5
Cancer Goes Viral
THE IRISH SURGEON Denis Parsons Burkitt was only eleven years old when an injury destroyed his vision in one eye. He threw himself into his studies, and after completing surgical training, he joined the Irish Army Medical Corps. He was stationed in Africa, where he would make his most important discoveries. What he lost in eyesight he made up for in insight, becoming one of the most influential doctors of his era.
In 1957, Burkitt was astounded to treat a five-year-old boy with multiple jaw tumors. In all his years of medical training, he had never seen such a thing. But this was only the first of many such patients with strange tumors. Shortly afterward, he saw a second child with four tumors in his jaw and multiple tumors in his abdomen. The biopsy showed “small round cell sarcoma.” It was cancer.
Two children presenting with this highly unusual (for him) cancer in short succession piqued Burkitt’s curiosity. Reviewing the local hospital records, he found a staggering twenty-nine other children with similar cancers. This type of cancer was apparently common in Africa, but Burkitt knew nothing of it, and there was no mention of it anywhere in the medical literature. In 1958, he published his findings in the British Journal of Surgery.1
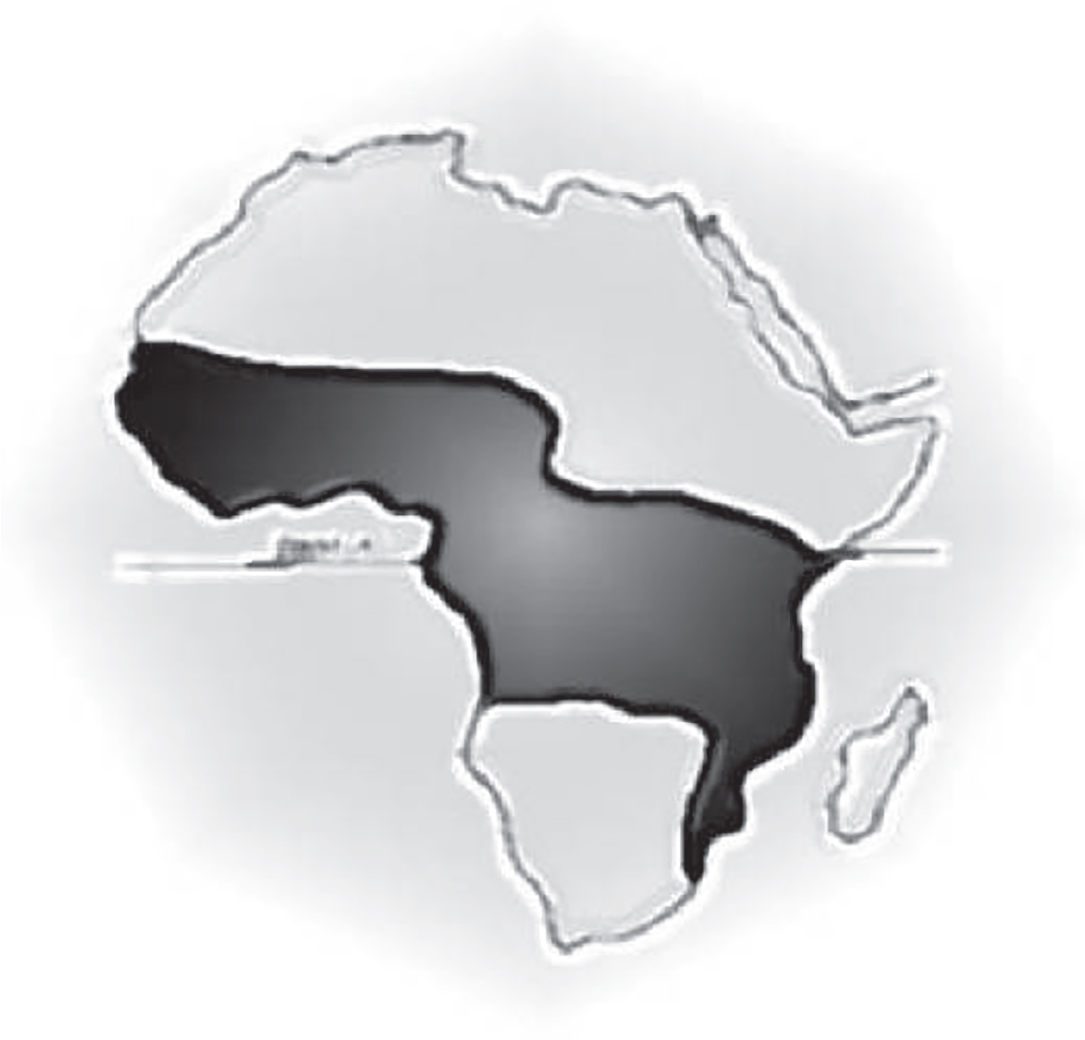
Not all of Africa was similarly afflicted. Shortly after the publication of his paper, local cancer specialists showed Burkitt that this particular cancer, common in certain parts of Africa, was simply not seen in South Africa. Intrigued, Burkitt began tracing Africa’s “lymphoma belt, which ran through the middle of the African continent” (see Figure 5.1).2 Sure enough, cancer followed a definite geographical distribution. In his mapping, he determined that elevation above sea level and distance from the equator were major factors in the incidence of cancer. This suggested that temperature was a key determinant of a population’s susceptibility to the disease. In Africa, this type of regional distribution of illness was not unusual. Infectious diseases spread by mosquitos, for example, followed an identical pattern. But this was a cancer, not an infection.
