 Chapter 7, ‘Clinical features of acquired syphilis’, pp. 120–122, for further details.
Chapter 7, ‘Clinical features of acquired syphilis’, pp. 120–122, for further details.Clinical features of acquired syphilis
Pregnancy and congenital infection
The origins of syphilis are unclear, but it became an epidemic in Europe in the late 15th century, although skeletal evidence suggests earlier endemic infection. The name originates from a poem about the infected shepherd Syphilis written by Fracastoro in 1530.
Syphilis is caused by a delicate spirochaete, Treponema pallidum (TP). It has a cylindrical nucleus and cytoplasm contained within a cell wall and outer envelope with flagella in the periplasmic space, 6–20 µm × 0.1–0.18 µm in size. Micro-aerophilic, so can only be grown on tissue culture. It has limited viability outside its human host, so is usually transmitted sexually through micro-abrasions in mucosal skin.
Currently high rates in Eastern Europe. In the UK, rates of transmission are highest amongst MSM, especially as outbreaks through anonymous sex in saunas, cruising sites, and geosocial networking apps, and in association with HIV infection. Entry site usually genital in heterosexuals, but often extra-genital (oral, anal, rectal) in MSM, via oro-genital, oro-anal, and anogenital contact.
Transmission routes are:
• Sexual—only from early syphilis: ~30–50% contacts are infected.
• Accidental infection by inoculation: e.g. healthcare professional.
• Blood-borne: needle sharing, blood transfusion (very rare as blood is screened and organisms die after 24–48 hours at 4°C.
• Transplacental: at any gestation. More common in early syphilis (80–90% risk) with RPR >1:8, rare after 4 years infection.
 Chapter 7, ‘Clinical features of acquired syphilis’, pp. 120–122, for further details.
Chapter 7, ‘Clinical features of acquired syphilis’, pp. 120–122, for further details.
1°: chancre develops 9-–90 days after infection (average 21 days), regional lymphadenopathy. Resolves by 3–8 weeks.
2°: dissemination of infection develops 6 weeks to 6 months after infection in 25% untreated. 25% relapse in first 2 years if untreated.
Asymptomatic infection. Early latent if infected <2 years. Late latent if > 2 years (</>1 year in USA), outcome in 2/3 untreated syphilis.
• Gummatous: musculoskeletal (10%), viscera/mucosa (15%) can occur <2 years, but usually after ~15 years.
• Cardiovascular (10%): occurs after 10–30 years.
• Neurosyphilis (10%): meningovascular after 2–7 years; general paresis after 10–20 years; tabes dorsalis after15–25 years.
Early congenital syphilis if <2 years old. Late congenital lesions usually develop after 2–3 years.
Box 7.1 outlines questions patients frequently ask.
Box 7.1 Frequently asked questions
Yes, you can catch syphilis from unprotected oral sex.
No. Having been infected once does not protect you from re-infection.
No. After infection with syphilis your body produces antibodies, which will usually remain detectable for life, and will be positive on screening tests. They do not provide protection against future infection. It is important to measure their nadir levels after treatment, as re-infection would cause a rise in their levels.
Your partner will require testing, and be offered treatment, either epidemiologically or if test is positive. Previous partners may also require testing and treating depending on how long you are likely to have had syphilis, and when your last sexual relationship was.
• 1°: all sexual partners within the past 3 months should be tested +/– treated
• 2° or early latent: all sexual partners in past 2 years need testing/treating.
• Late syphilis: this depends on estimated duration of infection.
Recommendation is to abstain from sex until signs of 1° syphilis have resolved and 2 weeks after completing treatment.
• Presentation: classically initial painless papule at inoculation site, which expands and ulcerates producing a usually solitary painless round/oval chancre 1–2 cm in diameter with indurated margin and clear, moist base, and serous exudate on pressure ( Plate 7), but may be atypical or ‘contemporary’ (
Plate 7), but may be atypical or ‘contemporary’ ( Plate 8). Bilateral painless regional lymphadenopathy, e.g. inguinal if genital lesion. In recent years, it has become more common to find multiple painful ulcers with little induration, mimicking herpes infection. Oral sex is a significant route of transmission with subsequent oropharyngeal ulceration.
Plate 8). Bilateral painless regional lymphadenopathy, e.g. inguinal if genital lesion. In recent years, it has become more common to find multiple painful ulcers with little induration, mimicking herpes infection. Oral sex is a significant route of transmission with subsequent oropharyngeal ulceration.
