8
Ascorbic acid acts at the foundation of life.
Since the identification of vitamin C as ascorbic acid and its chemical characterization in 1933, scientists have fleshed out its role in biology. This period of “normal science” has lacked drama, but the scientific advances have been substantial. Although questions remain, a host have been answered.
WHAT IS VITAMIN C?
The chemist Norman Haworth, Albert Szent-Gyorgyi’s collaborator, won the Nobel Prize in Chemistry in 1937 for finding the structure of ascorbic acid. It is a sugar-like molecule that contains six carbon, eight hydrogen, and six oxygen atoms (chemical formula C6H8O6).1 Four of the carbon atoms plus an oxygen atom form a ring, and the other two carbon atoms are in a side chain (see the following figure).Its resemblance to sugars made it difficult to purify from lemon juice, in which sugar molecules are much more abundant. This hurdle allowed Szent-Gyorgyi, who used bovine adrenal glands and Hungarian paprika as his starting materials, to leapfrog his competitors.
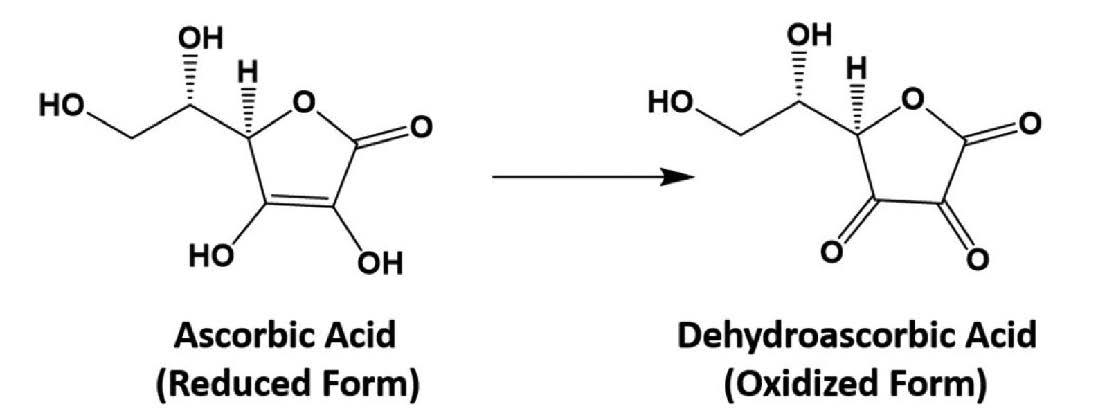
Chemical structures of the two forms of vitamin C.
Source: H. Abozenadah, A. Bishop, S. Bittner, and P. M. Flatt, “Chemistry and the Environment,” CC BY-NC-SA (2018), https://wou.edu/chemistry/courses/online-chemistry-textbooks/ch150-preparatory-chemistry/
When oxygen reacts with ascorbic acid, it removes two hydrogen atoms, forming dehydroascorbic acid. Inside cells, there are enzymes that convert dehydroascorbic acid back to ascorbic acid. Because of this interconversion, dehydroascorbic acid is also anti-scorbutic. Strictly, the term vitamin C refers to both compounds.
When ascorbic acid is not inside a cell—for example, in fruit juice or when pure ascorbic acid is in solution—oxygen in the air reacts with ascorbic acid, and the resulting dehydroascorbic acid is not converted back to ascorbic acid. Instead, it is unstable and breaks down into smaller, inactive molecules. This accounts for the loss of antiscorbutic efficacy of citrus juice in the presence of air. That loss of activity accelerates as the temperature is raised. James Lind’s method of preparing his rob—heating lemon juice in an open vessel for hours—rendered it useless to treat scurvy.
To store an ascorbic acid solution, it needs to be protected from oxygen. It should be made acidic, placed in a filled and tightly sealed container to minimize exposure to air, protected from light, and kept at a low temperature. Solid ascorbic acid, in the form of pills or powder, is stable if kept dry.
WHY ALL ANIMALS REQUIRE VITAMIN C
Ascorbic acid permits animals to live in an atmosphere that contains 20 percent oxygen.2 Oxygen is a double-edged sword for land-dwelling creatures. On one hand, they cannot live without it. Cells need oxygen to generate energy to support cellular metabolism, muscle contraction, and neuronal activity. In essence, cells burn carbon-containing foods. However, rather than the energy being dissipated as heat, it is retained to drive cellular processes. When this mechanism evolved, it permitted animals to generate sufficient energy to become mobile and manipulate their environment.
