Chapter 32
Eye Movements
As we learned in Chapter 26, the photoreceptor mosaic of the vertebrate retina transduces light energy in the form of photons into neural activity, ultimately in the form of action potentials. The spatial resolution of this transduction system is limited by the mosaic of photoreceptors and the optics of the eye, but only if the eye is held stationary with respect to the objects of interest in the external world. Thus, stabilizing the eyes with regard to the outside world and aiming the eyes toward moving or stationary targets are critical challenges to effective vision. Evolutionary pressures have shaped the eye movement systems of animals to meet this challenge in ways that are tailored to the visual structures and behavioral needs of each species. In this chapter we examine the behavioral and neural systems used by vertebrates to control eye movements in the support of vision.
As we shall see, the control of eye movements provides a window into several fundamental aspects of neuroscience. Because the eyes are a relatively simple mechanical system, eye movements provide an excellent system for investigating the neural mechanisms of motor control, and also offer some of the clearest views of other complex processes, such as sensory-motor interactions, and the higher-order control of behavior.
Eye Movements are Used to Stabilize Gaze or to Redirect Gaze
Similar to the handling of a camera, the images that fall on the retina depend on how the eyes are held and moved. The orientation of the eyes in the head and the orientation and position of the head in space together determine the gaze direction (i.e., gaze = eye + head) and, consequently, control the retinal image. These spatial arrangements underlie the two general classes of eye movements in vertebrates. One class is responsible for stabilizing gaze. When animals move with respect to their surroundings, they risk degrading their visual acuity, because moving the head necessarily moves the eyes, and can cause images to streak across the retina. Gaze stabilization mechanisms have evolved to solve this problem. They maintain visual acuity during self-motion by stabilizing the retinal image of the world with rotations of the eyes that exactly compensate for head and body movements. The neural mechanisms for gaze stabilization are highly conserved across vertebrates, reflecting the widespread need to stabilize visual inputs despite other sensory and motor differences between species. The second class is responsible for redirecting gaze. When animals visually inspect their surroundings, they may use these eye movements to aim the line of sight at objects or features of particular interest, even when those items move. In contrast to stabilizing gaze, the process of redirecting gaze involves selectively sampling the animal’s visual environment, because tracking one visual object often causes the retinal images of other objects to become blurred. Accordingly, the mechanisms for redirecting gaze are found only in animals that have retinal specializations, such as the primate fovea, that can be used to examine a limited region of visual space at a higher spatial resolution.
Stabilizing Gaze
There are two types of mechanisms for stabilizing gaze (Fig. 32.1): the vestibulo-ocular reflex (VOR) and the optokinetic response (OKR). The VOR uses signals from the vestibular labyrinth to counterrotate the eyeballs to keep retinal images stable during head movements. The rotational VOR is driven by head-rotation signals from the semicircular canals and represents a phylogenetically old reflex present in all vertebrate species. The fast transduction properties of the vestibular periphery, combined with a direct 3-neuron pathway, give the rotational VOR a very short latency (<10 ms). However, because the canals do not accurately transduce constant velocity head rotations, the output of the VOR decays over several seconds. Some vertebrates also possess a translational VOR, which is driven by head-movement signals from the otoliths. Unlike the image motion caused by head rotations, which is uniform across the visual field and can be fully compensated for by counterrotating the eyes, the image motion caused by translations are complex and can be only partly eliminated by eye movements. This explains why the translational VOR is found only in animals with central retinal specializations (e.g., foveal vision).
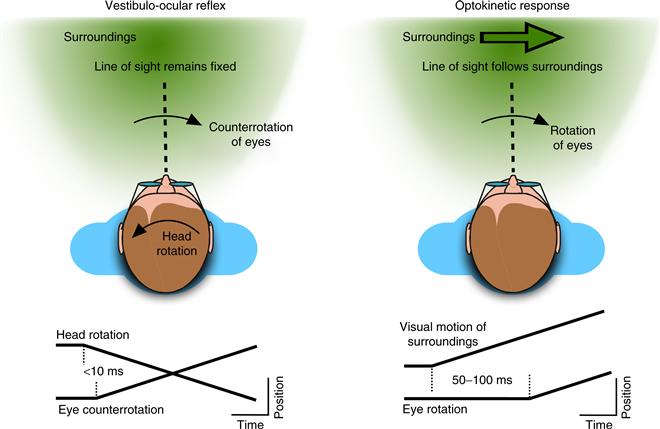
Figure 32.1 Eye movements that stabilize gaze. The vestibulo-ocular reflex keeps the line of sight fixed in the world by counterrotating the eyes during movements of the head. Here, the eyes rotate rightward at a short latency after the beginning of the leftward head movement. The optokinetic response stabilizes the line of sight with respect to the moving visual surround, but does so after a longer latency.
The optokinetic response (OKR) is driven by visual signals about the en masse movement of images across the retina. Because of the slow transduction of visual signals, the OKR has a longer latency (50–100 ms) than the VOR, but the use of visual motion signals makes it able to compensate for the constant motions and low speeds that the VOR cannot. The properties of the OKR therefore complement those of the VOR in maintaining stable gaze during movements, and the visual signals used by the OKR play a major role in guiding adaptive changes in the VOR. Animals with foveal vision also possess an ocular following response that is driven at very short latencies (~50 ms) by shifts in the retinal image, and that serves as the visual complement to the translational VOR (Miles, 1997).
Redirecting Gaze
Animals with foveal vision also possess several mechanisms for redirecting gaze (Fig. 32.2). Saccadic eye movements act to quickly move the image of a visual target from an eccentric retinal location to the center of the retina where it can be seen best. Saccades are very fast movements, reaching peak speeds greater than 500 deg/s and lasting only tens of milliseconds. Consequently, although saccades are often triggered by visual stimuli (at typical latencies of 150–250 ms), the trajectory of saccades is not guided by visual feedback, but instead follows a stereotyped profile that is largely determined by the size of the movement. Saccades do not require a visual stimulus, but can be guided by other modalities, and can even be directed toward the location of imagined or remembered targets.
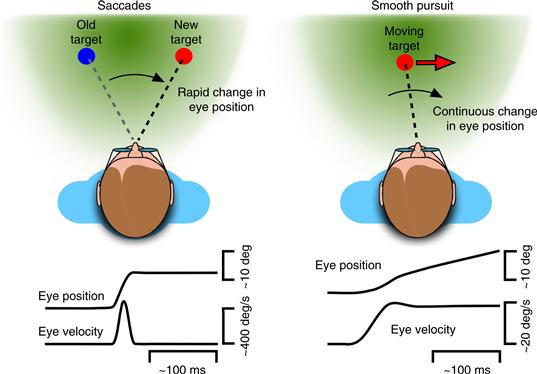
Figure 32.2 Eye movements that redirect gaze. Saccades change the line of sight to place the retinal image of visual targets onto the fovea. They are characterized by rapid changes in eye position (upward deflection in eye position trace) involving very high eye velocities (brief pulse in eye velocity trace). Smooth pursuit continuously changes the line of sight to minimize blurring of the target’s retinal image. These movements are characterized by smooth and continuous changes in eye position (ramp in eye position trace) involving lower eye velocities (smooth step in eye velocity trace).
Smooth pursuit eye movements slowly rotate the eyes to compensate for any motion of the visual target and thus act to minimize the blurring of the target’s retinal image that would otherwise occur. Smooth pursuit is a relatively slow movement whose trajectory is determined by the moving stimulus. Pursuit is triggered by visual stimuli at latencies similar to, but somewhat shorter than, the latencies of saccades (typically 100–200 ms), and generates eye velocities up to ~50 deg/s, continuously adjusted by visual feedback about the target’s retinal image. Unlike saccades, pursuit cannot be generated in the absence of a stimulus, although moving targets that are sensed through modalities other than vision can also guide pursuit.
