Chapter 9
Intracellular Signaling
Signaling Through G-Protein-Linked Receptors
Signal transduction through G-protein-linked receptors requires three membrane-bound components: (1) a cell surface receptor that determines to which signal the cell can respond; (2) a G protein on the intracellular side of the membrane that is stimulated by the activated receptor; and (3) either an effector enzyme that changes the level of a second messenger or an effector channel that changes ionic fluxes in the cell in response to the activated G protein. The human genome encodes for more than 800 receptors for catecholamines, odorants, neuropeptides, and light that couple to one or more of the 16 identified G proteins. These, in turn, regulate one or more of more than two dozen different effector channels and enzymes. The key feature of this information flow is the ability of G proteins to detect the presence of activated receptors and to amplify the signal by altering the activity of appropriate effector enzymes and channels. A nervous system with information flow by fast transmission alone would be capable of stereotyped or reflex responses. Modulation of this transmission and changes in other cellular functions by G-protein-linked systems and by receptor-tyrosine kinase-linked systems enables an orchestrated response. The large diversity of signaling molecules and their intracellular targets offer nearly unlimited flexibility of response over a broad time scale and with high amplification.
G proteins are GTP-binding proteins that couple the activation of seven-helix receptors by neurotransmitters at the cell surface to changes in the activity of effector enzymes and effector channels. A common effector enzyme is adenylate cyclase, which synthesizes cyclic AMP (cAMP)—an intracellular surrogate for the neurotransmitter, the first messenger. Phospholipase C (PLC), another effector enzyme, generates diacylglycerol (DAG) and inositol 1, 4, 5-trisphosphate (IP3), the latter of which releases intracellular stores of Ca2+. Information from an activated receptor flows to the second messengers that typically activate protein kinases, which modify a host of cellular functions. Ca2+, cAMP, and DAG have in common the ability to activate protein kinases with broad substrate specificities. They phosphorylate key intracellular proteins, ion channels, enzymes, and transcription factors taking part in diverse cellular biological processes. The activities of protein kinases and phosphatases are in balance, constituting a highly regulated process, as revealed by the phosphorylation state of these targets of the signal transduction process. In addition to regulating protein kinases, second messengers such as cAMP, cyclic GMP (cGMP), Ca2+, and arachidonic acid can directly gate, or modulate, ion channels. G proteins can also couple directly to ion channels without the interception of second messengers or protein kinases. In these diverse ways, a neurotransmitter outside the cell can modulate essentially every aspect of cell physiology and encode the history of cell stimuli in the form of altered activity and expression of its cellular constituents. An overview of G-protein signaling to protein kinases is presented in Fig. 9.1.
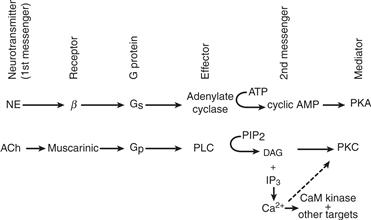
Figure 9.1 Overview of G-protein signaling to protein kinases. Norepinephrine (NE) and acetylcholine (ACh) can stimulate certain receptors that couple through distinct G proteins to different effectors, which results in increased synthesis of second messengers and activation of protein kinases (PKA and PKC). PLC, phospholipase C; PIP2, phosphatidylinositol bisphosphate; DAG, diacylglycerol; CaM, Ca2+- calmodulin dependent; IP3, inositol 1, 4, 5-triphosphate.
Receptors Catalyze the Conversion of G Proteins into the Active GTP-Bound State
G proteins undergo a molecular switch between two interconvertible states that are used to “turn on” or “turn off” downstream signaling. G proteins taking part in signal transduction utilize a regulatory motif that is seen in other GTPases engaged in protein synthesis and in intracellular vesicular traffic. G proteins are switched on by stimulated receptors, and they switch themselves off after a time delay. G proteins are inactive when GDP is bound and are active when GTP is bound. The sole function of seven-helix receptors in activating G proteins is to catalyze an exchange of GTP for GDP. This is a temporary switch because G proteins are designed with a GTPase activity that hydrolyzes the bound GTP and converts the G protein back into the GDP-bound, or inactive, state. Thus, a G protein must continuously sample the state of activation of the receptor, and it transmits downstream information only while the neuron or glial cell is exposed to neurotransmitter. The GTPase activity of G proteins thus serves both as a regulatable timer and as an amplifier (Fig. 9.2).
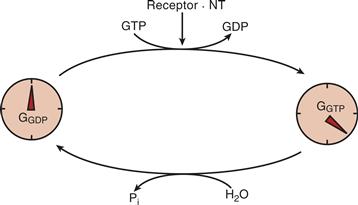
Figure 9.2 GTPase activity of G proteins serves as a timer and amplifier. Receptors activated by neurotransmitters (NT) initiate the GTPase timing mechanism of G proteins by displacement of GDP by GTP. Neurotransmitters thus convert G-GDP (“turned-off state”) to G-GTP (time-limited “turned-on” state).
The G-Protein Cycle
G proteins are trimeric structures composed of two functional units: (1) an α subunit (39–52 kDa) that catalyzes GTPase activity and (2) a βγ dimer (35 and 8 kDa, respectively) that interacts tightly with the α subunit when bound to GDP (Stryer & Bourne, 1986; Birnbaumer, 2007). The role of the three subunits in the G-protein cycle is depicted in Figures 9.3 and 9.4. In the basal state, GDP is bound tightly to the α subunit, which is associated with the βγ pair to form an inactive G protein. In addition to blocking interaction of the α subunit with its effector, the βγ pair increases the affinity of the α subunit for activated receptors. Binding of the neurotransmitter to the receptor produces a conformational change that positions previously buried residues that promote increased affinity of the receptors for the inactive G protein. A given receptor can interact with only one or a limited number of G proteins, and the α subunit generates most of this specificity. Coupling with the activated receptor reduces the affinity of the α subunit for GDP, facilitating its dissociation and replacement with GTP. Thus, the receptor effectively catalyzes an exchange of GTP for GDP. GTP-GDP exchange is inherently very slow and ensures that very little of the G protein is in the on state under basal conditions. The level of G protein in the “on” state can increase from being 1% to being more than 50% of all G protein (Stryer & Bourne, 1986).
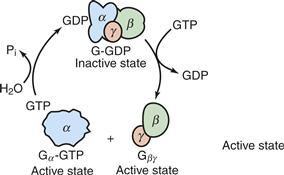
Figure 9.3 Interconversion, catalyzed by excited receptors, of G-protein subunits between inactive and active states. Displacement of GDP with GTP dissociates the inactive heterotrimeric G protein, generating α-GTP and βγ, both of which can interact with their respective effectors and activate them. The system converts into the inactive state after GTP has been hydrolyzed and the subunits have reassociated.
From Stryer (1995). Used with permission of W. H. Freeman and Company.
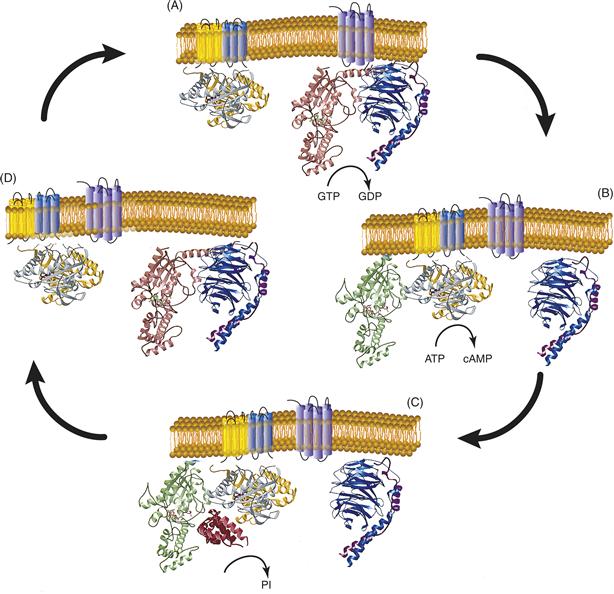
Figure 9.4 (A) G proteins are held in an inactive state because of very high affinity binding of GDP to their α subunits. When activated by agonist, membrane-bound seven helical receptors (Fig. 9.4 right, glowing magenta) interact with heterotrimeric G proteins (α, amber; β, teal; γ, burgundy) and stimulate dissociation of GDP. This permits GTP to bind to and activate α, which then dissociates from the high-affinity dimer of β and γ subunits. (B) Both activated (GTP-bound) α (lime) and βγ are capable of interacting with downstream effectors. Figure 9.4 shows the interaction of GTP-αs with adenylate cyclase (catalytic domains are mustard and ash). Adenylate cyclase then catalyzes the synthesis of the second messenger cyclic AMP (cAMP) from ATP. (C) Signaling is terminated when α hydrolyzes its bound GTP to GDP. In some signaling systems, GTP hydrolysis is stimulated by GTPase-activating proteins or GAPs (cranberry) that bind to α and stablize the transition state for GTP hydrolysis. (D) Hydrolysis of GTP permits GDP-α to dissociate from its effector and associate again with βγ. The heterotrimeric G protein is then ready for another signaling cycle if an activated receptor is present. This figure is based on the original work of Mark Wall and John Tesmer.
Information Flow through G-Protein Subunits
One of the more tense and public debates in signal transduction has been the question of whether the α subunit alone conveys information that specifies which effector is activated or whether the βγ pair can also interact with effectors. One of the contestants even paid for a vanity license plate proclaiming “α not βγ.” This notion was eventually changed because of the finding that βγ can directly activate certain K+ channels. It is now apparent that α and βγ subunits can both modify effector enzymes, but the historic association of G-protein function with α has persisted for the purpose of nomenclature, with Gs and αs referring to the G protein and its corresponding α subunit, which stimulates adenylate cyclase. The α subunits may act either independently or in concert with βγ (Clapham & Neer, 1993). Furthermore, β and γ subunits in a βγ pair can combine in many different ways. Other legacy terms include Gi, Gp, and Go used for G-protein activities that inhibited adenylate cyclase, stimulated phospholipase, or were presumed to have other effects, respectively.
Effector Enzymes, Channels, and Transporters Decode Receptor-Mediated Cell Stimulation in the Cell Interior
The function of the trimeric G proteins is to decode information about the concentration of neurotransmitters bound to appropriate receptors on the cell surface and convert this information into a change in the activity of enzymes and channels that mediate the effects of the neurotransmitter. The known effector functions of α include both stimulation and inhibition of adenylate cyclases that is sensitive to cholera toxin and pertussis toxin, respectively. In addition, it modulates activation of cGMP phosphodiesterase, PLC, and regulation of Na+/K+ exchange, PI3K, RhoGEF and rasGAP. The effector functions of βγ dimers include inhibition of many adenylate cyclases and co-stimulation of others. In addition, they regulate stimulation of phospholipase Cβ, K+ and Ca2+ channels, phospholipase A2, phosphatidylinositol-3-kinase, PKD and dynamin in vesicle budding.
