11
Systemic Hypertension
Michael Sutters, MD, MRCP (UK)
Based on the National Health and Nutrition Survey through 2016, about 45% of adults in the United States have a blood pressure greater than 140/90 mm Hg or are being treated for hypertension. About 80% of people with hypertension are aware of the diagnosis and 75% are receiving treatment, but hypertension is controlled in only 52% of those affected. Cardiovascular morbidity and mortality increase as both systolic and diastolic blood pressures rise, but in individuals over age 50 years, the systolic pressure and pulse pressure are better predictors of complications than diastolic pressure. The prevalence of hypertension increases with age, and it is more common in blacks than in whites. Adequate blood pressure control reduces the incidence of acute coronary syndrome by 20–25%, stroke by 30–35%, and heart failure by 50%.
HOW IS BLOOD PRESSURE MEASURED & HYPERTENSION DIAGNOSED?
Blood pressure should be measured with a well-calibrated sphygmomanometer. The bladder width within the cuff should encircle at least 80% of the arm circumference. Readings should be taken after the patient has been resting comfortably, back supported in the sitting or supine position, for at least 5 minutes and at least 30 minutes after smoking or coffee ingestion. Automated office blood pressure readings, made with office-based devices that permit multiple automated measurements after a pre-programmed rest period, produce data that are independent of digit preference bias (tendency to favor numbers that end with zero or five) and the “white coat” phenomenon (where blood pressure is elevated in the clinic but normal at home). Blood pressure measurements taken outside the office environment, either by intermittent self-monitoring (home blood pressure) or with an automated device programmed to take measurements at regular intervals (ambulatory blood pressure) are more powerful predictors of outcomes and are advocated in clinical guidelines. Home measurements are also helpful in differentiating white coat hypertension from hypertension that is resistant to treatment, and in diagnosis of “masked hypertension” (where blood pressure is normal in the clinic but elevated at home). The cardiovascular risk associated with masked hypertension is similar to that observed in sustained hypertension.
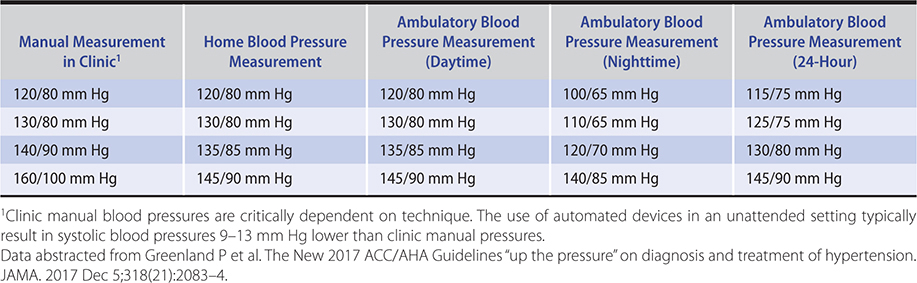
A single elevated blood pressure reading is not sufficient to establish the diagnosis of hypertension. The major exceptions to this rule are hypertensive presentations with unequivocal evidence of life-threatening end-organ damage, as seen in hypertensive emergency, or in hypertensive urgency where blood pressure is greater than 220/125 mm Hg but life-threatening end-organ damage is absent. In less severe cases, the diagnosis of hypertension depends on a series of measurements of blood pressure, since readings can vary and tend to regress toward the mean with time. Patients whose initial blood pressure is in the hypertensive range exhibit the greatest fall toward the normal range between the first and second encounters. However, the concern for diagnostic precision needs to be balanced by an appreciation of the importance of establishing the diagnosis of hypertension as quickly as possible, since a 3-month delay in treatment of hypertension in high-risk patients is associated with a twofold increase in cardiovascular morbidity and mortality. Based on epidemiological data, the conventional 140/90 mm Hg threshold for the diagnosis of hypertension has been revised. The 2017 guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) suggest that, for conventional office-based measurement, normal be defined as less than 120/80 mm Hg, elevated as 120–129/less than 80 mm Hg, stage 1 as 130–139/80–89 mm Hg and stage 2 as greater than or equal to 140/90 mm Hg. As exemplified by Hypertension Canada’s 2017 guidelines (Figure 11–1), automated and home blood pressure measurements have assumed greater prominence in the diagnostic algorithms published by many national hypertension workgroups. Equivalent blood pressure for these different modes of measurement are described in Table 11–1.
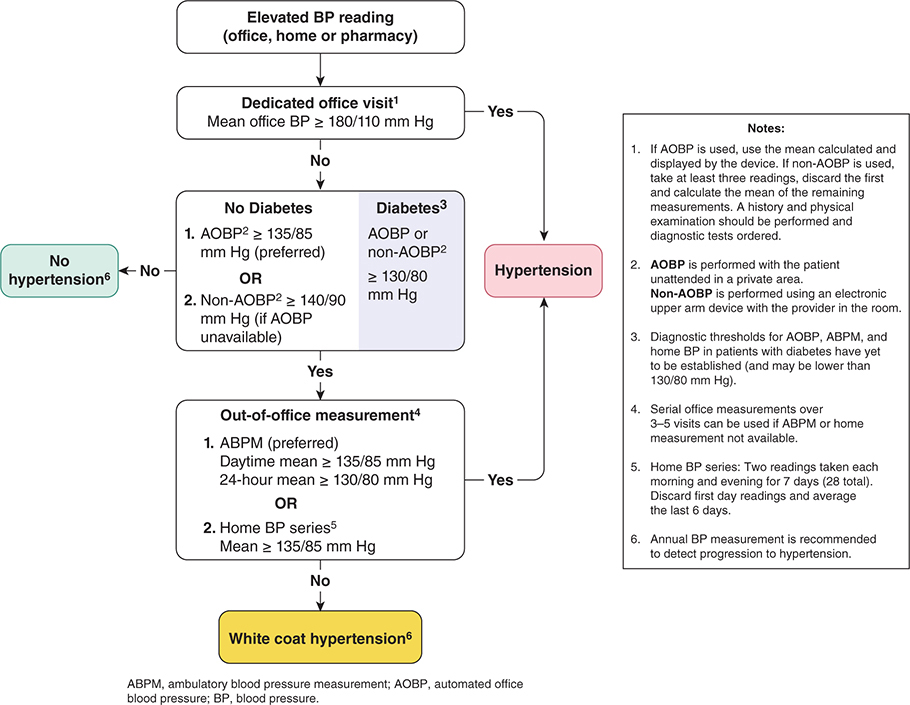
Figure 11–1. According to these recommendations, if AOBP measurements are not available, blood pressures recorded manually in the office may be substituted if taken as the mean of the last two readings of three consecutive readings. Note that the blood pressure threshold for diagnosing hypertension is higher if recorded manually in these guidelines. If home blood pressure monitoring is unavailable, office measurements recorded over three to five separate visits can be substituted. (Reproduced, with permission, from Leung AA et al; Hypertension Canada. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017 May;33(5):557–76. Erratum in: Can J Cardiol. 2017 Dec;33(12):1733–4. Copyright © 2017 Canadian Cardiovascular Society. Published by Elsevier Inc. All rights reserved.)
Table 11–1. Corresponding blood pressure values across a range of blood pressure measurement methods.
Ambulatory blood pressure readings are normally lowest at night and the loss of this nocturnal dip is a dominant predictor of cardiovascular risk, particularly risk of thrombotic stroke. An accentuation of the normal morning increase in blood pressure is associated with increased likelihood of cerebral hemorrhage. Furthermore, variability of systolic blood pressure predicts cardiovascular events independently of mean systolic blood pressure.
It is important to recognize that the diagnosis of hypertension does not automatically entail drug treatment; this decision depends on the clinical setting, as discussed below.
Greenland P et al. The New 2017 ACC/AHA Guidelines “up the pressure” on diagnosis and treatment of hypertension. JAMA. 2017 Dec 5;318(21):2083–4. [PMID: 29159417]
Jin J. JAMA patient page. Checking blood pressure at home. JAMA. 2017 Jul 18;318(3):310. [PMID: 28719694]
Leung AA et al; Hypertension Canada. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017 May;33(5):557–76. Erratum in: Can J Cardiol. 2017 Dec; 33(12):1733–4. [PMID: 28449828]
Melville S et al. Out-of-office blood pressure monitoring in 2018. JAMA. 2018 Nov 6;320(17):1805–6. [PMID: 30398589]
Myers MG. Automated office blood pressure—incorporating SPRINT into clinical practice. Am J Hypertens. 2017 Jan;30(1):8–11. [PMID: 27551025]
NCD Risk Factor Collaboration (NCD-RisC). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. 2019 Aug 24;394(10199):639–51. [PMID: 31327564]
APPROACH TO HYPERTENSION
 Etiology & Classification
Etiology & Classification
A. Primary Essential Hypertension
“Essential hypertension” is the term applied to the 95% of hypertensive patients in which elevated blood pressure results from complex interactions between multiple genetic and environmental factors. The proportion regarded as “essential” will diminish with improved detection of clearly defined secondary causes and with better understanding of pathophysiology. Essential hypertension occurs in 10–15% of white adults and 20–30% of black adults in the United States. The onset is usually between ages 25 and 50 years; it is uncommon before age 20 years. The best understood pathways underlying hypertension include overactivation of the sympathetic nervous and renin-angiotensin-aldosterone systems (RAAS), blunting of the pressure-natriuresis relationship, variation in cardiovascular and renal development, and elevated intracellular sodium and calcium levels.
Exacerbating factors include obesity, sleep apnea, increased salt intake, excessive alcohol use, cigarette smoking, polycythemia, nonsteroidal anti-inflammatory drug (NSAID) therapy, and low potassium intake. Obesity is associated with an increase in intravascular volume, elevated cardiac output, activation of the renin-angiotensin system, and, probably, increased sympathetic outflow. Lifestyle-driven weight reduction lowers blood pressure modestly, but the dramatic weight reduction following bariatric surgery results in improved blood pressure in most patients, and actual remission of hypertension in 20–40% of cases. In patients with sleep apnea, treatment with continuous positive airway pressure (CPAP) has been associated with improvements in blood pressure. Increased salt intake probably elevates blood pressure in some individuals so dietary salt restriction is recommended in patients with hypertension. Excessive use of alcohol also raises blood pressure, perhaps by increasing plasma catecholamines. Hypertension can be difficult to control in patients who consume more than 40 g of ethanol (two drinks) daily or drink in “binges.” Cigarette smoking raises blood pressure by increasing plasma norepinephrine. Although the long-term effect of smoking on blood pressure is less clear, the synergistic effects of smoking and high blood pressure on cardiovascular risk are well documented. The relationship of exercise to hypertension is variable. Aerobic exercise lowers blood pressure in previously sedentary individuals, but increasingly strenuous exercise in already active subjects has less effect. The relationship between stress and hypertension is not established. Polycythemia, whether primary, drug-induced, or due to diminished plasma volume, increases blood viscosity and may raise blood pressure. NSAIDs produce increases in blood pressure averaging 5 mm Hg and are best avoided in patients with borderline or elevated blood pressures. Low potassium intake is associated with higher blood pressure in some patients; an intake of 90 mmol/day is recommended.
The complex of abnormalities termed the “metabolic syndrome” (upper body obesity, insulin resistance, and hypertriglyceridemia) is associated with both the development of hypertension and an increased risk of adverse cardiovascular outcomes. Affected patients usually also have low high-density lipoprotein (HDL) cholesterol levels and elevated catecholamines and inflammatory markers such as C-reactive protein.
B. Secondary Hypertension
Approximately 5% of patients have hypertension secondary to identifiable specific causes (Table 11–2). Secondary hypertension should be suspected in patients in whom hypertension develops at an early age or after the age of 50 years, and in those previously well controlled who become refractory to treatment. Hypertension resistant to maximum doses of three medications is another clue, although multiple medications are usually required to control hypertension in persons with diabetes. Secondary causes include genetic syndromes; kidney disease; renal vascular disease; primary hyperaldosteronism; Cushing syndrome; pheochromocytoma; coarctation of the aorta and hypertension associated with pregnancy, estrogen use, hypercalcemia, and medications.
Table 11–2. Identifiable causes of hypertension.
Sleep apnea
Drug-induced or drug-related
Chronic kidney disease
Primary aldosteronism
Renovascular disease
Long-term corticosteroid therapy and Cushing syndrome
Pheochromocytoma
Coarctation of the aorta
Thyroid or parathyroid disease
Data from Chobanian AV et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72.
