1. Do you suffer from arthritis-like pain or inflammation?
2. Do you have night sweats?
3. Does a change in the weather produce joint pain?
4. Do your joints swell after physical activities?
5. Do you suffer from a feeling of low energy in the morning that takes until noon to overcome?
6. Do the stresses of your life affect your health?
7. Do you feel “wired and tired”?
8. Is your libido low for your age?
9. Are you chronically depressed—and do you feel you shouldn’t be?
10. Do you think you’re more forgetful than you should be—and does that concern you?
11. Is it difficult to get to sleep? To stay asleep?
12. Do you have chronic infections of the sinuses, tonsils, intestines, skin, or mouth?
13. Do you routinely take anti-inflammatory medications, either over-the-counter or by prescription?
14. Are you on blood pressure medication?
15. Do you take antidepressants?
Say wha’?
Ever been involved in a conversation in which something or other keeps you from communicating? Of course you have. We all have. The technical process of communication just gets interrupted. There you are, walking along the sidewalk with your friend—let’s call him Bill—and he has something to say. That means that his voice vibrates the air in a very specific pattern that you pick up through your receiver—namely, your eardrum and the sensitive parts of your inner ear. That receiver mechanism translates the movement of air Bill stirred up into a signal in your nervous system, and your brain translates the signal into a meaning you instantly apprehend.
Unless a car blaring its radio passes by in the middle of the process and you miss the last half of what Bill is saying. Or unless your brain is suddenly distracted by a guy on Rollerblades barreling toward you along the sidewalk and your attention focuses on that. Bill sees the guy too and raises his voice, increasing the intensity of the signal he’s sending to get your attention back on him. And it works.
But just as you’re ready to answer Bill, a bus lumbers along in the street beside you, and you have to raise your voice. And as cars honk and riders pour out of the bus and start talking on their cell phones, the ambient noise gets louder, and pretty soon you and Bill are yelling at the top of your lungs just to have a conversation.
In principle, much the same thing happens when one cell of the body wants to communicate with another. It sends a message—not by stirring the air, obviously, but in the form of a chemical substance or an electrical impulse through the nerves. There is a huge range of such messenger chemicals—hormones, neurotransmitters, inflammatory mediators, and more; think of them as different little language structures the cells can choose from to communicate with one another. They all carry the message through the bloodstream to the receptor on the cell for which the message is intended, and the receptor translates the signal just as your brain translated what Bill had to say.
And by the same token, just as your brain cut out when there was too much other noise to hear, cell receptors sometimes don’t get the message either. Physiologists call it resistance; the receptor can’t hear or isn’t listening. Then the messengers must, like Bill, intensify the content of their message—only they do it by sending more of the messenger chemical. The problem is that increasing the number of messenger chemicals may actually increase the resistance to the message—the equivalent of you and Bill yelling at the top of your lungs. Even worse, it can begin to damage other parts of the body.
What happens when two people have to shout to hear one another? The din becomes painful, and people around cover their ears. This result is mirrored, metaphorically, in cellular communications. One of the central realities of chronic illness is a cellular messaging system locked into a shouting match, an alarm state of communication that causes persistent collateral damage to innocent bystander cells.
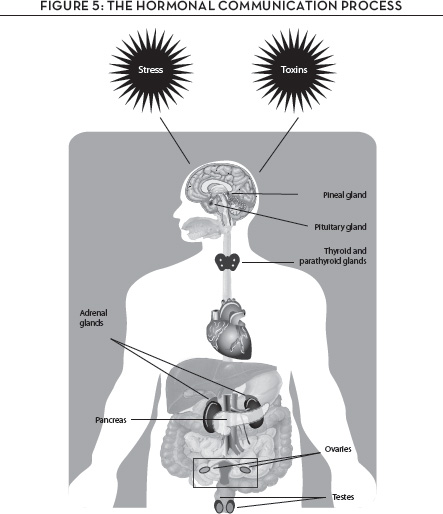
In this chapter, we’ll learn how the cellular communications process works and why tipping the balance into a shouting match underlies virtually every chronic illness. What can redress the balance? Follow along with Figure 5. We’ll follow the path from the vegetable garden and the fruit orchard to the very core of your genome, so you can see how some important compounds in fruits and vegetables can influence your cellular communications balance and keep you free of chronic disease.
