CHAPTER 141
Pneumonia, Bronchiectasis, and Lung Abscess
PNEUMONIA
Pneumonia, an infection of the lung parenchyma, is classified as community-acquired (CAP) or health care–associated (HCAP). The HCAP category is subdivided into hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP). HCAP is associated with hospitalization for ≥48 h, hospitalization for ≥2 days in the prior 3 months, residence in a nursing home or extended-care facility, antibiotic therapy in the preceding 3 months, chronic dialysis, home infusion therapy, home wound care, and contact with a family member who has a multidrug-resistant (MDR) infection.
PATHOPHYSIOLOGY
• Microorganisms gain access to the lower respiratory tract via micro-aspiration from the oropharynx (the most common route), inhalation of contaminated droplets, hematogenous spread, or contiguous extension from an infected pleural or mediastinal space.
• Before disease manifests, the size of the organism burden must overcome the ability of macrophages and other components of innate immunity (e.g., surfactant proteins A and D) to clear bacteria.
• Classic pneumonia (typified by that due to Streptococcus pneumoniae) presents as a lobar pattern and evolves through four phases characterized by changes in the alveoli:
– Edema: Proteinaceous exudates are present in the alveoli.
– Red hepatization: Erythrocytes and neutrophils are present in the intraalveolar exudate.
– Gray hepatization: Neutrophils and fibrin deposition are abundant.
– Resolution: Macrophages are the dominant cell type.
• In VAP, respiratory bronchiolitis can precede a radiologically apparent infiltrate.
COMMUNITY-ACQUIRED PNEUMONIA
Microbiology
Although many bacteria, viruses, fungi, and protozoa can cause CAP, most cases are caused by relatively few pathogens. In >50% of cases, a specific etiology is never determined.
• Typical bacterial pathogens include S. pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative bacteria such as Klebsiella pneumoniae and Pseudomonas aeruginosa.
• Atypical organisms include Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella species, and respiratory viruses (e.g., influenza viruses, adenoviruses, respiratory syncytial viruses).
– A virus may be responsible for up to 18% of cases of CAP that require hospital admission.
– 10–15% of CAP cases are polymicrobial and involve a combination of typical and atypical organisms.
• Involvement of anaerobes, which play a significant role in CAP only when aspiration precedes presentation by days or weeks, often results in significant empyemas.
Epidemiology
CAP affects ~4 million adults each year in the United States, 80% of whom are treated on an outpatient basis. CAP causes 45,000 deaths annually and is associated with an overall yearly cost of $9–10 billion.
• Incidence rates of CAP are highest at the extremes of age (i.e., <4 and >60 years).
• Risk factors for CAP include alcoholism, asthma, immunosuppression, institutionalization, and an age of ≥70 years (vs. 60–69 years).
• Many factors—e.g., tobacco smoking, chronic obstructive pulmonary disease, colonization with methicillin-resistant S. aureus (MRSA), recent hospitalization or antibiotic therapy—influence the types of pathogens that should be considered in the etiologic diagnosis.
Clinical Manifestations
Pts frequently have fever, chills, sweats, cough (either nonproductive or productive of mucoid, purulent, or blood-tinged sputum), pleuritic chest pain, and dyspnea.
• Other common symptoms include nausea, vomiting, diarrhea, fatigue, headache, myalgias, and arthralgias.
• Elderly pts may present atypically, with confusion but few other manifestations.
• Physical examination often reveals tachypnea; increased or decreased tactile fremitus; dull or flat percussion reflecting consolidation and pleural fluid, respectively; crackles; bronchial breath sounds; or a pleural friction rub.
Diagnosis
Both confirmation of the diagnosis and assessment of the likely etiology are required. Although no data have demonstrated that treatment directed at a specific pathogen is superior to empirical treatment, an etiologic diagnosis allows narrowing of the empirical regimen, identification of organisms with public safety implications (e.g., Mycobacterium tuberculosis, influenza virus), and monitoring of antibiotic susceptibility trends.
• Chest radiography is often required to differentiate CAP from other conditions, particularly since the sensitivity and specificity of physical exam findings for CAP are only 58% and 67%, respectively.
