Chapter 15
Pulmonary hypertension and right heart failure
Anastase Dzudie1, Friedrich Thienemann2, Okechukwu Samuel Ogah3, Dike Bevis Ojji4, and Mahmoud Sani5
1 Hospital General de Douala, Douala, Littoral, Cameroon
2 University of Cape Town, Cape Town, South Africa
3 University College Hospital, Ibadan, Oyo, Nigeria
4 University of Abuja Teaching Hospital, Abuja, Nigeria
5 Bayero University Kano/Aminu Kano Teaching Hospital, Kano, Nigeria
15.0 Introduction
Recent years have brought increasing awareness of the clinical significance of PH and cor pulmonale (RV dysfunction and RHF arising from pathological changes in the pulmonary vasculature/system) in Africa. This applies equally to the recognition of the importance of PH and RHF as both a primary diagnosis and as a poor prognostic marker for those primarily affected by left-sided HF [125,126]. However, data on the precursors and risk factors of those conditions are limited. As described in chapter 6, the largest study on PH/RHF in Africa was derived from the Heart of Soweto Study in South Africa, in which 2,505 cases presented with de novo HF during 2006 and 2008. Of these, 697 (28%) patients were diagnosed with PH/RHF, with PH/RHF as the primary diagnosis in 50% of cases. The majority presented with dyspnea and a mean right ventricular systolic pressure (RVSP) >50 mmHg. Left heart disease (31%), COPD and TB (26%), and PH (20%) due to HIV-related PH (HIV-PH), adult CHD, or idiopathic PH were the most common causes of RHF [127] in the Heart of Soweto cohort. Investigating the prevalence of PH in RHD, a review of 1,312 echocardiographic studies at a tertiary center in Nigeria found evidence of RHD in 10% of cases; in 80% of these, secondary PH was present [128]. The same center described an echocardiographic series of 80 HF admissions; in the 53 cases with PH, the most common causes were hypertensive heart disease (25%), PPCMO (25%), dilated CMO (17%), and RHD (13%) [129]. A study from Uganda, meanwhile, reported a 33% prevalence of PH in 309 patients with newly diagnosed RHD [130]. Investigating the prevalence of CVD in HIV-infection, a Nigerian study found HIV-PH in 1 of 100 patients [131], while an echocardiographic series of 102 HIV patients presenting with cardiac symptoms in Tanzania revealed PH in 13% [132]. PH was also present in 7% of long-term survivors in a cohort from Zimbabwe with vertically acquired HIV infection [133]. Hemolytic anemia is a known risk factor for PH; a screening study of patients with sickle-cell disease (SCD) in Nigeria found a PH prevalence of 25% [134], while an Egyptian study reported that patients with β-thalassemia were at risk for PH [135]. Another study from Egypt revealed PH in 9% of patients seropositive for schistosomal antibodies. Finally, a Sudanese group described 14 consecutive cases of PH in previously treated pulmonary TB, concluding that PH can occur after resolution of TB [136]. Combined, these data suggest that left heart disease, chronic lung disease, RHD, HIV-infection, schistosomiasis, and SCD may be the most common underlying causes of PH in the African context.
15.1 A prospective registry of PH in Africa: The landmark PAPUCO study
Thienemann F, Dzudie A, Mocumbi AO, Blauwet L, Sani MU, Karaye KM, et al. Rationale and design of the Pan African Pulmonary Hypertension Cohort (PAPUCO) study: implementing a contemporary registry on pulmonary hypertension in Africa. BMJ Open 2014; 4(10):e005950. [137]
15.1.1 Study aim and objectives
Given the paucity of African data on PH/RHF (<1% of published reports) in the context of its potentially rising burden, the PAPUCO study aims to describe the presentation, etiologies, comorbidities, and natural course of PH in Africa, as well as its diagnosis and therapeutic management. Specific secondary objectives are to determine the overall 6-month survival rate in PH/RHF and the 24-month survival rate in HIV-related PAH, and to compare 6-month survival rates between different diagnostic groups of PH (including PAH) while also determining the predictors of mortality across those groups. More broadly, the PAPUCO registry endeavours to develop sustainable clinical and research capacity across the African continent as well as raising awareness of PH and its risk factors.
