Chapter 29
Control of Movement
Four major pathways descend from the brain to the spinal cord to control muscles that move the skeleton. These pathways arise in the vestibular nuclei (vestibulospinal), in the brainstem reticular formation (reticulospinal), in the red nucleus (rubrospinal), and in the cerebral cortex (corticospinal). While these four descending pathways work together to provide seamless control of movements from postural reflexes to delicate manipulation, they can be separated broadly into two main systems. Vestibulospinal and reticulospinal pathways can be grouped together as a medial system: their axons descend through the brainstem and spinal cord close to the midline, and chiefly innervate axial and proximal limb musculature in which motoneurons lie medially in the ventral horn. The corticospinal and rubrospinal pathways together can be considered a lateral system: their axons descend in the lateral column of the spinal cord and chiefly innervate limb musculature, particularly distal musculature, in which motoneurons lie laterally in the ventral horn. More than just an anatomical distinction, the medial and lateral systems have different principal functions. The medial system provides postural control. Monkeys with medial system lesions fall over when they attempt to ambulate and climb, but when supported, they can use their hands and fingers adeptly in retrieving food pieces from narrow holes (Lawrence & Kuypers, 1968b). The lateral system provides fine control of voluntary movement. Monkeys with lateral system lesions rapidly recover the use of all four extremities in activities such as ambulation and climbing, but they are unable to make the fine finger movements needed to extract small pieces of food from narrow holes (Lawrence & Kuypers, 1968a). This chapter examines the contributions of first the medial and then the lateral systems to control of movement.
The Medial Postural System
Vestibular and Reticular Nuclei Control Posture and Reflex Behaviors
Early experimenters found that some degree of postural and reflex control remains after higher brain centers are completely cut off from the brainstem and spinal cord, and that the extent of preserved motor function is determined by the level of the transection that disconnects the upper portions of the brain. There is no functional regeneration of central neuronal pathways after damage, and when the spinal cord is separated from all of the brain above, as can occur in motor vehicle or equestrian accidents, the result is quadriplegia, loss of all voluntary movement and postural control in the arms and legs. Only the spinal reflexes discussed in the preceding chapter return, and those return only when spinal cord neurons recover excitability after a prolonged period of limp (flaccid) paralysis called spinal shock.
Transections at higher levels of the neuraxis leave part of the brainstem connected with the spinal cord. Chronic bulbospinal cats, those with medulla and spinal cord below the transection, show primitive attempts at righting themselves when placed on one side. Effective righting may be observed in chronically surviving animals subjected to transection that leaves the mesencephalon intact below the level of the cut. As the level of transection is raised, posture and balance become progressively closer to normal. The decorticate animal produced by the ablation of cerebral cortical tissue has many apparently normal postural reactions, lacking only certain placing and stepping reactions. Progressively higher brain transections enable more of the vestibular (balance) and proprioceptive (muscle and position sense) circuitry that participates in postural control, as well as improving the overall balance between excitatory and inhibitory influences on the spinal cord.
Nuclei of the brainstem reticular formation with intact spinal projections after high brainstem transaction include the locus ceruleus and raphe nuclei, the origins of descending noradrenergic and serotonergic neuromodulatory axonal projections that alter excitability of neurons in the spinal cord, as discussed in the preceding chapter. Parts of the raphe nuclei also have a role in circadian rhythms (Chapter 39), and projections of the locus ceruleus are involved in attention (Chapter 46) and internal reward (Chapter 41). Other areas of the reticular formation involved in motor control have specific functions in generation of the locomotor rhythm (MLR, mesencephalic locomotor region, Chapter 27) and rapid eye movements (PPRF and MRF, Chapter 32). Nuclei of the reticular formation are most often studied from the perspective of their contributions to specific functional systems described in other chapters and through the actions of their monoamine neuromodulators noradrenalin (norepinephrine) and serotonin. Vestibular nuclei influence several brain regions and are studied for their contributions to spatial cognition, autonomic function, eye movements, and postural reflexes.
The Vestibular Apparatus Senses Head Rotation and Tilt
The sensory inputs most important for postural responses are visual, proprioceptive, and vestibular. Vestibular sensory signals from the labyrinth of the inner ear provide direct information about rotations of the head and its orientation with respect to gravity. Two types of vestibular transducer organs—semicircular canals and otolithic maculae—are located within the labyrinth of the ear and respond to accelerations of the head in space. The three semicircular canals and the two otolithic maculae are stimulated by rotational and linear acceleration, respectively. The mechanical structure of the sense organ determines the nature of the effective stimulus (Wilson & Melvill-Jones, 1979).
The labyrinth (Fig. 29.1) is outlined by the bony labyrinth, a set of passages in the skull. The bony labyrinth is lined with membranes that contain endolymph and which make up the membranous labyrinth. The membranous labyrinth has a large central vessel called the utricle, another vessel called the saccule, and three narrow passages that emerge from the utricle and loop around to rejoin it. These three loops form circular passages for endolymph and are known as semicircular canals. The canals are oriented orthogonal to one another: there is a lateral or horizontal canal, an anterior or superior canal, and a posterior canal. A semicircular canal is excited by rotational accelerations, and the mechanical structure of the canal converts acceleration into a rotational velocity signal. The three canals in each labyrinth are oriented for excitation by rotations toward that side of the body, horizontally, diagonally forward, and diagonally backward for the horizontal, anterior, and posterior canals, respectively. Thus, each labyrinth is excited by head rotation toward its side, called ipsilateral rotation. Canal afferents fire action potentials at a rate approximately proportional to the velocity of head rotation in the direction of the canal orientation. In contrast with the auditory system, in which 8th nerve afferents are narrowly tuned to specific frequencies of sound (see Chapter 25), vestibular nerve afferents are broadly tuned, responding to the entire range of head motion frequencies.
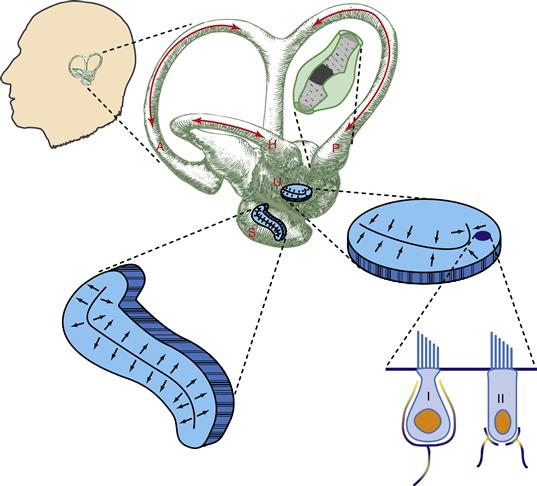
Figure 29.1 Vestibular canals and otoliths. The position and orientation of the labyrinth (not to scale) in the head are shown at the upper left of the figure. An enlarged view of the labyrinth shows the directions of head rotation and endolymph flow (red arrows) that excite each of the three semicircular canals. The horizontal orientation of the utricular macula and vertical orientation of the saccular macula are shown schematically. More highly magnified views of the receptor regions of a canal and of the otolith organs are shown with the cupula of the canal colored dark gray and the best directions for excitation of otolith hair cells marked by black arrows. At the lower right are two anatomical types of hair cell: the calyx or type I and bouton-ending or type II receptor. The tallest cilial extension on each cell is the kinocilium. A, anterior semicircular canal; H, horizontal semicircular canal; P, posterior semicircular canal; S, saccule containing saccular macula; U, utricule containing utricular macula; I, type I receptor; II, type II receptor. Based on studies reviewed by Wilson and Melvill-Jones (1979).
There are two otolith organs in mammals: the utricle (utriculus) and saccule (sacculus). The names apply to the fluid chambers in which they lie, and the specific locations of the receptors are called the maculae. The two maculae are small regions that contain hair cells innervated by vestibular afferents. Like auditory and semicircular canal hair cells, bending of their cilia is the excitatory stimulus for macular hair cells, and the cilia are imbedded in a gelatinous mass above the cell bodies. Unlike canal hair cells, the otolithic hair cell maculae rest in large chambers, in which fluid motion is thought to exert little or no force on the cilia. Instead, the gelatinous mass holding the cilia contains dense crystals, the otoconia or statoconia, and the entire mass above the hair cells of a macula is acted on by gravity or linear acceleration because of its higher density than the endolymph. The otoconial mass sags in the direction of a head tilt. This sagging, or the equivalent lagging of the mass behind a linear acceleration, provides the natural stimulus for otoliths.
The utricular macula lies horizontally on the floor of its cavity. When the head is still and held approximately erect with respect to gravity, there is no stimulus to this organ, but tilts from the erect posture will excite utricular afferents. Microscopic examination of the utricular macula shows that the hair cells are not all oriented with their cilia arranged in the same direction, and different directions of tilt excite different populations of utricular afferents. The direction of the hair cell, indicated by its structural orientation, corresponds to the best direction of tilt for exciting the receptor. Each utricular macula has hair cell orientations capable of responding to any direction of head tilt, but ipsilateral tilt excites a predominant number of afferents. Thus, as for the semicircular canals, we say that ipsilaterally directed head motion is excitatory. The saccular macula is placed vertically on the side of the saccule, approximately in a parasagittal plane. There is a strong acceleration stimulus to the saccular macula when the head is erect and little or no stimulus when the head is lying on either side. The sacculus responds vigorously when the head bobs up and down, as during locomotion. In summary, two types of vestibular signals are available to assist balance: ipsilaterally directed rotational head velocity is signaled by the semicircular canals and ipsilaterally directed head tilt or an equivalent linear acceleration is signaled by the utricular otoliths.