Response Specificity in G-Protein Signaling
How can a neurotransmitter produce a specific response if G-protein coupling has the potential for such a diversity of effectors? A given neuron has only a subset of receptors, G proteins, and effectors, thereby limiting possible signaling pathways. Transducin, for example, is confined to the visual system, where the predominant effector is the cGMP phosphodiesterase and not adenylate cyclase. Signal specificity is further refined by selective affinities between cognate sets of receptors, G proteins, and effector(s), and by spatial compartmentalization, such as at nerve terminals, where co-localization effects privileged signaling from a given receptor to a limited set of effectors. Furthermore, the intrinsic GTPase activity of G proteins can be modulated by GTPase-activating proteins (GAPs) that terminate its active state more quickly and selectively affect signal output.
Fine-Tuning of cAMP by Adenylate Cyclases
The level of cAMP is highly regulated due to a balance between synthesis by adenylate cyclases and degradation by cAMP phosphodiesterases (PDEs). Each of these enzymes can be regulated and manipulated independently. Adenylate cyclase was the first G-protein effector to be identified, and now a group of related adenylate cyclases are known to be regulated differentially by both α and βγ subunits (Taussig & Gilman, 1995). G proteins can both activate and inhibit adenylate cyclases either synergistically or antagonistically.
Adenylate cyclases are large proteins of approximately 120 kDa. They consist of a tandem repeat of the same structural motif—a short cytoplasmic region followed by six putative transmembrane segments and then a highly conserved catalytic domain of approximately 35 kDa on the cytoplasmic side that bind ATP and catalyze its conversion into cAMP. Some isoforms are activated by calmodulin, a Ca2+-binding protein that activates many enzymes after it binds Ca2+.
Differential Regulation of Adenylate Cyclase Isoforms
All adenylate cyclase isoforms are stimulated by Gs through its αs subunit. Known isoforms can be divided into four groups on the basis of additional regulatory properties (Fig. 9.5). Group I (AC1, 3, 8) possesses a calmodulin-binding domain and is activated by Ca2+-calmodulin. Group II (AC2, 4, 7) is weakly responsive to direct interaction with αs or βγ but is highly activated when both are present. As described later, this synergistic effect enables this cyclase to function as a coincidence detector. Group III cyclases (AC5, 6) are negatively regulated by inhibitory G proteins, whereas Group IV (AC9) has a forskolin-insensitive cyclase.
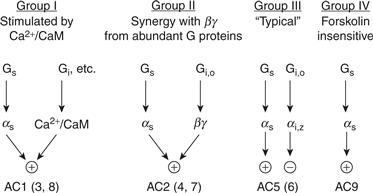
Figure 9.5 Isoforms of adenylate cyclase (AC). All isoforms are stimulated by αs but differ in the degree of interaction with Ca2+- calmodulin and with βγ derived from inhibitory G proteins. Not shown is the ability of excess βγ to complex with αs and inhibit Group I and Group III adenylate cyclases.
Updated from Taussig and Gilman (1995).
Inhibition of Adenylate Cyclases
Adenylate cyclases are also subject to several forms of inhibitory control. First, activation of all adenylate cyclases can be antagonized by βγ released from abundant G proteins, such as Gi, Go, and Gz. Released βγ complexes with αs-GTP and shift the equilibrium toward an inactive trimer by mass action. Second, either α or βγ subunits derived from Gi, Go, or Gz can directly inhibit group I cyclases, and the α subunit from Gi or Gz can inhibit Group III cyclases. The level of Gs in particular is low; thus, as derived from Gs is sufficient to activate adenylate cyclases, but the βγ derived from it is insufficient to directly inhibit or activate adenylate cyclases. This explains the apparent paradox that receptors that couple to Gs produce effects only through as, whereas receptors that couple to Gi produce effects through both αi and βγ. Additional inhibitory regulation of some groups is also effected by CaM kinases and by calcium.
Receptors Coupling to Adenylate Cyclase
Dozens of neurotransmitters and neuropeptides work through cAMP as a second messenger and by G protein-linked activation or inhibition of adenylate cyclase. Among the neurotransmitters that increase cAMP are the amines norepinephrine, epinephrine, dopamine, serotonin, and histamine and the neuropeptides vasointestinal peptide (VIP) and somatostatin. In the olfactory system, a special form of G-protein α subunit, termed αolf, serves the same function as αs and couples several hundred seven-helix receptors to AC3 adenylate cyclase in the neuroepithelium.
Adenylate Cyclases as Coincidence Detectors
Adenylate cyclases can integrate concurrent stimulation of neurons by two or more neurotransmitters (Bourne & Nicoll, 1993). AC1 adenylate cyclase is stimulated by neurotransmitters that couple to Gs and by neurotransmitters that elevate intracellular Ca2+. This adenylate cyclase can convert the depolarization of neurons into an increase in cAMP. Its role in associative forms of learning may be related to its ability to link cAMP-based and Ca2+-based signals. Stimulation of AC2 adenylate cyclase by αs is conditional on the presence of βγ derived from an abundant G protein—that is, other than Gs, thus enabling the cyclase to serve as a coincidence detector. Thus, activation of a second receptor, presumably coupled to the abundant Gi and Go, is needed to provide the βγ.
Sources of Second Messengers: Phospholipids
Two phospholipids—phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylcholine (PC)—are primary precursors for a G-protein-based second-messenger system. Three second-messengers—diacylglycerol, arachidonic acid and its metabolites, and elevated Ca2+—are ultimately produced. A single step converts inert phospholipid precursors into lipid messengers. DAG action is primarily mediated by protein kinase C (PKC) (Tanaka & Nishizuka, 1994). The elevation of Ca2+ levels is accomplished by the regulated entry of Ca2+ from a concentrated pool sequestered in the endoplasmic reticulum or from outside the cell. Ca2+ has many direct cellular targets but mediates most of its effects through Ca2+/calmodulin. One class of calmodulin-dependent enzymes is a family of protein kinases that enable Ca2+ signals to regulate a large number of cellular processes by phosphorylation (Wayman et al., 2008).
Generation of DAG and IP3 from Gq and Gi coupled to PLCβ
The phosphatidylinositide-signaling pathway is just as prominent in neuronal signaling as the cAMP pathway and is similar to it in overall design (Berridge, 2009). Stimulation of a large number of neurotransmitters and hormones [including acetylcholine (Ml, M3), serotonin (5HT2, 5HT1C), norepinephrine (α1A, α1B), glutamate (metabotropic), neurotensin, neuropeptide Y, and substance P] is coupled to the activation of a phosphatidylinositide-specific PLC.
Phosphatidylinositol (PI) is composed of a diacylglycerol backbone with myoinositol attached to the sn-3 hydroxyl by a phosphodiester bond (Fig. 9.6). The six positions of the inositol are not equivalent: the 1 position is attached by a phosphate to the DAG moiety. PI is phosphorylated by PI kinases at the 4 position and then at the 5 position to form PIP2. In response to the appropriate G-protein coupling, PLC hydrolyzes the bond between the sn-3 hydroxyl of the DAG backbone and the phosphoinositol to produce two second messengers: DAG, a hydrophobic molecule, and IP3, which is water soluble (Fig. 9.7). There are six classes of PLCs that hydrolyze PIP2 (PLCβ, γ, δ, ε, ζ, and η), with a common catalytic domain structure but different regulatory properties. G proteins couple to several variants of PLCβ. PLCγ is regulated by growth factor tyrosine kinases. Less is known about regulation of the others, but PKCε can be regulated by both G proteins and small GTPases.
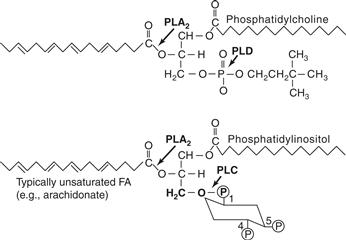
Figure 9.6 Structures of phosphatidylinositol and phosphatidylcholine. The sites of hydrolytic cleavage by PLC, PLD, and PLA2 are indicated by arrows. FA, fatty acid.
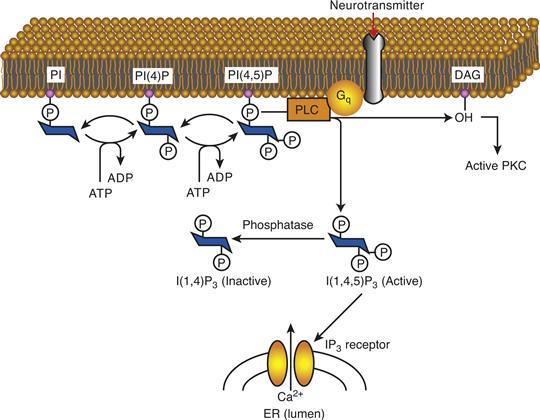
Figure 9.7 Schematic pathway of IP3 and DAG synthesis and action. Stimulation of receptors coupled to Gq activates PLCβ, which leads to the release of DAG and IP3. DAG activates PKC, whereas IP3 stimulates the IP3 receptor in the endoplasmic reticulum (ER), leading to mobilization of intracellular Ca2+ stores.
PLCβ is coupled to neurotransmitters by Gi and Gq. A pertussis toxin-sensitive pathway is mediated by a number of isoforms referred to as Gq and mediated by their αq. Gi is coupled to PLCβ via its βγ rather than α subunit in a pathway that is insensitive to pertussis toxin. Receptor tyrosine kinases can regulate PLCγ by a G-protein-independent pathway involving their recruitment to the receptor and activation via phosphorylation.
DAG Derived from Activation of Phospholipase D
A slower but larger increase in DAG can be generated by activation of phospholipase D (PLD), which cleaves phosphatidylcholine to produce phosphatidic acid and choline. Dephosphorylation of phosphatidic acid produces DAG. The PLD pathway may be used by some mitogens and growth factors and likely contains a variety of activation schemes that may include G proteins.
Additional Lipid Messengers
DAG is itself a source of another lipid messenger, by the action of phospholipase A2 (PLA2), which releases the fatty acid, typically arachidonic acid, from the sn-2 position of the DAG backbone (Fig. 9.6). Arachidonic acid has biological activity of its own in addition to serving as a precursor for prostaglandins and leukotrienes. Arachidonic acid and other cis-unsaturated fatty acids can modulate K+ channels, PLCγ, and some forms of PKC.
The phosphatidylinositides exist with modifications at the 3, 4, and 5 position to generate a family of seven lipids, [PI3P, PI4P, PI5P, PI(3,4)P2, PI(4,5)P2, PI(3,5)P2, and PI(3,4,5)P3] lipids that mediate important structural or enzymatic effects. They bind to specific protein binding domains to regulate vesicular traffic and protein kinases involved in survival and cell death. Mutations in their synthesis and metabolism have pathophysiological consequences (McCrea & De Camilli, 2009). There is evidence that another lipid, sphingomyelin, is a precursor for intracellular signals as well.
