18
Gynecologic Disorders
Jason Woo, MD, MPH, FACOG
Jill Long, MD, MPH, MHS, FACOG1
PREMENOPAUSAL ABNORMAL UTERINE BLEEDING
ESSENTIALS OF DIAGNOSIS

 Accurate diagnosis of abnormal uterine bleeding (AUB) depends on appropriate categorization and diagnostic tests.
Accurate diagnosis of abnormal uterine bleeding (AUB) depends on appropriate categorization and diagnostic tests.
 Pregnancy should always be ruled out as a cause of AUB in reproductive age women.
Pregnancy should always be ruled out as a cause of AUB in reproductive age women.
 The evaluation of AUB depends on the age and risk factors of the patient.
The evaluation of AUB depends on the age and risk factors of the patient.
 General Considerations
General Considerations
Normal menstrual bleeding lasts an average of 5 days (range, 2–7 days), with a mean blood loss of 40 mL per cycle. Abnormal uterine bleeding (AUB) refers to menstrual bleeding of abnormal quantity, duration, or schedule. The International Federation of Gynecology and Obstetrics (FIGO) introduced the current classification system for abnormal uterine bleeding in 2011, which was then endorsed by the American College of Obstetrics and Gynecology. This classification system pairs AUB with descriptive terms denoting the bleeding pattern (ie, heavy, light and menstrual, intermenstrual) and etiology (the acronym PALM-COEIN standing for Polyp, Adenomyosis, Leiomyoma, Malignancy and hyperplasia, Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified). In adolescents, AUB often occurs as a result of persistent anovulation due to the immaturity of the hypothalamic-pituitary-ovarian axis and represents normal physiology. Once regular menses has been established during adolescence, ovulatory dysfunction AUB (AUB-O) accounts for most cases. AUB in women aged 19–39 years is often a result of pregnancy, structural lesions, anovulatory cycles, use of hormonal contraception, or endometrial hyperplasia.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The diagnosis depends on the following: (1) confirming uterine source of the bleeding; (2) excluding pregnancy and confirming patient is premenopausal; (3) ascertaining whether the bleeding pattern suggests regular ovulatory bleeding or anovulatory bleeding; (4) determining contribution of structural abnormalities (PALM), including risk for malignancy/hyperplasia; (5) identifying risk of medical conditions that may impact bleeding (eg, inherited bleeding disorders, endocrine disease, risk of infection); and (6) assessing contribution of current medications, including contraceptives or natural product supplements or combinations that may affect bleeding.
B. Laboratory Studies
A complete blood count, pregnancy test, and thyroid tests should be done. For adolescents with heavy menstrual bleeding and adults with a positive screening history, coagulation studies should be considered, since up to 18% of women with severe menorrhagia have an underlying coagulopathy. Vaginal or urine samples should be obtained for polymerase chain reaction (PCR) or culture to rule out infectious causes. If indicated, cervical cytology should also be obtained.
C. Imaging
Transvaginal ultrasound is useful to assess for presence of fibroids, suspicion of adenomyosis, and to evaluate endometrial thickness. Sonohysterography or hysteroscopy may be used to diagnose endometrial polyps or subserous myomas. MRI is not a primary imaging modality for AUB but can more definitively diagnose submucous myomas and adenomyosis.
D. Cervical Biopsy and Endometrial Sampling
The purpose of endometrial sampling is to determine if hyperplasia or carcinoma is present. Sampling methods and other gynecologic diagnostic procedures are described in Table 18–1. Polyps, endometrial hyperplasia and, occasionally, submucous myomas are identified on endometrial biopsy. Endometrial sampling should be performed in patients with AUB who are 45 years and older, or in younger patients with a history of unopposed estrogen exposure or failed medical management and persistent AUB. If the Papanicolaou smear abnormality requires it or a gross cervical lesion is seen, colposcopic-directed biopsies and endocervical curettage are usually indicated.
Table 18–1. Common gynecologic diagnostic procedures.
Colposcopy
Visualization of cervical, vaginal, or vulvar epithelium under 5–50 × magnification with and without dilute acetic acid to identify abnormal areas requiring biopsy. An office procedure.
Dilation & curettage (D&C)
Dilation of the cervix and curettage of the entire endometrial cavity, using a metal curette or suction cannula and often using forceps for the removal of endometrial polyps. Can usually be done in the office under local anesthesia or in the operating room under sedation or general anesthesia. D&C is often combined with hysteroscopy for improved sensitivity.
Endometrial biopsy
Blind sampling of the endometrium by means of a curette or small aspiration device without cervical dilation. Diagnostic accuracy similar to D&C. An office procedure performed with or without local anesthesia.
Endocervical curettage
Removal of endocervical epithelium with a small curette for diagnosis of cervical dysplasia and cancer. An office procedure performed with or without local anesthesia.
Hysterosalpingography
Injection of radiopaque dye through the cervix to visualize the uterine cavity and oviducts. Mainly used in investigation of infertility, to identify a space-occupying lesion, or to confirm fallopian tube inserts (Essure®) sterilization.
Hysteroscopy
Visual examination of the uterine cavity with a small fiberoptic endoscope passed through the cervix. Curettage, endometrial ablation, biopsies of lesions, and excision of myomas or polyps can be performed concurrently. Can be done in the office under local anesthesia or in the operating room under sedation or general anesthesia. Greater sensitivity for diagnosis of uterine pathology than D&C.
Laparoscopy
Visualization of the abdominal and pelvic cavity through a small fiberoptic endoscope passed through a subumbilical incision. Permits diagnosis, tubal sterilization, and treatment of many conditions previously requiring laparotomy. General anesthesia is used.
Saline infusion sonohysterography
Introduction of saline solution into endometrial cavity with a catheter to visualize submucous myomas or endometrial polyps by transvaginal ultrasound. May be performed in the office with oral or local analgesia, or both.
 Treatment
Treatment
Treatment for premenopausal patients with AUB depends on the etiology of the bleeding, determined by history, physical examination, laboratory findings, imaging, and endometrial sampling. Patients with AUB due to submucosal myomas, infection, gestational trophoblastic conditions, thrombophilia, or pelvic neoplasms may require definitive therapy. A large proportion of premenopausal patients, however, have ovulatory dysfunction AUB (AUB-O).
Treatment for AUB-O should include consideration of potentially contributing medical conditions. Subsequently, AUB-O can be treated hormonally. For women amenable to using contraceptives, estrogen-progestin contraceptives or the 52-mg levonorgestrel-releasing intrauterine device (IUD) are both effective treatments. The choice between the two depends on whether any contraindications to these treatments exist as well as patient preference. High-dose oral or injectable progestin-only medications are also generally effective. For patients with irregular or light bleeding, medroxyprogesterone acetate, 10 mg/day orally, or norethindrone acetate, 5 mg/day orally, should be given for 10 days, following which withdrawal bleeding will occur. If successful, the treatment can be repeated for several cycles, starting medication on day 15 of subsequent cycles, or it can be reinstituted if amenorrhea or dysfunctional bleeding recurs. Nonhormonal options include nonsteroidal anti-inflammatory drugs (NSAIDs), such as naproxen or mefenamic acid, in the usual anti-inflammatory doses taken during menses, and tranexamic acid 1300 mg three times per day orally for up to 5 days. Both have been shown to decrease menstrual blood loss by about 40%, with tranexamic acid superior to NSAIDs in direct comparative studies.
Women who are experiencing heavier bleeding can be given a taper of any of the combination oral contraceptives (with 30–35 mcg of estrogen estradiol) to control the bleeding. There are several commonly used contraceptive dosing regimens, including four times daily for 1 or 2 days followed by two pills daily through day 5 and then one pill daily through day 20; after withdrawal bleeding occurs, pills are taken in the usual dosage for three cycles. In cases of intractable heavy bleeding, a gonadotropin-releasing hormone (GnRH) agonist such as depot leuprolide, 3.75 mg intramuscularly monthly, can be used for up to 6 months to create a temporary cessation of menstruation by ovarian suppression. These therapies require 2–4 weeks to down-regulate the pituitary and stop bleeding and will not stop bleeding acutely. In cases of heavy bleeding requiring hospitalization, intravenous conjugated estrogens, 25 mg every 4 hours for three or four doses, can be used, followed by oral conjugated estrogens, 2.5 mg daily, or ethinyl estradiol, 20 mcg orally daily, for 3 weeks, with the addition of medroxyprogesterone acetate, 10 mg orally daily for the last 10 days of treatment, or a combination oral contraceptive daily for 3 weeks. This will thicken the endometrium and control the bleeding.
For women with ineffective results from medical management or desiring definitive therapy, surgical options can be considered. Heavy menstrual bleeding due to structural lesions (eg, fibroids, adenomyosis) is the most common indication for surgery. Minimally invasive procedural options for fibroids include uterine artery embolization and focused ultrasound ablation. Surgical options include myomectomy or hysterectomy. For women without structural abnormalities, endometrial ablation has similar results compared to the levonorgestrel-releasing IUD in reducing menstrual blood loss. Hysteroscopic surgical approaches include endometrial ablation with laser photocoagulation or electrocautery. Nonhysteroscopic techniques include balloon thermal ablation, cryoablation, free-fluid thermal ablation, impedance bipolar radiofrequency ablation, and microwave ablation. The latter methods are well-adapted to outpatient therapy under local anesthesia. While hysterectomy was used commonly in the past for bleeding unresponsive to medical therapy, the low risk of complications and the good short-term results of both endometrial ablation and levonorgestrel-releasing IUD make them attractive alternatives to hysterectomy.
 When to Refer
When to Refer
• If bleeding is not controlled with first-line therapy.
• If expertise is needed for a surgical procedure.
 When to Admit
When to Admit
If bleeding is uncontrollable with first-line therapy or the patient is not hemodynamically stable.
Bryant-Smith AC et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018 Apr 15;4:CD000249. [PMID: 29656433]
Munro MG et al; FIGO Menstrual Disorders Committee. The Two FIGO Systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393–408. [PMID: 30198563]
Singh S et al. SOGC Clinical Practice Guideline No. 292. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can. 2018 May;40(5):e391–415. [PMID: 29731212]
POSTMENOPAUSAL UTERINE BLEEDING
ESSENTIALS OF DIAGNOSIS

 Any uterine bleeding in a postmenopausal woman (12 months or more following cessation of menstrual cycles).
Any uterine bleeding in a postmenopausal woman (12 months or more following cessation of menstrual cycles).
 Postmenopausal bleeding of any amount always should be evaluated.
Postmenopausal bleeding of any amount always should be evaluated.
 Transvaginal ultrasound measurement of the endometrium is an important tool in evaluating the cause of postmenopausal bleeding.
Transvaginal ultrasound measurement of the endometrium is an important tool in evaluating the cause of postmenopausal bleeding.
 General Considerations
General Considerations
The most common causes are endometrial atrophy, endometrial proliferation or hyperplasia, endometrial or cervical cancer, and administration of estrogens without or with added progestin. Other causes include atrophic vaginitis, trauma, endometrial polyps, abrasion of the cervix associated with prolapse of the uterus, and blood dyscrasias.
 Diagnosis
Diagnosis
The vulva and vagina should be inspected for areas of bleeding, ulcers, or neoplasms. Cervical cytology should be obtained, if indicated. Transvaginal sonography should be used to measure endometrial thickness. An endometrial stripe measurement of 4 mm or less indicates a low likelihood of hyperplasia or endometrial cancer. If the endometrial thickness is greater than 4 mm or there is a heterogeneous appearance to the endometrium, endometrial sampling is indicated. Sonohysterography may be helpful in determining if the endometrial thickening is diffuse or focal. If the thickening is global, endometrial biopsy or D&C is appropriate. If focal, guided sampling with hysteroscopy should be done.
 Treatment
Treatment
Management options for simple endometrial hyperplasia without atypia include surveillance, oral contraceptives, or progestin therapy. Surveillance may be used if the risk of occult cancer or progression to cancer is low and the inciting factor (eg, anovulation) has been eliminated. Progestin therapy may include cyclic or continuous therapy (medroxyprogesterone acetate, 10 mg/day orally, or norethindrone acetate, 5 mg/day orally) for 21 or 30 days of each month for 3 months or the use of a levonorgestrel-releasing IUD. Repeat sampling should be performed if symptoms recur. For complex hyperplasia without atypia, options include progestin therapy with scheduled repeat endometrial sampling or hysterectomy. Hysterectomy is indicated for endometrial hyperplasia with atypia (also called endometrial intraepithelial neoplasia) or carcinoma of the endometrium.
 When to Refer
When to Refer
• Expertise in performing ultrasonography is required.
• Endometrial hyperplasia with atypia is present.
• Hysteroscopy is indicated.
Bar-On S et al. Is outpatient hysteroscopy accurate for the diagnosis of endometrial pathology among perimenopausal and postmenopausal women? Menopause. 2018 Feb;25(2):160–4. [PMID: 28763396]
Schramm A et al. Value of endometrial thickness assessed by transvaginal ultrasound for the prediction of endometrial cancer in patients with postmenopausal bleeding. Arch Gynecol Obstet. 2017 Aug;296(2):319–26. [PMID: 28634754]
Turnbull HL et al. Investigating vaginal bleeding in postmenopausal women found to have an endometrial thickness of equal to or greater than 10 mm on ultrasonography. Arch Gynecol Obstet. 2017 Feb;295(2):445–50. [PMID: 27909879]
LEIOMYOMA OF THE UTERUS (Fibroid Tumor)
ESSENTIALS OF DIAGNOSIS

 Irregular enlargement of the uterus (may be asymptomatic).
Irregular enlargement of the uterus (may be asymptomatic).
 Heavy or irregular vaginal bleeding, dysmenorrhea.
Heavy or irregular vaginal bleeding, dysmenorrhea.
 Pelvic pain and pressure.
Pelvic pain and pressure.
 General Considerations
General Considerations
Uterine leiomyomas are the most common benign neoplasm of the female genital tract. They are discrete, round, firm, often multiple, uterine tumors composed of smooth muscle and connective tissue. The most convenient classification is by anatomic location: (1) intramural, (2) submucous, (3) subserous, and (4) cervical. Submucous myomas may become pedunculated and descend through the cervix into the vagina.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
In nonpregnant women, myomas are frequently asymptomatic. The two most common symptoms of uterine leiomyomas for which women seek treatment are AUB and pelvic pain or pressure. Occasionally, degeneration occurs, causing intense pain. Myomas that significantly distort the uterine cavity may affect fertility by interfering with implantation, rapidly distending in early pregnancy, or impairing uterine contractility.
B. Laboratory Findings
Iron deficiency anemia may result from blood loss.
C. Imaging
Ultrasonography will confirm the presence of uterine myomas and can be used sequentially to monitor growth. When multiple subserous or pedunculated myomas are being followed, ultrasonography is important to exclude ovarian masses. MRI can delineate intramural and submucous myomas accurately and is typically used prior to uterine artery embolization to determine fibroid size and location in relation to uterine blood supply. Hysterography or hysteroscopy can also confirm cervical or submucous myomas.
 Differential Diagnosis
Differential Diagnosis
Irregular myomatous enlargement of the uterus must be differentiated from the similar, but symmetric enlargement that may occur with pregnancy or adenomyosis. Subserous myomas must be distinguished from ovarian tumors. Leiomyosarcoma is an unusual tumor occurring in 0.5% of women operated on for symptomatic myomas. It is very rare under the age of 40 but increases in incidence thereafter.
 Treatment
Treatment
A. Nonsurgical Measures
Women who have small asymptomatic myomas can be managed expectantly and evaluated annually. In patients wishing to defer surgical management, nonhormonal therapies (such as NSAIDs and tranexamic acid) have been shown to decrease menstrual blood loss. Women with heavy bleeding related to fibroids often respond to estrogen-progestin oral contraceptives or the levonorgestrel IUD, although an IUD cannot be used with a distorted cavity. Hormonal therapies, such as GnRH agonists and selective progesterone receptor modulators (eg, low-dose mifepristone and ulipristal acetate), have been shown to reduce myoma volume, uterine size, and menstrual blood loss. Selective progesterone receptor modulators are not approved for fibroid treatment in the United States, however. Surgical intervention is based on the patient’s symptoms and desire for future fertility. Uterine size alone is not an indication for surgery. Cervical myomas larger than 3–4 cm in diameter or pedunculated myomas that protrude through the cervix can cause bleeding, infection, degeneration, pain, or urinary retention and often require removal. Submucous myomas can be removed by hysteroscopic resection.
Because the risk of surgical complications increases with the increasing size of the myoma, preoperative reduction of myoma size is sometimes desirable prior to hysterectomy. GnRH analogs such as depot leuprolide, 3.75 mg intramuscularly monthly, can be used preoperatively for 3- to 4-month periods to induce reversible hypogonadism, to temporarily reduce the size of myomas, and to reduce surrounding vascularity.
B. Surgical Measures
A variety of surgical measures are available for the treatment of myomas: myomectomy (hysteroscopic, laparoscopic, or abdominal) and hysterectomy (vaginal, laparoscopy-assisted vaginal, laparoscopic, abdominal, or robotic). Myomectomy is the treatment of choice for women who wish to preserve fertility. Uterine artery embolization is a minimally invasive treatment for uterine fibroids. In uterine artery embolization, the goal is to block the blood vessels supplying the fibroids, causing them to shrink. Magnetic resonance–guided high-intensity focused ultrasound, myolysis/radiofrequency ablation, and laparoscopic or vaginal occlusion of uterine vessels are newer interventions, with a smaller body of evidence.
 Prognosis
Prognosis
In women desiring future fertility, myomectomy can be offered, but patients should be counseled that recurrence is common, postoperative pelvic adhesions may impact fertility, and cesarean delivery may be necessary secondary to interruption of the myometrium. Approximately 80% of women have long-term improvement in symptoms following uterine artery embolization. Definitive surgical therapy (ie, hysterectomy) is curative.
 When to Refer
When to Refer
Refer to a gynecologist for treatment of symptomatic leiomyomata.
 When to Admit
When to Admit
For acute abdomen associated with an infarcted leiomyoma or for hemorrhage not controlled by outpatient measures.
Chudnoff S et al. Ultrasound-guided transcervical ablation of uterine leiomyomas. Obstet Gynecol 2019 Jan;133(1):13–22. [PMID: 30531573]
Donnez J et al. The current place of medical therapy in uterine fibroid management. Best Pract Res Clin Obstet Gynaecol. 2018 Jan;46:57–65. [PMID: 29169896]
Havryliuk Y et al. Symptomatic fibroid management: systematic review of the literature. JSLS. 2017 Jul–Sep;21(3). [PMID: 28951653]
Murji A et al. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev. 2017 Apr 26;4:CD010770. [PMID: 28444736]
Osuga Y et al. Oral gonadotropin-releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: a randomized clinical trial. Obstet Gynecol. 2019 Mar;133(3):423–33. [PMID: 30741797]
CERVICAL POLYPS
Cervical polyps commonly occur during the reproductive years, particularly after age 40, and are occasionally noted in postmenopausal women. The cause is not known, but inflammation may play an etiologic role. The principal symptoms are discharge and abnormal vaginal bleeding. However, abnormal bleeding should not be ascribed to a cervical polyp without sampling the endocervix and endometrium. The polyps are visible in the cervical os on speculum examination.
Cervical polyps must be differentiated from polypoid neoplastic disease of the endometrium, small submucous pedunculated myomas, large nabothian cysts, and endometrial polyps. Cervical polyps rarely contain foci of dysplasia (0.5%) or of malignancy (0.5%). Asymptomatic polyps in women under age 45 may be left untreated.
 Treatment
Treatment
Cervical polyps can generally be removed in the office by avulsion with uterine packing forceps or ring forceps.
 When to Refer
When to Refer
• Polyp with a wide base present.
• Inability to differentiate endocervical from endometrial polyp.
ENDOMETRIOSIS
ESSENTIALS OF DIAGNOSIS

