Chapter 9
Neuroscience Relevant to Core Processes in Psychotherapy
Greg J. Siegle, PhD
Western Psychiatric Institute and Clinic, University of Pittsburgh, Pittsburgh
James Coan, PhD
University of Virginia
The goal of this chapter is to provide translational bridges from the common vocabulary of core processes in psychotherapy described throughout this book to neural mechanisms, which are increasingly the lingua franca of the rest of medical science. Success in this endeavor will ideally allow clinicians in the psychological sciences to speak with and make use of insights from the rest of medicine more effectively. In the short term this may also allow clinicians to put neuroscience behind their explanations of mechanisms of change for clients. In the longer term this type of thinking could lead to the adoption of neuroscience methods in predicting response to psychological treatments, and in designing treatments.
In this chapter, we focus specifically on qualitative associations of brain networks with key concepts. We have chosen this granularity as it is likely to have direct clinical applicability given the recent emphasis on brain networks in understanding change processes (Chein & Schneider, 2005; Lane, Ryan, Nadel, & Greenberg, 2014; Tryon, 2014). More quantitative associations, for example, what neural reactivity best predicts response to what treatments (e.g., Hofmann, 2013; Siegle et al., 2012), involve solving technical hurdles of generalizability and societal issues, such as expenses that insurance companies do not currently reimburse. If clinicians understand basic units and principles of neural change, and the empirical associations of these units with clinical concepts, this knowledge may change how they explain interventions to clients and add to the skills they can capitalize on in current interventions, and eventually it may lead to the adoption of more neurally informed methods (prediction algorithms and treatments) as they become available. Our methodology for identifying clinically relevant networks utilizes whole-brain, meta-analytic (hence, quantitative) procedures so that our described intuitions are at least defensible and externally derivable.
Brain Networks
Increasingly, the field of cognitive neuroscience is moving away from a focus on specific brain areas putatively associated with specific discrete functions to one of networks of linked brain regions that accomplish various behavioral or psychological functions by interacting with each other (Sporns, 2010). For example, neural circuits associated with attention may modulate activity in circuits associated with emotion such that the reactions to attended emotional stimuli are different than those to unattended emotional stimuli. In this way, clinicians and therapists can conceive of a disorder not only as the activity or inactivity of a discrete neural region or circuit, but also in terms of abnormalities of communication between brain neural regions or circuits (Cai, Chen, Szegletes, Supekar, & Menon, 2015).
Change in Brain Networks
In this chapter we adopt the idea that change processes in psychotherapy are associated with neural change, generally described as “plasticity” or “learning” in the neuroscience literature. Neural change processes follow a few principles that are useful to highlight here. Hebbian learning (Choe, 2014) is the idea that when multiple brain mechanisms are active at the same time, the connection between them grows stronger. So, for example, the association of an event with an emotional quality could happen when neural representations of the memory for an event are coactive with neural representations of an emotion. Thus activity in brain systems associated with salience and emotion along with memory could be considered catalytic for emotional associative learning. Theoretically, change in psychotherapy could occur by systematically activating the memory without the emotional tone (extinction) when either of these associations is weakened. The idea of plasticity can seem redundant with learning, but the two terms are not conceptually identical. For example, the traditional belief that memories cannot change has largely been supplanted by the understanding that every time a memory is accessed, the neural representation of the memory itself becomes plastic and can change via reconsolidation (Axmacher & Rasch, 2017). With its emphasis on building new knowledge, lay notions of learning could be an imprecise description of memory reconsolidation. The practical outcome of this new understanding is that neurally informed therapies are increasingly working to intentionally optimize memory reconsolidation processes so as to maximize the potential for psychotherapeutic gains (Treanor, Brown, Rissman, & Craske, 2017), including the potential for integrating pharmacological and therapeutic mechanisms (Lonergan, Brunet, Olivera-Figueroa, & Pitman, 2013). In the remainder of this chapter, we concentrate on the potential effects of psychotherapeutic techniques in a handful of potential networks of interest and, in particular, the potential for change to how networks interact.
Brain Networks of Particular Interest
In this chapter we concentrate on a few canonical brain networks that have been identified across many studies (e.g., Bressler & Menon, 2010; K. L. Ray et al., 2013; Smith et al., 2009). Though there are many such networks, we will highlight only those that appear repeatedly in analyses of processes associated with therapeutic change, as described in the following sections. Three networks, shown in figure 1, derived using methods described in this section and consistent with those found in more traditional analyses (such as Bressler & Menon, 2010), have been particularly well characterized across multiple imaging modalities. A salience network is associated with monitoring the salience of external and internal stimuli. It consists of the insula, which is particularly associated with interoceptive processing (Craig, 2009); the dorsal anterior cingulate cortex, which is associated with the interface of emotional and cognitive information processing (Bush, Luu, & Posner, 2000); and regions traditionally considered to process emotional information, such as the amygdala (Armony, 2013). A central executive network is associated with executive control and task planning and execution. It is anchored by the dorsolateral prefrontal cortex and posterior parietal cortices. A default network (sometimes default mode) is associated with the brain’s resting state (Raichle et al., 2001); functional neuroimaging studies suggest that it activates, or becomes better synchronized, when there is no explicit task, and deactivates during explicit tasks. Its components are often detected in association with social information processing (Amodio & Frith, 2006), as well as self-referential processing (Davey, Pujol, & Harrison, 2016; Kim, 2012). It is anchored by the posterior cingulate cortex and the rostral anterior cingulate or more anterior medial structures in the orbitofrontal cortex. It also includes the hippocampus, which appears to be particularly involved in a subnetwork for learning and memory (Kim, 2012; Van Strien, Cappaert, & Witter, 2009).
