PUD occurs most commonly in duodenal bulb (duodenal ulcer, DU) and stomach (gastric ulcer, GU). It may also occur in esophagus, pyloric channel, duodenal loop, jejunum, Meckel’s diverticulum. PUD results when “aggressive” factors (gastric acid, pepsin) overwhelm “defensive” factors involved in mucosal resistance (gastric mucus, bicarbonate, microcirculation, prostaglandins, mucosal “barrier”), and from effects of Helicobacter pylori.
H. pylori is a spiral urease-producing organism that colonizes gastric antral mucosa in up to 100% of persons with DU and 80% with GU. It is also found in normals (increasing prevalence with age) and in those of low socioeconomic status. H. pylori is invariably associated with histologic evidence of active chronic gastritis, which over years can lead to atrophic gastritis and gastric cancer. The other major cause of ulcers (those not due to H. pylori) is nonsteroidal anti-inflammatory drugs (NSAIDs). Fewer than 1% are due to gastrinoma (Zollinger-Ellison syndrome). Other risk factors and associations: hereditary (? increased parietal cell number), smoking, hypercalcemia, mastocytosis, blood group O (antigens may bind H. pylori). Unproven: stress, coffee, alcohol.
Mild gastric acid hypersecretion resulting from (1) increased release of gastrin, presumably due to (a) stimulation of antral G cells by cytokines released by inflammatory cells and (b) diminished production of somatostatin by D cells, both resulting from H. pylori infection; and (2) an exaggerated acid response to gastrin due to an increased parietal cell mass resulting from gastrin stimulation. These abnormalities reverse rapidly with eradication of H. pylori. However, a mildly elevated maximum gastric acid output in response to exogenous gastrin persists in some pts long after eradication of H. pylori, suggesting that gastric acid hypersecretion may be, in part, genetically determined. H. pylori may also result in elevated serum pepsinogen levels. Mucosal defense in duodenum is compromised by toxic effects of H. pylori infection on patches of gastric metaplasia that result from gastric acid hypersecretion or rapid gastric emptying. Other risk factors include glucocorticoids, NSAIDs, chronic renal failure, renal transplantation, cirrhosis, chronic lung disease.
H. pylori is also principal cause. Gastric acid secretory rates are usually normal or reduced, possibly reflecting earlier age of infection by H. pylori than in DU pts. Gastritis due to reflux of duodenal contents (including bile) may play a role. Chronic salicylate or NSAID use may account for 15–30% of GUs and increase risk of associated bleeding, perforation.
Burning epigastric pain 90 min to 3 h after meals, often nocturnal, relieved by food.
Burning epigastric pain made worse by or unrelated to food; anorexia, food aversion, weight loss (in 40%). Great individual variation. Similar symptoms may occur in persons without demonstrated peptic ulcers (“nonulcer dyspepsia”); less responsive to standard therapy.
Bleeding, obstruction, penetration causing acute pancreatitis, perforation, intractability.
Upper endoscopy or upper GI barium radiography.
Upper endoscopy preferable to exclude possibility that ulcer is malignant (brush cytology, ≥6 pinch biopsies of ulcer margin). Radiographic features suggesting malignancy: ulcer within a mass, folds that do not radiate from ulcer margin, a large ulcer (>2.5–3 cm).
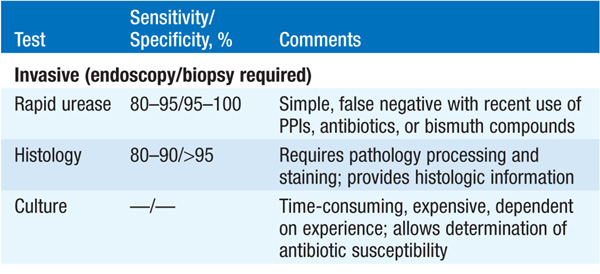
Detection of antibodies in serum (inexpensive, preferred when endoscopy is not required); rapid urease test of antral biopsy (when endoscopy is required). Urea breath test generally used to confirm eradication of H. pylori, if necessary. The fecal antigen test is sensitive, specific, and inexpensive (Table 158-1).
