“The limits of my language mean the limits of my world.”
—Ludwig Wittgenstein
Tractatus Logico-Philosophicus, 1922
“Bzzz.”
—Honey bee


The American poet A. R. Ammons (1926–2001) wrote, “Two things are dead giveaways in nature: one is moving, making a motion, and the other is making a sound. Because these two are so risky, wild nature is mostly still and quiet.” But life—and “wild nature”—are about risk. Whether we notice it or not, all around us species constantly take risks by both moving and making sounds. A crescendo of communication surrounds us, and while it is true that there are times when we might be stunned by the “deafening” silence of a forest like Ammons describes, there are many occasions on which we may feel deluged by the noise of abundant life—from the songs of birds to the symphonies of crickets. Nature is often neither still nor quiet. Yet, our noisy human world has left too many of us tone-deaf, so much so that when we find ourselves in the great outdoors we may fail to take notice of the variety of calls that envelope us. Each sound and each movement that alerts a predator is indeed risky, but wild nature must risk all in order to truly thrive. Only by doing so can animals—including insects—complete their lives: find food, locate and choose a mate, and keep their species thriving.
Our lives revolve around communication. We talk to our loved ones, to our coworkers, sometimes to ourselves, and even to our pets. Right now you are reading a silent form of communication involving an agreed-upon set of abstract scrawls to represent the sounds of words that would otherwise be spoken. Our other senses are also involved in communication: smells tell us of delicious meals, warn us of dangers, and fill us with memories; touch can similarly provide us with a flood of information. Our natural and cultural habit of gathering and sharing information is one of the defining traits of our civilizations and species.
Every insect species communicates in one form or another, and any nice summer’s evening attests to this profusion, with its background chorus of chirping crickets and roaring cicadas, and the gentle flashes of fireflies. The concert of sights and sounds goes well beyond these familiar signals—beetles stridulate, roaches hiss, stoneflies drum, moths send ultrasonic bursts, treehoppers buzz twigs, and any number of others dance. In fact, insects communicate via every modality imaginable—including some that we were scarcely aware of until recent decades. The most basic signal-receiver systems are those between male and female, parent and offspring, and prey and predator. Males and females must find each other within a varied and changing environment, often filled with hazards. Females signal to their broods when danger is near, and countless species warn potential attackers of their noxiousness through bright and distinctive palettes of color. Whether we “hear” it or not, there is a virtual cacophony of communication underway at all times in the insect world.

Crickets, grasshoppers, and katydids are among the most familiar “singing” insects. At top, the Asian sandy cricket (Schizodactylus monstrosus); in the center, the ant-loving cricket (Myrmecophilus acervorum), which lives in ants’ nests and more superficially resembles a roach nymph; and at bottom, the great green bush katydid (Tettigonia viridissima). From Georges Cuvier, Le règne animal distribué d’après son organisation (1836–1849).

The migratory locust (Locusta migratoria), the most widespread of locusts, has the potential to form massive swarms with tens of millions of individuals per square mile. From John Curtis, British Entomology (1823–1840).
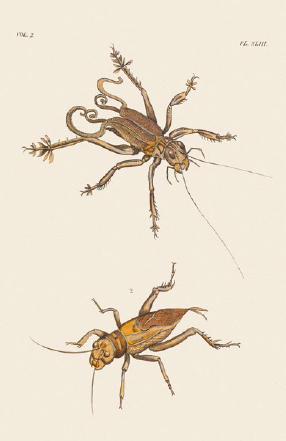
The large Asian sandy cricket (Schizodactylus monstrosus, top) has characteristically curled wing tips and flattened lobes that extend from the legs. They are a delicacy to some native peoples. The tobacco cricket (Brachytrupes membranaceus, below), native to Africa, feeds on young tobacco plants. From Dru Drury, Illustrations of Exotic Entomology (1837).
CHEMICAL SIGNALS

