Chapter 39
Circadian Timekeeping
Introduction
Life on Earth has adapted to the cyclic variation in conditions imposed by our planet’s daily rotation on its axis and its annual revolution around the sun. Almost all organisms studied, from cyanobacteria to Homo sapiens, have intrinsic time-keeping systems that adjust physiology and behavior to anticipate the daily cycles of light and darkness. Physiological processes that vary over the 24-hour day include activity, alertness, hormone secretion, organ physiology, and gene expression. These variations are not merely passive responses to a rhythmic environment, but instead reflect an underlying biological mechanism that can measure time in 24-hour increments. This mechanism orchestrates physiology to achieve predictive, rather than reactive, homeostasis.
Biological time measurement on the 24-hour time scale is achieved by a circadian timing mechanism. The term circadian is derived from the Latin words circa and diem, meaning “about a day,” referring to the cycle length of these rhythms. Circadian rhythms are defined as rhythms that persist with a cycle length of approximately 24 hours in constant environmental conditions (Figs. 39.1 and 39.2). These rhythms are generated by a biological timing mechanism that is normally synchronized (entrained) to the 24-hour day by environmental cues. Light is the most widely used signal for entrainment of circadian clocks, but temperature, hormone levels, nutrient availability, and other cues can also affect oscillations.
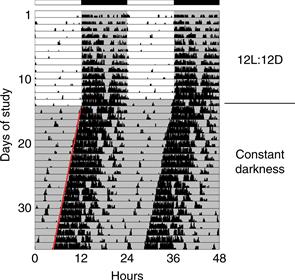
Figure 39.1 Locomotor activity rhythm of a mouse. This “double-plotted actogram” shows the activity pattern of an adult mouse housed individually in a cage with a running wheel. When the mouse rotates the wheel by running within it, a computerized system detects and records the wheel revolutions. Wheel revolutions are then plotted versus time, for weeks of data. Each horizontal line represents 48 hours of data. Wheel revolutions appear as bars coming up from the baseline, with their height proportional to the number of revolutions. On the first line of the record, the first 48 hours of data are shown. In the second line of the record, the data shown are from hours 24-72 of data collection. Thus, each 24-hour period of data after the first 24 hr is shown both to the right of the previous day’s data and below it; this artificial reproduction of the data (double-plotting) helps to see the rhythmicity when the cycle length of rhythmicity differs from 24 hours. When housed in a 12 hours light, 12 hours darkness (12L:12D) lighting cycle, the mouse is active almost exclusively at night (shaded region). When the lighting cycle is disabled so the animal is in constant darkness (DD, starting on day 14), the animal continues to show rhythmicity but with a cycle length slightly less than 24 hours. This record reveals two defining principles of circadian rhythms: (1) circadian rhythmicity is intrinsic, rather than being generated in response to environmental variation, and (2) the circadian clock is normally synchronized to the 24-hour day length by light. It is easy to draw a “best-fit” line through the onset of activity each day (red line). Note the precision of the rhythmicity: the actual time of activity onset does not deviate far from this “best-fit” line, indicating the cycle length is measured with great accuracy. Spend a few minutes to think about how you would design a biological timekeeping system that can measure a day while having a variation between successive cycles that is less than 1% (15 minutes per 24 hours; the actual variation is often considerably less than this amount).
Figure courtesy of Jason DeBruyne, Morehouse School of Medicine.
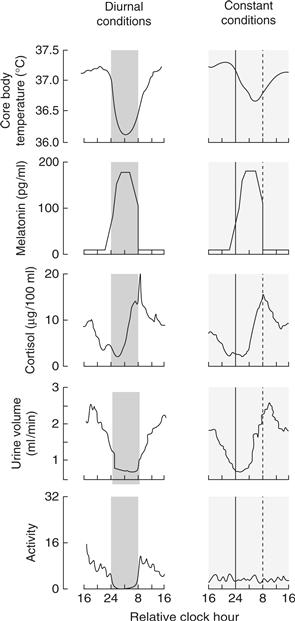
Figure 39.2 Diurnal and circadian rhythms in humans. The panels on the left show rhythms in several functions in human subjects maintained in a lighting cycle, with a period of sleep in the dark indicated by the shading. The panels on the right show the same endpoints studied in subjects maintained for 40 hours on a constant routine, with very low levels of illumination and with wakefulness maintained, while the subjects remain constantly in a semirecumbent posture and receive small meals at regular intervals throughout the study period. Persistence of the rhythms in constant conditions reveals they are true circadian rhythms. The apparent loss of the activity rhythm in constant conditions is due to the protocol-enforced inactivity of the subject.
Adapted from Czeisler, Buxton, & Khalsa, 2005, with permission Copyright 2005, Elsevier.
In this chapter, we describe how circadian rhythms are generated, how they become synchronized to environmental cues, and their impact on physiology and behavior.
Overview of the Mammalian Circadian Timing System
A biological timing system necessarily consists of an intrinsic clock mechanism that measures time, an input mechanism that allows the clock to become synchronized or reset by changes in the environment, and output pathways that lead to generation of overt rhythms such as daily changes in locomotor activity, sleep, and hormone levels. The mammalian circadian timing system is considerably more complicated than this simple linear scheme, because it is composed of a hierarchy of circadian oscillators (Fig. 39.3). At the pinnacle of this oscillatory system is a small brain area, the suprachiasmatic nuclei (SCN) of the anterior hypothalamus. The SCN are often called the master circadian clock, because these nuclei (one on each side of the brain) play a key role in coordinating oscillations in other tissues and in regulating behavior. Many other cells and tissues also have the capacity to display an approximately 24-hour rhythmicity. The molecular mechanism underlying these cell-autonomous circadian oscillations is a transcriptional feedback loop.
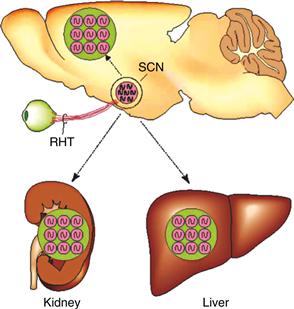
Figure 39.3 The mammalian circadian timing system consists of a hierarchy of oscillators. Oscillatory neurons in the SCN interact with each other to produce a set of coherent outputs. These outputs, which include behavioral and physiological rhythms, synchronize cell-autonomous oscillations in other brain regions and in peripheral tissues.
From Reppert & Weaver, 2002, with permission. Copyright 2002, Nature.
The primary input pathway to the SCN circadian clock is through retinal detection of light. Remarkably, the retinal photoreceptors that lead to visual image formation are not needed for circadian photoreception. Instead, a specialized population of retinal ganglion cells directly detect light, project to the SCN, and are necessary for photic entrainment of the SCN clock.
The main output of the SCN is encoded in neuronal firing rates. In addition, rhythmic neuropeptide secretion into the cerebrospinal fluid may be an important mechanism for regulation of downstream targets. Anatomically, the outputs of the SCN are focused within the hypothalamus but have widespread influence, consistent with the pervasive influence of the SCN on physiological functions ranging from the modulation of cognitive function to neuroendocrine responses. Output rhythms dependent on the SCN synchronize autonomous oscillators in other tissues. Local circadian oscillators regulate gene expression in a tissue-specific manner. Microarray studies conducted on brain, liver, heart, and kidney tissue in mammals reveal that 5 to 10% of the genome is rhythmically transcribed, and many of the genes that are rhythmically expressed represent key, tissue-specific metabolic control points. Thus, the circadian timing system has a pervasive impact on physiological processes via its output pathways.
The Suprachiasmatic Nuclei are the Site of the Primary Circadian Pacemaker in Mammals
The SCN have been recognized as the master circadian clock in rodents since the early 1970s (see Weaver, 1998). At that time, “new” techniques for anterograde tract-tracing were used to assess retinal projections, and they revealed a direct retina-to-SCN projection, the retinohypothalamic tract. To determine if the SCN were an important node on the anatomical pathway for detection of light for regulating rhythms, Moore and Eichler (1972) destroyed the rat SCN and observed loss of rhythmic serum corticosterone in a population of rats. Using a similar approach, Stephan and Zucker (1972) discovered that the rhythm of drinking in individual rats was lost following destruction of the SCN. Together, these two studies demonstrated the necessity of the SCN for circadian rhythmicity. Hundreds of subsequent studies have confirmed the necessity of the SCN for rhythmicity in physiology and behavior (Weaver, 1998; Welsh, Takahashi, & Kay 2010).
SCN: Oscillator and Pacemaker
Lesion studies leading to loss of rhythmicity do not necessarily show that the SCN function as the primary circadian pacemaker. An alternative possibility is that the SCN could be a necessary element on a key output pathway leading to physiological rhythmicity. The presence of rhythms in SCN metabolic activity, electrical activity, and gene expression profiles in vivo do not distinguish between these possibilities. Studies demonstrating rhythms in neuropeptide secretion, metabolic activity, and electrical firing rate in SCN tissue maintained in vitro do show that the SCN contain a functional oscillator. The SCN do not simply oscillate, however: through its outputs, the SCN regulate rhythms in physiology and behavior, and thus serve as a circadian pacemaker. This unique pacemaker role of the SCN is revealed most clearly by studies showing that rodents made arrhythmic by SCN lesion can have rhythmicity restored by transplantation of fetal hypothalamic tissue containing the SCN into the third ventricle of the lesioned adult. Furthermore, transplants between hamsters with different circadian cycle lengths revealed that the period of restored rhythmicity is dictated by the genotype of the SCN tissue donor (Ralph, Foster, Davis, & Menaker 1990). Thus, the SCN serve as a pacemaker that communicates rhythmicity to tissues regulating behavioral rhythms.
Summary
The SCN function as the master circadian pacemaker in the mammalian brain. The SCN oscillate in vivo and also when placed in vitro. More importantly, however, the SCN generate output signals that lead to physiological and behavioral rhythms. The SCN is positioned at the interface between the outside world (detected by retinal photoreceptors) and light-insensitive effector tissues.
A Hierarchy of Cell-Autonomous Circadian Oscillators
Single-Cell SCN Oscillators Are Coupled to Form a Coherent Clock
In rodents, the SCN contain approximately 10,000 neurons per side of the brain, with heterogeneity of neuropeptide phenotype, connectivity, and other properties (Silver & Schwartz, 2005; Morin, 2007). Despite this heterogeneity, most cells within the SCN are believed to have the capacity for cell-autonomous rhythmicity. Long-term rhythmicity in individual SCN neurons was first detected using extracellular recording of SCN neuronal firing rate (Fig. 39.4A). More recent studies reveal rhythmicity in single SCN cells using imaging methods designed to detect temporal variations in reporter gene expression (Fig. 39.4C).
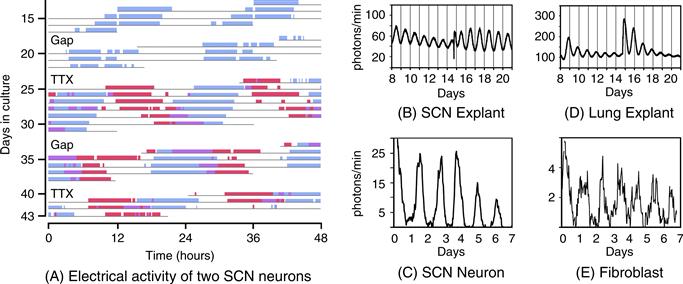
Figure 39.4 Cell-autonomous circadian rhythmicity in vitro. (A). Independently phased circadian rhythms in cultured SCN neurons. Spontaneous electrical activity of two SCN neurons in dispersed cell culture is shown in actogram-like format. Red bars represent one cell, while blue bars represent the other. The “activity” bars represent intervals when the neuronal firing rate for that cell is above its daily average. Where the active periods of the two cells overlap, they are shown in purple. Note that the two cells are cycling with independent period lengths. Gaps in the record represent days without recording. On two of these gaps, the culture was bathed with tetrodotoxin (TTX) for 2.5 days to block sodium-dependent action potentials. Note the molecular oscillations persist with unchanged period or phase despite TTX exposure. (Adapted from D.K. Welsh, Logothetis, Meister, & Reppert, 1995, with permission. Copyright 1995, Elsevier.) (B–E). Bioluminescence rhythms. Tissues and cells from mice with a rhythmically expressed luciferase reporter gene (encoding luciferase fused to PER2) were cultured in the presence of luciferin, and light emission was detected and plotted against time. Explants containing the SCN (B) or lung tissue (D) maintain rhythmicity in vitro. On the day labeled 14, culture media was changed, re-storing rhythm amplitude. Bioluminescence rhythms are also detectable, by imaging, from individual SCN neurons (C) and individual fibroblasts (E).
Adapted from A.C. Liu et al., 2007, with permission. Copyright 2007, Elsevier.
The cycle length of individual oscillating SCN cells is more variable than the period of animal behavior or the period of rhythms detected from slices of SCN tissue maintained in vitro. Thus, some mechanisms for interaction among the heterogeneous SCN neurons must exist, allowing SCN neurons to synchronize their diverse periods.
