CHAPTER 9

Science Review
REVIEW OF BIOLOGICAL SCIENCES
CELL STRUCTURE
Cell Theory
The cell is considered to be the basic unit of life. It can take on a variety of functions, depending on the organism and the tissue in which the cell is located. Although early scientists were able to see various parts of the cell, they did not understand the processes that took place within the cell. From what was known in the early 1900s, the cell theory was formed:
• All cells arise from preexisting cells.
• Cells can carry out the processes of life.
• Organisms are made of cells that function together.
Prokaryotes versus Eukaryotes
There are two basic types of cells, called prokaryotic cells and eukaryotic cells. Single-celled organisms, such as bacteria, are examples of prokaryotes, whereas the cells of multicellular organisms, such as plants and animals, are examples of eukaryotes. Both have deoxyribonucleic acid (DNA) as the material that carries the genetic code, but a prokaryotic cell does not have a nucleus. Although both have a cell membrane, only some eukaryotes have a cell wall, whereas all prokaryotes have a cell wall. Today, we know a great deal about cells and their functions.
Cell Structure and Organelles
The cell can be considered to be the basic unit of life. Within the cell, there are a number of organelles that help the cell carry out certain functions. They can be compared to the organs within your body that perform certain processes to keep you alive. Below is a diagram of a eukaryotic cell and table of the organelle functions.
The Eukaryotic Cell
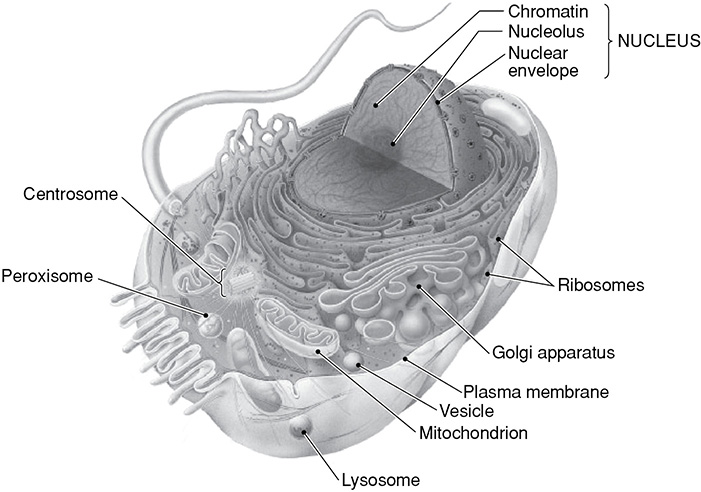
SOURCE: From Stephanie Zinn, ed., McGraw-Hill’s SAT Subject Test: Biology, McGraw-Hill, 2006; reproduced with permission of The McGraw-Hill Companies.

Transport Across the Plasma Membrane
Consider a tea bag placed into a hot cup of water. Without any stirring, the water eventually becomes colored due to the tea moving throughout the cup of water. Particles naturally move from an area of high concentration (the tea bag) to an area of low concentration (the clean, boiled water). This process is called diffusion. Diffusion also explains why you can smell an odor from across a room. Concentrations of solutes inside and outside of a cell also cause these substances to diffuse through the cell membrane. Oxygen and carbon dioxide are excellent examples of substances that can diffuse through the membrane based on their concentrations both inside and outside the cell.
Water can also diffuse across a membrane in a process called osmosis. By doing so, the water dilutes a solution that is inside or outside of a cell in an attempt to equalize the concentrations. In an isotonic solution, the concentrations inside and outside the cell are the same. In a hypotonic solution, the solution outside the cell has a lower concentration of solutes than the solution inside the cell. Water moves from the outside solution into the cell in an attempt to dilute the concentrations in the cell. This causes the cell to swell and/or burst. In a hypertonic solution, the outside solution has a higher concentration of solute than the cell. Water leaves the cell in an effort to dilute the outside solution. This causes the cell to shrivel and shrink.
Transport across the cell membrane can be passive or active. Passive transport is transportation that occurs from a high concentration to a low concentration naturally and without any additional energy input. Active transport takes place when materials need to be moved from an area of low concentration to one of higher concentration. Because this works against a favored process, the cell must use energy to carry out active transport.
Biochemistry
The molecules needed for life to carry out its functions can be classified as being both organic (carbon-based) and inorganic (without carbon). Some organic compounds include lipids, carbohydrates, proteins, and nucleic acids. Some inorganic substances include ions, iron, calcium, and water. Water is considered to be the universal solvent and can dissolve a range of substances that are polar or contain ions. Water molecules are polar and can attract other water molecules via cohesive forces (forces between the same molecules). Water molecules can also attract other polar molecules via adhesive forces (forces between different molecules).
Carbohydrates are organic compounds that contain carbon, oxygen, and hydrogen. They are major sources of energy in the body. The simplest sugars are called monosaccharides; examples are glucose and fructose. The combination of monosaccharides to form disaccharides and starches occurs with the removal of water via a dehydration synthesis. Hydrolysis is the reverse process, in which larger starches or polysaccharides have water added to them to break them down into simple sugars for use by cells.
Proteins contain carbon, hydrogen, oxygen, and nitrogen. Proteins are long chains of amino acids joined via a dehydration synthesis. The amine group of one molecule reacts with the carboxylic acid group of another amino acid to form a dipeptide. The bond that is formed from this reaction is called the peptide bond. Besides an amine group and a carboxylic acid group, each one of the 20 amino acids has a special “R” group that makes it one of the distinct amino acids.
Proteins make complex structures by organizing themselves at different levels. The primary sequence of proteins is the order in which amino acids have formed peptide bonds. This sequence causes the protein to form an alpha helix or a beta pleated sheet, creating its secondary structure. The three-dimensional shaping of a protein is called its tertiary structure. This structure can be held together with hydrogen bonds and disulfide “bridges.” A quaternary structure is made of many tertiary structures held together. Hemoglobin, with its four peptide chains held together, is an example of this.
Enzymes are examples of complex proteins that function to regulate the rate at which reactions occur. Enzymes are catalysts that aim to lower the amount of energy it takes for a reaction to occur. Once the activation energy of the reaction has been lowered, the reaction can take place at a faster rate. Each enzyme has a specific substrate on which it acts. Because of this, the fitting of a substrate into the active site of an enzyme is often compared to a lock and a key. The induced fit hypothesis tells a slightly different story, dictating that an enzyme changes the shape of its active site slightly to accommodate a substrate. Because enzymes can be denatured by certain temperatures and pH values, different structures of enzymes can function in various parts of the body and under different conditions. Outside of its ideal conditions, an enzyme can have its shape altered dramatically and become denatured.
Lipids are made of carbon, hydrogen, and oxygen (very little oxygen in comparison to carbohydrates). They are made from the dehydration synthesis of a glycerol molecule and three fatty acid molecules. Fats can contain carbon chains that contain single or double bonds. If a fat chain contains all single bonds, the fat is classified as saturated. If there is a double bond in the fat chain, the fat is classified as unsaturated. Lipids are stored by the body as an energy reserve, and they can provide about twice as much energy per gram as proteins or carbohydrates. Fats can also provide our bodies with insulation, preventing heat loss.
Nucleic acids are the building blocks of DNA and ribonucleic acid (RNA). Nucleic acids contain carbon, hydrogen, and oxygen, along with nitrogen and phosphorus atoms as well. Each nucleic acid contains a phosphate group, a five-carbon sugar, and a nitrogen base. The phosphate groups are what join the nucleic acids in a chain. The nitrogen bases can pair up with a complementary base to form the double-stranded DNA.
Pathways for Energy Synthesis
Energy is needed by all living things so that the processes of life can be carried out. The molecule that supplies energy is called adenosine triphosphate (ATP). The steps that the cells of an organism take to produce this needed energy can occur in a variety of ways. Some methods for producing energy require oxygen (aerobic), whereas some do not (anaerobic). The table below compares four processes for producing energy:

Genetic Material
Deoxyribonucleic acid, or DNA, is the macromolecule in cells that codes for how amino acids form proteins. DNA is a double-stranded helix that has complementary nucleic acids that are hydrogen bonded to each other. These pairings are thymine with adenine and guanine with cytosine. The nucleic acids can be classified as purines (adenine and guanine) or pyrimidines (cytosine and thymine). The fact that the nitrogen bases are hydrogen bonded to each other, and not covalently bonded, makes it easy for a segment of a DNA molecule to “unzip” and replicate without having to overcome strong covalent bonds.
Ribonucleic acid, or RNA, exists as messenger RNA (mRNA), transfer RNA (tRNA), or ribosomal RNA (rRNA). Each of these types of RNA has a function:

RNA has nitrogen bases that match up to the nucleic acids for DNA with one exception: RNA does not have the nitrogen base thymine and pairs up uracil opposite the adenine nitrogen base of DNA. Also, while DNA is double-stranded, RNA exists as a single strand.
In the environment, there are factors that can alter or damage genetic material. Radioactive isotopes, radiation, and carcinogens can alter or damage the sequence of nucleic acids in our genetic material. This is called a mutation. Mutations can lead to a number of disorders and/or cancers.
Cell Reproduction
There are two processes by which new cells are formed. One, called mitosis, is the process by which most new cells are produced in eukaryotes. At the completion of mitosis, two new daughter cells are produced from one preexisting cell. The other process, which produces sex sells (sperm and egg), is called meiosis. Let’s examine mitosis first. The steps of mitosis are shown in the table and figure that follow.

Meiosis is the process by which cell division takes place so that the sex cells, sperm and egg, are produced. Although meiosis is similar to mitosis, you should note the number of divisions involved and the number of chromosomes present in each cell. Although the human cell has 46 chromosomes, the sex cell needs to be produced so that each sex cell has just 23 chromosomes—all single chromosomes, no pairs. These cells are termed haploid. When a sperm cell and an egg cell join, the resulting zygote now has 46 chromosomes (diploid). The steps of meiotic division are shown in the table that follows.
Gametogenesis is different for males and females. In spermatogenesis, the primary spermatocyte develops into four sperm. In oogenesis, just one egg cell is formed from the oocyte along with three polar bodies.
Mitosis
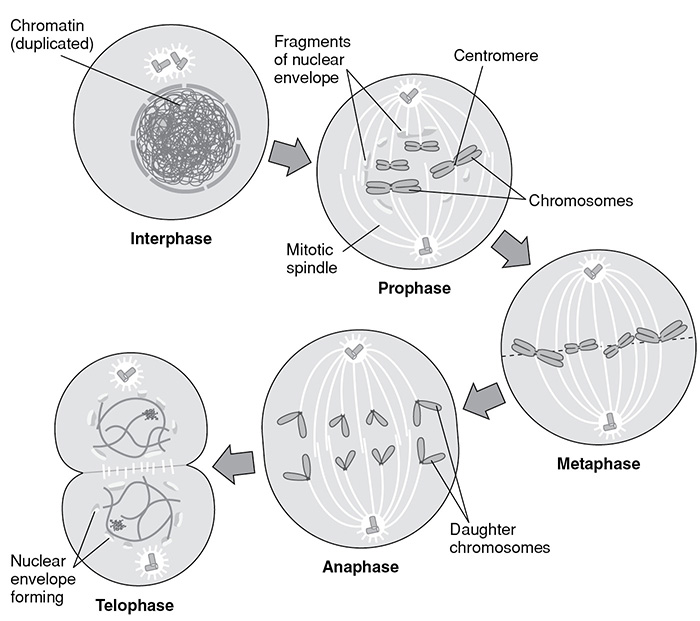
SOURCE: From Stephanie Zinn, ed., McGraw-Hill’s SAT Subject Test: Biology, McGraw-Hill, 2006; reproduced with permission of The McGraw-Hill Companies.

Meiosis

SOURCE: From Stephanie Zinn, ed., McGraw-Hill’s SAT Subject Test: Biology, McGraw-Hill, 2006; reproduced with permission of The McGraw-Hill Companies.
Spermatogenesis and Oogenesis
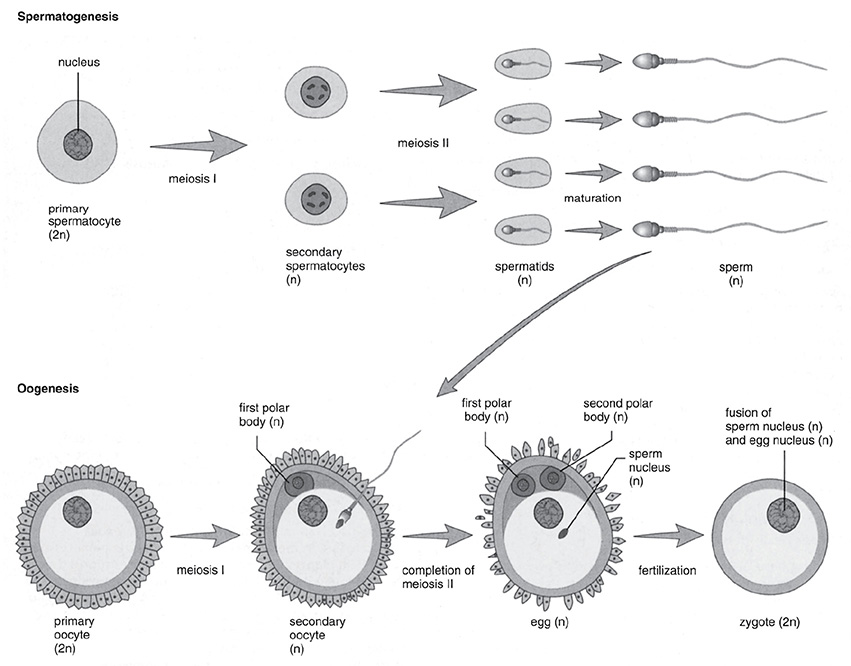
SOURCE: From Sylvia S. Mader, Biology, eighth edition, McGraw-Hill, 2004; reproduced with permission of the McGraw-Hill Companies.
CELL STRUCTURE QUIZ
1. Match the organelle on the left with the role the organelle takes on the right.

2. Enzymes
(A) are catalysts that are made from lipids.
(B) are made of proteins and can speed up reactions.
(C) form long chains to make double strands of DNA.
(D) cannot be denatured.
3. A cell is in a solution in which the concentration of solutes is higher inside the cell than outside the cell. The cell will likely
(A) swell up and possibly burst.
(B) shrivel and shrink.
(C) maintain its size.
(D) grow a cell wall for support.
4. Yeast is used to make bread rise because
(A) the yeast engages in photosynthesis, which produces oxygen gas.
(B) carbon dioxide forms while the yeast carries out photosynthesis.
(C) the yeast carries out fermentation, which produces ethanol and carbon dioxide.
(D) yeast breathes in oxygen and produces carbon dioxide as aerobic respiration takes place.
5. Which statement below does not have the same truth value as the others?
(A) RNA is single-stranded.
(B) RNA contains uracil.
(C) DNA codes for proteins.
(D) DNA cannot be altered.
6. Which of the following is a way in which mitosis differs from meiosis?
(A) Mitosis takes place to form sex cells.
(B) Meiosis creates cells with half the number of chromosomes than the original cell.
(C) Telophase does not take place in mitosis.
(D) Spermatogenesis and oogenesis occur via mitosis.
ORGAN SYSTEM ANATOMY
Locating the Organs
Because of the complexity of the organ systems located within the human body, a set of adjectives is used to locate the organs with reference to other parts of the body. Here is a brief summary of some of these terms:

Digestive System
Digestion, a process that takes approximately 24 hours, includes both chemical and mechanical means. At certain times during its travel through the digestive system, food is acted on by enzymes, acid, and other substances. At other times, food is chewed and churned to help break it down and continuously increase its surface area. The following table outlines the chemical and mechanical mechanisms for this breakdown as food moves through the digestive system.

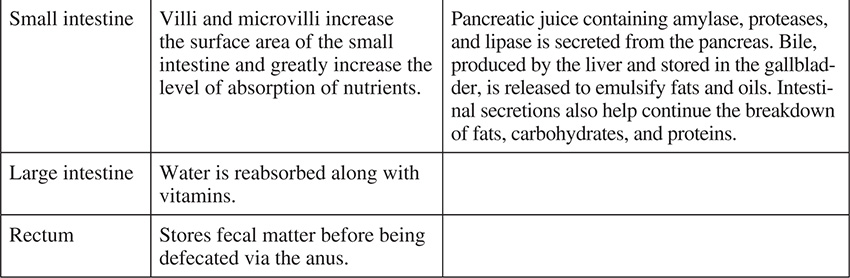
The Digestive System

SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
Circulatory System
The circulatory system provides transport for a variety of substances throughout the body. Every single cell in the body needs to remove wastes, take in nutrition, and be defended against foreign matter. The circulatory system takes on this huge task and can adjust the rate at which it performs to help maintain homeostasis.
There are three types of blood vessels responsible for transporting the blood. They are arteries, capillaries and veins. A comparison is made in the chart that follows.
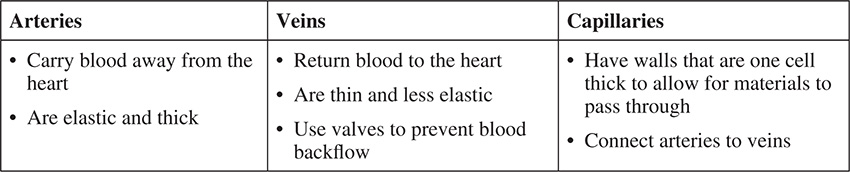
A major concern regarding the American diet is the consumption of high levels of fat and cholesterol. Cholesterol is known to cause a buildup of a plaque in the arteries causing atherosclerosis. The hardening of the arteries as they close can lead to a heart attack. The heart, made almost entirely of muscle, works to pump blood through the body. The muscle cells present are tightly locked together to provide contractions strong enough to force the blood to all areas in the body. The heart contains four chambers, the upper two being labeled atria (singular: atrium) and the bottom two labeled ventricles. The septum is a wall that separates the left and right sides of the heart, whereas valves ensure that the blood flows in only one direction through the heart, to and from the lungs, and throughout the body.
There are two periods of one beat of the heart. The time during which the heart is contracting is called the systole, whereas the time during which the heart relaxes is called the diastole. Deoxygenated blood enters the heart via the right atrium by flowing through the inferior vena cava and superior vena cava. Most of the blood will then flow into the right ventricle when the heart is relaxed and the atrioventricular (AV) valve is open. Next, the right atrium contracts slightly. The right ventricle then contracts, which forces the blood to flow through the pulmonary (pertaining to the lungs) artery. On return to the heart from the lungs, the blood once again enters into an atrium; however, this time it enters the left atrium. The blood is now oxygenated and ready to provide this oxygen to the rest of the body. After a relaxation allows the blood to enter the left ventricle, a contraction in the left atrium and then in the left ventricle pushes the blood through the aorta and to the rest of the body.
The Heart

SOURCE: From Stephanie Zinn, ed., McGraw-Hill’s SAT Subject Test: Biology, McGraw-Hill, 2006; reproduced with permission of The McGraw-Hill Companies.
The cardiac cycle is regulated by autorhythmic cells in the heart, the sinoatrial (SA) node (pacemaker), and the AV node. The SA node is responsible for starting the cardiac cycle by contracting both atria and by sending a signal that causes the AV node to signal to contract the ventricles.
The lymphatic system is another passageway by which fluids and wastes can be delivered into the circulatory system. The lymphatic system is composed of veins and capillaries in which lymph can travel via contractions of the muscles surrounding the veins. These veins, just like the ones that carry blood, contain valves that prevent the backflow of lymph. Finally, the lymph is cleaned and filtered by lymph nodes. Inside the lymph nodes, the body’s immune system works to respond to and defend against foreign invaders.
Blood is composed of plasma, red blood cells, white blood cells, and platelets. The red blood cells (erythrocytes) are responsible for carrying oxygen via the hemoglobin present in the cells. White blood cells (leukocytes) defend the body against foreign invaders such as bacteria and viruses. Platelets are responsible for helping the blood clot and seal breaks in the walls of blood vessels.
Immune System
The body takes a number of measures to prevent infection. The body’s primary defenses against infection include the skin, tears, stomach acid, urine, sweat, mucus, and saliva. By having this range of both physical and chemical defenses, the body is able to defend against a range of pathogens.
Secondary defenses bring about inflammation. The swelling, redness, and warmth of the infected area cause the body to call in macrophages and neutrophils to consume the bacteria. If the pathogen is a virus, interferon is produced so that other cells in that region of the body can block the virus from attacking any healthy cells.
The body’s third line of defense is the way the body remembers specific pathogens and their structures. If the pathogen enters the body again, the body’s response will be much quicker than the first time the pathogen invaded the body. Antibodies, specific to each pathogen, are ready to respond should this occur. The memorization and production of antibodies is called active immunity. In passive immunity, the antibodies have been obtained from outside the body, either from another animal or person.
A number of cells are involved in combating the invasion of viruses and bacteria. B cells have antigen receptors and antibodies, and they work to fight off bacteria. B cells can form plasma cells and memory cells. The plasma cells produce antibodies that bind to antigens, whereas the memory B cells form new plasma cells if the bacteria enter the body again. T cells are responsible for recognizing nonself cells. On engagement with nonself cells, they produce killer T cells and memory T cells. The killer T cells have the task of binding to cells that have been infected by viruses. The memory T cells are ready to produce more killer T cells if the virus enters the body again. In both cases, bacterial and viral infections, helper T cells are available to recognize the antigens that have been ingested and displayed by macrophages.
Respiratory System
The respiratory system allows for gases to enter and exit as needed by the body. The rate at which respiration occurs is governed by the level of carbon dioxide present in the body. Higher levels will trigger the body to increase breathing rate to dispose of this excess carbon dioxide.
The movement of air into the lungs first starts with the movement of the diaphragm downward. Because the chest cavity is sealed from air, the downward movement of the diaphragm creates a vacuum that causes air to flow into the respiratory system and fill this available space. The pathway of the airflow is shown in the following table.
The Respiratory System

SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.

Excretory System
The fatal buildup of toxins and waste in our bodies is countered by the continuous work of the excretory system. Again, a number of organs work together to maintain homeostasis in the body.

The urinary system can be diagrammed as follows:
The Urinary System

SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
The Kidney

SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
Endocrine System
The endocrine system contains a number of endocrine glands that secrete hormones that regulate a range of processes in the body. The rate at which hormones are released is determined by the needs of the body at a given time. In a positive feedback mechanism, a change in a hormone’s concentration causes the same kind of change in some other substance. In a negative feedback mechanism, a change in the concentration of a hormone causes a change in the concentration of another substance opposite to the change in the concentration of the hormone.

