21
Cancer Prevention and Screening
TODAY, HEART DISEASE is the leading cause of death in the United States, with cancer as the perpetual runner-up in this morbid race. But that may soon change. From 1969 to 2014, death from heart disease declined by 68.4 percent among men and 67.6 percent in women, due to both improved prevention and improved treatment.1
Yet, over that same period, cancer deaths declined by a relatively minimal 21.9 percent for men and 15.6 percent for women—less than one third the rate at which heart disease declined (see Figures 21.1 and 21.2). In 1969, the risk of dying from heart disease was two to three times higher than the risk of dying from cancer. In 2019, those risks were virtually equal.2 As recently as 2000, cancer was the leading cause of death in only two states (Alaska and Minnesota). In 2014, fully twenty-two states reported that cancer was the leading cause of death.3 According to the American Cancer Society, the lifetime risk of being diagnosed with cancer is more than one in three,4 and worse, many of the obesity-related cancers are rising in prevalence and considered avoidable.
While progress against cancer lags far behind that for heart disease, there are still some bright spots. Total cancer deaths peaked in 1991 and fell steadily by 27 percent in the twenty-five years to 2016, due mostly to the reduction in lung cancer deaths thanks to the increased regulation of the tobacco industry and effective antismoking campaigns.

Figure 21.1

Figure 21.2
Prevention is the surest route to defeating cancer and is responsible for this significant victory in the reduction in lung cancer deaths. The popularity of smoking tobacco in the United States began to increase around 1900, gaining momentum during World Wars I and II, and peaked in 1964, when 42 percent of Americans smoked.5 Smoking was fashionable, common, and seemingly harmless. Many physicians smoked, along with much of the rest of the male population. Smoking in women was decidedly less popular in the 1960s, but it gained steam afterward, ironically as a symbol of female empowerment.
The seminal event in the history of cancer prevention was the 1964 declaration by Luther Terry, then the surgeon general of the U.S. Public Health Service, that smoking caused lung cancer. Cigarette smoking is estimated to account for 81 percent of lung cancer (see Figure 21.3). Terry, himself a long-term smoker, almost singlehandedly saved hundreds of millions of lives.
Public perception of smoking changed gradually but irrevocably after the 1964 report. Just a year later, new legislation mandated that warning labels be placed on cigarette packaging, to let consumers know that smoking was hazardous to their health. Other public health measures included restricting cigarette advertising, especially to young people. Smoking rates in the United States steadily declined over the ensuing years, reaching 15.5 percent by 2016.6
Lung cancer deaths followed an identical trajectory, delayed by the twenty-five years or so that it takes to develop cancer. From 1990 to 2016, lung cancer deaths fell 48 percent in men. Smoking in women started later than in men, and therefore showed a slower uptake and decline.
But cigarette smoking contributes to more than just lung cancer. The chronic irritation caused by tobacco smoke causes at least twelve other cancers and increases the risk of heart disease, stroke, and chronic lung disease.7

Figure 21.3
Lung cancer was still responsible for most cancer deaths in 2019, for both men and women, although the numbers are dropping significantly. There is little doubt about the best way to treat lung cancer: stop smoking. Nothing else comes anywhere close. It’s a matter of execution, not knowledge.
Cancers caused by infectious agents like bacteria and viruses have also been declining steadily. Stomach cancer has fallen steadily since 1930, as improving public sanitation dramatically decreased the prevalence of H. pylori. Overcrowding and poor sanitation persisted in Asia for much of the first half of the twentieth century, leading to endemic H. pylori infection and high rates of stomach cancer. In Japan, for example, death from stomach cancer did not start to fall until the mid-1960s.8
Liver cancer fell steadily in America from 1930 to 1980, due to major advances in the identification and prevention of the viruses hepatitis B and C. Both viruses are less common in America compared to Asia, where hepatitis B has stayed endemic due to maternal transmission. Deaths from liver cancer in Japan did not peak until the 1990s.9 Today, widespread vaccination for hepatitis B and effective antiviral medications for hepatitis C have led to the hope that liver cancer may follow its persistent downtrend.
The recent news on liver cancer is not good, but for a completely different reason. Over the last forty years in the United States, liver cancer diagnoses have tripled and deaths have more than doubled. There is little mystery as to why this is so: liver cancer is one of the obesity-related cancers, with obese and overweight individuals suffering almost double the risk compared to healthy-weight individuals.10 Fatty liver disease can cause chronic inflammation, leading to cirrhosis and liver cancer. Pancreatic cancer, also an obesity-related cancer, is increasing in incidence, by about 1 percent per year from 2006 to 2015.
