10 Autonomic Nervous System
10.1 The Sympathetic and Parasympathetic Nervous Systems
The autonomic, or visceral, nervous system innervates the internal organs. It is divided into three parts: the sympathetic, parasympathetic, and enteric nervous systems. For didactic reasons these systems are discussed separately; however, they represent one functional unit.
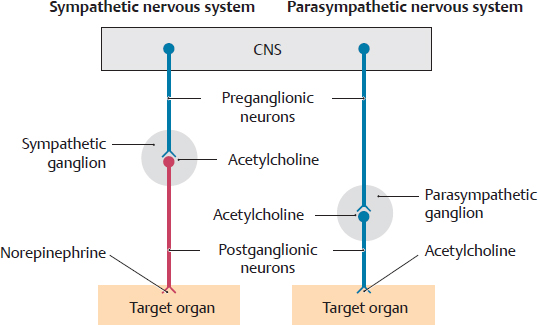
A Structure of the sympathetic (red) and parasympathetic (blue) nervous systems
Both the sympathetic and parasympathetic systems utilize a two-neuron pathway between the CNS and their targets. The first neuron is called the preganglionic neuron, and the second neuron is the postganglionic neuron. The preganglionic sympathetic neurons are located in the lateral horn of lower cervical, thoracic, and upper lumbar regions of the spinal cord. The preganglionic parasympathetic neurons are located in cranial nerve nuclei and the sacral region of the spinal cord. The vagus nerve, a cranial nerve, contains the preganglionic parasympathetic neurons that will innervate cervical, thoracic, and abdominal viscera. In both the sympathetic and parasympathetic nervous systems, the preganglionic neurons of the CNS synapse with the postganglionic neurons in ganglia of the peripheral nervous system (see C and D).
• In the sympathetic nervous system, the preganglionic neuron synapses with the postganglionic neuron in ganglia of the sympathetic trunk (for trunk and limbs), in prevertebral ganglia (for viscera) or directly in the organs (only suprarenal glands).
• In the parasympathetic nervous system, the vagus nerve terminates at ganglia close to or in the walls (intramural ganglia) of the organs.
According to Langley (1905), the terms sympathetic and parasympathetic nervous system originally referred only to efferent neurons and their axons (visceral efferent fibers, as shown above). It has now been shown that the sympathetic and parasympathetic nervous systems contain afferent fibers (visceral afferent fibers, pain and stretch receptors not shown here, see p. 72).
B Synopsis of the sympathetic and parasympathetic nervous systems
1. The sympathetic nervous system can be considered the excitatory part of the autonomic nervous system that prepares the body for a “fight or flight” response.
2. The parasympathetic nervous system is the part of the autonomic nervous system that coordinates the “rest and digest” responses of the body.
3. Although there are separate control centers for the two divisions in the brainstem and spinal cord, they have close anatomic and functional ties in the periphery.
4. The principal transmitter at the target organ is acetylcholine in the parasympathetic nervous system and norepinephrine in the sympathetic nervous organ.
5. Stimulation of the sympathetic and parasympathetic nervous systems produces the following different effects on specific organs:
Organ |
Sympathetic nervous system |
Parasympathetic nervous system |
Heart |
Increased heart rate |
Decreased heart rate |
Lungs |
Bronchodilation and decreased bronchial secretions |
Bronchoconstriction and increased bronchial secretions |
Gastrointestinal tract |
Decreased secretions and motor activity |
Increased secretions and motor activity |
Pancreas |
Decreased endocrine and exocrine secretions |
Increased exocrine secretions |
Male genitalia |
Ejaculation |
Erection |
C Circuit diagram of the autonomic nervous system
The synapse of the central, preganglionic neuron uses acetylcholine as a transmitter in both the sympathetic and parasympathetic nervous systems (cholinergic neuron, shown in blue). In the sympathetic nervous system, the transmitter changes to norepinephrine at the synapse of the postganglionic neuron with the target organ (adrenergic neuron, shown in red), while the parasympathetic system continues to use acetylcholine at that level.
Note: Various types of receptors for acetylcholine (neurotransmitter sensors) are located in the membrane of the target cells. As a result, acetylcholine can produce a range of effects depending on the receptor type.
