5
Palliative Care & Pain Management
Michael W. Rabow, MD
Steven Z. Pantilat, MD
Ann Cai Shah, MD
Lawrence Poree, MD, MPH, PhD
Scott Steiger, MD
PALLIATIVE CARE
DEFINITION & SCOPE
Palliative care is medical care focused on improving quality of life for people living with serious illness. Serious illness is defined as “a condition that carries a high risk of mortality, negatively impacts quality of life and daily function, and/or is burdensome in symptoms, treatments or caregiver stress.” Palliative care addresses and treats symptoms, supports patients’ families and loved ones, and through clear communication helps ensure that care aligns with patients’ preferences, values, and goals. Near the end of life, palliative care may become the sole focus of care, but palliative care alongside cure-focused treatment or disease management is beneficial throughout the course of a serious illness, regardless of its prognosis.
Palliative care includes management of physical symptoms, such as pain, dyspnea, nausea and vomiting, constipation, delirium, and agitation; emotional distress, such as depression, anxiety, and interpersonal strain; and existential distress, such as spiritual crisis. While palliative care is a medical subspecialty recognized by the American Board of Medical Specialties (“specialty palliative care”) and is typically provided by an interdisciplinary team of experts, all clinicians should have the skills to provide “primary palliative care” including managing pain; treating dyspnea; identifying mood disorders; communicating about prognosis and patient preferences for care; and helping address spiritual distress. The fourth edition of the National Consensus Project’s Clinical Practice Guidelines for Quality Palliative Care emphasizes that palliative care is the responsibility of all clinicians and disciplines caring for people with serious illness, in all health care settings (including hospitals, primary care and specialty clinics, nursing homes, and the community).
The scope of “generalist” palliative care and the ideal timing to begin “specialized” palliative care for patients with different illnesses is an evolving area of practice.
During any stage of illness, patients should be screened routinely for symptoms. Any symptoms that cause significant suffering are a medical emergency that should be managed aggressively with frequent elicitation and reassessment as well as individualized treatment. While patients at the end of life may experience a host of distressing symptoms, pain, dyspnea, and delirium are among the most feared and burdensome. Management of these common symptoms is described later in this chapter. Randomized studies have shown that palliative care provided alongside disease-focused treatment can improve quality of life, promote symptom management, and even prolong life.
Gärtner J et al. Early palliative care: pro, but please be precise! Oncol Res Treat. 2019;42(1–2):11–18. [PMID: 30685764]
Krikorian A et al. Patient’s perspectives on the notion of a good death: a systematic review of the literature. J Pain Symptom Manage. 2020 Jan;59(1):152–64. [PMID: 31404643]
Mechler K et al. Palliative care approach to chronic diseases: end stages of heart failure, chronic obstructive pulmonary disease, liver failure, and renal failure. Prim Care. 2019 Sep;46(3):415–32. [PMID: 31375190]
Ruiz M et al. Role of early palliative care interventions in hematological malignancies and bone marrow transplant patients: barriers and potential solutions. Am J Hosp Palliat Care. 2018 Nov;35(11):1456–60. [PMID: 29699418]
Schuler US. Early integration of palliative and oncological care: con. Oncol Res Treat. 2019;42(1–2):19–24. [PMID: 30572330]
Zhou K et al. Palliative care in heart failure: a meta-analysis of randomized controlled trials. Herz. 2019 Aug;44(5):440–4. [PMID: 29468259]
PALLIATION OF COMMON NONPAIN SYMPTOMS
DYSPNEA
Dyspnea is the subjective experience of difficulty breathing and may be characterized by patients as tightness in the chest, shortness of breath, breathlessness, or a feeling of suffocation. Up to half of people at the end of life may experience severe dyspnea.
Treatment of dyspnea is first directed at the cause (see Chapter 9) if a workup is consistent with the patient’s goals. At the end of life, dyspnea responds to opioids, which have been proven effective in multiple randomized trials. Starting doses are typically lower than would be necessary for the relief of moderate pain. Immediate-release morphine given orally (2–4 mg every 4 hours) or intravenously (1–2 mg every 4 hours) treats dyspnea effectively. Sustained-release morphine given orally at 10 mg daily is safe and effective for most patients with ongoing dyspnea. Supplemental oxygen may be useful for the dyspneic patient who is hypoxic. However, a nasal cannula and face mask are sometimes not well tolerated, and fresh air from a window or fan may provide relief for patients who are not hypoxic. Judicious use of noninvasive ventilation as well as nonpharmacologic relaxation techniques, such as meditation and guided imagery, may be beneficial for some patients. Benzodiazepines may be useful adjuncts for treatment of dyspnea-related anxiety.
NAUSEA & VOMITING
Nausea and vomiting are common and distressing symptoms. As with pain, the management of nausea may be optimized by regular dosing and often requires multiple medications targeting the four major inputs to the vomiting center (see Chapter 15).
Vomiting associated with opioids is discussed below. Nasogastric suction may provide rapid, short-term relief for vomiting associated with constipation (in addition to laxatives), gastroparesis, or gastric outlet or bowel obstruction. Prokinetic agents, such as metoclopramide (5–20 mg orally or intravenously four times a day), can be helpful in the setting of partial gastric outlet obstruction. Transdermal scopolamine (1.5-mg patch every 3 days) can reduce peristalsis and cramping pain, and H2-blocking medications can reduce gastric secretions. High-dose corticosteroids (eg, dexamethasone, 20 mg orally or intravenously daily in divided doses) can be used in refractory cases of nausea or vomiting or when it is due to bowel obstruction or increased intracranial pressure. Malignant bowel obstruction in people with advanced cancer is a poor prognostic sign and surgery is rarely helpful.
Vomiting due to disturbance of the vestibular apparatus may be treated with anticholinergic and antihistaminic agents (including diphenhydramine, 25 mg orally or intravenously every 8 hours, or scopolamine, 1.5-mg patch every 3 days).
Benzodiazepines (eg, lorazepam, 0.5–1.0 mg given orally every 6–8 hours) can be effective in preventing the anticipatory nausea and anxiety associated with chemotherapy. For emetogenic chemotherapy, therapy includes combinations of 5-HT3-antagonists (eg, ondansetron, granisetron, dolasetron, or palonosetron), neurokinin-1 receptor antagonists (eg, aprepitant, fosaprepitant, or rolapitant), the N-receptor antagonist netupitant combined with palonosetron (NEPA), olanzapine, dexamethasone, and prochlorperazine. In addition to its effect on mood, mirtazepine, 15–45 mg orally nightly, may help with nausea and improve appetite. Finally, dronabinol (2.5–20 mg orally every 4–6 hours) can be helpful in the management of nausea and vomiting. Patients report relief from medical cannabis, although the tetrahydrocannabinol (THC) or cannabidiol (CBD) strains that are most effective remain unclear.
CONSTIPATION
Given the frequent use of opioids, poor dietary intake, physical inactivity, and lack of privacy, constipation is a common problem in seriously ill and dying patients. Clinicians must inquire about any difficulty with hard or infrequent stools. Constipation is an easily preventable and treatable cause of discomfort, distress, and nausea and vomiting (see Chapter 15).
Constipation may be prevented or relieved if patients can increase their activity and their intake of fluids. Simple considerations, such as privacy, undisturbed toilet time, and a bedside commode rather than a bedpan may be important for some patients.
A prophylactic bowel regimen with a stimulant laxative (senna or bisacodyl) should be started when opioid treatment is begun. Table 15–4 lists other agents (including osmotic laxatives such as polyethylene glycol and lactulose) that can be added as needed. Docusate, a stool softener, is not recommended because it does not add benefit beyond stimulant laxatives in well-hydrated patients. Peripherally acting mu-receptor antagonists (including the oral agents naloxegol and naldemedine, and the subcutaneous methylnaltrexone) are recommended to treat opioid-induced constipation in laxative-refractory opioid-induced constipation. Evidence is insufficient to recommend lubiprostone or prucalopride for opioid-induced constipation. Patients who report being constipated and then have diarrhea typically are passing liquid stool around impacted stool.
FATIGUE
Fatigue, a distressing symptom, is the most common complaint among people with cancer. Specific abnormalities that can contribute to fatigue, including anemia, hypothyroidism, hypogonadism, cognitive and functional impairment, and malnutrition, should be corrected if possible (and desired by the patient). Because pain, depression, and fatigue often coexist, pain and depression should be managed appropriately in patients with fatigue. Fatigue from medication adverse effects and polypharmacy is common and should be addressed. For nonspecific fatigue, exercise and physical rehabilitation are safest and may be most effective. Although commonly used, strong evidence is lacking for psychostimulants, such as methylphenidate, 5–10 mg orally in the morning and afternoon, or modafinil, 200 mg orally in the morning, for cancer-related fatigue. American Ginseng (Panax quinquefolius) has been shown to be effective for cancer-related fatigue but may have an estrogenic effect. Corticosteroids may have a short-term benefit. Caffeinated beverages can help.
DELIRIUM & AGITATION
Many patients die in a state of delirium—a waxing and waning in level of consciousness and a change in cognition that develops over a short time and is manifested by misinterpretations, illusions, hallucinations, sleep-wake cycle disruptions, psychomotor disturbances (eg, lethargy, restlessness), and mood disturbances (eg, fear, anxiety). Delirium may be hyperactive, hypoactive, or mixed. Agitated delirium at the end of life has been called terminal restlessness.
Some delirious patients may appear “pleasantly confused,” although it is difficult to know what patients experience. In the absence of obvious distress in the patient, a decision by the patient’s family and the clinician not to treat delirium may be considered. More commonly, however, agitated delirium at the end of life is distressing to patients and family and requires treatment. Delirium may interfere with the family’s ability to interact with the patient and may prevent a patient from being able to recognize and report important symptoms. Common reversible causes of delirium include urinary retention, constipation, anticholinergic medications, and pain; these should be addressed whenever possible. There is no evidence that dehydration causes or that hydration relieves delirium. Careful attention to patient safety and nonpharmacologic strategies to help the patient remain oriented (clock, calendar, familiar environment, reassurance and redirection from caregivers) may be sufficient to prevent or manage mild delirium. A randomized trial of placebo compared to risperidone or haloperidol in delirious patients demonstrated increased mortality with neuroleptics. Thus, neuroleptic agents (eg, haloperidol, 1–10 mg orally, subcutaneously, intramuscularly, or intravenously twice or three times a day, or risperidone, 1–3 mg orally twice a day) generally should be avoided. When delirium is refractory to other treatments and remains intolerable, especially at the end of life, neuroleptic agents or frank sedation may be required to provide relief.
Adeboye OO et al. Nonpain symptom management. Prim Care. 2019 Sep;46(3):335–51. [PMID: 31375185]
Chen YJ et al. Exercise training for improving patient-reported outcomes in patients with advanced-stage cancer: a systematic review and meta-analysis. J Pain Symptom Manage. 2020 Mar;59(3):734–49. [PMID: 31546002]
Kleckner AS et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019 Aug 1;11:1758835919866362. [PMID: 31413731]
Rao VL et al. Medical management of opioid-induced constipation. JAMA. 2019 Dec 10;322(10):2241–2. [PMID: 31682706]
Sorathia L. Palliative care in chronic obstructive pulmonary disease. Med Clin North Am. 2019 May;103(3):517–26. [PMID: 30955518]
CARE OF PATIENTS AT THE END OF LIFE
In the United States, more than 2.8 million people die each year. Caring for patients at the end of life is an important responsibility and a rewarding opportunity for clinicians. From the medical perspective, the end of life may be defined as that time when death—whether due to terminal illness or acute or chronic illness—is expected within hours to months and can no longer be reasonably forestalled by medical intervention. Palliative care at the end of life focuses on relieving distressing symptoms and promoting quality of life (as in all other stages of illness). For patients at the end of life, palliative care may become the sole focus of care.
Emanuel EJ. The status of end-of-life care in the United States: the glass is half full. JAMA. 2018 Jul 17;320(3):239–41. [PMID: 30027232]
 Prognosis at the End of Life
Prognosis at the End of Life
Clinicians must help patients understand when they are approaching the end of life. Most patients, and their family caregivers, want accurate prognostic information. This information influences patients’ treatment decisions, may change how they spend their remaining time, and does not negatively impact patient survival. One-half or more of cancer patients do not understand that many treatments they might be offered are palliative and not curative.
While certain diseases, such as cancer, are more amenable to prognostic estimates regarding the time course to death, the other common causes of mortality—including heart disease, stroke, chronic lung disease, and dementia—have more variable trajectories and difficult-to-predict prognoses. Even for patients with cancer, clinician estimates of prognosis are often inaccurate and generally overly optimistic. The advent of new anticancer treatments including immunotherapy and targeted therapies has made prognosis more challenging in some cancers. Nonetheless, clinical experience, epidemiologic data, guidelines from professional organizations, and computer modeling and prediction tools (eg, the Palliative Performance Scale or http://eprognosis.ucsf.edu/index.php) may be used to help offer patients more realistic estimates of prognosis. Clinicians can also ask themselves “Would I be surprised if this patient died in the next year?” to determine whether a discussion of prognosis would be appropriate. If the answer is “no,” then the clinician should initiate a discussion. Recognizing that patients may have different levels of comfort with prognostic information, clinicians can introduce the topic by simply saying, “I have information about the likely time course of your illness. Would you like to talk about it?”
Chu C et al. Prognostication in palliative care. Clin Med (Lond). 2019 Jul;19(4):306–10. [PMID: 31308109]
Hui D et al. Prognostication in advanced cancer: update and directions for future research. Support Care Cancer. 2019 Jun;27(6):1973–84. [PMID: 30863893]
Smith-Uffen MES et al. Estimating survival in advanced cancer: a comparison of estimates made by oncologists and patients. Support Care Cancer. 2019 Nov 28. [Epub ahead of print] [PMID: 31781946]
 Expectations About the End of Life
Expectations About the End of Life
Death is often regarded by clinicians, patients, and families as a failure of medical science. This attitude can create or heighten a sense of guilt about the failure to prevent dying. Both the general public and clinicians often view death as an enemy to be battled furiously in hospitals rather than as an inevitable outcome to be experienced as a part of life at home. As a result, most people in the United States die in hospitals or long-term care facilities even though they may have wished otherwise. There is a trend of fewer deaths in hospitals and more deaths at home or in other community settings.
Relieving suffering, providing support, and helping the patient make the most of their life should be foremost considerations, even when the clinician and patient continue to pursue cure of potentially reversible disease. Patients at the end of life and their families identify a number of elements as important to quality end-of-life care: managing pain and other symptoms adequately, avoiding inappropriate prolongation of dying, communicating clearly, preserving dignity, preparing for death, achieving a sense of control, relieving the burden on others, and strengthening relationships with loved ones.
 Communication & Care of the Patient
Communication & Care of the Patient
Caring for patients at the end of life requires the same skills clinicians use in other tasks of medical care: diagnosing treatable conditions, providing patient education, facilitating decision making, and expressing understanding and caring. Communication skills are vitally important and can be improved through training. Higher-quality communication is associated with greater satisfaction and awareness of patient wishes. Clinicians must become proficient at delivering serious news and then dealing with its consequences (Table 5–1). Smartphone and Internet communication resources are available to support clinicians (www.vitaltalk.org), and evidence suggests that communication checklists and guides can be effective. When the clinician and patient do not share a common language, the use of a professional interpreter is needed to facilitate clear communication and help broker cultural issues.
Table 5–1. Suggestions for the delivery of serious news.
Prepare an appropriate place and time.