SCREENING
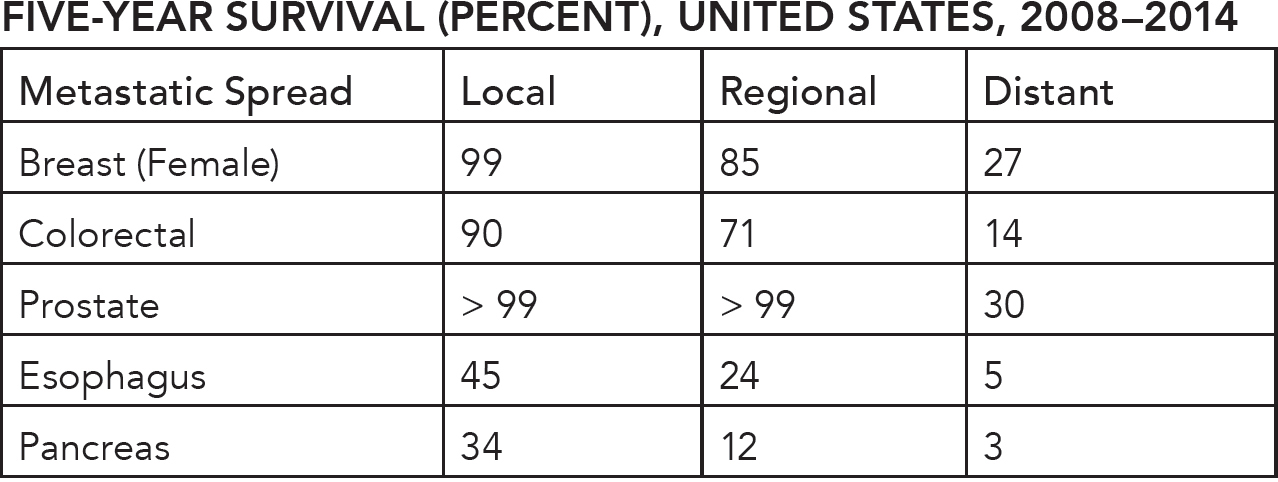
After lung cancer, the next biggest cancer killers are prostate cancer (men) or breast cancer (women) and then colorectal cancer in third place. In all three types of cancer, no single major causative agent has yet been identified, although breast and colon cancer are the two most prominent obesity-related cancers. Without knowing causation, these cancers cannot be entirely prevented. The next best step may be early detection and treatment through screening. When they are caught early, the five-year survival rate for breast, colorectal, and prostate cancer exceeds 90 percent (see Figure 21.4).
Once the cancers become metastatic, however, these survival rates drop to less than 30 percent. The key, then, is to reduce the number of patients who present with late-stage disease. One well-tested strategy is to screen for early disease with the hope that we can reduce the number of people presenting with the more lethal, late-stage disease. While several large population-based screening programs have been wildly successful in reducing the burden of cervical and colorectal cancer, screening for three other common cancers (breast, prostate, and thyroid) have been decidedly less successful. Unfortunately, simply catching more early-stage disease is not useful by itself. In the first two cases (breast and colorectal cancer), screening has reduced late-stage disease, but in the latter three cases (prostate, esophageal, and pancreatic cancer) it has not, and that makes all the difference in the world.
American Cancer Society, Facts & Figures, 2019.
Figure 21.4
CERVICAL CANCER
Deaths from cervical cancer have dropped dramatically since the 1940s, largely due to the introduction of the Pap smear. It’s now known that 70 percent of cervical cancer is attributable to two strains (16 and 18) of the human papillomavirus (HPV), a disease transmitted largely through sexual contact. After infection by these cancer-causing subtypes of HPV, the cervix sheds abnormal cells for several years prior to the development of invasive cervical cancer.11
In 1928, gynecologist Dr. George N. Papanicolaou discovered that when the cervix was scraped with a small brush and the sample cells obtained were examined under a microscope, previously hidden cancer cells could be detected.12 Women had no symptoms at this stage, and felt entirely well. By 1939, Papanicolaou was routinely sampling cells from all women admitted to the obstetrical and gynecological service at his New York hospital. In 1941, his landmark research paper described how the “Pap” smear could detect unsuspected precancerous lesions.13 With the cancer diagnosed early, removal of these lesions halted further progression toward a full-blown cancer. Today, cervical cancer serves as the poster child of screening programs, being one of the earliest and most successful cancer interventions to date.