Figure 1. Neurosynth meta-analyses highlighting networks associated with the search terms “default mode” (default network; 516 studies), “salience network” (60 studies), and “executive” (executive network; 588 studies), as well as networks using the terms “social” (social information processing network; 1,000 studies) and “reward” (reward network; 671 studies)
Two other networks appear key to change in psychological interventions. Building on structures in the default network, researchers have observed that an expanded social information processing network (Burnett, Sebastian, Cohen Kadosh, & Blakemore, 2011) contains not only the rostral cingulate but structures such as the temporoparietal junction and superior temporal sulcus, suggesting they are involved in the perception of others’ emotions and theory-of-mind. Often discussed in the literature is the reward network, which is really a set of networks that largely reflect the brain’s responses to rewarding or positive stimuli. They are centered on the dopamine-producing ventral-tegmental area and reward-monitoring ventral striatum, or nucleus accumbens (Camara, Rodriguez-Fornells, Ye, & Münte, 2009).
By appealing to the putative function of these networks it is easy to speculate on how brain function may relate to specific therapeutic interventions. Interventions devoted to increasing reward responses might be expected to activate the reward network. Interventions devoted to decreasing self-focused processing might decrease activity in the default network. And interventions devoted to increasing social communication might activate the social information processing network. That said, these associations have not been rigorously tested, and brain reactions are often unintuitive. Thus, the forthcoming sections consist of empirical investigations of how these brain networks respond to the types of interventions discussed in this book.
How Brain Networks Are Involved in Psychotherapeutic Change Processes
Methods. To describe brain networks involved in the concepts discussed in this book, we used the Neurosynth engine (http://neurosynth.org; Yarkoni, Poldrack, Nichols, van Essen, & Wager, 2011) to create meta-analytic images of associated concepts. We provide basic interpretations of the derived images with respect to the aforementioned brain networks. When other functional magnetic resonance imaging (fMRI) meta-analyses of similar concepts are available, we cite them as well and discuss similarities. Our searches used terms associated with each chapter in this book. When there were enough studies to create an interpretable map for a particular therapeutic or intervention technique, we included that map. That said, in general, neuroimaging studies of therapeutic techniques are sparse and in their infancy. Thus, we primarily report on studies of associated phenomena. So, for example, rather than reporting on studies of arousal reduction, we include neuroimaging meta-analytic maps for “arousal” and interpret what the associated networks might suggest about reducing arousal.
For the interested methodologist, in all cases maps are shown for reverse inference (chances that the term is used, given the presence of activation in the area), which is more conservative than typical fMRI strategies of forward inference (chances the area is observed, given the term that is used). We chose this strategy as many psychological terms tend to yield similar broad patterns of activation—reverse inference allows more specificity of network activity related to psychological constructs. We used a false discovery rate criterion of 0.01 as a threshold for the images.
The curious reader can directly access the neuroimaging meta-analyses reported in this chapter online. When primary Neurosynth terms were available, we used those. Otherwise, we did “custom” analyses based on Neurosynth’s “studies” analyses; these can be accessed via the URLs listed in the appendix. The reader can thus regenerate any maps we describe. We generally show only a single representative axial, coronal, and sagittal image for each analysis; by directly regenerating the analyses, readers can see and interact with full brain maps slice by slice, as well as examine each associated study and its specific contributions to the meta-analysis. References for individual studies in the reported meta-analyses can be accessed by regenerating the associated searches.
Contingency management and estimation. Contingency, in the neuroimaging literature, has primarily been used to understand action contingencies—that is, what the consequences of some action or behavior will likely be. Neurosynth-nominated studies of “contingency” (figure 2; custom search URL in the appendix) were associated with increased activation in the reward network (throughout the striatum) and default network, including both ventromedial and posterior cingulate aspects. Indeed it has been increasingly understood that individuals with psychopathology estimate reward contingencies differently than healthy individuals (e.g., having decreased reactivity to temporally distant rewards in brain networks associated with reward perception; Vanyukov et al., 2016) or systematically estimate the probability of reward to be low (Olino et al., 2014). We found initial support for the idea that such associations can be exploited to yield psychological change; in the absence of other repetitive training, the ability to estimate high probabilities of reward is associated with not only decreased neural reactivity to negative information but decreased depressive symptomatology (Collier & Siegle, 2015). The described map may suggest the utility of not only explicitly managing reward contingencies but working with clients to associate reward contingencies with the types of calculations thought to be associated with the default network—which is to say, those involving self-related processing and impressions of the self with respect to others (Olino, McMakin, & Forbes, 2016). For example, one might help an individual to understand that a compliment is not just a positive outcome, but also a statement of deeper, ongoing personal (and interpersonal) relevance.
 Figure 2. Neurosynth meta-analysis of “contingency” (eight studies)
Figure 2. Neurosynth meta-analysis of “contingency” (eight studies)
Stimulus control and shaping. Generally, stimulus control and shaping techniques in psychotherapeutic processes occur in the context of manipulating associations to promote specific associative learning or to extinguish learned associations. Thus we examined neural features of associative learning, revealed by the term “associative.” Neurosynth meta-analyses of both “associative” and “learning” (figure 3) primarily revealed activation of the bilateral hippocampus and parahippocampus, which is consistent with the hippocampus’s frequently described role in indexing associative memories. To the extent that stimulus control is associated with manipulating hippocampal processes, we can see stimulus control through the lens of helping individuals to write new associative memories in place of dysfunctional associations, as well as other processes that promote clinically meaningful reconsolidation (Da Silva et al., 2007; Inaba, Kai, & Kida, 2016; Schmidt et al., 2017).