The most prevalent means of communication among insects is via chemical cues. Pheromones are used to bring males and females together, facilitating propagation of their species. The feathered antennae of most moths are greatly attuned to specific chemical signatures. In fact, there are some male moths that are so sensitive to the chemical signature of the female that they can hone in on a single molecule emitted from a source miles away. The many branches and sometimes broad shapes of these antennae allow the flying male to have the greatest amount of air flow and circulation over the numerous microscopic receptors, of which there can be many tens of thousands.
A splash of colorful beetles and some examples of aposematic coloration. The large beetle at center (Meloe variegatus) and the black beetle at its far left (Meloe proscarabaeus) are blister beetles, the males of which secrete a defensive chemical called cantharidin, which they offer to the female as a nuptial gift at mating. The female covers her eggs with cantharidin as a protection against predators. Interestingly, the black-headed cardinal beetle (Pyrochroa coccinea) between the two blister beetles uses its red color to warn predators that it, too, is toxic, but it must collect its chemical defense by licking cantharidin off the backs of blister beetles. From Jules Rothschild, ed., Musée entomologique illustré: histoire naturelle des Coléoptères (1876).
Social insects use alarm pheromones to alert related individuals of danger. Such chemical signals are often employed to alert a colony of some invader, and these alarms can cause huge numbers of worker ants or bees to flow from their nests, either rearing to defend their nestmates, or simply to flee. Chemical signals may also be sent to individuals of a different species. Stink bugs, stick insects, and many other insects have glands that produce repugnant—and sometimes powerfully pungent or even caustic and harmful—fluids that are meant to repel an attacker. Blister beetles (see illustration on previous page) are so named because their defensive secretion, cantharidin, is particularly powerful and can cause chemical burns. Toxic species often advertise this aspect of themselves through some form of coloration, called aposematic coloration. Among blister beetles, for example, some may be black with prominent red, orange, or yellow bands or spots, signaling “do not touch.” Others, however, can be entirely black or blue and yet just as capable of inflicting a painful burn.
MOVEMENT AND LIGHT, VIBRATION AND SOUND

Because humans are such visual creatures, we are drawn to anything that may catch our eye, like behavioral displays and vibrant washes of color—whether or not the information conveyed is intended for us. The warning coloration of the aforementioned blister beetles, as well as those of harmful or toxic species ranging from wasps to monarch butterflies, certainly communicate loudly, and can do so even if the insect is at rest. Insect movement also conveys considerable meaning, and perhaps the most common visual repertoires are courtship displays, which are performed across the full spectrum of hexapod evolution, from springtails to bark lice to butterflies. Nuptial dances, consisting of choreographed and stereotypical movements, sometimes coupled with species-specific patterns of coloration, not only permit individuals to recognize whether or not they are of the same species, but they also allow females to be choosy. Depending on the vigor of the displays, a female will select among potential mates, and this finickiness can sometimes lead to the evolution of exaggerated traits among males as they compete for the favors of their beloved. Even the primitive springtails, bristletails, and silverfish—which do not copulate—perform ritualized dances with females that ultimately lead to her accepting a spermatophore. Most of these visual signals are made while the insects are in close proximity, owing to varying degrees of visual acuity for certain insect groups and sometimes the incorporation of tactile stimulatory elements to the dance. Otherwise, the males’ pirouettes and turns might go unseen. But this does not mean that all visual expressions are restricted to the myopic.
Fireflies, a worldwide group of beetles, are the insect world’s great telegraphers, with those that are luminescent sending characteristic flashes of light after dusk. Each species produces its own pattern of flashes, much like a glowing Morse code. The light is produced in special photic organs located in the beetle’s abdomen, the result of a chemical reaction that, depending on the species, appears as pink, yellow, or the more-familiar pale-green light. The duration, location, and even the visual shape of the flashes are sufficiently specific as to permit the proper pairing of males and females.

The beetles commonly known as fireflies (family Lampyridae) comprise approximately two thousand species, and while not all produce light, the flashes from those that do are distinctive to their individual species. From Biologia Centrali-Americana. Insecta. Coleoptera. (1880–1911).

