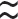 60%. Carriage of H. ducreyi without symptoms or signs has been reported in sex workers, who may be an important infection reservoir. No evidence of congenital or perinatal transmission.
60%. Carriage of H. ducreyi without symptoms or signs has been reported in sex workers, who may be an important infection reservoir. No evidence of congenital or perinatal transmission.Small Gram –ve anaerobic coccobacillus occurring in chains. Culture requires blood-enriched medium incubated in an atmosphere of 5–10% carbon dioxide. Most clinical isolates are β-lactamase producers.
Cases are now only diagnosed sporadically, even where there was a significant prevalence, such as tropical and subtropical countries (especially Africa, south-west Asia, South America, and the Caribbean). Genital ulcer disease (GUD) in these countries is commonly caused by HSV-2. Co-infections of H. ducreyi with Treponema pallidum and HSV infection found in over 10% in African studies. In UK, the majority of cases are either acquired abroad or from a partner who has been abroad. A few sporadic outbreaks were previously reported in Europe and the USA
Sexually transmitted (including oral sex), but also auto-inoculated, especially by fingers. Infection rate following single exposure from male to female  60%. Carriage of H. ducreyi without symptoms or signs has been reported in sex workers, who may be an important infection reservoir. No evidence of congenital or perinatal transmission.
60%. Carriage of H. ducreyi without symptoms or signs has been reported in sex workers, who may be an important infection reservoir. No evidence of congenital or perinatal transmission.
Associated with the prepuce (the uncircumcised twice as susceptible), sex work, crack cocaine use (in USA), HIV infection (GUD enhances the transmission of HIV).
• Incubation period: 4–7 days (range 1–14 days).
• Initial tender red papule, which progresses to a pustule then an ulcer after 2–3 days. Ulcers can be single or multiple (Plate 12), and are painful. Multiple ulcers may be facilitated by auto-inoculation—‘kissing’ lesions. Usually 1–2 cm in diameter, irregular margins, bleed on touch, non-indurated (soft chancre or sore). May coalesce into giant ulcers.
• Males—prepuce (may cause phimosis), coronal sulcus, frenulum and anus (MSM)
• Females—labia minora, fourchette, and rarely vagina, cervix, and perianal region
• Extragenital—very unusual (fingers, breasts, conjunctivae)
• Inguinal lymphadenopathy—usually unilateral occurring in  50% as a tender swelling, which may develop into a unilocular abscess (bubo) in 25%.
50% as a tender swelling, which may develop into a unilocular abscess (bubo) in 25%.
• Disseminated infection not reported.
Plate 12 Chancroid (18).
Bacterial superinfection with tissue destruction (phagedenic chancroid); chronic suppurative inguinal sinuses.
Most sensitive technique: 95% sensitive compared with culture (due to presence of polymerase inhibitors). Multiplex PCR test is available and detects H. ducreyi, T. pallidum, and HSV simultaneously.
Material obtained from ulcer base (remove superficial pus) or pus aspirated from the bubo. Many culture media are available and the use of more than one increases sensitivity to up to 80%. In endemic areas, positive culture is achievable in 60–80% of those clinically considered to have chancroid.
Low sensitivity and not recommended as a diagnostic test. Smear from cleaned ulcer or pus aspirate from bubo by rolling the swab through 180o on the slide and stain with Gram stain. Typically, small Gram +ve rods running in parallel and forming chains; ‘shoals of fish’ seen (unreliable due to bacterial contamination).
Only useful for epidemiological studies.
• Oral azithromycin 1g single dose.
• Oral ciprofloxacin 500 mg bd for 3 days (no effect against treponemes; therefore, will not mask developing syphilis).
• IM ceftriaxone 250 mg single dose.
• Oral erythromycin 500 mg qds for 7 days.
Ciprofloxacin and erythromycin are recommended for HIV +ve individuals.