 Figure 3. Neurosynth meta-analyses of “associative” (220 studies) and “learning” (876 studies)
Figure 3. Neurosynth meta-analyses of “associative” (220 studies) and “learning” (876 studies)
Self-management. Self-management involves a wide collection of techniques unified by the idea of individuals taking responsibility for their behavior and well-being (e.g., by setting goals and managing priorities). In this sense, self-management can be seen as a combination of skills described in other sections of this chapter, such as contingency management, problem solving, and emotion regulation, with the constraint that these strategies are directed toward management of the self. Thus, we considered brain function to be specifically associated with self-processing. A Neurosynth meta-analysis of “self” (figure 4) revealed activity in the default network, which is strongly implicated, along with the superior temporal sulcus region of the social information processing network, in uncontrolled attention being paid to the self and one’s self in relation to others. By “uncontrolled,” we mean to suggest that default network processing is largely free of executive control, as measured by activity in the executive network. Indeed, default network processing is reliably inversely associated with outwardly directed attention and executive control (Uddin, Kelly, Biswal, Castellanos, & Milham, 2009). Together, these considerations suggest a fundamental tension between self (default network) and management (largely executive network) activities. Thus it may be intuitive why default network–mediated thinking about the self, particularly regarding distressing topics, can be “sticky”—that is, hard to get free of and manage. Increasing evidence suggests that default network processing is particularly competitive with executive network processing in psychopathology (Delaveau et al., 2017; Di & Biswal, 2014; Hamilton et al., 2011; Maresh, Allen, & Coan, 2014).
 Figure 4. Neurosynth meta-analysis of “self” (903 studies)
Figure 4. Neurosynth meta-analysis of “self” (903 studies)
Arousal reduction. A Neurosynth meta-analysis of “arousal” (figure 5) revealed increased activation throughout the salience network (e.g., amygdala, insula, and subgenual cingulate). Indeed, psychological disorders are often characterized by increased and sustained neural reactivity to negative information (Siegle et al., 2015), particularly in these regions. The literature suggests that reducing arousal likely involves decreasing the salience of emotional stimuli, an effect that should be reflected in diminished or inhibited salience network processing. The extensive literature showing mutual inhibition between the executive and salience networks could also speak to the potential for arousal reduction strategies to capitalize on the involvement of executive control (e.g., purposeful redirection of attention, as done in reframing; see “Values Choice and Clarification” below).
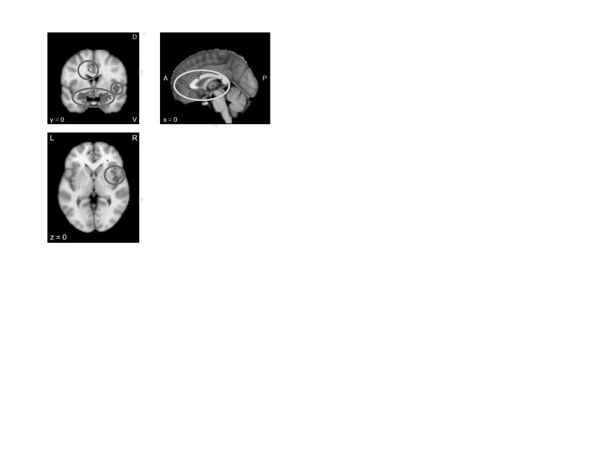 ]Figure 5. Neurosynth meta-analysis of “arousal” (227 studies)
]Figure 5. Neurosynth meta-analysis of “arousal” (227 studies)
Coping and emotion regulation. A Neurosynth meta-analysis of “emotion regulation” (figure 6) yielded activation in the salience network (particularly the amygdala but also the posterior insula) as well as the executive network, including bilateral dorsolateral prefrontal and parietal regions, but no medial prefrontal regions. Indeed activity in these two networks has been specifically associated with response to emotion regulation therapy (Fresco et al., 2017). Associations with these networks may suggest that emotion regulation involves both effortful control and active emotional processing. This formulation may be more relevant to putatively “voluntary” or effortful forms of cognitive emotion regulation (Gross & Thompson, 2007), as opposed to more “automatic” manifestations (resulting from, for example, interventions such as exposure therapy), which are likely to be mediated through more medial prefrontal activity (R. D. Ray & Zald, 2012). The disruption of salience or threat signals by executive control could help individuals override prepotent responses that would otherwise trigger uncontrolled emotional reactions.
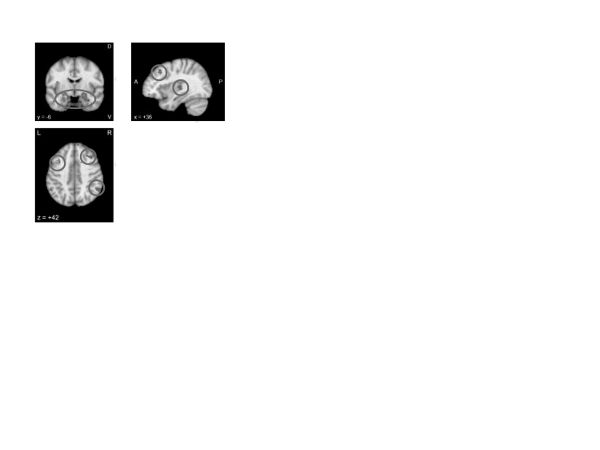 Figure 6. Neurosynth meta-analysis of “emotion regulation” (161 studies)
Figure 6. Neurosynth meta-analysis of “emotion regulation” (161 studies)
There were four Neurosynth-nominated studies of “coping,” but we didn’t report on them because they were not strongly related to therapeutic processes (e.g., two were on repressive coping style).
Problem solving. A Neurosynth meta-analysis of “problem solving” (figure 7; custom search URL in the appendix) revealed activations throughout aspects of the default network (posterior cingulate) and the rostrolateral prefrontal cortex (superior frontal gyrus)—a region strongly associated with relational integration and reasoning (Christoff et al., 2001; Davis, Goldwater, & Giron, 2017; Wendelken, Nakhabenko, Donohue, Carter, & Bunge, 2008), along with the caudate (part of the salience network), which, in combination with other regions, has also been associated with relational reasoning (Melrose, Poulin, & Stern, 2007). Taken together, these maps suggest that problem solving is likely a widely distributed activity requiring integration throughout multiple brain networks, consistent with the view that problem solving entails diverse cognitive operations, from conceptual encoding to the planning of contingencies and actions (Anderson & Fincham, 2014). Aspects of this wider network have been implicated in problem-solving failures, such as those observed in rumination in depression (Jones, Fournier, & Stone, 2017). Therapeutic interventions emphasizing problem solving may thus require the recruitment of systems associated with relating one domain to another, while preserving motivation for this type of activity.