1. Genetic causes—Hypertension can be caused by mutations in single genes, inherited on a Mendelian basis. Although rare, these conditions provide important insight into blood pressure regulation and possibly the genetic basis of essential hypertension. Glucocorticoid remediable aldosteronism is an autosomal dominant cause of early-onset hypertension with normal or high aldosterone and low renin levels. In the syndrome of apparent mineralocorticoid excess, early-onset hypertension with hypokalemic metabolic alkalosis is inherited on an autosomal recessive basis. Although plasma renin is low and plasma aldosterone level is very low in these patients, aldosterone antagonists are effective in controlling hypertension. Glycyrrhetinic acid, found in licorice, causes increased blood pressure through inhibition of 11beta-hydroxysteroid dehydrogenase. The syndrome of hypertension exacerbated in pregnancy is inherited as an autosomal dominant trait. In these patients, a mutation in the mineralocorticoid receptor makes it abnormally responsive to progesterone and, paradoxically, to spironolactone. Gordon syndrome, or pseudohypoaldosteronism type II, presents with early-onset hypertension associated with hyperkalemia, metabolic acidosis and relative suppression of aldosterone. Inheritance is most often autosomal dominant and the hypertension responds to a thiazide diuretic. The underlying mutations occur in one of several genes encoding proteins that regulate the thiazide-sensitive NaCl co-transporter in the distal nephron, leading to constitutive activation of sodium and chloride reabsorption.
2. Kidney disease—Renal parenchymal disease is the most common cause of secondary hypertension, which results from increased intravascular volume and increased activity of the RAAS. Increased sympathetic nerve activity may also contribute.
3. Renal vascular hypertension—Renal artery stenosis is present in 1–2% of hypertensive patients. The most common cause is atherosclerosis, but fibromuscular dysplasia should be suspected in women under 50 years of age. Excessive renin release occurs due to reduction in renal perfusion pressure, while attenuation of pressure natriuresis contributes to hypertension in patients with a single kidney or bilateral lesions. Activation of the renal sympathetic nerves may also be important.
Renal vascular hypertension should be suspected in the following circumstances: (1) the documented onset is before age 20 or after age 50 years, (2) the hypertension is resistant to three or more drugs, (3) there are epigastric or renal artery bruits, (4) there is atherosclerotic disease of the aorta or peripheral arteries (15–25% of patients with symptomatic lower limb atherosclerotic vascular disease have renal artery stenosis), (5) there is an abrupt increase (more than 25%) in the level of serum creatinine after administration of angiotensin-converting enzyme (ACE) inhibitors, or (6) episodes of pulmonary edema are associated with abrupt surges in blood pressure. (See Renal Artery Stenosis, Chapter 22.)
4. Primary hyperaldosteronism—Hyperaldosteronism should be considered in people with resistant hypertension, blood pressures consistently greater than 150/100 mm Hg, hypokalemia (although this is often absent), or adrenal incidentaloma, and in those with a family history of hyperaldosteronism. Mild hypernatremia and metabolic alkalosis may also occur. Hypersecretion of aldosterone is estimated to be present in 5–10% of hypertensive patients and, besides noncompliance, is the most common cause of resistant hypertension. The initial screening step is the simultaneous measurement of aldosterone and renin in blood in a morning sample collected after 30 minutes quietly seated. Hyperaldosteronism is suggested when the plasma aldosterone concentration is elevated (normal: 1–16 ng/dL) in association with suppression of plasma renin activity (normal: 1–2.5 ng/mL/h). However, the plasma aldosterone/renin ratio (normal less than 30) is not highly specific as a screening test. This is because renin levels may approach zero, which leads to exponential increases in the plasma aldosterone/renin ratio even when aldosterone levels are normal. Hence, an elevated plasma aldosterone/renin ratio should probably not be taken as evidence of hyperaldosteronism unless the aldosterone level is actually elevated.
During the workup for hyperaldosteronism, an initial plasma aldosterone/renin ratio can be measured while the patient continues taking usual medications. If under these circumstances the ratio proves normal or equivocal, medications that alter renin and aldosterone levels, including ACE inhibitors, angiotensin receptor blockers (ARBs), diuretics, beta-blockers, and clonidine, should be discontinued for 2 weeks before repeating the plasma aldosterone/renin ratio; spironolactone and eplerenone should be held for 4 weeks. Slow-release verapamil and alpha-receptor blockers can be used to control blood pressure during this drug washout period. Patients with a plasma aldosterone level greater than 16 ng/dL and an aldosterone/renin ratio of 30 or more might require further evaluation for primary hyperaldosteronism.
The lesion responsible for hyperaldosteronism is an adrenal adenoma or bilateral adrenal hyperplasia.
5. Cushing syndrome—Hypertension occurs in about 80% of patients with spontaneous Cushing syndrome. Excess glucocorticoid may act through salt and water retention (via mineralocorticoid effects), increased angiotensinogen levels, or permissive effects in the regulation of vascular tone.
Diagnosis and treatment of Cushing syndrome are discussed in Chapter 26.
6. Pheochromocytoma—Pheochromocytomas are uncommon; they are probably found in less than 0.1% of all patients with hypertension and in approximately two individuals per million population. However, autopsy studies indicate that pheochromocytomas are very often undiagnosed in life. The blood pressure elevation caused by the catecholamine excess results mainly from alpha-receptor–mediated vasoconstriction of arterioles, with a contribution from beta-1-receptor–mediated increases in cardiac output and renin release. Chronic vasoconstriction of the arterial and venous beds leads to a reduction in plasma volume and predisposes to postural hypotension. Glucose intolerance develops in some patients. Hypertensive crisis in pheochromocytoma may be precipitated by a variety of drugs, including tricyclic antidepressants, antidopaminergic agents, metoclopramide, and naloxone. The diagnosis and treatment of pheochromocytoma are discussed in Chapter 26.
7. Coarctation of the aorta—This uncommon cause of hypertension is discussed in Chapter 10. Evidence of radial-femoral delay should be sought in all younger patients with hypertension.
8. Hypertension associated with pregnancy—Hypertension occurring de novo or worsening during pregnancy, including preeclampsia and eclampsia, is one of the most common causes of maternal and fetal morbidity and mortality (see Chapter 19). Autoantibodies with the potential to activate the angiotensin II type 1 receptor have been causally implicated in preeclampsia, in resistant hypertension, and in progressive systemic sclerosis.
9. Estrogen use—A small increase in blood pressure occurs in most women taking oral contraceptives. A more significant increase of 8/6 mm Hg systolic/diastolic is noted in about 5% of women, mostly in obese individuals older than age 35 who have been treated for more than 5 years. This is caused by increased hepatic synthesis of angiotensinogen. The lower dose of postmenopausal estrogen does not generally cause hypertension but rather maintains endothelium-mediated vasodilation.
10. Other causes of secondary hypertension—Hypertension has been associated with hypercalcemia, acromegaly, hyperthyroidism, hypothyroidism, baroreceptor denervation, compression of the rostral ventrolateral medulla, and increased intracranial pressure. A number of medications may cause or exacerbate hypertension—most importantly cyclosporine, tacrolimus, angiogenesis inhibitors, and erythrocyte-stimulating agents (such as erythropoietin). Decongestants, NSAIDs, cocaine, and alcohol should also be considered. Over-the-counter products should not be overlooked, eg, a dietary supplement marketed to enhance libido was found to contain yohimbine, an alpha-2–antagonist, which can produce severe rebound hypertension in patients taking clonidine.
 When to Refer
When to Refer
Referral to a hypertension specialist should be considered in cases of severe, resistant or early-/late-onset hypertension or when secondary hypertension is suggested by screening.
Byrd JB et al. Primary aldosteronism. Circulation. 2018 Aug 21;138(8):823–35. [PMID: 30359120]
Owen JG et al. Bariatric surgery and hypertension. Am J Hypertens. 2017 Dec 8;31(1):11–7. [PMID: 28985287]
Raina R et al. Overview of monogenic or Mendelian forms of hypertension. Front Pediatr. 2019 Jul 1;7:263. [PMID: 31312622]
Raman G et al. Comparative effectiveness of management strategies for renal artery stenosis: an updated systematic review. Ann Intern Med. 2016 Nov 1;165(9):635–49. [PMID: 27536808]
 Complications of Untreated Hypertension
Complications of Untreated Hypertension
Elevated blood pressure results in structural and functional changes in the vasculature and heart. Most of the adverse outcomes in hypertension are associated with thrombosis rather than bleeding, possibly because increased vascular shear stress converts the normally anticoagulant endothelium to a prothrombotic state. The excess morbidity and mortality related to hypertension approximately doubles for each 6 mm Hg increase in diastolic blood pressure. However, target-organ damage varies markedly between individuals with similar levels of office hypertension; home and ambulatory pressures are superior to office readings in the prediction of end-organ damage.
A. Hypertensive Cardiovascular Disease
Cardiac complications are the major causes of morbidity and mortality in primary (essential) hypertension. For any level of blood pressure, left ventricular hypertrophy is associated with incremental cardiovascular risk in association with heart failure (through systolic or diastolic dysfunction), ventricular arrhythmias, myocardial ischemia, and sudden death.
The occurrence of heart failure is reduced by 50% with antihypertensive therapy. Hypertensive left ventricular hypertrophy regresses with therapy and is most closely related to the degree of systolic blood pressure reduction. Diuretics have produced equal or greater reductions of left ventricular mass when compared with other drug classes. Conventional beta-blockers are less effective in reducing left ventricular hypertrophy but play a specific role in patients with established coronary artery disease or impaired left ventricular function.
B. Hypertensive Cerebrovascular Disease and Dementia
Hypertension is the major predisposing cause of hemorrhagic and ischemic stroke. Cerebrovascular complications are more closely correlated with systolic than diastolic blood pressure. The incidence of these complications is markedly reduced by antihypertensive therapy. Preceding hypertension is associated with a higher incidence of subsequent dementia of both vascular and Alzheimer types. Home and ambulatory blood pressure may be a better predictor of cognitive decline than office readings in older people. Effective blood pressure control reduces the risk of cognitive dysfunction developing later in life.
C. Hypertensive Kidney Disease
Chronic hypertension is associated with injury to vascular, glomerular, and tubulointerstitial compartments within the kidney, accounting for about 25% of end-stage kidney disease. Nephrosclerosis is particularly prevalent in blacks, in whom susceptibility is linked to APOL1 mutations and hypertension results from kidney disease rather than causing it.
D. Aortic Dissection
Hypertension is a contributing factor in many patients with dissection of the aorta. Its diagnosis and treatment are discussed in Chapter 12.
E. Atherosclerotic Complications
Most Americans with hypertension die of complications of atherosclerosis, but antihypertensive therapy seems to have a lesser impact on atherosclerotic complications compared with the other effects of treatment outlined above. Prevention of cardiovascular outcomes related to atherosclerosis probably requires control of multiple risk factors, of which hypertension is only one.
Seccia TM et al. Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J Hypertens. 2017 Feb;35(2):205–12. [PMID: 27782909]
Supiano MA et al. New guidelines and SPRINT results: implications for geriatric hypertension. Circulation. 2019 Sep 17;140(12):976–8. [PMID: 31525101]
 Clinical Findings
Clinical Findings
The clinical and laboratory findings are mainly referable to involvement of the target organs: heart, brain, kidneys, eyes, and peripheral arteries.
A. Symptoms
Mild to moderate primary (essential) hypertension is largely asymptomatic for many years. The most frequent symptom, headache, is also nonspecific. Accelerated hypertension is associated with somnolence, confusion, visual disturbances, and nausea and vomiting (hypertensive encephalopathy).
Hypertension in patients with pheochromocytomas that secrete predominantly norepinephrine is usually sustained but may be episodic. The typical attack lasts from minutes to hours and is associated with headache, anxiety, palpitation, profuse perspiration, pallor, tremor, and nausea and vomiting. Blood pressure is markedly elevated, and angina or acute pulmonary edema may occur. In primary aldosteronism, patients may have muscular weakness, polyuria, and nocturia due to hypokalemia; malignant hypertension is rare. Chronic hypertension often leads to left ventricular hypertrophy and diastolic dysfunction, which can present with exertional and paroxysmal nocturnal dyspnea. Cerebral involvement causes stroke due to thrombosis or hemorrhage from microaneurysms of small penetrating intracranial arteries. Hypertensive encephalopathy is probably caused by acute capillary congestion and exudation with cerebral edema, which is reversible.
B. Signs
Like symptoms, physical findings depend on the cause of hypertension, its duration and severity, and the degree of effect on target organs.
1. Blood pressure—Blood pressure is taken in both arms and, if lower extremity pulses are diminished or delayed, in the legs to exclude coarctation of the aorta. If blood pressure differs between right and left arms, the higher reading should be recorded as the actual blood pressure and subclavian stenosis suspected in the other arm. An orthostatic drop of at least 20/10 mm Hg is often present in pheochromocytoma. Older patients may have falsely elevated readings by sphygmomanometry because of noncompressible vessels. This may be suspected in the presence of Osler sign—a palpable brachial or radial artery when the cuff is inflated above systolic pressure. Occasionally, it may be necessary to make direct measurements of intra-arterial pressure, especially in patients with apparent severe hypertension who do not tolerate therapy.
