G
Gambieric acids
Gambieric acids A-D are potent, antifungal compounds obtained from the marine dinoflagellate Gambierdiscus toxicus (Nagai et al., 1992). The antifungal activity of these ladder-shaped polyethers against Aspergillus niger is 2000 times greater than that of amphotericin B.Morohashi et al. (2000) determined the absolute configuration of gambieric acids A-D as shown in Scheme G.24.
Gambierdiscus toxicus also produces other polyethers, such as brevetoxins and ciguatoxins, which are highly toxic. These compounds exert their neurotoxicity by binding to a specific site on the voltage-gated sodium channels of excitable membranes (Catterall, 2000), refered to as site 5. Inoue and coworkers (2003) recently showed that gambieric acid-A, a nontoxic polyether, inhibited the binding of brevetoxin to site 5.
References
Catterall, W.A., From ionic currents to molecular mechanisms: The structure and function of voltagegated sodium channels, Neuron, 26:13–25, 2000.
Inoue, M., Hirama, M., Satake, M., Sugiyama, K., and Yasumoto, T., Inhibition of brevetoxin binding to the voltage-gated sodium channel by gambierol and gambieric acid-A, Toxicon, 41:469–414, 2003.
Morohashi, A., Satake, M., Nagai, H., Oshima, Y., and Yasumoto, T., The absolute configuration of gambieric acids A-D, potent antifungal polyethers, isolated from the marine dinoflagellate Gambierdiscus toxicus, Tetrahedron Lett., 56:8995–9001, 2000.
Nagai, H., Murata, M., Torigoe, K., Satake, M., and Yasumoto, T., Gambieric acids, new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gambierdiscus toxicus, J. Org. Chem., 57:5448–5453, 1992.
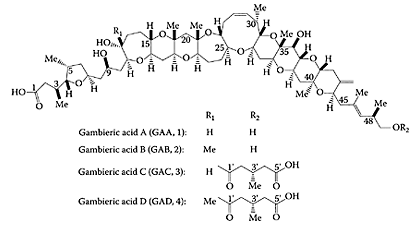
SCHEME G.24 Structures of gambieric acids A-D. (From Morohashi et al., Tetrahendron, 56:8995–9001, 2000. With permission.)
Gamma amino butyric acid (GABA)
The amino acid γ-amino butyric acid (GABA) is produced primarily by decarboxylation of glutamate by glutamate decarboxylase, a vitamin B6-dependent enzyme. Found in many fruits and vegetables, GABA was reported almost 50 years ago to reduce blood pressure in animals and man (Takahashi et al., 1953; Elliott and Hobbiger, 1959). This property was due, in part, to its ability to block peripheral ganglia (Stanton, 1963). Using hypertensive rats, Hayakawa and coworkers (2002) showed the antihypertensive effect of GABA involved possible inhibition of noradrenaline release from sympathetic nerve endings. Recent work by Inoue et al. (2003) examined the effect of a new, fermented-milk product containing GABA on mildly hypertensive patients. A randomized, placebo-controlled, single-blind trial on 39 mildly hypertensive patents (16 women and 23 men), ranging in age from 28–81 years, were fed the fermented milk product over a 12-week period. A significant (p<0.05) decrease in systolic, diastolic, and mean blood pressure was observed after four weeks for the group fed the fermented-milk product (Figure G.41). The reduction in systolic and mean blood pressures were significantly lower for the treated group over the entire 12 weeks of the study. No side effects were observed from the intake of the fermented product, suggesting the potential of GABA-containing products for controlling blood pressure in patients suffering from mild hypertension.
Gamma amino butyric acid (GABA). (From Mohorashi et al., Tetrahendron, 56:8995–9001, 2000. With permission.)
References
Elliott, K.A.C. and Hobbinger, F., Gamma aminobutyric acid: Circulatory and respiratory effects in different species: Re-investigation of the anti-strychnine action in mice, J. Physiol., 146:70–84, 1959.
Hayakawa, K., Kimura, M., and Kamata, K., Mechanism underlying gamma-aminobutyric acidinduced antihypertensive effect in spontaneously hypertensive rats, Eur. J. Pharmacol., 438:107–113, 2002.
Inoue, K., Shirai, T., Ochiai, H., Kaso, M., Hayakawa, H., Kimura, M., and Sansawa, H., Bloodpressure-lowering effect of a novel fermented milk product containing γ-aminobutyric acid (GABA) in mild hypertensives, Eur. J. Clin. Nutr., 57:490–495, 2003.
Stanton, H.C., Mode of action of gamma aminobutyric acid on the cardiovascular system, Arch. Int. Pharmacodyn., 143:195–204, 1963.
Takahashi, H., Tiba, M., Iino, M., and Takayasu, T., The effect of γ-aminobutyric acid on blood pressure, Jpn. J. Physiol., 5:334–341, 1955.
Gamma linolenic acid (GLA)
Gamma linolenic acid (GLA, C18:3 ω-6) occurs abundantly in such plant seeds as borage, evening primrose, and black currants (Eskin, 2002). The conversion of GLA from dietary linoleic acid (C18:2 ω-6) in humans is impaired, as the enzyme involved, Δ 6-desaturase, is either blocked or saturated. Consequently oral GLA has been shown to treat a number of inflammatory disorders, such as rheumatoid arthritis (Leventhal et al., 1993; Zurrier et al., 1996) and atopic dermatitis (Horrobin, 1993; Andreassi et al., 1997). The benefits associated with GLA are attributed to its interference in AA metabolism to bioactive eicosanoids (Miller and Ziboh, 1988). This appears paradoxical, as GLA is a precursor of AA, which can bring about proinflammatory events. However, Peterson et al. (1999) reported dietary GLA exerted immunoregulatory fuctions. These anti-inflammatory effects were found by Kaku et al. (2001) to be due to the suppression of leukotriene B4 production by high doses of GLA.
Gillis et al. (2002) showed GLA alone, or in combination with EPA, significantly induced neutrophil apoptosis and reduced cell viability in human promyelocytic leukemia HL-60 cells. This effect was attributed to GLA’s ability to attenuate the production of leukotriene B4 (LTB4) by inhibiting 5-lipoxygenase (Ziboh and Fletcher, 1992). LTB4 is crucial for neutrophil viability and chemotaxis. Using a rat-infusion glioma model, Leaver and coworkers (2002) found slow infusion of GLA of 2 mM/L over seven days stimulated regression in tumor size and cell death in tumor implants. The potential therapeutic, safe use of γ-linolenic acid for treating human gliomas was further verified by Bakshi et al. (2003). GLA (1 mg) was administered for seven days to nine patients via a cerebral reservoir or by direct, intratumoral delivery, with grade four disease and recurrent glioma. Some improvement in patient survival was observed, with no side effects reported.
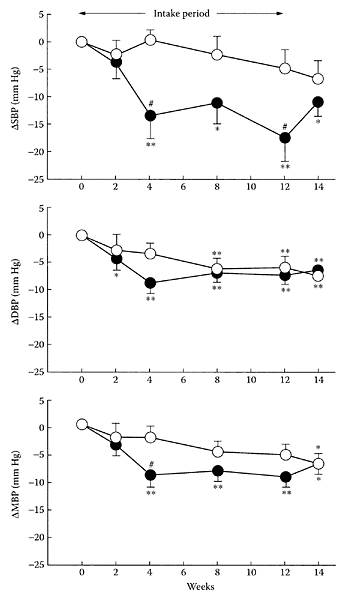
FIGURE G.41 Changes (relative to baseline values) in systolic blood pressure (ΔSBP), diastolic blood pressure (ΔDBP), and mean blood pressure (MBP) after intake of fermented-milk product with GABA (●) or placebo (○). (From Inoue et al., Eur. J. Clin. Nutr., 57:490–495, 2003. With permission.)
GLA has also been studied as a novel, intravesical cytotoxic agent against superficial bladder cancer (Crook et al., 2000). The instability of GLA led to its coupling with a number of agents to enhance its half-life. Harris and coworkers (2003) used the formulation of meglumine GLA (MeGLA), as meglumine is a nontoxic compound used in radiological-contrast media, to assess its efficacy in 30 patients with recurrent transitional-cell carcinoma. No local or systemic side effects were observed following treatment of 15 patients with 50 mL of either 50 mg (1 mg/mL) or 125 mg (2.5 mg/mL) of MeGLA in water. The safety and tolerability of MeGLA were confirmed, and the 43 percent response rate indicated significant cytotoxic effects against transitional-cell carcinoma.
References
Andreassi, M., Forleo, P., Di Lorio, A., Masci, S., Abate, G., and Amerio, P., Efficacy of γ- linolenic acid in the treatment of patients with atopic dermatitis, J. Int. Med. Res., 25:266–274, 1997.
Bakshi, A., Mukherjee, D., Bakshi, A., Banerji, A.K., and Das, U.N., γ-Linolenic acid therapy of human gliomas, Nutrition, 19:305–309, 2003.
Crook, T., Hall, S., Solomon, L., Bass, P., Cooper, A., and Birch, B., Intravesical meglumine gamma linolenic acid, phase 1 tolerability studies in patients undergoing cystetomy, Urooncol., 1:39–42, 2000.
Eskin, N.A.M., Authentication of evening primrose, borage and fish oils, in Oils and Fats Authentication, Jee, M., Ed., Blackwell Publishing and CRC Press, 2002, chap. 4, pp. 95–107.
Gillis, R.C., Daley, B.J., Enderson, B.L., and Karlstad, M.D., Eicosapentaenoic acid and γ-linolenic acid induce apoptosis in HL-60 lines, J. Surg. Res., 107:145–153, 2002.
Harris, N.M., Crook, T.J., Dyer, J.P., Cooper, A.J., and Birch, B.R., Intravesicular meglumine gammalinolenic acid in superficial bladder cancer: An efficacy study, Eur. Urol., 42:39–42, 2002.
Horrobin, D.F., Fatty acid metabolism in health and disease: the role of delta-6-desaturase, Am. J. Clin. Nutr., 57:732S-736S, 736S-737S, 1993.
Kaku, S., Ohkura, K., Yunoki, S., Nonaka, M., Tachibana, H., Sugano, M., and Yamada, K., Dietary γ-linolenic acid dose-dependently modifies fatty acid composition and immune parameters in rats, Prostaglandins Leukot. Essent. Fatty Acids, 65:205–210, 2001.
Leaver, H.A., Wharton, S.B., Bell, H.S., Leaver-Yap, M.M., and Whittle, I.R., Highly unsaturated fatty acid induced tumor regression in glioma pharmacodynamics and bioavailability of gamma linolenic acid in an implantation glioma model: Effects on tumor biomass, apoptosis and neuronal tissue histology, Prostaglandins, 67:283–292, 2002.
Leventhal, L.J., Boyce, E.G., and Zurrier, R.B., Treatment of rheumatoid arthritris with gamma lindenic acid, Ann. Intern. Med., 119:867–873, 1993.
Miller, C.C. and Ziboh, V.A., Gammalinolenic acid-enriched diet alters cutaneous eicosanoids, Biochem. Biophys. Res. Commun., 154:967–974, 1988.
Peterson, L.D., Thies, F., and Calder, P.C., Dosedependent effcts of dietary γ-linolenic acid on rat spleen lymphocyte functions, Prostaglandins Leukot. Essent. Fatty Acids, 61:19–24, 1999.
Ziboh, V.A. and Fletcher, M.P., Dose-response effects of dietary gamma linolenic acid-enriched oils on human polymorphonuclear-neutrophil biosynthesis of leukotriene B4, Am. J. Clin. Nutr., 55:39–45, 1992.
Zurier, R.B., Rosseti, R.G., Jacobson, E.W., DeMarco, D.M., Liu, N.Y., Temming, J.E., White, B.M., and Laposata, M., Gamma-linolenic acid treatment of rheumatoid arthritis, a randomized, placebocontrolled trial, Arthritis Rheum., 39:1808–1817, 1996.
