Initial care of critically ill pts must often be performed rapidly and before a thorough medical history has been obtained. Physiologic stabilization begins with the principles of advanced cardiovascular life support and frequently involves invasive techniques such as mechanical ventilation and renal replacement therapy to support organ systems that are failing. A variety of severity-of-illness scoring systems, such as APACHE (acute physiology and chronic health evaluation), have been developed. Although these tools are useful for ensuring similarity among groups of pts involved in clinical trials or in quality assurance monitoring, their relevance to individual pts is less clear. These scoring systems are not typically used to guide clinical management.
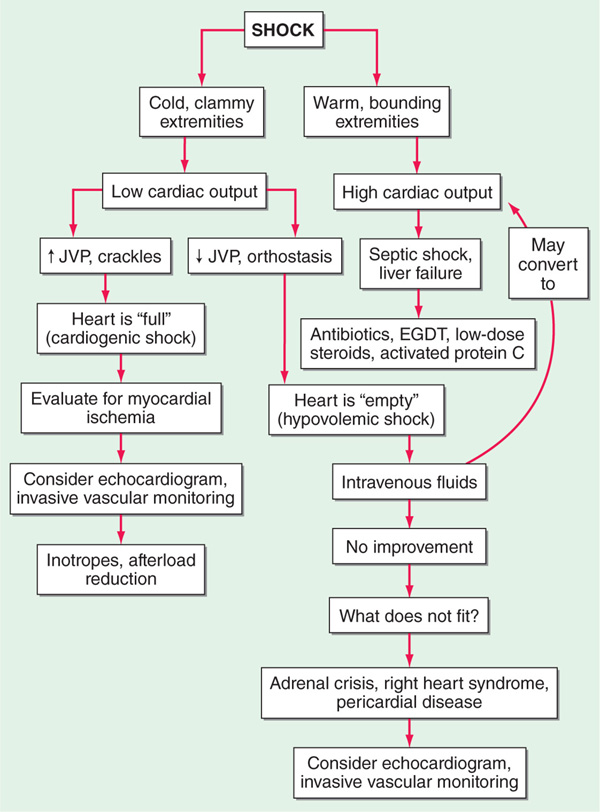
Shock, which is characterized by multisystem end-organ hypoperfusion and tissue hypoxia, is a frequent problem requiring ICU admission. A variety of clinical indicators of shock exist, including reduced mean arterial pressure, tachycardia, tachypnea, cool extremities, altered mental status, oliguria, and lactic acidosis. Although hypotension is usually observed in shock, there is not a specific blood pressure threshold that is used to define it. Shock can result from decreased cardiac output, decreased systemic vascular resistance, or both. The three main categories of shock are hypovolemic, cardiogenic, and high cardiac output/low systemic vascular resistance. Clinical evaluation can be useful to assess the adequacy of cardiac output, with narrow pulse pressure, cool extremities, and delayed capillary refill suggestive of reduced cardiac output. Indicators of high cardiac output (e.g., widened pulse pressure, warm extremities, and rapid capillary refill) associated with shock suggest reduced systemic vascular resistance. Reduced cardiac output can be due to intravascular volume depletion (e.g., hemorrhage) or cardiac dysfunction. Intravascular volume depletion can be assessed through the jugular venous pressure, changes in right atrial pressure with spontaneous respirations, or changes in pulse pressure during positive pressure mechanical ventilation. Reduced systemic vascular resistance is often caused by sepsis, but high cardiac output hypotension is also seen in pancreatitis, liver failure, burns, anaphylaxis, peripheral arteriovenous shunts, and thyrotoxicosis. Early resuscitation of septic and cardiogenic shock may improve survival; objective assessments such as echocardiography and/or invasive vascular monitoring should be used to complement clinical evaluation and minimize end-organ damage. The approach to the pt in shock is outlined in Fig. 5-1.
FIGURE 5-1 Approach to pt in shock. EGDT, early goal directed therapy; JVP, jugular venous pulse.
Critically ill pts often require mechanical ventilation. During initial resuscitation, standard principles of advanced cardiovascular life support should be followed. Mechanical ventilation should be considered for acute hypoxemic respiratory failure, which may occur with cardiogenic shock, pulmonary edema (cardiogenic or noncardiogenic), or pneumonia. Mechanical ventilation should also be considered for treatment of ventila-tory failure, which can result from an increased load on the respiratory system—often manifested by lactic acidosis or decreased lung compliance. Mechanical ventilation may decrease respiratory work, improve arterial oxygenation with improved tissue O2 delivery, and reduce acidosis. Reduction in mean arterial pressure after institution of mechanical ventilation commonly occurs due to reduced venous return from positive pressure ventilation, reduced endogenous catecholamine secretion, and administration of drugs used to facilitate intubation. Since hypovolemia often contributes to postintubation hypotension, IV volume administration should be considered. The major types of respiratory failure are discussed in Chap. 16.
Many pts receiving mechanical ventilation require treatment for pain (typically with opiates) and for anxiety (typically with benzodiazepines, which also have the benefit of providing amnesia). Less commonly, neuromuscular blocking agents are required to facilitate ventilation when there is extreme dyssynchrony between the pt’s respiratory efforts and the ventilator that cannot be corrected with manipulation of the ventilator settings; aggressive sedation is required during treatment with neuromuscular blockers. Neuromuscular blocking agents should be used with caution because a myopathy associated with prolonged weakness can result.