Throughout the 1940s and ’50s, the American Cancer Society enthusiastically promoted the Pap smear for mass screening. It trained physicians and pathologists in its use and established a series of cancer detection clinics, focused mainly on cervical cancer.14 Deaths from cervical cancer dropped an estimated 71 percent from 1969 to 2016. The good news is that large-scale, population-based vaccination programs have been developed and approved in many countries. Preventing viral transmission of HPV 16 and 18 promises to lower the rate of cervical cancer even further.
COLORECTAL CANCER
Population-based screening for colorectal cancer is another great example of how to increase the odds of successful cancer treatment, as deaths from colorectal cancer have trended steadily downward since the mid-1980s, due largely to population-wide screening programs.
In 1927, researchers discovered that normal colon tissue did not transition directly into colorectal cancer, but went through an initial precancerous phase called an adenomatous polyp.15 The polyp stage lasted for years or even decades before transforming into an invasive cancer. Before the 1960s, detecting adenomatous polyps was no easy task.
Digital rectal exams were historically the preferred method of screening. As part of the routine physical examination, patients heard the dreaded snapping of the latex glove and the stern instructions to bend over, just before the physician’s finger was inserted into their rectum, to feel around for anything unusual. Not only was this exam unpopular, it was also virtually useless, as the human colon is approximately five feet long, and the physician could feel only the first few inches. Luckily, advances in technology would eventually provide a better solution.
In the 1940s, a rigid camera called a sigmoidoscope was developed. It was inserted into the sigmoid colon, the last fifteen inches or so of the large intestine. It was a difficult and painful procedure, but nevertheless, starting in 1948, large-scale screening programs using this primitive technology resulted in a jaw-dropping 85 percent reduced rate of colorectal cancer over twenty-five years.16 The concept was proven, but the screening procedure was not generally acceptable to the public.
Improved medical technology changed the game once more. Polyps may bleed intermittently, allowing small amounts of blood into the stool that are not immediately visible to the naked eye. In the late 1960s, fecal occult blood testing (FOBT) was developed, allowing otherwise invisible amounts of blood to be detected and serving as an early warning sign of colorectal cancer. This required only a simple stool sample, and no tubes needed to be inserted into any orifices.
By the middle of the 1970s, flexible colonoscopy was developed. Instead of a rigid scope with limited reach, the colonoscope was flexible, allowing easier passage throughout the entire colon, with its many twists and turns. A positive test for fecal occult blood could now be followed up by a relatively simple colonoscopy that could both detect and remove polyps.17
In 1993, findings from the National Polyp Study proved that this combined approach reduced colorectal cancer by a stunning 76 to 90 percent18 and cancer deaths by 51 percent.19 The 1993 Minnesota Colon Cancer Control Study confirmed that screening with fecal occult blood and colonoscopy reduced death from colorectal cancer by 33 percent.20 This was not just a home run for cancer prevention and treatment; it was a grand slam.
Currently, the U.S. Preventive Services Task Force (USPSTF) recommends screening with FOBT or colonoscopy starting at age fifty and until age seventy-five. Colonoscopy has the advantage of being able to both detect the polyp and remove it immediately. Screening rates have increased from the 20 percent range in the 1990s to approximately 65 percent in the United States today.21
The increased screening has steadily decreased deaths from colorectal cancer, but the 2019 statistics contain some disturbing data: this cancer is increasing disproportionately in younger patients, likely related to the ongoing and increasing obesity crisis. The American Cancer Society estimates that 55 percent of colorectal cancers are attributable to modifiable risk factors, predominantly excess body weight. For those patients over age fifty-five, colorectal cancer decreased by 3.7 percent per year from 2006 to 2015; but for those younger than fifty-five, it increased by 1.8 percent.
Both cervical cancer and colorectal cancer progress in a fairly orderly fashion, from a premalignant condition to invasive cancer. This provides a window of opportunity where early detection and intervention can stop cancer’s progression. Hopes were high that these successes could be repeated for the other big killers, breast and prostate cancer, through mammography and prostate-specific antigen (PSA) blood testing, respectively.