Ganorderma
Ganorderma is a mushroom used in Oriental traditional medicine for treating many chronic diseases (Gao and Zhou, 2003). Polysaccharides present its fruit body, mycelia, or spores of Ganordema are pharmacologically active and include β-D-glucans, heteropolysaccharides, and glycoproteins. A fucose-containing glycoprotein isolated from Ganordema lucidum was shown to stimulate spleen-cell proliferation and cytokine expression (Wang et al, 2002). Over 100 triterpenoids were isolated from Ganordema lucidum including the highly oxidized lanostane-type triterpenoids, such as ganoderic and lucidenic acids. Cancer-preventive action by Ganordema was attributed to the antiproliferative properties of its triterpenoids by inducing apoptosis (Birt et al., 2001; Gan et al., 1998).
Ganordema products are sold as a single agent or with other herbal medicines. Clinical studies on prostate cancer patients using a combination of Ganordema and eight herbs (PC-SPES) showed a significant reduction in the prostate-specific antigen (PSA) levels (Small et al., 2000). In vitro and in vivo studies on Ganordema attributed its anticancer effects to antioxidative, free-radical scavenging, enhancement of the immune system, cell-cycle arrest, and apoptosis.
References
Birt, D.F., Hendrich, S., and Wang, W., Dietary agents in cancer prevention: Flavonoids and isoflavonoids, Pharmacol. Ther., 90:157–177, 2001.
Gan, K.H., Fann, Y.F., Hsu, S-H., Kuo, K.W., and Lin, C.N., Mediation of the cytotoxicity of lanostanoids and steroids of Ganordema tsugae through apoptosis and cell cycle, J. Nat. Prod., 61: 485–487, 1998.
Gao, Y. and Zhou, S., Cancer prevention and treatment by Ganordema, a mushroom with medicinal properties, Food Rev. Inter., 19:275–325, 2003.
Small, E.J., Frohlich, M.W., Bok, R., Shinohara, K., Grossfeld, G., Rozenblat, Z., Kelly, W.K., Corry, M., and Reese, D.M., Prospective trial of the herbal supplement PC-SPES in patients with progressive prostate cancer, J. Clin. Oncol., 18:3595–3603, 2000.
Wang, Y.Y., Khoo, K.H., Chen, S.T., Lin, C.C., Wong, C.H., and Lin, C.H., Stidies on the immunomodulating and antitumor activities Ganordema lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities, Bioorg. Med. Chem., 10:1057–1062, 2002.
Garden cress (Lepidium sativum)
Garden cress is fairly unique among the Brassica vegetables, as it contains only one glucosinolate, namely glucotropaeolin (GT) (Fenwick et al., 1983). This glucosinolate is then hydrolyzed by the enzyme myrosinase to yield the corresponding isothiocyanate, benzylisothiocyanate (BITC) (Scheme G.25). Glucosinolates have been shown to protect laboratory animals from chemically induced cancers by inhibition of phase I enzymes or by induction of glutathione S-transferase (Chung et al., 1992; Knasmuller et al., 1996; Zhang and Talalay, 1994).
Kassie and coworkers (2002) examined the chemoprotective properties of garden cress toward the genotoxic effects of the heterocyclic aromatic amine, 2-amino-3-methyl-imidazo[ 4,5-f] quinoline (IQ), in F344 rats. Garden-cress juice reduced the IQ-induced genotoxic effects and colonic-preneoplastic lesions by the induction of UDPglucurononsyltransferase (UDPGT), a key enzyme in the detoxification of heterocyclic aromatic amines. Kassie et al. (2002) found that the amount of juice needed to produce these changes was quite small but similar to the level of glucosinolates consumed in a regular salad.
References
Chung, F.L., Chemoprevention of lung carcinogenesis by aromatic isothiocyanates, in Cancer Chemoprevention, Wattenberg, L.W., Lipkin, M., Boone, C.W., and Kelloff, G.J., Eds., CRC Press, Boca Raton, Florida, 1992, pp. 227–245.
Fenwick, G.R., Heaney, R.K., and Mullin, W.J., Glucosinolates and their breakdown products in food and food plants, Crit. Rev. Food Sci. Nutr., 18:123–201, 1983.
Ishimoto, H., Fukushi, Y., and Tahara, S., Non-pathogenic Fusarum strains protect seedlings of Lepidium sativum and Pythium ultimum, Soil Biol. Biochem., 36:409–414, 2004.
Kassie, F., Rabot, S., Uhl, M., Huber, W., Qin, H.M., Helma, C., Schulte-Hermann, R.S., and Knasmuller, S., Chemoprotective effects of garden cress (Lepidium sativum) and its constituents towards 2-amino-3-methyl-imidazo [4,5-f]quinoline (IQ)-induced genotoxic effects and colonic preneoplastic lesions, Carcinogenesis, 23:1155–1161, 2002.
Knasmuller, S., Friesen, M.D., Holme, J.A., Alexander, J., Sanyal, R., Kassie, F., and Bartsch, H., Effects of phenethyl isothiocyanate on metabolism and on genotoxicity of dimethylnitrosamine and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), Mutat. Res., 350:93–102, 1996.
Zhang, Y. and Talalay, P., Anticarcinogenic activities of organic isothiocyanates: Chemistry and mechanisms, Cancer Res., 54:1976S-1981S, 1994.
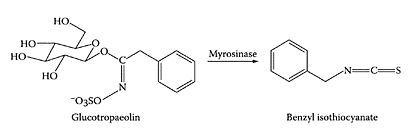
SCHEME G.25 Enzymic hydrolysis of glucotropaeolin to benzyl isothiocyanate. (From Ishimoto et al., Soil Biol. Biochem., 36:409–414, 2004. With permission.)
Garlic
In addition to being a flavoring agent, garlic (Allium sativum L.) is also pharmacologically active against microbial infection, thrombosis, hypertension, hyperglycemia, hyperlipidemia, and cancer. The pharmacological properties of garlic, such as lipid-lowering effects, appear to be related to sulfur-rich compounds, particularly allicin. Shukla and Taneja (2002) clearly showed the antimutagenic effects of garlic extract (GE) in Swiss albino mice using an “in vivo chromosomal aberration assay.” Pretreatment with 2.5 percent and 5 percent GE significantly suppressed chromosomal aberrations in cyclophosphamide (CP)-treated (a well-known mutagen) mice. The anticytoxic effects of GE were demonstrated by a significant increase in mitotoxic idex, as well as reduction in CP-induced clastogenicity. Sengupta et al. (2002) also showed that garlic constituents protected Swiss mice from DMBA-induced clastogenicity by significantly reducing chromosomal aberrations in the bone marrow, Iimuro and coworkers (2002) found that a garlic extract suppressed Helicobacter pyloriinduced gastritis in Mongolian gerbils. Infection by this organism has been associated with the development of stomach cancer so that garlic extract appeared to be useful for reducing the risk of gastric cancer. Patients with benign prostate hyperplasia and prostate cancer showed significant improvements after consuming an aqueous garlic extract (1 mL/kg weight) for a month (Durak et al., 2003). In addition to reducing the mass of prostate, the urinary frequency was decreased, while maximum and average rates of urine flow increased. Cancer patients had significantly lower PSA values after consumption of the garlic extract.
Epidemiological data showed an inverse relationship between garlic consumption and reduced risk of cardiovascular disease (Kendler, 1987; Keys, 1980). In their review of garlic and cardiovascular disease, Banerjee and Maulik (2002) pointed to the need to identify specific components responsible for its cardioprotective effects. Ozturk et al. (1994) observed the beneficial effects of garlic extract on vascular responsiveness in normal rats. A recent study by Baluchnejadmojarad et al. (2003) showed an aqueous garlic extract significantly improved impaired endothelium-dependent relaxations, as well as decreased the enhanced contractile response to phenyl epinephrine in diabetic rats.
Fresh garlic was reported to lower blood pressure in spontaneously hypertensive rats (Foushee et al., 1982). Further researchers confirmed the ability of garlic to control mild hypertension. Al-Quattan et al. (1999) examined the effectiveness of garlic in treating more severe hypertension, such as in unilateral renovascular hypertension (URVH). Using a 2K1C hypertensive rat, they showed that a single dose had a maximum antihypertensive effect 2–6 h after administration, continuing for up to 24 h (Table G.33). Multiple doses of garlic also controlled the rise in blood pressure in these hypertensive rats. Sharif et al. (2003) showed a negative correlation between garlic, blood pressure, and angiotensin-converting enzyme (ACE) using the same 2K1C hypertensive model. An enteric-coated garlic-powder supplement suggested that the ability of garlic to lower blood pressure was attributed, in part, to a reduction in ACE activity.
References
Al-Quattan, K.K., Alnaqeeb, M.A., and Ali, M., The antihypertensive effect of garlic (Allium sativum) in the rat two-kidney-one-clip Goldblatt model, J. Ethnopharmacol., 66:217–222, 1999.
Baluchnejadmojarad, T., Roghani, M., Homayounfar, H., and Hosseini, M., Beneficial effect of aqueous garlic extract on the vascular reactivity of streptozoticin-diabetic rats, J. Ethnopharmacol., 85:139–144, 2003.
Banerjee, S.K. and Maulik, S.K., Effect of garlic on cardiovascular disorders: A review, Nutr. J., 1:4, 2002.
Durak, I., Yllmaz, E., Devrim, E., Perk, H., and Kacmaz, M., Consumption of aqueous garlic extract leads to significant improvement in patients with benign prostate hyperplasia and prostate cancer, Nutr. Res., 23:199–204, 2003.
Foushee, D.B., Ruffn, J., and Banerjee, U., Garlic as a natural agent for the treatment of hypertension: A preliminary report, Cytobios, 34:142–152, 1982.
Iimuro, M., Shibata, H., Kawamori, T., Matsumoto, T., Arakawa, T., Sugimura, T., and Wakabayashi, K., Suppressive effects of garlic extract on Helicobacter pylori-induced gastritis in Mongolian gerbils, Cancer Lett., 187:61–68, 2002.
Kendler, B.S., Garlic (Allium sativum) and onion (Alliuim cepa): A review of their relationship to cardiovascular disease, Prev. Med., 16:670–685, 1987.
Keys, A., Wine, garlic and CHD in seven countries. Lancet, 1:145–146, 1980.
Ozturk, Y., Aydin, S., Kosar, M., and Baser, K.H.C., Endothelium-dependent and independent effects of garlic and rat aorta, J. Ethnopharmacol., 44:109–116, 1994.
Sengupta, A., Ghosh, S., and Das, S., Administration of garlic and tomato can protect from carcinogen induced clastogenicity, Nutr. Res., 22:859–866, 2002.
Sharifi, A.M., Darabi, R., and Akbarloo, N., Investigation of antihypertensive mechanism of garlic in 2K1C hypertensive rat, J. Ethnopharmacol., 86:219–224, 2003.
Shukla, Y. and Taneja, P., Antimutagenic effects of garlic extract on chromosomal aberrations, Cancer Lett., 176:31–36, 2002.
Zhang, X.-H., Lowe, D., Giles, P., Fell, S., Connock, M.J., and Maslin, D.J., Gender may affect the action of garlic oil on plasma cholesterol and glucose levels of normal subjects, J. Nutr., 131:1471–1478, 2001.