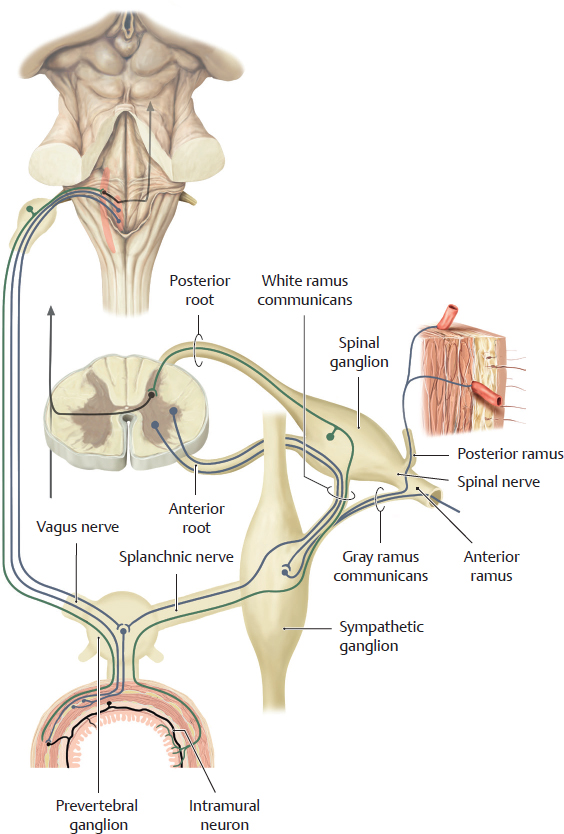
D Circuitry of the autonomic nervous system
Although the sympathetic and parasympathetic nervous systems emerge from two different regions of the CNS (see A), they form a close structural and functional unit close to the organs. The perikarya of the preganglionic sympathetic neurons are located in the lateral horn of the spinal cord. Their axons exit the spinal cord through the anterior root and travel in the white rami communicans (white because they are myelinated) to the sympathetic chain ganglia. The axons synapse with the postganglionic neurons at three different levels:
• For the sympathetic fibers going to the limbs and trunk wall, the preganglionic sympathetic neurons synapse with the postganglionic neurons in the sympathetic chain ganglia. The postganglionic fibers travel in the gray rami communicans (gray because they are unmyelinated) back to the spinal nerves.
• For the sympathetic fibers going to the viscera, the preganglionic sympathetic fibers usually pass through the sympathetic chain ganglia as splanchnic nerves. They synapse with the postganglionic sympathetic neurons in ganglia close to the organs (prevertebral ganglia). From there, the postganglionic fibers travel to the organs. The sympathetic nervous system also influences the enteric nervous system, which is referred to as the third part of the autonomic nervous system (see p. 73). In the colon, for example, sympathetic fibers will contact intramural neurons of the enteric system.
• For the sympathetic fibers going to the suprarenal medulla, the preganglionic sympathetic fibers terminate on the cells of the medulla (not shown here).
The preganglionic parasympathetic neurons of the viscera located in the body cavities originate from brainstem nuclei of the vagus nerves or from sacral spinal cord levels (not shown). They synapse in ganglia that are either very close to or embedded in the organ (intramural ganglia). Afferent pain fibers (marked in green) accompany both sympathetic and parasympathetic nerve fibers. The axons of these fibers originate in pseudounipolar neurons, which are located either in spinal ganglion or in ganglia of the vagus nerves (see p. 72).