Bugs produce varied communications, and water striders such as Limnoporus rufoscutellata (with its long legs, center), can produce ripples on the surface of the water that serve as calls to their mates or to ward off other individuals. Circling the water strider, clockwise from upper left: feather-legged bug (Holoptilus ursus), thread-legged bug (Empicoris vagabunda), masked hunter bug (Reduvius personatus), water measurer bug (Hydrometra stagnorum), and riffle bug (Velia rivulorum). From Cuvier, Le règne animal distribué d’après son organisation.
Other forms of communication are heard and felt. Outside of insects, we are all familiar with the sensitivity of spiders to the vibrations of their webs, which inform them of the arrival of an ensnared meal. The uses of similar surface vibrations is numerous among insects. Nymphs of the treehopper Umbonia crassicornis, or thorn bug, feed in small aggregations, usually through punctures made by the mother into a plant stem, while she sits not too far off. Upon the approach of a predator, such as a wasp, fly, or beetle, the nymphs produce vibrational calls that travel through the stem of the plant. The calls become synchronized as more and more alarmed nymphs join in the plea for help, and these vibrations are felt by the nearby mother who comes to their aid, beating away the attacker with her toughened wings and legs.
Water striders, those slender-legged bugs that skate along the edges of calm ponds, are general predators who will snatch helpless arthropods that accidentally drop to the water’s surface. One genus, Halobates, can quite remarkably stride over open-ocean water. Living on the surface of water possesses several challenges, not the least of which is avoiding drowning. Like the treehoppers, however, water striders communicate through vibrations, a form of ripple communication, with different frequencies conveying differences in intent. The most common of these is a ripple to let nearby striders know of an individual’s presence, each replying to the other with a similar ripple as a way of asking them to back off. If a male strider sends a ripple but receives no reply from the other strider, then he knows it to be a female who is open to mating. He will then sing to her with a different form of ripple as a means of courtship. If she is not impressed, she signifies with a ripple of her own letting him down. If she is taken by his charm, then she will orient herself such that he can join her on the water.
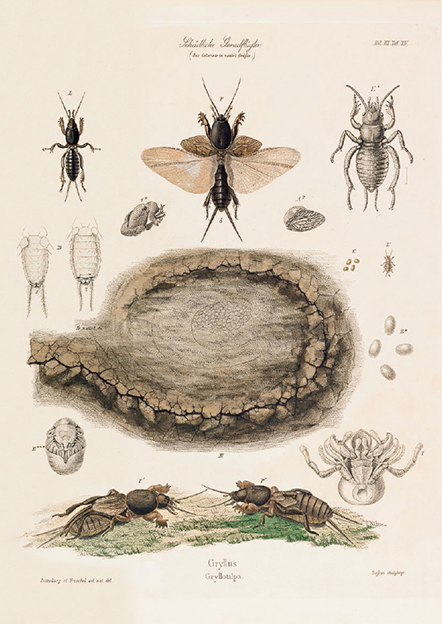
The more famous vibrations are the many trills, tweets, chirps, clicks, and clacks of the “singing” insects—the very melodies that led English poet John Keats (1795–1821) to write that, “the Poetry of earth is never dead.” The acoustic sounds of insects are never really “sung,” as none produces sounds vocally. Instead, insects are fiddlers and drummers, rubbing wings, legs, abdomens, or even mandibles together to create the choruses with which we are so familiar. These may be solo acts or generated by entire orchestras. Acoustic calls are most famous among the orthopteran insects, such as those of crickets, grasshoppers, and katydids. Most of these calls are made by the males singing for their mates, each sending forth long-distance arias. Crickets and katydids do this by rubbing together specialized files on their wings, the sound amplified by membranous areas outlined by veins, called mirrors. By contrast, those grasshoppers that call, and not all do, generate their sound by rubbing a scraper on their hind legs against a thickened file on the wing. In all of these, the sounds produced are unique to each species, and the trained ear can identify what species are present simply from the calls, without ever seeing the insect itself. So specific are the calls that specialists might first realize that a new species is present upon hearing its song—before the animal itself has been captured! Some mole crickets—whose forelegs, which are used for burrowing, resemble those of tiny moles—build small amphitheaters at the openings of their tunnels in order to better project their calls. Another subterranean group, related to grasshoppers but entirely wingless, are the Australian sandgropers, family Cylindrachetidae. Sandgropers have specialized mandibles with scrapers and files, and they generate their calls by rubbing these together.
Our summer evenings are awash with the loud song of crickets (family Gryllidae)—such as these Mesoamerican, Caribbean, and southwestern US species. Colored crickets, clockwise from top left: Phyllopalpus caeruleus, Phyllopalpus brunnerianus, Anurogryllus abortivus, Prosthacusta circumcincta, Gryllodes sigillatus, and Anurogryllus toltecus. From Henri de Saussure, Études sur les Myriapodes et les insects (1870).
As correctly figured by Rösel von Rosenhof, grasshoppers dig shallow burrows into which they extend their abdomen in order to lay a clutch of eggs. The eggs are then covered over while the embryos inside develop, and eventually emerge as nymphs. From Rösel von Rosenhof, De natuurlyke historie der insecten.
Long before the evolution of birds and their melodious calls, ancient forests were filled with the cacophony of insect songs. Remains of a katydid from 165 million years ago, complete with the scraper and file of the wings, were used to reconstruct the sound produced, revealing this ancient acoustician to produce a pure-tone call. We are well familiar with pure tones, as these comprise music. The ancient katydid did not call out with a nonmusical buzz or clack, but with music—a Jurassic-age love song.