STRESSED OUT
First of all, it’s essential to realize that our cells are communicating with one another all the time. Remember that our bodies are teeming factories of chemical reactions, which are stimulated by the messages of the cellular communications system, going on around the clock, whether we wake or sleep. These messages, signals, alerts, and in many cases alarms are what maintain the dynamic tension between inside and outside our bodies. Cellular communications sustain the interdependence of the core physiological processes and keep the human organism that is us in balance. The word for it is homeostasis, a kind of perfect equilibrium that is sustained precisely through constant messaging and adjusting, signal and response, alterations, rearrangements, corrections, repairs, renewals, and change.
The scientist who really turbocharged our clinical understanding of how the cellular communications process may get out of balance was Dr. Hans Selye, the world-renowned endocrinologist who coined the term “stress” to describe the physiological response of animals to a change in their environment. As we noted back in Chapter 2, the term has since become the most widely used English word in medicine and physiology. It’s pretty pervasive in daily speech as well, probably because Selye’s book, The Stress of Life, published in 1956, became an instant classic and has been reprinted in virtually every major language.*
Basically, it’s all about the fight-or-flight response; simply put, here’s what happens. Under conditions that an individual perceives as threatening and therefore stressful, the cellular communication system swings into action in all its locations: in the brain and in the hypothalamus within the brain, in the thyroid gland, in the adrenal glands that reside on your kidneys, and in your sex glands—ovaries in women and testes in men. Once activated, the system begins releasing the chemical messengers adrenaline and noradrenaline, which stimulate further message activity in numerous other cells.
All this responding to stressors is called allostasis, a concept developed by renowned stress researcher Dr. Bruce McEwen.* The term refers to that process by which the human organism makes adjustments and effects change precisely in order to regain the stability of homeostasis. In response to each of these stressor messages, your organism either fights or flees, either combats the stress or runs from it.
Fight-or-flight is a basic response mechanism. It helps us humans escape from danger or do things that seem beyond our normal capability; the classic example is of a mother lifting a car under which one of her children was pinned. And to the extent that our system is resilient enough to let us recover from its effects, it is a very important mechanism.
What Selye learned from his extensive studies on more than 15,000 animals, however, is that continual reliance on the fight-or-flight system to cope with daily life produces a resistance to the message; the body hears the message so much it stops listening. He termed it “adaptation,” and he tracked the sequence of events in the clinical process he dubbed the general adaptation syndrome (GAS).
First comes arousal as the communication system is activated and adrenaline and other stress hormones are released. You feel a nervous tension. Your mind races, your muscles tighten, you’re on adrenaline overload.
But after repeated instances of arousal in response to stress, the receptors on the stress hormone cells resist the message—they’ve heard it too often, and you fail to feel the jittery tenseness from high adrenaline that the threat may actually warrant. That is called allostatic load—the cumulative effect of too many responses.* It now takes a much higher level of chemical messengers to get the threat message through these now-resistant receptors, but that can be costly. Remember that too much of anything in the body can be toxic, and that is the case here. Higher levels of these messengers producing heavier allostatic load can have damaging effects on a whole bunch of innocent-bystander organs: the brain, heart, and blood vessels, your digestive system, your bones, skin, muscles, and kidneys. And these damaging effects often manifest themselves in the form of chronic illness. These effects are due to the production of stress-related substances in the body that are friends at low levels, but at high levels of exposure for a long time they become toxic to cells. This once again reminds us that we shouldn’t put labels on substances in our body, saying some are good and others are bad. Everything produced by the body can be either good or bad. It is all about amounts and balance. Too much of anything, including air and water, can be toxic.
What you feel now is disturbance manifesting itself as chronic sleep problems, mood swings, fatigue, inability to concentrate, worry over impending concerns in your life. The levels of fats and sugar in your blood rise too high, and you’re probably in chronic pain.
Finally, you reach a point of exhaustion. The still rising allostatic load has tipped the balance; it now overwhelms your body’s ability to produce stress hormones. Your communication system is unable to get back into balance, while the control mechanism that keeps trying for homeostasis is simply depleted. In some people, the result is what used to be called a nervous breakdown; today, we just call it burnout. In the third stage of the general adaptation syndrome, in other words, people just run out of GAS.
Because the body’s organs are so closely interconnected through the chemical communication system, however, when the system runs out of GAS, all sorts of other organs feel adverse effects. People often complain of low thyroid function. Women feel the loss of estrogen; studies of women going through menopause show that those under high allostatic load suffer more night sweats, hot flashes, sleep disturbances, mood swings, and depression than do women with a lower allostatic load.