Address basic information needs.
Be brief and direct; avoid jargon and euphemisms.
Allow for silence and expression of emotions.
Assess and validate patient reactions.
Respond to immediate discomforts and risks.
Listen actively and express empathy.
Achieve a common perception of the problem.
Reassure that care will continue.
Ensure follow-up and make specific plans for the future.
Three further obligations are central to the clinician’s role at this time. First, he or she must work to identify, understand, and relieve physical, psychological, social, and spiritual distress or suffering. Second, clinicians can serve as facilitators or catalysts for hope. While hope for a particular outcome such as cure may fade, it can be refocused on what is still possible. Although a patient may hope for a “miracle,” other more likely hopes can be encouraged and supported, including hope for relief of pain, for reconciliation with loved ones, for discovery of meaning, and for spiritual growth. With such questions as “What is still possible now for you?” and “When you look to the future, what do you hope for?” clinicians can help patients uncover hope, explore meaningful and realistic goals, and develop strategies to achieve them.
Finally, dying patients’ feelings of isolation and fear demand that clinicians assert that they will care for the patient throughout the final stage of life. The promise of nonabandonment is the central principle of end-of-life care and is a clinician’s pledge to serve as a caring partner, a resource for creative problem solving and relief of suffering, a guide during uncertain times, and a witness to the patient’s experiences—no matter what happens. Clinicians can say to a patient, “I will care for you whatever happens.”
Hanning J et al. Goals-of-care discussions for adult patients nearing end of life in emergency departments: a systematic review. Emerg Med Australas. 2019 Aug;31(4):525–32. [PMID: 31044525]
Paladino J et al. Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: a cluster randomized clinical trial of the Serious Illness Care Program. JAMA Oncol. 2019 Jun 1;5(6):801–9. [PMID: 30870556]
 Caring for the Family
Caring for the Family
Clinicians must be attuned to the potential impact of illness on the patient’s family: greater physical caregiving responsibilities and financial burdens as well as higher rates of anxiety, depression, chronic illness, and even mortality. The threatened loss of a loved one may create or reveal dysfunctional or painful family dynamics. Family caregivers, typically women, commonly provide the bulk of care for patients at the end of life, yet their work is often not acknowledged, supported, or compensated. Clinicians should recognize that in many cases patients and their families are the unit of care. Simply acknowledging and praising the caregiver can provide much needed and appreciated support.
Clinicians can help families confront the imminent loss of a loved one and often must negotiate amid complex and changing family needs. Identifying a spokesperson for the family, conducting family meetings, allowing all to be heard, and providing time for consensus may help the clinician work effectively with the family. Providing good palliative care to the patient can reduce the risk of depression and complicated grief in loved ones after the patient’s death. Palliative care support directly for caregivers improves caregiver depression.
Durepos P et al. What does death preparedness mean for family caregivers of persons with dementia? Am J Hosp Palliat Care. 2019 May;36(5):436–46. [PMID: 30518228]
 Clinician Self-Care
Clinician Self-Care
Many clinicians find caring for patients at the end of life to be one of the most rewarding aspects of practice. However, working with the dying is also sad and can invoke feelings of grief and loss in clinicians. Clinicians must be able to tolerate its uncertainty, ambiguity, and existential challenges. Clinicians also need to recognize and respect their own limitations, attend to their own needs, and work in sustainable health care systems in order to avoid being overburdened, overly distressed, or emotionally depleted.
Kamal AH et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manage. 2019 Nov 25. [Epub ahead of print] [PMID: 31778784]
Medisauskaite A et al. Reducing burnout and anxiety among doctors: randomized controlled trial. Psychiatry Res. 2019 Apr;274:383–90. [PMID: 30852432]
Zanatta F et al. Resilience in palliative healthcare professionals: a systematic review. Support Care Cancer. 2020 Mar;28(3):971–8. [PMID: 31811483]
 Decision Making, Advance Care Planning, & Advance Directives
Decision Making, Advance Care Planning, & Advance Directives
The idea that patients must choose between quality and quantity of life is an outmoded concept that presents patients with a false choice. Clinicians should discuss with patients that an approach that provides concurrent palliative and disease-focused care is the one most likely to achieve improvements in both quality and quantity of life. Patients deserve to have their health care be consistent with their values, preferences, and goals of care. Unfortunately, some evidence suggests that end-of-life care for some patients is determined more by local availability of services and physician comfort than by patient wishes. Well-informed, competent adults have a right to refuse life-sustaining interventions even if this would result in death. In order to promote patient autonomy, clinicians are obligated to inform patients about the risks, benefits, alternatives, and expected outcomes of medical interventions, such as cardiopulmonary resuscitation (CPR), mechanical ventilation, hospitalization and ICU care, and artificial nutrition and hydration. Advance directives are oral or written statements made by patients when they are competent that project their autonomy into the future and are intended to guide care should they lose the ability to make and communicate their own decisions. Advance directives are an important part of advance care planning—defined by an international Delphi panel as “a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care. The goal of advance care planning is to help ensure that people receive medical care that is consistent with their values, goals and preferences during serious and chronic illness.” Advance directives take effect when the patient can no longer communicate his or her preferences directly. While oral statements about these matters are ethically binding, they are not legally binding in all states. State-specific advance directive forms are available from a number of sources, including http://www.caringinfo.org.
Clinicians should facilitate the process for all patients—ideally, well before the end of life—to consider their preferences, to appoint a surrogate, to talk to that person about their preferences, and to complete a formal advance directive. There are numerous resources that can be helpful, such as https://prepareforyourcare.org. Most patients with a serious illness have already thought about end-of-life issues, want to discuss them with their clinician, want the clinician to bring up the subject, and feel better for having had the discussion. Patients who have such discussions with their clinicians are more satisfied with their clinician, perceived by their family as having a better quality of life at the end of life, less likely to die in the hospital, and more likely to utilize hospice care. The loved ones of patients who engage in advance care planning discussions are less likely to suffer from depression during bereavement. In the United States, Medicare provides payment to clinicians for having advance care planning discussions with patients.
One type of advance directive is the Durable Power of Attorney for Health Care (DPOA-HC) that allows the patient to designate a surrogate decision maker. The DPOA-HC is particularly useful because it is often difficult to anticipate what specific decisions will need to be made. The responsibility of the surrogate is to provide “substituted judgment”—to decide as the patient would, not as the surrogate wants. Clinicians should encourage patients to talk with their surrogates about their preferences generally and about scenarios that are likely to arise, such as the need for mechanical ventilation in a patient with end-stage emphysema. Clear clinician communication is important to correct misunderstandings and address biases. In the absence of a designated surrogate, clinicians usually turn to family members or next of kin. Regulations require health care institutions to inform patients of their rights to formulate an advance directive. Physician (or Medical) Orders for Life-Sustaining Treatment (POLST or MOLST) or Physician (or Medical) Orders for Scope of Treatment (POST or MOST) forms are clinician orders that document patient preferences and accompany patients wherever they are cared for—home, hospital, or nursing home. They are available in most states and used to complement advance directives for patients approaching the end of life.
Cauley CE et al. DNR, DNI, and DNO? J Palliat Med. 2019 Nov 12. [Epub ahead of print] [PMID: 31718398]
Kim JW et al. Completion rate of physician orders for life-sustaining treatment for patients with metastatic or recurrent cancer: a preliminary, cross-sectional study. BMC Palliat Care. 2019 Oct 22;18(1):84. [PMID: 31640677]
O’Halloran P et al. Advance care planning with patients who have end-stage kidney disease: a systematic realist review. J Pain Symptom Manage. 2018 Nov;56(5):795–807. [PMID: 30025939]
Pearse W et al. Advance care planning in the context of clinical deterioration: a systematic review of the literature. Palliat Care. 2019 Jan 19;12:1178224218823509. [PMID: 30718959]
 Do Not Attempt Resuscitation Orders
Do Not Attempt Resuscitation Orders
Because the “default” in US hospitals is that patients will undergo CPR in the event of cardiopulmonary arrest, as part of advance care planning, clinicians should elicit patient preferences about CPR. Most patients and many clinicians overestimate the chances of success of CPR. Only about 17% of all patients who undergo CPR in the hospital survive to hospital discharge and, among people with multisystem organ failure, metastatic cancer, and sepsis, the likelihood of survival to hospital discharge following CPR is virtually nil. Patients may ask their hospital clinician to write an order that CPR not be attempted on them. Although this order initially was referred to as a “DNR” (do not resuscitate) order, many clinicians prefer the term “DNAR” (do not attempt resuscitation) to emphasize the low likelihood of success. Some clinicians and institutions use an order to “Allow Natural Death” for situations in which death is imminent and the patient wishes to receive only those interventions that will promote comfort.
For most patients at the end of life, decisions about CPR may not be about whether they will live but about how they will die. Clinicians should correct the misconception that withholding CPR in appropriate circumstances is tantamount to “not doing everything” or “just letting someone die.” While respecting the patient’s right ultimately to make the decision—and keeping in mind their own biases and prejudices—clinicians should offer explicit recommendations about DNAR orders and protect dying patients and their families from feelings of guilt and from the sorrow associated with vain hopes. Clinicians should discuss what interventions will be continued and started to promote quality of life rather than focusing only on what is not to be done. For patients with implantable cardioverter defibrillators (ICDs), clinicians must also address issues of turning off these devices, while leaving the pacemaker function on, as death approaches to prevent the uncommon but distressing situation of the ICD discharging during the dying process.
 Hospice & Other Palliative Care Services
Hospice & Other Palliative Care Services
In the United States, hospice is a specific type of palliative care service that comprehensively addresses the needs of the dying, focusing on their comfort while not attempting to prolong their life or hasten their death. In the United States, 48.2% of people with Medicare who die use hospice, most at home or in a nursing home where they can be cared for by their family, other caregivers, and visiting hospice staff. Hospice care can also be provided in institutional residences and hospitals. As is true of all types of palliative care, hospice emphasizes individualized attention and human contact, and uses an interdisciplinary team approach. Hospice care can include arranging for respite for family caregivers and assisting with referrals for legal, financial, and other services. Patients in hospice require a physician, preferably their primary care clinician or specialist, to oversee their care.
Hospice care was used by 1.49 million Medicare beneficiaries in 2017 (the most recent year for which there are published data), about 30% of whom had cancer. Hospice is rated highly by families and has been shown to increase patient satisfaction and to decrease family caregiver mortality. In 2017, 48.2% of hospice patients died at home; 31.8% died in a skilled nursing facility. Despite evidence that suggests that hospice care does not shorten length of life, hospice care tends to be used very late, often near the very end of life. In 2017, the mean average length of stay in hospice care in the United States was 76.1 days, but the median length of stay was 24 days. More than half of patients died within 30 days of enrolling in a hospice, and 28% of patients died within 7 days of starting hospice.
In the United States, most hospice organizations require clinicians to estimate the patient’s prognosis to be less than 6 months, since this is a criterion for eligibility under the Medicare hospice benefit that is typically the same for other insurance coverage.
 Cultural Issues
Cultural Issues
The individual patient’s experience of dying occurs in the context of a complex interaction of personal, philosophic, and cultural values. Various religious, ethnic, gender, class, and cultural traditions influence a patient’s style of communication, comfort in discussing particular topics, expectations about dying and medical interventions, and attitudes about the appropriate disposition of dead bodies. While there are differences in beliefs regarding advance directives, autopsy, organ donation, hospice care, and withdrawal of life-sustaining interventions among patients of different ethnic groups, clinicians should be careful not to make assumptions about individual patients. Clinicians must appreciate that palliative care is susceptible to the same explicit and implicit biases documented in other medical disciplines. Being sensitive to a person’s cultural beliefs and respecting traditions are important responsibilities of the clinician caring for a patient at the end of life. A clinician may ask a patient, “What do I need to know about you and your beliefs that will help me take care of you?” and “How do you deal with these issues in your family?”
Mathew-Geevarughese SE et al. Cultural, religious, and spiritual issues in palliative care. Prim Care. 2019 Sep;46(3):399–413. [PMID: 31375189]
Wang SY et al. Racial differences in health care transitions and hospice use at the end of life. J Palliat Med. 2019 Jun;22(6):619–27. [PMID: 30615546]
 Nutrition & Hydration
Nutrition & Hydration
People approaching the end of life often lose their appetite and most stop eating and drinking in their last days. Clinicians should explain to families that the dying patient is not suffering from hunger or thirst; rather, the discontinuation of eating and drinking is part of dying. The anorexia-cachexia syndrome frequently occurs in patients with advanced cancer, and cachexia is common and a poor prognostic sign in patients with heart failure. Seriously ill people often have no hunger despite not eating at all and the associated ketonemia can produce a sense of well-being, analgesia, and mild euphoria. Although it is unclear to what extent withholding hydration at the end of life creates an uncomfortable sensation of thirst, any such sensation is usually relieved by simply moistening the dry mouth. Ice chips, hard candy, swabs, popsicles, or minted mouthwash may be effective. Although this normal process of diminishing oral intake and accompanying weight loss is very common, it can be distressing to patients and families who may associate the offering of food with compassion and love and lack of eating with distressing images of starvation. In response, patients and families often ask about supplemental enteral or parenteral nutrition.
Supplemental artificial nutrition and hydration offer no benefit to those at the end of life and rarely achieve patient and family goals. The American Geriatrics Society recommends against liquid artificial nutrition (“tube feeding”) in people with advanced dementia because it does not provide any benefit. Furthermore, enteral feeding may cause nausea and vomiting in ill patients and can lead to diarrhea in the setting of malabsorption. Artificial nutrition and hydration may increase oral and airway secretions as well as increase the risk of choking, aspiration, and dyspnea; ascites, edema, and effusions may be worsened. In addition, liquid artificial nutrition by nasogastric and gastrostomy tubes and parenteral nutrition impose risks of infection, epistaxis, pneumothorax, electrolyte imbalance, and aspiration—as well as the need to physically restrain the delirious patient to prevent dislodgment of tubes and catheters. A randomized trial of intravenous fluids found no benefit.
Individuals at the end of life have a right to voluntarily refuse all nutrition and hydration. Because they may have deep social and cultural significance for patients, families, and clinicians themselves, decisions about artificial nutrition and hydration are not simply medical. Eliciting perceived goals of artificial nutrition and hydration and correcting misperceptions can help patients and families make clear decisions.
Hoffman MR. Tracheostomies and PEGs: when are they really indicated? Surg Clin North Am. 2019 Oct;99(5):955–65. [PMID: 31446920]
Mayers T et al. International review of national-level guidelines on end-of-life care with focus on the withholding and withdrawing of artificial nutrition and hydration. Geriatr Gerontol Int. 2019 Sep;19(9):847–53. [PMID: 31389113]
 Withdrawal of Curative Efforts
Withdrawal of Curative Efforts
Requests from appropriately informed and competent patients or their surrogates for withdrawal of life-sustaining interventions must be respected. Limitation of life-sustaining interventions prior to death is common practice in ICUs in the United States. The withdrawal of life-sustaining interventions, such as mechanical ventilation, must be approached carefully to avoid patient suffering and distress for those in attendance. Clinicians should educate the patient and family about the expected course of events and the difficulty of determining the precise timing of death after withdrawal of interventions. Sedative and analgesic agents should be administered to ensure patient comfort even at the risk of respiratory depression or hypotension. While “death rattle,” the sound of air flowing over airway secretions, is common in actively dying patients and can be distressing to families, it is doubtful that it causes discomfort to the patient. Turning the patient can decrease the sound of death rattle. There is no evidence that any medications reduce death rattle, and suctioning should be avoided as it can cause patient discomfort.