Fluctuant buboes should be aspirated (by needle) from adjacent healthy skin under antibiotic cover. Patients should be re-examined 3–7 days after treatment to ensure symptomatic improvement. Re-epithelization occurs within 7 days of the onset of therapy. Resolution of fluctuant lymphadenopathy is slower than that of ulcers and may require repeated needle aspiration.
Sexual contacts within 10 days of disease onset should be examined and given epidemiological treatment as for a clinical case.
L1, L2, L3 serovars of Chlamydia trachomatis, a primitive obligatory intracellular bacterium ( Chapter 9, ‘Aetiology’, p. 150).
Chapter 9, ‘Aetiology’, p. 150).
Africa, India, Caribbean, central America, south-east Asia. However, since 2003 outbreaks and epidemics in the UK, Western Europe and America in MSM. New cases peaked in the UK in 2005, but are maintained at an endemic level (UK has highest rates in Europe).
Traditionally sexually acquired in tropical and subtropical areas. Highest rates in the 20–30-year age group and associated with sex work, multiple sexual partners, other STIs, and social deprivation. Congenital infection does not occur, but perinatal infection may be acquired from birth canal.
Western European/American cases almost entirely MSM, with high levels of co-infection with HIV (up to 80%) and other STIs, although heterosexual and female cases have been reported. Cases caused by the L2 serovar. Most infections have been acquired following local sexual contact, and factors implicated include MSM having sex at sex-on-premises venues or sex parties, Internet dating, unprotected anal sex, fisting, and use of enemas and sex toys. Mode of transmission unclear in view of the overwhelming predominance of rectal infection. Recent data indicate that new index cases report significantly fewer sexual partners, suggesting that LGV may be moving into MSM with lower risk status. In the UK, this is currently being monitored by Public Health England as an enhanced surveillance programme.
Incubation period: 3–30 days. Small painless papule at site of infection progressing to a pustule and asymptomatic ulcer that heals without scarring. Primary lesion reported by only 20–50% of men. Some ulcers in the recent MSM outbreak are indurated and painful, and durations lasting for weeks. Sites involved include coronal sulcus, prepuce, glans, scrotum, vulva, vagina, cervix (cervicitis). Extra-genital lesions such as perianal ulcers, lip and oral cavity lesions have been reported.
LGV serovars are lymphotropic infecting lymphocytes and macrophages. The pathological process is thrombolymphangitis and perilymphangitis. Lymphadenopathy follows the primary lesion by 10–30 days, rarely months. In men, usual presentation is unilateral lymphadenopathy (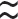 70%). Only 20–30% of women with LGV develop inguinal lymphadenopathy as lesions from the posterior vulva, anus, and vagina drain to the perirectal or pelvic nodes. Femoral nodes may also be involved (20%) and the ‘groove sign’ (enlargement of nodes above and below the inguinal ligament) is found in 15–33% (see Plate 14). Lymphadenopathy progressing to multilocular abscesses (buboes), which may rupture producing sinuses, occurs in
70%). Only 20–30% of women with LGV develop inguinal lymphadenopathy as lesions from the posterior vulva, anus, and vagina drain to the perirectal or pelvic nodes. Femoral nodes may also be involved (20%) and the ‘groove sign’ (enlargement of nodes above and below the inguinal ligament) is found in 15–33% (see Plate 14). Lymphadenopathy progressing to multilocular abscesses (buboes), which may rupture producing sinuses, occurs in  33%. Otherwise, they involute, forming firm inguinal masses. Constitutional features include fever, arthritis, aseptic meningitis, hepatitis, perihepatitis, pneumonia, erythema multiforme, and erythema nodosum.
33%. Otherwise, they involute, forming firm inguinal masses. Constitutional features include fever, arthritis, aseptic meningitis, hepatitis, perihepatitis, pneumonia, erythema multiforme, and erythema nodosum.
Plate 14 LGV “groove sign” (18).
Reprinted from Temesgen Z. (2012) Mayo Clinic Infectious Diseases Board Review © Mayo Foundation for Medical Education and Research by permission of Oxford University Press, USA.