10.2 Afferent Pathways of the Autonomic Nervous System and the Enteric Nervous System
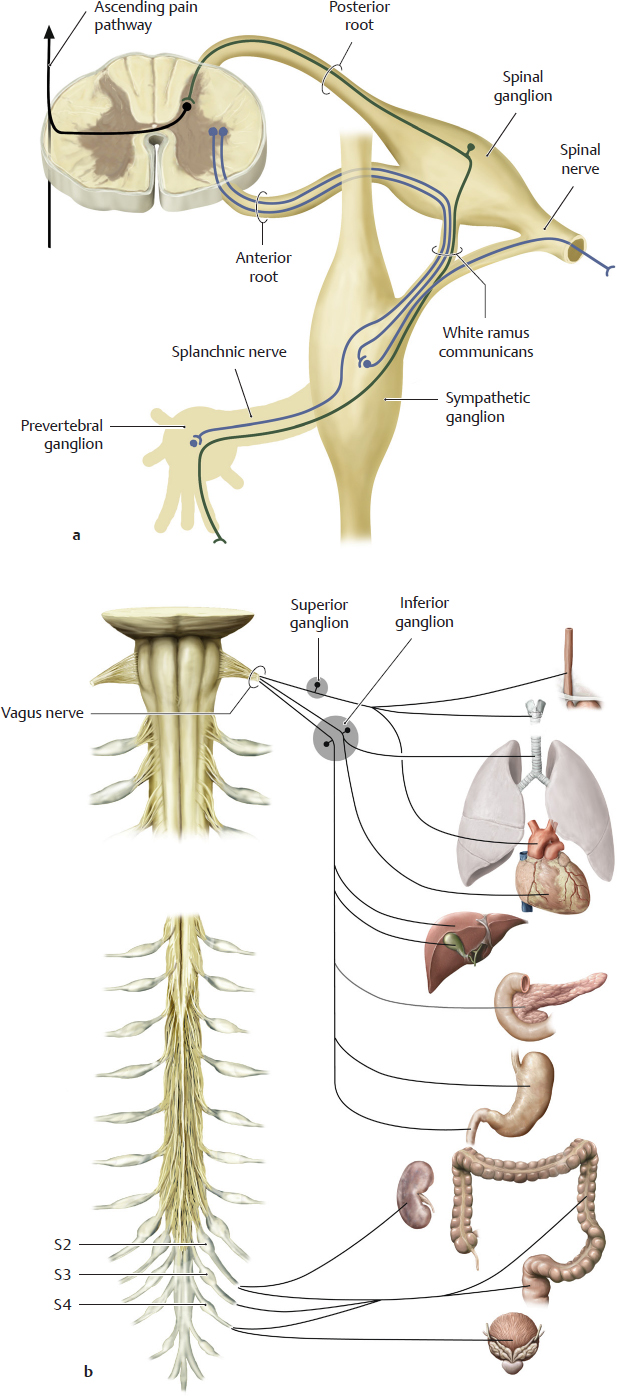
A Pain afferents from the viscera conducted by the sympathetic and parasympathetic pathways (after Jaenig)
a Sympathetic pain fibers, b Parasympathetic pain fibers.
Both nervous systems also carry axons of afferent pain fibers, which run parallel to the efferent pathways. They only make up about 5% of all afferent pain fibers and thus, quantatively, they play a minor role and become active mainly in response to organ lesions.
a The pain conducting (nociceptive) axons from the viscera run along with the splanchnic nerves to the sympathetic ganglia and reach the spinal nerves by way of the white rami communicans. They then run in the posterior roots of the spinal nerves to the spinal ganglia, where their perikarya are located. From the ganglia, their axons pass through the posterior roots to the posterior horn of the spinal cord, where they synapse and establish connections with ascending pain pathways.
Note: Unlike the efferent system, the afferent nociceptive fibers do not synapse in peripheral ganglia.
b The perikarya of the pain-conducting pseudounipolar neurons in the cranial part of the parasympathetic nervous system are located in the inferior or superior ganglia of the vagus nerves. Those of the sacral part of the parasympathetic system are located in spinal ganglia of sacral spinal cord levels S2–S4. Their fibers run parallel to the efferent vagal, or spinal, fibers and establish a central connection with the pain-processing systems.
B Referred pain
Pain afferents from the viscera (visceral pain) and dermatomes (somatic pain) terminate at the same pain processing neurons in the posterior horn of the spinal cord. The convergence of visceral and somatic afferent fibers confuses the relationship between the pain’s origin and its perception. Thus, the cortex may register pain impulses from the stomach as coming from the abdominal wall. This phenomenon is known as referred pain. The pain impulses from a particular internal organ are consistently projected to the same well-defined skin area (see dermatome levels on figure). Thus, the area of skin that the pain is projected to provides crucial information regarding what organ is affected. The skin areas to which a particular internal organ projects its pain are referred to as Head-zones, named after their discoverer, the English neurologist Sir Henry Head. This model considers only the peripheral processing of impulses, which the cortex perceives as pain. It is not clear why somatic pain is not perceived as visceral pain.
C The enteric nervous system in the small intestine
The enteric nervous system is considered a third and independent part of the autonomic nervous system (“The gut has a small brain”), and thus it is discussed separately after the sympathetic and parasympathetic nervous systems. The enteric nervous system consists of small groups of neurons that form interconnected, microscopically visible ganglia in the wall of the gut tube. The enteric system is organized into two plexuses: the myenteric plexus (Auerbach’s), located between the longitudinal and circular muscle layers of the muscularis externa, and the submucosal plexus (in the submucosa), which is subdivided into an external plexus (Schabadasch‘s) and an internal plexus (Meissner‘s). (For more details on the structure of the enteric nervous system see histology textbooks.) These networks of neurons form the basis for autonomic reflex pathways. In principle, they can function without external innervation but their activity is greatly influenced by the sympathetic and parasympathetic nervous systems. Activities regulated by the enteric nervous system include gastrointestinal motility, secretions into the gut tube and local blood flow.