The North American pygmy grasshoppers Tetrix ornata and Tettigidea lateralis. From Thomas Say, American Entomology (1828).

The miniatures of Rösel von Rosenhof are stunning for their level of accurate detail and for capturing of the insects in life, such that a reader might easily imagine the chirping of these crickets. From August Johann Rösel von Rosenhof, De natuurlyke historie der insecten (1764–1768).

Sandgropers (family Cylindrachetidae) are a lineage of greatly modified grasshoppers found only in Argentina, New Guinea, and Australia. Species such as the Australian Cylindracheta spegazzinii, figured here, use stridulatory files on their mandibles to produce their subterranean calls. From Ermanno Giglio-Tos, “Sulla posizione sistematica del gen. Cylindracheta Kirby,” Annali del Museo civico di storia naturale di Genova (1914).
Males of the European mole cricket (Gryllotalpa gryllotalpa), use their molelike forelegs to dig burrows in the soil that function as amphitheaters to amplify their mating calls. From Julius T. C. Ratzeburg, Die Forst-Insecten (1844).

Woodcuts depicting different views of European cicadas from Thomas Moffet’s Insectorum sive Minimorum Aniamalium Theatrum (1634).
The noisiest calls, of course, are those of cicadas. The roar of chorusing cicadas can be deafening, quite literally. The cumulative cater-wauling of cicadas in treetops can quickly exceed 100 decibels, a level equivalent to that of some jets at takeoff. Noises over 90 decibels can damage hearing, and at 110 decibels we experience noticeable pain. In fact, male cicadas are capable of disengaging their own “ears” during such choruses in order to avoid damaging their hearing. These impressive sounds are produced by plates (called tymbal plates, which are located on the sides of the abdomen) that are vibrated rapidly. Adjacent to the plates are large air sacs that serve to amplify the sound produced, and bordering the tymbals are the cicada’s ears, or tympana. The tympana act much like our eardrums and permit the insects to hear (see also page 62). As in the previous examples, the calls are specific to each species. Not all cicada sounds, however, are meant as courtship songs, and these insects will produce tones for other purposes, such as shrill shrieks to startle predators.

The drowning calls of cicadas, such as the little-owl cicada of Madagascar (Pycna strix, top) and the common European cicada (Lyristes plebejus, bottom), are common sounds during evenings after males and females emerge and begin the hunt to find one another. From Cuvier, Le règne animal distribué d’après son organisation.
Although insect sounds are made by drumming or scraping, there is one creature famous for its hiss. The Madagascar hissing cockroach, Gromphadorhina portentosa, is a large, wingless roach popular as a pet and in zoological exhibits. These harmless herbivores push air through special holes in their abdomen, creating the hissing sound for which they are famed. When the roach is disturbed it will emit this hiss, but males will also hiss to attract a female or to convey aggression toward competing males.