– CT of the chest may be helpful for pts with suspected postobstructive pneumonia.
– Some radiographic patterns suggest an etiology; e.g., pneumatoceles suggest S. aureus.
• Sputum samples must have >25 WBCs and <10 squamous epithelial cells per high-power field to be appropriate for culture. The sensitivity of sputum cultures is highly variable; in cases of proven bacteremic pneumococcal pneumonia, the yield of positive cultures from sputum samples is ≤50%.
• Blood cultures are positive in 5–14% of cases, most commonly yielding S. pneumoniae. Blood cultures are optional for most CAP pts, but should be performed for high-risk pts (e.g., pts with chronic liver disease or asplenia).
• Urine antigen tests for S. pneumoniae and Legionella pneumophila type 1 can be helpful.
• Serology: A fourfold rise in titer of specific IgM antibody can assist in the diagnosis of pneumonia due to some pathogens; however, the time required to obtain a final result makes serology of limited clinical utility.
TREATMENT Community-Acquired Pneumonia
DECIDING WHETHER TO HOSPITALIZE PTS
• Two sets of criteria identify pts who will benefit from hospital care. It is not clear which set is superior, and application of each tool should be tempered by a consideration of factors relevant to the individual pt.
– Pneumonia Severity Index (PSI): Points are given for 20 variables, including age, coexisting illness, and abnormal physical and laboratory findings. On this basis, pts are assigned to one of five classes of mortality risk.
– CURB-65: Five variables are included: confusion (C); urea >7 mmol/L (U); respiratory rate ≥30/min (R); blood pressure, systolic ≤90 mmHg or diastolic ≤60 mmHg (B); and age ≥65 years (65). Pts with a score of 0 can be treated at home, pts with a score of 2 should be hospitalized, and pts with a score of ≥3 may require management in the intensive care unit (ICU).
ANTIBIOTIC THERAPY
• For recommendations on empirical antibiotic treatment of CAP, see Table 141-1. U.S. guidelines always target S. pneumoniae and atypical pathogens. Retrospective data suggest that this approach lowers the mortality rate.
TABLE 141-1 EMPIRICAL ANTIBIOTIC TREATMENT OF COMMUNITY-ACQUIRED PNEUMONIA
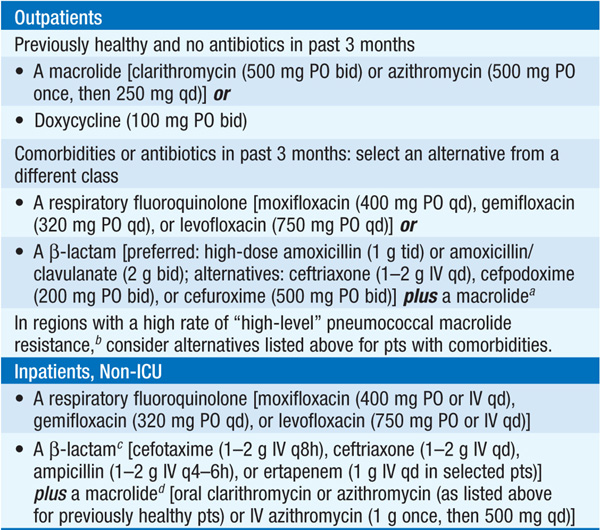
• Pts initially treated with IV antibiotics can be switched to oral agents when they can ingest and absorb drugs, are hemodynamically stable, and are improving clinically.
• CAP has historically been treated for 10–14 days, but a 5-day course of a fluoroquinolone is sufficient for cases of uncomplicated CAP. A longer course is required for pts with bacteremia, metastatic infection, or infection with a particularly virulent pathogen and in most cases of severe CAP.
• Fever and leukocytosis usually resolve within 2–4 days. Pts who have not responded to therapy by day 3 should be reevaluated, with consideration of alternative diagnoses, antibiotic resistance in the pathogen, and the possibility that the wrong drug is being given.