 Dysmenorrhea or pelvic pain is most common presenting complaint.
Dysmenorrhea or pelvic pain is most common presenting complaint.
 Dyspareunia.
Dyspareunia.
 Increased frequency among infertile women.
Increased frequency among infertile women.
 General Considerations
General Considerations
Endometriosis is an aberrant growth of endometrium outside of the uterus, particularly in the dependent parts of the pelvis and in the ovaries. Its principal manifestations are chronic pain and infertility. While retrograde menstruation is the most widely accepted cause, its pathogenesis and natural course are not fully understood. The overall prevalence in the United States is 6–10%.
 Clinical Findings
Clinical Findings
The clinical manifestations of endometriosis are variable and unpredictable in both presentation and course. Dysmenorrhea, chronic pelvic pain, and dyspareunia, are among the well-recognized manifestations. A significant number of women with endometriosis, however, remain asymptomatic and most women with endometriosis have a normal pelvic examination. However, in some women, pelvic examination can disclose tender nodules in the cul-de-sac or rectovaginal septum, uterine retroversion with decreased uterine mobility, uterine tenderness, or adnexal mass or tenderness.
Endometriosis must be distinguished from pelvic inflammatory disease (PID), ovarian neoplasms, and uterine myomas. Bowel invasion by endometrial tissue may produce blood in the stool that must be distinguished from bowel neoplasm.
Imaging is useful mainly in the presence of a pelvic or adnexal mass. Transvaginal ultrasonography is the imaging modality of choice to detect the presence of deeply penetrating endometriosis of the rectum or rectovaginal septum; MRI should be reserved for equivocal cases of rectovaginal or bladder endometriosis. A definitive diagnosis of endometriosis is made only by histology of lesions removed at surgery.
 Treatment
Treatment
A. Medical Treatment
Although there is no conclusive evidence that NSAIDs improve the pain associated with endometriosis, these agents are reasonable options in appropriately selected patients. Medical treatment, using a variety of hormonal therapies, is effective in the amelioration of pain associated with endometriosis. Most of these regimens are designed to inhibit ovulation over 4–9 months and to lower hormone levels, thus preventing cyclic stimulation of endometriotic implants and inducing atrophy. The optimum duration of hormonal therapy is not clear, and their relative merits in terms of side effects and long-term risks and benefits show insignificant differences when compared with one another and even, in mild cases, with placebo. Commonly used medical regimens include the following:
1. Estrogen-progestin contraceptives are first-line treatment and can be given cyclically or continuously; prolonged suppression of ovulation often inhibits further stimulation of residual endometriosis, especially if taken after one of the therapies mentioned here. Any of the combination oral contraceptives, the contraceptive patch, or the vaginal ring may be used continuously. Breakthrough bleeding can be treated with conjugated estrogens, 1.25 mg orally daily for 1 week, or estradiol, 2 mg daily orally for 1 week. Alternatively, a short hormone-free interval to allow a withdrawal bleed can be used whenever bothersome breakthrough bleeding occurs.
2. Progestins, specifically oral norethindrone acetate and subcutaneous depo-medroxyprogesterone acetate (DMPA) have been approved by the FDA for treatment of endometriosis-associated pain. The etonogestrel implant has also been shown to decrease endometriosis-related pain.
3. Intrauterine progestin, using the levonorgestrel-releasing IUD, has been shown to be effective in reducing endometriosis-associated pelvic pain, and it is recommended before surgery.
4. GnRH agonists are highly effective in reducing the pain syndromes associated with endometriosis. However, they are not superior to other methods such as combined hormonal contraceptives as first-line therapy. The GnRH analog (long-acting injectable leuprolide acetate, 3.75 mg intramuscularly monthly, used for 6 months) suppresses ovulation. Side effects of vasomotor symptoms and bone demineralization may be relieved by “add-back” therapy, such as conjugated equine estrogen, 0.625 mg orally daily, or norethindrone, 5 mg orally daily.
5. Danazol is an androgenic medication that has been used for the treatment of endometriosis-associated pain. It may be used for 4–6 months in the lowest dose necessary to suppress menstruation, usually 200–400 mg orally twice daily. However, danazol has a high incidence of androgenic side effects that are more severe than other medications available, including decreased breast size, weight gain, acne, and hirsutism.
6. Aromatase inhibitors (such as anastrozole or letrozole) in combination with conventional therapy have been evaluated with positive results in premenopausal women with endometriosis-associated pain and pain recurrence.
7. GnRH antagonists suppress pituitary gonadotropin production and create a hypoestrogenic state, like GnRH agonists, but they are effective immediately rather than requiring 7–14 days for GnRH suppression. Injectable and oral forms (eg, cetrorelix and elagolix, respectively) are available.
B. Surgical Measures
Surgical treatment of endometriosis—particularly extensive disease—is effective both in reducing pain and in promoting fertility. Laparoscopic ablation of endometrial implants significantly reduces pain. Ablation of implants and, if necessary, removal of ovarian endometriomas enhance fertility, although subsequent pregnancy rates are inversely related to the severity of disease. Women with disabling pain for whom childbearing is not a consideration can be treated definitively with total abdominal hysterectomy plus bilateral salpingo-oophorectomy. In premenopausal women, hormone replacement may then be used to relieve vasomotor symptoms.
 Prognosis
Prognosis
There is little systematic research regarding either the progression of the disease or the prediction of clinical outcomes. The prognosis for reproductive function in early or moderately advanced endometriosis appears to be good with conservative therapy. Hysterectomy, with bilateral salpingo-oophorectomy, often is regarded as definitive treatment of endometriosis associated with intractable pelvic pain, adnexal masses, or multiple previous ineffective conservative surgical procedures. However, symptoms may recur even after hysterectomy and oophorectomy.
 When to Refer
When to Refer
Refer to a gynecologist for laparoscopic diagnosis or surgical treatment.
 When to Admit
When to Admit
Rarely necessary except for acute abdomen associated with ruptured or bleeding endometrioma.
Brown J et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2017 Jan 23;1:CD004753. [PMID: 28114727]
Fu J et al. Progesterone receptor modulators for endometriosis. Cochrane Database Syst Rev. 2017 Jul 25;7:CD009881. [PMID: 28742263]
Rafique S et al. Medical management of endometriosis. Clin Obstet Gynecol. 2017 Sep;60(3):485–96. [PMID: 28590310]
Singh SS et al. Surgical outcomes in patients with endometriosis: a systematic review. J Obstet Gynaecol Can. 2019 Nov 9. [Epub ahead of print] [PMID: 31718952]
Taylor HS et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017 Jul 6;377(1):28–40. [PMID: 28525302]
Vilasagar S et al. A practical guide to the clinical evaluation of endometriosis-associated pelvic pain. J Minim Invasive Gynecol. 2020 Feb;27(2):270–9. [PMID: 31669551]
PELVIC PAIN
1. Primary Dysmenorrhea
Primary dysmenorrhea is menstrual pain associated with menstrual cycles in the absence of pathologic findings. Primary dysmenorrhea pain usually begins within 1–2 years after the menarche and may become progressively more severe. The frequency of cases increases up to age 20 and then decreases with both increasing age and parity. Fifty percent to 75% of women are affected by dysmenorrhea at some time and 5–6% have incapacitating pain.
 Clinical Findings
Clinical Findings
Primary dysmenorrhea is low, midline, wave-like, cramping pelvic pain often radiating to the back or inner thighs. Cramps may last for 1 or more days and may be associated with nausea, diarrhea, headache, and flushing. The pain is produced by uterine vasoconstriction, anoxia, and sustained contractions mediated by prostaglandins. The pelvic examination is normal between menses; examination during menses may produce discomfort, but there are no pathologic findings.
 Treatment
Treatment
NSAIDs (ibuprofen, ketoprofen, mefenamic acid, naproxen) and the cyclooxygenase (COX)-2 inhibitor (celecoxib) are generally helpful. The medication should be started 1–2 days before expected menses. Symptoms can be suppressed with use of combined hormonal contraceptives, DMPA, etonogestrel subdermal implant (Nexplanon), or the levonorgestrel-releasing IUD. Continuous use of oral contraceptives can be used to suppress menstruation completely and prevent dysmenorrhea. For women who do not wish to use hormonal contraception, other therapies that have shown at least some benefit include local heat; thiamine, 100 mg/day orally; vitamin E, 200 units/day orally from 2 days prior to and for the first 3 days of menses; and high-frequency transcutaneous electrical nerve stimulation.
2. Other Categories of Pelvic Pain
Unlike primary dysmenorrhea, other causes of pelvic pain may or may not be associated with the menstrual cycle but are more likely to be associated with pelvic pathology. Conditions such as endometriosis, adenomyosis, fibroids, PID, or other anatomic abnormalities of the pelvic organs, including the bowel or bladder, may present with symptoms during the menstrual cycle.
 Clinical Findings
Clinical Findings
The history and physical examination may suggest endometriosis, adenomyosis, or fibroids as causes of pelvic pain. Other causes include PID, tubo-ovarian abscess, submucous myoma(s), IUD use, cervical stenosis with obstruction, or blind uterine horn (rare). Careful review of bowel or bladder symptoms besides pain should be done to exclude another pelvic organ source.
 Diagnosis
Diagnosis
Targeted physical examination may help identify the anatomic source of pelvic pain. PID should be considered in sexually active women with pelvic pain and examination findings of cervical motion tenderness, uterine, or adnexal tenderness without another explanation for the pain. Pelvic imaging is useful for diagnosing the presence of uterine fibroids or other anomalies. Adenomyosis (the presence of endometrial glands and stroma within the myometrium) may be detected with ultrasound or MRI. Laparoscopy may help diagnose endometriosis or other pelvic abnormalities not visualized by imaging.
 Treatment
Treatment
A. Specific Measures
Combined estrogen and progestin and progestin-only hormonal contraceptives are first-line therapies for alleviating the symptom of dysmenorrhea. Periodic use of analgesics, including the NSAIDs given for primary dysmenorrhea, may be beneficial, particularly in pelvic pain due to endometriosis. GnRH agonists are also an effective treatment of endometriosis, although their long-term use may be limited by cost or side effects. Adenomyosis may respond to the levonorgestrel-releasing IUD, uterine artery embolization, or hormonal approaches used to treat endometriosis, but hysterectomy remains the definitive treatment of choice for women for whom childbearing is not a consideration.
B. Surgical Measures
If disability is marked or prolonged, diagnostic laparoscopy is usually warranted. Definitive surgery depends on the degree of disability and the findings at operation. Uterine fibroids may be removed or treated by uterine artery embolization. Hysterectomy may be done if other treatments have not worked but is usually a last resort.
 When to Refer
When to Refer
• Standard therapy fails to relieve pain.
• Suspicion of pelvic pathology, such as endometriosis, leiomyomas, or adenomyosis.
American College of Obstetrics and Gynecology. Committee Opinion No. 770: Dysmenorrhea and endometriosis in the adolescent. Obstet Gynecol. 2018 Dec;132(6):e249–258. [PMID: 30461694]
Bishop LA. Management of chronic pelvic pain. Clin Obstet Gynecol. 2017 Sep;60(3):524–30. [PMID: 28742584]
Brown J et al. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2018 May 22;5:CD001019. [PMID: 29786828]
Carey ET et al. Updates in the approach to chronic pelvic pain: what the treating gynecologist should know. Clin Obstet Gynecol. 2019 Dec;62(4):666–76. [PMID: 31524660]
Ferrero S et al. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother. 2018 Jul;19(10):1109–25. [PMID: 29975553]
Matsushima T et al. Efficacy of hormonal therapies for decreasing uterine volume in patients with adenomyosis. Gynecol Minim Invasive Ther. 2018 Jul–Sep;7(3):119–23. [PMID: 30254953]
Oladosu FA et al. Nonsteroidal anti-inflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol. 2018 Apr;218(4):390–400. [PMID: 28888592]
Smith SE et al. Interventional pain management and female pelvic pain: considerations for diagnosis and treatment. Semin Reprod Med. 2018 Mar;36(2):159–63. [PMID: 30566982]
PELVIC ORGAN PROLAPSE
 General Considerations
General Considerations
Pelvic organ prolapses, including cystocele, rectocele, and enterocele, are vaginal hernias commonly seen in multiparous women. Cystocele is a hernia of the bladder wall into the vagina, causing a soft anterior fullness. Cystocele may be accompanied by urethrocele, which is not a hernia but a sagging of the urethra following its detachment from the pubic symphysis during childbirth. Rectocele is a herniation of the terminal rectum into the posterior vagina, causing a collapsible pouch-like fullness. Enterocele is a vaginal vault hernia containing small intestine, usually in the posterior vagina and resulting from a deepening of the pouch of Douglas. Two or all three types of hernia may occur in combination. Risk factors may include vaginal birth, genetic predisposition, advancing age, prior pelvic surgery, connective tissue disorders, and increased intra-abdominal pressure associated with obesity or straining associated with chronic constipation or coughing.
 Clinical Findings
Clinical Findings
Symptoms of pelvic organ prolapse may include a sensation of a bulge or protrusion in the vagina, urinary or fecal incontinence, constipation, sense of incomplete bladder emptying, and dyspareunia. The cause of pelvic organ prolapse, including prolapse of the uterus, vaginal apex, and anterior or posterior vaginal walls, is likely multifactorial.
 Treatment
Treatment
The type of therapy depends on the extent of prolapse and associated symptoms, impact on the patient’s quality of life, the patient’s age, and her desire for menstruation, pregnancy, and coitus.
A. General Measures
Supportive measures include a high-fiber diet and laxatives to improve constipation. Weight reduction in obese patients and limitation of straining and lifting are helpful. Pelvic muscle training (Kegel exercises) is a simple, noninvasive intervention that may improve pelvic function; it has demonstrated clear benefit for women with urinary or fecal symptoms, especially incontinence. Pessaries may reduce a cystocele, rectocele, or enterocele and are helpful in women who do not wish to undergo surgery or who are poor surgical candidates.
B. Surgical Measures
The most common surgical procedure is vaginal or abdominal hysterectomy with additional attention to restoring apical support after the uterus is removed, by suspension either by vaginal uterosacral or sacrospinous fixation or by abdominal sacral colpopexy. Since stress incontinence is common after vault suspension procedures, an anti-incontinence procedure should be considered. Surgical mesh placed transvaginally for pelvic organ prolapse repair was introduced into clinical practice in 2002, but in 2011 the FDA issued warnings about concerns for serious complications associated with this practice (including mesh erosion and pain). Use of these methods subsequently declined significantly. In April 2019, the US FDA withdrew its previous (2016) class III (high risk) approval of surgical mesh intended for transvaginal repair of anterior compartment prolapse. Patients planning to have surgical repair of pelvic organ prolapse should discuss all treatment options, including the risks and benefits of using surgical mesh, with their clinician. If the patient desires pregnancy, the same procedures for vaginal suspension can be performed without hysterectomy, though limited data on pregnancy outcomes or prolapse outcomes are available. Generally, surgical repair of pelvic organ prolapse is reserved until after completion of childbearing. For elderly women who do not desire coitus, colpocleisis, the partial obliteration of the vagina, is surgically simple and effective. Uterine suspension with sacrospinous cervicocolpopexy may be an effective approach in older women who wish to avoid hysterectomy but preserve coital function. Women who have received transvaginal mesh for the surgical repair of pelvic organ prolapse but who are having no symptoms or complications related to it should continue with their annual check-ups and other routine follow-up care. They should let their clinician know that they have a surgical mesh implant, especially if they plan to have another pelvic surgery or related medical procedure. In addition, they should notify their clinician if they develop symptoms such as persistent vaginal bleeding or discharge, pelvic or groin pain, or dyspareunia.
 When to Refer
When to Refer
• Refer to urogynecologist or gynecologist for incontinence evaluation.
• Refer if nonsurgical therapy is ineffective.
• Refer for removal of mesh if symptoms develop.
Coolen AWM et al. Primary treatment of pelvic organ prolapse: pessary use versus prolapse surgery. Int Urogynecol J. 2018 Jan;29(1):99–107. [PMID: 28600758]
Meriwether KV et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systemic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018 Aug;219(2):129–46. [PMID: 29353031]
U.S. Food & Drug Administration. Urogynecologic surgical mesh implants, April 16 2019. https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants
Winkelman WD et al. U.S. Food and Drug Administration statements about transvaginal mesh and changes in apical prolapse surgery. Obstet Gynecol. 2019 Oct;134(4):745–52. [PMID: 31503162]
PREMENSTRUAL SYNDROME
 General Considerations
General Considerations
The premenstrual syndrome (PMS) is a recurrent, variable cluster of troublesome physical and emotional symptoms that develop during the 5 days before the onset of menses and subside within 4 days after menstruation occurs. PMS intermittently affects about 40% of all premenopausal women, primarily those 25–40 years of age. In about 5–8% of affected women, the syndrome may be severe. Although not every woman experiences all the symptoms or signs at one time, many describe bloating, breast pain, headache, swelling, irritability, aggressiveness, depression, inability to concentrate, libido change, lethargy, and food cravings. When emotional or mood symptoms predominate, along with physical symptoms, and there is a clear functional impairment with work or personal relationships, the term “premenstrual dysphoric disorder” (PMDD) may be applied. The pathogenesis of PMS/PMDD is still uncertain, and current treatment methods are mainly empiric. The clinician should provide support for both the patient’s emotional and physical distress, including the following:
1. Careful evaluation of the patient, with understanding, explanation, and reassurance.
2. Advice to keep a daily diary of all symptoms for 2–3 months, such as the Daily Record of Severity of Problems, to evaluate the timing and characteristics of her symptoms. If her symptoms occur throughout the month rather than in the 2 weeks before menses, she may have depression or other mental health problems instead of or in addition to PMS.
 Treatment
Treatment
For mild to moderate symptoms, a program of aerobic exercise; reduction of caffeine, salt, and alcohol intake; an increase in dietary calcium (to 1200 mg/day), vitamin D, or magnesium, and complex carbohydrates in the diet; and use of alternative therapies such as acupuncture and herbal treatments may be helpful, although these interventions remain unproven.
Medications that prevent ovulation, such as hormonal contraceptives, may lessen physical symptoms. These include continuous combined hormonal contraceptive methods (pill, patch, or vaginal ring); or GnRH agonist with “add-back” therapy (eg, conjugated equine estrogen, 0.625 mg orally daily with medroxyprogesterone acetate, 2.5–5 mg orally daily).
When mood disorders predominate, several serotonin reuptake inhibitors have been shown to be effective in relieving tension, irritability, and dysphoria with few side effects. First-line medication therapy includes serotonergic antidepressants (citalopram, escitalopram, fluoxetine, sertraline, venlafaxine) either daily or only on symptom days. There are few data to support the use of calcium, vitamin D, and vitamin B6 supplementation. There is insufficient evidence to support cognitive behavioral therapy.
Naheed B et al. Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome. Cochrane Database Syst Rev. 2017 Mar 3;3:CD010503. [PMID: 28257559]
Yonkers KA et al. Premenstrual disorders. Am J Obstet Gynecol. 2018 Jan;218(1):68–74. [PMID: 28571724]
MENOPAUSAL SYNDROME
ESSENTIALS OF DIAGNOSIS