Majority of patients recover after the secondary stage. In a few patients, infection persists or progresses in anogenital tissues leading to chronic inflammatory response with tissue destruction, manifesting as proctitis, proctocolitis mimicking Crohn’s disease, fistulae, strictures, and disfiguring fibrosis and scarring of the vulva with esthiomene (Greek word meaning ‘eating away’). In men, penile and scrotal oedema may develop causing distortion, which may produce the ‘saxophone penis”.
Tertiary complications of anorectal LGV have rarely been observed in the MSM outbreak.
Asymptomatic infection is uncommon. Most cases present with haemorrhagic proctitis following direct transmission to the rectal mucosa. Symptoms include rectal pain, anorectal bleeding, rectal discharge, tenesmus, constipation, and other symptoms of lower GI inflammation. About 25% have systemic symptoms. Genital ulcers and inguinal symptoms are rare (~2%).
Pharyngeal LGV have been reported causing ulcers and pharyngitis.
Genital lymphoedema (elephantiasis), suppurative fistulae and sinuses, rectal strictures leading to intestinal obstruction, rectal carcinoma.
Obtain samples containing cellular material from ulcer base exudate, rectal swabs and pharyngeal swabs, aspirated pus from lymph nodes or buboes and urethral swab/first catch urine. Test using:
• Nucleic acid amplification tests: If C. trachomatis detected, the specimen is tested for LGV-specific DNA.
• Cell culture from suspected LGV lesions: sensitivity  80% from ulcer,
80% from ulcer, 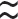 30% bubo pus. Not appropriate for urine testing.
30% bubo pus. Not appropriate for urine testing.
• Serology: less value and limited availability. Four techniques are used: complement fixation test (CFT), single L-type immunofluorescence test, micro-immunofluorescence (micro-IF) and anti-MOMP IgA assay. A 4-fold antibody rise or single-point titres of >1:64 or >1:128 for the micro-IF is considered positive as only invasive infection, such as that caused by LGV would achieve these levels. All serological tests have low sensitivities and specificities, and cannot distinguish past from current LGV infection.
• Other tests: patients must be tested for other STIs, including IHV and HCV.
• Oral doxycycline: 100 mg bd for 3 weeks
• Erythromycin: 500mg 4 times a day for 3 weeks
• Azithromycin 1 g stat and 1 g weekly for 3 weeks for rectal LGV in MSM
• Ofloxacin or Moxifloxacin for 2 weeks, expected to be effective and may be give if clinically necessary with test of cure 2 weeks after completion of therapy
• Repeat needle aspiration of buboes may be required.
As buboes are multilocular, surgical incision of fluctuant glands is contraindicated. Surgery may be required for late manifestations.
PN: sexual contacts within 30 days before the onset of symptoms or the last 3 months if asymptomatic LGV, they should be examined, tested for rectal, urethral, and cervical and pharyngeal chlamydial infection. They should receive epidemiological treatment with 3 weeks doxycycline 100 mg bd or an alternative regimen for the same duration.
• Formerly called Calymmatobacterium granulomatis.
• Human parasite (no animal model). Gram –ve pleomorphic bacterium with a well-defined capsule 1–1.5 µm long and 0.6 µm wide.
Papua New Guinea, South Africa, Southern India, and Brazil. Infection routes are unclear. Sexual transmission, especially anal intercourse, most likely, but accidental inoculation from skin contact and faecal contamination possible. Mother-to-child transmission at birth.
• Most commonly affects sexually active adults aged <30 years
• Genital infection usual (including cervix as sole site, anus with receptive intercourse)
• Associated with concurrent STIs, including HIV.
• Occurs in young children (allegedly from sitting on lap of infected adult) and the sexually inactive
• Relatively uncommon in sex workers
• Rare in partners of those with open lesions
• An outbreak has been linked to a HCP.
Incubation period: uncertain (1–360 days), probably  50 days. The genitals are affected in 90% and inguinal area in 10%. Extra-genital in 6%, including lips, cheek, palate, and pharynx. Lymphadenitis is uncommon. Haematogenous spread to bone, liver, and spleen rare.
