularly interesting. The GM crop is insect‐resistant Bt brinjal (aubergine or eggplant). It is a constant feature the rhetoric of those opposing adoption of GM crops in less‐developed countries that those countries are exploited by large multinationals such as Monsanto. However, the insect‐resistant Bt brinjal is owned and distributed by the public sector Bangladesh Agricultural Research Institute, with no involvement from Monsanto or any other multinational company.20 In that respect it typifies the way that locally relevant crop varieties are being developed within a number of less‐developed countries. Further, there is clear evidence that, in common with other countries where health and safety guidelines may not be very rigorous or may not be observed, the use of a GM insect‐resistant crop has led to improvements in the health of farmers and their families because they are no longer exposed to insecticide sprays. In 2016, the famers (numbering 200–300) who had been growing Bt brinjal for three years reported that results had been very good in terms of reduced pesticide use and of yield. Unfortunately no actual data were presented at that time.
On the wider scene, in a recent survey of both developed and less developed countries, it was shown that the vast majority of GM‐bred varieties in use or under development are not owned by large multinational companies. Another example is virus‐resistant papaya that has effectively saved the livelihoods of papaya farmers in Hawaii.21
Despite the growth in the number of countries in which GM crops are grown commercially, this trend is not repeated in the EU. The very extensive evaluation procedure employed in the EU has led to permission to grow just three GM crops, including insect‐resistant (‘Bt’) maize (a variety developed by Monsanto) and a potato variety (developed by the German company BASF) in which the starch has been modified for use in paper making. Only Spain has adopted Bt‐maize on a large scale (139,000 hectares, about 30% of the country’s total maize production), where it is used in animal feed, while it is grown commercially on a very small scale in four other EU countries, Portugal, Romania, Poland and Slovakia, again for animal feed. On the other hand, this GM crop variety has been banned in Germany, Greece, Austria, Luxembourg, Hungary, Bulgaria and Italy, while (at the time of writing) a moratorium is in place in France. Interestingly, there had previously been some commercial growth of this crop in France and Germany. The potato variety has been received even less enthusiastically. Initially grown on a relatively small scale in Germany and Sweden in 2011, the variety was withdrawn by BASF in 2012 because of ‘public’ protest. Further, it is rather ironic that the first successful plant GM experiments were carried out in Belgium but nevertheless, Belgium is also one of the countries where crops bred by GM techniques are not yet grown commercially.
The regulatory framework in the EU, regarded by many scientists as unnecessarily slow and cumbersome, has led to some strange situations. For example, GM herbicide‐tolerant soybean used to be grown (for animal feed) in Romania. However, now that Romania is part of the EU, Romanian farmers can no longer grow it because it has not yet been approved by the European Food Safety Authority. In the past, some of this crop was exported, thus benefitting Romanian farmers. The EU‐imposed ban on growth of GM soybean means that GM animal feed is now imported and Romanian farmers receive EU subsidies.
More detailed information is given on European (including British) public attitudes in the ‘Eurobarometer’ data, published every three to five years and based on questionnaires designed to elicit public opinion. The most recent edition in which questions were asked about biotechnology (2010) still shows some opposition to commercial growth of GM crops; even in Spain where Bt‐maize is widely grown, just over 50% of the population opposed their growth. It was therefore somewhat contradictory that 53% of respondents ‘expected a positive effect from biotechnology and genetic engineering’ over the 20 years between 2010 and 2030.
The reasons given for opposition to GM foods tend to be an amalgam of worries about food safety (despite all the assurances mentioned above), about the environment, the ‘unnaturalness’ of the technology and, very frequently, the supposed domination of the market by large American biotechnology companies (Monsanto in particular comes up very often in these discussions). All this leaves the farming community very frustrated. Across Europe, farmers wish to be able to grow appropriate GM crop varieties but are prevented from doing so (as discussed in relation to GM soybean in Romania). There is equal frustration among plant scientists and among scientists of many other disciplines that a very useful technique, developed in good faith, is effectively going to waste. As reported by the BBC, a GM variety of potato developed in the UK’s John Innes Centre is now being used in the United States. However, it is possible, given recent changes in EU regulations (see below), that another GM potato variety (resistant to late blight and to certain environmental stresses) being developed in the same laboratory may actually be grown in British fields.