 Menopause is a retrospective diagnosis after 12 months of amenorrhea.
Menopause is a retrospective diagnosis after 12 months of amenorrhea.
 Approximately 80% of women will experience hot flushes and night sweats.
Approximately 80% of women will experience hot flushes and night sweats.
 Elevated follicle-stimulating hormone (FSH) and low estradiol can help confirm the diagnosis.
Elevated follicle-stimulating hormone (FSH) and low estradiol can help confirm the diagnosis.
 General Considerations
General Considerations
The term “menopause” denotes the final cessation of menstruation, either as a normal part of aging or as the result of surgical removal of both ovaries. In a broader sense, as the term is commonly used, it denotes a 1- to 3-year period during which a woman adjusts to a diminishing, and then absent, menstrual flow and the physiologic changes that may be associated with lowered estrogen levels—hot flushes, night sweats, and vaginal dryness.
The average age at menopause in Western societies is 51 years. Premature menopause is defined as ovarian failure and menstrual cessation before age 40; this often has a genetic or autoimmune basis. Surgical menopause due to bilateral oophorectomy is common and can cause more severe symptoms owing to the sudden rapid drop in sex hormone levels.
There is no objective evidence that cessation of ovarian function is associated with severe emotional disturbance or personality changes. However, mood changes toward depression and anxiety can occur at this time. Disruption of sleep patterns associated with the menopause can affect mood and concentration and cause fatigue. Furthermore, the time of menopause often coincides with other major life changes, such as departure of children from the home, a midlife identity crisis, or divorce.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
1. Cessation of menstruation—Menstrual cycles generally become irregular as menopause approaches. Anovulatory cycles occur more often, with irregular cycle length and occasional menorrhagia. Menstrual flow usually diminishes in amount owing to decreased estrogen secretion, resulting in less abundant endometrial growth. Finally, cycles become longer, with missed periods or episodes of spotting only. When no bleeding has occurred for 1 year, the menopausal transition can be said to have occurred. Any bleeding after 6 months of the cessation of menses warrants investigation by endometrial curettage or aspiration to rule out endometrial cancer.
2. Hot flushes—Hot flushes (feelings of intense heat over the trunk and face, with flushing of the skin and sweating) occur in over 80% of women as a result of the decrease in ovarian hormones. Hot flushes can begin before the cessation of menses. Menopausal vasomotor symptoms last longer than previously thought, and there are ethnic differences in the duration of symptoms. Vasomotor symptoms last more than 7 years in more than 50% of the women. African-American women report the longest duration of vasomotor symptoms. The etiology of hot flushes is unknown. Occurring at night, they often cause sweating and insomnia and result in fatigue on the following day.
3. Vaginal atrophy—With decreased estrogen secretion, thinning of the vaginal mucosa and decreased vaginal lubrication occur and may lead to dyspareunia. The introitus decreases in diameter. Pelvic examination reveals pale, smooth vaginal mucosa and a small cervix and uterus. The ovaries are not normally palpable after the menopause.
4. Osteoporosis—Osteoporosis may occur as a late sequela of menopause. The US Preventive Services Task Force recommends screening for osteoporosis beginning at age 65. The most recent June 2018 USPSTF recommendation summary can be found at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening1.
B. Laboratory Findings
Serum FSH, luteinizing hormone (LH), and estradiol levels are of little diagnostic value because of unpredictable variability during the menopausal transition but can provide confirmation if the FSH remains consistently elevated and the estradiol, low. Vaginal cytologic examination will show a low estrogen effect with predominantly parabasal cells, indicating lack of epithelial maturation due to hypoestrogenism.
 Treatment
Treatment
A. Natural Menopause
Education and support from health providers, midlife discussion groups, and reading material will help most women having difficulty adjusting to the menopause. Physiologic symptoms can be treated with both hormonal or nonhormonal therapies. Hormonal replacement therapy has been shown to be effective in treating perimenopausal and menopausal symptoms but is not effective in prevention of chronic conditions such as coronary heart disease.
1. Vasomotor symptoms—For women with mild symptoms, lifestyle modification, such as increased hydration, decreased caffeine consumption, tobacco cessation, and dressing in layers are first-line therapies to be undertaken before consideration of other nonhormonal or hormonal medical treatments. For women with moderate to severe vasomotor symptoms, hormone replacement estrogen or estrogen/progestin regimens are the most effective approach to symptom relief. Conjugated estrogens, 0.3 mg, 0.45 mg, or 0.625 mg; 17-beta-estradiol, 0.5 or 1 mg; or estrone sulfate, 0.625 mg can be given once daily orally; or estradiol can be given transdermally as skin patches that are changed once or twice weekly and secrete 0.05–0.1 mg of hormone daily. Unless the patient has undergone hysterectomy, a combination regimen of an estrogen with a progestin such as medroxyprogesterone, 1.5 or 2.5 mg, or norethindrone, 0.1, 0.25, or 0.5 mg, should be used to prevent endometrial hyperplasia or cancer. There is also a patch available containing estradiol and the progestin levonorgestrel. Oral hormones can be given in several different regimens. Give estrogen on days 1–25 of each calendar month, with 5–10 mg of oral medroxyprogesterone acetate added on days 14–25. Withhold hormones from day 26 until the end of the month, when the endometrium will be shed, producing a light, generally painless monthly period. Alternatively, give the estrogen along with a progestin daily, without stopping. This regimen causes some initial bleeding or spotting, but within a few months it produces an atrophic endometrium that will not bleed. If the patient has had a hysterectomy, a progestin need not be given with the estrogen.
Postmenopausal women generally should not use combination progestin-estrogen therapy for more than 3 or 4 years. Women who cannot find relief with alternative approaches may wish to consider continuing use of combination therapy after a thorough discussion of the risks and benefits. Alternatives to hormone therapy for vasomotor symptoms include selective serotonin reuptake inhibitors (SSRIs), such as paroxetine, 12.5 mg or 25 mg/day orally, or venlafaxine, 75 mg/day orally. Gabapentin, an antiseizure medication, is also effective at 900 mg/day orally. Clonidine given orally or transdermally, 100–150 mcg daily, may also reduce the frequency of hot flushes, but its use is limited by side effects, including dry mouth, drowsiness, and hypotension. There is some evidence that soy isoflavones may be effective in treating menopausal symptoms.
2. Vaginal atrophy—A vaginal ring containing 2 mg of estradiol can be left in place for 3 months and is suitable for long-term use. Although serum estrogen level increases associated with vaginal rings are lower than other routes of administration, it is recommended that the ring be used with caution. ACOG suggests that vaginal estrogen is an option for urogenital symptoms even in breast cancer survivors. Short-term use of estrogen vaginal cream will relieve symptoms of atrophy, but because of variable absorption, therapy with either the vaginal ring or systemic hormone replacement is preferable. A low-dose estradiol tablet (10 mcg) is available and is inserted in the vagina daily for 2 weeks and then twice a week for long-term use. Testosterone propionate 1–2%, 0.5–1 g, in a vanishing cream base used in the same manner is also effective if estrogen is contraindicated. A bland lubricant such as unscented cold cream or water-soluble gel can be helpful at the time of coitus.
3. Osteoporosis—(See also discussion in Chapter 26.) Women should ingest at least 800 mg of calcium daily throughout life. Nonfat or low-fat milk products, calcium-fortified orange juice, green leafy vegetables, corn tortillas, and canned sardines or salmon consumed with the bones are good dietary sources. Vitamin D, at least 800 international units/day from food, sunlight, or supplements, is necessary to enhance calcium absorption and maintain bone mass. A daily program of energetic walking and exercise to strengthen the arms and upper body helps maintain bone mass. Screening bone densitometry is recommended for women beginning at age 65 (see Chapter 1). Women most at risk for osteoporotic fractures should consider bisphosphonates, raloxifene, or hormone replacement therapy. This includes white and Asian women, especially if they have a family history of osteoporosis, are thin, short, cigarette smokers, have a history of hyperthyroidism, use corticosteroid medications long term, or are physically inactive.
B. Risks of Hormone Therapy
Double-blind, randomized, controlled trials have shown no overall cardiovascular benefit with estrogen-progestin replacement therapy in a group of postmenopausal women, including women both with and without established coronary disease. In the Women’s Health Initiative (WHI) trial and in the Heart and Estrogen/Progestin Replacement Study (HERS), the overall health risks (increased risk of coronary heart events; strokes; thromboembolic disease; gallstones; and breast cancer, including an increased risk of mortality from breast cancer) exceeded the benefits from the long-term use of combination estrogen and progesterone. Ancillary analysis of the WHI study showed that not only did estrogen-progestin hormone replacement therapy not benefit cognitive function, but there was a small unanticipated increased risk of cognitive decline in that group compared with women in the placebo group. The unopposed estrogen arm of the WHI trial demonstrated a decrease in the risk of hip fracture, a small but not significant decrease of breast cancer, but an increased risk of stroke and no evidence of protection from coronary heart disease. The study also showed a small increase in the combined risk of mild cognitive impairment and dementia with estrogen use compared with placebo, similar to the estrogen-progestin arm. Women who have been receiving long-term estrogen-progestin hormone replacement therapy, even in the absence of complications, should be encouraged to stop, especially if they do not have menopausal symptoms. However, the risks appear to be lower in women starting therapy at the time of menopause and higher in previously untreated women starting therapy long after menopause. Therapy should be individualized as the risk-benefit profile varies with age and individual risk factors. (See also discussions of estrogen and progestin replacement therapy in Chapter 26.)
C. Surgical Menopause
The abrupt hormonal decrease resulting from oophorectomy generally results in severe vasomotor symptoms and rapid onset of dyspareunia and osteoporosis unless treated. If not contraindicated, estrogen replacement is generally started immediately after surgery. Conjugated estrogens 1.25 mg orally, estrone sulfate 1.25 mg orally, or estradiol 2 mg orally is given for 25 days of each month. After age 45–50 years, this dose can be tapered to 0.625 mg of conjugated estrogens or equivalent.
Del Carmen MG et al. Management of menopausal symptoms in women with gynecologic cancers. Gynecol Oncol. 2017 Aug;146(2):427–35. [PMID: 28625396]
Gartlehner G et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017 Dec 12;318(22):2234–49. [PMID: 29234813]
The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017 Jul;24(7):728–53. [PMID: 28650869]
POLYCYSTIC OVARY SYNDROME
ESSENTIALS OF DIAGNOSIS

 Clinical or biochemical evidence of hyper-androgenism.
Clinical or biochemical evidence of hyper-androgenism.
 Oligoovulation or anovulation.
Oligoovulation or anovulation.
 Polycystic ovaries on ultrasonography.
Polycystic ovaries on ultrasonography.
 General Considerations
General Considerations
Polycystic ovary syndrome (PCOS) is a common endocrine disorder of unknown etiology affecting 5–10% of reproductive age women. PCOS is characterized by chronic anovulation, polycystic ovaries, and hyperandrogenism. It is associated with hirsutism and obesity as well as an increased risk of diabetes mellitus, cardiovascular disease, and metabolic syndrome. Unrecognized or untreated PCOS is a risk factor for cardiovascular disease. The Rotterdam Criteria identify androgen production, ovulatory dysfunction, and polycystic ovaries as the key diagnostic features of the disorder in adult women; the emerging consensus is that at least two of these features must be present for diagnosis. The classification system has been endorsed by the National Institutes of Health.
 Clinical Findings
Clinical Findings
PCOS often presents as a menstrual disorder (ranging from amenorrhea to menorrhagia) and infertility. Skin disorders due to peripheral androgen excess, including hirsutism and acne, are common. Patients may also show signs of insulin resistance and hyperinsulinemia, and these women are at increased risk for early-onset type 2 diabetes mellitus and the metabolic syndrome. Patients who do become pregnant are at increased risk for perinatal complications, such as gestational diabetes and preeclampsia. In addition, they have an increased long-term risk of endometrial cancer secondary to unopposed estrogen secretion.
 Differential Diagnosis
Differential Diagnosis
Anovulation in the reproductive years may also be due to (1) premature ovarian failure (high FSH and LH levels); (2) functional hypothalamic amenorrhea, often associated with rapid weight loss or extreme physical exertion (low to normal FSH and LH levels for age); (3) discontinuation of hormonal contraceptives (return to ovulation typically occurs within 90 days); (4) pituitary adenoma with elevated prolactin (galactorrhea may or may not be present); and (5) hyperthyroidism or hypothyroidism. To rule out other etiologies in women with suspected PCOS, serum FSH, LH, prolactin, and thyroid-stimulating hormone should be checked. Because of the high risk of insulin resistance and dyslipidemia, all women with suspected PCOS should have a hemoglobin A1C and fasting glucose along with a lipid profile. Women with clinical evidence of androgen excess should have total testosterone, free (bioavailable) testosterone, and 17-hydroxyprogesterone measured. Women with stigmata of Cushing syndrome should have a 24-hour urinary free cortisol or a low-dose dexamethasone suppression test. Congenital adrenal hyperplasia and androgen-secreting adrenal tumors also tend to have high circulating androgen levels and anovulation with polycystic ovaries; these disorders must also be ruled out in women with presumed PCOS and high serum androgens.
 Treatment
Treatment
In obese patients with PCOS, weight reduction and exercise are often effective in reversing the metabolic effects and in inducing ovulation. For those women who do not respond to weight loss and exercise, metformin therapy may be helpful. Metformin is beneficial for metabolic or glucose abnormalities, and it can improve menstrual function. Metformin has little or no benefit in the treatment of hirsutism, acne, or infertility. Contraceptive counseling should be offered to prevent unplanned pregnancy in case of a return of ovulatory cycles. For women who are seeking pregnancy and remain anovulatory, clomiphene or other medications can be used for ovarian stimulation (see section on Infertility below). Clomiphene is the first-line therapy for infertility. If induction of ovulation fails, treatment with gonadotropins, but at low dose to lower the risk of ovarian hyperstimulation syndrome, may be successful. Second-line therapies such as use of aromatase inhibitors or laparoscopic “ovarian drilling” are of unproved benefit. Women with PCOS are at greater risk than normal women for twin gestation with ovarian stimulation.
If the patient does not desire pregnancy and does not want or is not a candidate for contraception, then medroxyprogesterone acetate, 10 mg/day orally for the first 10 days of every 1–3 months, should be given to ensure regular shedding of the endometrium and thus avoid endometrial hyperplasia. If contraception is desired, combination hormonal contraceptives (pill, ring, or patch) can be used; this is also useful in controlling hirsutism, for which treatment must be continued for 6–12 months before results are seen. The levonorgestrel-releasing IUD is another option to minimize uterine bleeding and protect against endometrial hyperplasia, but unlike the combined estrogen-progestin contraceptives, the IUD does not help control hirsutism.
Spironolactone is useful for hirsutism in doses of 25 mg three or four times daily. Flutamide, 125–250 mg orally daily, and finasteride, 5 mg orally daily, are also effective for treating hirsutism. Because these three agents are potentially teratogenic, they should be used only in conjunction with secure contraception. Topical eflornithine cream applied to affected facial areas twice daily for 6 months may be helpful in most women. Hirsutism may also be managed with depilatory creams, electrolysis, and laser therapy. The combination of laser therapy and topical eflornithine may be particularly effective.
Weight loss, exercise, and treatment of unresolved metabolic derangements are important in preventing cardiovascular disease. Women with PCOS should be managed aggressively and should have regular monitoring of lipid profiles and glucose. In adolescent patients with PCOS, hormonal contraceptives and metformin are treatment options.
 When to Refer
When to Refer
• If expertise in diagnosis is needed.
• If patient is infertile.
American College of Obstetricians and Gynecologists. Practice Bulletin No. 194: Polycystic ovary syndrome. Obstet Gynecol. 2018 Jun;131(6):e157–71. [PMID: 29794677]
Bednarska S et al. The pathogenesis and treatment of polycystic ovary syndrome: what’s new? Adv Clin Exp Med. 2017 Mar–Apr;26(2):359–67. [PMID: 28791858]
Gadalla MA et al. Medical and surgical treatment of reproductive outcomes in polycystic ovary syndrome: an overview of systematic reviews. Int J Fertil Steril. 2020 Jan;13(4):257–70. [PMID: 31710185]
INFERTILITY
A couple is said to be infertile if pregnancy does not result after 1 year of normal sexual activity without contraception. About 20% of couples experience infertility at some point in their reproductive lives; the incidence of infertility increases with age, with a decline in fertility beginning in the early 30s and accelerating in the late 30s. The male partner contributes to about 40% of cases of infertility, and a combination of factors is common. The most recent data from the CDC National Survey of Family Growth noted that 12% of women in the United States aged 15–44 have impaired fecundity.
A. Initial Testing
During the initial interview, the clinician can present an overview of infertility and discuss an evaluation and management plan. Private consultations with each partner separately are then conducted, allowing appraisal of psychosexual adjustment without embarrassment or criticism. Pertinent details (eg, sexually transmitted infection history or prior pregnancies) must be obtained. The ill effects of cigarettes, alcohol, and other recreational drugs on male fertility should be discussed. Prescription medications that impair male potency and factors that may lead to scrotal hyperthermia, such as tight underwear or frequent use of saunas or hot tubs, should be discussed. The gynecologic history should include the menstrual pattern, the use and types of contraceptives, frequency and success of coitus, and correlation of intercourse with time of ovulation. The American Society for Reproductive Medicine provides patient information on the infertility evaluation and treatment (https://www.reproductivefacts.org/topics/topics-index/infertility/).
General physical and genital examinations are performed on the female partner. Basic laboratory studies include assessment of ovarian reserve (eg, antimüllerian hormone, and day 3 FSH and estradiol) and thyroid function tests. If the woman has regular menses with minimal symptoms, the likelihood of ovulatory cycles is very high. A luteal phase serum progesterone above 3 ng/mL establishes ovulation. Couples should be advised that coitus resulting in conception occurs during the 6-day window around the day of ovulation. Ovulation predictor kits have largely replaced basal body temperatures for predicting ovulation, but temperature charting is a natural and inexpensive way to identify most fertile days. Basal body temperature charts cannot predict ovulation; they can only retrospectively confirm that ovulation occurred.
A semen analysis should be completed to rule out a male factor for infertility (see Chapter 29).
B. Further Testing
1. Gross deficiencies of sperm (number, motility, or appearance) require a repeat confirmatory analysis. Intracytoplasmic sperm injection is the treatment option available for sperm deficiencies except for azoospermia (absence of sperm). Intracytoplasmic sperm injection requires the female partner to undergo in vitro fertilization (IVF).
2. A screening pelvic ultrasound and hysterosalpingography to identify uterine cavity or tubal anomalies should be performed. Hysterosalpingography using an oil dye is performed within 3 days following the menstrual period if structural abnormalities are suspected. This radiographic study will demonstrate uterine abnormalities (septa, polyps, submucous myomas) and tubal obstruction. Oil-based (versus water-soluble) contrast media may improve pregnancy rates but reports of complications using oil-based media have resulted in a decrease in its usage. Women who have had prior pelvic inflammation should receive doxycycline, 100 mg orally twice daily, beginning immediately before and for 7 days after the radiographic study. IVF is recommended as the primary treatment option for tubal factor infertility. Surgery can be considered in women with mild tubal disease, but no rigorous research has evaluated surgical outcomes compared to IVF or expectant management.
3. Absent or infrequent ovulation requires additional laboratory evaluation. Elevated FSH and LH levels and low estradiol levels indicate ovarian insufficiency. Elevated LH levels in the presence of normal FSH levels may indicate the presence of polycystic ovaries. Elevation of blood prolactin levels suggests a pituitary adenoma. Women over age 35 may require further assessment of ovarian reserve. A markedly elevated FSH (greater than 15–20 international units/L) on day 3 of the menstrual cycle suggests inadequate ovarian reserve. Although less widely used clinically, a clomiphene citrate challenge test, with measurement of FSH on day 10 after administration of clomiphene from days 5–9, can help confirm a diagnosis of diminished ovarian reserve. The number of antral follicles during the early follicular phase of the cycle can provide useful information about ovarian reserve and can confirm serum testing. An antimüllerian hormone level can be measured at any time during the menstrual cycle and is less likely to be affected by hormones.
4. If all of the above testing is normal, unexplained infertility is diagnosed. In approximately 25% of women whose basic evaluation is normal, the first-line therapy is usually controlled ovarian hyperstimulation (usually with clomiphene citrate) and intrauterine insemination. IVF may be recommended as second-line therapy.
 Treatment
Treatment
A. Medical Measures
Fertility may be restored by treatment of endocrine abnormalities, particularly hypothyroidism or hyperthyroidism. Women who are anovulatory as a result of low body weight or exercise may become ovulatory when they gain weight or decrease their exercise levels; conversely, obese women who are anovulatory may become ovulatory with loss of even 5–10% of body weight.
B. Surgical Measures
Excision of ovarian tumors or ovarian foci of endometriosis can improve fertility. Microsurgical relief of tubal obstruction due to salpingitis or tubal ligation will reestablish fertility in a significant number of cases, although with severe disease or proximal obstruction, IVF is preferable. Peritubal adhesions or endometriotic implants often can be treated via laparoscopy.
In a male with a varicocele, sperm characteristics may be improved following surgical treatment. For men who have sperm production but obstructive azoospermia, trans-epidermal sperm aspiration or microsurgical epidermal sperm aspiration has been successful.
C. Induction of Ovulation
1. Clomiphene citrate—Clomiphene citrate stimulates gonadotropin release, especially FSH. It acts as a selective estrogen receptor modulator, similar to tamoxifen and raloxifene, and binds to the estrogen receptor. The body perceives a low level of estrogen, decreasing the negative feedback on the hypothalamus, and there is an increased release of FSH and LH. When FSH and LH are present in the appropriate amounts and timing, ovulation occurs.
After a normal menstrual period or induction of withdrawal bleeding with progestin, clomiphene 50 mg orally should be given daily for 5 days, typically on days 3–7 of the cycle. If ovulation does not occur, the clomiphene dosage is increased to 100 mg orally daily for 5 days. If ovulation still does not occur, the course is repeated with 150 mg orally daily for 5 days and then 200 mg orally daily for 5 days. The maximum dosage is 200 mg orally daily. Ovulation and appropriate timing of intercourse can be facilitated with the addition of chorionic gonadotropin, 10,000 units intramuscularly. Monitoring of the follicles by transvaginal ultrasound is usually necessary to time the hCG injection appropriately. The rate of ovulation following this treatment is 90% in the absence of other infertility factors. The pregnancy rate is high. Twinning occurs in 5% of these pregnancies, but three or more fetuses are rare (less than 0.5% of cases). Pregnancy is most likely to occur within the first three ovulatory cycles, and unlikely to occur after cycle six. In addition, several studies have suggested a twofold to threefold increased risk of ovarian cancer with the use of clomiphene for more than 1 year, so treatment with clomiphene is usually limited to a maximum of six cycles.
2. Letrozole—The aromatase inhibitor letrozole appears to be at least as effective as clomiphene for induction of ovulation in women with PCOS. There is a reduced risk of multiple pregnancy, a lack of antiestrogenic effects, and a reduced need for ultrasound monitoring. The dose of letrozole is 5–7.5 mg daily, starting on day 3 of the menstrual cycle. In women who have a history of estrogen dependent tumors, such as breast cancer, letrozole is preferred over other agents because the estrogen levels with this medication are much lower.
3. Cabergoline or bromocriptine—If prolactin levels are elevated, an evaluation for a pituitary mass should be conducted. In the absence of a pituitary mass, cabergoline or bromocriptine may be used if prolactin levels are elevated and there is no withdrawal bleeding following progesterone administration (otherwise, clomiphene is used). The initial cabergoline dosage is 2.5 mg orally once daily, increased to 2.5 mg two or three times daily by 1.25 mg increments of cabergoline. The medication is discontinued once pregnancy occurs. Cabergoline causes fewer adverse effects than bromocriptine. However, it is much more expensive. Cabergoline is often used in patients who cannot tolerate the adverse effects of bromocriptine or who do not respond to bromocriptine.
4. Human menopausal gonadotropins (hMG) or recombinant FSH—hMG or recombinant FSH is indicated in cases of hypogonadotropism and most other types of anovulation resistant to clomiphene treatment. Because of the complexities, laboratory tests, and expense associated with this treatment, these patients should be referred to an infertility specialist.
D. Treatment of Endometriosis
See above.
E. Artificial Insemination in Azoospermia
If azoospermia is present, artificial insemination by a donor usually results in pregnancy, assuming female function is normal. The use of frozen sperm provides the opportunity for screening for sexually transmitted infections, including HIV infection.
F. Assisted Reproductive Technology (ART)
Couples who have not responded to traditional infertility treatments, including those with tubal disease, severe endometriosis, oligospermia, and immunologic or unexplained infertility, may benefit from ART. Gamete intrafallopian transfer and zygote intrafallopian transfer are rarely performed, although they may be an option in a few selected patients. These techniques are complex and require a highly organized team of specialists. All ART procedures involve ovarian stimulation to produce multiple oocytes, oocyte retrieval by transvaginal sonography–guided needle aspiration, and handling of the oocytes outside the body. With IVF, the eggs are fertilized in vitro and the embryos transferred to the uterus. Intracytoplasmic sperm injection allows fertilization with a single sperm. While originally intended for couples with male factor infertility, it is now used in two-thirds of all IVF procedures in the United States.
The chance of a multiple gestation pregnancy (ie, twins, triplets) is increased in all assisted reproductive procedures, increasing the risk of preterm delivery and other pregnancy complications. To minimize this risk, most infertility specialists recommend transferring only one embryo in appropriately selected patients with a favorable prognosis. In women with prior failed IVF cycles who are over the age of 40 who have poor embryo quality, up to four embryos may be transferred. In the event of a multiple gestation pregnancy, a couple may consider selective reduction to avoid the medical issues generally related to multiple births. This issue should be discussed with the couple before embryo transfer.
 Prognosis
Prognosis
The prognosis for conception and normal pregnancy is good if minor (even multiple) disorders can be identified and treated; it is poor if the causes of infertility are severe, untreatable, or of prolonged duration (over 3 years).
It is important to remember that in the absence of identifiable causes of infertility, 60% of couples will achieve a spontaneous pregnancy within 3 years. Couples in which the woman is younger than 35 years who do not achieve pregnancy within 1 year of trying may be candidates for infertility treatment, and within 6 months for women age 35 years and older. Also, offering appropriately timed information about adoption is considered part of a complete infertility regimen.
 When to Refer
When to Refer
Refer to reproductive endocrinologist if ART is indicated, or surgery is required.
American College of Obstetricians and Gynecologists. Committee Opinion No. 781: Infertility workup for the women’s health specialist. Obstet Gynecol. 2019 Jun;133(6):e377–84. [PMID: 31135764]
Chua SJ et al. Surgery for tubal infertility. Cochrane Database Syst Rev. 2017 Jan 23;1:CD006415. [PMID: 28112384]
Hanson B et al. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017 Feb;34(2):167–77. [PMID: 27817040]
Jeelani R et al. Imaging and the infertility evaluation. Clin Obstet Gynecol. 2017 Mar;60(1):93–107. [PMID: 28106643]
CONTRACEPTION
Unintended pregnancies are a worldwide problem but disproportionately impact developing countries. Studies estimate that 40% of the 213 million pregnancies that occurred in 2012 were unintended. Globally, 50% ended in abortion, 13% ended in miscarriage, and 38% resulted in an unplanned birth. It is important for primary care providers to educate their patients about the benefits of contraception and to provide options that are appropriate and desirable for the patient.
1. Oral Contraceptives
A. Combined Oral Contraceptives
1. Efficacy and methods of use—Combined oral contraceptives have a perfect use failure rate of 0.3% and a typical use failure rate of 8%. Their primary mode of action is suppression of ovulation. The pills can be initially started on the first day of the menstrual cycle, the first Sunday after the onset of the cycle, or on any day of the cycle. If started more than 5 days after the first day of the cycle, a backup method should be used for the first 7 days. If an active pill is missed at any time, and no intercourse occurred in the past 5 days, two pills should be taken immediately and a backup method should be used for 7 days. If intercourse occurred in the previous 5 days, emergency contraception should be offered, and the pills restarted the following day. A backup method should be used for 5 days.
2. Benefits of oral contraceptives—Noncontraceptive benefits of oral contraceptives include lighter menses and improvement of dysmenorrhea symptoms, decreased risk of ovarian and endometrial cancer, and improvement in acne. Functional ovarian cysts are less likely with oral contraceptive use. The frequency of developing uterine myomas is lower in patients who have taken oral contraceptives for longer than 4 years. There is also a beneficial effect on bone mass.
3. Selection of an oral contraceptive—Any of the combination oral contraceptives containing 35 mcg or less of ethinyl estradiol or 3 mg of estradiol valerate are suitable for most women. There is some variation in potency of the various progestins in the pills, but there are essentially no clinically significant differences for most women among the progestins in the low-dose pills. There is insufficient evidence that triphasic oral contraceptives provide any benefit compared to monophasic oral contraceptives in terms of effectiveness, bleeding patterns or discontinuation rates. Therefore, monophasic pills are recommended as a first choice for women starting oral contraceptive use. Women who have acne or hirsutism may benefit from treatment with desogestrel, drospirenone, or norgestimate, since they are the least androgenic. A combination regimen with 84 active and 7 inert pills that results in only four withdrawal bleeds per year is available. There is also a combination regimen that is taken continuously with withdrawal bleeds. At the end of 1 year of use of this method, 58% of the women had amenorrhea, and nearly 80% reported no bleeding requiring sanitary protection. Studies have not shown any significant risk from long-term amenorrhea in patients taking continuous oral contraceptives. The low-dose oral contraceptives commonly used in the United States are listed in Table 18–2.
Table 18–2. Commonly used low-dose oral contraceptives (listed within each group in order of increasing estrogen dose).