Genistein
One of the main isoflavone components in soy is genistein. It is a phytoestrogen and an antioxidant and acts on osteoblast-like cells, increasing cellular proliferation. Lee and coworkers (2001) found genistein stimulated cell proliferation, as well as protected against oxidative damage to osteoblast-like cells from the action of free radicals. Epidemiological evidence suggests the low incidence of prostate cancer among Asians is associated with a high intake of soy. Studies conducted by Wang et al. (2002) showed that dietary genistein suppressed chemically induced prostate cancer in Lobund Wistar rats. Po and coworkers (2002a) found genistein was not as effective in suppressing estrogen-receptor sites or inducing apoptosis using a transient transfection mouse model. Confirmation of the inability of soybean genistein to exert its chemoprotective effect through antagonizing the estrogen receptor was provided by Po et al. (2002b). Soybean genistein was also found by Hewitt and Singletary (2003) to inhibit adenocarcinoma tumor growth in a syngeneic mouse model. However, the greater inhibition by the soy extract compared to genistein suggested the presence of other components besides genistein.
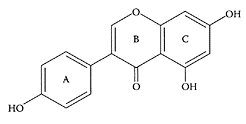
Genistein. (Adapted from Xu et al., Structure, 12:2197–2207, 2004.)
The induction of apoptosis in a variety of cancer-cell lines by genistein is well-established (Lian et al., 1998; Shao et al., 1998). Recent research by Baxa and and Yoshimura (2003) showed that genistein induced inhibition of NF-κB by cleaving IκBα (Figure G.42). The latter is a protein residing in the cytosol, where it associates with NF-κB. These researchers also showed that caspase activity was involved in cleaving IκBα in genistein-treated cells. This was evident by a significant elevation of caspase-3 activity observed in genistein-treated T lymphoma 92316T cells after 6 h compared to the untreated cells. Other possible activities by genistein, such as inhibition of tyrosine kinase, which also reduces NF-κB, cannot be ruled out.
References
Baxa, D.M. and Yoshimura, F.K., Genistein reduces NF-κB in T lymphoma cells via a caspase-mediated cleavage of IκBα, Biochem. Pharmacol., 66:1009–1018, 2003.
Hewitt, A.L. and Singletary, K.W., Soy extracts inhibits mammary adenocarcinoma growth in a syngeneic mouse model, Cancer Lett., 192:133–143, 2003.
Lee, Y.-S., Chen, X., and Anderson, J.J.B., Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells, Nutr. Res., 21:1287–1298, 2001.
Lian, F., Bhuiyan, M., Li, Y., Wall, N., Kraut, M., and Sarker, F.H., Genistein-induced G2-M arrest, p21WAFI upregulation, and apoptosis in a non-small cell lung cancer cell line, Nutr. Cancer, 31:184–191, 1998.
Po, L.S., Chen, Z.-Y., Tsang, D.S.C., and Leung, L.K., Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells, Cancer Lett., 187:33–40, 2002a.
Po, L.S., Wang, T.T., Chen, Z.-Y., and Leung, L.K., Genistein-induced apoptosis in MCF-7 cells involves changes in Bak and Bol-x without evidence of antioestrogenic effects, Br. J. Nutr., 88:463–469, 2002b.
Shao, Z.M., Alpaugh, M.L., Fontina, J.A., and Barsky, S.H., Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by p21WAF1/CIPl induction, G2.M arrest, and apoptosis, J. Cell Biochem., 69:44–54, 1998.
Wang, J.M., Eltoum, I.-E., and Lamartiniere, C.A., Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats, Cancer Lett., 186:11–18,2002.
Geraniol
Geraniol is an acyclic monoterpene alcohol found in the essential oils of fruits and herbs. Like many monoterpenes, geraniol has been reported to exert chemopreventive effects, including in vivo and in vitro antitumor activity against leukemia, hepatoma, and melanoma cells in experimental animals (Shoff et al., 1991; Yu et al., 1995; Burke et al., 1997). Carnesecchi and coworkers (2001) demonstrated for the first time that geraniol (400 μM) inhibited the growth of a human colon cancer-cell line (Caco-2) by 70 percent. No cytotoxic effects or apoptosis were observed. The potent, antiproliferative effects of geraniol were attributed, in part, to a 50 percent decrease in ornithine decarboxylase activity, a key enzyme in polyamine biosynthesis, which is normally enhanced during carcinogenesis. Further research by these researchers (Carnesecchi and coworkers, 2002) showed that the antiproliferative effect of geraniol on Caco-2 cells was directly linked to perturbation of cell-membrane function, resulting in a 60 percent reduction of protein kinase C (PKC) activity. In addition, there was a 50 percent decrease in the active forms of p44/p42 extracellular signalregulated protein kinases (ERK), suggesting perturbation in signal-transduction pathways.
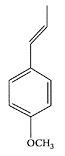
Geraniol. (From Duncan et al., Biochem. Pharmacol., 68:1739–1747, 2004. With permission.)
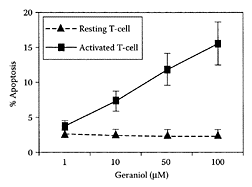
FIGURE G.43 The rat T-cells apoptosis assay, resting and activated T-cells were treated with varying concentrations of geraniol (between 1 μmol/L and 100 μmol/L), and the activated T-cells showed higher apoptosis compared to resting cells. (From Ji et al., Transpl. Proc., 34:1418–1419, 2002. With permission.)
The anticancer properties of monoterpenes were attributed to their ability to prevent iso-prenylation of GTPases (Steinmetz et al., 1991). Ji and coworkers (2003) demonstrated geraniol exhibited modest immunosuppressive activity both in vitro and in vivo. This study provided the first evidence that geraniol induced apoptosis preferentially in activated T-cells (Figure G.43). Because of its suppressive property, geraniol was able to prolong graft survival in a cardiac allograft transplant model.
References
Burke, Y.D., Stark, M.J., Roach, S.L., Sen, S.E., and Crowell, P.L., Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol, Lipids, 32:151–156, 1997.
Carnesecchi, S., Bradaia, A., Fischer, B., Coelho, D., Scholler-Guinard, M., Gosse, F., and Raul, F., Perturbation by geraniol of cell membrane permeability and signal transduction pathways in human colon cancer cells, J. Pharmacol. Exp. Ther., 303:711–715, 2002.
Carnesecchi, S., Schneider, Y., Ceraline, J., Duranton, B., Gosse, F., Seiler, N., and Raul, F., Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells, J. Pharmacol. Exp. Ther., 298:197–200, 2001.
Duncan, R.E., Lau, D., El-Sohemy, A., and Archer, M.C., Geraniol and β-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity, Biochem. Pharmacol., 68:1739–1747, 2004.
Ji, P., Si, M.-S., Podnos, Y., and Imagawa, D.K., Monoterpene geraniol prevents acute allograft rejection, Transpl. Proc., 34:1418–1419, 2002.
Shoff, S.M., Grummer., M., Yatvin, M.B., and Elson, C.E., Concentration-dependent increase of murine P388 and B16 population doubling time by the acyclic monoterpene geraniol, Cancer Res., 51:37–42, 1991.
Steinmetz, K.A. and Potter, J.D., Vegetables, fruit, and cancer, I. Epidemiology, Cancer Causes Cont., 2:325–327, 1991.
Yu, S.G., Hildebrandt, L.A., and Elson, C.E., Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice, J. Nutr., 125:2763–2767, 1995.
Ginger
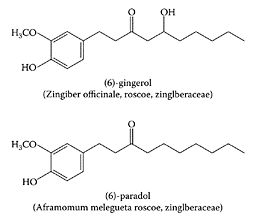
Ginger (Zingiber officinale Roscoe, Zingiberaceae) is used worldwide as a spice in the preparation of foods. In addition, the rhizome of ginger is a recognized treatment in traditional Oriental medicine for inflammation and rheumatism, as well as gastrointestinal problems. Koshimizu et al. (1988) found that the rhizome of ginger exerted antitumor properties by inhibiting 12-O-tetra-decanoylphor-bol-13-acetate (TPA)-induced Epstein-Barr virus activation in Raji cells. A later study by Katiyar and coworkers (1996) showed topical application of an ethanol extract of ginger suppressed TPA-mediated induction of ornithine decarboxylase and its mRNA expression in SENCAR mouse skin. These workers also reported that the ginger extract protected the mouse skin from 7,12-dimethylbenz[a]anthracene (DMBA)-induced carcinogenesis. The protective effects of ginger have been attributed to the presence of several phenolic compounds, or vanilloids, gingerol and paradol (Locksley et al., 1972; Lee and Surh, 1998; Park et al., 1998).
Abe et al. (2002) isolated a pungent principle present in Japanese ginger (Zingiber mioga Roscoe) as a labdane-type dialdehyde identified as galanals A and B.Miyoshi et al. (2003) recently showed galanals A and B both significantly inhibited cell proliferation of human T lymphoma Jurkat cells in a dose-dependent manner (IC50 of 18 and 32 μMm, respectively) (Figure G.44). The mechanism of apoptosis involved reduction of the Bcl-2: Bax ratio and caspase-3 activation, suggesting their potential as anticancer agents.
(From Lee and Suhr, Cancer Lett., 134:163–168, 1998. With permission.)
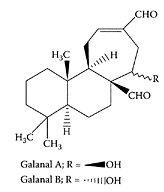
Structure of galanal A and B. (Adapted from Miyoshi et al., Cancer Lett., 199:113–119, 2003.)
A recent study by Hori and coworkers (2003) isolated five sulfonated compounds from Zingiberis rhizome (Shokyo), 4-gingesulfonic acid, and shogasulfonic acids A, B, C, and D, together with 6-gingesulfonic acid. Their potential bioactivity remains to be examined.
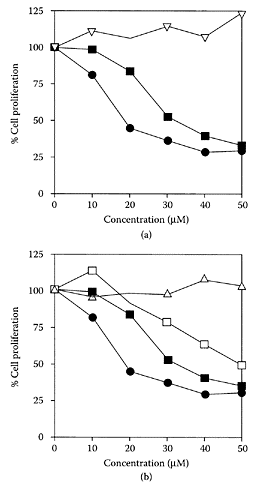
FIGURE G.44 Effect of compounds isolated from ginger plants on the cell growth of Jurkat cells. (A) Geranal A (●), galanal B (■), curcumin (□), or 6-gingerol (Δ). (Adapted from Miyoshi et al., Cancer Lett., 199:113–119, 2003. With permission.)
References
Abe, M., Ozawa, Y., Uda, Y., Yamada, Y., Morimitsu, Y., Nakamura, Y., and Osawa, T. Labdanetype dialdehyde, pungent principle of myoga, Zingiber mioga Roscoe, Biosci. Biotechnol. Biochem., 66:2698–2700, 2002.
Hori, Y., Miura, T., Hirai, Y., Fukumura, M., Nemoto, Y., Toriizuka, K., and Ida, Y., Pharmacognosticx studies on giner and related drugs-part 1: five sulfonated compounds from Zingiberis rhizome (Shokyo), Phytochemistry, 62:613–617, 2003.
Katiyar, S.K., Agarwal, R., and Mukhtar, H., Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome, Cancer Res., 56:1023–1030, 1996.
Koshimizu, K., Ohigashi, H., Tokuda, H., Kondo, A., and Yamaguchi, K., Screening of edible plants against possible anti-tumor promoting activity, Cancer Lett., 39:247–257, 1988.
Lee, E. and Surh, Y.-J., Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-generol and [6]-paradol, Cancer Lett., 134:163–168, 1998.
Locksley, H.D., Rainey, D.K., and Rohan, T.A., Pungent compounds, part 1, an improved synthesis of paradols (alkyl 4-hydroxy-3-methoxyphenyethyl ketones) and an assessment of their pungency, J. Chem. Soc. Perkin, I:3001–3006, 1972.
Miyoshi, N., Nakamura, Y., Ueda, Y., Abe, M., Ozawa, Y., Uchida, K., and Osawa, T., Dietary ginger constituents, galanals A and B, are potent apoptosis inducers in human T lymphoma Jurkat cells, Cancer Lett., 199:113–119, 2003.