Two specific examples illustrate the continuing tensions. The first was in 2012 when the UK’s publicly funded Rothamsted Research Institute announced that it was carrying out field trials with a GM aphid‐repellent variety of pasta wheat. The wheat contained a gene from peppermint that enables it to produce an aphid‐signalling molecule (a pheromone) to which the pests react by flying away. Announcement of the trial elicited the expected chorus of protest from Greenpeace and other organisations, including the Green Party. An organisation called Take the Flour Back planned to ‘decontaminate’ the site by tearing up the plants. As always, misinformation was rife, especially about the dangers of cross‐pollination (wheat is self‐pollinated) and about ‘contamination of the food chain’. In response, there was a nationwide campaign of support for the research, coordinated via the social media; those expressing support were from a much wider constituency than just the plant science community (notwithstanding Eurobarometer data – see above). The local authority obtained an injunction to prevent access to the site, which was then protected by the police and thus the trial was successfully completed. In the end, it turned out that the crop did not effectively deter aphids but without the trials this would not have been established.
The second example is the support given to GM technology by two UK government ministers in the spring of 2013. The then Environment Secretary Owen Paterson was especially supportive in a speech that was welcomed by the plant science and farming communities, albeit that he made some rather expansive claims for the usefulness of the technology. He also wished to free individual EU countries from central decisions on whether or not to adopt particular GM varieties (see below). And of course, government support for use of GM technology in agriculture was opposed by Greenpeace and Friends of the Earth and elicited from many ‘liberal thinkers’ the ‘politically correct’ opposition to which we referred earlier.
On the wider European scene, it takes a long time to get approval for growing a GM crop variety, as discussed earlier. For this reason Monsanto has withdrawn from any further attempt to obtain approval in the EU for any new GM varieties, even though another GM insect‐tolerant maize variety, developed by two other agricultural biotechnology companies, was, in 2014, approved for commercial growth in Europe. Indeed, the regulatory framework is perceived as so cumbersome that some companies operating in Europe have decided to return to ‘conventional’ breeding methods and in particular the generation of new mutants by treating plants either with ionising radiation or with chemical mutagens. This was first used in the 1920s but became much more popular in the second half of the 20th century. It is rather a ‘hit and miss’ method but has yielded some useful varieties of several crops, including, in the United Kingdom, a short‐stalked malting barley, ‘Golden Promise’. Some scientists have suggested that as a breeding tool it is much more risky than GM but despite this, as a ‘conventional’ method, it escapes the evaluation procedures imposed on GM (as has already been noted in respect of salt‐tolerant wheat, produced by introgression breeding: Section 10.3). In any case, even if it is more risky than GM, that risk must be very small indeed because no problems have been reported in nearly 90 years of use. That should put into perspective the perceived riskiness of GM crops.22
However, EU regulations have recently been relaxed so that individual countries have more autonomy in decisions about growth of GM crops. Whether this opens the door wide to the adoption of GM crops across the continent is doubtful, especially as one result of increased autonomy is that an individual country can ban a crop that has been approved. However it does make it likely that some countries will slowly adopt new GM‐bred crop varieties, including the blight‐resistant potato mentioned above.
Opposition to GM is not confined to Europe. We have already noted that some African countries have adopted GM technology. However, in Angola, Ethiopia, Kenya, Lesotho, Madagascar, Malawi, Mozambique, Swaziland, Tanzania, Zambia and Zimbabwe, import of GM crops is banned, although in some, ‘milled’ seeds are acceptable (note that some countries in the latter list are also in the list of those where field trials are in progress). In several African countries strong opinions in favour of or opposed to the commercial growth of GM crops have been expressed.23 A group led by Kofi Annan, the Ghanaian‐born former United Nations Secretary‐General, has stated that the growing opposition is farcical, whereas the Gaia Foundation wishes to close the door to American biotechnology interests and to support ‘food sovereignty’, the right of people to define their own food systems. The debate in Africa is becoming as polarised as that in the United Kingdom and EU (see also Chapter 15). However, there is now evidence that across the continent, the possible use of GM in breeding programmes for ‘climate‐resilient’ crops is now being viewed in a favourable light.24
Thus there is a complex pattern developing across the world, with some countries adopting the technology and others enacting legislation based on opposition to GM crops. What the legislation actually prohibits varies from country to country. For example, Peru has banned the import and growth of all GM crops and the sale of any products made from them (although the latter may be difficult: see Section 10.6). Japan bans the growth of GM crops but the import of seeds of GM oilseed rape (for extraction of lipids) is not banned. New Zealand has banned the growth of GM crops as have two states in Australia (Tasmania and South Australia). In Canberra, Australian Capital Territory, a trial of GM wheat (low sugar, high fibre) was totally destroyed by Greenpeace in 2011. Mexico, in a move that seems specifically directed at Monsanto, has banned the import and growth of GM maize, although the legislation leaves the door open for the country to develop and grow its own GM varieties.