2. Retinas—Narrowing of arterial diameter to less than 50% of venous diameter, copper or silver wire appearance, exudates, hemorrhages, or hypertensive retinopathy are associated with a worse prognosis. the typical changes of severe hypertensive retinopathy are shown in Figure 11–2.
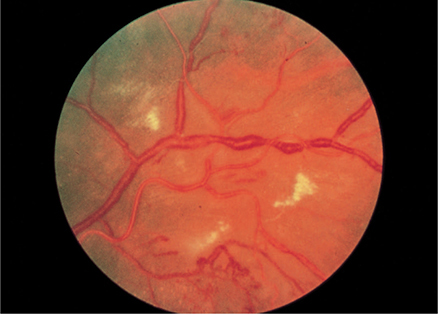
Figure 11–2. Severe, chronic hypertensive retinopathy with hard exudates, increased vessel light reflexes, and sausage-shaped veins. (Used, with permission, from Richard E. Wyszynski, MD, in Knoop KJ, Stack LB, Storrow AB, Thurman RJ. The Atlas of Emergency Medicine, 4th ed. McGraw-Hill, 2016.)
3. Heart—A left ventricular heave indicates severe hypertrophy. Aortic regurgitation may be auscultated in up to 5% of patients, and hemodynamically insignificant aortic regurgitation can be detected by Doppler echocardiography in 10–20%. A presystolic (S4) gallop due to decreased compliance of the left ventricle is quite common in patients in sinus rhythm.
4. Pulses—Radial-femoral delay suggests coarctation of the aorta; loss of peripheral pulses occurs due to atherosclerosis, less commonly aortic dissection, and rarely Takayasu arteritis, all of which can involve the renal arteries.
C. Laboratory Findings
Recommended testing includes the following: hemoglobin; serum electrolytes and serum creatinine; fasting blood sugar level (hypertension is a risk factor for the development of diabetes, and hyperglycemia can be a presenting feature of pheochromocytoma); plasma lipids (necessary to calculate cardiovascular risk and as a modifiable risk factor); serum uric acid (hyperuricemia is a relative contraindication to diuretic therapy); and urinalysis.
D. Electrocardiography and Chest Radiographs
Electrocardiographic criteria are highly specific but not very sensitive for left ventricular hypertrophy. The “strain” pattern of ST–T wave changes is a sign of more advanced disease and is associated with a poor prognosis. A chest radiograph is not necessary in the workup for uncomplicated hypertension.
E. Echocardiography
The primary role of echocardiography should be to evaluate patients with clinical symptoms or signs of cardiac disease.
F. Diagnostic Studies
Additional diagnostic studies are indicated only if the clinical presentation or routine tests suggest secondary or complicated hypertension. These may include 24-hour urine free cortisol, urine or plasma metanephrines, and plasma aldosterone and renin concentrations to screen for endocrine causes of hypertension. Renal ultrasound will detect structural changes (such as polycystic kidneys, asymmetry, and hydronephrosis); echogenicity and reduced cortical volume are reliable indicators of advanced chronic kidney disease. Evaluation for renal artery stenosis should be undertaken in concert with subspecialist consultation.
G. Summary
Since most hypertension is essential or primary, few studies are necessary beyond those listed above. If conventional therapy is unsuccessful or if secondary hypertension is suspected, further studies and perhaps referral to a hypertension specialist are indicated.
Katsi V et al. Impact of arterial hypertension on the eye. Curr Hypertens Rep. 2012 Dec;14(6):581–90. [PMID: 22673879]
 Nonpharmacologic Therapy
Nonpharmacologic Therapy
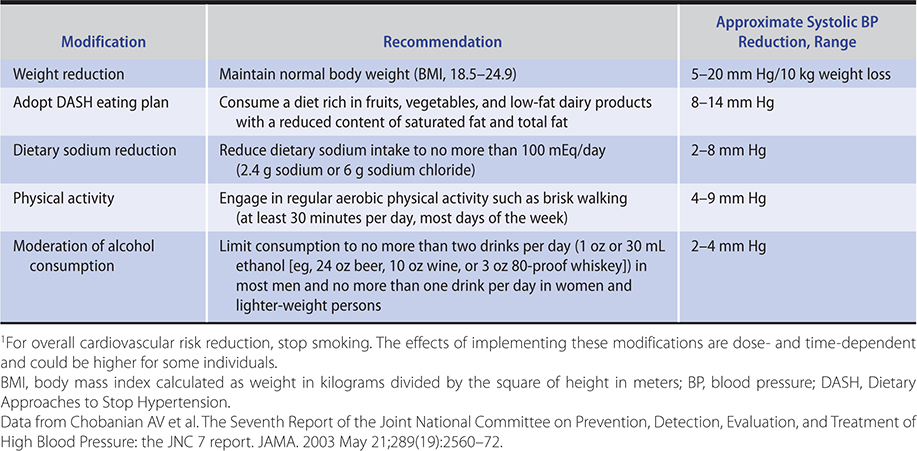
Lifestyle modification may have an impact on morbidity and mortality and is recommended in all patients with elevated blood pressure. A diet rich in fruits, vegetables, and low-fat dairy foods and low in saturated and total fats (DASH diet) has been shown to lower blood pressure. Increased dietary fiber lowers blood pressure. For every 7 g of dietary fiber ingested, cardiovascular risk could be lowered by 9%. The effect of diet on blood pressure may be mediated by shifts in the microbial species in the gut, the intestinal microbiota. Hand squeezing exercises three times a week can lower systolic blood pressure by 6 mm Hg. The protocol comprises four repeats of 2 minutes at 30% of maximum force (using a handheld dynamometer) with 1- to 3-minute rest intervals between squeezes. Additional lifestyle changes, listed in Table 11–3, can prevent or mitigate hypertension or its cardiovascular consequences.
Table 11–3. Lifestyle modifications to manage hypertension.1
Appel LJ. The effects of dietary factors on blood pressure. Cardiol Clin. 2017 May;35(2):197–212. [PMID: 28411894]
Marques FZ et al. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018 Jan;15(1):20–32. [PMID: 28836619]
Smart NA et al. An evidence-based analysis of managing hypertension with isometric resistance exercise—are the guidelines current? Hypertens Res. 2020 Apr;43(4):249–54. [PMID: 31758166]
 Who Should Be Treated With Medications?
Who Should Be Treated With Medications?
Treatment should be offered to all persons in whom blood pressure reduction, irrespective of initial blood pressure levels, will reduce cardiovascular risk with an acceptably low rate of medication-associated adverse effects. The American College of Cardiology and the American Heart Association (ACC/AHA), Hypertension Canada (HC), and the European Society of Hypertension and European Society of Cardiology (ESH/ESC) have developed independent guidelines for the evaluation and management of hypertension. There is broad agreement that drug treatment is necessary in those with office-based blood pressures exceeding 160/100 mm Hg, irrespective of cardiac risk. Similarly, the American, Canadian, and European guidelines agree that treatment thresholds should be lower in the presence of elevated cardiovascular risk. American guidelines stand apart in recommending initiation of antihypertensive pharmacotherapy in those with stage 1 hypertension (140–159/90–99 mm Hg) even if cardiovascular risk is not elevated. By contrast, the Canadian guidelines suggest lifestyle modifications in this low-cardiovascular-risk group, while the European guidelines recommend initiation of pharmacotherapy only if elevated pressure in this low-risk population persists after lifestyle modification. There is no outcomes evidence that mortality or risk of cardiovascular events can be reduced by treating mild hypertension (140/90–160/100 mm Hg) in low-risk individuals. Table 11–4 compares these three sets of guidelines. Since evaluation of total cardiovascular risk (Table 11–5) is important in deciding who to treat with antihypertensive medications, risk calculators are essential clinical tools. The ACC has an online toolkit relevant to primary prevention (https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/), and an associated app called ASCVD Risk Estimator Plus (downloadable at https://www.acc.org/ASCVDApp).
Table 11–4. Comparison of blood pressure treatment thresholds from the 2017 ACC/AHA guidelines, the 2018 Hypertension Canada guidelines, and the 2018 ESH/ESC guidelines.

Table 11–5. Cardiovascular risk factors.
Major risk factors
Hypertension1
Cigarette smoking
Obesity (BMI ≥ 30)1
Physical inactivity
Dyslipidemia1
Diabetes mellitus1
Microalbuminuria or estimated GFR < 60 mL/min
Age (> 55 years for men, > 65 years for women)
Family history of premature cardiovascular disease (< 55 years for men, < 65 years for women)
Target-organ damage
Heart
Left ventricular hypertrophy
Angina or prior myocardial infarction
Prior coronary revascularization
Heart failure
Brain
Stroke or transient ischemic attack
Chronic kidney disease
Peripheral arterial disease
Retinopathy
1Components of the metabolic syndrome.
BMI, body mass index; GFR, glomerular filtration rate.
Data from Chobanian AV et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21; 289(19):2560–72.
 Goals of Treatment
Goals of Treatment
Traditionally, the most widely accepted goal for blood pressure management has been less than 140/90 mm Hg. However, observational studies suggest that there does not seem to be a blood pressure level below which decrements in cardiovascular risk taper off, and a number of randomized controlled trials have suggested that treatment to blood pressure targets considerably below 140 mm Hg may benefit certain patient groups.
The SPRINT study suggests that outcomes improve in nondiabetic patients with considerably elevated cardiovascular risk when treatment lowers systolic pressure to less than 120 mm Hg compared to less than 140 mm Hg. On the other hand, in the HOPE3 study of largely nondiabetic patients at somewhat lower risk than those in SPRINT, reducing blood pressure by an average of 6/3 mm Hg systolic/diastolic from a baseline of 138/82 mm Hg provided no significant outcomes benefits. Therefore, it appears that blood pressure targets should be lower in people at greater estimated cardiovascular risk. In response to the SPRINT study, the 2018 Hypertension Canada guidelines urge prescribers to consider a blood pressure goal of less than 120/80 mm Hg in patients considered at elevated risk for cardiovascular events. The 2017 ACC/AHA guidelines take a different approach by defining a 130/80 mm Hg goal as “reasonable” in nonelevated risk patients, strengthening this to “recommended” in elevated risk hypertensive patients. The 2018 ESH/ESC guidelines specify a target of less than 140 mm Hg systolic for all, and less than 130 mm Hg for most if tolerated. There is a trend toward recommending similar treatment targets in the elderly; this topic is discussed in greater detail below. Some experts note that manual office measurements of around 130/80 mm Hg are likely to approximate the lower blood pressure targets specified in the SPRINT study, which used automated office blood pressure measuring devices that have been demonstrated to read as much as 16/7 mm Hg lower than manual office readings. The 2018 Canadian guidelines acknowledge this disparity in measurement methods by specifying that automated office devices should be used in the monitoring of patients selected for the aggressive blood pressure goal of less than 120/80 mm Hg. Table 11–4 compares the treatment threshold and target recommendations laid out in the American, Canadian, and European guidelines.
Treatment to blood pressures less than 130 mm Hg systolic seems especially important in stroke prevention. The ACCORD study examined the effect of treatment of systolic pressures to below 130–135 mm Hg in patients with diabetes. Although the lower treatment goal significantly increased the risk of serious adverse effects (with no additional gain in terms of heart, kidney, or retinal disease), there was a significant additional reduction in the risk of stroke. Lower targets might be justified in diabetic patients at high risk for cerebrovascular events.
Similarly, in the SPS3 trial in patients who have had a lacunar stroke, treating the systolic blood pressure to less than 130 mm Hg (mean systolic blood pressure of 127 mm Hg among treated versus mean systolic blood pressure 138 mm Hg among untreated patients) probably reduced the risk of recurrent stroke (and with an acceptably low rate of adverse effects from treatment). Blood pressure management in acute stroke is discussed below.
 How Low To Go?
How Low To Go?
Although observational studies indicate that the blood pressure-risk relationship holds up at levels considerably below 120 mm Hg, there is uncertainty about whether this is true for treated blood pressure. This question was addressed in a secondary analysis of data from the ONTARGET and TRANSCEND studies in which participants with elevated cardiovascular risk but no history of stroke were treated with telmisartan (plus or minus ramipril), or placebo. The risk of the composite cardiovascular endpoint was lowest at a treated systolic blood pressure range between 120 mm Hg and 140 mm Hg. Increased risk was observed at blood pressures below and above this range. The risk of stroke was the only exception, with incremental benefit observed below a treated systolic of 120 mm Hg. With respect to diastolic blood pressure on treatment, composite risk began to increase at levels below 70 mm Hg. This suggests that the blood pressure–cardiovascular risk relationship evident in observational studies may not hold in the case of treated blood pressure and that there are grounds for a degree of caution in treating below a systolic pressure of 120 mm Hg.