Vestibular Nuclei Convey Head Motion Signals to Many Brain Regions
Four brainstem vestibular nuclei receive inputs from the vestibular portion of the VIIIth nerve (Fig. 29.2). The medial, lateral (Deiter’s), and descending (inferior) nuclei contribute to the medial and lateral vestibulospinal tracts which provide direct vestibular influence on the spinal cord. Neurons in the superior and medial vestibular nuclei mediate vestibulo-ocular reflexes (Chapter 32). The medial vestibular nucleus also sends projections to brainstem autonomic nuclei and to the parabrachial nucleus, which integrates viscerosensory signals and has bidirectional connections with the amygdala. Ascending pathways from each of the vestibular nuclei project to thalamic nuclei, which influence widespread regions of the cortex.
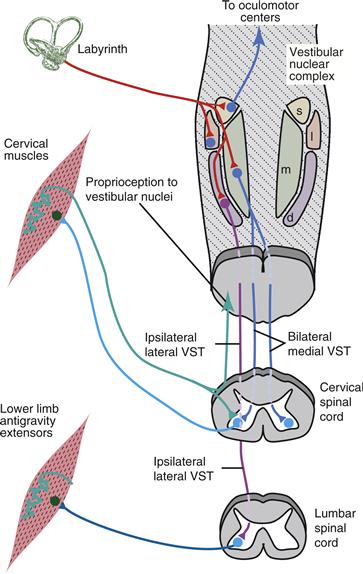
Figure 29.2 Vestibular and proprioceptive reflex signal inputs and major pathways from the brainstem vestibular nuclei. The medial vestibulospinal tract projects bilaterally to the cervical spinal cord to mediate the vestibulocollic reflex. The lateral vestibulospinal tract descends to lumbar levels of the spinal cord to influence limb extensors involved in balance. Neck muscle proprioceptors send signals to vestibular nuclei to participate in cervicocollic reflexes and interactions among reflexes. d, descending vestibular nucleus; 1, lateral vestibular nucleus; m, medial vestibular nucleus; s, superior vestibular nucleus; VST, vestibulospinal tract.
Neurons in the Vestibular System Are Specialized for Fast Behaviors
To effectively stabilize the body and eyes during self-motion, the vestibular system must rapidly convey head motion signals to motoneurons. The vestibular system is the fastest of all sensory systems; rapid head movements can evoke eye movements in less than 10 msec. Several mechanisms ensure that the central processing of head motion signals is both fast and accurate. Vestibular nerve afferents and central vestibular neurons fire tonically at high rates—about 50–100 Hz when the head is not moving—and modulate their firing rates in response to head movement. High firing rates enable head motion signals to be encoded both rapidly and bidirectionally; for example, vestibular nucleus neurons receiving inputs from horizontal canal afferents increase firing rates when the head turns to the same side (ipsiversively) and decrease firing rates when the head turns to the opposite side (contraversively). As with vestibular afferents, central vestibular nucleus neurons are broadly tuned for head movement frequencies and their firing rates are linearly modulated by head movement.
Central vestibular synapses and neurons are specialized for linear signaling over a wide range of firing rates. At well-studied synapses onto pyramidal cells in the hippocampus and cortex, synaptic efficacy is influenced strongly by the recent history of presynaptic activity (see Chapter 10). In contrast, across the behaviorally relevant range of afferent firing rates, central vestibular nerve synapses exhibit neither short-term depression nor short-term facilitation, enabling them to transmit signals that accurately reflect head motion (Bagnall, McElvain, Faulstich, & du Lac, 2008). Postsynaptic vestibular nucleus neurons fire spontaneously in the absence of synaptic stimulation, enabling rapid responses to synaptic inputs. They express sodium and potassium channels that enable them to sustain firing at high rates (see Chapter 31) as well as calcium-activated potassium channels which enable them to respond to synaptic inputs with linear changes in firing rates (Kolkman, McElvain, & du Lac, 2011). These synaptic and cell biological properties of vestibular neurons enable vestibulo-motor reflexes to respond rapidly and linearly to head motion.
Vestibulocervical and Vestibulospinal Reflexes Stabilize Head and Body Posture
Vestibulocervical (neck or collic reflexes) and vestibulospinal reflexes use canal and otolith signals to stabilize the posture of the head and body. These reflexes act as “negative feedback” systems (Schor, Kearney, & Dieringer, 1988). When the head and body rotate or tilt in any direction, the vestibular stimulus excites pathways that contract neck and limb muscles that oppose the motion so that the undesired movement is reduced or corrected. The importance of vestibulocervical and vestibulospinal reflexes is demonstrated by damage to the labyrinth or VIIIth cranial nerve. Unilateral labyrinthectomy causes an initial postural disability, with leaning or falling toward the side of the lesion. Bilaterally symmetric semicircular canal plugging, which removes head velocity signals with little loss or imbalance in tonic vestibular nerve activity, produces head instability with oscillations that may persist for several days.
Electrical activation of the vestibular pathways shows that vestibulocervical reflexes are mediated primarily by excitatory and inhibitory connections of the bilaterally projecting medial vestibulospinal tract and that ipsilateral excitation of leg antigravity muscles is carried via the lateral vestibulospinal tract. The bulk of the lateral vestibulospinal tract projects from the lateral vestibular nucleus to the lumbar spinal cord for vestibulospinal reflex control of upright body posture. Vestibular nuclei also project heavily to reticular nuclei, and only lesions that impinge on both vestibulospinal and reticulospinal pathways substantially blunt vestibular reflex responses.
The bilateral, mixed excitatory and inhibitory connections of the vestibulocervical reflex circuits indicate that its actions are complex, and considerable effort has been devoted to explicating the exact nature of these actions. The excitatory lateral vestibulospinal tract projection to ipsilateral extensor motoneurons offers a simple example of negative feedback: When the head and body tilt to one side, canals and otoliths of the labyrinth on that side are excited, and the lateral vestibulospinal tract carries this excitation to the ipsilateral leg extensor motoneurons. The ipsilateral leg is thus extended to oppose the tilt and maintain upright posture.
Initial quantitative studies of the vestibulocervical reflex used sinusoidally oscillating stimuli and concentrated on the timing of the reflex head torques or the electromyographic activity that accompanied neck muscle contractions The conclusion from these studies was that central vestibular circuits process low frequency (slow) head movements and high frequency (fast) movements in distinct ways. At low frequencies a portion of the velocity signal from the semicircular canals is converted to a head position signal appropriate for repositioning the head in response to a slow change in head angle. At high frequencies, however, vestibulocervical reflex circuitry introduces a “phase lead,” or anticipatory response, which works in conjunction with the canal afferent signal to produce movements with short response times. As such, the reflexive behavioral responses oppose head angular acceleration, as would be appropriate given that inertia of the head must be overcome for adequate compensation during rapid head motion.
The limbs and trunk represent a more complex mechanical system than the neck and head, complicating the analysis of vestibulo-spinal reflexes. Electromyographic recordings in decerebrated animals have shown that limb extensors at low frequencies of vestibular oscillation are excited in phase with head position. For example, when the head rolls slowly to the left, the left forelimb extends as if to brace against further displacement. The likely source of signals exciting these responses is the otolith organs. As the frequency of head oscillation increases, the timing of vestibulospinal responses corresponds more closely to head rotation velocity, indicating that semicircular canal signals begin to predominate over otolith signals in vestibulospinal responses at higher frequencies.
Body musculature could be coordinated to compensate for a particular direction of head and body rotation using a variety of strategies, but only general features of vestibulocervical and vestibulospinal synergies are known (Baker, Goldberg, & Peterson, 1985). Each of the major neck muscles responds to vestibular stimulation (rotation) in many different directions, specific for each neck muscle, that do not reflect the directional sensitivity of any single semicircular canal. Instead, the responses to rapid motions reflect a weighted sum of canal and otolith inputs. The appropriate postural response to a particular pattern of vestibular stimulation depends on many factors, including the position of the head, trunk, and limbs. The cerebellum is critical for the complex, context-dependent computations that mediate vestibulo-spinal reflexes (Chapter 31).
The Cervicocervical Reflex Stabilizes the Head by Opposing Lengthening of Neck Muscles
The proprioceptive contribution to reflex stabilization of the head and body has been examined in many of the same ways as vestibular contributions (Peterson, Goldberg, Bilotto, & Fuller, 1985). The use of proprioceptive signals is more complicated than for vestibular signals, as any of the muscles of the neck, trunk, or limbs could provide an input to influence any other muscle or synergistic group of muscles. The neck stretch or cervicocervical reflex has been studied most extensively. Like the stretch reflex for limb extensors, the cervicocervical reflex opposes lengthening of the muscles concerned and so is a negative feedback compensatory system.
The cervicocervical reflex has been analyzed by rotating the trunk of experimental animals while holding the head fixed in space. This stimulates neck proprioceptors without stimulating vestibular sense organs. Nevertheless, the results are reminiscent of the vestibulocervical reflex. At low frequencies of neck rotation cervicocervical reflex muscle contractions correspond to the extent of muscle stretch, but at higher frequencies the responses occur more rapidly, corresponding to the velocity or acceleration of muscle stretch. The directionality of neck muscle responses to stretch matches that of vestibulocervical responses fairly well. Common directionality implies that signals for vestibular and stretch reflexes to neck muscles share central circuitry.