Southeast Asia has also become part of the anti‐GM battle ground. Mention has already been made of Golden Rice, enhanced to produce elevated amounts of β‐carotene (metabolic precursor of vitamin A). This is currently still being field trialled at the International Rice Research Institute (IRRI) in the Philippines but the trials were severely disrupted four years ago, when, in August 2013, protestors broke into one of the trial areas and uprooted most of the plants. The protestors appeared to be concerned about both food safety and environmental safety, both of which had already subject to extensive evaluation. The reaction to this destruction of GM crops was interesting. Predictably, Greenpeace expressed support for the protestors but on the other side of the debate, it was as if the plant science community had had enough and that a dam of pent‐up frustration had burst; the opposition to the protests was spread across the globe and some of it was very fierce. The social media were used to organise online ‘letters’ of support for GM rice, ‘signed’ by thousands of scientists across the world, while the major front‐line science and plant science journals published editorials in favour of growing Golden Rice. Some of the comments published in writing and/or on the Internet were very angry indeed, equivalent to those of anti‐GM campaigners in the 1990s. Thus Mark Lynas, formerly opposed to but now in favour of GM crops, said on BBC Radio that Golden Rice could be saving lives right now. The reason it hasn’t happened is because of the large‐scale opposition of the environmental movement and that needs to stop. Patrick Moore (not the astronomer), an early member of Greenpeace but who now opposes their anti‐GM campaign, went even further. On his website and on a poster he stated, Greenpeace and its allies have blocked this cure [i.e. Golden Rice], resulting in 8 million deaths, mostly among poor children. We believe this is a crime against humanity as defined by the International Criminal Court. This is an astonishing claim and we need to state straight away that it cannot be true. It is another example of misinformation generated by one side or the other in this debate. In respect of Lynas’s claim, there is certainly a view that anti‐GM campaigning has slowed the adoption of Golden Rice but some commentators believe that these delays have been mainly caused by technical hurdles.25 So, once again, the plant science community, with its rather more nuanced but certainly very widespread support for IRRI and the Golden Rice field trials, finds itself in the crossfire between protagonists at either extreme in the debate. Meanwhile, in mid‐2017, Golden Rice is still not available to farmers and consumers.
Interestingly, during the preparation of this chapter, there has been an increase in the ‘visibility’ and activity of anti‐GM groups in the United States. This has stimulated the production of a range of books with titles such as ‘GMO – How to avoid foods that ruin your life’26 and ‘GMO Free Diet: The Ultimate Guide on Avoiding GMO Foods and keeping Your Family Healthy with a GMO‐free diet.’27 It has also led, among other things, to some states insisting that products made from GM‐bred varieties are labelled as ‘containing GMOs’, even if the products themselves are identical to those from conventionally bred varieties. In July 2016, the US Congress made it mandatory for companies to provide information on whether foods were derived from ‘GMOs’. However, that information can be hidden within a QR code or even require consulting a website or calling a phone number. The information is available but not overtly so. Further, the Bill makes it likely that products refined from GM‐bred plants, such as sugars or lipids, will not need to be labelled.
A number of food companies and restaurant chains are now attempting to go ‘GMO‐free’, albeit that it is often not possible to know whether, for example, products from GM‐bred crops have been used in feeding cattle from which beef is obtained (as we discuss in relation to the United Kingdom in Section 10.6). Indeed, based on problems such as these and on the increasing difficulty of separating GM techniques from non‐GM techniques (see Chapter 9), some commentators have suggested that the label is at best meaningless and at worst a lie.28
Nevertheless, we need to ask what has led to the rise of anti‐GM views in the United States, a country in which GM‐bred crops have been grown on an increasingly large scale for over 20 years. A significant group among the more vocal opponents are the ‘anti‐vaxers’ who refuse to have their children immunised, following the totally spurious but widely publicised claim that an almost universally used MMR triple vaccine caused autism. It is almost as if the latter claim generated an anti‐science sentiment, which also shows itself as anti‐GM crops (‘GMO foods’ in the United States usage).29 In this context, it is interesting that some American anti‐GM groups have been accused of extensively disseminating ‘dis‐information’, an accusation also made against some of the European campaigning groups back at the beginning of this century.