Saccades and smooth pursuit produce conjugate, or yoked, movements of the two eyes. These are also referred to as versional eye movements. In contrast, a third type of mechanism for redirecting gaze, called vergence eye movements, produces disconjugate movements; the two eyes move in opposite directions or by different amounts. Vergence eye movements act to change the depth at which the eyes’ lines of sight meet. When we look at a near object our eyes converge (rotate inward), and when we look at a far object, our eyes diverge (rotate outward). Before redirecting our gaze to an object at a different distance, the pair of images produced by that object will be spatially mismatched between the two eyes, causing binocular disparity. Also, because the curvatures of the two eyes’ lenses are set to focus the currently viewed object, the image of the new object will also be blurred. These two signals, binocular disparity and blur, drive the combination of vergence eye movements and accommodation (which changes the dioptric power of the lenses by contracting or relaxing the ciliary muscle) that bring the new object into focus on the fovea of both eyes. Vergence and accommodation are tightly linked and are often referred to together as the near response. When evoked by themselves (e.g., by binocular disparity), vergence movements are very slow, taking up to 1 second to converge or diverge the eyes. However, when they occur in combination with faster versional eye movements such as saccades, vergence movements speed up, and interestingly, so does accommodation.
Fixation and Fixational Eye Movements
In between gaze movements, the eyes are actively held steady by a fixation mechanism. By preventing the eyes from redirecting gaze to another visual target, fixation makes it possible to visually inspect a particular object at length. Periods of fixation typically last about 200 ms but vary depending on what the animal is doing. Fixation does not necessarily act to keep the eyes from moving, but to hold the eyes steady with respect to the environment. For example, gaze stabilization mechanisms such as the VOR are active during periods of fixation.
Even during periods of steady fixation of a stationary object, the eyes are not completely still. The eyes typically make tiny movements during fixation in seemingly random directions that cause very slight changes in how images fall on the retinal mosaic but without displacing images from the fovea. Microsaccades are very small saccades with amplitudes typically less than 0.1 degree of visual angle, and are interspersed with slow drifts (speeds less than ¼ degree per second) of similar amplitude. The function of these movements remains unclear. However, like other larger saccades, they can prevent the adaptation of retinal signals that would occur with perfectly stabilized images, they are influenced by shifts of attention, and they can be strategically targeted or suppressed depending on the task.
Box 32.1 Eye Movements and Reading
During reading, saccadic eye movements bring successive snippets of text images to the center of the retina. The amplitude of the saccades depends on the letter size—a typical reader makes saccades that shift the line of sight by about 8 character spaces. In common situations—for example, viewing 12-point font at a distance of about 18 inches—each saccade is therefore only a couple of degrees in amplitude. The majority of saccades (~85%) form a forward path along the line of text (e.g., left to right for English readers), but a substantial minority of saccades (~15%), referred to as regressions, direct the line of sight back to text that has already been foveated.
The periods of fixation between saccades are crucial for reading comprehension, and typically last only about one-fifth of a second (~200 ms). Fixation provides the high-acuity input about individual letters—for example, telling the difference between “o” and “c”—that is necessary to correctly identify words. The number of letters that can be processed during each fixation (the perceptual span) is a key factor in determining reading proficiency. In skilled readers, the perceptual span stretches ahead of the currently fixated point, covering about 14 letters forward and only about 4 letters backward. Letters at the center of the perceptual span are processed for meaning and identifying words; letters at the edges of the perceptual span appear to be important for parsing the text and perhaps determining the landing points for upcoming saccades.
The skillful deployment of saccades and fixation for reading requires a great deal of practice, and represents an important developmental milestone for visual-motor control and cognition. In typical individuals, as text becomes harder to read, the amplitudes of saccades decrease, the number of regressions increase, and the duration of fixations increase. In reading disorders, such as dyslexia, these patterns are exaggerated, and accompanied by other unusual scan path features, such as targeting the beginning rather than the middle of words. This situation poses a chicken-or-the-egg problem: are reading disorders caused by a problem with controlling eye movements, or are eye movement problems caused by the reading disorder?
The Mechanics of Moving the Eyes
Each Eye Is Controlled by Three Pairs of Muscles
Moving the eye involves rotating the globe of the eye in the orbital socket of the skull. Because the eye undergoes minimal translation during movement, it can be viewed as a spherical joint with an orientation defined by three axes of rotation (horizontal, vertical, and torsional). Correspondingly, the orientation and movement of each eye is accomplished by three complementary pairs of extraocular muscles (Fig. 32.3). The lateral and medial rectus muscles form an antagonistic pair that controls the horizontal position of the eye. Contraction of the lateral rectus (and commensurate relaxation of the medial rectus) causes the eye to abduct, or rotate outward. Conversely, contraction of the medial rectus (and relaxation of the lateral rectus) causes the eye to adduct, or rotate inward. The actions of the other two pairs of muscles are more complex. When the eye is centered in the orbit, the primary effect of the superior and inferior recti is to elevate (rotate up) or depress (rotate down) the eye. However, when the eye is deviated horizontally in the orbit, these muscles also contribute to torsion, the rotation of the eye around the line of site that determines the orientation of images on the retina. The primary effect of the superior and inferior obliques is intortion (the top of the eye rotates toward the nose) or extortion (the top of the eye rotates away from the nose), but depending on the horizontal deviation of the eye, these muscles may also help determine the vertical orientation of the eye. Eye movements therefore involve redefining the 3-dimensional orientation of the eye by changing the balance of forces applied to the globe by all three pairs of extraocular muscles.
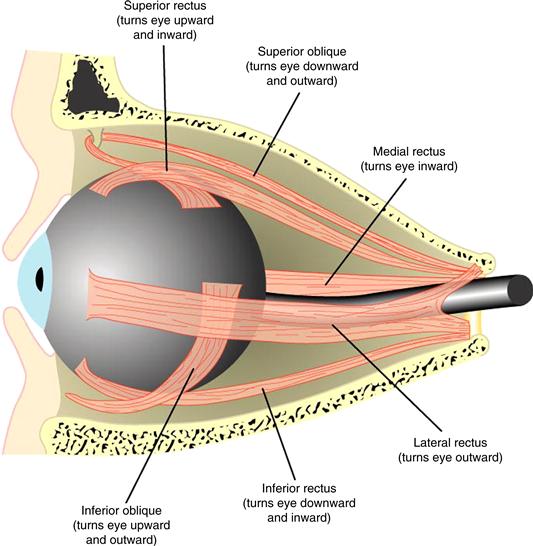
Figure 32.3 Muscles of the eye. Eye movements are controlled by six extraocular muscles arranged in three pairs, shown here in a cutaway view of the eye in its socket, or orbit.
The mechanics of moving the eye may seem simple, but actually provide a computationally complex challenge for the brain. Because the eye is free to rotate in 3-dimensions, the final orientation of the eye depends on the order in which the rotations are carried out—for example, “turn-then-spin” gives a different outcome than “spin-then-turn.” This property is referred to as noncommutativity. In fact, when the eye moves, the final 3-dimensional orientation of the eye and the axis of rotation used to get there are not arbitrary, but form an orderly set. Donder’s law states that the orientation of the eye is always the same when the eye is aimed in a particular direction. Listing’s law specifies these orientations by stating that the axes used to rotate the eye are confined to a single plane, referred to as Listing’s plane. These orderly relationships are achieved, at least in part, by a mechanism that involves the muscles themselves (Klier, Meng, & Angelaki, 2011). The extraocular muscles consist of two distinct layers: global fibers, which attach directly to the globe of the eye, and orbital fibers, which terminate in connective tissue that ensheathes the body of the muscle and acts like a moveable pulley for the global fibers. Because the pulleys themselves move when the muscle is contracted or relaxed, the pulling direction of the extraocular muscles changes with eye position. These gaze-dependent mechanical changes in muscle action largely account for Listing’s law (Demer, 2006).
Interestingly, Listing’s law is obeyed during saccades and smooth pursuit, but is violated during the VOR and the OKR. This difference presumably stems from the different inputs for these movements. Saccades and smooth pursuit may follow Listing’s law because they involve local 2-dimensional retinal inputs that do not uniquely specify the orientation of the eye. In contrast, the VOR is driven by 3-dimensional inputs from the semicircular canals for which the extra degree of freedom is not redundant. Thus, the mechanics of the eye muscles impose kinematic constraints that are helpful during visually guided eye movements, but these constraints can be altered or lifted by neural control signals during gaze stabilization.
Central Control of the Extraocular Muscles
The six extraocular muscles are innervated by three of the bilaterally paired cranial nerves (Fig. 32.4). The oculomotor nerve (cranial nerve III) innervates the medial rectus, the superior and inferior rectus, and the inferior oblique on one side of the head. The cell bodies for these motor neurons thus lie in the third cranial nerve nucleus. The oculomotor nerve also contains the fibers that control accommodation of the lens. The trochlear nerve (cranial nerve IV) innervates the superior oblique muscle, and the abducens nerve (cranial nerve VI) innervates the lateral rectus. These three pairs of nuclei, distributed through the brain stem, contain all of the oculomotor motor neurons and are heavily interconnected by a pathway called the medial longitudinal fasciculus (mlf). This interconnection is crucial for the precise coordination of the extraocular muscles that is necessary for the control of eye movements.