The Brainstem Controls Coordinated Postural Reactions
A variety of reflexes contribute to the maintenance of overall body posture. The tonic neck or cervicospinal reflexes are the best known of these, but the supporting reactions, placing reactions, righting reactions, hopping or stepping reactions, and others are also useful parts of the neural control of posture. The tonic neck reflex adjusts the extension of the limbs in response to the angle of the head on the trunk. Roberts (1967) showed that stretching the neck by rolling the spine to one side elicited tonic flexion and extension of the forelimbs, as if the support surface had been tilted to produce the rotation. The placement of a limb on the ground initiates a set of reflex reactions that stiffen the limb into a supporting pillar. This response is called the “positive supporting reaction” and depends on the integrity of the brainstem. As discussed earlier, righting reactions also depend on the brainstem, and “optical righting reflexes” mediated by vision require an intact cerebral cortex. Stable posture requires that the feet be not only rigid but placed correctly on a supporting surface, and the placing reactions of quadrupeds contribute to this by moving the feet toward a visible surface (visual placing reaction) or onto a surface that has tactile contact with the top of the foot, chin, or whiskers (tactile placing reactions). Although a tactile placing reaction may be evoked in primitive form in spinalized animals, it is generally held that at least the lower brainstem must be intact for tactile placing to occur effectively. Finally, if balance reactions are insufficient to maintain stable posture, as when the support surface is moved beneath the foot, the limb may hop to a new position where stable posture is possible (hopping reaction). The action of the righting reflex of cats during falling is familiar to all. Humans show a similar (but less graceful) reaction to an unexpected drop, measurable as a short latency electromyographic response in the gastrocnemius muscle. In cats, this response survives blockage of the semicircular canals, but not total labyrinthectomy, and so appears to be mediated by otoliths.
Balance Depends on Context-Dependent Postural Strategies
An important tool for exploring body stability is the posture platform, a base on which subjects can stand and be subjected to displacements of the underlying supporting surface. The posture platform is used with body position sensors and electromyography to evaluate standing posture (Horak & Nashner, 1986; Horak & Shupert, 1994; Nashner, 1982). Even on a perfectly stable support surface with vision, somatosensory, and vestibular senses all active, a standing person will sway slightly. When the eyes are closed, sway is greater. When the visual surround is linked to the subject’s head position, visual information is misleading and sway is increased. When the support surface tilts along with the body, sway increases because proprioceptive information is misleading. The vestibular system thus works in combination with proprioception and vision to promote postural stability. How multisensory signals are appropriately weighted and combined to produce a reliable and integrated sense of self motion is an area of active research (Angelaki & Cullen, 2008)
Sudden displacement of the platform on which a subject stands allows measurement of the latencies and patterns of electromyographic activity in the postural muscles of the legs. When the subject is standing on a large platform that is displaced forward or backward, the leg muscles contract in sequence from ankle to thigh to hip, and motion occurs primarily about the ankle joint. When the platform displacement is forward, the ankle flexor tibialis anterior on the front of the calf is first to be excited (flexion is the toe-up direction), at about 80–100 ms after the displacement. This is followed about 20 ms later by contraction of the quadriceps thigh muscles and then later still by contraction of trunk musculature. Backward displacement first elicits excitation of the gactrocnemius muscle at the back of the calf to extend the ankle, followed by excitation of thigh and trunk muscles antagonistic to those excited by forward displacement (Fig. 29.3). The overall postural reaction of distal-to-proximal excitation has been termed an “ankle strategy” for maintaining balance.
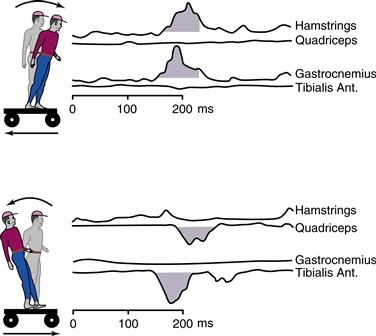
Figure 29.3 Sequencing of muscle activation in response to displacement of a supporting platform. When the supporting surface is displaced backward (at 0 ms), flexor muscles are excited first in the distal lower limb segments (gastrocnemius, about 80 ms latency) and then in the proximal segment (hamstrings, about 100 ms latency). Forward displacement of the platform activates lower limb extensors, again in a distal (tibialis anterior) to proximal (quadriceps) sequence. Black arrows mark the first detected electromyographic response to displacement. Based on studies by Horak and Nashner (1986).
Although the ankle strategy dominates in simple forward–backward displacement, other strategies are available, and postural reactions adapt to alterations in the support surface or sensory inputs. Displacement of a support surface that is short compared to the foot, or rotation of the support instead of displacement, demands a different postural response. In these cases a hip strategy is adopted, in which the body is bent at the hips so that the lower half of the body moves in the same direction as it does during ankle strategy reactions while the upper body moves in the opposite direction. Thus, during a forward, front downward tilt of a supporting platform, the hips move backward and the head forward. This shifts the center of gravity, which has moved forward due to the displacement, backward so that it is placed over the support again. Other work has documented a stiffening strategy of muscle cocontraction in response to base rotation and a multilink strategy that includes ankle, hip, and neck joint motions in response to translation. Stiffening, ankle, and hip or multilink strategies are applicable to the sway that occurs during normal standing on commonly encountered kinds of surfaces and may represent basic units of postural reaction. The range of postural reactions recorded from standing human subjects argues for a complexity beyond that observed in the vestibulocervical or vestibulospinal reflexes studied during rotation or tilting of animal subjects.
Vestibular Damage Results in Disorders of Postural Control
Control of posture involves many regions of the nervous system, and it is not surprising that disorders of posture can result from damage to the sensory periphery or to telencephalic, cerebellar, brainstem, or spinal centers. The striking motor consequences of lesions of the basal ganglia may include profound effects on posture, such as the rigidity and general poverty of movement associated with Parkinson’s disease (Chapter 30). Damage to the anterior vermis of the cerebellum can exaggerate decerebrate rigidity, and cerebellar patients show poorer performance in posture platform situations, with less adaptive modification of responses, as expected from our knowledge of cerebellar function (Chapter 31).
Box 29.1 Vestibular Plasticity
The experience of sailors getting their “sea legs” demonstrates that postural reflexes and balance can adapt over time to produce better performance in new circumstances. Clear evidence for this comes from the study of postural control in astronauts. After a space flight, astronauts rely more heavily than before on visual cues for postural orientation, and they show degraded performance in their responses to disturbances generated by a posture platform. Sway during standing is increased dramatically when visual cues are removed, and the body segments move in a less coordinated manner than before space flight. Nonetheless, the performance of astronauts after the experience of space flight is quite remarkable; they are able to balance adequately within hours of landing and regain their preflight postural performance within a few days.
The behavioral strategies and cellular mechanisms that mediate compensation for vestibular loss have been studied in animal models. Following unilateral labyrinthectomy (surgical removal of the inner ear), animals exhibit transient postural impairments, such as rolling and tilting toward the side of the lesion, which resolve within hours. Two distinct mechanisms account for the rapid recovery of postural stability despite the persistent impairment of vestibular function. First, the influence of the remaining senses that signal self-motion—proprioception and vision—increases rapidly following vestibular loss. Second, cellular mechanisms of plasticity in vestibular nucleus neurons enable the available sensory signals to recalibrate postural reflexes.
James F. Baker, Marc H. Schieber and Sascha du Lac
Reference
1. Cullen KE, Minor LB, Beraneck M, Sadeghi SG. Neural substrates underlying vstibular compensation: Contributions of peripheral versus central processing. Journal of Vestibular Research. 2009;19:171–182.
Many types of disease or damage of the vestibular system affect posture (and generally also cause dizziness), including vestibular neuritis, peripheral or central tumors or infarction, and Meniere’s syndrome (Baloh & Honrubia, 1990) (see Box 29.2). Bilateral involvement and acute, episodic, or chronic time courses occur in many vestibular disorders. The most obvious form of vestibular system damage is unilateral labyrinthectomy, performed either experimentally in animals or as the result of disease or surgical intervention against disease in humans. The postural symptoms immediately following loss of vestibular input to one side of the brain vary across species, with nonmammalian and “lower” mammalian species typically showing a more prominent tilting of the head and body toward the side of damage. Vestibular afferents have a high level of resting activity, and leaning toward the side of lost vestibular signals makes sense in that the spontaneous or resting activity of the remaining vestibular apparatus is signaling, in effect, motion toward the intact side because it is not balanced by equal resting activity from the damaged side. To counter this neural stimulus, the affected individual tilts away from the directions of apparent motion. This static postural effect of unilateral labyrinthectomy is accompanied by dynamic postural deficits, seen in experimental animals as weakened ipsilateral limb extensor responses and delayed responses to sudden drops. Remarkable compensation for unilateral labyrinthectomy occurs over a period of a few weeks, during which there is considerable recovery (see Box 29.1).
Box 29.2 Meniere Syndrome
The typical patient with Meniere syndrome develops a sensation of fullness and pressure along with decreased hearing and tinnitus in one ear. Vertigo follows rapidly, reaching a maximum intensity within minutes and then slowly subsiding over several hours. Often the patient is left with a sense of unsteadiness and nonspecific dizziness that can go on for days after the acute vertiginous spell. In the early stages, hearing loss is completely reversible, but as the disease progresses, residual hearing loss becomes a prominent feature. The tinnitus is typically described as a roaring sound similar to the sound of the ocean. These episodes occur at irregular intervals over years, with periods of remission unpredictably intermixed. Eventually, most patients reach the so-called “burnt-out phase,” where the episodic vertigo disappears and severe permanent hearing loss remains.