McPherson K et al. Limitation of life-sustaining care in the critically ill: a systematic review of the literature. J Hosp Med. 2019 May;14(5):303–10. [PMID: 30794145]
Reignier J et al. Withholding and withdrawing life-support in adults in emergency care: joint position paper from the French Intensive Care Society and French Society of Emergency Medicine. Ann Intensive Care. 2019 Sep 23;9(1):105. [PMID: 31549266]
 Physician-Assisted Death
Physician-Assisted Death
Physician-assisted death is the legally sanctioned process by which patients who have a terminal illness may request and receive a prescription from a physician for a lethal dose of medication that they themselves would self-administer for the purpose of ending their own life. Terminology for this practice varies. “Physician-assisted death” is used here to clarify that a willing physician provides assistance in accordance with the law (by writing a prescription for a lethal medication) to a patient who makes a request for it and who meets specific criteria. Patients, family members, nonmedical and medical organizations, clinicians, lawmakers, and the public frequently use other terms, such as “physician or medical aid in dying,” “aid in dying,” “death with dignity,” or “physician-assisted suicide.” This latter term is not preferred because when this action is taken according to the law, it is not considered suicide and people who are actively suicidal are not eligible for this process.
Although public and state support for physician-assisted death has grown in the United States, physician-assisted death remains an area of active and intense debate. To date, no state court has recognized physician-assisted death as a fundamental “right.” As of 2019, physician-assisted death has been legalized with careful restriction and specific procedures for residents in nine US states (Oregon, Washington, Montana, Vermont, Colorado, Hawaii, and California) and in the District of Columbia, making it available to 22% of the US population. Physician-assisted death remains illegal in all other states. Internationally, physician-assisted death (and/or euthanasia, the administration a lethal dose of medication by a clinician) is legal in nine countries (the Netherlands, Belgium, Luxembourg, Switzerland, Colombia, Canada, Germany, Japan, and the Australian state of Victoria). There are no universal standards about whether patients who request lethal medication for self-administration require a particular prognosis or about what types and levels of suffering qualify them for it, although the current US state laws permitting it generally require physician certification of a terminal disease with a prognosis of 6 months or less. These laws generally also require the individual to be an adult resident of the state, to be physically capable of self-administering the medication, and to be mentally competent (eg, physician or mental health professional certification that they are capable of making and communicating their own healthcare decisions). Laws in the United States authorizing physician-assisted death distinguish it from euthanasia, which is illegal in the United States. Any clinician that participates in physician-assisted death should be familiar with the laws governing its use in their jurisdiction and seek recommendations and help with writing the appropriate prescription.
Most requests for physician-assisted death come from patients with cancer. In the United States, most patients requesting it are male, college-educated, and receiving hospice care. Requests for physician-assisted death are relatively rare. Internationally, less than 5% of deaths are due to either physician-assisted death or euthanasia in locales where one or both of these are legal. In Oregon, the first US state to legalize physician-assisted death, approximately 0.39% of deaths in 2015 resulted from this practice. In California in 2017, just 0.21% of people who died did so through physician-assisted death. Patient motivations for physician-assisted death generally revolve around preserving dignity, self-respect, and autonomy (control), and maintaining personal connections at the end of life rather than experiencing intolerable pain or suffering. Some patients who have requested medication to self-administer for a physician-assisted death later withdraw their request when provided palliative care interventions.
Each clinician must decide his or her personal approach in caring for patients who ask about physician-assisted death. Regardless of the clinician’s personal feelings about the process, the clinician can respond initially by exploring the patient’s reasons and concerns that prompted the request. During the dialog, the clinician should inform the patient about palliative options, including hospice care; access to expert symptom management; and psychological, social, and spiritual support, as needed, and provide reassurance and commitment to address future problems that may arise. For clinicians who object to physician-assisted death on moral or ethical grounds, referral to another clinician may be necessary and may help the patient avoid feeling abandoned. That clinician must be willing to provide the prescription for lethal medication, to care for the patient until death (though it is not necessary to be present at the death), to sign the death certificate listing the underlying terminal condition as the cause of death, and in some jurisdictions to complete a mandatory follow-up form.
Hedberg K et al. Oregon’s Death with Dignity Act: 20 years of experience to inform the debate. Ann Intern Med. 2017 Oct 17;167(8):579–83. [PMID: 28975232]
Jansen LA et al. Drawing the line on physician-assisted death. J Med Ethics. 2019 Mar;45(3):190–7. [PMID: 30463933]
McClelland W et al. Withholding or withdrawing life support versus physician-assisted death: a distinction with a difference? Curr Opin Anaesthesiol. 2019 Apr;32(2):184–9. [PMID: 30817393]
Russell JA. Physician-hastened-death in patients with progressive neurodegenerative or neuromuscular disorders. Semin Neurol. 2018 Oct;38(5):522–32. [PMID: 30321890]
 Ethical & Legal Issues
Ethical & Legal Issues
Clinicians’ care of patients at the end of life is guided by the same ethical and legal principles that inform other types of medical care. Foremost among these are (1) truth-telling, (2) nonmaleficence, (3) beneficence, (4) autonomy, (5) confidentiality, and (6) procedural and distributive justice. Important ethical principles may come into conflict when caring for patients. For example, many treatments that promote beneficence and autonomy, such as surgery or bone marrow transplant, may violate the clinician’s obligation for nonmaleficence; thus, balancing the benefits and risks of treatments is a fundamental ethical responsibility. Similarly, while a patient may express his or her autonomy as a desire for a particular medical intervention such as CPR in the setting of multisystem organ failure, the clinician may decline to provide the intervention because it is futile (ie, of no therapeutic benefit and thus violates both beneficence and nonmaleficence). However, clinicians must use caution in invoking futility, since strict futility is rare and what constitutes futility is often a matter of controversy and subject to bias. While in the vast majority of cases clinicians and patients and families will agree on the appropriateness of and decisions to withdraw life-sustaining interventions, in rare cases, such as CPR in multisystem organ failure, clinicians may determine unilaterally that a particular intervention is medically inappropriate. In such cases, the clinician’s intention to withhold CPR should be communicated to the patient and family and documented, and the clinician must consult with another clinician not involved in the care of the patient. If differences of opinion persist about the appropriateness of particular care decisions, the assistance of an institutional ethics committee should be sought. Because such unilateral actions violate the autonomy of the patient, clinicians should rarely resort to such unilateral actions. Studies confirm that most disagreements between patients and families and clinicians can be resolved with good communication. Although clinicians and family members often feel differently about withholding versus withdrawing life-sustaining interventions, there is consensus among ethicists, supported by legal precedent, of their ethical equivalence.
The ethical principle of “double effect” argues that the potential to hasten imminent death is acceptable if it comes as the known but unintended consequence of a primary intention to provide comfort and relieve suffering. For example, it is acceptable to provide high doses of opioids if needed to control pain even if there is the known and unintended potential effect of depressing respiration.
Chessa F et al. Ethical and legal considerations in end-of-life care. Prim Care. 2019 Sep;46(3):387–98. [PMID: 31375188]
Rodrigues P et al. Palliative sedation for existential suffering: a systematic review of argument-based ethics literature. J Pain Symptom Manage. 2018 Jun;55(6):1577–90. [PMID: 29382541]
 Psychological, Social, & Spiritual Issues
Psychological, Social, & Spiritual Issues
Dying is not exclusively or even primarily a biomedical event. It is an intimate personal experience with profound psychological, interpersonal, and existential meanings. For many people at the end of life, the prospect of impending death stimulates a deep and urgent assessment of their identity, the quality of their relationships, the meaning and purpose of their life, and their legacy.
A. Psychological Challenges
In 1969, Dr. Elisabeth Kübler-Ross identified five psychological reactions or patterns of emotions that patients at the end of life may experience: denial and isolation, anger, bargaining, depression, and acceptance. Most patients will experience these reactions throughout the course of illness and not in an orderly progression. In addition to these five reactions are the perpetual challenges of anxiety and fear of the unknown. Simple information, listening, assurance, and support may help patients with these psychological challenges. Patients and families rank emotional support as one of the most important aspects of good end-of-life care. Psychotherapy and group support may be beneficial as well.
Despite the significant emotional stress of facing death, clinical depression is not normal at the end of life and should be treated. Cognitive and affective signs of depression, such as feelings of worthlessness, hopelessness, or helplessness, may help distinguish depression from the low energy and other vegetative signs common with advanced illness. Although traditional antidepressant treatments such as selective serotonin reuptake inhibitors are effective, more rapidly acting medications, such as dextroamphetamine (2.5–7.5 mg orally at 8 am and noon) or methylphenidate (2.5–10 mg orally at 8 am and noon), may be particularly useful when the end of life is near or while waiting for another antidepressant medication to take effect. Ketamine is now approved, with restrictions, as a treatment for depression and psychedelics are being explored as rapid-onset treatment for anxiety and depression at the end of life. Some research suggests a mortality benefit from treating depression in the setting of serious illness.
B. Social Challenges
At the end of life, patients should be encouraged to take care of personal, professional, and business obligations. These tasks include completing important work or personal projects, distributing possessions, writing a will, and making funeral and burial arrangements. The prospect of death often prompts patients to examine the quality of their interpersonal relationships and to begin the process of saying goodbye (Table 5–2). Concern about estranged relationships or “unfinished business” with significant others and interest in reconciliation may become paramount at this time.
Table 5–2. Five statements often necessary for the completion of important interpersonal relationships.

C. Spiritual Challenges
Spirituality includes the attempt to understand or accept the underlying meaning of life, one’s relationships to oneself and other people, one’s place in the universe, one’s legacy, and the possibility of a “higher power” in the universe. People may experience spirituality as part of or distinct from particular religious practices or beliefs.
Unlike physical ailments, such as infections and fractures, which usually require a clinician’s intervention to be treated, the patient’s spiritual concerns often require only a clinician’s attention, listening, and witness. Clinicians can inquire about the patient’s spiritual concerns and ask whether the patient wishes to discuss them. For example, asking, “How are you within yourself?” or “Are you at peace?” communicates that the clinician is interested in the patient’s whole experience and provides an opportunity for the patient to share perceptions about his or her inner life. Questions that might constitute an existential “review of systems” are presented in Table 5–3. Formal legacy work and dignity therapy have been shown to be effective in improving quality of life and spiritual well-being.
Table 5–3. An existential review of systems.
Intrapersonal
“What does your illness/dying mean to you?”
“What do you think caused your illness?”
“How have you been healed in the past?”
“What do you think is needed for you to be healed now?”
“What is right with you now?”
“What do you hope for?”
“Are you at peace?”
Interpersonal
“Who is important to you?”
“To whom does your illness/dying matter?”
“Do you have any unfinished business with significant others?”
Transpersonal
“What is your source of strength, help, or hope?”
“Do you have spiritual concerns or a spiritual practice?”
“If so, how does your spirituality relate to your illness/dying, and how can I help integrate your spirituality into your health care?”
“What do you think happens after we die?”
“What do you think is trying to happen here?”
Attending to the spiritual concerns of patients calls for listening to their stories. Storytelling gives patients the opportunity to verbalize what is meaningful to them and to leave something of themselves behind—a legacy, the promise of being remembered. Storytelling may be facilitated by suggesting that the patient share his or her life story with family members, make an audio or video recording, assemble a photo album, organize a scrapbook, or write or dictate an autobiography.
The end of life offers an opportunity for psychological, interpersonal, and spiritual development and to experience and achieve important goals. Individuals may grow—even experience a heightened sense of well-being or transcendence—in the process of dying. Through listening, support, and presence, clinicians may help foster this learning and be a catalyst for this transformation. Rather than thinking of dying simply as the termination of life, clinicians and patients may be guided by a developmental model of life that recognizes a series of lifelong developmental tasks and landmarks and allows for growth at the end of life.
Egan R et al. Spiritual beliefs, practices, and needs at the end of life: results from a New Zealand national hospice study. Palliat Support Care. 2017 Feb;31(2):140–6. [PMID: 27572901]
Gerson SM et al. Medical aid in dying, hastened death and suicide: a qualitative study of hospice professionals’ experiences from Washington State. J Pain Symptom Manage. 2020 Mar;59(3):679–86. [PMID: 31678464]
Puchalski CM et al. Spiritual considerations. Hematol Oncol Clin North Am. 2018 Jun;32(3):505–17. [PMID: 29729785]
Strada EA. Psychosocial issues and bereavement. Prim Care. 2019 Sep;46(3):373–86. [PMID: 31375187]
Wholihan D. Psychological issues of patient transition from intensive care to palliative care. Crit Care Nurs Clin North Am. 2019 Dec;31(4):547–56. [PMID: 31685121]
TASKS AFTER DEATH
After the death of a patient, the clinician is called upon to perform a number of tasks, both required and recommended. The clinician must plainly and directly inform the family of the death, complete a death certificate, contact an organ procurement organization, and request an autopsy. Providing words of sympathy and reassurance, time for questions and initial grief and, for people who die in the hospital or other health care facility, a quiet private room for the family to grieve is appropriate and much appreciated.
 The Pronouncement & Death Certificate
The Pronouncement & Death Certificate
In the United States, state policies direct clinicians to confirm the death of a patient in a formal process called “pronouncement.” The diagnosis of death is typically easy to make, and the clinician need only verify the absence of spontaneous respirations and cardiac activity by auscultating for each for 1 minute. A note describing these findings, the time of death, and that the family has been notified is entered in the patient’s medical record. In many states, when a patient whose death is expected dies outside of the hospital (at home or in prison, for example), nurses may be authorized to report the death over the telephone to a physician who assumes responsibility for signing the death certificate within 24 hours. For traumatic deaths, some states allow emergency medical technicians to pronounce a patient dead at the scene based on clearly defined criteria and with physician telephonic or radio supervision.
While the pronouncement may often seem like an awkward and unnecessary formality, clinicians may use this time to reassure the patient’s loved ones at the bedside that the patient died peacefully and that all appropriate care had been given. Both clinicians and families may use the ritual of the pronouncement as an opportunity to begin to process emotionally the death of the patient.
Physicians are legally required to report certain deaths to the coroner and to accurately report the underlying cause of death on the death certificate. This reporting is important both for patients’ families (for insurance purposes and the need for an accurate family medical history) and for the epidemiologic study of disease and public health. The physician should be specific about the major cause of death being the condition without which the patient would not have died (eg, “decompensated cirrhosis”) and its contributory cause (eg, “hepatitis B and hepatitis C infections, chronic alcoholic hepatitis, and alcoholism”) as well as any associated conditions (eg, “acute kidney injury”)—and not simply put down “cardiac arrest” as the cause of death. In relevant cases, it is prohibited (in some jurisdictions) to list either “physician-assisted death” (or any synonymous term) or the medication used to accomplish it (eg, secobarbital) on the death certificate; instead, the clinician prescribing the lethal dose of medication for this purpose and following the patient until death must (in most jurisdictions) complete and submit a follow-up form and list the cause of death as the underlying condition that led to death.