Park, K.-K., Chun, K.-S., Lee, J.-M., Lee, S.S., and Surh, Y.-J., Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice, Cancer Lett., 129:139–144, 1998.
Gingerol
Gingerols are the pungent oleoresin constituents present in ginger. The major gingerol component is [6]-gingerol (1-[4′-hydroxy-3′-methoxyphenyl]-5-hydroxy-3-decanone). Isolated [6]-gingerol appeared to be the active principle responsible for inhibiting secondary platelet activation and ATP release from platelets in human platelet-rich plasma. This inhibition is reversible, as it involves inhibition of arachidonic-acid metabolism and cyclooxygenase (COX) activity (Kiuchi et al., 1982; Guh et al., 1995). Koo and coworkers (2001) showed gingerols inhibited the arachidonic acid-induced platelet serotonin release reaction in a similar dose range as aspirin. Tjendraputra et al. (2001) examined 17 oleoresin principles of ginger (Zingiber officinale, Roscoe) together with synthetic analogues of gingerol. They all proved potent inhibitors of COX-2 and effective for treating inflammation. Based on their relative rates of inhibition, three important structural features were required for COX-2 inhibition: (1) lipophilicity of the alkyl side chain, (2) substitution pattern of hydroxy and carbonyl groups on the side chain, and (3) substitution of hydroxy and methoxy groups on the aromatic ring (Scheme G.26).
Tjendraputra, E., et al., Bioorg. Chem., 29:156–163, 2001. With permission.
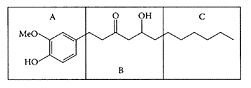
SCHEME G.26 Structural features of gingerol and synthetic analogues required for COX-2 inhibition. (A) Substitution pattern on the aromatic moiety, (B) functional group substitution pattern on the side chain, and (C) the lipophilic alkyl side chain. (From Tjendraputra et al., Bioorg. Chem., 29:156–163, 2001. With permission.)
[6]-Gingerol is a strong antioxidant, as evident by its ability to inhibit FeCl3-ascorbateinduced peroxidation of phospholipids (Aeschbach et al., 1994) or prevent the generation of reactive-oxygen species by xanthine oxidase (Chang et al., 1994). Park et al. (1998) demonstrated the antitumor activity of [6]-gingerol by its ability to significantly inhibit 7,12-dimethylbenz[a]-anthracene (DMBA)-induced skin papillomagenesis. This is shown in Table G.34 by its suppression of the tumor promoterinduced inflammation by reduction in mouseear edema. While [6]-gingerol reduced ear edema by 61 percent, it was much less than curcumin’s 91 percent reduction at the same concentration of 10 μmol.
References
Aesbach, R., Loliger, J., Scott, B.C., Murcia, A., Butler, J., Halliwell, B., and Aruoma, O.I., Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol, Food Chem. Toxicol., 32:31–36, 1994.
Chang, W.-S., Chang, Y.-H., Lu, F.-L., and Chiang, H.-C., Inhibitory effects of phenolics on xanthine oxidase, Anticancer Res., 14:501–506, 1994.
Guh, J.H., Ko, F.N., Jong, T.T., and Teng, C.M., Antiplatelet effect of gingerol isolated from Zingiber officinale, J. Pharm. Pharmacol., 47:329–332, 1995.
Kiuchi, F., Shibuya, M., and Sankawa, U., Inhibitors of prostaglandin biosynthesis from ginger, Chem. Pharm. Bull., 30:754–757, 1982.
Koo, K.L.K., Ammit, A.J., Tran, V.H., Duke, C.C., and Roufogalis, B.D., Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation, Thromb. Res., 103:387–397, 2001.
Park, K.-K., Chun, K.-S., Lee, J.-M., Lee, S.S., and Surh, Y.-J., Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phosbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice, Cancer Lett., 129:139–144, 1998.
Tjendraputra, E., Tran, V.H., Liu-Brennan, D., Roufogalis, B.D., and Duke, C.C., Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells, Bioor. Chem., 29:156–163, 2001.
TABLE G.34
Inhibition of Tumor Promoter-Induced Inflammation in Mouse Ear by [6]-Gingerola
Ginkgo biloba
Extracts from the leaves of Ginkgo biloba L., one of the oldest phytomedicines in China, is still used extensively in therapy. The dry extract EGb 761, the essential component of Ginkgo biloba, is based on the content of flavonoids (24 percent) and terpenoids (6 percent). These preparations are used to treat diseases associated with advanced age, such as cerebrovascular and peripheral circulatory insufficiences and memory disturbances (Newall et al., 1996). The extract is an effective free-radical scavenger, with antioxidant activity capable of protecting against free-radical damage associated with such diseases as arteriosclerosis, rheumatism, and cancer. The action of EGb 761 on the human brain was confirmed by electroencephalography with enhancement of the α-wave component (Itil and Martorano, 1995; Luthringer et al., 1995). Animal studies showed EGb 761 facilitated acquisition and retention of memory (Cohen-Salmon et al., 1997; Winter, 1998) through protection of the hippocampus (Barkats et al., 1995). Since Alzheimer’s disease is associated with a severely atrophied hippocampus, EGb 761 appeared to have potential as a treatment. The free-radical-scavenging effect of EGb 761 was demonstrated by the reduction of lipid peroxidation in a mouse-model brain with experimental cerebral ischemia (Pierre et al., 2002). EGb 761 prevents oxidative stress from destroying neurons, particularly by apoptosis, induced by glutamate, nitric oxide, and β-amyloid (Aβ) (Bastianetto and Quirion, 2002; Luo et al., 2002). Treatment of Wistar male rats with a Ginkgo biloba extract (GK 501) was shown by Hadjiivanova and Petkov (2002) to induce a significant decrease in the density (Bmax) of β-adrenoreceptors in the frontal cortex and hippocampus regions of the brain (Table G.35). Since both of these areas are involved in cognition, it suggests that treatment with Ginkgo biloba extract may have a beneficial effect on learning and memory.
Brayboy and coworkers (2001) showed EGb 761 protected osteoblast-like bone cells (MC3T3-E1) from free-radical damage, as well as enhanced the proliferation of these cells. Ellnain-Wojtaszek and coworkers (2002) recommended that the leaves of Ginkgo biloba be stored for as short time as possible to prevent loss of free-radical-scavenging activity. Several excellent reviews on Ginkgo biloba were recently published in a new journal on nutra ceuticals (Christen, 2003; Luo, 2003).
References
Barkats, M., Venault, P., Christen, Y., and CohenSalmon, C., Effects of long term treatment with EGb 761 on age-dependent structural changes in the hippocampi of three inbred mouse strains, Life Sci., 56:213–222, 1995.
Bastianetto, S. and Quirion, R., EGb 761 is a neuroprotective agent against β-amyloid toxicity, Cell. Mol. Biol., 48:693–697, 2002.
Brayboy, J.R., Chen, X.W., Lee, Y.S., and Anderson, J.J.B., The protective effects of Ginkgo biloba extract (EGb761) against free radical damage to osteoblastlike bone cells (MC3T3-E1) and the proliferative effects of Egb 761 on these cells, Nutr. Res., 21:275–1285, 2001.
Cohen-Salmon, C., Venault, P., Martin, B., RafalliSebille, M.J., Barkats, M., Clostre, F., Pardon, M.-C., Christen, Y., and Chapouthier, G., Effects of Ginkgo biloba extract EGb 761 on learning and memory, J. Physiol., 91:91–300, 1997.
Christen, Y., From clinical observations to molecular biology: Ginkgo biloba extract EGB 761, a success for reverse pharmacology, Curr. Top. Nutraceut. Res., 1:59–72, 2003.
Ellnain-Wojtaszek, M., Kruczynski, Z., and Kasprzak, J., Variations in the free radical scavenging activity of Ginkgo biloba L. leaves in the period of complete development of green leaves to fall of yellow ones, Food Chem., 79:79–84, 2002.
Hadjiivanova, Ch.I. and Petkov, V.V., Effect of Ginkgo biloba extract on β-adrenergic receptors in different rat brain regions, Phytother. Res., 16:488–492, 2002.
Itil, T. and Martirano, D., Natural substances in psychiatry (Ginkgo biloba in dementia), Psychopharmacol. Bull., 31:147–158, 1995.
Luo, Y., Contemporary neuroscience meets traditional medicine—towards understanding ginkgo biloba neuroprotection, Curr. Top. Nutraceut. Res., 1:49–58, 2003.
Lutheringer, R., d’Arbigny, P., and Macher, J.P., Ginkgo biloba extract (EGb 761), EEG and event-related potentials mapping profile, in Advances in Ginkgo Biloba Extract Research, Vol. A, Effects of Age-related Disorder, Christen, Y., Courtois, Y., and Droy-Lefaix, M.-T., Eds., Elsevier, Paris, 1995, pp. 107–118.
Newall, C.A., Anderson, L.A., and Phillipson, J.D., Herbal Medicines, The Pharmaceuticals Press, London, 1996.
Pierre, S., Jamme, I., Robert, K., Gerbi, A., Duran, M.-J., Sennoune, S., Droy-Lefaix, M.-T., Nouvelot, A., and Maixent, J.-M., Ginkgo biloba extract (EGb 761) protects Na, K-ATPase isoenzymes during cerebral ischemia, Cell. Mol. Biol., 48:671–679, 2002.
Winter, J.C., The effect of an extract of Ginkgo biloba EGb761, on cognitive behavior and longevity in the rat, Physiol. Behav., 63:425–433, 1998.
Ginseng
Ginseng, the root of Panax ginseng, has been used in Oriental medicine for many centuries to treat a wide range of ailments. In Europe, it is sold over the counter to enhance physical and mental performance. Ginseng products are either white or red. White ginseng is the dried root with the skin peeled off, whereas red ginseng is the steamed root, which is caramel-colored. White ginseng (includes lateral roots and root hairs) is commonly used in the European market, while red ginseng is the preferred form in Asia. The unique constituents identified in ginseng include several classes of compounds: triterpene saponins; essential oil-containing poly acetylenes and sesquiterpenes; polysaccharides; peptidoglycans; and nitrogen-containing compounds (Tang and Eisenbrand 1992). Triterpene saponins are referred to as ginsenosides, as their property appears to be a function of the number of monosaccharide residues in the sugar chain (Hostettmann and Marston, 1995). Thirty-one ginsenosides have been isolated from the roots of white and red ginseng that can be categorized into three groups, based on their aglycones, as protopanaxadiol-type, protopanaxatriol-type, and oleanolic acid-type saponins (Sticher, 1998). Ginseng is specified in the Swiss and German pharmacopeias on the total ginsensoside content, calculated as ginsenoside Rg1, as not less than 2.0 percent and 1.5 percent, respectively. The European pharmacopeia requires that the ginsenoside Rga and Rb1 content in ginseng must not be less than 0.3 percent.
Research on ginseng suggests ginsenosides have antiaging properties by enhancing the immune system by increasing serum-specific antibodies and IgG content and protective B-lymphocytes (Nah et al, 1995; Liu et al., 1995; Yamada et al., 1995). In addition to ginsenosides other isolated components, such as polyacetylenes, panaxytriol, panaxynol, and panaxydol have cytotoxic, antiplatelet, and antiinflammatory properties, respectively (Deng and Zhang, 1991; Matsunaga et al., 1995; Kobayashi et al., 1995). The hypoglycemic effect of ginseng is attributed to its polysaccharides, the panaxans, which are themselves peptidoglycans. Immunological activity is also associated with some of its polysaccharides, the ginsenans. While the precise structures of these polysaccharides are not fully known, their backbone chain is mainly β-1,3-linked D-galactoside (Tomoda et al., 1993).
References
Deng, H. and Zhang, J., Anti-lipid peroxidative effect of ginsenoside Rb1 and Rg1, Chin. Med. J., 104:395–398, 1991.