Complications
Common complications of severe CAP include respiratory failure, shock and multiorgan failure, coagulopathy, and exacerbation of comorbid disease. Metastatic infection (e.g., brain abscess, endocarditis) occurs rarely and requires immediate attention.
• Lung abscess may occur in association with aspiration or infection caused by single CAP pathogens [e.g., community-acquired MRSA (CA-MRSA) or P. aeruginosa]. Drainage should be established and proper antibiotics administered.
• Any significant pleural effusion should be tapped for diagnostic and therapeutic purposes. If the fluid has a pH <7, a glucose level <2.2 mmol/L, and a lactate dehydrogenase content >1000 U or if bacteria are seen or cultured, fluid should be drained; a chest tube is usually required.
Follow-Up
Chest x-ray abnormalities may require 4–12 weeks to clear. Pts should receive influenza and pneumococcal vaccines, as appropriate.
HEALTH CARE–ASSOCIATED PNEUMONIA (SEE ALSO CHAP. 87)
VENTILATOR-ASSOCIATED PNEUMONIA
Microbiology
Potential etiologic agents include MDR and non-MDR pathogens; the prominence of the various pathogens depends on the length of hospital stay at the time of infection.
Epidemiology, Pathogenesis, and Clinical Manifestations
Prevalence estimates of VAP are 6–52 cases per 100 pts, with the highest hazard ratio in the first 5 days of mechanical ventilation.
• Three factors important in the pathogenesis of VAP are colonization of the oropharynx with pathogenic microorganisms, aspiration of these organisms to the lower respiratory tract, and compromise of normal host defense mechanisms.
• Clinical manifestations are similar to those in other forms of pneumonia.
Diagnosis
Application of clinical criteria consistently results in overdiagnosis of VAP. Use of quantitative cultures to discriminate between colonization and true infection by determining bacterial burden may be helpful; the more distal in the respiratory tree the diagnostic sampling, the more specific the results.
TREATMENT Ventilator-Associated Pneumonia
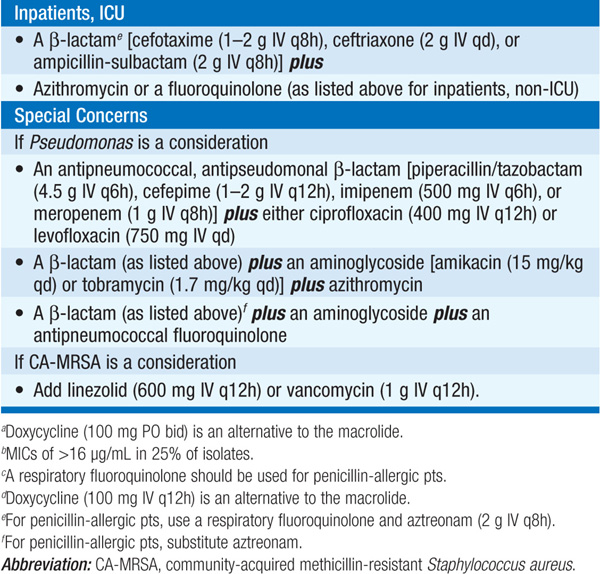
• See Table 141-2 for recommended options for empirical therapy for HCAP.
TABLE 141-2 EMPIRICAL ANTIBIOTIC TREATMENT OF HEALTH CARE–ASSOCIATED PNEUMONIA
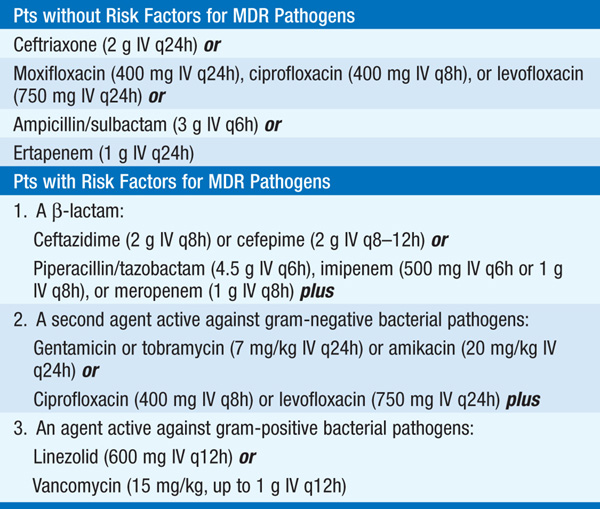
– Higher mortality rates are associated with inappropriate initial empirical treatment.