A cancer-causing virus? Perhaps the notion was not as silly as it first seemed. In 1910, Peyton Rous at the Rockefeller Institute, a chicken virologist, transmitted a sarcoma from one chicken to another. The cancer-causing agent was named the Rous sarcoma virus (RSV), for which Rous received the 1966 Nobel Prize in Medicine. In 1935, a papillomavirus was found to cause tumors in rabbits, and in the 1940s, leukemia-causing viruses were isolated in mice and cats. But could a virus cause cancer in humans? Viral cancer might be true for a few chickens in a few research laboratories at the frontiers of medicine, but this was practically unknown in clinical medicine. But the data is the data, and it does not much care what you think.
I. Magrath, “Denis Burkitt and the African Lymphoma,” Ecancermedicalscience 3, no. 159 (2009): doi: 10.3332/ecancer.2009.159.
Figure 5.1
The African children’s cancer was common in areas where the temperature did not fall below sixty degrees Fahrenheit and where they had at least twenty inches of rainfall per year—precisely the conditions required for mosquito breeding. Africa’s lymphoma belt was essentially identical to the endemic areas for malaria, yellow fever, and trypanosomiasis (sleeping sickness), all conditions spread by mosquitos. Burkitt suspected that this cancer, now renamed Burkitt’s lymphoma, was linked to an infection. In 1961, Burkitt sent some tumor samples to London to be examined by pathologist Michael Anthony Epstein, who had access to modern electron microscopy.
Laboriously growing the tumor cells in culture, Epstein identified a herpes-like virus particle.3 This previously unknown virus, and the first known human cancer-causing virus, is now called the Epstein-Barr virus (EBV). It turns out to be one of the most common viruses in the world, with an estimated 90 percent of adults having been exposed to it.4 In developed countries, initial EBV infection usually occurs during adolescence, sometimes accompanied by symptoms of infectious mononucleosis, or “mono.” Transmitted by saliva, EBV is sometimes called the “kissing disease.” In Africa, however, initial infection usually occurs at birth. In Uganda, for example, an estimated 80 percent of children under age one have been exposed to EBV, compared to less than 50 percent in America. If almost the entire world is infected with EBV, why did only some children get cancer? And why was lymphoma confined to the “lymphoma belt”? These are good questions for which firm answers still do not exist.