15.1.2 Study methods
15.1.2.1 Study design and setting
The PAPUCO study is a prospective observational registry on PH with nine actively recruiting specialist centers in four countries in sub-Saharan Africa (see Figure 15.1). The registry aims to recruit 250 patients with newly diagnosed PH based on echocardiography (noting a research report based on the successful achievement of this target will be submitted for publication in the last quarter of 2015). All participating centers are public health care institutions, and the majority of patients will represent socioeconomic disadvantaged populations with restricted access to health care. Center eligibility criteria include (a) availability of echocardiography and training in assessing right heart function, (b) experience in diagnosing PH according to WHO classification (see Table 15.1), (c) experience in clinical management of patients with RHF, and (d) resources to review patients at 6-month follow-up. All participating centers obtained ethical approval from their local ethics committee review board.

Figure 15.1 Country participation in the PAPUCO registry.
Adapted from Thienemann et al., 2014 [137].
Table 15.1 WHO classification for pulmonary hypertension [138].
| Pulmonary Hypertension Group Description | Clinical Correlate |
| Group 1: PAH | HIV-related PAH, schistosomiasis, drugs/toxins |
| Group 2: Pulmonary hypertension due to left heart disease | Mitral stenosis due to RHD, hypertensive VHD, PPCMO, VHD |
| Group 3: Pulmonary hypertension due to lung diseases and/or hypoxemia | COPD, posttuberculous lung bronchiectiasis, interstitial lung disease |
| Group 4: Chronic thromboembolic pulmonary hypertension | Chronic pulmonary embolism |
| Group 5: Pulmonary hypertension with unclear or multifactorial mechanisms | EMF, chronic hemolytic anemia (SCD) |
15.1.2.2 Patient enrollment
Consecutive patients per center will be included if newly diagnosed with PH according to prespecified clinical and echocardiographic criteria (see below), able or likely to return for 6-month follow-up, at least 18 years old (except for pediatric centers in Mozambique and Nigeria), and consented in writing to participate in the registry.
15.1.2.3 Definition of PH
For the purpose of this registry, PH is defined as >35 mmHg RVSP on trans-thoracic echocardiography in the absence of pulmonary stenosis and acute RHF; it is usually accompanied by dyspnea, fatigue, peripheral edema, and other cardiovascular symptoms, and there are possibly ECG and chest x-ray changes in keeping with PH [138]. The WHO classification system for PH will be applied to describe the different etiologies of PH presentation. Once definitive assessment and treatment has been applied, specific data are documented for each patient: (a) all major cardiovascular diagnoses according ICD-10 coding, (b) up to six noncardiovascular diagnoses according to ICD-10 coding, and (c) prescribed pharmacological therapy.
15.1.2.4 The PAPUCO research platform
A tailor-made database was developed to fulfill the study requirements. Open-source technology was used to develop the web-based system (www.papuco.org) that allows investigators to collect, store, analyze, report on, and export clinical research data in various formats. Simple and user friendly, the system anonymizes personal patient data, which are stored as electronic case report forms on a secure, encrypted, and backed-up server. It provides hierarchic permissions and validation at the point of data entry. Multimedia data formats (including echocardiographic images) can be uploaded on the platform, allowing storage of complete clinical records, which guarantees completeness of data. Tools for education, training, and communication are installed within the web portal, and documents such as paper case report forms, informed consent forms, study information sheets, and patient education sheets on PH are available for download. The platform has been developed to cater for mobile Internet connectivity available in most parts of Africa and represents a unique research platform far beyond a simple web-based database.