Cicadas amid their hemipteran relatives: in the center, the large Diceroprocta ruatana; and below it, Dorisiana amoena. From Biologia Centrali-Americana. Insecta. Rhynchota. Hemiptera-Homoptera. (1881–1909).
Any long-distance signal runs the risk of being intercepted, and it is not uncommon that some unwanted party may be “listening” in on a conversation. Certain predators and parasites have evolved to detect signals such as this. A mating call for one species can turn into a “dinner bell” to the right kind of predator. For example, species of the firefly genus Photuris are predators whose females have evolved to send out light displays that mimic those of other fireflies (see pages 148-149), thus luring in unsuspecting males of the other species, which they then devour. Similarly, the calls of male crickets are also heard by parasitic flies via their attuned ears. Ormiine tachinid flies listen in to the reproductive songs of crickets, follow the sound back to the singer, and then lay live larvae—rather than eggs—nearby. The fly’s larvae burrow into the body of the cricket, feeding within its body before becoming pupae.
Making a noise or emitting light certainly carries with it considerable risk, as birds and bats are also attracted to certain insect calls. Sometimes no communication is intended by a caller, and yet its signal is discerned by an animal who then reacts accordingly. Bats are famed for their echolocation, producing ultrasonic bursts as a natural form of sonar. With this sonar they develop an acoustic map that enables them to locate and hone in on an insect in flight, for those that are insectivorous. Of course, it is preferential to the bat if an insect is unaware that it is being tracked. Fortuitously, many groups of insects have evolved to hear these ultrasonic sounds and can understand precisely what such signals foretell. Moths, lacewings, and even praying mantises can hear ultrasonic sounds, giving them a fighting chance when it comes to facing such a fearsome predator. In moths, the ears are located on the sides of the abdomen, while in green lacewings they are on the base of the wings, and in mantises there is a single ear centered on its chest. A moth or lacewing in flight that detects the sonic burst of a bat will dive from the air, often moving in an irregular pattern so as to make itself more difficult to trace. There are even species of moths that can apparently “talk back” to the bat, producing their own ultrasonic clicks. In some species the clicks are meant to convey that the moths are toxic, either truthfully or as a form of deception. In the Southeast Asian hawk moth Theretra nessus, such sounds are made by rasping the genitalia against the abdomen. The tiger moth Bertholdia trigona generates ultrasonic clicks with tymbal plates, similar to those of cicadas, and the signal produced actually jams the bat’s sonar.

The hissing roaches of Madagascar (genus Gromphadorhina), at left (colored) and center, produce their distinctive sounds by forcing air through the spiracles of their abdomen. Other Malagasy roaches (colored), at right from top to bottom: Ateloblatta cambouini, Ateloblatta malagassa, and Thliptoblatta obtrita (two views). From Henri de Saussure, Histoire physique, naturelle et politique de Madagascar, Orthoptères (1895).

Excellent “ears”—used to detect the ultrasonic bursts of rapidly approaching bats—have evolved numerous times among moths, such as in the several species of hawk moths (family Sphingidae) seen here (except for the castniid moth, Athis clitarcha, at bottom). From Biologia Centrali-Americana. Insecta. Lepidoptera-Heterocera. (1881–1900).
THE DANCE OF THE HONEY BEES

The ultimate form of communication, however, is that of language. Language is set apart from other forms of communication in its use of arbitrary symbols in a structured form to convey ideas. We use sounds and symbols, placed together in specific patterns, to convey ideas. Thus, the letters printed on this page serve to represent sounds, and when placed together in a specific order they denote words, which when again arranged according to a set of rules form utterances that then pass along meaning.
Since antiquity we’ve known of a peculiar behavior among honey bees, where returning foragers will “dance.” Aristotle recorded the behavior over twenty-three hundred years ago, and through the ages writers have ascribed varied meanings to the action, including simply thinking the bees were so overjoyed at returning home they performed a little jig. In fact, the dance and its many nuanced components serve as a set of organized symbols, a language, through which one bee can inform others of the direction, distance, and quality of a particular resource, often of nectar and pollen but even of potential new nest sites. This remarkable reality was discovered through a series of intricate experiments by the German behavioral entomologist Karl von Frisch (1886–1982). The discovery was so considerable that it won von Frisch the Nobel Prize in Physiology or Medicine in 1973, a prize he shared with Austrian zoologist Konrad Lorenz (1903–1989) and Dutch biologist Nikolaas Tinbergen (1907–1988) “for their discoveries concerning organization and elicitation of individual and social behavior patterns”; von Frisch is the only entomologist to have ever won the award.

A species in the same genus as the Southeast Asian hawk moth Theretra clotho (top right) is capable of not only detecting but actually producing ultrasonic bursts. Other moths (clockwise): Agnosia orneus, Amplypterus panopus, Hayesiana triopus, and Lenyra ashtaroth. From John O. Westwood, The Cabinet of Oriental Entomology (1848).