Men suffer equivalently. Surely you’ve seen the rash of advertisements for testosterone-replacement medications for men with “low T.” It’s true enough that men who are going through the climacteric—hormonal changes similar to those experienced by menopausal women—experience worse loss of energy and muscle mass, a greater feeling of weakness, and more severe mood swings as a consequence of high allostatic load. Of course, the testosterone-replacement medications will treat only the symptoms of the general adaptation syndrome, which is precisely what this is, rather than addressing the cause of the syndrome—the imbalance in the core process of cellular communication.
Nor is it solely the feeling that the body really is running out of GAS. Dementia, heart attack and stroke, ulcers and inflammatory bowel disease, osteoporosis from bone loss, muscle loss, high blood pressure, diabetes, and chronic kidney disease are among the end products of the imbalance in cellular communications.
BEYOND THE STRESS RESPONSE
Yet the stress response, GAS, and the allostatic load are only the start of our understanding of how imbalance in cellular communications causes chronic illness. Emergent science has found the origin of many chronic diseases in the way the imbalance alters the body’s regulation of inflammation. Some scientists—notably physicians Paul Ridker and Peter Libby of Boston’s Brigham and Women’s Hospital and Harvard Medical School—have suggested that chronic inflammation associated with the alteration of cellular communication is a hallmark of most chronic diseases.
Ridker and his research group pioneered the identification of a substance produced by the liver that is a messenger of inflammation. It is called high-sensitivity C-reactive protein, or hs-CRP. The level of hs-CRP in the blood has been found to be a biomarker indicating a shift in the balance of cellular communication toward chronic inflammation. Levels of this blood biomarker above 2 milligrams per 100 milliliters of blood are also associated with an increased risk of heart disease, early stages of arthritis, obesity, and type 2 diabetes. Imbalance in the cellular communications process to inflammation to disease: the links are there.
Peter Libby’s group has found that chronic states of inflammation are associated with alteration in a number of substances that control cellular communication. These substances as a group are called cytokines, although they also have such individual names as interleukin 1, interleukin 6, and tumor necrosis factor alpha, and they are created by genes of cells within the immune system. Something in the environment activates these genes in the immune system; the genes control the production of cytokines; the cytokines control the communication of inflammation among organs. Once the cytokines are released into the bloodstream, that triggers a cascading release of other substances that control specific types of inflammation in specific parts of the body.
That’s how and why inflammation doesn’t stay isolated in one organ even though the symptoms—pain, redness, and swelling—might be seen in just one place in the body. Nevertheless, the inflammation messengers are zipping through the bloodstream, exerting their personality all over the body. Chronic inflammation is thus a systemic issue.*
But even systemic inflammation is a factor in chronic illness only when it is off balance, when something has altered the communication process that regulates it. Otherwise, the inflammation mechanism is an important part of healing—essential for the maintenance of health. Suppress that process, as many medications do, and you may also be turning off the body’s normal repair processes and unbalancing the healthy level of inflammation needed to prevent both infection and chronic illness.
THE NSAIDs Issue
A great deal has been written about the most common medications used to influence inflammation—nonsteroidal anti-inflammatory drugs, NSAIDs. They include aspirin, ibuprofen, indomethacin, and ketoprofen among others, and we take them for all sorts of ailments. Have a headache? Pop a pair of aspirins. Muscle pain? Whether it’s from tennis elbow or Parkinson’s, take ibuprofen. Gout, arthritis, fever, menstrual pain: NSAIDs are nonnarcotic and nonaddictive, and they relieve your pain.
They work by blocking one of the cellular communication processes associated with inflammation; this particular process is one that stirs increased activity of the enzyme in cells called cyclooxygenase, or COX. The resulting enzyme reaction converts one substance into another substance that amplifies the inflammation process, just like the amplifier in your sound system. If the inflammation messenger hs-CRP hits a certain level in your bloodstream, it means that COX activity is working overtime—your natural defense or self-healing mechanism is in high gear. Actually, there are two forms of COX. COX-1 helps maintain healthy function and keeps the inflammation process in balance; COX-2 is turned on selectively anytime an inflammation response is activated.