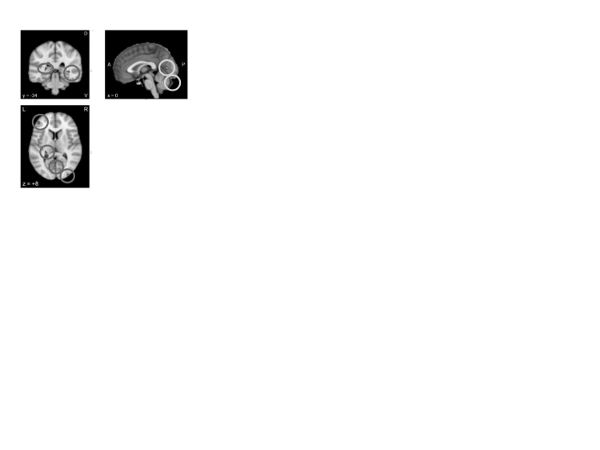 Figure 7. Neurosynth meta-analysis of “problem solving” (fifteen studies)
Figure 7. Neurosynth meta-analysis of “problem solving” (fifteen studies)
Exposure strategies. Exposure therapies generally rely on confronting individuals with situations or stimuli that they fear. While there are few neuroimaging studies of exposure per se (the Neurosynth engine has many references to “exposure” that are not relevant; e.g., drug cue exposure), the salience network was well represented in the Neurosynth meta-analysis of “fear” (figure 8), including the amygdala and dorsal anterior cingulate. It has been hypothesized that the salience network developed to prepare the brain for action in response to potential threat (Seeley et al., 2007); exposure therapies that signal a decreased need for action in response to threat likely reduce activity in this network. Contemporary investigations of pharmacological agents used to enhance exposure therapy, such as d-cycloserine (Hofmann, Mundy, & Curtiss, 2015), have shown that these drugs affect activity in the salience network (Wu et al., 2008), particularly during extinction (Portero-Tresserra, Martí-Nicolovius, Guillazo-Blanch, Boadas-Vaello, & Vale-Martínez, 2013; Wisłowska-Stanek, Lehner, Turzyńska, Sobolewska, & Płaźnik, 2010). A Neurosynth meta-analysis of “extinction” (figure 8) revealed activity in the ventromedial prefrontal cortex (vmPFC). This finding is consistent with work suggesting circuits of the vmPFC that inhibit activity in the salience network mediate the effects of exposure therapy (via extinction learning; Phelps, Delgado, Nearing, & LeDoux, 2004).
 Figure 8. Neurosynth meta-analyses of “fear” (298 studies) and “extinction” (59 studies)
Figure 8. Neurosynth meta-analyses of “fear” (298 studies) and “extinction” (59 studies)
Behavioral activation. Behavioral activation involves using goal-directed activity and reward to increase appetitive behavior and pleasure responses. Key to the success of these interventions is increasing reward anticipation. A Neurosynth meta-analysis of “reward anticipation” (figure 9) revealed activity throughout the reward network, particularly within the striatum, along with activity in the hippocampus, potentially reflecting reward associations in memory. Indeed psychopathologies such as depression are characterized by disruptions in the reward network (Smoski, Rittenberg, & Dichter, 2011) and its connectivity to other networks (Sharma et al., 2017). The reward network has long been implicated in behavioral activation (Kalivas & Nakamura, 1999). Thus, it is possible that behavioral activation therapies work to restore connections between the reward network and networks more strongly associated with intentional action.
 Figure 9. Neurosynth meta-analysis of “reward anticipation” (sixty-four studies)
Figure 9. Neurosynth meta-analysis of “reward anticipation” (sixty-four studies)
Interpersonal skills. Access to quality social relationships is a major challenge in many psychological disorders. Indeed, difficulty reading and interpreting social cues, as well as responding appropriately to those cues, could be considered defining characteristics of many personality disorders. Social cognition is a broad term encompassing everything from distinguishing self from others to identifying action intentions to detecting and assigning agency to empathizing. A Neurosynth meta-analysis of “social cognition” (figure 10) revealed activation of the central executive network (dorsolateral and anterior portions of the PFC) and default network (dorsal posterior cingulate) as well the social information processing network (fusiform gyrus and temporoparietal junction), suggesting the potential for using executive processing to modulate otherwise more automatic aspects of social perception and interaction.
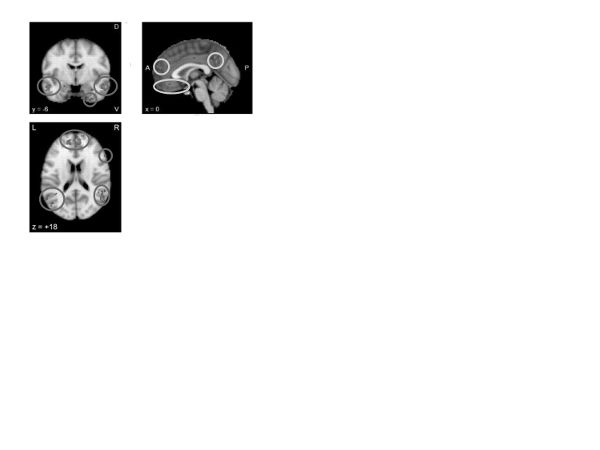 Figure 10. Neurosynth meta-analysis of “social cognition” (166 studies)
Figure 10. Neurosynth meta-analysis of “social cognition” (166 studies)
Cognitive restructuring, challenging, or reframing. Neuroimaging research has primarily studied cognitive restructuring and challenging using reappraisal designs in which participants are instructed to think differently about negative beliefs, images, or other stimuli. A Neurosynth meta-analysis of “reappraisal” (figure 11) revealed increased activation in aspects of the executive (e.g., dorsolateral prefrontal) and salience (e.g., amygdala, striatum) networks. These results largely match a recently published meta-analysis (Buhle et al., 2014; coordinates regenerated using Neurosynth), which also found deactivation in the salience network (insula, dorsal cingulate). These analyses could thus suggest that cognitive reframing/reappraisal represents an effortful but also emotional process, which appeals to voluntary cognitive, rather than body-based or more automatic, emotion regulation capabilities.