• Chancre site: Genital chancre may appear anywhere on genitals, but commonly on mucosal surfaces. Cervical lesions are usually asymptomatic and do not produce inguinal lymphadenopathy. Rarely intra-urethral chancres can present as urethritis. Balanitis has also been reported (rare, including balanitis of Follman). Extragenital chancres are common and have been reported on lips, tongue, tonsils, pharynx, anal margin (painful, resembling anal fissure), rectum, and rarely finger, hand/arm, nipple, eyelid, and supraclavicular.
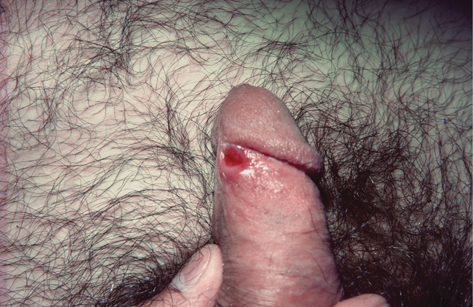
Plate 7 Classic chancre (7).
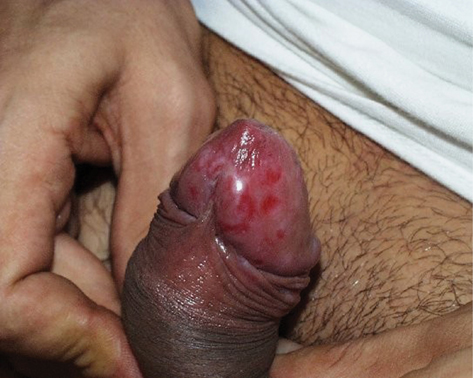
Plate 8 ‘Contemporary’ chancre (7).
Symptoms are a result of haematogenous dissemination of infection.
• Constitutional symptoms of malaise, fever, headache, anorexia, myalgia.
• Skin lesions may be polymorphic (80%), pruritic (40%):
• Macular—pink 1 cm diameter, mostly on trunk.
• Papular—dull red with shiny surface or papulosquamous with surface scale, often extensive, affecting flexor surfaces, palms, and soles (firm non-prominent papules). ( Plate 9).
Plate 9).
• Pustular and hyper- or hypo-pigmented lesions also been seen.
• Lympadenopathy: 75% inguinal, 60% generalized, splenomegaly.
• Mucous membrane lesions (30%) ‘mucous patch’: ulcer with white/grey border may coalesce or form snail track ulcer, found in oral cavity, larynx (sore throat, hoarse), nasal mucosa, genitalia, anus, rectum (diffuse distal proctitis).
• Alopecia: specific ‘moth eaten’ or non-specific diffuse telogen effluvium.
• Musculoskeletal: periostitis (25% pre-antibiotic era) causing bone pain, especially tibia, and bursitis (6%), causing arthralgia.
• Hepatitis (20%): usually subclinical raised enzymes, mainly alkaline phosphatase.
• Renal: glomerulonephritis (rare), nephrotic syndrome. Both mild and self-limiting.
• Neurological: meningism (1–2%), transitory cerebrospinal fluid (CSF) white cell and protein  (5–40%), rarely meningitis/meningovasculitis, nerve deafness, peripheral neuropathy.
(5–40%), rarely meningitis/meningovasculitis, nerve deafness, peripheral neuropathy.
• Eyes: iritis (<1%), anterior uveitis ( in HIV infection), choroidoretinitis, optic atrophy (usually asymptomatic).
in HIV infection), choroidoretinitis, optic atrophy (usually asymptomatic).
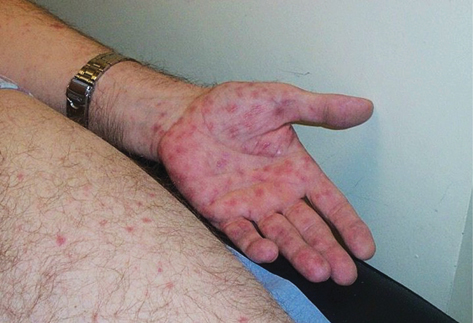
Plate 9 Secondary syphilis (7).
No signs/symptoms, but positive serology within 2 years of infection.
• Pityriasis rosea (has initial herald patch).
• Tinea/pityriasis versicolor (hypo- or hyperpigmented scaly, mostly over trunk. Culture scales for Malassezia furfur).
• Measles (oral Koplik spots).
• Rubella (posterior cervical lymphadenopathy).