Burkitt’s lymphoma might be caused by the coinfection of EBV with malaria.5 In the 1960s, the African islands of Zanzibar and Pemba sprayed the toxic insecticide DDT to eradicate mosquitos. Malaria rates dramatically plunged, from 70 percent to 5 percent, and lymphoma rates followed this steep decline. When the highly toxic DDT was banned, malarial rates gradually rose, along with lymphoma, as inseparable as salt from the ocean.
In Tanzania, malaria prophylaxis with chloroquine reduced Burkitt’s lymphoma by an astounding 82 percent. When increasing drug resistance forced the discontinuation of this program, malarial rates rose, and lymphoma rates increased 273 percent.6 The precise mechanism of disease is still uncertain, but malaria may stimulate overproduction of B lymphocytes (the malignant cells in lymphoma). These cells then become infected with EBV, which somehow triggers their transformation into cancerous cells.
In other parts of the world, EBV causes a completely different cancer, called nasopharyngeal cancer (NPC). It is a rare cancer worldwide, but common in Hong Kong, Taiwan, and in the native Inuit people of Alaska and Greenland. In 2012, it accounted for only 0.71 percent of cancers worldwide, but 71 percent of those cases occurred in Southeast Asia.7 It is unknown why EBV should cause different disease in different populations despite almost universal exposure. In southern China, it is the third most common cancer8 and more than ten times more common than in Europe and America.
As with Burkitt’s lymphoma, NPC is associated with EBV infection in early childhood. In Hong Kong, almost 100 percent of children have been exposed to EBV by age ten. Asian immigrants to other countries suffer far less NPC, a fact that argues against a purely genetic predisposition. The risk of NPC falls by about 50 percent in Chinese immigrants to America.9 Some have speculated that a once-popular diet staple in China, salted fish, may be the missing link. The salt preservation process was inefficient in China, allowing for significant putrefaction and the development of the chemical N-nitrosamine, a known carcinogen.
THE SPECIAL VIRUS CANCER PROGRAM
This discovery, that cancer could be caused by infections, was electrifying. It opened up the terrifying possibility that cancer was contagious. Yet, like the hope remaining in Pandora’s box, it also offered the possibility that it was curable. Bacteria could be killed with antibiotics. While antiviral medications had not yet been developed, vaccines were available, and once instituted widely, these became extremely effective at eradicating viruses and preventing viral outbreaks. Viral infections like measles, mumps, polio, and chicken pox, once a rite of passage in childhood, have largely faded.
The National Cancer Institute raced to investigate the exciting new possibilities. In 1964, the Special Virus Cancer Program (SVCP) was launched with the mandate of identifying additional viral causes of cancer. Over the next decade, the SVCP received over 10 percent of the total research funds earmarked for cancer—almost $500 million. By contrast, the research funds devoted to investigating the role of diet in cancer amounted to less than one twentieth that sum.
The SVCP was an enormous undertaking, and it was the beating heart of President Nixon’s war on cancer. Hundreds of monkeys were inoculated with human tumors to see if these could be transmitted. Yet, in the end, the project produced little useful data. The SVCP itself was held in rather low esteem by the scientific community and was regarded as having political rather than scientific goals.10 Scientists suspected that its true purpose was to give the appearance of making progress rather than making real progress. One prominent researcher noted that “The SVCP has been extremely ineffective and maybe has even had a negative effect.” Other researchers cynically opined that its unwritten motto should have been “Nothing too stupid to test.”
Lack of oversight led to contractors awarding themselves multimillion-dollar deals, and the New York Times reported that the managers of the SVCP “are also often the recipients of large amounts of money they dispense.” In 1974, due to sharp criticism by the National Cancer Advisory Board about conflicts of interests, the SVCP was reorganized.11 When one looks back at the project now, boondoggle is the word that comes to mind.
The program was formally terminated in 1980, with most of the scientific community convinced by the fiasco that infections and viruses had little or nothing to do with cancer. Then, only a few years later, new evidence surfaced that, once again, pointed to infections as the key cause of certain cancers.