The clinical syndrome was first described by Prosper Meniere in 1861, but Hallpike and Cairns made the initial clinical-pathological correlation with hydrops of the labyrinth in 1938. Patients with Meniere syndrome invariably show an increase in volume of endolymph associated with distention of the entire endolymphatic system. Herniations and ruptures in the membranous labyrinth commonly occur, which may explain the episodes of hearing loss and vertigo.
Delayed endolymphatic hydrops occurs in an ear that has been damaged years before, usually by infection. With this disorder, the patient reports a long history of hearing loss, typically since childhood, followed many years later by episodic vertigo but without the typical auditory symptoms. The pathologic findings are remarkably similar to idiopathic Meniere syndrome, suggesting a common etiology. A subclinical viral infection could damage the resorptive mechanism of the inner ear, leading to an eventual decompensation in the balance between secretion and resorption of endolymph.
The key to the diagnosis of Meniere syndrome is to document fluctuating hearing levels in a patient with the characteristic clinical history. In the early stages, the sensorineural hearing loss is usually greater in the low frequencies. Some patients with Meniere syndrome develop abrupt episodes of falling to the ground without loss of consciousness or associated neurologic symptoms. These episodes have been called “otolithic catastrophes” because they are thought to result from a sudden mechanical deformation of the otolith receptor organ. Patients often report feeling as though they were pushed to the ground by some external force.
Because the cause of Meniere syndrome is usually unknown, treatment is empiric. Medical management consists of symptomatic treatment with antivertiginous drugs and long-term prophylaxis with salt restriction and diuretics. Many different surgical procedures have been tried but none has been consistently effective. Shunt operations to decrease the endolymph pressure have not been successful because the implanted drain devices are encapsulated rapidly by fibrous tissue. Destructive surgeries (removing the labyrinth or cutting the vestibular nerve), can stop the episodes of vertigo but do not change the tinnitus and progressive hearing loss.
Robert W. Baloh
Patients with long-standing bilateral vestibular loss may perform well on posture platforms when visual and somatosensory cues are present, but fail completely to maintain upright stance when the support surface and visual surround both sway with the patient so that only vestibular information is accurate. Patients with recent bilateral vestibular loss or who have not yet compensated for vestibular loss also do poorly when vision and support surface are unreliable, but these patients perform poorly if only one sense, be it vision or somatosensory, is made an unreliable indicator of balance.
Summary
Axons from vestibular and reticular nuclei in the brainstem descend in pathways located ventromedially in the spinal cord to provide postural tone and balance. Balance is maintained by negative feedback reflexes that are stimulated by the vestibular canals and otoliths. The vestibulocervical reflex excites neck muscles via the medial vestibulospinal tract to oppose head tilt, and vestibulospinal reflexes carried by the lateral vestibulospinal tract excite ipsilateral limb extensors to oppose body tilt. Vestibular, muscle stretch, and other reflexes work together to coordinate posture and balance, and the medial system adopts different strategies for balance control depending on the body support surface and information from sensory inputs. The medial postural system can adapt to different postural situations, but its actions can be compromised by central neural damage or unbalanced by peripheral vestibular loss. The reflexive control of body stability and posture by the medial descending system allows the lateral descending system to specialize in execution of precise voluntary movements of the extremities.
The Lateral Voluntary System
While the medial system thus provides underlying postural control for stance, ambulation, and orientation of the head, the lateral system superimposes the ability to make more sophisticated, voluntary movements in response to complex features of the external environment (perceived through the senses) and internal state (stored memories, knowledge, and emotion). Much of this control is mediated by specialized regions of the cerebral cortex. Outflow from this motor cortex dominates the lateral descending system, particularly in primates and humans.
Components of the Lateral Voluntary System
The Corticospinal Projection Is the Most Direct Pathway from the Cerebral Cortex to Spinal Motoneurons
For more than a century, electrical stimulation of a limited portion of the frontal lobe of the cerebral cortex has been known to evoke movements. Electrical stimulation of this “excitable cortex” evokes movements readily because this region has relatively direct connections to spinal motoneurons. In primates with a central sulcus (Rolandic fissure) in the neocortex, cortical neurons with axons projecting to the spinal cord are found most densely in the anterior bank of the central sulcus. The density of such neurons decreases from there rostrally to the precentral sulcus (posterior bank of the arcuate sulcus in macaque monkeys) and medially to the cingulate sulcus (Fig. 29.4). This territory corresponds to cytoarchitectonic areas 4 and 6 of Brodmann. Additional corticospinal neurons in areas 1, 2, 3, 5, and 7 of the parietal lobe project to the dorsal horn of the spinal cord to regulate sensory inflow.
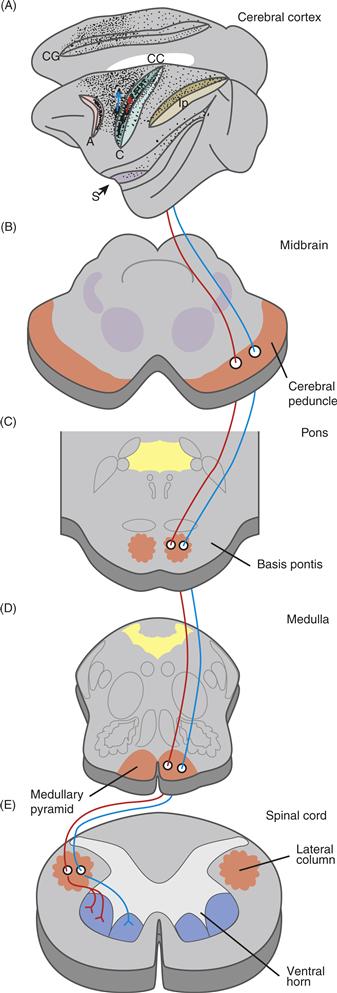
Figure 29.4 The corticospinal projection in the macaque monkey. (A) The density of corticospinal neuronal somata is shown by stippling in this lateral view of the left cerebral hemisphere; the superior medial surface of the hemisphere is also shown (above) as if reflected in a mirror. The central sulcus (C), arcuate sulcus (A), cingulate sulcus (Cg), intraparietal sulcus (Ip), and Sylvian fissure (S) are drawn as if pulled open to reveal the neurons in their banks. Two schematic corticospinal neurons, one relatively posterior and the other relatively anterior in area 4, send their axons down through the midbrain (B), pons (C), medulla (D), and spinal cord (E), which are drawn in cross section. In the spinal cord, the former corticospinal axon leaves the lateral column to terminate in the dorsolateral ventral horn, whereas the latter axon terminates in the ventromedial ventral horn.
The axons of neurons that project from the cerebral cortex to the brainstem and spinal cord have large pyramid-shaped somata in cortical layer V. Their axons leave the cortex, pass through the centrum semiovale, and enter the internal capsule, along with many other axons from other cortical areas. Axons from the motor cortex are concentrated most heavily in the middle third of the posterior limb of the internal capsule. As axons descend from the internal capsule below the thalamus, they come to lie on the ventral surface of the brainstem. Here, they form the cerebral peduncle of the midbrain, where the corticospinal axons are concentrated in the middle third. Some descending axons end in or send collaterals to the red nucleus. At the level of the pons, the descending axons of the peduncle become intermixed with pontine nuclear neurons in the base of the pons (basis pontis). Most of these axons synapse on pontine neurons, providing input from the cerebral cortex to the cerebellum. Other descending axons synapse in the pontomedullary reticular formation, providing input to the reticulospinal system and to brainstem motor pattern generators (Chapter 27). The continuing corticospinal axons collect to form the medullary pyramid (Box 29.3). As the medulla blends into the spinal cord, the vast majority of corticospinal axons cross the midline and enter the lateral column of white matter on the opposite side of the spinal cord. Because of this decussation of the pyramidal tract, the left motor cortex controls movements on the right side of the body and vice versa. A small minority of corticospinal axons remain uncrossed and continue caudally as the ventral (or anterior) corticospinal tract near the ventral midline of the spinal cord.
Box 29.3 Pyramids in the Brain
Inspection of the ventral surface of the medulla reveals a structure that looks like a small pyramid. This medullary pyramid turns out to be the collected bundle of corticospinal axons. The corticospinal tract therefore is often referred to as the pyramidal tract. Axons of noncorticospinal descending pathways do not pass through the medullary pyramid and therefore can be described collectively as extrapyramidal pathways. At one time these pathways were thought to receive their major controlling inputs from the basal ganglia. Human neurologic disorders that result from dysfunction of the basal ganglia thus came to be known as “extrapyramidal syndromes.” This term is misleading, however. More recent work has shown that many of the brainstem nuclei giving rise to extrapyramidal pathways receive cortical input and that much of the output of the basal ganglia is directed via the thalamus back to cortical motor areas. Many manifestations of extrapyramidal syndromes may therefore be played out through cortical motor areas, even via the pyramidal tract. Indeed, surgical lesions of the pyramidal tract once were used to ameliorate some extrapyramidal syndromes.