BREAST CANCER
Breast cancer screening focuses on mammography, a type of X-ray, because breast self-examination is too variable and unreliable. For decades, cancer societies recommended annual screening mammography for women starting at age forty, and by all accounts, early screening seemed like a successful intervention. Breast cancer deaths peaked in 1989 and declined by 40 percent from 1989 to 2016. So, it is somewhat surprising that, recently, many countries are suggesting less screening, particularly for those ages forty to fifty.
In 2013, the Cochrane Library, acknowledged as a world expert in evidence-based medicine, reviewed all available data on mammography and concluded that it provided no overall benefit in preventing breast cancer deaths.22 How could that be possible? But the Cochrane Library was not alone in its doubts.

Figure 21.5
In 2014, the Swiss Medical Board noted how “non-obvious it was that the benefits outweighed the harms.”23 It was not immediately apparent to these experts that screening mammography did any good whatsoever. For a fifty-year-old woman, the Swiss Medical Board estimated that screening prevented only 1 breast cancer death for every 1,000 women screened. This means that the other 999 (or 99.9 percent) women did not benefit directly from mammography, but may have suffered from overdiagnosis.
By contrast, it was obvious to any casual observer that the Pap smear screening had dramatically reduced the burden of cervical cancer. No randomized trial was ever needed because the benefits were so clear. Where was the disconnect?
There are three main problems with breast cancer screening: lead time bias (see Figure 21.6), cancer deaths versus overall deaths, and failure to reduce late-stage disease. Much of the benefit of screening is illusory due a phenomenon known as lead time bias. Imagine two women who both develop breast cancer at age sixty and who both die of the disease at age seventy. The first undergoes screening and finds the disease at age sixty-one. The other does not undergo screening and finds the disease at age sixty-five. The first woman “survived” cancer for nine years, whereas the second “survived” cancer for only five years. Screening improved cancer survival by an apparent four years, but this is only an illusion.

Figure 21.6
The second problem is a methodologic issue. Many screening programs declare themselves a success by reducing cancer deaths rather than overall deaths. Why is this important? Suppose a group of one hundred people with cancer will die of the disease within the next five years, and early screening is completely ineffective. However, the toxicity of treatment (surgery, radiation, and chemotherapy) causes heart attacks and infections, killing twenty-five of those patients. Without screening, one hundred patients died of cancer, but with screening, only seventy-five died of cancer and twenty-five died of other causes. Screening reduced cancer deaths by 25 percent, but the benefits were entirely illusory (see Figure 21.7). The patients didn’t actually derive any benefit from screening, and most were actually harmed (see Figure 21.5). For that reason, the only relevant outcome is overall survival, not cancer deaths.
The third problem, failure to reduce late-stage diagnosis, is much more serious. Mammography detects plenty of early-stage breast cancer, exactly as intended. From 1976 to 2008, screening caught more than twice the number of early-stage breast cancers compared to the prior period before widespread screening (see Figure 21.8). Logically, you would expect that catching and treating the disease early should reduce the amount of breast cancer diagnosed at a late stage. But it didn’t. The rate of late-stage cancer decreased by only a minuscule 8 percent.
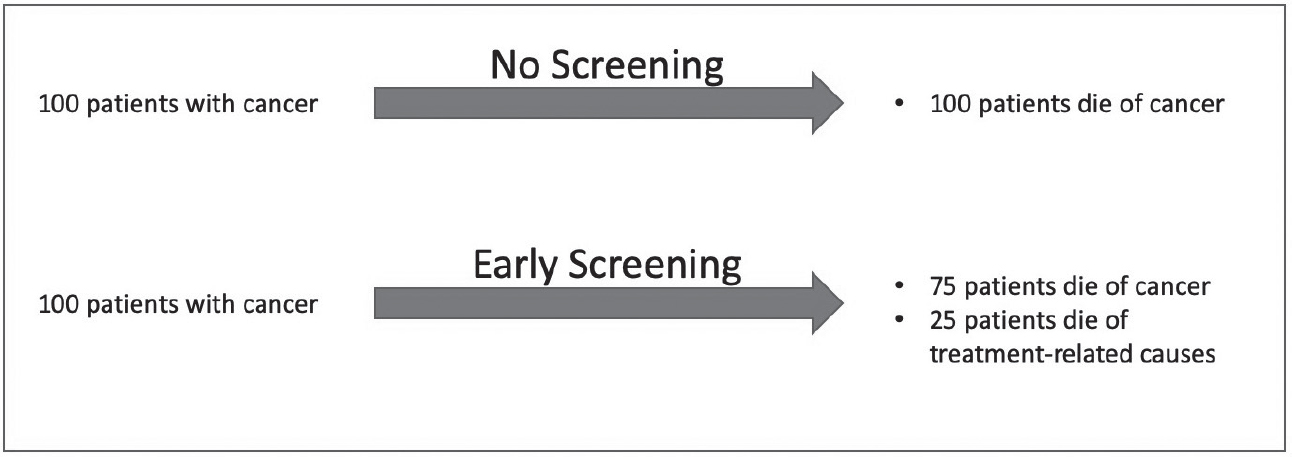
Figure 21.7
Late-stage disease is highly lethal, whereas early-stage disease is highly curable. The early-stage cases being detected were unlikely to progress to more advanced disease. Only a tiny 6.6 percent of those early-stage cases were expected to progress to invasive cancer. In other words, in 93.4 percent of the early-stage cases detected by screening, treatment offered no discernible benefit. We were finding cases that didn’t need to be treated. And we weren’t significantly reducing the number of late-stage cases that are the deadliest.24