• Infectious mononucleosis [may be associated with false positive VDRL/RPR (Venereal Disease Research Laboratory/rapid plasma reagin).
• Drug reaction (association with drug administration, pruritus).
• Psoriasis (extensor surfaces, knees, elbows, scalp, nail pitting).
No signs or symptoms, but positive syphilis serology >2 years after acquisition. More common now as active late syphilis  due to widespread use of antibiotics for other infections.
due to widespread use of antibiotics for other infections.
Gumma formation (syphilitic granulation tissue) is due to reactivation of residual treponemes in sensitized host. Gummata are nodules or nodulo-ulcers, indurated and indolent, single or few in number. They commonly heal with central scarring, while peripherally still active. Ulcers are described as ‘punched out’ with a basal ‘wash leather’ appearance due to slough. They are not contagious and resolve with treatment.
• Skin: especially below knee, buttocks, thighs, shoulders, scalp, face.
• Bones: gummatous periostitis (bony proliferation), e.g. sabre tibia; gummatous osteitis with bone destruction.
• Mouth and throat: palatal perforation; gumma of tongue or superfi-cial glossitis, associated with leucoplakia and malignancy; epiglot-tis destruction and laryngeal infiltration causing hoarseness.
• Other organs: gummata of liver, testis, oesophagus, stomach, intes-tine, cerebrum, spinal cord, aortic wall, myocardium. Also reported in bronchi and lungs, kidney, bladder adrenal glands, and breast.
• Conduction defects: if gummatous involvement (e.g. Stokes–Adams syndrome).
• Aortic aneurysm—proximal ascending aorta, fusiform, or saccular, without dissection. Presents as chest pain or signs of compression of adjacent structures, e.g. hoarseness, dysphagia.
• Aortic regurgitation (30% of those with cardiovascular syphilis)—insidious, so well compensated. Typical early diastolic murmur on forced expiration with patient inclined forward.
• Coronary ostia stenosis leading to angina and heart failure.
• Asymptomatic: just abnormal CSF findings. Found in up to 30% of 1° and 2° syphilis, and usually does not become clinically relevant. In the pre-antibiotic era 23–87% of cases progressed to clinical neuro-syphilis.
• Meningovascular: focal arteritis causing infarction and meningeal inflammation. Now the most common neurosyphilitic presentation. Consider if cerebrovascular accident in a young adult. Often sudden onset, preceded by prodromal headache, insomnia, emotional lability, and mental deterioration. Hemiplegia/paresis, aphasia, seizures are typical features, but ocular palsy and trigeminal neuralgia may occur.
• Pupillary abnormalities are frequent with a full Argyll Robertson pupil (constriction on accommodation, not to light) in 10%.
• General paralysis: cortical neuronal loss. Insidious, dulling of intellect, judgement, and insight. Memory loss, antisocial behaviour, grandiose delusions (rare), hand and facial tremor, depression, and dementia. Seizures and paresis (spastic) are late complications. Full Argyll Robertson pupil in 25%.
• Tabes dorsalis: selective inflammation and degeneration of the spinal dorsal columns and nerve roots. Lightning pains, paraesthesia, visceral crises (smooth muscle spasm), sensory ataxia with stamping gait, and positive Romberg sign. Diminished or absent reflexes, deep pain, vibration, and position sense. Trophic changes lead to neuropathic joints (Charcot) and painless perforating plantar ulcers. Optic atrophy and bilateral ptosis are common. Argyll Robertson pupil seen most commonly in tabes dorsalis, with at least 80% developing pupil abnormalities.
All pregnant ♀ should be screened for syphilis at initial booking visit. If new risk factors or symptoms become apparent, tests should be repeated later in pregnancy, with referral to Sexual Health services. Syphilis can be transmitted at any stage of pregnancy, with foetal infection reported from 9th week of gestation. However, it is most likely to arise after the 18th week. It may result in polyhydramnios, preterm labour, hydrops, congenital syphilis, miscarriage, and stillbirth, with spontaneous abortion most commonly occurring in 2nd and early 3rd trimester. Outcome in untreated infection varies with stage.
• 1° or 2° syphilis: up to 50% prematurity/perinatal death and 50% congenital infection.
• Early latent syphilis: up to 40% prematurity/perinatal death and 20% congenital infection.
• Late latent syphilis: up to 20% prematurity/perinatal death and 10% congenital infection.
• Normal control: 9% prematurity/perinatal death.
Despite treatment in early syphilis up to 14% may result in foetal death or congenital infection.
If untreated syphilis is detected, consideration should be given to testing children from earlier pregnancies.