 Figure 11. Neurosynth meta-analysis of “reappraisal” (sixty-four studies) and meta-analysis by Buhle and colleagues (2014)
Figure 11. Neurosynth meta-analysis of “reappraisal” (sixty-four studies) and meta-analysis by Buhle and colleagues (2014)
Modifying core beliefs. From the reappraisal discussion above we can suggest that modifying core beliefs has elements of voluntary thought modification. The additional element of modifying core beliefs may involve other brain mechanisms. A Neurosynth meta-analysis of “belief” (figure 12) revealed activation in aspects of the default network associated with self-referential processing (BA10, posterior cingulate) and parietal aspects of the executive network. Thus, changing beliefs could be said to differ from more general thought challenging, as it involves activations and modifications of neural mechanisms of self-representation.
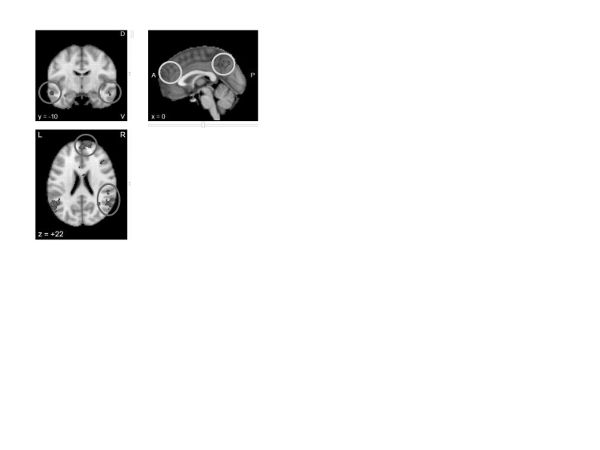 Figure 12. Neurosynth meta-analysis of “belief” (sixty-six studies)
Figure 12. Neurosynth meta-analysis of “belief” (sixty-six studies)
Defusion/distancing. To date, we are aware of a single study that investigated distancing as an emotion regulation strategy (Koenigsberg et al., 2009, 2010; Neurosynth reconstruction in figure 13); it appears none have been done that nominally reference defusion. The study considered distancing to be a special case of reappraisal, and, indeed, the same networks of the brain were activated for both the distancing and reappraisal studies.
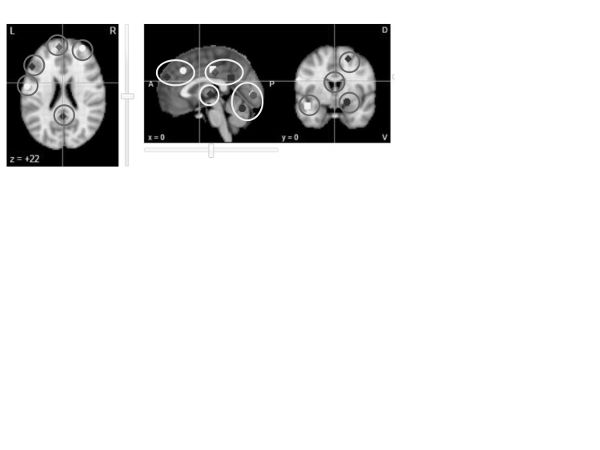 Figure 13. Neurosynth reconstruction of the map associated with “distancing” (Koenigsberg et al., 2010)
Figure 13. Neurosynth reconstruction of the map associated with “distancing” (Koenigsberg et al., 2010)
Psychological acceptance. The neuroimaging literature on psychological acceptance is sparse, with only two studies in the Neurosynth database through 2015 (Servaas et al., 2015; Smoski et al., 2015). Their aggregation (figure 14) revealed a variety of activations throughout the executive and salience networks. An additional study published after the Neurosynth database was completed (Ellard, Barlow, Whitfield-Gabrieli, Gabrieli, & Deckersbach, 2017) confirmed activations in the medial and ventrolateral frontal aspects of the executive network. To the extent that these findings are replicable, they may suggest that acceptance is an executive strategy that affects a wide range of cortical and subcortical functions, much like other executive regulatory strategies (e.g., reframing). The study by Ellard and colleagues (2017) specifically contrasted acceptance with other strategies, including suppression and worry, primarily finding that these other strategies required more prefrontal recruitment, possibly suggesting that acceptance can accomplish the same goals as these other regulatory strategies, but with less executive effort.
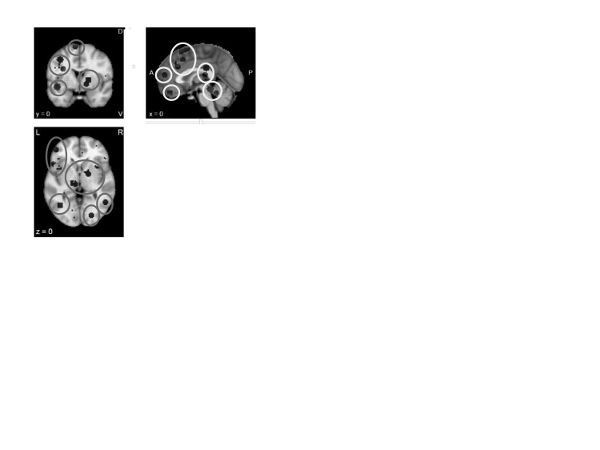 Figure 14. Neurosynth meta-analysis of “acceptance” (two studies)
Figure 14. Neurosynth meta-analysis of “acceptance” (two studies)
Values choice and clarification. We consider values choice and clarification to involve iterative processes associated with specifying one’s values and then reevaluating those specifications. There were 284 Neurosynth-nominated studies of “values” that primarily looked at unrelated concepts (e.g., “activation values”) or reward valuation, which may or may not be involved in values choice and clarification. Of these studies, a Neurosynth meta-analysis of seventeen of them—which appeared to author Greg Siegle as being more clearly related to “subjective values” (figure 15; custom search URL in the appendix)—revealed activations primarily in default network regions associated with self-referential processing, such as the orbitofrontal cortex, rostral anterior cingulate, and hippocampus. Thus, we conclude that intervening on one’s values may help individuals to evaluate self-relevant, if abstract, information.