The combs of honey bees also serve as the dance floors for their distinctive system of communication. The combs of dwarf and giant honey bees (Apis dorsata and Apis florea) in Southeast Asia are built in the open, hanging from branches, and the worker bees dance on the upper surface of the hive in order to “tell” their nestmates where to find resources. The large carpenter bee, Xylocopa chloroptera (upper right), is also depicted along with its nest composed of linear brood chambers in branches. From Horne and Smith, Transactions of the Zoological Society of London (1870).
At its simplest, a laden forager “speaks” to her nestmates through a series of movements called the waggle dance. The bee dances on the vertical comb in the hive in a figure-eight fashion, waggling her abdomen during the middle part of the eight before circling back to do so again, each return cycle alternating between left or right turns. The bee does not waggle during the return runs, only doing so during the middle part of the dance. In addition, she is very specific in the direction in which she orients herself for the waggle run of the dance. It is this orientation that conveys the direction to the source. The world outside is a horizontal landscape, and the bees are dancing on a vertical surface, which means they require an abstract point of reference that they can then convert and use out of the hive. This point of reference is the sun, and a waggle run that is pointed directly up means that the direction is toward the sun. Any deviation, either left or right, from direct up indicates the same degree of deviation outside and relative to the sun. The length of the waggle run correlates to a specific distance from the hive, while the vigor of the performance indicates the relative quality of the food. All of this is taking place within the confines of a dark and crowded hive, and the bees being recruited therefore crowd close to the dancer. Their antennae are placed in close proximity to her, and the Johnston’s organ—that specialized sensory structure that helps to define all insects (see page 8) detects the frequency of vibrations as well as the orientation of the dancer’s body relative to gravity. Collectively, these various elements give sufficient precision for a recruited worker to decide whether or not the food is good enough to visit, and, if so, to depart from the hive in the proper compass direction relative to the sun and fly the necessary distance to find it. There may also be odor cues, such as the scent of the flowers that were visited, that further aid the recruit upon its arrival at the correct location. The language is actually more varied and nuanced than this. For example, should a resource be close enough, then the bee abandons the figure-eight form and does a round dance.
An iconic honey bee (Apis mellifera) skep, or basket, hive from the frontispiece of Moffet’s Insectorum sive Minimorum Animalium Theatrum.

Just as with a human language where there are regional dialects, the same is found among honey bees. Subtle variations are known among distant populations, such as the duration and number of waggle runs correlating to different distances. Thus, a follower from one region “listening” to a dancer from another region will arrive at the wrong location—perhaps going too far or not far enough. They understand the language but don’t quite get the exact meaning. The same is true for all seven species of honey bees, each of which has its own variant of the waggle dance.
Linguists and semioticians forever debate what constitutes language, a concept easily understood but difficult to define. What criteria must be fulfilled in order to satisfy the high standards of a communicative form becoming so labeled? The great founder of semiology, Swiss linguist Ferdinand de Saussure (1857–1913), considered language to require a signifier (“sound-image”) and a signified (“concept”), components both achieved by honey bees. (Interestingly, Saussure’s father was the celebrated entomological taxonomist Henri de Saussure [1829–1905], renowned worldwide for his extensive monographs on Orthoptera and Hymenoptera. One has to wonder whether the communicative calls of the many crickets and katydids his father studied might have stimulated the junior Saussure toward his calling in linguistics.) The symbols and affiliated notions are then arranged according to syntax to form language. Do honey bees have syntax? Yes, since the dance proceeds in an organized and structured fashion, with the particular signs conveyed in an order. For example, waggling does not occur during the return loops, and waggling out of place would lead to confusion, just as writing the words of this paragraph in random order would lose their meaning. A titan among modern linguists, Noam Chomsky (b. 1928) once noted that language was a set of sentences of finite length and constructed of a finite set of elements, and these convey either a finite or infinite range of ideas. Our language is considered “open,” as our range and creativity permit us to generate a seemingly endless possibility of expressions. As far as we know, honey bees are fairly limited in the variety of information conveyed, their language therefore being “closed.” Bees don’t muse about the beauty of a tree or the comforting feel of warm sunshine, nor do they debate whether the squawking of Homo sapiens constitutes a language or not (that we know of, at least). Other schools of thought argue that true language is not innate, i.e., it is not an intrinsic behavior genetically conveyed like most insect communication systems, and yet today’s biolinguists have uncovered that our own foundational capacity for language is ingrained and heritable. Thus, our languages and those of the honey bees seem to sit on a spectrum, distinguished more by a mere degree of complexity and range. For the bees, their dances speak volumes.
The debate over how narrowly or broadly the term language should be used will continue to occupy linguists and philosophers for generations, but no matter how you slice it, an abstract language evolved first among insects. Honey bees have been using a language for at least thirty-five million years, and although the range of ideas that can be expressed is limited, it is nonetheless perhaps one of the most remarkable forms of communication across animals.