There are a number of glands that can release hormones into the blood, each with its own function. The chart on page 222 outlines some of the major glands and hormones that regulate our body’s processes.
Nervous System
The nervous system is composed of a network of nerve cells that allow nerve impulses to travel throughout the body. Neurons (nerve cells) are made of a cell body that contains a nucleus. A number of branched fibers called dendrites receive impulses that are carried through the axon. The axon is covered by a fatty substance called the myelin sheath. The final destination of the impulse after traveling the axon is the terminal branches and synaptic knobs. The impulses then reach a gap between neurons called a synapse, where neurotransmitters cross the gap and transmit the impulse to another neuron.
Nerve impulses travel at high speeds and are conducted via a sodium-potassium pump in the nerve cell membrane. Impulses travel along axons because the ions that are pumped cause a reverse in the polarity of the membrane.
There are a number of divisions in the nervous system, each with its own purpose and area of control in the body. The central nervous system is composed of the brain and the spinal cord. The brain is composed of the cerebrum, cerebellum, and medulla oblongata. The cerebrum is responsible for activities such as speech, memory, olfaction, and movement. The cerebellum controls voluntary movements and some involuntary movements. The medulla oblongata is responsible for involuntary commands.
The spinal cord runs along the spinal column and connects the peripheral nervous system to the brain (an electroencephalogram can be taken to measure the brain’s activity). The spinal cord also allows for certain automatic reflex responses. The peripheral nervous system is composed of sensory neurons that transmit nerve impulses toward the central nervous system, along with motor neurons that transmit impulses from the central nervous system.
Motor neurons can be further classified as part of the somatic nervous system or the autonomic nervous system. The somatic nervous system guides the actions of skeletal muscles, whereas the autonomic nervous system guides the actions of organs and involuntary muscles. The autonomic nervous system controls actions that raise the body’s level of activity. The parasympathetic nervous system guides the actions of the body that calm the body down.
The Nephron

SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
A Nerve Cell

SOURCE: From Stephanie Zinn, ed., McGraw-Hill’s SAT Subject Test: Biology, McGraw-Hill, 2006; reproduced with permission of The McGraw-Hill Companies.
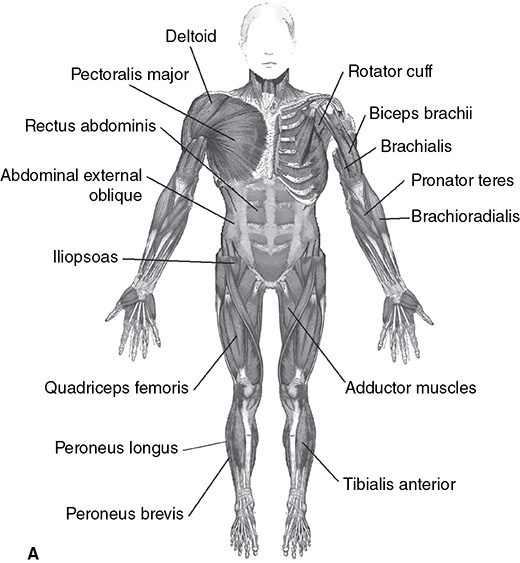
Musculoskeletal System
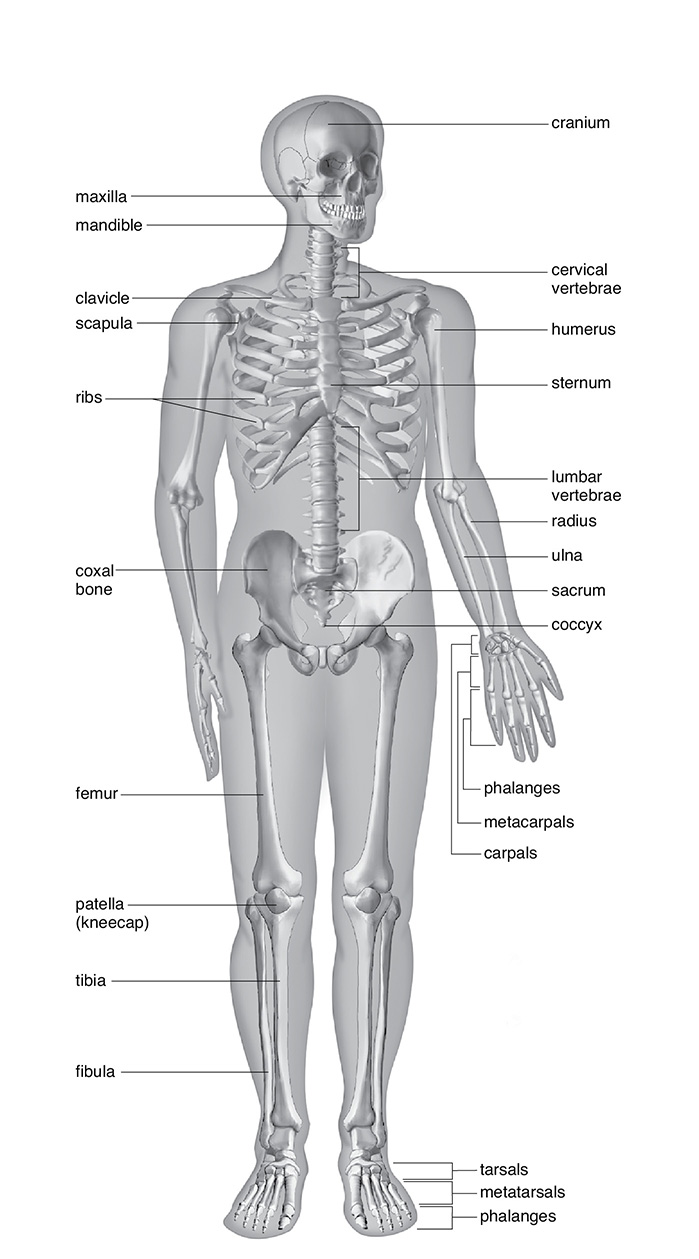
The human musculoskeletal system provides structure, and the muscles provide the strength and ability to move. Bones, besides providing structure, also provide protection for internal organs. The marrow of the bone is where red blood cells are formed along with white blood cells. Some parts of our bodies contain cartilage instead of bone, an example being the ear. Cartilage is flexible but serves the same purpose as bone, as a type of connective tissue. Cartilage allows for some degree of movement so that bones can bend easily at the joints. Cartilage also allows for some protection against impacts.
Of the parts of the skeletal system, the axial skeletal system includes the breastbone, skull, ribs, and vertebrae (organisms with a spine are called vertebrates). The appendicular skeleton includes the legs, arms, shoulder blades, collar bones, and the pelvic bone and girdle. The point where one bone meets another is called a joint. Movable joints have ligaments, keeping the bones held together. Ligaments should not be confused with tendons, which connect bones to muscle.
The element calcium is a metallic element, which accounts for the hardness of bones and teeth. Calcium needs to be obtained from foods to keep bones strong and healthy. Vitamin D also helps with the body’s uptake of calcium to maintain overall health in the bones.
There are three types of muscle present in the body: smooth, skeletal, and cardiac. As its name suggests, cardiac muscle is present in the heart. Skeletal muscle is attached to the skeleton of the body and is used for voluntary movements. Smooth muscle is used involuntarily and is present in the diaphragm, digestive system, and arteries. Some muscles cause the joints in the body to flex and are called flexors, whereas other muscles extend joints of the body and are called extensors.

SOURCE: (A) Häggström, Mikael (2014). “Medical gallery of Mikael Häggström 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN2002-4436. Public Domain (B) Häggström, Mikael (2014). “Medical gallery of Mikael Häggström 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN2002-4436. Public Domain
The Human Skeletal System
SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
Integumentary System
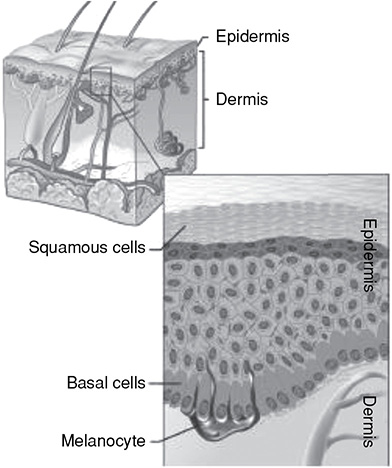
The integumentary system includes the skin and the appendages imbedded within that work together to take on a number of tasks. Some of these tasks are to regulate body temperature, provide protection against foreign bodies, make vitamin D, and provide a sense of touch so that the body can move away from harm.
There are several layers to the skin: the epidermis, dermis, and subcutis. Within these layers, we can find structures such as pores, fat cells, a pigment layer, hair follicles, sweat and oil glands, blood and lymph vessels, and nerves. Each of these has a specific function to help the organs within the body maintain homeostasis.

SOURCE: Illustration by Don Bliss from the National Cancer Institute
Human Reproductive Systems
Human reproduction takes place internally, where sperm and egg join inside the female reproductive system. (In many other species, eggs are fertilized and develop outside of body.) Before getting into the details of reproduction and development, let’s first review the parts of the human reproductive systems.
Human Male Reproductive System
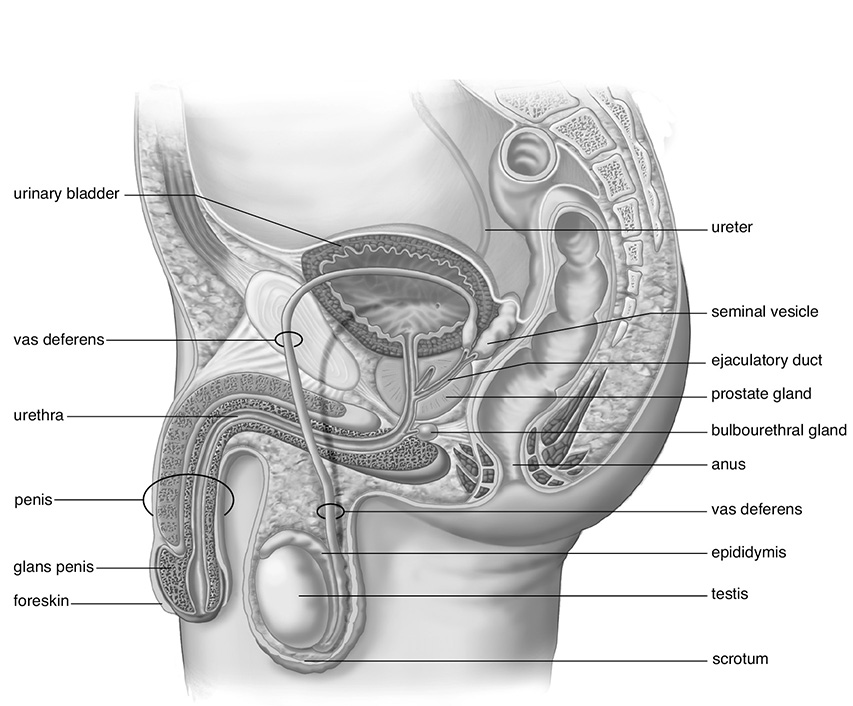
SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
In the male, the testes are located in the scrotum. After sperm are produced in the testes, they are then stored in the epididymis, where they mature. On leaving the epididymis, the sperm travel through the vas deferens. These two passageways lead the sperm into the urethra. As the sperm travel through the vas deferens, a number of glands, including the seminal and prostate glands, secrete fluids to create semen. The process by which semen exits the penis is called ejaculation.
In the female, eggs are produced in the ovaries; on maturation, they move with their follicles to the surface of the ovary. The process of ovulation is the point where the egg is released from its follicle. The egg is then moved through the fallopian tube (oviduct) via the action of cilia. If a sperm is present, it meets the egg in the fallopian tube. The egg then passes into the uterus. If the egg is fertilized, it remains at the uterine wall to develop into a fetus. The fetus then passes through the birth canal (vagina) during birth.
Human Female Reproductive System

SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
Upon fertilization, the sperm and egg become a zygote, which begins a number of cell divisions called cleavage. The cells do not grow during this time; instead they continue to divide. The cells form a ball of cells called a morula, then a hollow ball called a blastula. A second layer of cells forms, and the resulting cells make a gastrula. The germ layers then form what are called the ectoderm, mesoderm, and endotherm. These layers will develop into the different organs and organ systems in the body.
As the development continues in the uterus, the fetus receives nutrients from the mother via the umbilical cord, which is attached to the placenta. The fetus continues to develop while surrounded by amniotic fluid.
The Five Senses
The ear, besides allowing humans to hear sounds, helps humans keep their equilibrium and balance. Each part of the ear has a number of organs working together to convert air compressions into a sound that can be interpreted. These parts are shown in the following diagram:
The Human Ear
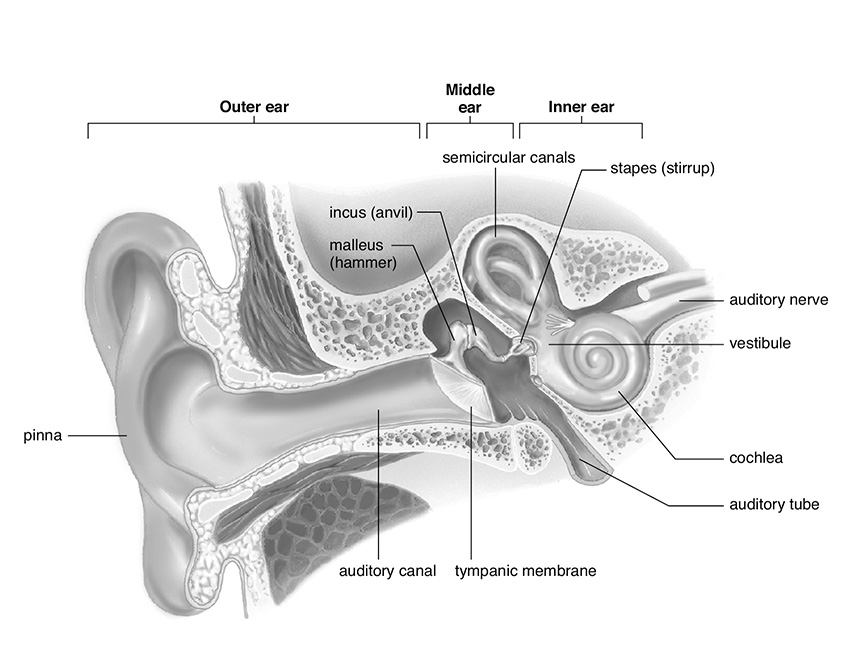
SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
The eye features the cornea, allowing light into the eye. Light then passes through the iris (the colored portion of the eye). The iris has an opening called the pupil, which can become larger or smaller depending on how much light is present. The lens then focuses the light on the retina, which is attached to the optic nerve. A layer of light-sensitive cells called the cones and rods make up one of the layers of the retina. Individuals with defects to this portion of the eye are color-blind and usually cannot see shades of greens and/or reds.
The Human Eye
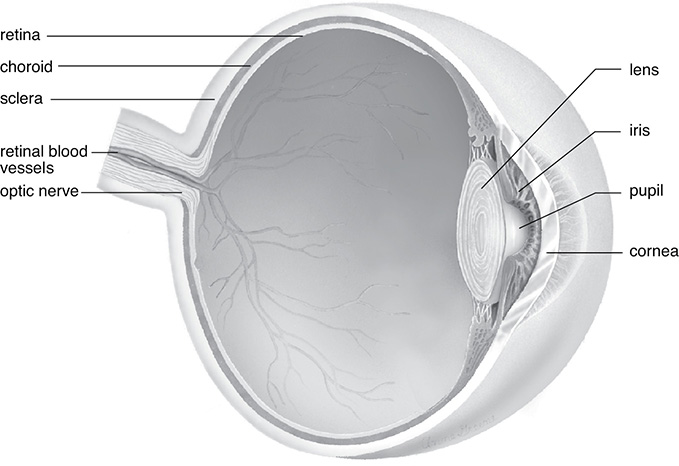
SOURCE: From Sylvia S. Mader, Biology, 8th ed., McGraw-Hill, 2004; reproduced with permission of The McGraw-Hill Companies.
The nose and tongue also have sensory nerves. Inside the nose, olfactory cells are embedded in a mucous membrane. When odors enter the nose, they enter this mucous lining, causing the olfactory nerves to be stimulated and then be interpreted by the brain. Inside the olfactory mucosa, we can find the olfactory glands or Bowman’s glands. These glands contain cells that have vesicles that secrete mucus to keep the olfactory surface moist.
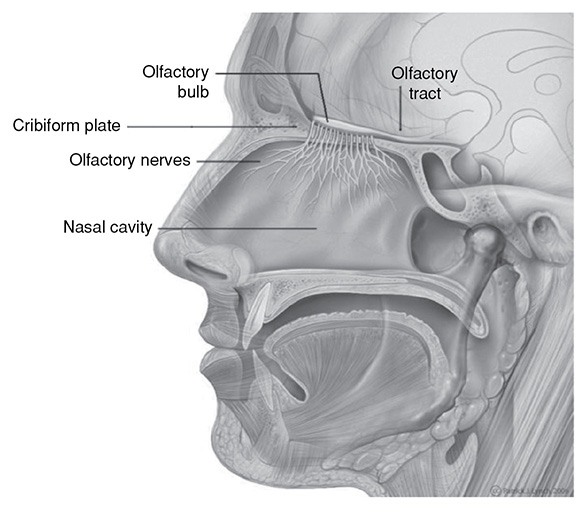
On the tongue, we have several types of papillae, circumvallate, foliate, fungiform, and filiform. All but the filiform type of papillae contain the taste buds. Taste buds are responsible for picking up tastes that are sweet, sour, bitter, salty, and umani. These sensory nerve fibers are located on various regions of the tongue.
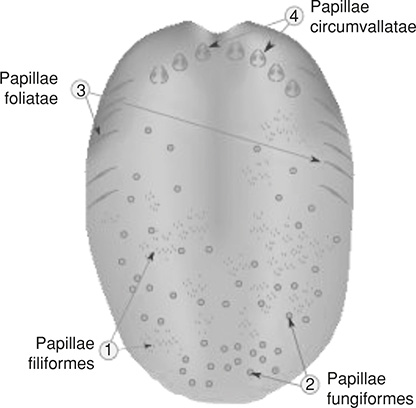
We have a sense of touch throughout the entire body, which is composed of a network of nerve endings and touch receptors. This somatosensory system makes us sensitive to many touch sensations such as hot, cold, vibrations, contact, and pain, etc. Within the skin, we find mechanoreceptors such as end-bulbs of Krause, Merkel nerve endings, Meissner corpuscles, Pacinian corpuscles, and Ruffini endings, which can detect these sensations. These receptors are connected to nerves that send information to the central nervous system. Once the signals reach the brain, our touch sensations are processed in the primary somatic sensory cortex. From there, the brain can further process and interpret these signals and send out signals to take appropriate action to maintain homeostasis.
ORGAN SYSTEMS QUIZ
Circle the letter of your choice.
1. The ribs are _____________ to the lungs in the human body.
(A) medial
(B) distal
(C) anterior
(D) deep
2. Which of the following statements is true?
(A) The digestive system can do its job without the use of accessory organs.
(B) The digestive system uses sphincters to contain substances being digested at certain points of the digestive tract.
(C) Mechanical digestion is more important than chemical digestion.
(D) The juices that are used to digest food can be effective at any point along the digestive tract.
3. The heart and veins have valves to ensure that
(A) blood flows in only one direction.
(B) oxygen and carbon dioxide can be exchanged.
(C) lymph can be directed through the arteries.
(D) platelets do not clot at the site of a wound.
4. Of the processes below, which one is a different level of defense from the other three?
(A) a low pH in the stomach
(B) cilia present in the trachea
(C) cells within the body recognizing a pathogen
(D) mucus present in the nasal cavity
5. The movement of air through the respiratory system depends on
(A) the movements of the diaphragm.
(B) how hard the muscles in the trachea contract.
(C) how hard the lungs push air out of the body.
(D) cilia along the respiratory tract pushing air in and out of the body.
6. Which pathway/order is correct?
(A) sperm: testes, epididymis, vas deferens, urethra
(B) egg: vagina, uterus, ovary, fallopian tube
(C) development: morula, blastula, zygote, egg
(D) development: fertilization, ovulation, ejaculation
7. Which of the following statements is false?
(A) Vitamin D and calcium are needed for strong healthy bones.
(B) Ligaments keep bones joined together.
(C) Skeletal muscle is needed for voluntary movements.
(D) Cartilage’s rigidity helps maintain structure.
8. Which of the following statements is false?
(A) The autonomic nervous system allows humans to decide how to use involuntary muscles.
(B) The brain and the spinal cord are part of the central nervous system.
(C) Nerve impulses can send signals faster than chemical signals traveling through the blood.
(D) Neurotransmitters are needed to help send signals between nerves.
9. Inside the kidney, one does not find
(A) nephrons.
(B) Bowman’s capsules.
(C) glomeruli.
(D) ureters.
10. The purpose of the endocrine system is to
(A) send nerve impulses throughout the body.
(B) aid in digestion.
(C) release chemical signals into the body to regulate functions.
(D) act as a primary defense against foreign invaders.
PLANTS
The structure of plants includes roots, leaves, stem, and flowers. The roots hold the plant in place and provide an enormous surface area for increased water uptake along with the uptake of minerals from the soil. This is made possible by capillary action and root pressure. From there, the water and minerals are carried up through the plant via a plant tissue called xylem. Another vascular tissue that plants have is phloem, which carries food and other materials both up and down in the plant. The leaves are where light is captured by the plant. This is why the leaves usually have a large surface area and develop in all directions around the plant. On the leaves are holes called stomates, which can be opened and closed by guard cells. The opening and closing of the stomates allows for carbon dioxide, oxygen, and water vapor to be exchanged with the air.
Plants have the ability to capture light and convert it into chemical energy. This is accomplished by carrying out photosynthesis. Because plants can make their own food, they are classified as autotrophs. It is the chloroplasts that contain the chlorophyll to help with this conversion. Chlorophyll absorbs light best when the wavelength of the light is approximately 430nm (blue) and 660nm (red). Plants have more than just one type of chlorophyll, and they also contain other pigments as well. This helps give plants additional wavelengths in which they can absorb light optimally. Besides this, plants also have the ability to carry out phototropism. This process allows them to bend toward a light source so that they can obtain the maximum amount of the available light.
The flowers of the plant are responsible for the sexual reproduction that many plants carry out. The stamen (composed of a filament and anther) is the male reproductive organ, whereas the pistil (composed of the ovary, style, and stigma) is the female reproductive organ. When pollen from the anther is transferred to a stigma, the two gametophytes form a zygote and develop a seed with an embryo inside. The ovaries of the pistil contain ovules. These ovules develop the seed and the ovary of the plant becomes a fruit. The fruit holds the seeds and protects them. This is why tomatoes are classified as fruits and not vegetables.
Reproductive Organs of a Flower
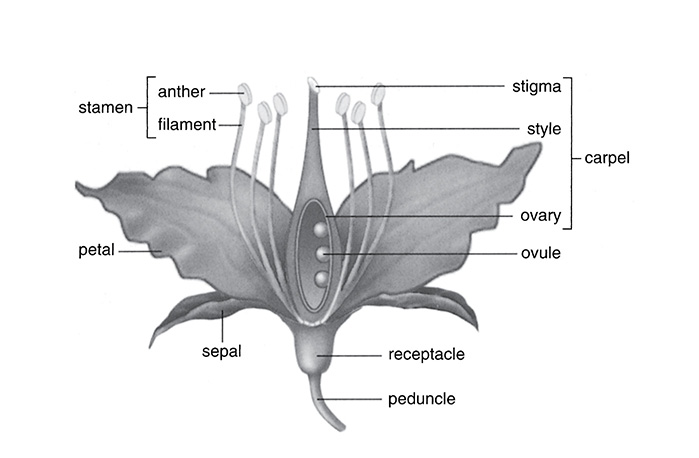
SOURCE: From Sylvia S. Mader, Biology, eighth edition, McGraw-Hill, 2004; reproduced with permission of the McGraw-Hill Companies.
PLANTS QUIZ
1. Plant cells are different from animal cells because plant cells
(A) have a nucleus.
(B) divide to form daughter cells.
(C) have a cell wall.
(D) have no need for chloroplasts.
2. The roots of a plant are not
(A) needed to uptake water.
(B) responsible for carrying out photosynthesis.
(C) responsible for anchoring the plant into the soil.
(D) needed to uptake minerals from the soil.
3. Which wavelength of light is best absorbed by chlorophyll?
(A) 500 nm
(B) 550 nm
(C) 660 nm
(D) 485 nm
4. Which part of the plant reproductive system is of a different “gender” from the other three?
(A) stamen
(B) pistil
(C) stigma
(D) style
MENDELIAN GENETICS
The Austrian botanist Gregor Mendel (1822–1884) is famous for his experiments with pea plants. Because of these experiments, humans began to understand the expression of the genes that living organisms carry. When Mendel crossed a tall pea plant with a short pea plant, the plants in the resulting generation, the F1 generation, were all tall. The F1 generation of pea plants is said to be a hybrid because it contains the genes for both tallness and shortness. The genes that are carried by an organism make up its genotype.
Why were the genes for tallness expressed and the genes for shortness were not? This refers to the organism’s phenotype, the physical traits that are actually expressed by an organism. Because the genes for tallness were the only ones expressed, Mendel described them as dominant. The genes for shortness that were not expressed (or “hidden”) he described as recessive. From these results, Mendel deduced that genes exist in varied forms called alleles. For each trait, an organism inherits two alleles, one from each parent. The resulting allele pair determines how the trait is expressed in the organism. According to Mendel’s law of segregation, these allele pairs separate (or segregate) during gamete formation and then randomly unite at fertilization. The new allele pair then determines how the trait is expressed in the new organism. This process can be diagrammed using what is called a Punnett square.
Cross of pure tall and pure short to produce F1 generation
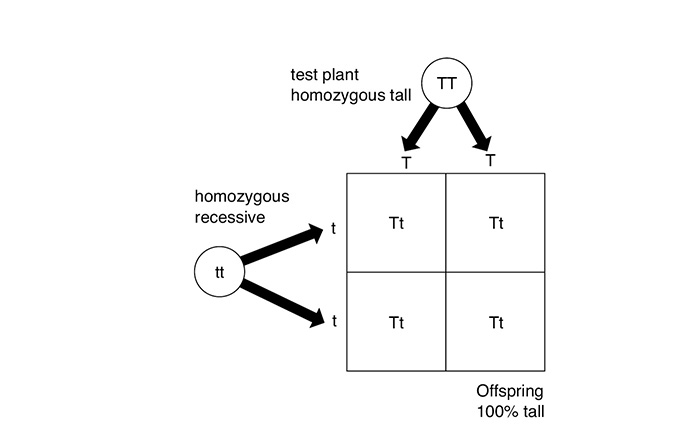
The Punnett square above shows Mendel’s experiment. The tall pea plant is said to be homozygous because it has two alleles for tallness (TT). The short pea plant is also homozygous, with two alleles for shortness (tt). However, the offspring are heterozygous, with alleles for both tallness and shortness. All of the offspring are tall because tallness is dominant, but each offspring also carries an allele for shortness that is not expressed.
When seeds from this F1 generation were crossed, in the F2 generation approximately three-fourths of the plants were tall and one-fourth of the plants were short. The short plants, a phenotype that was not expressed in the F1 generation, thus appeared again but in only 25 percent of the plants of the F2 generation.
Cross of F1 generation to produce F2 generation
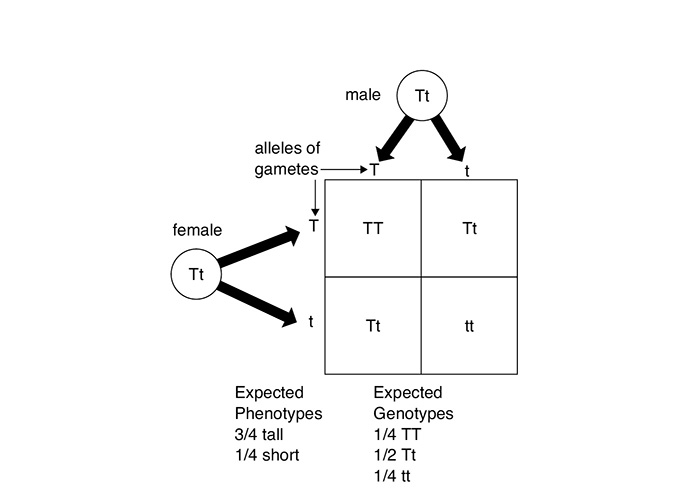
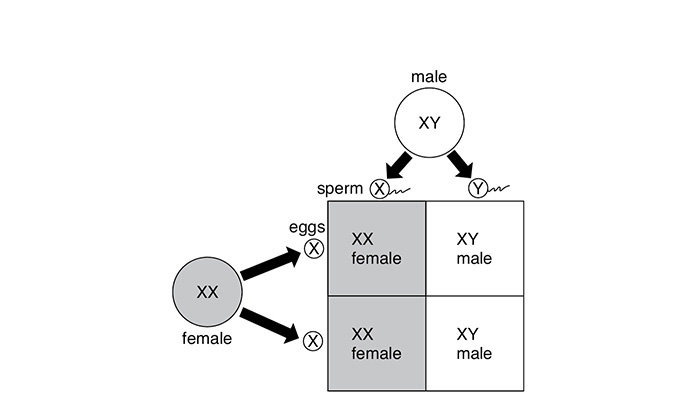
The cross between a male and a female shows that a couple has a 50 percent chance of having either a boy or a girl. Because the female always provides X chromosomes, it is the male who determines the sex of the child because the male can provide either an X chromosome or a Y chromosome. The Y chromosome is dominant. Although the Punnett square below shows more X chromosomes, it is the dominance of the Y chromosome that keeps the chance of producing a male at 50 percent.
Some traits are controlled by genes that are found on the sex chromosomes. These are called sex-linked traits. Sometimes diseases and conditions such as color blindness are the results of these traits being passed on to offspring. A sex-linked trait (allele) on an X sex chromosome, for example, is shown as X′. For color blindness to occur in an individual, all of the X chromosomes must have the allele for color blindness. For females to have the condition, both X chromosomes must have this allele (X′X′). For males, it is just the one X chromosome (X′Y). That is why color blindness occurs more often in males, whereas females can be carriers of the disorder without expressing it in their phenotype.
Cross of human male and female
Recessive defective alleles on chromosomes can lead to other disorders, such as sickle cell anemia, cystic fibrosis, and Tay-Sachs disease. Many people can carry these disorders and not show signs of the illness because they have a dominant allele that is expressed instead of the recessive one. However, should two carriers produce offspring, they have a 25 percent chance of having a child with the disorder.
MENDELIAN GENETICS QUIZ
1. A short pea plant that is homozygous recessive for tallness (tt) is crossed with a tall pea plant that is heterozygous for tallness (Tt). The expected outcome is
(A) 25 percent of the plants will be short.
(B) 75 percent of the plants will be short.
(C) 50 percent of the plants will be tall.
(D) 100 percent of the plants will be tall.
2. A male who is color-blind (X′Y) produces offspring with a female carrier of the same disorder (X′X). Which of the following is expected?
(A) All of the children will be color-blind.
(B) None of the children will be color-blind.
(C) The male offspring will be color-blind.
(D) The offspring have a 50 percent chance of being color-blind.
ANATOMY AND PHYSIOLOGY
Homeostasis and Bodily Equilibrium
Homeostasis is the body’s ability to maintain a stable environment. This is done by having many feedback loops constantly working to resist any major changes from homeostasis. These feedback loops can regulate conditions such as body temperature, blood pressure, and hormones, etc. All of our physiological conditions have what is called a setpoint, a desired target value for an essential variable. There is also the normal range, a set of values that is stable and healthful for us to maintain. An example of normal ranges can be found on one’s hemanalysis or urinalysis report.
In our brain, we have several control centers that regulate our systems and keep them within the normal range. Should there be a major deviation from the setpoint, this change is detected by receptors, cells and organs than can receive internal and external stimuli. These receptors then report the change to the control center. The control center will activate an effector, which works to reverse the change in conditions and restore homeostasis and the normal range.
There are two types of feedback loops, negative feedback and positive feedback. When a negative feedback loop responds to a stimulus, it works to turn off or reduce the intensity of the stimulus. When a positive feedback loop responds to a stimulus, it leads to an increase in that stimulus and moves the system even further from equilibrium. Two examples of negative feedback are regulating body temperature and regulating glucose levels. These are shown below:
Negative Feedback:
Body Temperature Rises → Body Produces More Sweat → Body Temperature Drops