Vasoactive Intestinal Peptide (VIP) plays a key role in synchronizing the activity of SCN neurons into a coherent, functional oscillator (Aton, Colwell, Harmar, Waschek, & Herzog, 2005; Maywood et al., 2006). VIP is expressed in a subset of neurons in the “core” region of the SCN (Fig. 39.5). VIP-containing neurons and other core neurons project heavily within the SCN core, to the surrounding “shell” region, and also beyond the SCN borders (Fig. 39.5). Mice lacking VIP, or the VPAC2 receptor for VIP, have severely disrupted circadian rhythms. At the cellular level, the amplitude of individual neuronal oscillators is reduced in the absence of VIP or VPAC2 signaling, and the neurons are not coordinated to one another (Aton & Herzog 2005; Maywood et al., 2006). These findings strongly suggest that a “coupling deficit” leads to altered circadian rhythmicity in these lines of mice. VIP/VPAC2 acts through elevation of cAMP levels in SCN (Atkinson et al., 2011). Interestingly, the receptor for the insect neuropeptide Pigment Dispersing Factor (PDF) is closely related in structure to the VIP receptor (Helfrich-Förster, 2005). Moreover, PDF plays a role in the Drosophila (fruit fly) circadian system, which is strikingly similar to the role played by VIP in the mammalian SCN (Box 39.1). This indicates that the mechanisms underlying the communication between circadian neurons were established very early during the evolution of animals, at least 600 million years ago.
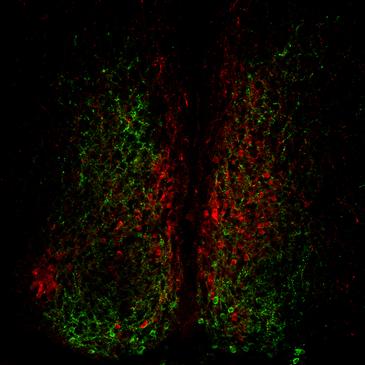
Figure 39.5 Neuropeptide expression in the mouse suprachiasmatic nucleus. Immunofluorescence image showing neurons expressing arginine vasopressin (AVP, red) in the dorsomedial aspect of the SCN, and neuronal cell bodies expressing VIP (green) in the ventral SCN. Note the extensive distribution of VIP-positive axons within the SCN.
Image courtesy of Nicola Smyllie and Mick Hastings, Laboratory of Molecular Biology, MRC, Cambridge, UK.
Box 39.1 Neuronal Control of Drosophila Circadian Behavior
One of the greatest strengths of Drosophila as a model to study circadian behavior (and more generally any behavior) is the remarkably sophisticated set of tools available to manipulate specific populations of neurons. Using tissue-specific “drivers” to express various genes, neurons can be eliminated, electrically silenced or forced to fire action potentials, for example. Levels of gene expression can also be manipulated by overexpression or RNA interference. With this wide panoply of genetic tools, researchers are beginning to understand the role of the complex mosaic of circadian neurons.
There are about 150 circadian neurons in the fly brain, divided in several groups named by their anatomical locations (see Figure B39.1). These clusters of circadian neurons can be further divided based on their morphology, and the neuropeptides or neurotransmitters they express. The small ventral Lateral Neurons (s-LNvs) function as pacemaker neurons in constant darkness (DD): they are necessary and sufficient for rhythmic locomotor behavior to persist in DD. These neurons secrete the neuropeptide Pigment Dispersing Factor (PDF). PDF is necessary for self-sustained behavioral rhythms and to synchronize molecular circadian rhythms in the brain. Interestingly, the phenotypes of flies mutant for either PDF or its receptor (PDFR, which is expressed on most circadian neurons) are remarkably similar to those of mice missing VIP or its receptor (VIPR): arrhythmicity and desynchronization between circadian neurons. Moreover, VIPR and PDFR share extensive sequences homologies. Thus, functional and molecular conservation of circadian rhythm mechanisms between mammals and insects is not limited to the circadian molecular pacemaker (see Box 39.2), but extend to circadian neural circuits.
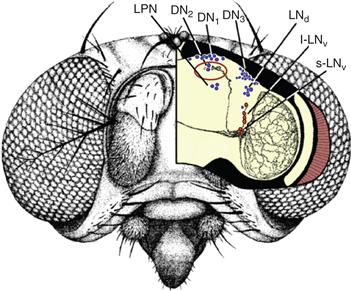
Figure B39.1 Circadian neurons in the brain of Drosophila melanogaster. Neurons expressing circadian proteins are shown in blue (Dorsal Neurons 1–3 [DN1, DN2, DN3], dorsal Lateral Neurons [LNd], and Lateral Posterior Neurons [LPN]). Neurons expressing circadian proteins and the circadian neuropeptide Pigment Dispersing Factor (PDF) are shown in red (small and large ventral Lateral Neurons [s-LNv and l-LNv]). The PDF-positive neural projections are drawn in black. The most important projections for the synchronization of the circadian network are the s-LNv projections directed toward the DN1 and DN2 groups. Indeed, PDF level oscillates in the termini of these projections (circled in red), presumably because PDF is rhythmically released.
Adapted from C. Helfrich-Förster, 2005, with permission. Copyright 2005, Elsevier.
Why have so many different circadian neurons if just 8 of them (the s-LNvs) can generate robust behavioral rhythms in DD? A first answer is the need to divide tasks. Fruit flies are mostly active at dawn and dusk under Light:Dark (LD) cycles. The PDF positive s-LNvs drive morning activity, but it is a different group of cells that drive evening activity: the dorsal Lateral Neurons (LNds). The s-LNvs and the LNds might thus increase their electrical activity at different times of the day to generate morning and evening bouts of activity.
A second reason for the need of a complex circadian neural network is adaptation to an ever-changing environment. Day length (photoperiod) and overall temperatures cycle during the course of the year at most latitudes, and random weather changes influence daily environmental conditions. It is becoming increasingly clear that one of the main purposes for the diversity of circadian neurons is the integration of environmental inputs and a proper adaptation to external conditions. For example, some circadian neurons (DN2s, LPNs) are particularly sensitive to temperature cycles. Others (l-LNvs, DN1s) appear to be important for responses to light inputs. Finally, the contribution of a subset of DN1s to circadian locomotor behavior is dependent on both ambient light intensity and temperature. Interestingly, the hierarchy between circadian neurons can change in response to light exposure. In the dark, or under short photoperiod, the PDF positive s-LNvs play a particularly important role in the control of circadian behavior, while under constant light or under long photoperiod PDF negative circadian neurons (LNds, DN1s) are dominant. This presumably helps flies to cope with seasonal changes in day length. Whether similar mechanisms act in mammals is not yet clear, but specific populations of SCN neurons adjust their firing cycles in response to changes in photoperiods.
Patrick Emery
Further Readings
1. Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Current Biology. 2008;18:R84–93.
2. Zhang Y, Emery P. Molecular and neural control of insect circadian rhythms. In: Gilbert LI, ed. Insect Molecular Biology and Biochemistry. San Diego: Academic Press; 2011:513–551.
The entire population of SCN neurons usually functions in concert as an orchestrating oscillator. Under specific conditions, however, SCN cells can become coupled into two functionally distinct subpopulations that can independently influence circadian behavior. For example, hamsters exposed to constant light for long periods of time display “splitting,” leading to two peaks of locomotor activity per 24 hours. Molecular analysis has revealed that the left and right SCN are oscillating in opposite phases in “split” hamsters, with each side regulating locomotor activity (de la Iglesia, Meyer, Carpino, & Schwartz, 2000). In rats, complex lighting cycles can be used to dissociate ventrolateral and dorsomedial subdivisions of the SCN from each other, resulting in simultaneous expression of rhythms with two different periods; each activity component is apparently driven by a different region of the SCN. Imaging studies reveal waves of gene expression progressing through the SCN in vitro, and subpopulations of neurons are coupled with differing relationships to the environmental lighting cycle. These experiments emphasize that the multi-oscillatory nature of the circadian system can be appreciated even within the SCN, and that coupling among SCN neurons normally prevents conflicting output signals.
Peripheral Oscillators
Despite sporadic reports of rhythmicity in isolated mammalian tissues, for years the dogma in the circadian field was that only the SCN possessed the capacity for self-sustaining, autonomous rhythmicity. The identification of a family of mammalian Period genes helped to change this perception. The mammalian Period genes are widely expressed (Zylka, Shearman, Weaver, & Reppert, 1998), suggesting that rhythmicity could occur in additional tissues. In addition, widespread, cell-automonous circadian clocks were demonstrated in Drosophila at about the same time (Plautz, Kaneko, Hall, & Kay, 1997).
That the capacity for circadian rhythmicity is not limited to the SCN in mammals was clearly demonstrated in a landmark study by Balsalobre, Damiola, and Schibler (1998). The field was shocked to learn that brief exposure to a high concentration of serum (“serum shock”) could lead to rhythmic gene expression in cultured fibroblasts. More recent single-cell analysis revealed that fibroblasts have ongoing (but uncoordinated) rhythms, and a serum pulse merely synchronizes these rhythms so that rhythmicity can be detected in the population (Nagoshi et al., 2004; Welsh et al., 2004; see Fig. 39.4). The serum shock is not a unique stimulus; acute activation of each of several pathways can induce rhythmicity in fibroblasts, suggesting that multiple signaling pathways converge upon the oscillator.
It is now generally believed that most mammalian cell types are capable of circadian oscillation. In normal circumstances, the rhythmicity in tissues outside the SCN is synchronized by SCN-dependent output signals. These signals include physiological and behavioral rhythms (e.g., body temperature and food intake) and daily fluctuations in hormone levels (e.g., glucocorticoids and melatonin). Thus, rhythms controlled by the SCN synchronize molecular rhythmicity among cells within a peripheral tissue (Fig. 39.3). In the absence of rhythmic input from the SCN, oscillators in some tissues become desynchronized, and thus the organ as a whole loses detectable rhythmicity. In the olfactory bulb, however, interactions among oscillating cells are sufficiently robust to maintain coordinated rhythmicity (Granados-Fuentes, Prolo, Abraham, & Herzog, 2004).
While the SCN function as the master circadian pacemaker in most circumstances, there is now compelling evidence that oscillators outside the SCN can regulate rhythmic behavior (Stephan, 2002; Taratoglu, Davidson, Benvenuto, & Menaker, 2006). While these oscillatory mechanisms are overshadowed in the presence of a functioning SCN, these studies reveal the potential impact of tissue-autonomous oscillators outside the SCN. Our understanding of the implications of the hierarchical nature of the mammalian circadian timing system is advancing rapidly.
Coordination of rhythms within a tissue may optimize organ-level physiological processes, while a circadian timing system in general may achieve “internal temporal order” among organs so that organs are prepared to function most efficiently. Circadian misalignment and disruption of internal coordination occur with shift work and following transmeridian travel (jet lag) (see Box 39.3).
Summary
Many cell types possess the capacity for oscillation with a cycle length near 24 hours. Neurons within the SCN oscillate and also communicate with one another, such that the SCN are able to provide a coherent output resulting in rhythmicity of other functions. Oscillating cells in other tissues generally require SCN-dependent signals, in the form of physiological rhythms, to remain synchronized, both within and between tissues.
The Molecular Basis for Circadian Oscillation is A Transcriptional Feedback Loop
The preceding discussion emphasizes that circadian rhythmicity occurs within single, isolated cells. But how do individual cells measure 24-hour time intervals? Studies conducted in many labs over the past 15 years have revealed a great deal about the molecular mechanisms underlying mammalian circadian rhythmicity (Fig. 39.6).
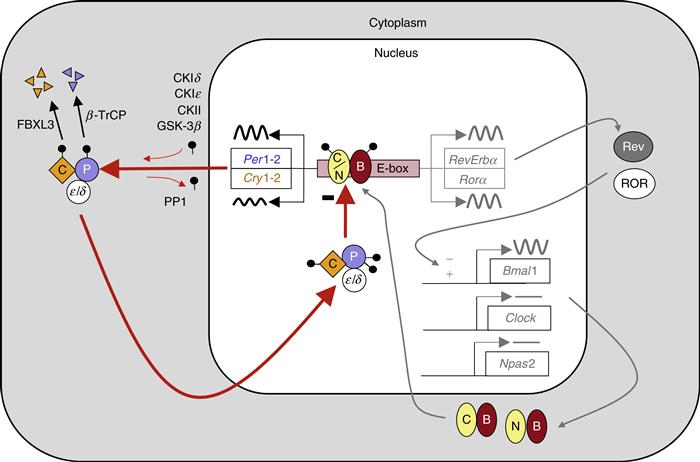
Figure 39.6 The molecular mechanism for circadian rhythms in mammals is a transcriptional feedback loop. Transcriptional activation leads to expression of Per and Cry genes. Posttranslational modifications, including phosphorylation (black lollipop) mediated in part by casein kinases (CKIδ, CKIε, CKII) and GSK-3β, affect the interactions of PER (P in blue circle) and CRY (C in gold diamond) proteins, promote their proteosomal degradation by F-box proteins (β-TrCP and FBXL3), and regulate nuclear entry of the inhibitory PER:CRY complex. The high-amplitude rhythm of PER production controls the timing of negative feedback. Collectively, these events lead to a delayed, negative feedback to shut off the transcriptional activation, resulting in a negative feedback loop (red arrows). The positive drive to the system comes from the transcription factors CLOCK (C in yellow oval) and BMAL1 (B in red oval), or NPAS2 (N in yellow oval) and BMAL1. The orphan nuclear receptors RORA (ROR) and REVERB-alpha (Rev) control the rhythmic expression of Bmal1, with peak levels occurring opposite to the peak in Per expression. The antiphase rhythmicity of this second feedback loop is not essential for rhythm generation, but BMAL1 itself is necessary.