FEMININE WIT AND INDUSTRY
A ristotle remained one of the primary sources for information on natural history through the early seventeenth century. Apiculture was already ancient by Aristotle’s age, and the Greek scholar was keeping with convention when he wrote that the leader of a honey bee’s hive was the “king.” The mere inclusion of this idea in Aristotle’s Historia animalium (see page 15) consecrated the concept in the minds of later naturalists. The work of one radical English vicar and beekeeper, however, would overturn this received wisdom, rightly revealing a hive to be a feminine monarchy. Charles Butler was born to an impoverished family in Buckinghamshire in 1560, but his humble origin did not hold him back. In his late teens he was admitted to Magdalen Hall at Oxford, working to support his studies, which in time likely included teaching courses of his own. After ten years of hard effort, Butler completed an arts degree in 1587. Trained in the ministry, following graduation he worked at Magdalen as a Bible clerk until 1593, when he became rector of Nately Scures in Hampshire.
Aside from his ministry, Butler was a logician, grammarian, phonetician, musician, and, most notably, a beekeeper. After moving a couple of times, in 1600, Butler settled down as vicar of the village of Wootton St. Lawrence, remaining there until his passing in 1647. In 1609, Butler published The Feminine Monarchie, the first comprehensive tract on beekeeping in English. The book was of considerable practical use, with instructions on capturing swarms, building hives, and managing the various enemies of the colony. In addition, he wrote of the importance of the bees to gardens and fruit pollination, and even how to use the tone of the bees’ buzzing to predict when a colony was about to abscond and swarm. As a musician, he was so inspired by the sounds of the hive that he even composed a four-part madrigal titled Melissomelos, a transliteration of the tones he perceived the bees to be making (see page 165).

The title page to the 1634 edition of Charles Butler’s The Feminine Monarchie (1609), in which he employed the phonetic alphabet he developed as a grammarian and phonetician.
Butler demonstrated that the workers produce their wax from the underside of the abdomen—from structures we today call wax mirrors but which are specialized glands—rather than collect it from mysterious sources in the environment. Most importantly, Butler popularized the seemingly heretical notion that the “king” of the hive was, in actuality, a queen, that the drones were the males of the species, and that the workers—whom he praised for their wit and industry (the solertia et labore motto on his book’s frontispiece illustration of a stylized hive, see page 163)—were themselves female. He did not have much empirical evidence for this, but such proof would be forthcoming by the end of the century, when Jan Swammerdam dissected a queen and discovered ovaries therein. Likely unbeknownst to Butler, a Spaniard, Luis Méndez de Torres (see page 129), had also advocated this idea in 1586, but it was Butler’s popular treatise that widely disseminated the feminine rule of bees.

The frontispiece of The Feminine Monarchie, with its stylized honey bee comb. Butler first disseminated the correct notion that the ruler of the hive was a queen, here, depicted with a crown at the top of the hive and surrounded by a retinue of workers and princes (drones).

Butler was so moved by the honey bees he tended and studied that he was inspired to compose a madrigal based on the sounds of the hive. The madrigal, the Melissomelos (opposite), was performed at the dedication of a stained glass window (right) honoring Butler in the parish over which he presided at Wootton St. Lawrence in Hampshire, nearly 350 years after the publication of The Feminine Monarchie. (Parts of the musical notations were printed upside down so two or four singers facing each other could share the book while singing.)
Butler’s The Feminine Monarchie ordained him as the “Father of English Beekeeping.” It is perhaps, however, his 1634 edition that is the most fascinating. As a phonetician and grammarian, Butler pushed for an overhaul of the English language, publishing The English Grammar in 1633, in which he invented an entirely new phonetic alphabet. The revised edition of The Feminine Monarchie that appeared the following year was printed in Butler’s new phonetic system. To commemorate the coronation of Queen Elizabeth II (b. 1926) in 1953, a new stained-glass window, opposite, was installed in the Wootton St. Lawrence church. The window, opposite, depicts Butler holding The Feminine Monarchie, a hexagonal comb surrounding his head and shoulders, much like a halo, with bees arranged in the same formation as in the illustration from the book’s frontispiece. Quite appropriately, the choir performed Melissomelos at the window’s dedication. It may finally be time for Butler to gain a new title as England’s patron saint of apiculture.

Detail of the stick insect Bacteria virgea, from John O. Westwood, The Cabinet of Oriental Entomology (1848) (also see page 172).