Two examples of positive feedback are child birth and blood clotting:
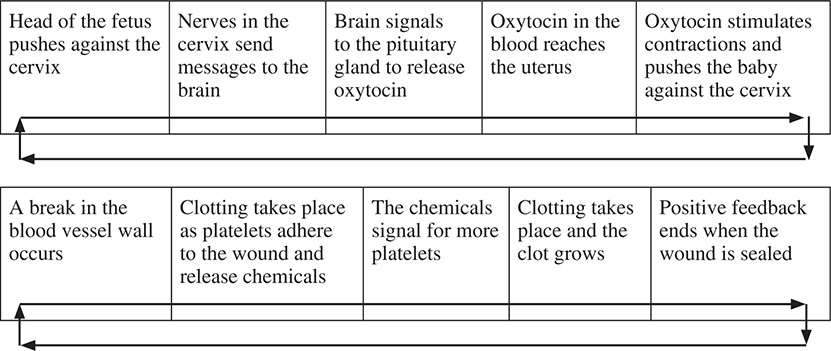
Positive feedback is used by the body when the results need to come quickly. This is helpful when we do not want the baby to get stuck in the birth canal or when we need to stop a blood vessel from losing blood.
Although the above examples of feedback loops are simplified, remember that it takes many systems within the body to work together to achieve the goals of the feedback loops. For example, think about how many systems are working to tell you that you have exercised too much when you have overexerted yourself during a hard aerobic workout.
Oxygenation
When looking at how our body takes in oxygen and exhales carbon dioxide, we have to first examine the percent composition of air and the partial pressure that each component of air has. On a typical day, the average barometric pressure is 30.00 inches of mercury. In millimeters, this turns out to be 760 millimeters of mercury or 760 mm Hg. Because air is approximately 78.6% nitrogen gas, 20.9% oxygen gas, 0.04% water, and 0.004 percent carbon dioxide, each of these gases will have a partial pressure that can be seen in the chart below:
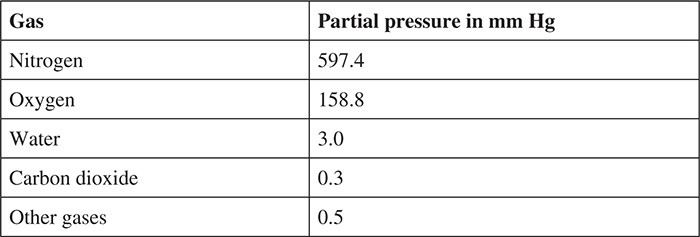
What does this have to do with gas exchange? Remember that “things will flow from high to low”—that is, substances will move from a high concentration to a low concentration. Gases will move from a higher partial pressure to a lower partial pressure.
When looking at gas exchange, we next have to understand ventilation and perfusion. Ventilation is the movement of air in and out of the lungs and is regulated by the diameter of the airways. Perfusion is the flow of blood through the body or circulatory system to the organs or tissues. Perfusion is regulated by the diameter of the blood vessels. Ideally, the volume of gas involved with ventilation and perfusion should be in a certain ratio, the V/Q ratio. However, disease or medical conditions can disturb this balance. The body can adjust the amount of blood that reaches the alveoli that are or are not receiving sufficient ventilation. Carbon dioxide, oxygen, and pH levels are examples of stimuli that can cause these changes to occur.
Gas exchange occurs in the lungs at the respiratory membrane and at the tissues in our body. When referring to the gas exchange in the lungs and alveoli, we call this external respiration. When referring to the exchange of gases in tissues, we call this internal respiration. External respiration features gas exchange in the pulmonary capillaries. When the gas is exchanged, they enter the red blood cells and bind to hemoglobin. At the same time, carbon dioxide is released from the blood to the alveoli. Going back to our chemistry lesson on partial pressures above, all of the exchanges occur due to the partial pressures of the gases in the alveoli and in the pulmonary capillaries. One more factor is worthy of mention with regards to gas exchange: solubility. Oxygen is not very soluble in blood, so it takes a higher difference in partial pressures for oxygen to cross the respiratory membrane. However, carbon dioxide is soluble in blood and requires a lower partial pressure for exchange than does oxygen.
Internal respiration, again, occurs at the body’s tissues. Partial pressure comes into play again when oxygen moves from the blood to the tissues. The partial pressure of oxygen in the tissues is lower than that in the blood, causing the oxygen to dissociate from the hemoglobin and enter the tissue. Because our cells produce carbon dioxide, the partial pressure of carbon dioxide will be high in the tissues and lower in the blood. The carbon dioxide can be returned to the lungs bound to a protein or hemoglobin, buffered with water as carbonic acid, or dissolved in the plasma.
Elimination
As our body performs metabolic activity, there is going to be a buildup of waste. The process of removing these wastes from our body is called excretion. The removal helps us maintain the homeostasis in the body. Homeostasis is maintained via a coordinated effort across many organs in the body and elimination is an excellent example of this.
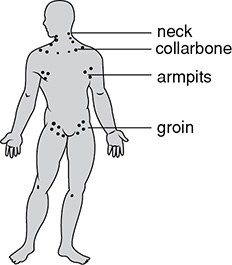
Our spleen is responsible for removing malformed or damaged red blood cells. However, it is very efficient at recycling any useful components of these cells, such as iron. The spleen also works with the lymph nodes to create lymphocytes which can fight foreign bodies.
The location of lymph nodes in the body.
During expiration, the lungs remove carbon dioxide from the body so that the body can maintain an appropriate pH in the body at a range of 7.35 to 7.45. The skin releases heat to help us maintain a body temperature of 36.5 degrees Celsius. If we should surpass this temperature, water is excreted to cool the skin down. In our sweat, there are also salts and urea that are excreted as well. Our digestive system not only breaks down food and absorbs nutrients, but it also eliminates solid wastes as well in the form of feces. Feces, besides containing the food material that was not digestible, also contains bacteria from the intestinal lining and bile. Although the kidneys produce urine, they are only part of the larger urinary system, which is responsible for eliminating water, ions, salts, and urea as well as regulating pH levels.
Nutrition
Although our bodies will need various levels of nutrition at various stages of our lives, all of the cells in our bodies will need a constant amount of nutrition to function properly and keep us healthy. For example, the diet of a growing child will differ from that of a well-trained athlete, which will differ from that of a senior citizen who has difficulty walking.
Macronutrients are larger organic molecules that give us both energy and the building blocks for growth. Proteins, carbohydrates, and lipids are all larger macronutrients that first need to be digested and undergo hydrolysis so that they can be broken down into the smaller building blocks of amino acids, monosaccharides, and fatty acids and glycerol, respectively. Once in their monomer form, the body can then best decide on how to use these building blocks.
Carbohydrates can be obtained from fruits, vegetables, and plants. These can provide us with starches, simple sugars, and fiber. Carbohydrates are a short-term source of energy and larger carbohydrates can be converted into glucose as needed. Any extra glucose in the body is converted to glycogen and stored in the liver and skeletal muscle for later use. Glucose can also be converted to fat via a complex chemical process and stored in our adipose tissue. Fiber, also a type of carbohydrate, can exist as soluble fiber or insoluble fiber. Soluble fiber absorbs water and can slow digestion. Insoluble fiber adds bulk to the stool and can move through the GI tract faster.
The term “lipids” can encompass more than just fats and include molecules such as fatty acids, waxes, phospholipids, cholesterol, and fatty acid derivatives. Fats are a subgroup of lipids and are called “triglycerides.” Saturated fats have long carbon chains that contain all single bonds, whereas unsaturated fats contain long chains that feature double bonds. Lipids are stored in our body in an unregulated manner and can provide energy to our bodies on a long-term basis. Carbohydrates and proteins provide us with 4 calories per gram, whereas lipids can provide us with 9 calories per gram. Although lipids and fat have developed a bad name over the years, they are still important components of cells and can act as nonpolar carriers for hydrophobic vitamins, such as vitamins A, D, K, and E. Fat on our body can serve as insulation, energy, and as a shock absorber. Too much fat stored on our body, however, can have many negative impacts on our health.
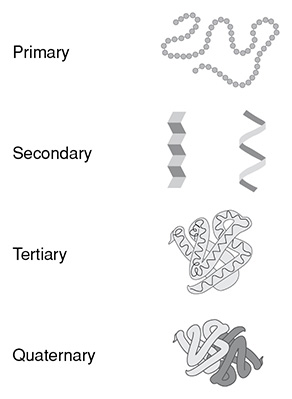
Proteins are macronutrients that are made from amino acids. Once proteins are broken down into simple amino acids, these amino acids can then be put back together in a different sequence to help us grow and repair. Our muscles, collagen, hormones, arteries and veins, skin, hair, fingernails, etc. are all built from protein. Structural proteins provide structure to the body, such as muscles and organs, whereas hormones, antibodies, and enzymes serve as examples of working proteins. Proteins can also have complex structures. The primary structure of a protein is simply the sequence of amino acids. The secondary structure of a protein is created from the amino acids forming an alpha helix or beta pleated sheet. When these structures fold over each other, they create the tertiary structure of a protein. Many units of tertiary structures can be put together the quaternary structure of a protein. These structures can be summarized by the image below.
The four levels of protein structure.
We also need a range of vitamins and minerals to help support the proper functioning of our body’s systems. Fat-soluble vitamins include, as mentioned above, vitamins A, D, E, and K, whereas vitamin C is an example of a vitamin that is water soluble. The amount of minerals needed by our body varies greatly. For example, we will need more sodium, calcium, and potassium because they are needed for proper nerve function, while we need lower amounts of certain minerals such as iodine, chromium, molybdenum, and selenium. Water is also essential to life. It is recommended that we consume at least 3 liters of water daily, barring conditions that say otherwise. Athletes and those who sweat heavily will need to consume more than this amount.
Fluid Balance
Water is a vital component to our lives. Without water, pending conditions, we can live anywhere from a few hours to a few days, while being able to live for weeks without food. Men tend to have a body percentage of water that is between 55 and 65 percent and women about 50 to 60 percent. Different tissues in our body differ as well, with muscle being about 75 percent water and fat tissue being about 10 percent water.
Fluid balance is maintained via our intake and excretion of water. Our water intake is almost all from our digestive system when we drink fluids. However, there are reactions in our body, such as condensation reactions, which release water when smaller monomers are used to make larger polymers. For example, amino acids can release water molecules when they join together into a protein. Water excretion can come from our urinary system, perspiration, or from breathing (your breath condensing in cold air serves as evidence). There can also be illnesses that cause diarrhea or vomiting, which can also lead to water severe water loss. Where this water is located in us varies greatly. The fluid within our cells is called intracellular fluid (ICF) and it makes up about 40% of our body weight. The fluid outside our cells is called extracellular fluid (ECF) and makes up about 20% of our weight. Of the ECF, about 75% of it is interstitial and 25% of it is in plasma.
We maintain our fluid levels within the cells not by any active transport, but by passive transport. The specific type of passive transport used is called osmosis. The movement of water is determined by the concentration of ions that are outside the cell as water will always move in an effort to dilute the higher concentration of ions. That is, the ECF is what determines the osmolality of the body fluid. Because the concentrations are what determine water movement, we can also say that there are no receptors that monitor any fluid or electrolyte levels.
There are several hormones that help us regulate water balance in the body. A antidiuretic hormone (ADH, or vasopressin) is controlled by the hypothalamus, which signals to the pituitary gland that the hormone needs to be released. This hormone causes the kidneys to increase water reabsorption. When we drink water, the ADH release is decreased and the kidneys will excrete more water, producing more urine. Aldosterone is a hormone secreted by the adrenal cortex that works to maintain our blood pressure and water and salt levels. It does so by helping the kidneys excrete potassium and retain sodium. Another hormone called atrial natriuretic peptide hormone (ANP) prevents sodium reabsorption. This hormone acts as a diuretic because it decreases water reabsorption and lowers blood pressure.
As mentioned above, medical illnesses such as vomiting or diarrhea can lead to severe water loss. So can profuse sweating or lack of water consumption. However, overhydration can also be of concern if there is renal failure. The drug ecstasy can interfere with our endocrine system and increase our levels of ADH, causing the kidneys to reabsorb too much water. Finally, we never want to drink pure water because pure water is a hypotonic solution. This will cause your cells to absorb water and cause a complete imbalance of electrolytes.
Finally, our body works to maintain a pH of about 7.4 in the ECF. To keep this pH balanced, we have a number of buffer systems in the body that help to resist changes in pH. One such system is the bicarbonate buffer system, which uses a weak acid and the conjugate base of that weak acid to neutralize any acid or base that enters the system. The phosphate buffer system works in the ICF of our cells. There is also a protein buffer system that helps to maintain the pH around the cells. Acidemia (the pH is too low) or alkalemia (the pH is too high) can result when the buffer systems do not function properly.
Movement and Activity
Before examining movements in detail, it is first important to examine the various types of joints in our body. These can be seen in the chart below, which summarizes their key features.

With regards to a synarthrosis, amphiarthrosis, and diarthrosis, we have the suture joints of the skull, intervertebral disks of the spine, and knee that serve as examples, respectively.
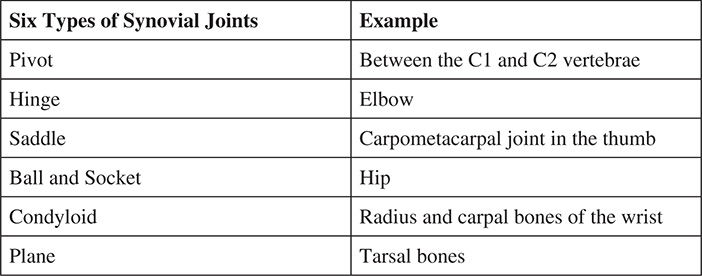
Because the synovial joint is most abundant in the body, we will focus on the six types that are present. These are summarized below:
The joints in our body allow us to make a range of movements. These will be summarized via a series of images that follow.
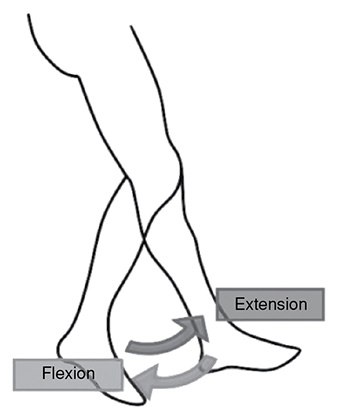
Flexion and Extension:
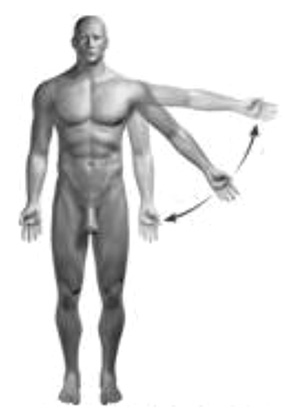
Abduction and Adduction:
Circumduction:
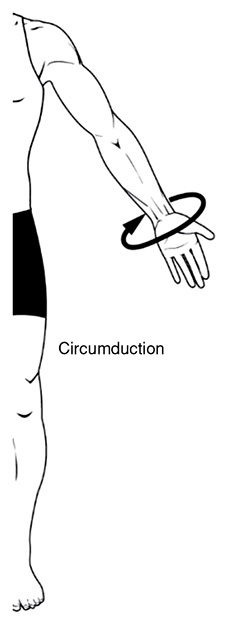
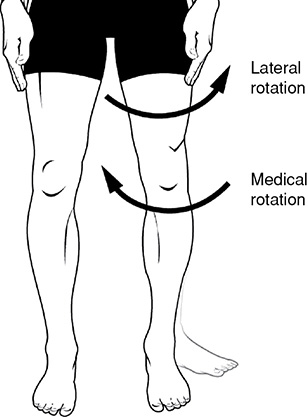
Rotation, both Lateral and Medial:
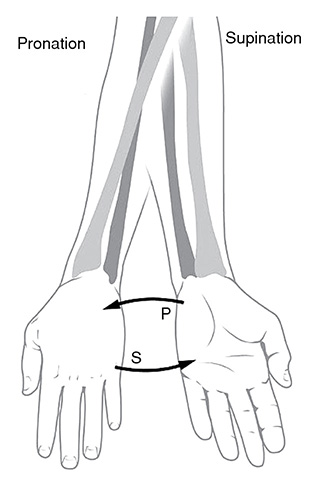
Pronation and Suponation:
Dorsiflexion and Plantarflexion:

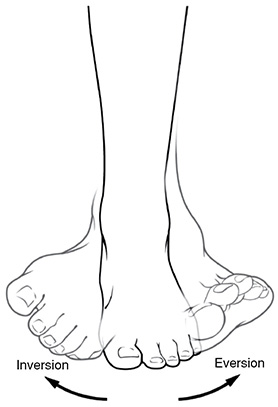
Inversion and Eversion:
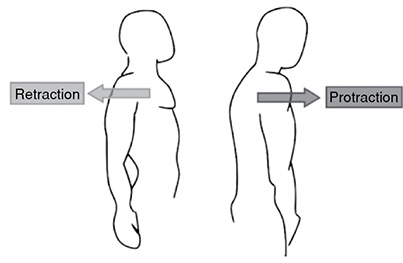
Retraction and Protraction:
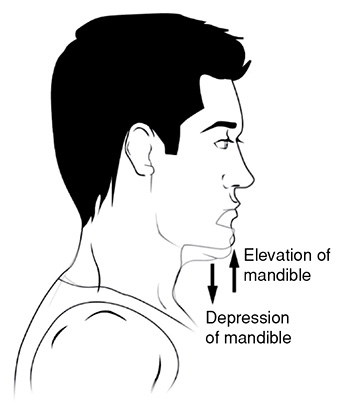
Elevation and Depression:
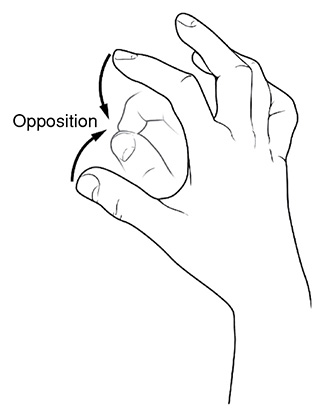
Opposition:
Fetal Development
After a long trip through the female reproductive system, sperm can unite with an egg in the fallopian tube. When sperm and egg unite, we can say that fertilization takes place. Each gamete provides 23 chromosomes so that the resulting zygote contains 46 chromosomes total and the information needed to start developing. The zygote will continue to divide via the process of cleavage to form a morula which is a ball of cells. Cell division continues and a space forms between the cells. At this point, the mass of cells is called a blastula.
Before the blastula reaches the uterus, the uterine lining has been prepared and thickened because of the hormone called progesterone. Estrogen also works to maintain pregnancy and prepares the mother’s body in many ways. When the blastula reaches the uterus, the trophectoderm cells of the blastula attach to the endometrial cells of the uterus, a process known as implantation. These trophectoderm cells will become the placenta. Growth will occur quickly and the embryo’s cells will undergo differentiation, allowing varied types of cells to form so that they can become various organs. This occurs during gastrulation and the embryo is now considered to be a gastrula. The germ layers that have formed are called the ectoderm, mesoderm, and endoderm. These layers will develop as follows:

The amniotic sac that developed around the fertilized egg is accompanied by a second membrane called the chorion, which develops between the fetus and the mother. The amniotic sac is filled with amniotic fluid, which acts to protect the fetus.
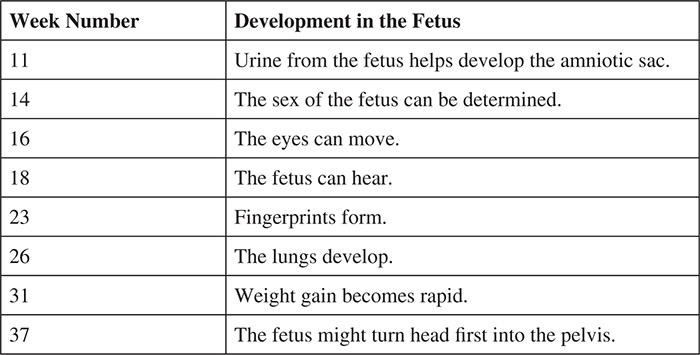
After eight weeks, the embryo is considered to be a fetus. While the fetus is still receiving nourishment from the yolk sac, the placenta is developing. Eventually, the placenta will be fully developed and the formation of the umbilical cord will allow the fetus to receive nutrients from the mother. In weeks 11 through 37 (approximately), one can expect the following:
Around week 39 or so, the mother will begin to go into labor. During the dilation stage, the amniotic sac will break and her cervix will dilate and increase in diameter. This is stimulated by the hormone estrogen. Uterine contractions will be stimulated by the hormone oxytocin. During the expulsion stage, the fetus will pass through the cervix and the vagina. Afterwards, the placenta, chorion, and endometrium are all expelled during the expulsion phase.
For the safety of the fetus and mother, there are several tests than can be performed during pregnancy. Some of these include:

Growth and Development
Once born, a baby is going to grow into a senior adult over many decades. A number of psychologists have outlined our growth over these years and the typical behaviors that we will exhibit during this time. The outlines below show the developmental stages defined by many psychologists and what is expected during growth.