IP3, a Potent Second Messenger That Produces Its Effects by Mobilizing Intracellular Ca2+
The main function of IP3 is to stimulate the release of Ca2+ from intracellular stores. Ca2+ levels are kept low in the cytosol by its sequestration in the ER where it is complexed with low-affinity-binding proteins. The ER is the major IP3-sensitive Ca2+ store in cells (Fig. 9.7) and Ca2+ readily flows down its concentration gradient into the cell lumen upon opening of Ca2+ channels in the ER.
The IP3 receptor is a macromolecular complex that functions as an IP3 sensor and a Ca2+ release channel. It has a broad tissue distribution but is highly concentrated in the cerebellum. The IP3 receptor is a tetramer of 313-kDa subunits with a single IP3-binding site at its N-terminal of each subunit, facing the cytoplasm. Ca2+ release by IP3 is highly cooperative so that a small change in IP3 has a large effect on Ca2+ release from the ER. The mouse mutants pcd and nervous have deficient levels of the IP3 receptor and exhibit defective Ca2+ signaling, and a genetic knockout of the IP3 receptor leads to motor and other deficits.
Termination of the IP3 Signal
IP3 is a transient signal terminated by dephosphorylation to inositol. Inactivation is initiated either by dephosphorylation to inositol 1,4-bisphosphate (Fig. 9.8) or by an initial phosphorylation to a tetrakisphosphate form that is dephosphorylated by a different pathway. Both pathways have in common an enzyme that cleaves the phosphate on the 1 position. Complete dephosphorylation yields inositol, which is recycled in the biosynthetic pathway. Recycling is important because most tissues do not contain de novo biosynthetic pathways for making inositol. Salvaging inositol may be particularly important when cells are actively undergoing PI turnover. It is intriguing that Li+, the simple salt used to treat bipolar disorder, selectively inhibits the salvage of inositol by inhibiting the enzyme that dephosphorylates the 1 position and is common to the two pathways. At therapeutic doses of Li+, the reduced salvage of inositol in cells with high phosphoinositide signaling may lead to a depletion of PIP2 and a selective inhibition of this signaling pathway in active cells.
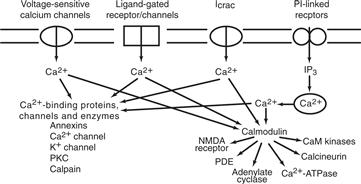
Figure 9.8 Multiple sources of Ca2+ converge on calmodulin and other Ca2+-binding proteins. Cellular levels of Ca2+ can rise either by influx (e.g., through voltage-sensitive channels or ligand-gated channels) or by redistribution from intracellular stores triggered by IP3. Calcium modulates dozens of cellular processes by the action of the Ca2+-calmodulin complex on many enzymes, and calcium has some direct effects on enzymes such as PKC and calpain. CaM kinase, Ca2+-calmodulin-dependent kinase.
Calcium Ion
Calcium has a dual role as a carrier of electrical current and as a second messenger. Its effects are more diverse than those of other second messengers such as cAMP and DAG because its actions are mediated by a much larger array of proteins, including protein kinases (Carafoli and Klee, 1999). Furthermore, many signaling pathways directly or indirectly increase cytosolic Ca2+ concentration from 100 nM to 0.5–1.0 mM. The source of elevated Ca2+ can be either the ER or the extracellular space (Fig. 9.8). In addition to IP3-mediated release, Ca2+ can activate its own mobilization through the ryanodine receptor on the ER. Mechanisms for Ca2+ influx from outside the cell include several voltage-sensitive Ca2+ channels and ligand-gated cation channels that are permeable to Ca2+ [e.g., nicotinic receptor and N-methyl-d-aspartate (NMDA) receptor].
Dynamics of Ca2+ Signaling Revealed by Fluorescent Ca2+ Indicators
We know a great deal about the spatial and temporal regulation of Ca2+ signals because of the development of fluorescent Ca2+ indicators. A variety of fluorescent compounds selectively bind Ca2+ at physiological concentration ranges and rapidly change their fluorescent properties upon binding Ca2+ to a fairly accurate measurement of ionized Ca2+.
Calmodulin-Mediated Effects of Ca2+
Ca2+ acts as a second messenger to modulate the activity of many mediators. The predominant mediator of Ca2+ action is calmodulin, a ubiquitous 17-kDa calcium-binding protein. Ca2+ binds to calmodulin in the physiological range and converts it into an activator of many cellular targets (Cohen & Klee, 1988). Binding of Ca2+ to calmodulin produces a conformational change that greatly increases its affinity for more than two dozen eukaryotic enzymes that it activates, including cyclic nucleotide PDEs, adenylate cyclase, nitric oxide synthase, Ca2+-ATPase, calcineurin (a phophoprotein phosphatase), and several protein kinases (Fig. 9.8). This activation of calmodulin allows neurotransmitters that change Ca2+ to affect dozens of cellular proteins, presumably in an orchestrated fashion.
Regulation of Guanylate Cyclase by Nitric Oxide
An important target of Ca2+-calmodulin is the enzyme nitric oxide synthase (NOS). This enzyme synthesizes one of the simplest known messengers, the gas NO (Sen & Snyder, 2010). In the pathway that led to its discovery, acetylcholine stimulates the PI signaling pathway in the endothelium to increase intracellular Ca2+, which activates NOS so that more NO is made. NO then diffuses radially from the endothelial cells across two cell membranes to the smooth muscle cell, where it activates guanylate cyclase to make cGMP. This in turn activates a cGMP-dependent protein kinase that phosphorylates proteins, leading to a relaxation of muscle. In 1998, Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad received the Nobel Prize for their discoveries concerning nitric oxide as a signaling molecule and therapeutic mediator in the cardiovascular system.
Let us now turn to the details of the NO pathway. Nitric oxide is derived from L-arginine in a reaction catalyzed by NOS, a complex enzyme that converts L-arginine and O2 into NO and L-citrulline. NO lasts only a few seconds in biological fluids and thus no specialized processes are needed to inactivate this particular signaling molecule. As a gas, NO is soluble in both aqueous and lipid media and can diffuse readily from its site of synthesis across the cytosol or cell membrane and affect targets in the same cell or in nearby neurons, glia, and vasculature (Sen & Snyder, 2010). NO produces a variety of effects, including relaxation of smooth muscle of the vasculature, relaxation of smooth muscle of the gut in peristalsis, and killing of foreign cells by macrophages. It was first recognized as a neuronal messenger that couples glutamate receptor stimulation to increases in cGMP. NO produced by Ca2+-calmodulin-dependent activation of NOS concentrated in cerebellar granule cells activates guanylate cyclase in nearby Purkinje cells during the induction of long-term depression in the cerebellum (see Chapter 32).
Activation of Guanylate Cyclases
Two types of guanylate cyclase, a soluble one regulated by NO and a membrane-bound enzyme regulated directly by neuropeptides, synthesize cGMP from GTP in a reaction similar to the synthesis of cAMP from ATP. NO activates the soluble enzyme by binding to the iron atom of the heme moiety. This is the basic mechanism for the regulation of soluble guanylate cyclases. A number of therapeutic muscle relaxants, such as nitroglycerin and nitroprusside, are NO donors that produce their effects by stimulating cGMP synthesis. Membrane-bound guanylate cyclases are transmembrane proteins with a binding site for neuroendocrine peptides on the extracellular side of the plasma membrane and a catalytic domain on the cytosolic side.
Cyclic GMP Phosphodiesterase, an Effector Enzyme in Vertebrate Vision
The versatility of G-protein signaling is illustrated in vertebrate phototransduction, in which a specialized G protein called transducin (Gt) is activated by light rather than by a hormone or neurotransmitter. Transducin stimulates cGMP phosphodiesterase, an effector enzyme that hydrolyzes cGMP and ultimately turns off the dark current (see Chapter 27). Nature has evolved an elegant mechanism for using photons of light to modify a hormone-like molecule, retinal, that activates a seven-helix receptor called rhodopsin. Activated rhodopsin dissociates αt from transducin which then activates a soluble cGMP phosphodiesterase.
Rods can detect a single photon of light because the signal-to-noise ratio of the system is very low and the amplification factor in phototransduction is quite high; one rhodopsin molecule stimulated by a single photon can activate 500 transducins. Transducin remains in the “on” state long enough to activate 500 PDEs. PDE can hydrolyze about 100 cGMP molecules in the second before it is deactivated. cGMP in rods regulates a cGMP-gated cation channel, leading to additional amplification of the signal.
Modulation of Ion Channels by G Protein
Each type of neuron has a repertoire of ion channels that give it a distinct response signature, and it is not surprising that several types of mechanisms regulate these channels. Channel modulation occurs via G proteins, second messengers, and their cognate protein kinases that phosphorylate ion channels as well as by direct effects of G proteins (Greengard, 2001).
The first ion channel demonstrated to undergo regulation by G proteins was the cardiac K+ channel that mediates slowing of the heart by acetylcholine released from the vagus nerve. When this IKACh channel is examined in a membrane patch delimited by the seal of a cell-attached electrode, the addition of acetylcholine within the electrode increases the frequency of channel opening dramatically, whereas the addition of acetylcholine to the cell surface outside the seal does not. The process is therefore described as membrane delimited, with a direct interaction between the G protein (either αi or βγ) and the channel.
Ca2+ channels are also modulated by G proteins. The central role played by Ca2+ in muscle contraction, in synaptic release, and in gene expression makes Ca2+ influx a compelling target for regulation by neurotransmitters. In the heart, where L-type Ca2+ channels are critical for the regulation of contractile strength, the Ca2+ current is enhanced by as formed by β-adrenergic stimulation of Gs. In contrast, N-type Ca2+ channels, which modulate synaptic release in nerve terminals, are often inhibited by muscarinic and α-adrenergic agents and by opiates acting at receptors coupled to Gi and Go.
G-Protein Signaling Gives Special Advantages in Neural Transmission
The G-protein-based signaling system provides several advantages over fast transmission (Hille, 1992; Birnbaumer, 2007). These advantages include amplification of the signal, modulation of cell function over a broad temporal range, diffusion of the signal to a large cellular volume, cross talk, and coordination of diverse cell functions. The sacrifice in speed relative to signaling by ligand gated ion channels is compensated by a broad range of signaling that facilitates integration of signals by the G-protein system. A slower time frame means that cellular processes that are quite distant from the receptor can be modulated. Diffusion of second messengers such as IP3, Ca2+, and DAG can extend neurotransmission through the cell body and to the nucleus to alter gene expression and via NO to other cells. Neurotransmitters acting through G proteins can elicit a coordinated response of the cell that can modulate synaptic release, resynthesis of neurotransmitter, membrane excitability, the cytoskeleton, metabolism, and gene expression.