Progress made in understanding the circadian clock mechanisms in the fruit fly, Drosophila melanogaster, was instrumental in developing our current understanding of mammalian clock mechanisms (Box 39.2). Most of the genes involved in the fly circadian mechanism have mammalian homologs, and their roles in circadian function have now been examined in mammals. While there have been some alterations in gene products performing specific functions, and gene duplication in mammals has led to greater complexity, the general features of the core clock mechanisms are similar between flies and mammals (Box 39.2). This demonstrates again the ancient origin of circadian timing mechanisms in the animal kingdom.
Box 39.2 Molecular Mechanisms of Circadian Timekeeping in Drosophila
Virtually nothing was known about the genetic and molecular mechanisms controlling circadian rhythms until the late 1960s, when Seymour Benzer and his graduate student, Ron Konopka, identified the first mutations affecting circadian behavior. Most scientists at the time were skeptical that individual genes could affect complex behaviors. Benzer was nevertheless determined to identify the genetic underpinnings of behavior, and embarked on an ambitious program using the animal model of choice for genetic studies, the fruit fly Drosophila melanogaster. Benzer and colleagues exposed large numbers of flies to a chemical mutagen to isolate mutant lines with abnormal behavior in various assays. To identify circadian genes, Konopka screened for mutant fly lines that had lost the ability to predict dawn, as assessed by observing the time of day at which the young flies emerged from their pupal cases. Using this approach, Konopka and Benzer isolated three mutant lines with abnormal circadian rhythms. In one line, the flies emerged from their pupal cases too early, in a second line they were too late, while in a third line the flies emerged at random times. All three mutations mapped to the same genetic locus, named period (per). Importantly, the three period mutants had similar circadian defects in their locomotor activity rhythms, which provided strong evidence that Konopka and Benzer had identified a gene central to the circadian pacemaker.
Benzer’s pioneering idea of using unbiased genetic screens to identify genes involved in circadian behavior has led to identification of additional “clock genes” by several other labs. The subsequent genetic, molecular, and biochemical studies have resulted in a remarkably detailed understanding of the Drosophila circadian pacemaker (see Figure B39.2). CLOCK (CLK) and CYCLE (CYC) are two basic helix-loop-helix transcription factors that heterodimerize and bind to E-boxes within the per and timeless (tim) promoters. PER and TIM dimerize in the cytoplasm, with TIM stabilizing PER. Several kinases (DOUBLETIME, CASEIN KINASE 2, NEMO, and SHAGGY [the Drosophila homolog of glycogen synthase kinase 3]) and at least two phosphatases (PROTEIN PHOSPHATASE 2A and PROTEIN PHOSPHATASE 1) regulate the stability and thus the rate of PER and TIM accumulation, and the timing of their nuclear entry. Once in the nucleus, the PER/TIM heterodimer binds to the CLK/CYC dimer and disrupts its transcriptional activity and its interaction with E-box sequences. The inhibition of per and tim gene expression is maintained for several hours until the hyperphosphorylated and unstable PER and TIM proteins decay. CLK/CYC can once again bind to E-box sequences and restart the circadian cycle. The kinase DOUBLETIME, which translocates into the nucleus with PER and TIM, also plays an important role for repression by regulating CLK phosphorylation and degradation. The CLK/CYC, PER/TIM feedback loop is essential for maintaining rhythms in Drosophila. A secondary feedback loop might contribute to the precision and robustness of circadian oscillations through the regulation of clk mRNA levels. The Drosophila molecular clock is primarily synchronized to environmental light/dark cycles by an intracellular photoreceptor, CRYPTOCHROME (CRY). Light activates CRY, and CRY then binds to and triggers the rapid degradation of TIM. Since TIM stabilizes PER, the degradation of TIM also results in a reduction in PER levels that resets the circadian pacemaker.
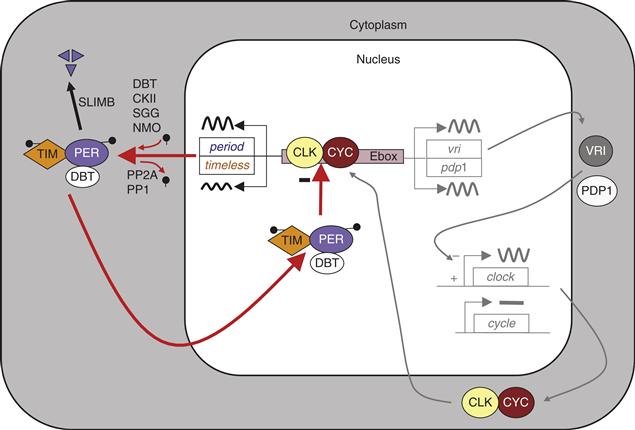
Figure B39.2 Model of the molecular circadian clock in Drosophila. In the core transcriptional feedback loop (left side of the figure), CLOCK (CLK) and CYCLE (CYC) positively regulate period (per) and timeless (tim) transcription. PER is phosphorylated by DOUBLETIME (DBT), CASEIN KINASE II (CKII) and NEMO (NMO), while TIM is phosphorylated by SHAGGY (SGG) and CKII. PER and TIM phosphorylation levels are negatively regulated by PROTEIN PHOSPHATASE 1 and 2A (PP1 and PP2A). Hyperphosphorylated PER is ubiquitinated by SLIMB and degraded by the proteasome. PER and TIM enter into the nucleus with DBT to inhibit the activity of CLK/CYC. In a secondary feedback loop shown in grey, VRILLE (VRI) and PAR DOMAIN PROTEIN 1ε (PDP1) regulate clk transcription.
As the first components of the mammalian molecular clock were identified in the late 1990s, it quickly became clear that important concepts learned from the Drosophila circadian clock also apply to mammals. The circadian pacemaker of both Drosophila and mammals involve homologous proteins in analogous transcriptional feedback loops. Strikingly, the first identified genetic cause of a human circadian rhythm disorder, Familial Advanced Sleep Phase Syndrome, is a mutation in human Per2, a homolog of the Drosophila per gene.
Ania Busza and Patrick Emery
Further Readings
1. Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster, Proc Natl Acad Sci USA. 1971;68:2112–2116.
2. Hardin PE. The circadian timekeeping system of Drosophila. Current Biology. 2005;15:R714–722.
3. Zhang Y, Emery P. Molecular and Neural Control of Insect Circadian Rhythms. In: Gilbert LI, ed. Insect molecular biology and biochemistry. Academic Press 2011:513–551.
4. Weiner J. Time, love, memory: a great biologist and his quest for the origins of behavior New York: Alfred A. Knopf; 2000.
The molecular mechanism for measuring time on a 24-hour time-scale is based on a self-sustaining transcriptional/translational feedback loop present within individual cells (Fig. 39.6). According to the current model for this molecular feedback loop (Reppert & Weaver, 2002; DeBruyne, 2008; Welsh et al., 2010), pairs of basic helix-loop-helix, PAS-domain containing (bHLH-PAS) transcription factors dimerize and activate transcription of several genes, including Period and Cryptochrome genes, through specific promoter elements called E-boxes. CLOCK and BMAL1 are the major transcriptional activator complex, but in a subset of SCN neurons, NPAS2 and BMAL1 heterodimers form and can maintain rhythmicity. High-amplitude rhythmicity in the expression of two Period genes (Per1 and Per2; Fig. 39.7) is believed to be rate-limiting for formation of complexes between the PERIOD (PER) and CRYPTOCHROME (CRY) proteins. PER:CRY-containing protein complexes recruit other proteins, and this complex disrupts the activity of the CLOCK:BMAL1 complex. The temporally regulated nuclear accumulation of PER:CRY complexes is thought to be critical for closing the feedback loop. Thus, molecular oscillations occur with a cycle length of approximately 24 hours, in which a phase of CLOCK/NPAS2:BMAL1-mediated transcriptional activation is followed by phase of PER:CRY-mediated repression. It is important to note that this transcriptional feedback loop regulates additional genes, including other transcription factors, thus generating rhythmic expression of a large number of genes (Jin et al., 1999; Ueda et al., 2005; Kornmann, Schaad, Bujard, Takahashi, & Schibler, 2007; Miller et al., 2007; Atwood et al., 2011).

Figure 39.7 High-amplitude circadian rhythms of mPer gene expression in the mouse SCN. Left: Adjacent sections through the SCN from brains collected at the time of peak Per transcript levels during the subjective day (left column) or during the middle of the subjective night (right column) were processed for in situ hybridization to detect mPer1 and mPer2 mRNAs. “Subjective” refers to the fact that the animals were in constant darkness on the day of tissue collection, rather than in a lighting cycle. The SCN are indicated by an arrowhead in each panel. Adapted from Shearman, Zylka, Weaver, Kolakowski, & Reppert, 1997, with permission. Copyright 1997, Elsevier. Right: Per RNA (open circles and dashed lines) and PER protein (filled circles and solid lines) rhythms in mouse SCN. mPer1 (upper panel) and mPer2 (lower panel) RNA levels were assessed by in situ hybridization from samples collected on the first day in constant darkness. Protein levels were determined by counting nuclei within the SCN from sections processed for immunohistochemical detection of mPER1 and mPER2. The bar below the panel represents the lighting cycle the animals were exposed to on days prior to the day of tissue collection. Circadian Time 0–12 corresponds to subjective day, when the lights would have been on had the animals remained in a lighting cycle.
Data from Shearman et al., 1997 and Hastings, Field, Maywood, Weaver, & Reppert, 1999.
Post-translational modifications build a time delay into the circadian oscillation. Without this delay, transcription of Per genes would rapidly lead to nuclear accumulation of PER:CRY complexes and a premature inhibition of transcription. In this case, the clock would either cycle with a very short period or the system would equilibrate to a nonrhythmic steady state. Phosphorylation of the PER proteins is critical for this time delay. Phosphorylation alters their intracellular localization, protein:protein interactions, and stability, and thereby contributes to regulating circadian cycle length. Rhythmic phosphorylation of PER proteins is due at least in part to the activity of casein kinase 1 delta (CK1δ) and CK1 epsilon (CK1ε) and mutations of these kinase genes or their pharmacological inhibition alters circadian cycle length in flies, hamsters, mice, and humans. A circadian-based sleep disorder in humans is due to alterations in CK1-mediated phosphorylation of PER2 proteins, leading to rhythms having an unusually early phase (Box 39.3). Phosphorylation of clock proteins alters their activity and can also target them for proteosomal degradation; mutations in the gene encoding FBXL3, which participates in phosphorylation-dependent ubiquitination of CRY proteins, slow the circadian cycle in mice (Gatfield & Schibler, 2007). β-TRCP is responsible for the ubiquitination and degradation of PER proteins. The fine balance between kinases (including CK1δ/ε and CK2) and phosphatases (notably protein phosphatase 1), and subsequent phosphorylation-dependent degradation contribute greatly to regulating circadian period length.
Box 39.3 Circadian-Based Sleep Disorders, Clock Genes and Neuropsychiatric Diseases
The timing of sleep observed in adult humans normally is from approximately midnight to 8 a.m. Most people will voluntarily move sleep to a later time when free of work constraints and readily readjust their schedule back to an earlier time when the workweek begins again. There are developmental changes in the “normal” timing of sleep, with adolescents being prone to a later phase and the elderly typically assuming an advanced (earlier) phase. These developmental changes in preferred phase of activity and sleep mirror the natural variation within adult populations, with early-rising “larks” and late-rising “night owls” being readily recognizable patterns. At the extreme, however, these differences in timing of activity and sleep can disrupt social life and employment. These extreme cases are referred to as circadian-based sleep disorders (see Figure B39.3).
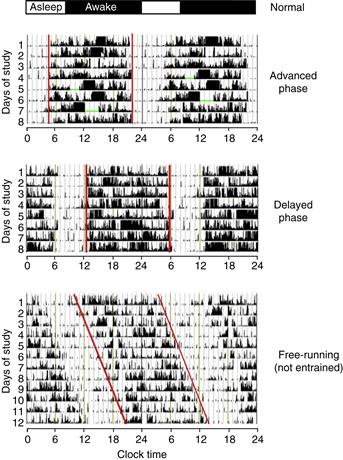
Figure B39.3 Disorders of sleep timing in humans. The typical timing of sleep and wake is shown at the top of the figure. Below are double-plotted activity records of three individuals. Activity was recorded by a motion-sensitive wristwatch. Activity appears as vertical deflections in the records. Intervals where the watch was taken off are indicated by green bars. Red lines mark activity onset and offset. The top actogram illustrates an individual with advanced sleep phase syndrome; activity occurs between 0400 and 2000. The second pattern shows delayed sleep phase syndrome; activity occurs from 1300 to 0600. The lower actogram shows an individual that is free-running due to failure to entrain to environmental cues. Modified from Sleep, Medicine, vol 8, Barion, A., & Zee, P.C., “A clinical approach to circadian rhythm sleep disorders,” pp. 566–677, Copyright 2007, with permission from Elsevier.