Hostettmann, K. and Marston, A., Saponins. Cambridge University Press, Cambridge, U.K., 1995, p. 50.
Kobayashi, M., Mahmud, T., Umezome, T., and Kitgawa, I., The absolute stereostructure of panaxytriol, a biologically active diacetylenic acetogenin, from Ginseng Radix Rubra, Chem. Pharm. Bull., 43:1595–1597, 1995.
Liu, J., Wang, S., Liu, H., Yang, L., and Nan, G., Stimulatory effect of saponin from Panax ginseng on immune function of lymphocytes in the elderly, Mech. Ageing Dev., 83:43–53, 1995.
Matsunaga, H., Saita, T., Nagumo, F., Mori, M., and Katano, M., A possible mechanism for the cytoxicity of a polyacetylenic alcohol, panaxtriol, inhibition of mitochondrial respiration, Cancer Chemother. Pharmacol., 35:291–296, 1995.
Nah, S., Park, H., and McCleskey, E.W., A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G-protein, Proc. Natl. Acad. Sci., 92:8739–8743, 1995.
Sticher, O., Getting to the root of ginseng, Chemtech, (April):26–32, 1998.
Tang, W. and Eisenbrand, G., Chinese Drugs of Plant Origin, Springer Verlag, Berlin, 1992, pp. 711–737.
Yamada, H., Otsuka, H., and Kiyohra, H., Fractionation and characterization of anticomplimentary and mitogenic substances from Panax ginseng extract G-115, Phytother. Res., 9:264–269, 1995.
Glabridin
Glabridin is the major isoflavan in licorice root (Glycyrrhiza glabra) with two hydroxyl groups at the 2′ and 4′ positions, a 2,2-dimethyl-γ-pyran ring fused to the B ring with a double bond between carbon 3 and 4 in the C ring. The conjugated double-bond system present in glabridin appeared to enhance its antioxidant properties, which accounted for it being the most active compound isolated from licorice that inhibited LDL oxidation (Vaya et al., 1997: Belinsky et al., 1998). Tamir et al. (2000) demonstrated the estrogenic properties of glabridin, as well as its antiproliferative properties against human breast-cancer cells. Further research by Tamir and coworkers (2001) confirmed the estrogen-like properties of glabridin but also identified a new phytoestrogen, glabrene, an isoflavene in licorice roots exhibiting stronger estrogenic agonist activity. Glabridin also affects the skin, as Yokota and coworkers (1998) found it inhibited melanogenesis and inflammation in cultured B16 murine-melanoma cells.
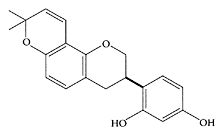
Glabridin. (From Fukai et al., Fitoterapia, 74:624–629, 2003. With permission.)
References
Belinsky, P.A., Aviram, M., Mahmood, S., and Vaya, J., Structural aspects of the inhibitory effect of glabridin on LDL oxidation, Free Rad. Biol. Med., 24: 1419–1429, 1998.
Fuai, T., Satoh, K., Nomura, T., and Sakagami, T., Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra, Fitoterapia, 74:624–629, 2003.
Tamir, S., Eizenberg, M., Somjen, D., Israel, S., and Vaya, J., Estrogen-like activity of glabrene and other constituents isolated from licorice root, J. Steroid Biochem. Mol. Biol., 78:291–298, 2001.
Tamir, S., Eizenberg, M., Somjen, D., Stern, N., Shelach, R., Kiaye, A., and Vaya, J., Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells, Cancer Res., 60:5704–5709, 2000.
Vaya, J., Belinsky, P.A., and Aviram, M., Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity toward LDL oxidation, Free Rad. Biol. Med., 23:302–313, 1997.
Yokota, T., Nishi, H., Kubota, Y., and Mizoguchi, M., The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation, Pigment Cell Res., 11:355–361, 1998.
Glucosamine sulfate
Glucosamine sulfate is a commonly used treatment for osteoarthritis. A three-year, randomized, placebo-controlled, double-blind study of 202 patients with knee osteoarthritis by Pavelka and coworkers (2002) showed that long-term treatment with glucosamine sulfate retarded the progression of the disease. Unlike systemic inflammatory diseases, such as rheumatoid arthritis, osteoarthritis is characterized by local inflammatory activity. A recent review by Amin et al. (1999) pointed to a local increase in the proinflammatory cytokine, interleukin-1 β (IL-1β) during progression of osteoarthritis. IL-β initiates a whole series of events leading to cartillage damage, including activation of nuclear factor kappa B (NfκB). Using cell cultures of human osteoarthritic chondrocytes, Largo and coworkers (2003) showed glucosamine significantly inhibited NfκB activity, as well as the nuclear translocation of p50 and p65 proteins involved in the inflammatory process. The use of glucosamine and chondroitin sulfate for the symptomatic treatment of osteoarthritis has been controversial. Nevertheless, a recent review by Hungerford and Jones (2003) pointed to the many in vitro and in vivo animal clinical and human clinical studies that confirm both the efficacy and safety of their treatment of osteoarthritis.
References
Amine, A.R., Attur, M.G., and Abramson, S.B., Regulation of nitric oxide and inflammatory mediators in human osteoaithritis-affected cartilage: Implication for pharmacological intervention, in The Pathophysiology and Clinical Applications of Nitric Oxide, Rubanyi, Ed., Harwood Academic Publishers, Newark, New Jersey, 1999, pp. 397–413.
Hungerford, D.S. and Jones, L.C., Glucosamine and chondroitin sulfate are effective in the management of osteoarthritis, J. Arthroplasty, 18:5–9, 2003.
Largo, R., Alvarez-Soria, M.A., Diez-Ortego, I., Calvo, E., Sanchez-Pernaute, O., Egido, J., and Herrero-Beaumont, G., Glucosamine inhibits IL-1β-induced NFκB activation in human osteoarthritic chondrocytes, Osteoarth. Cart., 11:290–298, 2003.
Pavelka, K., Gatterova, J., Olejarova, M., Machacek, S., Giacovelli, G., and Rovati, L.C., Glucosamine use and delay of progression of knee osteoarthritis, a 3 year, randomized, placebocontrolled, double-blind study, Arch. Intern. Med., 162:2113–2123, 2002.
Glucosinolates
The reduced risk of colorectal cancer associated with the consumption of cruciferous vegetables is attributed to the presence of a group of secondary plant metabolites known as glucosinolates (Verhoeven et al., 1996). These bioactive, sulfur-containing components are hydrolyzed by an endogenous plant enzyme myrosinase (thioglucoside glucohydrolase EC 3.2.3.1) to isothiocyanates. This only occurs when the cells are broken or damaged by cutting or chewing, as myrosinase and glucosinolates are separated from each other in the intact plant. Glucosinolates are broken down into isothiocyanates, nitriles, thiocyanates, indoles, and oxazolidinethiones (Scheme G.27). Some of these degradation products, particularly the isothiocyanates (ITCs) and some indolic compounds, have health-protective effects. A typical glucosinolate is singrin, which was shown to reduce the number of precancerous lesions in a dimethylhydrazine (DMH)-induced rat colon cancer model (Smith et al., 1998). Lund and coworkers (2001) compared the ability of four isothiocyanates (ITC), benzyl-ITC, allyl-ITC (AITC), phenylethyl-ITC (PEITC), and methyl-sulphinylbutyl-ITC (sulforaphane), to induce apoptosis in colorectal adenocarcinoma cells (HT9). The relative potency of these compounds to reduce adherent cell number was BITC=AITC> PEITC>>sulforaphane. The primary action of these ITCs was to block rapidly proliferating cancer cells at G2/M. These researchers commented that reducing the levels of glucosinolates by breeding to reduce the hot and bitter flavors associated with their degradation products might impair the health benefits associated with these vegetables.
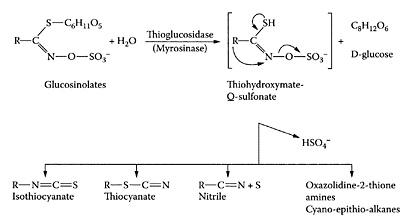
SCHEME G.27 Chemical structures of glucosinolates and their breakdown products following enzymic hydrolysis by myrosinase (Adapted from Pessina et al., Arch. Biochem. Biophys., 280:383–389, 1990, by Steinkeller et al., Mutat. Res., 480–481:285–287, 2001. With permission.)
Glucosinolates are activators of liver-detoxification enzymes; their mode of protection appears to involve modulation of carcinogen metabolism by inducing phase II detoxification enzymes, while inhibiting phase I detoxification enzymes (Hecht, 1999). Shapiro et al. (1980) showed substantial amounts of isothiocyanates were converted to dithiocarbamates. Smith and coworkers (1998) observed that the purified glucosinolate, sinigrin, reduced the number of precancerous lesions in rat colon induced by dimethylhydrazine (DMH) and was associated with an increase in apoptosis within 48 hours of exposure to DMH. The underlying mechanism responsible for cell death induced by four isothiocyanates (ITCs), benzyl-ITC, allyl-ITC, phenylethyl-ITC, and methylsulphinylbutyl-ITC (sulforaphane), was studied by Lund et al. (2001). The primary mechanism involved blocking the rapidly proliferating colorectal adenocarcinoma (HT29) cancer cells in G2/M.
References
Hecht, S.S., Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism, J. Nutr., 129:768S-774S, 1999.
Lund, E.K., Smith, T.K., Clarke, R.G., and Johnson, I.T., Cell death in the colorectal cancer cell line HT29 in response to glucosinolate metabolites, J. Sci. Food Agric., 81:959–961, 2001.
Pessina, A., Thomas, R.M., Palmieri, S., and Lussi, P.L., An improved method for purification of myrosinase and its physicochemical characterization, Arch. Biochem. Biophys., 280:383–389, 1990.
Smith, T.K., Lund, E.K., and Johnson, I.T., Inhibition of dimemylhydrazine-induced aberrant crypt foci and induction of apoptosis in rat colon following oral administration of the glucosinolate sinigrin, Carcinogenesis, 19:267–273, 1998.
Steinkeller, H., Rabot, S., Freyald, C., Nobis, E., Scharf, G., Chabicovsky, M., Knasmuller, S., and Kassie, F., Effects of cruciferous vegetables and their constituents on drug metabolizing enzymes involved in the bioactivation of DNA-reactive dietary carcinogens, Mutat. Res., 480– 481:285–297, 2001.
Verhoeven, D.T., Goldbohm, R.A., Poppel, G., Verhagen, H., and van den Brandt, P.A., Epidemiological studies on brassica vegetables and cancer risk, Cancer Epidemiol. Biomarkers Prevention, 5:733–748, 1996.
Glycyrrhin and 18 β -Glycyrrhetinic acid
Glycyrrhin, a pentacyclic triterpene derivative, is the main, water-soluble constituent of licorice roots (Glycyrrhizza glabra L.). Besides being a sweetening and flavoring agent in foods, glycyrrhin has been used in Asia and Europe for many years as an antidote, demulcent, and folk medicine. Glycyrrhin is hydrolyzed by the intestinal bacterial glucuronidase to the corresponding aglycone, 18β-glycyrrhetinic acid, which is then absorbed by the body. Both glycyrrhin and its aglycone have been associated with a number of health benefits, including antiulcerative (Doll et al., 1962), anti-inflammatory (Ohuchi et al., 1981), antiviral (Ito et al., 1988), antihepatitis (Kiso et al., 1984), and antitumor (Suzuki et al., 1992) properties. Jeong and coworkers (2002) recently showed that the potent hepatoprotective properties of 18β-glycerrhetinic acid were due to its ability to block bioactivation of carbon tetrachloride by inhibiting cytochrome P450 2E1 expression.
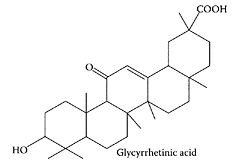
Glycyrrhetinic acid. (From Wang et al., J. Chromatogr. A., 811:219– 224, 1998. With permission.)