– Broad-spectrum treatment should be modified when a pathogen is identified.
– Clinical improvement, if it occurs, is usually evident within 48–72 h of the initiation of antimicrobial treatment.
• Treatment failure in VAP is not uncommon, especially when MDR pathogens are involved; MRSA and P. aeruginosa are associated with high failure rates.
• VAP complications include prolongation of mechanical ventilation, increased length of ICU stay, and necrotizing pneumonia with pulmonary hemorrhage or bronchiectasis. VAP is associated with significant mortality risk.
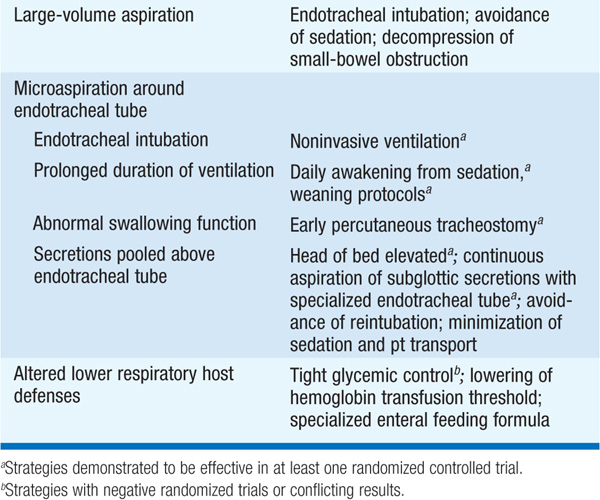
• Strategies effective for the prevention of VAP are listed in Table 141-3.
TABLE 141-3 PATHOGENIC MECHANISMS AND CORRESPONDING PREVENTION STRATEGIES FOR VENTILATOR-ASSOCIATED PNEUMONIA
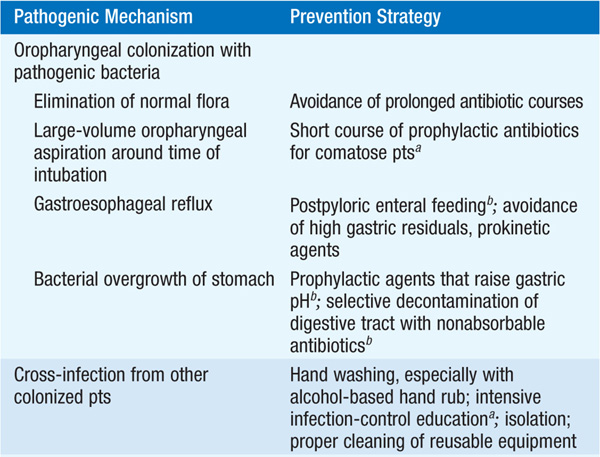
HOSPITAL-ACQUIRED PNEUMONIA
Less well studied than VAP, HAP more commonly involves non-MDR pathogens. Anaerobes may also be more commonly involved in non-VAP pts because of the increased risk of macroaspiration in pts who are not intubated.
BRONCHIECTASIS
Etiology and Epidemiology
Bronchiectasis is an irreversible airway dilation that involves the lung in either a focal (due to obstruction) or a diffuse (due to a systemic or infectious process) manner. Bronchiectasis can arise from infectious or noninfectious causes.
• The epidemiology varies greatly with the underlying etiology; in general, the incidence of bronchiectasis increases with age and is higher among women than among men.
• 25–50% of pts with bronchiectasis have idiopathic disease.
Pathogenesis
The most widely cited mechanism of infectious bronchiectasis is the “vicious cycle hypothesis,” in which susceptibility to infection and poor mucociliary clearance result in microbial colonization of the bronchial tree. Proposed mechanisms for noninfectious bronchiectasis include immune-mediated reactions that damage the bronchial wall and parenchymal distortion as a result of lung fibrosis (e.g., postradiation fibrosis or idiopathic pulmonary fibrosis).