4. Drug interactions—Several medications interact with oral contraceptives to decrease their efficacy, typically by causing induction of microsomal enzymes in the liver. Some commonly prescribed medications in this category are phenytoin, phenobarbital (and other barbiturates), primidone, topiramate, carbamazepine, rifampin, and St. John’s wort. Women taking these medications should use another means of contraception for maximum safety.
Antiretroviral medications, specifically ritonavir-boosted protease inhibitors, may significantly decrease the efficacy of combined oral contraceptives. Other antiretrovirals, such as nonnucleoside reverse transcriptase inhibitors have smaller effects on oral contraceptive efficacy.
5. Contraindications and adverse effects—Oral contraceptives have been associated with many adverse effects; they are contraindicated with some conditions and should be used with caution in others (Table 18–3).
Table 18–3. Contraindications to use of oral contraceptives.
Absolute contraindications
Pregnancy
Thrombophlebitis or thromboembolic disorders (past or present)
Stroke or coronary artery disease (past or present)
Cancer of the breast (known or suspected)
Undiagnosed abnormal vaginal bleeding
Estrogen-dependent cancer (known or suspected)
Hepatocellular adenoma (past or present)
Uncontrolled hypertension
Diabetes mellitus with vascular disease
Age over 35 and smoking > 15 cigarettes daily
Known thrombophilia
Migraine with aura
Active hepatitis
Surgery or orthopedic injury requiring prolonged immobilization
Relative contraindications
Migraine without aura
Hypertension
Heart or kidney disease
Diabetes mellitus
Gallbladder disease
Cholestasis during pregnancy
Sickle cell disease (S/S or S/C type)
Lactation
a. Myocardial infarction—The risk of heart attack is higher with use of oral contraceptives, particularly with pills containing 50 mcg of estrogen or more. Cigarette smoking, obesity, hypertension, diabetes mellitus, or hypercholesterolemia increases the risk. Young nonsmoking women have minimal increased risk. Smokers over age 35 and women with other cardiovascular risk factors should use other non-estrogen–containing methods of birth control.
b. Thromboembolic disease—An increased rate of venous thromboembolism is found in oral contraceptive users, especially if the dose of estrogen is 50 mcg or more. While the overall risk is very low (5–6 per 100,000 woman-years compared to 50–300 per 100,000 pregnancies), several studies have reported a twofold increased risk in women using oral contraceptives containing the progestins, gestodene (not available in the United States), drospirenone, or desogestrel, compared with women using oral contraceptives with levonorgestrel and norethindrone. Women in whom thrombophlebitis develops should stop using this method, as should those at increased risk for thrombophlebitis associated with surgery, fracture, serious injury, hypercoagulable condition, or immobilization. Women with a known thrombophilia should not use estrogen-containing contraceptives.
c. Cerebrovascular disease—Overall, a small increased risk of hemorrhagic stroke and subarachnoid hemorrhage and a somewhat greater increased risk of thrombotic stroke have been found; smoking, hypertension, and age over 35 years are associated with increased risk. Women should stop using estrogen-containing contraceptives if such warning symptoms as severe headache, blurred or lost vision, or other transient neurologic disorders develop.
d. Carcinoma—There is no increased risk of breast cancer in women aged 35–64 who are current or former users of oral contraceptives. Women with a family history of breast cancer or women who started oral contraceptive use at a young age are not at increased risk. Combination oral contraceptives reduce the risk of endometrial carcinoma by 40% after 2 years of use and 60% after 4 or more years of use. The risk of ovarian cancer is reduced by 30% with pill use for less than 4 years, by 60% with pill use for 5–11 years, and by 80% with use for 12 or more years. Oral contraceptives have been associated with the development of benign hepatocellular adenomas and peliosis hepatis (blood-filled cavities) (but not focal nodular hyperplasia or hepatocellular carcinoma); hepatocellular adenomas may rarely cause rupture of the liver, hemorrhage, and death. The risk of hepatocellular adenoma increases with higher dosage, longer duration of use, and older age.
e. Hypertension—Oral contraceptives may cause hypertension in some women; the risk is increased with longer duration of use and older age. Women in whom hypertension develops while using oral contraceptives should use other non-estrogen–containing contraceptive methods. However, with regular blood pressure monitoring, nonsmoking women with well-controlled mild hypertension may use oral contraceptives.
f. Headache—Migraine or other vascular headaches may occur or worsen with pill use. If severe or frequent headaches develop while using this method, it should be discontinued. Women with migraine headaches with an aura should not use oral contraceptives.
g. Lactation—Combined oral contraceptives can impair the quantity and quality of breast milk. While it is preferable to avoid the use of combination oral contraceptives during lactation, the effects on milk quality are small and are not associated with developmental abnormalities in infants. Combination oral contraceptives should be started no earlier than 6 weeks postpartum to allow for establishment of lactation. Progestin-only pills, levonorgestrel implants, and DMPA are alternatives with no adverse effects on milk quality.
h. Other disorders—Depression may occur or be worsened with oral contraceptive use. Fluid retention may occur. Patients who had cholestatic jaundice during pregnancy may develop it while taking birth control pills.
i. Obesity—Obese and overweight women have generally been excluded from oral contraceptive trials until recently. Obesity is an independent risk factor for thromboembolic complications. However, it is important that obese women are not denied effective contraception as a result of concerns about complications or efficacy of oral contraceptives. Current evidence suggests that efficacy is similar for normal weight as well as for overweight and obese women.
6. Minor side effects—Nausea and dizziness may occur in the first few months of pill use. Spotting or breakthrough bleeding between menstrual periods may occur, especially if a pill is skipped or taken late; this may be helped by switching to a pill of slightly greater potency. Missed menstrual periods may occur, especially with low-dose pills. A pregnancy test should be performed if pills have been skipped or if two or more expected menstrual periods are missed. Fatigue and decreased libido can occur. Chloasma may occur, as in pregnancy, and is increased by exposure to sunlight.
B. Progestin Minipill
1. Efficacy and methods of use—A formulation containing 0.35 mg of norethindrone alone is available in the United States. The efficacy is similar to that of combined oral contraceptives but is highly dependent on consistent use (eg, taking the pill within the same 3-hour window every day). The minipill is believed to prevent conception by causing thickening of the cervical mucus to make it hostile to sperm, by causing alteration of ovum transport (which may account for the slightly higher rate of ectopic pregnancy with these pills), and by causing inhibition of implantation. Ovulation is inhibited inconsistently with this method. The minipill is begun on the first day of a menstrual cycle and then taken continuously for as long as contraception is desired; there is no “placebo week.”
2. Advantages—The low dose of progestin and absence of estrogen make the minipill safe during lactation; it may increase the flow of milk. It is often tried by women who want minimal doses of hormones and by patients who are over age 35. The minipill lacks the cardiovascular side effects of combination pills. It can be safely used by women with sickle cell (S/S or S/C) disease.
3. Complications and contraindications—Minipill users often have bleeding irregularities (eg, prolonged flow, spotting, or amenorrhea); such patients may need regular pregnancy tests if there is a concern about contraceptive effectiveness. Many of the absolute contraindications and relative contraindications listed in Table 18–3 apply to the minipill; however, the contraceptive benefit of the minipill may outweigh the risks for patients who smoke, who are over age 35, or who have conditions such as superficial or deep venous thrombosis or known thromboembolic disorders or diabetes mellitus with vascular disease. Minor side effects of combination oral contraceptives such as mild headache may also occur with the minipill.
Brown EJ et al. Contraception update: oral contraception. FP Essent. 2017 Nov;462:11–9. [PMID: 29172411]
Serfaty D. Update on contraceptive contraindications. J Gynecol Obstet Hum Reprod. 2019 May;48(5):297–307. [PMID: 30796985]
Tepper NK et al. Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use, 2016: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection. MMWR Morb Mortal Wkly Rep. 2017 Sep 22;66(37):990–4. [PMID: 28934178]
2. Contraceptive Injections & Implants (Long-Acting Progestins)
The injectable progestin DMPA is approved for contraceptive use in the United States. There has been extensive worldwide experience with this method over the past 3 decades. The medication is given as a deep intramuscular injection of 150 mg every 3 months and has a contraceptive efficacy of 99.7%. Common side effects include irregular bleeding, amenorrhea, weight gain, and headache. It is associated with bone mineral loss that is reversible after discontinuation of the method. Users commonly have irregular bleeding initially and subsequently develop amenorrhea. Ovulation may be delayed after its discontinuation. Contraindications are similar to those for the minipill.
A single-rod, subdermal progestin implant, etonogestrel subdermal (Nexplanon), is approved for use in the United States. Nexplanon is a 40-mm by 2-mm rod containing 68 mg of the progestin etonogestrel that is inserted in the inner aspect of the nondominant arm. It remains effective for 3 years. Hormone levels drop rapidly after removal, and there is no delay in the return of fertility. In clinical trials, the pregnancy rate was 0.0% with 3 years of use. The side effect profile is similar to the minipill and DMPA. Irregular bleeding has been the most common reason for discontinuation.
American College of Obstetricians and Gynecologists. Practice Bulletin No. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017 Nov;130(5):e251–69. [PMID: 29064972]
American College of Obstetricians and Gynecologists. Practice Bulletin No. 206: Use of hormonal contraception in women with coexisting medical conditions. 2019 Feb;133(2):e128–150. Erratum in: Obstet Gynecol. 2019 Jun;133(6):1288. [PMID: 30681544]
Deshmukh P et al. Contraception update: progestin-only implants and injections. FP Essent. 2017 Nov;462:25–9. [PMID: 29172413]
Dianat S et al. Side effects and health benefits of depot medroxyprogesterone acetate: a systematic review. Obstet Gynecol. 2019 Feb;133(2):332–41. [PMID: 30633132]
Espey E et al. Barriers and solutions to improve adolescent intrauterine device access. J Pediatr Adolesc Gynecol. 2019 Sep;32(5S):S7–13. [PMID: 31585618]
Horvath S et al. From uptake to access: a decade of learning from ACOG LARC program. Am J Obstet Gynecol. 2020 Apr;222(4S):S866–8. [PMID: 31794720]
Hubacher D et al. Long-acting reversible contraceptive acceptability and unintended pregnancy among women presenting for short-acting methods: a randomized patient preference trial. Am J Obstet Gynecol. 2017 Feb;216(2):101–9. [PMID: 27662799]
Sothornwit J et al. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database Syst Rev. 2017 Apr 22;4:CD011913. [PMID: 28432791]
3. Complex Delivery System Contraceptives
A transdermal contraceptive patch containing norelgestromin (150 mcg) and ethinyl estradiol (20 mcg) and measuring 20 cm2 is available. The patch is applied to the lower abdomen, upper torso, or buttock once a week for 3 consecutive weeks, followed by 1 week without the patch. It appears that the average steady-state concentration of ethinyl estradiol with the patch is approximately 60% higher than with a 35-mcg pill. However, there is currently no evidence for an increased incidence of estrogen-related side effects. The mechanism of action, side effects, and efficacy are similar to those associated with oral contraceptives, although compliance may be better. However, discontinuation for side effects is more frequent.
A contraceptive vaginal ring that releases 120 mcg of etonogestrel and 15 mcg of ethinyl estradiol daily (Nuva-ring) is available. The ring is soft and flexible and is placed in the upper vagina for 3 weeks, removed, and replaced 1 week later, or can be removed and replaced after 4 weeks for continuous cycling, similar to oral contraceptives. The efficacy, mechanism of action, and systemic side effects are similar to those associated with oral contraceptives. Ring users may experience an increased incidence of vaginal discharge.
4. Intrauterine Devices
In the United States, the following IUDs are available: the levonorgestrel-releasing Mirena, Liletta, Kyleena, and Skyla IUDs and the copper-bearing TCu380A (Paragard). The mechanism of action of the copper IUD is thought to involve either spermicidal or inhibitory effects on sperm capacitation and transport. The levonorgestrel-containing IUDs also cause thickening of cervical mucus, prevent endometrial thickening, and can inhibit ovulation. IUDs are not abortifacients.
Skyla is FDA approved for 3 years, Mirena, Liletta, and Kyleena for 5 years, and the TCu380A for 10 years. The hormone-containing IUDs have the advantage of reducing cramping and menstrual flow.
The IUD is an excellent contraceptive method for most women. The devices are highly effective, with failure rates similar to those achieved with surgical sterilization. Nulliparity is not a contraindication to IUD use. Adolescents are also candidates for IUD use. Women who are not in mutually monogamous relationships should use condoms for protection from sexually transmitted diseases. Levonorgestrel-containing IUDs may have a protective effect against upper tract infection similar to that of oral contraceptives.
A. Insertion
Insertion can be performed during or after the menses, at midcycle to prevent implantation, or later in the cycle if the patient has not become pregnant. There is growing evidence to suggest that IUDs can be safely inserted in the immediate postabortal and postpartum periods.
Both types of IUDs (levonorgestrel-releasing and copper bearing) may be inserted up to 48 hours after vaginal delivery, or prior to closure of the uterus at the time of cesarean section. Insertion immediately following abortion is acceptable if there is no sepsis and if follow-up insertion a month later will not be possible; otherwise, it is wise to wait until 4 weeks postabortion. NSAIDs given as premedication may help insertions in nulliparous patients or when insertion is not performed during menses.
B. Contraindications and Complications
Contraindications to use of IUDs are outlined in Table 18–4.
Table 18–4. Contraindications to IUD use.
Absolute contraindications
Pregnancy
Acute or subacute pelvic inflammatory disease or purulent cervicitis
Significant anatomic abnormality of uterus
Unexplained uterine bleeding
Active liver disease (Mirena only)
Relative contraindications
History of pelvic inflammatory disease since the last pregnancy
Lack of available follow-up care
Menorrhagia or severe dysmenorrhea (copper IUD)
Cervical or uterine neoplasia
IUD, intrauterine device.
1. Pregnancy—A copper-containing IUD can be inserted within 5 days following a single episode of unprotected midcycle coitus as a postcoital contraceptive. An IUD should not be inserted into a pregnant uterus. If pregnancy occurs as an IUD failure, there is a greater chance of spontaneous abortion if the IUD is left in situ (50%) than if it is removed (25%). Spontaneous abortion with an IUD in place is associated with a high risk of severe sepsis, and death can occur rapidly. Women using an IUD who become pregnant should have the IUD removed if the string is visible. It can be removed at the time of abortion if that is desired. If the string is not visible and the patient wants to continue the pregnancy, she should be informed of the serious risk of sepsis and, occasionally, death with such pregnancies. She should be informed that any flu-like symptoms such as fever, myalgia, headache, or nausea warrant immediate medical attention for possible septic abortion.
Since the risk of ectopic pregnancy is increased in IUD users who become pregnant with an IUD in situ, clinicians should search for adnexal masses in early pregnancy and should always check the products of conception for placental tissue following abortion.
2. Pelvic infection—There is an increased risk of pelvic infection during the first month following insertion; however, prophylactic antibiotics given at the time of insertion do not appear to decrease this risk. The subsequent risk of pelvic infection appears to be primarily related to the risk of acquiring sexually transmitted infections. Infertility rates do not appear to be increased among women who have previously used the currently available IUDs. At the time of insertion, women with an increased risk of sexually transmitted diseases should be screened for gonorrhea and Chlamydia. Women with a history of recent or recurrent pelvic infection are not good candidates for IUD implantation.
3. Heavy menstrual bleeding or severe dysmenorrhea—The copper IUD can cause heavier menstrual periods, bleeding between periods, and more cramping, so it is generally not suitable for women who already suffer from these problems. Alternatively, the hormone-releasing IUD Mirena has been approved by the FDA to treat heavy menstrual bleeding. NSAIDs are also helpful in decreasing bleeding and pain in IUD users.
4. Complete or partial expulsion—Spontaneous expulsion of the IUD occurs in up to 10% of women during the first year of use. Any IUD should be removed if the body of the device can be seen or felt in the cervical os.
5. Missing IUD strings—If the transcervical tail cannot be seen, this may signify unnoticed expulsion, perforation of the uterus with abdominal migration of the IUD, or simply retraction of the string into the cervical canal or uterus owing to movement of the IUD or uterine growth with pregnancy. Once pregnancy is ruled out, a cervical speculum may be used to visualize the IUD string in the cervical canal. If not visualized, one should probe for the IUD with sterile sound or forceps designed for IUD removal. If the IUD cannot be detected, pelvic ultrasound will demonstrate the IUD if it is in the uterus. Alternatively, obtain anteroposterior and lateral radiographs of the pelvis with another IUD or a sound in the uterus as a marker to confirm an extrauterine IUD. If the IUD is in the abdominal cavity, it should generally be removed by laparoscopy or laparotomy. Perforations of the uterus are less likely if insertion is performed slowly, with meticulous care taken to follow directions applicable to each type of IUD.
American College of Obstetricians and Gynecologists. Practice Bulletin No. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017 Nov;130(5):e251–69. [PMID: 29064972]
Moniz MH et al. Inpatient postpartum long-acting reversible contraception and sterilization in the United States, 2008–2013. Obstet Gynecol. 2017 Jun;129(6):1078–85. [PMID: 28486357]
5. Diaphragm & Cervical Cap
The diaphragm (with contraceptive jelly) is a safe and effective contraceptive method with features that make it acceptable to some women and not others. Failure rates range from 6% to 16%, depending on the motivation of the woman and the care with which the diaphragm is used. The advantages of this method are that it has no systemic side effects and gives significant protection against pelvic infection and cervical dysplasia as well as pregnancy. The disadvantages are that it must be inserted near the time of coitus and that pressure from the rim predisposes some women to cystitis after intercourse.
The cervical cap (with contraceptive jelly) is similar to the diaphragm but fits snugly over the cervix only (the diaphragm stretches from behind the cervix to behind the pubic symphysis). The cervical cap is more difficult to insert and remove than the diaphragm. The main advantages are that it can be used by women who cannot be fitted for a diaphragm because of a relaxed anterior vaginal wall or by women who have discomfort with or in whom repeated bladder infections develop with the diaphragm. However, failure rates are 9% (perfect use) and 16% (typical use) in nulliparous women and 26% (perfect use) and 32% (typical use) in parous women.
Because of the small risk of toxic shock syndrome, a cervical cap or diaphragm should not be left in the vagina for over 12–18 hours, nor should these devices be used during the menstrual period.
6. Contraceptive Foam, Cream, Film, Sponge, Jelly, & Suppository
These products are available without prescription, are easy to use, and have typical failure rates of 10–22%. All contain the spermicide nonoxynol-9, which also has some viricidal and bactericidal activity. Nonoxynol-9 does not appear to adversely affect the vaginal colonization of hydrogen peroxide–producing lactobacilli. The FDA requires products containing nonoxynol-9 to include a warning that the products do not protect against HIV or other sexually transmitted diseases and that use of these products can irritate the vagina and rectum and may increase the risk of HIV acquisition from an infected partner. Low-risk women using a nonoxynol-9 product, with coital activity two to three times per week, are not at increased risk for epithelial disruption, compared with couples using condoms alone.
7. Condom
The male condom of latex, polyurethane or animal membrane affords protection against pregnancy—equivalent to that of a diaphragm and spermicidal jelly; latex and polyurethane (but not animal membrane) condoms also offer protection against many sexually transmitted diseases, including HIV. When a spermicide, such as vaginal foam, is used with the condom, perfect use failure rate is approximately 2% and typical use 15%. The disadvantages of condoms are dulling of sensation and spillage of semen due to tearing, slipping, or leakage with detumescence of the penis.
Two female condoms, one made of polyurethane and the other of synthetic nitrile, are available in the United States. The reported failure rates range from 5% to 21%; the efficacy is comparable to that of the diaphragm. These are the only female-controlled method that offers significant protection against both pregnancy and sexually transmitted diseases.
8. Contraception Based on Awareness of Fertile Periods
These methods are most effective when the couple restricts intercourse to the post-ovular phase of the cycle or uses a barrier method at other times. Well-instructed, motivated couples may be able to achieve low pregnancy rates with fertility awareness methods. However, properly done randomized clinical trials comparing the efficacy of most of these methods with other contraceptive methods do not exist.
9. Emergency Contraception
If unprotected intercourse occurs in midcycle and if the woman is certain she has not inadvertently become pregnant earlier in the cycle, the following regimens are effective in preventing implantation. These methods should be started as soon as possible and within 120 hours after unprotected coitus: (1) Levonorgestrel, 1.5 mg orally as a single dose (available in the United States prepackaged as Plan B and available over-the-counter (OTC) for women aged 17 years and older), has a 1–2% failure rate when taken within 72 hours. It remains efficacious up to 120 hours after intercourse, though less so compared with earlier use. (2) If the levonorgestrel regimen is not available, a combination oral contraceptive containing ethinyl estradiol and levonorgestrel given twice in 12 hours may be used. At least 20 brands of pills may be used in this way. For specific dosages and instructions for each pill brand, consult “not-2-late” at http://ec.princeton.edu/. Used within 72 hours, the failure rate of these regimens is approximately 3%, but antinausea medication is often necessary. (3) Ulipristal acetate, 30 mg orally as a single dose, has been shown to be more effective than levonorgestrel, especially when used between 72 and 120 hours, particularly among overweight women. It is available by prescription in the United States and Western Europe. (4) Copper IUD insertion within 5 days after one episode of unprotected midcycle coitus will also prevent pregnancy. Copper IUD use for emergency contraception is the most effective available method, with first cycle pregnancy rates of 0.1%. All victims of sexual violence should be offered emergency contraception.
Information on clinics or individual clinicians providing emergency contraception in the United States may be obtained by calling 1-888-668-2528.
American College of Obstetricians and Gynecologists. Committee Opinion No. 707: Access to emergency contraception. Obstet Gynecol. 2017 Jul;130(1):e48–52. [PMID: 28644339]
Goldstuck ND et al. The efficacy of intrauterine devices for emergency contraception and beyond: a systemic review update. Int J Womens Health. 2019 Aug 21;11:471–9. [PMID: 31686919]
Shen J et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2019 Jan 20;1:CD001324. [PMID: 30661244]
10. Abortion
Since the legalization of abortion in the United States in 1973, the related maternal mortality rate has fallen markedly, because illegal and self-induced abortions have been replaced by safer medical procedures. Abortions in the first trimester of pregnancy are performed by vacuum aspiration under local anesthesia or with medical regimens. Dilation and evacuation, a variation of vacuum aspiration is generally used in the second trimester. Techniques utilizing intra-amniotic instillation of hypertonic saline solution or various prostaglandins regimens, along with medical or osmotic dilators are occasionally used after 18 weeks. Several medical abortion regimens using mifepristone and multiple doses of misoprostol have been reported as being effective in the second trimester. Overall, legal abortion in the United States has a mortality rate of less than 1:100,000. Rates of morbidity and mortality rise with length of gestation. In the United States, more than 60% of abortions are performed before 9 weeks, and more than 90% are performed before 13 weeks’ gestation; only 1.2% are performed after 20 weeks. If abortion is chosen, every effort should be made to encourage the patient to seek an early procedure. In the United States, while numerous state laws limiting access to abortion and a federal law banning a rarely used variation of dilation and evacuation have been enacted, abortion remains legal and available until fetal viability (definition varies by state), under Roe v. Wade.
Complications resulting from abortion include retained products of conception (often associated with infection and heavy bleeding), uterine perforation, and unrecognized ectopic pregnancy. Immediate analysis of the removed tissue for placenta can exclude or corroborate the diagnosis of ectopic pregnancy. Women who have fever, bleeding, or abdominal pain after abortion should be examined; use of broad-spectrum antibiotics and reaspiration of the uterus are frequently necessary. Hospitalization is advisable if postabortal endometritis requires intravenous administration of antibiotics. Complications following illegal abortion often need emergency care for hemorrhage, septic shock, or uterine perforation.
Prophylactic antibiotics are indicated for surgical abortion; for example, a one-dose regimen of doxycycline, 200 mg orally, can be given 1 hour before the procedure. Many clinics prescribe tetracycline, 500 mg orally four times daily for 5 days after the procedure, as presumptive treatment for Chlamydia. Rh immune globulin should be given to all Rh-negative women following abortion. Contraception should be thoroughly discussed, and contraceptive supplies or pills provided at the time of abortion. There is growing evidence to support the safety and efficacy of immediate postabortal insertion of IUDs.
Mifepristone (RU 486) is approved by the FDA as an oral abortifacient at a dose of 600 mg on day 1, followed by 400 mcg orally of misoprostol on day 3. This combination is 95% successful in terminating pregnancies of up to 9 weeks’ duration with few complications. A more commonly used, evidence-based regimen is mifepristone, 200 mg orally on day 1, followed by misoprostol, 800 mcg vaginally either immediately or within 6–8 hours. Although not approved by the FDA for this indication, a combination of intramuscular methotrexate, 50 mg/m2 of body surface area, followed 3–7 days later by vaginal misoprostol, 800 mcg, is 98% successful in terminating pregnancy at 8 weeks or less. Minor side effects, such as nausea, vomiting, and diarrhea, are common with these regimens. There is a 5–10% incidence of hemorrhage or incomplete abortion requiring curettage. Medical abortion is generally considered as safe as surgical abortion in the first trimester but is associated with a lower success rate (requiring surgical abortion). Overall, the risk of uterine infection is lower with medical than with surgical abortion.
Bizjak I et al. Efficacy and safety of very early medical termination of pregnancy: a cohort study. BJOG. 2017 Dec;124(13):1993–9. [PMID: 28856829]
Mark KS et al. Risk of complication during surgical abortion in obese women. Am J Obstet Gynecol. 2018 Feb;218(2):238.e1–5. [PMID: 29074080]
11. Sterilization
In the United States, sterilization is the most popular method of birth control for couples who want no more children. Although sterilization is reversible in some instances, reversal surgery for both women and men is costly, complicated, and not always successful. Therefore, patients should be counseled carefully before sterilization and should view the procedure as permanent.
Female sterilization procedures include laparoscopic bipolar electrocoagulation, salpingectomy, or plastic ring application on the uterine tubes, or minilaparotomy with tubal resection. Salpingectomy may be preferred for the added benefit of decreasing ovarian cancer risk. The advantages of laparoscopy are minimal postoperative pain, small incisions, and rapid recovery. The advantages of minilaparotomy are that it can be performed with standard surgical instruments under local or general anesthesia. However, there is more postoperative pain and a longer recovery period. The cumulative 10-year failure rate for all methods combined is 1.85%, varying from 0.75% for postpartum partial salpingectomy and laparoscopic unipolar coagulation to 3.65% for spring clips; this fact should be discussed with women preoperatively. Some studies have found an increased risk of menstrual irregularities as a long-term complication of tubal ligation, but findings in different studies have been inconsistent. A method of trans-cervical sterilization, Essure, involving placement of an expanding nickel-titanium microcoil into the proximal uterine tube under hysteroscopic guidance, was approved by the FDA in 2002. The efficacy rate at 1 year was reported at 99.8% and required confirmation of tubal occlusion at 3 months with hysterosalpingogram. As of 2018, Essure was no longer marketed due to concerns related to complications and side effects reported by users.
Male sterilization by vasectomy is a safe, simple procedure in which the vas deferens is severed and sealed through a scrotal incision under local anesthesia. Long-term follow-up studies on vasectomized men show no excess risk of cardiovascular disease. Despite past controversy, there is no definite association of vasectomy with prostate cancer.
 When to Refer
When to Refer
Refer to experienced clinicians for etonogestrel subdermal (Nexplanon) insertion, IUD insertion, tubal occlusion or ligation, therapeutic abortion, or vasectomy.
Antell K et al. Contraception update: sterilization. FP Essent. 2017 Nov;462:30–4. [PMID: 29172414]
Bhindi B et al. The association between vasectomy and prostate cancer: a systematic review and meta-analysis. JAMA Intern Med. 2017 Sep 1;177(9):1273–86. [PMID: 28715534]
Zamorano AS et al. Postpartum salpingectomy: a procedure whose time has come. Am J Obstet Gynecol. 2019 Jan;220(1):8–9. [PMID: 30591122]
FEMALE SEXUAL DYSFUNCTION
 General Considerations
General Considerations
Female sexual dysfunction is a common problem. Depending on the questions asked, surveys have shown that from 35% to 98% of women report sexual concerns. Questions related to sexual functioning should be asked as part of the routine medical history. Three helpful questions to broach the topic are “Are you currently involved in a sexual relationship?,” “With men, women, or both?,” and “Do you have any sexual concerns or any pain with sex?” If the woman is not involved in a sexual relationship, she should be asked if there are any concerns that are contributing to a lack of sexual behavior. If a history of sexual dysfunction is elicited, a complete history of factors that may affect sexual function should be taken. These factors include her reproductive history (including pregnancies and mode of delivery) as well as history of infertility, sexually transmitted infection, rape or sexual violence, gynecologic or urologic disorders, endocrine abnormalities (such as diabetes mellitus or thyroid disease), neurologic problems, cardiovascular disease, psychiatric disease, and current prescription and over-the-counter medication use. A detailed history of the specific sexual dysfunction should be elicited, and a gynecologic examination should focus on findings that may contribute to sexual complaints.
 Etiology
Etiology
A. Disorders of Sexual Desire
Sexual desire in women is a complex and poorly understood phenomenon. Emotion is a key factor. Relationship conflict, fear or anxiety related to previous sexual encounters, or history of sexual abuse or violence may contribute to a lack of desire. Physical factors such as chronic illness, fatigue, depression, and specific medical disorders (such as diabetes mellitus, thyroid disease, or adrenal insufficiency) may also contribute. Menopause and attitudes toward aging may play a role. In addition, sexual desire may be influenced by other sexual dysfunction, such as arousal disorders, dyspareunia, or anorgasmia.
B. Sexual Arousal Disorders
Sexual arousal disorders may be both subjective and objective. Sexual stimulation normally leads to genital vasocongestion and lubrication. Some women may have a physiologic response to sexual stimuli but may not subjectively feel aroused because of factors such as distractions; negative expectations; anxiety; fatigue; depression; or medications, such as SSRIs or oral contraceptives. Other women with vaginal atrophy may lack both a subjective and physiologic response to sexual stimuli.
C. Orgasmic Disorders
In spite of subjective and physiologic arousal, women may experience a marked delay in orgasm, diminished sensation of an orgasm, or anorgasmia. The etiology of orgasmic disorders is complex and typically multifactorial, but the cause of a particular patient’s orgasmic disorder is usually amenable to treatment.
D. Sexual Pain Disorders
Dyspareunia and vaginismus are two subcategories of sexual pain disorders.
Dyspareunia is defined as recurrent or persistent genital pain associated with sexual intercourse that is not caused exclusively by lack of lubrication or by vaginismus and that causes marked distress or interpersonal difficulty. Vulvodynia is the most frequent cause of dyspareunia in premenopausal women. It is characterized by a sensation of burning along with other symptoms, including pain, itching, stinging, irritation, and rawness. The discomfort may be experienced as either constant or intermittent, focal or diffuse, and deep or superficial. There are generally no physical findings except minimal erythema that may be associated in a subset of patients with vulvodynia, ie, those with vulvar vestibulitis. Vulvar vestibulitis is normally asymptomatic but pain may be associated with touching or pressure on the vestibule, such as with vaginal entry of the examiner’s finger or even with insertion of a tampon. Pain occurring with deep thrusting during coitus is usually due to acute or chronic infection of the cervix, uterus, or adnexa; endometriosis; adnexal tumors; or adhesions resulting from prior pelvic disease or operation.
Vaginismus is defined as recurrent or persistent involuntary spasm of the musculature of the outer third of the vagina that interferes with sexual intercourse, resulting from fear, pain, sexual violence, or a negative attitude toward sex, often learned in childhood, and causing marked distress or interpersonal difficulty. Other medical causes of sexual pain may include vulvovaginitis; vulvar disease, including lichen planus, lichen sclerosus, and lichen simplex chronicus; pelvic disease, such as endometriosis or chronic PID; or vaginal atrophy.
 Treatment
Treatment
A. Disorders of Sexual Desire
In the absence of specific medical disorders, arousal or orgasmic disorders or dyspareunia, the focus of therapy is psychological. Cognitive behavioral therapy, sexual therapy, and couples therapy may all play a role. Success with pharmacologic therapy, particularly the use of dopamine agonists or testosterone with estrogen, has been reported, but data from large long-term clinical trials are lacking.
B. Sexual Arousal Disorders
As with disorders of sexual desire, arousal disorders may respond to psychological therapy. The phosphodiesterase inhibitors used in men do not appear to benefit the majority of women with sexual arousal disorders. However, there is some evidence to suggest a role for sildenafil in women with sexual dysfunction due to multiple sclerosis, type 1 diabetes mellitus, spinal cord injury, and antidepressant medications if other established approaches fail.
Flibanserin (Addyi), an antidepressant, was approved by the FDA in August 2015 as an effective treatment of hypoactive sexual desire disorder in premenopausal women; however, it must be used long term to be effective and has significant risks that require specific certifications of providers and pharmacies for dispensation to patients in the United States. While this medication remains available, it is not commonly prescribed.
C. Orgasmic Disorders
For many women, counseling or sex therapy may be adequate treatment. There is an FDA-cleared vacuum device that increases clitoral blood flow and may improve the likelihood of orgasm.
D. Sexual Pain Disorders
Specific medical disorders, such as endometriosis, vulvovaginitis, vulvar dermatoses, or vaginal atrophy, should be treated as outlined in other sections of this chapter.
Vaginismus may be treated initially with sexual counseling and education on anatomy and sexual functioning. The patient can be instructed in self-dilation, using a lubricated finger or dilators of graduated sizes. Before coitus (with adequate lubrication) is attempted, the patient—and then her partner—should be able to easily and painlessly introduce two fingers into the vagina. Penetration should never be forced, and the woman should always be the one to control the depth of insertion during dilation or intercourse. Injection of botulinum toxin has been used successfully in refractory cases.
Since the cause of vulvodynia is unknown, management is difficult. Few treatment approaches have been subjected to methodologically rigorous trials. A variety of topical agents have been tried, although only topical anesthetics (eg, estrogen cream and a compounded mixture of topical amitriptyline 2% and baclofen 2% in a water washable base) have been useful in relieving vulvodynia. Useful oral medications include tricyclic antidepressants, such as amitriptyline in gradually increasing doses from 10 mg/day to 75–100 mg/day; various SSRIs; and anticonvulsants, such as gabapentin, starting at 300 mg three times daily and increasing to 1200 mg three times daily. Biofeedback and physical therapy, with a physical therapist experienced with the treatment of vulvar pain, have been shown to be helpful. Surgery—usually consisting of vestibulectomy—has been useful for women with introital dyspareunia. See also Chapter e6.
 When to Refer
When to Refer
• When symptoms or concerns persist despite first-line therapy.
• For expertise in surgical procedures.
American College of Obstetricians and Gynecologists. Committee Opinion No 706: Sexual health. Obstet Gynecol. 2017 Jul;130(1):e42–7. [PMID: 28644338]
Clayton AH et al. Female sexual dysfunction. Psychiatr Clin North Am. 2017 Jun;40(2):267–84. [PMID: 28477652]
Dawson ML et al. The evaluation and management of female sexual dysfunction. J Fam Pract. 2017 Dec;66(12):722–8. [PMID: 29202143]
Kingsberg SA et al. Female sexual dysfunction—medical and psychological treatments, Committee 14. J Sex Med. 2017 Dec;14(12):1463–91. Erratum in: J Sex Med. 2018 Feb;15(2):270. [PMID: 29198504]
Rogers RG et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018 May;29(5):647–66. [PMID: 29577166]
Stenson AL. Vulvodynia: diagnosis and management. Obstet Gynecol Clin North Am. 2017 Sep;44(3):493–508. [PMID: 28778645]
Zhou ES et al. Hormonal changes and sexual dysfunction. Med Clin North Am. 2017 Nov;101(6):1135–50. [PMID: 28992859]
SEXUAL VIOLENCE
ESSENTIALS OF DIAGNOSIS