In seeking to simplify decision making in the treatment of hypertension, some authors have suggested that a systolic blood pressure goal in the 120–130 mm Hg range would be safe and effective in high-risk patients, and a systolic blood pressure of around 130 mm Hg would be reasonable in lower-risk patients.
Data from multiple studies indicate that statins should be part of the strategy to reduce overall cardiovascular risk. The HOPE3 study of persons at intermediate cardiovascular risk showed that 10 mg of rosuvastatin reduced average low-density lipoprotein (LDL) cholesterol from 130 mg/dL to 90 mg/dL (3.36–2.33 mmol/L), and significantly reduced the risk of multiple cardiovascular events, including myocardial infarction and coronary revascularization. Low-dose aspirin (81 mg/day) is likely to be beneficial in patients older than age 50 with either target-organ damage or elevated total cardiovascular risk (greater than 20–30%). Care should be taken to ensure that blood pressure is controlled to the recommended levels before starting aspirin to minimize the risk of intracranial hemorrhage. Data do not support the routine use of aspirin for prophylaxis in low-risk patients, including those over 65 years of age.
ACCORD Study Group; Cushman WC et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010 Apr 29;362(17):1575–85. [PMID: 20228401]
Böhm M et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017 Jun 3;389(10085):2226–37. [PMID: 28390695]
Lonn EM et al; HOPE-3 Investigators. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016 May 26;374(21):2009–20. [PMID: 27041480]
Ridker PM. Should aspirin be used for primary prevention in the post-statin era? N Engl J Med. 2018 Oct 18;379(16):1572–4. [PMID: 30332575]
Ruiz-Hurtado G et al. Has the SPRINT trial introduced a new blood-pressure goal in hypertension? Nat Rev Cardiol. 2017 Sep;14(9):560–6. [PMID: 28492286]
Sheppard JP et al. Benefits and harms of antihypertensive treatment in low-risk patients with mild hypertension. JAMA Intern Med. 2018 Dec; 178(12):1626–34. [PMID: 30383082]
SPRINT Research Group; Wright JT Jr et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015 Nov 26;373(22):2103–16. [PMID: 26551272]
Williams B et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). Blood Press. 2018 Dec;27(6):314–40. [PMID: 30380928]
Yusuf S et al; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016 May 26;374(21):2021–31. [PMID: 27040132]
DRUG THERAPY: CURRENT ANTIHYPERTENSIVE AGENTS
There are many classes of antihypertensive drugs of which six (ACE inhibitors, ARBs, renin inhibitors, calcium channel blockers, diuretics, and beta-blockers) are suitable for initial therapy based on efficacy and tolerability. A number of considerations enter into the selection of the initial regimen for a given patient. These include the weight of evidence for beneficial effects on clinical outcomes, the safety and tolerability of the drug, its cost, demographic differences in response, concomitant medical conditions, and lifestyle issues. The specific classes of antihypertensive medications are discussed below, and guidelines for the choice of initial medications are offered.
A. Angiotensin-Converting Enzyme Inhibitors
ACE inhibitors are commonly used as the initial medication in mild to moderate hypertension (Table 11–6). Their primary mode of action is inhibition of the RAAS, but they also inhibit bradykinin degradation, stimulate the synthesis of vasodilating prostaglandins, and can reduce sympathetic nervous system activity. These latter actions may explain why they exhibit some effect even in patients with low plasma renin activity. ACE inhibitors appear to be more effective in younger white patients. They are relatively less effective in blacks and older persons and in predominantly systolic hypertension. Although as single therapy they achieve adequate antihypertensive control in only about 40–50% of patients, the combination of an ACE inhibitor and a diuretic or calcium channel blocker is potent.
Table 11–6. Antihypertensive drugs: renin and ACE inhibitors and angiotensin II receptor blockers.


ACE inhibitors are the agents of choice in persons with type 1 diabetes with frank proteinuria or evidence of kidney dysfunction because they delay the progression to end-stage renal disease. Many authorities have expanded this indication to include persons with type 1 and type 2 diabetes mellitus with microalbuminuria who do not meet the usual criteria for antihypertensive therapy. ACE inhibitors may also delay the progression of nondiabetic kidney disease. The Heart Outcomes Prevention Evaluation (HOPE) trial demonstrated that the ACE inhibitor ramipril reduced the number of cardiovascular deaths, nonfatal myocardial infarctions, and nonfatal strokes and also reduced the incidence of new-onset heart failure, kidney dysfunction, and new-onset diabetes in a population of patients at high risk for vascular events. Although this was not specifically a hypertensive population, the benefits were associated with a modest reduction in blood pressure, and the results inferentially support the use of ACE inhibitors in similar hypertensive patients. ACE inhibitors are a drug of choice (usually in conjunction with a diuretic and a beta-blocker) in patients with heart failure with reduced ejection fraction and are indicated also in asymptomatic patients with reduced ejection fraction.
How to initiate therapy—A baseline metabolic panel should be drawn prior to starting medications that interfere with the RAAS, repeated 1–2 weeks after initiation of therapy to evaluate changes in creatinine and potassium. Minor dose adjustments of these medications rarely trigger significant shifts in these values.
Side effects—An advantage of the ACE inhibitors is their relative freedom from troublesome side effects (Table 11–6). Severe hypotension can occur in patients with bilateral renal artery stenosis; significant increases in creatinine may ensue but are usually reversible with the discontinuation of the ACE inhibitor. Hyperkalemia may develop in patients with kidney disease and type IV renal tubular acidosis (commonly seen in patients with diabetes) and in older adults. A chronic dry cough is common, seen in 10% of patients or more, and may require stopping the drug. Skin rashes are observed with any ACE inhibitor. Angioedema is an uncommon but potentially dangerous side effect of all agents of this class because of their inhibition of kininase. Exposure of the fetus to ACE inhibitors during the second and third trimesters of pregnancy has been associated with a variety of defects due to hypotension and reduced renal blood flow.
B. Angiotensin II Receptor Blockers
ARBs can improve cardiovascular outcomes in patients with hypertension as well as in patients with related conditions, such as heart failure and type 2 diabetes with nephropathy. ARBs have not been compared with ACE inhibitors in randomized controlled trials in patients with hypertension, but two trials comparing losartan with captopril in heart failure and post–myocardial infarction left ventricular dysfunction showed trends toward worse outcomes in the losartan group. By contrast, valsartan seems as effective as ACE inhibitors in these settings. Within group heterogeneity of antihypertensive potency and duration of action might explain such observations. The Losartan Intervention for Endpoints (LIFE) trial in nearly 9000 hypertensive patients with electrocardiographic evidence of left ventricular hypertrophy—comparing losartan with the beta-blocker atenolol as initial therapy—demonstrated a significant reduction in stroke with losartan. Of note is that in diabetic patients, death and myocardial infarction were also reduced, and there was a lower occurrence of new-onset diabetes. In a subgroup analysis from the LIFE trial, atenolol appeared to be superior to losartan in blacks, while the opposite was the case in non-blacks. A similar lack of efficacy of lisinopril compared to diuretics and calcium channel blockers was observed in blacks in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attach Trial (ALLHAT), suggesting that ACE inhibitors and ARBs may not be the preferred agents in black patients. In the treatment of hypertension, combination therapy with an ACE inhibitor and an ARB is not advised because it generally offers no advantage over monotherapy at maximum dose with addition of a complementary class where necessary.
Side effects—Unlike ACE inhibitors, the ARBs rarely cause cough and are less likely to be associated with skin rashes or angioedema (Table 11–6). However, as seen with ACE inhibitors, hyperkalemia can be a problem, and patients with bilateral renal artery stenosis may exhibit hypotension and worsened kidney function. Olmesartan has been linked to a sprue-like syndrome, presenting with abdominal pain, weight loss, and nausea, which subsides upon drug discontinuation. There is evidence from an observational study suggesting that ARBs and ACE inhibitors are less likely to be associated with depression than calcium channel blockers and beta-blockers.
C. Renin Inhibitors
Since renin cleavage of angiotensinogen is the rate-limiting step in the renin-angiotensin cascade, the most efficient inactivation of this system would be expected with renin inhibition. Conventional ACE inhibitors and ARBs probably offer incomplete blockade, even in combination. Aliskiren, a renin inhibitor, binds the proteolytic site of renin, thereby preventing cleavage of angiotensinogen. As a consequence, levels of angiotensins I and II are reduced and renin concentration is increased. Aliskiren effectively lowers blood pressure, reduces albuminuria, and limits left ventricular hypertrophy, but it has yet to be established as a first-line drug based on outcomes data. The combination of aliskiren with ACE inhibitors or ARBs in persons with type 2 diabetes mellitus offers no advantage and might even increase the risk of adverse cardiac or renal consequences.
D. Calcium Channel Blocking Agents
These agents act by causing peripheral vasodilation but with less reflex tachycardia and fluid retention than other vasodilators. They are effective as single-drug therapy in approximately 60% of patients in all demographic groups and all grades of hypertension (Table 11–7). For these reasons, they may be preferable to beta-blockers and ACE inhibitors in blacks and older persons. Verapamil and diltiazem should be combined cautiously with beta-blockers because of their potential for depressing atrioventricular (AV) conduction and sinus node automaticity as well as contractility.
Table 11–7. Antihypertensive drugs: calcium channel blocking agents.
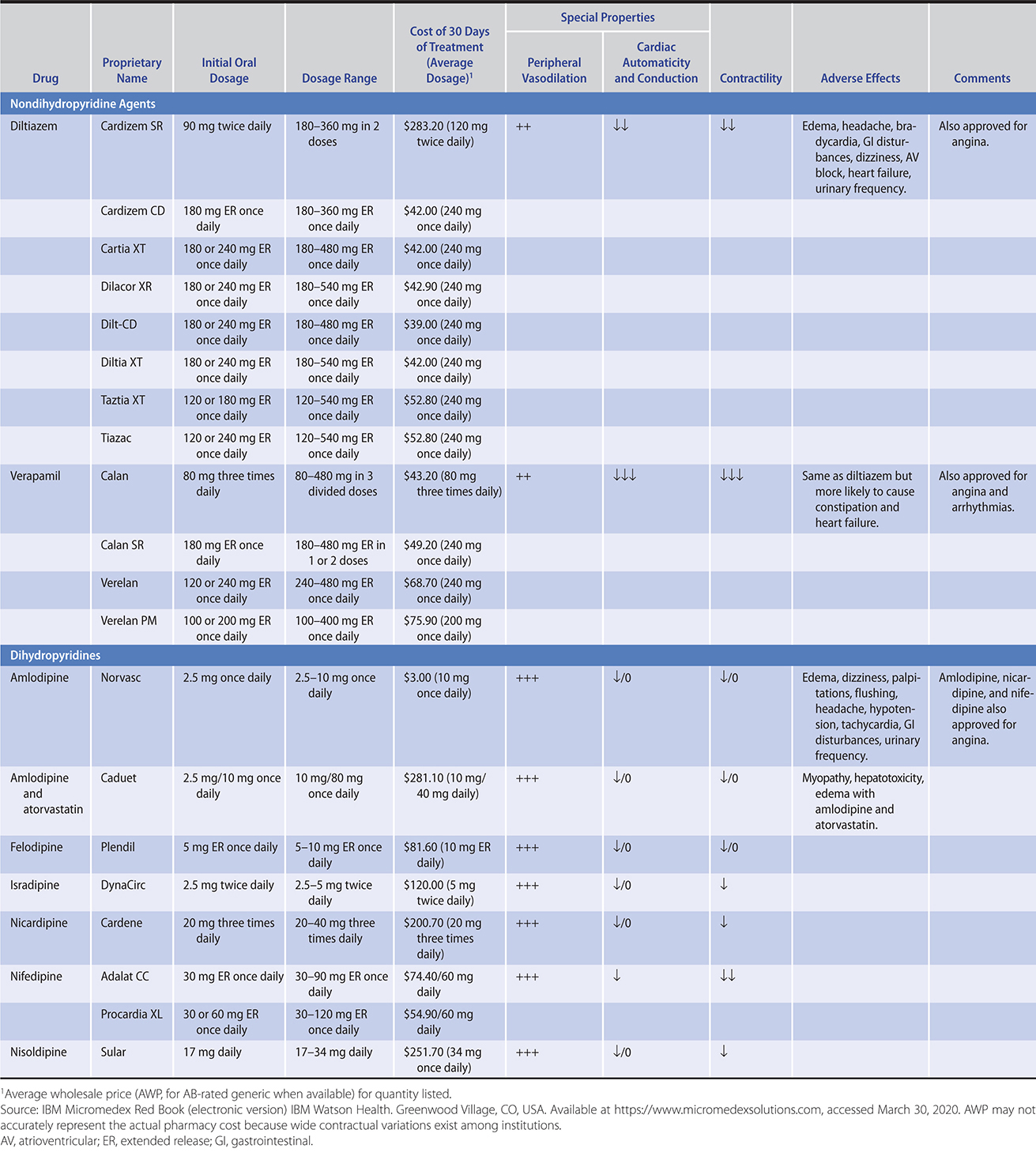
Initial concerns about possible adverse cardiac effects of calcium channel blockers have been convincingly allayed by several subsequent large studies that have demonstrated that calcium channel blockers are equivalent to ACE inhibitors and thiazide diuretics in prevention of coronary heart disease, major cardiovascular events, cardiovascular death, and total mortality. A protective effect against stroke with calcium channel blockers is well established, and in two trials (ALLHAT and the Systolic Hypertension in Europe trial), these agents appeared to be more effective than diuretic-based therapy.