Hatano Y et al. Physician behavior toward death pronouncement in palliative care units. J Palliat Med. 2018 Mar;21(3):368–72. [PMID: 28945507]
 Autopsy & Organ Donation
Autopsy & Organ Donation
Discussing the options and obtaining consent for autopsy and organ donation with patients prior to death is a good practice as it advances the principle of patient autonomy and lessens the responsibilities of distressed family members during the period immediately following the death. In the United States, federal regulations require that a designated representative of an organ procurement organization approach the family about organ donation if the organs are appropriate for transplantation because designated organ transplant personnel are more experienced and successful than treating clinicians at obtaining consent for organ donation from surviving family members. While most people in the United States support the donation of organs for transplants, organ transplantation is severely limited by the availability of donor organs. The families of donors experience a sense of reward in contributing, even through death, to the lives of others.
The results of an autopsy may help surviving family members and clinicians understand the exact cause of a patient’s death and foster a sense of closure. Despite the use of more sophisticated diagnostic tests, the rate of unexpected findings at autopsy has remained stable, and thus, an autopsy can provide important health information to families. Pathologists can perform autopsies without interfering with funeral plans or the appearance of the deceased. A clinician–family conference to review the results of the autopsy provides a good opportunity for clinicians to assess how well families are grieving and to answer questions.
Buja LM et al. The importance of the autopsy in medicine: perspectives of pathology colleagues. Acad Pathol. 2019 Mar 10;6:2374289519834041. [PMID: 30886893]
 Follow-Up & Grieving
Follow-Up & Grieving
Proper care of patients at the end of life includes following up with surviving family members after the patient has died. Contacting loved ones by telephone enables the clinician to assuage any guilt about decisions the family may have made, assess how families are grieving, reassure them about the nature of normal grieving, and identify complicated grief or depression. Clinicians can recommend support groups and counseling as needed. A card or telephone call from the clinician to the family days to weeks after the patient’s death (and perhaps on the anniversary of the death) allows the clinician to express concern for the family and the deceased.
After a patient dies, clinicians also grieve. Although clinicians may be relatively unaffected by the deaths of some patients, other deaths may cause feelings of sadness, loss, and guilt. These emotions should be recognized as the first step toward processing and healing them. Each clinician may find personal or communal resources that help with the process of grieving. Shedding tears, sharing with colleagues, taking time for reflection, and engaging in traditional or personal mourning rituals all may be effective. Attending the funeral of a patient who has died can be a satisfying personal experience that is almost universally appreciated by families and that may be the final element in caring well for people at the end of life.
Johannsen M et al. Psychological interventions for grief in adults: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2019 Jun 15;253:69–86. [PMID: 31029856]
Strada EA. Psychosocial issues and bereavement. Prim Care. 2019 Sep;46(3):373–86. [PMID: 31375187]
PAIN MANAGEMENT
TAXONOMY OF PAIN
The International Association for the Study of Pain (IASP) defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Acute pain resolves within the expected period of healing and is self-limited. Chronic pain persists beyond the expected period of healing and is itself a disease state. In general, chronic pain is defined as extending beyond 3–6 months, although definitions vary in terms of the time period from initial onset of nociception. Cancer pain is in its own special category because of the unique ways neoplasia and its therapies (such as surgery, chemotherapy, or radiation therapy) can lead to burdensome pain. Finally, related to cancer pain, there is pain at the end of life, for which measures to alleviate suffering may take priority over promoting restoration of function.
Pain is a worldwide burden; across the globe; one in five adults suffers from pain. In 2010, members from 130 countries signed the Declaration of Montreal stating that access to pain management is a fundamental human right. The first CDC guidelines on opioid prescribing for chronic pain, including chronic noncancer pain, cancer pain, and pain at the end of life, were published in March of 2016, and continue to be updated.
Centers for Disease Control and Prevention (CDC). CDC guideline for prescribing opioids for chronic pain. 2019 Aug 28. https://www.cdc.gov/drugoverdose/prescribing/guideline.html
Dowell D et al. No shortcuts to safer opioid prescribing. N Engl J Med. 2019 Jun 13;380(24):2285–7. [PMID: 31018066]
ACUTE PAIN
Acute pain resolves within the expected period of healing and is self-limited. Common examples include pain from dental caries, kidney stones, surgery, or trauma. Management of acute pain depends on comprehending the type of pain (somatic, visceral, or neuropathic) and on understanding the risks and benefits of potential therapies. At times, treating the underlying cause of the pain (eg, dental caries) may be all that is needed, and pharmacologic therapies may not be required for additional analgesia. On the other hand, not relieving acute pain can have consequences beyond the immediate suffering. Inadequately treated acute pain can develop into chronic pain in some patients. This transition from acute to chronic pain (so-called “chronification” of pain) depends on the pain’s cause, type, and severity and on the patient’s age, psychological status, and genetics, among other factors. This transition is an area of increasing study because chronic pain leads to significant societal costs beyond the individual’s experiences of suffering, helplessness, and depression. Emerging studies have shown that increased intensity and duration of acute pain may be correlated with a higher incidence of chronic pain, and regional anesthesia, ketamine, gabapentinoids, and cyclooxygenase (COX) inhibitors may be helpful for prevention of chronic postsurgical pain. These approaches are particularly important given concerns that exposure to opioids in the perioperative period can lead to chronic opioid dependence beyond the immediate postoperative period.
The Oxford League Table of Analgesics is a useful guide; for example, it lists the number-needed-to-treat for specific doses of various medications to relieve acute pain. Nonsteroidal anti-inflammatory drugs (NSAIDs) or COX inhibitors are at the top of the list, with the lowest number-needed-to-treat. These medications can be delivered via oral, intramuscular, intravenous, intranasal, rectal, and other routes of administration. They generally work by inhibiting COX-1 and -2 and therefore reduce the levels of prostaglandins involved in inflammatory nociception (eg, PGI2 and PGE2). These oxygenase enzymes also determine levels of other breakdown products such as other prostaglandins, thromboxane, and prostacyclins that play a role in renal, gastrointestinal, and cardiovascular homeostasis. For this reason, the primary limitation of the COX inhibitors is their side effect profile of gastritis, kidney dysfunction, bleeding, hypertension, and cardiovascular adverse events such as myocardial infarction or stroke. Ketorolac is primarily a COX-1 inhibitor that has an analgesic effect as potent as morphine at the appropriate dosage. Like most pharmacologic therapies, the limitation of COX inhibitors is that they have a “ceiling” effect, meaning that beyond a certain dose, there is no additional benefit.
Acetaminophen (paracetamol) is effective as a sole agent, or in combination with a COX inhibitor or an opioid in acute pain. Its mechanism of action remains undetermined but is thought to act centrally through mechanisms such as the prostaglandin, serotonergic, and opioid pathways. It is one of the most widely used and best tolerated analgesics; its primary limitation is hepatoxicity when given in high doses or to patients with underlying impaired liver function.
Postoperatively, patient-controlled analgesia (PCA) with intravenous morphine, hydromorphone, or another opioid can achieve analgesia faster and with less daily medication requirement than with standard “as needed” or even scheduled intermittent dosing. PCA has been adapted for use with oral analgesic opioid medications and for neuraxial delivery of both opioids and local anesthetics in the epidural and intrathecal spaces. The goal of PCA is to maintain a patient’s plasma concentration of opioid in the “therapeutic window,” between the minimum effective analgesic concentration and a toxic dose.
In order to prevent opioid use disorder and prolonged inappropriate opioid use, multimodal analgesia (including regional anesthesia) has been employed to decrease the need for postoperative opioids. Patients may undergo either neuraxial anesthesia with an epidural catheter, for example, or undergo regional anesthesia with a nerve block with or without a catheter. These techniques are effective for both intraoperative pain and postoperative pain management and can diminish the need for both intraoperative and postoperative opioids.
Helander EM et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017 Jan;21(1):3. [PMID: 28132136]
Polomano RC et al. Multimodal analgesia for acute postoperative and trauma-related pain. Am J Nurs. 2017 Mar;117(3 Suppl 1):S12–26. [PMID: 28212146]
Small C et al. Acute postoperative pain management. Br J Surg. 2020 Jan;107(2):e70–80. [PMID: 31903595]
CHRONIC NONCANCER PAIN
Chronic noncancer pain may begin as acute pain that then fails to resolve and extends beyond the expected period of healing or it may be a primary disease state, rather than the symptom residual from another condition. Common examples of chronic noncancer pain include chronic low-back pain and arthralgias (often somatic in origin), chronic abdominal pain and pelvic pain (often visceral in origin), and chronic headaches, peripheral neuropathy, and postherpetic neuralgia (neuropathic origin) as well as other less common but debilitating syndromes such as chronic trigeminal neuralgia (neuropathic origin) and complex regional pain syndrome (mixed origin). Chronic noncancer pain is common, with the World Health Organization estimating a worldwide prevalence of 20%. In the United States, 11% of adults suffer from chronic noncancer pain, and the Institute of Medicine estimates that it costs $635 billion annually in treatment and lost productivity costs.
Chronic noncancer pain requires interdisciplinary management. Generally, no one therapy by itself is sufficient to manage such chronic pain. Physical or functional therapy and cognitive behavioral therapy have been shown to be the most effective for treating chronic noncancer pain, but other modalities including pharmacologic therapy, interventional modalities, and complementary/integrative approaches are useful in caring for affected patients.
Chronic low-back pain is one example of a common chronic noncancer pain. It causes more disability globally than any other condition. Chronic low-back pain includes spondylosis, spondylolisthesis, and spinal canal stenosis (Chapter 24), and the “failed back surgery syndrome,” a term used to refer to patients in whom chronic pain develops, persists after lumbar spine surgery, or both. Also referred to as the post-laminectomy pain syndrome, it can affect 10–40% of patients after lumbar spine surgery.
The importance of clinicians knowing the many causes of chronic low-back pain and, in particular, understanding how anatomic structures relate to one another and how they can cause the different types of low-back pain, has been highlighted by the epidemic of opioid abuse in the United States since the year 2000. In fact, evidence-based practice does not support the use of prolonged opioid therapy for chronic low-back pain.
Manchikanti L et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017 Feb;20(2S):S3–92. [PMID: 28226332]
Qaseem A et al. Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Apr 4;166(7):514–30. [PMID: 28192789]
CANCER PAIN
Cancer pain deserves its own category because it is unique in cause and in therapies. Cancer pain consists of both acute pain and chronic pain from the neoplasm itself and from the therapies associated with it, such as surgery, chemotherapy, radiation, and immunotherapy. In addition, patients with cancer pain may also have acute or chronic non–cancer-related pain, and this possibility should not be overlooked when taking care of cancer patients.
Cancer pain includes somatic pain (eg, neoplastic invasion of tissue such as painful fungating chest wall masses in breast cancer), visceral pain (eg, painful hepatomegaly from liver metastases, stretching the liver capsule), neuropathic pain (eg, neoplastic invasion of sacral nerve roots), or pain from a paraneoplastic syndrome (eg, peripheral neuropathy related to anti-Hu antibody production). Chemotherapy can cause peripheral neuropathies, radiation can cause neuritis or skin allodynia, and surgery can cause persistent postsurgical pain syndromes such as post-mastectomy or post-thoracotomy pain syndromes.
Generally, patients with cancer pain do not exhibit a single type of pain—they may have multiple reasons for pain and thus benefit from a comprehensive and multimodal strategy. The WHO Analgesic Ladder, first published in 1986, suggests starting medication treatment with nonopioid analgesics, then weak opioid agonists, followed by strong opioid agonists. While opioid therapy can be helpful for a majority of patients living with cancer pain, therapy must be individualized depending on the individual patient, their family, and the clinician. For example, if one of the goals of care is to have a lucid and coherent patient, opioids may not be the optimal choice; interventional therapies such as implantable devices may be an option, weighing their risks and costs against their potential benefits. Alternatively, in dying patients, provided there is careful documentation of continued, renewed, or accelerating pain, use of opioid doses exceeding those recommended as standard for acute (postoperative) pain is acceptable.
One of the unique challenges in treating cancer pain is that it is often a “moving target,” with disease progression and improvements in disease progression or worsening pain directly stemming from chemotherapy, radiation, or immunotherapy. Therefore, frequent adjustments may be required to any pharmacologic regimen. Interventional approaches such as celiac plexus neurolysis and intrathecal therapy are well-studied and may be appropriate both for analgesia as well as reduction of side effects from systemic medications. Radiation therapy (including single-fraction external beam treatments) or radionuclide therapy (eg, strontium-89), which aims to decrease the size of both primary and metastatic disease, is one of the unique options for patients with pain from cancer.
Careskey H et al. Interventional anesthetic methods for pain in hematology/oncology patients. Hematol Oncol Clin North Am. 2018 Jun;32(3):433–45. [PMID: 29729779]
Lau J et al. Interventional anesthesia and palliative care collaboration to manage cancer pain: a narrative review. Can J Anaesth. 2020 Feb;67(2):235–46. [PMID: 31571119]
Neufeld NJ et al. Cancer pain: a review of epidemiology, clinical quality and value impact. Future Oncol. 2017 Apr;13(9):833–41. [PMID: 27875910]
Paice JA. Under pressure: the tension between access and abuse of opioids in cancer pain management. J Oncol Pract. 2017 Sep;13(9):595–6. [PMID: 28813190]
PAIN AT THE END OF LIFE
Pain is what many people say they fear most about dying, and pain at the end of life is consistently undertreated. Up to 75% of patients dying of cancer, heart failure, chronic obstructive pulmonary disease, AIDS, or other diseases experience pain. In the United States, the Joint Commission includes pain management standards in its reviews of health care organizations and, in 2018, it began mandating that each hospital have a designated leader in pain management.
The ratio of risk versus benefit changes in end-of-life pain management. Harms from the use of opioid analgesics, including death, eg, from respiratory depression (rare), are perhaps less of a concern in patients approaching the end of life. In all cases, clinicians must be prepared to use appropriate doses of opioids in order to relieve this distressing symptom for these patients. Typically, for ongoing cancer pain, a long-acting opioid analgesic can be given around the clock with a short-acting opioid medication as needed for “breakthrough” pain.
PRINCIPLES OF PAIN MANAGEMENT
The experience of pain is unique to each person and influenced by many factors, including the patient’s prior experiences with pain, meaning given to the pain, emotional stresses, and family and cultural influences. Pain is a subjective and multi-faceted phenomenon, and clinicians cannot reliably detect its existence or quantify its severity without asking the patient directly. A brief means of assessing pain and evaluating the effectiveness of analgesia is to ask the patient to rate the degree of pain along a numeric or visual pain scale (Table 5–4), assessing trends over time. Clinicians should ask about the nature, severity, timing, location, quality, and aggravating and relieving factors of the pain.
Table 5–4. Pain assessment scales.

General guidelines for diagnosis and management of pain are recommended for the treatment of all patients with pain but clinicians must comprehend that such guidelines may not be suited for every individual. Because of pain’s complexity, it is important to understand benefits and risks of treatment with growing evidence for each patient. Distinguishing between nociceptive (somatic or visceral) and neuropathic pain is essential to proper management.
In addition, while clinicians should seek to diagnose the underlying cause of pain and then treat it, they must balance the burden of diagnostic tests or therapeutic interventions with the patient’s suffering. For example, single-fraction radiation therapy for painful bone metastases or nerve blocks for neuropathic pain may obviate the need for ongoing treatment with analgesics and their side effects. Regardless of decisions about seeking and treating the underlying cause of pain, every patient should be offered prompt pain relief.
The aim of effective pain management is to meet specific goals, such as preservation or restoration of function or quality of life, and this aim must be discussed between provider and patient, as well as their family. For example, some patients may wish to be completely free of pain even at the cost of significant sedation, while others will wish to control pain to a level that still allows maximal cognitive functioning.