HEPATITIS B AND C
Viral hepatitis (inflammation of the liver) has been described in the medical literature for millennia. The most conspicuous manifestation is jaundice, a noticeable yellowing of the eyes and skin. The first virus identified, hepatitis A, is typically found in overcrowded cities and army barracks and is transmitted through fecal contamination. Hepatitis A causes an acute illness but not chronic disease. Other forms of infectious hepatitis that caused chronic liver disease were transmitted through contamination of body fluids, such as blood and sexual contact.
Through the early twentieth century, the increased use of syringes inadvertently increased the spread of viral hepatitis. Syringes and needles were expensive, so they were routinely reused, often without adequate sterilization. In 1885, an outbreak of jaundice followed a mass vaccination of shipyard workers in Bremen, Germany, and also occurred in an asylum for the mentally ill in Merzig, Germany, where 25 percent of the vaccine recipients developed jaundice. Blood transfusions, a practice that increased exponentially during World War II, were also a risk factor for viral hepatitis. By 1947, the distinction “hepatitis B” was accepted, but the virus itself had not yet been identified. Then came Dr. Barry Blumberg, who would eventually win the 1976 Nobel Prize in Medicine.
Blumberg was an American physician and geneticist whose primary research interest was the diversity of populations, not liver disease or viruses. As he studied the variety of proteins in human blood, it occurred to him that blood transfusion may cause new proteins to form. In 1961, he discovered a new protein that he called the Australia antigen, as it was discovered in the blood serum of an Australian aboriginal man.12 Following the trail of the Australia antigen would eventually lead Blumberg to his momentous discovery of the hepatitis B virus, one of the smallest DNA viruses to afflict humans. Endemic in Asia, the hepatitis B virus is often transmitted from mother to child, resulting in many asymptomatic children becoming chronically infected, which greatly increases their chance of developing liver cancer.
In 1981, studies found that chronic hepatitis B infection increased the risk of liver cancer by two hundred times.13 In 2008, liver cancer was the fifth most common cancer in men and the seventh most common in women, worldwide. China in particular accounts for about 50 percent of those cases and deaths.14
Hepatitis B vaccines became available by the early 1980s, and nationwide vaccination programs in Asia have virtually eradicated liver cancer in the pediatric population. Hepatitis B vaccination has now been incorporated into the national infant immunization programs of at least 177 countries worldwide. Chronic infections and liver disease have dramatically declined, with beneficial implications for the future of liver cancer.
After identification of hepatitis B in the 1960s, post-transfusion hepatitis decreased, but it did not disappear, which implied another yet-to-be-identified blood-borne virus that could cause chronic liver disease.15 This became known as “non-A, non-B hepatitis” because it was . . . well, not hepatitis A and not hepatitis B. (Scientists are sometimes a funny lot. It’s not clear to me why nobody immediately said, “Guys, it’s not hepatitis A, and it’s not hepatitis B. So, can we all agree to call it, I don’t know, hepatitis C?”)
The identification of the hepatitis C virus took until 1989 because the amount of virus in the blood is several thousandfold lower than in hepatitis B. Both hepatitis B and C cause chronic liver disease. At its peak, hepatitis C infected approximately 160 million people worldwide and caused liver cancer in many of those afflicted. It is predominantly transmitted by sharing infected needles. In the post–World War II era, the reusing of needles for vaccines, especially in Italy, caused early hepatitis C outbreaks. Thereafter, the main route of transmission was illicit drug users who shared needles. Today, cutting-edge antiviral drugs may cure up to 90 percent of those infected with the virus, offering significant hope for the future.
Liver cancer develops only after many decades of chronic infection and inflammation. About 80 percent of liver cancer is related to the hepatitis B (HBV) and hepatitis C (HCV) viruses. HBV is estimated to cause 50 to 55 percent of cancer and HCV, 25 to 30 percent.