References
Doll, R., Hill, I.D., Mutton, C., and Underwood, D.J., Clinical trial of a triterpenoid licorice compound in gastric and duodenal ulcer, Lancet, 2:793–796, 1962.
Itoh, M., Sato, A., Hirabayashi, K., Tanabe, F., Shigeta, S., Baba, M., De Clercq, E., Nakashima, H., and Yamamoto, N., Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV), Antiviral Res., 10:289–298, 1988.
Jeong, H.G., You, H.J., Park, S.J., Moon, A.R., Chung, Y.C., Kang, S.K., and Chun, H.Y., Hepato-protective effects of 18-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: Inhibition of cytochrome P450 2E1 expression, Pharmacol. Res., 46: 221–227, 2002.
Kiso, Y., Tohkin, M., Hikino, H., Hattori, M., Sakamoto, T., and Namba, T., Mechanism of antihepatotoxic activity of glycyrrhizin. I. Effect of free radical generation and lipid peroxidation, Planta Med., 50: 298–302, 1984.
Ohuchi, K., Kamada, Y., Levine, L., and Tsurufuji, S., Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats, Prostaglandin Med., 7:457–463, 1981.
Suzuki, F., Schmitt, D.A., Utsunomiya, T., and Pollard, R.B., Stimulation of host resistance against tumors by glycyrrhin, an active component of licorice roots, In-Vivo, 6:589–596, 1992.
Vaya, J. Bellinky, P.A., and Aviram, M., Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity towards LDL oxidation, Free Rad. Biol. Med., 23:302–313, 1997.
Glycyrrhizic acid
Glycyrrhizic acid, another component of licorice root, was found to be active against a number of viruses, such as herpes simplex type 1, varicella-zoster virus, human cytomegalovirus, hepatitis A, B, and C viruses, human immunodeficiency virus-1, and influenza virus (Crance et al., 1994; Sato et al., 1996; Arase et al., 1997; Utsunomiya et al., 1997). Recently, Lin and coworkers (2003) showed glycyrrhizic acid inhibited the replication of the Epstein-Barr virus, which differed from mode of action of nucleoside analogues, which inhibit DNA polymerase. The ability of glycyrrhizic acid to protect against aflatoxin-induced oxidative stress in hepatoma cells was recently reported by Chan and coworkers (2003).
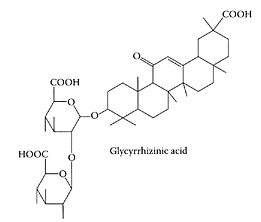
Glycyrrhizic acid. (From Wang et al., J. Chromatogr. A., 811:219–224, 1998. With permission.)
References
Arase, Y., Ikeda, K., Murashima, N., Chayama, K., Tsubota, A., Koida, I., Suzuki, Y., Saitoh, S., Kobayashi, M., and Kumada, H., The long term efficacy of glycyrrhizin in chronic hepatitis C patients, Cancer, 79:1494–1500, 1997.
Chan, H., Chan, C., and Ho, J.W., Inhibition of glycyrrhizic on aflatoxin B1 -induced cytoxicity in hepatoma cells, Toxicology, 188:211–217, 2003.
Crance, J.M., Leveque, F., Biziagos, E., van CuyckGandre, H., Jouan, A., and Deloince, R., Studies on mechanism of action of glycyrrhizin against hepatitis A virus replication in vitro, Antiviral Res., 23:63–76, 1994.
Lin, J.-C., Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro, Antiviral Res., 59:41–47, 2003.
Sato, H., Goto, W., Yamamura, J., Kurokawa, M., Kageyama, S., Takahara, T., Watanabe, A., and Shiraki, K., Therapeutic basis of glycyrrhizin on chronic hepatitis B, Antiviral Res., 30:171– 177, 1996.
Utsunomiya, T., Kobayashi, M., Pollard, R.B., and Suzuki, F., Glycyrrhizin an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus, Antimicrob. Agents Chemother., 41:551–556, 1997.
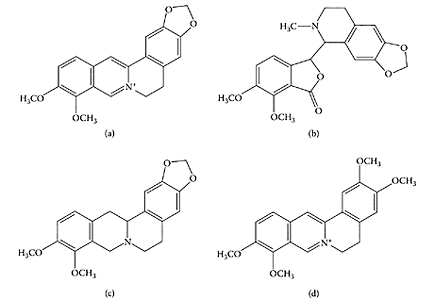
SCHEME G.28 Goldenseal isoquinoline alkalioids: berberine (a); hydrastine (b); canadine (c); palmatine (d). (From Weber et al., J. Agric. Food Chem., 51:7352–7358, 2003. With permission.)
Goldenseal (Hydrastis canadensis L.)
Goldenseal (Hydrastis canadensis L.) is a perennial, herbaceous plant native to eastern North America. It is a medicinal herb used primarily for its antimicrobial properties (Diamond and Towers, 1999; Upton, 2001). Goldenseal is a very popular supplement and is often sold together with Echinacea. The dried roots or rhizome of goldenseal contain a number of isoquinoline alkaloids, the major ones being hydrastine and berberine, together with smaller amounts of canadine and palmatine (Scheme G.28). The American Herbal Pharmacopoeai states that fresh or dried roots from goldenseal should not contain less than 2.5 percent berberine and 2.0 percent hydrastine, on a dry-weight basis (Upton, 2001). Berberine has been used to treat psoriasis and eye infections (Sabir et al., 1978; Muller et al., 1995).
Since goldenseal is used extensively in eyewashes and in skin lotions, Inbaraj and coworkers (2001) examined possible adverse interactions with light. In the presence of 50 μM berberine, UVA irradiation of transformed epidermal human-cell line, HaCaT keratinocytes, resulted in a decrease in cell viability of 80 percent, while tripling the amount of DNA damage. Based on these results, avoiding sunlight or artificial-light sources emitting UVA was recommended when using preparations containing goldenseal or berberine. However, these researchers pointed out that antiseptic properties associated with such topical preparations could be due to such interactions.
Rehman et al. (1999) showed that both Echinacea and goldenseal enhanced antibody production in rats, each acting on a different immunoglobulin subtype. Echinacea treatment improved the IgG immune response in rats one or two weeks after treatment with KLH, a novel antigen, keyhole limpet hemocyanin, while goldenseal augmented IgM response during the first two weeks of treatment. Such augmentation in antibody response could lead to identification of novel immune adjuvants.
References
Diamond, S. and Towers, G.H.N., in Herbs, Botanicals and Teas, Mazza, G. and Oomah, B.D., Eds., Technomic Publishing Co., Lancaster, Pennsylvania, 1999, pp. 177–211.
Inbaraj, J.J., Kukielczak, B.M., Bilski, Sandvik, S.L., and Chignell, C.F., Photochemistry and photocytotoxicity of alkaloids from goldenseal (Hydrastis Canadensis L.), 1. Berberine, Chem. Res. Toxicol., 14:1529–1534, 2001.
Muller, K., Ziereis, K. and Gawlik, I., The antipso riatic Mahonia aquifolium and its active constituents, II. Antiproliferative activity against cell growth of human keratinocytes, Planta Med., 61:74–75, 1995.
Rehman, J., Dillow, J.M., Carter, S.M., Chou, J., Le, B., and Maisel, A.S., Increased production of antigen-specific immunoglobulins G and M following in vivo treatment with Echinacea augustifolia and Hydrastis canadensis, Immunol. Lett., 68:391–395, 1999.
Sabir, M., Akhter, M.H., and Bhide, N.K., Further studies on the pharmacology of berberine, Ind. J. Physiol. Pharmacol., 22:9–23, 1978.
Upton, R., Goldseal root, Hydrastis canadensis, in Standards of Analysis, Quality Control and Therapeutics, American Herbal Pharmacopoeia, Santa Cruz, California, 2001.
Weber, H.A., Zart, M.K., Hodges, A.E., Molloy, H.M., O’Brien, B.M., Moody, L.A., Clark, A.P., Harris, R.K., Overstreet, J.D., and Smith, C.S., Chemical comparison of goldenseal (Hydrastis canadensis L.) root powder from three commercial suppliers, J. Agric. Food Chem., 51:7352– 7358, 2003.
Grapefruit
Grapefruit juice has been reported to interact and elevate the pharmacological efficacy of a number of medications, including cyclosporine (Ducharme et al., 1995), midazolam (Kupferschmidt et al., 1995), felodipine (Bailey et al., 1990), and lovastatin (Kantola et al., 1998). The bioavailability of these drugs was enhanced 1.5- to 15-fold following ingestion of grapefruit juice. The lipophilic nature of these drugs necessitates their oxidative transformation by cytochrome P450 (CYP3A4) prior to excretion. The major flavonoid in grapefruit is naringin, which, together with its aglycone naringenin, both inhibit CYP3A4, although naringenin was found to be the more potent inhibitor in vitro (Miniscalo et al., 1992). However, Schmiedlin-Ren et al. (1997) reported that the furanocoumarin derivative, dihydroxybergapten, was a far more potent inhibitor, which was later shown to have a very low IC40 (Ho et al., 1998). The marked differences between the levels of naringin, naringenin, and bergapten (5-methoxypsoralen) in grapefruit and grapefruit-juice products were attributed by Ho et al. (2000) to the contradictory drug-interaction results reported in the literature. Tassaneeyakul et al. (2000) showed that, in addition to the human microsomal CYP3A4, CYP2C19 was also inhibited by grapefruit extract or its isolated furancoumarins. Using the carageenan-induced paw oedema model, Mahgoub (2001) also reported grapefruit juice potentiated the effects of the nonsteroidal, anti-inflammatory drug diclofenac to inhibit other cytochrome P450 isoenzymes. A new CYP3A4 inhibitor, a furanocoumarin derivative, paradisin C, was recently identified by Ohta and coworkers (2002) in grapefruit juice (Scheme G.29).
In vitro studies by Goff-Klein et al. (2003) on human liver microsomes and rat hepatocytes and microsomes showed interspecies differences involved different CYP450 isoenzymes in the metabolism of simvastatin (SV), a drug used to treat hypercholesterolemia, in the presence of bergamottin, a grapefruit component. Inhibition of CPY450 isoenzymes by bergamottin can lead to prolonged exposure to SV with high serum simastavin-acid concentrations, increasing the risk of skeletal-muscle toxicity (Bogman et al., 2001). Prolonged exposure to some drugs may be beneficial, while drugs, such as simastavin, could be harmful. As a result, grapefruit is no longer served in hospitals.
Miyata et al. (2002) examined the effect of grapefruit juice on the heterocyclic amine, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), a component in cooked meat and fish implicated in the etiology of colon cancer. They showed that pretreatment of rats with grapefruit juice suppressed colon DNA damage using the comet assay. A 6.2-fold and 5.4-fold increase in migration of DNA and frequency of tailed nuclei were observed in colon nuclei of PhIP-treated rats compared to vehicle-treated rats (Figure G.45). In contrast, a reduction of 20 percent and 36 percent in DNA migration and frequency of tailed nuclei was evident in grapefruit juice-treated rats compared to the control rats, respectively.
SCHEME G.29 COSY (bold lines) and HMC (arrows) NMR Spectral data correlations observed for paradisin C. (From Ohta et al, Tetrahedron, 58:6631–6635, 2002. With permission.)