10.4.5 Genome Editing: A Special Case?
The relative ease with which genome editing can be carried out with the CRISPR–cas9 system was mentioned in the previous chapter. In brief, it is possible to remove very specific sections of DNA from anywhere in the genome. In most instances, the DNA is repaired by the end‐joining mechanism although in some applications the piece of DNA that has been removed may be replaced by another (e.g. to replace a ‘faulty’ gene with a fully functional version). If used just to remove a tract of DNA, it would be possible, for example, to prevent apples from going brown when they are sliced.
Is genome editing to be regarded as genetic modification if no new genes are added (or even if a ‘faulty’ gene is replaced with a functional copy)? Many campaigners have already lined themselves to oppose the commercial applications of genome editing in plants. However, as we mention in the previous chapter, the regulatory authorities in the United States have already allowed at least one application of genome editing in the food chain (non‐browning mushrooms) without insisting on the rigorous testing that is applied to GM‐bred crop varieties. It will be interesting to see what happens in the United Kingdom and the rest of Europe.
10.5 GM Crops: Is a Different Approach Possible?
The arguments and counter arguments set out above give a picture of two sides, entrenched in particular positions, lobbing verbal and written grenades at each other but with the science community, generally supportive of appropriate use of GM technology nevertheless caught in the middle. Focussing on the EU, including the United Kingdom, the continued attempts of supporters on both sides of the debate to score points off their opponents has actually made it very difficult for the public to discern what the real issues are. What is needed is a new approach that involves a much less heated debate that recognises on the one hand the good science that lies behind GM technology and the potential of the technology and on the other hand the genuine concerns of the campaigners and the wider public.
There is a strong tendency, at least in the public arena, to regard organisms in which a new gene (or genes) has been inserted by GM techniques as ‘new’ types of living organisms, GMOs. This has led to crops bred by GM techniques being regarded as almost a different class of crops. We wonder how the public would have reacted had Golden Promise and other crops bred via radiation‐induced mutation been designated as ‘mutant crops’! Of course GM plants are not new types of organism, any more than mutagenesis‐bred crops are new types of organism. No taxonomist has suggested that a maize plant carrying an insect‐resistance gene is a new species; it is simply a new variety of the familiar maize. Nevertheless, the term GMO conveys an idea that many members of the public are unhappy with, in particular in relation to food crops (although much less so for animals or microorganisms, but see Chapter 9).
One thing that would be helpful in the debate (although it may be too late to achieve) would be to regard GM technology as a sophisticated breeding technique, as an addition, albeit a very useful addition, to the plant breeder’s toolkit. Indeed, it is argued that GM techniques may be less invasive than other methods used routinely in plant breeding, such as forced hybridisations or the induction of mutations by exposing seeds to ionising radiation (as mentioned above). Perhaps it is a mistake to focus on the technique for introducing genes into plants rather than on the use to which the technique is put. Overall, a change of focus from the technique to the genetic trait has found favour with the scientific community and with some of the opponents of GM crops. However, there remain some campaigners either with intrinsic objections to all genetic modification or who regard GM techniques as being so far from what they regard as natural that they are unconvinced by this approach.
So, if we regard GM as a plant breeding technique, what should be our concerns and what should be evaluated? As has been hinted at in the discussion above, what really matters when it comes to environmental or food safety are the genetic traits of the crop, not the method by which the traits were bred into the crop. This approach was nicely illustrated in the United Kingdom by the farm‐scale evaluations of three herbicide‐tolerant crops bred by GM techniques. In the trials the GM‐bred herbicide‐tolerant strains were not compared with herbicide‐tolerant strains generated by non‐GM techniques. The comparison therefore was with non‐GM, non‐herbicide‐tolerant strains. It is immediately obvious firstly that there were no proper controls with respect to GM versus non‐GM plants carrying the same trait. Secondly, this in turn meant that if anything was being tested in these trials it was the herbicide‐tolerance trait and the associated system of crop husbandry. In all three, more efficient weed control was achieved but with two of them, there was some above ground reduction in biodiversity30 in and immediately around the fields of crops (no attempt was made to study below ground biodiversity), while with the third, an herbicide‐tolerant maize (corn), there was a slight increase in biodiversity. Genetic scientists may well join with the anti‐GM campaigners in suggesting that the trials have told us very little. However they do indicate that GM as a technique should not be a specific target of concern. Indeed, based on more 2000 independent evaluations, about 275 national and transnational science academies and organisations31 have stated that plant breeding by GM techniques is completely safe. It is what is done with it (or indeed with any other plant‐breeding technique) that should be evaluated.