Side effects—The most common side effects of calcium channel blockers are headache, peripheral edema, bradycardia, and constipation (especially with verapamil in older adults) (Table 11–7). The dihydropyridine agents—nifedipine, nicardipine, isradipine, felodipine, nisoldipine, and amlodipine—are more likely to produce symptoms of vasodilation, such as headache, flushing, palpitations, and peripheral edema. Edema is minimized by coadministration of an ACE inhibitor or ARB. Calcium channel blockers have negative inotropic effects and should be used cautiously in patients with cardiac dysfunction. Amlodipine is the only calcium channel blocker with established safety in patients with severe heart failure.
E. Diuretics
Thiazide diuretics (Table 11–8) are the antihypertensives that have been most extensively studied and most consistently effective in clinical trials. They lower blood pressure initially by decreasing plasma volume, but during long-term therapy, their major hemodynamic effect is reduction of peripheral vascular resistance. Most of the antihypertensive effect of these agents is achieved at lower dosages than used previously (typically, 12.5 mg of hydrochlorothiazide or equivalent), but their biochemical and metabolic effects are dose related. Chlorthalidone has the advantage of better 24-hour blood pressure control than hydrochlorothiazide in clinical trials. Thiazides may be used at higher doses if plasma potassium is above 4.5 mmol/L. The loop diuretics (such as furosemide) may lead to electrolyte and volume depletion more readily than the thiazides and have short durations of action. Because of these adverse effects, loop diuretics should be reserved for use in patients with kidney dysfunction (serum creatinine greater than 2.5 mg/dL [208.3 mcmol/L]; estimated glomerular filtration rate [eGFR] less than 30 mL/min) in which case they are more effective than thiazides. Relative to beta-blockers and ACE inhibitors, diuretics are more potent in blacks, older individuals, the obese, and other subgroups with increased plasma volume or low plasma renin activity (or both). They are relatively more effective in smokers than in nonsmokers. Long-term thiazide administration also mitigates the loss of bone mineral content in older women at risk for osteoporosis.
Table 11–8. Antihypertensive drugs: diuretics (in descending order of preference).
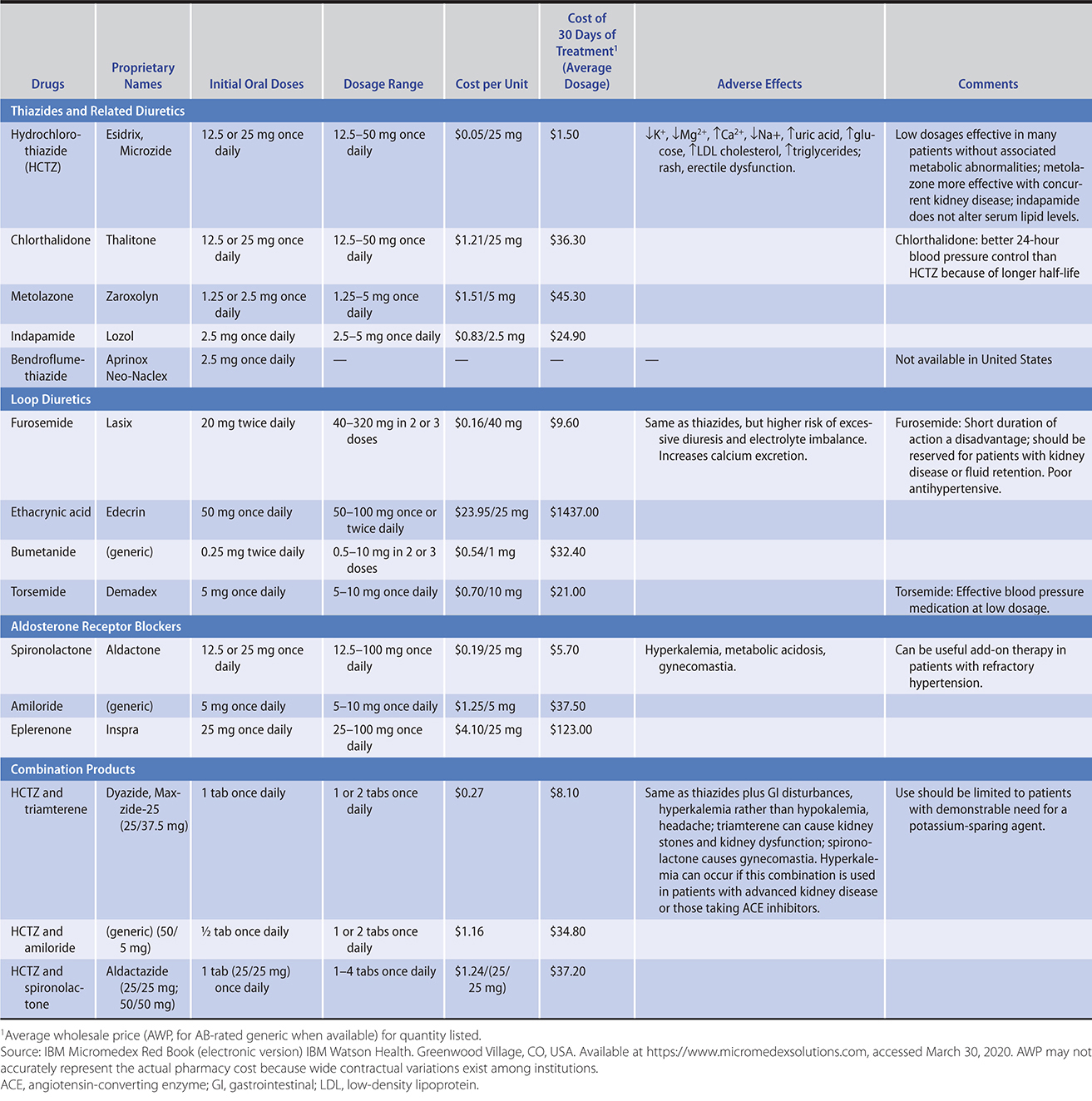
Overall, diuretics administered alone control blood pressure in 50% of patients with mild to moderate hypertension and can be used effectively in combination with all other agents. They are also useful for lowering isolated or predominantly systolic hypertension.
Side effects—The adverse effects of diuretics relate primarily to the metabolic changes listed in Table 11–8. Erectile dysfunction, skin rashes, and photosensitivity are less frequent. Hypokalemia has been a concern but is uncommon at the recommended dosages. The risk can be minimized by limiting dietary salt or increasing dietary potassium; potassium replacement is not usually required to maintain serum K+ at greater than 3.5 mmol/L. Higher serum K+ levels are prudent in patients at special risk from intracellular potassium depletion, such as those taking digoxin or with a history of ventricular arrhythmias in which case a potassium-sparing agent could be used. Compared with ACE inhibitors and ARBs, diuretic therapy is associated with a slightly higher incidence of mild new-onset diabetes. Diuretics also increase serum uric acid and may precipitate gout. Increases in blood glucose, triglycerides, and LDL cholesterol may occur but are relatively minor during long-term low-dose therapy. The potential for worsening of diabetes is outweighed by the advantages of blood pressure control, and diuretics should not be withheld from diabetic patients.
F. Aldosterone Receptor Antagonists
Spironolactone and eplerenone are natriuretic in sodium-retaining states, such as heart failure and cirrhosis, but only very weakly so in hypertension. These drugs have reemerged in the treatment of hypertension, particularly in resistant patients and are helpful additions to most other antihypertensive medications. Consistent with the increasingly appreciated importance of aldosterone in essential hypertension, the aldosterone receptor blockers are effective at lowering blood pressure in all hypertensive patients regardless of renin level, and are also effective in blacks. Aldosterone plays a central role in target-organ damage, including the development of ventricular and vascular hypertrophy and renal fibrosis. Aldosterone receptor antagonists ameliorate these consequences of hypertension, to some extent independently of effects on blood pressure.
Side effects—Spironolactone can cause breast pain and gynecomastia in men through activity at the progesterone receptor, an effect not seen with the more specific eplerenone. Hyperkalemia is a problem with both drugs, chiefly in patients with chronic kidney disease. Hyperkalemia is more likely if the pretreatment plasma potassium exceeds 4.5 mmol/L.
G. Beta-Adrenergic Blocking Agents
These drugs are effective in hypertension because they decrease the heart rate and cardiac output. The beta-blockers also decrease renin release and are more efficacious in populations with elevated plasma renin activity, such as younger white patients. They neutralize the reflex tachycardia caused by vasodilators and are especially useful in patients with associated conditions that benefit from the cardioprotective effects of these agents. These include individuals with angina pectoris, previous myocardial infarction, and stable heart failure as well as those with migraine headaches and somatic manifestations of anxiety.
Although all beta-blockers appear to be similar in antihypertensive potency, they differ in a number of pharmacologic properties (these differences are summarized in Table 11–9), including specificity to the cardiac beta-1-receptors (cardioselectivity) and whether they also block the beta-2-receptors in the bronchi and vasculature; at higher dosages, however, all agents are nonselective. The beta-blockers also differ in their pharmacokinetics, lipid solubility—which determines whether they cross the blood-brain barrier predisposing to central nervous system side effects—and route of metabolism. Metoprolol reduces mortality and morbidity in patients with chronic stable heart failure with reduced ejection fraction (see Chapter 10). Carvedilol and nebivolol maintain cardiac output and are beneficial in patients with left ventricular systolic dysfunction. Carvedilol and nebivolol may reduce peripheral vascular resistance by concomitant alpha-blockade (carvedilol) and increased nitric oxide release (nebivolol). Because of the lack of efficacy in primary prevention of myocardial infarction and inferiority compared with other drugs in prevention of stroke and left ventricular hypertrophy, traditional beta-blockers should not be used as first-line agents in the treatment of hypertension without specific compelling indications (such as active coronary artery disease). Vasodilating beta-blockers may emerge as alternative first-line antihypertensives, but this possibility has yet to be rigorously tested in outcome studies.
Table 11–9. Antihypertensive drugs: beta-adrenergic blocking agents.


Side effects—The side effects of beta-blockers include inducing or exacerbating bronchospasm in predisposed patients; sinus node dysfunction and AV conduction depression (resulting in bradycardia or AV block); nasal congestion; Raynaud phenomenon; and central nervous system symptoms with nightmares, excitement, depression, and confusion. Fatigue, lethargy, and erectile dysfunction may occur. The traditional beta-blockers (but not the vasodilator beta-blockers carvedilol and nebivolol) have an adverse effect on lipids and glucose metabolism. Beta-blockers are used cautiously in patients with type 1 diabetes, since they can mask the symptoms of hypoglycemia and prolong these episodes by inhibiting gluconeogenesis. These drugs should also be used with caution in patients with advanced peripheral vascular disease associated with rest pain or nonhealing ulcers, but they are generally well tolerated in patients with mild claudication. Nebivolol can be safely used in patients with stage II claudication (claudication at 200 m).
In treatment of pheochromocytoma, beta-blockers should not be administered until alpha-blockade (eg, phentolamine) has been established. Otherwise, blockade of vasodilatory beta-2-adrenergic receptors will allow unopposed vasoconstrictor alpha-adrenergic receptor activation with worsening of hypertension. For the same reason, beta-blockers should not be used to treat hypertension arising from cocaine use.
Great care should be exercised if the decision is made, in the absence of compelling indications, to remove beta-blockers from the treatment regimen because abrupt withdrawal can precipitate acute coronary events and severe increases in blood pressure.