On the other hand, oxygen can be dangerous. Unregulated oxidation degrades cellular proteins, lipids, and nucleic acids.3 Not only are we exposed to atmospheric oxygen, but normal cellular metabolism produces many oxidizing agents, termed reactive oxygen intermediates (ROI). Molecules essential to cellular function must be protected from these marauding, highly reactive molecules. Among other adverse effects, oxygen may facilitate the deposition of fats in blood vessel walls and cause the degeneration of neurons. Gradual and relentless oxidation may be a component of the aging process.
The major antioxidants in cells are ascorbic acid and glutathione (GSH), a molecule comprised of a chain of three amino acids. There is about ten times more glutathione than ascorbic acid in cells, and it mainly exists in its reduced, antioxidant form.4
Glutathione acts as a loyal bodyguard by initially taking the hit and shielding essential molecules from the attack of oxidizing agents. However, ascorbic acid is the eventual fall guy when the oxygen gets passed on from oxidized glutathione. Ascorbate participates in a reaction that returns the oxidized glutathione back to its antioxidant form while ascorbic acid is oxidized to dehydroascorbate. In scurvy, when there is insufficient ascorbic acid to react with the oxidizing agents, those agents may damage tissues and account for some features of the disease.
There is one additional complication. At high concentrations—higher than exist in the body under normal circumstances—ascorbic acid may become prooxidant. Therefore it may facilitate oxidation rather than protect against it.5 This only comes into play when high doses of ascorbic acid are administered intravenously. With oral ascorbate, not enough gets absorbed from the intestine to achieve prooxidant levels in the blood. This prooxidant action of ascorbic acid may be toxic to cancer cells, and clinical trials are testing high-dose intravenous ascorbate for that purpose.
HOW THE ABILITY, AND THEN THE INABILITY, TO MAKE ASCORBIC ACID EVOLVED
The first animals that lived in the oceans did not synthesize vitamin C, and today few species of marine animals make the substance. However, soon after the first amphibians ventured onto land, they evolved that ability and passed it on their descendants, including mammals.
There are exceptions. Several animal species have lost the ability to make vitamin C.6 In addition to primates, these include guinea pigs, almost all species of bats, and some species of birds. In these animals, the gene for the final enzyme in the synthetic pathway of ascorbic acid is mutated to an inactive form. This enzyme has the catchy name L-gulano-γ-lactoneoxidase (GULO).
Inactivating mutations in the GULO gene have occurred several times during evolution and have had no deleterious effects if the diet contains the vitamin in abundance. In technical terms, the mutations are neutral. Species that are primarily vegetarian and that have access to dietary sources of abundant vitamin C can prosper despite losing the ability to synthesize the molecule, although they are susceptible to developing scurvy when the diet is deficient.
WHAT SCURVY DOES TO ANIMALS THAT CANNOT SYNTHESIZE VITAMIN C
Axel Holst and Theodor Frølich fortuitously chose guinea pigs as their experimental animals. The guinea pig lacks an active GULO protein and develops signs of scurvy when limited to a diet lacking vitamin C. They first develop swelling of joints and lie on their sides with the affected limb held in the air, keeping their weight off it. Their teeth and gums become affected, and they may press the side of their face into the floor of the cage. They have hemorrhages into their limbs, and the growing ends of the bones separate from the shafts. This last finding was crucial in identifying the condition as scurvy. James Lind and Thomas Barlow described similar abnormalities of the bones in human scurvy.
Reflecting the more rapid metabolism of small animals, the time course of the disease is compressed compared to humans. Guinea pigs develop scurvy after two weeks on a vitamin C deficient diet.
Other mammalian species that normally can synthesize ascorbic acid may undergo spontaneous mutations that inactivate the GULO gene. Two such strains of mutant rats, termed ODS and Sfx, were discovered because of bone abnormalities.7 Molecular biologists have deleted the GULO gene in mice, and these mice are now commonly used in vitamin C research. Monkeys and other apes also lack an active GULO gene, but the expense and difficulties of caring for monkeys have limited their use as experimental animals.
WHY THERE IS SO MUCH VITAMIN C IN PLANTS
All plants synthesize their own vitamin C, and many—including fruits, berries, and green vegetables—make lots of it (see appendix). One orange contains more than five times the daily amount of vitamin C necessary to prevent scurvy in an adult human.