Most NSAIDs block both. That means even if you are taking the NSAID for a temporary acute ailment, you are nevertheless blocking the good features of COX-1. Do it often enough, and you may be setting yourself up for such predicaments as ulcers, kidney problems, and heart disease. Since more than 100,000 people per year are brought to a hospital emergency room with internal bleeding due to NSAID overuse, and since more than 10,000 die each year from this cause, we can say with some certainty that way too many of us are taking way too many NSAIDs way too often.
What can we do about this? How can we relieve pain and let the body manage the inflammation process in its own way? To find the answer, we need to dig still deeper into how that process works.
Keep in mind that the process of inflammation is initiated when something somewhere triggers an alarm reaction in the immune system. It is not unlike what happens when exposure to stress activates the release of stress hormones. In the case of stress, however, the hormone messengers are released by the endocrine system; in the case of inflammation, the cellular communication messengers, the cytokines, are released by the immune system.
They then travel in the bloodstream to receptors on the surface of cells. The surface receptors pick up the inflammation signal and transfer the message through the cellular membrane to the inside of the cell. This triggers a process called intercellular signal transduction—basically, converting a signal from outside the cell into a set of responses inside the cell.
It happens like a relay race within the cell. When the surface receptors push the inflammation signal through the cellular membrane, the gun goes off, and runner after runner—substances known as kinases—carries the signal deeper and deeper into the cell, each kinase handing off the message to the next kinase. The kinase-messenger for the anchor leg of the relay—the final leg—is a substance called nuclear factor kappa beta. NF-kB carries the inflammation signal smack-dab into the nucleus of the cell where the genome resides, and it crosses the finish line when it gains access to the coding in your book of life that controls the production of inflammation communication agents. That’s when it activates the cell’s gene expression, and the message of inflammation is sent out globally throughout the body.
The whole relay-race process of signal transduction is very carefully controlled within the cell because maintaining a balance of inflammation is so important for health. Each kinase-runner in the race is controlled by a specific gene, and each of these genes is unique to the individual. So here it is once again: response to the environment by the cellular communication system is absolutely individualized. That’s key because it is in the gene expression part of the process that inflammation is regulated—by turning on or off the release of NF-kB, by letting it go or preventing it from going.
Yet NSAIDs work by blocking the final action of signal transduction—the action of one of the last substances that create inflammation—thus stepping right into the middle of the individual’s natural process of controlling inflammation. Whereas the body controls inflammation upstream of gene expression, anti-inflammatory drugs control inflammation at the symptom level downstream of the genetic regulatory process. In a sense, the anti-inflammatory drugs hijack the process by which inflammation messaging is so intricately controlled.
PHYTONUTRIENTS AND THEIR EFFECT
Back in 2002, our functional medicine research group took a look at how the body manages the control of cellular information messaging so that we could perhaps find a way to regulate it at the point of genetic expression rather than downstream in blockading COX. The idea was to maintain the proper balance of inflammation and anti-inflammation control while reducing the risk of the adverse side effects seen with NSAIDs. We felt, to put it very simply, that this was a case where nature knew best and where we should try to find a way to mimic nature’s grand design.
We initiated our first screening studies in 2003. The objective was to determine whether certain natural substances could mimic the natural inflammation process. It was what is called a high-risk research project, meaning that we had absolutely no idea if the screening could work at all.
The question for each substance we screened was whether it worked as a traditional NSAID by blocking COX-1 and COX-2, or whether it worked upstream by influencing the way the inflammatory signal influenced gene expression. We started with natural substances that had a history of use by various cultures for anti-inflammatory effects; among these were ginger, frankincense (Boswellia serrata), and curcumin from the spice turmeric. The results of the study were revealing—and fascinating. Some of the nearly three hundred natural substances we analyzed did indeed exert their mild anti-inflammatory activity by blocking COX-1 and COX-2; they worked like NSAIDs. Another group, however, did not block COX directly but rather controlled the expression of genes that regulate the production of the inflammatory mediators; they mimicked nature’s design.
The surprise of the study—its real revelation—was that the best in class of all the natural substances screened was a family of compounds called isohumulones derived from hops. The anti-inflammatory activity of the hops concentrate containing the isohumulones was significantly higher than that of the natural products that blocked COX-1 and COX-2.