 The legal definition of rape varies by state and geographic location. The term “sexual violence” is used by the CDC and will be used in this discussion. It can be committed by a stranger, but more commonly the assailant is known to the victim, including a current or former partner or spouse (a form of intimate partner violence [IPV]).
The legal definition of rape varies by state and geographic location. The term “sexual violence” is used by the CDC and will be used in this discussion. It can be committed by a stranger, but more commonly the assailant is known to the victim, including a current or former partner or spouse (a form of intimate partner violence [IPV]).
 All victims of sexual violence should be offered emergency contraception.
All victims of sexual violence should be offered emergency contraception.
 The large number of individuals affected, the enormous health care costs, and the need for a multidisciplinary approach make sexual violence and IPV important health care issues.
The large number of individuals affected, the enormous health care costs, and the need for a multidisciplinary approach make sexual violence and IPV important health care issues.
 Knowledge of state laws and collection of evidence requirements are essential for clinicians evaluating possible victims of sexual violence, including IPV.
Knowledge of state laws and collection of evidence requirements are essential for clinicians evaluating possible victims of sexual violence, including IPV.
 General Considerations
General Considerations
Rape, or sexual assault, is legally defined in different ways in various jurisdictions. Clinicians and emergency department personnel who deal with victims of sexual violence should be familiar with the laws pertaining to sexual assault in their own state. From a medical and psychological viewpoint, it is essential that persons treating victims of sexual violence recognize the nonconsensual and violent nature of the crime. About 95% of reported victims of sexual violence are women. Each year in the United States, 4.8 million incidents of physical or sexual assault are reported by women. Penetration may be vaginal, anal, or oral and may be by the penis, hand, or a foreign object. The absence of genital injury does not imply consent by the victim. The assailant may be unknown to the victim or, more frequently, may be an acquaintance or even the spouse.
“Unlawful sexual intercourse,” or statutory rape, is intercourse with a female before the age of majority even with her consent.
Health care providers can have a significant impact in increasing the reporting of sexual violence and in identifying resources for the victims. The International Rescue Committee has developed a multimedia training tool to encourage competent, compassionate, and confidential clinical care for sexual violence survivors in low-resource settings. They have studied this intervention in over 100 healthcare providers and found that knowledge increased from 49% to 62% (P < 0.001) and confidence, from 58% to 73% (P < 0.001) in clinical care for sexual violence survivors following training. There was also a documented increase in eligible survivors receiving emergency contraception from 50% to 82% (P < 0.01), HIV postexposure prophylaxis from 42% to 92% (P < 0.001), and sexually transmitted infection prophylaxis and treatment from 45% to 96% (P < 0.01). This training encourages providers to offer care in the areas of pregnancy and sexually transmitted infection prevention as well as assistance for psychological trauma.
Because sexual violence is a personal crisis, each patient will react differently, but anxiety disorders and posttraumatic stress disorder (PTSD) are common sequelae. The rape trauma syndrome comprises two principal phases. (1) Immediate or acute: Shaking, sobbing, and restless activity may last from a few days to a few weeks. The patient may experience anger, guilt, or shame or may repress these emotions. Reactions vary depending on the victim’s personality and the circumstances of the attack. (2) Late or chronic: Problems related to the attack may develop weeks or months later. The lifestyle and work patterns of the individual may change. Sleep disorders or phobias often develop. Rarely, loss of self-esteem can lead to suicide.
Clinicians and emergency department personnel who deal with victims of sexual violence should work with community rape crisis centers or other sources of ongoing psychological support and counseling.
 General Office Procedures
General Office Procedures
The clinician who first sees the alleged victim of sexual violence should be empathetic and prepared with appropriate evidence collection and treatment materials. Standardized information and training, such as the program created by the International Rescue Committee, can be a helpful resource to the providers caring for these patients. Many emergency departments have a protocol for sexual violence victims and personnel who are trained in interviewing and examining victims of sexual violence.
 Treatment
Treatment
1. Give analgesics or sedatives if indicated. Administer tetanus toxoid if deep lacerations contain soil or dirt particles.
2. Give ceftriaxone, 250 mg intramuscularly plus azithromycin 1 g orally, to prevent gonorrhoea and chlamydia. In addition, give metronidazole, 2 g orally as a single dose to treat trichomoniasis. Incubating syphilis will probably be prevented by these medications, but the VDRL test should be repeated 6 weeks after the assault.
3. Prevent pregnancy by using one of the methods discussed under Emergency Contraception.
4. Vaccinate against hepatitis B.
5. Offer HIV prophylaxis (see Chapter 31).
6. Because women who are sexually assaulted are at increased risk for long-term psychological sequelae, such as PTSD and anxiety disorders, it is critical that the patient and her family and friends have a source of ongoing counseling and psychological support.
 When to Refer
When to Refer
All women who seek care for sexual assault should be referred to a facility that has expertise in the management of victims of sexual violence and is qualified to perform expert forensic examination, if requested.
Adams JA et al. Interpretation of medical findings in suspected child sexual abuse: an update for 2018. J Pediatr Adolesc Gynecol. 2018 Jun;31(3):225–31. [PMID: 29294380]
American College of Obstetricians and Gynecologists. Sexual assault. ACOG Committee Opinion No. 777. Obstet Gynecol. 2019 Apr;133:e296–302. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/04/sexual-assault
Crawford-Jakubiak JE et al; Committee on Child Abuse and Neglect; Committee on Adolescence. Care of the adolescent after an acute sexual assault. Pediatrics. 2017 Mar;139(3). Erratum in: Pediatrics. 2017 Jun;139(3):e20164243. [PMID: 28242861]
Vrees RA. Evaluation and management of female victims of sexual assault. Obstet Gynecol Surv. 2017 Jan;72(1):39–53. [PMID: 28134394]
BARTHOLIN DUCT CYSTS & ABSCESSES
Trauma or infection may involve the Bartholin duct, causing obstruction of the gland. Drainage of secretions is prevented, leading to pain, swelling, and abscess formation (Figure 18–1).

