
Diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS) are acute complications of diabetes mellitus (DM). DKA is seen primarily in individuals with type 1 DM and HHS in individuals with type 2 DM. Both disorders are associated with absolute or relative insulin deficiency, volume depletion, and altered mental status. The metabolic similarities and differences in DKA and HHS are summarized in Table 24-1.
TABLE 24-1 LABORATORY VALUES IN DIABETIC KETOACIDOSIS (DKA) AND HYPERGLYCEMIC HYPEROSMOLAR STATE (HHS) (REPRESENTATIVE RANGES AT PRESENTATION)

DKA results from insulin deficiency with a relative or absolute increase in glucagon and may be caused by inadequate insulin administration, infection (pneumonia, urinary tract infection, gastroenteritis, sepsis), infarction (cerebral, coronary, mesenteric, peripheral), surgery, trauma, drugs (cocaine), or pregnancy. A common precipitating scenario is the pt with type 1 DM who erroneously stops administering insulin because of anorexia/lack of food intake caused by a minor illness, followed by lipolysis and progressive ketosis leading to DKA.
The initial symptoms of DKA include anorexia, nausea, vomiting, polyuria, and thirst. Abdominal pain, altered mental function, or frank coma may ensue. Classic signs of DKA include Kussmaul respirations and an acetone odor on the pt’s breath. Volume depletion can lead to dry mucous membranes, tachycardia, and hypotension. Fever and abdominal tenderness may also be present. Laboratory evaluation reveals hyperglycemia, ketosis (β-hydroxybutyrate > acetoacetate), and metabolic acidosis (arterial pH 6.8–7.3) with an increased anion gap (Table 24-1). The fluid deficit is often 3–5 L and can be greater. Despite a total-body potassium deficit, the serum potassium at presentation may be normal or mildly high as a result of acidosis. Similarly, phosphate may be normal at presentation despite total body phosphate depletion. Leukocytosis, hypertriglyceridemia, and hyperlipoproteinemia are common. Hyperamylasemia is usually of salivary origin but may suggest a diagnosis of pancreatitis. The measured serum sodium is reduced as a consequence of osmotic fluid shifts due to hyper-glycemia [1.6-meq reduction for each 5.6-mmol/L (100-mg/dL) rise in the serum glucose].
TREATMENT Diabetic Ketoacidosis
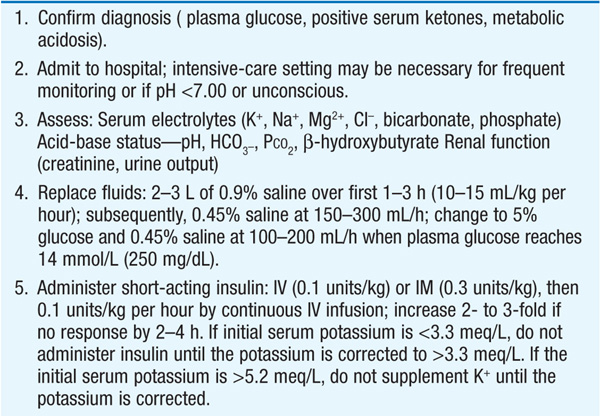
The management of DKA is outlined in Table 24-2.
TABLE 24-2 MANAGEMENT OF DIABETIC KETOACIDOSIS

Relative insulin deficiency and inadequate fluid intake are the underlying causes of HHS. Hyperglycemia induces an osmotic diuresis that leads to profound intravascular volume depletion. HHS is often precipitated by a serious, concurrent illness such as myocardial infarction or sepsis and compounded by conditions that impede access to water.
Presenting symptoms include polyuria, thirst, and altered mental state, ranging from lethargy to coma. Notably absent are symptoms of nausea, vomiting, and abdominal pain and the Kussmaul respirations characteristic of DKA. The prototypical pt is an elderly individual with a several week history of polyuria, weight loss, and diminished oral intake. The laboratory features are summarized in Table 24-1. In contrast to DKA, acidosis and ketonemia are usually not found; however, a small anion gap may be due to lactic acidosis, and moderate ketonuria may occur from starvation. Prerenal azotemia is typically present. Although the measured serum sodium may be normal or slightly low, the corrected serum sodium is usually increased [add 1.6 meq to measured sodium for each 5.6-mmol/L (100-mg/dL) rise in the serum glucose]. HHS, even when adequately treated, has a significant mortality rate (up to 15%), which is in part explained by comorbidities and pt age.
TREATMENT Hyperglycemic Hyperosmolar State
The precipitating problem should be sought and treated. Sufficient IV fluids (1–3 L of 0.9% normal saline over the first 2–3 h) must be given to stabilize the hemodynamic status. The calculated free water deficit (usually 9–10 L) should be reversed over the next 1–2 days, using 0.45% saline initially then 5% dextrose in water. Overly rapid fluid replacement should be avoided to prevent worsening of neurologic status. Potassium repletion is usually necessary. The plasma glucose may drop precipitously with hydration alone, though insulin therapy with an IV bolus of 0.1 units/kg followed by a constant infusion rate (0.1 units/kg per hour) is usually required. If the serum glucose does not fall, the insulin infusion rate should be doubled. Glucose should be added to IV fluid and the insulin infusion rate decreased when the plasma glucose falls to 13.9 mmol/L (250 mg/dL). The insulin infusion should be continued until the pt has resumed eating and can be transitioned to a subcutaneous insulin regimen.

For a more detailed discussion, see Powers AC: Diabetes Mellitus, Chap. 344, p. 2968, in HPIM-18.