Weaning from mechanical ventilation should be considered when the disease process prompting intubation has improved. Daily screening of intubated pts for weaning potential should be performed. Stable oxygenation (at low PEEP levels), intact cough and airway reflexes, and lack of requirement for vasopressor agents are required before considering a trial of weaning from mechanical ventilation. The most effective approach for weaning is usually a spontaneous breathing trial, which involves 30–120 min of breathing without significant ventilatory support. Either an open T-piece breathing system or minimal amounts of ventilatory support (pressure support to overcome resistance of the endotracheal tube and/or low levels of CPAP) can be used. Failure of a spontaneous breathing trial has occurred if tachypnea (respiratory rate >35 breaths/min for >5 min), hypoxemia (O2 saturation <90%), tachycardia (>140 beats/min or 20% increase from baseline), bradycardia (20% reduction from baseline), hypotension (<90 mmHg), hypertension (>180 mmHg), increased anxiety, or diaphoresis develop. At the end of the spontaneous breathing trial, the rapid shallow breathing index (RSBI or f/VT), which is calculated as respiratory rate in breaths/min divided by tidal volume in liters, can be used to predict weanability. A f/VT <105 at the end of the spontaneous breathing test warrants a trial of extubation. Daily interruption of sedative infusions in conjunction with spontaneous breathing trials can limit excessive sedation and shorten the duration of mechanical ventilation. Despite careful weaning protocols, up to 10% of pts develop respiratory distress after extubation and may require reintubation.
Multiorgan system failure is a syndrome defined by the simultaneous dysfunction or failure of two or more organs in pts with critical illness. Multiorgan system failure is a common consequence of systemic inflammatory conditions (e.g., sepsis, pancreatitis, and trauma). To meet the criteria for multiorgan system failure, organ failure must persist for >24 h. Prognosis worsens with increased duration of organ failure and increased number of organ systems involved.
With critical illness, close and often continuous monitoring of multiple organ systems is required. In addition to pulse oximetry, frequent arterial blood gas analysis can reveal evolving acid-base disturbances and assess the adequacy of ventilation. Intra-arterial pressure monitoring is frequently performed to follow blood pressure and to provide arterial blood gases and other blood samples. Pulmonary artery (Swan-Ganz) catheters can provide pulmonary artery pressure, cardiac output, systemic vascular resistance, and oxygen delivery measurements. However, no morbidity or mortality benefit from pulmonary artery catheter use has been demonstrated, and rare but significant complications from placement of central venous access (e.g., pneumothorax, infection) or the pulmonary artery catheter (e.g., cardiac arrhythmias, pulmonary artery rupture) can result. Thus, routine pulmonary artery catheterization in critically ill pts is not recommended.
For intubated pts receiving volume-controlled modes of mechanical ventilation, respiratory mechanics can be followed easily. The peak airway pressure is regularly measured by mechanical ventilators, and the plateau pressure can be assessed by including an end-inspiratory pause. The inspiratory airway resistance is calculated as the difference between the peak and plateau airway pressures (with adjustment for flow rate). Increased airway resistance can result from bronchospasm, respiratory secretions, or a kinked endotracheal tube. Static compliance of the respiratory system is calculated as the tidal volume divided by the gradient in airway pressure (plateau pressure minus PEEP). Reduced respiratory system compliance can result from pleural effusions, pneumothorax, pneumonia, pulmonary edema, or auto-PEEP (elevated end-expiratory pressure related to insufficient time for alveolar emptying before the next inspiration).
Critically ill pts are prone to a number of complications, including the following:
• Sepsis—Often related to the invasive monitoring performed of critically ill pts.
• Anemia—Usually due to chronic inflammation as well as iatrogenic blood loss. A conservative approach to providing blood transfusions is recommended unless pts have active hemorrhage.
• Deep-vein thrombosis—May occur despite standard prophylaxis with SC heparin or lower extremity sequential compression devices and may occur at the site of central venous catheters. Low-molecular-weight heparins (e.g., enoxaparin) are more effective for high-risk pts than is unfractionated heparin.
• GI bleeding—Stress ulcers of the gastric mucosa frequently develop in pts with bleeding diatheses, shock, or respiratory failure, necessitating prophylactic acid neutralization in such pts.
• Acute renal failure—A frequent occurrence in ICU pts, exacerbated by nephrotoxic medications and hypoperfusion. The most common etiology is acute tubular necrosis. Low-dose dopamine treatment does not protect against the development of acute renal failure.
• Inadequate nutrition and hyperglycemia—Enteral feeding, when possible, is preferred over parenteral nutrition, since the parenteral route is associated with multiple complications including hyperglycemia, cholestasis, and sepsis. The utility of tight glucose control in the ICU is controversial.
• ICU-acquired weakness—Neuropathies and myopathies have been described—typically after at least one week of ICU care. These complications are especially common in sepsis.
A variety of neurologic problems can develop in critically ill pts. Most ICU pts develop delirium, which is characterized by acute changes in mental status, inattention, disorganized thinking, and an altered level of consciousness. Use of dexmedetomidine was associated with less ICU delirium than midazolam, one of the conventional sedatives. Less common but important neurologic complications include anoxic brain injury, stroke, and status epilepticus.
Withholding or withdrawing care commonly occurs in the ICU. Technological advances have allowed many pts to be maintained in the ICU with little or no chance of recovery. Increasingly, pts, families, and caregivers have acknowledged the ethical validity to withhold or withdraw care when the pt or surrogate decision-maker determines that the pt’s goals for care are no longer achievable with the clinical situation.

For a more detailed discussion, see Kress JP, Hall JB: Approach to the Patient With Critical Illness, Chap. 267, p. 2196, in HPIM-18.