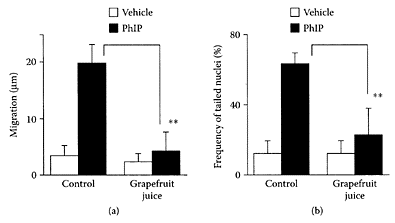
FIGURE G.45 Effect of pretreatment with grapefruit juice on PhIP-induced DNA damage in rat colon, (a) Migration, (b) Frequency of tailed nuclei. Rats had free access to grapefruit juice five days prior to administration of PhIP (60 mg/kg). Colon nuclei were isolated 3 h after PhIP treatment. Migration measured as the difference between the length of the whole comet and the diameter of the head. Values represent the mean± SD (n=3 in each group). **p<0.01, significant differences from corresponding control group. (From Miyata et al., Cancer Lett., 183:17–22, 2002. With permission.)
References
Bailey, D.G., Arnold, J.M.O., and Spence, J.D., Grapefruit juice-drug interaction, Br. J. Clin. Pharmacol., 46:101–110, 1998.
Bogmann, K., Peyer, A.K., Torok, M., Kusters, E., and Drewe, J., HMG-CoA reductase inhibitors and P-glycoprotein modulation, Br. J. Pharmacol., 132: 1183–1192, 2001.
Ducharme, M.P., Warbasse, L.H., and Edwards, D.J, Disposition of intravenous and oral cyclosporine after administration with grapefruit juice, Clin. Pharmacol Ther., 57:485–491, 1995.
Goff-Klein, N.L., Koffell, J.-C., Jung, L., and Ubeaud, G., in vitro inhibition of simvastatin metabolism, an HMG-CoA reductase inhibitor in human and rat liver by bergamottin, a component of grapefruit juice, Eur. J. Pharm. Sci., 18:31–35, 2003.
Ho, P.C., Saville, D.J., Coville, P.F., and Wanwimolruk, S., Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products, Pharm. Acta Helv., 74:379–385, 2000.
Ho, P.C., Wanwimolruk, S., and Saville, D.J., Inhibition of CYP3A4 in human liver microsomes by bioflavonoids and coumarin derivatives found in grapefruit juice, Pharmacol. Sci., 1:S38, 1998.
Kantola, T., Kivisto, K.T., and Neuvonen, P.J., Grapefruit juice greatly increases serum concentration of lovastatin and lovastatin acid, Clin. Pharmacol. Ther., 63:397–402, 1998.
Kupferschmidt, H.H.T., Ha, H.R., Ziegler, W.H., Meier, P.J., and Krahenbuhl, S., Interaction between grapefruit juice and oral midazolam in humans, Clin. Pharmacol. Ther., 58:20–28, 1995.
Mahgoub, A.A., Grapefruit juice potentiates the antiinflammatory effects of diclofenac on the carrageenan-induced rat’s paw model, Pharmacol. Res., 45: 1–4, 2002.
Miniscalco, A., Lundahl, J., Regardh, C.G., Edgar, B., and Eriksson, U.G., Inhibition of dihydropyridine metabolism in rat and human liver microsomes by flavonoids found in grapefruit juice, J. Pharmacol. Exp. Ther., 261:1195–1199, 1992.
Miyata, M., Takano, H., Takahashi, K., Sasaki, Y.F., and Yamazoe, Y., Suppression of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced DNA damage in rat colon after grapefruit juice intake, Cancer Lett., 183:17–22, 2002.
Ohta, T., Maruyama, T., Nagahashi, M., Miyamoto, Y., Hosoi, S., Kiuchi, F., Yamagoe, Y., and Tsukamoto, S., Paradisin C: A new CYP3A4 inhibitor from grapefruit juice, Tetrahedron, 58:6631–6635, 2002.
Schmiedlin-Ren, P., Edwards, D.J., Fitzsimmons, M.E., He, K., Lown, K.S., Woster, P.M., Rahman, A., Thummel, K.E., Fisher, J.M., Hollenberg, P.F., and Watkins, P.B., Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents—decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins, Drug Metab. Dispos., 25:1228–1233, 1997.
Tassaneeyakul, W., Guo, L-Q, Fukuda, K., Ohta, T., and Yamazoe, Y., Inhibition selectivity of grapefruit juice components on human cytochrome P450. Arch. Biochem. Biophys., 378:356– 363, 2000.
Grape juice
Grapes are one of the richest sources of phenolic compounds compared to other fruit (Machiex et al., 1990). They contribute flavonoids and polyphenolic tannins, in addition to nonflavonoid hydroxy-cinnamic acids, hydroxybenzoic acids, and stilbene. Many of these compounds were found to be potent antioxidants inhibiting the in vitro oxidation of LDL cholesterol. Frankel and coworkers (1998) found the commercial Concord and blends of grape juices had comparable inhibitory activity to that of red wine, with respect to the in vitro oxidation of human low-density lipoproteins. Using a hamster model of atherosclerosis, Vinson et al. (2001) showed grape juice was twice as effective in lowering cholesterol compared to dealcoholized wine or red wine, two to six times more effective in decreasing LDL, and twice as effective as red wine in preventing atherosclerosis (Figure G.46). Grape juice was a more efficient ex vivo antioxidant, as it was more effective in increasing the lag time by four times compared to either dealcoholized wine or red wine. Park et al. (2003) showed grape juice reduced total free-radical levels and DNA damage in 67 healthy Koreans (16 women and 51 men) maintained on a daily grape-juice regime of 480 mL pure grape juice twice a day for eight weeks. The results from this study combined, with antiplatelet and antioxidant benefits reported previously for grape consumption (O’Byrne et al., 2002; Vinson et al., 2000), suggests grape juice should become a regular part of a healthy diet.
References
Frankel, E.N., Bosanek, C.A., Meyer, A.S., Silliman, K., and Kirk, L.L., Commercial grape juices inhibit the in vitro oxidation of human low-density lipoproteins, J. Agric. Food Chem., 46:834– 838, 1998.
Machiex, J.J., Fleuriet, A., and Billot, J., The main phenolics of fruits, in Fruit Phenolics, CRC Press, Boca Raton, Florida, pp. 1–98, 1990.
O’Byrne, D.J., Devaraj, S., Grundy, S.M., and Jialal, I., Comparison of the antioxidant effects of concord grape juice flavonoids and α-tocopherol on markers of oxidative stress in healthy adults, Am. J. Clin. Nutr., 76:1367–1374, 2002.
Park, Y.K., Park, E., Kim, J.-S., and Kang, M.-H., Daily grape juice consumption reduces oxidative DNA damage and plasma free radical levels in healthy Koreans, Mutat. Res., 529:77–86, 2003.
Vinson, J.A., Teufel, K., and Wu, N., Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model, Atherosclerosis, 156:67–72, 2001.
Vinson, J.A., Yang, J., Proch, J., and Liang, X., Grape juice, but not orange juice, has in vitro, ex vivi, and ex vivo antioxidant properties, J. Med. Food, 2:167–171, 2000.
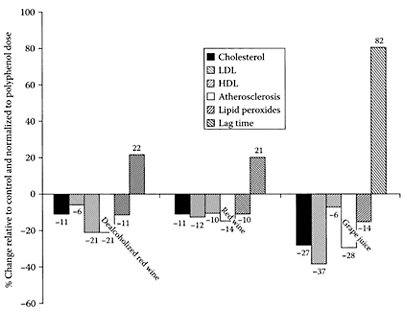
FIGURE G.46 Comparative polyphenol efficiency of beverages with respect to atherosclerosis, lipid, and antioxidant parameters. (Vinson et al., Atherosclerosis, 156:67–72, 2003. With permission.)
Grape seeds
Grape-seed extract is widely consumed in the United States as a dietary supplement because of a number of related health benefits associated with its bioflavonoids or procyanidins. Zhao et al. (1999) showed that a polyphenolic fraction from grape seeds exhibited antitumor-promoting activity by reducing tumor incidence in a 7,12-dimethylbenz-[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol 13 acetate (TPA)-promoted SENCAR mouse skin two-stage carcinogenesis model system. The antitumor-promoting activity was attributed to the potent antioxidant properties of the grape-seed polyphenols. Procyanidin B5–3′ gallate was identified with the most potent antioxidant activity and potential as a cancer chemopreventive and anticarcinogenic agent. Agarwal and coworkers (2000) also showed grape-seed extract inhibited growth and induced apoptosis in human prostate-cancer cells, both in culture and in nude mice. These researchers (Agawal et al., 2002) found grapeseed extract induced apoptosis in humanprostate carcinoma DU145 cells by activation of caspases.
Yamakoshi and coworkers (1999) showed that feeding a proanthocyanidin-rich grape-seed extract to cholesterol-fed rabbits significantly reduced severe atherosclerosis in the aorta. It decreased the number of oxidized LDL-positive, macrophage-derived foam cells in athertosclerotic lesions in the aorta of cholesterol-fed rabbits, as a result of its ability to trap reactive-oxygen species in aqueous solution. Shao et al. (2003) confirmed the cardioprotective activity of grape-seed proanthocyanidin by its ability to attenuate oxidant injury in chick embryonic ventricular myocytes. The molecular mechanisms involved in cardioprotection by a novel grape-seed proanthocyanidin extract (IH636) were shown by Bagchi et al. (2003) to include:
potent hydroxyl and other free-radicalscavenging activities
antiapoptotic, antinecrotic, and antiendonucleolytic potentials
modulation of apoptotic regulators, bcl-XL, p53, and c-myc genes
inhibition of cytochrome P450 2E1
inhibition of adhesion molecules
modulation of proapoptotic and cardioregulatory genes, c-JUN, JNK-1, and CD36.
References
Argawal, C., Sharma, Y., and Argawal, R., Anticarcinogenic effect of a polyphenolic fraction isolated from grape seeds in human protate carcinoma DU145 cells: Modulation of mitogenic signaling and cell cycle regulators and induction of G1 arrest and apoptosis, Mol. Carcinog., 28:129–138, 2000.
Agarwal, C., Singh, R.P., and Agarwal, R., Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release, Carcinogenesis, 23:1869–1876, 2002.
Bagchi, D., Sen, C.K., Ray, S.D., Das, D.K., Bagchi, M., Preuss, H.G., and Vinson, J.A., Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract, Mutat. Res., 523–524:87–97, 2003.
Shao, Z.-H., Becker, L.B., Vanden Hoek, T.L., Schumacker, P.T., Li, C.-Q., Zhao, D., Wojcik, K., Anderson, T., Qin, Y., Dey, L., and Yuan, C.-S., Grape seed proanthocyanidin extract attenuates oxidant injury in cardiomyocytes, Pharmacol. Res., 47:463–469, 2003.
Yamakoshi, J., Kataoka, S., Koga, T., and Ariga, T., Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits, Atherosclerosis, 142:139–149, 1999.
Zhao, J., Wang, J., Chen, Y., and Agarwal, R., Antitumor promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol, and identification of procyanidin B5–3′-gallate as the most effective antioxidant constituent, Carcinogenesis, 20: 1737–1745, 1999.
Green tea
Green tea, a popular beverage in Japan made from the leaves of Camellia sinensis, is recognized for its health benefits. It is a nonfermented product obtained by leaf dessication that contains potent, polyphenolic antioxidants, with a flavan-3-olic structure, referred to as green-tea catechins. They include seven types, (−)-gallocatechin (GC), (−)-epigallocat-echin (EGC), (+)-catechin (C), (−)-epigallocat-echin-3-gallate (EGCG), (−)-epigallocatechin (EC), (−)-gallocatechingallate (GCG), and (−) epicatechingallate (ECG) (Bonoli et al., 2003).