Figure 18–1. Right-sided Bartholin cyst (abscess). The Bartholin gland is located in the lower two-thirds of the introitus. (From Susan Lindsley, Public Health Image Library, CDC.)
The principal symptoms are periodic painful swelling on either side of the introitus and dyspareunia. A fluctuant swelling 1–4 cm in diameter lateral to either labium minus is a sign of occlusion of a Bartholin duct. Tenderness is evidence of active infection.
Purulent drainage or secretions from the gland should be cultured for Chlamydia and other pathogens and treated accordingly (see Chapter 33); frequent warm sitz baths may be helpful. All cysts and abscesses greater than 3 cm should undergo incision and drainage with additional efforts to keep the drainage tract open (eg, Word catheter or marsupialization). Marsupialization should be considered for recurrence after two prior Word catheters placements. Antibiotics are unnecessary unless cellulitis is present. In women under 40 years of age, asymptomatic cysts do not require therapy; in women over age 40, biopsy or removal are recommended to rule out vulvar carcinoma.
 When to Refer
When to Refer
Surgical therapy (marsupialization) is indicated.
VAGINITIS
ESSENTIALS OF DIAGNOSIS

 Vaginal irritation.
Vaginal irritation.
 Pruritus.
Pruritus.
 Abnormal or malodorous discharge.
Abnormal or malodorous discharge.
 General Considerations
General Considerations
Inflammation and infection of the vagina are common gynecologic complaints, resulting from a variety of pathogens, allergic reactions to vaginal contraceptives or other products, vaginal atrophy, or friction during coitus. The normal vaginal pH is 4.5 or less, and Lactobacillus is the predominant organism. Normal secretions during the middle of the cycle, or during pregnancy, can be confused with vaginitis.
 Clinical Findings
Clinical Findings
When the patient complains of vaginal irritation, pain, or unusual or malodorous discharge, a history should be taken, noting the onset of the LMP; recent sexual activity; use of contraceptives, tampons, or douches; recent changes in medications or use of antibiotics; and the presence of vaginal burning, pain, pruritus, or unusually profuse or malodorous discharge. The physical examination should include careful inspection of the vulva and speculum examination of the vagina and cervix. A vaginal, cervical, or urine sample can be obtained for detection of gonococcus and Chlamydia, if clinically indicated. A specimen of vaginal discharge is examined under the microscope in a drop of 0.9% saline solution to look for trichomonads or clue cells and in a drop of 10% potassium hydroxide to search for Candida. The vaginal pH should be tested; it is frequently greater than 4.5 in infections due to trichomonads and bacterial vaginosis. A bimanual examination to look for evidence of pelvic infection, namely cervical motion, uterine, or adnexal tenderness, should follow. Point-of-care testing is available for all three main organisms that cause vaginitis and can be used if microscopy is not available or for confirmatory testing of microscopy.
A. Vulvovaginal Candidiasis
Pregnancy, diabetes, and use of broad-spectrum antibiotics or corticosteroids predispose patients to Candida infections. Heat, moisture, and occlusive clothing also contribute to the risk. Pruritus, vulvovaginal erythema, and a white curd-like discharge that is not malodorous are found (Figure 18–2). Microscopic examination with 10% potassium hydroxide reveals hyphae and spores. A swab for cultures with Nickerson medium or for PCR testing may be performed if Candida is suspected but not demonstrated.

Figure 18–2. Cervical candidiasis. (Public Health Image Library, CDC.)
B. Trichomonas vaginalis Vaginitis
This sexually transmitted protozoal flagellate infects the vagina, Skene ducts, and lower urinary tract in women and the lower genitourinary tract in men. Pruritus and a malodorous frothy, yellow-green discharge occur, along with diffuse vaginal erythema and red macular lesions on the cervix in severe cases (“strawberry cervix,” Figure 18–3). Motile organisms with flagella seen by microscopic examination of a wet mount with saline solution is confirmatory but is identified in only 60–70% of cases. Nucleic acid amplification tests are highly sensitive and specific to identify T vaginalis. Other commercially available rapid diagnostic tests (eg, Affirm VP III and OSOM Trichomonas Rapid Test) have high sensitivity.
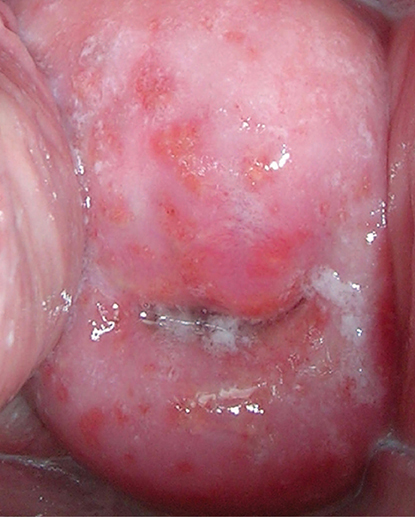
Figure 18–3. Strawberry cervix in Trichomonas vaginalis infection, with inflammation and punctate hemorrhages. (Used, with permission, from Richard P. Usatine, MD.)
C. Bacterial Vaginosis
Bacterial vaginosis is a polymicrobial disease that is not considered a sexually transmitted infection, but sexual activity is a risk factor for it. An overgrowth of Gardnerella and other anaerobes is often associated with increased malodorous discharge without obvious vulvitis or vaginitis. The discharge is grayish and sometimes frothy, with a pH of 5.0–5.5. An amine-like (“fishy”) odor is present if a drop of discharge is alkalinized with 10% potassium hydroxide. On wet mount in saline, epithelial cells are covered with bacteria to such an extent that cell borders are obscured (clue cells, Figure 18–4). Vaginal cultures are generally not useful in diagnosis; however, PCR testing is available.
Figure 18–4. Clue cells seen in bacterial vaginosis due to Gardnerella vaginalis. (Reproduced, with permission, from Richard P. Usatine, MD.)
 Treatment
Treatment
A. Vulvovaginal Candidiasis
A variety of topical and oral regimens are available to treat vulvovaginal candidiasis. Women with uncomplicated vulvovaginal candidiasis will usually respond to a 1- to 3-day regimen of a topical azole or a one-time dose of oral fluconazole. Women with complicated infection (including four or more episodes in 1 year [recurrent vulvovaginal candidiasis], severe signs and symptoms, non-albicans species, uncontrolled diabetes, HIV infection, corticosteroid treatment, or pregnancy) should receive 7–14 days of a topical regimen or two doses of oral fluconazole 3 days apart. In recurrent non-albicans infections, 600 mg of boric acid in a gelatin capsule intravaginally once daily for 2 weeks is approximately 70% effective. If recurrence occurs, referral to a gynecologist or an infectious disease specialist is indicated.
1. Single-dose regimens—Effective single-dose regimens include miconazole (1200-mg vaginal suppository), tioconazole (6.5% cream, 5 g vaginally), sustained-release butoconazole (2% cream, 5 g vaginally), or fluconazole (150-mg oral tablet).
2. Three-day regimens—Effective 3-day regimens include butoconazole (2% cream, 5 g vaginally once daily), clotrimazole (2% cream, 5 g vaginally once daily), terconazole (0.8% cream, 5 g, or 80-mg vaginal suppository once daily), or miconazole (200-mg vaginal suppository once daily).
3. Seven-day regimens—The following regimens are given once daily: clotrimazole (1% cream), miconazole (2% cream, 5 g, or 100-mg vaginal suppository), or terconazole (0.4% cream, 5 g).
4. Fourteen-day regimen—An effective 14-day regimen is nystatin (100,000-unit vaginal tablet once daily).
5. Recurrent vulvovaginal candidiasis (maintenance therapy)—Clotrimazole (500-mg vaginal suppository once weekly or 200 mg cream twice weekly) or fluconazole (100, 150, or 200 mg orally once weekly) is an effective regimen for maintenance therapy for up to 6 months.
B. Trichomonas vaginalis Vaginitis
Treatment of both partners simultaneously is recommended; metronidazole or tinidazole, 2 g orally as a single dose or 500 mg orally twice a day for 7 days, is usually used.
In the case of treatment failure with metronidazole in the absence of reexposure, the patient should be re-treated with metronidazole, 500 mg orally twice a day for 7 days, or tinidazole, 2 g orally as a single dose. If treatment failure occurs again, give metronidazole or tinidazole, 2 g orally once daily for 5 days. If this is not effective in eradicating the organisms, metronidazole and tinidazole susceptibility testing can be arranged with the Centers for Disease Control and Prevention (CDC) at 404-718-4141 or at https://www.cdc.gov/std. Women infected with T vaginalis are at increased risk for concurrent infection with other sexually transmitted diseases (STDs) and should be offered comprehensive STD testing.
C. Bacterial Vaginosis
The recommended regimens are metronidazole (500 mg orally, twice daily for 7 days), clindamycin vaginal cream (2%, 5 g, once daily for 7 days), or metronidazole gel (0.75%, 5 g, twice daily for 5 days). Alternative regimens include clindamycin (300 mg orally twice daily for 7 days), clindamycin ovules (100 g intravaginally at bedtime for 3 days), tinidazole (2 g orally once daily for 3 days), or tinidazole (1 g orally once daily for 7 days). The National STD Curriculum offers a helpful training module to clinicians to review current recommendations for treatment of vaginitis (https://www.std.uw.edu/custom/self-study/vaginitis).
Giovanini AF et al. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2019 Mar 14;380(11):1088–9. [PMID: 30865815]
PELVIC INFLAMMATORY DISEASE (Salpingitis, Endometritis)
ESSENTIALS OF DIAGNOSIS