H. Alpha-Adrenoceptor Antagonists
Prazosin, terazosin, and doxazosin (Table 11–10) block postsynaptic alpha-receptors, relax smooth muscle, and reduce blood pressure by lowering peripheral vascular resistance. These agents are effective as single-drug therapy in some individuals, but tachyphylaxis may appear during long-term therapy. Unlike beta-blockers and diuretics, alpha-blockers have no adverse effect on serum lipid levels. In fact, alpha-blockers increase HDL cholesterol while reducing total cholesterol; whether this is beneficial in the long term has not been established.
Table 11–10. Alpha-adrenoceptor blocking agents, sympatholytics, and vasodilators.

Side effects—Side effects are relatively common (Table 11–10). These include marked hypotension after the first dose which, therefore, should be small and given at bedtime. Post-dosing palpitations, headache, and nervousness may continue to occur during long-term therapy; these symptoms may be less frequent or severe with doxazosin because of its more gradual onset of action. In ALLHAT, persons receiving doxazosin as initial therapy had a significant increase in heart failure hospitalizations and a higher incidence of stroke relative to those receiving diuretics, prompting discontinuation of this arm of the study. Cataractectomy in patients exposed to alpha-blockers can be complicated by the floppy iris syndrome, even after discontinuation of the drug, so the ophthalmologist should be alerted that the patient has been taking the drug prior to surgery.
To summarize, alpha-blockers should generally not be used as initial agents to treat hypertension—except perhaps in men with symptomatic prostatism or nightmares linked to posttraumatic stress disorder.
I. Drugs With Central Sympatholytic Action
Methyldopa, clonidine, guanabenz, and guanfacine (Table 11–10) lower blood pressure by stimulating alpha-adrenergic receptors in the central nervous system, thus reducing efferent peripheral sympathetic outflow. There is considerable experience with methyldopa in pregnant women, and it is still used for this population. Clonidine is available in patches, which may have particular value in noncompliant patients. All of these central sympatholytic agents are effective as single therapy in some patients, but they are usually used as second- or third-line agents because of the high frequency of drug intolerance.
Side effects—Side effects include sedation, fatigue, dry mouth, postural hypotension, and erectile dysfunction. An important concern is rebound hypertension following withdrawal. Methyldopa also causes hepatitis and hemolytic anemia and should be restricted to individuals who have already tolerated long-term therapy.
J. Peripheral Sympathetic Inhibitors
These agents are now used infrequently and usually in refractory hypertension. Reserpine remains a cost-effective antihypertensive agent (Table 11–10). Its reputation for inducing mental depression and its other side effects—sedation, nasal stuffiness, sleep disturbances, and peptic ulcers—has made it unpopular, though these problems are uncommon at low dosages. Guanethidine and guanadrel inhibit catecholamine release from peripheral neurons but frequently cause orthostatic hypotension (especially in the morning or after exercise), diarrhea, and fluid retention.
K. Arteriolar Dilators
Hydralazine and minoxidil (Table 11–10) relax vascular smooth muscle and produce peripheral vasodilation. When given alone, they stimulate reflex tachycardia; increase myocardial contractility; and cause headache, palpitations, and fluid retention. To counteract these effects, the agents are usually given in combination with diuretics and beta-blockers in resistant patients. Hydralazine produces frequent gastrointestinal disturbances and may induce a lupus-like syndrome. Minoxidil causes hirsutism and marked fluid retention; this very potent agent is reserved for the most refractory of cases.
 Antihypertensive Medications & the Risk of Cancer
Antihypertensive Medications & the Risk of Cancer
A number of observational studies have examined the association between long-term exposure to antihypertensive medications and cancer. Weak associations have been suggested by some of these studies, but results have been very mixed. Examples of positive studies include an association between ACE inhibitors and lung cancer (hazard ratio 1.14) and between photosensitizing drugs and squamous skin cancer (the use of alpha-2-receptors blockers and diuretics was associated with a 17% increased risk of squamous cell cancer). The relationship between calcium channel blockers and breast cancer remains uncertain. In the absence of large-scale prospective studies with cancer as a prespecified outcome measure, the effect of antihypertensive drugs on the risk of cancer remains uncertain. By contrast, the beneficial effect of these drugs on cardiovascular outcomes has been clearly established. Concern about increased risk of cancer should not be minimized, but at present there are no compelling data to prompt a change in prescribing patterns.
Hicks BM et al. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ. 2018 Oct 24;363:k4209. [PMID: 30355745]
Ostrov DA et al. Rationally designed small molecules to prevent type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2019 Apr;26(2):90–5. [PMID: 30694829]
Su KA et al. Photosensitizing antihypertensive drug use and risk of cutaneous squamous cell carcinoma. Br J Dermatol. 2018 Nov;179(5):1088–94. [PMID: 29723931]
Wright CM et al. Calcium channel blockers and breast cancer incidence: an updated systematic review and meta-analysis of the evidence. Cancer Epidemiol. 2017 Oct;50(Pt A):113–24. [PMID: 28866282]
 Developing an Antihypertensive Regimen
Developing an Antihypertensive Regimen
Historically, data from large placebo-controlled trials supported the overall conclusion that antihypertensive therapy with diuretics and beta-blockers had a major beneficial effect on a broad spectrum of cardiovascular outcomes, reducing the incidence of stroke by 30–50% and of heart failure by 40–50%, and halting progression to accelerated hypertension syndromes. The decreases in fatal and nonfatal coronary heart disease and cardiovascular and total mortality were less dramatic, ranging from 10% to 15%. Similar placebo-controlled data pertaining to the newer agents are generally lacking, except for stroke reduction with the calcium channel blocker nitrendipine in the Systolic Hypertension in Europe trial. However, there is substantial evidence that ACE inhibitors, and to a lesser extent ARBs, reduce adverse cardiovascular outcomes in other related populations (eg, patients with diabetic nephropathy, heart failure, or postmyocardial infarction and individuals at high risk for cardiovascular events). Most large clinical trials that have compared outcomes in relatively unselected patients have failed to show a difference between newer agents—such as ACE inhibitors, calcium channel blockers, and ARBs—and the older diuretic-based regimens with regard to survival, myocardial infarction, and stroke. Where differences have been observed, they have mostly been attributable to subtle asymmetries in blood pressure control rather than to any inherent advantages of one agent over another. Recommendations for initial treatment identify ACE inhibitors, ARBs, and calcium channel blockers as valid choices. Because of their adverse metabolic profile, initial therapy with thiazides might best be restricted to older patients. Thiazides are acceptable as first-line therapy in blacks because of specific efficacy in this group.
As discussed above, beta-blockers are not ideal first-line drugs in the treatment of hypertension without compelling indications for their use (such as active coronary artery disease and heart failure). Vasodilator beta-blockers (such as carvedilol and nebivolol) may produce better outcomes than traditional beta-blockers; however, this possibility remains a theoretical consideration.
The American Diabetes Association has advocated evening dosing of one or more antihypertensive medications to restore nocturnal blood pressure dipping. Outcomes data to support this proposal are limited. The Spanish MAPEC study of such nocturnal antihypertensive dosing showed a significant reduction in a range of major cardiovascular events in 2156 participants over 5.6 years. However, there are concerns that ischemic optic neuropathy may be triggered by profound nocturnal hypotension. Thus, larger studies are necessary before this approach can be firmly recommended.
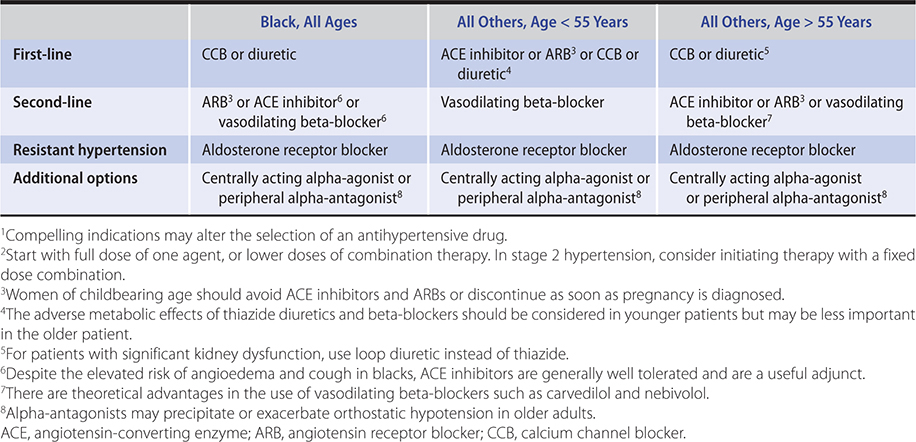
Drugs that interrupt the renin-angiotensin cascade are more effective in young, white persons, in whom renin tends to be higher. Calcium channel blockers and diuretics are more effective in older or black persons, in whom renin levels are generally lower. Many patients require two or more medications and even then a substantial proportion fail to achieve the goal blood pressure. A stepped care approach to the drug treatment of hypertension is outlined in Table 11–11. In diabetic patients, three or four drugs are usually required to reduce systolic blood pressure to less than 140 mm Hg. In many patients, blood pressure cannot be adequately controlled with any combination. As a result, debating the appropriate first-line agent is less relevant than determining the most appropriate combinations of agents. The mnemonic ABCD can be used to remember four classes of antihypertensive medications. These four classes can be divided into two categories: AB and CD. AB refers to drugs that block the RAAS (ACE/ARB and beta-blockers). CD refers to those that work in other pathways (calcium channel blockers and diuretics). As discussed above, beta-blockers are no longer considered first-line drugs in the absence of compelling indications. Combinations of drugs between the two categories are more potent than combinations from within a category. Many experts recommend the use of fixed-dose combination (between two categories) antihypertensive agents as first-line therapy in patients with substantially elevated systolic pressures (greater than 160/100 mm Hg) or difficult-to-control hypertension (which is often associated with diabetes or kidney dysfunction). In light of unwanted metabolic effects, calcium channel blockers might be preferable to thiazides in the younger hypertensive patient requiring a second antihypertensive drug following initiation of therapy with an ACE inhibitor or ARB. Furthermore, based on the results from the ACCOMPLISH trial, a combination of ACE inhibitor and calcium channel blocker may also prove optimal for patients at high risk for cardiovascular events. The initial use of low-dose combinations allows faster blood pressure reduction without substantially higher intolerance rates and is likely to be better accepted by patients. Data from the ALTITUDE study (in patients with type 2 diabetes and chronic kidney disease or cardiovascular disease or both) indicate that the addition of aliskiren to either ARB or ACE inhibitor was associated with worse outcomes and cannot be recommended, at least in this population. A suggested approach to treatment, tailored to patient demographics, is outlined in Table 11–12.
Table 11–11. A step care approach to the initiation and titration of antihypertension medications.1,2
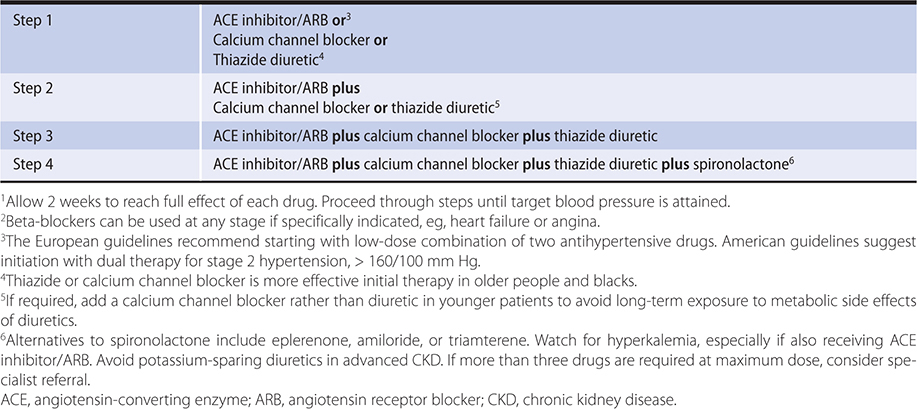
Table 11–12. Choice of antihypertensive agent based on demographic considerations.1,2
In sum, as a prelude to treatment, the patient should be informed of common side effects and the need for diligent compliance. In patients with mild or stage 1 hypertension (less than 160/90 mm Hg) in whom pharmacotherapy is indicated, treatment should start with a single agent or two-drug combination at a low dose. Follow-up visits should usually be at 4- to 6-week intervals to allow for full medication effects to be established (especially with diuretics) before further titration or adjustment. If, after titration to usual doses, the patient has shown a discernible but incomplete response and a good tolerance of the initial drug, another medication should be added. See Goals of Treatment, above. As a rule of thumb, a blood pressure reduction of 10 mm Hg can be expected for each antihypertensive agent added to the regimen and titrated to the optimum dose. In those with more severe hypertension (stage 2), or with comorbidities (such as diabetes) that are likely to render them resistant to treatment, initiation with combination therapy is advised and more frequent follow-up is indicated.