15.1.2.5 Diagnostic algorithm and data collection in the PAPUCO registry
A diagnostic algorithm to diagnose PH in resource-limited settings without access to right heart catheterization (Figure 15.2) has been developed following the guidelines for the diagnosis of PH in the African context. Key information collected includes information on socioeconomic background, medical history, comorbidities, cardiac risk factors, and environmental exposures. The clinical aspects of the assessment include symptom scoring, a full clinical examination, and functional tests including WHO functional class and 6-minute walk test [139,140]. Further investigations are at the discretion of the treating physician and typically include pulmonary function tests, radio nucleotide perfusion scans, chest x-ray, chest CT, and right heart catheterization if available. HF treatment and comedication, hospitalization, and mortality data will also be collected.
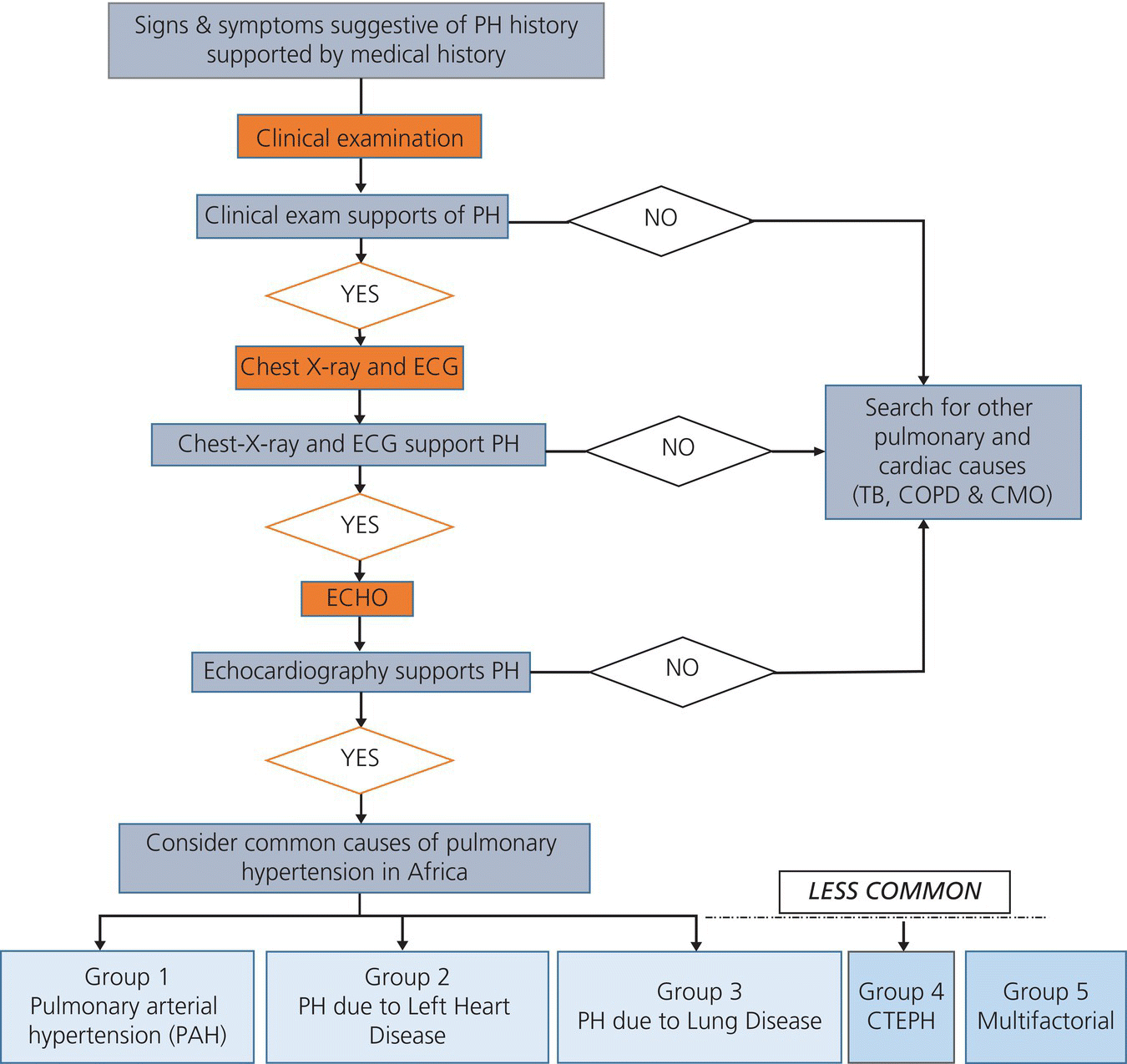
Figure 15.2 Standardized pathway to diagnose PH in the PAPUCO registry.