Whenever possible, the oral route of analgesic administration is preferred because it is easier to manage at home, is not itself painful, and imposes no risk from needle exposure. In unique situations, or near the end of life, transdermal, subcutaneous, rectal, and intravenous routes of administration are used; intrathecal administration is used when necessary.
Finally, pain management should not automatically indicate opioid therapy. While many individuals fare better with opioid therapy in specific situations, this does not mean that opioids are the answer for every patient. There are situations where opioids actually make the quality of life worse for individuals, due to a lack of adequate analgesic effect or due to their side effects.
 Barriers to Good Care
Barriers to Good Care
One barrier to good pain control is that many clinicians have limited training and clinical experience with pain management and thus are reluctant to attempt to manage severe pain. Lack of knowledge about the proper selection and dosing of analgesic medications carries with it attendant and typically exaggerated fears about the side effects of pain medications. Consultation with a palliative care or a pain management specialist may provide additional expertise.
PHARMACOLOGIC PAIN MANAGEMENT STRATEGIES
Pain generally can be well controlled with nonopioid and opioid analgesic medications, complemented by nonpharmacologic adjunctive and interventional treatments. For mild to moderate pain, acetaminophen, aspirin, and NSAIDs (also known as COX inhibitors) may be sufficient. For moderate to severe pain, especially for those with acute pain, short courses of opioids are sometimes necessary; for those with cancer pain or pain from advanced, progressive serious illness, opioids are generally required and interventional modalities should be considered. In all cases, the choice of an analgesic medication must be guided by careful attention to the physiology of the pain and the benefits and risks of the particular analgesic being considered.
 Acetaminophen, Aspirin, Celecoxib, & NSAIDs (COX Inhibitors)
Acetaminophen, Aspirin, Celecoxib, & NSAIDs (COX Inhibitors)
Table 5–5 provides comparison information for acetaminophen, aspirin, the COX-2 inhibitor celecoxib and the NSAIDs. An appropriate dose of acetaminophen may be just as effective an analgesic and antipyretic as an NSAID but without the risk of gastrointestinal bleeding or ulceration. Acetaminophen can be given at a dosage of 500–1000 mg orally every 6 hours, not to exceed 4000 mg/day maximum for short-term use. Total acetaminophen doses should not exceed 3000 mg/day for long-term use or 2000 mg/day for older patients and for those with liver disease. Hepatotoxicity is of particular concern because of how commonly acetaminophen is also an ingredient in various over-the-counter medications and because of failure to account for the acetaminophen dose in combination acetaminophen-opioid medications such as Vicodin or Norco. The FDA has limited the amount of acetaminophen available in some combination analgesics (eg, in acetaminophen plus codeine preparations).
Table 5–5. Acetaminophen, aspirin, and useful nonsteroidal anti-inflammatory drugs and COX inhibitors.


Aspirin (325–650 mg orally every 4 hours) is an effective analgesic, antipyretic, and anti-inflammatory medication. Gastrointestinal irritation and bleeding are side effects that are lessened with enteric-coated formulations and by concomitant use of proton pump inhibitor medication. Bleeding, allergy, and an association with Reye syndrome in children and adolescents further limit its use.
NSAIDs are antipyretic, analgesic, and anti-inflammatory. Treatment with NSAIDs increases the risk of gastrointestinal bleeding 1.5 times; the risks of bleeding and nephrotoxicity are both increased in elders. Gastrointestinal bleeding and ulceration may be prevented with either the concurrent use of proton pump inhibitors (eg, omeprazole, 20–40 mg orally daily) or the use of celecoxib (100 mg orally daily to 200 mg orally twice daily), the only COX-2 inhibitor available. Celecoxib and the NSAIDs can lead to fluid retention, kidney injury, and exacerbations of heart failure and should be used with caution in patients with that condition. Topical formulations of NSAIDs (such as diclofenac 1.3% patch or 1% gel), placed over the painful body part for treatment of musculoskeletal pain, are associated with less systemic absorption and fewer side effects than oral administration and are likely underutilized in patients at risk for gastrointestinal bleeding.
Chang AK et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017 Nov 7;318(17):1661–7. [PMID: 29114833]
Wiffen PJ et al. Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017 Jul 12;7:CD012637. [PMID: 28700092]
 Opioids
Opioids
A. A Shared Decision-Making Approach to Clinical Opioid Use
For many patients at the end of life, opioids are the mainstay of pain management (Tables 5–6 and 5–7). Opioids are appropriate for managing severe pain at the end of life due to any cause, including neuropathic pain, cancer pain, and pain from other serious illnesses. Using opioids long-term in other settings requires careful consideration.
In an effort to treat chronic pain more aggressively, clinicians in the United States dramatically increased the prescription of opioids beginning in the mid-1990s and peaking in 2010. More attention to treating chronic noncancer pain undoubtedly improved the lives of many patients, but the increase in prescribed opioids had a deleterious effect on the health of the population as a whole. The increased population exposure to prescription opioids appears to have expanded the market for illicit opioids (heroin, fentanyl and its derivatives), with concomitant increase in opioid use disorder and in opioid overdoses, which caused nearly 50,000 deaths in 2017. The CDC named both misuse of prescription medications and opioid overdoses as epidemics in the United States and released guidelines in 2016 to limit the risks of prescribed opioids (https://www.cdc.gov/drugoverdose/prescribing/guideline.html). Also in 2016, the US Surgeon General directly appealed to prescribing physicians to focus on combating the opioid epidemic and issued a report titled “Facing Addiction in America” (https://addiction.surgeongeneral.gov/sites/default/files/surgeon-generals-report.pdf). The CDC later issued a follow up clarification to their guidelines to encourage clinicians and insurers to avoid the unintended consequence of denying opioids to patients with cancer, sickle cell disease, and other conditions not targeted in the guidelines for chronic, noncancer pain. As of late 2019, experts recommend prescribing a limited supply of opioids to patients with severe, acute pain (fracture, postoperative), avoiding initiation of opioids for chronic noncancer pain, careful monitoring of patients already on opioid therapy for chronic noncancer pain, and evidence-based treatment of opioid use disorder if it is diagnosed.
Taking the approach of carefully evaluating benefits and risks in individual cases allows the opportunity for shared decision making between patient and clinician. Clinical trials do suggest more harms than benefits for the population of people prescribed opioids for chronic noncancer pain. While observational data indicate that a majority of patients taking high doses of opioids long-term have improved pain control when they voluntarily reduce their doses, they also suggest that forced or involuntary tapers may have significant negative consequences. It is incumbent upon the clinician to provide frank advice to patients prescribed long-term opioids for chronic noncancer pain and to offer safer alternatives when the benefit is insufficient or the risks are too high.
B. Assessing Benefits of Opioids
Opioids have long been known to be effective in managing acute pain. The potential benefits of daily opioid therapy for patients with chronic noncancer pain are less impressive. For example, research demonstrates that the beneficial effect of opioids for chronic noncancer pain is modest in magnitude and limited in duration. No measures have been identified to predict a good response. The improvements are generally measured in terms of a reduction in the analog pain score of 2–3 points on a 10-point scale (see Table 5–4) or in improvements in the important but less precise outcome of function. For patients already receiving daily, long-term opioid therapy, clinicians should discuss these modest benefits with patients to help set realistic goals of therapy (eg, moving from an average pain level of a “7” to a “4”). Many experts recommend developing a specific goal of improved function (eg, return to work or to an exercise regimen) and tracking the patient’s progress toward achieving this goal.
For the many patients who do not have specific measurable goals, monitoring response to treatment over time can be difficult. A useful tracking measure derived from the Brief Pain Inventory and validated for use in primary care is the “PEG,” which directs patients to quantify on a scale of 0–10 the following three outcomes over the last week: average pain intensity, how much the pain has affected their enjoyment of life, and how much their pain has impacted their general activity. Patients who do not progress toward their goal or whose PEG scores remain high over time may have pain that is unresponsive to opioids, and clinicians should reconsider the original diagnosis and use other modalities (both pharmacologic and nonpharmacologic) to provide analgesia. Without a clear analgesic benefit from opioids for chronic noncancer pain, the risks may predominate, and the ineffective therapy should be discontinued in a patient-centered manner.
C. Formulations and Regimens
Full opioid agonists such as morphine, hydromorphone, oxycodone, methadone, fentanyl, hydrocodone, tramadol, and codeine are used most commonly (Table 5–6). Hydrocodone and codeine are typically combined with acetaminophen or an NSAID, although acetaminophen in these combinations is restricted to 300–325 mg per unit dose due to the risk of hepatotoxicity. Extended-release hydrocodone without acetaminophen is also available. Short-acting formulations of oral morphine sulfate (starting dosage 4–8 mg orally every 3–4 hours), hydromorphone (1–2 mg orally every 3–4 hours), or oxycodone (5 mg orally every 3–4 hours) are useful for severe acute pain not controlled with other analgesics. The transmucosal intermediate-release fentanyl products, such as oral transmucosal fentanyl (200 mcg oralet dissolved in the mouth) or buccal fentanyl (100 mcg dissolved in the mouth), can be used for treating patients with cancer pain that breaks through long-acting medications, or it can be administered before activity known to cause more pain (such as burn wound dressing changes). Buprenorphine as a short-acting analgesic generally should be reserved for use by pain management specialists.
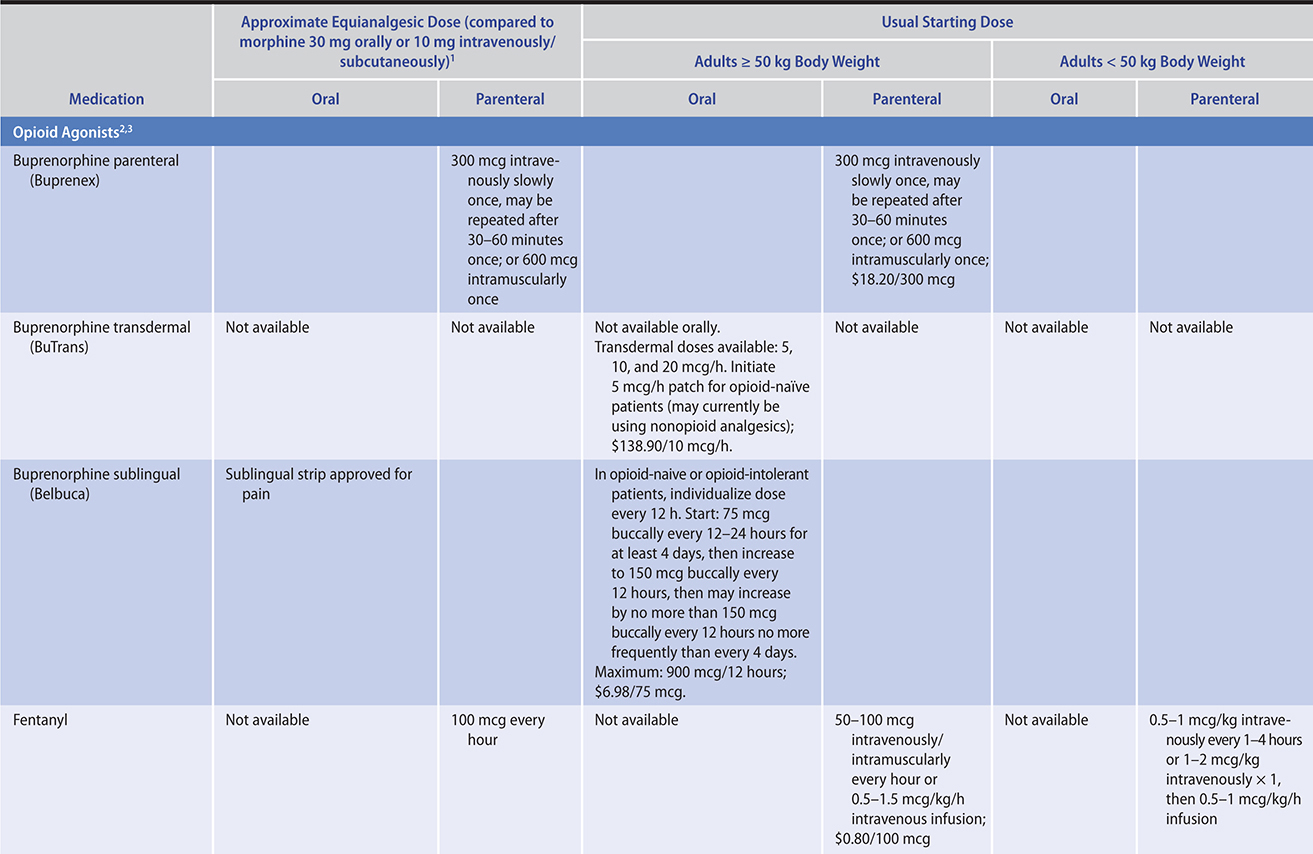
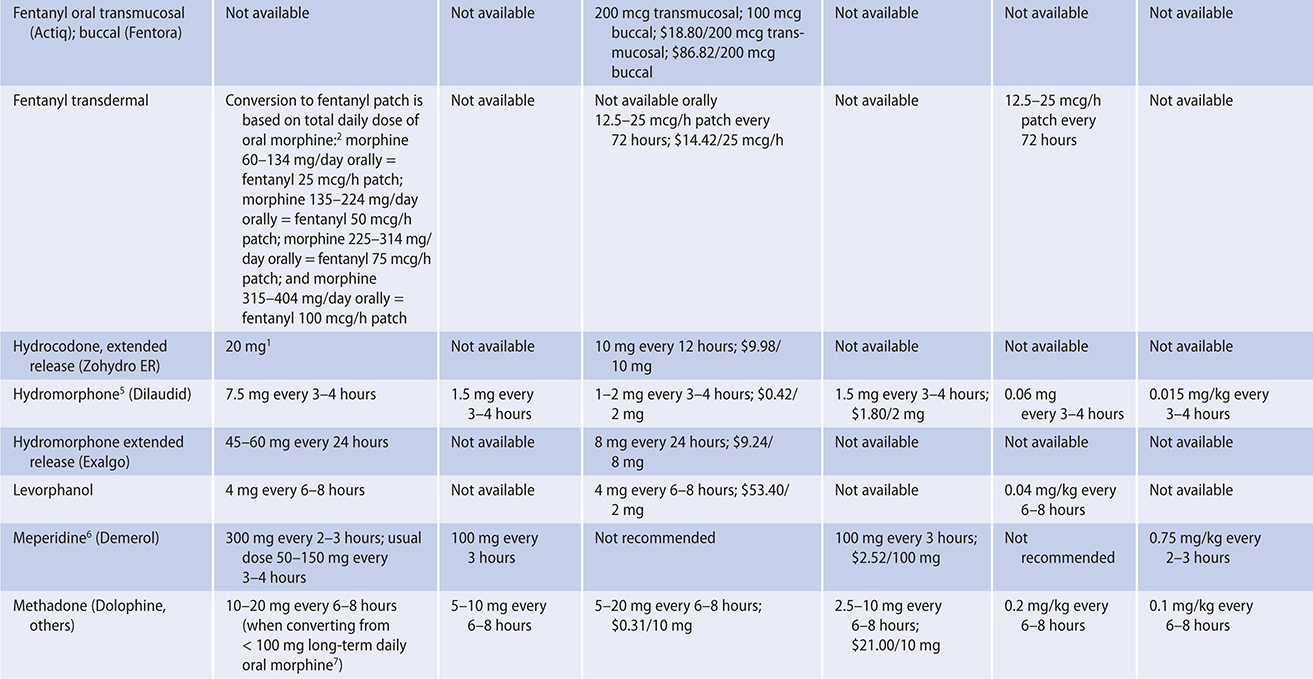
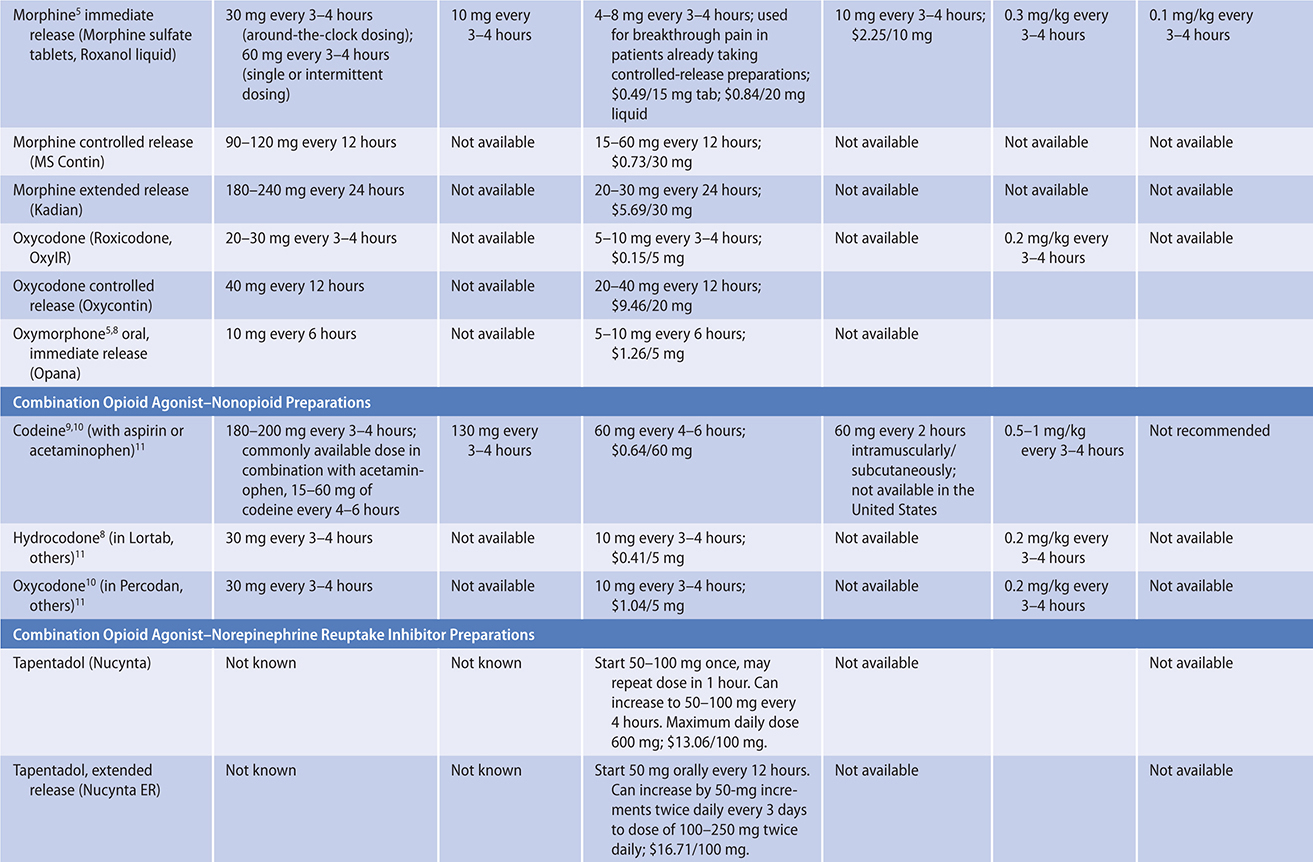

Table 5–7. Opioids advantages and disadvantages.