Marc H. Schieber and James F. Baker
As descending corticospinal axons reach their target levels in the spinal cord, they enter the spinal gray matter, where they ramify and synapse. The majority of corticospinal axons synapse on premotor interneurons in the intermediate zone (Rexed’s laminae VII and VIII), and relatively few synapse directly on spinal motoneurons in Rexed’s lamina IX. The somata of most corticospinal neurons with monosynaptic connections to motoneurons lie posteriorly in area 4, and their monosynaptic connections are made on the motoneurons of distal limb muscles, whose somata are clustered in the dorsolateral ventral horn. Corticospinal axons from neurons located more anteriorly typically synapse in the ventromedial portion of the ventral horn, where the motoneurons of proximal limb muscles and axial muscles are located. Some corticospinal axons cross the midline spinal gray matter to reach the ventromedial ventral horn ipsilateral to their origin; such doubly decussating corticospinal axons, along with the uncrossed ventral corticospinal tract, may be partly responsible for the relative preservation of trunk and proximal limb movements after unilateral damage to the cortex.
Indirect Pathways to the Spinal Cord Involve Centers in the Brainstem
The corticospinal tract is not the only output pathway through which the cerebral cortex contributes to motor control (Kuypers, 1987). The presence of other, less direct pathways in the macaque has been demonstrated by cutting the medullary pyramid on one side. Stimulation of the cortex on that side still evoked contralateral movements. The somatotopic organization of the cortex in the operated animals was similar to that in normal macaques, although the thresholds for stimulation were raised and distal movements were evoked less often.
Intermixed with corticospinal neurons in layer V of areas 4 and 6 are corticorubral neurons, whose axons project to the red nucleus (RN). Some corticospinal axons also send collaterals to the RN. In addition to cortical inputs, the RN also receives considerable input from the cerebellum (Chapter 31). Many RN neurons, particularly those in the caudal, magno-cellular portion, in turn send their axons across the midline and into the lateral column of the spinal cord, terminating most heavily in the dorsolateral region of the ventral horn. These descending axons from the RN constitute the rubrospinal tract. A pathway thus exists from the cortex to the RN to the spinal cord. The activity of rubrospinal neurons indicates that they play a significant role during voluntary movements of the arm, hand, and fingers. The rubrospinal pathway thus works synergistically with the corticospinal pathway in controlling limb movements.
A second pathway involves neurons scattered in the medial reticular formation of the pons and medulla. The medial reticular formation receives input from cortical motor areas and projects via the ventral column of the spinal cord to the ventral horn, chiefly its ventromedial portion. The axons that make this projection constitute the reticulospinal tract. Although the reticulospinal tract is also part of the medial descending system, the activity of some reticular formation neurons indicates that they also participate in controlling reaching and grasping movements (Baker, 2011).
Organization of the Motor Cortex
The original “motor cortex” is now appreciated to be comprised of several cortical areas. These areas are considered “motor” because (1) they project to other motor structures, (2) their ablation causes deficits in movement, and (3) their stimulation evokes or alters movements.
The Motor Cortex Is Subdivided into Multiple Cortical Motor Areas
In addition to its descending projections to spinal motoneurons, the motor cortex has cytoarchitectonic features that distinguish it from other regions of the cerebral cortex. The somata of many pyramidal neurons in layer V, the output layer, are exceptionally large (Betz cells). In addition, the neurons of layer IV, the granular layer that receives thalamic input, are very sparse compared to other areas of the neocortex, and hence the motor cortex has been described as dysgranular (area 6) or agranular (area 4) cortex. Based on cytoarchitectonics, myeloarchitectonics, and histochemical features, the motor cortex of macaque monkeys can be subdivided into the cortical motor areas shown in Figure 29.5A. In general, these subdivisions show three mediolaterally oriented strips of cortex, with the primary motor cortex (M1) most posterior and two premotor strips progressively more anterior. Each of these premotor strips has a subdivision on the medial wall of the hemisphere, a second subdivision on the dorsal convexity, and a third on the ventral convexity. In addition to these subdivisions of Brodmann’s areas 4 and 6, areas 23 and 24 in the banks of the cingulate sulcus and on the medial surface of the hemisphere in the cingulate gyrus contain at least two additional cortical motor areas. Cortical motor areas differ, not only in their intrinsic composition, but also in their interconnections with other parts of the central nervous system.
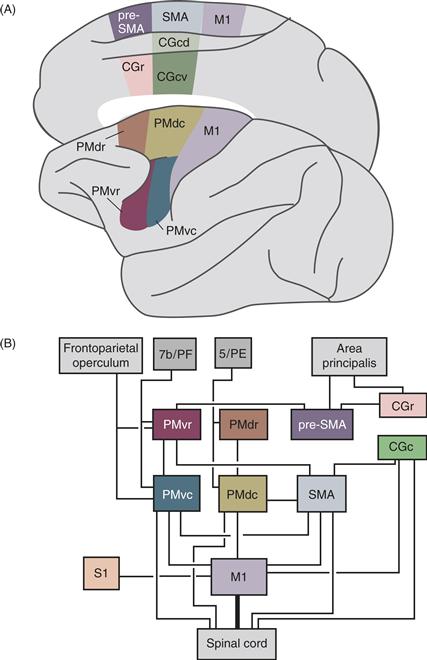
Figure 29.5 Cortical motor areas. (A) Diagram of a macaque brain showing current parcellation of cortical motor areas in the frontal lobe. Modified from Matelli, Luppino, and Rizzolatti (1991). (B) Connections of the cortical motor areas. Most corticocortical connections are reciprocal. CGc,-cingulate motor area, caudal; CGr,-cingulate motor area, rostral; M1, primary motor cortex; PMdc, premotor cortex, dorsal caudal; PMdr, premotor cortex, dorsal, rostral; PMvc, premotor cortex, ventral, caudal; PMvr, premotor cortex, ventral, rostral; Pre-SMA, presupplementary motor area; SMA supplementary motor area proper. Thin lines to the spinal cord from PMvc, PMdc, SMA, and CGc indicate that corticospinal projections from these areas are not as strong as that from M1.
Cortical motor areas differ in their connections with the thalamus. M1, PMv, and SMA, for example, connect primarily with VPLo/VLc, area X, and VLo in the thalamus, respectively. These differences are significant because thalamic nuclei VPLo/VLc and area X receive major inputs from the cerebellum, whereas VLo receives major input from the basal ganglia. Thus, information processed by the cerebellum is directed largely to M1 and PMv, whereas information from the basal ganglia is sent largely to SMA.
Cortical motor areas also differ in their connections with one another and with other regions of the cortex (Fig. 29.5B). For example, when horseradish peroxidase (HRP) is injected into the hand region of M1, separate patches of retrogradely labeled neuronal somata are found in PMv, PMd, SMA, CGc, and CGr, indicating that each of these areas sends a separate projection to the hand region of M1. In contrast, other motor cortical regions do not project directly to M1. Instead, PMvr projects to PMvc, PMdr projects to PMdc, and pre-SMA projects to PMvr. The extensive connectivity between motor cortical areas subserves the dynamic visuomotor transformations required to successfully grasp an object (Davare, Kraskov, Rothwell, & Lemon, 2011).
Different cortical motor areas also receive input from different cortical sensory and association areas: M1 receives input from the primary somatosensory area (S1), PMv receives input from visual association area 7b, particularly the anterior intraparietal area (AIP), and PMd receives input from somatosensory association area 5 and the parietal reach region. Because of these inputs, many motor cortex neurons have somatosensory receptive fields. Neurons located caudally in M1 tend to respond to cutaneous modalities, whereas neurons located rostrally in M1 tend to respond to deep modalities. The somatosensory input of an M1 neuron is generally related to the output function of that neuron. For example, caudally located M1 neurons that control the fingers may have cutaneous receptive fields on the fingers (Fig. 29.6A), whereas more rostrally located M1 neurons that control the biceps or triceps may have deep receptive fields in those muscles.
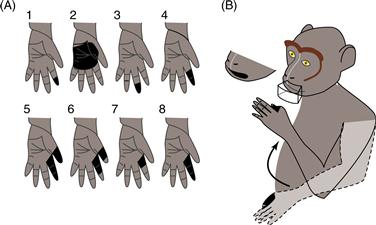
Figure 29.6 Sensory receptive fields in M1 and PMv. (A) Black regions show the tactile receptive fields of eight M1 neurons recorded at loci where intracortical microstimulation evoked flexion of the monkey’s index and middle fingers. Other neurons at the same loci responded to passive extension of those fingers. From Rosen and Asanuma (1972). (B) A single PMv neuron responded to visual stimuli moving near the mouth, to tactile stimulation of the lips and of the skin between the thumb and the index finger, and to flexion of the elbow. From Rizzolatti, Scandolara, Matelli, and Gentilucci (1981).
PMv receives both somatosensory and visual input via areas 7b and AIP. Both the somatosensory and the visual receptive fields of PMv neurons tend to be large, and when single PMv neurons receive both types of sensory information, the fields tend to be related (Fig. 29.6B). A neuron with a somatosensory receptive field covering the forearm, for instance, may respond to visual stimuli moving near the forearm. Interestingly, if the forearm is moved to a different position, the visual receptive field of the PMv neuron moves with the forearm. The somatosensory inputs to both PMv and M1, plus the visual inputs to PMv, reflect the role of sensory inputs in guiding ongoing movements as they are being generated by cortical motor areas.
Somatotopic Organization in the Motor Cortex Is Not a One-to-One Map of Body Parts, Muscles, or Movements
The organization of the primary motor cortex, M1, has been studied more extensively than that of other cortical motor areas. Within the M1 of primates, the face is represented laterally, the lower extremity (or hindlimb) and tail medially, and the upper extremity (or forelimb) in between (Fig. 29.7). This overall organization of M1 according to major body parts is termed somatotopic. The somatotopic organization of M1 becomes evident in clinical neurology. Lesions on the lateral convexity of human M1 cause weakness or paralysis of the contralateral face, more medial lesions on the convexity affect the contralateral hand and arm, and lesions on the medial wall of the hemisphere affect the leg and foot.