Advanced sleep phase syndrome (ASPS) is characterized by early-morning awakening and an inability to maintain wakefulness into the evening (the typical wake interval is from 0400 to 2000; see top panel of figure). Studies of familial cases of ASPS have identified two genes that, when mutated, contribute to ASPS. Remarkably, these genes were already discussed in the context of the core circadian feedback loop in mice. The mutations defined in humans that lead to familial ASPS are a Serine to Glycine mutation at position 662 of human PER2 (within the casein kinase interacting domain of PER2), and a Threonine to Alanine mutation at position 44 of casein kinase I delta (a mutation that increases the ability of the kinase to phosphorylate PER2).
Delayed sleep phase syndrome (DSPS) is characterized by late awakening and late bedtimes (typical wake interval from noon to 0400), and an apparent inability to reset the clock to earlier times of day. This disorder frequently begins in adolescence or young adulthood and tends to be sporadic rather than familial.
“Non-24-hour sleep wake syndrome” has also been described, in which individuals fail to entrain to the 24-hour day (see bottom panel of figure). This condition is relatively frequent in blind individuals. Affected individuals have intervals where their biological day and night coincide with the solar day and night, but then their free-running period (usually ~24.5 hours) causes them to drift out of phase with the environment. This results in periods of insomnia and daytime drowsiness (when the individuals are out-of-phase) alternating with periods of coordination with the environment. Melatonin, administered at 24-hour intervals, is useful in synchronizing some blind individuals to a 24-hour cycle length.
Several studies have identified genetic differences between “owls” and “larks.” Specific haplotypes of the Per3 gene, in particular, have been associated with delayed diurnal preference and with delayed sleep phase syndrome. Thus, even within the normal range of sleep timing, phase preference may have a significant genetic component.
Circadian rhythms are phase-advanced in depression, and rhythm alterations occur in other neuropsychiatric and neurodegenerative diseases. Recent studies reveal increased prevalence of certain disorders in subjects with specific alleles of circadian-relevant diseases. Circadian rhythm alterations may be a risk factor for neuropsychiatric disease.
David R. Weaver
Further Reading
1. Dijk D-J, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Medicine Reviews. 2010;14:151–160.
2. Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nature Medicine. 1999;5:1062–1065.
3. Kennaway DJ. Clock genes at the heart of depression. Journal of Psychopharmacology. 2010;24(2 Suppl):5–14.
4. Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043.
5. Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644.
6. Menet JS, Rosbash M. When brain clocks lose track of time: cause or consequence of neuropsychiatric disorders. Current Opinion in Neurobiology. 2011;21:1–9.
An interlocked, second feedback loop is important for the rhythmic regulation of BMAL1. Two members of the orphan nuclear receptor gene family, Rev-erb-alpha and Rora, are among the genes regulated by CLOCK and BMAL1. The protein products of these genes compete for binding to an orphan nuclear receptor binding site in the promoter of the Bmal1 gene and have antagonistic effects on Bmal1 gene expression. Overexpression of the repressor, REV-ERB-α in the liver greatly reduces Bmal1 expression, leading to liver-specific loss of rhythmicity (Kornmann et al., 2007). While BMAL1 is necessary for rhythmicity, rhythmic BMAL1 production appears relatively unimportant for maintaining rhythmicity.
Molecular Redundancies Revealed by Studies of Knockout Mice
The importance of the genes discussed above in the circadian mechanism has been revealed in part through studies of mice with targeted gene disruption. These studies reveal that, in most cases, there is at least partial redundancy of function among members of these gene families (Table 39.1). Mice homozygous for mutation of either mPer1 or mPer2 alone have period defects and/or a gradual loss of rhythmicity, while mice homozygous for disruption of both mPer1 and mPer2 become arrhythmic immediately in constant darkness. Notably, however, the phenotype of mice homozygous for mutation of either Per gene is markedly affected by genetic background; single Per mutations are almost without effect on the C57BL/6J background (Pendergast, Friday, & Yamazaki, 2010), indicating redundancy between the PER proteins.
Table 39.1 Gene Families Regulating Mammalian Circadian Rhythms

Mutations and mutant phenotypes listed were studied in mice, except as noted by asterisks: * indicates Human, ** indicates Syrian hamster. Missense mutations are those that lead to a change in a single amino acid residue; the residue and the change are indicated in single letter format: S662G indicates that a Serine residue at position 662 of human PER2 is mutated to Glycine.
Alleles designated as “Deletion” produce some protein products, but the deletion of genomic material results in deletion-mutant protein products that appear to lack functional activity. From a functional point of view, these are viewed as null alleles. “Null” alleles are those where there is either no product or the product is so truncated that it is irrelevant. “Dominant-negative” alleles result in protein products that are altered in activity, such that the mutant product interferes with the activity of the product from the normal allele, thus producing a mutant phenotype in heterozygotes and a more severe phenotype in homozygotes (semidominant inheritance). “Gain-of-function” alleles are hyperactive in performing the function of the mutated gene. Additional genes not listed may modify the circadian feeback loop in more subtle ways. Results of overexpression studies are not included.
Mice with targeted disruption of either mCry1 or mCry2 have altered circadian period lengths (~22.5 and ~24.5 hours, respectively), but the double-mutant mice completely lose circadian rhythmicity immediately upon placement in constant darkness.
While mice homozygous for a semidominant mutation in the Clock gene (ClockΔ19) have significant behavioral and molecular phenotypes, mice completely lacking CLOCK protein retain behavioral rhythmicity (Fig. 39.8). As noted earlier, the closely related bHLH-PAS transcription factor NPAS2 (also called MOP4) maintains circadian rhythms in the SCN, even in the absence of CLOCK (Fig. 39.8). CLOCK appears to be much more abundant than NPAS2 in the SCN, and transcriptional regulation occurs primarily through CLOCK:BMAL1 heterodimers in SCN and other tissues. BMAL1 is necessary for maintenance of behavioral rhythmicity (Bunger et al., 2000). Overexpression of BMAL2, a protein closely related to BMAL1, can restore rhythmicity in BMAL1-deficient mice (Shi et al., 2010). BMAL2 expression is greatly reduced in BMAL1-deficient tissues; the BMAL1-deficient mouse may be a functional double-knockout, explaining this apparent departure from the trend for functional redundancy among gene family members.

Figure 39.8 Molecular redundancy in the maintenance of mouse circadian rhythms. Panels A–D show double-plotted actograms from (A) a wild-type mouse, (B) an NPAS2-deficient mouse, (C) a CLOCK-deficient mouse, and (D) a double-mutant (CLOCK-deficient, NPAS2-deficient) mouse. The animals were initially housed in a light:dark cycle (LD), and then the lights were disabled and they entered constant darkness (DD). Note that the double-mutant mouse becomes arrhythmic in DD, while the mice of the other genotypes “free run” with a cycle length of less than 24 hours. The apparent rhythmicity when the double-mutant mouse is housed in LD is due to partial suppression of locomotor activity by light, a phenomenon called negative masking. Plotting conventions as in Fig. 1.
Modified from DeBruyne, Weaver, & Reppert, 2007, with permission from Nature Neuroscience.
Posttranslational modifications were discussed briefly above. Disruption of CK1δ cause period lengthening, while null mutations of CK1ε have only a very modest effect. Pharmacological inhibition of both CK1δ and CK1ε greatly lengthens period, indicating compensation by one kinase in the absence of the other is occurring in the genetic models. “Activating mutations” of CK1δ or CK1ε shorten cycle length. Mutation or inhibition of ubiquitin ligases (Fbxl3 or β–TrCP) lengthens period.
The preceding discussion addresses the phenotype of intact mutant mice, using behavioral and physiological rhythms to assess the functional state of the SCN circadian clock. Notably, however, studies of dissociated SCN neurons reveal that a more severe phenotype is observed at the level of single SCN neurons from knockout mice, relative to SCN explants maintained under similar conditions or the in vivo phenotype (Liu et al., 2007; Welsh et al., 2010). A small population of weakly functional oscillators apparently can use the network properties of the SCN to generate coherent rhythmicity (DeBruyne, 2008; Welsh et al., 2010).
Clock-Controlled Genes: From the Core Clockwork to Cellular Rhythms
Genes that participate in the core negative feedback loop and whose gene family are necessary for its function are called “clock genes.” The impact of the molecular feedback loop occurs through the rhythmic regulation of “clock-controlled genes,” which are distinguished from clock genes by the fact that they do not feed back on the core oscillatory mechanism in a manner that makes them necessary for rhythmicity. Some clock-controlled genes with E-box elements in their regulatory regions are controlled in the same manner as Pers and Crys: their transcription is directly activated by CLOCK:BMAL1 and inhibited by the activity of the PER/CRY complexes disrupting this transcriptional activation. The neuropeptide arginine vasopressin (AVP) is a prototype for this mechanism (Jin et al., 1999). The rhythmic expression of AVP mRNA in the SCN leads to a high-amplitude circadian rhythm of AVP peptide in the cerebrospinal fluid that is insulated from the more well-known osmotic regulation of AVP in blood (see Chapters 37 and 38). The rhythmic synthesis and secretion of AVP from SCN neurons may modulate electrical activity of nearby neurons, increasing the amplitude of the ensemble SCN neuronal firing rhythm and thus affecting many rhythms. AVP of SCN origin also appears to play a role in regulation of the hypothalamo-pituitary-adrenal axis (Chapter 38).
Notably, products of some genes regulated by the CLOCK:BMAL1 complex are themselves able to regulate transcription through additional regulatory elements. For example, a family of D-element binding proteins (DBP/HLF/TEF and E4BP4) are controlled via E-box elements, and these proteins compete for occupation of elements in responsive genes. Regulation of Rev-erb-α and Rora, noted previously, also occurs through E-box mediated activation. Thus, the rhythmic regulation of these transcription factors, and the multiple regulatory element combinations that can occur in responsive genes, results in a cascade leading to rhythmic gene expression with many possible peak times of expression and with tissue-specific regulation (Ueda et al., 2005).
Summary
The basis for circadian oscillation is a molecular feedback loop. A phase of transcriptional activation alternates with a phase of transcriptional repression, and the cycle length of this rhythm is approximately 24 hours. The bHLH-PAS proteins CLOCK and NPAS2 heterodimerize with BMAL1 to produce E-box-dependent transcriptional activation. Among the genes controlled primarily by E-box regulation are Per1 and Per2; their high-amplitude temporal regulation is critical to determining the timing of transcriptional repression. PER protein abundance regulates the formation of inhibitory complexes containing PERs, CRYs, and casein kinase proteins. These complexes recruit transcriptional repressors and negate the activity of the activator complex. Post-translational events including phosphorylation and ubiquitination of PERs and CRYs are important for building a time delay into the feedback loop; altering these activities alters circadian cycle length. In parallel with the regulation of “clock genes,” the molecular feedback loop regulates the expression of effector genes and also of transcription factors. Through these concentric waves of transcriptional activity, the circadian clock can affect the expression of key (often rate-limiting) genes, in a tissue-specific manner.
Circadian Photoreception in Mammals
In the following sections, we discuss the mechanisms by which the circadian clock in the mammalian SCN is synchronized to the daily light:dark cycle.
Entrainment of the SCN Pacemaker Occurs through Daily Phase Shifts
The intrinsic period of the circadian pacemaker is revealed by measuring it in constant environmental conditions, or in animals made insensitive to light by disruption of anatomical pathways from the eye to the SCN. Rhythms that are not entrained (e.g., not synchronized by a rhythmic environmental cue) are referred to as “free-running” rhythms. The period length of rhythms that are free-running is usually not exactly 24 hours (Fig. 39.1). Instead, the circadian oscillator has a species-typical intrinsic period. Rats housed in constant darkness have a free-running period of approximately 24.3 hr, while mice under the same conditions free-run with a period of approximately 23.6 hr. There are strain variations within these species as well.
For an animal to entrain to a light:dark cycle that has a 24-hour cycle length, light must shift the animal’s clock each day by an amount equal to the difference between their intrinsic, free-running period and the 24-hour period of the lighting cycle. While it might seem simpler for the clock to have a cycle length of exactly 24 hours (and forget about daily readjustments), there is seasonal variation in lighting conditions in natural environments, most notably a seasonal change in the length of the light period each day. A timekeeping system that tracks dusk and dawn each day is needed for accurate entrainment under these circumstances.