Summary
A major class of signaling utilizing G-protein-linked signals affords the nervous system a rich diversity of modulation, amplification, and plasticity. Signals are mediated through second messengers activating proteins that modify cellular processes and gene transcription. A key feature is the ability of G proteins to detect the presence of activated receptors and to amplify the signal through effector enzymes and channels. Phosphorylation of key intracellular proteins, ion channels, and enzymes activates diverse, highly regulated cellular processes. The specificity of response is ensured through receptors reacting only with a limited number of G proteins. Coupling between receptor, G protein, and its effector(s), and spatial compartmentalization of the system enables specificity and localized control of signaling. Phospholipids and phosphoinositols provide substrates for second-messenger signaling for G proteins. Stimulation of release of intracellular calcium is often the mediator of the signal. Calcium itself has a dual role as a carrier of electrical current and as a second messenger. Calmodulin is a key regulator that provides complexity and enhances specificity of the signaling system. Sensitivity of the system is imparted by an extremely robust amplification system, as seen in the visual system, which can detect single photons of light.
Modulation of Neuronal Function by Protein Kinases and Phosphatases
Protein phosphorylation and dephosphorylation are key processes that regulate cellular function. They play a fundamental role in mediating signal transduction initiated by neurotransmitters, neuropeptides, growth factors, hormones, and other signaling molecules (Fig. 9.9). The functional state of many proteins is modified by phosphorylation-dephosphorylation, the most ubiquitous post-translational modification in eukaryotes. More than a fifth of all proteins serve as targets for kinases and phosphatases. Phosphorylation or dephosphorylation can rapidly modify the function of enzymes, structural and regulatory proteins, receptors, and ion channels taking part in diverse processes, without a need to change the level of their expression. It can also produce long-term alterations in cellular properties by modulating transcription and translation and changing the complement of proteins expressed by cells.
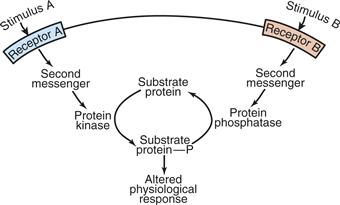
Figure 9.9 Regulation by protein kinases and protein phosphatases. Enzymes and other proteins serve as substrates for protein kinases and phosphoprotein phosphatases, which modify their activity and control them in a dynamic fashion. Multiple signals can be integrated at this level of protein modification.
Adapted from Svenningsson et al. (2004).
Protein kinases catalyze the transfer of the terminal, or γ, phosphate of ATP to the hydroxyl moieties of Ser, Thr, or Tyr residues at specific sites on target proteins. Most protein kinases are either Ser/Thr kinases or Tyr kinases, with only a few designed to phosphorylate both categories of acceptor amino acids. Protein phosphatases catalyze the hydrolysis of the phosphoryl groups from phosphoserine, phosphothreonine, phosphotyrosine, or both types of phosphorylated amino acids on phosphoproteins.
The activity of protein kinases and protein phosphatases is often regulated either by a second messenger (e.g., cAMP or Ca2+) or by an extracellular ligand (e.g., nerve growth factor). In general, second-messenger-regulated kinases modify Ser and Thr, whereas receptor-linked kinases modify Tyr. Among the many protein kinases and protein phosphatases in neurons, a relatively small number serve as master regulators to orchestrate neuronal function. The cAMP-dependent protein kinase (PKA) is a prototype for the known regulated Ser/Thr kinases; they are similar in overall structure and regulatory design. PKA is the predominant mediator for signaling through cAMP, the only other being a cAMP-liganded ion channel in olfaction and an exchange protein directly activated by cAMP (Epac), which modulate GDP–GTP exchange for the small GTPases Rap1 and Rap2. Epacs are activated by cAMP independent of PKA action and regulate cell adhesion, cell junction formation, and exocytosis.
In a similar fashion, the related cGMP-dependent protein kinase (PKG) mediates most of the actions of cGMP. Ca2+-calmodulin-dependent protein kinase II (CaMKII) and several other kinases mediate many of the actions of stimuli that elevate intracellular Ca2+. Finally, the PI signaling system increases both DAG and Ca2+, which activate any of a family of protein kinases collectively called protein kinase C (PKC).
The activities of these second messenger-regulated protein kinases are countered by a relatively small number of phosphatases, exemplified by protein phosphatase 1 (PP–1), protein phosphatase 2A (PP–2A), and protein phosphatase 2B (PP–2B, or calcineurin). Phosphorylation and dephosphorylation are reversible processes, and the net activity of the two processes determines the phosphorylation state of each substrate. The Nobel Prize for Physiology and Medicine was awarded to Edwin G. Krebs and Edmund H. Fischer in 1992 for their pioneering work on the regulation of cell function by protein kinases and phosphatases.
Certain Principles are Common in Protein Phosphorylation and Dephosphorylation
Protein kinases and protein phosphatases are described either as multifunctional if they have a broad specificity and therefore modify many protein targets or as dedicated if they have a very narrow substrate specificity. Spatial positioning of kinases and their substrates in the cell further increases or decreases the likelihood of phosphorylation-dephosphorylation of a given substrate.
The amplification of signal transduction described earlier is continued during transmission of the signal by protein kinases and protein phosphatases. In some cases, the kinases are themselves subject to activation by phosphorylation in a cascade in which one activated kinase phosphorylates and activates a second, and so on, to provide amplification and a switch-like response termed ultrasensitivity.
Kinases and phosphatases integrate cellular stimuli and encode the stimuli as the steady-state level of phosphorylation of a large complement of proteins in the cell (Hunter, 1995). Distinct signal transduction pathways can converge on the same or different target substrates. In some cases, these substrates can be phosphorylated by several kinases at distinct sites. Phosphorylation can alter cellular processes over broad time scales, from milliseconds to hours and even much longer by altering gene expression.
Phosphorylation produces specific changes in the function of a target protein, such as increasing or decreasing the catalytic activity of an enzyme, conductance of an ion channel, or desensitization of a receptor. Kinases and phosphatases modulate proteins by regulating the presence of a highly charged and bulky phosphoryl moiety on Ser, Thr, or Tyr at a precise location on the substrate protein. The phosphate may elicit a conformational change or alter interaction with other proteins.
Finally, each of the three kinases exemplified here can function as a cognitive kinase—that is, a kinase capable of a molecular memory. Although the activity of each kinase requires a second messenger for initial activation, each can become persistently active and independent of its second messenger. This molecular memory potentiates the activity of these kinases and may enable them to participate in aspects of neuronal plasticity.
cAMP-Dependent Protein Kinase was the First Well-Characterized Kinase
Neurotransmitters that stimulate the synthesis of cAMP exert their intracellular effects primarily by activating PKA. The functions (and substrates) regulated by PKA include gene expression [cAMP response element-binding protein (CREB)], catecholamine synthesis (tyrosine hydroxylase), carbohydrate metabolism (phosphorylase kinase), cell morphology [microtubule-associated protein 2 (MAP–2)], postsynaptic sensitivity (AMPA receptor), and membrane conductance (K channel). Paul Greengard and Eric Kandel received the Nobel Prize for Medicine in 2000 (along with Arvid Carlsson) for their discoveries concerning signal transduction via PKA and phosphoprotein phosphatases in the nervous system. PKA is a tetrameric protein composed of two types of subunits: (1) a dimer of regulatory (R) subunits (either two RI subunits for type I PKA or two RII subunits for type II PKA) and (2) two catalytic subunits (C subunit). Two or more isoforms of the RI, RII, and C subunits have distinct tissue and developmental patterns of expression but appear to function similarly. The C subunits are 40-kDa proteins that contain the binding sites for protein substrates and ATP. The R subunits are 49-to 51-kDa proteins that contain two cAMP-binding sites. In addition, the R subunit dimer contains a region that interacts with cellular anchoring proteins that serve to localize PKA appropriately within the cell.
The binding of second messengers by PKA and the other second-messenger-regulated kinases relieves an inhibitory constraint and thus activates the enzymes (Fig. 9.10). cAMP binding leads to subunit dissociation, thereby relieving the C subunit of its inhibitory R subunits and activating the kinase. The steady-state level of cAMP determines the fraction of PKA that is in the dissociated or active form. In this way PKA decodes cAMP signals into the phosphorylation of proteins and the resultant change in various cellular processes.
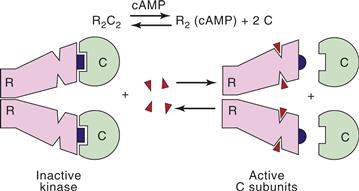
Figure 9.10 Activation of PKA by cAMP. An autoinhibitory segment (blue) of the regulatory subunit (R) dimer interacts with the substrate-binding domain of the catalytic (C) subunits of PKA, blocking access of substrates to their binding site. Binding of four molecules of cAMP reduces the affinity of R for C, resulting in dissociation of constitutively active C subunits.
PKA is a member of a large family of protein kinases that have in common a significant degree of homology in their catalytic domains and are likely derived from an ancestral gene (Fig. 9.11). This homology extends to the three-dimensional crystal structure based on x-ray crystallography of PKA and other kinases. The catalytic domain may be in a subunit distinct from the regulatory domain, as in PKA, or in the same subunit, as in PKC and the CaM kinases. The crystal structure of the C subunit complexed to a segment of protein kinase inhibitor (PKI), a selective high-affinity inhibitor of PKA, reveals that the C subunit is composed of two lobes. The N-terminal lobe contains a highly conserved region that binds Mg2+-ATP in a cleft between the two lobes. A larger C-terminal lobe contains the protein-substrate recognition sites and the appropriate amino acids for catalyzing transfer of the γ-phosphoryl moiety from ATP to the substrate. Inhibition by PKI is diagnostic of PKA involvement; PKI contains an autoinhibitory sequence resembling PKA substrates and is positioned in the catalytic site like a substrate, thus blocking access for substrates.
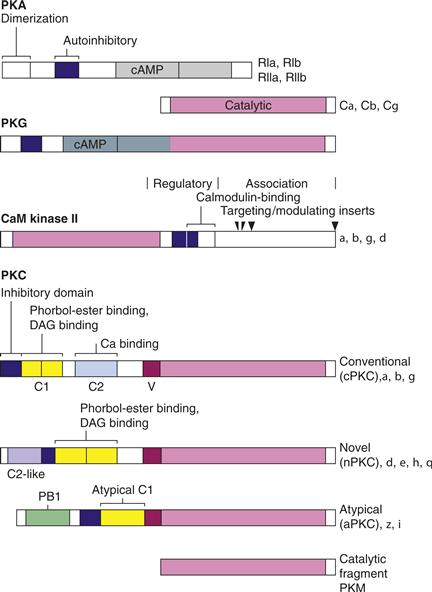
Figure 9.11 Domain structure of protein kinases. Protein kinases are encoded by proteins with recognizable structural sequences that encode specialized functional domains. Each of the kinases [PKA, PKG, CaMKII, and PKC] has homologous catalytic domains that are kept inactive by the presence of an autoinhibitory segment (blue lines). Regulatory domains contain sites for binding second messengers such as cAMP, cGMP, Ca2+-calmodulin, DAG, and Ca2+- phosphatidylserine. Alternative splicing creates additional diversity.