Did this mean we should all just go out and drink as much beer as possible to control any inflammation? Not quite. Instead, the finding spurred further research, and it produced what I believe to have been the most significant discovery of my forty years in scientific research. It came about thanks to the scientific intuition of my colleague Dr. Matthew Tripp, at the time the vice president of research and development at MetaProteomics, at whose lab the research was proceeding. Matt is a cellular biologist who, while working on the control of gene expression decades before, had made discoveries that contributed to the recognition of what was then a major new family of regulatory substances in cells—our friends, the kinases, the runners on the relay race team for signal transduction. More than 300 kinases have now been discovered; they control how chemical messengers outside the cell can speak to the genes inside and regulate gene expression. So that kind of gives you an idea of where Matt Tripp’s head is.
On this particular day, Matt walked into my office and set a colored chart down on my desk. He didn’t say anything, just stood there to see how I would react. So I leaned over and took a good hard look at the data on the chart. And what I saw there hit me like a ton of bricks: I was looking at a representation of how specific substances in plants—called phytonutrients—selectively influence the way substances outside the cell can communicate with specific regions of the genome. To me, it was like having a private audience with God.
What was so stunning about the data on the chart? First, let’s back up for a second to understand what phytonutrients are and do. They are chemical compounds in plants—phyto is Greek for plant—that react and interact. They evolved to become the protective capacity of the plant world. After all, a plant cannot run away and hide from sun, pests, mold, or blight, so it must produce substances that defend it from its own hazardous environment—and by the way that also give fruits and vegetables their color, fragrance, and taste.
Many phytonutrients are not well absorbed by cells—that is, they do not pass through the cellular membrane; yet the data showed clearly they could selectively affect cellular function. How? Matthew Tripp’s chart suggested that they bind to specific receptors on the cell surface, then translate their message into the cell via the kinase network, which takes specific information in the message to the appropriate specific regions in your genome to be read or expressed by your genes. Since different plants have different phytonutrients, they influence the kinase network in different ways.
It means that different plant foods—as well as the botanical medicines derived from plants—can influence cellular function in different ways based on how they modulate the kinase network. And thus we humans eating those plant foods can reap the benefits of a range of protective capabilities built into the plants through evolution. That was the great discovery of 2005, the most significant scientific discovery in which I have been personally involved. As scientists do, we of course gave a name to this effect—selective kinase response modulation—and called the phytonutrients that produce the effect SKRMs, selective kinase response modulators.
Since then, numerous other researchers have confirmed the SKRM influence of specific phytonutrients in such plant foods as licorice, green tea, red grape skins, cranberries, pomegranate, and blueberries, to take just a few examples. And the many publications from scientists in Matt Tripp’s team of PhDs and MDs* have expanded our knowledge and understanding of the potentially important role that these SKRMs play in balancing cellular communication—and in designing a personalized program to address an imbalance in the core process of communication.
THE KINASE NETWORK: COMPLEXITY AND DIVERSITY
That deeper understanding of the kinase network showed how very complex it is—a network of many interacting components. There isn’t just a single route a signal travels from the cell surface to the genome; rather, there are numerous intersecting routes. But there is a purpose to that complexity; it answers nature’s compelling interest in stability. The more complex a system needs to be to do its job, the greater the diversity of pathways and methods for getting it done, and the less vulnerable it will therefore be to catastrophe. Diversity means stability. This is true inside a cell, and it is mirrored in the environment at large.
Have you ever been in a rain forest? From forest floor to the top of the canopy, it swarms with species diversity—plants, flowers, epiphytes, trees of every sort, and animals ranging from the tiniest insects to the biggest predators, from beetles and butterflies to amphibians and reptiles, from birds of every size and shape and color to mammals of nearly every description. If one organism in the rain forest’s ecological system becomes extinct, that’s sad, but the rain forest can still survive because it contains so many other organisms that can fill the functional niche left by the extinct organism.
Now go stand in a cornfield. There is no diversity at all; one species of plant stretches as far as the eye can see on the prairie of Iowa or the sandy flatlands of New Jersey. If a pest or disease attacks the corn, the whole ecosystem is in jeopardy, and the entire crop may be doomed. That is why farmers turn to pesticides, herbicides, and other plant drugs. These synthetic killers of pests and disease compensate for the absence of diversity and protect against the instability that is a result of that absence of diversity.
Diversity means stability in human physiology too. The heart rhythm of a trained athlete is much more complex—has far greater diversity of resources—than that of a sedentary couch potato. In fact, the simplest heart rhythms are in people with heart disease. The sicker the heart becomes, the more it loses complexity; the less complexity it has, the more unstable it becomes in the face of any changes in the person’s environment; it lacks the diverse resources that might be able to adapt to or defy such changes. As people go from a state of wellness to that of disease, the complexity of their physiological system continues to decline until, in an end-stage disease state, just a few of the critical characteristics that support basic physiology remain. If one of these is jeopardized, the person probably dies; more than one and death is certain.