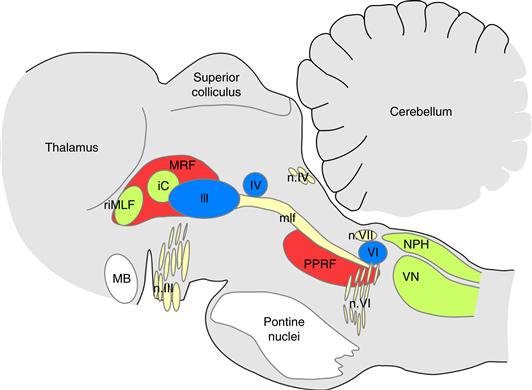
Figure 32.4 Oculomotor nuclei in the brainstem. Parasagittal section through the brainstem, cerebellum, and thalamus of a rhesus monkey, showing the location of the major brainstem nuclei involved in the control of eye movements. Motor neurons for the eye muscles are located in the oculomotor nucleus (III), trochlear nucleus (IV), and abducens nucleus (VI), and reach the extraocular muscles via the corresponding nerves (n. III, n. IV, n. VI). Premotor neurons for controlling eye movements are located in the paramedian pontine reticular formation (PPRF), the mesencephalic reticular formation (MRF), rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), the interstitial nucleus of Cajal (IC), the vestibular nuclei (VN), and the nucleus prepositus hypoglossi (NPH). The medial longitudinal fasciculus (mlf) is a major fiber tract containing the axons of these neurons. Other abbreviation: MB, mammillary body of the hypothalamus.
The firing rates of the oculomotor motor neurons determine the forces applied by the extraocular muscles and, consequently, whether the eye moves or is held in place (Fuchs & Luschei, 1970). When the eye is stationary, the firing rate of oculomotor motor neurons is proportional to eye position, because under static conditions the balance of forces exerted on the globe determines the orientation of the eye (Fig. 32.5). Each motor neuron has a recruitment point (an eye position at which the motor neuron begins to fire) and a characteristic slope (the change in firing rate as the eye rotates in the pulling direction of the relevant muscle). Thus, as in the skeletal system, oculomotor motor neurons have a fixed recruitment order. When the eye moves in the pulling direction of the motor unit, oculomotor motor neurons exhibit a high-frequency pulse of activity that drives the high-velocity phase of the eye movement. The high-frequency pulse is followed by a sustained step-like increase in firing rate appropriate for the new static position of the eye. When the eye moves in the opposite direction, the motor neurons pause their firing during the movement and then resume their activity at a lower sustained level.
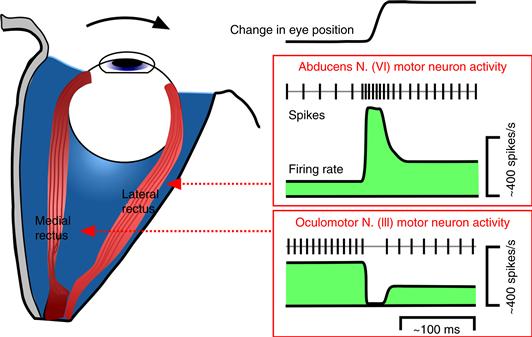
Figure 32.5 The firing rate patterns of eye movement motor neurons during horizontal eye movements. The rapid rightward change in eye position, indicated by the upward deflection in the eye position trace, involves contraction of the right lateral rectus and relaxation of the right medial rectus. This is accomplished by a pulse-step increase in activity of the agonist motor neurons in the abducens nucleus, and a pause-step decrease in activity of the antagonist motor neurons in the oculomotor nucleus.
The neural commands from these motor neurons therefore take the form of a pulse-step during rapid eye movements such as saccades. The amplitude and duration of the pulse determine the speed and duration of the movement, and the amplitude of the step determines the final static eye position. The need for the pulse derives from the sluggish dynamics of the eye and orbital tissues, which is dominated by the viscosity of the extraocular muscles. If the eye were moved by only a step change in force, it would take nearly a second for the eye to settle at its new orientation, which would obviously be incompatible with the goal of supporting vision. Similarly, during slow eye movements such as smooth pursuit, the neural commands from the motor neurons consist of a ramp-like change in activity to drive the smooth changes in eye position, plus an additional increment to overcome the sluggish dynamics of the eye. These patterns of activity are precisely adjusted by central mechanisms involving the brainstem and cerebellum, and illustrate a general problem in motor control—the need to convert desired actions into appropriately formed motor commands. The solution appears to involve the use of internal models that allow central mechanisms to take into account the dynamic properties of the body part or object to be moved.
Oculomotor motor units are also unusual in several ways (Buttner-Ennever, 2007). Unlike skeletal motor units, eye motor neurons participate equally in all types of eye movements, regardless of the speed involved. The extraocular muscles contain muscle spindles, but no Golgi tendon organs, and there are no ocular stretch reflexes. Instead, eye proprioception appears to mainly come from palisade endings on the eye muscle tendons. The function of this input is unclear, but it is distributed via the trigeminal nucleus to many key structures in the oculomotor system (including the vestibular nuclei, cerebellum, superior colliculus, and frontal eye fields) and may play a role in the long-term adaptive control of eye movements.
The Fundamental Circuits for Stabilizing Gaze
The Vestibulo-Ocular Reflex (VOR) Stabilizes Gaze during Head Movements
The function of the VOR is to stabilize retinal images during head movements by using head-motion signals from the vestibular organs. A purely rotational VOR can be elicited in complete darkness by rotating the head roughly about its center (e.g., the interaural axis). Head rotations stimulate the semicircular canals of the inner ear (Chapter 29), which provide the sensory signals about head motion that drive this highly conserved mechanism for counterrotating the eyes. During sustained rotations of the head, the eyes cannot continue to counterrotate endlessly in the orbit, but are intermittently and rapidly reset to a central position during the ongoing compensatory eye rotation. The profile of eye position over time during the VOR therefore exhibits a characteristic sawtooth pattern consisting of the slow-phase counterrotations and quick-phase resetting eye movements. This pattern is called nystagmus, and is described by the direction of the quick phases (Fig. 32.6 is an example of right-beat nystagmus).
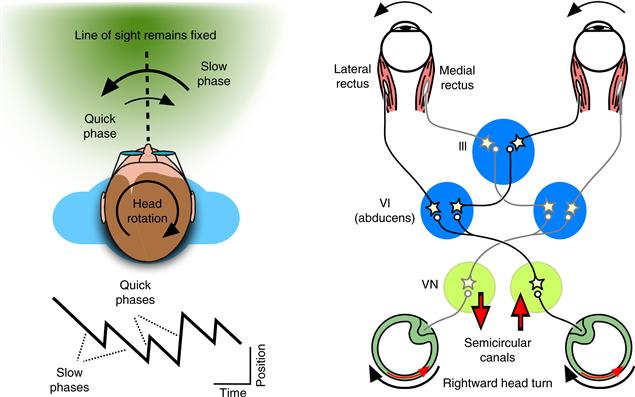
Figure 32.6 The eye movements and neural circuits for the VOR. During a sustained rotation of the head (here, rightward), the eyes rotate slowly to the left (downward deflections in eye position trace labeled “slow phases”), and then abruptly shift rightward to reset the eyes toward a central position (upward deflections in eye position trace labeled “quick phases”). This is an example of right-beat nystagmus. The diagram of the basic VOR circuit shows the push-pull organization of the horizontal VOR. When the head rotates rightward, activity increases in the right semicircular canal, right vestibular nucleus (VN), and left abducens nucleus (VI). This leads to increased activation of the left eye lateral rectus and, through a crossed projection to the oculomotor nucleus (III), increased activation of the right eye medial rectus. The complementary set of pathways (colored in gray) show decreases in activity.