PKA phosphorylates Ser or Thr at specific sites in dozens of proteins. The sequences of amino acids at the phosphorylation sites are not identical. Each kinase has a characteristic consensus sequence that forms the basis for distinct substrate specificities.
A regulatory theme common to PKA, CaMKII, and PKC is that their second messengers activate them by displacing an inhibitory domain from the active site. In PKA, binding of the R subunit distorts the active site of the C subunit and blocks access of substrates by positioning a pseudosubstrate or inhibitory sequence in the catalytic site. Binding of cAMP to the R subunit produces a large conformation change in R that disrupts its binding to the C subunit, thus leading to dissociation of an active C subunit. CaMKII and PKC have the catalytic domain and inhibitory sequence in the same polypeptide with binding of their respective second-messengers similarly de-inhibiting the kinase by displacement of the autoinhibitory domain (Fig. 9.11).
Multifunctional CaMKII Decodes Diverse Signals That Elevate Intracellular Ca2+
Most of the effects of Ca2+ in neurons and other cell types are mediated by calmodulin, and many of the effects of Ca2+-calmodulin are mediated by protein phosphorylation-dephosphorylation (Wayman et al., 2008). The Ca2+-signaling system contains a family of Ca2+-calmodulin-dependent protein kinases with broad substrate specificity, including CaM kinases I, II, and IV; of these, CaMKII is the best characterized. CaMKII phosphorylates tyrosine hydroxylase, MAP-2, synapsin I, calcium channels, Ca2+-ATPase, transcription factors, and glutamate receptors and thereby regulates synthesis of catecholamines, cytoskeletal function, synaptic release in response to high-frequency stimuli, calcium currents, calcium homeostasis, gene expression, and synaptic plasticity, respectively. This kinase is found in every tissue but is particularly enriched in neurons. It is found in the cytosol, in the nucleus, in association with cytoskeletal elements, and in postsynaptic thickening termed the postsynaptic density found in asymmetric synapses. It is a large multimeric enzyme, consisting of 12 subunits derived from four homologous genes (a, β, γ, and δ) that encode different isoforms of the kinase that range from 54 to 72 kDa per subunit.
The catalytic, regulatory, and targeting domains of CaMKII are all contained within a single polypeptide (Fig. 9.11). Following the catalytic domain on the N-terminal half of each isoform is the regulatory domain, which contains an autoinhibitory domain with an overlapping calmodulin-binding sequence. The C-terminal end contains an association domain that allows 12 subunits (two rings of six catalytic domains each) to assemble into a multimer, as well as targeting sequences that direct the kinase to distinct intracellular sites (Chao et al., 2011).
Regulation of the kinase by autophosphorylation is a critical feature of CaMKII. The kinase is inactive in the basal state because an autoinhibitory segment distorts the active site and sterically blocks access to its substrates. Binding of Ca2+-calmodulin to the calmodulin-binding domain of the kinase displaces the autoinhibitory domain from the catalytic site, thus activating the kinase by enabling ATP and protein substrates to bind. Displacement of this domain also exposes a binding site for anchoring proteins that the activated kinase can bind. If the kinase is activated, it can autophosphorylate Thr-286 (in α-CaMKII). Phosphorylation disables the autoinhibitory segment by preventing it from reblocking the active site after calmodulin dissociates and thereby locks the kinase in a partially active state that is independent, or autonomous, of Ca2+-calmodulin and can anchor to additional targets. Autophosphorylation prolongs the active state of the kinase, a potentiation that led to its description as a cognitive kinase.
CaMKII is targeted to distinct cellular compartments. Differences between the four genes encoding CaMKII and between the two or more isoforms that are encoded by each gene by apparent alternative splicing reside primarily in a variable region at the start of the association domain (Fig. 9.11). In some isoforms, this region contains an additional sequence that targets those isoforms to the nucleus. The major neuronal isoform, α-CaMKII, is largely cytosolic but is also found associated with postsynaptic densities and synaptic vesicles. Targeting to the NMDA type glutamate receptor occurs only after calmodulin activates the kinase and exposes a binding site. The kinase also serves as a scaffold for binding of other proteins, including the recruitment of the ubiquitin proteasome to dendritic spines.
Protein Kinase C is the Principal Target of the PI Signaling System
Protein kinase C is a collective name for members of a relatively diverse family of protein kinases most closely associated with the PI-signaling system. PKC is a multifunctional Ser/Thr kinase capable of modulating many cellular processes, including exocytosis and endocytosis of neurotransmitter vesicles, neuronal plasticity, gene expression, regulation of cell growth and cell cycle, ion channels, and receptors. The role of DAG generated during PI signaling was unclear until its link to PKC was established. Many PKC isoforms also require an acidic phospholipid such as phosphatidylserine for appropriate activation. The kinase is also of interest because it is the target of a class of tumor promoters called phorbol esters. They activate PKC by simulating the action of DAG, bypassing the normal receptor-based pathway, and inappropriately stimulating cell growth.
We now understand that the PKC family of kinases is diverse in structure and regulatory properties (Newton, 2010). PKC is monomeric (78–90 kDa) with catalytic, regulatory, and targeting domains all on one polypeptide (Fig. 9.11). The conventional isoforms (or cPKC), have all of the following domains:
• an autoinhibitory or pseudosubstrate sequence
• C1, a cysteine-rich domain that binds DAG and phorbol esters
• C2, a region necessary for Ca2+ sensitivity and for binding to phosphatidylserine and to anchoring proteins
Another class of isoforms, termed novel PKCs (nPKC), lacks a true C2 domain and is therefore not Ca2+ sensitive. Another class is considered atypical (aPKC) because it lacks C2 and the first of two cysteine-rich domains that are necessary for DAG (or phorbol ester) sensitivity. This class is neither Ca2+ nor DAG sensitive but has a PB1 domain involved in protein interaction. Not included is a family of closely related kinases termed PKN.
Activation of PKC is best understood for the conventional isoforms. Generation of DAG resulting from stimulation of the PI-signaling pathway increases the affinity of cPKC isoforms for Ca2+ and phosphatidylserine. DAG, or specifically its sn-1,2-diacylglycerol isomer, is derived only from PI turnover, and it is the only isomer effective in activating PKC. Cell stimulation results in the translocation of cPKC from a variety of sites to the membrane or cytoskeletal elements where it interacts with PS-Ca2+- DAG at the membrane. Binding of the second messengers to the regulatory domain disrupts the nearby autoinhibitory domain, leading to a reversible activation of PKC by deinhibition. Translocation is not restricted to the plasma membrane. Upon activation some PKC isoforms reversibly translocate to intracellular sites enriched with anchoring proteins, termed receptors for activated C kinase (RACK).
Prolonged activation of PKC can be produced by the addition of phorbol esters, which simulate activation by DAG but remain in the cell until they are washed out. In a matter of hours to days, such persistent activation by phorbol esters leads to a degradation of PKC. This phenomenon is sometimes used experimentally to produce a PKC-depleted cell (at least for phorbol ester-binding isoforms) and thereafter to test for a loss of putative PKC functions.
Spatial Localization Regulates Protein Kinases and Phosphatases
Protein kinases and protein phosphatases are often positioned spatially near their substrates or they translocate to their substrates upon activation to improve speed and specificity in response to neurotransmitter stimulation. For example, A Kinase Anchoring Protein 79 (AKAP79) while first identified with PKA binding, also has binding site for PKC and calcineurin (Logue & Scott, 2010). Another example of a signaling complex is the protein termed yotiao, which binds to the NMDA type glutamate receptor and serves as an anchor for both PKA and a phosphatase (PP–1).
The use of anchoring proteins has several consequences. First, rate of phosphorylation and specificity are enhanced when kinases or phosphatases are concentrated near intended substrates. Second, it increases the signal-to-noise ratio for substrates that are not near anchoring proteins by reducing basal state phosphorylation. For example, PKA is anchored on the Golgi away from the nucleus so that phosphorylation in the basal state or even after a brief stimulus produces little phosphorylation of nuclear proteins. Prolonged stimuli, however, enable some C subunits to diffuse passively through nuclear pores and regulate gene expression. Termination of the nuclear action of C subunits is aided by PKI, which acts to inhibit and export it back out of the nucleus. Third, anchoring can enable significant phosphorylation of nearby substrates at basal cAMP, such as a Ca2+ channel phosphorylated when its phosphorylation site is exposed during depolarization.
The Cognitive Kinases
The ability of three major Ser/Thr kinases (PKA, CaMKII, and PKC) in brain to initiate or maintain synaptic changes that underlie learning and memory may require that they themselves undergo some form of persistent change in activity. Both their functional and molecular properties led to their description as cognitive kinases.
cAMP-Dependent Protein Kinase
A role for PKA as a cognitive kinase can be seen in long-term facilitation of the gill-withdrawal reflex in Aplysia and in long-term potentiation in the rodent hippocampus. In motor neuron cultures, repeated or prolonged exposure to serotonin or cAMP leads to long-term facilitation because PKA becomes persistently active despite the fact that cAMP is no longer elevated (Chain et al., 1999). During such activation there is a preferential degradation and decrease in the inhibitory RII subunits and thus a slight excess of C subunits that remain persistently active because of insufficient RII subunits. The C subunit then enters the nucleus and induces expression of one protein that facilitates further proteolysis of RII. In this interesting process, a molecular memory of appropriate stimulation by serotonin is encoded by a persistence of PKA activity that is regenerative.
Ca2+-Calmodulin-Dependent Protein Kinase
CaMKII has features of a cognitive kinase because it has a molecular memory based on autophosphorylation and it phosphorylates proteins that modulate synaptic plasticity (Lisman, Schulman, & Cline, 2002). The biochemical properties of CaMKII suggest mechanisms by which appropriate stimulus frequencies can generate an autonomous enzyme (Fig. 9.12). At low stimulus frequency, the time between stimuli is sufficient for calmodulin to dissociate and the kinase to be dephosphorylated, and the same submaximal activation will occur with each stimulus. At higher frequencies, however, some subunits will remain autophosphorylated and bound to calmodulin so successive stimuli will result in more calmodulin bound per holoenzyme, which will make autophosphorylation more probable because it requires two active proximate neighboring subunits. The enzyme is therefore able to decode the frequency of cellular stimulation and translate this into a prolonged activated state.