All of which is simply to emphasize that the complexity of the kinase network, the network that controls communication within the cell, is essential to its ability to fine-tune its translation of the messages coming in from outside and headed for the genome. Families of kinases work together within the cell to regulate that translation. They do it through a rich and highly diverse vocabulary that allows for very precise communication to the genes. Such precision is what provides physiological resilience in the face of changing environmental situations.
We spoke earlier of resilience vis-à-vis recovering from the fight-or-flight response to stress, and how a failure of resilience signaled adaptation to stress and an imbalance in the cellular communication process. Lowered resilience is also associated with diminished organ reserve and an increased biological age, so it is a key factor in our health or lack of health. It is here at the cellular level that resilience is created—in the highly intricate communications along the kinase network. The functional state of that network is therefore essential to human health.
And indeed it has been found that in severe diseases like cancer, the cause may be that a gene for a specific kinase has become damaged or has mutated. That single change to one single kinase in the regulatory network is then capable of changing the physiology of the cell from normal to cancerous.
As I write this, addressing that change has become the breakthrough thrust of cancer research and cancer treatment. The technology is straightforward: A genetic analysis of the genome of the cancer cell can diagnose the presence of a rogue kinase. A specific kinase-inhibiting drug can then be administered, tailored to that rogue kinase in that patient. There are now genomic diagnostic laboratories at virtually every major cancer treatment center in the country, and the newer cancer drugs now being developed are specific inhibitors of mutated kinases that have gone rogue. Meanwhile, research in this area is advancing rapidly at the Van Andel Institute in Grand Rapids, Michigan, at the Broad Institute in Cambridge, Massachusetts, and in many other academic and commercial cancer laboratories. It represents the increasing application of personalized medicine in the treatment of acute diseases.
SKRMs and Human Health
Once our research group understood how the natural phytonutrients in food substances could influence the kinase network, we began to look at whether specific phytonutrients could influence a specific family of kinases. The answer was that they did not affect any one kinase in their kinase family as strongly as a kinase-inhibiting drug could; rather, the phytonutrients influenced many kinases in the family, and the influence was mild, not dramatic as with the drug. In the language of traditional pharmacology, the bioactive agents in the phytonutrients qualified as low-potency “dirty drugs,” meaning they didn’t have the level of pinpoint control of a kinase that a good drug could achieve.
We wondered why evolution should have favored the development of such a response to these bioactive agents in food—namely, a response that altered many kinases within a family, and did so mildly. The answer seemed obvious: if the bioactive agents in our food produced a druglike effect, then every meal and snack would jolt our physiology, changing it dramatically and pushing and pulling our cellular functioning up and down like a yo-yo. The fact that the phytonutrients in our food speak to our genes more gently and quietly, unraveling the needed information and parceling it out to the genes at different locations around the regulatory network, provides the stability of cellular function we equate with resilience—and therefore with good health.
It’s a different effect from that of vitamins; their absence from our diet can result in nutrient-deficiency diseases. Instead, the effects of phytonutrients are more subtle and more intricate as they control how our genes respond to environmental changes. Remember that these are chemicals the plants produce to defend themselves against the stresses of their hostile environment. We know for a fact that the greater the stress on them, the more phytonutrients plants manufacture. It’s one reason why organic fruits and vegetables are considered preferable to nonorganic. Since organic farmers eschew pesticides or herbicides or any other plant medicines, organic plants are forced to defend themselves more vigorously from environmental threats. Hence they produce more phytonutrients. When we humans consume the food of plants, these antistress agents go to work for us, in their converted form as messengers to our genes, to increase our physiological resilience.
So the next question was whether the SKRM effect from particular foods had any clinical value in improving cellular function in humans and diminishing the symptoms of chronic illness. Our first clinical proof-of-concept studies, as they are called, were performed at the Functional Medicine Clinical Research Center.* From 2005 through 2012, a series of human clinical trials evaluated the influence of specific isohumulone phytonutrients derived from hops on such inflammatory disorders as osteoarthritis and degenerative joint disease, two of the most common conditions treated with NSAIDs. (As is standard with all clinical trials using human participants, an outside institutional review board examined and approved all protocols of these trials for safety and scientific rigor.)