Two-thirds are asymptomatic at birth, but most develop signs by 2–12 weeks after birth. Features include:
• snuffles: mucosal lesions with pharyngeal and nasal involvement progressing to local destruction and perforation
• rashes similar to 2° syphilis, but prominent around the mouth and body orifices leading to scarring (rhagades)
• blistering bullous eruption of palms and soles (syphilitic pemphi-gus)
• condylomata lata around anus and genitalia (see Plate 10)
• sparse hair and brittle, atrophic nails
• hepatosplenomegaly and moderate generalized lymphadenopathy
• osteochondritis and later periostitis especially of the long bones (may present as pseudoparalysis)
• other features may include meningitis, nephrotic syndrome, choroidoretinitis, anaemia, thrombocytopenia.
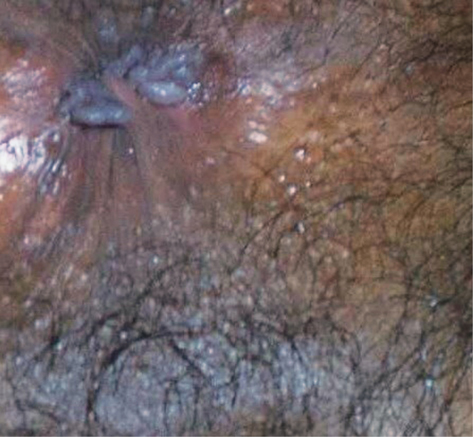
Plate 10 Condolymata lata (7).
No clinical features in about 60% (diagnosed on serology). Otherwise, many features are similar to late-stage-acquired syphilis (although the cardiovascular system is usually spared) with clinical manifestations usually appearing around the time of puberty.
Interstitial keratitis: most common late feature, 20–50%.
• Deafness 2° to otolabyrinthitis.
• Clutton’s joints: bilateral painless effusion of the knee joints.
• Gummata of nasal septum, palate and throat; skin and bone.
• Neurosyphilis: seizures, mental deficiency, juvenile tabes dorsalis and general paralysis.
• Paroxysmal cold haemoglobinuria.
• Craniofacial: frontal bossing; ‘bulldog’ appearance—hypoplastic maxilla, high-arched palate, and prominent mandible; ‘saddle-nose’; rhagades (peri-oral fissures).
• Hutchinson’s incisors (usually upper central)—conical, tapered towards the apex and notched.
• Hutchinson’s triad—Hutchinson’s incisors, interstitial keratitis, and nerve deafness.
• Mulberry molars—1st lower molars dome-shaped with hypoplastic cusps.
• Skeletal: bony sclerosis (generalized) or nodules (localized). Long bones primarily affected, especially the tibia with anterior bowing (‘sabre tibia’).
Syphilis in pregnancy is associated with a high risk of spontaneous abortion, premature delivery, perinatal death, and congenital syphilis. Risks are greater with early syphilis. Despite treatment of early syphilis during pregnancy, up to 14% will have a foetal death or a baby with congenital syphilis. Early congenital syphilis occurs within the first 2 years of life, late congenital manifestations occur after 2 years of age.
 Chapter 5, ‘Slide preparation’, p. 70.
Chapter 5, ‘Slide preparation’, p. 70.
• 1° syphilis: serum from chancres (79–86% sensitivity)—less reliable for non-genital lesions, especially oral, because of other treponemes.
• Aspiration of a regional lymph node (especially if the chancre is secondarily infected).
• 1° and early congenital syphilis: serum from mucous patches, ulcers, and condylomata lata.
• Treponemes are very slender with tight spirals moving forwards and backwards, rotating about longitudinal axis and angulating to ~90°.
• If negative and there is clinical suspicion, send for syphilis PCR and early repeat serology.
• If 2° infection of ulcer, saline lavage or antibiotics inactive against T. pallidum to clear contaminants (e.g. co-trimoxazole, quinolones).
See Table 7.1.