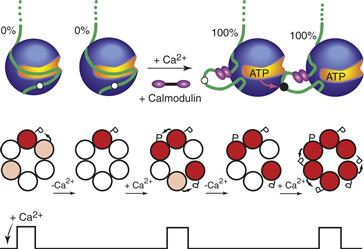
Figure 9.12 Frequency-dependent activation of CaMKII. Autophosphorylation occurs when both neighboring subunits in a holoenzyme are bound to calmodulin. At a high frequency of stimulation (rapid Ca2+ spikes), the interspike interval is too short to allow significant dephosphorylation or dissociation of calmodulin, thereby increasing the probability of autophosphorylation with each successive spike. In a simplified CaMKII with only one ring of six subunits, calmodulin-bound subunits are shown in pink and autophosphorylated subunits with trapped calmodulin are shown in red.
Adapted from Hudmon and Schulman (2002).
CaMKII phosphorylates a number of substrates that affect synaptic strength. Inhibition of CaMKII in hippocampal slices or just elimination of its autophosphorylation by an α-CaMKII mouse knock-in in which the critical Thr was replaced by Ala blocks autonomy and the induction of long-term potentiation. These mice are deficient in learning spatial navigational cues, one of the functions of the rodent hippocampus. The basis for its role involves a net shift of AMPA receptors to the synapse that may be mediated by phosphorylation of both GluA1 receptors and their accessory protein(s), leading to a greater postsynaptic response (Lisman, Schulman, & Cline, 2002; Opazo et al., 2010). The autophosphorylated kinase also serves as a scaffold to recruit the ubiquitin proteasome to synaptic sites that enable dynamic changes at the synapse.
Protein Kinase C
PKC can also be converted into a form that is independent, or autonomous, of its second messenger and can be described as a cognitive kinase. During the persistent phase of long-term potentiation, some of the inhibitory domain of some PKC molecules is proteolyically removed, thus converting it to a constitutively active kinase termed protein kinase M (PKM). PKCζ can also be transcribed from an internal promoter to produce PKM, whose translation in dendrites is unblocked during LTP and whose activity then disables the translational block, an interesting form of molecular memory (Sacktor, 2011). PKC (and PKM) substrates associated with long-term potentiation include NMDA and AMPA receptors.
Protein Tyrosine Kinases Take Part in Cell Growth and Differentiation
Protein kinases that phosphorylate tyrosine residues are usually associated with the regulation of cell growth and differentiation. Signal transduction by protein tyrosine kinases often includes a cascade of kinases phosphorylating other kinases, eventually activating Ser/Thr kinases, which carry out the intended modification of a cellular process. There are both receptor tyrosine kinases, activated by the binding of extracellular growth factors such as nerve growth factor and epidermal growth factor, and soluble ones, activated indirectly by extracellular ligands such as c-Src (Lemmon & Schlessinger, 2010).
Protein Phosphatases Undo What Kinases Create
Protein phosphatases in neuronal signaling are categorized as either phosphoserine-phosphothreonine phosphatases (PSPs) or phosphotyrosine phosphatases (PTPs) (Hunter, 1995; Mansuy & Shenolikar, 2006). The enzymes catalyze the hydrolysis of the ester bond of the phosphorylated amino acids to release inorganic phosphate and the unphosphorylated protein. A limited number of multifunctional PSPs account for most of such phosphatase activity in cells. They are categorized based on their domain structure, inhibitor sensitivity, and catalytic mechanism (Table 9.1). There are three families of protein serine/threonine phosphatases, designated as phosphoprotein phosphatases (PP-1, -2B (calcineurin), -2A, -4, -6, -5, -7), metal-dependent phosphatases (PP2C), and aspartate-based phosphatases (FCP and SCP). Protein phosphatase 2B (PP-2B, or calcineurin) responds directly to a second messenger, Ca2+. The specificity of PP-1 and PP-2A is particularly broad in isolation, but both their protein substrates and cellular localization are refined by interaction with many regulatory and scaffold proteins. Phosphotyrosine phosphatases constitute a distinct and larger class of phosphatases, including PTPs with dual specificity for both phosphotyrosines and phosphoserine-phosphothreonines. PTPs are either soluble enzymes or membrane proteins with variable extracellular domains that enable regulation by extracellular binding of either soluble or membrane-bound signals.
Table 9.1 Categories of Protein Phosphatasesa
| Phosphatase | Characteristic | Other Inhibitors |
| PP-1 | Sensitive to phospho-inhibitor-1, phospho-DARPP-32, and inhibitor-2 | Weakly sensitive to okadaic acid |
| PP-2B (calcineurin) | Ca2+/calmodulin-dependent | FK506, cyclosporin |
| PP-2A | Highly abundant | Highly sensitive to okadaic acid |
| PP-4/PP-6 | Nuclear | Highly sensitive to okadaic acid |
| PP-5 | Nuclear | Mildly sensitive to okadaic acid |
| PP-7 | Nuclear | Mildly sensitive to okadaic acid |
| PP-2C | Requires metal (Mg2+/Mn2+) | EDTA |
| FCP/SCPb | Aspartic acid-based catalysis | |
| Receptor PTPsc | Plasma membrane | Vanadate |
| Nonreceptor PTPs | Various cellular compartments | Vanadate |
| Dual specificity PTPs | Nuclear (e.g., cdc25A/B/C and VH family) | Vanadate |
aUpdated from Hunter (1995).
bTFIIF-associated component of RNA polymerase II CTD phosphatase/small CTD phosphatase.
cProtein phosphotyrosine phosphatases.
Structure and Regulation of PP-1 and Calcineurin
PP-1 and calcineurin are the best characterized phosphatases with regard to both structure and regulation. The domain structures of the catalytic subunits of PP-1 and calcineurin are depicted in Figure 9.13. PP-1 is a protein of 35–38 kDa; most of the sequence forms the catalytic domain, and its C-terminal is the site of regulatory phosphorylation. The catalytic domains of PP-1, PP-2A, and calcineurin are highly homologous.
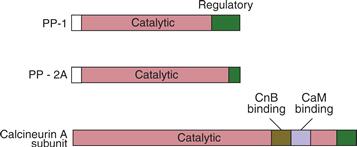
Figure 9.13 Domain structure of the catalytic subunits of some Ser/Thr phosphatases. The three major phosphoprotein phosphatases, PP-1, PP-2A, and calcineurin, have homologous catalytic domains but differ in their regulatory properties.
PP-1 and PP-2A are normally complexed in cells with specific targeting or regulatory subunits. Targeting of PP-1 can be modulated by phosphorylation of its targets. As PP-1 dissociates from targeting subunits, it becomes susceptible to inhibition by inhibitor-2.
Inhibition of PP-1 by two other inhibitors, inhibitor-1 and its homologue DARPP-32 (dopamine and cAMP-regulated phosphoprotein; Mr 32,000), is conditional on their phosphorylation by either PKA or PKG (Fig. 9.14). Because the substrates for PKA and PP-1 overlap to a great extent, the rate and extent of phosphorylation of such substrates are enhanced by the ability of PKA to catalyze their phosphorylation while blocking their dephosphorylation via PP-1. Inhibitor-1, DARPP-32, and inhibitor-2 are all selective for PP-1. Highly selective inhibitors capable of penetrating the cell membrane are available for these phosphatases. Okadaic acid, a natural product of marine dinoflagellates, is a tumor promoter but, unlike phorbol esters, it acts on PP-2A and PP-1 rather than on PKC.
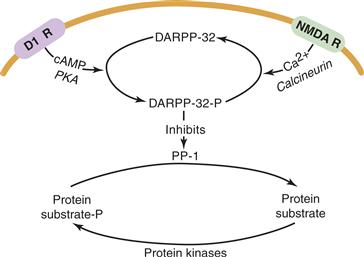
Figure 9.14 Cross talk between kinases and phosphatases. The state of phosphorylation of protein substrates is regulated dynamically by protein kinases and phosphatases. In the striatum, for example, dopamine stimulates PKA, which converts DARPP-32 into an effective inhibitor of PP-1. This increases the steady-state level of phosphorylation of a hypothetical substrate subject to phosphorylation by a variety of protein kinases. This action can be countered by NMDA receptor stimulation by another stimulus that increases intracellular Ca2+ and activates calcineurin. PP-1 is deinhibited and dephosphorylates the phosphorylated substrate when calcineurin deactives DARPP-32-P.
Adapted from Svenningsson et al. (2004).
Protein Phosphatase 1
The x-ray structure of the catalytic subunit of PP-1 bound to the toxin microcystin, a cyclic peptide inhibitor, reveals PP-1 to be a compact ellipsoid with hydrophobic and acidic surfaces forming a cleft for binding substrates. PP-1 is a metalloenzyme requiring two metals in the active site that likely take part in electrostatic interactions with the phosphate on substrates that aid in catalyzing the hydrolytic reaction. Substrate binding is blocked when phospho-inhibitor-1 or microcrystin LR binds to this surface.
Calcineurin (PP-2B)
Calcineurin is a Ca2+-calmodulin-dependent phosphatase that is highly enriched in the brain. It is a heterodimer with a 60-kDa subunit (CnA) that contains an N-terminal catalytic domain similar to PP-1 and a C-terminal regulatory domain that includes an autoinhibitory segment, a calmodulin-binding domain, and a binding site for the 19-kDa regulatory B subunit (CnB). CnB is a calmodulin-like Ca2+-binding protein that binds to a hinge region of CnA. Some activation of calcineurin is attained by binding of Ca2+ to CnB. Stronger activation is obtained by the binding of Ca2+-camodulin. Additional regulation may be accorded by interaction of its hinge region with cyclophilin and FKBP (FK506-binding protein), proteins that bind the immunosuppressive agents cyclosporin and FK506, respectively.
The Ca2+-calmodulin sensitivities of calcineurin and CaMKII are quite different. Weak or low-frequency stimuli may selectively activate calcineurin, whereas strong or high-frequency stimuli activate CaMKII and calcineurin. This difference may play a role in the bidirectional control of synaptic strength (depression vs. potentiation) by low- and high-frequency stimulation.
Protein Kinases, Protein Phosphatases, and Their Substrates are Integrated Networks
Cross talk between protein kinases and protein phosphatases is critical to their ability to integrate inputs into neurons. Such cross talk is exemplified by the interaction of cAMP and Ca2+ signals through PKA and calcineurin, respectively. The medium spiny neurons in the neostriatum receive cortical inputs from glutamatergic neurons that are excitatory and nigral inputs by dopaminergic neurons that inhibit them. A possible signal transduction scheme for this regulation is shown in Figure 9.14. The key to regulation is the bidirectional control of DARPP-32 phosphorylation (Svenningsson et al., 2004). Glutamate activates calcineurin by increasing intracellular Ca2+, leading to the dephosphorylation and inactivation of phospho-DARPP-32. This releases inhibition of PP-1, which can then dephosphorylate a variety of substrates, including Na+, K+-ATPase, and lead to membrane depolarization. This is countered by dopamine, which stimulates cAMP formation and activation of PKA, which then converts DARPP-32 into its phosphorylated (i.e., PP-1 inhibitory) state. There are many other receptors and signaling integrated by these pathways. For example, adenosine, serotonin, and VIP act through their cognate receptors to elevate cAMP, similarly to dopamine at D1 receptors, while opiates can signal to inhibit the action of dopamine at D1 and adenosine at A2A receptors. Although PKA and calcineurin are acting in an antagonistic manner, they are not doing it by phosphorylating and dephosphorylating the ATPase. By their actions upstream, at the level of DARPP-32, the regulation of numerous target enzymes (e.g., Ca2+ channels and Na+ channels) in addition to the ATPase can be coordinated.