The speed of the slow phases during nystagmus indicates how effectively the VOR is compensating for the head rotation. The degree of compensation is often characterized by the VOR gain, which is defined as the ratio of slow-phase eye speed over the speed of head rotation. Perfect compensation occurs when the eye rotates in the opposite direction at exactly the same speed as the head rotation, which corresponds to a gain of 1. The ability of the VOR to compensate for head motion is limited by the dynamics of the signals from the semicircular canals, which act as “high-pass” sensors of head velocity. Thus, the VOR is very effective (its gain is near 1) for fast back-and-forth head rotations that take a second or less to complete (i.e., frequencies over 1 Hz), but it is much less effective for slow head rotations that continue over many seconds (e.g., 0.1 Hz). Under natural conditions, this limitation of the VOR does not pose a problem for vision, because at these lower speeds visual signals can be used to stabilize gaze very effectively through the optokinetic response.
The fundamental neural mechanism for the VOR consists of brainstem circuits linking the vestibular afferents to the extraocular muscles, including a direct 3-neuron arc pathway (Fig. 32.6). For example, during a rightward head rotation vestibular afferents from the horizontal semicircular canal on the right side increase their firing rates, and this increase is passed on to neurons in the vestibular nucleus (VN) on the same side. These vestibular nucleus neurons, in turn, increase the activity of motor neurons and interneurons in the abducens nucleus (VI) on the opposite (left) side. Motor neurons in the abducens innervate the lateral rectus of the left eye, completing the 3-neuron arc. Interneurons in the abducens provide a crossed projection back to the right oculomotor nucleus, where motor neurons innervate the medial rectus of the right eye. By contracting both the left lateral rectus and the right medial rectus, the two eyes rotate leftward together in response to the rightward head rotation. In addition, because the vestibular afferents on the left side decrease their activity during a rightward rotation, a complementary circuit causes relaxation of the right lateral rectus and the left medial rectus. The VOR is therefore controlled in a push-pull fashion that yokes the movements of the two eyes through the bilateral symmetry of these basic circuits.
These direct pathways are key components of the VOR mechanism, but the VOR also requires indirect pathways to function correctly. As discussed in the previous section, the firing rate of oculomotor motor neurons is primarily related to eye position, but the afferent signals from the semicircular canals are primarily related to the head velocity. In order to construct the motor commands needed to hold, as well as move, the eyes during the VOR, the brain needs to transform the dynamic signals provided by the vestibular afferents into the combination of dynamic and static commands found on motor neurons. Similar to the operation of mathematical integration in calculus, this process involves the temporal integration of velocity inputs into position signals.
The neural integrator for eye movements is accomplished by neurons in the vestibular nuclei and two other brainstem nuclei—the nucleus prepositus hypoglossi (for horizontal components) and the interstitial nucleus of Cajal (for vertical)—and is fine-tuned by connections between these brainstem nuclei and the vestibulocerebellum (Cannon & Robinson, 1987). The output from the neural integrator pathway sums with the signals from the direct pathway to provide the motor neurons with the complete set of velocity and position commands needed to accomplish the VOR. Importantly, all conjugate eye movements also use this same indirect integrator pathway. Thus, although the source of the velocity signals for the VOR, OKR, saccades, and pursuit are quite different, they appear to share a common brainstem mechanism for transforming these signals into the final motor commands.
The Optokinetic Response (OKR) Uses Visual Inputs to Stabilize Gaze
The OKR also functions to stabilize retinal images during head movements, but uses visual inputs to infer the direction and speed of head motion rather than responding directly to head-velocity signals. The optimal stimulus for the OKR is full-field motion of the visual environment that, like head rotations, causes a saw-tooth pattern of eye movements referred to as optokinetic nystagmus. Like the VOR, the slow phase movements during nystagmus compensate for the head rotation that presumably caused the full-field motion. However, because the OKR is driven by visual signals, which are processed much more slowly than vestibular signals, the range of motions that are effectively compensated by the OKR is quite different from the VOR. The OKR is very effective (its gain is near 1) for slow rotations that continue over many seconds, but it is much less effective for very fast back-and-forth rotations. These properties of the OKR therefore complement those of the VOR and together, the two types of mechanisms can effectively stabilize gaze over a much wider range of speeds and behavioral conditions than either could achieve alone.
The interlocking nature of the two systems is also evident in the neural pathways for the OKR, despite the difference in their input signals. In vertebrate species without foveal vision, such as the rabbit, the visual processing for the OKR is accomplished entirely within the brainstem. Direction-selective retinal ganglion cells project directly to a set of visual nuclei in the midbrain called the pretectum and accessory optic nuclei. In species with foveal vision, the visual properties of these nuclei are determined not only by inputs from the retina, but are also shaped by inputs from visual areas of the cerebral cortex. Neurons in the accessory optic nuclei have very large receptive fields and respond selectively to global visual motion that would result from rotating the head about a particular spatial axis. Remarkably, the selectivity of individual neurons is restricted to one of the three axes of rotation that are also detected by the semicircular canals. This common rotational coordinate system makes it easier to combine visual reafferent signals about head motion from the accessory optic nuclei with the vestibular afferent signals provided by the labyrinths. Accordingly, neurons in the pretectum and accessory optic nuclei project to the vestibular nuclei and nucleus prepositus hypoglossi, where these visual signals join the same final pathways used by the VOR. These nuclei also project to the vestibulocerebellum, where the visual signals contribute to the velocity commands for the OKR and other smooth eye movements, and also act as feedback signals that play a critical role in the long-term adjustment of the VOR.
The Commands for Redirecting Gaze are Formed in the Brainstem
The commands for redirecting gaze use the same final motor pathways that are used for stabilizing gaze, but the signals and mechanisms giving rise to the movement commands are quite different. For smooth pursuit, they are based on motion signals about the visual target that are first extracted in specialized areas of the cerebral cortex and then used to commandeer the brainstem and cerebellar pathways for the OKR. For saccades, which are not guided by visual feedback, a specialized circuit in the brainstem reticular formation produces the stereotyped velocity command that determines the trajectory of the saccade.
The Motor Commands for Saccades Are Constructed in the Brainstem Reticular Formation
The control signals for saccades descending from higher centers specify the location of the target, which is then transformed by circuits in the brainstem reticular formation into the motor commands for saccades (Fig. 32.7). The horizontal and vertical components of saccades are formed by separate but interconnected circuits—the horizontal commands are formed in the paramedian pontine reticular formation (PPRF), and the vertical commands are formed in a part of the mesencephalic reticular formation called the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF).
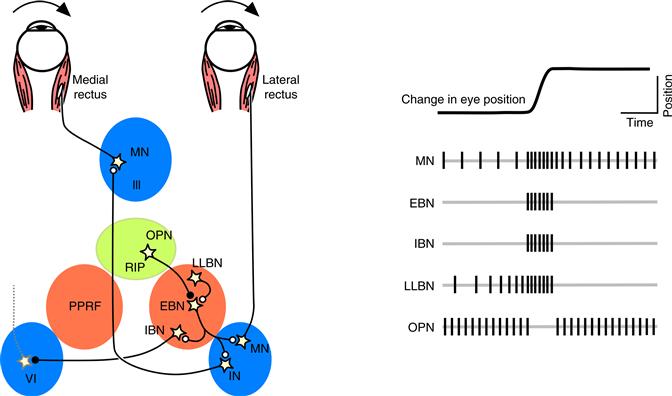
Figure 32.7 The brainstem circuits for controlling saccadic eye movements. This diagram of the saccade burst generator outlines the major classes of neurons involved in constructing the motor command for horizontal saccades. Omnipause neurons (OPN) located near the midline in the nucleus raphe interpositus (RIP) tonically inhibit excitatory burst neurons (EBN) located in the paramedian pontine reticular formation (PPRF). When OPNs pause, the EBNs emit a burst of spikes, which activate motor neurons (MN) in the abducens nucleus (VI) innervating the lateral rectus muscle. The burst also activates interneurons (IN) which, in turn, activate motor neurons on the oculomotor nucleus (III) on the opposite side, innervating the medial rectus. Inhibitory burst neurons (IBN) show a pattern of activity similar to EBNs, but provide inhibitory inputs to decrease activation in the complementary circuits and antagonist muscles. Long-lead burst neurons (LLBN) show activity long before movement onset and provide an excitatory input to EBNs.