 Lower abdominal pain with uterine, adnexal, or cervical motion tenderness.
Lower abdominal pain with uterine, adnexal, or cervical motion tenderness.
 Absence of a competing diagnosis.
Absence of a competing diagnosis.
 General Considerations
General Considerations
Pelvic inflammatory disease (PID) is a polymicrobial infection of the upper genital tract associated with the sexually transmitted organisms Neisseria gonorrhoeae and Chlamydia trachomatis as well as endogenous organisms, including anaerobes, Haemophilus influenzae, enteric gram-negative rods, and streptococci. It is most common in young, nulliparous, sexually active women with multiple partners and is a leading cause of infertility and ectopic pregnancy. The use of barrier methods of contraception may provide significant protection.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Patients with PID most commonly present with lower abdominal pain. Additional complaints may include abnormal uterine bleeding and abnormal vaginal discharge. Systemic features such as fever typically indicate more severe disease, including pelvic abscess. Right upper quadrant pain (Fitz-Hugh and Curtis syndrome) may indicate an associated perihepatitis. Diagnosis of PID is complicated by the fact that many women may have subtle or mild symptoms that are not readily recognized as PID, such as postcoital bleeding, urinary frequency, or low back pain.
B. Minimum Diagnostic Criteria
Women with cervical motion, uterine, or adnexal tenderness meet diagnostic criteria for PID and should be treated with antibiotics unless there is a competing diagnosis, such as ectopic pregnancy or appendicitis.
C. Additional Criteria
No single historical, physical, or laboratory finding is definitive for acute PID. The following criteria may be used to enhance the specificity of the diagnosis: (1) oral temperature higher than 38.3°C, (2) abnormal cervical or vaginal discharge with white cells on saline microscopy (greater than 1 leukocyte per epithelial cell), (3) elevated erythrocyte sedimentation rate, (4) elevated C-reactive protein, and (5) laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis. Testing for gonorrhea and chlamydia should be performed routinely, but treatment should not be delayed while awaiting results.
 Differential Diagnosis
Differential Diagnosis
Appendicitis, ectopic pregnancy, septic abortion, hemorrhagic or ruptured ovarian cysts or tumors, torsion of an ovarian cyst, degeneration of a myoma, and acute enteritis must be considered. PID is more likely to occur when there is a prior history of PID, recent sexual contact, recent onset of menses, recent insertion of an IUD, or recent intercourse with a partner who has a sexually transmitted disease. Acute PID is highly unlikely when recent (within 60 days) intercourse has not taken place. A sensitive serum pregnancy test should be obtained to rule out ectopic pregnancy. Pelvic ultrasonography is helpful to rule out a tubo-ovarian abscess. Laparoscopy should be considered when imaging is not informative and the patient has not responded to outpatient treatment for PID or has not improved after 72 hours of inpatient treatment; it should also be considered when an acutely ill patient has with a high suspicion of a competing diagnosis requiring surgical intervention (eg, appendicitis). The appendix should be visualized at laparoscopy to rule out appendicitis. Cultures obtained at the time of laparoscopy are often specific and helpful.
 Treatment
Treatment
A. Antibiotics
Early treatment with appropriate antibiotics effective against N gonorrhoeae, C trachomatis, and the endogenous organisms listed above is essential to prevent long-term sequelae. The sexual partner should be treated appropriately. Most women with mild to moderate disease can be treated successfully as an outpatient. The recommended outpatient regimen is a single dose of cefoxitin, 2 g intramuscularly, with probenecid, 1 g orally, plus doxycycline 100 mg orally twice a day for 14 days, or ceftriaxone 250 mg intramuscularly plus doxycycline, 100 mg orally twice daily, for 14 days. Metronidazole 500 mg orally twice daily for 14 days may also be added to either of these two regimens and will also treat bacterial vaginosis that is frequently associated with PID. For patients with severe disease or those who meet the other criteria for hospitalization, there are two recommended regimens. One regimen includes either cefotetan, 2 g intravenously every 12 hours, or cefoxitin, 2 g intravenously every 6 hours, plus doxycycline, 100 mg orally or intravenously every 12 hours. The other recommended regimen is clindamycin, 900 mg intravenously every 8 hours, plus gentamicin, a loading dose of 2 mg/kg intravenously or intramuscularly followed by a maintenance dose of 1.5 mg/kg every 8 hours (or as a single daily dose, 3–5 mg/kg). These regimens should be continued for a minimum of 24 hours after the patient shows significant clinical improvement. Then, an oral regimen should be given for a total course of antibiotics of 14 days with either doxycycline, 100 mg orally twice a day, or clindamycin, 450 mg orally four times a day. If a tubo-ovarian abscess is present, clindamycin or metronidazole should be used with doxycycline to complete the 14-day treatment for better anaerobic coverage.
B. Surgical Measures
Tubo-ovarian abscesses may require surgical excision or transcutaneous or transvaginal aspiration. Unless rupture is suspected, institute high-dose antibiotic therapy in the hospital, and monitor therapy with ultrasound. In 70% of cases, antibiotics are effective; in 30%, there is inadequate response in 48–72 hours, and surgical intervention is required. Unilateral adnexectomy is acceptable for unilateral abscess. Hysterectomy and bilateral salpingo-oophorectomy may be necessary for overwhelming infection or in cases of chronic disease with intractable pelvic pain.
 Prognosis
Prognosis
In spite of treatment, long-term sequelae, including repeated episodes of infection, chronic pelvic pain, dyspareunia, ectopic pregnancy, or infertility, develop in one-fourth of women with acute disease. The risk of infertility increases with repeated episodes of salpingitis: it is estimated at 10% after the first episode, 25% after a second episode, and 50% after a third episode.
 When to Admit
When to Admit
The following patients with acute PID should be admitted for intravenous antibiotic therapy:
• The patient has a tubo-ovarian abscess (direct inpatient observation for at least 24 hours before switching to outpatient parenteral therapy).
• The patient is pregnant.
• The patient is unable to follow or tolerate an outpatient regimen.
• The patient has not responded clinically to outpatient therapy within 72 hours.
• The patient has severe illness, nausea and vomiting, or high fever.
• Another surgical emergency, such as appendicitis, cannot be ruled out.
Curry A et al. Pelvic inflammatory disease: diagnosis, management and prevention. Am Fam Physician. 2019 Sep 15;100(6):357–64. [PMID: 31524362]
Ross J et al. 2017 European guideline for the management of pelvic inflammatory disease. Int J STD AIDS. 2018 Feb;29(2):108–14. [PMID: 29198181]
CONDYLOMA ACUMINATA
Warty growths on the vulva, perianal area, vaginal walls, or cervix are caused by various types of the human papillomavirus (HPV). Pregnancy and immunosuppression favor growth. Ninety percent of genital warts are caused by HPV 6 and 11. With increasing use of a quadrivalent HPV vaccine in the United States, the prevalence of HPV types 6, 11, 16 and 18 decreased from 11.5% in 2003–2006 to 4.3% in 2009–2012 among girls aged 14–19 years, and from 18.5% to 12.1% in women aged 20–24 years. Vulvar lesions may be obviously wart-like or may be diagnosed only after application of 4% acetic acid (vinegar) and colposcopy, when they appear whitish, with prominent papillae. Vaginal lesions may show diffuse hypertrophy or a cobblestone appearance.
Recommended treatments for vulvar warts include podophyllum resin 10–25% in tincture of benzoin (do not use during pregnancy or on bleeding lesions) or 80–90% trichloroacetic or bichloroacetic acid, carefully applied to avoid the surrounding skin. The pain of bichloroacetic or trichloroacetic acid application can be lessened by a sodium bicarbonate paste applied immediately after treatment. Podophyllum resin must be washed off after 2–4 hours. Freezing with liquid nitrogen or a cryoprobe and electrocautery are also effective. Patient-applied regimens, useful when the entire lesion is accessible to the patient, include podofilox 0.5% solution or gel, imiquimod 5% cream, or sinecatechins 15% ointment. Vaginal warts may be treated with cryotherapy with liquid nitrogen or trichloroacetic acid. Extensive warts may require treatment with CO2 laser, electrocautery, or excision under local or general anesthesia.
Grennan D. JAMA patient page. Genital warts. JAMA. 2019 Feb 5;321(5):520. [PMID: 30721297]
Meites E et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019 Aug 16;68(32):698–702. [PMID: 31415491]
Mills BB. Vaginitis: beyond the basics. Obstet Gynecol Clin North Am. 2017 Jun;44(2):159–77. [PMID: 28499528]
CERVICAL INTRAEPITHELIAL NEOPLASIA (CIN) (Dysplasia of the Cervix)
ESSENTIALS OF DIAGNOSIS

 The presumptive diagnosis is made by an abnormal Papanicolaou smear of an asymptomatic woman with no grossly visible cervical changes.
The presumptive diagnosis is made by an abnormal Papanicolaou smear of an asymptomatic woman with no grossly visible cervical changes.
 Diagnose by colposcopically directed biopsy.
Diagnose by colposcopically directed biopsy.
 General Considerations
General Considerations
The squamocolumnar junction of the cervix is an area of active squamous cell proliferation. In childhood, this junction is located on the exposed vaginal portion of the cervix. At puberty, because of hormonal influence and possibly because of changes in the vaginal pH, the squamous margin begins to encroach on the single-layered, mucus-secreting epithelium, creating an area of metaplasia (transformation zone). Infection with HPV (see Prevention, below) may lead to cellular abnormalities, which over time may develop into squamous cell dysplasia or cancer. There are varying degrees of dysplasia (Table 18–5), defined by the degree of cellular atypia; all atypia must be observed and treated if persistent or worsening.
Table 18–5. Classification systems for Papanicolaou smears.
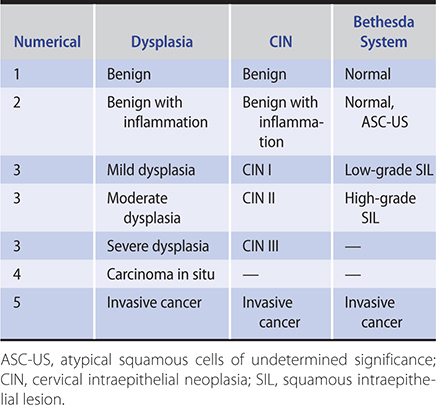
 Clinical Findings
Clinical Findings
There are no specific symptoms or signs of CIN. The presumptive diagnosis is made by cytologic screening of an asymptomatic population with no grossly visible cervical changes. All visible abnormal cervical lesions should be biopsied (Figure 18–5).

Figure 18–5. Erosion of the cervix due to cervical intraepithelial neoplasia (CIN), a precursor lesion to cervical cancer. (Public Health Image Library, CDC.)
 Screening & Diagnosis
Screening & Diagnosis
A. Cytologic Examination (Papanicolaou Smear)
In immunocompetent women, cervical cancer screening should begin at age 21. The recommendation to start screening at age 21 years regardless of the age of onset of sexual intercourse is based on the very low incidence of cancer in younger women and the potential for adverse effects associated with treatment of young women with abnormal cytology screening results. In contrast to the high rate of infection with HPV in sexually active adolescents, invasive cervical cancer is very rare in women younger than age 21 years. The US Preventive Services Task Force (USPSTF) 2018 statement recommends screening for cervical cancer in women aged 21 to 65 years as follows: for women aged 21 to 29 years, screening with cytology (conventional [Papanicolaou smear] or liquid based) alone every 3 years; and for women aged 30 to 65 years, screening with cytology alone every 3 years, with high-risk HPV testing alone every 5 years, or with a combination of cytology and high-risk HPV testing (cotesting) every 5 years. These recommendations apply to women who have a cervix, regardless of their sexual history or HPV vaccination status. They do not apply to women who have previously been diagnosed with cervical cancer or a high-grade precancerous cervical lesion (ie, CIN grade II or III) or to women with immune compromise (eg, living with HIV) or with in utero exposure to diethylstilbestrol; such women may require more frequent screening.
The USPSTF recommends against screening for cervical cancer for women younger than age 21 years, for women older than age 65 years who have had adequate prior screening and are not otherwise at high risk for cervical cancer, and for women who have had a hysterectomy with removal of the cervix and who have no history of cervical cancer or a high-grade precancerous lesion.
The goal of screening is to identify high-grade precancerous cervical lesions to prevent their progression to cervical cancer. These high-grade cervical lesions may be treated with excisional and ablative therapies (see below). Early-stage cervical cancer may be treated with surgery (hysterectomy) or chemotherapy (see next section). Online guidelines are available for the management of abnormal screening test results at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening2 (August, 2018) and https://www.asccp.org/guidelines (April, 2019).
Cytologic reports from the laboratory may describe findings in one of several ways (see Table 18–5). The Bethesda System uses the terminology “atypical squamous cells of unknown significance” (ASC-US) and “squamous intraepithelial lesions,” either low-grade (LSIL) or high-grade (HSIL). HPV DNA testing can be used as an adjunct in cervical cancer screening as a triage test to stratify risk in women age 21 years and older with a cytologic diagnosis of ASC-US and in postmenopausal women with a cytologic diagnosis of ASC-US or LSIL.
B. Colposcopy
Women with ASC-US and a negative HPV screening may be followed up in 1 year for a repeat Papanicolaou smear and HPV co-testing. If the HPV screen is positive, colposcopy is indicated. If HPV screening is unavailable, repeat cytology may be done at 12 months. Women between ages 21–24 with LSIL should have repeat Papanicolaou smear in 1 year. Women age 25 and older with SIL or atypical glandular cells should undergo colposcopy. Viewing the cervix with 10–20 × magnification allows for assessment of the size and margins of an abnormal transformation zone and determination of extension into the endocervical canal. The application of 3–5% acetic acid (vinegar) dissolves mucus, and the acid’s desiccating action sharpens the contrast between normal and actively proliferating squamous epithelium. Abnormal changes include white patches and vascular atypia, which indicate areas of greatest cellular activity.
C. Biopsy
Colposcopically directed biopsy and endocervical curettage are office procedures. Data from both cervical biopsy and endocervical curettage are important in deciding on treatment.
 Prevention
Prevention
Cervical infection with the HPV is associated with virtually all cervical dysplasias and cancers. There are over 100 recognized HPV subtypes. Types 6 and 11 tend to cause genital warts and mild dysplasia and rarely progress to cervical cancer; types 16, 18, 31, and others cause higher-grade dysplasia. The HPV 9-valent (Gardasil-9) recombinant vaccine (9vHPV) is indicated for the prevention of cervical, vaginal, and vulvar cancers (in women) and anal cancers (in women and men) caused by HPV types 16, 18, 31, 33, 45, 52, and 58; genital warts (in women and men) caused by HPV types 6 and 11; and precancerous/dysplastic lesions of cervix, vagina, vulva (in women), and anus (in women and men) caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Gardasil-9 is recommended for vaccination of females and males ages 9-45 years old. The earlier HPV 4-valent vaccine known as Gardasil that was indicated for prevention of diseases related to HPV types 6, 11, 16, and 18 has been discontinued in the United States. The use of HPV vaccination in the United States continues to increase; however, the HPV vaccination continues to lag far behind other vaccines recommended for adolescents. In 2018, 51% of adolescents were up to date with the three-dose HPV vaccine series compared with 48% in 2017.
Because complete coverage of all carcinogenic HPV types is not provided by either vaccine, all women need to have regular cervical cancer screening as outlined above. In addition to vaccination, preventive measures include limiting the number of sexual partners and thus exposure to HPV, using a diaphragm or condom for coitus, and smoking cessation and avoiding exposure to secondhand smoke.
 Treatment
Treatment
Treatment varies depending on the degree and extent of CIN. Biopsies should precede treatment, except in cases of HSIL where it may be appropriate to proceed directly to a LEEP.
A. Cryosurgery
The use of freezing (cryosurgery) is effective for noninvasive small lesions visible on the cervix without endocervical extension.
B. CO2 Laser
This well-controlled method minimizes tissue destruction. It is colposcopically directed and requires special training. It may be used with large visible lesions. In current practice, it involves the vaporization of the transformation zone on the cervix and the distal 5–7 mm of endocervical canal.
C. Loop Excision
When the CIN is clearly visible in its entirety, a wire loop can be used for excisional biopsy. This office procedure, called LEEP (loop electrosurgical excision procedure), done with local anesthesia is quick and straightforward. Cutting and hemostasis are achieved with a low-voltage electrosurgical machine.
D. Conization of the Cervix
Conization is surgical removal of the entire transformation zone and endocervical canal. It is reserved for cases of severe dysplasia (CIN III) or carcinoma in situ, particularly those with endocervical extension. It can be performed with scalpel, CO2 laser, needle electrode, or large-loop excision.
 Follow-Up
Follow-Up
Because recurrence is possible—especially in the first 2 years after treatment—and because the false-negative rate of a single cervical cytologic test is 20%, close follow-up after colposcopy and biopsy is imperative. Following excisional or ablative procedure, co-testing (cytology and HPV DNA) should be repeated at 12-month intervals for 2 years. If CIN II or III is identified at the margins of an endocervical curettage procedure, however, repeat cytology with endocervical curettage is preferred at 4–6 months. If follow-up testing is normal, routine cytologic screening can be resumed. Colposcopy and endocervical sampling should be performed for any abnormality.
The American Society for Colposcopy and Cervical Pathology Guidelines for cervical cancer screening and management of abnormal Papanicolaou smears are available online (April, 2019, https://www.asccp.org/consensus-guidelines).
 When to Refer
When to Refer
• Patients with CIN II/III should be referred to an experienced colposcopist.
• Patients requiring conization biopsy should be referred to a gynecologist.
Arbyn M et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018 May 9;5:CD009069. [PMID: 29740819]
Melnikow J et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018 Aug 21;320(7):687–705. [PMID: 30140883]
Perkins RB et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020 Apr;24(2):102–31. [PMID: 32243307]
Ogilvie GS et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018 Jul 3;320(1):43–52. [PMID: 29971397]
Oshman LD et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices (ACIP). JAMA. 2020 Feb 4;323(5):468–9. [PMID: 31930397]
Smith RA et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019 May;69(3):184–210. [PMID: 30875085]
Stumbar SE et al. Cervical cancer and its precursors: a preventative approach to screening, diagnosis, and management. Prim Care. 2019 Mar;46(1):117–34. [PMID: 30704652]
US Preventive Services Task Force; Curry SJ et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Aug 21;320(7):674–86. [PMID: 30140884]
CARCINOMA OF THE CERVIX
ESSENTIALS OF DIAGNOSIS