One of its functions in plants is the same as in animals: to protect the cells from oxygen.8 Whereas animals burn carbon-containing food to produce energy and generate carbon dioxide as a waste product, plants operate in reverse. They absorb carbon dioxide from the air and use energy from sunlight to split the carbon atom from the two oxygens. The carbon gets incorporated into molecules that make up their tissues, and the oxygen is released into the air. This process is termed photosynthesis—the use of light energy to make molecules.
Within plant cells, photosynthesis takes place in chloroplasts, the intracellular organelles that contain chlorophyll and make plants green. This process generates the oxygen we breathe and, at the same time, other highly reactive oxidizing agents. Ascorbate is synthesized in the mitochondria of plants and transported into the chloroplast, where it protects essential cellular machinery from oxidation. For example, the most abundant oxidizing agent produced during photosynthesis is hydrogen peroxide. Ascorbic acid is a cofactor in an enzyme that converts the hydrogen peroxide to oxygen and water, which pass harmlessly into the atmosphere. Since ascorbic acid is present in high concentrations in the green, photosynthesizing parts of plants, it is abundant in dark green, leafy vegetables such as kale and spinach and in the leaves of trees.
Ascorbic acid is also abundant in the pulp of fruits and berries, tissues that do not engage in photosynthesis. Szent-Gyorgyi became interested in oxidation-reduction chemistry when he investigated why some fruits turn brown after exposure to the air and others keep their natural color. Scientific curiosity about a seemingly trivial question can lead to major discoveries. Szent-Gyorgyi quickly found that the fruits and vegetables that did not turn brown contained high concentrations of a reducing agent, which he eventually identified as ascorbic acid. Although that explains why lemon juice prevents a cut avocado from turning brown, Szent-Gyorgyi never explained why ascorbic acid is present in high concentrations in certain fruits but not others, and to this day that is understood only in general terms.
The fruit of a plant contains its seeds and has evolved to be tasty to animals. The animals eat the seeds along with the fruit and then spread the seeds around their territory, mainly in their feces. The antioxidant ascorbic acid in the fruit preserves its tastiness. Pure ascorbate itself has a tart taste that would be unlikely to make the fruit an inviting snack, but it can protect tastier substances, mainly sugars. Hence, vitamin C helps preserve the flavor and texture of fruits and berries. The meat of the apple, for example, oxidizes and turns brown when bruised or exposed to air. This makes the fruit less tasty, and bruised, damaged, or soft apples are avoided. Even in the supermarket, vitamin C is important in counteracting the adverse effects of oxidation.
WHAT VITAMIN C DOES WITH OXYGEN OTHER THAN FIGHT IT
Besides protecting cellular machinery from the damaging effects of oxygen, ascorbic acid also helps harness the beneficial properties of oxygen by acting as a cofactor for enzymes.9 Enzymes are proteins that catalyze biochemical reactions, and cofactors are small molecules that bind to the enzyme and allow it to exert its full catalytic activity. Ascorbic acid is a cofactor for many enzymes that contain iron or copper. These metal atoms are easily oxidized, as is seen when iron rusts or copper acquires a green patina. Ascorbic acid acts as a reducing agent to maintain the metal atoms in their active, reduced (unoxidized) state. Many of these metal-containing enzymes catalyze the transfer of oxygen atoms to proteins or other molecules in a highly regulated manner.
The role of ascorbic acid in the synthesis of collagen is of direct relevance to key features of scurvy.10 Collagen is the most abundant protein in animals, and it is the major molecule holding our bodies together. Hair and nails are mainly composed of collagen, and collagen makes skin, cartilage, and tendons tough. It helps maintain the integrity of blood vessels. Collagen synthesis is impaired in scorbutic animals. Many of the manifestations of scurvy result from defective connective tissues: soft gums and loss of teeth, bone defects, breakdown of wounds, broken blood vessels with resulting hemorrhages, and abnormal hair fibers.
Ascorbate stimulates collagen synthesis in multiple ways. The best understood is that it allows the collagen protein to attain its proper shape. Collagen is a fiber composed of three amino acid chains wound around each other to form a triple helix. For the three collagen protein chains to fold and entwine correctly, hydroxyl groups (an oxygen plus a hydrogen atom) must be attached. This reaction is catalyzed by an iron-containing enzyme that uses ascorbic acid as a cofactor.