50 days. The genitals are affected in 90% and inguinal area in 10%. Extra-genital in 6%, including lips, cheek, palate, and pharynx. Lymphadenitis is uncommon. Haematogenous spread to bone, liver, and spleen rare.
• Ulcerogranulomatous: the most common. Single or multiple, non-tender, fleshy, exuberant, beefy red ulcers that bleed easily when touched (Plate 13).
• Hypertrophic or verrucous type: an ulcer or growth with raised irregular edge, sometimes with a walnut appearance.
• Necrotic, usually deep foul smelling ulcer causing tissue destruction.
• Sclerotic with extensive scarring.
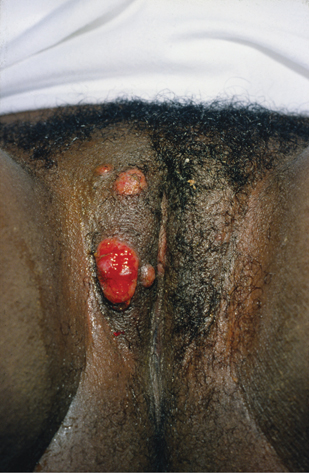
Plate 13 Granuloma inguinale (18).
Include lymphatic genital oedema (elephantiasis) in 15–20%, stenosis (anus, urethra, vagina), and local skin malignancy.
• Direct microscopy: tissue smear or biopsy from lesion stained with Giemsa stain. Donovan bodies (bipolar, resembling ‘closed safety-pins’) within mononuclear leucocytes.
• Histological examination: Donovan bodies and chronic inflammatory picture.
• PCR and culture are not routinely available.
• Recommended: oral azithromycin 1 g weekly or 500 mg od for 3 weeks, or until lesions healed completely.
• Alternative: oral co-trimoxazole 960 mg bd, doxycycline 100 mg bd, erythromycin 500 mg ×4 daily (all for 3 weeks or until lesions healed).
• Pregnancy: oral erythromycin 500 mg ×4 daily (minimum 3 weeks or until lesions healed) and Caesarean section if active cervical lesions (which may complicate delivery).
• PN: all sexual contacts in the last 6 months should have clinical examination for lesions.
• Schistosomiasis (Schistosoma haematobium): from swimming in freshwater lakes in Africa (e.g. Lake Malawi). Usual urinary symptoms include dysuria and haematuria. Other features:
• urethritis, lumpy semen, haematospermia
• friable polyps and ulcers of cervix, vagina, and vulva; groin/scrotal cutanea tarda.
• Bancroftian filariasis (E. Africa): spermatic cord inflammation (funiculitis), hydrocele, scrotal, and vulval elephantiasis.
• Onchocerciasis (tropical Africa, Central and South America): itchy papules and nodules around genitalia similar to scabies.
• Guinea worm infestation (dracunculiasis): remote parts of Africa, especially South Sudan; presents as a blister from which the worm’s head protrudes. Usually affects lower limbs, but may involve the scrotum.
• Amoebiasis (Entamoeba histolytica): especially Africa, Asia, Central and South America; peri-anal, cervical, and penile ulceration especially in MSM.
• Leishmaniasis (Middle East, Indian subcontinent): genital ulceration and annular perigenital skin lesions.
• Cutaneous larva migrans (Ancyclostoma caninum, dog hookworm), tropical and subtropical countries: usually acquired from bare skin contact (e.g. nude sunbathing) with infected tropical beaches. Pruritic erythematous papules or tracts moving several millimetres a day may be seen affecting the glutei and genitalia.
• Myiasis (Central and South America): infestation by the larvae of certain fly species, e.g. Dermatobia hominis (botfly); may rarely affect healthy external genital tissue, especially the scrotum. Presents as a nodular inflammatory lesion.
Further information
British Association for Sexual Health and HIV guidelines for Chancroid, Donovanosis and LGV  https://www.bashh.org/guidelines
https://www.bashh.org/guidelines