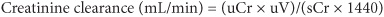
Azotemia is the retention of nitrogenous waste products excreted by the kidney. Increased levels of blood urea nitrogen (BUN) [>10.7 mmol/L (>30 mg/dL)] and creatinine [>133 μmol/L (>1.5 mg/dL)] are ordinarily indicative of impaired renal function. Renal function can be estimated by determining the clearance of creatinine (CLcr) (normal >100 mL/min); this can be directly measured from a 24-h urine collection using the following equation:
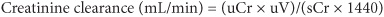
1. Where uCr is urine creatinine in mg/dL
2. Where sCr is serum creatinine in mg/dL
3. Where uV is 24-h urine volume in mL
4. Where 1440 represents number of minutes in 24 h
The “adequacy” or “completeness” of the collection is estimated by the urinary volume and creatinine content; creatinine is produced from muscle and excreted at a relatively constant rate. For a 20- to 50-year-old man, creatinine excretion should be 18.5–25.0 mg/kg body weight; for a woman of the same age, it should be 16.5–22.4 mg/kg body weight. For example, an 80-kg man should excrete between ~1500 and 2000 mg of creatinine in an “adequate” collection. Creatinine excretion is also influenced by age and muscle mass. Notably, creatinine is an imperfect measure of glomerular filtration rate (GFR), since it is both filtered by glomeruli and secreted by proximal tubular cells; the relative contribution of tubular secretion increases with advancing renal dysfunction, such that creatinine clearance will provide an overestimate of the “true” GFR in pts with chronic kidney disease. Isotopic markers that are filtered and not secreted (e.g., iothalamate) provide more accurate estimates of GFR.
A formula that allows for an estimate of creatinine clearance in men that accounts for age-related decreases in GFR, body weight, and sex has been derived by Cockcroft-Gault:
Creatinine clearance (mL/min)
= (140 age) × lean body weight (kg)/plasma creatinine (mg/dL) × 72
This value should be multiplied by 0.85 for women.
GFR may also be estimated using serum creatinine–based equations derived from the Modification of Diet in Renal Disease Study. This “eGFR” is now reported with serum creatinine by most clinical laboratories in the United States and is the basis for the National Kidney Foundation classification of chronic kidney disease (Table 52-1).
TABLE 52-1 THE CLASSIFICATION OF CHRONIC KIDNEY DISEASE (NATIONAL KIDNEY FOUNDATION GUIDELINES)

Manifestations of impaired renal function include volume overload, hypertension, electrolyte abnormalities (e.g., hyperkalemia, hypocalcemia, hyper-phosphatemia), metabolic acidosis, and hormonal disturbances (e.g., insulin resistance, functional vitamin D deficiency, secondary hyperparathyroidism). When severe, the symptom complex of “uremia” may develop, encompassing one or more of the following symptoms and signs: anorexia, dysgeusia, nausea, vomiting, lethargy, confusion, asterixis, pleuritis, pericarditis, enteritis, pruritus, sleep and taste disturbance, nitrogenous fetor.
An approach to the pt with azotemia is shown in Fig. 52-1.
FIGURE 52-1 Approach to the pt with azotemia. FeNa, fractional excretion of sodium; GBM, glomerular basement membrane; RBC, red blood cell; WBC, white blood cell. (From Lin J and Denker BM: HPIM-18.)
This refers to reduced urine output, usually defined as <400 mL/d. Oligoanuria refers to a more marked reduction in urine output, i.e., <100 mL/d. Anuria indicates the complete absence of urine output. Oliguria most often occurs in the setting of volume depletion and/or renal hypoperfusion, resulting in “prerenal azotemia” and acute renal failure (Chap. 148). Anuria can be caused by complete bilateral urinary tract obstruction; a vascular catastrophe (dissection or arterial occlusion); renal vein thrombosis; renal cortical necrosis; severe acute tubular necrosis; nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme (ACE) inhibitors, and/or angiotensin receptor blockers; and hypovolemic, cardiogenic, or septic shock. Oliguria is never normal, since at least 400 mL of maximally concentrated urine must be produced to excrete the obligate daily osmolar load.
Polyuria is defined as a urine output >3 L/d. It is often accompanied by nocturia and urinary frequency and must be differentiated from other more common conditions associated with lower urinary tract pathology and urinary urgency or frequency (e.g., cystitis, prostatism). It is often accompanied by hypernatremia (Chap. 2). Polyuria (Table 52-2) can occur as a response to a solute load (e.g., hyperglycemia) or to an abnormality in argi-nine vasopressin [AVP; also known as antidiuretic hormone (ADH)] action. Diabetes insipidus is termed central if due to the insufficient hypothalamic production of AVP and nephrogenic if the result of renal insensitivity to the action of AVP. Excess fluid intake can lead to polyuria, but primary polydipsia rarely results in changes in plasma osmolality unless urinary diluting capacity is impaired. Tubulointerstitial diseases, lithium therapy, and resolving acute tubular necrosis or urinary tract obstruction can be associated with nephrogenic diabetes insipidus, which is more rarely caused by mutations in the V2 AVP receptor or the AVP-regulated water channel, aquaporin 2.
TABLE 52-2 MAJOR CAUSES OF POLYURIA

The approach to the pt with polyuria is shown in Fig. 52-2.
FIGURE 52-2 Approach to the pt with polyuria. ADH, antidiuretic hormone; ATN, acute tubular necrosis. (From Lin J and Denker BM: HPIM-18.)