Also of possible relevance to the symptoms of scurvy, vitamin C participates in the synthesis of carnitine, a molecule essential for muscle cells—both skeletal and cardiac muscles—to generate the energy required to contract.11 Muscles depleted of carnitine are weak. Guinea pigs placed on a vitamin C deficient diet have low carnitine levels in multiple organs, including heart and skeletal muscles. The weakness and shortness of breath, which are prominent early symptoms of scurvy, may result from a deficiency of carnitine in muscles.
Another mechanism that uses ascorbic acid is that by which cells respond to low oxygen levels.12 The discoverers of this mechanism won the Nobel Prize in Physiology or Medicine in 2019. When oxygen is abundant, an enzyme that requires ascorbic acid adds oxygen to a signaling molecule called hypoxia-inducible factor (HIF). When the oxygen is attached, it is a signal that there is plenty of oxygen. The cell does not need HIF and chews it up. This is the normal state of most of the cells in our body.
When oxygen levels are low, the oxygen cannot be added, and the HIF builds up inside the cell. It tells the cell to make more enzymes that can generate energy from glucose without needing oxygen and to use less of the machinery that requires oxygen. Without oxygen, the cell must make do with less energy than is optimal. This is stressful to the cell, and the HIF induces the cell to make proteins that help the cell to survive this stress. In GULO knockout, vitamin C deficient mice, glutathione can substitute for ascorbic acid and the mechanism is preserved.13
Vitamin C is necessary to produce many hormones. Szent-Gyorgyi found his reducing substance, which turned out to be ascorbic acid, in high concentrations in the mammalian adrenal gland. The adrenal gland sits on top of the kidney and has two layers: the core, or medulla, manufactures and secretes steroid hormones, including cortisol; the outer layer, or cortex, manufactures and secretes catecholamines, mainly epinephrine. The synthesis of both classes of hormones requires ascorbic acid as a cofactor.14 Ascorbic acid is stored within the same subcellular vesicles, the chromaffin granules, in which epinephrine is stored and secreted. Epinephrine is easily oxidized, and the ascorbate, besides participating in its synthesis, helps to maintain it in its active form.
Vitamin C is also present in high concentration in the pituitary gland, which sits at the base of the brain and secretes several “master hormones” that control the activity of other hormone-secreting organs, including the adrenal and thyroid glands. Ascorbic acid is required for the finishing step in the synthesis of several pituitary hormones.
The brain has high levels of vitamin C compared to other organs, second only to the adrenal gland. In the brain, just as in the adrenal gland, ascorbic acid is a cofactor for the synthesis of catecholamines and protects them from oxidation. In guinea pigs deprived of vitamin C, the brain and adrenal gland hold on to the vitamin more avidly than other organs, reflecting the importance of the vitamin in their function.15
VITAMIN C AND INFECTIONS
Claims that vitamin C boosts immunity to infections have generated interest in the role of vitamin C in the immune system. If the claims are true, one would expect that people with scurvy would have been prone to infections. There is little evidence of that.16 Victims dying of scurvy frequently had pneumonia terminally, but that is true of any debilitating illness.17 Wound infections were also common, but they were much more likely to have resulted from a failure of wound healing than from immune deficiency.
Two observations indirectly support a role of vitamin C in immune function. First, ascorbic acid concentrations are high in white blood cells—the blood cells that form the first line of defense against invading microorganisms.18 The concentration of ascorbate inside of the most abundant type of white blood cell, the neutrophil, is fifteen to thirty times greater than the concentration in blood plasma. The concentration is even higher in another white blood cell, the lymphocyte.
The presumed role of vitamin C in neutrophils is to act as a protective antioxidant. A key mechanism by which neutrophils kill invading bacteria and viruses is by engulfing them, walling them off in an intracellular organelle called a phagosome, and releasing a burst of oxidizing agents into the phagosome to kill the invaders.High concentrations of ascorbate protect the neutrophil from committing suicide in the process.
The second observation is that plasma and white blood cell concentrations of ascorbic acid fall in response to acute infection. In people with colds and animals injected with viruses, blood and white blood cell levels of the vitamin fall rapidly and return to normal upon recovery from the infection.
These observations have prompted a series of studies in tissue culture to examine the effects of ascorbic acid on white blood cells. Replacement of ascorbic acid has several effects on the behavior of leukocytes, which are first made deficient in the vitamin. However, white blood cells with normal stores of the vitamin do not function better if given an extra dose of the vitamin, nor do high doses of vitamin C protect animals against infection or increase the production of antibodies. So if vitamin C protects against infections, the mechanism is unknown.