In assessing the response of the circadian system to light, it is often more informative to assess the response of animals to brief light pulses rather than to full light-dark cycles. A brief pulse of light is provided when an animal is free-running in constant conditions, allowing control over the timing, intensity, and even wavelength of the stimulus. Experiments of this type reveal that the response of the circadian system to light depends strongly on the time of the day at which the light exposure occurs (Fig. 39.9). In constant conditions, the time when the animal “thinks” it is night (as reported by its locomotor activity rhythm) is referred to as “subjective night,” while the time that corresponds to internal daytime is “subjective day.” Light falling early in the subjective night leads to a phase delay—for example, activity on subsequent days begins at a later time, consistent with a light-induced shift in the timing of the oscillator (Fig. 39.9C). Conversely, light exposure late in the night will cause a phase advance (Fig. 39.9D, E). During large portions of the animal’s subjective day, light exposure does not lead to a phase shift (Fig. 39.9A). Plotting the response to brief pulses of light against the time of the light exposure leads to a phase response curve (PRC; Fig. 39.9F). In general, the shape of the PRC to brief light pulses is very similar across species, whether nocturnal, diurnal, or crepuscular, and regardless of whether their intrinsic period is less than or greater than 24 hours. With very strong stimulation (e.g., long duration or bright light exposure) the clock can be reset to a specific point in the circadian cycle (so-called “type 0 resetting”). Many pharmacological stimuli also cause phase-dependent phase shifts of the SCN oscillator, and the shape of the PRC to nonphotic stimuli differs from the PRC to light.

Figure 39.9 Phase-dependent effects of light on circadian rhythmicity. (A–E) Schematic representation of the phase-dependent phase shifts that occur following exposure of free-running animals to brief pulses of light. Each panel represents an actogram of one animal housed in constant darkness and exposed to a single 1-hour light pulse (yellow box). The phase shift is the difference, on the day of the light pulse, between a line drawn through activity onsets before the light pulse (blue lines) with a line through activity onsets after the light pulse (red line). In some cases, the behavioral shift is not complete on the first cycle after the light exposure, and the shift seems to occur over several days. The intermediate activity onsets are called “transients” and are ignored when determining the phase after the shift. (F) Phase Response Curve. The phase shift induced by a brief light pulse is highly dependent on the time at which the light exposure occurred. Circadian Time (CT)12 is defined as the time of activity onset in a nocturnal animal. Light exposure occurring during the biological daytime (CT0–12) does not cause a shift. Responses occur only during the animals’ biological nighttime. Early in the subjective night, light exposure delays the clock, so activity begins at a later time on subsequent cycles. Late in the subjective night, light exposure causes a phase advance, as detected by earlier activity onsets on the days after the light pulse. Phase-dependent phase shifts allow the circadian oscillator to be entrained to environmental cycles.
A Specific Retinal Photoreceptive System Mediates Circadian Entrainment to Light
In mammals, detection of light by the circadian system requires the presence of the retina. A direct anatomical connection from the retina to the SCN was first identified in the early 1970s. This retino-hypothalamic tract is necessary and sufficient for photic entrainment of circadian rhythms.
Remarkably, the retinal photoreceptors required for visual image formation are not necessary for circadian photoreception. Studies of mutant mice show that circadian sensitivity to light persists even with virtually complete loss of the rods and cones. Other nonvisual responses to light (inhibition of locomotor activity, suppression of nocturnal melatonin secretion, and constriction of the pupil in response to light) also occur in the absence of rods and cones. These findings suggested that a nonrod, noncone photoreceptive cell mediates these “nonvisual” responses to light.
An opsin-like pigment, melanopsin, was initially identified as a putative photopigment in the light-responsive melanophores of frog skin, and was later found to be expressed in a subset of retinal ganglion cells (RGCs) in the mammalian retina (Provencio, Jiang, De Grip, Hayes, & Rollag, 1998; Provencio, 2011). More recently, melanopsin has been shown to contribute to circadian photoreception in the mammalian retina. Melanopsin is expressed in a relatively small population (~2400) of RGCs in each mouse eye (Fig. 39.10A). Melanopsin-containing retinal ganglion cells respond directly to light, even when isolated from the rods and cones. Their intrinsic photosensitivity requires the presence of melanopsin.
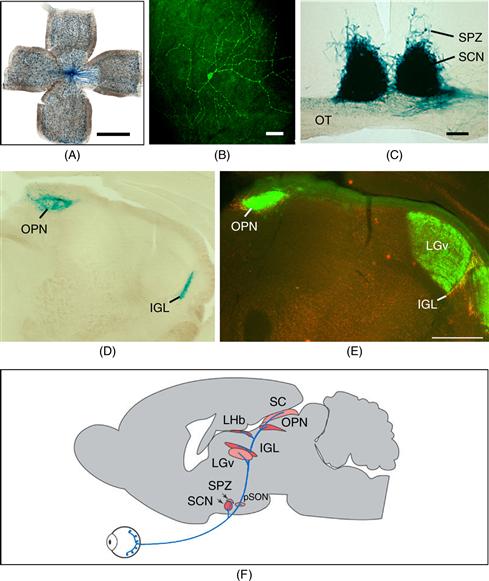
Figure 39.10 Retinal ganglion cells containing melanopsin innervate the SCN and other “nonvisual” targets. (A) Melanopsin-expressing retinal ganglion cells. Melanopsin-expressing retinal ganglion cells were detected in this whole-mount preparation of a postnatal day 5 mouse retina using a beta-galactosidase reporter gene inserted into the melanopsin locus. Tissue was processed for histochemical detection of beta-galactosidase activity, resulting in deposition of blue reaction product in cells and processes containing melanopsin. Note the sparse distribution of labeled retinal ganglion cells (individual blue dots); axons course toward the head of the optic nerve in the center of the field. Scale bar is 1 mm. Images in panels A, C, D, E, and F provided by Samer Hattar, Johns Hopkins University. (B) Immunofluorescence image of a ganglion cell in the human retina stained for melanopsin. Melanopsin immunoreactivity appears green. Note the large dendritic field of the cell, the localization of melanopsin throughout the dendritic tree, and dendritic varicosities. These cells are ill-suited for spatial localization, but are ideally suited for luminance detection because the entire cell is photosensitive. Scale bar = 100 microns. Image provided by Ignacio Provencio, University of Virginia. (C) The SCN receives a strong, bilateral input from melanopsin-containing RGC’s. Central projections of melanopsin-expressing retinal ganglion cells were detected using a beta-galactosidase reporter gene inserted into the melanopsin locus as described in Panel A. Melanopsin-containing cell bodies are present only in the retina, so the blue staining seen in the brain represents axonal projections of these retinal neurons. The right eye of the animal had been removed over 2 weeks prior to staining. Labeled, retinal fibers in the optic tract (OT) have degenerated on the apparent left side of the image, leaving weak, granular labeling. The staining in the SCN remains strong on both sides, indicating the extensive, bilateral projections to the SCN. Melanopsin-containing processes also extend into the subparaventricular zone (SPZ). Scale bar = 100 microns. (D) Central projections of melanopsin-containing retinal ganglion cells, revealed by beta-galactosidase staining. The olivary pretectal nucleus (OPN) and intergeniculate leaflet (IGL) receive a crossed input from melanopsin-expressing retinal ganglion cells. (E) Melanopsin is localized within a subset of retinal projections. The anatomical level is comparable to Panel D. Projections of the entire population of retinal ganglion cells were revealed by intraocular injection of cholera toxin B subunit three days prior to tissue collection, allowing anterograde labeling of the fibers of all retinal ganglion, followed by detection of the toxin which appears green. Note the intense labeling in the dorsal lateral geniculate nucleus (LGd). Melanopsin expression in retinal terminals was detected by red immunofluorescence; in this merged image, the overlap of red and green channels in the melanopsin-containing retinal ganglion cells yields yellow labeling in the IGL and OPN. Scale bar (for panels D and E) = 500 microns. (F) Schematic illustration of the axonal projections of the M1 class of melanopsin-expressing retinal ganglion cell as revealed by beta-galactosidase staining. Principle targets (SCN, OPN, IGL, and lateral habenula [LHb]) are indicated by dark red shading. Less extensively innervated structures indicated by light red shading are the SPZ, superior colliculus, peri-supraoptic nucleus, and ventral subdivision of the lateral geniculate nucleus. Minor targets (not shown) include the preoptic area, bed nucleus of the stria terminalis, medial amygdaloid nucleus, anterior hypothalamic area, lateral hypothalamus, LGd, and periaqueductal gray. (See Hattar et al., 2006.)
The population of intrinsically photosentitive RGCs (ipRGCs) is heterogeneous in laminar distribution of dendritic arborization, cellular morphology, level of expression of melanopsin, central projections, and also in function (Schmidt, Chen, & Hattar, 2011). Five subtypes (M1–M5) are recognized (Ecker et al., 2010). Melanopsin can be detected in the M1 subtype by immunohistochemistry (Fig. 39.10B). Mice in which lacZ is inserted into the melanopsin locus provide another way to view melanopsin-expressing cells, although beta-galactosidase staining is detectable only in the M1 subtype. This blue stain is also readily detected in the M1 cells’ projections into the central nervous system (Fig. 3.10C,D). The M1’s densely innervate the SCN, the intergeniculate leaflet (IGL), and the olivary pretectal nucleus (OPN), a site critical for pupillary constriction in response to light. Additional projections of the M1 class provide innervation to the ventrolateral preoptic area, important in the regulation of sleep, and to the ventral subparaventricular zone (vSPZ), implicated in the inhibition of locomotor activity by light. The M1 class (~700 RGC’s) can be further subdivided based on expression of the transcription factor Brn3b. The Brn3b-negative M1 group (~200 cells) is critical for circadian photoentrainment (Chen, Badea, & Hattar, 2011). The Brn3b-expressing M1’s mediate pupillary constriction in response to light (Güler et al., 2008). Remarkably, ipRGCs are sufficient for visual pattern discrimination and photic induction of immediate-early gene expression in visual cortex, even in the absence of rod-cone signaling (Ecker et al., 2010).
The phototransduction mechanism in melanopsin-containing cells appears to be more similar to the invertebrate visual signaling cascade (in which phosphoinositide signaling activates cation channels of the transient receptor potential channel type), rather than through the typical vertebrate photoreceptor cascade involving transducin, cGMP, and cyclic nucleotide gated ion channels.
Despite being well positioned to serve as the circadian photoreceptive molecule, melanopsin is in fact not the only photopigment involved in circadian photoreception. Melanopsin-deficient mice have a reduction in the effects of light on phase shifting, negative masking of locomotor activity, and pupillary responses at high light intensities, but these responses to light are not completely absent (Provencio, 2011). Importantly, mice that lack rod and cone function and also lack melanopsin appear completely blind with respect to both image formation and nonimage forming responses to light (Fig. 39.11). Rods and cones can signal through the melanopsin-expressing RGC’s, even if the latter cells are melanopsin-deficient. Destruction of the melanopsin-expressing cells, however, blocks nonvisual photoreception (Güler et al., 2008; Hatori et al., 2008). Thus, rod-cone input converges on the melanopsin-containing retinal ganglion cells, and parallel pathways that control different nonvisual image formation functions arise from defined subsets of these ipRGCs (Schmidt et al., 2011).

Figure 39.11 Rods, cones, and intrinsically photosensitive retinal ganglion cells expressing melanopsin mediate circadian entrainment. (A) is a double-plotted actogram of a control mouse showing normal entrainment to the light:dark cycle. (B) shows a double-plotted actogram from a triple-mutant mouse, lacking rod and cone phototransduction mechanisms and also lacking melanopsin. Triple-mutant animals free-run as though there is no lighting cycle. Triple-mutant animals also lack pupillary constriction responses and acute inhibition of locomotor activity by light (Hattar et al., 2003). The disruption of rod and cone signaling is accomplished by breeding to generate animals homozygous for disruption of the alpha subunit of rod transducin and the cone cyclic nucleotide gated channel A3 subunit. Actograms provided by Samer Hattar, Johns Hopkins University.
Retinal Input to the SCN: Anatomy and Neurochemistry
The melanopsin-expressing retinal ganglion cells that project to the SCN use glutamate as their primary neurotransmitter. A large percentage of these retinal neurons also contain the neuropeptide Pituitary Adenylate Cyclase Activating Peptide (PACAP). Application of these agents to SCN slices in vitro causes phase-dependent phase shifts of intrinsic SCN rhythms. Furthermore, glutamate receptor antagonists block light-induced phase shifts in vivo, revealing the functional importance of glutamatergic input to the SCN.
Axon collaterals of the melanopsin-positive retinal ganglion cells innervate neurons in the intergeniculate leaflet (IGL) that express neuropeptide Y (NPY). These NPY-containing neurons project to the SCN as the geniculohypothalamic tract. The geniculohypothalamic tract is thought to play a major role in nonphotic phase shifting, a phenomenon in which behavioral arousal (usually accompanied by intense locomotor activity) during the circadian day leads to increased NPY release within the SCN and leads to a phase shift of activity rhythms. The indirect retinal pathway through the intergeniculate leaflet also appears to modulate photic entrainment. The direct retino-hypothalamic tract and the indirect retino-geniculohypothalamic pathway converge in the SCN, in the “core” region defined by the dense retinal input and by a subpopulation of neurons expressing vasoactive intestinal peptide (VIP). Another major input to the SCN core is a serotonergic input from the raphe nuclei, which modulates the impact of light input to the SCN through presynaptic serotonin receptors on retinohypothalamic tract terminals.