Figure 29.7 Somatotopic maps in M1. (A) Map by Woolsey et al. (1952) in which each figurine represents in black and gray the body parts that moved a lot or a little, respectively, when the cortical surface at that site was stimulated. In addition to the primary representation on the convexity, their map shows a secondary representation on the medial surface of the hemisphere, called the supplementary motor area (SMA). As defined in this study, M1 and SMA each included several of the currently defined cortical motor areas. (B) Intracortical microstimulation of M1 in an owl monkey produced this map, consisting of a complex mosaic of different body parts. In this species, the central sulcus is only a shallow dimple, and M1 is entirely on the surface of the hemisphere. Each dot represents a stimulated locus, and lines surround adjacent loci from which movement of the same body part was evoked. Note that the forepaw digits (purple) and the hindpaw digits (green) are represented in multiple areas separated by areas representing nearby body parts. Modified from Gould, Cusic, Pons, and Kaas (1986). In both A and B, the inset at the top indicates the region of the frontal lobe enlarged below.
Moreover, distal parts of the extremities and acral parts of the face (lips and tongue) are most heavily represented caudally, whereas proximal parts of the extremities and axial movements are most heavily represented rostrally. Those parts of the body that are used for fine manipulative movements (such as lips, tongue, and fingers in primates) are generally represented over a wider cortical territory than body parts used in gross movements such as ambulation. Penfield, in his cartoon of the motor homunculus (little man), and Woolsey, in his cartoon of the motor simiusculus (little monkey), conveyed this apparent magnification of certain body parts with respect to cortical territory by distorting the size of body parts relative to their normal proportions.
The somatotopic organization of M1 is not, however, a one-to-one mapping of body parts, muscles, or movements. Within the arm representation, for example, the cortical territory representing any particular part of the arm overlaps considerably with the territory representing nearby parts. This overlap results from three features of M1’s organization: convergence, divergence, and horizontal interconnection.
M1 outputs arising from a wide cortical territory converge on the spinal motoneuron pool of any given muscle. Convergence of M1 outputs has been shown by delivering intracortical microstimulation while observing the body for evoked movements and/or recording electromyographic (EMG) activity from multiple muscles. Maps of which body parts move or which muscles contract upon threshold stimulation at different cortical sites look like a complex mosaic (Fig. 29.8A). As the stimulation current is increased, the mosaic pieces representing a given muscle coalesce into a larger and larger territory that overlaps more and more with the territories of other muscles. These findings indicate that any given muscle is controlled by a large territory in M1 and that the territories for different muscles overlap (Fig. 29.8B). Using retrograde transneuronal tracing with rabies virus, these large and overlapping cortical territories for different muscles recently have been demonstrated anatomically as well.
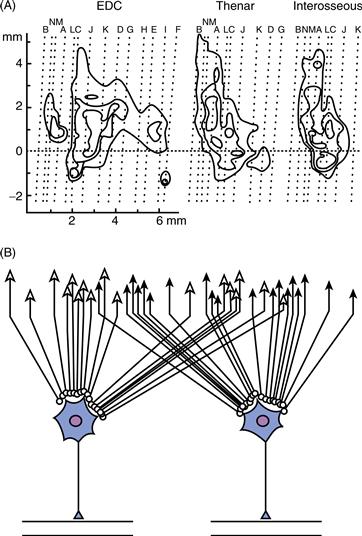
Figure 29.8 Convergence of M1 outputs to single muscles. (A) Isothreshold contours show the points at which EMG responses were evoked in three different muscles—extensor digitorum communis (EDC), thenar, and first dorsal interosseus—by intracortical microstimulation in the anterior bank of the central sulcus. (B) Data shown in A indicate that the cortical input to the motoneurons of any muscle originates from a wide territory in M1 and that the cortical territory providing input to a given muscle overlaps extensively with the cortical territory providing input to other muscles in the same part of the body. Modified from Andersen, Hagan, Phillips, and Powell (1975).
A second factor contributing to the overlap of territories in M1 is the divergence of output from single cortical neurons to multiple motoneuron pools. Intracellular staining has shown that a single corticospinal axon may have terminal ramifications within the motoneuron pools of multiple muscles over several segmental levels of the spinal cord (Fig. 29.9A). Moreover, spike-triggered averaging of EMG activity has demonstrated that single M1 neurons can have relatively direct effects on the motoneuron pools of multiple muscles (Fig. 29.9B). These findings indicate that single M1 neurons have output connections that diverge to innervate the spinal motoneuron pools of multiple muscles (Fig. 29.9C).
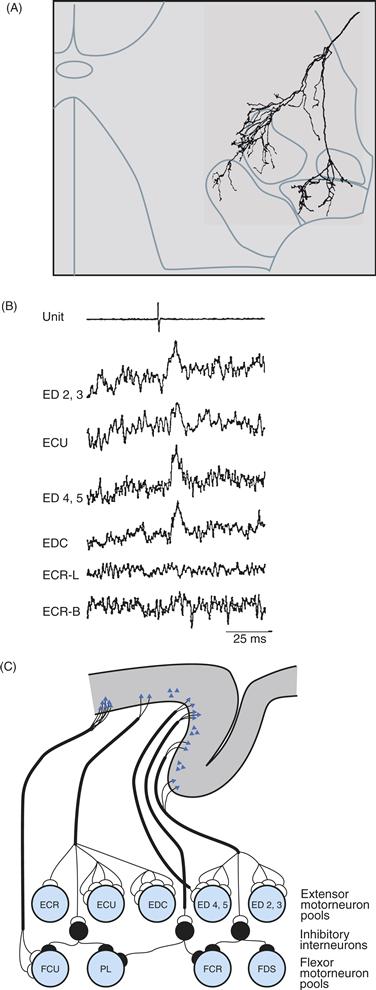
Figure 29.9 Divergence of M1 outputs to multiple muscles. (A) Tracing of a single corticospinal axon ramifying in the ventral horn of the spinal cord shows terminal fields in the motoneuron pools of four forearm muscles. From Shinoda, Yokota, and Futami (1981). (B) Action potentials in a cortical neuron (top trace) are followed at a fixed latency by peaks of postspike facilitation in EMGs recorded from four of six recorded forearm muscles (lower traces), consistent with monosynaptic excitation of all four motoneuron pools by that cortical neuron. The EMGs are rectified and averaged responses to 7051 action potentials in the cortical neuron. From Fetz and Cheney (1980). (C) These anatomic and physiologic findings indicate that the output of single corticospinal neurons often diverges to influence multiple muscles. From Cheney, Fetz, and Palmer (1985).
A third factor contributing to overlapping representation is the horizontal interconnectivity intrinsic to M1. Although most horizontal axon collaterals of neurons in layer V extend only 1–2 mm, some extend across the upper extremity representation, interconnecting even the territories of proximal and distal muscles
Because of convergence, divergence, and horizontal interconnections, neuronal activity is widely distributed in M1 during natural movements, even discrete movements of single body parts. In monkeys trained to perform individuated movements of each finger, individual M1 neurons are active during movements of multiple fingers, and neurons throughout the M1 hand area are active during movements of any given finger. Likewise, in humans performing movements of different fingers, activity is distributed over the entire M1 hand representation whether the subject is moving a single finger or the whole hand. Although the entire hand representation may be activated for movement of any given finger, monkey and human studies have shown a tendency for the center of activation during movements of the thumb to be located laterally to that for movements of other fingers, consistent with the somatotopic orientation of the hand in the classic simiusculus and homunculus.
The somatotopic representation in M1, like that in the primary somatosensory cortex, has a certain degree of plasticity. The cortical territory representing a given muscle can enlarge when nearby body parts are denervated experimentally. Threshold stimulation in the cortical territory that had represented a denervated body part then comes to evoke movements in nearby body parts. Enlargement of a given muscle’s cortical territory occurs as well when that muscle is stretched passively or used intensely for a prolonged period. Furthermore, in normal humans who are actively practicing a complex sequence of finger movements, the cortical territory representing a given finger muscle enlarges as the subjects become skilled at performing the sequence. Because such changes can occur within several minutes, they probably are mediated by long-term potentiation and/or depression at existing synapses. This ability of the M1 cortex to reorganize may in part underlie motor recovery seen in humans after damage to M1 or the corticospinal tract.
Control of Voluntary Movements by the Motor Cortex
Several methods have enabled investigators to explore how cortical motor areas contribute to the production and control of voluntary movement in awake, behaving subjects. Whereas microelectrodes record the action potentials of single neurons, measurements made by functional imaging or by recording surface potentials reflect the net activity of thousands of cortical neurons and millions of synapses.
Multiple Cortical Areas Are Active When the Brain Generates a Voluntary Movement
These different methodologies now make it clear that many cortical areas in addition to the primary motor cortex are active during the planning and execution of voluntary movements. During performance of either a simple keypress or a complex movement sequence, for example, functional imaging studies in humans have shown bilateral activation of the primary sensorimotor hand representation, the supplementary motor area, and the ventrolateral premotor cortex, plus contralateral activation of the dorsolateral premotor cortex and the medial cortex rostral to the SMA. Whereas such techniques provide information on the parts of the cortex that are active in a given situation, studies of electrical potentials provide information on the time course of activation. For simple self-paced movements, cortical electrical potentials over the SMA and M1 begin to change as early as 1 s prior to the movement. As the time of movement onset approaches, the amplitude of these bilateral electrical potential shifts increases (the Bereitschaft potential). At the time of the movement, a further increase in amplitude occurs over the somatotopically appropriate region of M1 contralateral to the moving body part. Given that many cortical areas are active in controlling movement raises the question: What does each area contribute to control?