TABLE 158-1 TESTS FOR DETECTION OF H. PYLORI

TREATMENT Peptic Ulcer Disease
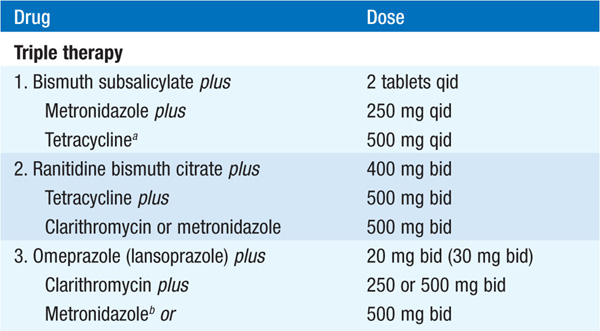
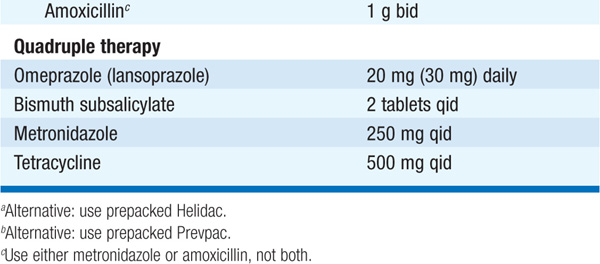
MEDICAL Objectives: pain relief, healing, prevention of complications, prevention of recurrences. For GU, exclude malignancy (follow endoscopically to healing). Dietary restriction unnecessary with contemporary drugs; discontinue NSAIDs; smoking may prevent healing and should be stopped. Eradication of H. pylori markedly reduces rate of ulcer relapse and is indicated for all DUs and GUs associated with H. pylori (Table 158-2). Acid suppression is generally included in regimen. Reinfection rates are <1%/year. Standard drugs (H2 receptor blockers, sucralfate, antacids) heal 80–90% of DUs and 60% of GUs in 6 weeks; healing is more rapid with omeprazole (20 mg/d).
TABLE 158-2 REGIMENS RECOMMENDED FOR ERADICATION OF H. PYLORI INFECTION
SURGERY Used for complications (persistent or recurrent bleeding, obstruction, perforation) or, uncommonly, intractability (first screen for surreptitious NSAID use and gastrinoma). For DU, see Table 158-3. For GU, perform subtotal gastrectomy.
TABLE 158-3 SURGICAL TREATMENT OF DUODENAL ULCER
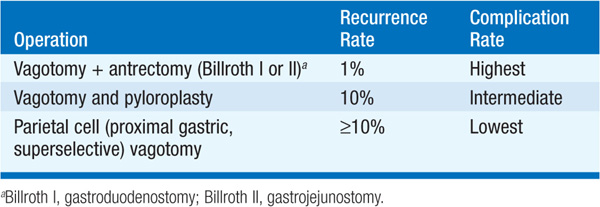
COMPLICATIONS OF SURGERY (1) Obstructed afferent loop (Billroth II), (2) bile reflux gastritis, (3) dumping syndrome (rapid gastric emptying with abdominal distress + postprandial vasomotor symptoms), (4) post-vagotomy diarrhea, (5) bezoar, (6) anemia (iron, B12, folate malabsorption), (7) malabsorption (poor mixing of gastric contents, pancreatic juices, bile; bacterial overgrowth), (8) osteomalacia and osteoporosis (vitamin D and Ca malabsorption), (9) gastric remnant carcinoma.
Optimal approach is uncertain. Serologic testing for H. pylori and treating, if present, may be cost-effective. Other options include trial of acid-suppressive therapy, endoscopy only in treatment failures, or initial endoscopy in all cases.
Hemorrhagic gastritis, multiple gastric erosions may be caused by aspirin and other NSAIDs (lower risk with newer agents, e.g., nabumetone and etodolac, which do not inhibit gastric mucosal prostaglandins) or severe stress (burns, sepsis, trauma, surgery, shock, or respiratory, renal, or liver failure). Pt may be asymptomatic or experience epigastric discomfort, nausea, hematemesis, or melena. Diagnosis is made by upper endoscopy.
Removal of offending agent and maintenance of O2 and blood volume as required. For prevention of stress ulcers in critically ill pts, hourly oral administration of liquid antacids (e.g., Maalox 30 mL), IV H2 receptor antagonist (e.g., cimetidine, 300-mg bolus + 37.5–50 mg/h IV), or both is recommended to maintain gastric 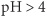 . Alternatively, sucralfate slurry, 1 g PO q6h, can be given; does not raise gastric pH and may thus avoid increased risk of aspiration pneumonia associated with liquid antacids. Pantoprazole can be administered IV to suppress gastric acid in the critically ill. Misoprostol, 200 μg PO qid, or profound acid suppression (e.g., famotidine, 40 mg PO bid) can be used with NSAIDs to prevent NSAID-induced ulcers.