Molecular Mechanisms of Entrainment: Light-Induced Per Expression
Per1 and Per2 gene expression levels are rhythmic in the SCN, with high levels during the daytime (Fig. 39.7). These molecular rhythms persist in constant darkness. Exposure to light at night causes a large, transient increase in both Per1 and Per2 transcript levels. Per gene expression in the SCN is unresponsive to light exposure during the subjective daytime, when light exposure does not cause phase shifts. The temporally “gated” induction of Per1 and Per2 gene expression is likely mediated by activation of MAP kinase and CREB phosphorylation by retinal input, leading to induction of Per1 and Per2 expression through Ca2+/cAMP response elements (CRE) in the promoters of these genes. (A third Period gene, Per3, lacks a promoter CRE element and is unresponsive to light even at night. Per3 has, at most, a very modest contribution to the core circadian oscillatory mechanism or the resetting mechanism).
Increased Per1 and Per2 expression are the only widely reproducible changes in clock gene expression that occur in response to light, focusing attention on these genes as important transducers of the effects of light on the molecular clock. Studies in mutant mice reveal that neither gene is necessary for phase shifting responses to light, however (Pendergast et al., 2010). Per1 and Per2 may be redundant in function for phase shifting, with either gene able to mediate the re-setting response in the absence of the other.
Peak nuclear PER1 and PER2 protein levels occur at the time of the light-to-dark transition (Fig. 39.7). Light-induced increases in PER1 and PER2 protein early in the night would extend the period of transcriptional inhibition by the PER/CRY complex. This may be the mechanism by which light exposure early in the night causes a phase delay. In contrast, light-induced PER expression late in the night would lead to an earlier-than-normal initiation of PER/CRY-mediated inhibition, resulting in an advance of SCN molecular rhythmicity in subsequent cycles. Thus, light-induced alterations of PER expression and interaction of the light-induced PER with the molecular feedback loop may explain the phase-dependent phase shifting effect of light shown in Figure 39.9.
While light does not influence Per levels during the animal’s subjective daytime and does not cause phase shifts at this time, nonphotic manipulations can cause phase shifts when administered during the daytime (e.g., novelty-induced voluntary locomotor activity, arousal, serotonin receptor activation). These manipulations lead to reduced Per gene expression in the SCN. Thus, acute regulation of Per gene expression may be the mechanism by which a variety of manipulations affect circadian phase.
Summary
The circadian clock in the SCN is entrained to the daily light-dark cycle by light causing phase-dependent phase shifts each day. While the retinae are required for entrainment, the retinal photoreceptors necessary for visual image formation, the rods and cones, are not needed. Instead, a population of intrinsically photoreceptive retinal ganglion cells conveys information about the level of light to the SCN and to other brain regions that regulate nonvisual photoreception. The photopigment in these retinal ganglion cells is melanopsin, and they utilize glutamate and PACAP as their transmitters. In the absence of melanopsin, rods and cones appear capable of signaling through the ipRGC’s to result in nonvisual responses to light, including entrainment. Specific subsets of melanopsin-expressing cells have distinct functions. The impact of nocturnal light exposure on SCN physiology includes numerous signaling responses and induction of Per gene expression. Light-induced alterations in Per gene expression likely provide a key role in resetting of the molecular feedback loop within SCN neurons.
Circadian Output Mechanisms
From Molecular Feedback Loops to Firing Rate Rhythms
The molecular feedback loop within individual SCN neurons ultimately has its impact on physiological processes through regulation of SCN neuronal firing rate (Kuhlman & McMahon, 2006). Rhythmic regulation of potassium channels in the membrane of SCN neurons appears to be critical for producing circadian rhythms in membrane potential and firing rate.
Large-conductance, calcium-activated potassium channels (BK channels) are rhythmically expressed in the SCN, with high levels of transcript levels and BK channel activity during the night contributing to the reduction in firing rate at night (Meredith et al., 2006). Disruption of the BK channel (Kcnma1-/-) results in higher SCN firing rate selectively at night, and the mutant mice have greatly reduced amplitude of several circadian output rhythms.
In addition, the fast delayed rectifier (FDR) current is critical for robust circadian rhythms in behavior. The Kv3.1 and Kv3.2 potassium channels and their activity are more abundant in the SCN during the circadian daytime, and blocking their activity reduces SCN rhythmicity (Itri, Michel, Vansteensel, Meijer, & Colwell, 2005). Mice lacking both the Kcnc1 and Kcnc2 genes do not express the Kv3.1 and 3.2 channels in the SCN, have greatly reduced FDR current, and have severely disrupted circadian behavioral rhythms (Kudo, Kuljis, Constance, & Colwell, 2011). It thus appears that multiple ionic mechanisms contribute to rhythmicity in SCN neuronal firing rate, and regulation of channel activity is probably accomplished through both transcriptional and post-transcriptional mechanisms.
Rhythmic firing of SCN neurons not only serves as its mechanism of communication with downstream target regions, but also plays an important role in synchronizing the cellular oscillators within the SCN to each other. When sodium-dependent action potentials are blocked for several days, SCN neurons become desynchronized from one another. The mechanism for this may be as simple as the loss of rhythmic, synaptic release of neurotransmitters, including VIP. Indeed, synaptic vesicle cycling plays an important role in the regulation of circadian rhythms (Deery et al., 2009).
Humoral Outputs from the SCN Also Influence Locomotor Activity Rhythms
The rhythmic electrical activity of SCN neurons likely leads to rhythmic neurotransmitter release and modulation of downstream, synaptic targets along defined anatomical pathways as discussed in greater detail below. Several lines of evidence indicate that the SCN also influences locomotor activity through secretion of humoral output molecules. Among these lines of evidence are the findings that (1) transplants of fetal SCN tissue that restore locomotor activity rhythms do not restore other rhythms, including endocrine rhythms, and (2) transplanted SCN tissue encapsulated so as to allow diffusion of small molecules while preventing axonal outgrowth nevertheless can restore locomotor activity rhythms in SCN-lesioned hamsters (Silver, LeSauter, Tresco, & Lehman, 1996). The latter finding suggests that substances secreted by the SCN act in regions near the SCN (but not in the SCN itself) to regulate locomotor activity. Three substances proposed to play a role in regulating locomotor activity rhythms in rodents are transforming growth factor alpha, cardiotropin-like cytokine 1, and prokineticin 2.
Summary
For oscillations in the abundance of circadian clock gene products in SCN neurons to affect physiology and behavior, the molecular feedback loop must impact on more overt aspects of cellular rhythmicity. One mechanism for this to occur is through circadian regulation of transcripts encoding ion channels; indeed, potassium channels have been identified that contribute to circadian rhythmicity. Through regulation of potassium channel activity, rhythmic transcription factor activity is translated into rhythmicity in neuronal firing rate and neurotransmitter release. While much of the output of the SCN comes via synaptic interactions to downstream targets, substances released from the SCN by a humoral mechanism can also influence locomotor activity.
Diversity of Output Pathways Leading to Physiological Rhythms
Animals that lack circadian rhythms (those with destruction of the SCN or genetic disruption of key circadian clock genes) survive, and these animals generally can reproduce. Nevertheless, the circadian timing system is thought to optimize “internal temporal order” by coordinating rhythms in different tissues, and to allow anticipation of physiological demands imposed by the predictably changing environment. The impact of the circadian oscillator is exerted through the rhythmicity it imposes on physiological functions.
The following sections address the regulation of several physiological rhythms. In most cases, we emphasize how these rhythms are “wired” to the SCN through neuroanatomical pathways and emphasize rhythms for which the SCN can be viewed as a driving pacemaker. It is important to note that SCN-generated physiological rhythms can also entrain peripheral oscillators, without a direct SCN-to-target output (Kornmann et al., 2007; Vollmers et al., 2009; Buhr, Yoo, Takahashi, 2010; see Fig. 39.3). Subsequent tissue-autonomous rhythmicity can profoundly influence physiology and response to perturbations (Box 39.4) (Kornmann et al., 2007; Marcheva et al., 2010; Sadacca, Lamia, deLemos, Blum, & Weitz, 2011)
Box 39.4 Chronopharmacology
Many drugs, toxins, and stressors have effects that vary over the 24-hour day. Many of these variations represent true circadian rhythms in responses—for example, they persist in constant environmental conditions. Chronopharmacology is the subdiscipline of chronobiology that seeks to identify and understand these rhythms, and it is an important field, since altering the time of drug administration can have a profound impact on its efficacy, toxicity, and the ratio between these two measures (the therapeutic index). For this reason, there is often a specific time for optimal response to a chemotherapeutic agent, and this optimal timing varies between agents depending on their mechanism of action. Rhythmicity in drug effects could be due to alterations in bioavailability (absorption, metabolism, or excretion) or in end organ sensitivity. Rhythmic variation in therapeutic index has been well documented for several chemotherapeutic agents and anesthetics. There are also robust rhythms in responses to toxic agents, including infectious agents such as E. coli and endotoxin derived from bacterial cell walls. Recent studies have begun to unravel the molecular mechanisms underlying rhythms in detoxification and drug sensitivity, and focus attention on rhythmicity within target cell populations.
David R. Weaver
Further Readings
1. Gachon F, Firsov D. The role of the circadian timing system on drug metabolism and detoxification. Expert Opin Drug Metab Toxicol. 2011;7:147–158.
2. Innominato PF, Lévi FA, Bjarnason GA. Chronotherapy and the molecular clock: Clinical implications in oncology. Advance Drug Delivery Review. 2010;62:979–1001.
3. Lévi F, Schibler U. Circadian rhythms: Mechanisms and therapeutic implications. Annual Review of Pharmacology Toxicology. 2007;47:593–628.
4. Lévi F, Filipski E, Iurisci I, Li XM, Innominato P. Cross-talks between circadian timing and cell division cycle determine cancer biology and therapeutics. Cold Spring Harbor Symposia Quantitative Biology. 2007;72:465–475.
5. Paschos GK, Baggs JE, Hogenesch JB, FitzGerald GA. The role of clock genes in pharmacology. Annual Review Pharmacology Toxicology. 2011;50:187–214.
Amplification of SCN Output and Divergence of Output Pathways
Considering the pervasive influence of the SCN on rhythmic physiology, the direct anatomical output of the SCN is surprisingly limited. SCN efferents project heavily to a column of cells dorsal and caudal of the SCN, including the subparaventricular zone (SPZ) and the dorsomedial nucleus of the hypothalamus (DMH) (Figs. 39.12 and 39.13). Neurons in the SPZ and DMH in turn project to brain regions that ultimately regulate rhythmic activities (Fig. 39.13). Many effector regions receive a small, direct innervation from the SCN that converges with the larger, indirect output via the SPZ. The SPZ thus appears to relay and reinforce the direct output of the SCN, and allows convergence of influences from other brain areas. Lesions that kill cell bodies in the ventral SPZ (while leaving axons passing through the region intact) disrupt the circadian rhythms of sleep/wakefulness and locomotor activity (Lu et al., 2001). Amplification of SCN output in the vSPZ is thus necessary for these rhythms; direct SCN output to target sites is insufficient. In a similar manner, fiber-sparing lesions of the dorsal part of the SPZ (dSPZ) selectively disrupt the body temperature rhythm while leaving sleep and activity rhythms intact.
Figure 39.12 Distribution of vasoactive intestinal peptide (VIP) immunoreactivity in the rat brain reveals the major output pathways from the SCN. Staining reveals VIP-immunoreactive cell bodies in the SCN and their projections. The major projection of the SCN is to a cell column dorsal and caudal of the SCN, including the subparaventricular zone (SPZ) and the dorsomedial nucleus of the hypothalamus (DMH).
Reprinted from Lu et al., 2001, with permission from the Society for Neuroscience.
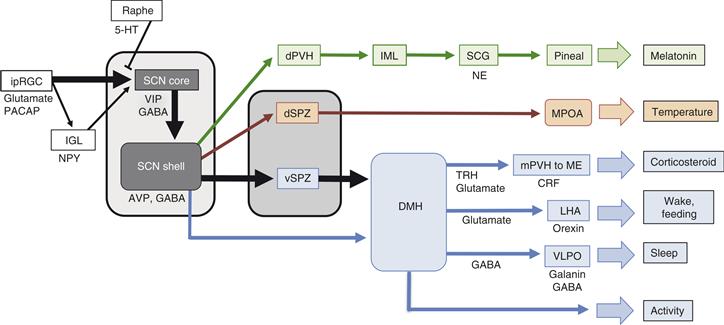
Figure 39.13 Schematic of the rodent circadian system, with emphasis on the divergence of output pathways. Rhythmically regulated events are listed on the right, and the pathways leading to their circadian regulation are shown.