The studies concluded that the SKRM-capable phytonutrients played a clinically important role in reducing the pain and inflammation of osteoarthritis and were found to be safe for the digestive tract, kidneys, and blood vessels. In other words, we had shown that nature’s intelligence was built into these substances. In the study participants with osteoarthritis, we observed declines in the biomarkers of inflammation in those taking the SKRM phytonutrients versus those consuming a placebo. But the study made it clear that these phytonutrients are not potent inhibitors of COX like the NSAIDs.
The bottom line? The studies confirmed that there are substances in certain foods that send messages to our genes through the influence of the kinase regulatory network, and that these messages control the production and release of inflammatory mediators. Food really is information for our genes, and its role in modulating the expression of genes associated with inflammation has a clear and certain effect on our physiological resilience and our overall health.
WHAT FOOD PROCESSING DOES TO PHYTONUTRIENTS
That is precisely why food processing can be so harmful to our health, for one of its core purposes is to remove phytonutrients from the products. Why? Phytonutrients often impart a bitter taste; remember, they are a plant’s stress fighters, and they “bite.” To the food industry, there is therefore a flavor advantage in removing them. Indeed, the phytonutrient index defining the amount of phytonutrients present in the diet makes it clear that the more processed food a person consumes, the more his or her phytonutrient index declines.*
By contrast, high phytonutrient indexes are the norm among populations in which chronic diseases least frequently occur. In blue zones like our island of Ikaria, Costa Rica, Sardinia, Okinawa, and Loma Linda, California, all places where people live longer, healthier lives, the population consumes many more vegetable products in their diets.
I know what you’re thinking: Loma Linda? Right smack in the middle of San Bernardino County? The city with a defense plant that has leached chemicals into the water and westerly winds bringing pollutants from Los Angeles? A place you’d expect would share the disease patterns of all the other populations in the region? That Loma Linda?
None other—for the reason that Loma Linda is home to a large population of Seventh-Day Adventists who follow a vegetarian diet rich in nuts and beans and are kosher, eschewing certain foods they deem “unclean.” They also do not smoke, drink alcohol, or do drugs, and they scrupulously observe their Sabbath day of rest. Even though the Loma Linda Adventists represent diverse genetic backgrounds, these practices are enough to make their city stand out statistically for greater longevity despite the polluted air and water. And it demonstrates once again that it isn’t our genes that determine our life span and life’s health, but rather how we communicate with our genes.
Processed foods have very few phytonutrients. Foods high in sugar and fat, animal foods, and white-flour food products have the very lowest phytonutrient content. Some foods, on the other hand, are particularly high in phytonutrients. As noted, organic fruits and vegetables have a higher phytonutrient index than their nonorganic equivalents. Remember that an organic vegetable or fruit has to work harder to defend itself from the stress of its environment. It therefore manufactures more phytonutrient stress-fighters than do foods coddled by pesticides, herbicides, and fungicides that do the stress-fighting for them. If you really want to send some powerful health messages to influence the kinase network, try organic berries, red grapes, celery, green pepper, capers, dill, watercress, cruciferous vegetables, hops, tomato, rhubarb, garlic, spinach, and adzuki beans.
In situations where the phytonutrient intake from the diet is not sufficient to influence the kinase cellular communication network, it is now possible to provide a nutritional supplement containing concentrated forms of SKRMs. It is not uncommon for this phytonutrient nutritional gap to go unrecognized, but the development of this supplement represents an exciting new chapter in our ability to use specific nutrients to address health issues.
It is also important to observe once again that the need for specific phytonutrients can vary considerably from person to person based upon genetic predisposition. Dr. Kenneth Kornman and his colleagues at the laboratory of Interleukin Genetics in Waltham, Massachusetts, reported in 2007 that people with a specific SNP—a particular variant—of the gene that codes the receptor of the inflammatory mediator interleukin-1 have increased response thanks to a phytonutrient supplement of particular vegetable concentrates. These concentrates contain specific SKRMs that modulate the cellular communication of this particular inflammatory gene SNP. This is a direct application of the newly emergent field known as nutrigenomics, which charts the level of specific nutrients an individual should intake to promote optimal function. It opens a new era of nutrition, one in which nutritional products can be formulated to meet specific genetic needs based on the evaluation of each individual’s genome pattern.