But why didn’t the detection of more early-stage disease translate into fewer overall late-stage cancers? In both cervical and colorectal cancer, progression follows an orderly and defined path, from microscopic tumor to larger tumor to metastasis. Therefore, virtually each small precancerous lesion detected and treated was one fewer late-stage cancer in the future. But breast cancer works differently.

Figure 21.8
The evolutionary model of cancer can help us understand why removing small cancers does not reduce the incidence of fully matured cancers. Cancer does not follow linear evolution, where one event follows the next in an orderly fashion. Instead, cancer follows branched-chain evolution. Thus, just as a tree may grow through a fence, blocking a single branch may not stop overall progression of the cancer.
Metastasis accounts for the majority of deaths from cancer. If metastasis occurs late, then early detection and treatment will lower the risk of metastasis. But the new paradigm of cancer recognizes that metastasis is an early event. Very early in the course of cancer’s development, often when the primary cancer is itself undetectable, metastatic cancer cells are shed into the blood. Detecting more early cancers does not always reduce the number of late cancers, and thus the benefits of mammographic screening are far less than we’d once hoped and believed.
Sadly, there is worse news to come. Screening is not just expensive (for both patients and health care providers); it leads to overdiagnosis, defined as the detection of tumors at screening that might never have progressed to become symptomatic or life-threatening. A whopping 31 percent of all breast cancers diagnosed are estimated to be cases of overdiagnosis, totaling 1.3 million U.S. women over the age of thirty. When detected on mammography, almost all tumors are treated, leading to overtreatment, defined as treatments that are unnecessary or harmful.
One in ten women will have a positive mammogram, but only 5 percent of those positives are actually found to be cancer. Put another way, 95 percent of women with a positive mammogram are subjected to invasive procedures for no eventual benefit. This includes biopsies, lumpectomies, and sometimes unnecessary chemotherapy. Women who get screening mammography are also treated more often with mastectomy and radiation. In the United States, the false positive rate is 30 to 50 percent.25 In addition, it has been well established that women testing positive on mammography suffer psychological problems and poor quality of life, even up to three years after screening.

Figure 21.9
The majority of breast cancers diagnosed from mammogram screening are classified as ductal carcinoma in situ (DCIS), a very early stage of cancer. This diagnosis represents approximately 20 percent of all breast cancers, and its incidence has risen dramatically with the onset of widespread screening.26 From 1983 to 2004, its incidence has risen tenfold. Many of these early-stage breast cancers are unlikely ever to progress to a dangerous state—as the evolutionary model explains, a cancer can be fully contained by the body’s own anticancer mechanisms. Aggressive treatment of early cancers is simply unnecessary.
Mammography is particularly problematic in women ages forty to forty-nine, whose firmer breast tissue makes the images more difficult to interpret, resulting in false positive rates of over 12 percent.27 These women, most of whom have no evidence of cancer, often need repeat mammograms or invasive procedures such as biopsies.
The growing recognition of the harm of false-positive screening tests led the USPSTF to change its recommendation on mammography. The 2016 USPSTF update on breast cancer found no reduction in death for those ages thirty-nine to forty-nine, and no longer recommends routine screening for that age group.28 Early screening benefits less than 0.1 percent of women, while the risk of overdiagnosis is 31 percent. We were doing more harm than good (see Figure 21.9).