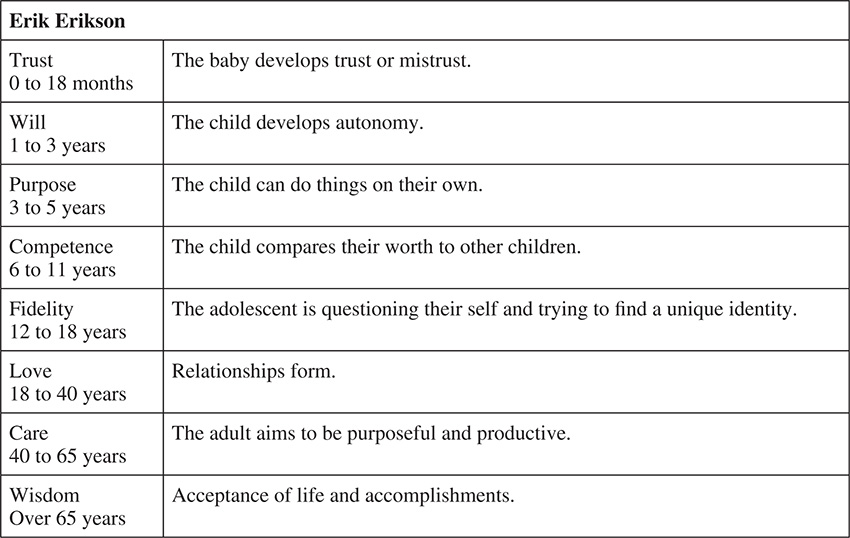




Anatomy and Physiology Quiz
1. Which type of papillae does not contain taste buds?
(A) Circumvallate
(B) Filiform
(C) Fungiform
(D) Foliate
2. Meissner corpuscles, Pacinian corpuscles, Ruffini endings, and end-bulbs of Krause assist allow us to have a sense of
(A) touch.
(B) taste.
(C) sight.
(D) smell.
3. You put more money in a bank account, which allows you to earn more interest, which puts more money in your bank account, which allows you to earn more interest. This is an example of
(A) homeostasis.
(B) equilibrium.
(C) positive feedback.
(D) negative feedback.
4. A hyperbaric chamber is used when someone has a severe case of carbon monoxide poisoning. The hyperbaric chamber will
(A) remove carbon monoxide from the victim.
(B) remove carbon dioxide from the victim.
(C) introduce a high level of inert nitrogen gas to the victim.
(D) force oxygen into the victim at high pressures.
5. The __________ will remove malformed or damaged red blood cells. However, it is also very efficient in recycling any useful components from the red blood cells.
(A) lungs
(B) digestive system
(C) lymphatic system
(D) spleen
6. The __________ can store glycogen in the body, secrete bile, and detoxify the blood.
(A) liver
(B) spleen
(C) kidneys
(D) heart
7. Water reabsorption in the kidneys can be increased when ___________ levels are increased in the body.
(A) Aldosterone
(B) ADH
(C) ANP
(D) none of the above
8. The type of joint that connects adjacent bone by either hyaline cartilage or fibrocartilage is called a __________ joint.
(A) diarthrosis
(B) fibrous
(C) cartilaginous
(D) synovial
9. Implantation involves joining of the endometrial cells of the uterus and
(A) a zygote.
(B) the ectoderm.
(C) the trophectoderm.
(D) the placenta.
10. Leaving the parents, reflecting on one’s future, finding a unique identity, and seeking independence most likely describes someone in
(A) infancy.
(B) adolescence.
(C) adulthood.
(D) childhood.
11. The Bowman’s gland is found in the __________, whereas the Bowman’s capsule is found in the __________.
(A) nose, ear
(B) kidney, stomach
(C) nose, kidney
(D) eye, ear
12. A major change in our setpoint is detected by
(A) receptors.
(B) effectors.
(C) stimulus.
(D) control center.
13. The exchange of gases in tissues is called
(A) ventilation.
(B) perfusion.
(C) internal respiration.
(D) external respiration.
14. The pH of our blood can be maintained by
(A) our lungs.
(B) buffers in the blood.
(C) the kidneys.
(D) All of the above
15. The building blocks of hormones, muscles, antibodies, and skin are
(A) monosaccharides.
(B) amino acids.
(C) fatty acids.
(D) enzymes.
16. Which type/example of transport is different from the other three?
(A) Osmosis
(B) Active
(C) Passive
(D) Diffusion
17. Someone makes the “ok” sign to a friend by touching their pointer finger to their thumb while holding up their three other fingers. This best describes
(A) opposition.
(B) protraction.
(C) depression.
(D) supination.
18. Without the use of chromosomal analysis, the sex of a fetus can be determined by approximately week __________ of pregnancy.
(A) 1
(B) 14
(C) 23
(D) 31
19. Of the following, which one will not examine the genotype of a fetus as to check for chromosomal abnormalities?
(A) Amniocentesis
(B) Triple screen testing
(C) Ultrasound
(D) Chorionic villus sampling
20. Acceptance of life, physical deterioration, and one’s accomplishments would most likely come in our
(A) midlife years.
(B) adult years.
(C) early adulthood years.
(D) senior years.
21. Which example of a feedback loop differs from the other three?
(A) The role of oxytocin during childbirth
(B) The role of sweat in cooling us down
(C) The role of chemicals and platelets during blood clotting
(D) The role of ethylene in fruit ripening
22. Which of the following is true with regards to oxygen and carbon dioxide exchange?
(A) Oxygen is not soluble in blood and needs a low difference in partial pressure to be exchanged.
(B) Oxygen is very soluble in blood and needs a high difference in partial pressure to be exchanged.
(C) Carbon dioxide is very soluble in blood and needs a low difference in partial pressure to be exchanged.
(D) Carbon dioxide is very soluble in blood and needs a high difference in partial pressure to be exchanged.
23. The lymphatic system and lymph nodes have a role of
(A) transporting fluid in the body.
(B) working to fight infections.
(C) fat absorption in the gastrointestinal tract.
(D) All of the above
24. Which of the following vitamins will not be carried by lipids?
(A) Vitamin A
(B) Vitamin B
(C) Vitamin C
(D) Vitamin E
ECOLOGY
Ecology is the study of relationships between organisms and their surroundings. A number of factors can affect an organism and its surroundings. These can be classified as biotic or abiotic. Abiotic factors are the factors that are not living, such as amount of light available, temperature, amounts of water available, and levels of minerals present in the soil. Biotic factors are factors that are living.
Many organisms live in symbiosis, a relationship in which two or more organisms live close to each other and at least one of them benefits from the relationship. If both organisms benefit, the relationship is called mutualism. Commensalism is a symbiotic relationship in which one organism benefits and the other is not affected. Parasitism is a symbiotic relationship in which an organism benefits while harming another.
A group of organisms of the same type living together is called a population; for example, the population of humans in the United States is roughly 360 million people. A community includes many different organisms living in the same area. An ecosystem includes a community and the biotic and abiotic factors that govern the exchange of living and nonliving parts within that ecosystem. Biomes are large regions inhabited by ecologically similar communities of living organisms. Terrestrial (land-based) biomes include deserts and tropical rainforests. Aquatic (water-based) biomes include oceans and lakes. Different biomes have different animal and plant life as well as different climates and weather patterns.
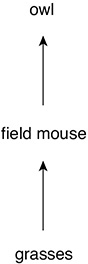
Within ecosystems, materials are passed from one organism to another. Repeated patterns of materials being exchanged are called cycles. Cycles exist for water, oxygen, carbon dioxide, and nitrogen. Producers and consumers also pass materials to one another in a food chain. For example, grass (producer) might be consumed by a mouse (primary consumer), which could then be consumed by a snake (secondary consumer). Because many animals can consume a range of living organisms, we can combine the many food chains together to produce a food web.
A Food Chain
A Food Web
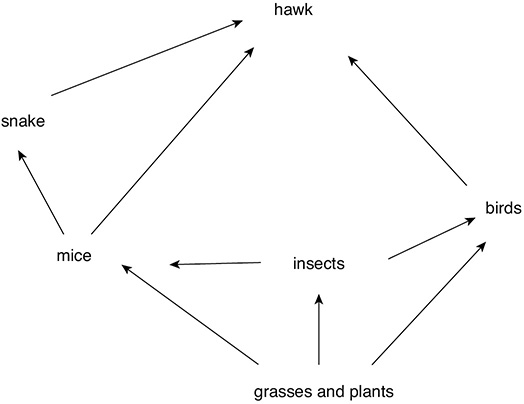
One other important member of the ecosystem is the decomposer. Decomposers break down the remains of dead animals and plants. By doing so, they release substances into the ecosystems that can be reused by other organisms. Examples of decomposers are bacteria, fungi, and detritivores (earthworms, for example).
Humans have had a tremendous impact on the Earth and the environment. The Earth’s population of humans is believed to be more than 6 billion people. Because of this, humans have urbanized areas that once belonged to other species. In addition, pollution of the water, air, and soil has made living conditions harsh for many organisms. Places on Earth that were once habitable by organisms can no longer support life. Efforts to combat the problem include recycling, use of renewable energy sources, pollution control laws, and conservation.
ECOLOGY QUIZ
1. Two organisms live in a symbiotic relationship from which both benefit. This is best described as
(A) mutualism.
(B) parasitism.
(C) commensalism.
(D) conservation.
2. Which of the following is not helpful in protecting the environment?
(A) recycling plastic, glass, and paper
(B) making use of solar energy
(C) burning fossil fuels
(D) driving hydrogen fuel cell cars
3. This question is based on the following:
Grass → Insect → Bird → Hawk
This diagram shows
(A) a food web.
(B) a food chain.
(C) decomposers in action.
(D) what happens when sunlight is not available.
4. Examples of biomes do not include
(A) deserts.
(B) tundra.
(C) tropical rainforests.
(D) neighborhoods in a city.
DIVERSITY OF LIFE
Evolution has produced millions and millions of different types of organisms over a few billion years. Only a fraction of all of the organisms that have ever made an appearance on Earth still exist today. Fossils within rocks, amber, and ice provide the remains of organisms that were once on earth but are now extinct. In addition to providing fossils and clues about the past, rock layers provide clues to the relative ages of fossils and the sequence of which they were once living organisms. Methods of radioactive dating and measuring the decay of certain isotopes can provide a better estimate of the age of a fossil. The isotopes usually used are C‑14 for living organisms and U‑235 for rocks.
The English naturalist Charles Darwin (1809–1882) proposed the theory of natural selection and described it in his book On the Origin of Species in the mid-1800s. According to this theory, all organisms compete for food and survival. Only the organisms that possess the best genes to survive live to reproduce and pass on those “best survival genes” to their offspring. Those organisms that cannot compete die off and are not able to pass on genes that are not favorable to survival. The term natural selection is sometimes described as “survival of the fittest.”
Because of the vast number of organisms that are alive today or that have lived in the past, scientists use a system of taxonomy for classifying organisms. In this system, each organism is classified as a member of a kingdom, phylum, class, order, family, genus, species, and subspecies. To name organisms, scientists use a binomial system that includes the organism’s genus and species. Because the diversity of life is so great and new species are being discovered all the time, the classifications of many organisms have changed over the years.
DIVERSITY OF LIFE QUIZ
1. The scientific name for a house cat is Felis catus. This indicates the house cat’s
(A) kingdom and family.
(B) order and subspecies.
(C) phylum and class.
(D) genus and species.
2. Which of the following statements is false?
(A) The exact age of a fossil can be determined by examining rock layers.
(B) Radioisotope dating can help determine the approximate age of a fossil.
(C) It is possible to determine the approximate age of a rock.
(D) Just a small number of organisms that ever lived on Earth are alive today.
3. Natural selection does not include the idea that
(A) only the genes of the best fit organisms will be passed on.
(B) all organisms have a fair and equal chance of surviving.
(C) only the fittest organisms survive.
(D) there is a competition between organisms for survival.
ANSWERS TO BIOLOGICAL SCIENCES QUIZZES
CELL STRUCTURE
1. B, E, D, A, C
Mitochondria are the sites of energy production. Vacuoles provide storage space in the cell. The nucleus holds the DNA of the cell. The cell membrane is selectively permeable and allows only certain substances to pass through. Ribosomes are the sites of protein synthesis.
2. B Enzymes are proteins and catalysts that can speed up a reaction.
3. A Because the cell is in a solution where the concentration of solutes is higher inside the cell than outside the cell, water is expected to flow into the cell to dilute the concentration of solutes inside the cell. This causes the cell to swell up and possibly burst.
4. C The products of fermentation are ethanol and carbon dioxide. It is the carbon dioxide that causes the dough to rise.
5. D “DNA cannot be altered” is a false statement because DNA can be mutated and changed. The other answer choices are true statements.
6. B Mitosis creates cells that are exact copies of each other, including the number of chromosomes in the cell. Meiosis, which creates sex cells, forms cells with half the number of chromosomes in the original cell.
ORGAN SYSTEMS
1. C The ribs are in front of the lungs in the human body, making them closer to the front of the body. This is best described as being anterior.
2. B Sphincters help keep food in the stomach while being digested, and the anus is a sphincter that holds fecal matter from being eliminated until the organism is ready to do so.
3. A The heart and veins have valves to ensure that the blood flows in only one direction.
4. C A low pH in the stomach, cilia present in the trachea, and mucus present in the nasal cavity are primary defenses. Cells within the body recognizing a pathogen are an example of a third line of defense.
5. A The movement of air through the respiratory system depends on the movements of the diaphragm. It is the movement of this muscle that creates a void space, allowing air to rush in.
6. A Sperm are produced in the testes, stored in the epididymis, and then travel through the vas deferens and urethra.
7. D Cartilage is a soft type of bone that lacks rigidity. This is why you are able to bend your ears.
8. A The autonomic nervous system guides the actions of organs and involuntary muscles. However, because involuntary muscles are involved, you cannot control those actions.
9. D The ureter, although part of the excretory system, is not part of the kidney.
10. C The purpose of the endocrine system is to release hormones (chemical signals) into the body to regulate the body’s functions.
PLANTS
1. C Plant cells are different from animal cells because plant cells have cell walls and contain chloroplasts to carry out photosynthesis.
2. B The roots of a plant are not responsible for carrying out photosynthesis. This is the job of the chloroplasts.
3. C Red and blue light are best absorbed by chlorophyll. This corresponds to wavelengths of 660nm and 430nm, respectively.
4. A The stamen is considered to be a male part of the plant reproductive system, whereas the pistil, stigma, and style (and ovary) are considered to be female parts of the plant reproductive system.
MENDELIAN GENETICS
1. C Fifty percent of the plants will be tall because, as a Punnett square would show, two of the new allele sets will be Tt and the other two will be tt.
2. D If a Punnett square is created showing the cross between a color-blind male (X′Y) and a female carrier (X′X), the resulting combinations will be X′X′, X′Y, X′X, and XY. The underlined offspring are color-blind.