Major inputs to the SCN are the melanopsin-containing ipRGC’s, neuropeptide Y (NPY)-containing neurons in the intergeniculate leaflet (IGL), and serotonergic afferents from the raphe that influence the RHT input. Inputs converge primarily in the core region, although many additional regions project into the SCN (not shown). The SCN core region projects extensively to the shell. The major output of the SCN arises from the shell and is directed caudally to the subparaventricular zone (SPZ). The dorsal subparaventricular zone (dSPZ) projects to the medial preoptic area (MPOA), and is important for circadian regulation of core body temperature. Axons from the ventral SPZ (vSPZ) and direct SCN projections converge in the dorsomedial hypothalamic nucleus (DMH). From the DMH arise distinct projections critical for the circadian regulation of the hypothalamic-pituitary-adrenal axis, sleep/wake and feeding; the next anatomical site along each of these pathways is indicated. The DMH is also critical for regulation of locomotor activity rhythms, but effector structures are not clear. Regulation of the adrenal rhythm and of pineal melatonin secretion occur through the medial parvicellular paraventricular nucleus (mPVH) and dorsal parvicellular PVH (dPVH), respectively. Where known, neurotransmitters are indicated. Abbreviations: LHA, lateral hypothalamic area; VLPO, ventrolateral preoptic area; ME, median eminence; NE, norepinephrine; PACAP, Pituitary adenylate cyclase activating peptide; TRH, thyrotropin releasing hormone.
Another level of amplification and integration occurs in the DMH, which is heavily innervated by the ventral SPZ as well as receiving direct innervation from the SCN (Figs. 39.12 and 39.13). Lesions of the DMH specifically disrupt circadian rhythms of sleep, locomotor activity, corticosterone secretion and feeding, but not body temperature (Chou et al., 2003).
Circadian Regulation of Sleep and Wake
Among the most widely recognized roles of the circadian timing system is in the regulation of behavioral state. Our level of alertness is strongly influenced by two biological factors: the time previously spent awake and the circadian time (see Chapter 40). There is a strong and opposing influence of circadian time and the time spent awake, a phenomenon described as the “opponent-process” model for behavioral state control. With increasing time awake, subjects experience a greater homeostatic drive to sleep. The circadian timing system opposes this drive, by providing an arousing signal that reaches peak levels late in the afternoon/early evening in humans.
The influence of the circadian timing system on sleep is mediated by SCN efferents that indirectly reach the brain regions regulating sleep. As noted above, a pathway from the SCN via the vSPZ and DMH is necessary for circadian regulation of sleep. In contrast to the sparse connections of the SPZ to sleep-regulatory areas, the DMH is a primary source of input to the ventrolateral preoptic area (VLPO) and to the neurons of the lateral hypothalamus that secrete the neuropeptide orexin, that are critical for regulation of sleep. The output from the DMH to the VLPO is GABAergic, therefore promoting wakefulness by inhibiting the “sleep switch” neurons. The output from the DMH to the orexin neurons in the lateral hypothalamus contains glutamate and thyrotropin-releasing hormone, and is presumably excitatory, thus promoting wakefulness. While the DMH has few direct projections to the brainstem areas that comprise the ascending arousal system, the orexin neurons have extensive, excitatory projections to these regions (Saper, Lu, Chou, & Gooley, 2005). Thus, a multistage pathway from the SCN to sleep regulatory centers allows integration of other inputs (emotional and cognitive inputs as well as the homeostatic drive for sleep) and promotes sharp transitions between states, rather than direct control of behavioral state. The importance of orexin neurons in coordinating the activity of this system is revealed by narcolepsy, a sleep disorder caused by autoimmune destruction of the orexin-containing neurons in the lateral hypothalamus, and mimicked in animal models by disruption of orexin or orexin receptors (see Chapter 40).
One relatively small subclass of sleep disorders are the circadian-based sleep disorders (Box 39.3). While most insomnias are due to difficulty in initiation or maintenance of sleep, or are related to airway patency during sleep (sleep apnea), circadian-based sleep disorders involve problems with the relative timing of sleep. Transient disruption of sleep often occurs with attempts to shift the relative timing of sleep, as occur in shift work and jet lag (Box 39.5)
Box 39.5 Jet Lag and Shift Work
Jet lag is induced by flying across several time zones and represents the lack of coordination of the flyer’s body to the new local time. Individuals usually entrain to a phase-shifted environment at the rate of about 1 hour per day, although careful control over when light exposure occurs may improve this rate.
Another environmentally imposed disruption of circadian rhythms occurs with shift work. Most people working the night shift remain entrained to the solar day, so that their biological rhythms (e.g., alertness, temperature and hormonal rhythms) are in opposition to the demands of the work schedule. Some of the negative health effects of shift work may be due to the stress caused by trying to sleep when the biological clock dictates it is time to be awake, and trying to be alert and productive when the body expects rest. Clear performance deficits occur when individuals attempt to work for extended periods, ignoring the need for sleep. Performance deficits also occur at specific times of day, irrespective of prior sleep history.
In rodent models, repeated shifting of the light-dark cycle, as occurs with jet lag or shift work, can have significant adverse consequences on health. Circadian clocks normally coordinate internal rhythms to each other and to the environment. Disrupting this coordination may similarly have adverse consequences on human health.
David R. Weaver
Further Readings
1. Castanon-Cervantes O, Wu M, Ehlen JC, et al. Dysregulation of inflammatory responses by chronic circadian disruption. Journal of Immunology. 2010;185:5796–5805.
2. Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Current Biology. 2006;16:R914–916.
3. Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2008;295:R2034–2040.
4. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4453–4458.
5. Wang X-S, Armstrong MEG, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occupational Medicine (Lond). 2011;61:78–89.
6. Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2008;294:R1675–1683.
Circadian Regulation of Neuroendocrine Functions
The SCN output pathway leading to rhythms in adrenal corticosteroid secretion starts with a projection to the paraventricular nucleus of the hypothalamus (PVN). A subset of neurons in the PVN contain corticotrophin releasing hormone (CRH), and project to the external zone of the median eminence. CRH, released into the pituitary portal system, regulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH, in turn, is carried in the bloodstream to the adrenal gland, where it stimulates secretion of adrenal steroids. Adrenal steroids feed back to inhibit CRH and ACTH release in a typical neuroendocrine feedback loop (see Chapter 38). This feedback loop is modulated by the circadian timing system, however: the set point for steroid feedback apparently varies over the course of the day (e.g., higher corticosteroid levels are required to reduce ACTH release at some times of day than at others). As a result, corticosteroid levels vary over the course of the day with a sinusoidal rhythm. Light exposure at night can activate the sympathetic innervation of the adrenal gland via the SCN, bypassing the pituitary and directly increasing corticosteroid production (Ishida et al., 2005).
The SCN also regulates circadian rhythms in other neuroendocrine activities. In rodents, the timing of ovulation is strictly regulated and occurs early in the active period on the day of behavioral estrus (de la Iglesia & Schwartz, 2006). Ovulation is blocked by injection of the anesthetic pentobarbital early in the afternoon on the day of estrus. Remarkably, ovulation then occurs the following day—exactly 24 hours after it should have occurred. Ovariectomized, estradiol-treated female rats also have daily surges of luteinizing hormone in the late afternoon. This is the neuroendocrine equivalent of ovulation, occurring in the absence of the ovaries. These luteinizing hormone surges persist when animals are housed in constant darkness and are not present in animals with SCN lesions. An SCN-dependent neuronal output leads to daily activation of gonadotropin releasing hormone-containing neurons (de la Iglesia, Meyer, Schwartz, 2003).
Twice-daily prolactin surges can be induced in female rats by mating (or by vagino-cervical stimulation that mimics mating, induces pseudopregnancy). The prolactin surges are timed by the SCN. These prolactin surges are ultimately regulated by dopamine released from the median eminence. Feedback of prolactin on the hypothalamic dopamine neurons is also important. VIP neurons in the SCN and oxytocin neurons in the PVN appear to be involved in regulating the hypothalamic dopamine neurons and thus the prolactin surges (Egli, Bertram, Sellix, Freeman, 2004).
Circadian Regulation of Autonomic Function
Many physiological rhythms are regulated by autonomic function. Pathways resulting in rhythmic autonomic activity originate in the SCN, utilize GABA as a neurotransmitter, and generally project to the paraventricular nucleus of the hypothalamus (PVN). Neurons in the PVN (many containing oxytocin) have direct projections to the spinal cord, where they regulate the activity of autonomic preganglionic neurons. Additional pathways include modulation of vagal activity. These pathways regulate autonomic responses leading to circadian variation in blood pressure and heart rate. These pathways also influence metabolism through direct innervation of fat pads and the liver, the latter of which contributes to the circadian rhythm in plasma glucose levels (Cailotto et al., 2005; Kalsbeek et al., 2006).
Circadian Regulation of Pineal Function
One very well-characterized output rhythm regulated via the autonomic nervous system is the rhythmic production of melatonin. This rhythm is regulated by an anatomical pathway pathway from the SCN via the PVN to the pineal gland (Fig. 39.14A). GABA released from SCN neurons inhibits neurons in the PVN during the day, while the reduction in SCN GABA release at night apparently allows the PVN neurons to be more active, driving melatonin synthesis (Kalsbeek et al., 2000). The PVN neurons project to autonomic preganglionic neurons in the intermediolateral cell column of the spinal cord. The preganglionic neurons project to the superior cervical ganglion, whose fibers innervate the pineal gland. Norepinephrine from these fibers increases the activity of the penultimate enzyme in melatonin biosynthesis, arylalkylamine N-acetyltransferase (AANAT), leading to melatonin synthesis (Fig. 39.14B). AANAT activity is controlled by a combination of transcriptional mechanisms and switch-like regulation of proteasomal degradation of AANAT protein.
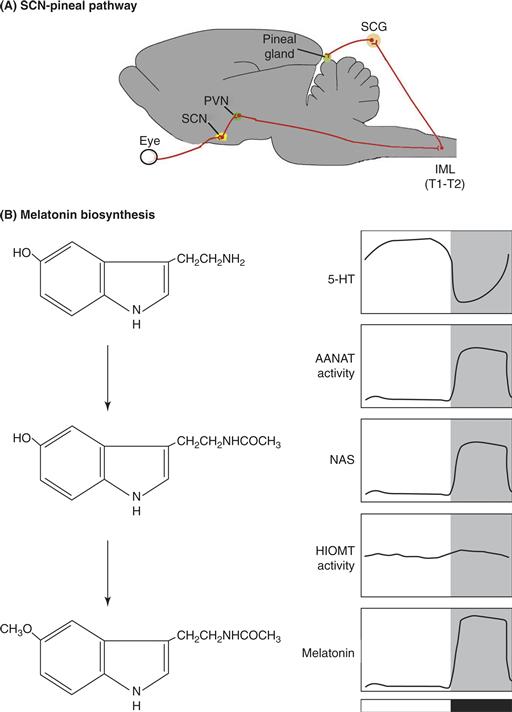
Figure 39.14 (A) Schematic illustration of the neuroanatomical circuit regulating pineal melatonin secretion. Photic input detected in the retina is relayed to the suprachiasmatic nucleus (SCN), and then to the paraventricular nucleus of the hypothalamus (PVN). A subset of PVN neurons projects to the intermediolateral cell column (IML) of the spinal cord. Preganglionic sympathetic cell bodies at thoracic levels T1 and T2 project to the superior cervical ganglion (SCG), which innervates the pineal gland. Norepinephrine released from the terminals of SCG neurons is the primary input to the pineal responsible for stimulating melatonin production at night. Light entrains the SCN clock controlling melatonin, but light at night also acutely inhibits melatonin synthesis by disrupting the sympathetic input to the gland. Blood melatonin levels drop within minutes after exposure to light at night. (B) Biosynthesis of melatonin from serotonin (5-hydroxytryptamine, 5-HT). The rate-limiting and highly rhythmic step is the regulation of arylalkylamine N-acetyltransferase activity, which modestly depletes the pineal gland of 5-HT at night as it converts 5HT to N-acetyl-serotonin (NAS). Hydroxy-indole-O-methyltransferase (HIOMT) converts NAS to melatonin (N-acetyl,5-methoxytryptamine). There is no storage mechanism for melatonin; melatonin diffuses into the cerebrospinal fluid and bloodstream as it is produced, making blood melatonin levels a good reflection of the sympathetic input to the pineal gland.
Circadian Regulation of Melatonin Is Critical for Seasonal Reproduction
Several strategies exist to deal with the varying environmental conditions and varying levels of resources that occur over the course of the year. These strategies include seasonal migration to allow highly mobile species to escape challenging conditions (Box 39.6), and, for those species that remain, seasonal regulation of physiology and behavioral adaptation (see Box 39.1).
Box 39.6 Lost Without a clock
A most fascinating function of circadian clocks is their use in time-compensated sun compass orientation, a phenomenon that was first described by Karl von Frisch in foraging honeybees and by Gustav Kramer in migratory birds. Time-compensated sun compass orientation is also used by eastern North American monarch butterflies (Danaus plexippus) during their spectacular fall migration. During the fall journey, the butterflies use their navigation skills to fly south from the United States and southern Canada to overwinter in a few restricted sites atop the Transvolcanic Mountains in central Mexico, some having traveled distances approaching 4000 kilometers.