Adapted from Thienemann et al., 2014 [137].
15.1.3 Study findings
At the time of publication of this book, the findings from this landmark study were not far from being published, the collaboration having successfully reached its goal of collecting data on >250 cases of PH across a range of African countries and in adult and pediatric patients. Any future editions of this book will, of course, provide the specific details.
15.1.4 Study interpretation
Newly released guidelines on the management of PH [141] provide an important contrast and backdrop to the future findings of the PAPUCO study given the emphasis on right heart catheterization (as opposed to the heavy reliance on clinical presentation and echocardiography in the African context) and the extremely expensive/niche first-line therapeutics that impose a burden on even the richest of countries. Establishing a definitive diagnosis of PH is an enormous challenge when relying on bedside tools, including history and physical examination. Right heart catheterization is indeed the gold standard to diagnose and confirm PH, but performing this procedure in all patients with dyspnea would be costly, apply excessive risk, and be impractical in any cost-constrained environment such as sub-Saharan Africa. The question of a better test that is widely available and supplies accuracy, safety, simplicity, and cost-effectiveness therefore becomes vital, particularly in an African-specific context. Echocardiography is more readily available in Africa and provides an estimate of RVSP, functional and morphologic cardiac sequelae of PH, and identification of possible cardiac causes. However, the accuracy of RVSP determination by echocardiography remains open to question, with some contemporary studies suggesting that echocardiography may not accurately estimate pulmonary arterial pressures [142,143]. Possible explanations for this inaccuracy include the presence of sufficient tricuspid regurgitation to produce a Doppler envelope and appropriate gain adjustments [142,143]. An undergained spectral signal will tend to result in underestimation of pulmonary pressures, while an overgained signal might significantly overestimate the measurement. Furthermore, careful adjustment of the transducer position and the use of color-flow Doppler are critical in order to reduce the Doppler angle and to obtain the maximal regurgitant flow velocity. Moreover, in the case of severe tricuspid regurgitation, the potential for laminar flow is high, invalidating the application of the Bernoulli equation. Volume status and systemic BP are other confounding factors. Finally, although it is commonly assumed the highest value of right atrial pressure is 15 mm Hg, values commonly exceed this in clinical practice. In the first systematic review and meta-analysis addressing the diagnostic accuracy of echocardiography for PH, the correlation of pulmonary arterial systolic pressure by echocardiography compared with right heart catheter was revealed to be reasonable, with a summary correlation coefficient of 0.70 (95% CI 0.67–0.73) [144]. The diagnostic accuracy of echocardiography to detect PH was also acceptable with a summary sensitivity and specificity of 83% (95% CI 73–90) and 72% (95% CI 53–85), respectively. Furthermore, pulmonary pressure measurements provided by echocardiography in experienced hands have been demonstrated as a strong predictor of mortality [145]. Finally, easily obtainable echocardiographic parameters such as E/A ratio and left atrial size can reliably distinguish between PH due to lung disease and PH due to left heart disease, allowing for rapid triage of patients for right heart catheterization if required. The results of the PAPUCO study will largely rely on echocardiography and will need to be evaluated using the best available resources in a resource-poor environment.