10.6 Closing Comments: Consumer Choice
In the late 1990s and the early years of this century, consumer choice – whether or not to consume GM crops or their products – was seen as an important part of the GM debate. If people did not want to consume these products, they should be able to avoid them. Many food retailers, noting the public antipathy to GM crops and their products displayed statements that their foods were GM‐free. Some of these statements were somewhat bizarre. Thus the restaurants in a particular chain of department stores all displayed signs stating, ‘In order to provide consumer choice, all our foods are guaranteed to be GM‐free’. It seems to have escaped their notice that customers who wished to consume the products of GM crops were being given no choice at all.
In relation to consumer choice, labelling became a key issue. In the EU, legislation introduced in 2004 requires that if a food contains or consists of GMOs, or contains ingredients produced from GMOs, this must be indicated on the label. For GM products sold ‘loose’, information must be displayed immediately next to the food to indicate that it is GM. Ethically this is a virtuous action, giving space to others to exercise their scruples. Thus the UK’s Food Standards Agency states that The Agency supports consumer choice. We recognise that some people will want to choose not to buy or eat genetically modified (GM) foods, however carefully they have been assessed for safety.
However, it turns out to be more complicated than at first thought. In several countries including the United States, the ‘nature‐identical’ products of GM plants do not need to be labelled (although three American states, Connecticut, Maine and Vermont) have recently adopted legislation that requires labelling). Nature‐identical products are products extracted from GM plants but which themselves are completely unaltered by the GM process (see Section 10.1). They include soya oil and protein (extensively used both in animal feed and in human nutrition), rapeseed oil, flour from maize and wheat and the non‐food product, cotton. The only way that a retailer can guarantee that such products are not from GM plants is if the crop processors have bought their produce from growers who certify their crops to be GM‐free (in the United Kingdom, e.g. ‘organic’ crops). But it is getting more and more difficult. The products from GM crops have already achieved widespread penetration of world markets. Thus, in 2016, it was estimated that 78% of soya oil and protein and 65% of cotton came from GM plants. The very high market penetrance of GM soybean and cotton means that is often impossible for a processor (and hence a retailer) to avoid products from GM crops (or even to know whether particular batches of product have or have not been derived from GM plants). Thus in 2013, several of the UK’s major supermarket chains stated that, first, they were no longer demanding that meat (including chicken) came from animals fed on ‘GM‐free’ food and secondly that they were no longer able to guarantee that any individual processed food product or cotton garment was ‘GM‐free’. Thus, labelling has become meaningless. The second of the two statements initially referred only to ‘non‐own brand’ products but three supermarkets have now extended it to their own brands. Thus, those who, for various reasons, wish to avoid consuming (or wearing) products from GM plants are finding it increasingly difficult to do so.
The supermarkets regarded this as a matter of honesty and transparency: it would be untrue to state that particular items were definitely GM‐free, except for those from certified organic sources.
As may be imagined, some anti‐GM campaigners were not happy about this and some reacted very negatively. Thus in a blog entitled ‘The Therapy Book’ (based on a book of the same name, specialising in information on ‘alternative’ medicine and similar topics), we find the following statement made in March 2013 in response to the supermarkets’ announcements: We at The Therapy Book have been developing a phone app32 which will enable you to easily identify, in one swipe, which foods in UK supermarkets contain GM ingredients, or animals that are reared on GM feed. At least that way, you and your family will have a choice about what you eat. And if we ‘vote with our wallets’, that may at least have some effect, the profit bottom line being the only language these sociopathic purveyors of Frankenfoods understand. The blog has since been taken down but very strong anti‐GM views are still posted in articles on the Therapy Book website33 and on the website of its parent organisation, The Holistic Works.34 Interestingly, among the articles posted by the Therapy Book, we also see support for the claim that the MMR triple vaccine causes autism (see Section 10.4.4). In the opinion of many scientists, this association between ‘anti‐vax’ and anti‐GM, also seen in the United States, makes it hard to take the anti‐GM case seriously. Nevertheless, many anti‐GM campaigners are very thoughtful and sincere. The debate is not over.
Key References and Suggestions for Further Reading
- Barnes B (2002) The public evaluation of science and technology. In Bioethics for Scientists, eds Bryant J, la Velle LB, Searle J. Wiley, Chichester, pp 19–36.
- Bryant JA, Hughes SG (2017) Food safety issues. In Encyclopaedia of Applied Plant Sciences, Vol 2, eds Thomas B, Murray BG, Murphy DJ. Elsevier, Amsterdam.