Mammography is not the holy grail of cancer screening we expected it to be. In men, a similar story has played out for prostate cancer.
PROSTATE CANCER
First identified in the 1960s, the protein called prostate-specific antigen (PSA) liquefies semen to allow sperm to swim freely. The PSA assay, which measures the amount of the antigen in the blood, was developed for law enforcement agencies in rape cases. By 1980, PSA was also found in the blood of patients with prostate cancer, raising the hope that PSA-based blood testing could become the male equivalent of the Pap smear.29
High PSA levels are not specific for prostate cancer, as they are also commonly found in men with enlarged or inflamed prostates. The FDA approved PSA-based screening for prostate cancer in 1986, setting the cutoff level at 4.0 ng/mL, as 85 percent of men had values lower than this. This meant that fifteen men out of one hundred had levels higher than 4.0 ng/mL and would undergo further prostate biopsy, of which between four and five would be diagnosed with aggressive prostate cancer.30
Enthusiasm for PSA-based screening exploded in the 1990s and 2000s. More than twenty million tests were performed each year, and more early prostate cancer was diagnosed than ever. In 1986, fewer than one third of men were diagnosed with early-stage disease confined to the prostate. By 2007, over two thirds of prostate cancer cases were early stage (see Figure 21.10). Death from prostate cancer began to drop, and it was looking like yet another feel-good cancer success story. But alas, the PSA story was not so simple.
Screening detected more early-stage prostate cancer, but did it improve overall survival? Three very large long-term studies of PSA-based screening answered that very question. In the United States, there was the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which involved more than 76,000 men.31 In Europe, there was the European Randomized Study of Screening for Prostate Cancer (ERSPC) study, with almost 182,000 study participants.32 And in the United Kingdom, there was the PROTECT (Prostate Testing for Cancer and Treatment) study, with 408,825 participants.33 With between 10 and 14.8 years of follow-up, none of these three enormous trials detected a discernible benefit with regard to overall survival to PSA-based testing. The USPSTF estimated the rate of overdiagnosis at 16.4 to 40.7 percent. The screening detected early, less aggressive prostate cancer, but did not reduce advanced disease. Once again, it seemed that screening was detecting the cancers that didn’t require treatment.
Patients with a positive PSA are subjected to invasive procedures with significant side effects. Approximately 10 percent of men screened received at least one false-positive PSA screening result, leading to more than a million prostate biopsies being done per year.34 Of the men in the PLCO screening group, 12.6 percent underwent one or more biopsies. Of those, 2 to 5 percent experienced biopsy-related complications. Given the huge numbers of men undergoing screening, this is a massive number of complications. As with mammography, overdiagnosis is a major problem. Men diagnosed with prostate cancer are more likely to have a heart attack or commit suicide in the year after diagnosis.35

Figure 21.10
In 2012, the USPSTF recommended against PSA-based screening, noting with moderate certainty that the benefits do not outweigh the harms. In 2018, the USPSTF again reviewed the evidence and recommended against routine screening.36 The task force felt moderately certain that screening with PSA-based methods is worse than doing nothing at all.
According to USPSTF guidelines, men younger than fifty-five or older than seventy should not be tested for PSA. Between ages fifty-five and sixty-nine, it is an individual choice. They note that “Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening.”37 Not exactly a screaming endorsement.
THYROID CANCER
In 1999, South Korea embraced national screening in an effort in its own “war on cancer.” Screening for breast, cervical, colorectal, gastric, and hepatic cancer was provided free of charge to the entire population. Screening for thyroid cancer was not included, but for a small surcharge, approximately thirty to fifty dollars, patients could elect to have a neck ultrasound performed as well. By 2011, thyroid cancer was being diagnosed fifteen times more frequently than in 1993 (see Figure 21.11).38 In almost all diagnosed cases, the thyroid was either partially or completely removed. This treatment was not without consequences. There was an 11 percent risk of reduced parathyroid function and a 2 percent risk of paralysis of the vocal cords due to nerve damage.