. Alternatively, sucralfate slurry, 1 g PO q6h, can be given; does not raise gastric pH and may thus avoid increased risk of aspiration pneumonia associated with liquid antacids. Pantoprazole can be administered IV to suppress gastric acid in the critically ill. Misoprostol, 200 μg PO qid, or profound acid suppression (e.g., famotidine, 40 mg PO bid) can be used with NSAIDs to prevent NSAID-induced ulcers.
Identified histologically by an inflammatory cell infiltrate dominated by lymphocytes and plasma cells with scant neutrophils. In its early stage, the changes are limited to the lamina propria (superficial gastritis). When the disease progresses to destroy glands, it becomes atrophic gastritis. The final stage is gastric atrophy, in which the mucosa is thin and the infiltrate sparse. Chronic gastritis can be classified based on predominant site of involvement.
This is the body-predominant and less common form. Generally asymptomatic, common in elderly; autoimmune mechanism may be associated with achlorhydria, pernicious anemia, and increased risk of gastric cancer (value of screening endoscopy uncertain). Antibodies to parietal cells present in >90%.
This is antral-predominant disease and caused by H. pylori. Often asymptomatic but may be associated with dyspepsia. Atrophic gastritis, gastric atrophy, gastric lymphoid follicles, and gastric B cell lymphomas may occur. Infection early in life or in setting of malnutrition or low gastric acid output is associated with gastritis of entire stomach (including body) and increased risk of gastric cancer. Eradication of H. pylori (Table 158-2) not routinely recommended unless PUD or gastric mucosa-associated lymphoid tissue (MALT) lymphoma is present.
Alcoholic gastropathy (submucosal hemorrhages), Ménétrier’s disease (hypertrophic gastropathy), eosinophilic gastritis, granulomatous gastritis, Crohn’s disease, sarcoidosis, infections (tuberculosis, syphilis, fungi, viruses, parasites), pseudolymphoma, radiation, corrosive gastritis.
Consider when ulcer disease is severe, refractory to therapy, associated with ulcers in atypical locations, or associated with diarrhea. Tumors are usually pancreatic or in duodenum (submucosal, often small), may be multiple, slowly growing; >60% malignant; 25% associated with MEN 1, i.e., multiple endocrine neoplasia type 1 (gastrinoma, hyperparathyroidism, pituitary neoplasm), often duodenal, small, multicentric, less likely to metastasize to liver than pancreatic gastrinomas but often metastasize to local lymph nodes.
Basal acid output >15 mmol/h; basal/maximal acid output >60%; large mucosal folds on endoscopy or upper GI radiograph.
Serum gastrin > 1000 ng/L or rise in gastrin of 200 ng/L following IV secretin and, if necessary, rise of 400 ng/L following IV calcium (Table 158-4).
TABLE 158-4 DIFFERENTIAL DIAGNOSTIC TESTS
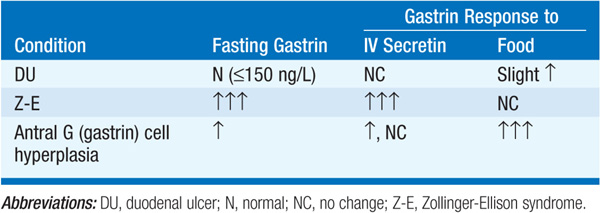
Z-E syndrome, antral G cell hyperplasia or hyperfunction (? due to H. pylori), postgastrectomy retained antrum, renal failure, massive small bowel resection, chronic gastric outlet obstruction.
Pernicious anemia, chronic gastritis, gastric cancer, vagotomy, pheochromocytoma.
TREATMENT Zollinger-Ellison Syndrome
Omeprazole (or lansoprazole), beginning at 60 mg PO q A.M. and increasing until maximal gastric acid output is <10 mmol/h before next dose, is drug of choice during evaluation and in pts who are not surgical candidates; dose can often be reduced over time. Radiolabeled octreotide scanning has emerged as the most sensitive test for detecting primary tumors and metastases; may be supplemented by endoscopic ultrasonography. Exploratory laparotomy with resection of primary tumor and solitary metastases is done when possible. In pts with MEN 1, tumor is often multifocal and unresectable; treat hyperparathyroidism first (hypergastrinemia may improve). For unresectable tumors, parietal cell vagotomy may enhance control of ulcer disease by drugs. Chemotherapy is used for metastatic tumor to control symptoms (e.g., streptozocin, 5-fluorouracil, doxorubicin, or interferon α); 40% partial response rate. Newer agents effective in pancreatic neuroendocrine tumors have not been evaluated.

For a more detailed discussion, see Del Valle J: Peptic Ulcer Disease and Related Disorders, Chap. 293, p. 2438, in HPIM-18.