 Increased risk in women who smoke and those with HIV or high-risk HPV types.
Increased risk in women who smoke and those with HIV or high-risk HPV types.
 Gross lesions should be evaluated by colposcopically directed biopsies and not cytology alone.
Gross lesions should be evaluated by colposcopically directed biopsies and not cytology alone.
 General Considerations
General Considerations
Cervical cancer is the third most common cancer in the world and the leading cause of cancer death among women in developing countries. It is considered a sexually transmitted disease as both squamous cell and adenocarcinoma of the cervix are secondary to infection with HPV, primarily types 16 and 18. Women infected with HIV and other forms of immunosuppression are at an increased risk for high-risk HPV infection and CIN. Smoking appears to be a cofactor for squamous cell carcinoma (SCC). SCC accounts for approximately 80% of cervical cancers, while adenocarcinoma accounts for 15%, and adenosquamous carcinoma for 3–5%; neuroendocrine or small cell carcinomas are rare.
SCC appears first in the intraepithelial layers (the preinvasive stage, or carcinoma in situ). Preinvasive cancer (CIN III) is a common diagnosis in women 25–40 years of age. Two to 10 years are required for carcinoma to penetrate the basement membrane and become invasive. While cervical cancer mortality has declined steadily in the United States due to high rates of screening and improved treatment, the rate of decline has slowed in recent years. In general, black women experienced much higher incidence and mortality than white women. The 5-year survival rate ranges from 63% for stage II cervical cancer to less than 20% for stage IV.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Early cervical cancer is often asymptomatic. The most common signs are irregular or heavy bleeding and postcoital spotting. Bladder and rectal dysfunction or fistulas and pain are late symptoms.
B. Cervical Biopsy and Endocervical Curettage or Conization
These procedures are necessary steps after a positive Papanicolaou smear to determine the extent and depth of invasion of the cancer. Even if the smear is positive, treatment with additional surgery or radiation is never justified until definitive diagnosis has been established through biopsy.
C. “Staging” or Estimate of Gross Spread of Cancer of the Cervix
Staging of invasive cervical cancer is achieved by clinical evaluation, usually conducted under anesthesia. Further examinations, such as ultrasonography, CT, MRI, lymphangiography, laparoscopy, and fine-needle aspiration, are valuable for treatment planning.
 Complications
Complications
Metastases to regional lymph nodes occur with increasing frequency from stage I to stage IV. Paracervical extension occurs in all directions from the cervix. The ureters may become obstructed lateral to the cervix, causing hydroureter and hydronephrosis and consequently impaired kidney function. Almost two-thirds of patients with untreated carcinoma of the cervix die of uremia when ureteral obstruction is bilateral. Pain in the back, in the distribution of the lumbosacral plexus, is often indicative of neurologic involvement. Gross edema of the legs may be indicative of vascular and lymphatic stasis due to tumor. Vaginal fistulas to the rectum and urinary tract are severe late complications. Hemorrhage is the cause of death in 10–20% of patients with extensive invasive carcinoma.
 Prevention
Prevention
Vaccination with the recombinant 9-valent HPV vaccine (Gardasil-9) can prevent cervical cancer by targeting the HPV types that pose the greatest risk as well as protect against low-grade and precancerous lesions caused by other HPV types (see Cervical Intraepithelial Neoplasia).
 Treatment
Treatment
A. Emergency Measures
Vaginal hemorrhage originates from gross ulceration and cavitation in later stage cervical carcinoma. Ligation and suturing of the cervix are usually not feasible, but emergent vaginal packing, cautery, tranexamic acid, and irradiation are helpful to stop bleeding temporarily. Ligation, resection, or embolization of the uterine or hypogastric arteries may be lifesaving when other measures fail.
B. Specific Measures
1. Carcinoma in situ (stage 0)—In women for whom childbearing is not a consideration, total hysterectomy is the definitive treatment. In women who wish to retain the uterus, acceptable alternatives include cryosurgery, laser surgery, LEEP, or cervical conization. Follow-up co-testing (cytology and HPV DNA) should be repeated at 12-month intervals for 2 years after excisional or ablative treatment.
2. Invasive carcinoma—Microinvasive carcinoma (stage IA1) is treated with simple, extrafascial hysterectomy. Stages IA2, IB1, and IIA cancers may be treated with either radical hysterectomy with concomitant radiation and chemotherapy or with radiation plus chemotherapy alone. Women with stage IB1 may be candidates for fertility-sparing surgery that includes radical trachelectomy and lymph node dissection with preservation of the uterus and ovaries. Stages IB2, IIB, III, and IV cancers are treated with radiation therapy plus concurrent chemotherapy.
 Prognosis
Prognosis
The overall 5-year relative survival rate for carcinoma of the cervix is 68% in white women and 55% in black women in the United States. Survival rates are inversely proportionate to the stage of cancer: stage 0, 99–100%; stage IA, more than 95%; stage IB–IIA, 80–90%; stage IIB, 65%; stage III, 40%; and stage IV, less than 20%.
 When to Refer
When to Refer
All patients with invasive cervical carcinoma (stage IA or higher) should be referred to a gynecologic oncologist.
American Cancer Society. Survival rates for cervical cancer, by stage, January 3, 2020. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html
Cantillo E et al. Less is more: minimally invasive and quality surgical management of gynecologic cancer. Obstet Gynecol Clin North Am. 2019 Mar;46(1):55–66. [PMID: 30683266]
Johnson CA et al. Cervical cancer: an overview of pathophysiology and management. Semin Oncol Nurs. 2019 Apr;35(2):166–74. [PMID: 30878194]
US Preventive Services Task Force; Curry SJ et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Aug 21;320(7):674–86. [PMID: 30140884]
Van Dyne EA et al. Trends in human papillomavirus-associated cancers—United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018 Aug 24;67(33):918–24. [PMID: 30138307]
CARCINOMA OF THE ENDOMETRIUM
ESSENTIALS OF DIAGNOSIS

 Abnormal bleeding is the presenting sign in 90% of cases.
Abnormal bleeding is the presenting sign in 90% of cases.
 Papanicolaou smear is frequently negative.
Papanicolaou smear is frequently negative.
 After a negative pregnancy test, endometrial tissue is required to confirm the diagnosis.
After a negative pregnancy test, endometrial tissue is required to confirm the diagnosis.
 General Considerations
General Considerations
Adenocarcinoma of the endometrium is the second most common cancer of the female genital tract. It occurs most often in women 50–70 years of age. Obesity, nulliparity, diabetes, polycystic ovaries with prolonged anovulation, unopposed estrogen therapy, and the extended use of tamoxifen for the treatment of breast cancer are also risk factors. Women with a family history of colon cancer (hereditary nonpolyposis colorectal cancer, Lynch syndrome) are at significantly increased risk, with a lifetime incidence as high as 30%.
Abnormal bleeding is the presenting sign in 90% of cases. Any postmenopausal bleeding requires investigation. Pain generally occurs late in the disease, with metastases or infection.
Papanicolaou smears of the cervix occasionally show atypical endometrial cells but are an insensitive diagnostic tool. Endocervical and endometrial sampling is the only reliable means of diagnosis. Simultaneous hysteroscopy can be a valuable addition in order to localize polyps or other lesions within the uterine cavity. Pelvic ultrasonography may be used to determine the thickness of the endometrium as an indication of hypertrophy and possible neoplastic change. The finding of a thin endometrial lining on ultrasound is clinically reassuring in cases where very little tissue is obtainable through endometrial biopsy.
Pathologic assessment is important in differentiating hyperplasias, which often can be treated hormonally.
 Prevention
Prevention
Prompt endometrial sampling for patients who report abnormal menstrual bleeding or postmenopausal uterine bleeding will reveal many incipient as well as clinical cases of endometrial cancer. Younger women with chronic anovulation are at risk for endometrial hyperplasia and subsequent endometrial cancer; they can significantly reduce the risk of hyperplasia with the use of oral contraceptives or cyclic progestin therapy.
 Staging
Staging
Staging and prognosis are based on surgical and pathologic evaluation only. Examination under anesthesia, endometrial and endocervical sampling, chest radiography, intravenous urography, cystoscopy, sigmoidoscopy, transvaginal sonography, and MRI will help determine the extent of the disease and its appropriate treatment.
 Treatment
Treatment
Treatment consists of total hysterectomy and bilateral salpingo-oophorectomy. Peritoneal washings for cytologic examination are routinely taken and lymph node sampling may be done. Women with high-risk endometrial cancer (serous adenocarcinoma, clear cell carcinoma, grade 3 deeply invasive endometrioid carcinoma, and stages III/IV disease) are generally treated with surgery followed by chemotherapy and/or radiation therapy.
 Prognosis
Prognosis
With early diagnosis and treatment, the overall 5-year survival is 80–85%. With stage I disease, the depth of myometrial invasion is the strongest predictor of survival, with a 98% 5-year survival with less than 66% depth of invasion and 78% survival with 66% or more invasion.
 When to Refer
When to Refer
All patients with endometrial carcinoma should be referred to a gynecologic oncologist.
Bodurtha Smith AJ et al. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017 May;216(5):459–76. [PMID: 27871836]
McDonald ME et al. Endometrial cancer: obesity, genetics and targeted agents. Obstet Gynecol Clin North Am. 2019 Mar;46(1):89–105. [PMID: 30683268]
Passarello K et al. Endometrial cancer: an overview of pathophysiology, management and care. Semin Oncol Nurs. 2019 Apr;35(2):157–65. [PMID: 30867105]
Visser NCM et al. Accuracy of endometrial sampling in endometrial carcinoma: a systematic review and meta-analysis. Obstet Gynecol. 2017 Oct;130(4):803–13. [PMID: 28885397]
CARCINOMA OF THE VULVA
ESSENTIALS OF DIAGNOSIS

 Two independent pathways for development: HPV or chronic inflammation.
Two independent pathways for development: HPV or chronic inflammation.
 History of prolonged vulvar irritation, with pruritus, local discomfort, or slight bloody discharge.
History of prolonged vulvar irritation, with pruritus, local discomfort, or slight bloody discharge.
 Early lesions may suggest or include non-neoplastic epithelial disorders.
Early lesions may suggest or include non-neoplastic epithelial disorders.
 Late lesions appear as a mass, an exophytic growth, or a firm, ulcerated area in the vulva.
Late lesions appear as a mass, an exophytic growth, or a firm, ulcerated area in the vulva.
 Biopsy is necessary for diagnosis.
Biopsy is necessary for diagnosis.
 General Considerations
General Considerations
The majority of cancers of the vulva are squamous lesions that classically have occurred in women over 50 years of age. Several subtypes (particularly 16, 18, and 31) of HPV have been identified in some but not all vulvar cancers. About 70–90% of vulvar intraepithelial neoplasias (VIN) and 40–60% of vulvar cancers are HPV associated. Vulvar lichen sclerosus also is associated with an increased risk of developing vulvar cancer. As with squamous cell lesions of the cervix, a grading system of VIN from mild dysplasia to carcinoma in situ is used.
 Differential Diagnosis
Differential Diagnosis
Other vulvar lesions must be considered. Vulvar intraepithelial neoplasia may resemble vulvar cancer and must be distinguished by histology. Benign vulvar disorders that must be excluded in the diagnosis of carcinoma of the vulva include inflammatory vulvar dermatoses (psoriasis, lichen sclerosus, lichen planus), chronic granulomatous lesions (eg, lymphogranuloma venereum, syphilis), condylomas, epidermal inclusion cysts, hidradenomas, or neurofibromas. Lichen sclerosus and other associated leukoplakic changes in the skin should be biopsied. The likelihood that a superimposed vulvar cancer will develop in a woman with a non-neoplastic epithelial disorder is very low (1–5%).
 Diagnosis
Diagnosis
Biopsy is essential for the diagnosis of VIN and vulvar cancer and should be performed with any localized atypical vulvar lesion, including white patches. Multiple skin-punch specimens can be taken in the office under local anesthesia, with care to include tissue from the edges of each lesion sampled. Colposcopy of vulva, vagina, and cervix can help in identifying areas for biopsy and in planning further treatment.
 Staging
Staging
Vulvar cancer generally spreads by direct extension into the vagina, urethra, perineum, and anus, with discontinuous spread into the inguinal and femoral lymph nodes. Staging is based on a combined clinical and surgical/pathologic system.
 Treatment
Treatment
Invasive carcinoma confined to the vulva without evidence of spread to adjacent organs or to the regional lymph nodes is treated with wide local excision and inguinal lymphadenectomy or wide local excision alone if invasion is less than 1 mm. To avoid the morbidity of inguinal lymphadenectomy, some guidelines recommend sentinel lymph node sampling for women with early-stage vulvar cancer. Patients with more advanced disease may receive preoperative radiation, chemotherapy, or both.
 Prognosis
Prognosis
Basal cell vulvar carcinomas very seldom metastasize. With adequate excision, the prognosis is excellent. Patients with invasive vulvar SCC 2 cm in diameter or less, without inguinal lymph node metastases, have an 85–90% 5-year survival rate. If the lesion is larger than 2 cm and lymph node involvement is present, the likelihood of 5-year survival is approximately 40%.
 When to Refer
When to Refer
All patients with invasive vulvar carcinoma should be referred to a gynecologic oncologist.
Allbritton JI. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017 Sep;44(3):339–52. [PMID: 28778635]
Dellinger TH et al. Surgical management of vulvar cancer. J Natl Compr Canc Netw. 2017 Jan;15(1):121–8. [PMID: 28040722]
Halec G et al. Biological relevance of human papillomaviruses in vulvar cancer. Mod Pathol. 2017 Apr;30(4):549–62. [PMID: 28059099]
Koh WJ et al. Vulvar cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017 Jan;15(1):92–120. [PMID: 28040721]
OVARIAN CANCER & OVARIAN TUMORS
ESSENTIALS OF DIAGNOSIS

 Symptoms include vague gastrointestinal discomfort, pelvic pressure, or pain.
Symptoms include vague gastrointestinal discomfort, pelvic pressure, or pain.
 Many cases of early-stage cancer are asymptomatic.
Many cases of early-stage cancer are asymptomatic.
 Pelvic examination and ultrasound are mainstays of diagnosis.
Pelvic examination and ultrasound are mainstays of diagnosis.
 General Considerations
General Considerations
Ovarian tumors are common. Most are benign, but malignant ovarian tumors are the leading cause of death from gynecologic cancer. The wide range of types and patterns of ovarian tumors is due to the complexity of ovarian embryology and differences in tissues of origin.
In women with no family history of ovarian cancer, the lifetime risk is 1.6%, whereas a woman with one affected first-degree relative has a 5% lifetime risk. Ultrasound or tumor marker screening for women with one or no affected first-degree relatives has not been shown to reduce mortality from ovarian cancer, and the risks associated with unnecessary prophylactic surgical procedures outweigh the benefits in low-risk women. With two or more affected first-degree relatives, the risk is 7%. Approximately 3% of women with two or more affected first-degree relatives will have a hereditary ovarian cancer syndrome with a lifetime risk of 40%. Women with a BRCA1 gene mutation have a 45% lifetime risk of ovarian cancer and those with a BRCA2 mutation, a 25% risk. Consideration should be given to screening with transvaginal sonography and serum CA 125 testing, starting at age 30–35 years for women with BRCA1 or age 35–40 for women with BRCA2 or 5–10 years earlier than the earliest age that ovarian cancer was first diagnosed in any family member. Of note, this screening regimen has not been shown to reduce mortality; thus, prophylactic oophorectomy should be considered at conclusion of childbearing.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Most women with both benign and malignant ovarian neoplasms are either asymptomatic or experience only mild nonspecific gastrointestinal symptoms or pelvic pressure. Women with advanced malignant disease may experience abdominal pain and bloating, and a palpable abdominal mass with ascites is often present.
B. Laboratory Findings
Serum CA 125 is elevated in 80% of women with epithelial ovarian cancer overall but in only 50% of women with early disease. However, CA 125 may be elevated in premenopausal women with benign disease (such as endometriosis), minimizing its usefulness in ovarian cancer screening. In premenopausal women with ovarian masses, other tumor markers (such as human chorionic gonadotropin [hCG], lactate dehydrogenase, or alpha-fetoprotein) may be indicators of the tumor type.
C. Imaging
Transvaginal sonography is useful for screening high-risk women but has inadequate sensitivity for screening low-risk women. Ultrasound is helpful in differentiating ovarian masses that are benign and likely to resolve spontaneously from those with malignant potential. Color Doppler imaging may further enhance the specificity of ultrasound diagnosis.
 Differential Diagnosis
Differential Diagnosis
Once an ovarian mass has been detected, it must be categorized as functional, benign neoplastic, or potentially malignant. Predictive factors include age, size of the mass, ultrasound configuration, serum CA 125 level, the presence of symptoms, and whether the mass is unilateral or bilateral. Simple cysts up to 5 cm in diameter are almost universally benign in both premenopausal and postmenopausal patients. Most will resolve spontaneously and may be monitored without intervention. If the mass is larger or unchanged on repeat pelvic examination and transvaginal sonography, surgical evaluation is warranted.
Laparoscopic or robotic approaches may be used for oophorectomy and staging in early-stage ovarian cancer when the ovarian mass is small. In these cases, malignancy is often not diagnosed prior to the surgery. Data (retrospective) comparing open with laparoscopic approaches to ovarian cancer staging are limited. Laparotomy is generally preferred for staging and debulking if malignancy is suspected from findings on transvaginal ultrasound with morphologic scoring, color Doppler assessment of vascular quality, and CA 125 level.
 Treatment
Treatment
If a malignant ovarian mass is suspected, surgical evaluation should be performed by a gynecologic oncologist. For benign neoplasms, tumor removal or unilateral oophorectomy is usually performed. For ovarian cancer in an early stage, the standard therapy is complete surgical staging followed by hysterectomy and bilateral salpingo-oophorectomy with omentectomy and selective lymphadenectomy. With more advanced disease, in addition, aggressive removal of all visible tumor improves survival. Except for women with low-grade ovarian cancer in an early stage, postoperative chemotherapy is indicated (see Table 39–3). Several chemotherapy regimens are effective, such as the combination of cisplatin or carboplatin with paclitaxel, with clinical response rates of up to 60–70% (see Table 39–13).
 Prognosis
Prognosis
Advanced disease is diagnosed in approximately 75% of women with ovarian cancer. The overall 5-year survival is approximately 17% with distant metastases, 36% with local spread, and 89% with early disease.
 When to Refer
When to Refer
If a malignant mass is suspected, surgical evaluation should be performed by a gynecologic oncologist.
American College of Obstetricians and Gynecologists. Committee Opinion No. 716: The role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer in women at average risk. Obstet Gynecol. 2017 Sep;130(3):e146–9. [PMID: 28832487]
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 793: Hereditary cancer syndromes and risk assessment. Obstet Gynecol. 2019 Dec;134(6):e143–9. [PMID: 31764758]
Centers for Disease Control and Prevention (CDC). Ovarian cancer screening. 2019 Aug 15. https://www.cdc.gov/cancer/ovarian/basic_info/screening.htm
US Preventive Services Task Force, Grossman DC et al. Screening for ovarian cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Feb 13;319(6):588–94. [PMID: 29450531]
1Dr. Long is an employee of the National Institutes of Health (NIH). The views expressed in this chapter are hers and Dr. Woo’s and do not necessarily represent the views of the NIH or the US government.