The clarification of values involves an iterative process of belief refinement, which may be considered to reflect the large neuroscience literature on the adjustments of beliefs in response to errors in prediction (i.e., realizing that something you thought was incorrect and, thus, changing thinking). A Neurosynth meta-analysis of “prediction error” (figure 15) revealed reactivity almost exclusively in the basal ganglia, a key element of the reward network. Thus, we suggest that values clarification may involve the iterative refinement of what one views as rewarding or punishing, and how rewarding or punishing it is, with respect to the self.
 Figure 15. Neurosynth meta-analyses of (subjective) “values” (seventeen studies) and “prediction error” (sixty-six studies)
Figure 15. Neurosynth meta-analyses of (subjective) “values” (seventeen studies) and “prediction error” (sixty-six studies)
Mindfulness. A Neurosynth meta-analysis of “mindfulness” (figure 16; custom search URL in the appendix) revealed activations in the salience network (anterior insula) and frontal structures often implicated in attention (rostral cingulate). These results largely match a recent meta-analysis (Tomasino, Chiesa, & Fabbro, 2014) that also implicated a network of frontal structures associated with attention. Thus, mindfulness interventions appear to recruit brain networks consistent with often-described increases in attentional control and focus on internal body sensations.
 Figure 16. Neurosynth meta-analysis of “mindfulness” (fifteen studies)
Figure 16. Neurosynth meta-analysis of “mindfulness” (fifteen studies)
Motivational strategies. Neurosynth meta-analyses of “motivation” and “motivational” (figure 17) revealed nearly identical maps. These data suggest that, much like the behavioral activation strategies discussed earlier, motivational features are associated with the activation of the reward network, particularly the basal ganglia (especially the striatum), subgenual anterior cingulate, and sublenticular extended amygdala, all of which have been associated with emotion/reward-based preparation for action, along with evaluation of the extent to which possible outcomes are estimated to be rewarding. Thus, the neural data could suggest that motivational strategies capitalize on the brain’s ability to conceive of otherwise difficult actions as being rewarding.
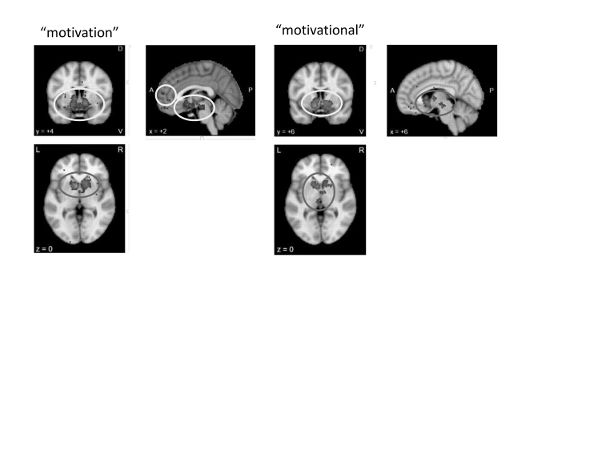 Figure 17. Neurosynth meta-analyses of “motivation” (135 studies) and “motivational” (149 studies)
Figure 17. Neurosynth meta-analyses of “motivation” (135 studies) and “motivational” (149 studies)
Conclusion
We highlighted brain networks that are associated with concepts addressed in therapeutic change generally and the contents of this book specifically. The similarities of maps and identified networks across the sections of this chapter suggest that different therapeutic techniques may share key elements and may have critical similarities despite their nominal differences. In particular, the evidence highlights increased executive control, increased reward, and the use of somatic processing as possible routes to emotional change. Taking advantage of inherent tensions between executive control and automatic processing of salient information, as well as the potential use of executive control to increase reward valuation, are common mechanisms across intervention techniques. Keeping such common principles in mind may help clinicians to unify and promote a translational appreciation of what they are doing in the therapy room.
Appendix: Custom Neurosynth Meta-Analyses
These custom Neurosynth meta-analyses are not among Neurosynth’s stored canonical meta-analyses. They represent searches of terms from article texts.
Acceptance: http://neurosynth.org/analyses/custom/69f0107f-ea71–437c
Alexithymia: http://neurosynth.org/analyses/custom/d6d48d7d-00ac-43a6
Contingency: http://neurosynth.org/analyses/custom/e7a9cb5c-e0f3–4fae
Dissociation: http://neurosynth.org/analyses/custom/ffaa34e4-d75e-4355
Mindfulness: http://neurosynth.org/analyses/custom/62bf31de-285b-4239
Problem solving: http://neurosynth.org/analyses/custom/9fbbed1a-9078–45e3
Subjective values: http://neurosynth.org/analyses/custom/ab283af2–32f0–49b6
References
Amodio, D. M., & Frith, C. D. (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–277.
Anderson, J. R., & Fincham, J. M. (2014). Extending problem-solving procedures through reflection. Cognitive Psychology, 74, 1–34.
Armony, J. L. (2013). Current emotion research in behavioral neuroscience: The role(s) of the amygdala. Emotion Review: Journal of the International Society for Research on Emotion, 5(1), 104–115.
Axmacher, N., & Rasch, B. (2017). Cognitive neuroscience of memory consolidation. Charm, Switzerland: Springer.
Bressler, S. L., & Menon, V. (2010). Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290.
Buhle, J. T., Silvers, J. A., Wager, T. D., Lopez, R., Onyemekwu, C., Kober, H., et al. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990.