Clinical Manifestations
Presenting pts typically have a persistent productive cough with ongoing production of thick, tenacious sputum.
• Physical exam usually reveals crackles and wheezing on lung auscultation and occasionally reveals digital clubbing.
• Acute exacerbations are associated with increased purulent-sputum production.
Diagnosis
The diagnosis of bronchiectasis is based on clinical presentation with consistent radiographic findings, such as parallel “tram tracks,” a “signet-ring sign” (a cross-sectional area of the airway with a diameter at least 1.5 times that of the adjacent vessel), lack of bronchial tapering, bronchial wall thickening, or cysts emanating from the bronchial wall.
Treatment of infectious bronchiectasis is directed at the control of active infection and at improvements in secretion clearance and bronchial hygiene.
• Acute exacerbations should be treated with a 7- to 10-day course of antibiotics targeting the causative or presumptive pathogen; H. influenzae and P. aeruginosa are isolated commonly.
• Hydration and mucolytic administration, aerosolization of bronchodilators and hyperosmolar agents (e.g., hypertonic saline), and chest physiotherapy can be used to enhance secretion clearance.
• For pts with ≥3 recurrences per year, suppressive antibiotic treatment to minimize the microbial load and reduce the frequency of exacerbations has been proposed.
• In select cases, surgery (including lung transplantation) should be considered.
LUNG ABSCESS
Microbiology
Lung abscess—infection of the lung that results in necrosis of the pulmonary parenchyma—can be caused by a variety of microorganisms. The etiology depends, in part, on the characteristics of the host.
• Previously healthy pts are at risk for infection with bacteria (e.g., S. aureus, Streptococcus milleri, K. pneumoniae, group A Streptococcus) and parasites (e.g., Entamoeba histolytica, Paragonimus westermani, Strongyloides stercoralis).
• Aspiration-prone pts are at risk for infection with anaerobic bacteria; S. aureus, P. aeruginosa, and F. necrophorum (embolic lesions); endemic fungi; and mycobacteria.
• Immunocompromised pts are susceptible to M. tuberculosis, Nocardia asteroides, Rhodococcus equi, Legionella species, Enterobacteriaceae, Aspergillus species, and Cryptococcus species.
Clinical Manifestations
Nonspecific lung abscesses—typically presumed to be due to anaerobic organisms—present as an indolent infection with fatigue, cough, sputum production, fever, weight loss, and anemia. Pts may have foul-smelling breath or evidence of periodontal infection with pyorrhea or gingivitis.
Diagnosis
A chest CT is the preferred radiographic study to precisely delineate the lesion.
• Sputum samples can be cultured to detect aerobic bacteria but are unreliable for culture of anaerobic bacteria.
• Pleural fluid or bronchoalveolar lavage specimens may be helpful if they are processed promptly and appropriately for anaerobic bacteria.
Treatment depends on the presumed or established etiology.
• Most infections caused by anaerobic bacteria should be treated initially with clindamycin (600 mg IV qid). Any β-lactam/-β-lactamase inhibitor combination is an alternative.
• A transition from parenteral to oral therapy is appropriate once pts become afebrile and improve clinically.
• Although the duration of therapy is arbitrary, continuation of oral treatment is recommended until imaging shows that chest lesions have cleared or have left a small, stable scar.
• Fever persisting for 5–7 days after antibiotics have been started suggests failure of therapy and a need to exclude factors such as obstruction, complicating empyema, and involvement of antibiotic-resistant bacteria.
– Clinical improvement with decreased fever usually occurs within 3–5 days, with defervescence in 5–10 days.
– Pts with fevers persisting for 7–14 days should undergo bronchoscopy or other diagnostic tests to better define anatomic changes and microbiologic findings.

For a more detailed discussion, see Mandell LA, Wunderink R: Pneumonia, Chap. 257, p. 2130; and Baron RM, Bartlett JG: Bronchiectasis and Lung Abscess, Chap. 258, p. 2142, in HPIM-18.