Patients who are compliant with their medications and who do not respond to conventional combination regimens should usually be evaluated for secondary hypertension before proceeding to more complex regimens.
 Medication Nonadherence
Medication Nonadherence
Adherence to antihypertensive treatment is alarmingly poor. In one European study of patients’ antihypertensive medication compliance, there was a 40% discontinuation rate at 1 year after initiation. Only 39% of patients were found to be taking their medications continuously over a 10-year period. Collaborative care, using clinicians, pharmacists, social workers, and nurses to encourage compliance, has had a variable and often rather modest effect on blood pressure control. Adherence is enhanced by patient education and by use of home blood pressure measurement. The choice of antihypertensive medication is important. Better compliance has been reported for patients whose medications could be taken once daily or as combination pills. Adherence is best with ACE inhibitors and ARBs, and worse with beta-blockers and diuretics.
 Consideration of Gender in Hypertension
Consideration of Gender in Hypertension
Because of the preponderance of male recruitment into large-scale clinical trials, the impact of gender on the evaluation and management of hypertension remains uncertain. The limited data that exist suggest a steeper relationship in women between 24-hour ambulatory and night time systolic blood pressure and the risk of cardiovascular events. There are many gender-specific effects on the mechanisms and end organ impact of hypertension. In younger adults, men are more likely to be hypertensive than women, a relationship that reverses in later life. Regression of left ventricular hypertrophy in response to ACE inhibitors is less pronounced in women. Women are more likely to have isolated systolic hypertension, probably because they develop more active left ventricular systolic function and greater vascular stiffness than men. Fibromuscular dysplasia of the renal artery is much more common in women than men. The side effects of many antihypertensive drugs are more pronounced in women than men, including ACE inhibitor–associated cough and hyponatremia and hypokalemia in response to diuretics. Conversely, thiazides can help preserve bone density. Dependent edema due to amlodipine is more likely in women, and women are more sensitive to beta-blockers. There are no data to support a different blood pressure target in women, but this question has not been examined in dedicated clinical trials.
 Special Considerations in the Treatment of Diabetic Hypertensive Patients
Special Considerations in the Treatment of Diabetic Hypertensive Patients
Hypertensive patients with diabetes are at particularly high risk for cardiovascular events. Data from the ACCORD study of diabetic patients demonstrated that most of the benefits of blood pressure lowering were seen with a systolic target of less than 140 mm Hg. Although there was a reduction in stroke risk at a systolic target below 120/70 mm Hg, treatment to this lower target was associated with an increased risk of serious adverse effects. US and Canadian guidelines recommend a blood pressure goal of less than 130/80 mm Hg in diabetic patients. Because of the beneficial effects of ACE inhibitors in diabetic nephropathy, they should be part of the initial treatment regimen. ARBs or perhaps renin inhibitors may be substituted in those intolerant of ACE inhibitors. While the ONTARGET study showed that combinations of ACE inhibitors and ARBs in persons with atherosclerosis or type 2 diabetes with end-organ damage appeared to minimize proteinuria, this strategy slightly increased the risks of progression to dialysis and of death; thus, it is not recommended. Most diabetic patients require combinations of three to five agents to achieve target blood pressure, usually including a diuretic and a calcium channel blocker or beta-blocker. In addition to rigorous blood pressure control, treatment of persons with diabetes should include aggressive treatment of other risk factors.
 Treatment of Hypertension in Chronic Kidney Disease
Treatment of Hypertension in Chronic Kidney Disease
Hypertension is present in 40% of patients with a GFR of 60–90 mL/min, and 75% of patients with a GFR less than 30 mL/min. The rate of progression of chronic kidney disease is markedly slowed by treatment of hypertension. In the SPRINT trial, the reduction in cardiovascular risk associated with lower blood pressure targets was also observed in the subgroup with a GFR of less than 60 mL/min. However, an effect of lower blood pressure targets on the slowing of chronic kidney disease progression appears to be restricted to those with pronounced proteinuria. In the SPRINT trial, the lower blood pressure goal was associated with increased risk of acute kidney injury, but this was generally reversible and not associated with elevated biomarkers for ischemic injury. Most experts recommend a blood pressure target of less than 130/80 mm Hg in patients with chronic kidney disease, with consideration of more intensive lowering if proteinuria greater than 1 g per 24 hours is present. Medications that interrupt the renin-angiotensin cascade can slow the progression of kidney disease and are preferred for initial therapy, especially in those with albuminuria of greater than 300 mg/g creatinine. Transition from thiazide to loop diuretic is often necessary to control volume expansion as the eGFR falls below 30 mL/min. ACE inhibitors remain protective and safe in kidney disease associated with significant proteinuria and serum creatinine as high as 5 mg/dL (380 mcmol/L). However, the use of drugs blocking the RAAS cascade in patients with advanced chronic kidney disease should be supervised by a nephrologist. Kidney function and electrolytes should be measured 1 week after initiating treatment and subsequently monitored carefully in patients with kidney disease. An increase in creatinine of 20–30% is acceptable and expected; more exaggerated responses suggest the possibility of renal artery stenosis or volume contraction. Although lower blood pressure levels are associated with acute decreases in GFR, this appears not to translate into an increased risk of developing end-stage renal disease in the long term. Persistence with ACE inhibitor or ARB therapy as the serum potassium level exceeds 5.5 mEq/L is probably not warranted, since other antihypertensive medications are renoprotective as long as goal blood pressures are maintained. However, diuretics can often be helpful in controlling mild hyperkalemia, and there are novel cation exchange polymers (such as patiromer) that sequester potassium in the gut and are more effective and better tolerated than sodium polystyrene sulfonate.
 Hypertension Management in Blacks
Hypertension Management in Blacks
Substantial evidence indicates that blacks are not only more likely to become hypertensive and more susceptible to the cardiovascular and renal complications of hypertension—they also respond differently to many antihypertensive medications. The REGARDS study illustrates these differences. At systolic blood pressures less than 120 mm Hg, black and white participants between 45 and 64 years of age had equal risk of stroke. For a 10 mm Hg increase in systolic blood pressure, the risk of stroke was threefold higher in black participants. At the level of stage 1 hypertension, the hazard ratio for stroke in black compared to white participants between 45 and 64 years of age was 2.35. This increased susceptibility may reflect genetic differences in the cause of hypertension or the subsequent responses to it, differences in occurrence of comorbid conditions such as diabetes or obesity, or environmental factors such as diet, activity, stress, or access to health care services. In any case, as in all persons with hypertension, a multifaceted program of education and lifestyle modification is warranted. Early introduction of combination therapy has been advocated, but there are no clinical trial data to support a lower than usual blood pressure goal in blacks. Because it appears that ACE inhibitors and ARBs—in the absence of concomitant diuretics—are less effective in blacks than in whites, initial therapy should generally be a diuretic or a diuretic in combination with a calcium channel blocker. However, inhibitors of the RAAS do lower blood pressure in black patients, are useful adjuncts to the recommended diuretic and calcium channel blockers, and should be used in patients with hypertension and compelling indications such as heart failure and kidney disease (especially in the presence of proteinuria) (Table 11–13). Black patients have an elevated risk of ACE inhibitor–associated angioedema and cough, so ARBs would be the preferred choice.
Table 11–13. Recommended antihypertensive medications.
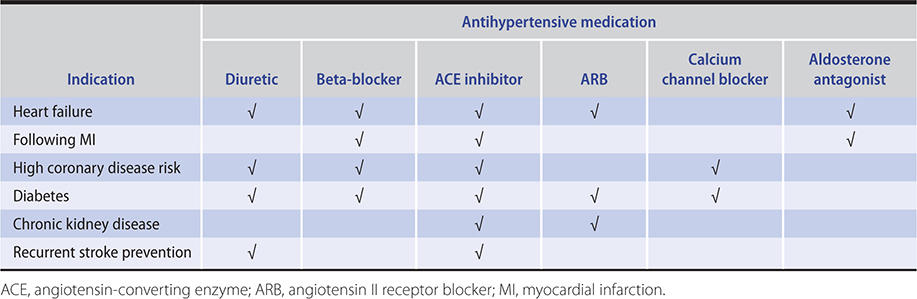
 Treating Hypertension in Older Adults
Treating Hypertension in Older Adults
Several studies in persons over 60 years of age have confirmed that antihypertensive therapy prevents fatal and nonfatal myocardial infarction and reduces overall cardiovascular mortality. The HYVET study indicated that a reasonable ultimate blood pressure goal is 150/80 mm Hg. Updated guidelines suggest that blood pressure goals should not generally be influenced by age alone. An exploratory subgroup analysis of the SPRINT study found that people older than age 75 years showed benefit at the 120 mm Hg systolic treatment target. Importantly, these benefits were also evident in patients classified as frail. This more aggressive approach was, however, associated with greater risk of falls and worsening kidney function, indicating that close monitoring is required in elderly patients treated to lower blood pressure goals. It is also important to note the exclusion criteria of the SPRINT study, which included diabetes mellitus, stroke, and orthostatic hypotension.
Blood pressure treatment goals should be individualized in the very elderly. In the SPRINT MIND study, the lower systolic blood pressure target of 120 mm Hg was associated with a 15% reduction in the incidence of mild cognitive impairment and probable all cause dementia compared to the 140 mm Hg in the target group. Based upon this data, aggressive control of hypertension in high-risk individuals would have a significant impact on the prevalence of dementia. As discussed above, it is important to note that blood pressure measurements in the SPRINT study were made by automated devices, which are known to read lower than conventional office measurements.
How to initiate antihypertensive therapy in older patients—The same medications are used in older patients but at 50% lower doses. Pressure should be reduced more gradually with a safe intermediate systolic blood pressure goal of 160 mm Hg. As treatment is initiated, older patients should be carefully monitored for orthostasis, altered cognition, and electrolyte disturbances. The elderly are especially susceptible to problems associated with polypharmacy, including drug interactions and dosing errors.
 Follow-Up of Patients Receiving Hypertension Therapy
Follow-Up of Patients Receiving Hypertension Therapy
Once blood pressure is controlled on a well-tolerated regimen, follow-up visits can be infrequent and laboratory testing limited to those appropriate for the patient and the medications used. Yearly monitoring of blood lipids is recommended, and an electrocardiogram could be repeated at 2- to 4-year intervals depending on whether initial abnormalities are present and on the presence of coronary risk factors. Patients who have had excellent blood pressure control for several years, especially if they have lost weight and initiated favorable lifestyle modifications, might be considered for a trial of reduced antihypertensive medications.
Boal AH et al. Monotherapy with major antihypertensive drug classes and risk of hospital admissions for mood disorders. Hypertension. 2016 Nov;68(5):1132–8. [PMID: 27733585]
Burnier M. Drug adherence in hypertension. Pharmacol Res. 2017 Nov;125(Pt B):142–9. [PMID: 28870498]
Carnethon MR et al; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017 Nov 21;136(21):e393–423. [PMID: 29061565]
Chang AR et al. Blood pressure goals in patients with chronic kidney disease: a review of evidence and guidelines. Clin J Am Soc Nephrol. 2019 Jan 7;14(1):161–9. [PMID: 30455322]
Gudsoorkar PS et al. Changing concepts in hypertension management. J Hum Hypertens. 2017 Dec;31(12):763–7. [PMID: 28748919]
Supiano MA et al. New guidelines and SPRINT results: implications for geriatric hypertension. Circulation. 2019 Sep 17;140(12):976–8. [PMID: 31525101]
Wenger NK et al. Hypertension across a woman’s life cycle. J Am Coll Cardiol. 2018 Apr 24;71(16):1797–813. [PMID: 29673470]
RESISTANT HYPERTENSION
Resistant hypertension is defined as the failure to reach blood pressure control in patients who are adherent to full doses of an appropriate three-drug regimen (including a diuretic). Adherence is a major issue: the rate of partial or complete noncompliance probably approaches 50% in this group of patients; doxazosin, spironolactone, and hydrochlorothiazide were particularly unpopular in one Eastern European study based on drug assay. In the approach to resistant hypertension, the clinician should first confirm compliance and rule out “white coat hypertension,” ideally using ambulatory or home-based measurement of blood pressure. Exacerbating factors should be considered (as outlined above). Finally, identifiable causes of resistant hypertension should be sought (Table 11–14). The clinician should pay particular attention to the type of diuretic being used in relation to the patient’s kidney function. Aldosterone may play an important role in resistant hypertension and aldosterone receptor blockers can be very useful. If goal blood pressure cannot be achieved following completion of these steps, consultation with a hypertension specialist should be considered. Procedure-based approaches to resistant hypertension are being developed, but the Symplicity HTN 3 study failed to show that renal sympathetic ablation improved blood pressure compared to a sham-operated control group.