10.3 Paraganglia
A Definition, function, and classification of paraganglia
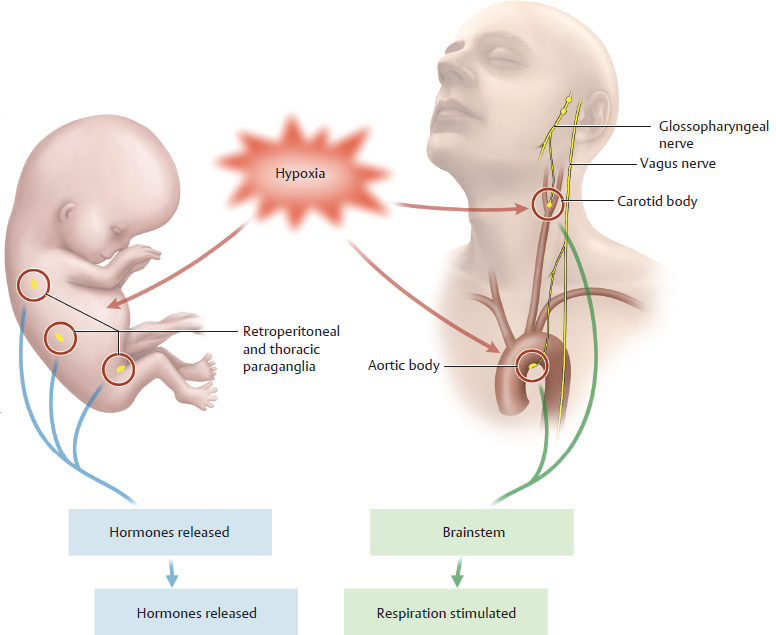
Ganglia are aggregations of nerve cell bodies in the peripheral nervous system. Paraganglia are aggregations of specialized neurons in the peripheral nervous system which in part have an endocrine function. As such they are between the nervous system and the hormone system. Measuring only a few millimeters in size, the paraganglia act as an “early warning and control system” for lack of oxygen in arterial blood (hypoxia). They continuously measure the intra-arterial partial pressure of O2 and CO2 as well as the pH value. Thus they act as chemosensors. In the fetus, paraganglia respond to hypoxia by releasing hormones that increase circulatory activity. In the fully developed body after birth, certain paraganglia respond to hypoxia by transmitting a signal to the brainstem via nerves. The brainstem reflexively increases respiration, improving the supply of oxygen. This means that paraganglia are of paramount importance for regulating the supply of oxygen in the body. Two types are differentiated according to their location in the body:
• Retroperitoneal ganglia: In the embryo and small child they are found in large numbers in the thorax and in the retroperitoneal space of the abdomen next to (= para) the ganglia of the sympathetic trunk. Such ganglia are also found in the genital region. Their number decreases markedly in the adult. In the fetal period and at birth, they respond to hypoxia by secreting the hormone norepinephrine, which increases the unborn child’s blood pressure and pulse rate. Their functional significance in adults is the subject of discussion. A comparatively large group of paraganglia ususally located at the level of the origin of the inferior mesenteric artery where it arises from the abdominal aorta are known as the organs of Zuckerkandl.
• Glomus bodies (especially the carotid body and the aortic body; glomus = ball): These extravascular structures lie at the bifurcation of the common carotid artery (carotid body; at the level of the C4 vertebra, one on each side) and at the aortic arch (usually multiple aortic bodies are present). Functionally, they are directly associated with the ninth cranial nerve (glossopharyngeal nerve; carotid body) and the tenth cranial nerve (vagus nerve; aortic bodies) through which their signal reaches the brainstem. The hypoxia signal of the glomus bodies leads to increased respiratory activity. In unborn fetuses, which do not yet have independent respiration, they have no functional significance.
• A jugular body lying near the base of the skull along the internal jugular vein is occasionally observed. Little is known about its function. It is not shown here.