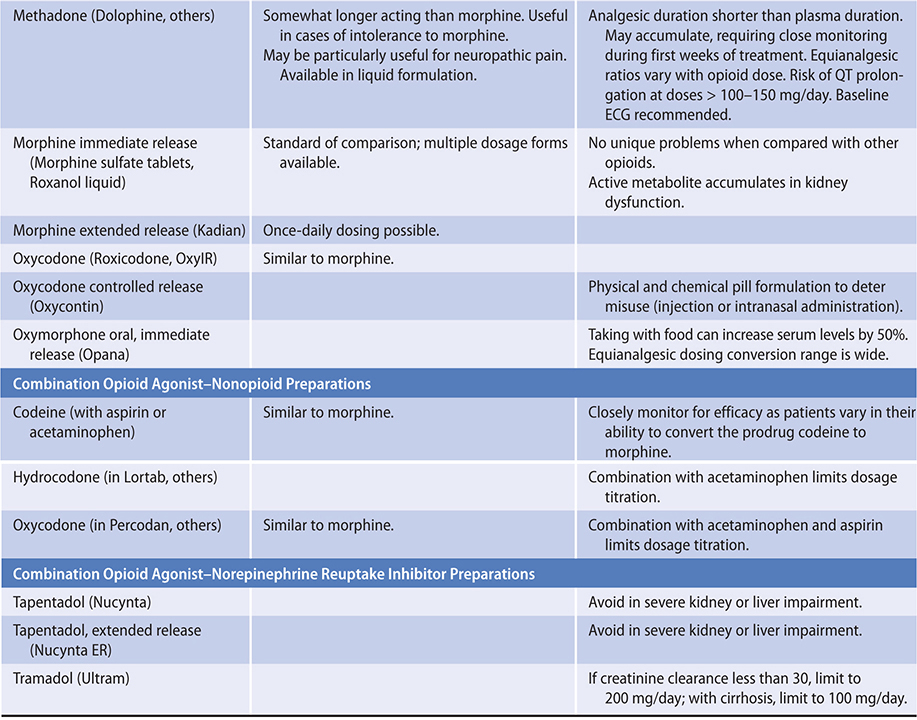
Clinicians prescribing opioids must understand the concept of equianalgesic dosing. The dosages of any full opioid agonists used to control pain can be converted into an equivalent dose of any other opioid. This approach is helpful in estimating the appropriate dose of a long-acting opioid based on the amount of short-acting opioid required over the preceding days. For example, 24-hour opioid requirements established using short-acting opioid medications can be converted into equivalent dosages of long-acting medications or formulations. Cross-tolerance is often incomplete, however, so generally only two-thirds to three-quarters of the full, calculated equianalgesic dosage is administered initially when switching between opioid formulations.
For chronic stable cancer pain, long-acting medications are preferred, such as oral sustained-release formulations of morphine (one to three times a day), hydromorphone (once daily), oxymorphone (two times a day), oxycodone (two or three times a day), hydrocodone (two times a day), or methadone (three or four times a day), all of which have long half-lives. In chronic noncancer pain, long-acting full opioid agonists increase risks of complication (see section Common Side Effects of Opioids, below) without demonstrable improvement in pain control.
The partial agonist buprenorphine is effective in the treatment of moderate to severe chronic pain and is available in parenteral, transdermal, and buccal formulations. Attractive benefits of buprenorphine compared to other opioids include its long half-life, lower risk of sedation and respiratory depression during treatment, and lower likelihood of withdrawal upon discontinuation.
The transdermal patch of buprenorphine (BuTrans) is available in dosages of 5, 10, and 20 mcg/h. The buccal buprenorphine strip formulation (Belbuca) is sometimes used by pain management specialists for moderate to severe constant pain. It can be more frequently uptitrated since it is given twice daily. Depending on the patient’s current opioid usage, it can be started at 75–300 mcg once or twice daily, then escalated by 150- to 450-mcg doses twice daily to a maximum of 900 mcg twice daily. The parenteral formulation of buprenorphine (Buprenex) can be used for more acute pain in settings where more rapid onset or higher peak is required. The usual dosages are 300 mcg intravenously once (may be repeated once after 30–60 minutes) or as 600 mcg intramuscularly once.
In addition, buprenorphine comes in significantly more potent formulations generally reserved for the treatment of opioid use disorder with or without comorbid constant pain: a sublingual tablet (Subutex and others), a sublingual film (Suboxone and others) in which the buprenorphine is combined with naloxone, a subdermal implant of buprenorphine alone (Probuphine), and a subcutaneous depot injection (Sublocade). These are used in maintenance treatment to reduce problematic use of other opioids but should be considered for off-label analgesic use in patients who have been maintained on high doses of other opioids, since available evidence indicates that most patients experience improvement in their pain control after transition.
Methadone deserves special consideration among the long-acting opioids because it is inexpensive, available in a liquid formulation, and may have added efficacy for neuropathic pain. However, equianalgesic dosing is complex because it varies with the patient’s opioid dose, and caution must be exercised at higher methadone doses (generally more than 100–150 mg/day) because of the risk of QT prolongation. Baseline electrocardiography should be considered before starting methadone and repeated up to monthly, except at the end of life where comfort is the only goal. Given the complexities of management, consultation with a palliative medicine or pain specialist may be appropriate.
Transdermal fentanyl is only appropriate to use with patients already tolerant to other opioids for at least 1 week at a dose equivalent to at least 60 mg/day of oral morphine (equivalent to a transdermal fentanyl 25 mcg/h patch applied topically every 72 hours). Therefore, it should not be used in the postoperative setting. It should not be the first opioid used with any patient. Since transdermal fentanyl can require 24–48 hours to achieve a pharmacologic “steady state,” patients should be weaned off their current opioid and given short-acting opioids while awaiting the full analgesic effect of a newly prescribed transdermal fentanyl patch. Changes in dose of transdermal fentanyl should be made no more frequently than every 6 days.
While some clinicians inexperienced with the management of severe pain at the end of life may be more comfortable prescribing combined nonopioid-opioid agents, full agonist opioids are typically a better choice in patients with such severe pain because the dose of opioid is not limited by the toxicities of the acetaminophen, aspirin, or NSAID component of combination preparations. In end-of-life care, there may be no maximal allowable or effective dose for full opioid agonists, but for patients with longer life expectancy or for patients suffering from chronic noncancer pain, expert guidelines recommend avoiding long-term opioids when possible, and limiting total daily dose to less than the equivalent of 90 mg of morphine. When titrating, clinicians should confirm that increasing doses of opioid provide additional pain relief and remember that not all pain is opioid sensitive and that certain types of pain, such as neuropathic pain, may respond better to agents other than opioids, or to combinations of opioids with coanalgesic medications for neuropathic pain.
While physiologic tolerance is expected with opioids, failure of a previously effective opioid dose to adequately relieve pain in a patient with cancer is usually due to worsening of the underlying condition causing pain, such as tumor growth or new metastasis. In this case, for moderate unrelieved pain, the dose of opioid can be increased by 25–50%. For severe unrelieved pain, a dose increase of 50–100% may be appropriate. The frequency of dosing should be adjusted so that pain control is continuous. Long-term dosing may then be adjusted by adding the average daily amount of short-acting opioid necessary for breakthrough pain over the preceding 72–96 hours to the long-acting medication dose. In establishing or reestablishing adequate dosing, frequent reassessments of the patient’s pain and medication side effects are necessary.
D. Common Side Effects of Opioids
At higher doses or with long-term use of opioids, patients may experience increasing difficulty with the side effects. Opioid-related constipation should be anticipated and prevented in all patients. Constipation is common at any dose of opioid, and tolerance to this side effect does not develop over time. Prescribing a bowel regimen (see Chapter 15) to a patient taking opioids long term is a quality of care measure supported by the National Quality Forum.
Sedation can be expected with opioids, although tolerance to this effect and to side effects other than constipation typically develops within 24–72 hours at a stable dose. Sedation typically appears well before significant respiratory depression. If treatment for sedation is desired, dextroamphetamine (2.5–7.5 mg orally at 8 am and noon) or methylphenidate (2.5–10 mg orally at 8 am and noon) may be helpful. Caffeinated beverages can also ameliorate minor opioid sedation. For patients with noncancer pain who experience sedation, decreasing the available dose is recommended.
Opioid-induced neurotoxicity, including myoclonus, hyperalgesia, delirium with hallucinosis, and seizures, may develop in patients who take high doses of opioids for a prolonged period. Opioid-induced hyperalgesia appears to be a result of changes in both the peripheral and central nervous systems such that typically benign or even soothing stimuli (eg, light massage) may be perceived as painful (allodynia); increasing the opioid dose may exacerbate the problem. Opioid-induced neurotoxicity symptoms typically resolve after lowering the dose or switching opioids (“opioid rotation”). While waiting for the level of the offending opioid to fall in patients receiving end-of-life care, low doses of clonazepam, baclofen, or gabapentin may be helpful for treating myoclonus; haloperidol may be useful for treating delirium. Avoiding or correcting dehydration may be helpful for avoiding opioid-induced neurotoxicity.
Nausea may occur with initiation of opioid therapy and resolve after a few days. Notably, unrelieved constipation may be a more likely cause of nausea in the setting of opioid use than opioid-induced nausea. Severe or persistent nausea despite treatment of constipation can be managed by switching opioids or by giving haloperidol, 0.5–4 mg orally, subcutaneously, or intravenously every 6 hours or prochlorperazine, 10 mg orally or intravenously or 25 mg rectally every 6 hours. Ondansetron, 4–8 mg orally or intravenously every 6 hours, also relieves nausea but can contribute to constipation. Mirtazapine and medical cannabis may each have a role in treating opioid-induced nausea. Most antiemetic treatments can contribute to sedation.
The risk of respiratory depression with opioids may be decreased by initiating the opioid drug at a low dose and titrating it upward slowly, taking advantage of physiologic tolerance. Patients at particular risk for respiratory depression include those with obstructive or central sleep apnea, chronic obstructive pulmonary disease, and baseline CO2 retention; those with liver or kidney or combined liver-kidney failure; and those with adrenal insufficiency or frank myxedema. Yet, even patients with severe pulmonary disease and obstructive sleep apnea can tolerate low-dose opioids, although these patients should be monitored carefully. Hospitalized patients with these conditions who require increased doses of opioids should be monitored with continuous pulse oximetry.
While physiologic tolerance (requiring increasing dosage to achieve the same analgesic effect) and dependence (requiring continued dosing to prevent symptoms of medication withdrawal) are expected with regular opioid use, the use of opioids at the end of life for relief of pain and dyspnea is not generally associated with a risk of psychological addiction (use of a substance despite negative health or social consequences, cravings to use a substance, compulsive use or loss of control over level or time of use). The risk of addiction increases the longer the duration and the higher the dose of opioid exposure. If opioid use disorder is diagnosed in a patient, then the appropriate treatment is opioid agonist therapy with either methadone or buprenorphine, often accompanied by psychosocial interventions. Patients with opioid use disorder or other substance use disorders may need pain relief and may benefit from additional opioids, but their pain control will be inadequate without proper treatment of the substance use disorder.
Additional adverse side effects of long-term opioid use include hypogonadism, falls, fractures, and difficult interactions with the health care system. Finally, diversion of medication from patients to whom they are prescribed into other hands is an additional risk that must be considered when prescribing long-term opioids. Diversion can represent opportunism, eg, when a patient sells medication in order to make money. In addition, family members (including children), acquaintances, or strangers may steal or extort medication for their own use or monetary gain.
E. Limiting Risks of Opioids
A number of interventions have been used in an effort to limit the risks of opioids for patients with chronic noncancer pain. Data demonstrating the effectiveness of such measures are limited, but nearly all medical society consensus panels and expert guidelines recommend using risk assessment tools, patient-provider agreements, urine drug testing, dose limitations, limits on the use of some medications, and medication treatments of opioid use disorder.