Burnett, S., Sebastian, C., Cohen Kadosh, K., & Blakemore, S.-J. (2011). The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews, 35(8), 1654–1664.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222.
Cai, W., Chen, T., Szegletes, L., Supekar, K., & Menon, V. (2015). Aberrant cross-brain network interaction in children with attention-deficit/hyperactivity disorder and its relation to attention deficits: A multisite and cross-site replication study. Biological Psychiatry. Retrieved from http://dx.doi.org/10.1016/j.biopsych.2015.10.017.
Camara, E., Rodriguez-Fornells, A., Ye, Z., & Münte, T. F. (2009). Reward networks in the brain as captured by connectivity measures. Frontiers in Neuroscience, 3(3), 350–362.
Chein, J. M., & Schneider, W. (2005). Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Cognitive Brain Research, 25(3), 607–623.
Choe, Y. (2014). Hebbian learning. In D. Jaeger & R. Jung (Eds.), Encyclopedia of computational neuroscience (pp. 1–5). New York: Springer Verlag.
Christoff, K., Prabhakaran, V., Dorfman, J., Zhao, Z., Kroger, J. K., Holyoak, K. J., et al. (2001). Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage, 14(5), 1136–1149.
Collier, A., & Siegle, G. J. (2015). Individual differences in response to prediction bias training. Clinical Psychological Science, 3(1), 79–90.
Craig, A. D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1): 59–70.
Da Silva, W. C., Bonini, J. S., Bevilaqua, L. R. M., Medina, J. H., Izquierdo, I., & Cammarota, M. (2007). Inhibition of mRNA synthesis in the hippocampus impairs consolidation and reconsolidation of spatial memory. Hippocampus, 18(1), 29–39.
Davey, C. G., Pujol, J., & Harrison, B. J. (2016). Mapping the self in the brain’s default mode network. NeuroImage, 132, 390–397.
Davis, T., Goldwater, M., & Giron, J. (2017). From concrete examples to abstract relations: The rostrolateral prefrontal cortex integrates novel examples into relational categories. Cerebral Cortex, 27(4), 2652–2670.
Delaveau, P., Arruda Sanchez, T., Steffen, R., Deschet, K., Jabourian, M., Perlbarg, V., et al. (2017). Default mode and task-positive networks connectivity during the N-Back task in remitted depressed patients with or without emotional residual symptoms. Human Brain Mapping, 38(7), 3491–3501. Retrieved from http://dx.doi.org/10.1002/hbm.23603.
Di, X., & Biswal, B. B. (2014). Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ, 2, e367.
Ellard, K. K., Barlow, D. H., Whitfield-Gabrieli, S., Gabrieli, J. D. E., & Deckersbach, T. (2017). Neural correlates of emotion acceptance versus worry or suppression in generalized anxiety disorder. Social Cognitive and Affective Neuroscience, 12(6), 1009–1021. Retrieved from http://dx.doi.org/10.1093/scan/nsx025.
Fresco, D. M., Roy, A. K., Adelsberg, S., Seeley, S., García-Lesy, E., Liston, C., et al. (2017). Distinct functional connectivities predict clinical response with emotion regulation therapy. Frontiers in Human Neuroscience, 11, 86.
Gross, J. J., & Thompson, R. A. (2007). Emotion regulation: Conceptual foundations. In J. J. Gross (Ed.), Handbook of emotion regulation (pp. 3–24). New York: Guilford Press.
Hamilton, J. P., Furman, D. J., Chang, C., Thomason, M. E., Dennis, E., & Gotlib, I. H. (2011). Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry, 70(4), 327–333.
Hofmann, S. G. (2013). Can fMRI be used to predict the course of treatment for social anxiety disorder? Expert Review of Neurotherapeutics, 13(2), 123–125.
Hofmann, S. G., Mundy, E. A., & Curtiss, J. (2015). Neuroenhancement of exposure therapy in anxiety disorders. AIMS Neuroscience, 2(3), 123–138.
Inaba, H., Kai, D., & Kida, S. (2016). N-glycosylation in the hippocampus is required for the consolidation and reconsolidation of contextual fear memory. Neurobiology of Learning and Memory, 135, 57–65.
Jones, N. P., Fournier, J. C., & Stone, L. B. (2017). Neural correlates of autobiographical problem-solving deficits associated with rumination in depression. Journal of Affective Disorders, 218, 210–216.
Kalivas, P. W., & Nakamura, M. (1999). Neural systems for behavioral activation and reward. Current Opinion in Neurobiology, 9(2), 223–227.
Kim, H. (2012). A dual-subsystem model of the brain’s default network: Self-referential processing, memory retrieval processes, and autobiographical memory retrieval. NeuroImage, 61(4), 966–977.
Koenigsberg, H. W., Fan, J., Ochsner, K. N., Liu, X., Guise, K. G., Pizzarello, S., et al. (2009). Neural correlates of the use of psychological distancing to regulate responses to negative social cues: A study of patients with borderline personality disorder. Biological Psychiatry, 66(9), 854–863.
Koenigsberg, H. W., Fan, J., Ochsner, K. N., Liu, X., Guise, K., Pizzarello, S., et al. (2010). Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia, 48(6), 1813–1822.
Lane, R. D., Ryan, L., Nadel, L., & Greenberg, L. (2014). Memory reconsolidation, emotional arousal, and the process of change in psychotherapy: New insights from brain science. Behavioral and Brain Sciences, 38, e1. Retrieved from http://dx.doi.org/10.1017/s0140525x14000041.
Lonergan, M. H., Brunet, A., Olivera-Figueroa, L. A., & Pitman, R. K. (2013). Disrupting consolidation and reconsolidation of human emotional memory with propranolol: A meta-analysis11. In C. M. Alberni (Ed.), Memory Reconsolidation (pp. 249–272). Amsterdam: Elsevier.
Maresh, E. L., Allen, J. P., & Coan, J. A. (2014). Increased default mode network activity in socially anxious individuals during reward processing. Biology of Mood and Anxiety Disorders, 4, 7.