This is the hallmark of glomerular disease. Levels up to 150 mg/d are considered within normal limits. Typical measurements are semiquantitative, using a moderately sensitive dipstick that estimates protein concentration; therefore, the degree of hydration may influence the dipstick protein determination. Most commercially available urine dipsticks detect albumin and do not detect smaller proteins, such as light chains, that require testing with sulfosalicylic acid. More sensitive assays can in turn be used to detect microalbuminuria, an important screening tool for diabetic nephropathy. A urine albumin to creatinine ratio >30 mg/g defines the presence of micro-albuminuria.
Formal assessment of urinary protein excretion requires a 24-h urine protein collection (see “Abnormalities of Renal Function, Azotemia,” above). The ratio of protein to creatinine in a random, “spot” urine can also provide a rough estimate of protein excretion; for example, a protein/creati-nine ratio of 3.0 correlates to ~3.0 g of proteinuria per day.
Urinary protein excretion rates between 500 mg/d and 3 g/d are nonspecific and can be seen in a variety of renal diseases (including hypertensive nephrosclerosis, interstitial nephritis, vascular disease, and other primary renal diseases with little or no glomerular involvement). Transient, lesser degrees of proteinuria (500 mg/d to 1.5 g/d) may be seen after vigorous exercise, changes in body position, fever, or congestive heart failure. Protein excretion rates >3 g/d are termed nephrotic range proteinuria in that they may be accompanied by hypoalbuminemia, hypercholesterolemia, and edema (the nephrotic syndrome). Nephrotic syndrome can be associated with a variety of extrarenal complications (Chap. 152). Massive degrees of proteinuria (>10 g/d) can be seen with minimal change disease, primary focal segmental sclerosis (FSGS), membranous nephropathy, collapsing glomerulopathy (a subtype of primary FSGS), and HIV-associated nephropathy.
Pharmacologic inhibition of ACE or blockade of angiotensin II should be employed to reduce proteinuria; successful reduction of proteinuria decreases the rate of progression to end-stage renal disease in diabetic nephropathy and other glomerulopathies. Specific therapy for a variety of causes of nephrotic syndrome is discussed in Chap. 152.
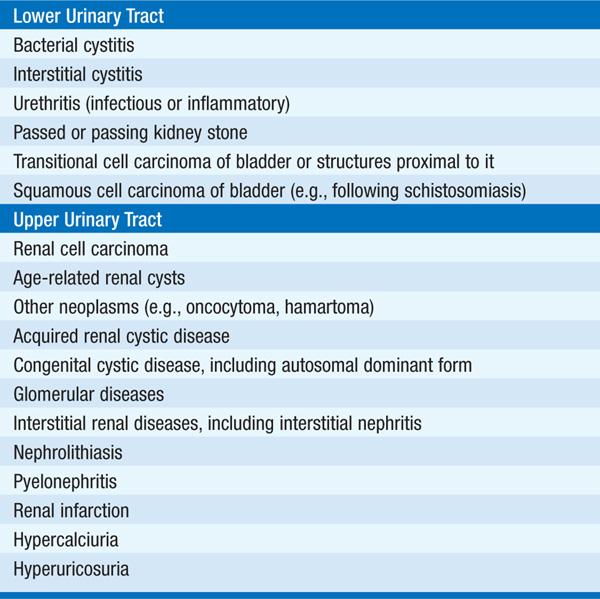
Gross hematuria refers to the presence of frank blood in the urine and is more characteristic of lower urinary tract disease and/or bleeding diatheses than intrinsic renal disease (Table 52-3). Cyst rupture in polycystic kidney disease and postpharyngitic flares of IgA nephropathy are exceptions. Microscopic hematuria [>1–2 red blood cells (RBCs) per high-powered field] accompanied by proteinuria, hypertension, and an active urinary sediment (the “nephritic syndrome”) is most likely related to an inflammatory glomerulonephritis, classically poststreptococcal glomerulonephritis (Chap. 152).
TABLE 52-3 MAJOR CAUSES OF HEMATURIA
Free hemoglobin and myoglobin are detected by dipstick; a negative urinary sediment with strongly heme-positive dipstick is characteristic of either hemolysis or rhabdomyolysis, which can be differentiated by clinical history and laboratory testing. RBC casts are not a sensitive finding but when seen are highly specific for glomerulonephritis. Specificity of urinalysis can be enhanced by examining urine with a phase contrast microscope capable of detecting dysmorphic red cells (“acanthocytes”) associated with glomerular disease.
The approach to the pt with hematuria is shown in Fig. 52-3.
FIGURE 52-3 Approach to the pt with hematuria. ANCA, antineutrophil cytoplasmic antibody; ASLO, antistreptolysin O; CT, computed tomography; GBM, glomerular basement membrane; IVP, intravenous pyelography; RBC, red blood cell; UA, urinalysis; VDRL, Venereal Disease Research Laboratory; WBC, white blood cell. (From Lin J and Denker BM: HPIM-18.)
This may accompany hematuria in inflammatory glomerular diseases. Isolated pyuria is most commonly observed in association with an infection of the upper or lower urinary tract. Pyuria may also occur with allergic interstitial nephritis (often with a preponderance of eosinophils), transplant rejection, and noninfectious, nonallergic tubulointerstitial diseases, including atheroembolic renal disease. The finding of “sterile” pyuria (i.e., urinary white blood cells without bacteria) in the appropriate clinical setting should raise suspicion of renal tuberculosis.
For a more detailed discussion, see Lin J, Denker BM: Azotemia and Urinary Abnormalities, Chap. 44, p. 334, in HPIM-18.