Within these brainstem circuits, often referred to as the saccadic burst generator, several different classes of neurons work together to construct the saccade motor command (Keller, 1974). Burst neurons fire a high-frequency volley of action potentials that begins ~10 ms before the onset of the saccade and ends slightly before the saccade lands on the target. The firing rate during the burst is related to the instantaneous velocity of the saccade, and the number of spikes in the burst is related to the amplitude of the saccade. There are several types of burst neurons. Excitatory burst neurons (EBN), sometimes called “medium-lead burst neurons,” provide the velocity signal for the eye muscles that will contract during the saccade, and specify the “pulse” portion of the saccade motor command. The EBNs provide a copy of this velocity signal to the brainstem nuclei of the neural integrator, which then generates the “step” portion of the motor command, just like they do for other eye movements. EBNs also contact inhibitory burst neurons (IBN), which provide a crossed projection that inhibits motor neurons for the antagonist eye muscles that will relax during the saccade. Finally, long-lead burst neurons (LLBN) show changes in their activity well in advance of the saccade, presumably reflecting inputs received from higher centers, and provide an excitatory input to the EBNs.
The other major class of neurons in the saccadic burst generator is the omnipause neurons (OPN). These neurons fire at a constant high rate (~100 spikes/s) during fixation, but completely pause their activity just before and during saccades in all directions. Because the OPNs directly inhibit burst neurons, their tonic activity prevents unwanted saccades during fixation, and the pause in their activity unleashes the synchronized bursts of action potentials that initiate and drive the saccade. The onset of the pause in OPN activity appears to be triggered by inhibitory signals originating from higher brain centers, but the duration of the pause does not determine when the saccade ends. Instead, stopping saccades involves another inhibitory influence on burst neurons. One likely candidate is the oculomotor cerebellum (lobules VI and VII of the vermis and the caudal fastigial nucleus). The oculomotor vermis is interconnected with the saccadic burst generator and plays a crucial role in adjusting the metrics of saccades so that the eyes land accurately on the target at the end of the gaze movement.
The Motor Commands for Pursuit Are Formed in the Brainstem and Cerebellum
The control signals for pursuit descending from higher centers continuously specify the motion of the target to be followed with the eyes. Thus, in contrast to saccades, pursuit does not require motor circuitry to construct the velocity command from scratch. Instead, the pursuit pathways in the brainstem and cerebellum sculpt the descending control signals to form an appropriately shaped velocity command, and provide a copy of this velocity signal to the neural integrator to generate the position component of the motor command.
The velocity commands for pursuit arise in areas of the cerebral cortex and reach the final motor circuits through several routes (Fig. 32.8). One pair of pathways involves projections from the cerebral cortex to the cerebellum via a large set of relay neurons on the floor of the brainstem called the basal pontine nuclei. Areas in extrastriate visual cortex provide signals related to visual motion through the dorsolateral pontine nuclei to a portion of the vestibulocerebellum called the ventral paraflocculus. The frontal cortex provides signals related to target and eye velocity through the dorsomedial pontine nuclei, and the adjacent nucleus reticularis tegmenti pontis, to the oculomotor cerebellum (vermis), the same portion of the cerebellum involved in adjusting the metrics of saccades. Both of these cerebellar regions provide inputs to parts of the brainstem motor pathways that we have already discussed. The output of the ventral paraflocculus is closely related to eye velocity and terminates in the vestibular nuclei, where it contributes to the velocity command for pursuit, and also provides an input to the neural integrator. The output of the oculomotor vermis is less well defined but appears to be related to eye acceleration as well as eye velocity. The outputs of the vermis and the associated deep cerebellar nucleus (the caudal fastigial nucleus) are sent to neurons in the brainstem reticular formation, and may play a role in shaping the velocity command appropriately when the target motion changes.
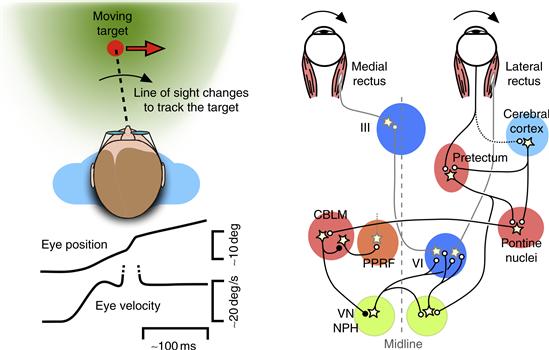
Figure 32.8 The brainstem and cerebellar circuits for controlling pursuit eye movements. During pursuit, the line of sight changes smoothly to follow the motion of the target. The smooth changes in eye velocity are often accompanied by small “corrective” saccades, indicated by the upward deflection in the eye velocity trace (dotted lines). The diagram outlines the circuits involved in constructing the motor command for horizontal pursuit. The major pathways involve projections from areas of the cerebral cortex via the pontine nuclei to the cerebellum (CBLM), which then modulates activity in nuclei associated with the VOR—namely, the vestibular nuclei (VN) and nucleus prepositus hypoglossi (NPH)—and also modulates activity in nuclei associated with saccades, such as the paramedian pontine reticular formation (PPRF). A second pathway involves projections from the pretectum to the pontine nuclei, as well as a direct projection to the vestibular nuclei and prepositus hypoglossi.
Another route for conveying motion signals for pursuit involves the pretectum. As described above, the pretectum plays a key role in the OKR, but in primates the pretectum receives inputs from areas of the cerebral cortex that are important for pursuit. Accordingly, some neurons in the primate pretectum have smaller receptive fields and respond well to the small moving stimuli typically used to elicit pursuit. The pretectum provides an input to the cerebellum through the basal pontine nuclei, but also projects directly to the vestibular nuclei and nucleus prepositus hypoglossi, offering a relatively direct route to the final motor pathways.
The brainstem control of pursuit also appears to involve some of the same neurons that are part of the saccadic burst generator. The EBNs and IBNs are not involved in forming the pursuit motor command, but some of the long-lead burst neurons in the brainstem reticular formation increase their tonic firing during pursuit, and show a buildup of activity before the onset of pursuit and saccades. Even more surprisingly, OPNs—the “gatekeepers” for saccades—are also modulated during pursuit. They do not completely pause their activity as they do during saccades, but decrease their activity by about one-third. Moreover, electrical stimulation applied to the OPNs slows down pursuit eye movements, in addition to stopping saccades (Missal & Keller, 2002). The function of this overlap between saccades and pursuit in these premotor circuits is not yet fully understood, but one possibility is that descending information about the target does not directly trigger saccades and pursuit, but instead a motor decision process at the level of the brainstem and cerebellum determines which type or combination of movements is most appropriate under the particular circumstances.
Box 32.2 Eye Movement Disorders
Disorders of eye movements arise from damage to widespread regions of the brain, and the symptoms reflect the contributions of the affected structures to eye motor control.
One common disorder of eye motor control is strabismus, which occurs when the movements of the two eyes are not properly yoked. This disorder typically occurs in children or infants (about 2% of children are affected), but can also affect adults. Strabismus is caused by deficits in any of the several factors that are necessary to properly align the eyes, including problems with eye muscles or oculomotor nerves, the presence of a large refractive error in one or both eyes, and lesions in central structures. If strabismus occurs during infancy, while connections in the visual system are developing, it can result in amblyopia, in which case visual inputs from the affected eye are mostly ignored.
Many eye movement disorders are caused by tumors, stroke, or other damage to the pathways responsible for forming the eye motor commands. Patients with damage to structures in the brainstem or cerebellum often have difficulty maintaining fixation, and exhibit nystagmus—a pattern of involuntary drifts in eye position, interspersed with centering saccades. Nystagmus can be caused by peripheral damage to the vestibular labyrinth or the eighth cranial nerve that transmits signals about head motion to the central nervous system. The imbalance in signals received from the left and right vestibular organs causes a slow drift of the eyes, away from the damaged side. If the nystagmus is caused by peripheral damage, then voluntary saccades and smooth pursuit are mostly unaffected. However, nystagmus can also be caused by central damage to brainstem or cerebellar structures. In these cases, voluntary saccades and smooth pursuit are also impaired, because the functions of the neural integrator and final motor pathways are affected.
Because gaze movements are controlled by descending signals from the cerebral cortex and basal ganglia, eye movement disorders also occur in diseases that affect higher-order brain functions, including schizophrenia, Huntington’s disease, and Parkinson’s disease. These disorders are characterized by problems with voluntary eye movements, most notably a difficulty in suppressing unwanted saccades during fixation or smooth pursuit eye movements. In Huntington’s disease, in addition to difficulty with suppressing unwanted saccades during fixation, patients also have difficulty initiating voluntary saccades. This problem can be partly overcome by blinking at the same time, because the circuits for controlling eye blinks and saccades overlap. In schizophrenia, smooth pursuit eye movements do not correctly match the speed of the moving target, and are interrupted by back-and-forth saccades that repeatedly take the eyes off the target. For reasons that are not yet understood, these deficits in smooth pursuit ability also occur in relatives of schizophrenics who are themselves asymptomatic for the disease.