Melrose, R. J., Poulin, R. M., & Stern, C. E. (2007). An fMRI investigation of the role of the basal ganglia in reasoning. Brain Research, 1142, 146–158.
Olino, T. M., McMakin, D. L., & Forbes, E. E. (2016). Toward an empirical multidimensional structure of anhedonia, reward sensitivity, and positive emotionality: An exploratory factor analytic study. Assessment. Retrieved from http://dx.doi.org/10.1177/1073191116680291.
Olino, T. M., McMakin, D. L., Morgan, J. K., Silk, J. S., Birmaher, B., Axelson, D. A., et al. (2014). Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Developmental Cognitive Neuroscience, 8, 55–64.
Phelps, E. A., Delgado, M. R., Nearing, K. I., & LeDoux, J. E. (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43(6), 897–905.
Portero-Tresserra, M., Martí-Nicolovius, M., Guillazo-Blanch, G., Boadas-Vaello, P., & Vale-Martínez, A. (2013). D-cycloserine in the basolateral amygdala prevents extinction and enhances reconsolidation of odor-reward associative learning in rats. Neurobiology of Learning and Memory, 100, 1–11.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682.
Ray, K. L., McKay, D. R., Fox, P. M., Riedel, M. C., Uecker, A. M., Beckmann, C. F., et al. (2013). ICA model order selection of task co-activation networks. Frontiers in Neuroscience, 7, 237.
Ray, R. D., & Zald, D. H. (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience and Biobehavioral Reviews, 36(1), 479–501.
Schmidt, S. D., Furini, C. R. G., Zinn, C. G., Cavalcante, L. E., Ferreira, F. F., Behling, J. A. K., et al. (2017). Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus. Neurobiology of Learning and Memory, 142(Part A), 48–54.
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356.
Servaas, M. N., Aleman, A., Marsman, J.-B. C., Renken, R. J., Riese, H., & Ormel, J. (2015). Lower dorsal striatum activation in association with neuroticism during the acceptance of unfair offers. Cognitive, Affective and Behavioral Neuroscience, 15(3), 537–552.
Sharma, A., Wolf, D. H., Ciric, R., Kable, J. W., Moore, T. M., Vandekar, S. N., et al. (2017). Common dimensional reward deficits across mood and psychotic disorders: A connectome-wide association study. American Journal of Psychiatry, 174(7), 657–666.
Siegle, G. J., D’Andrea, W., Jones, N., Hallquist, M. N., Stepp, S. D., Fortunato, A., et al. (2015). Prolonged physiological reactivity and loss: Association of pupillary reactivity with negative thinking and feelings. International Journal of Psychophysiology, 98(2, Part 2), 310–320.
Siegle, G. J., Thompson, W. K., Collier, A., Berman, S. R., Feldmiller, J., Thase, M. E., et al. (2012). Toward clinically useful neuroimaging in depression treatment: Prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Archives of General Psychiatry, 69(9), 913–924.
Smith, S. M., Laird, A. R., Glahn, D., Fox, P. M., Mackay, C. E., Filippini, N., et al. (2009). FMRI resting state networks match BrainMap activation networks. NeuroImage, 47, S147.
Smoski, M. J., Keng, S.-L., Ji, J. L., Moore, T., Minkel, J., & Dichter, G. S. (2015). Neural indicators of emotion regulation via acceptance vs. reappraisal in remitted major depressive disorder. Social Cognitive and Affective Neuroscience, 10(9), 1187–1194.
Smoski, M. J., Rittenberg, A., & Dichter, G. S. (2011). Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Research: Neuroimaging, 194(3), 263–270.
Sporns, O. (2010). Networks of the brain. Cambridge, MA: MIT Press.
Tomasino, B., Chiesa, A., & Fabbro, F. (2014). Disentangling the neural mechanisms involved in Hinduism- and Buddhism-related meditations. Brain and Cognition, 90, 32–40.
Treanor, M., Brown, L. A., Rissman, J., & Craske, M. G. (2017). Can memories of traumatic experiences or addiction be erased or modified? A critical review of research on the disruption of memory reconsolidation and its applications. Perspectives on Psychological Science, 12(2), 290–305.
Tryon, W. (2014). Cognitive neuroscience and psychotherapy: Network principles for a unified theory. Amsterdam: Elsevier.
Uddin, L. Q., Kelly, A. M., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637.
Van Strien, N. M., Cappaert, N. L. M., & Witter, M. P. (2009). The anatomy of memory: An interactive overview of the parahippocampal–hippocampal network. Nature Reviews Neuroscience, 10(4), 272–282.
Vanyukov, P. M., Szanto, K., Hallquist, M. N., Siegle, G. J., Reynolds, C. F., III, Forman, S. D., et al. (2016). Paralimbic and lateral prefrontal encoding of reward value during intertemporal choice in attempted suicide. Psychological Medicine, 46(2), 381–391.
Wendelken, C., Nakhabenko, D., Donohue, S. E., Carter, C. S., & Bunge, S. A. (2008). “Brain is to thought as stomach is to ??”: Investigating the role of rostrolateral prefrontal cortex in relational reasoning. Journal of Cognitive Neuroscience, 20(4), 682–693.
Wisłowska-Stanek, A., Lehner, M., Turzynska, D., Sobolewska, A., & Płaznik, A. (2010). The influence of D-cycloserine and midazolam on the release of glutamate and GABA in the basolateral amygdala of low and high anxiety rats during extinction of a conditioned fear. Pharmacological Reports, 62, 68–69.
Wu, S. L., Hsu, L. S., Tu, W. T., Wang, W. F., Huang, Y. T., Pawlak, C. R., et al. (2008). Effects of d-cycloserine on the behavior and ERK activity in the amygdala: Role of individual anxiety levels. Behavioural Brain Research, 187(2), 246–253.
Yarkoni, T., Poldrack, R. A., Nichols, T. E., van Essen, D. C., & Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670.