HUMAN PAPILLOMAVIRUS
In the 1970s, Dr. Harald zur Hausen, at the German Cancer Research Center in Heidelberg, noted a large number of scientific reports of genital warts “converting” into cancers in women. Human papillomavirus (HPV), of which there are hundreds of different subtypes, was known to cause genital warts. Based on this observation, he reasonably proposed that HPV caused both genital warts and cervical cancer. Cancer researchers, stung by the recent fiasco of the Special Virus Cancer Program, were not particularly receptive to his theory. Later, in an interview with the Nobel Prize committee, zur Hausen recalled, “My proposal was not welcome at the time.”16
Excited by his observation, zur Hausen focused his research on HPV. In 1979, he first isolated HPV subtype 6 from genital warts, but this subtype was not linked in any way to cervical cancer. Tenaciously, he next isolated HPV subtype 11, but this, too, was largely irrelevant to cervical cancer. In 1983, he isolated HPV subtype 16. Bingo! Viral DNA from HPV subtype 16 was found in about half of all cases of cervical cancer. Zur Hausen had just uncovered incontrovertible evidence that HPV subtype 16 infection played a major role in cervical cancer. A year later, he cloned both HPV 16 and 18, the two subtypes of HPV now known to cause the majority of cervical cancers.
By 1999, HPV was found in 99.7 percent of invasive cervical cancer.17 There are more than a hundred types of HPV, of which thirteen are cancer causing. Types 16 and 18 are the most common in North America, accounting for 70 to 80 percent of cervical cancers. It would take zur Hausen more than a decade to amass the scientific proof that would earn him the Nobel Prize in Medicine in 2008.
The revolutionary cycle had come full circle—from identifying and isolating the virus, to detection within the cancer cell, to the development of vaccines that protect up to 95 percent against HPV. Even today, cervical cancer is a significant worldwide burden. In 2012, there were an estimated 500,000 new cases worldwide and 266,000 deaths,18 but vaccination programs starting in 2007 against HPV subtypes 16 and 18 have already reduced infection and the risk of premalignant warts by over 50 percent.19 The long-held dream of cancer vaccination is quickly coming true.
HELICOBACTER PYLORI
One of the most bewildering successes in the war on cancer was the startling worldwide progress against stomach cancer. What made it puzzling was that for decades, researchers had absolutely no idea why stomach cancer was retreating. It was like winning Wimbledon without even knowing how to play tennis. Stomach cancer is particularly deadly because of the lack of early warning symptoms. By the time it is diagnosed, it is often too late.
It was not a trivial success, either. In the 1930s, stomach cancer was the most common cause of cancer death in the United States and Europe.20 Yet, by 2019, it ranked only as the seventh leading cause of cancer-related deaths in the United States. One of the most virulent cancers in the world was steadily losing momentum, but we had just about no idea why.
Rates of stomach cancer vary greatly worldwide. The Japanese suffer ten times more stomach cancer than Americans, but when they move to the United States, their risk of stomach cancer drops dramatically, a fact that points to an environmental rather than a genetic problem. The risk of stomach cancer is much higher for a Japanese person in Japan compared to a Japanese person in America. What could account for the wide variation in stomach cancer and for its steady decline? The answer came from an unlikely source: two obscure Australian physicians studying stomach ulcers.
In 1981, Drs. Barry Marshall and Robin Warren were looking at some strange-looking bacteria on pathology slides taken from the stomachs of patients. These bacteria had been observed for over a century, but they were casually dismissed as random stains created while slides were being processed. At the time, everybody believed that the stomach was a completely sterile environment. It was thought that stomach acid created a harsh, hostile, and highly acidic environment that killed all bacteria. In the 1980s, any scientist would have considered the possibility that a bacterium could survive in the stomach to be laughable—any scientist except for Marshall and Warren, that is. Convinced that these bacteria were real, Marshall tried to grow them from biopsy specimens. He failed with his first thirty-three attempts, but on patients thirty-four and thirty-five, the lab techs made a fortuitous mistake.