ANATOMY AND PHYSIOLOGY
1. B The filiform type of papillae does not contain taste buds, while the other three forms do.
2. A The mentioned structures are all located in the skin, giving us a sense of touch.
3. C Because the amount of money in the bank continues to grow the amount is not at equilibrium or stable. This example shows a positive feedback loop.
4. D When needing treatment for carbon monoxide poisoning or decompression sickness, the hyperbaric chamber can force oxygen into their lungs to reverse the effects of these cases.
5. D The spleen has many functions, including removing damaged red blood cells and recycling their components. When one looks at the size of the spleen and what it accomplishes, this little organ really “packs a punch.”
6. A All of these processes best describe the liver.
7. B ADH, antidiuretic hormone, does just what the name suggests: it prevents the passing of urine.
8. C As the name suggests, hyaline cartilage and fibrocartilage are found in cartilaginous joints. Synovial joints have a cavity that is filled with fluid while fibrous joints connect bone with fibrous connective tissue.
9. C Implantation involves the joining the trophectoderm cells of the blastula and the endometrial cells of the uterus.
10. B During the teen years, we begin to find a unique identity, seek independence, and look towards a future without our parents.
11. C The Bowman’s gland is part of the nose and involves the secretion of mucus. The Bowman’s capsule is located in the kidney and is the first step in producing urine from the filtering of blood.
12. A Receptors will detect a stimulus (or change in conditions). They then send a signal to the hypothalamus which then signals an effector (muscles, organ, etc.) to respond to the stimulus.
13. C Internal respiration happens at the tissue level whereas external respiration occurs in the pulmonary capillaries. Perfusion relates to the flow of blood, not gases. Ventilation refers to the movement of air in and out of the lungs.
14. D It takes many organs and organ systems to work together to maintain homeostasis. An example of this is our lungs, kidneys, and buffers working to maintain a pH of about 7.35 to 7.45.
15. B Amino acids are the building blocks of proteins. Hormones, enzymes, muscles, antibodies, and skin are just a few examples of what is made of protein in our body.
16. B The diffusion of gases and osmosis (movement of water) are both examples of passive transport—both requiring no energy. However, active transport requires energy.
17. A When we move one finger to touch another, we have engaged in a movement called opposition.
18. B Depending upon how the fetus is positioned, an ultrasound can be used to determine the sex of a fetus. Because the genetic tests are more invasive and carry some risk, they are not used just to determine the sex of the fetus.
19. C Building off of the previous question, we can look at the chromosomes of a fetus via amniocentesis, triple screen testing, and chorionic villus sampling.
20. D According to some psychologists, in order to live better as seniors, we should have a mindset that accepts what we did with our life, that we are going to deteriorate, and that we are nearing death.
21. B Sweat cooling us down is a negative feedback loop, whereas the other three examples are examples of positive feedback loops.
22. C Carbon dioxide is very much soluble in blood. This is why such as low partial pressure difference is needed for it to leave our tissues and enter our blood stream. Oxygen is not very soluble in blood and requires a much higher difference in partial pressure to be exchanged at this level.
23. D The lymphatic system is known for transporting fluids throughout the body. However, it is also part of the immune system and, lymph nodes near the gastrointestinal tract can participate in fat absorption.
24. C Vitamin C is water soluble due to it being a polar molecule. Vitamins A, B, and E are all nonpolar in nature and need a nonpolar lipid to carry them.
ECOLOGY
1. A Mutualism is a relationship in which two organisms live symbiotically and both benefit.
2. C The burning of fossil fuels has raised levels of carbon dioxide, a greenhouse gas, in the atmosphere. This is believed to be the cause of global warming.
3. B This example shows a food chain because only one pathway of energy transfer has been diagramed. A food web would show multiple pathways by which the energy from an organism could be passed along.
4. D Biomes are large regions inhabited by ecologically similar communities of living organisms. Examples are deserts, the tundra, and tropical rainforests. A city neighborhood is not a biome.
DIVERSITY OF LIFE
1. D The scientific name of an organism reflects its genus and species.
2. A Finding the exact age of a fossil by examining a rock layer is impossible. A rock layer can, however, can give an approximate age of a fossil.
3. B The idea that all organisms have a fair and equal chance of surviving is not the case. Organisms must compete for survival and resources. Only those that survive can pass on their genes to offspring, and those genes will produce physical characteristics that aid survival.
REVIEW OF HEALTH AND NUTRITION
Living a Healthy Life and Building Health Skills
One’s overall health takes into account many factors. These factors include physical, mental, emotional, and social well being. With making proper decision making and practicing certain behaviors, one can achieve wellness, making one feel and perform at one’s best. Our physical and social environments can play a major role in developing overall wellness as do our behaviors and attitudes. When we minimize risk behaviors, we can lower potentially threatening factors that impact us negatively. At the heart of building good health skills are decision-making skills, values, and goal setting. Some goals can be long-term or short-term. Either way, goals require an action plan. Also, when one practices good character building, it can have a positive impact both on you and others around you.
Being a Health-Literate Consumer
There are many factors that come into play when making choices regarding food and beverages. Some of these are being influenced by advertisements, cost, quality, and convenience. Food labels have come a long way and have begun to display more information in a clearer, easier to identify manner. Yet, it can still be confusing to read a food label when presented with so much data. Plus, one must consider what they are consuming for the entire day, and not just one meal. Away from food and beverage we also have to be concerned with products that promise certain claims such as weight loss and reversing the aging process. There are, however, a number of agencies in the United States that work to counter deceptive claims and to test the products and medications that we use. Even with these agencies in place, there are drugs and chemicals banned by other government agencies across the globe, but are still used in the United States.
Physical Activity for Life
Physical activity is a vital part in achieving one’s lifelong goal of good health. Physical activity and fitness make you stronger, increase your energy, and positively impact many of your body systems. Physical activity and fitness can also reduce stress and have a positive impact on your emotional and mental health. They can benefit your social health by making your more confident by giving you the chance to work as part of a team. Setting short and long term physical activity goals will lead to many years of quality health. One must be sure to engage in physical activity safely by choosing and using the right equipment and by preparation the body as to prevent injury. Preparation includes rest, hydration, and nutrition, among others. Also, one must remember to include a variety of exercises in their routines as to provide a well rounded impact on your body. Some of these exercises can be moderate, aerobic, flexibility, and anaerobic in nature. Sedentary activities, such as surfing the internet or watching television, should be kept infrequent.
Nutrition and Your Health
The process of your body taking in food and using it is called nutrition. The nutrients your body needs are carbohydrates, proteins, and fats. We also need the right amount of vitamins, minerals, and water. The nutrients obtained from food help you repair and grow, and they provide energy. The foods that one eats can be influenced by culture, ethnic background, cost, convenience, the people around you, and emotions, among others. While there are guidelines provided to us, specific amounts of nutrients that are needed will vary as we move from infancy, childhood, adolescence, adulthood, senior, and our elderly years. With healthful eating patterns and consuming certain nutrients in moderation, it is possible to create a healthy balance.
Managing Weight and Body Composition
One important factor to keep in mind when managing weight is one’s body mass index, BMI. BMI takes into account gender, age, height, and weight and uses a formula to assign you a number. A BMI chart will give one a sense of where they stand (as a percentile) against others who are similar in age, gender, height, and weight. Body composition can also be used when assessing how well someone is managing their weight. When this is calculated, one looks at the ratio of body fat to lean body tissue. While we have concerns about being overweight and obesity, there are also concerns about being underweight. Underweight people might have disorders, such as anorexia nervosa or bulimia, making them stop eating or purge, respectively, because of their fear of being obese.
Achieving Good Mental Health
When a person can handle a range of situations and feelings, they are considered to be emotionally and mentally healthy. Consider what you might encounter on a daily basis as a nurse in an emergency room. You will have to manage and adapt to emotions, be able to accept yourself and others, and handle all of the demands and challenges that you face. Meeting these challenges comes from having a positive self esteem, sense of belonging and purpose, a positive outlook on life, and autonomy. Abraham Maslow created a hierarchy of needs which places a ranking of human needs that are essential to our growth and development. These include our physical needs, safety, belonging, feeling recognized, and reaching our full potential. One’s personality and behavior are also signs of one’s mental health. One who works towards self acceptance and self improvement is someone who is working towards a healthy identity. How one deals with emotion also says much about their mental health.
Managing Stress and Anxiety
A stress is a change in our body’s conditions in reaction to the challenges and demands that we meet every day. Our nervous and endocrine systems are active in our response to a stressor. With the right amounts of sleep, exercise, nutrition, and advanced planning, stress can be minimized. We can also seek support, redirect our nervous energy into a fun activity, relax, laugh, and think positive thoughts to combat stress. When we fear that something will happen, we can develop the condition called anxiety. A prolonged feeling of being hopeless, sad, and helpless is called depression. These two conditions might require professional intervention. Those who can adapt and recover from feelings that impact us negatively are said to be resilient.
Mental and Emotional Problems
When one is mentally ill, there are feelings and thoughts that prevent them from leading a life that is happy, healthy, and productive. Some of these can be organic and brought upon someone by a physical injury or illness while others can be functional and brought upon by stress, conflict, fear, and disturbing events, among others. The types of mental disorders that one can develop range greatly. Even worse, one might respond to stressors with suicide. In the most desperate times, one must remember that seeking help is a sign of being strong and not being weak.
Skills for Healthy Relationships
Relationships are connections that you have with others. A few factors in keeping healthy relationships are communication, respect, honesty, cooperation, compromise, and commitment. While we always want to provide others with constructive criticism and acknowledge their progress, there are times when we will have conflict. If needed, a mediator can assist in finding a conflict resolution. When it comes to family relationships many of the same values listed above hold. However, changes can occur within a family that impact us in a negative way. Some examples are financial issues, the stresses of moving, death of a loved one, or a divorce. These challenging situations can escalate into domestic violence or abuse. There are professionals who can help families overcome these challenges and strengthen relationships. Peer relationships hold many of the same characteristics that family relationships have. However, while there is such a thing as positive peer pressure, one could fall victim to negative peer pressure and make unhealthy decisions. Two ways that this can be done is by harassment or by manipulation. Being assertive in a positive and firm manner about one’s choices is how one can resist these negative impressions.
Violence Prevention
When it comes to preventing violent crime, common sense is one’s first line of preventing problems. Avoiding problematic areas such as a dark parking lot or practicing “safety in numbers” are two examples of preventing crime. Other common sense practices include locking doors and safeguarding valuables as not to attract attention. More recently we have faced issues with school safety. While attacks on students via bullying have increased, we have also witnessed many violent incidents involving guns. Reporting potential threats along with prevention are key in preventing problems before they can occur. One might also want to engage in self-defense training as to be prepared should harm come their way. Finally, when it comes to the home, domestic violence can take its toll on all family members. Reporting the problem and seeking professional help can make matters improve over time.
Personal Care and Healthy Behaviors
When it comes to staying healthy, we are in control of our decisions and behaviors. Part of this is maintaining cleanliness. Taking care of our skin with daily bathing and UV protection are examples. This can help prevent body odor and skin cancer. Our hair needs daily attention to prevent conditions such as dandruff, and our nails need regular attention as to avoid becoming ingrown nails. Oral hygiene can be maintained by brushing and flossing as to prevent tartar, periodontal disease, and bad breath. Like our skin, we want to avoid UV light expose to the eyes by using sunglasses with the proper UV protection. When it comes to listening to our portable devices and/or music, we want protect our ears and avoid loud sounds to prevent tinnitus and deafness. Finally, seeing a specialist such as a dermatologist, eye doctor, or ENT doctor can help prevent and treat problems before they become chronic or life threatening.
Drugs, Alcohol, Tobacco, Medications, and Vaping
There are times in life when we might come across a substance that could possibly cause us to become dependent upon it. This dependency can be physiological or psychological. Substances that cause this dependence are called addictive drugs. The risks of smoking have been well documented. Even worse, we now face a number of people who think that vaping is a “healthy alternative” to smoking or using tobacco products. One must also realize that smoking poses a danger to those around you. Besides excessive alcohol consumption impacting our organs internally, such as our liver, alcohol consumption can increase the chances of engaging in violent or risky behaviors, such as unprotected sexual activity, birth defects, or driving under the influence. Prescription pain killers can become addictive if misused. However, one must not rule out the potential dangers that over the counter drugs can have if not used properly. There is also the battle that society has with drugs such as cocaine, marijuana, crack, and methamphetamines, which are not just costly to our heath but also tax our society with crime. Not using drugs or abusive substances is the practice that one can engage in as the best way to prevent their addiction.
Diseases and Disabilities
Despite our bodies having a number of defense systems, pathogens, such as bacteria or viruses, can penetrate our defenses and cause infections. There are a number of ways in which these pathogens can be transmitted. Indirect methods include air, water, and food, while direct contact methods involve kissing, sexual activity, and sneezing, among others At the heart of prevention is hand washing. Also, one can make sure that common areas, such as bathrooms, are kept clean and disinfected. We can avoid risky behaviors and not come into contact with the bodily fluids of others. Food preparation needs to be done safely. Vaccinations and boosters can also prevent diseases. We can manage stress and get plenty of rest so that our immune system can function properly. There are also diseases that we can develop that are not communicable. Some common examples are cardiovascular disease, cancer, diabetes, asthma, Alzheimer’s, lung disease, kidney disease, obesity, and stroke. Again, focusing on overall health and nutrition and avoiding risky behaviors can keep these diseases at bay. There are also disabilities that might be beyond one’s control. Many people may have a physical disability such as a motor, sight, or hearing impairment. The earlier in childhood that these impairments are recognized, the sooner they can be addressed. With proper accommodations, many people can engage in activities that at one time were considered out of reach.
Sexually Transmitted Diseases, Including HIV and AIDS
Sexually transmitted diseases can be a higher risk for those who are sexually active with more than one person, have unprotected sex, choose to have high risk partners, or are under the influence of drugs or alcohol. The human papillomavirus, HPV, can lead to genital warts and possibly cervical cancer or cancer of the penis. Genital herpes is another type of STD, which can cause sores on the body. HIV, human immunodeficiency virus, was discovered about 35 years ago and been a major health pandemic. Transmission methods include blood, semen, vaginal fluids, and breast milk. While those who are at high risk can take a dose of PrEP every day to keep HIV from establishing itself, avoiding risky behaviors and taking proper preventative measures are still best.
Injury Prevention and First Aid
While accidents may make entertaining videos to watch online, many accidents and injuries that people have are not funny and are plenty preventable. The home is the first place to start when it comes to being safe. Fire extinguishers, smoke and carbon dioxide detectors, and alarm systems are just the beginning. We also need to keep mindful of safety with the stove, keeping household chemicals out of reach, keeping volatile organics out of the house, electrical safety, ladder safety, and avoid tripping hazards. At the workplace, we want to be mindful of the same hazards as well. When it comes to recreational hazards, we want to choose the right equipment that will protect us. We also want to have water to prevent dehydration and clothing to prevent hypothermia. When driving, we want to be free of alcohol or drugs and we want to be well rested so that we can stay alert. Finally, we want to be prepared for all weather emergencies such as blizzards, tornadoes, or hurricanes. Being prepared with the right supplies and having knowledge of first aid can make a difference between life and death.
Environmental Health
One last word on keeping healthy is keeping the environment that we live in clean. While there are laws in place to keep air pollution down, we also need to focus on indoor air pollutants such as radon, asbestos, smoke, aerosol sprays, and volatile organics. These pollutants can cause asthma and other respiratory conditions. We also need to avoid noise pollution both indoors and outdoors because high decibel levels can lead to hearing loss over time. This pollutant can range from frequent trips to concerts to having earphones that are used with the volume too high. Water pollution can occur as well. There have been times when certain communities have been told to boil their water or not drink the water at all. Water filtration systems can help with the purity level of our water. We also want to make sure that local waste sites are not impacting the quality of our water, food, and air. While we do have many systems in place for recycling materials, keeping our carbon footprints low will mean that we are having a less taxing impact on the Earth.
CHAPTER REVIEW QUESTIONS ON HEALTH AND NUTRITION
1. Decision-making skills, values, and goal setting are all
(A) needed when building good health skills.
(B) intended to derail us from accomplishing tasks.
(C) negative influences on others.
(D) factors that increase risky behaviors.
2. When reading a food label on a box or bottle
(A) we only read the data on the front of the package/bottle.
(B) we only read the chart containing the nutritional facts.
(C) we read the nutritional facts, the ingredients, and the entire food label.
(D) we just consume the product knowing that exercise later on can overcome the calories consumed.
3. Physical activity can
(A) increase your social well being.
(B) be dangerous if the right precautions and equipment is not used.
(C) be enhanced with rest, hydration, and proper nutrition.
(D) All of the above
4. __________ can be influenced by cost, culture, convenience, and emotions.
(A) Stress
(B) Nutrition
(C) Disease
(D) Accidents
5. BMI calculations take into account
(A) age and gender only.
(B) height, ethnic background, and age.
(C) weight, height, age, and gender.
(D) ethnic background, weight, height, age, and gender.
6. When one can handle stress, challenges, manage and adapt to emotions, and be able to accept themselves and others, are considered to be
(A) in good physical health.
(B) in good mental health.
(C) in a state of disarray.
(D) managing obesity.
7. Feelings of being helpless, sad, and hopeless are signs of
(A) anxiety.
(B) stress.
(C) resiliency.
(D) depression.
8. An organic mental illness can be brought upon by
(A) an injury.
(B) fear.
(C) conflict.
(D) stress.
9. Harassment, bullying, and manipulation are all examples of
(A) positive peer pressure.
(B) healthy relationships.
(C) negative peer pressure.
(D) conflict resolution.
10. Violence can be prevented by
(A) keeping one’s home secured.
(B) reporting bullying.
(C) seeking help for domestic issues.
(D) All of the above
11. Preventing tartar building, periodontal disease, and gingivitis can be assisted by
(A) a general practitioner.
(B) a gastroenterologist.
(C) an ENT.
(D) a dentist.
12. Alcohol consumption
(A) carries more carcinogens than cigarette smoking.
(B) can lead to risky behaviors.
(C) cannot lead to dependency.
(D) is not harmful to the liver if one drinks plenty of water.
13. Which of the following diseases is different from the other three because it is communicable?
(A) HIV/AIDS
(B) Diabetes
(C) Asthma
(D) Cancer
14. Which of the following is least likely to spread HIV?
(A) Semen
(B) Hand shaking
(C) Vaginal fluids
(D) Breast milk
15. Bottled water, canned goods, a first aid kit, and flashlights with fresh batteries will best prepare us for
(A) a car accident.
(B) a fire in the home.
(C) a hurricane.
(D) an accident while walking down steps.
16. Which of the following is most likely going to impact the indoor air quality of your home?
(A) Using a roach spray
(B) Filtering the water
(C) Keeping the radio volume down
(D) Avoiding power strips from being overloaded
17. In setting health goals, we can
(A) set short-term goals.
(B) set long-term goals.
(C) create an action plan.
(D) All of the above
18. A prepared food item is consumed by a person daily for lunch. A particular ingredient is suspected of leading them to high blood pressure. They most likely were eating a food that was high in
(A) sodium.
(B) iron.
(C) fat.
(D) sugar.
19. To improve our emotional health, mental health, and fitness, we can engage in
(A) smoking.
(B) drinking alcohol.
(C) physical activity.
(D) drug use.
20. Which of the following has a low nutritional value?
(A) Fruits and vegetables
(B) Sweets and candies
(C) Meats, beans, fish, and poultry
(D) Milk, yogurt, and cheeses
21. Which might be the BMI of a person who is doing an excellent job in maintaining their BMI?
(A) 16.5
(B) 20.4
(C) 28.5
(D) 31.1
22. Reaching our full potential, feeling recognized, belonging, safety, and physical needs are parts of the hierarchy presented by
(A) Bloom.
(B) Pavlov.
(C) Einstein.
(D) Maslow.
23. One who can overcome from negative feelings is considered to be
(A) resilient.
(B) stressed.
(C) depressed.
(D) anxious.
24. Someone who seeks counseling or professional help
(A) must have been impacted by an organic mental illness.
(B) must have been impacted by a functional mental illness.
(C) is showing signs of being strong.
(D) is showing signs of being weak.
25. Professionals can help us deal with
(A) suicidal thoughts.
(B) domestic violence.
(C) addictions.
(D) All of the above
26. Which of the following is least likely to address a problem with cyber bullying?
(A) Monitoring what your child is subjected to on social media
(B) Telling your child that they should just ignore it
(C) Getting the school’s administration involved
(D) Reporting it to local authorities
27. Tinnitus is a condition that impacts your
(A) skin.
(B) eyes.
(C) ears.
(D) scalp.
28. Lung cancer and addiction to nicotine can be caused by
(A) smoking.
(B) excessive alcohol consumption.
(C) methamphetamines.
(D) pain killers.
29. In helping one overcome a disability, we can
(A) pass laws to provide accommodations.
(B) provide early intervention.
(C) detect the disability early in life.
(D) All of the above
30. PrEP has been found to help to prevent
(A) HIV/AIDS.
(B) HPV.
(C) STD.
(D) All of the above
31. A helmet, knee pads, and wrist guards are something you might purchase when participating in
(A) camping.
(B) rollerblading.
(C) handball.
(D) speed walking.
32. Nuclear energy has risks and benefits of use. Which one of the following is different from the other three?
(A) Food preservation
(B) Energy that does not contribute to greenhouse gases
(C) Medical diagnostics
(D) A meltdown at a facility
33. Good character building can positively impact
(A) you only.
(B) others only.
(C) neither you nor others.
(D) both you and others.
34. Why is it important to eat fruits and vegetables that vary in color?
(A) Different pigments indicate different nutrients being present.
(B) Different pigments will have more appeal to your palate.
(C) Different pigments will help you develop your artistic abilities.
(D) Different pigments will be more appealing to your eyes.
35. Rest, hydration, and nutrition can help you with
(A) recovery only.
(B) preparation only.
(C) both recovery and preparation.
(D) neither recovery nor preparation.
36. Which of the following is properly paired up with the number of calories per gram?
(A) Sugar, 9 calories per gram
(B) Fat, 9 calories per gram
(C) Protein, 9 calories per gram
(D) Fat, 4 calories per gram
37. Besides BMI, managing weight can be achieved by monitoring one’s
(A) anorexic index.
(B) shape in a mirror.
(C) body composition.
(D) bulimia index.
38. An anemic person lacks
(A) iron.
(B) potassium.
(C) DNA.
(D) calcium.
39. A change in conditions is called a
(A) relaxer.
(B) stimulant.
(C) depressor.
(D) stressor.
40. Someone who chooses a diet high in fat and cholesterol is most likely to develop
(A) coronary heart disease.
(B) obesity.
(C) cancer.
(D) All of the above
ANSWERS TO HEALTH AND NUTRITION CHAPTER REVIEW QUESTIONS
1. A Those who set goals, have values, and have good decision making skills will have good health skills.
2. C When reading a food label, we want to review the ingredients, the nutritional facts, and other information (such as a warning about containing nuts) that is presented.
3. D Being physically active can improve your social skills, be dangerous if not engaged in properly, and be enhanced with the right preparation and recovery.
4. B There are a number of factors that influence what we eat. This is why we see such variation from country to country and even variation within different regions of a country.
5. C BMI takes into account weight, height, age, and gender.
6. B The question addresses many quality traits of those who are in good mental health.
7. D While choices B and C not incorrect, one must not confuse the signs of depression with the signs of anxiety, which is the fear that something will happen.
8. A An organic mental illness is brought upon by an injury. This can be compared to a functional mental illness which can be brought upon by many factors.
9. C Harassment, bullying, and manipulation are all types of negative peer pressure and do not contribute to the betterment of others.
10. D Recognizing and addressing possible precursors to violence can be a way to prevent problems and violence.
11. D All of the conditions listed involve the teeth and/or mouth. Besides seeing a dentist to address these issues, halitosis (bad breath) can also be addressed by a dentist.
12. B Alcohol consumption can negatively impact our liver, and become addictive, and can lead to risky behaviors such as drunk driving, violence, and unprotected sex.
13. A HIV/AIDS is a communicable disease while the other three choices are not.
14. B HIV is spread via bodily fluids. Shaking the hand of someone who is HIV positive carries little risk.
15. C Everyone needs to have a roadside assistance plan when driving, a plan in case of fire, and a plan for when a natural disaster strikes. The listed items will help best during a natural disaster.
16. A Prolonged exposure to airborne particles in the home can have a negative impact on health. Filtering the air and allowing fresh air to enter the home can minimize the impacts of these substances.
17. D Having a concrete set of goals and a plan will help people reach their short- and long-term health goals.
18. A Sodium consumed in excessive amounts has been linked to high blood pressure. Excessive consumption of fat can lead to obesity and heart disease while excessive consumption of sugar can lead to diabetes.
19. C Engaging in physical activity and having good blood circulation can improve our health in so many ways.
20. B Sweets and candies have little to no nutritional value. This is why they are often referred to as “empty calories”.
21. B A person with a BMI value under 19 is considered to be underweight. From 20-24, they are considered normal while 25-29 puts them in a category of overweight. Those who have a BMI of 30 or above are considered obese.
22. D All of the mentioned are parts of Maslow’s hierarchy.
23. A Being able to overcome obstacles in one’s life is what makes them resilient.
24. C There are many things in our life that can impact our mental well being. While it might look weak to seek help, those who seek help are strong in that they are taking action to overcome these negative feelings.
25. D There are a range of professionals, counselors, and agencies to help us in any stage of our lives from infancy to being elderly. It is best to seek assistance when the need arises.
26. B Bullying is not something that should be ignored. Local authorities and school administrators have procedures in place to combat this problem. In addition, parents need to be mindful of possible sources of bullying that students can encounter, such as social media.
27. C Tinnitus is a “ringing in the ears” that can occur from prolonged subjection to loud noises.
28. A Smoking can lead to lung cancer and get someone addicted to nicotine. However, smoking has also been shown to impact so many of our organs in a negative manner.
29. D Many laws have been passed over the years that allow those who disabilities to have better access. Lower water fountains, specially sized bathroom stalls, and ramps are just a few examples. For those who do have a disability, the earlier in life that it is detected and addressed, the less likely the disability will hold back the child.
30. A PrEP is a combination of drugs that can stop HIV from establishing itself. However, the drug needs to be taken daily to be most effective.
31. B While each of the activities listed has proper equipment needed, the listed equipment in the question best suits someone who is rollerblading or engaging in a sport which involves them moving on wheels.
32. D While the word “nuclear” has many negative connotations to it, there are medical and beneficial uses to radiation. If radiation is used properly and in the right amounts, it can be beneficial. However, there have been accidents in the past that remind us of the harm that nuclear radiation can do.
33. D Having a healthy attitude can positively impact you and others. This can range from a simple smile to an act of kindness.
34. A Different colors appear in different fruits and vegetables because of the different nutrients present. For example, a red tomato contains more lycopene while an orange carrot contains more beta carotene.
35. C The proper amount of rest, hydration, and nutrition can prepare us for physical activity or help us recover from physical activity.
36. B Sugars and proteins each provide 4 calories per gram while fats provide 9 calories per gram. It takes twice as much work to “burn off” one gram of fat than it does one gram of sugar or protein.
37. C When managing weight, one could use a calculated BMI or calculate their body composition.
38. A A person who is anemic lacks iron in their diet.
39. D A stress is a change in conditions. What is important is how mentally, emotionally, and physically fit we are to address those changes.
40. D Diets high in fat and cholesterol have been shown to increase the risk of what is listed in the question and other health problems.
REVIEW OF PHYSICAL SCIENCES: CHEMISTRY
SCIENTIFIC NOTATION AND THE METRIC SYSTEM
Because science often deals with measurements that are much larger or smaller than our usual measurements, scientists use scientific notation to express those measurements.
Scientific Notation
Suppose you wanted to express the number of carbon atoms in one mole of carbon. One way is to say that there are 602,000,000,000,000,000,000,000 atoms of carbon present, but it is much easier to write that number in scientific notation as follows: 6.02×1023. In scientific notation, the number is simplified. The zeros are replaced by a power of 10, which indicates the number of places to move the decimal point left or right to write the actual number. Note the use of powers of 10 in the following examples:
1×100=1
1×102=100
1×104=10,000
1×10-1=0.1
1×10-3=0.001
When you write a very large or very small number, count the number of zeros or decimal places in the number and use a power of 10 to express the number in scientific notation.
The Metric System
When expressing measurements in the metric system, often you can indicate powers of ten by adding common prefixes to the base units. Some examples are shown below:
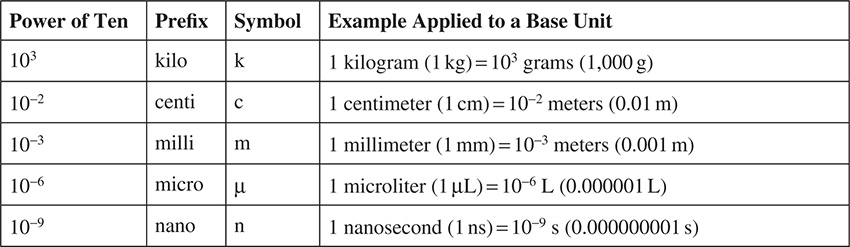
SCIENTIFIC NOTATION AND THE METRIC SYSTEM QUIZ
1. The number 1,000,000 is what power of 10?
(A) 10-6
(B) 106
(C) 16
(D) 0.000001
2. The quantity 6,180 meters can be rewritten as
(A) 6.180 × 103 meters.
(B) 6,180 kilometers.
(C) 6,180 × 103 meters.
(D) 180 × 103 meters.
3. How many millimeters are there in one centimeter?
(A) 10,000
(B) 1,000
(C) 100
(D) 10
ATOMIC STRUCTURE
Dalton’s Atomic Theory
A number of theories about the structures of the atom have been proposed over the years. At one time, it was thought that an atom was the point where a piece of matter could no longer be divided. Hence, atoms were named after the Greek word atomos, meaning “indivisible.”
The English chemist John Dalton (1766–1844) first developed the modern atomic theory. His theory, which summarized what was known about the atom in his time, was as follows:
1. All elements/matter are composed of atoms.
2. All atoms of the same element are exactly alike.
3. Atoms of different elements are different.
4. Compounds are formed by the joining of atoms in different ratios, and chemical reactions cause atoms to rearrange into different compounds.
Elements, Compounds, and Allotropes
Elements are substances that cannot be broken down chemically. Elements can bond together to form compounds. The bonds that form can later be broken so that the atoms can arrange again to form new compounds. Here is an example:
CH4+2O2→2H2O+CO2
In this example, the elements carbon (C), hydrogen (H), and oxygen (O) are present. The reactants (CH4 and O2) and products (H2O and CO2) are composed of the same elements. However, rearrangement of these elements has formed different compounds.
Allotropes are different forms of the same element. For example, oxygen can be found as diatomic oxygen, O2, or ozone, O3. Allotropes of carbon include diamonds, graphite, or buckminsterfullerene.
Subatomic Particles
Although the atom is considered the basic unit of matter, it is composed of three smaller subatomic particles. These are called protons, neutrons, and electrons, and they can be summarized as follows:
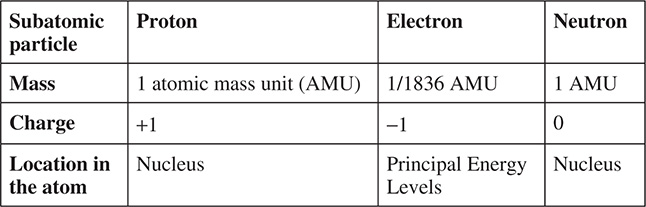
The Nucleus of the Atom
The nucleus of an atom contains the element’s protons and neutrons. The chemical symbol for the atom will tell you how many of each are present. Here is an example using the symbol for oxygen (O):
This symbol gives you the mass number (16) and the atomic number (8). The atomic number is the number of protons in the nucleus of the atom. In an atom of neutral charge, the number of electrons is the same as the number of protons. The mass number is the total number of nucleons (protons plus neutrons) in the nucleus of the atom. In the example above, there are eight protons in the nucleus of the oxygen atom. The number of neutrons is also eight (16 nucleons –8 protons=8 neutrons).
Isotopes
Even though Dalton’s atomic theory states that all atoms of a given element are alike, this idea has been modified by later discoveries. Consider two atoms of chlorine: chlorine-35 and chlorine-37. Let’s compare them with respect to their subatomic particles in the table that follows.