1. Risk assessment tool—No highly predictive models adequately predict harms or benefits from long-term opioids for chronic noncancer pain. Nevertheless, most published guidelines recommend using an instrument like the Opioid Risk Tool (available at https://www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf) to determine how closely to monitor patients who are receiving opioids long term, or whether to offer long-term opioids at all.
2. Patient-provider agreements—Also known as “pain contracts,” these agreements have a modest effect, with a 7–23% reduction in aberrant behaviors reported. They do represent an opportunity for the clinician to discuss explicitly the risks and benefits of opioids for chronic noncancer pain, protocols and procedural requirements for refills and monitoring, and consequences of worrisome behaviors.
3. Urine drug testing—Urine drug testing is a toxicology tool borrowed from addiction treatment programs with goals of limiting diversion and identifying risky secondary drug use. Guidelines recommend more frequent urine drug testing with any increased risk as determined by dose, risk assessment tool, or recent behavior. It is imperative that clinicians choose the tests appropriately and understand the limitations of toxicology testing when using this tool. Universal testing is recommended, given provider inability to judge misuse of medication and documented racial biases in monitoring.
4. Dose limitations—Risk of overdose increases approximately linearly with dose in observational studies. The CDC considers doses above the equivalent of 50 mg of morphine per day to be risky, and specifically recommends against prescribing more than 120 mg of morphine per day. To avoid withdrawal, clinicians must be cautious when tapering a patient’s long-term dose. No data support one tapering regimen over another, but for patients taking opioids for years, the CDC recommends no more than a monthly decrease of 10% of the original daily dose. Tapering too quickly may result in dissolution of the therapeutic relationship or may cause patients to engage in risky behaviors, such as use of nonprescribed opioids. It is notable that the most rapid rise of opioid overdose deaths in the United States occurred following the institution of restrictions on prescription opioids.
5. Special medication limitations—Many guidelines recommend that the prescription of methadone and fentanyl be limited to pain management, addiction, or palliative care specialists. Because of the increasing incidence of opioid overdoses, recent professional guidelines recommend against concurrent prescription of opioids with benzodiazepines.
6. Antidote to overdose—Distributing naloxone, a quick-onset opioid-receptor antagonist, has long been known to reduce overdose deaths in people who use heroin. More recently, prescribing naloxone to patients taking opioids for chronic noncancer pain has been demonstrated to reduce rates of opioid overdose deaths. Educating both patients and their caregivers on the use of “rescue” naloxone is important, since those experiencing sedation and respiratory suppression from opioid overdose will not be able to self-administer the naloxone. In addition to preloaded needle-tipped syringes, intranasal and intramuscular autoinjector naloxone preparations are approved for sale in the United States, where an increasing number of states authorize pharmacies to dispense naloxone in the absence of a prescription. CDC guidelines recommend, and some state laws require, prescribing naloxone for any patient with history of overdose, substance use disorder, concomitant benzodiazepine use, or daily doses above 50 mg morphine equivalent.
7. Medication treatment of opioid use disorder—Some patients who have been treated with long-term opioids will develop an opioid use disorder, and when this diagnosis is made, their opioid management should transition to appropriate treatment with methadone or buprenorphine maintenance. Both of these options have demonstrated a mortality benefit for patients with opioid addiction. Depending on jurisdiction, restrictions on how these—or other opioid agonist—treatments are delivered will apply.
Frank JW et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017 Aug 1;167(3):181–91. [PMID: 28715848]
George B et al. Opioids in cancer-related pain: current situation and outlook. Support Care Cancer. 2019 Aug;27(8):3105–18. [PMID: 31127436]
Manchikanti L et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017 Feb;20(2S):S3–92. [PMID: 28226332]
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Medication-Assisted Treatment for Opioid Use Disorder; Mancher M et al (editors). Medications for Opioid Use Disorder Save Lives. Washington (DC): National Academies Press, 2019 Mar 30. [PMID: 30896911]
Tucker HR et al. Harms and benefits of opioids for management of non-surgical acute and chronic low back pain: a systematic review. Br J Sports Med. 2019 Mar 22. [Epub ahead of print] [PMID: 30902816]
Wood E et al. Pain management with opioids in 2019–2020. JAMA. 2019;322(19):1912–3. [PMID: 31600370]
 Medications for Neuropathic Pain
Medications for Neuropathic Pain
When taking a patient’s history, listening for pain descriptions such as “burning,” “shooting,” “pins and needles,” or “electricity,” and for pain associated with numbness is essential because such a history suggests neuropathic pain. Studies are mixed with regard to efficacy of opioids for neuropathic pain. However, a number of nonopioid medications have also been found to be effective in randomized trials (Table 5–8). Successful management of neuropathic pain often requires the use of more than one effective medication. Since these medications bind to receptors on a large variety of neurons, they often have central nervous system side effects. These side effects often limit reaching therapeutic doses and may be the reason for higher numbers needed to treat (NNT 4–7) as compared to NSAIDs (NNT 2–4) (Table 5–9).
Table 5–8. Pharmacologic management of neuropathic pain.
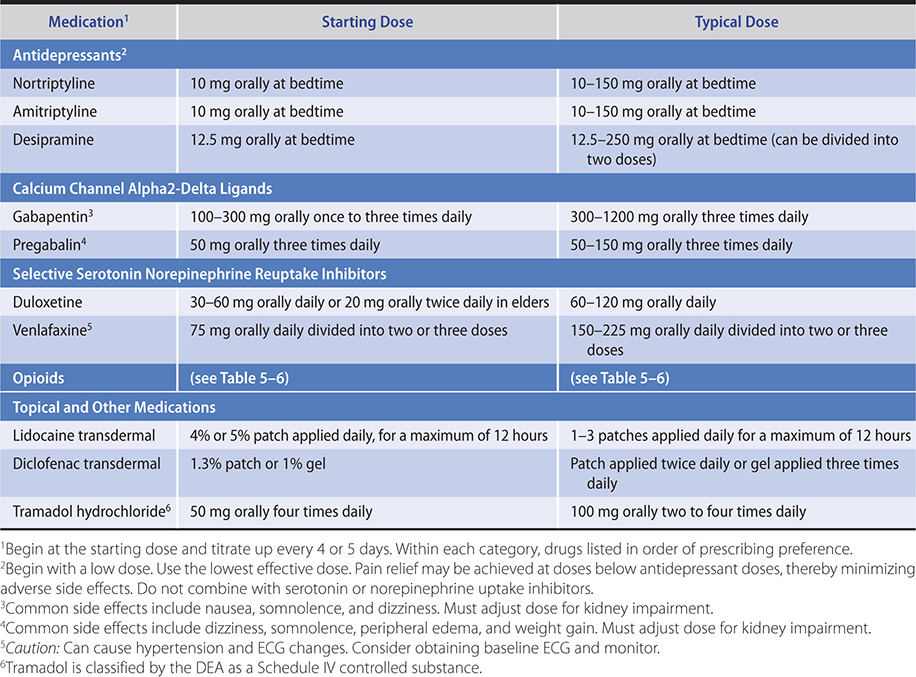
Table 5–9. Medications used for treatment of peripheral neuropathic pain.
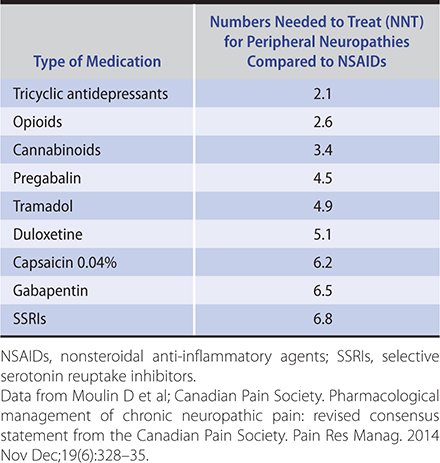
The calcium channel alpha2-delta ligands, gabapentin and pregabalin, are first-line therapies for neuropathic pain. Both medications have no significant medication interactions. However, they can cause sedation, dizziness, ataxia, and gastrointestinal side effects. Both gabapentin and pregabalin require dose adjustments in patients with kidney dysfunction. Gabapentin should be started at low dosages of 100–300 mg orally once daily and titrated upward by 300 mg/day every 4–7 days by adding additional doses throughout the day with a typical effective dose of 1800–3600 mg/day in three divided doses. Pregabalin should be started at 40–150 mg/day in two or three divided doses. If necessary, the dose of pregabalin can be titrated upward to 300–600 mg/day in two or three divided doses. Both medications are relatively safe in accidental overdose and may be preferred over tricyclic antidepressants (TCAs) for a patient with a history of heart failure or arrhythmia or if there is a risk of suicide. Prescribing both gabapentin and an opioid for neuropathic pain may provide better analgesia at lower doses than if each is used as a single agent.
The serotonin norepinephrine reuptake inhibitors (SNRIs) duloxetine and venlafaxine are also first-line treatments for neuropathic pain. Patients should be advised to take duloxetine on a full stomach because nausea is a common side effect. Duloxetine may provide increased benefit for neuropathic pain up to a total daily dose of 120 mg, beyond the 60-mg limit used for depression. Duloxetine generally should not be combined with other serotonin or norepinephrine uptake inhibitors, but it can be combined with gabapentin or pregabalin. Lower doses of venlafaxine have more serotonin than norepinephrine activity; therefore, higher doses may be required to treat neuropathic pain. Because venlafaxine can cause hypertension and induce ECG changes, patients with cardiovascular risk factors should be carefully monitored when starting this medication. Desvenlafaxine, the active metabolite of venlafaxine, is also available and may be tolerated better than venlafaxine.
TCAs are another class of medications for neuropathic pain that work through the norepinephrine and serotonin pathways. Among the TCAs that are effective for neuropathic pain, nortriptyline and desipramine are preferred over amitriptyline because they cause less orthostatic hypotension and have fewer anticholinergic effects. Start with a low dosage (10–25 mg orally daily) and titrate upward in 10-mg increments every 4 or 5 days aiming to use the lowest effective dose and to titrate up to a maximum of no greater than 100 mg daily. It may take several weeks for a TCA to have its full analgesic effect for neuropathic pain. Because TCAs and SNRIs both work through the serotonin and norepinephrine pathways, they generally should not be co-prescribed, particularly due to concerns for the serotonin syndrome.
Topical medications, such as 5% lidocaine patch and capsaicin 8% patches, are considered second-line therapies. The 5% lidocaine patch is particularly effective in postherpetic neuralgia and may be effective in other types of localized neuropathic pain. Due to its relatively minimal adverse effects, it is commonly used despite being considered second-line. The 4% lidocaine patch is available over the counter. Other medications effective for neuropathic pain include tramadol and tapentadol, both of which are opioids with norepinephrine activity. Medical cannabis strains high in cannabidiol have proven efficacy for some types of neuropathic pain.
Alles SRA et al. Etiology and pharmacology of neuropathic pain. Pharmacol Rev. 2018 Apr;70(2):315–47. [PMID: 29500312]
Fornasari D. Pharmacotherapy for neuropathic pain: a review. Pain Ther. 2017 Dec;6(Suppl 1):25–33. [PMID: 29178034]
 Adjuvant Pain Medications & Treatments
Adjuvant Pain Medications & Treatments
If pain cannot be controlled without intolerable medication side effects, clinicians should consider using lower doses of multiple medications, which is done commonly for neuropathic pain, rather than larger doses of one or two medications.
For metastatic bone pain, the anti-inflammatory effect of NSAIDs can be helpful. Furthermore, bisphosphonates (such as pamidronate and zoledronic acid) and receptor activator of NF-kappa-B ligand (RANKL) inhibitors (such as denosumab) may relieve such bone pain, although they are generally more useful for prevention of bone metastases than for analgesia.
Corticosteroids, such as dexamethasone, prednisone, and methylprednisolone, can be helpful for patients with headache due to increased intracranial pressure, pain from spinal cord compression, metastatic bone pain, and neuropathic pain due to invasion or infiltration of nerves by tumor. Because of the side effects of long-term corticosteroid administration, they are most appropriate for short-term use and in patients with end-stage disease. Low-dose intravenous, oral, buccal, and nasal ketamine has been used successfully for neuropathic and other pain syndromes refractory to opioids, although research data are limited.
Michelet D et al. Ketamine for chronic non-cancer pain: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur J Pain. 2018 Apr;22(4):632–46. [PMID: 29178663]
Rasu RS et al. Assessing prescribing trends of adjuvant medication therapy in outpatients with a diagnosis of noncancer chronic pain. Clin J Pain. 2017 Sep;33(9):786–92. [PMID: 28002095]
INTEGRATIVE THERAPIES & OTHER PAIN MANAGEMENT
Nonpharmacologic and noninterventional therapies are valuable in treating pain. In fact, physical or functional therapy and cognitive behavioral therapy have been shown to be the most effective for management of chronic pain. In particular, cognitive behavioral therapy has been proven effective in multiple randomized controlled studies as a primary evidence-based treatment for chronic pain. Hot or cold packs, massage, and physical therapy can be helpful for musculoskeletal pain. Similarly, integrative medicine therapies of acupuncture, chiropractic care, biofeedback, meditation, music therapy, guided imagery, cognitive distraction, and framing may be of help in treating pain. Because mood and psychological issues play an important role in the patient’s perception of and response to pain, psychotherapy, support groups, prayer, and pastoral counseling can also help in pain management. Depression and anxiety, which may be instigated by chronic pain or may alter the response to pain, should be treated aggressively with antidepressants and anxiolytics.
Martorella G et al. Tailored web-based interventions for pain: systematic review and meta-analysis. J Med Internet Res. 2017 Nov 10;19(11):e385. [PMID: 29127076]
Polatin P et al. Pharmacological treatment of depression in geriatric chronic pain patients: a biopsychosocial approach integrating functional restoration. Expert Rev Clin Pharmacol. 2017 Sep;10(9):957–63. [PMID: 28590144]
Zhao M et al. Acupressure therapy for acute ankle sprains: a randomized clinical trial. PM R Phys Med Rehab. 2018 Jan;10(1):36–44. [PMID: 28634002]
SELECTED INTERVENTIONAL MODALITIES FOR PAIN RELIEF
Pain management specialists are physicians who have completed a residency in anesthesiology, physical medicine and rehabilitation, neurology, internal medicine, emergency medicine, or psychiatry and usually also a fellowship in pain management to learn medication management and interventional techniques for acute, chronic, and cancer pain. Interventional pain management modalities performed by pain management specialists involve neuromodulation of specific targets to alleviate pain. The procedures they perform include percutaneous needle injection of local anesthetics and/or corticosteroids, radiofrequency (thermal) lesioning, cryotherapy, chemical neurolysis, or surgical implantation of intrathecal medication delivery pump systems or neurostimulation devices. While invasive procedures carry their own inherent risks such as bleeding or infection, they can drastically reduce or even obviate the need for conventional pharmacological therapies that may have side effects or be burdensome to the individual.
For some patients, a nerve block, such as a celiac plexus block for pain from pancreatic cancer, can provide substantial relief. Intrathecal pumps may be most useful for patients with severe pain responsive to opioids but who require such large doses that systemic side effects (eg, sedation, urinary retention, and constipation) become limiting. In the palliative care setting, these pumps are appropriate when life expectancy is long enough to justify the discomfort and cost of surgical implantation.