- Devos Y, Aguilera J, Diveski Z, et al. (2014) EFSA’s scientific activities and achievements on the risk assessment of genetically modified organisms (GMOs) during its first decade of existence: looking back and ahead. Transgenic Research 23, 1–25.
- Dzirutwe M (2016) Africa takes a fresh look at GMO crops as drought blights the continent. Reuters Environment, 7 January 2016. http://uk.reuters.com/article/us‐africa‐gmo‐idUKKBN0UL1UN20160107 (accessed 30 September 2017).
- Edwards J (2015) GMO – How To Avoid Foods That Ruin Your Life. CreateSpace Independent Publishing Platform/Amazon US, Seattle.
- Everding G (2016) https://source.wustl.edu/2016/06/genetically‐modified‐golden‐rice‐falls‐short‐lifesaving‐promises (accessed 30 September 2017).
- Food Standards Agency (2013) GM Labelling. http://www.food.gov.uk/policy‐advice/gm/gm_labelling#.Up4KcWdFDIU (accessed 30 September 2017).
- Greenpeace (2011) Environmental and Health Impacts of GM Crops – the Science. http://www.greenpeace.org/eu‐unit/Global/eu‐unit/reports‐briefings/2011%20pubs/9/Health%20and%20Environmental%20Impacts%20of%20GM%20crops.pdf (accessed 30 September 2017).
- Herrera‐Estrella L, Depicker A, van Montagu M, Schell J (1983) Expression of chimaeric genes transferred into plant cells using a Ti‐plasmid‐derived vector. Nature 303, 209–213.
- Hughes S, Bryant J (2002) GM crops and food: a scientific perspective. In Bioethics for Scientists, eds Bryant J, la Velle LB, Searle J. Wiley, Chichester, pp 115–140.
- ISAAA (2017) Biotech Crop Highlights in 2016. http://www.isaaa.org/resources/publications/pocketk/16/default.asp (accessed 30 September 2017).
- Lynas M (2011) The God Species: How the Planet Can Survive the Age of Humans. Fourth Estate/Harper Collins, London.
- Lynas M (2014) Bt Brinjal in Bangladesh – the True Story. www.marklynas.org/2014/05/bt‐brinjal‐in‐bangladesh‐the‐true‐story/ (accessed 30 September 2017).
- Martineau B, Gresshoff PM (1997) From Lab Bench to Market‐Place: The Calgene FLAVR SAVR Tomato. CRC Press, Boca Raton, FL.
- Mayer S (2002) Questioning GM foods. In Bioethics for Scientists, eds Bryant J, la Velle LB, Searle J. Wiley, Chichester, pp 141–152.
- Munns R, James RA, Xu B, et al. (2012) Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nature Biotechnology 30, 360–364.
- Nuffield Council on Bioethics (1999) Genetically Modified Crops. www.nuffieldbioethics.org/gm‐crops (accessed 30 September 2017).
- Roberts A (2017) Tamed: Ten Species that Changed our World. Penguin/Random House, London.
- Savage S (2016) The non‐GMO food label is a lie. Forbes, 11 June 2016. https://www.forbes.com/sites/stevensavage/2016/06/11/the‐non‐gmo‐food‐label‐is‐a‐lie/#4299e0b34b70 (accessed 19 October 2017).
- Skinner M (2015) GMO Free Diet: The Ultimate Guide on Avoiding GMO Foods and Keeping Your Family Healthy with a GMO‐Free Diet. CreateSpace Independent Publishing Platform/Amazon US, Seattle.
- Smyth S (2015) How GM papaya saved Hawaii’s papaya industry. SAIFood, 2 June 2015. http://www.saifood.ca/gm‐papaya/ (accessed 30 September 2017).
- The Editors (2013) Editorial: Labels for GMO foods are a bad idea. Scientific American, 1 September 2013.
- Tran M (2013) GM crops: African opposition is a farce, says group led by Kofi Annan. The Guardian, 5 September 2013. https://www.theguardian.com/global‐development/2013/sep/05/africa‐gm‐genetically‐modified‐crops (accessed 30 September 2017).
- US National Academies of Science, Medicine and Engineering (2016) Genetically Engineered Crops: Experience and Prospects. National Academies Press, Washington, DC.
- Ziemienowicz A, Tinland B, Bryant J, et al. (2000) Plant enzymes but not Agrobacterium VirD2 mediate T‐DNA ligation in vitro. Molecular and Cellular Biology 20, 6317–6322.