Note that the two atoms have different numbers of neutrons present. As a result, they have different mass numbers. Because the two atoms have the same number of protons, they are both the element chlorine. We say that atoms like these, which have the same number of protons but different numbers of neutrons and thus different mass numbers, are isotopes of each other.
Mass Number Compared to Atomic Mass
Do not confuse mass number with atomic mass. Although the mass number indicates the number of protons and neutrons for one particular isotope, the atomic mass accounts for all of the isotopes of an element. For example, the atomic mass of chlorine is 35.45. This is because the atomic mass takes into account the relative amounts of Cl-35 and Cl-37. A similar pattern holds true for bromine. The atomic mass of bromine 79.90 takes into account its two most abundant isotopes, Br-81 and Br-79.
Nuclear Reactions
Although the root meaning of atom is “indivisible,” by no means is the atom indivisible. Nuclear reactions can cause transmutations (changes in the nucleus). A fusion reaction is a nuclear reaction in which the nuclei of atoms are joined. An example is the joining of hydrogen nuclei in the sun to produce heavier elements and energy. A fission reaction is a nuclear reaction in which a nucleus or nuclei is/are split. Energy is released in this type of reaction as well. Fission reactions can be found in atomic weapons and nuclear energy reactors. Fusion reactions can be found in our sun and in stars.
Radioactive Nuclear Decay
Some atomic nuclei are unstable and lose particles or radiation by emitting radioactive alpha particles, beta particles, or gamma radiation. This process is called radioactive decay; when it takes place, the atom is transformed from one type to another. The rate of transformation is called the half-life (the time it takes for half of a sample of a radioactive substance to decay), and it is different for each radioactive isotope of an element. Below are three examples of radioactive decay. In each case, a difference radioactive particle or radiation is emitted. Note the formulas and symbols used to denote each of the two types of particles as well as the gamma radiation.
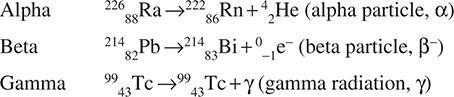
Uses for Radioisotopes
Certain radioactive isotopes (radioisotopes) have medical uses. These substances are used by the body as usual because the body cannot distinguish between isotopes of elements. Once in the body, they can be used via a scan to create pictures of the body’s internal organs. In addition, the radioactive particles emitted by radioisotopes can be used to fight cancer. Examples are radioactive “seeds” that are placed in the prostate to fight prostate cancer and radiation therapy that is used to counter a tumor.
Electron Configurations
The electrons are located in regions around the nucleus called orbitals and are arranged in a series of principal energy levels (PELs). Each PEL can hold up to a certain maximum number of electrons. You can calculate each maximum using the formula 2n2, where n is the number of the PEL. Thus, the maximum numbers of electrons in the first four PELs are as follows:

To picture how the electrons are arranged, you can imagine them filling the PELs starting with the level 1. When each level is filled to its maximum, the electrons begin filling the next level until all the electrons are in place. If you were to write the electron configuration of an atom of sulfur (sixteen electrons) it would look like this: 2-8-6. In other words, the sulfur atom has two electrons in the first PEL, eight in the second, and six in the third. Here is the electron configuration of nitrogen with its seven electrons: 2-5.
Atoms versus Ions
As atoms interact with each other, they sometimes gain or lose electrons. As a result, the number of electrons in the atom will be different from the number of protons. An atom of this kind is called an ion. An ion with more electrons than protons has a negative change and is called an anion. An atom with more protons than electrons has a positive charge and is called a cation. For example, an atom of nitrogen normally has seven protons and seven electrons. However, it may gain three electrons to form N3–, an ion with seven protons (7 positive charges) but ten electrons (10 negative charges), giving a total charge of –3. An ion with a positive charge is the sodium ion Na1+. This ion has lost one electron and now has eleven protons (11 positive charges) but only ten electrons (10 negative charges), giving a total charge of +1.
ATOMIC STRUCTURE QUIZ
1. Explain how isotopes are inconsistent with Dalton’s theory that “all atoms of the same element are alike.”

Circle the letter of your choice.
2. Which isotope below contains the greatest number of neutrons?
(A) 3517Cl
(B) 188O
(C) 4018Ar
(D) 4120Ca
3. Aluminum (Al) has 13 protons in its nucleus. What is the number of electrons in an Al3+ ion?
(A) 13
(B) 10
(C) 16
(D) 3
4. How many electrons can be held in the third PEL of an atom?
(A) 2
(B) 8
(C) 18
(D) 32
5. Fill in the chart below.

6. An atom with more protons in its nucleus has _________ an atom with fewer protons in its nucleus.
(A) a lesser nuclear charge than
(B) a greater nuclear charge than
(C) the same nuclear charge as
(D) no nuclear charge compared to
7. The half-life of a certain radioactive isotope is 5 days. How much of a 100-gram sample of this radioactive isotope will remain after 10 days?
(A) 25
(B) 50
(C) 75
(D) 100
8. What is the name of particle X in the following reaction?
23290 Th→22888Ra+X
(A) deuterium
(B) gamma radiation
(C) beta particle
(D) alpha particle
9. The electron configuration for neon is
(A) 2-5.
(B) 2-8.
(C) 2-18.
(D) 2-10.
THE PERIODIC TABLE
In the periodic table, the different elements are shown in order by increasing atomic number. The table is configured in such a way that different locations on the table correspond to different properties of the elements. As you move from one location to another on the table, the elements possess or lack different properties, or possess those properties to a greater or lesser extent. That is why the table is said to show “trends” among the elements. By knowing the location of an element on the periodic table, you can tell the properties of that element.
Each horizontal row in the periodic table is called a period. The periods correspond to the PELs that are filled by increasing numbers of electrons as you move from one element to the next across the row from left to right. The vertical columns are called groups or families. Each one contains elements with similar properties.
Periodic Table of the Elements

Metals, Nonmetals, and Semimetals
Metals make up about two-thirds of the elements in the periodic table. They are mainly located on the left and center areas of the table (hydrogen, a nonmetal, is an exception; it is located on the left). On the upper right side of the table are the nonmetals. In between are the semimetals: B, Si, As, Te, At, Ge, and Sb. Note how they form a “staircase” that separates the metals from the nonmetals. The properties of metals and nonmetals are outlined below.
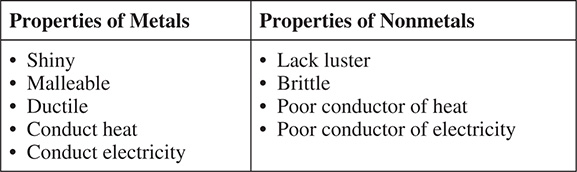
Atomic Radius
As you move from left to right across a period on the table, each element has a smaller atomic radius (distance from the nucleus to the outermost electron) than the one before. For example, in period two, lithium (Li) on the left side of the table has a larger atomic radius than fluorine (F), which is located on the right side of the periodic table. As you proceed from top to bottom in a group/family, the atomic radius increases as well.
Ionization Energy
The ionization energy of an atom is the energy required to remove the outermost electron from the atom. This energy is needed to overcome the attraction between the positively charged protons and negatively charged electrons. As you proceed from left to right in a period, the ionization energy of the elements increases, indicating their greater ability to attract or “hold on to” electrons. As you proceed from top to bottom in a group/family, the ionization energy of the elements decreases and electrons are lost more easily.
Electronegativity
The ability to attract electrons is called an element’s electronegativity. As you proceed from left to right in a period, the electronegativity of the elements increases. As you proceed down a group/family, the electronegativity of the elements decreases. One element to keep in mind is fluorine (top right corner of the periodic table) because it has the highest electronegativity of all of the elements. Use it as a reference for trends in electronegativity.
Groups and Families
The elements in the groups or families (vertical columns) of the periodic table exhibit similar chemical properties. That is because they have similar electron configurations, form ions of the same charge, and react in a similar fashion. For example, fluorine and chlorine each have seven electrons in their outermost PELs, 2-7 and 2-8-7, respectively. This places fluorine and chlorine in the same family. Both atoms will also form ions with the same charge of 1–. The names and properties of some selected families are shown in the following chart.
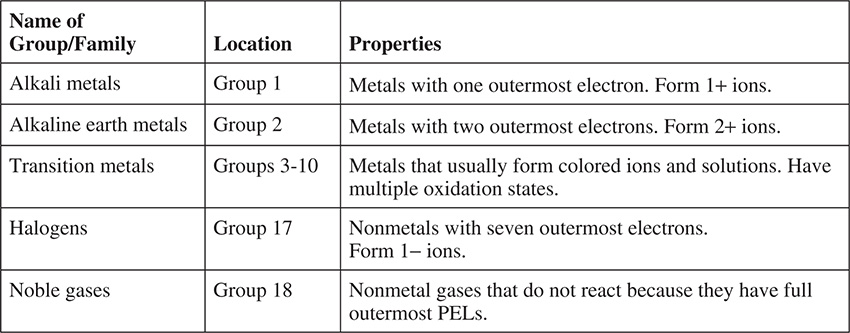
PERIODIC TABLE QUIZ
Circle the letter of your choice.
1. The arrangement of the modern periodic table is based on atomic
(A) mass.
(B) number.
(C) radius.
(D) electronegativity.
2. Where on the periodic table are the nonmetals located?
(A) upper right
(B) upper left
(C) lower right
(D) lower left
3. Which two elements have chemical properties that are similar?
(A) H and He
(B) Fe and W
(C) Li and Be
(D) Mg and Ca
4. Which element has the lowest electronegativity?
(A) F
(B) Cl
(C) Br
(D) I
5. Which of the following elements has the greatest atomic radius?
(A) strontium
(B) fluorine
(C) neon
(D) cobalt
6. Which statement below is false?
(A) Hydrogen is a nonmetal.
(B) Aluminum is a semimetal.
(C) Calcium is a metal.
(D) Argon is a gas at room temperature.
BONDING
Covalent Bonds and Ionic Bonds
Bonds between atoms can be formed when electrons are transferred or shared. The types of atoms involved in the bond also determine what type of bond will form. Two kinds of bonds are covalent bonds and ionic bonds. The differences between these two types are outlined in the chart below.

Examples of covalently bonded substances are H2O (water), CH4 (methane), and NH3 (ammonia). Ionic substances are salts such as NaCl (sodium chloride), KI (potassium iodide), and CaCl2 (calcium chloride). Noting the types of elements present in the covalent compounds and the ionic compounds, you see that covalently bonded substances have all nonmetal atoms, whereas the ionic substances contain metals and nonmetals.
If you further examine the electron configuration of Na and Cl before bonding and after bonding, you see that when one electron is transferred, each atom ends up with the full quota of eight electrons in its outermost principal energy level (this is the “octet rule”).
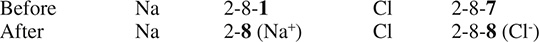
If you look at covalent bonding in ammonia (NH3), you see how two nonmetals share electrons. The three hydrogen atoms each have one electron in their outermost PEL, and nitrogen has five electrons in its outmost PEL. After they bond, there will be eight electrons around the nitrogen atom, again fulfilling the octet rule and becoming more stable due to the eight electrons present.
If two atoms of the same nonmetal element, such as the two hydrogen atoms in H2, should bond, then the covalent bond is termed nonpolar. The easy way to identify nonpolar bonds is in diatomic molecules where there is no difference in electronegativity between the elements. Examples of these diatomic molecules are O2, F2, and Br2. If the elements in the covalent bond have different electronegativity values, the unequal sharing of electrons causes the bond to be polar. Familiar substances that have polar covalent bonds are H2O, NH3, and HCl.
Formation of Covalent Bonds in Ammonia
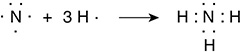
Types of Reactions
Reactions between substances do not cause matter to be created or destroyed. Instead, new compounds are formed by the rearrangement of atoms. There are four general categories of reactions, depending on how the substances involved rearrange. (As you study more chemistry, you will learn that there are also names for many specific types of chemical reactions.) The four basic categories are as follows:
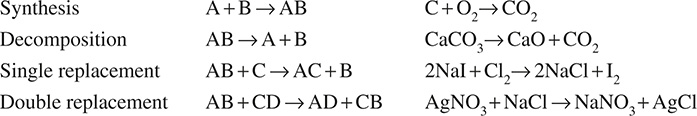
Endothermic versus Exothermic Reactions
As discussed earlier, there are reactions, such as fusion and fission reactions, that can release energy. These reactions are classified as exothermic reactions (literally, “exit heat”). Reactions or processes that absorb heat are classified as endothermic reactions (literally, “enter heat”). Placing a cold pack on an injured leg is an example of an endothermic process because the ice pack allows the heat from the leg to enter the ice pack.
What is it that causes the absorption or release of heat energy? It all comes back to the breaking or formation of bonds. Breaking bonds causes energy to be absorbed, whereas the formation of bonds causes energy to be released. What makes a reaction exothermic or endothermic is the overall amount of energy that is needed to break and create bonds; one process is sure to involve more heat than the other. This heat energy can be measured in calories or joules.
BONDING QUIZ
Circle the letter of your choice.
1. Which compound contains a bond with no ionic character?
(A) CO
(B) CaO
(C) K2O
(D) Na2O
2. When K bonds with I, the
(A) electrons are shared.
(B) potassium gains electrons which are lost by iodine.
(C) two elements form a covalent compound.
(D) potassium loses one electron to iodine.
3. Which compound below has a nonpolar bond in which the electrons are being shared equally?
(A) H2O
(B) NH3
(C) Cl2
(D) CH4
4. Which of the following processes is endothermic?
(A) ice melting
(B) a piece of paper burning
(C) a bomb exploding
(D) an organism’s metabolism producing a certain amount of heat
5. Which sentence best describes the following reaction?
2H2(g)+O2(g)→2H2O(l)+heat
(A) It is an endothermic reaction.
(B) It is an exothermic double replacement reaction.
(C) It is a synthesis reaction that is also exothermic.
(D) It is a decomposition reaction that is also endothermic.
PHASES OF MATTER
Temperature Scales
Temperature is the measure of the average kinetic energy of a sample. In other words, temperature measures the amount of motion of a sample’s molecules. In the United States, Fahrenheit is the temperature scale that is used. In this scale, 32 degrees, or 32°F, is the freezing point of water, and 212 degrees, or 212°F, is the boiling point of water. Elsewhere, the Celsius scale is generally used. The Celsius temperature scale is based on the freezing and boiling points of water; 0 degrees Celsius, or 0°C, is the freezing point of water, and 100 degrees Celsius, or 100°C, is the boiling point of water. There is also a third scale, the Kelvin scale. In the Kelvin scale, water freezes at 273°K and water boils at 373°K. The Kelvin scale is based on the fact that a temperature below absolute zero cannot be achieved. Kelvin is the preferred scale for calculations involving temperature because there cannot be a zero or negative number for the Kelvin temperature. The relationship between Kelvin and Celsius is K=C+273.
Gases, Liquids, and Solids
The three phases of matter are gas, liquid, and solid. As energy is added to or removed from a sample, its phase can change. The following table compares the three phases.

You can also outline the processes of changes in phases as follows:
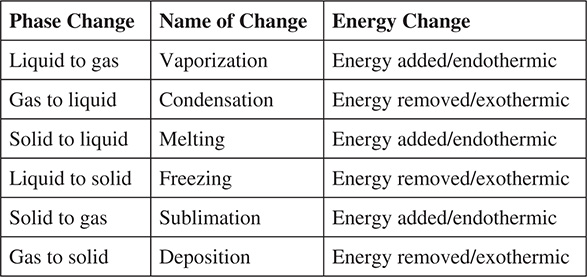
A phase change diagram can help you understand how energy affects changes in phase and changes in temperature. The phase change diagram below is also called a heating curve. In this curve, you are examining the effect of heat on phase and temperature as you follow a sample from its solid state to its gaseous state. A few points should be noted as you follow the curve upward:
• As the temperature (average kinetic energy) of the sample increases, the phase remains the same.
• As the phase of the sample changes (change in potential energy), the temperature remains the same.
• Two phases can exist at the same temperature (in equilibrium with each other).
With regard to the vaporization of liquids, liquids that evaporate at a very high rate, given the same temperature, are called volatile.
Heating Curve for Water
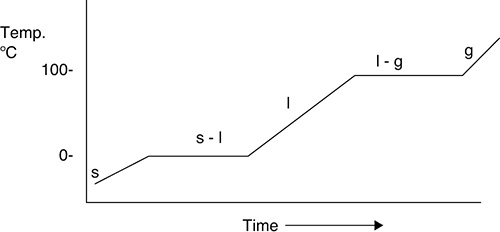
Density of Solids, Liquids, and Gases
Consider 1.0 kilograms of lead and 1.0 kilograms of feathers. Which one makes for more fun at a pillow fight? Should there be a difference if both samples have the same mass of 1.0 kilograms? The clear difference is in the density of the materials in question. When calculating the density of a solid or liquid, the mass of the sample is divided by the volume of the sample. This shows how much mass is “packed” into a certain amount of space. The equation for the density of solids and liquids is D=mass/volume. The units are in grams/milliliters. When determining the density of a gas, a larger volume needs to be considered because of the nature of gases. The density of a gas is calculated by dividing its gram formula mass (GFM, from the periodic table) by 22.4 liters giving Dgas=GFM/22.4L. The density of water is 1g/mL. This is helpful in determining if another liquid or solid will sink or float once placed in water.
The Gas Laws
Gases, because of their compressibility, behave according to a number of specific laws. Although it would be too much to look at each one in depth, a general outline helps review the major concepts. These laws are summarized in the chart below.

PHASES OF MATTER QUIZ
Circle the letter of your choice.
1. Which one of the following substances can be compressed the most?
(A) NaCl(aq)
(B) O2(g)
(C) H2O(s)
(D) Br2(l)
2. Definite shape and definite volume best describes a sample of
(A) I2(s).
(B) Br2(l).
(C) Cl(g).
(D) F2(g).
3. In which part of the heating curve below do water and ice exist at the same time?
(A) AB
(B) BC
(C) CD
(D) DE
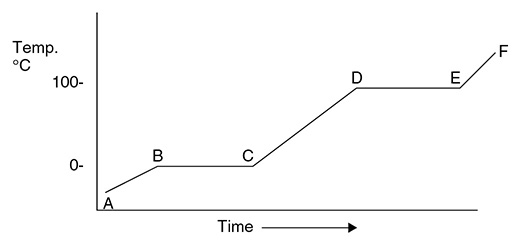
4. Which gas law below involves a variable not present in the other three?
(A) Boyle’s Law
(B) Charles’ Law
(C) Graham’s Law
(D) Combined Gas Law
THE MOLE
Moles Related to Mass and Volume
The mole is another name for Avogadro’s number. Just as the word dozen refers to 12 of something, the mole has its own value, which is equal to 6.02×1023. More important is what the mole can do for you when working quantitatively in chemistry. One important mole concept to remember is that one mole of atoms of any element has a weight equal to its atomic mass. For example, if you have 12.011 grams of carbon, then you have one mole of carbon atoms. If you were to weigh out 55.8 grams of iron, you would have a sample of 6.02×1023 atoms of iron or one mole of iron atoms.
Another concept that relates to the mole is that one mole of gas particles at 0°C (273°K) at 1 atmosphere (standard temperature and pressure, or STP) will occupy a volume of 22.4 liters. For example, 1 mole of nitrogen gas molecules, N2, occupies 22.4 liters, whereas 1 mole of neon gas molecules also occupies 22.4 liters, provided that both samples are at STP.
Percent Composition
If you were to look at a molecule of methane, CH4, you might think that because the hydrogen atoms outnumber the carbon atom, the hydrogen makes up more of the molecule. However, if you consider the atomic masses of the elements, you see that the opposite is true. You can set up a simple fraction to find the percent composition of each element. First, there are four hydrogen atoms present, and each one has an atomic mass of 1.0 gram (from the periodic table). There is only one carbon atom, and it has an atomic mass of 12.0 grams. Summarizing and calculating, you get:
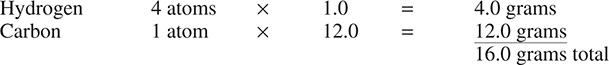
Because the hydrogen atoms make up 4.0 of the 16.0 grams, the amount of hydrogen by mass in methane is 4.0/16.0=0.25, or 25%. Because the carbon atom makes up 12.0 of the 16.0 grams, the amount of carbon by mass in methane is 12.0/16.0=0.75, or 75%.
A similar calculation can be performed to find the percent composition of potassium in KHCO3:

The percent potassium in the sample is 39.0/100.0=0.39, or 39%.
Moles Quiz
Circle the letter of your choice.
1. What is the molar mass (the mass of one mole of a substance) of ammonia, NH3?
(A) 10
(B) 17
(C) 8
(D) 15
2. What is the molar mass of calcium oxide, CaO?
(A) 56
(B) 28
(C) 640
(D) 320
3. Given one mole of O2 gas at STP, what is the mass of this sample?
(A) 8 grams
(B) 16 grams
(C) 32 grams
(D) 64 grams
4. Exactly 2.0 moles of an element weigh 8.0 grams. Which of the following is true?
(A) One mole of the element weighs 16.0 grams, and the element is sulfur, S.
(B) The element in question is beryllium, Be.
(C) One mole of the element also weighs 8.0 grams, and the element is oxygen, O.
(D) One mole of the element weighs 4.0 grams, and the element is helium, He.
5. Given 3.0 moles of krypton gas, Kr(g), how many liters will this sample occupy at STP?
(A) 11.2
(B) 22.4
(C) 44.8
(D) 67.2
6. What is the percent of oxygen in H2O?
(A) 11.1
(B) 33.3
(C) 88.9
(D) 100.0
SOLUTIONS
Solute and Solvents
A solution is a homogeneous mixture that combines a solute and a solvent. A solute is the substance that dissolved in the solvent. A solute changes phase when dissolved, whereas the solvent does not change its phase. Salt water, NaCl(aq), is an excellent example of this. The water is the solvent because it did not change its phase, but the salt changes its phase and is no longer visible as a white solid.
Molarity
As its name suggests, molarity makes use of the mole in calculating the concentration of a solution. To find the molarity of the solution, divide the number of moles of solute by the total volume of the solution:
M=Moles of solute/Liters of solution
For example, if 2.0 moles of KI (332 grams of KI) are dissolved in enough water to make 3.0 liters of solution, what is the molarity of this solution? Dividing 2.0 moles/3.0 liters gives a molarity of 0.67 M KI(aq).
Dilutions
If your glass of iced tea is too strong, one quick remedy is to add more water. By adding more water, the volume increases while the number of moles of tea mix in the drink does not change. What you have done is diluted the tea by increasing the total number of liters of solution. Dividing by this larger number causes the molarity of the iced tea to decrease. To calculate changes in molarity, use the equation:
M1V1=M2V2
Tinctures and Emulsifications
Solutions do not necessarily have to involve water. Although water is termed the “universal solvent,” it is not the only substance in which other substances can be dissolved. When alcohol is used as a solvent, the solution is termed a tincture. An example of this is an iodine tincture, which is used to kill bacteria and prevent infection at the site of a wound.
Emulsifications are formed when two substances that have opposite polarities are mixed. For example, a detergent can emulsify the oils and grease on a shirt with the water in a washing machine. Normally, water and oils do not mix, but because the detergent works to emulsify the oils, the water and oils/grease can now mix together. Bile, formed in the liver and secreted from the gallbladder, works to emulsify fats while they are being digested.
Solubility of Gases and Solids
Temperature can affect the amount of a solid or gas that dissolves in a certain amount of solvent. Evidence of this can be seen in the purchase of a cold, bottled ice tea from a convenience store. One look at the bottom of the bottle and you see the extra tea mix (solute) that has settled out. Why? As the temperature of a solvent decreases, the solubility of a solid decreases because there is less space between the solvent molecules in which the solute can dissolve. The opposite holds true for gases. Gases are more soluble at colder temperatures and less soluble at higher temperatures. When you heat a pot of water, you notice the bubbles that appear on the inside of the pot because the gases that are dissolved in the water are becoming less soluble with the increase in temperature.
Colligative Properties
A winter storm blows in overnight and leaves a sheet of ice on the sidewalk in front of your house. The remedy? You quickly reach for the rock salt in your garage and spread it all over the ice. The result: a few minutes later the ice has melted! Colligative properties are properties that change with the addition of a solute to a solvent. Two of these properties are melting point and boiling point. As a solute is added to a solvent, two things happen: the boiling point increases, making it harder for the solvent to boil. Also, the freezing point of the solvent decreases, so colder temperatures are needed for the solvent to freeze.
Consider antifreeze added to the radiator of a car. The ethylene glycol and water mixed together can lower the freezing point of the radiator to below 0°C and the boiling point to more than 100°C! If you live in the extreme cold or heat, you will be happy to have this protection rather than have your car break down in extreme climates.
SOLUTIONS QUIZ
Circle the letter of your choice.
1. Given a sample of C6H12O6(aq), which of the following is true?
(A) The glucose is the solvent and water is the solute.
(B) The glucose is the solvent and water is the solvent.
(C) The glucose is the solute and water is the solute.
(D) The glucose is the solute and water is the solvent.
2. If 58.5g of NaCl (1 mole of NaCl) are dissolved in enough water to make 0.500L of solution, what is the molarity of this solution?
(A) 2.0 M
(B) 11.7 M
(C) 1.0 M
(D) The answer cannot be determined from the information above.
3. A salt solution has a molarity of 1.5 M. How many moles of this salt are present in 2.0L of this solution?
(A) 1.5 moles
(B) 2.0 moles
(C) 3.0 moles
(D) 0.75 moles
4. As water is evaporated from a solution, the concentration of the solute in the solution will
(A) increase.
(B) decrease.
(C) remain the same.
(D) the answer cannot be determined from the information given.
5. Which substance shows a decrease in solubility in water with an increase in temperature?
(A) NaCl
(B) O2
(C) KI
(D) CaCl2
6. Salt is added to sample of water. Which of the following is true?
(A) The boiling point will increase and the freezing point will decrease.
(B) The boiling point will increase and the freezing point will increase.
(C) The boiling point will decrease and the freezing point will decrease.
(D) The boiling point will decrease and the freezing point will increase.
ORGANIC CHEMISTRY
Hydrocarbons
Organic compounds are the foundation for life as we know it. We are a carbon-based life form, and long carbon chains are the foundation of the molecules that make up life. Carbon atoms can form long and stable chains and can bond with other atoms, such as oxygen and nitrogen, to form various functional groups, such as alcohols, carboxylic acids, and ketones. It is this variety of lengths, shapes, and functions of organic molecules that creates the incredible diversity of organic substances which, in turn, allows for diversity among living things.
Organic hydrocarbons are easily named depending on the number of carbon atoms present in the longest chain of the hydrocarbon. For example, if the longest carbon chain is three carbon atoms long, the prefix to the name is prop-. If the longest carbon chain is four carbon atoms long, the prefix to the name is but-. The following chart summarizes the prefixes for the longest carbon chains. The other factor that helps determine the name of a hydrocarbon is the presence of all single bonds, a double bond, or a triple bond. Hydrocarbons that have all single bonds end in -ane, whereas those that have double or triple bonds end in -ene or -yne, respectively.
Aromatics
Another class of organic compounds that are made of hydrogen and carbon are the aromatics. At the heart of these ringed compounds is a simple, six-carbon aromatic compound called benzene, C6H6.
Although benzene itself is a known carcinogen, many benzene derivatives are important compounds that we use on a daily basis. Two examples are phenol (a disinfectant) and naphthalene (mothballs).
Three Ways to Draw Benzene
Oxygen, Nitrogen, and Sulfur
Although carbon and hydrogen are the elements most common in organic compounds, they are not the only ones. It is not uncommon to find an oxygen atom or nitrogen atom in the compound as well. Some popular functional groups containing these elements are:
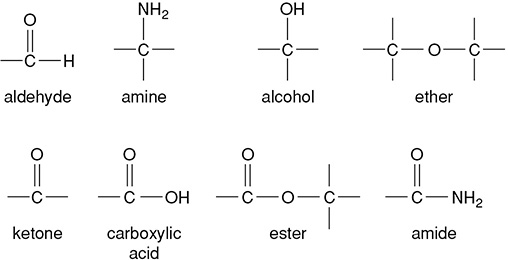
Although sulfur is not the most common element present in organic compounds, it is important in making disulfide bridges. These structures are needed to help proteins and enzymes maintain their structure.
Isomers
Many organic compounds contain the same number of certain elements but have different structures. Compounds that have the same molecular formula but different structures are termed isomers. For example, C2H6O is the molecular formula of ethyl (drinking) alcohol, but it is also the molecular formula for dimethyl ether. Comparing the two functional groups for these compounds, you see the different structures for these compounds, CH3CH2OH and CH3OCH3. The location makes all the difference as the ethyl alcohol can be consumed while the ether is highly toxic.
ORGANIC CHEMISTRY QUIZ
Circle the letter of your choice.
1. Organic compounds are the basis for life as we know it because
(A) carbon-to-carbon bonds are strong.
(B) carbon can form long chains.
(C) carbon chains can include other elements to give rise to different functional groups.
(D) all of the above are correct.
2. The name of the compound CH3-CH2-CH2-CH3 is most likely
(A) cyclobutane.
(B) butane.
(C) butene.
(D) butyne.
3. The compound CH2=CH-CH2-CH2-CH3 is an example of
(A) a pentane.
(B) a hexene.
(C) an alkene.
(D) an organic macromolecule.
4. An amino acid is expected to contain which two functional groups?
(A) R-NH2 and R-COOH
(B) R-CHO and R-CO-NH2
(C) R-OH and R-COOR
(D) R-O-R and R-COOH
5. Which element is most likely to be found in an organic compound?
(A) carbon
(B) oxygen
(C) nitrogen
(D) calcium
ACIDS AND BASES
Properties
Before examining acids and bases, it is important to first describe electrolytes. The Swedish chemist Arrhenius, who is famous for his definition of acids and bases, was actually an electrochemist. This makes sense because electrolytes conduct electricity in solution. This occurs because the mobile ions are able to carry an electrical current. The same occurs in a molten salt. Once the solid salt is heated to a liquid, the ions are able to move freely and conduct electricity. When a salt is in its solid state, its ions cannot conduct electricity. Reading on, you will see the connection between acids, bases, and electrolytes.
Acids and bases can be defined and identified in a number of ways. The operational definition relates to the properties that you can see, feel, or taste. These properties are as follows:

Chemically, acids are thought of as substances that produce H+ ions (hydronium ions) in solution, whereas bases yield OH- ions (hydroxide ions). Acidic substances are easily identified by their chemical formulas because they start with H, as in HCl and H2SO4. Basic substances have chemical formulas that usually end in OH, as in NaOH or Ca(OH)2. Some substances can act like an acid or a base, depending on the environment. Water is one of these substances, and it is termed amphoteric because of its ability to act as either an acid or a base.
Indicators
Indicators are substances that change color to indicate the presence of an acid or a base. Indicators can also be used to determine the pH of a substance. Common acid-base indicators are litmus and phenolphthalein. This chart shows their colors in acids and bases:

Reactions
The most common acid-base reaction that you will encounter is the neutralization reaction. In this reaction, an acid and a base react to form a salt and water. The water is formed from the combination of the hydronium ion from the acid and the hydroxide ion from the base. A simple reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) demonstrates this:
HCl+NaOH→NaCl+H2O
You might see the equation written as HCl+NaOH→NaCl+HOH to emphasize the formation of the water.
Acids can also react with active metals to form hydrogen gas. Samples of this type of reaction feature zinc and magnesium:
2HCl(aq)+Zn(s)→ZnCl2(aq)+H2(g)
2HCl(aq)+Mg(s)→MgCl2(aq)+H2(g)
Not every metal undergoes this type of reaction. Metals such as gold and silver do not lose electrons to hydronium ions, which explains why gold and silver are able to resist tarnishing and reaction with other substances.
pH
The pH of a solution indicates just how acidic or basic a solution is. The pH scale ranges from 1 through 14, with the number 7 indicating a neutral solution. As the number of hydronium ions increases, the pH value decreases as the solution becomes more acidic. As the number of hydroxide ions increases, the pH value increases as the solution becomes more basic. The pH scale below summarizes these trends:
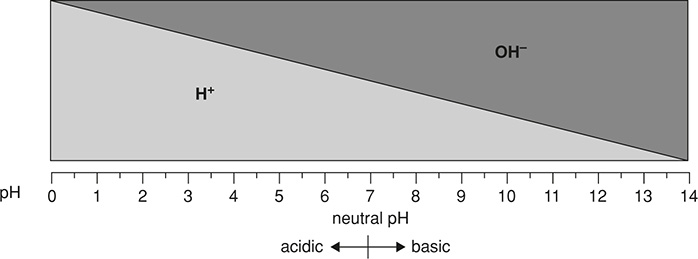
The calculation of pH requires that you understand the molarity of the hydronium ions in solution and also understand powers of ten. For starters, the equation for calculating the pH of a solution is pH=–log[H+]. Although this equation seems intimidating because of the use of logarithms, it is actually quite simple because logarithms are based on powers of ten. Bypassing the math involved, let’s look at a solution with a pH of 2 and a solution with a pH of 4. Using the pH scale above, we should be able to see that the solution with a pH of 2 is more acidic than a solution with a pH of 4 and that there are more hydronium ions in the solution with a pH of 2. Just how much more acidic is the pH of 2 than the pH of 4? Your first reaction might be to answer “two” or “twenty.” Keeping in mind that the pH scale is based on powers of 10, the pH of 2 is 100 (102) times more acidic than the pH of 4. Because they are two pH values apart, they are two powers of ten apart in their strength.
More intricate calculations involving pH can be accomplished by knowing the concentration of hydronium ions. When concentrations of hydronium ions are written in scientific notation, the exponent present in the number can give a clue as to what the pH of the solution will be. The equation pH=–log[H+] can be rearranged to read [H+]=1.0×10-pH. This means that if you were to negate the exponent of the concentration, you would obtain the value for the pH. For example, if the concentration of the hydronium ion in a solution is 1.0×10-6 M, the pH would be 6 (the negation of the exponent). If you were to examine the concentration of hydronium ion for a solution with a pH of 7 (neutral), you would see that the concentration of [H+]=1.0×10-7 M. Because this solution is neutral, the concentration of hydroxide ions must be the same at this pH and [OH-] must also be equal to 1.0×10-7 M.
Buffers
Buffers are substances that are added to a solution so that the solution resists changes in pH. A number of medications are designed to have a buffer around them so that they do not upset the lining of the stomach with a change in the pH of the stomach on ingestion. Also, the blood contains carbonic acid and bicarbonate working as buffers to help the blood maintain its pH at approximately 7.4.
Titration
Titrations are performed to determine the concentration of an acidic or a basic solution. During this process, an acid or a base is added to the solution so that the acid and base neutralize each other. Before the experiment is started, an indicator is added to the solution being titrated. As the acid or base is added drop by drop, the indicator changes color. This indicates the endpoint of the titration. At this point, the number of moles of acid is equal to the number of moles of base present in solution.
A calculation involving the number of milliliters of acid and base used along with a known concentration of either the acid or base will provide the concentration of the acidic or basic solution. This calculation follows the equation MaVa=MbVb.
ACIDS AND BASES QUIZ
Circle the letter of your choice.
1. Blue litmus paper turns red when the pH of a solution is
(A) 12.
(B) 8.
(C) 6.
(D) 7.
2. When an acid reacts with an active metal such as zinc, the products are
(A) water and salt.
(B) an acid and a base.
(C) neutralized.
(D) a salt and H2(g).
3. Which of the following substances is most likely to taste sour?
(A) NaOH
(B) NaCl
(C) NH3
(D) HC2H3O2
4. Which reaction below demonstrates a neutralization reaction?
(A) 23290 Th→22888Ra+42He
(B) NaCl+H2O→HCl+NaOH
(C) 2HNO3+Mg(OH)2→2H2O+Mg(NO3)2
(D) CH4+2O2→CO2+2H2O
5. How many times stronger is an acid with a pH of 3 than an acid with a pH of 6?
(A) A pH of 3 is three times as strong.
(B) A pH of 3 is one thousand times as strong.
(C) A pH of 3 is thirty times as weak.
(D) A pH of 3 is one thousand times as weak.
6. Which of the following is true for a basic solution?
(A) The hydroxide concentration equals zero.
(B) The hydronium concentration equals the hydroxide concentration.
(C) The hydronium concentration is less than the hydroxide concentration.
(D) The hydronium concentration is greater than the hydroxide concentration.
Rates of Reaction
The rate (or speed) at which a reaction proceeds can depend on a number of factors. As we have already examined, enzymes are catalysts that can alter the speed of a reaction. How do they do this? All reactions need energy to get the reaction started. This energy is called the activation energy. Enzymes and catalysts work to lower the activation energy of a reaction. Because this energy barrier has been lowered, it is easier and less time consuming to put in enough energy to start the reaction.
Besides enzymes and catalysts, other factors can also modify reaction rates. These factors:
• Cause molecules to collide more frequently
• Causes molecules to collide with more energy
The factors that affect rates of reaction are as follows:
RATES OF REACTION QUIZ
Circle the letter of your choice.
1. A reaction takes place between an acid and 0.5 grams of solid magnesium ribbon. Another reaction takes place between an acid and 0.5 grams of powdered magnesium. Which statement is true?
(A) The powdered magnesium reacts faster because the activation energy has been lowered.
(B) The magnesium strip reacts faster because it has a higher concentration of magnesium.
(C) The powdered magnesium reacts faster because it has a greater surface area.
(D) The magnesium strip reacts faster because it will create a higher temperature once the reaction starts.
2. Two different solutions, A and B, are in separate beakers. These solutions are mixed together into a third beaker that contains pure water. Which statement is true?
(A) The two solutions would have reacted faster if they were mixed together directly and not with water.
(B) The two solutions would react faster if they were under higher pressure.
(C) The two solutions would react faster if they had a greater activation energy.
(D) The two solutions would react faster after being mixed with water because their surface area will be increased.
ANSWERS TO CHEMISTRY QUIZZES
SCIENTIFIC NOTATION AND THE METRIC SYSTEM
1. B The number 1,000,000, or 1 million, has six zeros, making it 106.
2. A The number 6,180 meters can be rewritten as 6.180×103 meters.
3. D The prefix milli- means one-thousandth and centi- means one-hundredth. The prefixes differ by one power of ten. Because the prefix milli- is a smaller unit than centi-, ten millimeters are in one centimeter.
ATOMIC STRUCTURE
1. According to Dalton’s atomic theory, “All atoms of the same element are alike.” This is inconsistent with the concept of isotopes because the same element can differ in its mass number or number of neutrons. This explains why carbon can exist as three isotopes: C-12, C-13, and C-14.
2. C The atomic number of Ar is 18. Subtracting 18 protons from its mass number of 40 gives 22; Ar-40 has 22 neutrons in its nucleus.
3. B Aluminum as an atom has thirteen electrons. The aluminum ion with a positive three charge indicates that the atom lost three electrons and now has ten electrons.
4. C Eighteen electrons can be held in principal energy level number three.
5. See chart. Subtracting the number of protons from the mass number gives the number of neutrons: 10 for oxygen and 8 for carbon.
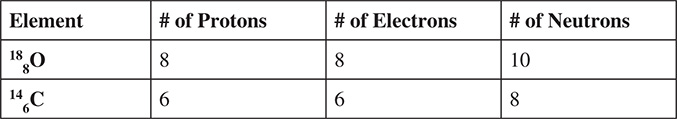
6. B Atoms with more protons in the nucleus have a greater nuclear charge than atoms with fewer protons.
7. A The initial sample starts with 100 grams. Because the half-life is five days, every five days only half of the sample will remain. After the first five days only 50 grams will remain. Because the problem calls for the mass after ten days, in another five days only 25 grams of the original sample will remain. This process can be outlined as follows:
100 grams–5 days→50 grams–5 more days (10 days total)→25 grams
8. D The reaction, 23290Th→22888Ra+X shows that a particle was produced as a result of nuclear decay. This transmutation shows an original mass number of 232 before the reaction and 228 after the reaction. This is a loss of 4 AMU. The number of protons before the decay was 90 and it became 88 after the decay, resulting in a net loss of two protons.
9. B Neon has 10 electrons. This is a configuration of 2-8.
PERIODIC TABLE
1. B The modern periodic table is arranged by atomic number.
2. A The nonmetals are located in the upper right of the periodic table.
3. D Elements in the same group/family are chemically similar. Mg and Ca are in the same group on the periodic table.
4. D Fluorine has the highest electronegativity. Because iodine (I) is the element farthest from fluorine (F), it has the lowest electronegativity of the choices.
5. A Fluorine and neon are nonmetals and are expected to have smaller atomic radii because they are located next to each other on the periodic table. Because strontium is located on the lower left of the periodic table, it has a larger atomic radius than cobalt.
6. B Aluminum is a metal. Further proof lies in the aluminum pan in your kitchen, which is shiny and conducts heat.
BONDING
1. A The compound CO is formed from two nonmetals and is a covalent compound.
2. D Potassium (K) is a metal, and iodine (I) is a nonmetal. This means that an ionic compound forms. To do this, the metal K loses an electron to the nonmetal I.
3. C All of the compounds listed are covalently bonded because they contain all nonmetals. The equal sharing occurs when the electronegativity of the elements is the same.
4. A The melting of ice requires the ice to absorb heat, an endothermic process.
5. C The reaction synthesizes water from hydrogen and oxygen. The reaction also indicates the release of heat energy—an exothermic reaction.
PHASES OF MATTER
1. B Oxygen has been labeled as a gas, which is the phase that is most easily compressed.
2. A The compound I2(s) indicates solid iodine, which has a definite shape and volume.
3. B Portion BC of the graph shows a phase change and no temperature change. Because it is occurring at the melting point of the substance, this is where the solid and liquid phase will exist together.
4. C Graham’s Law takes into account masses of gases and their speed. The other three choices all take into account the volume, pressure, and temperature of a sample of gas.
MOLES
1. B The atomic mass of nitrogen is 14.0 and the atomic mass of a hydrogen atom is 1.0. Because there are three hydrogen atoms, it is necessary to add 14.0 and 3.0, which gives 17.0.
2. A CaO has a gram formula mass of 56 because the atomic mass of Ca is 40 and the gram atomic mass of oxygen is 16.
3. C One mole of a substance weighs its gram formula mass. Because there are two oxygen atoms (16 grams each) present, the weight is 32.0 grams per mole.
4. D Because 2 moles weigh 8.0 grams, 1 mole of the substance will weigh 4.0 grams. The weight of one mole of a substance is its gram atomic mass. This corresponds to helium, He.
5. D The volume of 1 mole of a gas at STP is 22.4 liters, and 3 moles of the gas is three times the molar volume, or which is 67.2 liters.
6. C The gram formula mass of water is 18 grams per mole. The weight of the oxygen present makes up 16 of 18 grams per mole. Dividing 16 by 18 gives 0.888 or 88.9%.
SOLUTIONS
1. D The glucose is the solute and water is the solvent because the glucose changes its phase and the water does not.
2. A There are 58.5 grams of NaCl in 1 mole of NaCl. Setting up to calculate the molarity of the solution, you divide 1.0 moles by 0.500 liters: 1.0 moles/0.500 liters=2.0M.
3. C Using the equation for molarity, the number of moles divided by 2.0 liters equals 1.5. If 3.0 moles are dissolved in the 2.0 liters of solution, the concentration is 1.5M.
4. A As water is evaporated from a solution, the concentration of the solute in the solution increases because there is less water present.
5. B Gases show a decrease in solubility in water with an increase in temperature. The other three choices are salts, and they show an increase in solubility as temperature increases.
6. A As a solute is added to a solvent, the solvent undergoes a freezing point depression and a boiling point elevation.
ORGANIC CHEMISTRY
1. D The ability of carbon to form strong bonds and long chains and to have various functional groups allows for millions of organic compounds that are essential to life.
2. B The compound CH3-CH2-CH2-CH3 has four carbon atoms that are joined by single bonds. This makes for a molecule of butane.
3. C The compound CH2=CH-CH2-CH2-CH3 has five carbon atoms (pent-) and it has a double bond present as well (-ene). Because pentene is not a choice, this compound is an alkene.
4. A An amino acid has an amine and a carboxylic acid group present. These are represented by R-NH2 and R-COOH.
5. A Organic chemistry is the study of carbon.
ACIDS AND BASES
1. C Blue litmus paper turns red when the pH of a solution is acidic. The number 6 corresponds to this type of pH.
2. D A salt and H2 gas form when an acid reacts with an active metal.
3. D HC2H3O2 is acetic acid, the major ingredient in vinegar. It is an acid and tastes sour, the way citric acid can make lemons taste sour.
4. C The reactions are labeled as shown below:
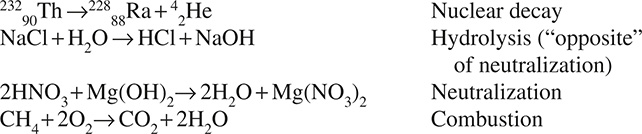
5. B A pH of 3 is one thousand times as strong as a pH of 6 because the pH scale works on powers of ten. Three pH units means a difference of 103 or 1,000 times.
6. C A basic solution has fewer hydronium ions and more hydroxide ions.
RATES OF REACTION
1. C Because the powdered magnesium has a greater surface area, it reacts faster than one solid strip of magnesium.
2. A Because water can dilute a solution, adding water lowers the concentration of the substances. This would cause the reactants to react slowly. Instead, it would have been better to mix the reactants directly.
REVIEW OF PHYSICAL SCIENCES: PHYSICS
KINEMATICS
As its name suggests, kinematics involves objects that are in motion (kinetic). We will start with the basics, distance and displacement, before looking into the rates at which objects move.
Distance and Displacement
A car travels one mile east on on a highway. After traveling one mile east, the car then makes a U-turn and continues two miles west. The car has traveled a total of three miles, but it has also traveled only one mile from its starting point. Both views are correct, depending on whether you are considering the car’s displacement or its distance. The car did travel a distance of three miles, as its odometer would indicate. However, because the car ended up only one mile from its starting point, the displacement (distance of final destination from the starting point) is one mile west. The distance is said to be a scalar quantity because it involves just a number, three miles total. The displacement is said to be a vector quantity because it involves a direction, one mile west.
Speed, Velocity, and Acceleration
The rate of motion at which an object travels in a certain amount of time is called the object’s speed. Speed is calculated using the formula s=d/t, and the units are usually measured in meters per second (m/s). When traveling by car, the units are usually measured in kilometers per hour (km/h) or miles per hour (mph). Because speed does not indicate a direction, it is a scalar quantity. Speed does have a magnitude (a number), but it lacks a direction, such as east or north.
Velocity, in contrast, takes into account speed and direction. The formula for velocity is the same as the formula for speed: v=d/t. Velocity also takes into account the direction in which the object travels. For example, if a car is heading east at 65km/h, the speed of the car is 65km/h, but the velocity of the car is 65km/h east. Because velocity takes into account a magnitude (65km/h) and a direction (east), velocity is said to be a vector quantity.
Acceleration calculates the change in an object’s velocity over time. The formula is a=∆v/t. The delta symbol (Δ) is read as “change in.” To calculate the change in velocity, you subtract the initial velocity from the final velocity. In other words, Δv=(final velocity–initial velocity). An object that is increasing in velocity is said to be accelerating. An object that is decreasing in velocity is said to be decelerating. The unit of measure for acceleration is meters per second (m/s) per second (t). The units are written as m/s2. Acceleration due to gravity (ag or just g) has a value of 9.8m/s2. This is the acceleration of an object near the Earth’s surface, disregarding air friction. If two objects of different masses are in a vacuum (no air present to introduce friction), they will both fall with the same acceleration of 9.8m/s2.
Momentum and Impulse
Compare a car traveling at a speed of 5m/s with a bird flying at 10m/s. Although the bird has a greater speed, the car has a greater momentum. The difference between the two is the masses involved. Momentum is calculated by multiplying the mass of an object by its velocity, p=mv.
An impulse is a force (we will discuss forces shortly) that is applied to an object over time, Ft. Impulse can also be thought of as a change in momentum, the mass times the change in velocity, m(vf-vi). Because the two equations both represent impulse, we can set them equal to each other, Ft=mΔv.
NEWTONIAN MECHANICS
According to a (probably fictitious) story, the English physicist Sir Isaac Newton (1642–1727) once was sitting under a tree when an apple fell from the tree and hit him on the head. What Newton encountered in that story was the force of gravity and what it does to the motion of objects. Newton is most famous for his three laws of motion.
1. Newton’s First Law involves inertia, a property of matter by which an object in motion tends to continue traveling in a straight line, whereas an object at rest stays at rest unless that object is acted on by a force. To put it simply, it takes a force to move an object or change its direction of motion.
2. Newton’s Second Law concerns the relationship between the forces applied to an object and the acceleration of the object. The greater the force applied to the object, the greater is its acceleration. The equation that shows this relationship is F=ma. The unit of measure for force is the Newton (N). This unit is a derived unit, which comes from multiplying the unit of mass, kg, by the unit of acceleration, m/s2. Multiplying the two, you get kg·m/s2. One caution: if an object is moving at a constant velocity (Δv=0), then it is not being accelerated and the force needed to achieve this constant velocity is also equal to zero.
3. Newton’s Third Law says that when one object applies a force to a second object, that second object applies an opposite and equal force to the first object. For example, if you were to push against a wall but neither you nor the wall moves as a result, then both you and the wall are applying an equal force to each other.
WORK AND ENERGY
The work done on an object is defined as the force applied to the object multiplied by the distance the object moves. The equation for calculating work is W=Fd. The unit of measure for force is the newton, etc., and the unit of measure for distance is the meter, so the unit of measure for work is the newton·meter (N·m). Power is related to work in that power is the amount of work done in a certain amount of time. The formula for calculating power is P=W/t. When you divide newton·meters by seconds, you get N·m/s (or the unit called the watt, W).
Energy is defined as the capacity to do work. Energy can be either potential or kinetic. Potential energy (PE) is energy that is stored within an object for later release. For example, a book held in the air above some surface, such as a table top, is considered to have PE (the energy that would be released when the book is dropped). Kinetic energy (KE) is energy that is in motion, The energy released when the book falls is KE. The amount of potential or kinetic energy an object has can be quantified. The formula for calculating gravitational PE (the kind stored up in the book in the example above) is PE=mgh where m=mass, g=gravity, and h=the height of the object above a particular surface. The formula for calculating kinetic energy includes velocity (v) because the object is moving. The formula for calculating KE is KE=½mv2. The unit of measure for both kinetic and potential energy is the joule (kg·m2/s2).
One important concept to remember is that energy is conserved; that is, it is never either created or destroyed. For example, consider a book that is held two meters above a table top. As you saw above, the book has PE. If the book is released and starts falling, the PE is converted into KE. Once the book hits the table top, the KE is converted into sound energy (the noise made by the impact). In this example, the energy has taken three forms: potential, kinetic, and sound. What does this show? It shows that although energy can never be created or destroyed, it can be converted from one form to another.
WAVES
Take a good look at a stadium full of fans who have started a “wave” as they cheer for the home team. What are they doing to create this “wave”? One group of people after another stands, and those around them remain seated. This pattern moves around the stadium, making it look as though a wave is rolling across the seats. This type of “wave” is similar to what we will discuss in this section.
Amplitude, Frequency, and Wavelength
Waves, which are continuous, have an amplitude, frequency, and wavelength, as follows:
Components of a Wave
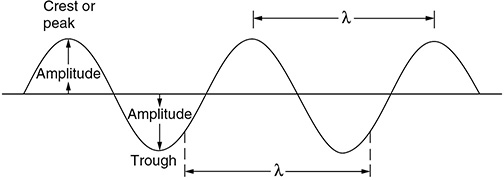
The highest and lowest points of a wave are called crests and troughs, respectively. The distance that crests and troughs are from the normal is called the amplitude. The distance between two crests (or two troughs, for that matter) is called the wavelength and is represented by the Greek letter lambda (λ). The wavelength is can also be thought of as the distance for one wave to be completed. The frequency is the number of complete waves that pass through a point over a given amount of time. Multiplying the frequency of a wave by its wavelength gives the velocity of the wave as shown by the formula v=fλ.
Longitudinal and Transverse Waves
The difference between transverse and longitudinal waves is shown below. Transverse waves have vibrations that are perpendicular (at right angles) to the direction in which the wave is moving. Longitudinal waves have vibrations that are moving in the same direction as the wave. In a longitudinal wave, there are no crests and troughs. Instead, there are areas of compression and expansion.
Transverse Wave
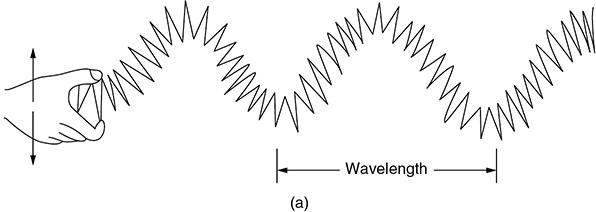
Longitudinal Wave
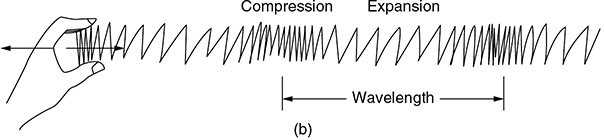
Light: Wave versus Particle
Light can be thought of as being either a wave or a particle because it exhibits the properties of both. This is called the “wave-particle duality of light.” Light particles are called photons, and their existence helps explain why processes such as photosynthesis, the photoelectric effect, and the Compton effect can take place (no need to worry about this in detail).
Reflection, Diffraction, Dispersion, and Refraction
When light hits a surface, some of the light energy is absorbed by the object, some is reflected back, and if the object is transparent, some light passes through. Look into a mirror, at a shiny new car, at polished furniture, or into a still pool of water and you will see your reflection. These surfaces allow for the light to be reflected or “bounced back,” because the surface allows the angle at which the light hits it to equal the angle at which the light is reflected. Rough surfaces do not allow you to see yourself in them because the angle at which the light hits the surface is different from the angle at which the light is “bounced back.”
When light goes from one medium (air, for example) into another medium (water, for example), the velocity of the wave changes. If you fill a glass halfway with water and drop a spoon into it, you notice that the image of the spoon under the water looks different from the image of the spoon above the water—because of the refraction of the light.
Diffraction takes place when a wave that is spreading out hits an object and, as it continues to travel, bends around the object. This is not to be confused with dispersion, which is the breaking up of white light into its colors. Dispersion occurs when white light passes through a prism, which breaks it up into a range of colors.
ELECTRICITY
Charges
As explained earlier in the chemistry portion of this chapter in the section on ions, the transfer of electrons from one object to another causes the objects to become charged. The object that loses electrons becomes positively charged, and the one that gains electrons becomes negatively charged. Like charges repel each other. If two positive charges come near each other, they repel each other. The same holds true for two negative charges placed near each other. If a positive charge comes near a negative charge, the two unlike charges attract.
Current, Voltage, Resistance, and Power
Every day, you take advantage of electricity flowing through wires to run appliances and other devices. When you were younger, you probably had fun with static electricity by rubbing a balloon against your hair and then watching as the balloon clung to a wall. Electricity is a flow of electrons through a wire. Static electricity results when a buildup of electrons causes a buildup in charge without the electrons flowing through a wire.
A current (I) is a flow of charge from one terminal of a circuit to another. To be more specific, it is the amount of charge that passes through a point per second. The unit of measure for current is coulombs/second or just amperes (A). Voltage (V) is the electrical potential (stored energy) that a power source has. For example, air conditioners for your home can run on 110 volts, whereas others need a potential of 220 volts. Resistance (R) is the ability of a substance to stop the flow of a current. For example, copper wire has low resistance and is a good conductor of electricity, whereas rubber has high resistance and cannot conduct electricity. The unit of measure for resistance is the ohm (Ω). Ohm’s law relates voltage, current, and resistance with this formula: V=IR. Power, which is measured in watts (W), can be calculated by multiplying the current by the voltage. The formula for calculating power is P=IV.
Series and Parallel Circuits
Some circuits have their components connected in series, and others have their components connected in parallel. The following diagram shows light bulbs connected in series on the left and in parallel on the right. In the series circuit, if one light bulb is removed, the entire set of lights goes out because of the break in the circuit. This can be countered by connecting the light bulbs in a parallel configuration. If one light goes out or is removed, the other light bulbs continue to glow. This configuration is appropriate for houses, buildings, and sets of lights that are usually used during the holiday season.
Series and Parallel Circuits
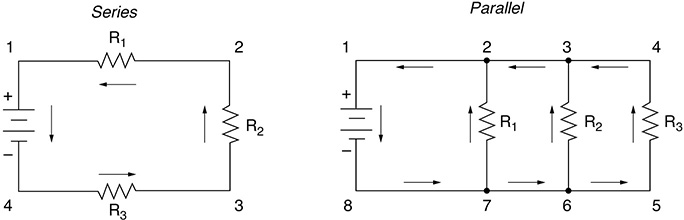
Physics Quiz
Circle the letter of your choice.
1. A car travels 3 miles north, 6 miles south, 2 miles east, 2 miles west, and then 3 miles north. Which of the following is true?
(A) The displacement of the car is 16 miles, and the distance traveled is 0 miles.
(B) The displacement of the car is 16 miles, and the distance traveled is 16 miles.
(C) The displacement of the car is 0 miles, and the distance traveled is 0 miles.
(D) The displacement of the car is 0 miles, and the distance traveled is 16 miles.
2. A car is traveling at 5m/s. A squirrel jumps into the middle of the road, and the car comes to a screeching halt over 2.5 seconds. What is the car’s acceleration over this time period?
(A) −2 m/s2
(B) 2 m/s2
(C) 1.25 m/s2
(D) 0 m/s2
3. A car, starting from rest, accelerates at 10 m/s2 for 5 seconds. What is the velocity of the car after 5 seconds?
(A) 2 m/s
(B) 50 m/s
(C) 0 m/s
(D) The answer cannot be determined from the information given.
4. Which of these objects has the greatest momentum?
(A) a 1,250-kg car moving at 5 m/s
(B) a 0.5-kg rock moving at 40 m/s
(C) a 10-kg piece of meteorite moving at 600 m/s
(D) a 80-kg person running at 4 m/s
5. A 1.0-kg block on a table is given a push so that it slides along the table. If the block is accelerated at 6m/s2, then what was the force applied to the block?
(A) 3 N
(B) 0 N
(C) 6 N
(D) The answer cannot be determined from the information above.
6. A box is moved by a 15 N force over a distance of 3m. What is the amount of work that has been done?
(A) 45°C
(B) 5 J
(C) 45 W
(D) 45 N·m
7. Which of the following is true?
(A) The amplitude of a wave is the distance that one complete wave covers.
(B) The frequency of a wave is the distance the crests and troughs are from the normal.
(C) The wavelength of a wave is how many waves pass a point in a given amount of time.
(D) The velocity of a wave can be found by multiplying the frequency by the wavelength.
8. Pair up each term with its correct definition:
(A) Reflection _____ White light is broken into the colors of the rainbow.
(B) Dispersion _____ Light bounces back at the same angle at which it hit an object.
(C) Refraction _____ A wave hits an object and bends around the object.
(D) Diffraction _____ Light changes speed as it goes from one medium to another.
9. Referring to Ohm’s law, you can conclude that
(A) voltage and current are inversely proportional when the resistance is constant.
(B) an electronic device that is connected to a 6-volt source and draws 3 amperes has a total resistance of 2 ohms.
(C) resistance is measured in volts.
(D) voltage is the amount of charge that passes through a point per second.
ANSWERS TO PHYSICS QUIZ
1. D If a car travels 3 miles north, 6 miles south, 2 miles east, 2 miles west, and then 3 miles north, it has traveled a total distance of 16 miles. Because of the directions the car has traveled in, it has ended up in the same place from which it started. This means that the displacement of the car is zero.
2. A Using the equation a=Δv/t, the car has a final velocity of 0m/s and an initial velocity of 5m/s. The change in velocity is –5m/s. Dividing this by the time, 2.5 seconds, the acceleration is –2m/s2.
3. B The velocity of the car is equal to the time multiplied by the acceleration. Multiplying 5 seconds by 10m/s2, you get a velocity of 50m/s.
4. A Momentum is calculated by multiplying mass and velocity, p=mv. Although the meteorite sounds intimidating, it is the car that has the greatest momentum at 6,250kg·m/s.
5. C Applying Newton’s Second Law, F=ma, a 1.0-kg block accelerated at 6m/s2 requires a 6-N force to move it.
6. D Work is the force that is applied to an object over a distance, W=Fd. The force is 15 N and the distance is 3m, giving a total amount of work equal to 45 N·m.
7. D The equation of the velocity of a wave is v=fλ. This is the multiplication of frequency by wavelength.
8. B, A, D, C
Reflection takes place when light bounces back at the same angle at which it hit an object. Refraction takes place when light changes speed as it goes from one medium to another. Dispersion takes place when white light is broken into the colors of the full spectrum. Diffraction is the bending of waves around obstacles in their path.
9. B Using the equation V=IR or R=V/I, an electronic device connected to a 6-volt source drawing 3 amperes has a total resistance of 2 ohms.
SCIENTIFIC METHOD, SCIENTIFIC REASONING, AND EXPERIMENTAL METHODOLOGY
How scientists go about solving problems involves a number of steps. When done properly, a systematic method for investigating a problem can lead to conclusions or raise further questions and investigation into the problem. Think about how far science has come in the last century. With each discovery that we make, someone out there wants to ask more “What if we …” type questions, which further what we know. In addition to this, with new advances in technology, we can answer more questions, which raise more questions to be answered. The result is human progress that far exceeds what was made over thousands of years.
The first step in the scientific method is to make an observation. There are so many phenomena occurring about us. The scientific method is designed to help us uncover as to why something occurs. This is followed by asking a question about the observations made such as, “Why is this occurring?” or “How does it happen?” This is then followed by research. Research provides us with a foundation to build upon. Research also makes sure that we do not answer a question that is already answered. Research may also direct you to ask additional questions or modify the original question posed.
The next step is to generate a hypothesis. There are two key words to use in one’s hypothesis, “if” and “then.” The hypothesis tells others what the experimenter believes will happen when the independent variable is changed. This is the “if” part of the hypothesis. The “then” portion of the hypothesis tells us what is going to happen. This is called the dependent variable because it depends upon what we do during the experimentation. The hypothesis also needs to be testable and it needs to make a prediction. Finally, there is something called the null hypothesis where we predict that the observed difference is due to pure chance and only pure chance. This type of hypothesis is best tested by a statistical analysis. Afterwards, it is either rejected or accepted.
During the experimentation, we want to use larger sample sets and record data. An experiment can include changing a number of factors at once to see the impact these changes have on the dependent variable. However, there are times when we might want to change just one factor and see what results we get. When we do an experiment with changes in any number of factors, we want to make sure we have a control group—a sample in which no factors were changed so that we can compare our results to group that was not impacted at all by our variations. When we have a case where one factor is modified and it is compared to a control group, then experiment is then called a controlled experiment. The variables that are held constant in an experiment, controlled or not, are called controlled variables (or just experimental constants).
A full analysis of the data is then done and we can draw conclusions. When the results of an experiment lie close together after repeated experimentation, we can say that the data is precise. When the data averages out to what is expected, we can say that the data is accurate. A very popular diagram that is used to remember this follows:
When data or a data point is outside an interval of confidence, those data are said to be uncertain. We also want our data and analysis to be supported by graphs. All graphs are expected to have a title, labeled axes that include units, clearly marked data points, and consistent scales.
When the conclusions are in agreement with the hypothesis, we are then ready to publish/communicate the results with others. However, there will be times when the conclusions do not agree with the hypothesis. This then raises a new question, which then raises a new hypothesis, and then requires further experimentation.
REVIEW QUESTIONS ON SCIENTIFIC METHOD, SCIENTIFIC REASONING, AND EXPERIMENTAL METHODOLOGY
1. A scientist is listening to the calls that animals use when they see a predator. The skill that is being used by the scientist is called
(A) creating a graph of the data.
(B) drawing a conclusion.
(C) making observations.
(D) posing a question.
2. What is the order that one takes when using the scientific method?
(A) Observation, question, research, hypothesis, record data, data analysis, draw conclusion
(B) Draw conclusion, data analysis, record data, hypothesis, research, question, observation
(C) Record data, data analysis, draw conclusion, observation, question, research, hypothesis
(D) Observation, research, question, record data, hypothesis, data analysis, draw conclusion
3. A hypothesis should be
(A) testable.
(B) an if/then statement.
(C) a prediction.
(D) All of the above
4. A controlled experiment
(A) tests many variables at once and is then compared to a control group.
(B) tests just one variable and is then compared to a control group.
(C) has all of the independent variables compared to the dependent variable.
(D) uses a smaller sample size to compare results to.
5. When doing an experiment with plants, the amount of sunlight and amount of water given are the same for all of the plants but the amount of plant food given to the plants differs. Which of the following is true?
(A) The amounts of sunlight and water are the dependent variables
(B) The sunlight and water are the experiment’s constants
(C) The amount of plant food given is the hypothesis
(D) The amount of plant food given is the dependent variable
6. A good __________ should have a title, labeled axes that include units, clearly marked data points, and consistent scales.
(A) experiment
(B) hypothesis
(C) graph
(D) theory
7. A student wants to show that carbon dioxide helps plants grow. He places four plants in a room and lights a cigarette in the room and leaves it in an ash tray. This is repeated for many days. His experiment is not valid because
(A) his sample size is too small.
(B) there is no control group present to compare to.
(C) cigarette smoke has other components that might alter the results.
(D) All of the above
8. The scientific method is
(A) a series of steps that we take to solve a problem.
(B) a set of guidelines for experimenting safely.
(C) a way of generating a theory.
(D) a way of generating a law.
9. The ___________________ attempts to show that there is no relationship that exists between variables.
(A) percent error
(B) precision
(C) accuracy
(D) null hypothesis
10. The graph below best shows

(A) high accuracy and high precision.
(B) low accuracy and high precision.
(C) high accuracy and low precision.
(D) low accuracy and low precision.
11. The statement, “If I change the pH of the soil, then the color of the flower’s pedals will change” is an example of
(A) a theory.
(B) an inquiry.
(C) a hypothesis.
(D) a controlled experiment.
12. An experiment is conducted by a student where a number of resistors which are connected in series. The student then connects the wire to a 12-volt battery and measures the current. The dependent variable is
(A) the current.
(B) the voltage.
(C) the resistance.
(D) the resistivity of the wire.
13. A large sample of a species is fish is genetically altered in the laboratory as to see if those fish can become resistant to a certain bacteria. These fish survived being exposed to the bacteria. When experimenting, there is another set of fish that were not genetically altered. They were subjected to the same bacteria and were shown to die from the bacteria. All of the fish tanks were experimented upon using the same conditions, other then the bacteria. The fish that were not genetically altered
(A) are not considered to be part of the experiment because they died.
(B) are considered to be the control group.
(C) should not be considered when reaching the conclusion of the experiment.
(D) probably died because of factors other than being subjected to the bacteria.
14. The diagram below shows
(A) a mistake in the data collection.
(B) an error in the experiment.
(C) that the scientific model produced doesn’t address the most essentials variables of the experiment.
(D) low accuracy and low precision.
15. When we use our senses to obtain information, we call this
(A) a theory.
(B) a conclusion.
(C) an experiment.
(D) an observation.
16. A __________ is what makes us decide whether or not the data generated from an experiment supports the hypothesis.
(A) question
(B) observations
(C) conclusion
(D) set of variables
17. Which of the following asks a scientific question?
(A) In what year did the Chinese observe a supernova?
(B) Does driving with the widows up impact the fuel economy of my car?
(C) When can we see the next lunar eclipse?
(D) How many square feet are in there in this backyard?
18. The term, “an educated guess” best describes
(A) the hypothesis.
(B) the conclusions that we generate from experimental data.
(C) the uncertainty in the data that has been collected.
(D) the variables that are controlled.
19. In order for the results of an experiment to be accepted, others must be able to
(A) repeat the experiment.
(B) steal the data from the laboratory notebooks of those who worked on the experiment.
(C) argue whether the experiment is valid.
(D) see the actual experiment for themselves.
20. A controlled experiment will examine how many variables at once?
(A) Many
(B) Up to three
(C) Up to two as to look at cause and effect
(D) One
21. A null hypothesis should be rejected or retained based upon
(A) the observations made.
(B) the questions posed.
(C) the statistical significance.
(D) the number of independent variables.
22. A scientific ___________ requires evidence to make a point whether a scientific idea is either accurate or inaccurate.
(A) variable
(B) experiment
(C) argument
(D) control
23. The diagram below shows
(A) that the experiment needs to be done over again.
(B) high accuracy and low precision.
(C) low accuracy and high precision.
(D) low accuracy and low precision.
24. The last step in the scientific method is to
(A) communicate the results with others.
(B) do the experiment over again.
(C) change the variables to see what happens.
(D) make an observation.
ANSWERS TO REVIEW QUESTIONS ON SCIENTIFIC METHOD, SCIENTIFIC REASONING, AND EXPERIMENTAL METHODOLOGY
1. C One uses their senses when making an observation. All of the other choices utilize other skills.
2. A This is the correct order in using the scientific method.
3. D A hypothesis has to be testable by experimentation. It also needs to be an if/then statement where the “then” portion of the statement is used as a prediction.
4. B In a controlled experiment just one variable is tested and it is compared to a control group. As always, a larger sample size is preferred.
5. B The constants in an experiment are not varied. This helps keep the experiment fair for the other factors being tested.
6. C The listed items in the question are all quality portions of a graph.
7. D An experiment needs to have a larger sample size and a control group to compare the results to. Also, the experimenter did not consider eliminating other factors (beyond carbon dioxide) in the cigarette smoke that could alter the results.
8. A The series of steps that we take to solve a problem is called the scientific method. While there may be guidelines for experimenting safely, this is only a smaller portion of the scientific method.
9. D This question shows the definition of a null hypothesis, where the variables have no relationship. This allows for all possibilities to be presumed true in the absence of other data.
10. B This image shows that the points are precise (together) but are not accurate because they miss the intended target.
11. C The statement provided is testable, makes a prediction, and is an if/then statement. All of these describe a hypothesis.
12. A The dependent variable is the variable that is the outcome of the independent variables (the ones that we change). The student can changed the number of resistors in the wire and then chose a battery of a certain voltage. The current is measured is the result of the combination of voltage and resistance.
13. B The group of fish that were not genetically altered serve as the control group. They show that not being genetically altered makes this species of fish vulnerable to the bacteria.
14. D None of the data in this image are shown together/precise. Also, the accuracy is low because they did not hit the intended target.
15. D Observations are made with our sense of smell, taste, touch, hearing, or sight. This can include detecting an odor, getting a taste in our mouth, feeling heat on our skin, hearing a sound, or seeing a flash of light.
16. C The conclusion brings together everything that we have done throughout the scientific method. This is where our experimental data is used to make a conclusion that is consistent with the hypothesis.
17. B The question posed in choice B forces us to design an experiment, collect data, and see if the data supports the question posed. All of the other questions are either recall or questions that do not require experimentation.
18. A Traditionally, the term, “educated guess” is used synonymously with the word, “hypothesis.” We say “traditionally” because many are now challenging the use of the words “educated guess”: the hypothesis is now being termed as an “uncertain explanation.”
19. A The beautiful thing about an experiment is that it is repeatable. Because science works the same way for all of us on Earth, we should get the same results when the experiment is repeated.
20. D A controlled experiment examines just one variable. An example of an experiment that examines many variables at once is an experiment that seeks to determine the rate law of a reaction.
21. C Statistics and data are used to determine if the null hypothesis should be rejected or retained.
22. C In resolving a scientific argument, one uses evidence to make their case for whether an idea is accurate or not.
23. B Although the data points in this image are not tightly together, they are still very much accurate in hitting the middle of the target. This makes them accurate, but not precise.
24. A After completing one’s work regarding the scientific method, one needs to publish their findings in a scientific journal or via another scientific forum as to communicate the results, take credit for the work, and further educate the scientific community.