Studying Cellular Processes Controlled by Phosphorylation-Dephosphorylation
Major goals of signal transduction research are to delineate pathways by which signals such as neurotransmitters transduce their signals to modify cellular processes. This is often the start of a process to identify targets for therapeutic intervention in disease. Cellular and biochemical assays can often identify the entire signaling pathway, from stimulation of receptor, to generation of a second-messenger activation of a kinase or phosphatase, change in the phosphorylation state of the substrate, and an ultimate change in its functional state. Such investigations utilize a variety of pharmacological inhibitors or activators of the signaling molecules complemented by genetic approaches that utilize transfection of activated forms of the kinases or phosphatases in question, siRNAs, transgenic animals, and mice with individual signaling components knocked out.
Summary
The morphology of a cell is determined by protein constituents. Its function is regulated by the phosphorylation or dephosphorylation of the proteins. Phosphorylation modifies the function of regulatory proteins subsequent to their genetic expression. The activities of the protein kinases and protein phosphatases are typically regulated by second messengers and extracellular ligands. Kinases and phosphatases integrate and encode stimulation of a large group of cellular receptors. The number of possible effects is almost limitless and enables the tuning of cellular processes over a broad time scale. Most of the effects of Ca2+ in cells are mediated by calmodulin, which in turn mediates changes in protein phosphorylation-dephosphorylation. The phosphoinositol signaling system is mediated through PKC, which modulates many cellular processes from exocytosis to gene expression. All three classes of enzymes discussed have been described as cognitive kinases because they are capable of sustaining their activated states after their second-messenger stimuli have returned to basal levels. PKA has been implicated in learning and memory in Aplysia and in hippocampus, where it is involved in long-term potentiation. Protein phosphatases play an equally important role in neuronal signaling by dephosphorylating proteins. Cross talk between protein kinases and protein phosphatases is key to their ability to integrate inputs into neurons.
Intracellular Signaling Affects Nuclear Gene Expression
For all living cells, regulation of gene expression by intracellular signals is a fundamental mechanism of development, homeostasis, and adaptation to the environment. Protein phosphorylation and regulation of gene expression by intracellular signals are the most important mechanisms underlying the remarkable degree of plasticity exhibited by neurons. Alterations in gene expression underlie many forms of long-term changes in neural functioning, with a time course that ranges from hours to many years. Signaling can also regulate translation of mRNA, including translation that is localized to dendrites and synaptic spines, as well as protein degradation that facilitates dynamic regulation of cell function and shape.
Interactions of Specific DNA Sequences with Regulatory Proteins Control Both Basal and Signal-Regulated Transcription
Information contained within DNA must be expressed through other molecules: RNA and proteins. The human genome contains approximately 25,000 genes that encode structural RNAs or protein-coding messenger RNAs (mRNAs). Regulated gene expression conferred by the nucleotide sequence of the DNA itself is called cis regulation because the control regions are linked physically on the DNA to regions that can potentially be transcribed. The cis regulatory sequences function by serving as high-affinity binding sites for regulatory proteins called transcription factors.
The transcription of specific genes into mRNA is carried out by a complex enzyme called RNA polymerase II. Roger Kornberg won the Nobel Prize in Chemistry in 2006 for his studies on the molecular basis for eukaryotic transcription. Transcription is often divided into three steps: initiation of RNA synthesis, RNA chain elongation, and chain termination. Extracellular signals, such as neurotransmitters, hormones, drugs, and growth factors generally control the transcription initiation step.
Transcription initiation requires two critical processes: (1) positioning of RNA polymerase II at the correct start site of the gene to be transcribed and (2) controlling the efficiency of initiations to produce the appropriate transcriptional rate for the circumstances of the cell (Tjian & Maniatis 1994). The cis-regulatory elements that set the transcription start sites of genes are called the basal promoter. Other cis-regulatory elements tether additional activator and represser proteins to the DNA to regulate the overall transcriptional rate (Fig. 9.15).
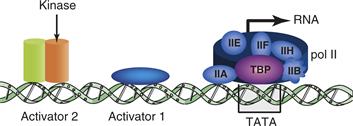
Figure 9.15 Schematic of a generalized RNA polymerase II promoter showing three separate cis-regulatory elements along a stretch of DNA. These elements are two hypothetical activator protein-binding sites and the TATA element. The TATA element is shown binding the TATA-binding protein (TBP). Multiple general transcription factors (IIA, IIB, etc.) and RNA polymerase II (pol II) associate with TBP. Each transcription factor comprises multiple individual proteins complexed together. This basal transcription apparatus recruits RNA polymerase II into the complex and also forms the substrate for interactions with the activator proteins binding to the activator elements shown. Activator 2 is shown to be a substrate for a protein kinase.
Sequence-Specific Transcription Factors
The promoters for RNA polymerase II transcribed genes contain a distinct basal promoter element on which a basal transcription complex is assembled. The basal promoter of most of these genes contains a sequence called a TATA box that is rich in the nucleotides adenine (A) and thymine (T) located between 25 and 30 bases upstream of the transcription start site.
To achieve significant levels of transcription, this multiprotein assembly requires help from sequence-specific transcriptional activators that recognize and bind distinct cis-regulatory elements. Functional cis-regulatory elements are generally 7–12 bp in length and structured as a palindrome, each of which is a specific binding site for one or more transcription factors. Each gene has a particular combination of cis-regulatory elements, the nature, number, and spatial arrangement of which determine the gene’s unique pattern of expression, including the cell types in which it is expressed, the times during development in which it is expressed, and the level at which it is expressed in adults both basally and in response to physiological signals (Tjian & Maniatis, 1994).
Many transcription factors are active only as dimers or higher order complexes formed via a multimerization domain. Both partners in a dimer commonly contribute jointly to both the DNA-binding domain and the activation domain. Dimerization can be a mechanism of either positive or negative control of transcription. The effects of sequence-specific transcriptional activator and represser factors are frequently mediated by adapter proteins (Fig. 9.16). In many cases, these adapter proteins are enzymes, such as histone acyl transferases and deacetylases, with the ability to modify the structure of the proteins associated with the DNA and modulate transcription (Fig. 9.16).
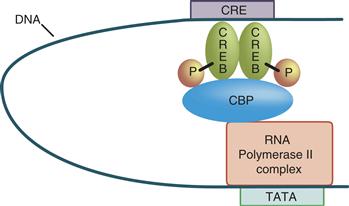
Figure 9.16 Looping of DNA permits activator (or represser) proteins binding at a distance to interact with the basal transcription apparatus. The basal transcription apparatus is shown as a single box (pol II complex) bound at the TATA element. The activator protein (CREB) is shown as having been phosphorylated. On phosphorylation, many activators, such as CREB, are able to recruit adaptor proteins that mediate between the activator and the basal transcription apparatus. An adaptor protein that binds phosphorylated CREB is called a CREB-binding protein (CBP).
A Significant Consequence of Intracellular Signaling is the Regulation of Transcription
Intracellular signals play a major role in the regulation of gene expression—for example, via nuclear translocation and/or phosphorylation of activator proteins. Signal-directed change in location or conformation of these proteins permits information obtained by the cell from its different signaling systems to regulate gene expression appropriate to the status of the cell.
Transcriptional Regulation by Intracellular Signals
Extracellular control of transcription requires a translocation step by which the signal is transmitted through the cytoplasm to the nucleus. Some transcription factors are themselves translocated to the nucleus. For example, the transcription factor NF-κB is retained in the cytoplasm by its binding protein IκB, which masks the NF-κB nuclear localization signal. Signal-regulated phosphorylation of IκB by PKC and other protein kinases leads to dissociation of NF-κB, permitting it to enter the nucleus. Other transcription factors must be directly phosphorylated or dephosphorylated to bind DNA. In many cytokine-signaling pathways, plasma membrane receptor tyrosine phosphorylation of transcription factors known as signal transducers and activators of transcription (STATs) permits their multimerization, which in turn permits both nuclear translocation and construction of an effective DNA-binding site within the multimer. Yet other transcription factors, such as CREB (Fig. 9.16), are already bound to their cognate cis-regulatory elements and become able to activate transcription after phosphorylation by a kinase that translocates to the nucleus.
Role of cAMP and Ca2+ in the Activation Pathways of Transcription
The cAMP second-messenger pathway regulates expression of a large number of genes via cAMP response elements (CREs) with the consensus palindromic sequence of TGACGTCA. Phosphorylation of CRE-bound CREB on its Ser-133 by activated PKA that translocates to the nucleus recruits the adapter protein CBP. This, in turn, interacts with the basal transcription complex and modifies histones to enhance the efficiency of transcription. CREB also serves to illustrate the convergence of signaling pathways (Fig. 9.17). CREB Ser-133 phosphorylation is not only mediated by PKA but also by CaMKII and CaMKIV and by RSK2, a kinase activated in growth factor pathways, including Ras and MAP kinase (Sakamoto, Karelina & Obrietan, 2011).
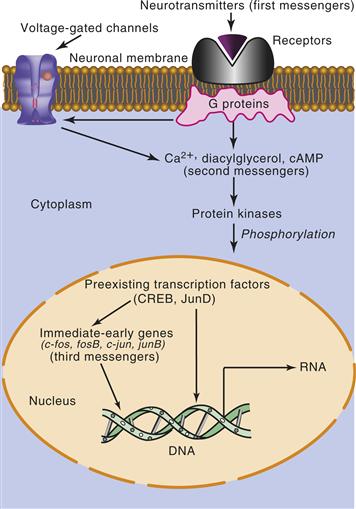
Figure 9.17 Signal transduction to the nucleus. In this schematic, activation of a neurotransmitter receptor activates cellular signals (G proteins and second-messenger systems). These signals, in turn, regulate the activation of protein kinases, which translocate to the nucleus. Within the nucleus, protein kinases can activate genes regulated by constitutively synthesized transcription factors. A subset of these genes encodes additional transcription factors (third messengers), which can then activate multiple downstream genes.