Bacterial cultures were routinely discarded after two days, based on the assumption that there were no viable bacteria. Accidently left in the incubator too long, the cultures of patients thirty-four and thirty-five turned positive. The bacteria were there, but took much longer than usual to grow. Marshall identified the slow-growing bacteria Helicobacter pylori (H. pylori) as the causative agent of peptic ulcer disease.
Remarkably, H. pylori can persist for decades in the stomach. It uses the protein urease to neutralize its highly acidic surroundings and grows in its own protective cloaking device. Genetic studies show that H. pylori has been colonizing human stomachs for more than 58,000 years.21 H. pylori was hiding in plain sight this whole time.
Marshall had a problem, though. Nobody believed him. In desperation, he cultured the bacteria from a patient with gastritis, “swizzled the organisms around in a cloudy broth, and drank it.”22 Super gross—but effective. Five days later, Marshall developed a stomach infection, proving that H. pylori caused the inflammation that led to an ulcer.
This was a stunning revelation. Until the 1980s, practically every physician and researcher in the world believed that stomach ulcers were caused by too much stress. Treatment of peptic ulcer disease consisted mainly of trying to relax. As you might imagine, long walks in the woods and meditation were not particularly effective against this infection.
Understanding that most stomach ulcers were caused by bacteria meant that antibiotics were curative. A cocktail of three medications, including two different antibiotics taken for one to two weeks, now cures about 80 percent of cases of H. pylori infection.23 For these insights, Marshall and Warren were awarded the 2005 Nobel Prize in Medicine.
Approximately half the world’s population is infected with H. pylori, although the vast majority of those afflicted have no symptoms. The urban overcrowding and poor sanitation in many parts of Asia laid the groundwork for much higher rates of H. pylori infestation. By the mid-1990s, the striking similarity between the worldwide prevalence of H. pylori infection and stomach cancer was noted. In Korea, for example, a nation with one of the highest rates of stomach cancer, 90 percent of adults over age twenty have H. pylori.24 It soon became clear that H. pylori caused not only chronic infection and ulcers but also stomach cancer.
H. pylori infection is associated with up to a sixteenfold increased risk of cancer.25 H. pylori infection starts with chronic inflammation (gastritis), which progresses to atrophy, metaplasia, dysplasia, and then finally, cancer. In 1994, the International Agency for Research on Cancer (IARC) listed H. pylori as a group 1 carcinogen (definite) in humans. It is estimated to be responsible by itself for 5.5 percent of the global cancer burden.26
H. pylori infestation has receded in recent decades, thanks to improved sanitation and housing conditions. Less H. pylori means less stomach cancer—the likely key to our stunning success in reducing stomach cancers. We were winning the war without even knowing why. Eradication of H. pylori with antibiotics reduced the chronic inflammation that leads to premalignant lesions of the stomach.27 A rarer form of stomach cancer, known as mucosa-associated lymphoid tissue (MALT lymphoma) also occurs in those infected with H. pylori. In its early stages, MALT lymphomas can be completely cured by eradication of H. pylori.28 Of those infected with H. pylori, only 10 percent will develop peptic ulcer disease, 1 to 3 percent develop stomach cancer, and less than 1 percent develop MALT lymphoma.29 But when multiplied by half the world’s population, these numbers become significant.
CANCER PARADIGMS
Let’s return to our original question: what causes cancer? Chemical carcinogens such as asbestos, tobacco, and soot cause cancer. Physical carcinogens such as radiation cause cancer.
Infections, both viral and bacterial, are also carcinogens—and they’re not so rare, with an estimated 18 percent of cancers having infectious disease origins.30 The main agents are Helicobacter pylori, human papillomaviruses, hepatitis B and C viruses, Epstein-Barr virus, HIV, and a few others.

Figure 5.2
By the 1960s, all the pieces seemed to be falling into place. We knew many of the underlying factors that cause cancer. But in considering cancer as a whole, what do these diverse factors have in common? What is the unifying mechanism? To this important question, cancer paradigm 1.0 had no answer (see Figure 5.2). But by the 1970s, a new paradigm of understanding was being constructed.