Gaze Movements are Controlled by the Midbrain and Forebrain
The motor commands for gaze movements formed in the brainstem depend on control signals descending from higher centers, in particular, from the superior colliculus (SC) and several eye-movement related areas of the cerebral cortex (Fig. 32.9). These brain regions make it possible to bring the well-honed motor circuits in the brainstem under the control of higher-order processes such as attention, perception, and cognition.
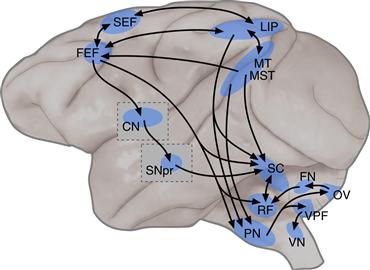
Figure 32.9 The descending pathways for controlling gaze movements, depicted schematically on a lateral view of the monkey brain. The superior colliculus (SC), located on the roof of the midbrain, is a major source of descending control signals. In the cerebral cortex, the major areas involved are the frontal eye fields (FEF), supplementary eye fields (SEF), lateral intraparietal area (LIP), middle temporal area (MT), and medial superior temporal area (MST). In the basal ganglia, a cascade through the caudate nucleus (CN) and substantia nigra pars reticulata (SNpr) provides inhibitory control over activity in the superior colliculus. These descending control signals are then converted into motor commands by circuits involving regions such as the reticular formation (RF), pontine nuclei (PN), vestibular nuclei (VN), and parts of the cerebellum such as the ventral paraflocculus (VPF), oculomotor vermis (OV), and fastigial nucleus (FN).
The Superior Colliculus Contains a Retinotopic Map for Controlling Gaze
The superior colliculus (SC) is a multilayered structure lying on the roof of the midbrain. In nonmammals, the homologous structure is called the optic tectum, which serves as the primary center for processing retinal inputs and for transforming sensory inputs into commands for orienting movements. In mammals, the SC has a similar functional role, and consists of superficial layers that receive direct inputs from the retina and striate cortex, and intermediate and deep layers that receive inputs from extrastriate, parietal and frontal cortex, and the basal ganglia. Neurons in the superficial layers have responses to visual stimuli that are enhanced when the stimulus is the target of a saccade. Neurons in the intermediate and deep layers may also respond to visual stimuli, and also to auditory stimuli from corresponding locations, so that the visual and auditory maps of the animal’s surroundings are in register. It is these intermediate and deep layers that also possess the movement-related activity that plays a crucial role in the control of gaze movements (Wurtz & Goldberg, 1972). These movement-related layers provide a major descending projection to the oculomotor nuclei in the brainstem reticular formation, and also a major ascending projection to the frontal cortex through the medial dorsal nucleus of the thalamus.
The most distinctive feature of the superior colliculus is that it contains a retinotopic map of contralateral visual space and this topography applies to the movement-related activity in the deeper layers as well as to the visual activity in the superficial layers (Fig. 32.10). The significance of this retinotopic map becomes evident when neural activity is artificially manipulated by passing small amounts of electrical current through the tip of an electrode placed in the intermediate layers (Robinson, 1972). Such stimulation elicits a saccadic eye movement with an amplitude and direction that depends on the placement of the electrode in the map. If the stimulation is applied as a long train, rather than as a brief pulse, a series of saccades is elicited, forming a “staircase” of eye movements, each with the same characteristic amplitude and direction. Thus, activity in the superior colliculus map is related to changes in eye position and not to absolute eye position, consistent with the organization of the superior colliculus in retinotopic (or oculocentric) coordinates.
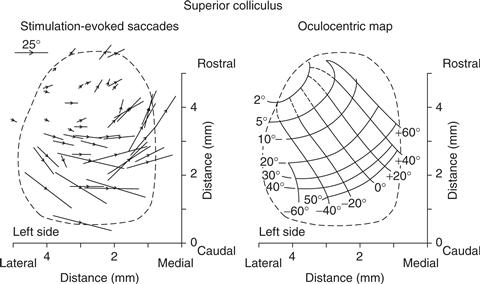
Figure 32.10 The superior colliculus and its retinotopic (or oculocentric) map of saccades and target positions. The left plot shows that electrical stimulation in the superior colliculus causes eye movements whose direction and amplitude depend on the location. Each arrow corresponds to a stimulation site in the left superior colliculus, and the length and direction of the arrow indicate the metrics of the evoked saccade. The evoked saccades are all directed rightward, but rostral sites are associated with smaller movements than caudal sites, and medial sites are associated with upward movements whereas lateral sites are associated with downward movements. The right plot summarizes these results in the form of a continuous map of saccades and target positions. As in the visual cortex, the representation of the center of the visual field is amplified.
Adapted from Robinson (1972).
During naturally occurring eye movements, activity in the superior colliculus is not restricted to just the few neurons that best match the endpoint of the saccade, but is distributed across a large number of neurons across the retinotopic map. This point was elegantly demonstrated by testing what happens to saccades when the injection of chemical agents into the superior colliculus temporarily inactivates neurons at a particular location in the map (Lee, Rohrer, & Sparks, 1988). Remarkably, it is still possible to make saccades directed to the center of the inactivated region, because the broad distribution of activity across the map leaves a halo of neurons that can still accurately guide the saccade. Moreover, saccades directed to locations slightly beyond the inactivated site become hypermetric (bigger than normal), because the inactivation removes the contribution from neurons that code for more proximal locations and smaller saccades. These results show that saccades are guided by the vector average of population activity in the superior colliculus, and were the first demonstration of vector averaging in a mammalian motor system.
The intermediate layers of the superior colliculus contain two main classes of neurons related to the control of gaze (Munoz & Wurtz, 1995). Burst neurons in the superior colliculus fire a volley of action potentials that begins ~20 ms before the onset of saccades, somewhat earlier than the burst neurons located downstream in the reticular formation, and often also have a visual response to the onset of visual targets. Unlike the burst neurons in the reticular formation, those in the superior colliculus fire for saccades directed to a portion of the visual field referred to as the neuron’s movement field, and the size of the burst depends on where the target falls with respect to the neuron’s movement field. Buildup neurons also have visual responses and saccade-related activity, but they are distinguished by the presence of sustained activity when the subject is required to delay their eye movement response after the presentation of a visual stimulus. This sustained activity typically increases over time as the time of the saccade draws near, and is believed to be important for the preparation of saccades, perhaps by facilitating the occurrence of the saccade-related burst. Recent results show that the activity of neurons in these layers of the superior colliculus is not only involved in motor preparation, but is also involved in the antecedent step of selecting the visual target, and is part of the neural circuits that control visual attention.
The retinotopic map in the superior colliculus includes the central visual field. Like the rest of the map, this part also contains neurons that fire during saccades, although the preferred saccades are smaller, as would be expected from the topography, and even include microsaccades (Hafed, Goffart, & Krauzlis, 2009). However, these same neurons can also show tonic activity during fixation and changes in firing rate for pursuit eye movements. These neurons therefore appear to represent the foveal region of a single “priority” map extending across the superior colliculus that supports multiple eye motor outputs.
The Frontal Eye Field Is the Primary Cortical Area for Controlling Gaze Movements
The frontal eye field (FEF) exerts its control on gaze movements through several descending pathways, including a direct projection to the intermediate layers of the superior colliculus, an indirect projection to the superior colliculus via the basal ganglia, and also projections to the cerebellum and the brainstem reticular formation. The FEF is best known for its role in the generation of saccades, but it also contains zones that are involved in the control of pursuit and vergence eye movements. Like the superior colliculus, electrical stimulation of the FEF causes saccades to a particular location in the contrateral visual hemifield, although the topography in the FEF is less orderly than in the superior colliculus. Electrical stimulation of the pursuit zone of the FEF causes smooth eye movements towards the ipsilateral side, and is the only cortical area where stimulation can directly evoke smooth eye movements. Damage to the FEF produces deficits in saccades, and the impairments are most noticeable when the target stimulus is accompanied by irrelevant distracter stimuli. For pursuit, disruption of the FEF reduces pursuit eye velocity and, in particular, affects the ability of pursuit to follow targets using prediction. The impairments after FEF damage are usually temporary, but combined damage to the FEF and SC results in a permanent deficit in the ability to generate voluntary gaze movements (Schiller, True, & Conway, 1980).