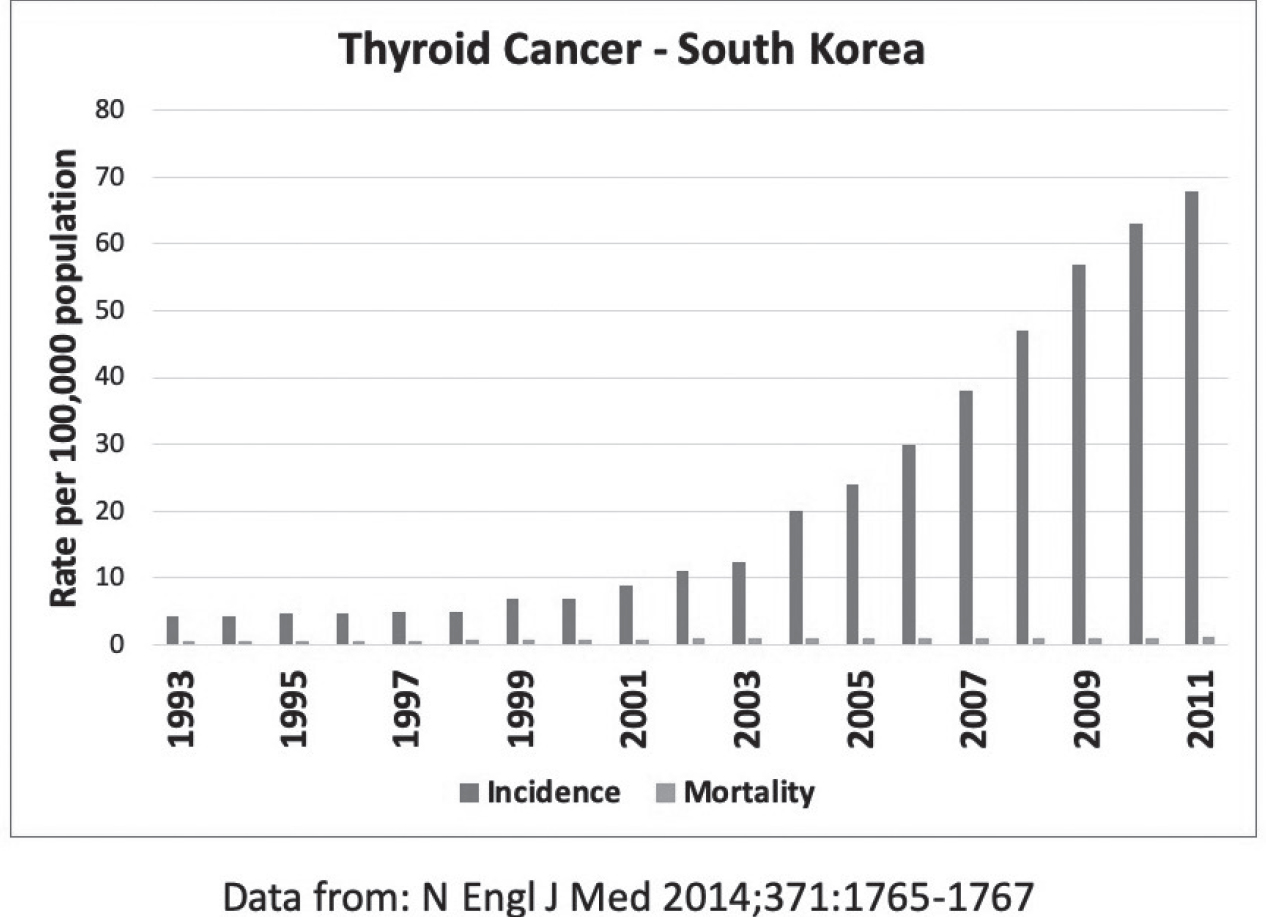
Figure 21.11
Despite this intensive effort to eradicate early-stage thyroid cancer, the risk of death from thyroid cancer was virtually unchanged. Simply put, it was a classic case of overdiagnosis—most of the thyroid cancers detected in screening did not require treatment. Finding and treating early disease is not useful. Only reducing the incidence of late-stage disease is useful, and these are not necessarily the same thing due to the occurrence of early metastasis. By some estimates, as many as one third of all adults have evidence of thyroid cancer, but the vast majority of these cancers do not produce symptoms or cause health problems.39 Finding and treating cancers that don’t need to be treated is not a useful strategy.
CONCLUSIONS
The evolutionary model of cancer explains the successes and failures of some screening programs. When cancers progress in an orderly manner from precancerous stage to small tumor to large tumor to metastasis, screening succeeds (see Figure 21.12). Screening to remove early cancers prevents the development of late cancers, and this saves lives.