Table 7.1 Syphilis serological response: confirm with second sample!
| Stage | EIA | TPPA | VDRL/RPR | IgM |
| Primary | +ve (90%) | +ve (90–100%) | +ve (60–90%) | +ve |
| Usual titre 80–320 | Usual titre Neat-1:16 | |||
| Secondary | +ve | +ve | +ve | +ve |
| High titres ~5120 | Usual titre 1:32–1:256 | |||
| Early latent | +ve | +ve | +ve | Usually +ve |
| Titres still usually high | Usual titre 1:16–1:64 | |||
| Late syphilis | +ve | +ve | +ve (50–65%) | Usually –ve |
| Low titres (80–640) unless active syphilis | ||||
| Old treated syphilis | Usually +ve | Usually +ve | Usually –ve or very low titre | –ve (unless recently treated) |
| Congenital syphilis | The demonstration of IgM is important as it does not cross the placenta and usually represents active infection. May take up to 3 months to appear. | |||
Positive serology usually found 4 weeks after infection, but may take up to 3 months to develop. Negative in up to 15% of those with chancre. Similar response with endemic treponematoses ( Chapter 19, ‘Endemic syphilis (bejel, dichuchwa)’, p. 258). Positive results should always be confirmed by testing a second sample.
Chapter 19, ‘Endemic syphilis (bejel, dichuchwa)’, p. 258). Positive results should always be confirmed by testing a second sample.
E.g. VDRL test, RPR test. It is inexpensive, readily quantifiable, and useful in assessing serial titres, but has potential for biological false positives (Box 7.2), and poor sensitivity in late syphilis. Usually positive 4 weeks after infection. Prozone phenomenon (false-negative results from strongly positive samples due to blocking antibodies) is excluded by specimen dilution.
Box 7.2 Biological false-positive reactions
VDRL/RPR (biological) tests have false positive reaction in <1% of the general population.
• Acute (lasting < 6 months): usually <30 years of age:
• acute febrile illnesses (e.g. EBV, HIV, viral hepatitis).
• Chronic (persists >6 months): usually >30 years of age:
• chronic infections (e.g. leprosy)
• autoimmune conditions (e.g. lupus erythematosus) injecting drug use.
• Agglutination: T. pallidum particle assay (TPPA) is now recommended in preference to the TP haemagglutination assay (TPHA) or microhaemagglutination assay for TP (MHA-TP), as it is more sensitive in 1° infection. Titre does not correlate with disease activity or treatment response, but distinguishes weak from strong reactivity.
• Enzyme immunoassay: simple, can be automated, widely used for screening and confirmation. EIAs that detect both IgG (4–5
weeks post-infection) and immunoglobulin M (IgM; 2–3 weeks post-infection) recommended as more sensitive for 1° syphilis.
• Specific antitreponemal IgM detection (EIA): usually first serological response. May remain reactive for 1–2 years. Important in diagnosing early congenital syphilis.
• Immunoblot test (also known as Western blot): antibody detection by response to recombinant antigens. Used in cases with discrepant isolated EIA and TPPA results.
• Fluorescent treponemal antibody absorbed (FTA abs) test: previously used in cases of discrepant EIA and TPPA results. No longer recommended. Has been replaced by immunoblot.
• EIA (ideally IgM plus IgG) or TPPA recommended for routine screening.
• If  syphilis risk (e.g. contact, suspicious oro-anogenital ulceration), request anti-treponemal IgM as well.
syphilis risk (e.g. contact, suspicious oro-anogenital ulceration), request anti-treponemal IgM as well.
• Rapid POCTs (EIA +/– non-treponemal test) are available. Used in outreach projects or field conditions in developing countries.
• Quantitative TPPA should be used to confirm +ve EIA.
• EIA should be used to confirm +ve TPPA.
• If discrepant results, test with IgG immunoblot.
• Quantitative VDRL/RPR testing and EIA IgM should be performed prior to treatment if screening tests positive.
• VDRL/RPR >1:16 suggests active syphilis.
• In patients previously treated for syphilis a 4-fold  VDRL titre and/or confirmed change in IgM to +ve suggests reinfection/relapse.
VDRL titre and/or confirmed change in IgM to +ve suggests reinfection/relapse.
FTA abs is not recommended, but sometimes used in specialist labs.
Can be performed on lesions of 1° or 2° syphilis, CSF, vitreous, blood. Most useful in assessing oral or other lesions, where contamination with commensal treponemes is likely (rendering dark ground microscopy unhelpful).
• Sensitivities: primary chancre 78%, plasma 39–55%, CSF 6–63%
• Specificities: primary chancre 96.6%, plasma 83–98%, CSF 85–96%
• Multiplex PCR has lower sensitivity and high HSV loads could lead to a false-negative PCR for T. pallidum.
• Recommended if neurosyphilis or treatment failure suspected.
• Serum RPR >1:32 after treatment predictive of neurosyphilis.
• CSF tests better at excluding than diagnosing.