The FEF contains neurons with different combinations of motor and visual activity. Movement-related neurons have movement fields like those found in the superior colliculus; they are active for saccades within a particular range of directions and amplitudes. Movement-related neurons project to the superior colliculus and show changes in activity that are related with when and if voluntary gaze movements will be initiated. In particular, the variability in the timing of saccade initiation is closely correlated with when the firing rates of these neurons reach a fairly constant “threshold” value (Hanes & Schall, 1996). In the pursuit zone of the FEF, neurons exhibit directionally selective responses to moving visual stimuli that are appropriate for determining the velocity command for pursuit eye movements.
Most neurons in the FEF also have visual responses, including a large class of visuo-movement neurons that show activity for both visual stimuli and the eye movements that follow. These visually responsive neurons often show activity related to the selection of the target. Prior to the gaze movement, the activity evoked by the nonselected stimulus is rapidly suppressed, whereas the activity for the selected stimulus remains conspicuous. Some FEF neurons discriminate visual targets even in the complete absence of an eye movement response, indicating that their activity is related to the allocation of visual attention rather than to the preparation of eye movements.
Another region of frontal cortex, the supplementary eye field (SEF), plays a less direct role in the control of saccades but is important for movements that are guided by cognitive factors. Neurons in the SEF are preferentially modulated in tasks that involve more abstract instructions, such as when saccades are directed to a particular side of an object rather than to a spatial location, or when saccades are directed to the location opposite the visual stimulus (the “anti-saccade” task). During pursuit eye movements, SEF neurons are especially active when the target moves along a predictable trajectory.
The Parietal Cortex Contributes to Eye Movements by Controlling Visual Attention
The lateral intraparietal area (LIP) plays a major role in the process of visual selection that precedes the generation of gaze movements. Reversible inactivation of neurons in area LIP does not cause large deficits in the timing or accuracy of gaze movements, but it dramatically reduces the frequency of movements toward the affected portions of the visual field, especially when competing stimuli are present elsewhere (Wardak, Olivier, & Duhamel, 2002). These deficits suggest a visual neglect of stimuli in the affected region, rather than a motor deficit in the generation of eye movements. Damage to the parietal cortex in humans, especially to the right hemisphere, also produces a neglect syndrome with similar deficits in attention.
Neurons in LIP show activity related to visual attention and to the intention to redirect gaze to particular locations in visual space. When an attention-grabbing, but irrelevant, distracter stimulus appears while the animal is preparing a saccade directed elsewhere, the activity of LIP neurons closely tracks the short-lived shift of attention to the distracter and its subsequent return to the intended location of the saccade. Activity in LIP is therefore often described as a salience map that helps to determine the location of spatial attention, as well as the endpoint of gaze movements (Bisley & Goldberg, 2010).
Motion-Processing Areas of Cortex Provide Crucial Signals for Pursuit Eye Movements
The middle temporal area (MT) and the medial superior temporal area (MST) are the primary sources of visual motion information that are used to construct the motor commands for pursuit. This motion information is also used to adjust the size of saccades made to moving visual targets. These cortical areas provide these inputs through several pathways, including projections to other cortical areas involved in controlling gaze movements (in particular, the FEF), descending projections to the vestibulocerebellum via the basal pontine nuclei, and projections to the pretectum, which contains neurons with activity related to the OKR and pursuit.
Neurons in MT and MST have activity related to the velocity of visual stimuli. In MT, neurons have smaller receptive fields and activity that depends on the presence of a visual stimulus. Lesions in MT produce blind spots for seeing motion that affect the motor commands for pursuit and saccades, and the perception of motion as well. In MST, neurons have larger receptive fields and prefer more complex patterns of visual motion. Lesions in MST also produce blind spots for motion, but in addition, cause a directional deficit in pursuit for movements directed to the ipsilateral side (Dürsteler & Wurtz, 1988). Stimulation of MT and MST can affect pursuit eye movements, but only if pursuit is already ongoing, unlike the effects of stimulation in the FEF. Thus, MT and MST are crucial sources of the motion signals needed to drive pursuit eye movements, but other areas appear necessary to determine when and if the movement should be started.
The Basal Ganglia Regulate Saccades by Inhibiting the Superior Colliculus
The caudate nucleus (CN) and the substantia nigra pars reticulata (SNpr) are parts of the basal ganglia that form an important circuit for controlling when gaze movements are initiated. The SNpr applies tonic inhibition to the superior colliculus using GABA-ergic synapses, suppressing the response to the many excitatory inputs to the superior colliculus that might otherwise evoke gaze movements. When SNpr neurons reduce or pause their activity, there is a disinhibition of activity in the superior colliculus, which then permits gaze movements to be initiated. The SNpr itself is under inhibitory control from the CN, which contains neurons that increase their activity with gaze movements. Together, this series of inhibitory connections through the basal ganglia acts to gate the generation of gaze movements by the superior colliculus.
The basal ganglia appear to be especially important for gaze movements involving reward and motivation (Hikosaka, Nakamura, & Nakahara, 2006). For example, the visual responses of CN neurons are larger for stimuli that are associated with larger rewards. This modulation in excitability may be caused by dopaminergic inputs from neurons in the substantia nigra pars compacta (SNpc), the same neurons whose degeneration is implicated in Parkinson’s disease.
The Thalamus Provides Feedback about the Control of Gaze Movements
In addition to these descending pathways, there are also ascending pathways to eye movement-related areas of cortex from nuclei in the central thalamus. These nuclei receive inputs from a wide range of oculomotor structures, including the superior colliculus, substantia nigra pars reticulata, cerebellum, the nucleus prepositus hypoglossi, and the brainstem reticular formation. Feedback through the thalamus appears to convey corollary discharge signals about the gaze movements that are executed by downstream motor structures, and is used to update the control signals formed in the cortex. Thus, when the central thalamus is inactivated by chemical agents, single saccades can still be made accurately, even in the dark, but subsequent saccades are spatially inaccurate, as though the intervening movement had not been taken into account (Sommer & Wurtz, 2002).
The Control of Gaze Movements Involves Higher-Order Processes
Saccades and Pursuit Are Coordinated during Redirecting of Gaze
The functions of saccades and pursuit are distinct, and yet during normal gaze movements the two types of movements are tightly coordinated and almost always select the same visual target. For example, when subjects are presented with two moving stimuli simultaneously, they almost always begin smooth pursuit in a direction that is a weighted average of the two motions. After a brief period of this “vector average” pursuit, subjects make a saccade toward one stimulus or the other and then pursue that one selectively. However, pursuit can selectively follow one stimulus in the presence of distracters without a targeting saccade, showing that saccades are not strictly necessary for pursuit target selection. Pursuit and saccades also exhibit nearly the same tradeoff between speed and accuracy, with saccades tending to be delayed and somewhat more accurate. This suggests that target selection for the two movements is guided by the same decision signals, but saccades use a somewhat more stringent decision criterion (Krauzlis, 2004).
Voluntary Gaze Movements Are Tightly Linked to Shifts of Visual Attention
A crucial aspect of voluntary gaze movements is that they are selectively guided by objects of interest to the observer, despite the fact that our surroundings usually contain many other distracting signals. This selectivity is achieved by a tight functional link between the control of gaze movements and the mechanisms of visual attention. When gaze is redirected, the movement of the eyes is always preceded by a shift of visual attention to the new location. For example, when subjects are asked to detect visual targets that appear just before a saccade is made, performance is much better when the target and saccade locations coincide than when they do not (Deubel & Schneider, 1996). Similarly during pursuit, subjects make more accurate perceptual judgments about the stimulus they are smoothly tracking with their eyes than about other, nontracked stimuli in the visual display. However, judgments about nontracked stimuli are still better than chance, showing that the selection of targets for gaze movements can occur in parallel with the perceptual processing of other visual stimuli. Also, although redirecting gaze typically requires visual attention, the converse is not true: visual attention can shift to new locations in the absence of gaze movements, and these are referred to as covert shifts of attention.
The tight linkage between visual attention and the planning of eye movements was a major rationale for the premotor theory of attention, which posited that the mechanisms for spatial attention and eye motor programming are essentially the same. Although it is generally recognized that the neural mechanisms for attention and eye movements are not identical, there is overlap in their control. Specifically, stimulation within the FEF with currents too weak to directly cause eye movements can nonetheless enhance the visual responses of neurons located in other visual areas of cortex, and can improve the behavioral performance on visual discrimination tasks (Moore & Fallah, 2004). Also, reversible inactivation of the SC causes profound deficits in the performance of spatial attention tasks, even in the absence of targeting eye movements (Lovejoy & Krauzlis, 2010).