Table 11–14. Causes of resistant hypertension.
Improper blood pressure measurement
Nonadherence
Volume overload and pseudotolerance
Excess sodium intake
Volume retention from kidney disease
Inadequate diuretic therapy
Drug-induced or other causes
Inadequate doses
Inappropriate combinations
Nonsteroidal anti-inflammatory drugs; cyclooxygenase-2 inhibitors
Cocaine, amphetamines, other illicit drugs
Sympathomimetics (decongestants, anorectics)
Oral contraceptives
Adrenal steroids
Cyclosporine and tacrolimus
Erythropoietin
Licorice (including some chewing tobacco)
Selected over-the-counter dietary supplements and medicines (eg, ephedra, ma huang, bitter orange)
Associated conditions
Obesity
Excess alcohol intake
Identifiable causes of hypertension (see Table 11–2)
Data from Chobanian AV et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72.
Wei FF et al. Diagnosis and management of resistant hypertension: state of the art. Nat Rev Nephrol. 2018 Jul;14(7):428–41. [PMID: 29700488]
HYPERTENSIVE URGENCIES & EMERGENCIES
Hypertensive emergencies have become less frequent in recent years but still require prompt recognition and aggressive but careful management. A spectrum of urgent presentations exists, and the appropriate therapeutic approach varies accordingly.
Hypertensive urgencies are situations in which blood pressure must be reduced within a few hours. These include patients with asymptomatic severe hypertension (systolic blood pressure greater than 220 mm Hg or diastolic pressure greater than 125 mm Hg that persists after a period of observation) and those with optic disk edema, progressive target-organ complications, and severe perioperative hypertension. Elevated blood pressure levels alone—in the absence of symptoms of new or progressive target-organ damage—rarely require emergency therapy. Parenteral drug therapy is not usually required; partial reduction of blood pressure with relief of symptoms is the goal. Effective oral agents are clonidine, captopril, and slow-release nifedipine.
Hypertensive emergencies require substantial reduction of blood pressure within 1 hour to avoid the risk of serious morbidity or death. Although blood pressure is usually strikingly elevated (diastolic pressure greater than 130 mm Hg), the correlation between pressure and end-organ damage is often poor. It is the presence of critical multiple end organ injury that determines the seriousness of the emergency and the approach to treatment. Emergencies include hypertensive encephalopathy (headache, irritability, confusion, and altered mental status due to cerebrovascular spasm), hypertensive nephropathy (hematuria, proteinuria, and acute kidney injury due to arteriolar necrosis and intimal hyperplasia of the interlobular arteries), intracranial hemorrhage, aortic dissection, preeclampsia-eclampsia, pulmonary edema, unstable angina, or myocardial infarction. Encephalopathy or nephropathy accompanying hypertensive retinopathy has historically been termed malignant hypertension, but the therapeutic approach is identical to that used in other hypertensive emergencies.
Parenteral therapy is indicated in most hypertensive emergencies, especially if encephalopathy is present. The initial goal in hypertensive emergencies is to reduce the pressure by no more than 25% (within minutes to 1 or 2 hours) and then toward a level of 160/100 mm Hg within 2–6 hours. Excessive reductions in pressure may precipitate coronary, cerebral, or renal ischemia. To avoid such declines, the use of agents that have a predictable, dose-dependent, transient, and progressive antihypertensive effect is preferable (Table 11–15). In that regard, the use of sublingual or oral fast-acting nifedipine preparations is best avoided.
Table 11–15. Treatment of hypertensive emergency depending on primary site of end-organ damage. See Table 11–16 for dosages.
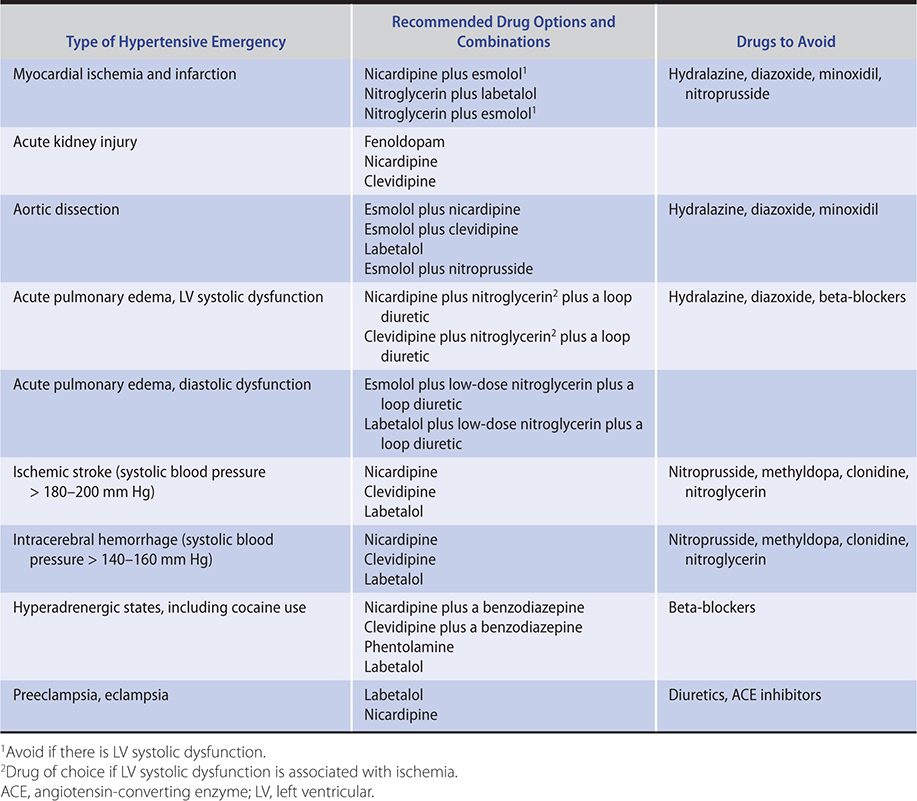
Acute ischemic stroke is often associated with marked elevation of blood pressure, which will usually fall spontaneously. In such cases, antihypertensives should only be used if the systolic blood pressure exceeds 180–200 mm Hg, and blood pressure should be reduced cautiously by 10–15% (Table 11–15). If thrombolytics are to be given, blood pressure should be maintained at less than 185/110 mm Hg during treatment and for 24 hours following treatment.
In intracerebral hemorrhage, the aim is to minimize bleeding by reducing the systolic blood pressure in most patients to 130–140 mm Hg within the first 6 hours. In acute subarachnoid hemorrhage, as long as the bleeding source remains uncorrected, a compromise must be struck between preventing further bleeding and maintaining cerebral perfusion in the face of cerebral vasospasm. In this situation, blood pressure goals depend on the patient’s usual blood pressure. In previously normotensive patients, the target should be a systolic blood pressure of 110–120 mm Hg; in hypertensive patients, blood pressure should be treated to 20% below baseline pressure. In the treatment of hypertensive emergencies complicated by (or precipitated by) central nervous system injury, labetalol or nicardipine are good choices, since they are nonsedating and do not appear to cause significant increases in cerebral blood flow or intracranial pressure. Patients with subarachnoid hemorrhage should receive nimodipine for 3 weeks following presentation to minimize cerebral vasospasm. In hypertensive emergencies arising from catecholaminergic mechanisms, such as pheochromocytoma or cocaine use, beta-blockers can worsen the hypertension because of unopposed peripheral vasoconstriction; nicardipine, clevidipine, or phentolamine is preferred. Labetalol is useful in these patients if the heart rate must be controlled. Table 11–15 provides guidelines for the choice of antihypertensive agent based on the site of end-organ damage. ACE inhibitors are specifically indicated for hypertensive crisis from scleroderma.
 Pharmacologic Management
Pharmacologic Management
A. Parenteral Agents
Sodium nitroprusside is no longer the treatment of choice for acute hypertensive problems; in most situations, appropriate control of blood pressure is best achieved using combinations of nicardipine or clevidipine plus labetalol or esmolol. (Table 11–16 lists drugs, dosages, and adverse effects.)
Table 11–16. Drugs for hypertensive emergencies and urgencies (in descending order of preference).

1. Nicardipine—Intravenous nicardipine is the most potent and the longest acting of the parenteral calcium channel blockers. As a primarily arterial vasodilator, it has the potential to precipitate reflex tachycardia, and for that reason it should not be used without a beta-blocker in patients with coronary artery disease.
2. Clevidipine—Intravenous clevidipine is an L-type calcium channel blocker with a 1-minute half-life, which facilitates swift and tight control of severe hypertension. It acts on arterial resistance vessels and is devoid of venodilatory or cardiodepressant effects.
3. Labetalol—This combined beta- and alpha-blocking agent is the most potent adrenergic blocker for rapid blood pressure reduction. Other beta-blockers are far less potent. Excessive blood pressure drops are unusual. Experience with this agent in hypertensive syndromes associated with pregnancy has been favorable.
4. Esmolol—This rapidly acting beta-blocker is approved only for treatment of supraventricular tachycardia, but is often used for lowering blood pressure. It is less potent than labetalol and should be reserved for patients in whom there is particular concern about serious adverse events related to beta-blockers.
5. Fenoldopam—Fenoldopam is a peripheral dopamine-1 (DA1) receptor agonist that causes a dose-dependent reduction in arterial pressure without evidence of tolerance, rebound, withdrawal, or deterioration of kidney function. In higher dosage ranges, tachycardia may occur. This drug is natriuretic, which may simplify volume management in acute kidney injury.
6. Enalaprilat—This is the active form of the oral ACE inhibitor enalapril. The onset of action is usually within 15 minutes, but the peak effect may be delayed for up to 6 hours. Thus, enalaprilat is used primarily as an adjunctive agent.
7. Diuretics—Intravenous loop diuretics can be very helpful when the patient has signs of heart failure or fluid retention, but the onset of their hypotensive response is slow, making them an adjunct rather than a primary agent for hypertensive emergencies. Low dosages should be used initially (furosemide, 20 mg, or bumetanide, 0.5 mg). They facilitate the response to vasodilators, which often stimulate fluid retention.
8. Hydralazine—Hydralazine can be given intravenously or intramuscularly, but its effect is less predictable than that of other drugs in this group. It produces reflex tachycardia and should not be given without beta-blockers in patients with possible coronary disease or aortic dissection. Hydralazine is used primarily in pregnancy and in children, but even in these situations, it is not a first-line drug.
9. Nitroglycerin, intravenous—This agent should be reserved for patients with accompanying acute coronary ischemic syndromes.
10. Nitroprusside sodium—This agent is given by controlled intravenous infusion gradually titrated to the desired effect. It lowers the blood pressure within seconds by direct arteriolar and venous dilation. Monitoring with an intra-arterial line avoids hypotension. Nitroprusside—in combination with a beta-blocker—is useful in patients with aortic dissection.
B. Oral Agents
Patients with less severe acute hypertensive syndromes can often be treated with oral therapy. Suitable drugs will reduce the blood pressure over a period of hours. In those presenting as a consequence of noncompliance, it is usually sufficient to restore the patient’s previously established oral regimen.
1. Clonidine—Clonidine, 0.2 mg orally initially, followed by 0.1 mg every hour to a total of 0.8 mg, will usually lower blood pressure over a period of several hours. Sedation is frequent, and rebound hypertension may occur if the drug is stopped.
2. Captopril—Captopril, 12.5–25 mg orally, will also lower blood pressure in 15–30 minutes. The response is variable and may be excessive. Captopril is the drug of choice in the management of scleroderma hypertensive crisis.
3. Nifedipine—The effect of fast-acting nifedipine capsules is unpredictable and may be excessive, resulting in hypotension and reflex tachycardia. Because myocardial infarction and stroke have been reported in this setting, the use of sublingual nifedipine is not advised. Nifedipine retard, 20 mg orally, appears to be safe and effective.
C. Subsequent Therapy
When the blood pressure has been brought under control, combinations of oral antihypertensive agents can be added as parenteral drugs are tapered off over a period of 2–3 days.
Cordonnier C et al. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018 Oct 6;392(10154):1257–68. [PMID: 30319113]
Ipek E et al. Hypertensive crisis: an update on clinical approach and management. Curr Opin Cardiol. 2017 Jul;32(4):397–406. [PMID: 28306673]
Lawton MT et al. Subarachnoid hemorrhage. N Engl J Med. 2017 Jul 20;377(3):257–66. [PMID: 28723321]
Peixoto AJ. Acute severe hypertension. N Engl J Med. 2019 Nov 7;381(19):1843–52. [PMID: 31693807]