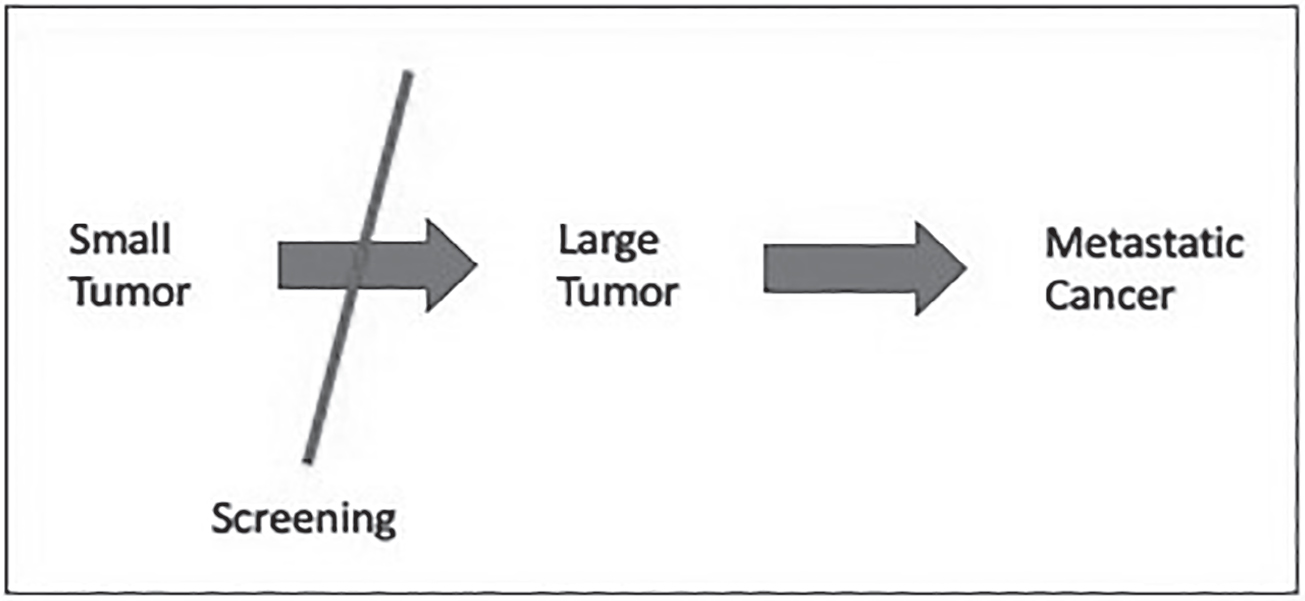
Figure 21.12
But if removal of early-stage cancer does not reduce late-stage cancer, screening is unsuccessful, and overdiagnosis becomes a problem. Not every early-stage cancer needs to be destroyed, as many small cancers are contained by the immune system and will never pose a serious health threat. With toxic treatments such as surgery, radiation, and chemotherapy, the treatment may be worse than the disease.
Consider the normal bacteria in your gut, known as the microbiome. Must every bacterium in your body be eradicated? No. Most bacteria living in your gastrointestinal tract are neutral or even good. Probiotic supplements and foods containing live cultures (e.g., yogurt) derive much of their proposed benefits by encouraging the growth of these “good bacteria.” Killing every bacterium with powerful antibiotics almost certainly does more harm than good. In the same manner, more people die with prostate cancer than of prostate cancer. Drastic treatments to eradicate every prostate cancer cell found may do more harm than good.
While the public perceives that cancer screening “saves lives,” the truth is far more nuanced. Some cancer screening does save lives. Some cancer screening does not. Even then, looking only at the number of “cancers prevented” with screening is highly misleading because it considers only the positives. How many lives are harmed by screening? Suppose you find a tiny breast cancer that was not destined to progress any further. Finding it on screening tests leads to mastectomy, chemotherapy, and a lifetime of worry. You might have disfiguring surgery that leaves you with a swollen arm for the rest of your life. Chemotherapy increases the risk of heart failure and a future cancer. The risks involved with early screening and detection are very real, but the public rarely hears about them.
Without good evidence of the benefits of screening, and a better understanding of why it may fail, we must rely on the ancient medical guiding principle of Primum non nocere, which means, “First, do no harm.” The perspective offered by today’s cancer paradigm explains why many national agencies are beginning to scale back on the amount of screening they recommend.