15.1.5 Study limitations
As with all observational registries, the PAPUCO initiative is reliant upon the flow of cases through participating centers and the systematic application of standardized clinical profiling. The risk of selection bias remains ever present in this situation. More important, from a PAH perspective, is the selective (if available) use of right heart catheterization. As always there are pragmatic considerations to be made when deriving a clinical diagnosis in limited resource settings—not applying a diagnosis (and treatment) is not an option.
15.1.6 Study conclusions
The PAPUCO study represents an important step in understanding the prevalence and clinical spectrum (including outcomes) of PH/RHF from a uniquely African standpoint. It joins such initiatives as the THESUS-HF study (see Chapter 12) in illuminating the burden of HF from a pan-African perspective. The ability to derive practical guidelines to inform the management and future pragmatic treatment trials of PH/RHF will surely arise, thereby improving what are otherwise likely to be very poor health outcomes in mainly young and disadvantaged individuals with limited resources to cope with a devastating condition.
S6.2 An increasingly complex picture of HF in Africa
As described in various parts of this book (particularly Section 3), profound sociodemographic and economic change on the African continent (particularly in the form of epidemiological transition) has transformed the landscape of heart disease and its most common chronic manifestatio—HF. This change is best summarized in Falase and Ogah’s [7] comparison of underlying causes of HF presentations in patients of African ancestry to the University College Hospital in Ibadan, Nigeria, between the late 1960s and 2010 (see Figure S6.1 and Figure S6.2).
As was thoroughly illustrated in Chapters 12, 13, and 14, acute and chronic manifestations of HF are predominantly driven by hypertensive heart disease. Conversely, HF cases due to ischemic CMO remain at historical lows, despite the prospect of more cases as African lifestyles begin to mirror those of Western countries. The Heart of Soweto Study first published in 2006 [30] made a key contribution to our understanding of the current spectrum of HF in urban African communities in epidemiological transition. It highlighted in particular the importance of maintaining detailed case presentations records underpinned by echocardiography and standardized case reporting. However, these data are limited both in scope and geographic representation. As the insights from data derived from a wide body of research across Western countries attest, it is clear that Africa needs a more wide-scale and consistent approach to monitoring the evolving burden of HF, in addition to developing its own evidence base to improve health outcomes. Fortunately, this process has commenced (even as this book was written and went to press other important studies, such as those published by Makubi and colleagues from Tanzania were published [146,147]) and will continue to expand as pan-African collaborations are initiated and begin to inform clinicians and health administrators alike on how to combat an evolving burden of HF in sub-Saharan Africa. In Chapter 12, the pattern of acute forms of HF, as captured by the international THESUS-HF Registry, was described. Chapter 13 concentrated on the contribution and pattern of hypertensive HF in an array of African countries (from Cameroon to Nigeria and then South Africa). Although there has been a natural tendency to focus on hospitalized cases of HF (predominantly acute episodes of HF) in a resource-poor, tertiary health care–dominated system, it is important to acknowledge that HF is primarily a chronic condition. In this context, Chapter 14 examined the pattern and cost of chronic HF from an individual to societal perspective. Finally, Chapter 15 broadly surveyed the pattern of RHF/cor pulmonale and PAH in the sub-Saharan African setting. Importantly, given the paucity of data that can be derived from European, pan-Pacific, and North American registries (typically reporting far fewer cases derived from right-sided heart disease [37,148–150] this chapter describes a pan-African initiative to better understand this important contributor to HF cases on the continent. Rather than purporting to present definitive data on RHF in Africa, therefore, Chapter 15 concludes fittingly with the illustration of an increasingly common phenomenon—the creation of pan-African collaborations to derive robust, representative, and meaningful clinical data that will inform public health policy and clinical practice initiatives across the African continent for decades to come.
In summary, therefore, HF (as with all forms of heart disease) on the African continent has reached a historically important turning point that will exert a profound effect on African communities from an individual to a whole society perspective, particularly given its propensity to strike both men and women in the prime of life.