Many studies have shown that drinking EGCG and green tea prevents carcinogenesis in rodent organs (Wang et al., 1992; Fujiki, 2002). Kavanagh et al. (2001) showed green tea had a significant chemoprotective effect against 7, 12-dimethyl(a)anthracene (DMBA)-induced mammary tumorigenesis in Sprague-Dawley rats. Inhibition of human breast cancer Hs578T cell proliferation by green tea appeared to be mediated, in part, by induction of p27Kip cyclindependent kinase inhibitor (CKI) expression. Gupta et al. (2002) summarized the antimutagenic and anticlastogenic properties of green and black teas. Green tea inhibited mutagenesis at concentration levels equivalent to human daily consumption. Using human umbilicalvein endothelial cells, Kojima-Yuasa et al. (2003) demonstrated, for the first time, that green-tea extracts reduced expression of vascular endothelial growth factor (VEGF) receptors fms-like tyrosine kinase (Flt-1) and fetal liver kinase-1/Kinase insert domain containing receptor (Flk-1/KDR). The antiangiogenic property of green-tea extracts has therapeutic potential in preventing the development of new microvascular networks (angiogenesis) needed for tumor growth. Maiti and coworkers (2003) also found green-tea polyphenols inhibited angiogenesis by reducing vascularization of chicken chorioallantoic membrane (CAM) by an angiogenin-like protein isolated from goat serum. Kemberling et al. (2003) showed greentea EGCG effectively inhibited bladder-tumor implantation growth in a Fischer 344 rat model, pointing to its potential as an intravesical chemotherapeutic agent.
Reducing the risk of coronary artery disease is associated with a number of factors, including inhibition of platelet function. EGCG was reported to inhibit platelet aggregation, possibly by involving inhibition of cytoplasmic calcium increase (Kang et al., 1999). Lill and coworkers (2003) found that only those green catechins with a galloyl group in the 3′ position inhibited platelet aggregation, while those without a galloyl group (catechin and epicatechin) or with the galloyl group in the 2′ position (epigallo-catechin) did not. EGCG proved to be the most effective in reducing thrombrin-induced aggregation of washed human platelets (Figure G.47).
TABLE G.36
The Antiviral Effect of EGCG and Green Tea
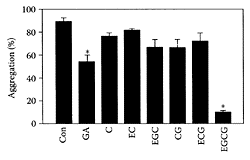
FIGURE G.47 Effect of various green-tea catechins (100 μmol/L) on thrombrin-induced platelet aggregation (means±S.E.M., n=5, *p<0.05 versus control). (From Lill et al., FEBS Lett., 546:265–270, 2003. With permission.)
The ability of green-tea catechins to inhibit adenovirus infection and adenain, the human adenovirus 2 endopeptidase, was reported by Weber and coworkers (2003). EGCG proved the most potent inhibitor of four green-tea catechins tested with a IC50 of 200 μM (Table G.36). Since the viral protease, adenain, appeared to be the target of EGCG, it is possible that all adenoviruses are sensitive to its action.
References
Bono, M., Colabufalo, P., Pelillo, M., Toschi, T.G. and Lerker, G., First determination of catechins and xanthines in tea beverage by micellar electrokinetic chromatograph, J. Agric. Food Chem., 51:1141–1147, 2003.
Fujiki, H., Two stages of cancer prevention with green tea, J. Cancer Res. Clin. Oncol., 125:589– 597, 1999.
Fujiki, H., Suganuma, M., Imai, K., and Nakachi, K., Green tea: Cancer preventive beverage and/or drug, Cancer Lett., 188:1–2, 9–13, 2002.
Gupta, S., Saha, B. and Giri, A.K., Comparative antimutagenic and anticlastogenic effects of green tea and black tea. Mutat. Res., 412:37–65, 2002.
Kang, W.S., Lim, I.H., Yuk, D.Y., Chung, K.H., Park, J.B., Yoo, H.S. and Yu, Y.P., Antithrombotic activities of green tea catechins and C-1-epigallocatechin gallate, Thromb. Res., 96:229–237, 1999.
Kavanagh, K.T., Hafer, L.J., Kim, D.W., Mann, K.K., Sherr, D.H., Rogers, A.E. and Sonenshein, G.E., Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture, J. Cell Biochem., 82:387–398, 2001.
Kemberling, J.K., Hampton, J.A., Keck, R.W., Gomez, M.A. and Selman, S.H., Inhibition of bladder tumor growth by the green tea derivative epigallocatechin-3-gallate, J. Urol., 170:773– 776, 2003.
Lill, G., Voit, S., Schror, K., and Weber, A.-A., Complex effects of different green tea catechins on human platelets, FEBS Lett., 546:265–270, 2003.
Maiti, T.K., Chatterjee, J. and Dasgupta, S., Effect of green tea polyphenols on angiogenesis induced by an angiogenin-like protein, Biochem. Biophys. Res. Commun., 308:64–67, 2003.
Toschi, T.G., Bordoni, A., Hrelia, S., Bendini, A., Lercker, G., and Biagi, P.L., The protective role of different green tea extracts after oxidative damage is related to their catechin composition, J. Agric. Food Chem., 48:3973–3978, 2000.
Wang, Z.Y., Hong, J.-Y., Huang, M.T., Ruehl, K.R., Conney, A.H., and Yang, C.S., Inhibition of N-nitrosodiethylamine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced tumorigenesis in A/J mice by green tea and black tea, Cancer Res., 52: 1943–1947, 1992.
Weber, J.M., Ruzindana-Umunyana, A., Imbeault, L., and Sircar, S., Inhibition of adenovirus infection and adenain by green tea catechins, Antiviral Res., 58:167–173, 2003.
Guava
Guava (Psidium guajava L.) is an evergreen native to Mexico and Cenral American countries. Guava leaves, roots, and fruits have been used to prevent and treat diarrhea (Lutterodt, 1989) and diabetes (Cheng and Yang, 1983). Studies have also reported that guava has antimutagenic activity, including the identification of (+)-gallocatechin, an antimutagenic compound in guava leaves (Grover and Bala, 1993; Matsuo et al., 1993). Popular Mexican medicine recommends the use of guava leaf water decoction to treat acute diarrhea, colic, flatulence, and gastric pain (Aguilar et al., 1994). Arima and Danno (2002) recently isolated four antibacterial compounds in guava leaves. In addition to guajavarin and quercetin, two new flavonoid glycosides were characterized. The latter were identified as morin-3-O-α-L-lyxopyranoside and morin-3-O-α-L-arabopyranoside, both of which were effective against Salmonella enteritidis and Bacillus cereus. Flavonoids, such as quercetin, are also associated with spasmolytic effect and antidiarrheic capacity of guava-leaf products. Lozoya and coworkers (2002) conducted a randomized, double-blinded clinical study that established the safety and efficacy of a phytodrug made from guava leaves standardized in its quercetin content. Decreased abdominal pain was experienced by adult patients suffering from acute diarrheic disease after taking a capsule containing 500 mg of the product every eight hours for three days. The research ers suggested it could be a useful alternative as an antispasmodic product.
References
Aguilar, A., Argueta, A., and Cano, L., Flora medicinal indigena de Mexico, Treinta y cinco monografias del Atlas de las Plantas de la Medicina Tradicional Mexicana, INI, Mexico, 245, 1994.
Arima, H. and Danno, G., Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation, Biosci. Biotechnol. Biochem. 66:1727–1730, 2002.
Cheng, J.T. and Yang, R.S., Hypoglycemic effect of guava juice in mice and human subjects, Am. J. Chin. Med., 11:74–76, 1983.
Grover, I.S. and Bala, S., Studies on antimutagenic effects of guava (Psidium guajava) in Salmonella typhimurium, 300:1–4, 1993.
Lozoya, X., Reyes-Morales, H., Chavez-Soto, M.A., Martinez-Garcia, M del C., Soto-Conzalez, Y., and Doubova, S.V., Intestinal anti-spasmodic effect of a phytodrug of Psidium guajava folia in the treatment of acute diarrheic disease, J. Ethnopharmacol., 83: 19–24, 2002.
Lutterodt, G.D., Inhibition of gastrointestinal release of acetylcholine by quercetin as a possible mode of action of Psidium guajava leaf extracts in the treatment of acute diarrhea disease, J. Ethnopharmacol., 25:235–247, 1989.
Matsuo, T., Hanamura, N., Shimoi, K., Nakamura, Y., and Tomita, I., Identification of (+)- gallocatechin as a bio-antimutagenic compound in Psidium guajava leaves, Phytochemistry, 36:1027–1029, 1993.
Gum acacia
see Acacia gum
Guar gum
Guar gum, a soluble fiber extracted from the endosperm of the Indian cluster bean (Cyanopsis tetragonoloba L.), is a summer legume grown mainly in Western India and Eastern Pakistan. It is a nongelling gum similar to locust-bean gum used extensively in the food industry. Guar gum is a galactomannan in which the molar ratio of galactose to mannose is approximately 1:2. Viscous fibers are very effective for glycemic control (Jenkins et al., 1979; Wolever et al., 1979; Wursch and PiSunyer, 1997). Thus, guar gum, a viscous fiber, is considered to be effective as long as it is not hydrolyzed (Cabre, 2004). A partially hydrolyzed guar gum was shown by Slavin and Greenberg (2003) to have a number of clinical uses, including reducing the incidence of diarrhea in septic patients maintained on enteral nutrition, reducing symptoms of irritable-bowel syndrome, and increasing bifidobacterium in the gut.
Early studies by Jenkins et al. (1979) suggested guar gum could lower total plasma total cholesterol and LDL cholesterol. Castro and coworkers (2003), however, were unable to find any changes in serum lipids of hypocholesterolemic rats maintained on a diet containing 1.5 percent of several hydrocolloids, including guar gum.
The suggestion that guar gum may be ben eficial for reducing weight is attributed to increasing viscosity of the bowel contents and the feeling of postprandial satiety (Blackburn et al., 1984; Van de Yen et al., 1994). Based on a number of clinical trials, guar gum is recommended for treating obese patients. Pittler and Ernst (2001) conducted a meta-analysis of randomized trials in which dietary guar gum was used for reducing body weight. Based on their findings they could not recommend guar gum as a treatment for reducing body weight.
References
Blackburn, N.A., Holgate, A.M., and Read, N.W., 1984. Does guar gum improve post-prandial hyperglycaemia in human by reducing small intestine area, Br. J. Nutr., 52:197–204, 1984.
Cabre, E., Fibre supplementation of enetral formuladiets: a look to the evidence, Clin. Nutr. Suppl., 1: 63–74, 2003
Castro, I.A., Tirapegui, J., and Benedicto, M.L., Effect of diet supplementation with three soluble polysaccharides on serum lipid levels of hypercholesterolemic rats, Food Chem., 80:323–330, 2003.
Jenkins, D.J, Wolever, T.M.S., Leeds, A.R., Gassull, M.A., Haisman, P., Dilawari, J., Goff, D.K., Metz, G.L., and Alberti, K.G.M.M., Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity, Br. Med. J., 1:1392–1394, 1978.
Jenkins, D.J.A., Leeds, A.R., Slavin, B., Mann, J., and Jepson, E.M., Dietary fiber and blood lipids: Reduction in serum cholesterol in type II hyperlipidemia by guar gum, Am. J. Clin. Nutr., 32:16–18, 1979.
Pittler, M.H. and Ernst, E., Guar gum for body weight reduction: Meta-analysis of randomized trials, Am. J. Med., 110:724–730, 2001.
Slavin, J.L. and Greenberg, N.A., Partially hydrolyzed guar gum: Clinical nutrition uses, Nutrition, 19:549–552, 2003.
Van de Ven, M.L.H.M., Westerterp-Plantenga, M.S., Wouters, L., and Saris, W.H.M., Effects of liquid preloads with different fructose/fibre concentrations on subsequent food intake and ratings of hunger in women, Appet., 23:129–146, 1994.
Wolever, T.M.S., Jenkins, D.J., Nineham, R., and Alberti, K.G., Guar gum and reduction of postprandial glycaemia: Effect of incorporation into solid food, liquid food and both, Br. J. Nutr., 41:505–510, 1979.
Wursch, P. and Pi-Sunyer, F.X., The role of viscous soluble fiber in metabolic control of diabetes: A review with special emphasis on cereals rich in beta glucan, Diabet. Care, 20:1774–1780, 1997.