The amazing navigation abilities of monarch butterflies are part of a genetic program initiated in the migrants, as those that make the trip south are at least two generations removed from the previous generation of migrants. Time-compensated sun compass orientation is believed to be an essential component of navigation required for successful migration. Time compensation is provided by the butterfly’s circadian clock. The clock allows the butterflies to continually correct their flight direction, relative to changing skylight parameters, to maintain a fixed flight bearing as the sun moves across the sky during the day (Fig. B39.6A). The importance of the circadian clock in regulating the time-compensated component of flight orientation in monarch butterflies has been shown in two ways. First, clock-shift experiments, in which the light-dark cycle is either advanced or delayed, cause predictable alterations in the direction the butterflies fly (Fig. B39.6B). Second, removing the time compensation clocks abolishes the time-compensated component of flight orientation (Fig. B39.6C). It is thus clear that time compensation is a requirement for successful migration.
Figure B39.6 (A) Monarch butterflies use a time-compensated sun compass to orient south during their fall migration. The butterfly circadian clock allows the butterflies to compensate for the movement of the sun. They are thereby able to maintain a constant bearing in the southerly direction over the course of the day. (B) Clock-shift experiment. When monarch butterflies are housed in a light-dark cycle that is advance by 6 hours, relative to ambient lighting, the butterflies perceive the 10:00 a.m. sun to be a 4:00 p.m. sun and shift their orientation counterclockwise to the southeast. (C) Removal of the time-compensation clocks, which reside in the antennae and not in brain, disrupts the migration south, and the butterflies are not able to successfully travel to their overwintering grounds. The arrows depict disrupted flight directions.
Steven M. Reppert
Further Reading
1. Reppert SM, Gegear RJ, Merlin C. Navigational mechanisms of migrating monarch butterflies. TINS. 2010;33:399–406.
Many mammalian species restrict breeding activity to specific times of year, resulting in birth of young in the spring and summer months when food is abundant and conditions are most favorable for their survival. Responses to seasonal changes in day length (so-called “photoperiodic responses”) include seasonal reproduction, but also extend to an array of physiological responses including seasonal molting of the coat (mediated by seasonal alterations in prolactin secretion), thermoregulatory adjustments (daily torpor and hibernation), alterations in metabolism and body weight, and alterations in behavior. These adjustments improve the probability of survival through challenging times. Like circadian rhythms, these seasonal responses are driven by a system that anticipates predictable environmental changes and prepares for them in a predictive, rather than reactive, manner.
The SCN-controlled circadian rhythm of pineal melatonin production controls photoperiodic responses in mammals. Melatonin is secreted into the bloodstream at night by the pineal gland. The nocturnal duration of melatonin secretion changes in parallel with the length of the night: the melatonin secretory duration thus encodes season. Removal of the pineal gland prevents seasonal responses to changes in day length, while administration of melatonin in patterns that mimic the profiles occurring at different times of year can predictably drive reproduction and other photoperiodic responses.
The actions of melatonin are mediated by specific, G-protein coupled receptors. Two receptors subtypes with high affinity for melatonin have been identified in mammals. The MT1 (Mel1a) receptor subtype is responsible for the majority of melatonin receptor binding found in SCN and elsewhere in brain, while the MT2 (Mel1b) receptor is expressed at considerably lower levels. Several photoperiodic species lack a functional MT2 receptor gene, suggesting that the MT1 receptor may be key for regulating seasonal responses.
A site containing a high concentration of MT1 receptors is the pituitary pars tuberalis (PT). Melatonin acting in the PT affects prolactin release from the anterior pituitary gland. In addition, melatonin acts in the PT to affect gonadotrophin secretion by a circuitous route that has just been unraveled. Melatonin regulates thyroid stimulating hormone (TSH) beta expression in the PT by controlling the expression of Eya3. This transcriptional coactivator interacts with the circadian transcription factor, TEF, which binds to a D-box in the TSHβ promoter, stimulating expression (Dardente et al., 2010). TSH from the PT then acts through TSH receptors located in the mediobasal hypothalamus, altering the balance between synthetic and catabolic enzymes regulating thyroid hormone. A local increase in the biologically active form of thyroid hormone, T3, within the mediobasal hypothalamus promotes GnRH secretion, leading to gonadotropin secretion and reproduction function. Melatonin, by regulating this local TSH-to-T3 cascade, has a profound impact on reproductive function in seasonally breeding species.
Summary
The proximate output of the SCN is a rhythm of neuronal firing, which results in rhythmic synaptic and perhaps humoral activation of additional brain regions. The major SCN target is a region dorsal and caudal of the SCN, including the subparaventricular zone (SPZ) and extending into the dorsomedial hypothalamic nucleus (DMH). This extensive projection appears to amplify SCN synaptic output and provides a mechanism for the SCN to influence a wide variety of downstream targets in a manner that can be site-specific. The anatomical pathways leading to specific behavioral and physiological rhythms have been defined to varying extents. Already within the subparaventricular zone, there is divergence of the output pathway controlling rhythms in sleep/wake and locomotor activity (which requires the ventral SPZ) from the pathway regulating body temperature (which requires the dorsal SPZ).
One of the most extensively studied outputs is the pathway regulating melatonin production, involving the SCN, PVN, and sympathetic innervation of the pineal gland. The circadian regulation of melatonin secretory duration regulates reproduction and other photoperiodic responses in seasonally breeding species. The regulation of photoperiodic responses is the most obviously adaptive role for circadian rhythmicity in mammals.
General Summary
Behavioral and physiological rhythmicity is widespread throughout the animal kingdom. A capacity for cell-autonomous rhythmicity exists in many cell types. Circadian rhythmicity in vertebrates and flies is based on an intracellular molecular feedback loop. Many components of this molecular feedback loop have been elucidated in the past 20 years. There is frequently redundancy of function between closely related genes in mammals. The SCN serve as the coordinating pacemaker in the mammalian circadian timing system. In the absence of the SCN, rhythms in other tissues generally degrade because the synchronization between oscillators is lost. The SCN clock is entrained primarily by light. Lighting information is detected by a specific luminance-coding retinal system that involves intrinsically photosensitive retinal ganglion cells that also serve as a conduit for information originating in rod and cone photoreceptors. SCN axonal output is focused dorso-caudally to the subparaventricular zone, and then diverges along specific anatomical pathways to control a diverse array of rhythmic functions. Among the most physiologically important output rhythms in mammals is the regulation of pineal melatonin, as this hormone is key to regulating photoperiodic adjustments in response to changing day length. Other circadian outputs are of less obvious survival value, but an increasing understanding of the importance of the circadian influence on sleep/wake, metabolism, cardiovascular and neuroendocrine function, and resistance to toxicological challenges indicates that the circadian system dictates an “internal temporal order” that improves physiological and mental performance.
References
1. Aton SJ, Herzog ED. Come together, right … now: Synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534.
2. Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature Neuroscience. 2005;8:476–483.
3. Atkinson SE, Maywood ES, Chesham JE, et al. Cyclic AMP signaling control of action potential firing rate and molecular circadian pacemaking in the suprachiasmatic nucleus. Journal of Biological Rhythms. 2011;26:210–220.
4. Atwood A, DeConde R, Wang SS, et al. Cell-autonomous circadian clock of hepatocytes drives rhythms in transcription and polyamine synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18560–18565.
5. Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937.
6. Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Medicine. 2007;8:566–577.
7. Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385.
8. Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017.
9. Cailotto C, La Fleur SE, Van Heijningen C, et al. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: Are the clock genes involved?. European Journal of Neuroscience. 2005;22:2531–2540.
10. Chen S-K, Badea TC, Hattar S. Photoentrainment and pupillary light reflexes are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95.
11. Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. Journal of Neuroscience. 2003;23:10691–10702.
12. Czeisler CA, Buxton OM, Khalsa SBS. The humans circadian timning system and sleep-wake regulation. In: Kryger M, Roth T, Dement W, eds. Principles and practice of sleep medicine. 4th ed New York: WB Saunders; 2005;375–394.
13. Dardente H, Wyse CA, Birnie MJ, et al. A molecular switch for photoperiod responsiveness in mammals. Current Biology. 2010;20:2193–2198.
14. DeBruyne JP. Oscillating perceptions: The ups and downs of the CLOCK protein in the mouse circadian system. Journal of Genetics. 2008;87:437–446.
15. DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nature Neuroscience. 2007;10:543–545.
16. Deery MJ, Maywood ES, Chesham JE, et al. Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Current Biologly. 2009;19:2031–2036.
17. de la Iglesia HO, Schwartz WJ. Timely ovulation: Circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153.
18. de la Iglesia HO, Meyer J, Carpino Jr A, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801.
19. de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. Journal of Neuroscience. 2003;23:7412–7414.
20. Ecker JL, Dumitrescu ON, Wong KY, et al. Melanopsin-expressing retinal ganglion cell photoreceptors: Cellular diversity and role in pattern vision. Neuron. 2010;67:49–60.
21. Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: Action of oxytocin coordinated by vasoactive intestinal polypeptide of uprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–3394.
22. Gatfield D, Schibler U. Proteasomes keep the circadian clock ticking. Science. 2007;316:1135–1136.
23. Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. Journal of Neuroscience. 2004;24:615–619.
24. Güler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105.
25. Hastings MH, Field MD, Maywood ES, Weaver DR, Reppert SM. Differential regulation of mPER1 and mTIM proteins in mouse suprachiasmatic nuclei: new insights into a core clock mechanism. Journal of Neuroscience. 1999;19:RC11.
26. Hatori M, Le H, Vollmers C, et al. Indicuble ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451.
27. Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81.
28. Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology. 2006;497:326–349.
29. Helfrich-Förster C. PDF has found its receptor. Neuron. 2005;48:161–163.
30. Ishida A, Mutoh T, Ueyama T, et al. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metabolism. 2005;2:297–307.
31. Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nature Neuroscience. 2005;8:650–656.
32. Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68.
33. Kalsbeek A, Garidou ML, Palm IF, et al. Melatonin sees the light: Blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. European Journal of Neuroscience. 2000;12:3146–3154.
34. Kalsbeek A, Ruiter M, La Fleur SE, Cailotto C, Kreier F, Buijs RM. The hypothalamic clock and its control of glucose homeostasis. Progress in Brain Research. 2006;153:283–307.
35. Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34.
36. Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: Critical for input and output of the circadian system. Journal of Neuroscience. 2011;31:2746–2755.
37. Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. Journal of Biological Rhythms. 2006;21:470–481.
38. Liu AC, Welsh DK, Ko CH, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616.
39. Lu J, Zhang YH, Chou TC, et al. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. Journal of Neuroscience. 2001;21:4864–4874.
40. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631.
41. Maywood ES, Reddy AB, Wong GK, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Current Biology. 2006;16:599–605.
42. Meredith AL, Wiler SW, Miller BH, et al. BK calciumactivated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nature Neuroscience. 2006;9:1041–1049 1193.
43. Miller BH, McDearmon EL, Panda S, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3342–3347.
44. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research. 1972;42:201–206.
45. Morin LP. SCN organization reconsidered. J Biological Rhythms. 2007;22:3–13.
46. Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: Cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705.
47. Pendergast JS, Friday RC, Yamazaki S. Photic entrainment of Period mutant mice is predicted from their phase response curves. Journal of Neuroscience,. 2010;30:12179–12184.
48. Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science. 1997;278:1632–1635.
49. Provencio I. The hidden organ in our eyes. Scientific American. 2011;304:57–61.
50. Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:340–345.
51. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978.
52. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941.
53. Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124.
54. Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends in Neuroscience. 2005;28:152–157.
55. Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends in Neuroscience. 2011;34:572–580.
56. Shearman LP, Zylka MJ, Weaver DR, Kolakowski Jr LF, Reppert SM. Two Period homologs: Circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269.
57. Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; Functional replacement with its paralog, Bmal2. Current Biology. 2010;20:316–321.
58. Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813.
59. Silver R, Schwartz WJ. The suprachiasmatic nucleus is a functionally heterogeneous timekeeping organ. Methods Enzymol. 2005;393:451–465.
60. Stephan FK. The “other” circadian system: Food as a Zeitgeber. Journal of Biology Rhythms. 2002;17:284–292.
61. Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1583–1586.
62. Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. Jouranl of Biology Rhythms. 2006;21:185–194.
63. Ueda HR, Hayashi S, Chen W, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature Genetics. 2005;37:187–192.
64. Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21453–21458.
65. Weaver DR. The suprachiasmatic nucleus: A 25-year retrospective. Journal of Biology Rhythms. 1998;13:100–112.
66. Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706.
67. Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Current Biology. 2004;14:2289–2295.
68. Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annual Review of Physiology. 2010;72:551–577.
69. Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110.
Suggested Readings
1. Arendt J. Melatonin and the mammalian pineal gland London: Chapman & Hall; 1995; 1995.
2. Benca R, Duncan MJ, Frank E, McClung C, Nelson RJ, Vicentic A. Biological rhythms, higher brain function, and behavior: Gaps, opportunities, and challenges. Brain Research Reviews. 2009;62:57–70.
3. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annual Review of Physiology. 2010;72:517–549.
4. Dunlap JC, Loros J, DeCoursey PJ, eds. Chronobiology: Biological timekeeping. Sunderland, MA: Sinauer Associates; 2004.
5. Klein DC, Moore RY, Reppert SM, eds. Suprachiasmatic nucleus: The mind’s clock. New York: Oxford University Press; 1991.