CREB illustrates yet another important principle of transcriptional regulation: CREB is a member of a family of related proteins. Many transcription factors are members of families; this permits complex forms of positive and negative regulation. CREB is closely related to other proteins called activating transcription factors (ATFs) and CRE modulators (CREMs). The dimerization domain used by CREB-ATF proteins and several other families of transcription factors is called a leucine zipper. The dimerization motif is an α helix in which every seventh residue is a leucine; based on the periodicity of α helices, the leucines line up along one face of the helix two turns apart. Dimerization juxtaposes the adjacent basic, DNA binding, regions of each of the partners. This combination of motifs is why this superfamily of proteins is referred to as basic leucine zipper proteins (bZIPs).
AP-1 Transcription Factors
Activator protein 1 (AP-1) is another family of bZIP transcription factors that play a central role in the regulation of neural gene expression by extracellular signals. The AP-1 family comprises multiple proteins that bind as heterodimers (and a few as homodimers) to the DNA sequence TGACTCA. While the AP-1 sequence differs from the CRE sequence (TGACGTCA) by only a single base, this one-base difference strongly biases protein binding away from the CREB family of proteins. AP-1 sequences confer responsiveness to the PKC pathway. AP-1 proteins generally bind DNA as heterodimers composed of one member each of two different families of related bZIP proteins, the Fos family (c-Fos, Fra-1, Fra-2, and FosB) and the Jun family (c-Jun, JunB, and JunD) providing for a multiplicity of regulatory control.
Cellular Immediate-Early Genes
Genes that are activated transcriptionally by synaptic activity, drugs, and growth factors have often been classified roughly into two groups. Genes, such as the c-fos gene itself, that are activated rapidly (within minutes), transiently, and without requiring new protein synthesis are often described as cellular immediate-early genes (IEGs). Genes that are induced or repressed more slowly (within hours) and are dependent on new protein synthesis have been described as late-response genes. Several IEGs have been used as cellular markers of neural activation because they are markedly induced by depolarization (the critical signal being Ca2+ entry) and second-messenger and growth factor pathways, permitting novel approaches to functional neuroanatomy.
The protein products of those cellular IEGs that function as transcription factors bind to cis-regulatory elements contained within a subset of late-response genes to activate or repress them (Fig. 9.17). In sum, neural genes that are regulated by extracellular signals are activated or repressed with varying time courses by reversible phosphorylation of constitutively synthesized transcription factors and by newly synthesized transcription factors, some of which are regulated as IEGs.
Activation of the c-fos Gene
The c-fos gene is activated rapidly by neurotransmitters or drugs that stimulate the cAMP pathway or Ca2+ elevation. Both pathways produce phosphorylation of transcription factor CREB. The c-fos gene contains three binding sites for CREB. The c-fos gene can also be induced by the Ras/MAP kinase pathway, which is activated by a number of growth factors. For example, neurotrophins, such as nerve growth factor (NGF), bind a family of receptor tyrosine kinases (Trks); NGF interacts with Trk A, which activates Ras. Ras then acts through a cascade of protein kinases. Cross talk between neurotransmitter and growth factor–signaling pathways has been documented with increasing frequency and likely plays an important role in the precise tuning of neural plasticity to diverse environmental stimuli.
Regulation of c-Jun
Expression of most of the proteins of the Fos and Jun families that constitute transcription factor AP-1 and the binding of AP-1 proteins to DNA is regulated by extracellular signals. Phosphorylation and activation of c-Jun can result from the action of Jun N-terminal kinase (JNK). JNK is a member of the mitogen activated protein kinase (MAPK) family of protein kinases. JNK has also been shown to be activated by neurotransmitters, including glutamate. Thus, AP-1–mediated transcription within the nervous system requires multiple steps, beginning with the activation of genes encoding AP-1 proteins.
Cytokines as Inducers of Gene Expression in the Nervous System
With regard to function, the boundary between trophic, or growth, factors and cytokines in the nervous system has become increasingly arbitrary. However, cell-signaling mechanisms offer a useful means of distinction. Growth factors, such as neurotrophins (e.g., nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3), epidermal growth factor (EGF), and fibroblast growth factor (FGF), act through receptor protein tyrosine kinases, whereas cytokines, such as leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), and interleukin-6 (IL-6), act through nonreceptor protein tyrosine kinases.
LIF, CNTF, and IL-6 subserve a wide array of overlapping functions inside and outside the nervous system, including hematopoietic and immunologic functions outside the nervous system and regulation of neuronal survival, differentiation, and, in certain circumstances, plasticity within the nervous system. Their receptors contain a common signal-transducing subunit, gp130. Receptors for these cytokines consist of a signal-transducing β component, which includes gp130, and interacts with nonreceptor protein tyrosine kinases (PTKs) of the Janus kinase (Jak) family (e.g., Jak1, Jak2, and Tyk2). Some cytokine receptors interact only with a single Jak PTK, whereas others interact with multiple Jak PTKs.
Signal transduction to the nucleus includes tyrosine phosphorylation by the Jak PTKs of one or more of the STAT proteins mentioned earlier. Upon phosphorylation, STAT proteins form dimers through the association of SH2 domains, an important type of protein interaction domain. Dimerization is thought to trigger translocation to the nucleus, where STATs bind their cognate cytokine response elements. Different STATs become activated by different cytokine receptors, not because of differential use of Jak PTKs but because of specific coupling of certain STATs to certain receptors. Thus, for example, the IL-6 receptor preferentially activates STAT1 and STAT3; the CNTF receptor preferentially activates STAT3. Cytokine response elements, to which STATs bind, have now been identified within many neural genes, including vasoactive intestinal polypeptide and several other neuropeptide genes.
Steroid Hormone Receptors
The differentiation of many cell types in the brain is established by exposure to steroids. Steroid hormones, including glucocorticoids, sex steroids, mineralocorticoids, retinoids, thyroid hormone, and vitamin D, are small lipid-soluble ligands that can diffuse across cell membranes. They act on their receptors within the cell cytoplasm in marked distinction to the other types of intercellular signals described herein. Another unique feature of steroid hormones is that their receptors are themselves transcription factors. Each has a transcriptional-activation domain at its amino terminus, a DNA-binding domain, and a hormone-binding domain at its carboxy terminus. DNA-binding domains recognize specific palindromic DNA sequences, steroid hormone response elements, within the regulatory regions of specific genes.
After having been bound by hormone, activated steroid hormone receptors translocate into the nucleus, where they bind to their cognate response elements. Such binding then increases or decreases the rate at which these target genes are transcribed, depending on the precise nature and DNA sequence context of the element.
Summary
The formation of long-term memories requires changes in gene expression and new protein synthesis. It is at transcription initiation that extracellular signals such as neurotransmitters, hormones, drugs, and growth factors exert their most significant control. The transcription is modulated by transcription factors that recruit the RNA polymerases to the DNA. For example, the critical nuclear translocation step in the activation of transcription factor CREB involves the catalytic subunit of PKA, which can phosphorylate CREB on entering the nucleus. In addition, increasing evidence indicates that at least some forms of long-term memory require new gene expression.
Genes that encode the transcription factors themselves may respond quickly or slowly. These genes have been coined third messengers in signal transduction cascades. Cross talk between neurotransmitter and growth factor-signaling pathways is likely to play an important role in the precise tuning of neuronal plasticity to diverse environmental stimuli.
The active, mature transcription complex is a remarkable architectural assembly of RNA polymerase II, transcription factors, and adaptors assembled at the basal promoter. Cells can exert exquisite control of the genes being transcribed in a variety of situations—for example, to govern appropriate entry or exit from the cell cycle, to maintain appropriate cellular identity, and to respond appropriately to extracellular signals.
Transcription can be regulated by many different extracellular signals modulated by a large array of signaling pathways (many including reversible phosphorylation) and a complex array of typically dimeric transcription factors. In this chapter, regulation has been illustrated by only a few of the families of transcription factors. Those chosen appear to play important roles in the nervous system and illustrate many of the basic principles of gene regulation.
References
1. Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Acta Biochimica et Biophysica. 2009;1793:933–940.
2. Birnbaumer L. Expansion of signal transduction by G proteins The second 15 years or so: From 3 to 16 α subunits plus βγ dimers. Acta Biochimica et Biophysica. 2007;1768:772–793.
3. Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993;72:65–75.
4. Carafoli E, Klee C. Calcium as a Cellular Regulator New York: Oxford University Press; 1999.
5. Chain DG, Casadio A, Schacher S, et al. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron. 1999;22:147–156.
6. Chao LH, Stratton MH, Lee I-H, et al. A Mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin-dependent kinase II holoenzyme. Cell. 2011;146:732–745.
7. Clapham DE, Neer EJ. New roles for G-protein βγ-dimers in transmembrane signalling. Nature. 1993;365:403–406.
8. Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030.
9. Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annual Review of Biochemistry. 2002;71:473–510.
10. Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992;9:187–195.
11. Hunter T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236.
12. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134.
13. Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic plasticity and behavioural memory. Nature Review Neuroscience. 2002;3:175–190.
14. Logue JS, Scott JD. Organizing signal transduction through A-kinase anchoring proteins (AKAPs). The FEBS Journal. 2010;277:4370–4375.
15. Mansuy IM, Shenolikar S. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends in Neuroscience. 2006;29:679–686.
16. McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda). 2009;24:8–16.
17. Newton AC. Protein kinase C: Poised to signal. American Journal of Physiology Endocrinology and Metabolism. 2010;298:E395–402.
18. Opazo P, Labrecque S, Tigaret CM, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252.
19. Sacktor TC. How does PKMζ maintain long-term memory?. Nature Review Neuroscience. 2011;12:9–15.
20. Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. Journal of Neurochemistry. 2011;116:1–9.
21. Sen N, Snyder SH. Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends in Neuroscience. 2010;33:493–502.
22. Stryer L. Biochemistry 4th ed. New York: Freeman; 1995.
23. Stryer L, Bourne HR. G proteins: A family of signal transducers. Annual Review of Cell Biology. 1986;2:391–419.
24. Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: An integrator of neurotransmission. Annual Review of Pharmacology and Toxicology. 2004;44:269–296.
25. Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annual Review of Neuroscience. 1994;17:551–567.
26. Taussig R, Gilman AG. Mammalian membrane-bound adenylyl cyclases. Journal of Biological Chemistry. 1995;270:1–4.
27. Tjian R, Maniatis T. Transcription activation: A complex puzzle with few easy pieces. Cell. 1994;77:5–8.
28. Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Silva A, Soderling TR. Calmodulin-kinases: Modulators of neuronal development and plasticity. Neuron. 2008;59:914–931.
Suggested Readings
1. Coba MP, Pocklington AJ, Collins MO, et al. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Science signaling. 2009;2 ra19.
2. Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030.
3. Hagenston AM, Bading H. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harbor Perspectives in Biology 2011; http://dx.doi.org/10.1101/cshperspect.a004564.
4. Rellos P, Pike ACW, Niesen FH, et al. Structure of the CaMKIIδ/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biology. 2010;8:e1000426.
5. Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Molecular Cell. 2009;33:537–545.