• CT head if abnormal neurology or clinical evidence of  intracranial pressure prior to lumbar puncture.
intracranial pressure prior to lumbar puncture.
• CSF white cell count >5 × 106/L in the majority of symptomatic neurosyphilis, or >20 if HIV+ or 6–20 if HIV+ on ART with VL<20 or CD4>200, supports a diagnosis of neurosyphilis.
• CSF protein >0.45 g/L usually seen in neurosyphilis.
• CSF VDRL/RPR (without blood contamination):
• Sensitivity depends on stage and symptomatology—varies from10% (asymptomatic) to 90% (symptomatic).
• Positive result in symptomatic patient considered diagnostic.
• CSF treponemal tests have high specificity, but low sensitivity:
• low specificity due to transudation from serum
• highly sensitive, so negative test makes neurosyphilis unlikely
• CSF TPPA >1:320 supports diagnosis of neurosyphilis.
• PCR has poor sensitivity and specificity so is unhelpful.
See Table 7.2 for CSF results supporting neurosyphilis diagnosis.
Table 7.2 CSF results supporting diagnosis of neurosyphilis
| CSF parameter | HIV –ve patients | HIV +ve patients |
| WBC | >5 µL | >20 µL or 6–20 µL if on ART/CD4 > 200 and VL < 20 |
| Protein | >0.45 g/L | >0.45 g/L |
| RPR/VDRL | Positive | Positive |
| TPPA | >1:320 | >1:320 |
• Biopsy and histology: for gummata, e.g. to exclude malignancy.
• X-ray: not recommended routinely, but as part of full assessment according to signs and symptoms:
• Cardiovascular—aortic dilatation with linear ‘egg-shell’ calcification.
• Tabes dorsalis—neuropathic joints (bone destruction, osteophytes).
• Bone gummata—osteomyelitic lesions sometimes hidden by reactive osteosclerosis; periosteal thickening.
• Early congenital—periostitis (new bone formation), metaphyseal calcification, dactylitis (spindle-shaped finger swelling).
• CT angiography: cardiovascular syphilis (level of ventricular reflux).
• Ophthalmic slit lamp examination: if eye pathology suspected.
• Neurological imaging: consider CT or MRI if neurological clinical features and certainly before lumbar puncture, if risk of  intracranial pressure.
intracranial pressure.
• Ultrasonography: to identify intrauterine congenital syphilis. Signs include hepatomegaly, splenomegaly, placentomegaly, scalp oedema, polyhydramnios.
All infants born to mothers with active syphilis during pregnancy require thorough clinical assessment with following investigations:
• Dark ground microscopy: e.g. from suspect lesions, nasal discharge.
• Infant serum (NB: not cord blood): should be tested for syphilis IgM and VDRL/RPR, in parallel with maternal serum if positive due to passive transfer of maternal treponemal IgG and non-treponemal antibodies, i.e. RPR/VDRL.
• Positive IgM EIA test ± sustained VDRL/RPR titres >4 times maternal level diagnostic of congenital infection (confirmed on repeat testing). Further investigations should include full blood count (FBC), electrolytes, liver function tests (LFTs), long-bone X-rays, and ophthalmic assessment.
• If IgM EIA –ve, VDRL/RPR titre <4 times maternal level, and no signs of congenital syphilis, repeat IgM and RPR/VDRL 3-monthly until –ve. No further follow-up once –ve.
• RPR/VDRL usually –ve by 6–12 months.
Offer screening for other STIs, including HIV. Examine for signs of syphilis according to history. Give clear explanation of their diagnosis. Advise to abstain from sex until symptoms resolved or 2 weeks after treatment complete
Optimal treponemal antibody sensitivity occurs during bacterial division (every 33 hours). Penicillin (1st line treatment) levels must be >0.018 mg/L for 7–10 days for early syphilis and 14–21 days for late syphilis. Desensitization should be considered as an option in those with penicillin allergy. Antibiotic free time or suboptimal levels should not exceed 24–30 hours.
• To reduce the pain associated with benzathine benzylpenicillin IM injections using 1% lidocaine as a diluent is recommended. Reconstitute vial of antibiotic with 8 mL of 1% lidocaine solution, divide resultant solution into two equal volumes, and administer by deep IM injection into two different sites, usually both gluteal muscles.
• If drug administration is interrupted for more than 1 day at any point during treatment course, the entire course should be re-started.
Benzathine benzylpenicillin 2.4 MU IM, single dose.
• Procaine benzylpenicillin 600,000 units IM daily for 10 days.