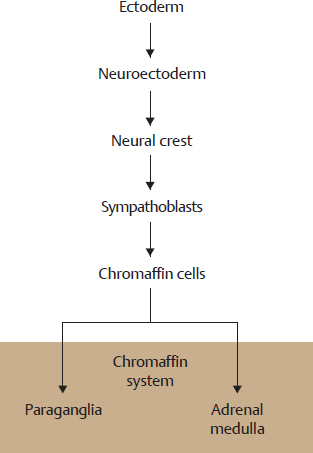
B Origin of the paraganglia
Paraganglia arise from the neural crest and thus from the neuroectoderm. They share a key histologic characteristic with the adrenal medulla, which also arises from the neural crest: so-called “chromaffin cells” (see C). Chromaffin cells contains granules (chromaffin granules) in which catecholamines (adrenaline, norepinephrine, dopamine) are stored as hormones or neurotransmitters. If one histologically fixes such cells with chromic salts, the granules (and thus indirectly the cells) stain grayish brown. The histophysiologic term chromaffin cell is a synonym for catecholamine-synthesizing cell. As both the adrenal medulla and the paraganglia synthesize catecholamines and their cells contain chromaffin granules, both of them are histologically classified as belonging to the c hromaffin system. Chromaffin cells arise in the embryo from a special neuroectodermal cell, the sympathoblast. Because of their embryonal relationship and their common histochemical characteristics, the adrenal medulla is generally understood to be a special form of paraganglia, although the literature is not consistent in this regard.
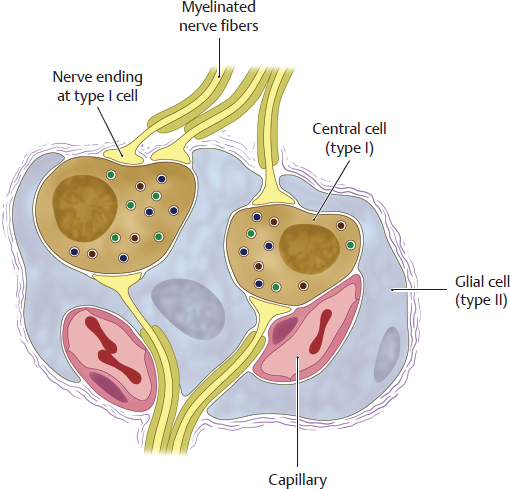
C Structure of the paraganglia
Schematic diagram of a glomus body. Paraganglia contain at least two types of cells:
• Central cells = type I cells (brown): They store catecholamines (especially dopamine), serotonin, and enkephalins in small granules and secrete the neurotransmitter dopamine. Functionally, they are the true chemosensors and morphologically they are the chromaffin cells. As secondary sensory cells, they have no axons but directly contact the afferent fibers of the glossopharyngeal or vagus nerves.
• Glial cells = type II cell (blue): They envelop the type I cells and are regarded as part of the peripheral glia. They are not chemosensitive but are thought to aid in signal transmission from the central cell to the nerve cell.
• Glomus bodies contain numerous sensory nerve endings (yellow) of the glossopharyngeal and vagus nerves and are connected via the inferior ganglion of the glossopharyngeal nerve (carotid body) or the vagus nerve (aortic body) to the nucleus of the solitary tract which in turn projects onto neurons of the respiratory center of the medulla oblongata (see D). Glomus bodies are highly vascularized with a fenestrated epithelium. Their relative perfusion is 25 times as high as that of the brain. This extreme perfusion ensures that representative quantities of blood can continuously be evaluated for their O2 and CO2 content.
D Functional synopsis of the glomus bodies
Type I cells detect a drop in intra-arterial O2 partial pressure (actual value is below target value), a drop in the pH value, or an increase in intra-arterial CO2 partial pressure. They then release the neurotransmitter dopamine from their granules. Afferent fibers of the glossopharyngeal nerve (carotid body) or the vagus nerve (aortic bodies) project via the inferior ganglion of the respective cranial nerves to the nucleus of the solitary tract which in turn activates respiratory centers in the medulla oblongata. These act on α-motor neurons in the spinal cord. The respiratory musculature is activated via the phrenic nerves. The increased respiratory activity then leads to an increase in O2 partial pressure and a decrease in CO2 partial pressure.
Retroperitoneal paraganglia in the embryo stimulate the circulatory system because it is not possible to increase respiration in an embryo (see A). Little is known about the function of the retroperitoneal paraganglia in the fully developed body.
Clinical note: Similar to the adrenal medulla, tumors can also arise from the paraganglia (pheochromocytomas or paragangliomas). Their spontaneous release of catecholamines can lead to episodic blood pressure crises. The clinical course of such tumors in children is usually unfavorable. It is also thought that spontaneously occurring “incorrect readings” of the oxygen content of the blood reported by the glomus bodies can lead to a sensation of asphyxiation and panic attacks.