Clinicians do not need to know all the details of interventional pain procedures but should consider referring their patients to pain management specialists if such procedures may be beneficial. For example, a common question is whether prolonged opioid therapy with its inherent risks is better than an injection or an implanted device. Beyond knowing the benefits and risks, fiscal considerations may be key.
Tables 5–10 and 5–11 list the procedures and the agents typically used in interventional pain modalities.
Table 5–10. Interventional techniques for chronic pain by anatomic location.
Neuraxial
Intrathecal
Epidural (caudal, lumbar, thoracic, cervical; interlaminar vs. transforaminal)
Paraneuraxial (planar blockade)
Paravertebral (intercostal)
Transversus abdominis plane/quadratus lumborum
Pectoralis and serratus anterior
Peripheral nerve (perineural blockade)
Brachial plexus and branches
Lumbar plexus and branches
Joints
Intra-articular injections
Joint denervation procedures
Sympathetic ganglion
Gasserian ganglion
Sphenopalatine ganglion
Cervical sympathetic blockade (stellate ganglion)
Lumbar sympathetic blockade
Celiac plexus
Superior hypogastric plexus
Ganglion impar
Continuous neuraxial drug delivery
Epidural (tunneled catheter, port)
Intrathecal (implanted intrathecal pump)
Neurostimulation
Dorsal column stimulation (spinal cord stimulation)
Dorsal root ganglion stimulation
Peripheral nerve or field stimulation
Table 5–11. Agents used1 in neuromodulatory therapies.
Voltage-gated sodium channel blockade—local anesthetics
Lidocaine
Mepivacaine
Bupivacaine
Ropivacaine
Corticosteroids
Triamcinolone
Methylprednisolone
Dexamethasone
Opioids
Morphine
Hydromorphone
Fentanyl
Adjuvants
Clonidine
Dexmedetomidine
Others
Chemical neurolysis
Alcohol
Phenol
Glycerol
Thermal neurolysis
Radiofrequency ablation
Cryoanalgesia
Neurostimulation
Various patterns, frequency, amplitude, pulse width
1Injected or applied.
List is not comprehensive but includes most commonly used agents.
INTRATHECAL DRUG DELIVERY SYSTEM
A. Indications
Intrathecal drug delivery therapy is indicated for patients with both malignant and nonmalignant pain and has been shown to be effective, cost-effective, and safe. It is generally accepted that intrathecal opioids have a 100- to 300-fold efficacy compared with oral opioids; therefore, the best candidates may be patients with good analgesic benefit from opioids but burdensome side effects. Common indications include cancer pain, chronic low-back pain (in particular, post-laminectomy syndrome), complex regional pain syndrome, and other causes of neuropathic pain. In a randomized controlled trial comparing intrathecal therapy with comprehensive medication management in cancer pain, intrathecal therapy was shown to be superior in both analgesia as well as to have fewer side effects. Due to the cost of implanting the device as well as the recovery time needed from surgical implantation, it is recommended that patients have a life expectancy of at least 2–3 months.
B. Procedure
Intrathecal drug delivery systems consist of a pump with a drug reservoir, typically implanted in the abdominal wall, connected to a catheter that delivers medications into the intrathecal space. Initial percutaneous trialing is indicated for patients with noncancer or cancer pain; such percutaneous trialing may consist of either epidural or intrathecal delivery of bolus or continuous medication to determine efficacy and side effect profiles of planned therapeutic agent(s). Some cancer patients may not undergo a trial to avoid delaying final implantation. Subsequent implantation of an intrathecal drug delivery system involves two incisions: one in the spine to accommodate the catheter and anchor, and another in the lower abdominal region to create a pocket to hold the pump. The catheter is tunneled through the lower abdominal and flank subcutaneous tissues to connect to the pump. Both trial and implantation are typically performed under sedation with local anesthetic infiltration; spinal anesthesia delivered from the pump itself can also be utilized for pump implantation. Some patients may require general anesthesia to tolerate the implantation procedure.
C. Medications Used
According to the Polyanalgesic Conference Consensus (PACC) guidelines for both malignant and nonmalignant pain, first-line intrathecal delivery medications include monotherapy with either morphine or ziconotide, a calcium channel inhibitor. However, the PACC guidelines also state that de facto practice includes combination therapy with opioids (eg, fentanyl, hydromorphone) and local anesthetic (eg, bupivacaine) and may include other medications (eg, baclofen or clonidine). Respiratory depression and sedation are two of the most concerning side effects of many intrathecal medications. Ziconotide may cause myositis and polyarthralgias as well as psychiatric and neurologic adverse effects (it is contraindicated in patients with preexisting psychosis). Side effects of morphine and fentanyl include nausea, edema, constipation, urinary retention, and pruritus.
D. Advantages and Disadvantages
The main advantage of intrathecal delivery therapy is targeted delivery of medication to the spinal cord with increased efficacy and diminished side effects compared with systemic analgesic medications. The increased efficacy is due to the 100- to 300-fold increased concentration of intrathecal drug compared with systemic medication. However, intrathecal therapy requires regular pump refills and may be complicated by infections, catheter or pump malfunctions requiring surgical revision, or development of catheter tip granulomas, potentially leading to inadequate analgesia or neurologic deficits. Pump batteries may last from 5 years to 10 years depending on usage. Fatalities surrounding intrathecal therapy have been linked to respiratory depression; patients must be monitored for respiratory depression or sedation when initiating or increasing intrathecal therapeutic agents. Some intrathecal pumps need to be emptied prior to MRI; due to the magnetic forces of the MRI, the entirety of the drug reservoir could inadvertently open. Therefore, it is critical that the type of pump is known prior to placing the patient and pump in an MRI machine. Additionally, anticoagulants and NSAIDs need to be stopped prior to pump implantation and need to be held briefly after the implantation as well; this temporary cessation imposes the risk of potentially causing blood clots.
E. Alternatives
For patients with limited life expectancy, continuous epidural drug delivery via an external pump or subcutaneous port may be more appropriate. Systemic medication delivered orally, intravenously, topically, or even by a subcutaneous infusion (as in palliative care settings) are alternatives to intrathecal therapy.
Careskey H et al. Interventional anesthetic methods for pain in hematology/oncology patients. Hematol Oncol Clin North Am. 2018 Jun;32(3):433–45. [PMID: 29729779]
Deer TR et al. The Polyanalgesic Consensus Conference (PACC): recommendations for intrathecal drug delivery: guidance for improving safety and mitigating risks. Neuromodulation. 2017 Feb;20(2):155–76. [PMID: 28042914]
Deer TR et al. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017 Feb;20(2):96–132. Erratum in: Neuromodulation. 2017 Jun;20(4):405–6. [PMID: 28042904]
Patel N et al. ‘Was it worth it?’ Intrathecal analgesia for cancer pain: a qualitative study exploring the views of family carers. Palliat Med. 2018 Jan;32(1):287–93. [PMID: 28832240]
Zheng S et al. Evaluation of intrathecal drug delivery system for intractable pain in advanced malignancies: a prospective cohort study. Medicine (Baltimore). 2017 Mar;96(11):e6354. [PMID: 28296770]
SPINAL STIMULATION
A. Indications
Spinal stimulation targets neuropathic pain in the trunk and limbs, such as failed back surgery syndrome, complex regional pain syndrome, and radiculopathy. There is also growing literature around its use for neuropathic pain associated with cancer.
B. Procedure
Neurostimulation devices consist of an implantable pulse generator typically placed in the flank or abdomen just under the skin and an array of electrical contacts on small cylindrical or paddle leads placed in the epidural space. Neurostimulation devices transmit electrical pulses to the spinal cord or dorsal root ganglion to block pain transmission. Paddle leads require neurosurgical implantation with laminotomy (and general anesthesia), while percutaneous wire leads may be implanted under sedation. Patients undergo a 3- to 7-day trial during which the leads are attached to an external battery source and undergo programming with different pulse waveforms to assess therapeutic efficacy prior to surgical implantation of permanent leads and implantable pulse generator.
C. Frequencies Used
Traditional neurostimulation resulted in paresthesias that were used to mask pain. It was presumed that these paresthesias were the result of stimulation of the dorsal column axons. Recent studies have revealed that analgesia can be obtained independent of paresthesias by altering a variety of spinal cord stimulation parameters, including constant high-frequency stimulation and burst high-frequency stimulation. More recent double-blind, randomized, controlled trials have revealed that both functional status and pain scores could be significantly improved in spinal cord stimulation systems that were capable of adapting the output to the patient’s individual neural response in a closed loop fashion. For more focal neuropathic pain conditions such as postoperative inguinal nerve injuries or thoracic post herpetic neuralgias, stimulation of the dorsal root ganglion is able to provide focal analgesia. These newer, more versatile systems deliver paresthesia-free analgesia with analgesic response rates that have steadily increased from about 50% with the traditional devices to about 80%. The newer devices also have greater longevity and most are MRI compatible.
D. Advantages and Disadvantages
Spinal cord stimulation is a reversible technology that may provide superior analgesic efficacy while eliminating the need for systemic medications. In fact, spinal cord stimulation has now advanced to a higher position in the treatment continuum; it can be considered before using long-term moderate doses of systemic opioids. On the other hand, because it is a surgical procedure, it may be associated with complications, such as infection, lead migration, device malfunction, or neurologic deficits. While MRIs were contraindicated with some older systems, most newer systems allow for limited MRI imaging. Batteries may require daily charging but typically do not require replacement for 5–10 years. Similar to intrathecal pumps, anticoagulants and NSAIDs need to be stopped prior to implantation of spinal cord stimulation devices because of the potential risks (eg, bleeding). The implanting surgeon, prescribing physician, and patient need to discuss the benefits and risks before proceeding.
E. Alternatives
In addition to medication management for pain, two neuromodulatory techniques may serve as alternatives to dorsal horn and dorsal root ganglion stimulation. Peripheral nerve stimulation is an emerging technology; it targets peripheral nerves using a similar system of a lead connected to a pulse generator. It may be most appropriate when there is a very specific neurologic target. Transcutaneous electrical nerve stimulators (TENS) and systemic pharmacologic therapies are alternatives.
Transcranial magnetic stimulation (TMS) is a noninvasive therapy to provide focused stimulation to various regions of the brain. TMS is approved for the treatment of intractable depression. However, a number of recent studies have been conducted and prompted the development of guidelines for the use of TMS for pain. As these guidelines are implemented and more robust studies emerge, this noninvasive neuromodulation therapy might eventually receive FDA approval for pain.
Deer TR et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017 Apr;158(4):669–81. [PMID: 28030470]
Deer TR et al. The Neuromodulation Appropriateness Consensus Committee on best practices for dorsal root ganglion stimulation. Neuromodulation. 2019 Jan;22(1):1–35. [PMID: 30246899]
Deer TR et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations for infection prevention and management. Neuromodulation. 2017 Jan;20(1):31–50. Erratum in: Neuromodulation. 2017 Jul;20(5):516. [PMID: 28042909]
Eghtesadi M et al. Neurostimulation for refractory cervicogenic headache: a three-year retrospective study. Neuromodulation. 2018 Apr;21(3):302–9. [PMID: 29178511]
Lefaucheur JP et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020 Feb;131(2):474–528. [PMID: 31901449]
Leung et al. Left dorsolateral prefrontal cortex rTMS in alleviating MTBI related headaches and depressive symptoms. Neuromodulation. 2018 Jun;21(4):390–401. [PMID: 28557049]
Mekhail N et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020 Feb;19(2):123–34. [PMID: 31870766]
Sharan A et al. An overview of chronic spinal pain: revisiting diagnostic categories and exploring an evolving role for neurostimulation. Spine (Phila Pa 1976). 2017 Jul 15;42(Suppl 14):S35–40. [PMID: 28441315]
CELIAC PLEXUS BLOCK & NEUROLYSIS
A. Indications
A celiac plexus block refers to injection of a long-acting anesthetic (eg, bupivacaine) with or without a corticosteroid (eg, methylprednisolone); with steroids, the block can provide relief for a few weeks to months. Celiac plexus neurolysis involves injection of a neurolytic agent (eg, alcohol or phenol); it may provide pain relief more consistently for 2–6 months. The most common indication is pancreatic cancer pain, but it can be used for pain from other malignancies (eg, stomach, liver, spleen, kidney, and gastrointestinal tract) or from chronic pancreatitis. Multiple randomized controlled trials and meta-analyses have shown superiority of celiac plexus neurolysis to medication management for pancreatic cancer, but evidence of its efficacy for chronic pancreatitis is more mixed.
B. Procedure
The most common approach is a percutaneous posterior approach under fluoroscopy guidance, with bilateral needles targeted to the celiac plexus at the level of T12–L1. Alternatively, ultrasound, CT, or endoscopic guidance can be used. Minimal sedation is required for the percutaneous approaches, while heavy sedation or general anesthesia may be required for endoscopic guidance.
C. Medications Used
Chemical neurolysis with alcohol or phenol is used to extend the duration of the analgesia to 2 or more months compared to a block with local anesthetic (eg, bupivacaine) and corticosteroid (eg, methylprednisolone), which produces an analgesic duration of weeks to months. For chemical neurolysis, alcohol is used most often because it does not require compounding, and importantly has a lower chance of permanent neurologic damage compared with phenol; however, it is more painful on injection.
D. Advantages and Disadvantages
The primary advantage is improved analgesia without need for systemic medications and their untoward effects. Common side effects of celiac plexus interventions include transient hypotension and transient diarrhea. Transient or permanent spinal cord damage is rare, although there is an increased risk of its occurrence with plexus (chemical) neurolysis compared with plexus (anesthetic) block.
E. Alternatives
Standard pain management is with oral or transdermal systemic medication. Intrathecal therapy is also an alternative, especially for cancer pain.
Ashlock K. Celiac plexus block: management of abdominal pain in patients with late-stage cancer. Clin J Oncol Nurs. 2018 Dec 1;22(6):663–5. [PMID: 30451994]
Careskey H et al. Interventional anesthetic methods for pain in hematology/oncology patients. Hematol Oncol Clin North Am. 2018 Jun;32(3):433–45. [PMID: 29729779]
Filippiadis DK et al. Percutaneous neurolysis for pain management in oncological patients. Cardiovasc Intervent Radiol. 2019 Jun;42(6):791–9. [PMID: 30783779]
Sachdev AH et al. Celiac plexus block and neurolysis: a review. Gastrointest Endosc Clin N Am. 2018 Oct;28(4):579–86. [PMID: 30241645]
Wyse JM et al. Endoscopic ultrasound-guided management of pain in chronic pancreatitis and pancreatic cancer: an update. Curr Treat Options Gastroenterol. 2018 Dec;16(4):417–27. [PMID: 30209676]
 When to Refer
When to Refer
Patients should be referred to pain management specialists if they have:
• Pain that does not respond to opioids at typical doses or causes major adverse effects at typical doses.
• Pain that cannot be controlled expeditiously or safely by other clinicians.
• Neuropathic pain that does not respond to first-line treatments.
• Complex medication management that uses buprenorphine or methadone.
• Severe pain from malignancy, including primary disease (eg, pancreatic cancer) or metastatic disease (eg, bony metastases).
 When to Admit
When to Admit
Patients should be hospitalized if they have:
• Severe exacerbation of pain not responsive to previous stable oral opioids given around-the-clock plus breakthrough doses.
• Pain that is so severe that it cannot be controlled at home.
• Uncontrollable side effects from opioids, including nausea, vomiting, myoclonus, and altered mental status.
• Need for a surgical procedure, such as implantation of an intrathecal drug delivery pump or neurostimulation device.