• Doxycycline 100 mg oral bd for 14 days.
• Ceftriaxone 500 mg IM daily for 10 days if no penicillin anaphylaxis.
• Amoxicillin 500 mg oral qds plus probenecid 500 mg oral qds for 14 days.
• Azithromycin 2 g od or 500 mg od for 10 days.
• Erythromycin 500 mg oral qds for 14 days (poor CSF and placental penetration).
▶ Treatment failure with macrolides reported so only use if no alternative, with close follow up.
▶ If ophthalmic involvement, treat as for neurosyphilis.
Benzathine benzylpenicillin 2.4 MU IM for 3 doses on days 1, 8, 15.
▶ For cardiovascular syphilis give 40–60 mg prednisolone daily for 3 days starting 24 hours before anti-treponemal antibiotics.
Doxycycline 100 mg oral bd for 28 days.
Amoxicillin 2 g oral tid plus probenecid 500 mg oral qds for 28 days
Procaine benzylpenicillin 1.8–2.4 MU IM daily plus oral probenecid 500 mg qds for 14 days (Box 7.3).
Benzylpenicillin 1.8–2.4 g IV 4-hourly for 14 days.
▶ Prednisolone 40–60 mg daily for 3 days should be started 24 hours before initiation of anti-treponemal antibiotics for neurosyphilis
Doxycycline 200 mg oral bd for 28 days.
Amoxicillin 2 g oral tid plus probenecid 500 mg oral qds for 28 days
Ceftriaxone 2 g IV (diluted in water) or IM (diluted in lidocaine) daily for 10–14 days (if no penicillin anaphylaxis).
Benzathine and procaine penicillins and probenecid are unlicensed in the UK. Practically, this means:
• use is justified by the clinical condition (recommended treatment)
• prescribers hold legal responsibility for the prescription
• the patient should be informed that the medication is unlicensed
• any adverse reactions should be reported via yellow card scheme.
Box 7.3 How to administer procaine benzylpenicillin
UK availability limited but may be imported as 1.2 MU/vial.
To produce standard dose of 1.8 MU:
• Reconstitute the powder in each vial with the 5 mL 1% lidocaine, making ~2 × 6mL = 2 × 1.2 MU procaine benzylpenicillin.
• After placing needle, aspirate to avoid inadvertent IV injection.
Inject 4.5 mL (0.9 MU) in each buttock =1.8 MU in total.
Timely referral to a sexual health physician is essential, with clear communication between sexual health, obstetrics, and midwifery, GP, paediatrics, and foetal medicine (if indicated), with consent.
• Pro forma for syphilis in pregnancy and birth plan are recommended.
• Early syphilis: as for non-pregnant in 1st and 2nd trimester with a second dose of benzathine benzylpenicillin a week later if treated after 28 weeks gestation (physiological changes in pregnancy alter drug concentrations). If second line treatment used, congenital infection has been reported with macrolides, so neonatal treatment is required after delivery. Doxycycline contra-indicated.
• Late syphilis: as for non-pregnant patients, except procaine benzylpenicillin 600,000 units IM daily for 10 days instead of doxycycline.
• Previously treated syphilis: consider retreatment if doubt about initial treatment, high re-infection risk, 4-fold drop in VDRL/RPR not achieved, serofast VDRL/RPR >1:8.
• Refer to foetal medicine if diagnosed after 26 weeks gestation for evaluation of foetal involvement and assessment of foetal distress during the first 24 hours ( if foetus has stigmata of congenital syphilis).
if foetus has stigmata of congenital syphilis).
• Jarisch–Herxheimer reaction may cause uterine contractions, foetal distress, and preterm labour, especially if the foetus is infected. No evidence that steroid administration reduces these complications.
• Serological cure may take several months and may not be demonstrated prior to delivery. Repeat serology in third trimester to assess fall in titres gives some reassurance.
• Treatment for congenital syphilis should be provided if:
• suspected or serological evidence of congenital syphilis
• mother has untreated syphilis
• mother treated <4 weeks prior to delivery
• mother treated with non-penicillin regimens
• Special care required if maternal serology remains serofast, especially if re-infection risk.
• First-line treatment: benzylpenicillin 30 mg/kg IV daily12-hourly in first 7 days of life, then 8-hourly, for a total of 10 days.
• Alternative: procaine benzylpenicillin 50,000 units/kg IM daily for 10 days.
Patients should be warned about potential adverse side effects,
• Facilities to manage anaphylaxis should be available.