Visual Perception and Cognition Contribute to the Control of Gaze Movements
Much of what we know about the neural control of gaze movements was learned using very simple visual, often small bright spots in an otherwise dark room. These studies made it possible to test and identify the basic feedback mechanisms and motor circuits that control gaze. However, under more natural conditions eye movements show properties that are more complex and flexible. One striking set of examples is provided by the work of Yarbus (1967), who showed that the pattern of saccades made while looking at visual scenes depends heavily on the instructions given to the observer (Fig. 32.11). These results illustrate that gaze movements are normally not just reactions to retinal events, but instead are guided by longer-term goals and serve to collect relevant visual information from the environment.

Figure 32.11 The scan path depends on the goals of the observer. Among other experiments, Yarbus (1967) monitored the eye movements of subjects as they viewed Repin’s painting The Unexpected Visitor and found that the pattern of eye movements depended on the task. When subjects were instructed to give the ages of the people, the scan path mostly settled on the faces, whereas when subjects were instructed to surmise what the family had been doing, the scan path included many more objects in the room.
Gaze movements also reveal properties of the visual processing steps required to segment and interpret the visual scene. In one demonstration, a few lights are attached to the rim of a wagon wheel, which is then rolled along in an otherwise dark display. Although the retinal stimulus consists of several spots each undergoing cycloidal motion, subjects perceive the horizontal motion of the wheel and are able to easily track this motion with their eyes (Steinbach, 1976). More recent studies have examined how these processes evolve over time. For many moving objects, the true trajectory of the object cannot be calculated locally in the retinal image, but requires integrating information over regions of visual space. This integration evidently takes some time (for example, ~100 ms), and the transition from local-motion signals to object-motion signals can be found in both the eye motor output and perceptual judgments.
Visual perception is also changed during eye movements. In particular, the world is not perceived as moving during saccadic eye movements, despite the rapid motion of the retinal image. You can demonstrate this to yourself by watching yourself make saccades in a mirror: you can see that your eyes change position, but you cannot see them actually move. This perception of a stable world is achieved through a combination of mechanisms, including the use of a corollary discharge of the outgoing eye motor command, saccadic suppression of visual signals during the eye movement, and visual masking by the advent of new visual signals on the retina (Wurtz, 2008). Together, these mechanisms help the visual system reduce its sensitivity to the unreliable visual signals that occur during gaze movements.
Conclusions
Eye movements serve two different functions. First, eye movements stabilize gaze when animals move about in their environment. Unpredictable high-speed rotations of the head, which are produced whenever an animal moves, change the line of gaze and, by smearing the optical image, reduce the resolution of the visual system. These high-velocity movements are compensated for by counterrotations of the eyes generated by the VOR. Working in tandem with the VOR is the optokinetic system. The optokinetic system compensates for the slower movements of the head, which happen so gradually that the vestibular system cannot accurately detect them. Second, in animals with retinas that have a small region of high resolution, eye movements redirect gaze to aim the line of sight at objects or features in the visual scene that are of particular interest. Saccades are fast, discrete movements that quickly move the image of a visual target to the center of the retina where acuity is best. Pursuit is a slow, continuous movement that compensates for the motion of the visual target, minimizing the blurring that would otherwise limit acuity. Although the properties of saccades and pursuit are distinct, the two types of movements are closely coordinated during visual search of the environment and both are tightly linked to visual attention. Together, the different types of eye movements make it possible to efficiently gather the visual information that is important for achieving other, longer-term behavioral goals.
Eye movements are controlled by circuits that span the brain. The eyes themselves are a relatively simple motor apparatus, and they are controlled by a final motor pathway in the brainstem that is shared by all classes of eye movements. The construction of the motor commands involves generating an eye velocity command to move the eyes, and an integrated version of this command to hold the eyes at their new position. These motor commands are constructed by circuits in the brainstem reticular formation, and fine-tuned by additional circuits involving several parts of the cerebellum. The descending control signals that drive these motor commands are provided by multiple brain regions, but the superior colliculus in the midbrain and the frontal eye fields in the cortex are especially important. These brain regions help integrate sensory inputs with a variety of higher-order influences so that eye movements are directed to the appropriate visual stimuli. Overall, eye movements provide a window into the brain mechanisms that accomplish motor control, and they also involve sensory-motor interactions and higher-order processes like attention and perception.
References
1. Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annual Review of Neuroscience. 2010;33:1–21.
2. Buttner-Ennever JA. Anatomy of the oculomotor system. Developments in Ophthalmology. 2007;40:1–14.
3. Cannon SC, Robinson DA. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. Journal of Neurophysiology. 1987;57:1383–1409.
4. Demer JL. Current concepts of mechanical and neural factors in ocular motility. Current Opinion in Neurology. 2006;19:4–13.
5. Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Research. 1996;36:1827–1837.
6. Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. Journal of Neurophysiology. 1988;60:940–965.
7. Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. Journal of Neurophysiology. 1970;33:382–392.
8. Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science. 2009;323:940–943.
9. Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430.
10. Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. Journal of Neurophysiology. 2006;95:567–584.
11. Keller EL. Participation of medial pontine reticular formation in eye movement generation in monkey. Journal of Neurophysiology. 1974;37:316–332.
12. Klier EM, Meng H, Angelaki DE. Revealing the kinematics of the oculomotor plant with tertiary eye positions and ocular counterroll. Journal of Neurophysiology. 2011;105:640–649.
13. Krauzlis RJ. Recasting the smooth pursuit eye movement system. Journal of Neurophysiology. 2004;91:591–603.
14. Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature. 1988;332:357–360.
15. Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nature Neuroscience. 2010;13:261–266.
16. Miles FA. Visual stabilization of the eyes in primates. Current Opinion in Neurology. 1997;7:867–871.
17. Missal M, Keller EL. Common inhibitory mechanism for saccades and smooth-pursuit eye movements. Journal of Neurophysiology. 2002;88:1880–1892.
18. Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. Journal of Neurophysiology. 2004;91:152–162.
19. Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus I Characteristics of burst and buildup cells. Journal of Neurophysiology. 1995;73:2313–2333.
20. Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Research. 1972;12:1795–1808.
21. Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye field and superior colliculus ablations. Journal of Neurophysiology. 1980;44:1175–1189.
22. Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482.
23. Steinbach M. Pursuing the perceptual rather than the retinal stimulus. Vision Research. 1976;16:1371–1376.
24. Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. Journal of Neuroscience. 2002;22:9877–9884.
25. Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey: III Cells discharging before eye movements. Journal of Neurophysiology. 1972;35:575–586.
26. Wurtz RH. Neuronal mechanisms of visual stability. Vision Research. 2008;48:2070–2089.
27. Yarbus AL. Eye movements and vision New York: Plenum; 1967.
Suggested Readings
1. Angelaki DE, Hess BJM. Self-motion-induced eye movements: effects on visual acuity and navigation. Nature Reviews Neuroscience. 2005;6:966–976.
2. Fuchs AF, Kaneko CRS, Scudder CA. Brainstem control of saccadic eye movements. Annual Review of Neuroscience. 1985;8:307–337.
3. Gandhi N, Katnani HA. Motor functions of the superior colliculus. Annual Review of Neuroscience. 2011;34:205–231.
4. Kowler E. Eye movements: the past 25 years. Vision Research. 2011;51:1457–1483.
5. Krauzlis RJ. The control of voluntary eye movements: new perspectives. Neuroscientist. 2005;11:124–137.
6. Leigh RJ, Zee DS. The neurology of eye movements 4th ed. Philadelphia, PA: F.A. Davis; 2006.
7. Lisberger SG. Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron. 2010;66:477–491.
8. Purcell BA, Heitz RP, Cohen JY, Schall JD, Logan GD, Palmeri TJ. Neurally constrained modeling of perceptual decision making. Psychological Review. 2010;117:1113–1143.
9. Schutz AC, Braun DI, Gegenfurtner KR. Eye movements and perception: a selective review. Journal of Vision. 2011;11:1–30.
10. Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annual Review of Neuroscience. 2008;31:317–338.
11. Sparks DL. The brainstem control of saccadic eye movements. Nature Reviews Neuroscience. 2002;3:952–964.