M1 Neurons Control Movement Kinematics and Dynamics
In one of the earliest studies of single neuron activity in awake behaving animals, Evarts recorded the activity of M1 pyramidal tract neurons in monkeys trained to raise and lower weights using flexion and extension wrist movements (Fig. 29.10). The discharge frequency of many M1 neurons changed systematically in temporal relation to either flexion or extension. A typical flexion-related neuron, for example, began to discharge several hundred milliseconds before wrist flexion began. As the onset of a flexion movement approached, the discharge frequency of the neuron increased, accelerating still more as the flexion movement was made. The neuron was silent during extension. The time course of these movement-related changes in single neuron activity parallels the time course of cortical electrical potential shifts.

Figure 29.10 Discharge of a single M1 neuron in a monkey making flexion and extension wrist movements with wrist flexors loaded (A) and unloaded (B) and with wrist extensors loaded (C). The discharge rate of this neuron was greatest when the monkey used its wrist flexor muscles against a load. Modified from Evarts (1968).
Evarts went on to demonstrate that the discharge frequency of M1 pyramidal tract neurons (PTNs) varied in relation to a number of mechanical parameters of the movement the monkey was making. By changing the weights the monkey had to lift, Evarts showed that PTN discharge frequency varied with the force the monkey exerted. Comparison of PTN discharge with simultaneous force recordings revealed that bursts of PTN firing were correlated with sudden increases in the exerted force, indicating a relationship between firing frequency and the rate of change of force.
Subsequent studies revealed that the discharge of M1 neurons can be related to the direction of movement, the position of a particular joint, or the velocity of movement (Kalaska, 2009). Many neurons in the SMA, PMd, and parietal area 5 (which projects to SMA and PMd) also fire in relation to movement direction, and some PMv neurons fire in relation to movement force. Therefore, neurons in several nonprimary cortical motor areas participate with those in M1 to control movement parameters such as direction, force, position, and velocity.
Although very strong correlations can be found between the discharge frequency of a given neuron and a particular movement parameter, an M1 neuron does not simply encode a single parameter. Rather, single M1 neurons show various degrees of correlation with direction, force, position, and velocity. Thus, the discharge of a single neuron in the motor cortex may influence several movement parameters.
Conversely, any given parameter or other feature of a movement is represented not by the discharge of a single M1 neuron, but by the ensemble activity of a large population of cortical neurons. Although a single M1 neuron typically discharges during reaching movements in several directions, for example, when the activity of many M1 neurons is combined appropriately, the population of M1 neurons can be shown to represent movement direction precisely (Fig. 29.11). Researchers have correlated the force, rate of change of force, position, and velocity of movements more accurately with the summed, weighted activity of a number of simultaneously recorded M1 neurons than with the discharge of any single neuron (see Box 29.4).
Box 29.4 Motor Neuroprosthetics
The fact that a subject’s movements can be decoded from populations of neurons, combined with the recently developed ability to implant devices in the brain that record populations of neurons simultaneously, has led to the development of brain-computer interfaces (BCIs) and brain machine interfaces (BMIs). These are devices in which a number of simultaneously recorded neurophysiological signals are decoded as a population, and the decoded output then is used to control either a cursor on a computer screen (BCI) or a physical device such as a robotic arm (BMI). While recordings of neuron spikes generally provide the best decoding, other types of neurophysiological signals—local field potentials recorded from penetrating microelectrodes (LFPs), recordings made from various sites on the surface of the brain (electrocorticographic, ECoG), or recordings obtained from the scalp (electroencephalographic, EEG)—all can provide decodable information about a subject’s movements. With current systems, subjects can control the movement of an artificial arm in three-dimensional space, and can open and close the hand. The hope is that with further development of such neuroprosthetic systems, amputees may be able to have dexterous control of a prosthetic arm and hand, and paralyzed patients may be able to work on a computer or move in a normal fashion by driving electrical stimulation of their own, otherwise intact muscles (Lebedev & Nicolelis, 2006).
In addition to the potential for useful devices controlled directly from the brain, the field of neuroprosthetics is expanding our understanding of the motor cortex. In a typical BCI session, neurophysiological activity is recorded while a normal subject performs arm movements that control a cursor on a computer screen, like when you move a computer mouse to control a cursor. A decoding algorithm then is derived from the recorded data to predict which patterns of neurophysiological activity correspond to which movements. Next, the decoding algorithm is used to drive a computer cursor. If in this “brain control” mode the subject again is asked to move the cursor to different locations on the screen, however, a number of interesting things often happen. The tuning relationship between neurophysiological activity and movement may change, and the subject may stop moving entirely, even though the computer cursor continues to move under direct control by the cortical activity. Exactly how the cortical activity becomes dissociated from movement of the limb (Schieber, 2011), and how the cortical activity adapts to control the BCI, are currently questions under investigation (Green & Kalaska, 2011).
Marc H. Schieber
References
1. Green AM, Kalaska JF. Learning to move machines with the mind. Trends in Neurosciences. 2011;34:61–75.
2. Lebedev MA, Nicolelis MA. Brain-machine interfaces: Past, present and future. Trends in Neurosciences. 2006;29:536–546.
3. Schieber MH. Dissociating motor cortex from the motor. Journal of Physiology. 2011;589:5613–5624.
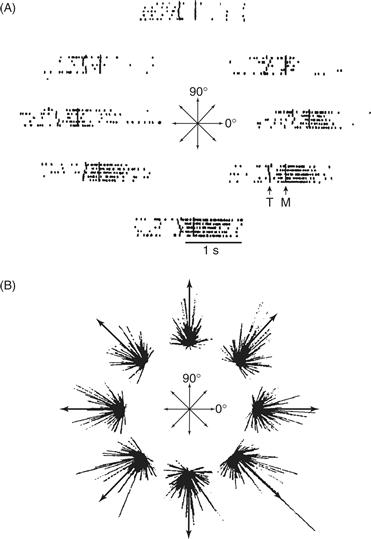
Figure 29.11 (A) Discharge of a single M1 neuron before and during arm movements in a monkey. Movements (represented by arrows) started from the same central point and ended at eight different points on a circle. The eight rasters show that the activity of this neuron was related to movements in four of the eight directions. The neuron discharged most intensely for movements down and to the right and was inhibited during movements up and to the left. (B) For each of the eight movements, the discharge of each M1 neuron is shown as a line pointing in the preferred direction of the neuron. Each line starts at the movement end point, and its length is proportional to the intensity of the discharge of that neuron during movement in that direction. Although the discharge of single neurons rarely identified any single movement direction with accuracy, the population vectors (arrows) summing the discharge of an ensemble of M1 neurons adequately specify each of the eight movement directions. From Georgopoulos (1988).
Cortical Motor Areas Prepare Voluntary Movements Based on a Variety of Cues
Cortical areas outside the primary motor cortex seem to be especially concerned with using a wide variety of sensory and other information as “cues” to select and guide movements. Insight into these cortical processes has been obtained by separating in time a cue instructing which movement to make from a second cue to execute the instructed movement. This creates a period between the two cues during which the subject knows what movement to make, but is not making the movement per se. During such an instructed delay period, many neurons in M1, SMA, and PMd discharge at their highest rate while the subject waits to move in a particular direction (Fig. 29.12). Such directional delay period activity is more common in PMd and SMA than in M1, where activity during movement execution predominates. During the delay between instruction and trigger, PMd, SMA, and other areas appear to store information on the direction of the impending movement. Indeed, neurons sometimes discharge in error during the delay, as if the monkey has seen a cue other than the one actually given and is preparing to move in the wrong direction. When such error discharges occur, the monkey often does make the wrong movement. The delay period discharge of such neurons thus represents stored information, not about which cue the monkey has seen, but rather about what movement the monkey will make.
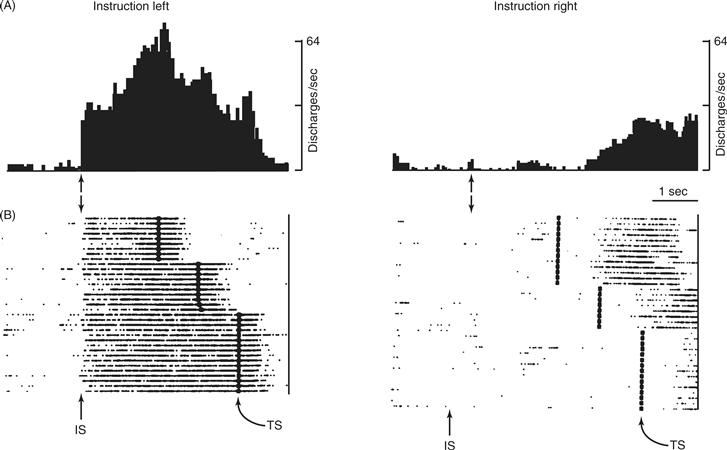
Figure 29.12 Directional delay period activity in a PM neuron. (A) As a monkey performed a delayed-reaction paradigm, this neuron began to discharge shortly after receiving instructions (IS) to perform a leftward movement. Discharge continued until after the monkey had subsequently received a separate triggering signal (TS, which occurred at three different time intervals after the IS) and performed the movement. During the delay between IS and TS, while the monkey did not move, the discharge of the neuron encoded the direction of the instruction, the direction of the impending movement, or both. (B) When the instruction was for rightward movement, this neuron did not discharge until after the movement had been made, presumably as the monkey was then preparing to move back to its original position. From Wise and Strick (1996).