THE RIGHT TOOL FOR THE JOB
Until the turn of the millennium there was little interest in how nutrition could influence cellular communications. But since 2000 there has been a virtual explosion of information in this field. As we’ve learned in this chapter, it is now well known that imbalances in the cellular communications network are associated with virtually every chronic disease. And we also now recognize the impact of diet, lifestyle, and environmental factors on kinase function and thus on influencing balance in the body’s cellular communication process. The bottom line is a new and elemental understanding of the role of phytonutrients and their SKRM capabilities in changing gene expression and thus restoring or maintaining balance in core processes.
So is there still a place for pharmaceutical products in the management of chronic disease? The answer is a resounding yes—with the qualification that the health-care consumer must ask the right questions about their use. Remember that these medications have been approved by the Food and Drug Administration specifically for the symptoms of a particular illness or condition. Remember also that they are designed to deliver a specific effect with high potency—most often the effect of blocking a specific, overly active physiological process downstream of the process of genetic expression. Examples include the blocking by statins of an enzyme in the body that controls the manufacture of cholesterol in the liver, the blocking by antacids of the physiological process by which stomach acid is secreted, the blocking by certain blood pressure medications of the activity of hormones that control the elasticity of the blood vessels, the blocking by certain antidepressants of the way the neurotransmitter serotonin is eliminated from the body, and the blocking by NSAIDs of the COX enzyme effects on the production of inflammatory substances. All of these medications do their job very well, but precisely because they are so potent, their effect can range off-target to touch other functions in the body and thereby possibly cause or contribute to adverse drug reactions.
They are also meant to be used on a temporary basis; most receive FDA approval based on clinical studies performed over one or two years of use. If use continues past that point, the body may become accustomed to the medication, and it may require a bigger dose to achieve the same effect as initially achieved with a small dose. This also increases the possibility of an adverse reaction.
This problem is particularly pertinent for sufferers of chronic illness, the medications for which may be used for tens of years even though data on their long-term safety doesn’t go beyond the two years of the clinical studies. Potentially adverse effects from using the drugs over the longer time periods are simply a black box; nobody knows what might happen. The concern becomes even more acute against the background of the significant genetic variation in how medication affects us.
The bottom line on all this? Pharmaceutical medications are very effective in managing short-term health problems, but their long-term use in managing chronic illness raises some critical concerns about safety and effectiveness. Phytonutrients can influence the expression of genes in ways that can beneficially affect our physiological resilience and our overall health, although diet and lifestyle changes may have no immediate impact on short-term health problems or acute pain.
It’s a trade-off.
And it is why the functional medicine approach to imbalances in the cellular communications process is to begin with a program of lifestyle and nutritional changes that can mediate the way that specific genes are expressed while minimizing the potential for long-term adverse effects. Start early with such a program and reverse the chronic illness or avoid it altogether, but if you can’t, save the drugs for later on if and when the condition becomes more severe.
Such a personalized plan is in keeping with what we might call the longest-running human experiment to design safe medical treatments—evolution. Over millions of years, living organisms have selectively survived to arrive at the right fit between the bioactive substances in food and the maintenance of health, creating a relationship between certain anti-stress substances in food and their use as anti-stress substances in humans. The bioactive substances in food just might turn out to be better at modulating chronic illness over the years than drugs, which are good for short-term therapies but have potential adverse long-term effects. Further studies will clarify that point.
For now, we have clarified how cellular messages are created and what their effects are in modulating the function of the body, and we have learned that managing stress through lifestyle, diet, and environment is a primary therapeutic objective for the management of chronic illnesses caused by an imbalance in our cellular communications process. Well might we ask, therefore, how these cellular messages get from one part of the body to another. It is this question that leads us to the fifth core physiological process, which we will discuss in the next chapter: cellular transport.
CHAPTER 7 TAKEAWAY
1. Complex symptoms associated with chronic diseases are caused by altered cellular communication processes.
2. Influenced by stress or allostatic load, environmental toxins, diet, fitness levels, and specific phytochemicals, altered cellular communication can produce inflammatory responses that are part of almost all chronic diseases.
3. Inflammation, which is a function of genetic expression in response to lifestyle, diet, and environment, will vary widely from individual to individual.
4. Food processing removes many of the important regulatory substances that positively influence cellular communication.
5. Altered cellular communication can cause imbalances in the levels of such hormones as estrogen, testosterone, and insulin.
6. A program to improve cellular communication through the evaluation of hs-CRP, insulin, estrogen, testosterone, and other biomarkers can be effective in averting or curing many chronic diseases.