• Consider desensitization for those with history of penicillin allergy.
A febrile illness developing within 4 hours of antibiotic treatment and resolving within 24 hours. Most common in 2° (~75%) and 1° (~50%) syphilis. Rare in late syphilis, but potentially life-threatening if strategic sites involved (e.g. coronary ostia, larynx, nervous system).
• Features: myalgia, rigors/chills, flush/fever/hypotension/deterioration of clinical lesions (therapeutic paradox), then resolution.
• Management: warn and reassure, bed rest, aspirin/paracetamol. If involvement of coronary ostia, larynx, optic neuritis, uveitis, nerve deafness (severe deterioration), and pregnancy (especially if foetal infection) special care is required. Steroid therapy initiated 24 hours before anti-treponemal antibiotics in the following situations (see also ‘Late latent, cardiovasular and gummatous syphilis’, p. 130, ‘Neurosyphilis’, p. 131, ‘Management of positive syphilis serology in pregnancy’, pp. 131–132).
• interstitial keratitis—0.1% betamethasone eye drops
• neurological involvement—incl. optic atrophy, optic neuritis, 8th nerve deafness
• cardiac disease—e.g. involvement of coronary ostia
• laryngeal involvement—e.g. gumma
• if none of the above—give reassurance, bed rest, and antipyretics.
Characterized by fear of impending death, hallucinations, or seizures, immediately following inadvertent IV injection of procaine benzylpenicillin. Lasts <20 minutes.
Follow-up is essential in order to assess serological response and detect recurrence or reinfection. Minimum serological review at 3, 6, and 12 months, or until serofast.
Treponemal antibody tests (except IgM) often remain +ve for life. It is important to communicate this to patients (as they may be tested elsewhere) and ensure that latest results and titres are clearly documented.
• Consider re-infection or relapse if VDRL/RPR antibody titres  4-fold (or greater), ideally compared with previous samples run in parallel or clinical evidence of infection.
4-fold (or greater), ideally compared with previous samples run in parallel or clinical evidence of infection.
• CSF analysis and re-treatment recommended if VDRL/RPR titres do not have 4-fold drop in 12 months.
• Lifelong annual syphilis serology recommended for people with HIV.
• Primary syphilis: all sexual partners within previous 3 months.
• Secondary and early latent syphilis: consider all sexual contacts within previous 2 years, or 3 months prior to last negative screen.
• Asymptomatic contacts of early syphilis either epidemiological treatment or re-screen 12 weeks after last exposure.
• Epidemiological treatment is with benzathine benzylpenicillin 2.4MU IM single dose or doxycycline 100mg BD for 14 days or azithromycin 2g single dose
• 46-60% of contactable partners are infected.
As index case is usually not sexually infectious at diagnosis an estimate of when the infection was acquired should be made (e.g. from previous negative serology) and contacts from within 2 years of this time notified. However, vertical infection, although most common in early syphilis, can occur for at least 10 years after infection. Therefore it is recommended that where prior serology is unavailable all children of women with late latent syphilis are screened.
• Temporary  of CD4 count and
of CD4 count and  viral load and shedding with new syphilis infection.
viral load and shedding with new syphilis infection.
•  risk of HIV acquisition with infectious syphilis 3–5-fold.
risk of HIV acquisition with infectious syphilis 3–5-fold.
• Maternal co-infection with HIV may  transmission risk of syphilis.
transmission risk of syphilis.
• Increased incidence of neurosyphilis in HIV-infected people
• CSF abnormalities  in people with HIV especially if not using ART and CD4<200 or detectable VL even in absence of syphilis.
in people with HIV especially if not using ART and CD4<200 or detectable VL even in absence of syphilis.
• Patients with CD4 <350 and VDRL/RPR >1:32 more likely to have clinical and CSF evidence of neurosyphilis.
• Careful neurological examination should be performed. Lumbar puncture only if neurological abnormality, with prior head CT/MRI.
• More rapid progression to gummatous syphilis.
• Syphilis serological response is usually normal, but rarely atypical reactions arise with a tendency for VDRL/RPR titres to be lower in 1° syphilis and higher in 2° syphilis.
• Prozone phenomenon is more likely.
• Testing and treatment should be as for HIV negative, but assess and monitor closely for signs of neurosyphilis or recurrence.
• Annual syphilis screening should be offered to all patients with HIV, or more often depending on sexual history.
Further information
British Association for Sexual Health and HIV Syphilis guideline  https://www.bashh.org/guidelines
https://www.bashh.org/guidelines