The direction instructed usually is the same as the direction of the actual movement. To pick up a pencil, for example, you look at the pencil and then move your hand to the same place. Your brain transforms the visual location of the pencil into the location to which your hand is moved. Insight into how cue direction is transformed into movement direction has come from tasks in which these two features were experimentally dissociated, as if you looked at a pencil in a mirror and then reached to pick up the real pencil instead of the mirror image. To dissociate cue direction and movement direction experimentally, researchers have trained monkeys to perform mental rotation tasks in which a cue at one location instructs a movement to a different location.
Such tasks have been used to study neurons in the area principalis of the dorsolateral frontal lobe, in PMd, in SMA, and in M1. In each of these cortical areas, the activity of some neurons correlates best with the direction of the instructional cue, whereas the activity of other neurons correlates best with the direction of the actual movement. In general, from the area principalis, through the PMd and SMA to M1, the percentage of cue-related neurons decreases and the percentage of movement-related neurons increases. Moreover, as time progresses from the appearance of the instructional cue to the execution of the movement, the discharge of the neuronal populations encode cue direction initially and movement direction subsequently. The transformation of cue direction into movement direction thus involves many cortical motor areas and progresses throughout the reaction time of the subject.
Some Cortical Motor Areas are Involved in Movements in Response to Either Internal or External Cues
Another aspect of movement preparation that differentially involves certain cortical motor areas has to do with whether the instructions about what to do come from internally remembered or externally delivered cues. In one experiment, for example, monkeys were trained to touch three of four pads in randomly selected sequences that were cued when the pads were lit in sequence. Once a monkey was accustomed to a given sequence, the lights were gradually dimmed until the monkey was performing the correct sequence based only on internally remembered cues. After several of these remembered trials, the pads were lit in a different sequence for several trials. Whereas neurons in M1 showed similar discharge rates during the internally remembered and externally cued trials for a given sequence, SMA neurons were generally more active during the internally remembered trials, and PMv neurons were generally more active during the externally cued trials (Fig. 29.13).
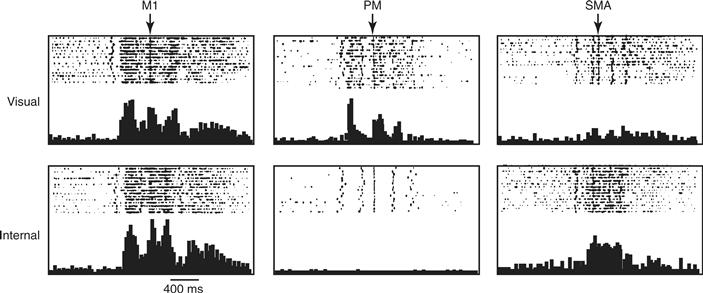
Figure 29.13 Activity of three neurons—one in M1, one in PM, and one in SMA—recorded as a monkey pressed three buttons in sequence. The sequence was first cued visually by lighting the buttons and was then cued internally. The M1 neuron showed similar activity whether the monkey performed from visual or internal cues. The PM neuron, however, was much more active in response to visual than internal cues, whereas the opposite was true for the SMA neuron. Modified from Mushiake, Inase, and Tanji (1991).
Summary
Axons from the cerebral cortex and from the red nucleus in the brainstem descend in pathways located dorsolaterally in the spinal cord to control voluntary movements. In addition to corticospinal and rubrospinal pathways, the motor cortex also sends projections to the red nucleus and to the pontomedullary reticular formation, providing additional pathways for voluntary control. Multiple cortical motor areas—distinguished by their inputs, their cytoarchitecture, and their interconnections with other cortical areas and with the thalamus—make different contributions to the control of voluntary movements. The most detailed motor map is found in the primary motor cortex, M1, but the somatotopic organization is limited by convergence and divergence in the corticospinal projection. Populations of M1 neurons are also in most direct control of movement kinematics and dynamics. Other cortical motor areas participate differentially in the selection of, and preparation for, voluntary movements based on a variety of internal and external cues.
Summary
The motor cortex, the red nucleus, the pontomedullary reticular formation, and the vestibular nuclei each send major axonal projections descending from the brain to the spinal cord to control bodily movements. Although these pathways normally provide seamless movement control, different contributions of the medial and lateral descending pathways have been identified experimentally.
The medial system—vestibulospinal and reticulospinal—receives sensory input from the vestibular apparatus and mediates postural responses that keep the head and body stabilized for stance and gait. Additional visual and proprioceptive inputs that reach the vestibular and reticular nuclei via brainstem pathways or after processing in the cerebellum (Chapter 31) also influence postural control. Context-dependent strategies established via centers including the cerebellum and motor cortex further adapt postural responses to the needs of particular complex situations.
The lateral system—corticospinal and rubrospinal—receives, in addition to somatosensory inputs, information processed by the cerebellum (Chapter 31), the basal ganglia (Chapter 30), and association cortical areas to mediate voluntary movements of the face and extremities. Different cortical motor areas receive different portions of these inputs and therefore make different contributions to controlling voluntary movements. The most elaborate somatotopic representation is found in the primary motor cortex, M1. While the convergence, divergence and horizontal interconnections of the intrinsic organization of M1 result in considerably distributed activation during voluntary movements, the same substrates enable substantial plasticity. Whereas M1 neurons most directly control the kinematics and dynamics of movement execution, other cortical motor areas participate in selecting which movement(s) to make on the basis of external cues and internal states.
References
1. Andersen P, Hagan PJ, Phillips CG, Powell TP. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon’s hand. Proceedings of the Royal Society of London B Biological Sciences. 1975;188:31–36.
2. Angelaki DE, Cullen KE. Vestibular system: The many facets of a multimodal system. Annual Review of Neuroscience. 2008;31:125–150.
3. Bagnall MW, McElvain LE, Faulstich M, du Lac S. Frequency-independent synaptic transmission supports a linear vestibular behavior. Neuron. 2008;60:343–352.
4. Baker SN. The primate reticulospinal tract, hand function and functional recovery. Journal of Physiology. 2011;589:5603–5612.
5. Baker J, Goldberg J, Peterson B. Spatial and temporal response properties of the vestibulocollic reflex in decerebrate cats. Journal of Neurophysiology. 1985;54:735–756.
6. Baloh R, Honrubia V. Clinical neurophysiology of the vestibular system 2nd ed. Philadelphia, PA: F. A. Davis; 1990.
7. Cheney PD, Fetz EE, Palmer SS. Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. Journal of Neurophysiology. 1985;53:805–820.
8. Davare M, Kraskov A, Rothwell JC, Lemon R. Interactions between areas of the cortical grasping network. Current Opinion in Neurobiology. 2011;21:565–570.
9. Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. Journal of Neurophysiology. 1968;31:14–27.
10. Fetz EE, Cheney PD. Postspike facilitation of fore-limb muscle activity by primate corticomotoneuronal cells. Journal of Neurophysiology. 1980;44:751–772.
11. Georgopoulos AP. Neural integration of movement: Role of motor cortex in reaching. FASEB Journal. 1988;2:2849–2857.
12. Gould HJ, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. The Journal of Comparative Neurology. 1986;247:297–325.
13. Horak F, Nashner L. Central programming of postural movements: Adaptation to altered support-surface configurations. Journal of Neurophysiology. 1986;55:1369–1381.
14. Horak F, Shupert C. Role of the vestibular system in postural control. In: Herdman S, ed. Vestibular rehabilitation. Philadelphia, PA: F. A. Davis; 1994.
15. Kalaska JF. From intention to action: Motor cortex and the control of reaching movements. Advances in Experimental Medicine and Biology. 2009;629:139–178.
16. Kolkman KE, McElvain LE, du Lac S. Diverse precerebellar neurons share similar intrinsic excitability. Journal of Neuroscience. 2011;31:16665–16674.
17. Kuypers HG. Some aspects of the organization of the output of the motor cortex. Ciba Foundation Symposium. 1987;132:63–82.
18. Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey I The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14.
19. Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey II The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36.
20. Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. Journal of Comparative Neurology. 1991;311:445–462.
21. Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. Journal of Neurophysiology. 1991;66:705–718.
22. Nashner L. Adaptation of human movement to altered environments. Trends in Neurosciences. 1982;5:358–361.
23. Peterson B, Goldberg J, Bilotto G, Fuller J. Cervico-collic reflex: Its dynamic properties and interaction with vestibular reflexes. Journal of Neurophysiology. 1985;54:90–109.
24. Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys II Visual responses. Behavioural Brain Research. 1981;2:147–163.
25. Roberts T. Neurophysiology of postural mechanisms New York, NY: Plenum Press; 1967.
26. Rosen I, Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: Input-output relations. Experimental Brain Research. 1972;14:257–273.
27. Schor R, Kearney R, Dieringer N. Reflex stabilization of the head. In: Peterson B, Richmond F, eds. Control of head movement. New York, NY: Oxford University Press; 1988.
28. Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neuroscience Letters. 1981;23:7–12.
29. Wilson V, Melvill-Jones G. Mammalian vestibular physiology New York, NY: Plenum Press; 1979.
30. Wise SP, Strick PL. Anatomical and physiological organization of the non-primary motor cortex. TINS. 1996;7:442–446.
31. Woolsey CN, Settlage PH, Meyer DR, Sencer W, Hamuy TP, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Research Publications - Association for Research in Nervous and Mental Disease. 1952;30:238–264.